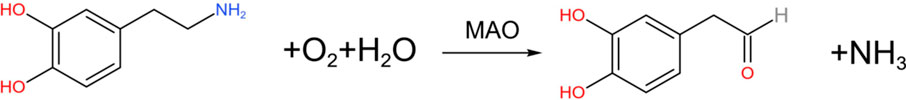- 1School of Materials Science and Engineering, Hefei University of Technology, Hefei, China
- 2Department of Ophthalmology, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 3Laboratory of Spinal and Spinal Cord Injury Regeneration and Repair, Department of Orthopedics (Spinal Surgery), The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 4Department of Neurosurgery, The First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital), Hefei, China
- 5MOE Key Lab for Cellular Dynamics, School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, China
- 6Clinical Research Experimental Center, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, China
- 7Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 8Intelligent Manufacturing Institute of HFUT, Hefei, China
Dopamine (DA) is one of the most important neurotransmitters in the human body, which is becoming a key breakthrough for addressing myopia, neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease, and mental diseases such as depression and schizophrenia. However, the activity of DA shows diurnal and seasonal variations, which may be due to the influence of solar activity time on the biological clock of the suprachiasmatic nucleus. By irradiating ARPE-19 cells with red and near-infrared light of different wavelengths, we studied and confirmed that the secretion and transformation of the light-induced neurotransmitter DA significantly depend on light wavelength and light dose. LED-chip light sources with emission peaks at 620, 680, 730, 800, and 850 nm and phosphor-converted LED light sources with emission peaks at 710 and 830 nm were used. It was confirmed that both the red and near-infrared light with variant wavelengths and doses can induce DA secretion to some extent. Yet, the concentrations of DA induced by the wideband spectral light of W710 and SW830 are higher than those induced by the narrowband single-LED-chip light and remain relatively stable under variant light doses. Among all the light sources, the model SW830 light source is the best one. This paper proposes a noninvasive way to induce the secretion of neurotransmitter DA and paves a reliable way to treat myopia, neurodegenerative diseases, and other diseases by using the neurotransmitter DA and the basic knowledge of photophysiology.
1 Introduction
Dopamine (DA) is one of the most important neurotransmitters in the human body that widely regulates motor control, emotion expression, cognitive function, and reward feedback (Lu et al., 2019). There are many methods to promote DA secretion. For example, regular aerobic exercise and strength training can directly stimulate the release of DA (Çinar et al., 2025), and listening to cheerful music can quickly activate the reward circuit of the brain (Reybrouck et al., 2021). As far as the synthesis pathway of DA is concerned, consuming substances rich in tyrosine, such as bananas, nuts, and fish, as well as an appropriate amount of caffeine and antioxidant substances, will provide the raw materials for the synthesis of DA (Mozaffarian, 2019). In addition, ensuring adequate good sleep (Andrea et al., 2017) and exposure to sunlight (Stern, 2013) can also help maintain the normal rhythm of the DA system. Moreover, the dopaminergic signaling pathway is the key to maintaining normal physiological functions. An imbalance in the synthesis and metabolism of the neurotransmitter DA may lead to some disorders of normal functions of the human body related to mental disturbance and neurodegenerative diseases (Klein et al., 2019).
DA dysfunction is closely related to various neurological and psychological diseases, such as Parkinson’s disease (Hirano, 2021), schizophrenia (Petty et al., 2019), depression (Taylor et al., 2022), addictive behaviors (Wise and Jordan, 2021), and attention-deficit hyperactivity disorder (Madras et al., 2005). It has been widely recognized among the ophthalmology community that DA regulates the elongation of the eye axis in axial myopia (Nebbioso et al., 2014). All these demonstrate the crucial role of DA in behavioral control and the necessity of understanding DA secretion and transformation for developing new treatment strategies for brain diseases.
In addition to Parkinson’s disease and myopia, the neurotransmitter DA could potentially offer a new treatment pathway for Alzheimer’s disease since it can increase the production of neprilysin, an enzyme that breaks down beta-amyloid plaques in the brain. Yet, levodopa (L-DOPA) is the direct precursor of DA. L-DOPA treatment can help reduce harmful beta-amyloid plaques and improve the memory function in a mouse model of Alzheimer’s disease (Watamura et al., 2024). Nevertheless, it is known that L-DOPA treatment causes serious side effects in patients. Notably, alterations in the photoperiod, circadian rhythm, and light exposure can cause anxiogenic and depressive behavior in diurnal adult mammals (Dulcis et al., 2013). Changes in the light–dark cycle have profound effects on human mood and behavior as well, contributing to a variety of disorders such as seasonal affective disorder (Birren and Marder, 2013). A diurnal rhythm of DA metabolism has been reported in various animal species (Nir and Michael Iuvone, 1994). Stimulation of DA biosynthesis in light is due to the activation of tyrosine hydroxylase (TH) to produce 3,4-dihydroxyphenylalanine (DOPA) and the induction of aromatic L-amino acid decarboxylase (AAAD), which catalyzes the formation of DA (Nir and Michael Iuvone, 1994). In many biological models, the effect of light on the release of DA in the retina has been verified through various experimental methods (Pozdeyev and Lavrikova, 2000; Ke et al., 2017; Lu and Tong, 2024).
Human beings have lived under the sun for approximately 200,000 years, evolving from a primitive society to modern civilization. In the long process of evolution, humans have adapted to sunlight. Therefore, one prominent advantage of light-induced DA secretion is that it is safe and has no side effects. In the past, the discussion on light exposure and DA secretion mainly focused on violet and visible light, aiming at myopia control (Nguyen et al., 2019; Carpena-Torres et al., 2023; Tian et al., 2021; Strickland et al., 2020). However, recent research shows that visible fluorescent light is harmful to DA neurons and induces neurodegeneration (Fasciani et al., 2020; Romeo et al., 2017; Ahamed et al., 2014). On delivering the 710 nm far-red light into the substantia nigra pars compacta of mice using a micro-optical fiber, it was found that the firing rate of DA neurons was enhanced four-fold from 2.2 impulse/s to 8.8 impulse/s. In photobiomodulation, the wavelength in the region of 600 nm–1,100 nm is the most used, which differs from the visible light that is mainly in the wavelength region of 400 nm–700 nm for indoor and outdoor lighting. Therefore, it is important to study the secretion and transformation of the neurotransmitter DA induced by light in the wavelength region of 600 nm–1,100 nm for photobiomodulation.
