Abstract
Intestinal mucositis is a clinically relevant side effect of anticancer therapies. It is experienced by 60–100% of patients undergoing treatment with high doses of chemotherapy, radiation therapy, and bone marrow transplantation. Intestinal mucositis can manifest as pain, weight loss, inflammation, diarrhea, rectal bleeding, and infection; affecting normal nutritional intake and intestinal function. It often impacts adherence to anticancer therapy as it frequently limits patient’s ability to tolerate treatment, causing schedule delays, interruptions, or premature discontinuation. In some cases, local and systemic secondary infections are observed, increasing the costs toward medical care and hospitalization. Several strategies for managing mucositis are available which do not always halt this condition. In this context, new therapeutic strategies are under investigation to prevent or treat intestinal mucositis. Polysaccharides from natural resources have recently become promising molecules against intestinal damage due to their ability to promote mucosal healing and their anti-inflammatory actions. These effects are associated with the protection of intestinal mucosa and regulation of microbiota and immune system. This review aims to discuss the recent advances of polysaccharides from natural resources as potential therapies for intestinal mucositis. The source, species, doses, treatment schedules, and mechanisms of action of polysaccharides will be discussed in detail.
Introduction
Intestinal mucositis is a clinically significant side effect of anti-cancer therapies, characterized by ulcerative lesions along the mucous membrane lining the gastrointestinal tract (Kwon, 2016). It occurs in approximately 40% of patients undergoing standard-dose chemotherapy (Naidu et al., 2004); and 60–100% of patients receiving high doses of chemotherapy, radiation therapy, and bone marrow transplantation (Köstler et al., 2001; Logan et al., 2007). Intestinal mucositis has been associated not only with a range of significant adverse symptoms, including severe diarrhea in 5–44% of patients (Krishnamurthi and Macaron, 2021), marked weight loss, and reduced nutrient absorption, but also with limited patient’s ability to tolerate treatment, thus, delaying subsequent cycles or leading to premature discontinuation (Basile et al., 2019).
Mucositis clinical symptoms result from epithelial injury, followed by a complex series of biological events that occur in the different cellular and tissue compartments of the mucosa (Sonis, 2004). In some cases, local and systemic secondary infections are observed, increasing costs with medical care and hospitalization (Gibson et al., 2013). Thus, intestinal mucositis has a notable negative impact on patient’s clinical outcome, and its complications can even lead to death in more severe cases (Al-Dasooqi et al., 2013). Currently, there are no preventive strategies or adequate treatments for intestinal mucositis (Gibson et al., 2013). Therapy mainly focuses on attenuating mucositis symptoms (Kuiken et al., 2017). To aid the clinical management of intestinal mucositis, the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) developed a guideline containing updated information on therapeutic alternatives for this disorder. These include mucosal coating agents, anesthetics and analgesics, growth factors and cytokines, antimicrobials, cryotherapy, and natural agents (Elad, 2020).
In this context, polysaccharides extracted from natural products represent an interesting strategy for the development of novel therapies and interventions for intestinal mucositis. This article summarizes potential therapeutic polysaccharides or polysaccharide extracts that have been studied for the treatment of this disorder induced by conventional chemotherapy. The review discusses the main animal models used for the study of intestinal mucositis and the mechanisms involved in the protective actions of polysaccharides.
Animal Models of Intestinal Mucositis
Gastrointestinal mucositis is a comprehensive term used to refer to the mucosal damage caused by antineoplastic treatment (i.e., chemotherapy and/or radiotherapy) (Cinausero et al., 2017). Treatment regimen can induce lesions in the oral cavity, pharynx, larynx, stomach, and intestine, with severe consequences for the morbidity and mortality of cancer patients (Sonis et al., 2015).
Rodent models of intestinal mucositis have been essential to allow the dissection of the mechanisms involved in chemotherapy-induced toxicity (Bowen et al., 2011). The different treatment schemes, including dosing schedules, routes of administration, duration of treatments, and period of experimental follow-up, generally reflect the five-phase model of intestinal mucositis: initiation, messenger signaling, signal amplification, ulceration with inflammation, and healing (Sonis et al., 2004; Sougiannis et al., 2021). The main drugs used in rodent models of intestinal mucositis include 5-fluorouracil (5-FU) and irinotecan; however, cyclophosphamide, cisplatin, and etoposide are also used (Ribeiro et al., 2016; Wardill et al., 2019).
Although each drug previously mentioned has a specific mechanism of action, in general, they trigger mucosal injury by similar mechanisms (Figure 1). The generation of reactive oxygen species (ROS) (Bhattacharyya et al., 2014), and inflammation – characterized by the production of cytokines, chemokines, and prostaglandins, trigger direct damage to cellular components such as nucleic acids, proteins, and lipids (Sonis, 2002; Logan et al., 2007). The activation of pathways such as caspase, protein kinases, as well as an unregulated expression of metalloproteinase, contribute to the damaging process of the intestinal mucosa, leading to apoptosis of basal and submucous epithelial cells and atrophic changes (Cinausero et al., 2017). All these irregular responses contribute to the intestinal tissue’s vulnerability to ulcerations and infections (Blijlevens and Sonis, 2007; Al-Dasooqi et al., 2010).
