- 1Department of Anatomy, Histology, Cytology, and Biology, Section “Biology”, Medical University – Pleven, Pleven, Bulgaria
- 2Dr Stefan Cherkezov Regional Hospital, Veliko Tarnovo, Bulgaria
Ophthalmomyiasis is an uncommon parasitic infestation of the human eye caused by dipteran larvae, most frequently Oestrus ovis. It is rarely reported in temperate countries such as Bulgaria. We present a case of external ophthalmomyiasis in a 35-year-old urban resident, with no livestock exposure, caused by O. ovis. Larvae were morphologically identified after extraction. Approximately 30 motile larvae were mechanically removed over several days. Microscopy confirmed first-instar O. ovis larvae. Treatment included systemic and topical antibiotics, anti-inflammatory agents, and eye irrigation, resulting in complete recovery. This case of ophthalmomyiasis demonstrated that infestation can occur among urban residents without direct contact with livestock. Early recognition and mechanical removal are essential to prevent ocular damage.
Highlights
● Ophthalmomyiasis, although rare in Bulgaria, occurs in both rural and urban populations.
● Awareness among ophthalmologists, general practitioners, and parasitologists is essential for early recognition and prevention of complications.
● Climate change and reduced vector control may increase future incidence.
Introduction
Myiasis is an infestation caused by the larvae of insects belonging to the order Diptera. Members of this order deposit eggs or, in the case of larviparous species, live larvae onto the skin, within open wounds, or into various natural body orifices of vertebrates, including humans (1). The most important families capable of causing myiasis in various hosts are Oestridae, Calliphoridae, Muscidae, and Sarcophagidae (2, 3). Globally, the most common etiological agents of human myiasis are Dermatobia hominis and Cordylobia anthropophaga.
Depending on the localization of the larvae within the human body, myiasis can present as cutaneous, nasopharyngeal, ophthalmomyiasis, intestinal, or urogenital forms (4). The clinical case presented herein describes ophthalmomyiasis caused by Oestrus ovis larvae.
The family Oestridae comprises 28 genera and 151 species causing myiasis. Morphologically, they are classified into four subfamilies: Oestrinae, Gasterophilinae, Hypodermatinae, and Cuterebrinae (4, 5). Oestrinae includes four genera, among which the genus Oestrus contains O. ovis, the sheep nasal botfly, which infests sheep, goats, and occasionally humans (6, 7). These flies typically deposit eggs or larvae on the skin or near body openings and, in some cases, directly into or around the host’s eyes.
Ophthalmomyiasis is the infestation of the eye by dipteran larvae. It is categorized into external (involving the eyelids and conjunctiva), internal (with penetration of larvae into the globe), and orbital (affecting the optic nerve and surrounding orbital tissues).
External ophthalmomyiasis usually presents with an acute onset, often within hours of contact with the fly. Symptoms include redness, burning, tearing, swelling, and a foreign body sensation in the eye. A characteristic complaint is the feeling of movement between the globe and the conjunctiva. Live, motile larvae can often be visualized by slit-lamp examination. Mild corneal involvement (keratitis) has been reported (8).
If not diagnosed in time, larvae may penetrate ocular structures, including the anterior chamber, vitreous body, and retina, and cause internal ophthalmomyiasis (9). This rarer but more severe form may present with floaters, photopsia, ocular pain, decreased visual acuity, vitreous haemorrhage, and retinal degeneration.
Orbital involvement damages the optic nerve and surrounding tissues, requiring aggressive treatment. If left untreated, it can lead to severe complications, including vision loss (10).
The most frequent cause of external ophthalmomyiasis in humans is O. ovis, particularly among individuals living or working in proximity to sheep and goats. Other species capable of causing both external and internal ophthalmomyiasis include D. hominis, Chrysomya bezziana, Hypoderma tarandi, and Musca domestica (11).