In order to explore the light wavelengths and light doses that can effectively induce the secretion of DA, we studied the photophysiological effects of red and near-infrared light on its secretion and transformation using a cell model that was adopted with the retinal pigment epithelia (ARPE-19) cells. We believe that red and near-infrared light can immediately stimulate the secretion of DA and also induce multiple transformations in neurotransmitters through a series of enzymatic reactions.
2 Methods
2.1 Experimental cell models
The ARPE-19 cells, which were bought from Wuhan Pricella Biotechnology Co, Ltd., were inoculated in cell culture flasks and then grown at 37 °C in a humid atmosphere of 95% air and 5% CO2 in high-glucose DMEM supplemented with heat-inactivated 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were passaged regularly using 0.25% trypsin-EDTA (Sigma). To examine the effect of light exposure on the cells, the ARPE-19 cells in the logarithmic growth phase were first re-inoculated in a culture flask at approximately 1 × 106 cells/mL. At approximately 24 h after replating, the medium was replaced with fresh medium, and the cells were placed under the same culture conditions.
2.2 Photobiomodulation
The ARPE-19 cells were irradiated with two types of light source. One was made with a single LED chip with emission peaks at 620 nm, 680 nm, 730 nm, 800 nm, and 850 nm, respectively; another was the phosphor-converted LEDs with two product models, i.e., the W710 and SW830. The product model W710 has an emission peak at 710 nm in the wavelength range of 600 nm–850 nm, and the product model SW830 has a main emission peak at 830 nm and a secondary peak at 710 nm in the wavelength range of 600 nm–1,100 nm. During the irradiation process, the cells were taken out of the incubator and placed on the experimental table. The height from the lamp surface to the bottom of the cell culture dish was fixed at 10 cm, ensuring that light evenly illuminated the cells. At this height, the radiation light power density was 3 mW/cm2, and the exposure times were 333, 667, 1,333, and 2,667 s, respectively, resulting in energy densities of 1, 2, 4, and 8 J/cm2. To prevent interference from other light sources, the outside of the cell culture dish was wrapped with tin foil, and the experiment was conducted in dark, as shown in Supplementary Figure S1. The cells in the control group were treated in the same way, but not exposed to light.
2.3 Enzyme-linked immunosorbent assay (ELISA) on dopamine
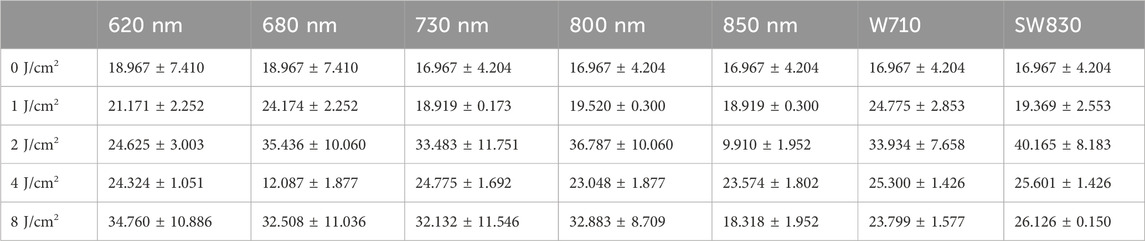
After the cells were first exposed to light, they were cultured in the incubator for 24 h. Then, the cells were irradiated with light for a second time. One hour after the second light treatment, the ELISA test was conducted. The quantity of the neurotransmitter DA in the cells’ supernatant of each group was detected by indirect ELISA, and the DA ELISA kit was used. Each test was replicated two times, and then the average values and errors were concluded, as shown in Table 1. The standard product or samples were added to the 96-well plate, and then the biotinylated antibody working solution was added and incubated at 37 °C for 45 min. After washing three times, 100 μL of the enzyme conjugate working solution was added, incubated at 37 °C for 30 min, washed five times, 90 μL substrate solution was added, incubated at 37 °C for 15 min, and finally, 50 μL termination solution was added. The absorbance of each hole was immediately measured by a microplate reader at a wavelength of 450 nm.
2.4 Neurotransmitter detection on other neurotransmitters
Other neurotransmitters including glutamine, glutamic acid, tyrosine, DA hydrochloride, norepinephrine hydrochloride, and kynurenic acid were measured using the high-performance liquid chromatography (HPLC) method and merely tested one time. First, the solution of the standard product was prepared. Then, the metabolites were extracted. An appropriate amount of the samples was taken into a 2-mL centrifuge tube, and 100 μL 2% formic acid and methanol solution was accurately added and mixed in a vortex. Then, 400 μL of aqueous solution was accurately added and swirled well. The solution was centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was passed through a 0.22-μm filter membrane. Next, 100 μL of the supernatant was taken and added to 20 ng/mL of Trp-d5 internal standard 100 μL, swirled well, and added into the test bottle as a low-concentration substance detection sample. An appropriate amount of the supernatant and 2% formic acid methanol solution were taken, diluted 200 times with water, and swirled well. Then, 100 μL of the diluent solution was taken and added to 20 ng/mL of Trp-d5 internal standard 100 μL, swirled well, and added into the test bottle as a sample for high-concentration substance detection. Finally, the neurotransmitters and their concentration were tested on a HPLC instrument (AB Sciex, ExionLC) using an ACQUITY UPLC® HSS T3 column (2.1*150 mm, 1.8 μm, from Waters, United States).
