- 1Department of Ophthalmology, Tokushima Red Cross Hospital, Komatsushima, Japan
- 2Department of Ophthalmology, Tokushima University Graduate School, Tokushima, Japan
Background: Central retinal artery occlusion (CRAO) is a vision-threatening emergency with no established effective treatment. Prostaglandin E1 (PGE1), known for its vasodilatory and cytoprotective properties, may offer therapeutic benefits for retinal ischemia.
Methods: In this retrospective study, we compared visual outcomes between CRAO patients who received intravenous PGE1 within 24 hours of symptom onset (followed by oral administration) and those who received conventional therapy. PGE1 was administered intravenously for 5 days.
Results: At one month, the PGE1 group showed significantly better best-corrected visual acuity compared to the control group. Baseline structural retinal parameters, including maximal retinal thickness (MRT) and central retinal thickness (CRT), did not differ significantly between groups. In the PGE1 group, baseline MRT was negatively correlated with visual acuity at one month. Retinal arteriovenous diameters showed no significant change post-treatment. No adverse events were observed in either group.
Conclusion: Early administration of PGE1 may improve visual outcomes in CRAO. These findings support further investigation into PGE1 as a potential treatment for acute retinal ischemia.
Introduction
Central retinal artery occlusion (CRAO) is caused by obstruction of the central retinal artery, leading to retinal ischemia and sudden vision loss (1, 2). The prognosis for vision recovery is generally poor, often resulting in permanent visual impairment that significantly affects quality of life (1–3). Existing treatments for CRAO, such as ocular message (4, 5), anterior chamber paracentesis (6, 7), intraocular pressure-lowering agents (5, 8), thrombolytic therapy (2, 9), hyperbaric oxygen therapy (10, 11), vitrectomy (12, 13), and neodymium-doped yttrium aluminum garnet laser (14, 15), have shown limited effectiveness in improving visual outcomes, and no established treatment has been confirmed (1, 2, 16).
Prostaglandin E1 (PGE1) is known for its vasodilatory effects, which improve blood flow in peripheral arterial diseases (17, 18), and has drawn attention as a potential treatment for CRAO (19–21). PGE1 acts on the vascular endothelium to increase oxygen supply to the retina, potentially aiding visual recovery (22). It also has neuroprotective properties by reducing oxidative stress and inflammation in ischemic tissue (23, 24).
This study aimed to evaluate the therapeutic effects of PGE1 in patients with CRAO. A control group was included to compare outcomes with conventional treatments. We also assessed PGE1’s vasodilatory effects by measuring changes in the diameters of the main branches of the central retinal artery and vein before and after administration. Additionally, we analyzed potential prognostic indicators of visual outcomes—including maximal retinal thickness (MRT), central retinal thickness (CRT), and optical-intensity ratio (OIR)—to explore their associations with structural changes and treatment response in the acute phase of CRAO.
Materials and methods
Participants
The patient database at Tokushima Red Cross Hospital was searched for individuals diagnosed with acute CRAO between April 2018 and May 2024. Inclusion criteria were cases in which treatment began within 24 h of symptom onset. Exclusion criteria included lack of follow-up data beyond 1 month from onset and suspected arteritic CRAO. From the medical records, we collected data on age at initial visit, sex, time from CRAO onset to PGE1 initiation (time to treatment), presence of ocular and systemic diseases, BCVA, and any documented ocular or systemic adverse events. Age, sex, and treatment time were compared between the PGE1 and control groups.
PGE1 treatment protocol
Acute CRAO was defined as the rapid onset of vision loss within 24 hours of presentation, with characteristic fundus findings such as ischemic retinal edema and a cherry-red spot in the macula. Subjects in the PGE1 group received intravenous infusions of 40 μg PGE1 (Alprostadil Alfadex; Takata Pharmaceutical, Saitama, Japan) in 250 mL saline, administered at 125 mL/hour twice daily (80 μg/day) for 5 days, based on prior studies (19). Patients also received 10 μg oral PGE1 (Limaprost Alfadex; Sawai Pharmaceutical, Osaka, Japan) three times daily (30 μg/day) for at least 1 month. During the study, no additional treatments (e.g., intraocular pressure-lowering, vasodilating, or thrombolytic agents) were administered. Ophthalmic exams, including slit-lamp biomicroscopy, applanation tonometry, and indirect ophthalmoscopy, were performed daily during the first 5 days and every 1–4 weeks during oral PGE1 treatment.
From April 2021 to May 2024, patients who presented within 24 hours of symptom onset were treated with PGE1 per this protocol. Before this period, patients received conventional therapy without PGE1. Thus, group allocation was based on treatment period rather than physician discretion.
Outcome measures
The primary endpoint was BCVA comparison at initial visit and at 1 month. Visual acuity was converted to logMAR for statistical analysis. For patients with very poor vision, approximated logMAR values were used: counting fingers = 2.0, hand motion = 2.3, light perception = 2.6, and no light perception = 2.9 (25, 26). Secondary outcomes included: (1) correlations between initial OIR and 1-month BCVA, initial MRT and 1-month BCVA, CRT and 1-month BCVA, and time to treatment and 1-month BCVA; (2) retinal vessel diameters at baseline and 1 month; and (3) adverse events.
Control group
The control group included CRAO patients who received conventional therapy, including thrombolytics, intraocular pressure-lowering agents, or no PGE1. Like the PGE1 group, control patients were diagnosed and treated within 24 hours of symptom onset. Inclusion and exclusion criteria were identical to those of the PGE1 group. The control group served to assess PGE1 efficacy relative to standard treatments.
