- 1Aier Eye Institute, Changsha, China
- 2Aier Academy of Ophthalmology, Central South University, Changsha, Hunan, China
- 3Wellcome-Wolfson Institute for Experimental Medicine, School of Medicine, Dentistry and Biomedical Sciences, Queen’s University Belfast, Belfast, United Kingdom
High myopia is a global health concern, often leading to degenerative retinal changes known as myopic retinopathy. Although mechanical stress, hypoperfusion, extracellular matrix remodeling, and growth factor dysregulation have been implicated in the pathogenesis of myopic retinopathy, emerging evidence highlights the critical role of chronic low-grade inflammation. Both innate and adaptive immune systems participate in myopic retinopathy through systemic and local inflammation. Systemically, immune dysregulation is marked by elevated levels of complement proteins C3, autoantibodies anti-LIM and senesce nt cell antigen-like-containing domain protein 1 (anti-LIMS1), and altered circulating immune cells (increased neutrophils and basophils). Locally, retinal homeostasis disruption triggers intraocular inflammation, evidenced by higher levels of interleukin-6 (IL−6), IL−8, tumor necrosis factor α (TNF−α), C-C motif chemokine ligand-2 (CCL2), C−X−C motif chemokine ligand 10 (CXCL10) and activating the complement system. The inflammatory response involves signaling pathways such as JAK-STAT and complement cascades. This review summarizes recent advances in understanding immunological mechanisms underlying myopic retinopathy, offering insights to guide future research.
1 Introduction
Myopia is a refractive error where, with accommodation relaxed, parallel light rays focus in front of the retina. This typically results from axial length (axial myopia), but can also stem from excessive refractive power of the cornea or lens (1). Myopia has become a serious global public health concern, particularly in Asia (2–8), driven by lifestyle changes, including reduced time in outdoor activities and prolonged exposure to electronic devices (3). Given current trends, this upward trajectory in myopia prevalence is expected to persist. Epidemiological projections suggest that by 2050, nearly 50% of the global population will be affected by myopia, and approximately 10% will suffer from high myopia (9).
High myopia is associated with multiple degenerative changes within the posterior segment of the eye (10), which may progress to pathologic myopia. According to the International Myopia Institute (IMI), pathologic myopia is defined as excessive axial elongation of the eye associated with myopia, resulting in structural alterations within the posterior segment (1). A key complication is “myopic retinopathy,” encompassing a spectrum of retinal and choroidal changes, including myopic peripheral retinal degeneration (mPRD), posterior staphyloma (PS), leopard-pattern fundus, lacquer cracks, arcuate spots, chorioretinal atrophy, Fuchs spots, myopic rhegmatogenous retinal detachment (mRRD), myopic maculopathy (MM), and myopic choroidal neovascularization (mCNV) (10–12). Myopic retinopathy is influenced by a variety of parameters, including axial length (11, 13), degree of myopia (14), age (14–16), previous ocular disease history (17), choroidal thickness (18), as well as environmental and lifestyle factors (19–22). The pathophysiology of myopic retinopathy is complicated and not completely understood. Potential mechanisms include mechanical stress (11), choroidal hypoperfusion (23), aberrant extracellular matrix remodeling (24), inflammatory and oxidative stress responses (25, 26), dysregulated growth factor expression (27, 28), and dysfunction of the Bruch’s membrane–RPE–photoreceptor complex (29, 30). Currently, there is no effective treatment for this disease.
Immune response is a protective action of the immune system to harmful stimuli, such as pathogens, damaged cells, or irritants, aimed at eliminating the threat and initiating repair. However, when exaggerated or unresolved, it can become dysregulated, leading to tissue damage and pathologies (31). The retina maintains a delicate balance of cellular, metabolic, and structural homeostasis. Disruption of the balance, for example, due to oxygen and nutrient imbalance, waste accumulation, or interstitial fluid dysregulation, can trigger inflammation (32). Although the primary cause of myopic retinopathy is high myopia-mediated structural changes in the eye, growing evidence links inflammation to the onset and progression of retinal complications (33–37). We highlight recent advances in understanding the role of inflammation in myopic retinopathy, offering insights to guide future research.
2 Systemic inflammation and myopic retinopathy
The immune system safeguards the body, and its alterations can affect the health of the eye. Accumulating evidence suggests that systemic inflammation may contribute to the development and progression of myopia and myopic retinopathy (34). Studies have shown an increased prevalence of myopia in individuals with inflammatory or autoimmune diseases, such as allergic conjunctivitis, systemic lupus erythematosus, type 1 diabetes, and other chronic inflammatory disorders (14, 34, 38). The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in the peripheral blood of patients with high myopia are significantly increased (39). We reported a higher neutrophil fraction and an elevated NLR, together with lower absolute counts of lymphocytes, eosinophils, and platelets in people with myopic retinopathy (40). We further found that higher levels of circulating basophils are associated with severe myopic retinopathy, such as mCNV (40). This suggests that changes in circulating immune cells are associated with different degrees of myopic retinopathy. As retinal degeneration progresses, microglia are activated, which may recruit circulating immune cells to remove debris (32), and systemic immune activation may affect retinal inflammation.
Apart from immune cells, modified circulating soluble factors were also detected in individuals with myopic retinopathy. For instance, the concentrations of high-sensitivity C-reactive protein (hs-CRP) and complement protein C3 in the peripheral blood were markedly elevated in myopic retinopathy, and C3 may serve as a predictive risk factor for mCNV (41). This indicates that systemic complement activation may play a role in myopic retinopathy. Moreover, it has been documented that serum concentrations of anti-LIM and senescent cell antigen-like-containing domain protein 1 (anti-LIMS1) autoantibodies were markedly increased and significantly associated with the severity of myopic macular degeneration (42).
In addition to alterations in peripheral immune cells and complement proteins, human genetic studies also suggest a role for systemic immune dysregulation in myopic disease. Large CREAM (Consortium for Refractive Error And Myopia) meta–GWAS (genome-wide association study) for myopia mapped the complement regulator CD55 and the T–cell–related transcription factor TOX among risk loci (43). In addition, bidirectional Mendelian–randomization analyses reported that genetically higher circulating IL–1RA and IL–2 are associated with refractive error (44). A GWAS in Chinese populations identified VIPR2 (which has anti−inflammatory roles in immune cells) as a robust susceptibility locus for high myopia (45). For pathological myopia, a Japanese GWAS study identified BLID (a pro−apoptotic regulator in retina) as a risk gene (46), although it was confirmed neither in Chinese cohorts (47) nor the CREAMs myopic macular degeneration analysis (48). Another GWAS identified LILRB2 (an inhibitory immune receptor on myeloid cells) as a susceptibility gene for pathological myopia (49). Functional studies suggested that LILRB2 overexpression may impair choroidal homeostasis and promote atrophic changes, and the major histocompatibility complex (MHC) pathway was found to be involvement (49). In mCNV, a recent meta-GWAS uncovered a new locus (near TEX29/LINC02337) as a shared genetic susceptibility with age-related macular degeneration (AMD), along with other risk variants at CETP and the ARMS2 regions (50).