3 Results
3.1 Light sources and their spectra
Figure 1 displays the emission spectra of the light sources used in this study. Figure 1A shows the emission spectra of the red and far-red light sources with emission peaks at 620, 680, and 730 nm; Figure 1B shows the emission spectra of near-infrared light sources with emission peaks at 800 and 850 nm, respectively. The light sources in Figures 1A, B with narrowband spectral configuration are all made using a single LED chip. The light sources in Figures 1C, D with broadband emission are made from phosphors by coating the mixture of different phosphors on the 450-nm blue-emitting LED chips, i.e., the phosphor-converted LEDs. In photobiomodulation, the cellular and molecular mechanisms of cells’ cytochrome c oxidase (CCO), located in the mitochondria, were mostly utilized. During the biological activities, the photons absorbed by CCO were used to trigger the biochemical reactions and mitochondrial signaling reactions (Karu, 2014). The absorption peaks of the reduced CuA, the oxidized CuB, the reduced CuB, and the oxidized CuA in CCO are located at 620, 680, 760, and 825 nm, respectively (Nguyen et al., 2019). The selected narrowband peaks at 620, 680, 730, and 850 nm in this work are consistent or close to the absorption peaks of the reduced CuA, the oxidized CuB, the reduced CuB, and the oxidized CuA. In addition, a narrowband peak at 800 nm, located between the absorption peak of the reduced CuB at 760 nm and that of the oxidized CuA at 825 nm, was chosen. The broadband W710 in 600 nm–850 nm could mainly cover the absorption peak of the oxidized CuB and the reduced CuB and partly cover the main absorption peak of the reduced CuA and the oxidized CuA. Nevertheless, the broadband SW830 in 600 nm–1,100 nm can not only fully cover the absorption peaks of the reduced CuA, the oxidized CuB, the reduced CuB, and the oxidized CuA at 620, 680, 760, and 825 nm, respectively, but may also stimulate some other unknown mechanisms, such as ion channels and nano water in the cell membrane (Chamkouri et al., 2024a; Chamkouri et al., 2024b).

Figure 1. The emission spectrum of the light sources utilized in this work. (A) The light sources made using single-type LED chip with emission peak at 620, 680, and 730 nm, respectively, in red and far-red wavelength region, (B) the light sources made using single-type LED chip with emission peak at 800 and 850 nm, respectively, in near-infrared wavelength region, (C) the phosphor-converted LED light source with emission spectrum in red and far-red wavelength region of 600-850 nm (product code W710), and (D) the phosphor-converted LED light source with emission spectrum in red and near-infrared wavelength region of 600-1100 nm (product code SW830).
3.2 Light induces the secretion of the neurotransmitter dopamine
Figure 2A presents the relationship of DA concentration as a function of light energy. Figure 2B plots the curves of DA concentration as a function of light wavelength at each energy level of 0, 1, 2, 4, and 8 J/cm2. The values of DA concentration, as corresponding to Figure 2A, B, are provided in Table 1. On the whole, the DA concentration of the ARPE-19 cells that were exposed to different light sources at variant energy was higher than that of the sample without light irradiation in the dark. As can be seen from Figure 2A, among all the seven light sources of 620 nm, 680 nm, 730 nm, 800 nm, 850 nm, W710, and SW830, except for 850 nm, six of them efficiently enhance the DA concentration at 2 J/cm2. In addition, there is another abnormal point at 4 J/cm2 for the 850-nm source, wherein the DA concentration is far lower than that of other energy levels. Either at 1, 2, 4, or 8 J/cm2 is relatively, it can be distinguished clearly from Figure 2B that the DA concentration induced by 850 nm is lower than that of other wavelengths at each corresponding energy level. When irradiated with the light wavelength of 850 nm, the changing trend was opposite to that of other wavelengths. Regarding the 680 nm wavelength, only the DA concentration at 2 J/cm2 is lower than that of other energy levels. These phenomena show the dependence of light-induced DA concentration on light wavelength and light doses. As seen in Figure 2A or Figure 2B, the DA concentration induced by the broadband spectra of W710 and SW830 is relatively high and remains stable over variant energy levels. Furthermore, from the perspective of different wavelengths, the light source SW830 is optimal. Although there are certain changes at different doses, the overall changes are relatively stable, and the secretion of DA is relatively high.

Figure 2. Relationship between the dopamine content in ARPE-19 cells and light doses of different wavelengths. (A) Content of dopamine as a function of light dose. (B) Content of dopamine as a function of light wavelength.
Figure 2B shows that the DA concentrations induced by 680 nm at 4 J/cm2 and that by 850 nm at 2 J/cm2 are lower than those in the dark condition. These abnormal data provide important clues for understanding the secretion and transformation of the neurotransmitter DA induced by light. Based on the above results, we selected the light sources of 680 nm, 850 nm, and SW830 and the supernatant of ARPE-19 cells for further detection.
3.3 The influence of light on cell morphology
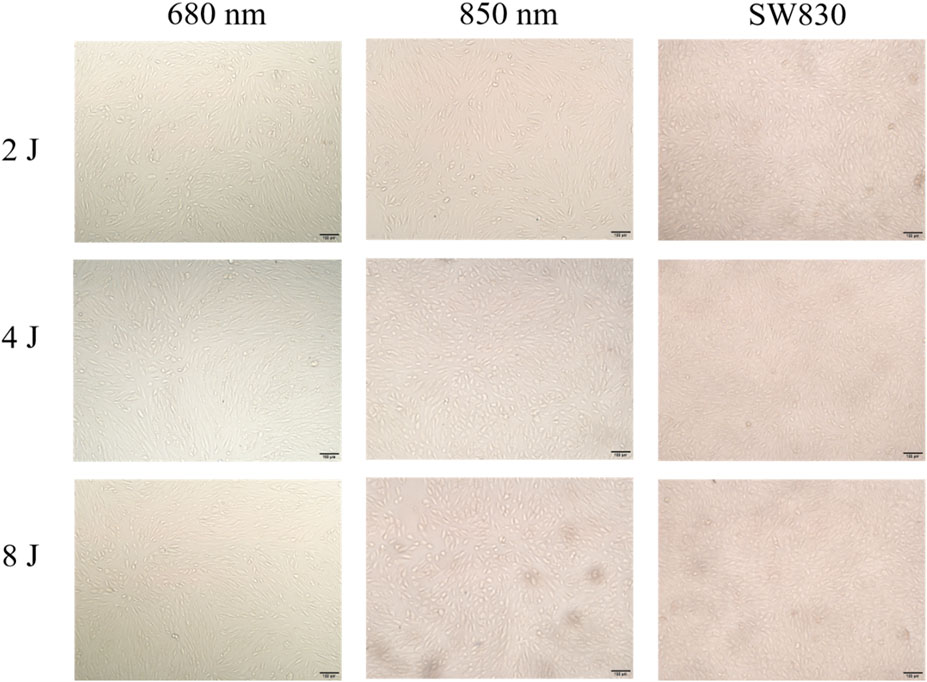
The cell morphology of the SW830 lamp-irradiated cells was compared with that of cells irradiated with wavelengths of 680 nm and 850 nm under dark conditions under an optical microscope (Figure 3), and it was further compared with the cell morphology of the control group in the dark without light (Supplementary Figure S2).