In Bulgaria, O. ovis is present throughout the country. According to an online publication from 2015 (12), its prevalence among sheep flocks ranges from 70% to 100%, as reported in surveys in Sardinia (13). Warm, dry weather favours parasite development. In Bulgaria and the Balkan Peninsula, O. ovis is larviparous (14) and produces two generations per year: one from early June to late July and the other from late August to October–November (12). Adult flies are grey-brown, 10–12 mm in body length, with sparse hair, small black spots on the thorax, and dull yellow head and legs. The mouthparts are vestigial, as adults do not feed during their approximately 2-week lifespan (14). After mating, the males die, while the females remain immobile for 10–20 days on walls, fences, and cracks where larval development occurs.
Females deposit between 8 and 40 live larvae into or around the nostrils of sheep and goats. Over their lifetime, each female releases approximately 350–500 larvae. Once inside the nasal passages, the larvae attach to the mucosa and migrate slowly toward the frontal sinuses and horn cavities, where they remain for 8–9 months throughout autumn and winter. During this time, they moult from first (L1) to second (L2) and third (L3) instars, reaching lengths of 20–30 mm. In spring, mature L3 larvae exit the sinuses and pupate, and after 38–65 days, they emerge as adults, restarting the cycle (13).
Usually, O. ovis larvae parasitize the nasal cavities, frontal sinuses, and, occasionally, the maxillary sinuses of sheep and goats (6), but have also been reported in other ruminants, including llamas (15).
First-instar larvae (L1) are translucent white, fusiform, dorsoventrally flattened, and elongated, measuring approximately 1–5 mm in length. The body consists of 11 segmented metameres, a relatively large cephalothoracic skeleton (Figure 1), and a pair of strongly curved oral hooks (Figure 1, BH) visible under a microscope. The hooks are small and serve to facilitate mucosal penetration and attachment. On the ventral side of the anterior segments, two to three complete rows of spines are present. The posterior end bears 22–25 terminal hooks arranged in two pads, with small dorsal spines on the third segment. A pair of dark yellow spiracles is visible posteriorly. L1 larvae actively migrate through the nasal mucosa.
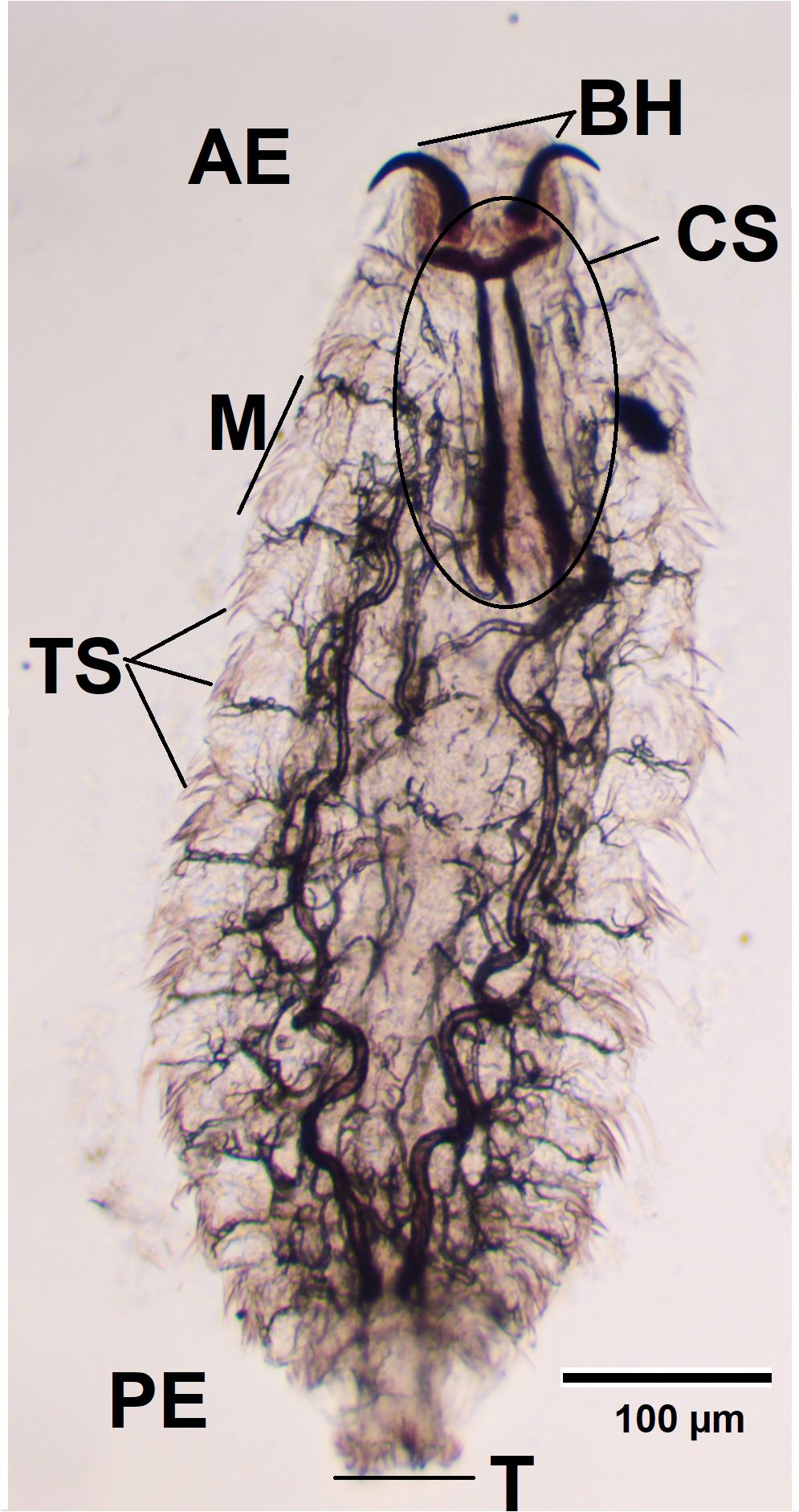