The causal relationship between systemic immune dysregulation and myopic retinopathy remains elusive. When retinal damage becomes extensive or persistent, intraocular clearance mechanisms become inadequate, and this may trigger the recruitment of circulating immune cells to the lesion site to maintain homeostasis. However, if the recruited cells are malfunctional due to genetic predispositions or other systemic inflammatory disorders, they may accelerate retinal pathology and contribute to myopic retinopathy.
3 Local ocular inflammation, myopia development, and myopic retinopathy
3.1 Local ocular inflammation in myopia development
Excessive axial length elongation in high myopia results from progressive remodeling of the sclera and choroid. It is now recognized that this process is mediated, at least in part, by immune dysregulation (51). Under hypoxic conditions, scleral fibroblasts secrete high levels of IL−6, which activates the TGF−β1/Smad2/matrix metallopeptidase 2 (MMP-2) signaling axis, promoting fibroblast differentiation, apoptosis, and extracellular matrix (ECM) degradation, leading to decreased scleral stiffness and axial length elongation (52, 53). Inflammatory mediators such as IL−1β trigger scleral fibroblast upregulation of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and various MMPs (54). This inflammatory activation converts scleral fibroblasts into both modulators and amplifiers of local immune responses, creating a feedback loop between structural remodeling and inflammation. In the choroid, a recent study identified two subsets of macrophages: prenatally derived FOLR2+ resident macrophages and infiltrating circulating CD14+/CD16+ monocytes (55). Resident macrophages support lipid handling and vascular maintenance, and their selective depletion precipitates choriocapillaris vasodegeneration and structural collapse. The thinning of the choroid driven by resident macrophage depletion resembles the choroidal attenuation seen in progressive high myopia. The results suggest that dysregulation of choroidal resident macrophages may facilitate the biomechanical and vascular changes that promote axial length elongation (55).
In the mouse model of simple myopia, scleral NLRP3 inflammasome activation is linked with MMP-2 upregulation, scleral matrix remodeling, and myopia progression (56). The C-C motif chemokine ligand-2 (CCL2) can recruit monocytes to differentiate into macrophages, and the infiltrating macrophages express high levels of MMP-2, which promotes myopia progression (57). Other studies have detected M2 macrophages in the sclera during myopia development, and inhibiting M2 macrophages can significantly alleviate myopia progression (58). Collectively, scleral fibroblasts and choroidal macrophages serve as dynamic, inflammation-responsive agents that mediate ECM alteration, vascular remodeling, and axial length elongation in myopia development and progression.
3.2 Local ocular inflammation in myopic retinopathy
Ocular immune privilege is maintained through a multi-layered defense system, including (i) physical barriers, primarily the blood–retina barrier (BRB), (ii) immunological and biochemical barriers, including various immunosuppressive molecules secreted by retinal neurons and RPE cells, and (iii) systemic immune regulation, such as the induction of regulatory T cells (Tregs) through mechanisms such as anterior chamber-associated immune deviation (ACAID) (59–61). In addition, the retina actively promotes immune tolerance. Upon encountering retinal antigens, naïve T cells differentiate into antigen-specific Tregs and anergic T cells. The Tregs suppress local autoimmune responses, while anergic T cells become unresponsive to further stimulation, both contributing to immune homeostasis (60, 62–65).
At the early stages of retinal disease, where the BRB remains intact, intraocular inflammatory mediators originate mainly from diseased retinal cells (66). However, if the BRB is damaged, choroidal and circulating immune cells can infiltrate the retina (67). When neurons degenerate, the neuron-immune cross-talk is disrupted and the immunological barrier may fail, leading to dysregulated intraocular inflammation (32). Growing evidence supports the role of ocular inflammation in the pathogenesis of myopic retinopathy, particularly the dysregulation of inflammatory cytokines and complement components.
3.2.1 Inflammatory cytokines and chemokines in myopic retinopathy
Studies from patient-derived samples support the role of ocular inflammation in myopic retinopathy. Higher intraocular levels of inflammatory factors have been detected in eyes with various types of myopic retinopathy. The aqueous humor from mCNV patients contained elevated levels of IL-8 and C−X−C motif chemokine ligand 10 (CXCL10) (68), platelet−derived growth factor (PDGF), IL-2, IL-5, IL-13, IL-15, IL-17A and TNF-α compared to the aqueous humor from simple myopia (69). A meta-analysis reported that the levels of VEGF and IL-8 in the aqueous humor of mCNV patients were higher than those of high myopia without CNV (70). The increasing inflammatory cytokines and chemokines indicate the potential involvement of JAK-STAT, MAPK, PI3K-AKT, and NF−κB signaling in mCNV. Indeed, proteomic analysis of the aqueous humor of mCNV patients showed that, compared with the myopic atrophic maculopathy group and the myopic non-maculopathy group, the differential proteins were significantly enriched in the JAK-STAT signaling pathway (71). Interestingly, apolipoprotein A-1 (APOA1), a protein known for its anti-inflammatory properties and association with chronic inflammation (72), was found to be elevated in the aqueous humor of patients with pathologic myopia (35).
We found that the intraocular inflammatory factors are related to the severity of myopic retinopathy. We investigated the aqueous humor inflammatory factors in patients with different degrees of myopic retinopathy: simple myopia, posterior scleral staphyloma, and posterior scleral staphyloma combined with chorioretinal atrophy. The pro-inflammatory cytokines (Chi3l1, IL-6Rα, IL-8, IL-12, IL-27) and inflammation-related cytokines (A proliferation-inducing ligand (April), B-cell activating factor (BAFF), IL-34) increased, and the anti-inflammation cytokines (IL-11 and aggrecan) decreased progressively with the severity of myopic retinopathy (73). The JAK-STAT signaling pathway was also found to be potentially involved in myopic retinopathy progression (73). Although in-depth studies of the JAK−STAT signaling pathway in myopic retinopathy are limited, it has been explored in other retinal degenerative diseases. Cell−specific STAT3 activation by deleting its inhibitor SOCS3 in rod photoreceptors upregulated anti−apoptotic genes and markedly slowed photoreceptor degeneration and preserved visual function in rd10 and rds mice (74). Transient STAT3 activation in RPE cells curbed oxidative stress; however, when driven chronically by IL−6, it amplified complement factor B (CFB) expression and sterile inflammation (75, 76). Conversely, mice with sustained STAT3 activation across the entire retina develop progressive photoreceptor loss and worsening uveitis, underscoring the neurotoxic potential of the JAK-STAT pathway when left unchecked (77). Thus, proper control of the JAK-STAT3 signaling is neuroprotective, and sustained activation drives inflammatory damage. The JAK−STAT3 signaling pathway may be targeted to control myopic retinopathy.