The influence of light on the cell morphology was examined by taking photographs of the ARPE-19 cells that were cultured under different wavelengths of light in combination with different light doses. Figure 3 presents the pictures, taken using an optical microscope, of the cells cultured under 680 nm, 850 nm, and SW830 at the doses of 2, 4, and 8 J/cm2. The ARPE-19 cells are a type of typical adherent cell. As can be seen from Figure 3 and Supplementary Figure S2, the cells exhibit a typical epithelial cell morphology, and the cells form a monolayer structure through tight junctions. After being exposed to light, the cells in each group showed polygonal atypia. The cells cultured under 680 nm have long-egg or slender-body morphology, the cells cultured under 850 nm have round-egg or spheroid-body morphology, and the cells cultured under SW830 also have round-egg or spheroid-body morphology. Comparatively, the cells cultured under SW830 are rounder or more regular than those cultured under 850 nm and 680 nm. Moreover, with the light exposure energy increasing from 2 to 4 and then to 8 J/cm2, the cells’ density increased on the whole. In comparison, the cells cultured under SW830 at 8 J have the largest density, while the cells cultured under 850 nm at 2 J/cm2 have the smallest density. See Supplementary Figure S2 for a comparison of the cells cultured under different light conditions with the control group grown in dark,
3.4 The light-induced transformation of dopamine and other neurotransmitters
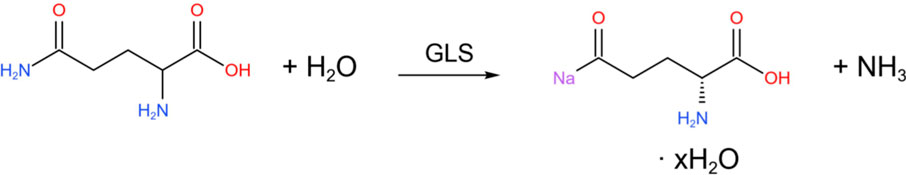
During physiological reactions, glutamine (Gln) can be hydrolyzed by glutaminase (GLS) into glutamic acid (Glu).
In addition, glutamic acid is one of the precursors for the synthesis of DA. It can generate α-ketoglutaric acid (α-KG) through transamination, enter the citric acid cycle (TCA) to provide energy, or serve as an intermediate in amino acid metabolism.
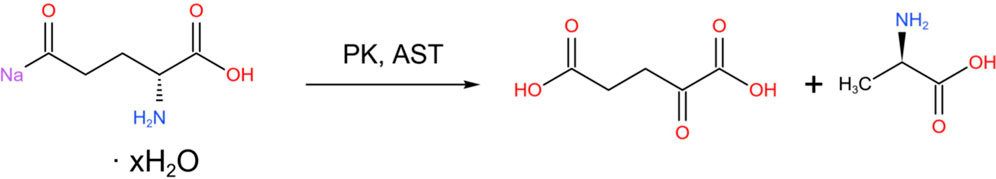
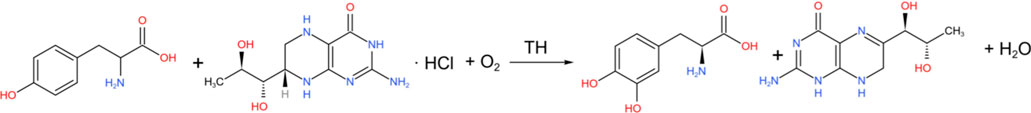
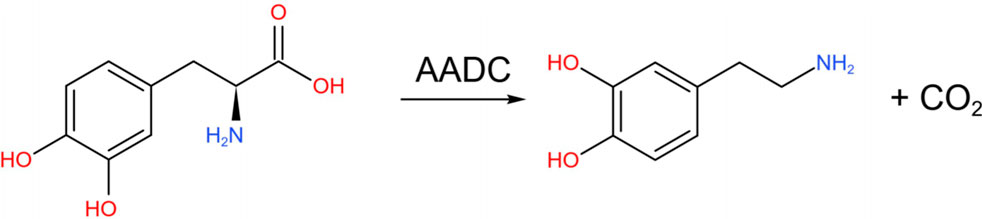
More directly, tyrosine is catalyzed by tyrosine hydroxylase (TH) to L-DOPA (Baytas et al., 2024).
Subsequently, levodopa is converted into DA under the action of aromatic L-amino acid decarboxylase (AADC) (Maini Rekdal et al., 2019).
Among them, TH is a key rate-limiting enzyme in the process of DA synthesis. It catalyzes the first step of the conversion of tyrosine to levodopa, which is generally regarded as the rate-limiting step in catecholamine biosynthesis. Studies have shown that in rats, the secretion of DA and the activity of TH, the rate-limiting enzyme in DA biosynthesis, are regulated by light (Inukai et al., 2022).