Retinal thickness and OIR measurements
Swept-source OCT images were acquired using the Mirante system (Nidek Co., Ltd., Aichi, Japan). MRT, CRT, and OIR were measured only at baseline. MRT was defined as the greatest vertical distance from the inner limiting membrane to the retinal pigment epithelium within a 1.5-mm radius centered on the fovea, manually identified on horizontal B-scans. CRT was the vertical distance at the foveal center. OIR was calculated as the ratio of mean pixel intensity in the inner retina (from the inner limiting membrane to the outer plexiform layer) to that in the outer retina (from the outer nuclear layer to the retinal pigment epithelium), following previously described methods (20), using ImageJ software (National Institutes of Health, Bethesda, MD, USA). A representative illustration of the measurement procedure is provided in Supplementary Figure S1.
Measurement of the retinal vessel diameter
Fundus photographs were used to assess arterial-to-disk (A/D) and venous-to-disk (V/D) diameter ratios. Vessel diameters were measured at the narrowest point of the first superotemporal branch of the central retinal artery between the optic disk and the second branch, and at the corresponding segment of the adjacent central retinal vein. The method was based on previously described techniques (19). Diameters were divided by the vertical optic disk diameter to calculate A/D and V/D ratios, following established methods (20). Measurements were performed at baseline and at 1 month. A representative illustration of this measurement is provided in Supplementary Figure S2.
Measurement protocol and blinding
All parameters (MRT, CRT, OIR, A/D, and V/D) were independently measured by two retinal specialists. One examiner (KH), blinded to visual outcomes, provided measurements used for statistical analysis. The other examiner (HS) was not blinded and contributed data for interrater reproducibility assessment.
Statistical analysis
Fisher’s exact test was used for categorical variables, such as sex distribution. The Mann–Whitney U test was used to compare continuous variables, including age, time to treatment, BCVA, MRT, CRT, OIR, and A/D and V/D ratios between the PGE1 and control groups. To explore prognostic factors for visual acuity in the PGE1 group, Pearson correlation coefficients were calculated for BCVA and related parameters. Pre- and post-treatment BCVA changes were evaluated using the Wilcoxon signed-rank test. Paired t-tests were also performed for exploratory analysis, acknowledging the small sample size. Statistical significance was set at p <0.05. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html).
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Japanese Red Cross Tokushima Hospital (Approval No. 504). The study was conducted in accordance with local regulations and institutional requirements. The requirement for written informed consent was waived by the ethics committee in accordance with national guidelines and the Declaration of Helsinki, as the study used anonymized retrospective data and obtaining consent was impracticable.
Results
Background factors
The baseline characteristics of the PGE1 group (n = 4) and the control group (n = 6) are summarized in Table 1.
No significant differences were observed between the groups in age, sex distribution, time to treatment, or systemic comorbidities, including hypertension, diabetes, and cardiovascular disease. Further granular, individual-level data—detailing baseline and follow-up BCVA, OCT parameters, specific comorbidities, and the treatments administered within the control group are additionally provided in Supplementary Table S1.
Comparison of MRT, CRT, and OIR
At the initial visit, the mean MRT was 495.5 (± 174.2) μm in the PGE1 group and 446.5 (± 73.8) μm in the control group (p = 1.00). CRT and OIR were also similar: CRT, 331.8 (± 206.9) μm vs. 264 (± 85.1) μm (p = 0.91); OIR, 140.9 (± 36.3) vs. 148.2 (± 18.0)% (p = 0.91). No significant differences in structural parameters were observed (Figure 1).
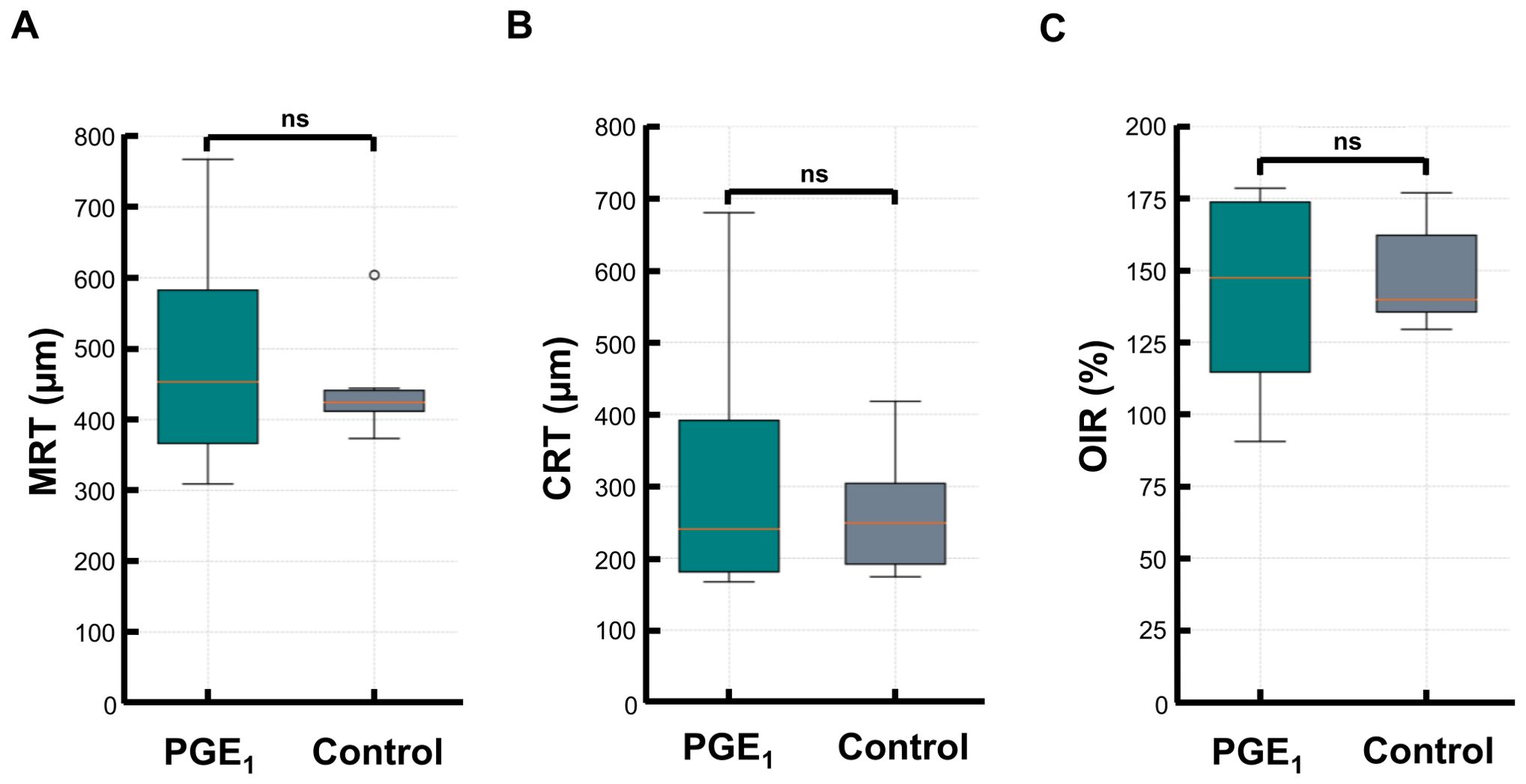
Figure 1. Comparison of MRT, CRT, and OIR at baseline between the PGE1 and control groups. Box plots comparing baseline retinal measurements in the PGE1 and control groups: (A) Maximal retinal thickness (MRT) (B) Central retinal thickness (CRT) (C) Optical-intensity ratio (OIR) No significant differences were observed between the groups for any parameter.