FIGURE 1
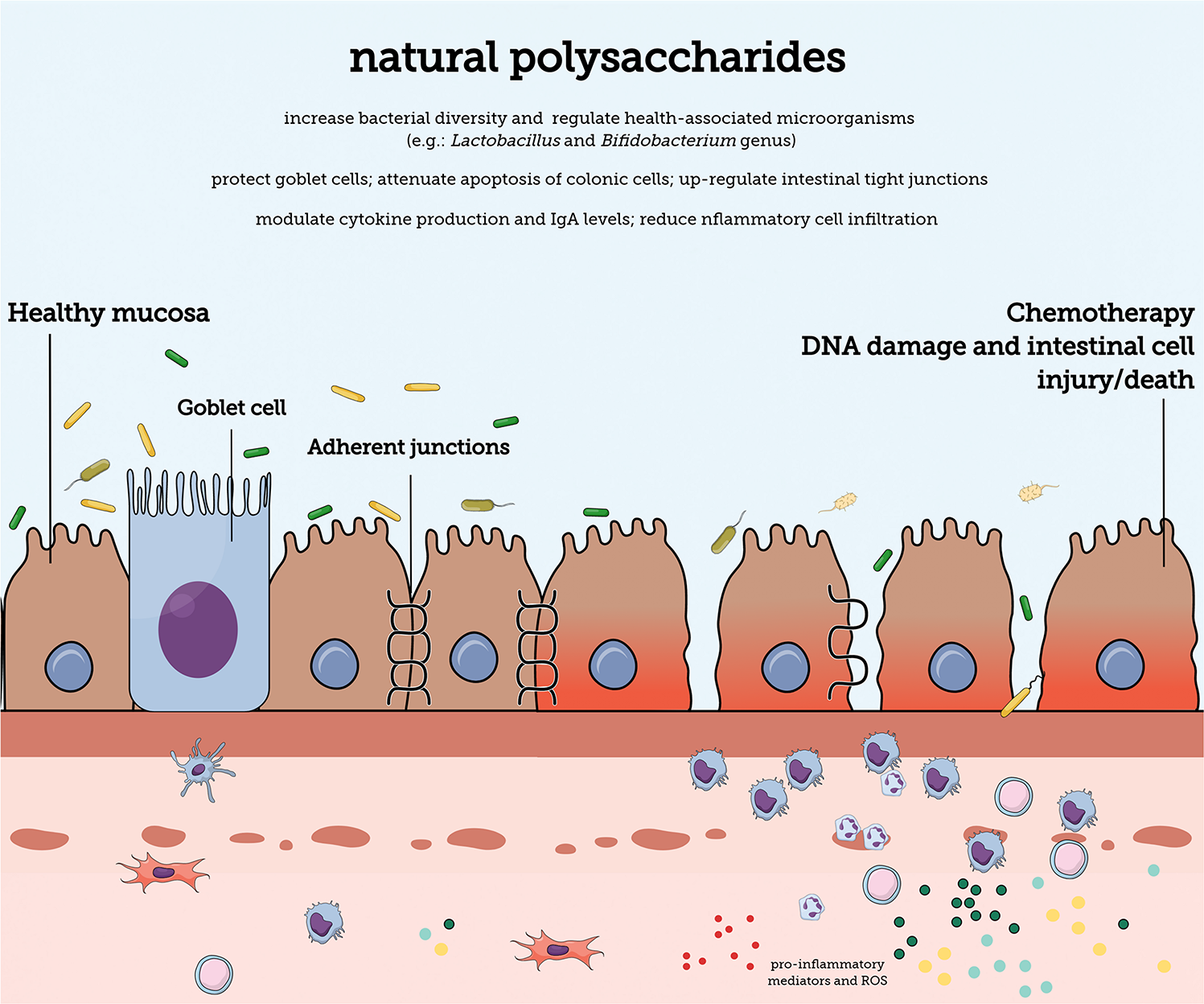
The gastrointesti nal mucosal barrier. Natural polysaccharides can contain different structural characteristics and can protect the mucosa against damage caused by chemotherapy antineoplastic treatment. The mucosal barrier contains diverse barriers (e.g., mucus layer and adherent junction proteins) that promote protection against internal and external harmful substances. A single epithelial cell layer forms the physical barrier against the entry of antigens. The lamina propria is densely populated by cells of the immune system that provide protection against damaging substances.
5-Fluorouracil is commonly used for the treatment of malignant tumors. It is an analog of uracil with a fluorine atom at the C-5 position in place of hydrogen. Intracellularly, 5-FU is converted into active metabolites that disrupt RNA synthesis and the action of thymidylate synthase, thus, killing malignant cells (Longley et al., 2003). In animals, 5-FU administration (25–450 mg/kg) results in weight loss, diarrhea, and increased mortality (Araújo et al., 2015; Pereira et al., 2015). 5-FU triggers inflammation of the small intestine, characterized by the infiltration of immune cells (Chang et al., 2012). Decreased cellularity in the small intestine and increased apoptosis in both the ileum and colon are also observed by TUNEL and western blot analysis (Justino et al., 2014; Yeung et al., 2015). Similarly, irinotecan, a topoisomerase I inhibitor, has also been broadly employed to investigate the pathogenesis of mucositis (Wardill et al., 2013). By binding to the topoisomerase I-DNA complex, irinotecan prevents the rebinding of the DNA strand, interfering with the replication fork thus, inducing replication arrest and lethal double-stranded breaks in the DNA (Fujita et al., 2015). Its administration to animals (75–300 mg/kg) causes severe diarrhea, increases intestinal motility, and triggers mucosal injury (Di Paolo et al., 2006; Gibson et al., 2007), associated with histopathological alterations and increased intestinal inflammation mediated by enhanced cytokine production (Melo et al., 2008). Cyclophosphamide is also widely used for the treatment of malignancies and known to induce intestinal mucositis. It is an alkylating agent of the nitrogen mustard class and in its activated form, phosphoramide mustard, binds to the DNA interfering with its normal function. Its main cytotoxic effect is due to cross-linking of nucleic acid strands and inhibition of protein synthesis (Iqubal et al., 2018). In animals, cyclophosphamide (50–400 mg/kg) induces changes similar to those described for 5-FU, such as diarrhea and dyskinesia (Wardill et al., 2019). Some studies also observed this chemotherapy leads to increased intestinal permeability (Xiang et al., 2010) and epithelial destruction, in addition to dysfunction of the intestinal immunity (Owari et al., 2012). Cisplatin (alkylating agent) and etoposide (topoisomerase II inhibitor), either alone or in combination (Safonova et al., 2019), and radiation therapy (Bowen et al., 2011) are less commonly used but are also valuable models (Wardill et al., 2019) which provide information regarding the underlying mechanisms of cancer therapy-induced mucosal damage.