Figure 1. Larva fixed in formalin, observed using a light microscope (×100). АЕ, anterior end; BH, buccal hooks; CS, cephalopharyngeal skeleton; M, metamere; TS, tufts of spines; T, tubercles; PE, posterior end.
Second-instar larvae (L2) are whitish to pale yellow, 9–15 mm long, with several small dorsal spines on the second segment and fine spines in the median area of the post-anal swelling. The ventral segments carry multiple rows of backwards-pointing comb-like spines, aiding in attachment and locomotion. At this stage, the posterior spiracles are well-developed, and the oral hooks are more prominent (16).
Third-instar larvae (L3) measure 14–27 mm, initially white to yellowish in colour, and gradually darken to light brown with a transverse reddish to black dorsal band in mature specimens. Numerous irregular small denticles are found dorsally on the second segment, while the subsequent segment is smooth. The ventral surfaces of all segments have regularly arranged spine rows, except the third segment, where they are irregular. The pre-anal swelling is spineless, whereas the post-anal swelling carries a few spines. Posterior spiracles turn black at this stage (17).
Case report
A 35-year-old man presented to the ophthalmologist after an incident in which a small object, seemingly a fly, struck his eye while he was walking past a meadow. Following the incident, he experienced ocular irritation, pain, and a sensation of movement inside the eye. The initial examination revealed conjunctival hyperemia and corneal defects, but no foreign bodies were detected.
The patient was immediately admitted to Dr Stefan Cherkezov Regional Hospital in Veliko Tarnovo. On subsequent examinations for persistent complaints of a moving foreign body sensation in his eye, several small, rapidly motile, translucent objects were detected. It was then presumed that, upon striking the patient’s eye, the suspected fly had deposited its larvae. Over several days, the attending ophthalmologist removed about 30 such objects. On the first day, these were less than 1 mm in size (Figure 2), barely visible to the naked eye (no microscopic measurements were performed). By the second day, they had visibly increased in size. They could not be removed by irrigation or swabbing and were extracted only with forceps. The second-day specimens were translucent, with darkened areas at one end, and were still no more than 1 mm in size.