Best-corrected visual acuity improvement
At the initial visit, BCVA was 2.4 (± 0.33) logMAR units in the PGE1 group and 2.3 (± 0.24) logMAR units in the control group, with no significant difference (p = 0.825). At 1 month, BCVA in the PGE1 group was significantly better than in the control group (0.67 ± 0.41 vs. 2.3 ± 0.3 logMAR units, p = 0.013) (Figure 2).
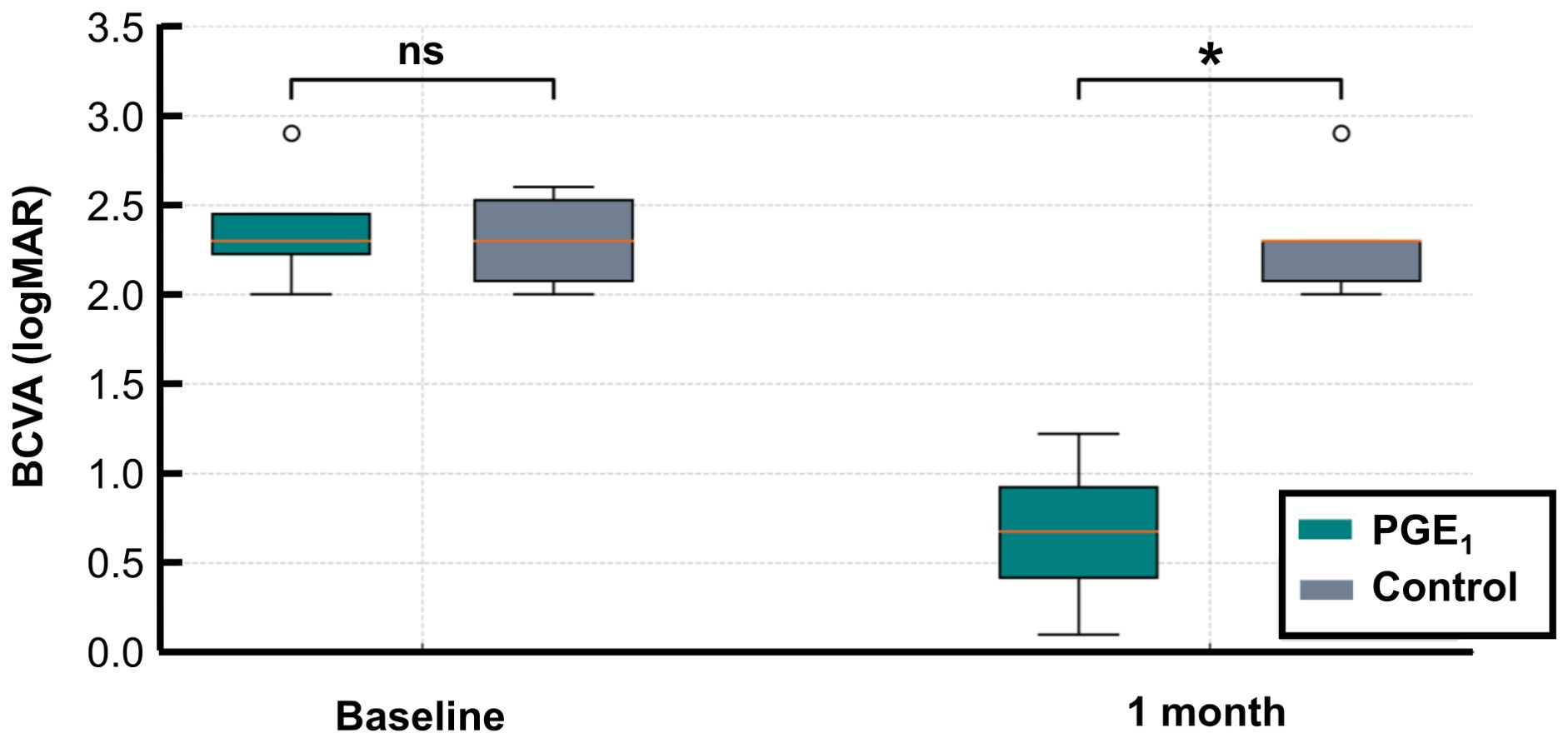
Figure 2. BCVA before and after treatment in the PGE1 and control groups. LogMAR BCVA values at baseline and at 1 month are shown for both groups: At 1 month, BCVA was significantly better in the PGE1 group than in the control group (*p = 0.013). No significant difference was observed at baseline. *p < 0.05.
