- 1Glaucoma of Ophthalmology, Ningxia Aier Eye Hospital, Yinchuan, Ningxia, China
- 2Aier Academy of Ophthalmology, Central South University, Changsha, Hunan, China
- 3Aier Glaucoma Institute, Hunan Engineering Research Center for Glaucoma with Artificial Intelligence in Diagnosis and Application of New Materials, Changsha Aier Eye Hospital, Changsha, Hunan, China
- 4Eye Center of Xiangya Hospital, Central South University, Changsha, Hunan, China
The iridocorneal endothelial syndrome encompasses a spectrum of ocular disorders predominantly affecting one eye in young to middle-aged women, typically without a familial predisposition. The hallmark feature of iridocorneal endothelial syndrome is the migration of corneal endothelial cells towards the iridocorneal angle and onto the iris. This syndrome comprises three distinct clinical variations: progressive essential atrophy of the iris (including corectopia, iris atrophy, or iris hole), Chandler syndrome (characterized by corneal edema with mild to absent changes in the iris), and Cogan-Reese syndrome (manifesting as nodular pigmented lesions on the front surface of the iris). In cases involving corneal manifestations, such as corneal edema or decompensation, options like Descemet’s stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty may be considered for optimal management. For instance, conditions affecting the iris, such as an iris cavity, multiple pupils, or photophobia, may make femtosecond-assisted keratopigmentation a treatment option. In cases of glaucoma secondary to iridocorneal endothelial syndrome, trabeculectomy with mitomycin C and the implantation of a glaucoma drainage device have been shown to reduce intraocular pressure effectively. At the same time, retrocorneal membrane interception-enhanced peripheral iridectomy has demonstrated significant efficacy.
1 Introduction
The iridocorneal endothelial (ICE) syndrome encompasses a collection of ocular disorders, initially proposed by Eagle and Yanoff in 1979 (1). Consequently, ICE mainly occurs unilaterally in young women without a family history (2, 3). While ICE syndrome is relatively uncommon in children, there are few reports that have documented its occurrence in the pediatric population (4–7). The hallmark of ICE syndrome is the migration of corneal endothelial cells towards the iridocorneal angle and onto the iris (8). Thus, ICE is commonly associated with glaucoma, either due to the obstruction of the angle by the proliferating cells or the contraction of their basement membrane over the iris. As such, this results in peripheral traction of the iris and potential development of peripheral anterior synechiae (PAS) (8).
The most significant clinical findings in ICE syndrome are the pathological elements observed in the endothelium, particularly the presence of “the ICE cell” (1, 8). These cells exhibit abnormal enlargement and increased pleomorphism (1, 8, 9). Immunohistochemistry studies have demonstrated the expression of vimentin and cytokeratin, which are typical of epithelial cells (10). Abnormal endothelial cells may increase the risk of corneal edema and decompensation, and they may also migrate posteriorly. Consequently, this leads to the formation of a membrane that extends to cover adjacent structures, such as the iris and trabecular meshwork (11). Moreover, membrane contraction leads to iris changes, iridotrabecular synechiae, corectopia with the pupil being drawn towards the area where the synechiae are most widespread, and to secondary angle-closure glaucoma (11, 12).
Therefore, the ICE syndrome encompasses three clinical variants: progressive essential atrophy of the iris (including corectopia, iris atrophy, or iris hole), Chandler syndrome (characterized by corneal edema with mild to absent iris change), and Cogan-Reese syndrome (manifesting nodular pigmented lesions on the anterior surface of the iris) (13).
2 Etiology
The pathogenesis of ICE is not fully understood, and several hypotheses have been proposed. Among the proposed pathogenic mechanisms, the neural crest theory suggests that ICE is associated with the abnormal proliferation of neural crest cells (14). The membrane theory, proposed in 1978, suggests that the initial pathological insult is characterized by corneal endothelial degeneration. Subsequently, this condition progresses to the abnormal over-proliferation of the endothelial membrane surrounding the iridocorneal angle. The contraction of the endothelial membrane, along with the resulting obstruction of the trabecular meshwork, leads to structural alterations in the iris, secondary glaucoma, and ectropion uveae (15). Another pathogenic mechanism, regarded as a viral trigger factor, was proposed when herpes simplex virus DNA was detected in ICE samples (16). In recent study, Findings from multiple sequencing assays uniformly demonstrate that there is no convincing evidence to support viral infection in ICE syndrome. Nevertheless, the transcriptional analysis of ICE cells indicates a suppressed immune response (17).
3 Clinical presentation and characteristics
3.1 Chandler syndrome
Chandler syndrome (CS) is defined by unilateral reduced vision, corneal edema, epithelial bullae, a distinctive appearance of the corneal endothelium resembling hammered silver (Figure 1B), normal intraocular pressure (IOP) at initial examination, and slight iris atrophy (8). Compared to other types of ICE syndrome, CS typically involves less damage to the iris; however, it is frequently observed that the pupil shows ectopia (Figure 1A). When the diagnosis is made at advanced stages, more noticeable iris anomalies may be present, and areas of atrophy can be observed. However, these typically do not result in a complete iris hole (11).
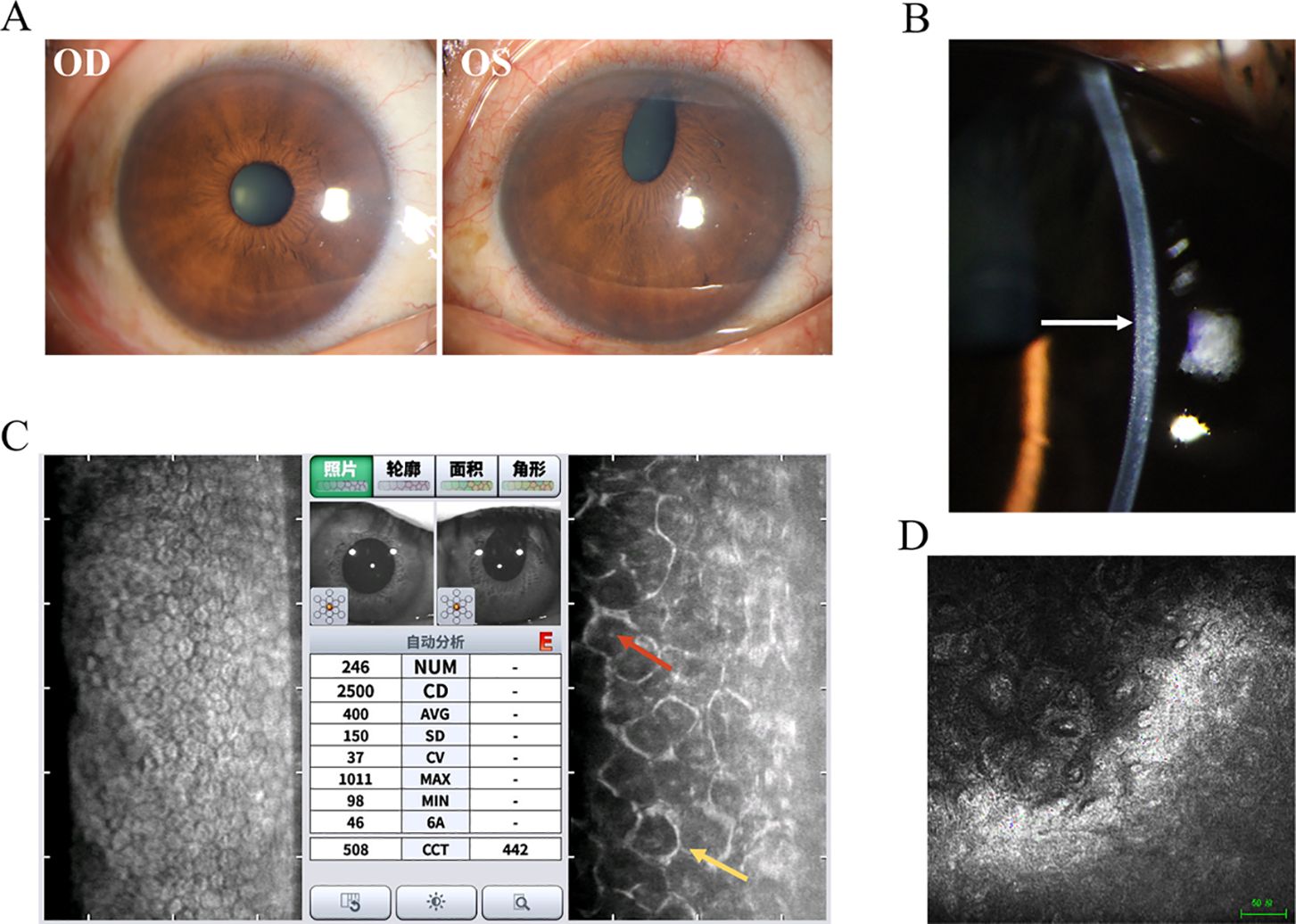
Figure 1. Clinical presentation and ancillary examination findings of Chandler syndrome. (A) Slit-lamp image of the left eye demonstrates an obliterated iris with a distorted pupil, while the right eye appears normal. (B) Slit-lamp image of the left eye reveals a distinct appearance of the corneal endothelium resembling hammered silver (white arrow). (C) Specular microscopy unveils characteristic dark-light reversal pattern (red and yellow arrow) in the corneal endothelial cells, known as "ICE cells". (D) Confocal microscopy identifies epithelioid endothelial cells with hyperreflective nuclei.