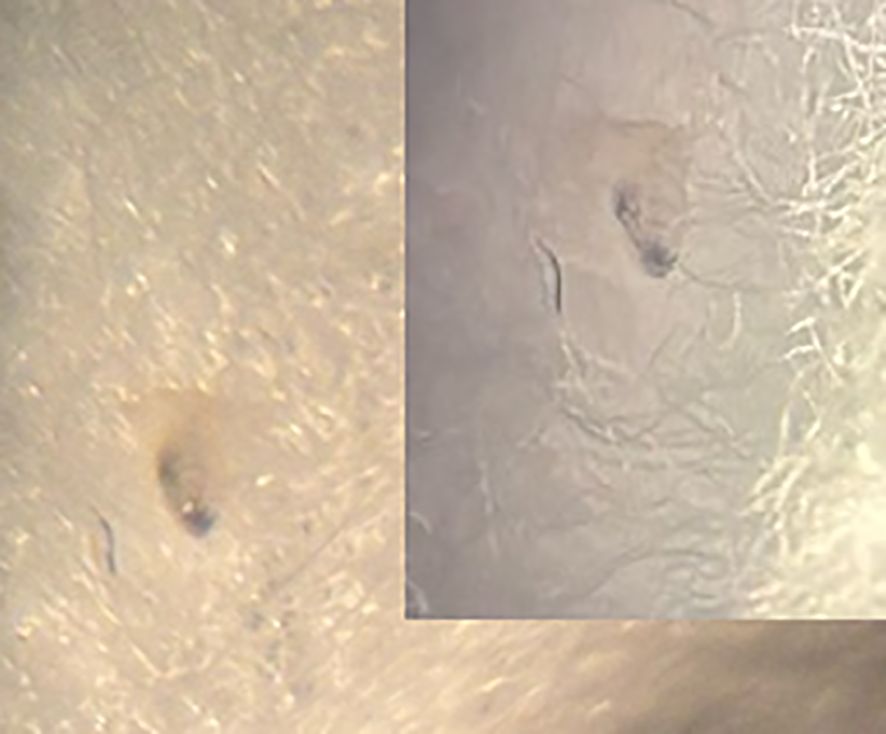
Figure 2. First-instar Oestrus ovis larva extracted from the conjunctiva showing translucent bodies and dark anterior ends.
Clinically, it was first assumed that these were Diptera larvae. Over the following days, the larvae grew to several millimetres. On the second day, three of the extracted specimens were preserved in formalin and sent to the Department of Anatomy, Histology, Cytology, and Biology at the Medical University – Pleven for microscopic identification. The specimens were examined using an Optica light microscope (Optica, Italy) at ×40 and ×100 magnification and mounted in Eukitt® Quick-hardening medium (Sigma-Aldrich, St. Louis, Missouri (MO), USA; cat. no. 03989) for permanent slides.
Macroscopically, the larvae were milky white and less than 1 mm in length (Figure 2). To measure the length and width of the larvae, we used an eyepiece micrometre and found that they were 0.7–1 mm long and 0.26–0.35 mm wide. Microscopically, they were fusiform and segmented (11 metameres; Figure 1, M) with tufts of spines between segments (Figure 1, TS). A pair of dark brown buccal hooks was located anteriorly (Figure 1, BH), connected to a cephalopharyngeal skeleton (Figure 1, CS). Comparison of these microscopic features (presence of morphological characteristics such as the cephalopharyngeal skeleton and anterior hooks) and their size with published descriptions (18, 19) identified the specimens as first-instar larvae of O. ovis.
Treatment included intravenous administration of ceftriaxone and gentamicin, non-steroidal anti-inflammatory agents, and topical ocular therapy with levofloxacin, netilmicin, ozonated oil drops (Ozodrop, FB Vision, Ascoli Piceno, Italy), tropicamide, and ofloxacin ointment. The eye was irrigated with saline and diluted povidone-iodine solution. Treatment with intravenous antibiotics was administered due to the pronounced inflammatory process in the eye—mixed infection, purulent discharge, multiple superficial and deep defects, and corneal edema—leading to a significant reduction in vision. In addition, there was hyperemia, a smooth iris structure, and a narrow pupil that did not respond to medical mydriasis. The described eye status indicated a secondary bacterial infection and spread of the inflammatory response to the anterior chamber of the eye, requiring antibiotic therapy.
Treatment was carried out in the hospital for 5 days, with two examinations per day using light biomicroscopy. During the first 3 days, parasites were removed, and by the fourth day, all parasites had been completely eliminated.