BCVA improved in all four eyes in the PGE1 group. However, the Wilcoxon signed-rank test did not show statistical significance (p = 0.125). The paired t-test showed significant improvement (p = 0.001), but this result should be interpreted cautiously due to the small sample size. No significant BCVA change was noted in the control group using either test.
Correlation between prognostic factors and visual outcome
In the PGE1 group, baseline MRT showed a significant positive correlation with logMAR BCVA at 1 month (r = 0.965, p = 0.034), indicating that a greater retinal thickening in the acute phase was associated with worse visual outcomes.
Baseline CRT (r = 0.91, p = 0.08), OIR (r = 0.89, p = 0.11), and treatment time (r = 0.74, p = 0.25) showed moderate positive correlations with BCVA but did not reach statistical significance (Figure 3).
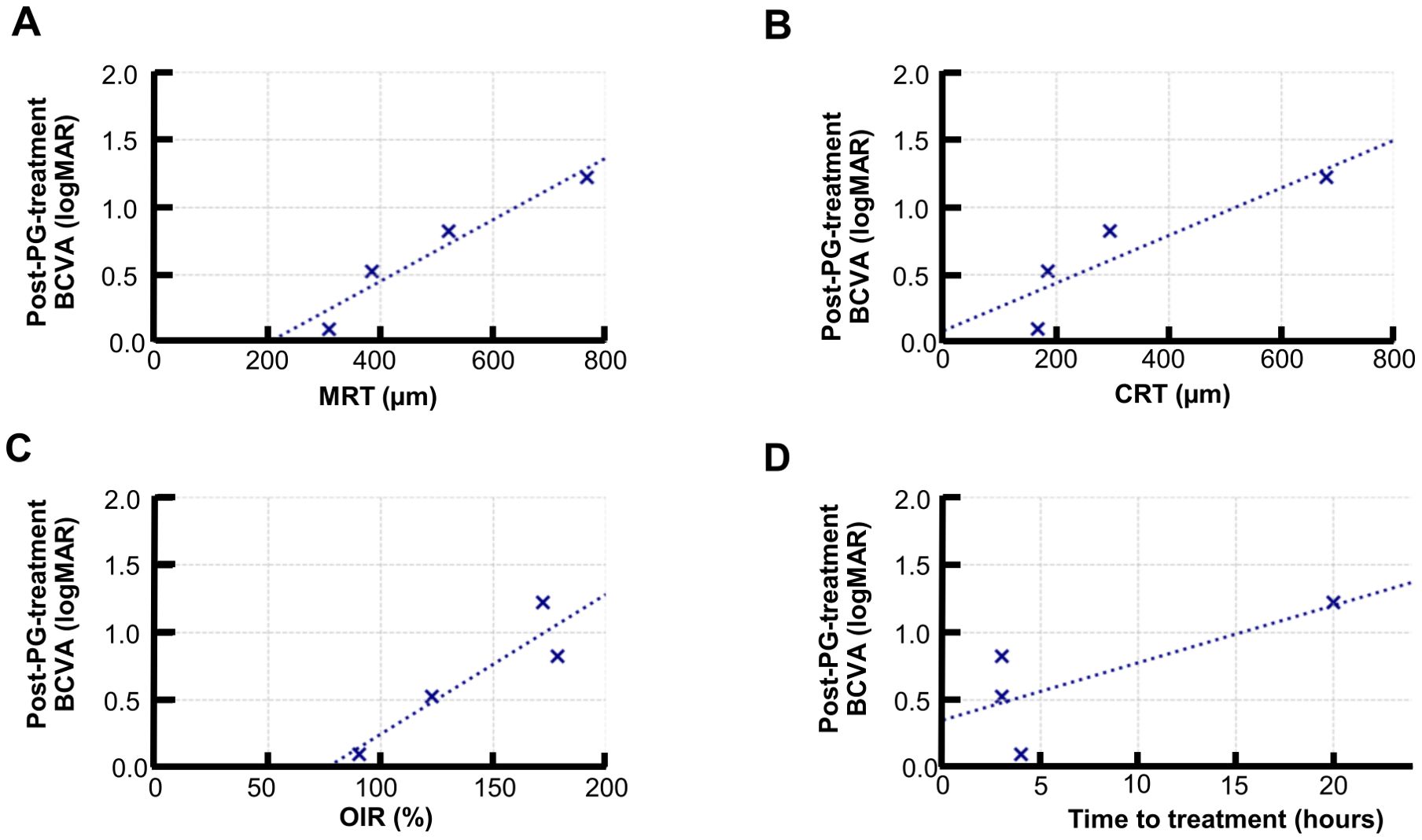
Figure 3. Correlation between baseline retinal structural parameters and visual outcomes in the PGE1 group. Scatter plots showing the correlation between baseline parameters and logMAR BCVA at 1 month: (A) MRT: strong, significant correlation (r = 0.965, p = 0.035) (B) CRT: moderate-to-nonsignificant correlation (r = 0.888, p = 0.112) (C) OIR: moderate, nonsignificant correlation (r = 0.914, p = 0.086) (D) Time to treatment: moderate-to-nonsignificant correlation (r = 0.745, p = 0.255).
Conversely, in the control group, none of the assessed parameters, including MRT (r = 0.14, p = 0.791), CRT (r = −0.0215, p = 0.968), OIR (r = 0.043, p = 0.936), and time to treatment (r = −0.377, p = 0.462), demonstrated a significant correlation with 1-month BCVA, uniformly showing only weak or negligible associations.