Natural Polysaccharides
As defined by the European Nutraceutical Association (Ena) (2016), nutraceuticals are “nutritional products that provide health and medical benefits, including the prevention and treatment of diseases.” Nutraceuticals include biologically active molecules present in food such as those of extracts and herbs, as well as minerals, vitamins, carbohydrates, lipids amongst other nutrients. The global nutraceutical market reached USD 382.51 billion in 2019 and it is expected to continue growing 8.3% by 2027 (Global Market Research, 2021). Those found in the market have a range of applications from appetite suppression to immunomodulation (Sachdeva et al., 2020).
Carbohydrates are mostly found as polysaccharides – macromolecules of high molecular weight formed by polymers of monosaccharides such as pentoses and hexoses linked by glycosidic bonds. Recent studies have identified various biological activities of polysaccharides with wide structural diversity from different sources including algae (Xu et al., 2017; Manlusoc et al., 2019; Van Weelden et al., 2019), herbs (Zhang et al., 2013, 2015; Li et al., 2018a; Maria-Ferreira et al., 2018), fruits (Potterat, 2010; Ahmadi Gavlighi et al., 2018), squids (Zuo et al., 2014a, 2015a,2015b; Gu et al., 2017; Chen et al., 2020a), bacteria (Ciszek-Lenda et al., 2011; Jones et al., 2014), and mushrooms (Kothari et al., 2018; Chen et al., 2020b; Jiang et al., 2020).
Polysaccharides can be classified according to their shape, monomeric units, and electric charge (BeMiller, 2018). By shape, polysaccharides can be linear, branched, short branches on an essentially linear backbone, or branch-on-branch structures. By monomeric units, they can be either homo- or heteroglycans, and by charge, neutral or anionic. Polysaccharides are also classified according to their source: (i) starch, cellulose, and exudate gums from plants; (ii) alginates and galactans from algae; (iii) chitin, chitosan, and glycosaminoglycans from animals; (iv) dextran, pullulan and, gellan and xanthan gums from microorganisms; and (v) glucans from mushrooms (Friedman, 2016; Choong et al., 2018; Li et al., 2018b; Yadav and Karthikeyan, 2019).
Polysaccharides are non-digestible fibers (prebiotics) which may confer health benefits through different mechanisms (Figure 1). In the context of intestinal mucositis, their protective effects may be due to the following actions: (i) modulation of immune and inflammatory responses (Zhao et al., 2020), (ii) antioxidant (Wang H. et al., 2013) and (iii) antibacterial potential (Coma et al., 2015), (iv) protection of the mucosa and mucin regulation (Zuo et al., 2015a,b; Meldrum et al., 2017), and (v) alterations in the intestinal microbiota (Tang et al., 2019). The later, comprises a positive regulation of health-associated microorganisms such as those of the Lactobacillus and Bifidobacterium genus, and increased bacterial diversity (Tang et al., 2019).
The potential of different natural polysaccharides to protect against the inflammatory/oxidative damage of the intestinal mucosa and their impacts on the histopathological changes of intestinal mucositis will now be discussed.
Effects of Natural Polysaccharides on Chemotherapy-Induced Intestinal Histopathological Changes
Animal models are reliable in mimicking the human intestinal mucositis (Wardill et al., 2019) and have been essential for the research of novel therapeutic and prevention strategies for this condition. Animals with intestinal mucositis typically show a range of gastrointestinal symptoms, including bloody diarrhea, ulcerations, and diverse structural tissue alterations (Cinausero et al., 2017). Antineoplastics such as 5-FU and cyclophosphamide induce villus atrophy, crypt apoptosis and decrease the number of intestinal cells (Pritchard et al., 1998; Logan et al., 2009; Yang et al., 2013; Cinausero et al., 2017). Mucus secretion, tight junction integrity and intestinal permeability are also altered by chemotherapy (Song et al., 2013; Yang et al., 2013; Yeung et al., 2015; Cinausero et al., 2017).
Polysaccharides isolated from different sources have been explored as potential treatments for intestinal mucositis. The identified mechanisms of action of these macromolecules in the histological alterations of mucositis are summarized in Table 1.