Altered cytokine levels in other ocular fluids, including the vitreous humor and tears, of patients with high myopia have also been examined. IL-5 and CXCL10 were significantly higher in high myopia and rhegmatogenous retinal detachment (78). Similarly, the levels of CCL2, IL-6, interferon−γ (IFN-γ), eotaxin, macrophage inflammatory protein−1 α (MIP-1α), IL-4, granulocyte−colony–stimulating factor (G-CSF), and CXCL10 in the vitreous of patients with high myopia with macular holes were significantly higher than those in patients with non-high myopia macular holes (78, 79). The levels of IL-6 and CCL2 in the tears from patients with high myopia were significantly increased, and correlated with the severity of myopic maculopathy (80). The authors suggested that these cytokines may be used as biomarkers to predict myopic maculopathy.
3.2.2 The complement system in myopic retinopathy
The complement system can be activated through the classical pathway, mannan-binding lectin (MBL) pathway, and the alternative pathway (81). The cleavages of C3 and C5 are two critical steps for the full activation of the complement system. The resulting fragments, such as C4b, C3a, C3b, and C5a, drive inflammation by opsonizing dead cells and debris for phagocytic clearance and participating in immune activation (81). The complement system has been implicated in the pathogenesis of myopic retinopathy. In the form-deprivation-induced mild/moderate myopia in guinea pigs, whereby the retina develops peripheral photoreceptor degeneration, we detected significant upregulation of the complement-related genes using RNA-seq technology (82). Higher expression levels of complement genes, such as C2, C3, and C4a, have been reported in other myopic models (83), including the chick FDM model (36) and the guinea pig model of negative lens-induced myopia (84). Proteomic analysis of aqueous humor from patients with pathologic myopia demonstrated prominent involvement of complement and coagulation cascades in disease progression (27). Additionally, our study of 147 myopic patients has shown significantly elevated levels of complement proteins involved in both the classical pathway (C1q, C2, C3, C4, and C4b) and alternative pathway (CFB, CFI, and C3b/iC3b) in the aqueous humor of patients with myopic retinopathy (83). C3b/iC3b and C4 showed a strong negative correlation with retinal neuronal thickness and vascular density in the macula and optic nerve head (83). These results suggest that intraocular complement activation may contribute to retinal vascular and neuronal degeneration in myopic eyes (Figure 1).
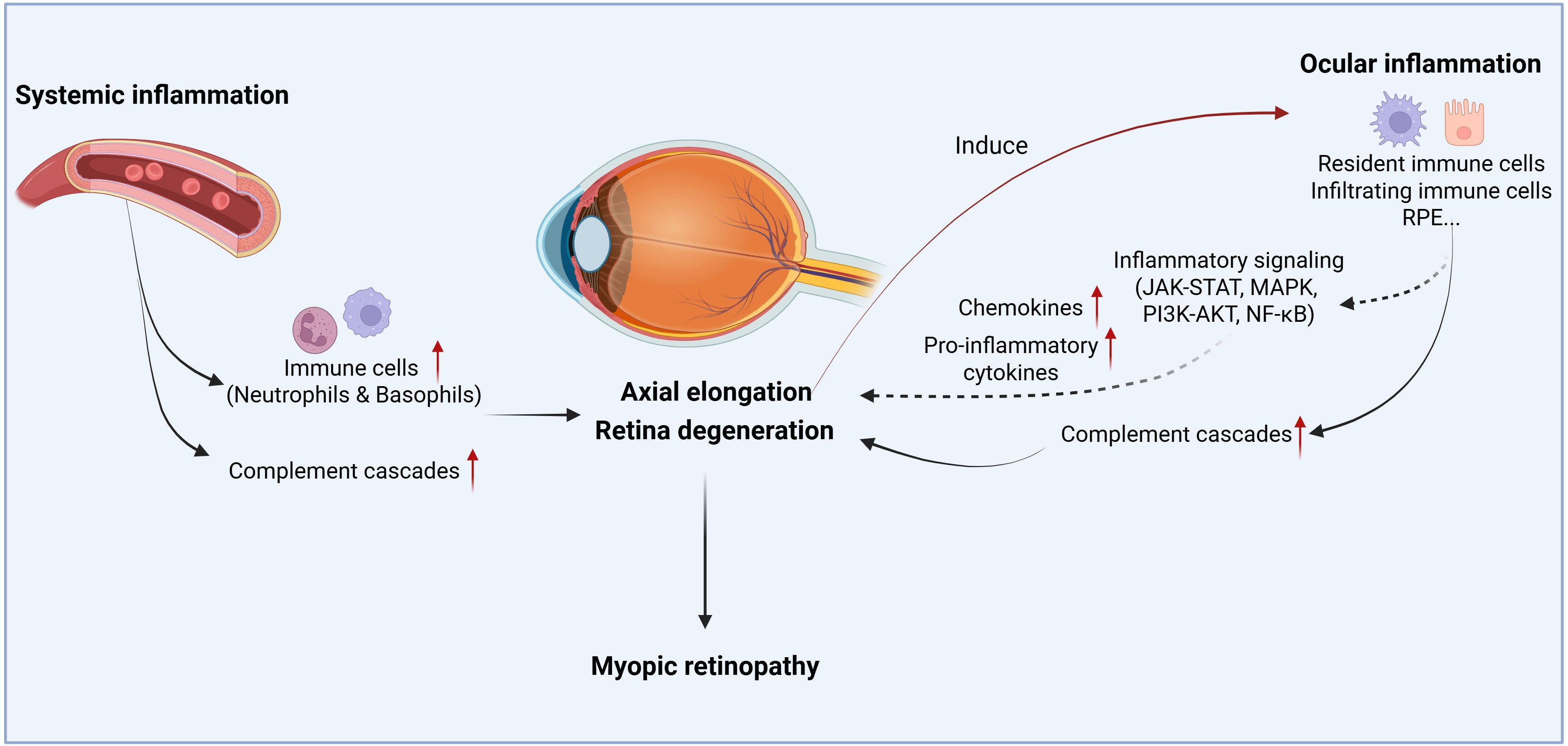
Figure 1. The schematic overview illustrates the role of inflammation in myopic progression and myopic retinopathy. Both systemic and local inflammatory responses participate in the disease progression. Circulating immune cells or inflammatory cytokines/chemokines can infiltrate the eye and participate in scleral remodeling, axial elongation, or retinal degeneration. When retina degenerates from pathological myopia, it induces intraocular inflammation, which in turn, may further promote retinal degeneration. Created with BioRender.com.