Change in the retinal vessel diameter (A/D ratio, V/D ratio)
At baseline, the A/D ratio in the PGE1 group was 4.5 (± 0.9), and the V/D ratio was 7.0 (± 2.0). At 1 month, the A/D ratio increased to 4.6 (± 1.0), and the V/D ratio to 8.0 (± 2.0). However, no significant changes were observed in A/D or V/D ratios (p = 0.886, p = 0.486) (Figure 4).
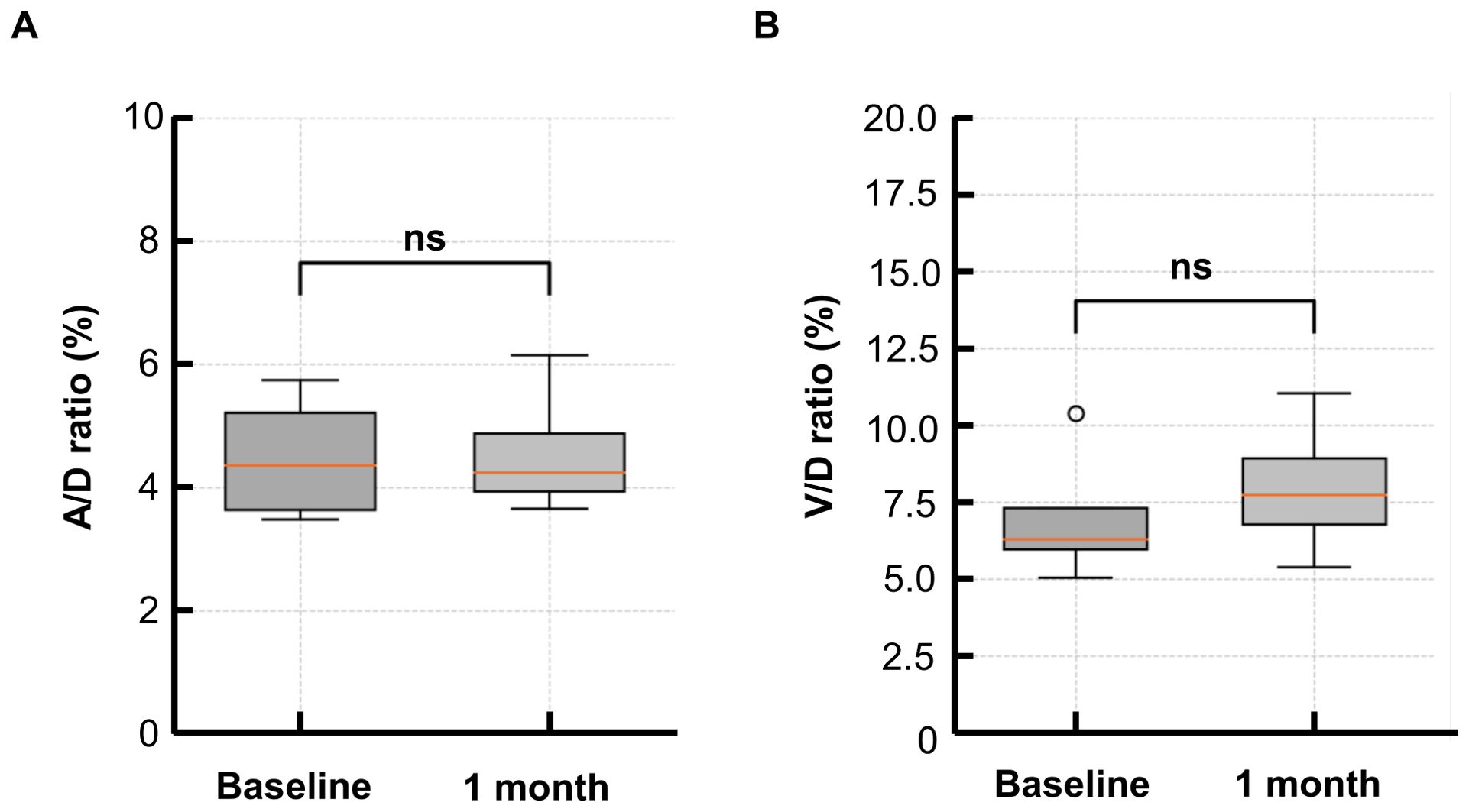
Figure 4. Changes in the A/D and V/D ratios from baseline to 1 month in the PGE1 group. (A) Arterial-to-disk (A/D) ratio before and after treatment (B) Venous-to-disk (V/D) ratio before and after treatment. No significant differences were observed at 1 month for either parameter.
Adverse events
Comprehensive monitoring revealed an absence of adverse events or treatment-related complications in the PGE1 group, during the administration phase and throughout the follow-up period. Likewise, no serious adverse events were noted in the control group.
Discussion
This study aimed to evaluate the therapeutic effect of PGE1 in patients with CRAO and to identify prognostic factors related to visual recovery. BCVA at 1 month was significantly better in the PGE1 group than in the control group. Among structural indicators, baseline MRT showed a statistically significant positive correlation with 1-month BCVA, suggesting that greater retinal thickening in the acute phase was linked to worse visual outcomes. Other factors, such as earlier treatment initiation, lower baseline CRT, and higher OIR, showed moderate correlations with better visual improvement, though not statistically significant.
Several previous studies have reported intravenous PGE1 administration for CRAO. Takai et al. administered 40 μg of intravenous PGE1 twice daily in 10 patients and observed significant BCVA improvements in all cases (19). Similarly, Malbin et al. reported visual improvement in six patients with acute CRAO after PGE1 infusion, with only mild vascular pain and no serious adverse effects (21). Suzuki et al. demonstrated significant visual improvement at 1 and 3 months in 21 patients who received intravenous liposomal PGE1 for 7–14 days (20). Chacko et al. documented retinal reperfusion via fluorescein angiography within 48 hours of PGE1 infusion in two CRAO cases (27). Our findings support these reports and further strengthen the evidence by including a control group, enabling clearer evaluation of treatment efficacy. Furthermore, it is well-established that intravenous PGE1 is generally well tolerated, with commonly recognized side effects including transient flushing, headache, hypotension, and nausea (19–21, 27). Our study likewise observed no adverse events or complications within the PGE1 treatment group, thereby further substantiating the favorable safety profile of this intervention in the setting of acute CRAO.