TABLE 1
| Animal model of intestinal mucositisy | Polysaccharide | References | |||||||||
| Species | Strain | Antineoplastic chemotherapy | Antineoplastic dose | Source | Polysaccharide compound/preparation | Dose | Route and frequency of administration | Effects on tissue histology | Effects on inflammation | Effects on oxidative stress | |
| Mice | Male Balb/c | Cyclophosphamide | 50 mg/kg | Acaudina molpadioides | Fucoidan or fucoidan products | 50 mg/kg | Gavage, once a day, for 28 days | Reduction of intestinal mucosa injury with preservation of villus length and crypt depth | Increase of intestinal IFN-γ/IL-4 ratio, IL-6/IL-10 production, and mucosal IgA levels | – | Zuo et al., 2014b |
| Cyclophosphamide | 50 mg/kg | Ommastrephes bartrami | Squid ink polysaccharide extract | 50–200 mg/kg | Gavage, once a day for 28 days | Up-regulation of intestinal epithelium barrier tight (occluding and ZO-1) and adherens junctions (E-cadherin) | – | – | Zuo et al., 2015a | ||
| Cyclophosphamide | 50 mg/kg | Ommastrephes bartrami | Squid ink polysaccharide extract | 50–200 mg/kg | Gavage, once a day for 28 days | Protection of the intestinal layer due to up-regulation of cytokeratin 18 and mucin 2 mRNA by goblet cells | – | – | Zuo et al., 2015b | ||
| 5-fluorouracil | 25 mg/kg | Poria cocos | Carboxymethyl pachyman | 50–100 mg/kg | Gavage, once a day, for 14 days | Prevention of colon and villi shortening, crypt disruption, goblet cell reduction, mucosa and muscle layer thinning, attenuation of the apoptosis of colonic cells | Reduction of inflammatory cell infiltration with attenuation of the expression of NF-κB and p38 MAPK | Suppression of ROS production and increase of the levels of CAT, GSH-Px, and GSH | Wang et al., 2018 | ||
| Female C57BL/6 | 5-fluorouracil | 50 mg/kg | Crab shell | Chitin or chitosan | 2 mg/animal | Intragastric, once a day, for 10 days | Reduction of mucosal ulceration and villus height, prevention of the apoptosis of intestinal crypt cells | Attenuation of the influx of MPO-positive cells | – | Koizumi et al., 2017 | |
| Male C57Bl/6 | Cisplatin + paclitaxel and cisplatin + irinotecan | Cisplatin (2.5 mg/kg) Paclitaxel (12 mg/kg) Cisplatin + irinotecan (10 mg/kg) | Tussilago farfara L. | Monosaccharide composition, uronic acid content | 20 mg/kg | Intraperitoneal, for 14 days | Decrease of DNA damage in intestinal cells | – | – | Safonova et al., 2019 | |
| Rats | Male Sprague-Dawley | 5-fluorouracil | 50 mg/kg | Crassostrea hongkongensis | Polysaccharide-based nutrition formula | 2.0 g/kg | Gavage, once a day, for 13 days | Reduction of ulceration with diminished villus atrophy and increase of the mucosa thickness | Increase of plasma levels of IFN-γ and IL-10, and augmentation of IL-2 production | – | Cai et al., 2018 |
| Female Dark Agouti | 5-fluorouracil | 150 mg/kg | – | Fructo-, galacto-, or mannan-oligosaccharides | 50 mg/kg | Gavage, once a day, for 13 days | Improvement of the intestinal integrity by galacto- oligosaccharides with no effects on mucin or in the number of goblet cells | No effects on tissue MPO-positive nor in peripheral blood cells | – | Yazbeck et al., 2019 | |
| Human | Male and female patients with colorectal cancer | Oxaliplatin, 5-fluorouracil, and folinic acid combination therapy | – | – | β-glucan | 50 mg/day | Oral, for at least 7-days | – | Prevention of the chemotherapy-induced peripheral blood leukocyte and neutrophil number reduction | – | Karaca et al., 2014 |
In vivo effects of natural polysaccharides on chemotherapy-induced intestinal mucositis.
Poria cocos is a saprophytic fungus, popularly used in traditional East-Asian medicine (Wang Y. Z. et al., 2013). Wang Y. Z. et al. (2013) modified its main constituent, pachyman, to a carboxymethylated pachyman (CMP), and tested it in the 5-FU-induced intestinal mucositis model. CMP (50–100 mg/kg) reduced colon injury in CT26 tumor-bearing male Balb/c mice treated with 5-FU. CMP also decreased the disease score and reversed intestinal shortening (Wang et al., 2018).
The protective effects of chitosan, a linear polysaccharide of the chitin family, in the digestive system were previously demonstrated (Kimura and Okuda, 1999; Kimura et al., 2001). In the 5-FU intestinal mucositis model, chitosan (2 mg/animal) prevented tissue damage, by reducing the loss of the intestinal architecture, as well as mucosal ulceration, villus height, and inflammatory cell infiltration, in female C57BL/6 mice (Koizumi et al., 2017).
In addition to the protective effects of isolated polysaccharides, immunonutrition formulas containing polysaccharides were also shown to improve the outcome of intestinal mucositis. In fact, the administration of OPNF (Crassostrea hongkongensis oyster polysaccharide-based nutrition formula, 2 g/kg) reduced the edema of the lamina propria and jejunum villi atrophy in Sprague-Dawley male rats receiving 5-FU chemotherapy. OPNF restored villus height, width, and mucosal thickness; and reduced the injury of the intestinal mucosa as shown by scanning electron microscopy (Cai et al., 2018). The same authors also demonstrated that OPNF inhibits tumor growth by improving leukocyte and lymphocyte indexes in S180 tumor-bearing mice undergoing therapy with 5-FU, suggesting that oyster polysaccharides may act as immunological stimulants (Cai et al., 2016).
Fucoidan is a fucose-enriched and sulfated polysaccharide, mainly sourced from seaweed which has been extensively investigated (Zhao et al., 2018) and suggested to present anticancer, antiviral, and anti-diabetic properties (Luthuli et al., 2019). The oral administration of different Acaudina molpadioides fucoidans (10–500 kDa) at a dose of 50 mg/kg, improved the histological morphology of the small intestine in male Balb/c mice challenged with cyclophosphamide. The length of the small intestine was restored by fucoidan. Also, the length and the villus/crypt ratio were increased, while the crypt depth was diminished in mice that received fucoidan. Interestingly, the results indicate that fucoidans of molecular weights ranging from 50 to 500 kDa present the greatest protective effects in intestinal mucositis (Zuo et al., 2014b).