Auxiliary examination plays an irreplaceable role in the clinical diagnosis of ICE. Specular microscopy reveals the presence of ICE cells, characterized by a distinctive pattern of dark-light reversal (9, 18, 19). Notably, the cell surface appears dark rather than light, often exhibiting a centrally located, highly reflective nucleus. In contrast, the intercellular junctions appear light instead of dark (Figure 1C). Four different patterns of ICE cell distribution have been identified in the cornea. In “complete ICE,” the normal endothelium is entirely replaced by ICE cells. In “partial ICE (+),” ICE cells replace some of the endothelium, with the remaining portion consisting of small cells. In “partial ICE (–)”, ICE cells replace part of the endothelium. Still, the remaining portion consists of enlarged cells. Lastly, “scattered ICE” refers to individual or small clusters of ICE cells dispersed among normal endothelial cells (18, 20). In the setting of corneal edema, the specular microscope cannot adequately visualize the endothelial changes. However, revealing hyperreflective nuclei, confocal microscopy can still detect epithelioid endothelial cells while maintaining the tissue organization of the corneal endothelium and without the presence of inflammatory cells (21–23) (Figure 1D). Confocal microscopy also reveals two distinct patterns of alterations in the epithelioid-like endothelial cells in ICE syndrome: 1. The cells exhibit relatively regular size and shape, resembling normal endothelial cells. However, these are accompanied by a loss of normal hexagonality and prominent, uniform nuclei arranged in a “cobblestone-like” pattern at the center. 2. The endothelial cells display more irregular size and shape, with hyperreflective nuclei of diverse shapes located near the cell boundaries. The stromal nerves seem to exhibit a pseudo-thickening in comparison to the unaffected side (22, 23). Other imaging techniques, such as ultrasound biomicroscopy and anterior-segment optical coherence tomography (AS-OCT), can also be utilized to evaluate the angles in instances of ICE syndrome accompanied by corneal edema (22, 24).
3.2 Progressive iris atrophy
Progressive iris atrophy (PIA) is a disease that progresses slowly over time. Typically, the first clinical occurrence is the development of focal PAS (25). Consequently, proliferation of endothelial cells may play a role in the development of focal adhesions in a previously unobstructed angle, leading to further complications such as increased intraocular pressure and iris retraction (8) (Figures 2A, B). The clinical presentation of the cornea can exhibit significant variability. In some cases, a modification in its posterior aspect was demonstrated, while others may additionally manifest corneal edema (26). Changes in the iris are usually detected in the later stages of the disease, marked by the emergence of corectopia and regions of stromal thinning. The pupil is also generally pulled towards a noticeable PAS accompanied by ectropion uveae (15). The iris undergoes stretching on the side opposite to the direction of pupillary distortion, resulting in stromal atrophy and the formation of a “stretch hole” (27). As membranes shrink and lead to progressive synechia formation and pupillary displacement, tension is exerted on the iris. Ultimately, as synechial closure increases, tension on the stroma rises, leading to the breakdown of stromal collagen and the collapse of vessels. Thus, this process results in iris atrophy and the formation of full-thickness holes (11, 27). As mentioned previously, AS-OCT can be utilized for the visualization and accurate documentation of iris atrophy and iridocorneal synechiae caused by ICE syndrome (22).
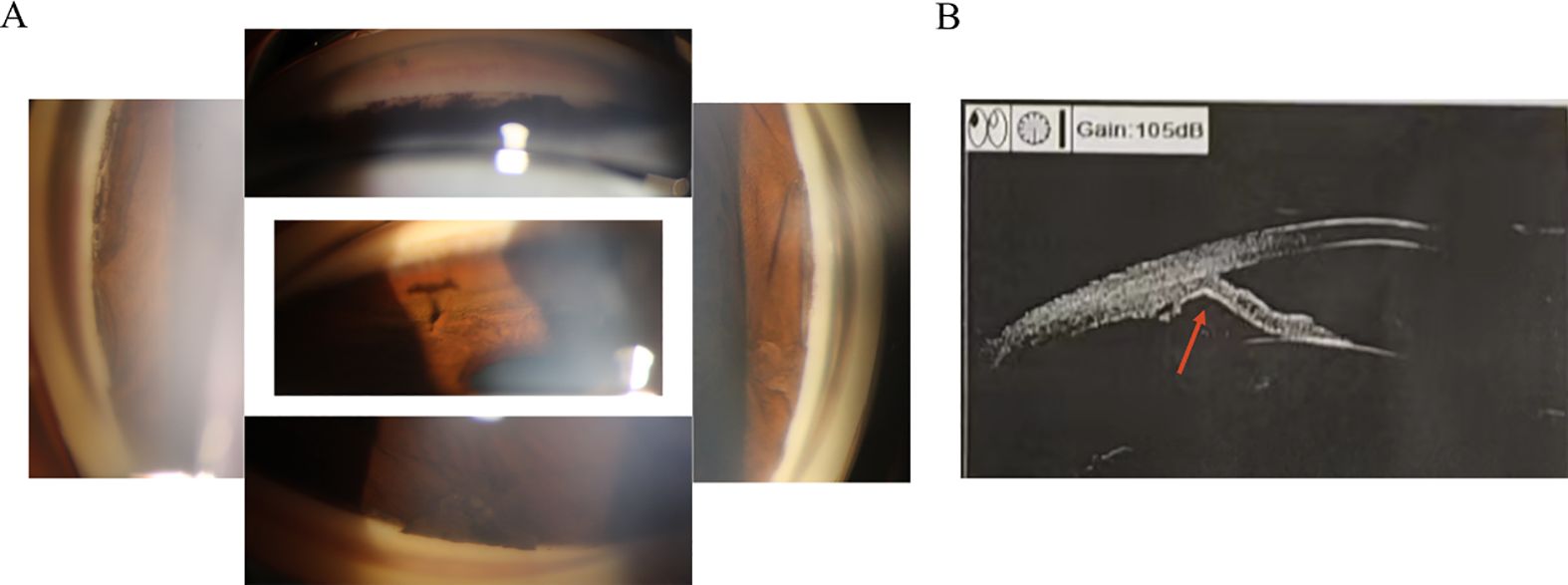
Figure 2. The development of focal peripheral anterior synechiae and causing angle closure of the iridocorneal endothelial syndrome. (A) Gonioscopy reveals the presence of local adhesion resulting in closed inspection angle. (B) Ultrasound biomicroscopy indicates iris local anterior adhesion (red arrow).
3.3 Cogan-Reese syndrome
The Cogan-Reese syndrome (CRS) is identified by distinctive pigmented nodules present on the iris and a disruption of the typical iris structure. The nodules might initially appear as a small number of delicate, light tan, or yellow bumps on the surface of the iris. The underlying tissue in the area where the nodules are located exhibits a distinct, tangled appearance and has lost its standard features. Iris nodules found in eyes with CRS are usually different, circular or flat, irregular, hyperpigmented spots (28). They are limited to the translucent membrane area and do not appear elsewhere on the iris surface (29). Scheie and Yanoff identified two different kinds of pigmented nodules on the iris in CRS. One type presents small, stalk-like nodules on the surface of the iris. The other type is characterized by a dense appearance within the iris stroma, with a velvety swirling surface and a loss of iris crypts. It is rare for both types of iris lesions to be present in the same eyes (30). Revealing iris nodules composed of polyhedral-to-fusiform melanocytic cells with surface microvilli and long, delicate interweaving dendritic-like processes are observed using transmission electron microscopy. These stromal cells cover long, stout, branching processes that appear to represent the underlying stromal cells of the iris (31). Teekhasaenee and Ritch noted a higher frequency of CRS in Asian eyes, particularly in those with dark brown irides. It is known that darker irides contain a greater amount of melanin pigment granules in the superficial stromal melanocytes compared to lighter irides. Likewise, it appears that a higher concentration of melanin in the iris is associated with an increased likelihood of nodules (29). This is due to alterations in the iris stroma and PAS, resulting in a loss of the typical iris pattern. The iris may appear smoother, with fewer crypts and a reduced pupillary ruff, while circular folds along the pupillary border become less prominent (30). The distortion of pupils is more pronounced in CRS and PIA compared to CS. Typically, the pupils tend to shift towards the location of the membrane and PAS. In CRS, the frequency and intensity of iris atrophy fall between those observed in CS and PIA (8).