In our study, baseline MRT showed a significant positive correlation with logMAR BCVA at 1 month in the PGE1 group, indicating that more severe retinal thickening in the acute phase is associated with poorer visual outcomes. Such a finding positions MRT as a potential structural biomarker reflecting the severity of ischemic injury, particularly relevant in the context of early active therapeutic intervention. Remarkably, this correlation was not observed in the control group, which we attribute to their generally limited visual improvement and the inherent constraints of a small sample size. While MRT offers promising structural insights, comprehensive validation through larger-scale studies is warranted to firmly establish its prognostic utility across a broader spectrum of treatment environments.
The significance of MRT in assessing the severity of CRAO has been underscored in earlier publications. Hayreh et al. emphasized that inner retinal ischemia and subsequent cytotoxic edema are the primary causes of irreversible vision loss in CRAO (28). Supporting this, Ochakovski et al. reported that MRT increased with ischemic edema severity and correlated with visual function, suggesting its value as a functional outcome predictor (29).
Wang et al. showed that visual improvement after intra-arterial thrombolysis was linked to reduced CRT and preserved retinal layer architecture, underscoring the value of OCT structural assessment in CRAO (30). However, Suzuki et al. found no significant correlation between CRT and visual outcomes, highlighting CRT’s limitations as a prognostic indicator (20). In contrast, Kim et al. reported that perifoveal rather than central retinal thickness was more strongly associated with visual recovery, implying that MRT, which reflects the most edematous macular area, may have greater prognostic value than CRT (31). Furthermore, Fouad et al. described that macular fluid in CRAO mainly affects the inner nuclear and outer plexiform layers, reinforcing MRT’s anatomical basis as a marker of ischemic swelling on OCT (32).
Although CRT and OIR showed moderate positive correlations with visual outcomes in our study, neither reached statistical significance. Collectively, these results suggest that MRT may be a more sensitive and clinically relevant structural biomarker for evaluating disease severity and predicting visual prognosis in CRAO than either CRT or OIR.
PGE1 is also widely used in other clinical contexts, including treating intermittent claudication in peripheral arterial disease and maintaining ductal patency in congenital heart disease, primarily due to its vasodilatory effects (33, 34). It also protects against ischemia-reperfusion injury in lung transplantation (24). These effects are mediated through E prostanoid receptor activation in vascular endothelial cells, stimulating the cyclic adenosine monophosphate/protein kinase A pathway and relaxing vascular smooth muscle (35). PGE1 further activates endothelial nitric oxide synthase and GTP cyclohydrolase I, aiding microcirculation maintenance (36).
However, our study did not detect any significant increase in retinal vessel diameter after PGE1 treatment. Recently, PGE1 has also shown neuroprotective effects. Zhang et al. reported that PGE1 inhibits the c-Jun N-terminal kinase/Bcl-2 interacting mediator of cell death pathway and reduces apoptosis in ischemic tissues (23). Rajan et al. showed PGE1 activation of Nurr1, a neuroprotective nuclear receptor (37). Yamamoto et al. found that PGE1 upregulates thioredoxin expression via cyclic adenosine monophosphate signaling (38, 39). Additionally, de Perrot et al. demonstrated PGE1’s anti-inflammatory effects by suppressing interleukin-1β and tumor necrosis factor-α while increasing interleukin-10 under ischemic stress (24).
Taken together, these pleiotropic effects of PGE1 may help explain the better visual outcomes observed in our study, despite the absence of detectable changes in retinal vessel diameter.
This study has several limitations. First, it was a retrospective analysis with a relatively small sample size, which may limit the generalizability of the findings. Second, although OCT-based structural parameters such as MRT were measured by a single examiner (KH) blinded to visual outcomes, manual measurements may still introduce subjectivity. Third, only short-term follow-up data were included, preventing evaluation of long-term effects. Additionally, OCT or fluorescein angiography was not performed to assess retinal perfusion, limiting direct evaluation of vascular changes and reperfusion. Moreover, although BCVA was assessed at one month post-treatment, OCT imaging was not consistently performed at this time point, precluding longitudinal morphological analysis. Previous studies have demonstrated progressive inner retinal thinning following CRAO, which may have prognostic implications (40). Finally, although we evaluated the A/D ratio, we did not assess the ratio of lumen diameter to arterial wall thickness, which might better reflect structural vascular changes. Future studies employing advanced imaging modalities such as adaptive optics scanning laser ophthalmoscopy may help clarify vascular remodeling in response to treatment (41).
Further prospective studies with larger sample sizes, standardized and automated imaging assessments, and longer follow-up are needed to validate these findings and clarify the prognostic value of OCT biomarkers such as MRT.