Another investigated polysaccharide is a marine glycosaminoglycan isolated from the squid ink (Ommastrephes bartrami, OBP). It was demonstrated in two studies, that OBP (50–200 mg/kg) protects male Balb/c mice against cyclophosphamide-induced intestinal damage (Zuo et al., 2015a,b). This effect was associated with an enhanced intestinal mRNA and protein expression of myosin, occludin, E-cadherin, and zonula occludens (ZO)-1. This was especially noted in animals treated with 200 mg/kg of OBP (Zuo et al., 2015a). OBP (100–200 mg/kg) was also found to prevent weight loss and decrease diarrhea severity (Zuo et al., 2015b); an effect associated with increased mRNA expression of cytokeratin 18 and mucin 2, and larger mucin-positive areas in the intestinal villi. The same study also demonstrated that OBP enhances the quantity of goblet cells in the epithelium.
Since mushrooms and fruits are routinely present in the human diet, polysaccharides isolated from them have been extensively explored as sources of compounds in recent years. Ganoderma atrum-derived polysaccharide (PSG-1, 25–100 mg/kg) was assessed in specific pathogen-free female BALB/c mice challenged with cyclophosphamide. PSG-1 (50 and 100 mg/kg) enhanced the number of goblet cells. Moreover, all tested doses of PSG-1 led to an increase of intestinal claudin-1, occluding, and ZO-1 protein levels, in comparison to vehicle animals receiving cyclophosphamide. The highest tested dose of PSG-1 presented the most pronounced effects on claudin-1 and ZO-1 expressions; whilst the lowest dose presented the greatest effect on occludin levels (Ying et al., 2020).
A polysaccharide isolated from Longan (LP), a fruit derived from Dimocarpus longan Lour, was also studied. LP (100–400 mg/kg) was tested in the cyclophosphamide mucositis model and found to partially protect the intestinal structure by reducing damage in comparison to vehicle-treated mice with cyclophosphamide-induced mucositis. The highest tested dose of LP (400 mg/kg) enhanced mucin 2 mRNA expression. LP also upregulated the mRNA and protein expressions of claudin-1, claudin-4, and ZO-1, especially at the highest dose, without altering occludin expression. On the other hand, E-cadherin mRNA and protein expressions were increased by LP, with no dose-effect relationship (Bai et al., 2019).
Finally, Safonova et al. (2019) used a combination therapy of cisplatin and irinotecan to evaluate the protective effects of Tussilago farfara L. polysaccharides on the small intestinal epithelium. T. farfara L. polysaccharides reduced the DNA damage caused by the chemotherapy denoted by increased percentage of DNA in the tail of the comet and apoptotic DNA in the small intestinal epithelium in comparison with those of the negative control group (Safonova et al., 2019).
Effects of Natural Polysaccharides on Intestinal Mucositis-Associated Inflammation and Oxidative Stress
Inflammation and oxidative stress account for as underlying mechanisms of the intestinal pathological changes caused by chemotherapy (Sougiannis et al., 2021). Chemotherapy triggers an initial tissue response in which DNA damage occurs, followed by an intense production of ROS, up-regulation of inflammatory genes, inflammasome activation and influx of activated immune cells. The enhanced generation of cytokines such as tumor necrosis factor-α, IL-1β, and IL-6, and chemokines amplify the accumulation of inflammatory cells in the damaged intestinal mucosa. The intense inflammation favors the occurrence of ulcerations in the intestinal epithelial layer, increasing ROS production. In parallel, the ulcers may become colonized by gut bacteria, amplifying tissue inflammation. These local changes as well as the systemic anemia and lymphocytopenia triggered by chemotherapy contribute to an inefficient healing of the intestine.
Pieces of evidence (Table 1) indicate a potential use of natural polysaccharides as therapeutic modulators of inflammation in intestinal mucositis. Indeed, apart from a single study which did not observe any benefits from prebiotics in rats with 5-FU-induced intestinal mucositis (Yazbeck et al., 2019), a variety of natural polysaccharides exhibited protective effects in different studies. Of note, these have been employed as orally administered preventive approaches to avoid intestinal inflammation and subsequent damage.
Wang et al. (2018) demonstrated the ability of P. cocos CMP (50–100 mg/kg) to protect the colon tissue against 5-FU-induced damage in Balb-c mice, by reducing inflammatory cell infiltration and pro-inflammatory gene expression and reducing oxidative stress. The protective effects of natural polysaccharides on chemotherapy-induced intestinal mucositis have been confirmed by different studies with other antineoplastics and animal strains including C57BL/6 and rats (Koizumi et al., 2017; Cai et al., 2018; Safonova et al., 2019), in addition to humans (Zhang et al., 2018). In animals with intestinal damage caused by 5-FU, the administration of crab shell chitin/chitosan (2 mg/animal) in C57BL/6 mice (Koizumi et al., 2017) or a polysaccharide-based nutrition formula (2 g/kg) from C. hongkongensis in rats impaired inflammation by reducing the influx of MPO-positive cells and increasing cytokine production (Cai et al., 2018).
Similarly, A. molpadioides fucoidan and its products (50 mg/kg) protected against the intestinal damage caused by cyclophosphamide in Balb-c mice by increasing intestinal IFN-γ/IL-4 ratio, IL-6/IL-10 production, and mucosal IgA levels (Zuo et al., 2014b). Another study with β-glucans in patients with colorectal cancer undergoing combination chemotherapy with oxaliplatin, 5-FU, and folinic acid, prevented drug-induced peripheral blood leukocyte and neutrophil number reduction (Karaca et al., 2014).