During hospital treatment, visual acuity was reduced to 0.3 (30%). On discharge, the condition of the eye was described as follows: the infection had completely subsided, the corneal defects had almost completely epithelialized, the edema had been resorbed, the iris had regained its structure, and the pupil was in maximum medicated mydriasis. Visual acuity was completely restored. After discharge, three follow-up examinations were performed within 1 month. The eye was completely healthy.
Discussion
Myiasis was first described by Hope in 1840 (20), and the first reported case of human ophthalmomyiasis caused by D. hominis (“the human botfly”) was published by Keyt in 1900 (21).
O. ovis, the sheep nasal botfly, is primarily an obligate parasite of sheep and goats, with larvae usually inhabiting the nasal passages and sinuses of these hosts. However, it can occasionally cause ophthalmomyiasis or, less frequently, nasopharyngeal myiasis in humans. Worldwide, approximately 70% of human ophthalmomyiasis cases are attributed to O. ovis (Martinez-Rojano, 2023).
Human ophthalmomyiasis and nasopharyngeal myiasis caused by O. ovis have been documented globally—in France (18, 22), Nepal (23), India (24, 25), Egypt (26), Turkey (27, 28), Saudi Arabia (29), Mexico (30), Bosnia (31), Peru (32), and many other countries.
The present case is only the second reported in Bulgaria. The first, described by Velev and Mikov (33), involved combined ophthalmomyiasis and nasopharyngeal myiasis. Humans are accidental hosts, and the primary risk groups include individuals raising sheep or goats and those living near livestock facilities. Most cases have a history of contact with farm animals or rural exposure. Additional risk factors include poor hygiene, periorbital wounds, and specific comorbidities such as obesity, diabetes, or necrotic/malignant lesions near the orbit, which may facilitate larval invasion (10).
Recent years have seen an increase in reported cases among urban residents without occupational or rural exposure (8). Our patient had a good social status, no occupation-related risk factors, and no close contact with livestock, indicating that ophthalmomyiasis can occur in individuals across all social and occupational backgrounds.
|The diagnosis of external ophthalmomyiasis is established by slit-lamp visualization of larvae on the conjunctiva or cornea. In suspected internal ophthalmomyiasis, fundus photography, ocular ultrasonography, or MRI may be indicated. Larval identification under microscopy remains challenging due to morphological similarities among Oestrus species (34).
Treatment consists of mechanical removal with sterile forceps under local anaesthesia. Topical anaesthetic agents may paralyse larvae, facilitating extraction (35, 36). O. ovis larvae are photophobic, tending to migrate toward shaded areas (37), so ectropionization is essential during examination and removal (26). The larvae’s buccal hooks anchor firmly to conjunctival and nasal mucosa, making irrigation alone insufficient (31). In our case, saline irrigation and swabbing were ineffective, requiring forceps extraction.
Post-extraction management includes topical antibiotics (e.g., tobramycin or moxifloxacin) and anti-inflammatory drops. Ivermectin, administered orally or topically, has been used with variable success but is considered to facilitate removal and reduce the risk of recurrence (38–40). A follow-up examination within 24–48 hours is recommended to ensure complete removal, as even remnants of larvae may cause persistent inflammation or secondary infection.
Preventive measures include avoiding direct contact with livestock, particularly in endemic rural areas, wearing protective eyewear during outdoor work, maintaining good personal hygiene, and implementing fly control programs.
Conclusion
Ophthalmomyiasis and other parasitic ocular infestations in humans are exceptionally rare in Bulgaria but do occur, regardless of patient age or sex. Importantly, the intermediate vector is not always a domestic animal. Some clinical cases result from accidental contact with flies that enter through open windows or from outdoor exposure.
Only one other Bulgarian case of ophthalmomyiasis—combined with nasopharyngeal myiasis—has been reported (33), diagnosed several days after infestation. Our report, together with the earlier one, confirms the presence of this parasitosis in Bulgaria despite its rarity. Even in a predominantly urban, non-tropical country, ophthalmomyiasis should be considered in the differential diagnosis of conjunctivitis-like symptoms, particularly against the background of a history of ocular foreign body entry.