4 Differential diagnosis
4.1 Axenfeld-Rieger syndrome
A hereditary condition affecting both eyes, usually inherited from a parent but can also occur randomly, and involves abnormalities in the iris such as irregular pupil shape, multiple pupils, and connections between the iris and the cornea leading to a corneal posterior embryotoxon (32, 33) (Figure 3A). The prominent histopathologic features of ARS include the anterior displacement of Schwalbe’s line and the presence of tissue strands that connect the peripheral iris to the corneal limbus (34). Although ICE and AR syndromes exhibit a single layer of cells resembling endothelial cells. They also exhibit a Descemet-like membrane that stretches from the cornea, through the anterior chamber angle, and onto the front surface of the iris. In ARS, it is believed that the membrane originates from the retention of the primordial endothelial layer lining the anterior chamber during gestation, rather than from the abnormal corneal endothelium acquired after birth, as in ICE syndrome (35). Different from ICE, ARS does not exhibit corneal endothelial changes. A notable distinction between the two conditions is the presence of a posterior embryotoxon with iris strands in ARS (8). In ICE syndrome, instead of iris strands, there may be PAS to the Schwalbe line or beyond, and a posterior embryotoxon is rarely observed (34). Furthermore, the mechanisms of glaucoma vary between the two conditions. A membrane over the trabecular meshwork or PAS in ICE syndrome causes secondary glaucoma. In contrast, maldevelopment of the trabecular meshwork and Schlemm canal, rather than associated iris strands, leads to secondary glaucoma in ARS (32, 34) (Figure 3B).
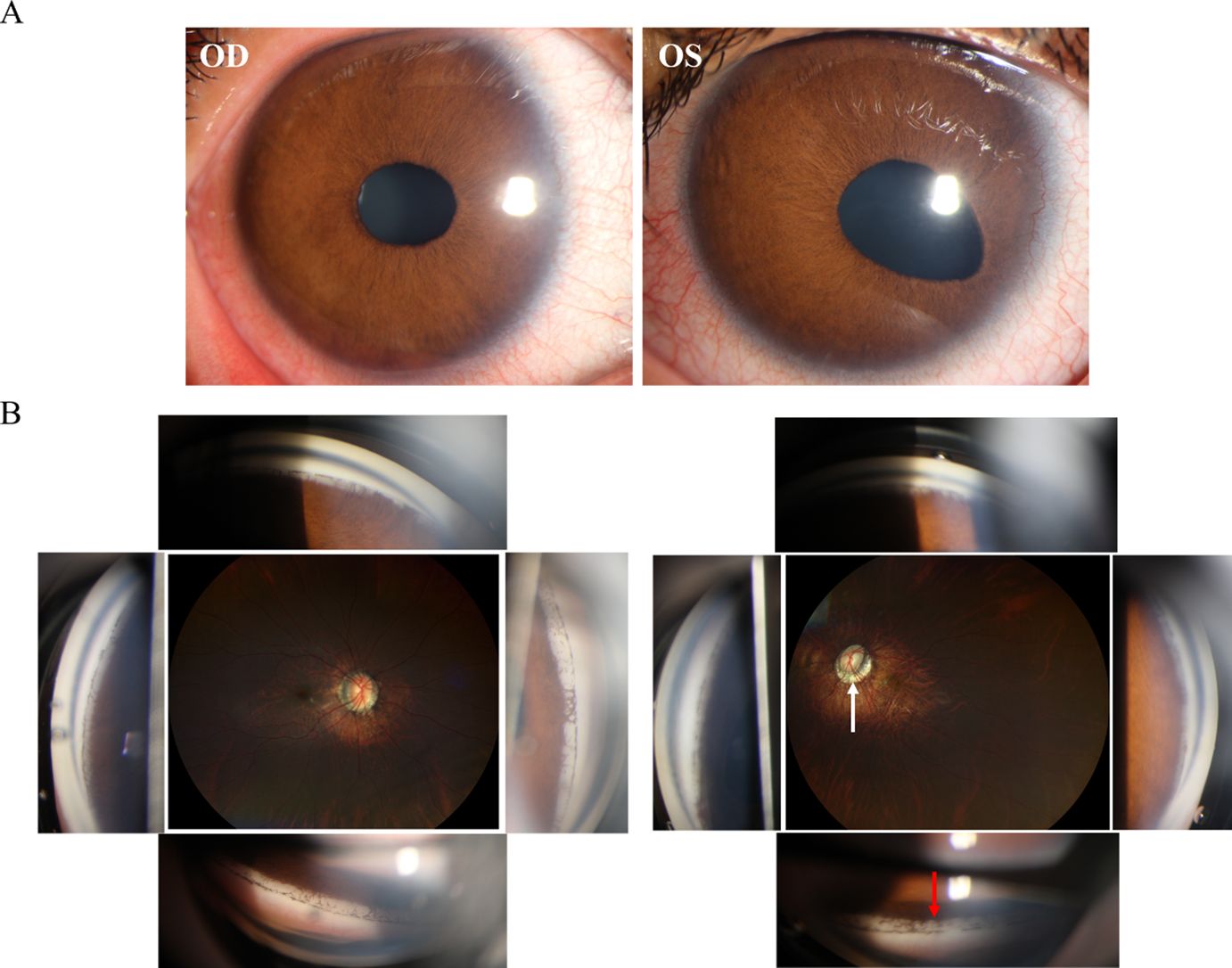
Figure 3. Clinical presentation and ancillary examination findings of Axenfeld-Rieger syndrome (ARS). (A) Slit-lamp image of both eyes special the left eye demonstrates an abnormal iris retraction with a displaced pupil. (B) Gonioscopy of an eye with ARS reveals distinctive iris strands connected to Schwalbe's line (red arrow).
Unlike ICE, ARS in pediatric patients displays a range of systemic observations that indicate abnormal neural crest cell development. Distinctive facial characteristics consist of an underdeveloped upper jaw, wide-set eyes, increased distance between the eyes, a thin upper lip, and a prominent forehead (33, 36) (Figures 4A–C). Referred to as maxillary and malar hypoplasia, dental abnormalities often manifest as hypodontia, oligodontia, and microdontia (36) (Figure 4C). These are commonly observed characteristics in patients. In addition to other systemic diseases associated with ARS, there may be abnormalities in the gastrointestinal system, excessive umbilical skin, skeletal and cranial abnormalities, as well as cardiac abnormalities (33, 36).
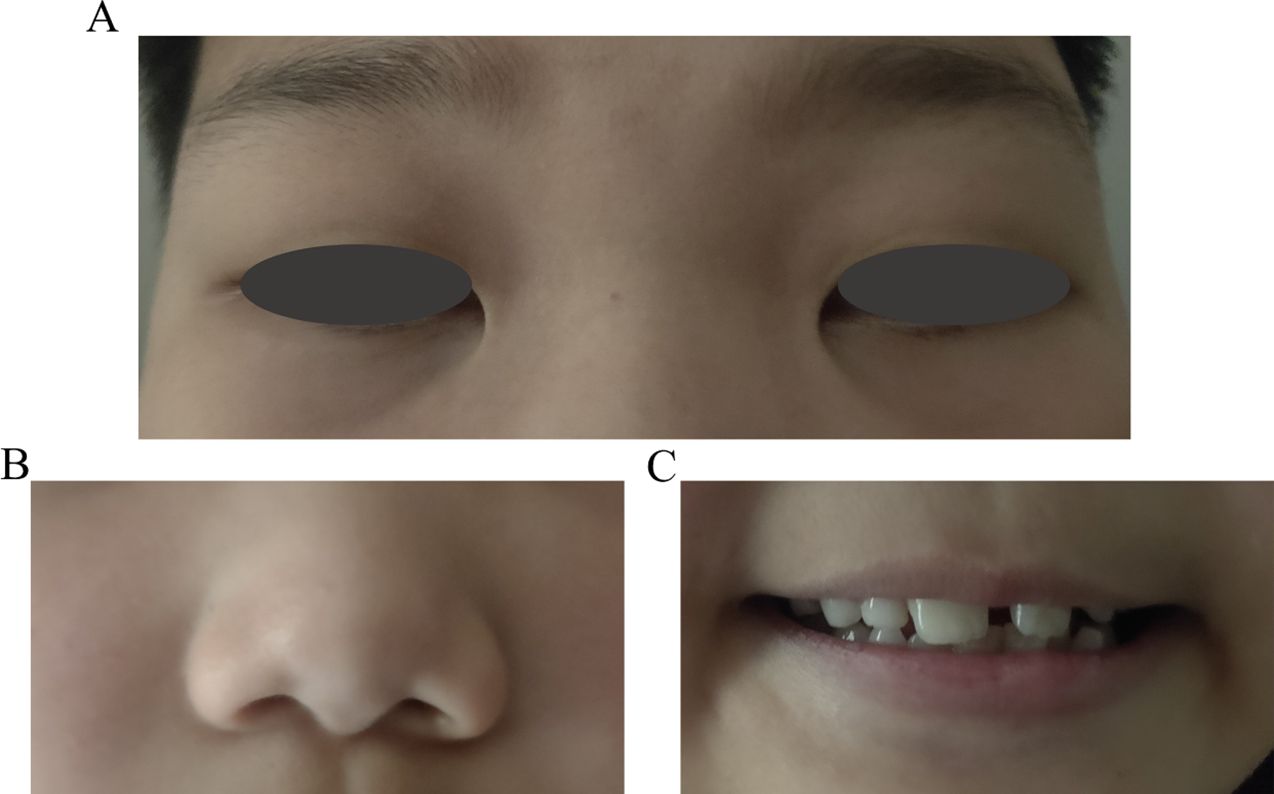
Figure 4. Pediatric patient’s facial characteristics in Axenfeld-Rieger syndrome (ARS). (A) The wide-set eyes of facial characteristics in ARS. (B) Thin upper lip of facial characteristics in ARS. (C) Dental abnormalities often manifest as hypodontia, oligodontia, and microdontia of ARS.