In conclusion, intravenous PGE1 may improve visual outcomes in patients with acute CRAO. Among structural OCT parameters, MRT showed a significant association with visual prognosis and may serve as a useful marker of ischemic severity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Japanese Red Cross Tokushima Hospital (Approval No. 504). Written informed consent from the [patients/participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. RY: Supervision, Writing – review & editing. HK: Data curation, Validation, Writing – review & editing. YM: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the medical staff at Tokushima Red Cross Hospital for their support in data collection. We also thank Enago (www.enago.jp) for the English language review. Portions of the manuscript text were edited with the assistance of ChatGPT (OpenAI), a large language model. The authors reviewed and approved all content. No AI tools were used for data analysis or image generation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Portions of the manuscript text were edited with the assistance of ChatGPT (OpenAI), a large language model. The authors reviewed and approved all content. No AI tools were used for data analysis or image generation.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2025.1665519/full#supplementary-material
References
1. Varma DD, Cugati S, Lee AW, and Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye. (2013) 27:688–97. doi: 10.1038/eye.2013.25
2. Mac Grory B, Schrag M, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, et al. Management of central retinal artery occlusion: a scientific statement from the American Heart Association. Stroke. (2021) 52:e282–94. doi: 10.1161/STR.0000000000000366
3. Weger M, Pichler T, Franke GH, Haas A, Thaler HV, Kraigher-Krainer N, et al. Assessment of vision-related quality of life in patients with central retinal artery occlusion. Retina. (2014) 34:539–45. doi: 10.1097/IAE.0b013e3182a0e42e
4. Cugati S, Varma DD, Chen CS, and Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. (2013) 15:63–77. doi: 10.1007/s11940-012-0202-9
5. Rumelt S, Dorenboim Y, and Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. (1999) 128:733–8. doi: 10.1016/s0002-9394(99)00359-1
6. Atebara NH, Brown GC, and Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. (1995) 102:2029–35. doi: 10.1016/s0161-6420(95)30758-0
7. Fieß A, Cal Ö, Kehrein S, Halstenberg S, Frisch I, and Steinhorst UH. Anterior chamber paracentesis after central retinal artery occlusion: a tenable therapy? BMC Ophthalmol. (2014) 14:28. doi: 10.1186/1471-2415-14-28
8. Duxbury O, Bhogal P, Cloud G, and Madigan J. Successful treatment of central retinal artery thromboembolism with ocular massage and intravenous acetazolamide. BMJ Case Rep 2014. (2014), bcr2014207943. doi: 10.1136/bcr-2014-207943
9. Huang L, Wang Y, and Zhang R. Intravenous thrombolysis in patients with central retinal artery occlusion: a systematic review and meta-analysis. J Neurol. (2022) 269:1825–33. doi: 10.1007/s00415-021-10838-6
10. Wu X, Chen S, Li S, Zhang J, Luan D, Zhao S, et al. Oxygen therapy in patients with retinal artery occlusion: a meta-analysis. PloS One. (2018) 13:e0202154. doi: 10.1371/journal.pone.0202154
11. Youn TS, Lavin P, Patrylo M, Schindler J, Kirshner H, Greer DM, et al. Current treatment of central retinal artery occlusion: a national survey. J Neurol. (2018) 265:330–5. doi: 10.1007/s00415-017-8702-x
12. García-Arumí J, Martinez-Castillo V, Boixadera A, Fonollosa A, and Corcostegui B. Surgical embolus removal in retinal artery occlusion. Br J Ophthalmol. (2006) 90:1252–5. doi: 10.1136/bjo.2006.097642
13. Cisiecki S, Bonińska K, and Bednarski M. Vitrectomy with arteriotomy and neurotomy in retinal artery occlusion – a case series. Indian J Ophthalmol. (2022) 70:2072–6. doi: 10.4103/ijo.IJO_1566_21
14. Man V, Hecht I, Talitman M, Hilely A, Midlij M, Burgansky-Eliash Z, et al. Treatment of retinal artery occlusion using transluminal Nd: YAG laser: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. (2017) 255:1869–77. doi: 10.1007/s00417-017-3777-8
15. Mehboob MA, Khan A, and Mukhtar A. Efficacy of YAG laser embolysis in retinal artery occlusion. Pak J Med Sci. (2021) 37:71–5. doi: 10.12669/pjms.37.1.3196
16. Sharma RA, Newman NJ, and Biousse V. Conservative treatments for acute nonarteritic central retinal artery occlusion: do they work? Taiwan J Ophthalmol. (2020) 11:16–24. doi: 10.4103/tjo.tjo_61_20
17. Creutzig A, Lehmacher W, and Elze M. Meta-analysis of randomised controlled prostaglandin E1 studies in peripheral arterial occlusive disease stages III and IV. Vasa. (2004) 33:137–44. doi: 10.1024/0301-1526.33.3.137
18. Brass EP, Anthony R, Dormandy J, Hiatt WR, Jiao J, Nakanishi A, et al. Parenteral therapy with lipo-ecraprost, a lipid-based formulation of a PGE1 analog, does not alter six-month outcomes in patients with critical leg ischemia. J Vasc Surg. (2006) 43:752–9. doi: 10.1016/j.jvs.2005.11.041
19. Takai Y, Tanito M, Matsuoka Y, Hara K, and Ohira A. Systemic prostaglandin E1 to treat acute central retinal artery occlusion. Invest Ophthalmol Vis Sci. (2013) 54:3065–71. doi: 10.1167/iovs.12-11445
20. Suzuki T, Obata R, Inoue T, Ueta Y, Azuma K, Tate H, et al. Intravenous lipo-prostaglandin E1 administration for patients with acute central retinal artery occlusion. BMJ Open Ophthalmol. (2022) 7:e001014. doi: 10.1136/bmjophth-2022-001014