Since the neurotransmitters of glutamine, glutamic acid, and tyrosine are direct or indirect precursors for the synthesis of the neurotransmitter DA, the change in the glutamine, glutamic acid, and tyrosine concentration as a function of light energy under the irradiation of 680 nm, 850 nm, and SW830 in Figures 4A–C can be used to explain the variation in DA concentration upon exposure to various light wavelengths and light doses in Figure 1. On the whole, the concentrations of glutamine, glutamic acid, and tyrosine decrease rapidly with the increase in light energy upon irradiating the ARPE-19 cells with 680 nm and SW830, although there is some difference in the efficacy between 680 nm and SW830 light sources. The concentration of glutamine and tyrosine decreases slightly by irradiating with 850 nm at the energy level of 4J. However, the concentrations of glutamine, glutamic acid, and tyrosine remain relatively stable and almost do not vary with the change in light energy upon stimulation with 680 nm, 850 nm, and SW830. Therefore, the light of 680 nm and SW830 could promote the physiological reactions to produce the neurotransmitter DA, whereas it is difficult to activate the reaction with the 850 nm light.

Figure 4. Change of neurotransmitters’ concentration upon irradiation of light dose on ARPE-19 cells with 680 nm, 850 nm, and SW830 light sources. (A) Glutamine; (B) glutamic acid; (C) tyrosine; (D) dopamine hydrochloride; (E) norepinephrine hydrochloride; (F) kynurenic acid. In this figure, the curves were connected with modified Bezier function.
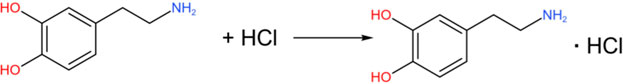
DA (in the free base form) is an organic base. The amino group (-NH2) in its molecule can undergo a neutralization reaction with hydrochloric acid (HCl) to form DA hydrochloride.
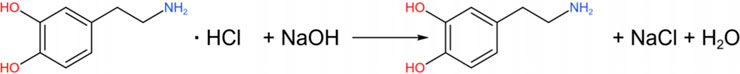
Under alkaline conditions, DA hydrochloride can be neutralized to release free DA again.
In the central nervous system, DA is synthesized through anabolic pathways shared with other catecholamines, such as L-demethyladrenaline (NE) and epinephrine (E) (Zhang et al., 2024). DA is the precursor of norepinephrine, and norepinephrine can be further converted into epinephrine (Forero-Girón and Toro-Labbé, 2025).
Quinolinic acid (kynurenic acid, Kyna) and DA are two molecules with important functions in the nervous system, and their interaction involves multiple aspects, such as neurotransmitter regulation, oxidative stress, and the mechanism of mental disorders (Kondo et al., 2024). Quinolinic acid indirectly regulates the activity of dopaminergic neurons by antagonizing NMDA receptors and α7 nicotinic acetylcholine receptors, especially playing a key role in the reward, cognitive, and motor functions (Toyoda, 2019). Kyna and DA are two different neuroactive substances that belong to the products of the tryptophan metabolism pathway and the tyrosine metabolism pathway, respectively. Their conversion relationship is not direct, but they can be indirectly related through common metabolic pathways or interactions.
Kyna is derived from the kynurenine pathway of tryptophan metabolism.
DA is derived from the catecholamine pathway of tyrosine metabolism.
The metabolism of tryptophan and tyrosine depends on aromatic amino acid hydroxylase (AAAH) and cofactors (such as tetrahydrobiopterin, BH4). Thus, the conversion of DA and Kyna is competitive. When tryptophan metabolism is enhanced (such as during inflammation), more cofactors may be consumed, indirectly inhibiting TH and reducing DA synthesis.
As can be seen from Figures 4D–F, with 850 nm irradiation, the concentrations of DA hydrochloride (DH), norepinephrine hydrochloride (NH), and Kyna almost do not change with light energy increasing from 1 to 8 J/cm2. However, the concentration of DH, NH, and Kyna increases rapidly with light energy increasing from 0 via 1 J/cm2–2 J/cm2. It reaches a maximum at 2 J/cm2 and then decreases a little when light energy further increases via 4 J/cm2–8 J/cm2.
On the whole, the concentration of glutamine, glutamic acid, and tyrosine decreases rapidly with the increase in light energy upon irradiation of the ARPE-19 cells with 680 nm and SW830, although there is some difference on the efficacy between 680 nm and SW830 light sources. The concentration of glutamine and tyrosine decreases a little on irradiation with 850 nm at the energy level of 4 J. However, the concentrations of glutamine, glutamic acid, and tyrosine remain relatively stable and almost do not vary with the change in light energy upon stimulation with 680 nm, 850 nm, and SW830. Therefore, the light of 680 nm and SW830 could promote the physiological reactions to produce the neurotransmitter DA, whereas it is difficult to activate the reaction with the light of 850 nm. The increase in these metabolites of DH, NH, and Kyna from 2 J to 8 J in Figure 4 can explain the decrease in DA concentration in Figure 2. It has to be noted that the concentration of DH in Figure 4D still increases with the light energy from 1 J to 8 J under the stimulation of SW830. The reaction between DA and DH is not a typical chemical transformation but involves processes such as acid–base neutralization or salt formation. In the human body, the DA hydrochloride dissociates into DA and chloride ions, exerting the function of a neurotransmitter. In other words, the conversion of DA into DH is beneficial for the long-term functioning of DA, which can explain how the high concentration of DA was obtained by inducing with SW830 from 1 J to 8 J in Figure 2.
Notably, DA (especially the free base) is easily oxidized to quinone compounds by high-energy photons. Yet, the low-energy photons of near-infrared light at 850 nm or 830 nm cannot activate the reaction, as concluded form Figure 4. In photobiomodulation, the deep red light of 670 nm was mostly used, which makes use of the cellular mechanism of CCO by exciting the absorption of oxidized CuBoxid in the mitochondria to produce adenosine triphosphate (ATP), which is the energy source for cells (Karu, 2014). In addition to the mechanism of CCO, the exceptional low concentration of DA observed in Figure 2 under irradiation with 680 nm at 2 J/cm2 may be due to the activation of other related metabolic enzymes (such as MAO or COMT). Light exposure may affect the intracellular signaling pathways, promoting the enhancement of the activity of these enzymes, thereby accelerating the metabolic transformation of DA.