4 Limitations of animal models
Animal models are essential for understanding the mechanism of myopic retinopathy. Several animal models have been reported. Chicks, whose eyelids were sutured for 8 weeks, showed retinal changes similar to lacquer cracks (85). G-protein subunit beta 1 (GNB3) gene global knockout chicks showed fundus lacquer cracks in the early stages, which developed into circular lesions with patchy of atrophy at 134 weeks (86). Since the chicken sclera is composed of cartilage and fibrous layers, which are quite different from those of humans (87), these chicken models have not been widely used to study the pathogenesis of myopic retinopathy. The Lumican-Fibromodulin double knockout mice displayed features of pathological myopia, including scleral thinning and retinal detachment (88). This mouse model is hampered by corneal opacity, systemic connective−tissue abnormalities, and only a modest (~10 %) axial−length increase, making it not an ideal model for human pathological myopia (88). Conditional Lrp2 knockout mice (KO in neural retina, RPE and ciliary body epithelium) develop retinal thinning and posterior scleral staphylomas (89). Furthermore, the RPE−specific Lrp2 knockout mice exhibited significant ocular axial length elongation, severe pan−retinal thinning/degeneration with vision loss, and typical RPE anomalies such as macromelanosome formation (90). These phenotypes mirror retinal complications observed in pathological myopia patients (90). This RPE-specific Lrp2 knockout mouse model may be a useful tool for investigating the mechanism of myopic retinopathy.
5 Clinical translation for myopic retinopathy
Standardization and clinical feasibility of detecting inflammatory markers are critical for successful clinical translation. Validating non−invasive biomarkers and incorporating them into early−intervention trials can bridge the gap between mechanistic insights and clinical application. Levels of tear IL−6 and CCL2/MCP−1 have been shown to correlate with axial length and may serve as predictive biomarkers for myopic macular degeneration (80). Moreover, omics-based approaches have identified additional innate−immune signatures, such as intraocular soluble intercellular adhesion molecule 1 (sICAM−1) (91). This biomarker−guided, early−intervention strategy may facilitate the evaluation of anti−inflammatory therapies for myopic retinopathy.
In animal models, various anti-inflammatory agents, such as lactoferrin, diacerein, resveratrol−based botanicals, and the NLRP3 inhibitor MCC950 have been shown to reduce the expression of IL−6, TNF−α, MMP−2 and related mediators, resulting in reduced axial elongation (92–96). Low-concentration of atropine eyedrop, which is widely used for myopic control, also suppresses the expression of c−Fos, IL−6, NF−κB and TNF−α in a hamster model (34). To enhance patient compliance, a self−powered eyelid−activated delivery system for atropine administration has been proposed (97). Interestingly, the immunosuppressive medication cyclosporine A has also demonstrated the ability to slow myopic progression (34). In laser−induced CNV, infliximab reduced retinal oedema (98), while anti−VEGF agents are thought to suppress myopic CNV partly through downregulation of inflammatory cytokines (99). Targeting the JAK−STAT signaling pathway represents another promising therapeutic avenue. Tofacitinib (targets JAK1/3), ruxolitinib (targets JAK1/2), and AG490 (targets JAK2) have demonstrated neuroprotective and anti-angiogenic effects in preclinical models (100–102). Complement-based therapies, including a C3 inhibitor and a C5-targeting aptamer, have been approved by the FDA for treating geographic atrophy type of AMD (103) and may be repurposed for the management of myopic retinopathy.
6 Summary
Myopic retinopathy is associated with changes in the immune system, both systemically and locally within the intraocular microenvironment (Figure 1). When retinal degeneration develops, ocular immune privilege may be compromised and circulating immune cells such as monocytes, neutrophils, and soluble factors, including complement proteins, may infiltrate the retina, leading to dysregulated intraocular inflammation, which may further promote retinal degeneration (Figure 1).
Emerging evidence suggests a bidirectional relationship between chronic inflammation and myopic retinal degeneration (33) (Figure 1). On one hand, a pro-inflammatory microenvironment may exacerbate myopia progression. Epidemiological data show that children with systemic inflammatory diseases have significantly higher rates of myopia compared to healthy controls (34). In experimental models, induced inflammation accelerates pathologic axial elongation, whereas anti-inflammatory interventions mitigate myopic eye growth (34). On the other hand, high myopia-mediated retinal degeneration can, in turn, trigger intraocular inflammation. Pathologic myopic eyes exhibit elevated levels of inflammatory cytokines and complement components, which correlate with the extent of retinal thinning, indicating that more severe myopic retinal degeneration is associated with greater inflammatory activities (83, 104).
Therefore, inflammation may not be the primary initiator of myopic retinopathy, retinal degeneration disrupts immune privilege and precipitates inflammatory cascades that further accelerate tissue damage. Controlling inflammation, along with conventional strategies to limit axial elongation, may offer a dual approach to slow the progression of myopic retinopathy.
7 Future directions
Despite significant progress in understanding the role of inflammation in myopic retinopathy, critical knowledge gaps remain. Clinical research is unlikely to definitively establish the causal role of inflammation or fully elucidate the underlying mechanisms due to confounding variables and the chronic nature of disease progression. Meanwhile, basic science research is hindered by the lack of reliable animal models that accurately recapitulate the pathological features of myopic retinopathy. Future investigations should prioritize the development of physiologically relevant models of pathological myopia or myopic retinopathy, including human induced pluripotent stem cells (iPSCs)-derived ocular organoids or animal models, delineation of the cellular sources and regulatory pathways of inflammation, and clarification of the precise role of inflammation in the onset and progression of myopic retinal degeneration. It also remains uncertain whether targeted anti−inflammatory therapies can effectively prevent or reverse the course of the disease. Addressing these challenges will be essential for advancing our understanding of the pathogenesis of myopic retinopathy and for developing effective therapeutic strategies.