Climate change and reduced insect population control may increase the likelihood of future cases. Rapid diagnosis and intervention are essential to prevent complications and vision loss. The gold standard of treatment remains prompt mechanical removal of larvae combined with antibiotic and local therapy (41).
Awareness should be raised not only among ophthalmologists but also among general practitioners, pathologists, parasitologists, and infectious disease specialists to facilitate timely recognition and management.
Although ophthalmomyiasis is generally self-limiting and usually resolves rapidly after treatment, it can lead to severe complications if diagnosis or treatment is delayed. Thus, it must be included among provisional diagnoses in patients presenting with acute conjunctivitis symptoms and unilateral foreign body sensation. Increased migration to rural areas, including among individuals seeking isolation or remote living, may lead to more frequent encounters with such cases in the future.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
MA: Conceptualization, Writing – original draft, Writing – review & editing. AB: Visualization, Writing – original draft. EM: Visualization, Writing – review & editing. LP: Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is financed by the European Union–NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project no. BG-RRP-2.004-0003.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jallow BJJ, Gassara G, Bajinka O, Luo Y, Liu M, Cai J, et al. Human myiasis in Sub-Saharan Africa: A systematic review. PloS Negl Trop Dis. (2024) 18:e0012027. doi: 10.1371/journal.pntd.0012027
2. Pezzi M, Scapoli C, Chicca M, Leis M, Marchetti MG, Del Zingaro CNF, et al. Cutaneous myiasis in cats and dogs: Cases, predisposing conditions and risk factors. Vet Med Sci. (2020) 7:378–84. doi: 10.1002/vms3.370
3. Zumpt F. Myiasis in man and animals in the Old World: a textbook for physicians, veterinarians and zoologists. In: Butterworths (London: Butterworths) (1965). Available online at: https://cir.nii.ac.jp/crid/1970023484934113183 (Accessed November 14, 2025).
4. Francesconi F and Lupi O. Myiasis. Clin Microbiol Rev. (2012) 25:79–105. doi: 10.1128/CMR.00010-11
5. Robbins K and Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. (2010) 49:1092–8. doi: 10.1111/j.1365-4632.2010.04577.x
6. Ahaduzzaman M. The global and regional prevalence of oestrosis in sheep and goats: a systematic review of articles and meta-analysis. Parasit Vectors. (2019) 12:346. doi: 10.1186/s13071-019-3597-2
7. Borade RR, Pradhan NS, and Bhat PM. Ophthalmomyiasis externa: A case report. Indian J Clin Exp Ophthalmol. (2023) 5:260–1. doi: 10.18231/j.ijceo.2019.061
8. Martinez-Rojano H, Huerta H, Sámano R, Chico-Barba G, Mier-Cabrera J, and Plascencia-Nieto ES. Ophthalmomyiasis externa and importance of risk factors, clinical manifestations, and diagnosis: review of the medical literature. Diseases. (2023) 11:180. doi: 10.3390/diseases11040180
9. Tamponi C, Pasini C, Ahmed F, Dessì G, Contu E, Porcu F, et al. External ophthalmomyiasis by Oestrus ovis in tourists visiting Italy. Report of three cases and a literature review. Travel Med Infect Dis. (2022) 46:102279. doi: 10.1016/j.tmaid.2022.102279
10. Ranjan R and Jain A. External ophthalmomyiasis. Oman J Ophthalmol. (2014) 7:160. doi: 10.4103/0974-620X.142607
11. Singh P and Tripathy K. Ophthalmomyiasis. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023). Available online at: https://www.ncbi.nlm.nih.gov/sites/books/NBK576408/.
12. Ivanov V. Desant. Oestrus seriously harasses sheep (2015). Available online at: http://www.desant.net/show-news/33668 (Accessed November 14, 2025).