DA is oxidized and deaminated by MAO to form 3, 4-dihydroxyphenylacetaldehyde (DOPAL) (Goldstein, 2021), and the chemical reaction formula is:
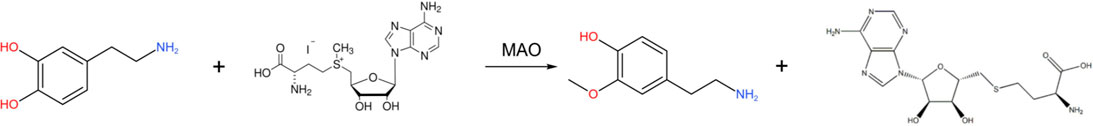
In addition, DA can also react with S-adenosine methionine (SAM) under the action of COMT to generate 3-methoxytyramine (3-MT) (Casal et al., 2016), and the chemical reaction formula is as follows:
Notably, different from the illumination results of other wavelengths, after being exposed to light with a wavelength of 850 nm, the changing trend is opposite to that of other groups, which suggests that the 850 nm wavelength plays an important role in storing DA although it cannot stimulate the transformation and digestion of DA. It likely helps maintain the redox state, supply water for synthesis, or activate other signaling paths.
In summary, the red light at 680 nm can effectively stimulate the generation of DA, but its concentration is not stable due to the transformation into other compounds; is hard to induce the secretion of DA under the near-infrared light of 850 nm, but it plays an important role in maintaining the stable concentration of DA; interestingly, the concentration of DA induced by broadband spectral light of SW830 is high and stable. For these phenomena, we have to consider some deeper mechanisms that are involved in the activation by light in the secretion and transformation of neurotransmitters in which the reactive oxygen species or membrane photoreceptors are most probably involved (Karu, 2014). The occurrence and development of myopia is strongly related with the neurotransmitter DA, and the elongation of the eye axis in axial myopia is responsible for the deficiency of DA. In terms of suppressing myopia progression in lens-induced guinea pigs, we observed that the action involved regulating the photoreceptor-related genes to improve photoreceptor function by transcriptomics analysis (Ding et al., 2025). The decrease in the concentration of glutamate along with the increase in DA produced in the subthalamic nucleus (STN) area, stimulated by delivering the near-infrared light of 840 nm wavelength with 130 Hz pulse repetition deep in the brain to rats using a fiber-coupled laser stimulator, was found by Kuo et al., which demonstrated the potential of a therapeutic strategy for DA-related diseases such as Parkinson’s disease using deep-brain light stimulation. The dependence of DA concentration on laser photon power was also observed (Kuo et al., 2014). Thus, the results in Kuo et al. (2014) support our conclusions presented in Figures 4A, B. Nevertheless, compared with the invasive method adopted in Kuo et al. (2014), it appears that a non-invasive method by radiating mice skin can perform the same function (Ding et al., 2025), exhibiting significant progress. Further research on this point will be presented in our upcoming paper on the Parkinson’s disease model mice.
Although the interesting results and some useful data have been achieved in this study, we are aware of the limitations of this work. Herein, the cells’ model and the test on DA concentration with a kit method were doped. If animal or human models were used and the test on DA concentration was conducted by analyzing the blood or cerebrospinal fluid using the HPLC method, the experimental values would be more accurate and evaluable. Further studies are necessary in future for extrapolating the in vitro results to biological or clinical settings.
4 Conclusion
Through the study of irradiation of ARPE-19 cells with different light wavelengths and light doses from different light sources, we confirm that the secretion and transformation of the light-induced neurotransmitter DA significantly depend on the light wavelength and light dose. Two types of light sources were used. One type is the single-LED chip light source, with emission peak wavelengths at 620, 680, 730, 800, and 850 nm, respectively, and the other type is the broadband spectral configuration phosphor-converted LED light sources. As for the phosphor-converted LEDs light sources, one with model W710 has an emission wavelength range of 600 nm–850 nm with a peak at 710 nm, and the other with model SW830 has an emission wavelength range of 600 nm–1,100 nm showing a main peak at 830 nm and a secondary peak at 710 nm. The use of different wavelengths and different light doses can induce the secretion of the neurotransmitter DA to a certain extent, but some light sources have obvious effects, while others do not. The concentration of DA induced by some light sources can remain relatively stable, while for others, it undergoes rapid transformation. Specifically, when ARPE-19 cells were irradiated with a narrowband light source fabricated from a single-chip LED with emission peak wavelength of 680 nm at the light dose of 2 J/cm2, the activity of the enzymes related to DA synthesis could be effectively activated, thereby promoting DA production. However, when the light dose increased to 4 J/cm2, it triggered the metabolic decomposition of DA, resulting in a decrease in DA content. When the ARPE-19 cells were irradiated with a narrowband light source fabricated from a single-chip LED with a peak emission wavelength of 850 nm, the changing trend was opposite to that of other groups. It decreased evidently at 2 J/cm2, yet, it increased at 4 J/cm2. Overall, the concentrations of DA induced by the broadband phosphor-converted LEDs light sources of W710 and SW830 are relatively high and remain stable, compared with those of single-LED chip light sources. Among them, the effect of the SW830 light source is better. This indicates that the secretion of the neurotransmitter DA induced by light involves the synergistic effect of multiple signals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for these studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