Author contributions
TY: Formal analysis, Investigation, Writing – original draft. JQ: Data curation, Investigation, Writing – original draft. HX: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Foreign Experts Program of Hunan Province Innovation Platform and Specialist Project (2022WZ1023), Science Research Foundation of Aier Eye Hospital Group (AMF2501D05), Natural Science Foundation of Hunan Province (2025JJ90270). Xiangjiang Philanthropy Foundation (KY24008, KY25012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, et al. IMI – defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Visual Sci. (2019) 60:M20. doi: 10.1167/iovs.18-25957
2. Dong L, Kang YK, Li Y, Wei WB, and Jonas JB. Prevalence and time trends of myopia in children and adolescents in China: A systemic review and meta-analysis. Retina. (2020) 40:399–411. doi: 10.1097/IAE.0000000000002590
3. Tsai T-H, Liu Y-L, Ma I-H, Su C-C, Lin C-W, Lin LL-K, et al. Evolution of the Prevalence of Myopia among Taiwanese Schoolchildren: A Review of Survey Data from 1983 through 2017. Ophthalmology. (2021) 128:290–301. doi: 10.1016/j.ophtha.2020.07.017
4. Choy BNK, You Q, Zhu MM, Lai JSM, Ng ALK, and Wong IYH. Prevalence and associations of myopia in Hong Kong primary school students. Jpn J Ophthalmol. (2020) 64:437–49. doi: 10.1007/s10384-020-00733-4
5. Nakao S, Miyake M, Hosoda Y, Nakano E, Mori Y, Takahashi A, et al. Myopia prevalence and ocular biometry features in a general Japanese population: the nagahama study. Ophthalmology. (2021) 128:522–31. doi: 10.1016/j.ophtha.2020.08.023
6. Matsumura S, Lanca C, Htoon HM, Brennan N, Tan C-S, Kathrani B, et al. Annual myopia progression and subsequent 2-year myopia progression in Singaporean children. Trans Vision Sci Technol. (2020) 9:12. doi: 10.1167/tvst.9.13.12
7. Hopf S, Korb C, Nickels S, Schulz A, Münzel T, Wild PS, et al. Prevalence of myopic maculopathy in the German population: results from the Gutenberg health study. Br J Ophthalmol. (2020) 104:1254–9. doi: 10.1136/bjophthalmol-2019-315255
8. Bikbov MM, Gilmanshin TR, Kazakbaeva GM, Zainullin RM, Rakhimova EM, Rusakova IA, et al. Prevalence of myopic maculopathy among adults in a Russian population. JAMA Netw Open. (2020) 3:e200567. doi: 10.1001/jamanetworkopen.2020.0567
9. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 123:1036–42. doi: 10.1016/j.ophtha.2016.01.006
10. Saw S-M, Gazzard G, Shih-Yen EC, and Chua W-H. Myopia and associated pathological complications. Ophthalmic Physiol Optics. (2005) 25:381–91. doi: 10.1111/j.1475-1313.2005.00298.x
11. Ohno-Matsui K, Lai TYY, Lai C-C, and Cheung CMG. Updates of pathologic myopia. Prog Retinal Eye Res. (2016) 52:156–87. doi: 10.1016/j.preteyeres.2015.12.001
12. Ohno-Matsui K, Wu P-C, Yamashiro K, Vutipongsatorn K, Fang Y, Cheung CMG, et al. IMI pathologic myopia. Invest Ophthalmol Visual Sci. (2021) 62:5. doi: 10.1167/iovs.62.5.5
13. An G, Dai F, Wang R, Liu Z, Guo J, Pan M, et al. Association between the types of posterior staphyloma and their risk factors in pathological myopia. Transl Vis Sci Technol. (2021) 10:5. doi: 10.1167/tvst.10.4.5
14. Vongphanit J, Mitchell P, and Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. (2002) 109:704–11. doi: 10.1016/S0161-6420(01)01024-7
15. Wong Y-L, Sabanayagam C, Ding Y, Wong C-W, Yeo AC-H, Cheung Y-B, et al. Prevalence, risk factors, and impact of myopic macular degeneration on visual impairment and functioning among adults in Singapore. Invest Ophthalmol Visual Sci. (2018) 59:4603–13. doi: 10.1167/iovs.18-24032
16. Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, et al. Long-term pattern of progression of myopic maculopathy: A natural history study. Ophthalmology. (2010) 117:1595–1611.e4. doi: 10.1016/j.ophtha.2009.11.003
17. Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, et al. Myopic choroidal neovascularization: A 10-year follow-up. Ophthalmology. (2003) 110:1297–305. doi: 10.1016/S0161-6420(03)00461-5
18. Fujiwara T, Imamura Y, Margolis R, Slakter JS, and Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. (2009) 148:445–50. doi: 10.1016/j.ajo.2009.04.029
19. Wu P-C, Huang H-M, Yu H-J, Fang P-C, and Chen C-T. Epidemiology of myopia. Asia-Pacific J Ophthalmol. (2016) 5:386–93. doi: 10.1097/APO.0000000000000236
20. Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. (2008) 115:1279–85. doi: 10.1016/j.ophtha.2007.12.019
21. Rose KA, French AN, and Morgan IG. Environmental factors and myopia: paradoxes and prospects for prevention. Asia-Pacific J Ophthalmol. (2016) 5:403–10. doi: 10.1097/APO.0000000000000233
22. Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, and Foster PJ. The association between time spent outdoors and myopia in children and adolescents: A systematic review and meta-analysis. Ophthalmology. (2012) 119:2141–51. doi: 10.1016/j.ophtha.2012.04.020
23. Wakabayashi T and Ikuno Y. Choroidal filling delay in choroidal neovascularisation due to pathological myopia. Br J Ophthalmol. (2010) 94:611–5. doi: 10.1136/bjo.2009.163535
24. Summers Rada JA, Shelton S, and Norton TT. The sclera and myopia. Exp Eye Res. (2006) 82:185–200. doi: 10.1016/j.exer.2005.08.009
25. Wu H, Chen W, Zhao F, Zhou Q, Reinach PS, Deng L, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U.S.A. (2018) 115:E7091–100. doi: 10.1073/pnas.1721443115
26. Zhao F, Wu H, Reinach PS, Wu Y, Zhai Y, Lei Y, et al. Up-regulation of matrix metalloproteinase-2 by scleral monocyte–derived macrophages contributes to myopia development. Am J Pathol. (2020) 190:1888–908. doi: 10.1016/j.ajpath.2020.06.002
27. Tong J-P, Chan W-M, Liu DTL, Lai TYY, Choy K-W, Pang C-P, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium–derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. (2006) 141:456–62. doi: 10.1016/j.ajo.2005.10.012
28. Yamada M, Hiratsuka Y, Roberts CB, Pezzullo ML, Yates K, Takano S, et al. Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol. (2010) 17(1):50–7. doi: 10.3109/09286580903450346
29. Curcio CA, Johnson M, Rudolf M, and Huang J-D. The oil spill in ageing Bruch membrane. Br J Ophthalmol. (2011) 95:1638–45. doi: 10.1136/bjophthalmol-2011-300344
30. Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmol. (2008) 2:413–24. doi: 10.2147/opth.s2151
31. Medzhitov R. Origin and physiological roles of inflammation. Nature. (2008) 454:428–35. doi: 10.1038/nature07201
32. Xu H and Chen M. Immune response in retinal degenerative diseases – Time to rethink? Prog Neurobiol. (2022) 219:102350. doi: 10.1016/j.pneurobio.2022.102350
33. Xu R, Zheng J, Liu L, and Zhang W. Effects of inflammation on myopia: evidence and potential mechanisms. Front Immunol. (2023) 14:1260592. doi: 10.3389/fimmu.2023.1260592
34. Lin H-J, Wei C-C, Chang C-Y, Chen T-H, Hsu Y-A, Hsieh Y-C, et al. Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. eBioMedicine. (2016) 10:269–81. doi: 10.1016/j.ebiom.2016.07.021
35. Xue M, Ke Y, Ren X, Zhou L, Liu J, Zhang X, et al. Proteomic analysis of aqueous humor in patients with pathologic myopia. J Proteomics. (2021) 234:104088. doi: 10.1016/j.jprot.2020.104088
36. Giummarra L, Crewther SG, Riddell N, Murphy MJ, and Crewther DP. Pathway analysis identifies altered mitochondrial metabolism, neurotransmission, structural pathways and complement cascade in retina/RPE/choroid in chick model of form-deprivation myopia. PeerJ. (2018) 6:e5048. doi: 10.7717/peerj.5048