13. Scala A, Paz-Silva A, Suárez JL, López C, Díaz P, Díez-baños P, et al. Chronobiology of oestrus ovis (Diptera: oestridae) in sardinia, Italy: guidelines to chemoprophylaxis. J Med Entomol. (2002) 39:652–7. doi: 10.1603/0022-2585-39.4.652
14. Silva BFD, Bassetto CC, and Amarante AFTD. Epidemiology of Oestrus ovis (Diptera: Oestridae) in sheep in Botucatu, State of São Paulo. Rev Bras Parasitol Vet. (2012) 21:386–90. doi: 10.1590/S1984-29612012000400008
15. Gomez-Puerta LA, Alroy KA, Ticona DS, Lopez-Urbina MT, and Gonzalez AE. A case of nasal myiasis due to Oestrus ovis (Diptera: Oestridae) in a llama (Lama glama). Rev Bras Parasitol Vet. (2013) 22:608–10. doi: 10.1590/S1984-29612013000400026
16. Adhem NA and Kreem S. Morphological and molecular detection of oestrus ovis isolated from goats in babylon province, Iraq. UTTAR PRADESH J Zool. (2024) 45:424–30. doi: 10.56557/upjoz/2024/v45i134171
17. Kiran AV, Murthy GS, Kumar MU, and Kalyani P. Morphobiological characterisation of Oestrus ovis. Int J Vet Sci Anim Husb. (2024) 9:625–8. doi: 10.22271/veterinary.2024.v9.i3i.1488
18. Basmaciyan L, Gabrielle PH, Valot S, Sautour M, Buisson JC, Creuzot-Garcher C, et al. Oestrus ovis external ophtalmomyiasis: a case report in Burgundy, France. BMC Ophthalmol. (2018) 18:335. doi: 10.1186/s12886-018-1003-z
19. Shankar MK, Diddapur SK, Nadagir SD, and Kota SG. Ophthalmomyiasis externa caused by oestrus ovis. J Lab Physicians. (2012) 4:43–4. doi: 10.4103/0974-2727.98671
20. Hope FW. On insect and their larvae occacionally found in human body. Trans R Ent Soc. (1840) 2:256–71.
21. Keyt FT. A case of “Beef worm” (Dermatobia noxialis) in the orbit. Br Med J. (1900) 1:316. doi: 10.1136/bmj.1.2041.316
22. Bonzon L, Toga I, Piarroux M, and Piarroux R. Clustered Cases of Oestrus ovis Ophthalmomyiasis after 3-Week Festival, Marseille, France, 2013. Emerg Infect Dis. (2015) 21:375–7. doi: 10.3201/eid2102.140974
23. Maharjan N. External ophthalmomyiasis by oestrus ovis: two case reports from Nepal. J Lumbini Med Coll. (2021) 9. Available online at: https://nepjol.info/index.php/JLMC/article/view/41326 (Accessed November 14, 2025).
24. Sucilathangam G, Meenakshisundaram A, Hariramasubramanian S, Anandhi D, Palaniappan N, and Anna T. External Ophthalmomyiasis which was Caused by Sheep Botfly (Oestrus ovis) Larva: A Report of 10 Cases. | EBSCOhost. J Clin Diagn Res. JCDR (2013) 7:539.
25. Vedpathak M, Chatterjee N, Baradkar V, and Shastri J. Ophthalmomyiasis externa: A case report. Trop Parasitol. (2020) 10:147. doi: 10.4103/tp.TP_2_20
26. Schrecker J and Ahnert R. Ophthalmomyiasis Externa due to Oestrus ovis Larvae: A Case Report. Case Rep Ophthalmol. (2022) 13:1010–4. doi: 10.1159/000527440
27. Gur OE, Ozturk Yilmaz G, and Arslan MO. Human nasal myiasis caused by oestrus ovis: two case reports and review of the literature. B-ENT. (2021) 17:121–3. doi: 10.5152/B-ENT.2021.21301
28. Kunduracı MS, Kutlutürk I, Duman GT, Polat E, Kunduracı MS, Kutlutürk I, et al. Ophthalmomyiasis externa: A case report. Turk J Parasitol. (2020) 44(1):261–3.