SW: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. CL: Data curation, Formal Analysis, Investigation, Visualization, Writing – review and editing. ZW: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – review and editing. JS: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review and editing. XH: Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review and editing. CS: Data curation, Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – review and editing. YZ: Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – review and editing. DL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review and editing. LC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation (21875058), the Natural Science Foundation of Anhui Province (2208085J13), the Major Science and Technology project of Anhui Province (202103a05020025), and the Major Science and Technology Project of Zhongshan City of Guangdong Province on the Strategic Emerging Industries Technology Research Topic (2022A1007), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphot.2025.1647467/full#supplementary-material
References
Ahamed, B. A., Mathangi, D. C., Shyamala, R., and Ramesh Rao, K. (2014). Protective effect of light emitting diode phototherapy on fluorescent light induced retinal damage in wistar strain albino rats. Ann. Anat. 196 (5), 312–316. doi:10.1016/j.aanat.2014.04.004
Andrea, H.-S., Wendy, H.-M., Luis, N.-J., and Oscar, A.-C. (2017). Dopaminergic modulation of sleep-wake states. CNS and Neurological Disord. - Drug Targets 16 (4), 380–386. doi:10.2174/1871527316666170320145429
Baytas, O., Davidson, S. M., Kauer, J. A., and Morrow, E. M. (2024). Loss of mitochondrial enzyme Gpt2 leads to reprogramming of synaptic glutamate metabolism. Mol. Brain 17 (1), 87. doi:10.1186/s13041-024-01154-x
Birren, S. J., and Marder, E. (2013). Plasticity in the neurotransmitter repertoire. Sci. (1979). 340 (6131), 436–437. doi:10.1126/science.1238518
Carpena-Torres, C., Schilling, T., Huete-Toral, F., Bahmani, H., and Carracedo, G. (2023). Increased ocular dopamine levels in rabbits after blue light stimulation of the optic nerve head. Exp. Eye Res. 234, 109604. doi:10.1016/j.exer.2023.109604
Casal, E., Palomo, L., Cabrera, D., and Falcon-Perez, J. M. (2016). A novel sensitive method to measure Catechol-O-Methyltransferase activity unravels the presence of this activity in extracellular vesicles released by rat hepatocytes. Front. Pharmacol. 7, 501. doi:10.3389/fphar.2016.00501
Chamkouri, H., Si, J., Chen, P., Ni, H., Bragin, D. E., Ahmadlouydarab, M., et al. (2024a). Overcoming challenges of clinical cell therapies for parkinson's disease with photobiomodulation. Interdiscip. Med. 2, e20240013. doi:10.1002/INMD.20240013
Chamkouri, H., Liu, Q., Zhang, Y., Chen, C., and Chen, L. (2024b). Brain photobiomodulation therapy on neurological and psychological diseases. J. Biophot. 17 (1), e202300145. doi:10.1002/jbio.202300145
Çinar, V., Bağ, M. F., Aslan, M., Çınar, F., Gennaro, A., Akbulut, T., et al. (2025). Impact of different exercise modalities on neuroendocrine well-being markers among university students: a study of renalase and catecholamine responses. Front. Physiol. 16, 1591132. doi:10.3389/fphys.2025.1591132
Ding, J., Wang, X., Cao, F., Gao, Y., Wang, C., Xu, T., et al. (2025). Therapeutic effect and mechanism of far-red/near-infrared light on lens-induced Myopia Guinea pigs. Biochem. Biophysical Res. Commun. 777, 152282. doi:10.1016/j.bbrc.2025.152282
Dulcis, D., Jamshidi, P., Leutgeb, S., and Spitzer, N. C. (2013). Neurotransmitter switching in the adult brain regulates behavior. Sci. (1979). 340 (6131), 449–453. doi:10.1126/science.1234152
Fasciani, I., Petragnano, F., Aloisi, G., Marampon, F., Rossi, M., Coppolino, M. F., et al. (2020). A new threat to dopamine neurons: the downside of artificial light. Neuroscience 432, 216–228. doi:10.1016/j.neuroscience.2020.02.047
Forero-Girón, A. C., and Toro-Labbé, A. (2025). How does dopamine convert into norepinephrine? insights on the key step of the reaction. J. Mol. Model. 31 (1), 32. doi:10.1007/s00894-024-06256-w
Goldstein, D. S. (2021). The catecholaldehyde hypothesis for the pathogenesis of catecholaminergic neurodegeneration: what we know and what we do not know. Int. J. Mol. Sci. 22 (11), 5999. doi:10.3390/ijms22115999
Hirano, S. (2021). Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of parkinson’s disease. Mol. Med. 27 (1), 40. doi:10.1186/s10020-021-00301-7
Inukai, S., Hara, S., and Ichinose, H. (2022). Tyrosine hydroxylase activity is regulated through the modification of the 176th cysteine residue. Biochem. Biophysical Res. Communications 589, 209–214. doi:10.1016/j.bbrc.2021.12.024
Karu, T. I. (2014). Cellular and molecular mechanisms of photobiomodulation (low-power laser therapy). IEEE J. Sel. Top. Quantum Electron. 20 (2), 143–148. doi:10.1109/JSTQE.2013.2273411
Ke, Y., Li, W., Tan, Z., and Yang, Z. (2017). Induction of dopamine D1 and D5 receptors in R28 cells by light exposures. Biochem. Biophysical Res. Commun. 486 (3), 686–692. doi:10.1016/j.bbrc.2017.03.099
Klein, M. O., Battagello, D. S., Cardoso, A. R., Hauser, D. N., Bittencourt, J. C., and Correa, R. G. (2019). Dopamine: functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 39 (1), 31–59. doi:10.1007/s10571-018-0632-3