37. Herbort CP, Papadia M, and Neri P. Myopia and inflammation. J Ophthalmic Vis Res. (2011) 6:270–83.
38. Wei C-C, Kung Y-J, Chen CS, Chang C-Y, Lin C-J, Tien P-T, et al. Allergic conjunctivitis-induced retinal inflammation promotes myopia progression. eBioMedicine. (2018) 28:274–86. doi: 10.1016/j.ebiom.2018.01.024
39. Wang X, He Q, Zhao X, Li H, Liu L, Wu D, et al. Assessment of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with high myopia. BMC Ophthalmol. (2022) 22:464. doi: 10.1186/s12886-022-02688-1
40. Qi J, Pan W, Peng T, Zeng L, Li X, Chen Z, et al. Higher circulating levels of neutrophils and basophils are linked to myopic retinopathy. IJMS. (2022) 24:80. doi: 10.3390/ijms24010080
41. Long Q, Ye J, Li Y, Wang S, and Jiang Y. C-reactive protein and complement components in patients with pathological myopia. Optometry Vision Sci. (2013) 90:501. doi: 10.1097/OPX.0b013e31828daa6e
42. Qi J, Li H, Du Y, Liu Y, He W, Meng J, et al. Circulating autoantibody profiling identifies LIMS1 as a potential target for pathogenic autoimmunity in pathologic myopia. Mol Cell Proteomics. (2024) 23:100783. doi: 10.1016/j.mcpro.2024.100783
43. Consortium for Refractive Error and Myopia (CREAM), Verhoeven VJM, The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group, Wellcome Trust Case Control Consortium 2 (WTCCC2), The Fuchs’ Genetics Multi-Center Study Group, Hysi PG, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. (2013) 45:314–8. doi: 10.1038/ng.2554
44. Xu Y, Dong X-X, Wang Y, Zhuang X-Y, Chen Y-J, Zhang X-F, et al. Association between inflammatory cytokines and refractive errors: A bidirectional mendelian randomization study. Trans Vis Sci Tech. (2025) 14:1–1. doi: 10.1167/tvst.14.5.1
45. Yiu WC, Yap MKH, Fung WY, Ng PW, and Yip SP. Genetic susceptibility to refractive error: association of vasoactive intestinal peptide receptor 2 (VIPR2) with high myopia in chinese. PloS One. (2013) 8:e61805. doi: 10.1371/journal.pone.0061805
46. Nakanishi H, Yamada R, Gotoh N, Hayashi H, Yamashiro K, Shimada N, et al. A genome-wide association analysis identified a novel susceptible locus for pathological myopia at 11q24.1. PloS Genet. (2009) 5:e1000660. doi: 10.1371/journal.pgen.1000660
47. Zhao F, B J, C W, X A, L C, Y Z, et al. Evaluation of BLID and LOC399959 as candidate genes for high myopia in the Chinese Han population. Mol Vision. (2010) 16:1920–27.
48. Wong Y-L, Hysi P, Cheung G, Tedja M, Hoang QV, Tompson SWJ, et al. Genetic variants linked to myopic macular degeneration in persons with high myopia: CREAM Consortium. PloS One. (2019) 14:e0220143. doi: 10.1371/journal.pone.0220143
49. Jiang L, Huang L, Dai C, Zheng R, Miyake M, Mori Y, et al. Genome-wide association analysis identifies LILRB2 gene for pathological myopia. Adv Sci (Weinh). (2024) 11:2308968. doi: 10.1002/advs.202308968
50. Morino K, Miyake M, Nagasaki M, Kawaguchi T, Numa S, Mori Y, et al. Genome-wide meta-analysis for myopic macular neovascularization identified a novel susceptibility locus and revealed a shared genetic susceptibility with age-related macular degeneration. Ophthalmol Retina. (2025) 9:367–77. doi: 10.1016/j.oret.2024.09.016
51. Xin J, Bao B, Liu J, Ma Z, Zhang M, Bi H, et al. Crosstalk between myopia and inflammation: A mini review. Int J Med Sci. (2024) 21:1589–603. doi: 10.7150/ijms.94826
52. Yin X and Ge J. The role of scleral changes in the progression of myopia: A review and future directions. Clin Ophthalmol. (2025) 19:1699–707. doi: 10.2147/OPTH.S523283
53. Liu L, Zhou W, Fan Y, Zhang L, Liu S, Song S, et al. Effect of interleukin 6 on scleral fibroblast proliferation, differentiation, and apoptosis involved in myopic scleral remodeling. Ophthalmic Res. (2022) 65:529–39. doi: 10.1159/000524502
54. Atta G, Schroedl F, Kaser-Eichberger A, Spitzer G, Traweger A, Heindl LM, et al. Scleraxis expressing scleral cells respond to inflammatory stimulation. Histochem Cell Biol. (2021) 156:123–32. doi: 10.1007/s00418-021-01985-y
55. Fortmann SD, Frey BF, Rosencrans RF, Adu-Rutledge Y, Ready VE, Kilchrist KV, et al. Prenatally derived macrophages support choroidal health and decline in age-related macular degeneration. J Exp Med. (2025) 222:e20242007. doi: 10.1084/jem.20242007
56. Chen Z, Xiao K, and Long Q. Up-regulation of NLRP3 in the sclera correlates with myopia progression in a form-deprivation myopia mouse model. FBL. (2023) 28:27. doi: 10.31083/j.fbl2802027
57. Zhao F, Wu H, Reinach PS, Wu Y, Zhai Y, Lei Y, et al. Up-regulation of matrix metalloproteinase-2 by scleral monocyte-derived macrophages contributes to myopia development. Am J Pathol. (2020) 190:1888–908. doi: 10.1016/j.ajpath.2020.06.002
58. Zheng B, Cui D, Deng B, Long W, Ye G, Zhang S, et al. Form-deprivation myopia promotes sclera M2-type macrophages polarization in mice. Biochem Biophys Res Commun. (2024) 737:150490. doi: 10.1016/j.bbrc.2024.150490
59. Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. (2006) 213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x
60. Forrester JV and McMenamin PG. Evolution of the ocular immune system. Eye (Lond). (2025) 39:468–77. doi: 10.1038/s41433-024-03512-4
61. Koevary SB. Ocular immune privilege: a review. Clin Eye Vision Care. (2000) 12:97–106. doi: 10.1016/S0953-4431(00)00041-2