21. Malbin B, Padidam S, Burke M, Akhter M, Eby A, Mishulin A, et al. Intravenous prostaglandin E1 infusion for acute central retinal artery occlusion. Ophthalmic Surg Lasers Imaging Retina. (2019) 50:S5–8. doi: 10.3928/23258160-20190108-02
22. Stark AK and Penn JS. Prostanoid signaling in retinal vascular diseases. Prostaglandins Other Lipid Mediat. (2024) 174:106864. doi: 10.1016/j.prostaglandins.2024.106864
23. Zhang YH, Zhang YQ, Guo CC, Wang LK, Cui YJ, Dong JJ, et al. Prostaglandin E1 attenuates high glucose-induced apoptosis in proximal renal tubular cells by inhibiting the JNK/Bim pathway. Acta Pharmacol Sin. (2020) 41:561–71. doi: 10.1038/s41401-019-0314-9
24. de Perrot M, Fischer S, Liu M, Jin R, Bai XH, Waddell TK, et al. Prostaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokines. Transplantation. (2001) 72:1505–12. doi: 10.1097/00007890-200111150-00006
25. Lange C, Feltgen N, Junker B, Schulze-Bonsel K, and Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. (2009) 247:137–42. doi: 10.1007/s00417-008-0926-0
26. Moussa G, Bassilious K, and Mathews N. A novel excel sheet conversion tool from Snellen fraction to LogMAR including ‘counting fingers’, ‘hand movement’, ‘light perception’ and ‘no light perception’ and focused review of literature of low visual acuity reference values. Acta Ophthalmol. (2021) 99:e963–5. doi: 10.1111/aos.14659
27. Chacko JA, Broyles HV, Chacko JG, and Uwaydat SH. Documented reperfusion of the retina on fluorescein angiography after administration of intravenous prostaglandin E1 for central retinal artery occlusion: a case report. Case Rep Ophthalmol. (2023) 14:469–76. doi: 10.1159/000533404
28. Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. (2011) 30:359–94. doi: 10.1016/j.preteyeres.2011.05.001
29. Ochakovski GA, Wenzel DA, Spitzer MS, Poli S, Härtig F, Fischer MD, et al. Retinal oedema in central retinal artery occlusion develops as a function of time. Acta Ophthalmol. (2020) 98:e680–4. doi: 10.1111/aos.14375
30. Wang W, Lei T, Wang R, Zhang B, Wang X, and Ma T. Correlation of visual acuity changes and optical coherence tomography imaging in patients with central retinal artery occlusion post-arterial thrombolysis. Sci Rep. (2025) 15:7962. doi: 10.1038/s41598-025-92890-0
31. Kim H, Kim HK, Yang JY, and Kim SS. Optical coherence tomography measurement and visual outcome in acute central retinal artery occlusion. Korean J Ophthalmol. (2018) 32:303–11. doi: 10.3341/kjo.2017.0093
32. Fouad YA, Hamza MN, and Wessam MM. Prevalence and distribution of macular fluid with central retinal artery occlusion and anterior ischemic optic neuropathy. Clin Ophthalmol. (2024) 18:887–93. doi: 10.2147/OPTH.S457503
33. Schainfeld RM. Management of peripheral arterial disease and intermittent claudication. J Am Board Fam Pract. (2001) 14:443–50.
34. Akkinapally S, Hundalani SG, Kulkarni M, Fernandes CJ, Cabrera AG, Shivanna B, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev. (2018) 2:CD011417. doi: 10.1002/14651858.CD011417.pub2
35. Lai YJ, Hsu HH, Chang GJ, Lin SH, Chen WJ, Huang CC, et al. Prostaglandin E1 attenuates pulmonary artery remodeling by activating phosphorylation of CREB and the PTEN signaling pathway. Sci Rep. (2017) 7:9974. doi: 10.1038/s41598-017-09707-y
36. Liu L, Zhang H, Shi Y, and Pan L. Prostaglandin E1 improves cerebral microcirculation through activation of endothelial NOS and GRPCH1. J Mol Neurosci. (2020) 70:2041–8. doi: 10.1007/s12031-020-01610-y
37. Rajan S, Jang Y, Kim CH, Kim W, Toh HT, Jeon J, et al. PGE1 and PGA1 bind to Nurr1 and activate its transcriptional function. Nat Chem Biol. (2020) 16:876–86. doi: 10.1038/s41589-020-0553-6
38. Yamamoto M, Ohira A, Honda O, Sato N, Furuke K, Yodoi J, et al. Analysis of localization of adult T-cell leukemia-derived factor in the transient ischemic rat retina after treatment with OP-1206 alpha-CD, a prostaglandin E1 analogue. J Histochem Cytochem. (1997) 45:63–70. doi: 10.1177/002215549704500109
39. Yamamoto M, Sato N, Tajima H, Furuke K, Ohira A, Honda Y, et al. Induction of human thioredoxin in cultured human retinal pigment epithelial cells through cyclic AMP-dependent pathway; involvement in the cytoprotective activity of prostaglandin E1. Ex. Eye Res. (1997) 65:645–52. doi: 10.1006/exer.1997.0370
40. Mangla R, Venkatesh R, Sangoram R, Acharya I, Parmar Y, Prabhu V, et al. Retinal OCT findings in acute central retinal artery occlusion of varying severity at different disease stages - a retrospective, observational study. Int J Retina Vitreous. (2023) 9:37. doi: 10.1186/s40942-023-00475-8
Keywords: central retinal artery occlusion, prostaglandin E1, retinal ischemia, optical coherence tomography, visual outcome, neuroprotection
Citation: Sano H, Yanai R, Kondo H and Mitamura Y (2025) Early prostaglandin E1 treatment improves visual outcomes in central retinal artery occlusion: a retrospective study. Front. Ophthalmol. 5:1665519. doi: 10.3389/fopht.2025.1665519
Received: 14 July 2025; Accepted: 04 August 2025;
Published: 20 August 2025.
Edited by:
Dong Ho Park, Kyungpook National University Hospital, Republic of KoreaReviewed by:
Ryo Mukai, Fukushima Medical University, JapanYong Koo Kang, Kyungpook National University, Republic of Korea
Jae Rock Do, Kyungpook National University, Republic of Korea
Copyright © 2025 Sano, Yanai, Kondo and Mitamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroki Sano, aGFrYW5heXVraUB5YWhvby5jby5qcA==
 Hiroki Sano
Hiroki Sano Ryoji Yanai
Ryoji Yanai Hirotaka Kondo1
Hirotaka Kondo1 Yoshinori Mitamura
Yoshinori Mitamura