Kondo, T., Okada, Y., Shizuya, S., Yamaguchi, N., Hatakeyama, S., and Maruyama, K. (2024). Neuroimmune modulation by tryptophan derivatives in neurological and inflammatory disorders. Eur. J. Cell Biol. 103 (2), 151418. doi:10.1016/j.ejcb.2024.151418
Kuo, J. R., Lin, S. S., Liu, J., Chen, S. H., Chio, C. C., Wang, J. J., et al. (2014). Deep brain light stimulation effects on glutamate and dopamine concentration. Biomed. Opt. Express 6 (1), 23–31. doi:10.1364/BOE.6.000023
Lu, Y., and Tong, M. (2024). Impact of red and blue monochromatic light on the visual system and dopamine pathways in juvenile zebrafish. BMC Ophthalmol. 24 (1), 475. doi:10.1186/s12886-024-03742-w
Lu, W., Ding, Z., Liu, F., Shan, W., Cheng, C., Xu, J., et al. (2019). Dopamine delays articular cartilage degradation in osteoarthritis by negative regulation of the Nf-Κb and Jak2/Stat3 signaling pathways. Biomed. and Pharmacother. 119, 109419. doi:10.1016/j.biopha.2019.109419
Madras, B. K., Miller, G. M., and Fischman, A. J. (2005). The dopamine transporter and attention-deficit/hyperactivity disorder. Biol. Psychiatry 57 (11), 1397–1409. doi:10.1016/j.biopsych.2004.10.011
Maini Rekdal, V., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J., and Balskus, E. P. (2019). Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Sci. (1979). 364 (6445), eaau6323. doi:10.1126/science.aau6323
Mozaffarian, D. (2019). The microbiome, plasma metabolites, dietary habits, and cardiovascular risk unravelling their interplay. Circ. Res. 124 (12), 1695–1696. doi:10.1161/CIRCRESAHA.119.315206
Nebbioso, M., Plateroti, A. M., Pucci, B., and Pescosolido, N. (2014). Role of the dopaminergic system in the development of myopia in children and adolescents. J. Child. Neurol. 29 (12), 1739–1746. doi:10.1177/0883073814538666
Nguyen, M. T., Vemaraju, S., Nayak, G., Odaka, Y., Buhr, E. D., Alonzo, N., et al. (2019). An opsin 5-dopamine pathway mediates light-dependent vascular development in the eye. Nat. Cell Biol. 21 (4), 420–429. doi:10.1038/s41556-019-0301-x
Nir, I., and Michael Iuvone, P. (1994). Alterations in light-evoked dopamine metabolism in dystrophic retinas of mutant rds mice. Brain Res. 649 (1), 85–94. doi:10.1016/0006-8993(94)91051-0
Petty, A., Cui, X., Tesiram, Y., Kirik, D., Howes, O., and Eyles, D. (2019). Enhanced dopamine in prodromal schizophrenia (Edips): a new animal model of relevance to schizophrenia. npj Schizophr. 5 (1), 6. doi:10.1038/s41537-019-0074-z
Pozdeyev, N. V., and Lavrikova, E. V. (2000). Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J. Mol. Neurosci. 15 (1), 1–10. doi:10.1385/JMN:15:1:1
Reybrouck, M., Vuust, P., and Brattico, E. (2021). Neural correlates of music listening: does the music matter? Brain Sci. 11 (12), 1553. doi:10.3390/brainsci11121553
Romeo, S., Vitale, F., Viaggi, C., di Marco, S., Aloisi, G., Fasciani, I., et al. (2017). Fluorescent light induces neurodegeneration in the rodent nigrostriatal system but near infrared LED light does not. Brain Res. 1662, 87–101. doi:10.1016/j.brainres.2017.02.026
Stern, P. R. (2013). Daylight determines dopamine. Sci. Signal. 6(273): ec95-ec. doi:10.1126/scisignal.2004276
Strickland, R., Landis, E. G., and Pardue, M. T. (2020). Short-wavelength (violet) light protects mice from myopia through cone signaling. Invest Ophthalmol. Vis. Sci. 61 (2), 13. doi:10.1167/iovs.61.2.13
Taylor, W. D., Zald, D. H., Felger, J. C., Christman, S., Claassen, D. O., Horga, G., et al. (2022). Influences of dopaminergic system dysfunction on late-life depression. Mol. Psychiatry 27 (1), 180–191. doi:10.1038/s41380-021-01265-0
Tian, T., Zou, L., Wang, S., Liu, R., and Liu, H. (2021). The role of dopamine in emmetropization modulated by wavelength and temporal frequency in Guinea pigs. Invest Ophthalmol. Vis. Sci. 62 (12), 20. doi:10.1167/iovs.62.12.20
Toyoda, H. (2019). Interaction of nicotinic acetylcholine receptors with dopamine receptors in synaptic plasticity of the mouse insular cortex. Insul. Cortex 73 (7), e22094. doi:10.1002/syn.22094
Watamura, N., Kakiya, N., Fujioka, R., Kamano, N., Takahashi, M., Nilsson, P., et al. (2024). The dopaminergic system promotes neprilysin-mediated degradation of amyloid-β in the brain. Sci. Signal 17 (848), eadk1822. doi:10.1126/scisignal.adk1822
Wise, R. A., and Jordan, C. J. (2021). Dopamine, behavior, and addiction. J. Biomed. Sci. 28 (1), 83. doi:10.1186/s12929-021-00779-7
Keywords: dopamine, neurotransmitter, light, photobiomodulation, photophysiology, secretion, metabolism
Citation: Wei S, Liu C, Wu Z, Si J, Huo X, Shen C, Zhen Y, Liu D and Chen L (2025) Light-induced secretion and transformation of neurotransmitter dopamine. Front. Photonics 6:1647467. doi: 10.3389/fphot.2025.1647467
Received: 15 June 2025; Accepted: 15 August 2025;
Published: 21 October 2025.
Edited by:
Shuo Chen, Northeastern University, ChinaReviewed by:
Rodrigo Alvaro Brandão Lopes-Martins, University Center of Anápolis, BrazilShimaa Hussein Kotb, Sphinx University, Egypt
Copyright © 2025 Wei, Liu, Wu, Si, Huo, Shen, Zhen, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianmin Si, c2lqaWFubWluQHVzdGMuZWR1LmNu; Xingxing Huo, aHh4MDUyMEBhaHRjbS5lZHUuY24=; Cailiang Shen, c2hlbmNhaWxpYW5nQGFobXUuZWR1LmNu; Yi Zhen, ZHJfemhlbnlpQDE2My5jb20=; Dongwei Liu, bGR3ZXllQDE2My5jb20=; Lei Chen, c2hhbmdnYW4yMDA5QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shuanghong Wei1†
Shuanghong Wei1† Zuomeng Wu
Zuomeng Wu Xingxing Huo
Xingxing Huo Cailiang Shen
Cailiang Shen Yi Zhen
Yi Zhen Dongwei Liu
Dongwei Liu Lei Chen
Lei Chen