62. McPherson SW, Heuss ND, and Gregerson DS. Local “On-demand” Generation and function of antigen specific foxP3+ Regulatory T cells. J Immunol. (2013) 190:4971–81. doi: 10.4049/jimmunol.1202625
63. McPherson SW, Heuss ND, Pierson MJ, and Gregerson DS. Retinal antigen-specific regulatory T cells protect against spontaneous and induced autoimmunity and require local dendritic cells. J Neuroinflamm. (2014) 11:205. doi: 10.1186/s12974-014-0205-4
64. Gregerson DS, Heuss ND, Lew KL, McPherson SW, and Ferrington DA. Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest Ophthalmol Vis Sci. (2007) 48:4654. doi: 10.1167/iovs.07-0286
65. Peng Z, Nagarajan V, Horai R, Jittayasothorn Y, Mattapallil MJ, and Caspi RR. Ocular immune privilege in action: The living eye imposes unique regulatory and anergic gene signatures on uveitogenic T cells. Cell Rep. (2025) 44. doi: 10.1016/j.celrep.2025.115780
66. Murakami Y, Ishikawa K, Nakao S, and Sonoda K-H. Innate immune response in retinal homeostasis and inflammatory disorders. Prog Retin Eye Res. (2020) 74:100778. doi: 10.1016/j.preteyeres.2019.100778
67. Tomkins-Netzer O, Niederer R, Greenwood J, Fabian ID, Serlin Y, Friedman A, et al. Mechanisms of blood-retinal barrier disruption related to intraocular inflammation and Malignancy. Prog Retin Eye Res. (2024) 99:101245. doi: 10.1016/j.preteyeres.2024.101245
68. Zhang S, Mao J, Chen N, Fang Y, Chen Y, Zheng Z, et al. Difference in aqueous concentration and vitreous mass of cytokines in high myopias with and without choroidal neovascularization. Front Med (Lausanne). (2022) 9:1029425. doi: 10.3389/fmed.2022.1029425
69. Shchuko AG, Zaitseva NV, Yurieva TN, Chernykh ER, Mikhalevich IM, Shevela EY, et al. Intraocular cytokines and their correlations with clinical parameters in patients with myopic choroidal neovascularization. Ophthalmologica. (2017) 237:96–104. doi: 10.1159/000455271
70. Zhang XJ, Chen XN, Tang FY, Szeto S, Ling XT, Lin ZX, et al. Pathogenesis of myopic choroidal neovascularization: A systematic review and meta-analysis. Surv Ophthalmol. (2023) 68:1011–26. doi: 10.1016/j.survophthal.2023.07.006
71. Yu H, Zhong Z, Zhao Y, Luo H, Sun J, Wang R, et al. Insights into myopic choroidal neovascularization based on quantitative proteomics analysis of the aqueous humor. BMC Genomics. (2023) 24:767. doi: 10.1186/s12864-023-09761-z
72. Tao X, Tao R, Wang K, and Wu L. Anti-inflammatory mechanism of apolipoprotein A-I. Front Immunol. (2024) 15:1417270. doi: 10.3389/fimmu.2024.1417270
73. Zeng L, Yang Z, Pan W, Lin D, Tang Y, Chen B, et al. Higher intraocular levels of inflammatory factors are related to retinal vascular and neurodegeneration in myopic retinopathy. JIR. (2024) 17:10889–900. doi: 10.2147/JIR.S484338
74. Wang Y, Nusinowitz S, and Yang X-J. Elevating Jak-STAT signaling via SOCS3 deletion sustains photoreceptor viability and visual function in mouse models of retinitis pigmentosa. Res Sq [Preprint]. (2025). doi: 10.21203/rs.3.rs-7089882/v1
75. Patel AK, Syeda S, and Hackam AS. Signal transducer and activator of transcription 3 (STAT3) signaling in retinal pigment epithelium cells. JAKSTAT. (2013) 2:e25434. doi: 10.4161/jkst.25434
76. Chou W-W, Wang Y-S, Chen K-C, Wu J-M, Liang C-L, and Juo S-HH. Tannic acid suppresses ultraviolet B-induced inflammatory signaling and complement factor B on human retinal pigment epithelial cells. Cell Immunol. (2012) 273:79–84. doi: 10.1016/j.cellimm.2011.11.003
77. Marrero B, He C, Oh H-M, Ukwu UT, Yu C-R, Dambuza IM, et al. Persistent activation of STAT3 pathway in the retina induced vision impairment and retinal degenerative changes in ageing mice. Adv Exp Med Biol. (2019) 1185:353–8. doi: 10.1007/978-3-030-27378-1_58
78. Wei Q, Zhuang X, Fan J, Jiang R, Chang Q, Xu G, et al. Proinflammatory and angiogenesis-related cytokines in vitreous samples of highly myopic patients. Cytokine. (2021) 137:155308. doi: 10.1016/j.cyto.2020.155308
79. Ando Y, Keino H, Inoue M, Hirota K, Takahashi H, Sano K, et al. Circulating vitreous microRNA as possible biomarker in high myopic eyes with macular hole. Int J Mol Sci. (2022) 23:3647. doi: 10.3390/ijms23073647
80. Guo D, Qi J, Du Y, Zhao C, Liu S, Lu Y, et al. Tear inflammatory cytokines as potential biomarkers for myopic macular degeneration. Exp Eye Res. (2023) 235:109648. doi: 10.1016/j.exer.2023.109648
81. Xu H, Yi C, and Chen M. The complement pathway as a therapeutic target for neovascular age-related macular degeneration-mediated subretinal fibrosis. Curr Opin Pharmacol. (2024) 76:102448. doi: 10.1016/j.coph.2024.102448
82. Zeng L, Li X, Liu J, Liu H, Xu H, and Yang Z. RNA-seq analysis reveals an essential role of the tyrosine metabolic pathway and inflammation in myopia-induced retinal degeneration in Guinea pigs. IJMS. (2021) 22:12598. doi: 10.3390/ijms222212598
83. Zeng L, Li X, Pan W, Tang Y, Lin D, Wang M, et al. Intraocular complement activation is related to retinal vascular and neuronal degeneration in myopic retinopathy. Front Cell Neurosci. (2023) 17:1187400. doi: 10.3389/fncel.2023.1187400
84. Ting-Ting Gao QL. Complement factors C1q, C3 and C5b-9 in the posterior sclera of Guinea pigs with negative lens-defocused myopia. qwer. (2015) 8:675–80. doi: 10.3980/j.issn.2222-3959.2015.04.06