4.2 Posterior polymorphous corneal dystrophy
Also known as Schlichting dystrophy, PPCD is a typically bilateral and rare autosomal dominant disorder characterized by epithelial-like endothelial cells and a thickened, multilaminar posterior non-banded portion of Descemet’s membrane (37–40) (Figure 5A). Confocal microscopy reveals that the corneal epithelium is in a highly reflective state. At the level of the Descemet's membrane, hyperreflective, placoid or homocentric lesions can be detected, along with hyporeflective, oval or round ones. Additionally, at the level of the endothelial cell layer, hyporeflective, crater-like lesions are evident (40) (Figures 5B, C). Clinically, patients may demonstrate clustered vesicles, gray-white plaques, or broad bands with serrated edges on the endothelium (37–39). However, ICE syndrome is typically unilateral, does not have a hereditary pattern, and affects young women more frequently.
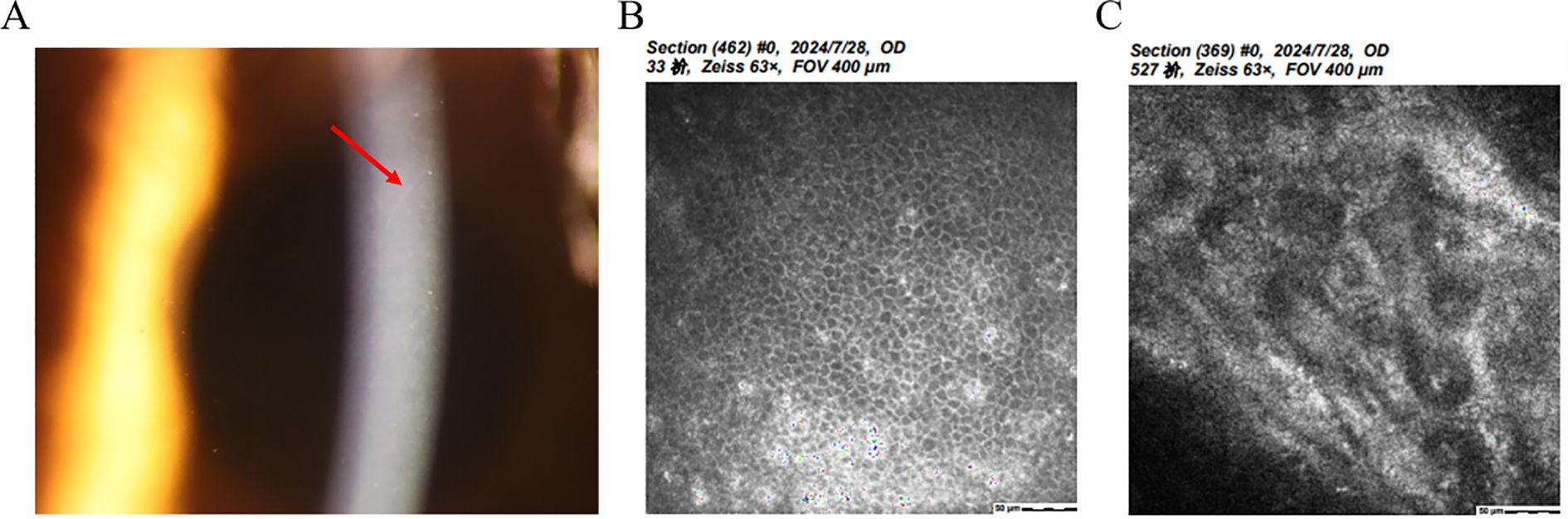
Figure 5. Clinical presentation and ancillary examination findings of Posterior polymorphous corneal dystrophy. (A) Slit-lamp image showing a thickened and multilaminar posterior non-banded portion of Descemet’s membrane and epithelium. (B) Specular microscopy reveals abnormal reflected signal of corneal epithelium. (C) Confocal microscopy demonstrates the presence of characteristic endothelial vesicular structures.
4.3 Fuchs endothelial corneal dystrophy
Fuchs Endothelial Corneal Dystrophy is a bilateral disease that progresses slowly and affects both eyes, with a higher prevalence in women aged between 50 and 60 years (41). Initially identified by Ernst Fuchs in 1910, the initial cases showed central clouding of the cornea, decreased corneal sensitivity, and development of epithelial bullae (42) (Figure 6A). Upon closer inspection, there is an evident reduction in endothelial cells and the development of guttae, which are abnormal deposits of extracellular matrix components in the Descemet membrane (41–43) (Figure 6B).
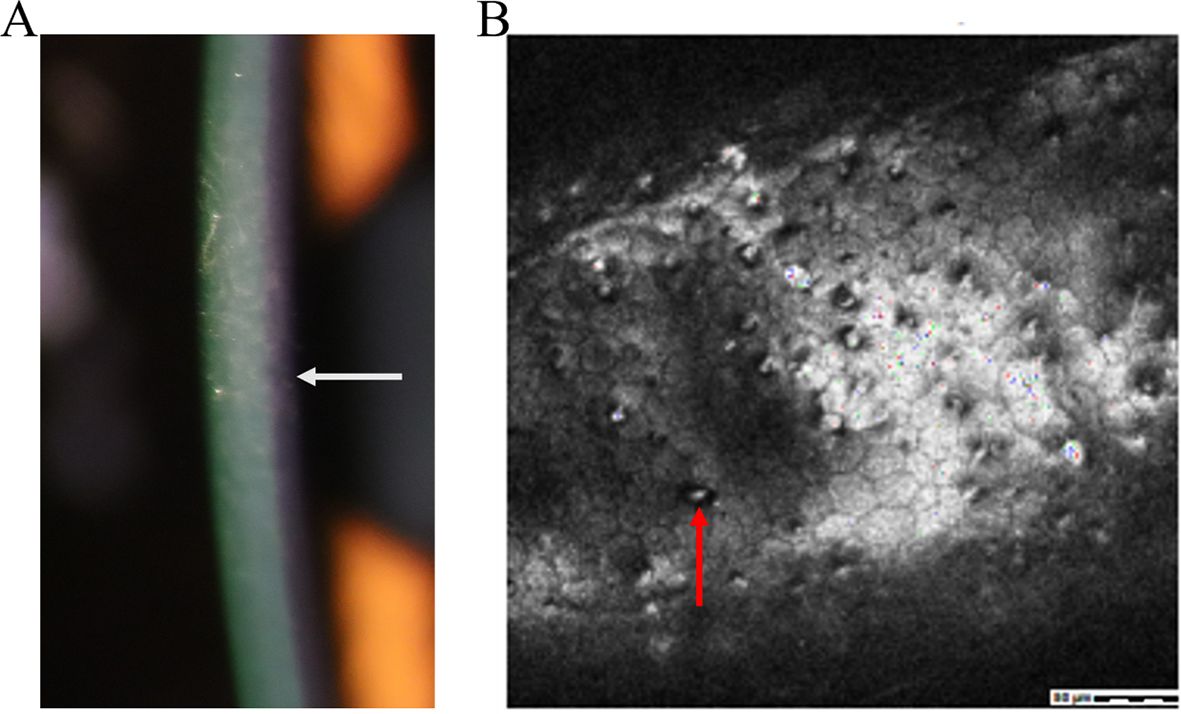
Figure 6. Clinical presentation and ancillary examination findings of Fuchs Endothelial Corneal Dystrophy. (A) Slit-lamp image the development of epithelial bullae (white arrow). (B) Confocal microscopy of epithelial bullae. abnormal deposits of extracellular matrix components in Descemet membrane (red arrow).