Effects of Natural Polysaccharides on Human Intestinal Mucositis
For the best of our knowledge, there are few reports on the effects of polysaccharides in patients with intestinal mucositis. Oral β-glucan, for instance, was found to improve immunity in patients undergoing chemotherapy, with no evidence on its ability to protect against intestinal damage (Steimbach et al., 2020). Also, a retrospective study demonstrated that the same compound reduces diarrhea in patients suffering from colorectal cancer undergoing chemotherapy (Karaca et al., 2014); this study did not assess any other relevant aspect of intestinal mucositis (Karaca et al., 2014). None of the aforementioned studies offered sufficient information on whether polysaccharides are beneficial in human intestinal mucositis. The limited information on the effects of polysaccharides in patients undergoing chemotherapy highlights the need for high-quality trials using multiple measures to assess their impact on all side effects induced by antineoplastics. In this context, further investigations aimed at determining the unknown molecular/underlining mechanisms of polysaccharides as well as how these macromolecules affect the intestinal microbiota are of great value for the clinical management of the intestinal mucositis.
Conclusion and Perspectives
All the non-clinical studies discussed here, indicate that regardless of the polysaccharide source or type, these, when given orally, present a great ability to prevent the intestinal damage caused by antineoplastic drugs such as 5-FU and cyclophosphamide, as well as polychemotherapy. Their effects are suggested to be due to their ability to regulate the inflammatory/immune responses and attenuate oxidative stress, resulting in enhanced integrity of the intestinal mucosa via regulation of mucin production and increased expression of different tight junction molecules. Despite the exciting evidence, their clinical benefits remain to be confirmed in patients with intestinal mucositis, highlighting the need for further detailed studies with these macromolecules.
Statements
Author contributions
KS, BC, LB, LM, and LS did the bibliographic survey and extracted data. DM-F and EF contributed to the conception and design of the manuscript and drafted and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 305676/2019-9 and 408053/2018-6), Instituto de Pesquisa Pelé Pequeno Príncipe, and INCT-INOVAMED. KS, BC, LM and LB were master students receiving a grant from the Instituto de Pesquisa Pelé Pequeno Príncipe.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Ahmadi GavlighiH.TabarsaM.YouS.SurayotU.Ghaderi-GhahfarokhiM. (2018). Extraction, characterization and immunomodulatory property of pectic polysaccharide from pomegranate peels: Enzymatic vs conventional approach.Int. J. Biol. Macromol.116698–706. 10.1016/j.ijbiomac.2018.05.083
2
Al-DasooqiN.GibsonR. J.BowenJ. M.LoganR. M.StringerA. M.KeefeD. M. (2010). Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat.Exp. Biol. Med.2351244–1256. 10.1258/ebm.2010.010082
3
Al-DasooqiN.SonisS. T.BowenJ. M.BatemanE.BlijlevensN.GibsonR. J.et al (2013). Mucositis study group of the multinational association of supportive care in Cancer/International society of oral oncology (MASCC/ISOO). emerging evidence on the pathobiology of mucositis.Support Care Cancer213233–3241. 10.1007/s00520-013-1900-x
4
AraújoC. V.LazzarottoC. R.AquinoC. C.FigueiredoI. L.CostaT. B.AlvesL. A.et al (2015). Alanyl-glutamine attenuates 5-fluorouracil-induced intestinal mucositis in apolipoprotein E-deficient mice.Braz. J. Med. Biol. Res.48493–501. 10.1590/1414-431X20144360
5
BaiY.HuangF.ZhangR.DongL.JiaX.LiuL.et al (2019). Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression.Carbohydr. Polym.229:115475. 10.1016/j.carbpol.2019.115475
6
BasileD.Di NardoP.CorvajaC.GarattiniS. K.PelizzariG.LisantiC.et al (2019). Mucosal injury during anti-cancer treatment: from pathobiology to bedside.Cancers (Basel)11:857. 10.3390/cancers11060857
7
BeMillerJ. N. (2018). Carbohydrate Chemistry for Food Scientists.Amsterdam: Elsevier.
8
BhattacharyyaA.ChattopadhyayR.MitraS.CroweS. E. (2014). Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases.Physiol. Rev.94329–354. 10.1152/physrev.00040.2012
9
BlijlevensN.SonisS. (2007). Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis.Ann. Oncol.18817–826. 10.1093/annonc/mdl332
10
BowenJ. M.GibsonR. J.KeefeD. M. (2011). Animal models of mucositis: implications for therapy.J. Support Oncol.9161–168. 10.1016/j.suponc.2011.04.009
11
CaiB.ChenH.SunH.WanP.SunH.PanJ. (2016). Production of immunoregulatory polysaccharides from Crassostrea hongkongensis and their positive effects as a nutrition factor in modulating the effectiveness and toxicity of 5-FU chemotherapy in mice.Food Funct.7390–397. 10.1039/c5fo00885a
12
CaiB.WanP.SunH.ChenD.ChenH.ChenX.et al (2018). Protective effects of enteral nutrition supplemented with crassostrea hongkongensis Polysaccharides against 5-Fluorouracil-induced intestinal mucosal damage in rats.J. Med. Food21348–355. 10.1089/jmf.2017.4025
13
ChangC. T.HoT. Y.LinH.LiangJ. A.HuangH. C.LiC. C.et al (2012). 5-Fluorouracil induced intestinal mucositis via nuclear factor-κB activation by transcriptomic analysis and in vivo bioluminescence imaging.PLoS One7:e31808. 10.1371/journal.pone.0031808
14
ChenY.LiuH.HuangH.MaY.WangR.HuY.et al (2020a). Squid ink polysaccharides protect human fibroblast against oxidative stress by regulating NADPH oxidase and connexin43.Front. Pharmacol.10:1574. 10.3389/fphar.2019.01574
15
ChenM.XiaoD.LiuW.SongY.ZouB.LiL.et al (2020b). Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats.Int. J. Biol. Macromol.155890–902. 10.1016/j.ijbiomac.2019.11.047
16
ChoongY.-K.EllanK.ChenX.-D.MohamadS. A. (2018). Extraction and Fractionation of Polysaccharides from a Selected Mushroom Species, Ganoderma lucidum: A Critical Review.London: IntechOpen.