85. Hirata A and Negi A. Lacquer crack lesions in experimental chick myopia. Graefe’s Arch Clin Exp Ophthalmol. (1998) 236:138–45. doi: 10.1007/s004170050054
86. Tummala H, Ali M, Getty P, Hocking PM, Burt DW, Inglehearn CF, et al. Mutation in the guanine nucleotide–binding protein β-3 causes retinal degeneration and embryonic mortality in chickens. Invest Ophthalmol Visual Sci. (2006) 47:4714–8. doi: 10.1167/iovs.06-0292
87. Glasser A, Murphy CJ, Troilo D, and Howland HC. The mechanism of lenticular accommodation in chicks. Vision Res. (1995) 35:1525–40. doi: 10.1016/0042-6989(94)00211-4
88. Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, and Birk DE. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest Ophthalmol Vis Sci. (2003) 44:2422. doi: 10.1167/iovs.02-0783
89. Cases O, Joseph A, Obry A, Santin MD, Ben-Yacoub S, Pâques M, et al. Foxg1-cre mediated lrp2 inactivation in the developing mouse neural retina, ciliary and retinal pigment epithelia models congenital high myopia. PloS One. (2015) 10:e0129518. doi: 10.1371/journal.pone.0129518
90. Storm T, Burgoyne T, Dunaief JL, Christensen EI, Futter C, and Nielsen R. Selective ablation of megalin in the retinal pigment epithelium results in megaophthalmos, macromelanosome formation and severe retina degeneration. Invest Ophthalmol Vis Sci. (2019) 60:322–30. doi: 10.1167/iovs.18-25667
91. Pan S, Yuan J, Jin Y, Liu X, Wu S, Wang Y, et al. Innate immune responsive inflammation in development of progressive myopia. Eye (Lond). (2024) 38:1542–8. doi: 10.1038/s41433-024-02947-z
92. Ikeda S-I, Kurihara T, Toda M, Jiang X, Torii H, and Tsubota K. Oral bovine milk lactoferrin administration suppressed myopia development through matrix metalloproteinase 2 in a mouse model. Nutrients. (2020) 12:3744. doi: 10.3390/nu12123744
93. Tien P-T, Lin C-H, Chen C-S, Chang C-Y, Ku H, Gan D, et al. Diacerein inhibits myopia progression through lowering inflammation in retinal pigment epithelial cell. Mediators Inflammation. (2021) 2021:6660640. doi: 10.1155/2021/6660640
94. Chen C-S, Hsu Y-A, Lin C-H, Wang Y-C, Lin E-S, Chang C-Y, et al. Fallopia Japonica and Prunella vulgaris inhibit myopia progression by suppressing AKT and NFκB mediated inflammatory reactions. BMC Complement Med Ther. (2022) 22:271. doi: 10.1186/s12906-022-03747-2
95. Hsu Y-A, Chen C-S, Wang Y-C, Lin E-S, Chang C-Y, Chen JJ-Y, et al. Anti-inflammatory effects of resveratrol on human retinal pigment cells and a myopia animal model. Curr Issues Mol Biol. (2021) 43:716–27. doi: 10.3390/cimb43020052
96. Chen Z, Xiao K, and Long Q. Intraperitoneal injection of MCC950 inhibits the progression of myopia in form-deprivation myopic mice. Int J Mol Sci. (2023) 24:15839. doi: 10.3390/ijms242115839
97. Jiang L, Zhang L, Dai C, Zhao B, Yang Y, Wu Z, et al. A self-generated electricity-driven drug delivery system for precision management of myopia. Nano Energy. (2024) 119:109040. doi: 10.1016/j.nanoen.2023.109040
98. Olson JL, Courtney RJ, and Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch Ophthalmol. (2007) 125:1221–4. doi: 10.1001/archopht.125.9.1221
99. Chan W-M, Lai TYY, Chan K-P, Li H, Liu DTL, Lam DSC, et al. Changes in aqueous vascular endothelial growth factor and pigment epithelial-derived factor levels following intravitreal bevacizumab injections for choroidal neovascularization secondary to age-related macular degeneration or pathologic myopia. Retina. (2008) 28:1308. doi: 10.1097/IAE.0b013e31818358b2
100. Byrne EM, Llorián-Salvador M, Lyons TJ, Chen M, and Xu H. Tofacitinib ameliorates retinal vascular leakage in a murine model of diabetic retinopathy with type 2 diabetes. Int J Mol Sci. (2021) 22:11876. doi: 10.3390/ijms222111876
101. Mercau ME, Akalu YT, Mazzoni F, Gyimesi G, Alberto EJ, Kong Y, et al. Inflammation of the retinal pigment epithelium drives early-onset photoreceptor degeneration in Mertk-associated retinitis pigmentosa. Sci Adv. (2023) 9:eade9459. doi: 10.1126/sciadv.ade9459
102. Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Remé C, Grimm C, et al. Differential role of Jak-STAT signaling in retinal degenerationsDifferential role of Jak-STAT signaling in retinal degenerations. FASEB J. (2006) 20:2411–3. doi: 10.1096/fj.06-5895fje
103. Shakeel L, Khan A, and Akilimali A. Izervay (avacincaptad pegol): paving the way for vision preservation in geographic atrophy. Ann Med Surg (Lond). (2024) 86:2413–6. doi: 10.1097/MS9.0000000000002021
Keywords: inflammation, pathologic myopia, myopic retinopathy, retinal degeneration, complement system, immune cells
Citation: Yang T, Qi J and Xu H (2025) The role of inflammation in myopic retinopathy. Front. Ophthalmol. 5:1632047. doi: 10.3389/fopht.2025.1632047
Received: 20 May 2025; Accepted: 05 August 2025;
Published: 20 August 2025.
Edited by:
Cheng-Rong Yu, National Eye Institute (NIH), United StatesReviewed by:
Zhang Shian, Zhejiang Provincial People’s Hospital, ChinaShanu Markand, A.T. Still University, United States
Zixuan Peng, National Eye Institute (NIH), United States
Copyright © 2025 Yang, Qi and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heping Xu, aGVwaW5nLnh1QHF1Yi5hYy51aw==; eHVoZXBpbmdAYWllcmNoaW5hLmNvbQ==
 Tianxiang Yang
Tianxiang Yang Jinyan Qi1,2
Jinyan Qi1,2 Heping Xu
Heping Xu