5 Treatment
The management of ICE syndrome can be classified into three primary domains, each addressing distinct facets of the condition. First, it is imperative to effectively address the degeneration of the cornea and any associated complications to preserve visual acuity and mitigate further ocular damage. Secondly, it is crucial to address iris atrophy and its impact on both the aesthetic appearance and visual function of patients with ICE syndrome. This may involve implementing cosmetic interventions to enhance the aesthetic appearance of the affected eye, as well as therapeutic measures to mitigate any visual disturbances resulting from alterations in iris morphology. Finally, it is imperative to manage glaucoma associated with ICE syndrome to prevent further damage to the optic nerve and preserve visual function. This may involve the use of pharmacological agents to reduce intraocular pressure or undergoing surgical interventions.
It is crucial to take a comprehensive approach that encompasses all three primary areas of treatment to effectively manage ICE syndrome and minimize its impact on a patient’s quality of life.
5.1 Corneal decompensation and its complications
As for corneal decompensation, penetrating keratoplasty (PK) has demonstrated favorable therapeutic outcomes. However, due to the relatively high rates of rejection and late endothelial failure, repeated surgeries are often indispensable for maintaining corneal clarity (44). When feasible, endothelial keratoplasty techniques are the preferred choices for addressing corneal edema associated with ICE syndrome. Compared to PK, Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) are considered more advantageous for ICE syndrome (45). DMEK is generally the preferred option over DSAEK due to its demonstrated superior outcomes, faster recovery, reduced risk of rejection, and minimized postoperative hyperopic shift in refraction. However, DSAEK remains the preferred option for ICE syndrome with significant iris abnormalities or extensive iridocorneal synechiae (46–48). In terms of graft survival, one study indicated that the median graft survival duration for PK was 8.9 years, whereas for DSAEK it was 4.9 years. Nevertheless, due to the insufficient incorporation of data in this research, the median graft survival rate of DMEK lacks statistical significance (45). Previously, DMEK has been demonstrated to have a 0.7% rejection rate, which is lower than PK, along with rapid visual recovery (49). Meanwhile, Chaurasia S and Jing Hong reported that Spokewise Iridotomy, used as an adjunctive surgery for DSAEK in ICE syndrome, resulted in significantly reduced short-term postoperative IOP, decreased AGMs, and PAS recurrence after EK, improved postoperative best corrected visual acuity, and an increased cumulative survival rate of grafts (50, 51). Furthermore, triple procedures (phacoemulsification, intraocular lens insertion, and DSAEK) have shown successful outcomes in CS cases (52). Recently, a case report was published regarding an ICE patient who successfully underwent phacoemulsification, intraocular lens and iris prosthesis implantation into the capsular bag, followed by DSAEK surgery (53). Quek and colleagues found a higher rate of graft failure in essential iris atrophy compared to CS and CRS. This is expected, as eyes with crucial iris atrophy tend to be more aggressive and increase PAS formation (54). (Figure 7 shows the surgical method and resection range of PK, DSEK, or DMEK).
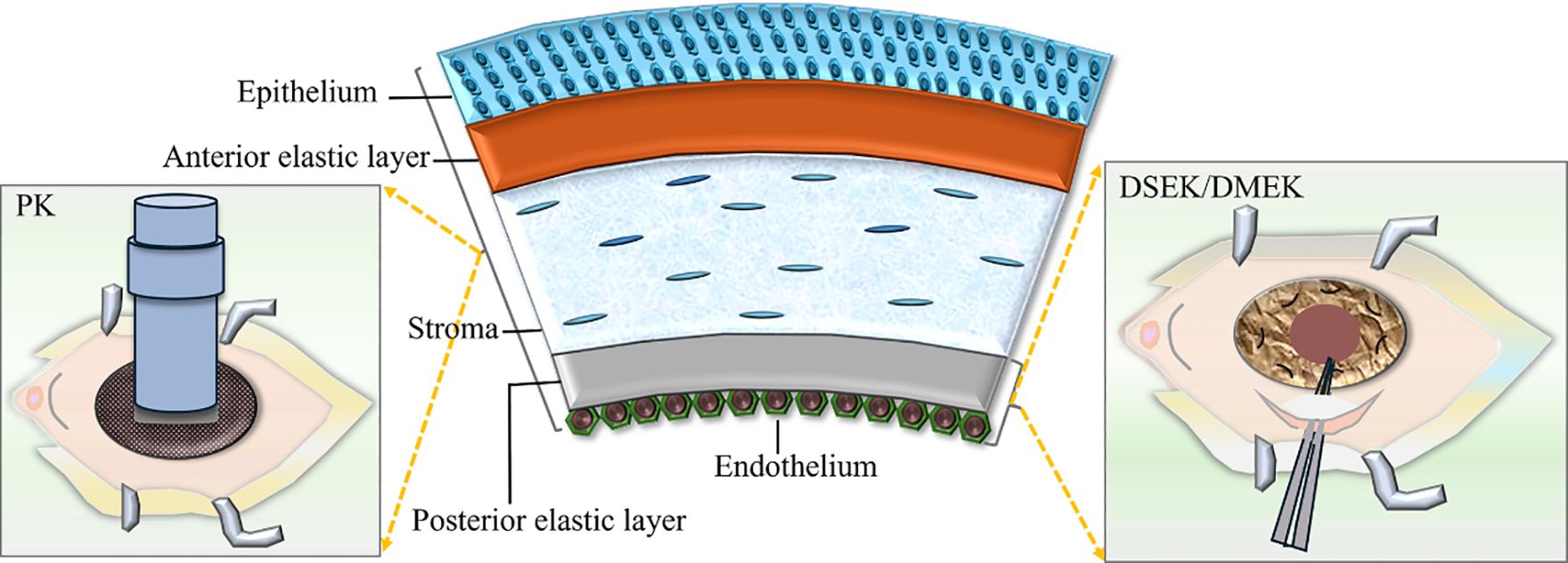
Figure 7. The surgical method and resection range of Penetrating keratoplasty (PK), Descemet stripping endothelial keratoplasty (DSEK) or Descemet membrane endothelial keratoplasty (DMEK).
Patients who have undergone multiple unsuccessful keratoplasties may consider keratoprosthesis as a potential solution for vision restoration (55). Furthermore, cell substitution therapy has demonstrated promising therapeutic outcomes in animal experiments. Notably, this approach involves the injection of cultured human corneal endothelial cells into the anterior chamber, showing promise in eliminating ICE cells by replacing diseased corneal endothelial cells with healthy ones after scraping them (56).
5.2 Iris atrophy on appearance and vision
Femtosecond-assisted keratopigmentation is a novel method for improving the appearance of opaque corneas for cosmetic objectives (57). Femtosecond laser technology, which involves intralamellar dissections, can enter the anterior chamber to eliminate degenerated sections of the iris that obstruct the visual pathway. Remarkable postoperative visual acuity outcomes have been reported (13, 58). Pupil ectopia or stroma opacity may lead to issues such as glare and monocular diplopia; a multipiece endocapsular prosthesis for iris reconstruction is an alternative treatment option. And a studies reported that combining cataract extraction with iris reconstruction using a multipiece endocapsular iris prosthesis can help alleviate symptoms and improve appearance (59).
5.3 Secondary glaucoma linked to ICE syndrome
The management of glaucoma secondary to iridocorneal endothelial syndrome (GS-ICE) is highly complex, and achieving therapeutic efficiency through simple pharmacological interventions is a significant challenge. In many cases, surgical intervention is frequently warranted. Common surgical modalities include trabeculectomy, implantation of a glaucoma drainage valve, and penetrating canaloplasty (PCP), among others. (Figure 8 shows the optic nerve damage caused by high IOP in glaucoma (Figure 8A) and the surgical type and primary method of GS-ICE (Figure 8B)).