17
CinauseroM.AprileG.ErmacoraP.BasileD.VitaleM. G.FanottoV.et al (2017). New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury.Front. Pharmacol.8:354. 10.3389/fphar.2017.00354
18
Ciszek-LendaM.NowakB.SróttekM.GamianA.MarcinkiewiczJ. (2011). Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37: effects on the production of inflammatory mediators by mouse macrophages.Int. J. Exp. Pathol.92382–391. 10.1111/j.1365-2613.2011.00788.x
19
ComaV.FreireC. S. R.SilvestreA. J. D. (2015). “Recent Advances on the Development of Antibacterial Polysaccharide-Based Materials,” in Polysaccharides, edsRamawatK.MérillonJ. M. (Cham: Springer).
20
Di PaoloA.BocciG.DanesiR.Del TaccaM. (2006). Clinical pharmacokinetics of irinotecan-based chemotherapy in colorectal cancer patients.Curr. Clin. Pharmacol.1311–323. 10.2174/157488406778249307
21
EladS. (2020). The MASCC/ISOO mucositis guidelines 2019: the second set of articles and future directions. Support.Care Cancer282445–2447. 10.1007/s00520-019-05153-w
22
European Nutraceutical Association (Ena). (2016). Science behind Nutraceuticals.Basel, Switzerland: European Nutraceutical Association.
23
FriedmanM. (2016). Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans.Foods5:80. 10.3390/foods5040080
24
FujitaK.KubotaY.IshidaH.SasakiY. (2015). Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer.World J. Gastroenterol.2112234–12248. 10.3748/wjg.v21.i43.12234
25
GibsonR. J.BowenJ. M.AlvarezE.FinnieJ.KeefeD. M. (2007). Establishment of a single-dose irinotecan model of gastrointestinal mucositis.Chemotherapy53360–369. 10.1159/000107458
26
GibsonR. J.KeefeD. M.LallaR. V.BatemanE.BlijlevensN.FijlstraM.et al (2013). Systematic review of agents for the management of gastrointestinal mucositis in cancer patients.Support Care Cancer21313–326. 10.1007/s00520-012-1644-z
27
Global Market Research. (2021). Available Online at: (https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market)
28
GuY. P.YangX. M.DuanZ. H.ShangJ. H.LuoP.XiaoW.et al (2017). Squid ink polysaccharide prevents autophagy and oxidative stress affected by cyclophosphamide in Leydig cells of mice: a pilot study.Iran. J. Basic Med. Sci.201194–1199. 10.22038/IJBMS.2017.9491
29
IqubalA.IqubalM. K.SharmaS.AnsariM. A.NajmiA.ZhangQ. Y.et al (2018). Natural product interventions for chemotherapy and radiotherapy-induced side effects.Front Pharmacol.9:1253. 10.3389/fphar.2018.01253
30
JiangX.MengW.LiL.MengZ.WangD. (2020). Adjuvant therapy with mushroom polysaccharides for diabetic complications.Front. Pharmacol.11:168. 10.3389/fphar.2020.00168
31
JonesS. E.PaynichM. L.KearnsD. B.KnightK. L. (2014). Protection from intestinal inflammation by bacterial exopolysaccharides.J. Immunol.1924813–4820. 10.4049/jimmunol.1303369
32
JustinoP. F.MeloL. F.NogueiraA. F.CostaJ. V.SilvaL. M.SantosC. M.et al (2014). Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice.Br. J. Nutr.1111611–1621. 10.1017/S0007114513004248
33
KaracaH.BozkurtO.OzaslanE.BaldaneS.BerkV.InancM.et al (2014). Positive effects of oral β-glucan on mucositis and leukopenia in colorectal cancer patients receiving adjuvant FOLFOX-4 combination chemotherapy.Asian. Pac. J. Cancer Prev.153641–3644. 10.7314/apjcp.2014.15.8.3641
34
KimuraY.OkudaH. (1999). Prevention by chitosan of myelotoxicity, gastrointestinal toxicity and immunocompetent organic toxicity induced by 5-fluorouracil without loss of antitumor activity in mice.Jpn. J. Cancer Res.90765–774. 10.1111/j.1349-7006.1999.tb00813.x
35
KimuraY.SawaiN.OkudaH. (2001). Antitumour activity and adverse reactions of combined treatment with chitosan and doxorubicin in tumour-bearing mice.J. Pharm. Pharmacol.531373–1378. 10.1211/0022357011777873
36
KoizumiR.AzumaK.IzawaH.MorimotoM.OchiK.TsukaT.et al (2017). Oral administration of surface-deacetylated chitin nanofibers and chitosan inhibit 5-Fluorouracil-induced intestinal mucositis in mice.Int. J. Mol. Sci.18:279. 10.3390/ijms18020279
37
KöstlerW. J.HejnaM.WenzelC.ZielinskiC. C. (2001). Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment.CA. Cancer J. Clin.51290–315. 10.3322/canjclin.51.5.290
38
KothariD.PatelS.KimS. K. (2018). Anticancer and other therapeutic relevance of mushroom polysaccharides: A holistic appraisal.Biomed. Pharmacother.105377–394. 10.1016/j.biopha.2018.05.138
39
KrishnamurthiS. S.MacaronC. (2021). Management of Acute Chemotherapy-Related Diarrhea. Up to Date. Topic 98342 Version 11.0.