29. Sharma K. Ophthalmomyiasis externa: A case report from Alkharj, Saudi Arabia. Saudi J Ophthalmol. (2018) 32:250–2. doi: 10.1016/j.sjopt.2017.11.005
30. García Guerrero CJ, Garza Quiñones J, Reza Caballero J, and González Treviño JL. External ophthalmomyiasis by Oestrus ovis treated with tobacco: clinical case report and medical literature review. Med Univ. (2014) 16:181–3.
31. Pejic R, Jakovac S, and Beck R. The first genetically confirmed case of human ophthalmomyasis due to oestrus ovis (Diptera oestridae) in Bosnia and Herzegovina. Acta Parasitol. (2020) 65:1011–3. doi: 10.1007/s11686-020-00249-4
32. Hoyer P, Williams RR, Lopez M, and Cabada MM. Human nasal myiasis caused by oestrus ovis in the highlands of cusco, Peru: report of a case and review of the literature. Case Rep Infect Dis. (2016) 2016:2456735. doi: 10.1155/2016/2456735
33. Velev V and Mikov O. Ophthalmomyiasis caused by larvae of the parasite Oestrus ovis. QJM Int J Med. (2018) 111:727–8. doi: 10.1093/qjmed/hcy106
34. Metwally DM, Albasyouni SA, Barakat IAH, Al-Turaiki IM, Almuhanna AM, Bashir MA, et al. Prevalence rate and molecular characteristics of oestrus ovis L. (Diptera, oestridae) in sheep and goats from riyadh, Saudi Arabia. Animals. (2021) 11:689. doi: 10.3390/ani11030689
35. Maurya RP and Shankar. Oculomyiasis: an overview. Indian J Clin Exp Ophthalmol. (2022) 8:439–41. doi: 10.18231/j.ijceo.2022.089
36. Tabatabaei SA, Soleimani M, Nikmanesh B, Mahmoudzadeh R, Vahedian Z, Salabati M, et al. Human subconjunctival dirofilariasis presenting as the daytime photophobia: A case report. Iran J Public Health. (2017) 46:1430–4.
37. Vogt R, Holzmann T, and Jägle H. Ophthalmomyiasis externa. Ophthalmol. (2015) 112:61–3. doi: 10.1007/s00347-014-3087-z
38. Adiri WN, Basil B, Onyia CP, Ugwuanyi JO, Azifuaku C, Iheukwumere SC, et al. Persistent intestinal myiasis caused by Oestrus ovis in a Nigerian university student: a case report. Egypt J Intern Med. (2025) 37:26. doi: 10.1186/s43162-025-00422-z
39. Kumar M, Joseph N, Srikanth K, and Stephen S. Conjunctival Myiais caused by Oestrus ovis in a medical college student which responded to Ivermectin. Ann Trop Med Public Health. (2013) 6:315–5. doi: 10.4103/1755-6783.121027
40. Macdonald PJ, Chan C, Dickson J, Jean-Louis F, and Heath A. Ophthalmomyiasis and nasal myiasis in New Zealand: a case series. N Z Med J. (1999) 112:445–7.
Keywords: ophthalmomyiasis, Oestrus ovis, parasitic eye infestation, Bulgaria, case report, myiasis
Citation: Atanasova MA, Blazhev AB, Milanova ET and Petrova LP (2025) Human ophthalmomyiasis caused by Oestrus ovis in Bulgaria: Case Report. Front. Ophthalmol. 5:1689524. doi: 10.3389/fopht.2025.1689524
Received: 20 August 2025; Accepted: 07 November 2025; Revised: 05 November 2025;
Published: 25 November 2025.
Edited by:
Mohammad Soleimani, University of Illinois Chicago, United StatesReviewed by:
Aynur Aliyeva, Yeditepe University, TürkiyeJasmeet Singh, Chhattisgarh Kamdhenu Vishwavidyalaya, India
Copyright © 2025 Atanasova, Blazhev, Milanova and Petrova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milena Atanasova Atanasova, bWlsZW5hYXIyMDAxQHlhaG9vLmNvbQ==
 Milena Atanasova Atanasova
Milena Atanasova Atanasova Alexander Bozhidarov Blazhev1
Alexander Bozhidarov Blazhev1 Lidiya Plamenova Petrova
Lidiya Plamenova Petrova