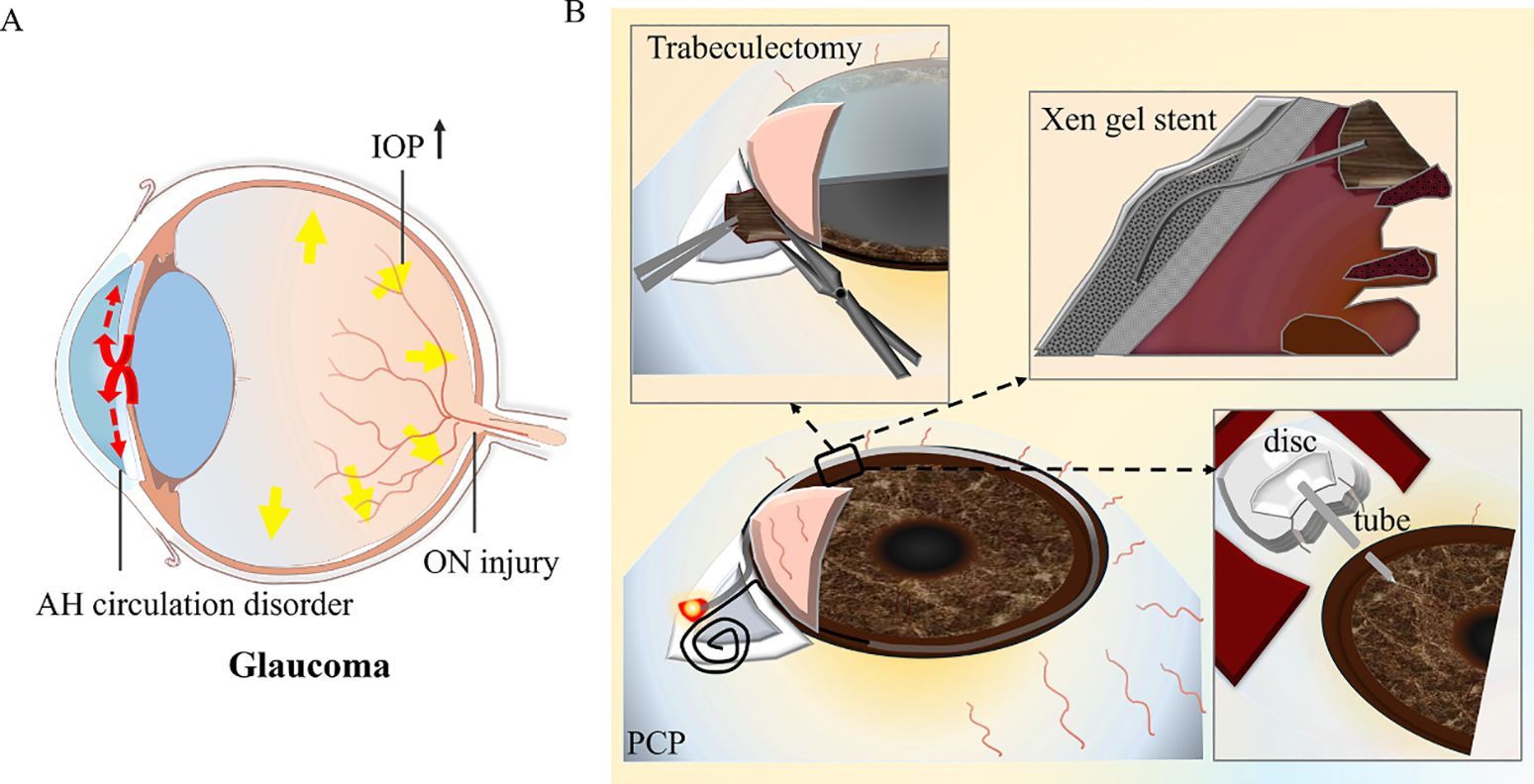
Figure 8. The surgical method and resection range of glaucoma secondary to iridocorneal endothelial syndrome. (A) The diagram depicts the optic nerve damage caused by elevated intraocular pressure in glaucoma. (B) The diagram indicates the surgical type and primary method. AH, aqueous humor; IOP, intraocular pressure; ON, optical nerve; PCP, penetrating canaloplasty.
Trabeculectomy with mitomycin C or 5-fluorouracil shows a reasonable level of success in the intermediate term for GS-ICE; However, its outcomes are less favorable when compared to juvenile, pigmentary, or primary open-angle glaucoma, likely due to the proliferation of endothelium and the production of abnormal basement membrane within the filtering blebs (18, 60). Glaucoma drainage devices (GDDs) provide an alternative pathway for the outflow of aqueous humor from the anterior chamber, guiding it through a tube to a subconjunctival bleb or to the suprachoroidal space. This approach circumvents the primary concern associated with filtration procedures, namely, the formation of an ICE membrane over the ostium for filtration. It appears to yield a higher success rate for GS-ICE compared to trabeculectomy (61). The implantation of the Ahmed glaucoma valve, Ex-PRESS mini-shunt, or microbypass Xen gel stent (collagen-based gelatin tube) has been demonstrated as a safe and effective approach for the management of GS-ICE (62–64). The primary cause for the failure of aqueous shunt surgery is the blockage of the tube opening by the iris, ICE membrane, or tube displacement. In pseudophakic eyes, we recommend inserting the tube through the ciliary sulcus to reduce the risk of corneal decompensation due to tube-cornea contact and lower the risk of iris damage or iris dialysis in eyes with significant PAS, which is also common in GS-ICE, particularly PIA or CRS (65). Repositioning the tube posterior to the iris may also have the potential to avoid blockage of the inner opening by ICE membrane movement from the iridocorneal angle, as previously reported.
PCP, a novel internal drainage procedure proposed by Yuanbo Liang in 2015, which expands the collapsed Schlemm tube and communicates the anterior chamber and Schlemm tube by excising local trabecular reticulum and peripheral iris, effectively reducing intraocular pressure in ICE syndrome (66, 67). Recently, retrocorneal membrane interception-enhanced PCP has exhibited promising outcomes in the treatment of GS-ICE with open-angle or small PAS for up to one year of follow-up, demonstrating both effectiveness and safety (25).
6 Prognosis
The prognosis of the ICE syndrome depends on the disease stage at diagnosis and the presence of secondary complications. Although a variety of treatment modalities are available, corneal surgery may not completely eradicate abnormal endothelial cells. As a result, it is difficult to prevent the progression of PAS or the occurrence of secondary glaucoma (18).
7 Conclusion
The ICE syndrome is a complex condition affecting both the cornea and iris, predominantly observed in young females. The hallmark of ICE syndrome is that the corneal endothelial cells migrate towards the iridocorneal angle and onto the iris. Diagnosis is primarily based on the identification of corneal and iris lesions, with auxiliary diagnostic tools including specular microscopy and confocal microscopy. As for CS, Specular microscopy and in vivo confocal microscopy may reveal the presence of “ICE cells”. When the cornea is involved, options such as DSAEK and DMEK may be considered for optimal management of conditions like corneal edema or decompensation. In instances where the iris is affected by an iris cavity, multiple pupils, or photophobia, Femtosecond-assisted keratopigmentation may be considered a viable treatment modality. In cases of GS-ICE, trabeculectomy and glaucoma drainage device implantation have been shown to effectively reduce IOP, while PCP, especially retrocorneal membrane interception-enhanced PCP, has demonstrated significant efficacy.
Author contributions
HM: Writing – original draft, Writing – review & editing. MX: Writing – original draft, Writing – review & editing. QG: Writing – original draft. LZ: Writing – original draft. SH: Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This review was funded by the Natural Science Foundation of Ningxia Province of China (Grant No. 2024AAC303822, 2024AAC03823), the Natural Science Foundation of Hunan Province of China (Grant No. 2024JJ9020), and Clinic Research Foundation of Aier Eye Hospital Group (Grant No. AMF2406D03).
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Conflict of interest
Author MX was employed by Aier Eye Hospital Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2025.1655669/full#supplementary-material
References
1. Beganovic AP, Vodencarevic AN, Halilbasic M, and Medjedovic A. Iridocorneal endothelial syndrome: case report of essential progressive iris atrophy. Med Arch. (2022) 76:224–8. doi: 10.5455/medarh.2022.76.224-228
2. Dubey S, Jain K, Singh S, and Mukhejee S. Iridocorneal endothelial syndrome with coexisting macular edema and neurosensory detachment: an unusual case report. J Curr Glaucoma Pract. (2021) 15:149–52. doi: 10.5005/jp-journals-10078-1315
3. Malhotra C, Seth NG, Pandav SS, Jain AK, Kaushik S, Gupta A, et al. Iridocorneal endothelial syndrome: Evaluation of patient demographics and endothelial morphology by in vivo confocal microscopy in an Indian cohort. Indian J Ophthalmol. (2019) 67:604–10. doi: 10.4103/ijo.IJO_1237_18
4. Aponte EP, Ball DC, and Alward WL. Iridocorneal endothelial syndrome in a 14-year-old male. J Glaucoma. (2016) 25:e115–6. doi: 10.1097/IJG.0000000000000288
5. Tang W, Wang Q, Zhang Q, Sun S, Zhang Y, and Wu Z. Iridocorneal endothelial syndrome in a Chinese child. Eye Sci. (2013) 28:153–6. doi: 10.3969/j.issn.1000-4432.2013.03.009
6. Olawoye O, Teng CC, Liebmann JM, Wang FM, and Ritch R. Iridocorneal endothelial syndrome in a 16-year-old. J Glaucoma. (2011) 20:294–7. doi: 10.1097/IJG.0b013e3181e664b0
7. Salim S, Shields MB, and Walton D. Iridocorneal endothelial syndrome in a child. J Pediatr Ophthalmol Strabismus. (2006) 43:308–10. doi: 10.3928/01913913-20060901-06
8. Silva L, Najafi A, Suwan Y, Teekhasaenee C, and Ritch R. The iridocorneal endothelial syndrome. Surv Ophthalmol. (2018) 63:665–76. doi: 10.1016/j.survophthal.2018.01.001
9. Levy SG, McCartney AC, Baghai MH, Barrett MC, and Moss J. Pathology of the iridocorneal-endothelial syndrome. ICE-cell. Invest Ophthalmol Vis Sci. (1995) 36:2592–601. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2179822
10. Hirst LW, Bancroft J, Yamauchi K, and Green WR. Immunohistochemical pathology of the corneal endothelium in iridocorneal endothelial syndrome. Invest Ophthalmol Vis Sci. (1995) 36:820–7. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2161231
11. Azari AA, Rezaei Kanavi M, Thompson MJ, Altaweel MM, Potter HD, and Albert DM. Iridocorneal endothelial syndrome. JAMA Ophthalmol. (2014) 132:56. doi: 10.1001/jamaophthalmol.2013.247
12. Bourne WM and Brubaker RF. Decreased endothelial permeability in the iridocorneal endothelial syndrome. Ophthalmology. (1982) 89:591–5. doi: 10.1016/s0161-6420(82)34745-4
13. Walkden A and Au L. Iridocorneal endothelial syndrome: clinical perspectives. Clin Ophthalmol. (2018) 12:657–64. doi: 10.2147/OPTH.S143132
14. Bahn CF, Falls HF, Varley GA, Meyer RF, Edelhauser HF, and Bourne WM. Classification of corneal endothelial disorders based on neural crest origin. Ophthalmology. (1984) 91:558–63. doi: 10.1016/s0161-6420(84)34249-x
15. Campbell DG, Shields MB, and Smith TR. The corneal endothelium and the spectrum of essential iris atrophy. Am J Ophthalmol. (1978) 86:317–24. doi: 10.1016/0002-9394(78)90232-5
16. Li F, Liu Y, Sun Y, and Zhang X. Etiological mechanism of iridocorneal endothelial (ICE) syndrome may involve infection of herpes simplex virus (HSV) and integration of viral genes into human genome. Med Hypotheses. (2018) 110:50–2. doi: 10.1016/j.mehy.2017.10.023
17. Zhang J, Yuan B, Peng R, Zhang P, Liu X, Qu Y, et al. Etiology of iridocorneal endothelial syndrome: viral infection and immune suppression. Cornea. (2025) 00:1-13. doi: 10.1097/ICO.0000000000003911
18. Sacchetti M, Mantelli F, Marenco M, Macchi I, Ambrosio O, and Rama P. Diagnosis and management of iridocorneal endothelial syndrome. BioMed Res Int. (2015) 2015:763093. doi: 10.1155/2015/763093
19. Chaurasia S, Choudhari NS, and Mohamed A. Clinical and Specular microscopy characteristics and corelation in Iridocorneal endothelial syndrome without corneal edema. Semin Ophthalmol. (2021) 36:561–8. doi: 10.1080/08820538.2021.1900285
20. Malhotra C, Pandav SS, Gupta A, and Jain AK. Phenotypic heterogeneity of corneal endothelium in iridocorneal endothelial syndrome by in vivo confocal microscopy. Cornea. (2014) 33:634–7. doi: 10.1097/ICO.0000000000000122
21. Chaurasia S and Vanathi M. Specular microscopy in clinical practice. Indian J Ophthalmol. (2021) 69:517–24. doi: 10.4103/ijo.IJO_574_20
22. Güler Canözer G, Tınkır Kayıtmazbatır E, Öztürk E, Bozkurt Oflaz A, and Bozkurt B. In vivo confocal microscopy and anterior segment optical coherence tomography findings of patients with iridocorneal endothelial syndrome. Turk J Ophthalmol. (2024) 54:170–4. doi: 10.4274/tjo.galenos.2024.78861
23. Ratra V. Commentary: In vivo confocal microscopy in iridocorneal endothelial syndrome. Indian J Ophthalmol. (2019) 67:610–1. doi: 10.4103/ijo.IJO_154_19
24. Ichhpujani P, Kaushik S, Gupta A, and Pandav SS. Bilateral Chandler’s syndrome: Uncommon entity diagnosed by ultrasound biomicroscopy and confocal microscopy. Indian J Ophthalmol. (2020) 68:528–9. doi: 10.4103/ijo.IJO_1123_19
25. Zhou M, Zhu S, Li H, Ye W, Xu S, Lin H, et al. Retrocorneal membrane interception enhanced penetrating canaloplasty for patients with open angle glaucoma secondary to ICE syndrome. Int Ophthalmol. (2024) 44:395. doi: 10.1007/s10792-024-03211-9
26. Lee GA. Management of iridocorneal endothelial syndrome from a corneal and glaucoma perspective. Clin Exp Ophthalmol. (2024) 52:491–2. doi: 10.1111/ceo.14372
28. Díaz Barrón A, Hervás Hernandis JM, and Duch-Samper AM. Clinical description of a Cogan-Reese type iridocorneal endothelial syndrome using anterior segment optical coherence tomography and specular microscopy. Arch Soc Esp Oftalmol (Engl Ed). (2020) 95:e72. doi: 10.1016/j.oftal.2020.03.006
29. Teekhasaenee C and Ritch R. Iridocorneal endothelial syndrome in Thai patients: clinical variations. Arch Ophthalmol. (2000) 118:187–92. doi: 10.1001/archopht.118.2.187
30. Scheie HG and Yanoff M. Iris nevus (Cogan-Reese) syndrome. A cause unilateral glaucoma. Arch Ophthalmol. (1975) 93:963–70. doi: 10.1001/archopht.1975.01010020761004
31. Behera G, Nag TC, Khokhar SK, and Sangaraju S. Electron microscopy in Cogan-Reese syndrome. Indian J Ophthalmol. (2022) 70:2666–8. doi: 10.4103/ijo.IJO_2777_21
32. Michels K and Bohnsack BL. Ophthalmological manifestations of axenfeld-rieger syndrome: current perspectives. Clin Ophthalmol. (2023) 17:819–28. doi: 10.2147/OPTH.S379853
33. Khandwala NS, Ramappa M, Edward DP, and Mocan MC. Axenfeld-Rieger syndrome in the pediatric population: A review. Taiwan J Ophthalmol. (2023) 13:417–24. doi: 10.4103/tjo.TJO-D-23-00089
34. Yu T, Dai Z, Peng R, Xiao G, Zhang P, Ma S, et al. Axenfeld-Rieger syndrome: a novel histopathologic finding associated with corneal abnormalities. BMC Ophthalmol. (2022) 22:514. doi: 10.1186/s12886-022-02754-8
35. Seifi M and Walter MA. Axenfeld-rieger syndrome. Clin Genet. (2018) 93:1123–30. doi: 10.1111/cge.13148
36. Reis LM, Maheshwari M, Capasso J, Atilla H, Dudakova L, Thompson S, et al. Axenfeld-Rieger syndrome: more than meets the eye. J Med Genet. (2023) 60:368–79. doi: 10.1136/jmg-2022-108646
37. Liskova P, Hafford-Tear NJ, Skalicka P, Malinka F, Jedlickova J, Ďuďáková ĽChecktae, et al. Posterior corneal vesicles are not associated with the genetic variants that cause posterior polymorphous corneal dystrophy. Acta Ophthalmol. (2022) 100:e1426–1426e1430. doi: 10.1111/aos.15114
38. Del Turco C, Pierro L, Querques G, Gagliardi M, Corvi F, Manitto MP, et al. Posterior polymorphous corneal dystrophy concomitant to large colloid drusen. Eur J Ophthalmol. (2015) 25:177–9. doi: 10.5301/ejo.5000526
39. Fernández-Gutiérrez E, Fernández-Pérez P, Boto-De-Los-Bueis A, García-Fernández L, Rodríguez-Solana P, Solís M, et al. Posterior polymorphous corneal dystrophy in a patient with a novel ZEB1 gene mutation. Int J Mol Sci. (2022) 24:209. doi: 10.3390/ijms24010209
40. Gu SF, Peng RM, Xiao GG, and Hong J. Imaging features of posterior polymorphous corneal dystrophy observed by in vivo confocal microscopy. Zhonghua Yan Ke Za Zhi. (2022) 58:103–11. doi: 10.3760/cma.j.cn112142-20210228-00099
41. Ong Tone S, Kocaba V, Böhm M, Wylegala A, White TL, and Jurkunas UV. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog Retin Eye Res. (2021) 80:100863. doi: 10.1016/j.preteyeres.2020.100863
42. Elhalis H, Azizi B, and Jurkunas UV. Fuchs endothelial corneal dystrophy. Ocul Surf. (2010) 8:173–84. doi: 10.1016/s1542-0124(12)70232-x
43. Matthaei M, Hribek A, Clahsen T, Bachmann B, Cursiefen C, and Jun AS. Fuchs endothelial corneal dystrophy: clinical, genetic, pathophysiologic, and therapeutic aspects. Annu Rev Vis Sci. (2019) 5:151–75. doi: 10.1146/annurev-vision-091718-014852
44. Tóth G, Váncsa S, Kói T, Kormányos K, Hegyi P, and Szentmáry N. Outcomes of penetrating keratoplasty versus lamellar endothelial keratoplasty in iridocorneal endothelial syndrome: A systematic review and meta-analysis. Am J Ophthalmol. (2025) 276:218–29. doi: 10.1016/j.ajo.2025.04.017
45. Roberts PK, Keane M, Yang G, Chan E, Harkin DG, McKirdy N, et al. Comparison of penetrating and endothelial keratoplasty in patients with iridocorneal endothelial syndrome: A registry study. Clin Exp Ophthalmol. (2023) 51:663–72. doi: 10.1111/ceo.14283
46. Fajgenbaum MA and Hollick EJ. Descemet stripping endothelial keratoplasty in iridocorneal endothelial syndrome: postoperative complications and long-term outcomes. Cornea. (2015) 34:1252–8. doi: 10.1097/ICO.0000000000000530
47. Ao M, Feng Y, Xiao G, Xu Y, and Hong J. Clinical outcome of Descemet stripping automated endothelial keratoplasty in 18 cases with iridocorneal endothelial syndrome. Eye (Lond). (2018) 32:679–86. doi: 10.1038/eye.2017.282
48. Ghaznawi N and Chen ES. Descemet’s stripping automated endothelial keratoplasty: innovations in surgical technique. Curr Opin Ophthalmol. (2010) 21:283–7. doi: 10.1097/ICU.0b013e32833a8cc9
49. Saleki M, Lee P, Thaung C, and Ashena Z. Descemet’s membrane endothelial keratoplasty in an eye with iridocorneal endothelial syndrome and rare association of corneal ectasia. Ther Adv Ophthalmol. (2025) 17:25158414251343968. doi: 10.1177/25158414251343968
50. Chaurasia S, Senthil S, and Choudhari N. Outcomes of Descemet stripping endothelial keratoplasty combined with near total iridectomy in iridocorneal endothelial syndrome. BMJ Case Rep. (2021) 14:e240988. doi: 10.1136/bcr-2020-240988
51. Zhang J, Peng R, Xiao G, Wang M, and Hong J. Spokewise iridotomy combined with Descemet stripping automated endothelial keratoplasty in iridocorneal endothelial syndrome. Front Med (Lausanne). (2023) 10:1187009. doi: 10.3389/fmed.2023.1187009
52. Kymionis GD, Kontadakis GA, Agorogiannis GI, Bennett M, and Angelidou F. Descemet stripping automated endothelial keratoplasty combined with phacoemulsification in Chandler syndrome. Eur J Ophthalmol. (2011) 21:495–7. doi: 10.5301/EJO.2010.6210
53. Rahimi M, Panahi Bazaz M, Sharifipour F, Hajizadeh M, and Cheraghian B. Corneal biomechanical changes after Descemet stripping automated endothelial keratoplasty, penetrating keratoplasty, and phacoemulsification. Int Ophthalmol. (2022) 42:3183–90. doi: 10.1007/s10792-022-02318-1
54. Quek DT, Wong CW, Wong TT, Han SB, Htoon HM, Ho CL, et al. Graft failure and intraocular pressure control after keratoplasty in iridocorneal endothelial syndrome. Am J Ophthalmol. (2015) 160:422–9.e1. doi: 10.1016/j.ajo.2015.05.024
55. Phillips DL, Goins KM, Greiner MA, Alward WL, Kwon YH, and Wagoner MD. Boston type 1 keratoprosthesis for iridocorneal endothelial syndromes. Cornea. (2015) 34:1383–6. doi: 10.1097/ICO.0000000000000616
56. Ng XY, Peh G, Yam GH, Tay HG, and Mehta JS. Corneal endothelial-like cells derived from induced pluripotent stem cells for cell therapy. Int J Mol Sci. (2023) 24:12433. doi: 10.3390/ijms241512433
57. Hasani H, Es’haghi A, Rafatnia S, Alilou S, and Abolmaali M. Keratopigmentation: a comprehensive review. Eye (Lond). (2020) 34:1039–46. doi: 10.1038/s41433-019-0750-2
58. Alió JL, Rodriguez AE, Toffaha BT, Piñero DP, and Moreno LJ. Femtosecond-assisted keratopigmentation for functional and cosmetic restoration in essential iris atrophy. J Cataract Refract Surg. (2011) 37:1744–7. doi: 10.1016/j.jcrs.2011.08.003
59. Gour A, Tibrewal S, Garg A, Vohra M, Ratna R, and Sangwan VS. New horizons in aniridia management: Clinical insights and therapeutic advances. Taiwan J Ophthalmol. (2023) 13:467–78. doi: 10.4103/tjo.TJO-D-23-00140
60. Chandran P, Rao HL, Mandal AK, Choudhari NS, Garudadri CS, and Senthil S. Outcomes of primary trabeculectomy with mitomycin-C in glaucoma secondary to iridocorneal endothelial syndrome. J Glaucoma. (2016) 25:e652–6. doi: 10.1097/IJG.0000000000000268
61. Imamoglu S, Sevim MS, Yıldız HE, Vural ET, Bardak H, and Bardak Y. Surgical outcomes of patients with iridocorneal endothelial syndrome: a case series. Int Ophthalmol. (2017) 37:607–13. doi: 10.1007/s10792-016-0317-1
62. Mao Z, Guo X, Zhong Y, and Liu X. Surgical outcomes of Ahmed glaucoma valve implantation in patients with glaucoma secondary to iridocorneal endothelial syndrome. Eye (Lond). (2021) 35:608–15. doi: 10.1038/s41433-020-0912-2
63. Hohberger B, Welge-Lüen UC, and Lämmer R. ICE-syndrome: A case report of implantation of a microbypass xen gel stent after DMEK transplantation. J Glaucoma. (2017) 26:e103–103e104. doi: 10.1097/IJG.0000000000000584
64. Colás-Tomás T and López Tizón E. Ex-PRESS mini-shunt implanted in a pregnant patient with iridocorneal endothelial syndrome. Eur J Ophthalmol. (2020) 30:NP25–25NP28. doi: 10.1177/1120672118820508
65. Sun Y, Duan X, Fang Y, and Tang X. Long-term surgical outcomes of combined Ahmed glaucoma valve implantation and phacoemulsification with intraocular lens implantation for patients with glaucoma secondary to iridocorneal endothelial syndrome. BMC Ophthalmol. (2024) 24:476. doi: 10.1186/s12886-024-03740-y
66. Zhang S, Hu C, Cheng H, Gu J, Samuel K, Lin H, et al. Efficacy of bleb-independent penetrating canaloplasty in primary angle-closure glaucoma: one-year results. Acta Ophthalmol. (2022) 100:e213–213e220. doi: 10.1111/aos.14869
Keywords: iridocorneal endothelial syndrome, progressive iris atrophy, Chandler syndrome, Cogan-Reese syndrome, glaucoma
Citation: Ma H, Xia M, Gu Q, Zheng L and Ha S (2025) Iridocorneal endothelial syndrome. Front. Ophthalmol. 5:1655669. doi: 10.3389/fopht.2025.1655669
Received: 28 June 2025; Accepted: 15 September 2025;
Published: 07 October 2025.
Edited by:
Luciano Quaranta, University of Pavia, ItalyReviewed by:
Parul Ichhpujani, Government Medical College and Hospital, IndiaSebastião Cronemberger, Federal University of Minas Gerais, Brazil
Copyright © 2025 Ma, Xia, Gu, Zheng and Ha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoping Ha, aGFzaGFvcGluZ0AxNjMuY29t
†These authors have contributed equally to this work
 Hua Ma
Hua Ma Mingfang Xia
Mingfang Xia Qing Gu1
Qing Gu1