40
KuikenN. S. S.RingsE. H. H. M.van den Heuvel-EibrinkM. M.van de WeteringM. D.TissingW. J. E. (2017). Feeding strategies in pediatric cancer patients with gastrointestinal mucositis: a multicenter prospective observational study and international survey.Support. Care Cancer.253075–3083. 10.1007/s00520-017-3715-7
41
KwonY. (2016). Mechanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy.Onco Targets Ther.52007–2016. 10.2147/OTT.S96899
42
LiQ.NiuY.XingP.WangC. (2018a). Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair.Chin. Med.13:7. 10.1186/s13020-018-0166-0
43
LiF.LuoP.LiuH. (2018b). A potential adjuvant agent of chemotherapy: sepia ink polysaccharides.Mar. Drugs.16:106. 10.3390/md16040106
44
LoganR. M.StringerA. M.BowenJ. M.GibsonR. J.SonisS. T.KeefeD. M. (2009). Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered?Cancer Chemother. Pharmacol.63239–251. 10.1007/s00280-008-0732-8
45
LoganR. M.StringerA. M.BowenJ. M.YeohA. S.GibsonR. J.SonisS. T.et al (2007). The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs.Cancer Treat. Rev.33448–460. 10.1016/j.ctrv.2007.03.001
46
LongleyD. B.HarkinD. P.JohnstonP. G. (2003). 5-fluorouracil: mechanisms of action and clinical strategies.Nat. Rev. Cancer.3330–338. 10.1038/nrc1074
47
LuthuliS.WuS.ChengY.ZhengX.WuM.TongH. (2019). Therapeutic effects of fucoidan: a review on recent studies.Mar. Drugs17:487. 10.3390/md17090487
48
ManlusocJ. K. T.HsiehC. L.HsiehC. Y.SalacE. S. N.LeeY. T.TsaiP. W. (2019). Pharmacologic application potentials of sulfated polysaccharide from Marine Algae.Polymers (Basel)11:1163. 10.3390/polym11071163
49
Maria-FerreiraD.NascimentoA. M.CiprianiT. R.Santana-FilhoA. P.WatanabeP. D. S.SantAnaD. M. G.et al (2018). Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells.Sci. Rep.8:12261. 10.1038/s41598-018-30526-2
50
MeldrumO. W.YakubovG. E.GartaulaG.McGuckinM. A.GidleyM. J. (2017). Mucoadhesive functionality of cell wall structures from fruits and grains: Electrostatic and polymer network interactions mediated by soluble dietary polysaccharides.Sci. Rep.7:15794. 10.1038/s41598-017-16090-1
51
MeloM. L.BritoG. A.SoaresR. C.CarvalhoS. B.SilvaJ. V.SoaresP. M.et al (2008). Role of cytokines (TNF-alpha, IL-1beta and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide.Cancer Chemother. Pharmacol.61775–784. 10.1007/s00280-007-0534-4
52
NaiduM. U.RamanaG. V.RaniP. U.MohanI. K.SumanA.RoyP. (2004). Chemotherapy-induced and/or radiation therapy-induced oral mucositis–complicating the treatment of cancer.Neoplasia6423–431. 10.1593/neo.04169
53
OwariM.WasaM.OueT.NoseS.FukuzawaM. (2012). Glutamine prevents intestinal mucosal injury induced by cyclophosphamide in rats.Pediatr. Surg. Int.28299–303. 10.1007/s00383-011-3023-0
54
PereiraV. B.MeloA. T.Assis-JúniorE. M.WongD. V.BritoG. A.AlmeidaP. R.et al (2015). A new animal model of intestinal mucositis induced by the combination of irinotecan and 5-fluorouracil in mice.Cancer Chemother. Pharmacol.77323–332. 10.1007/s00280-015-2938-x
55
PotteratO. (2010). Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity.Planta Med.767–19. 10.1055/s-0029-1186218
56
PritchardD. M.JackmanA.PottenC. S.HickmanJ. A. (1998). Chemically-induced apoptosis: p21 and p53 as determinants of enterotoxin activity.Toxicol Lett.102-10319–27. 10.1016/s0378-4274(98)00273-2
57
RibeiroR. A.WanderleyC. W.WongD. V.MotaJ. M.LeiteC. A.SouzaM. H.et al (2016). Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives.Cancer Chemother. Pharmacol.78881–893. 10.1007/s00280-016-3139-y
58
SachdevaV.RoyA.BharadvajaN. (2020). Current prospects of nutraceuticals: A review.Curr. Pharm. Biotechnol.21884–896. 10.2174/1389201021666200130113441
59
SafonovaE. A.LopatinaK. A.RazinaT. G.ZuevaE. P.SadrikinaL. A.Gur’evA. M.et al (2019). Correction of damaging effects of cisplatin-containing polychemotherapy on the intestinal epithelium with Tussilago farfara L. Polysaccharides.Bull. Exp. Biol. Med.167616–620. 10.1007/s10517-019-04582-1
60
SongM. K.ParkM. Y.SungM. K. (2013). 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice.J. Cancer Prev.18322–329. 10.15430/jcp.2013.18.4.322
61
SonisS. T. (2002). The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy.Crit. Rev. Oral Biol. Med.13380–389. 10.1177/154411130201300502
62
SonisS. T. (2004). The pathobiology of mucositis.Nat. Rev. Cancer4277–284. 10.1038/nrc1318
63
SonisS. T.EltingL. S.KeefeD.PetersonD. E.SchubertM.Hauer-JensenM.et al (2004). Mucositis study section of the multinational association for supportive care in cancer; International Society for Oral Oncology.Cancer1002026–2046. 10.1002/cncr.20163
64
SonisS.EltingL.EltingL.KeefeD.NguyenH.GrunbergS.et al (2015). Unanticipated frequency and consequences of regimen-related diarrhea in patients being treated with radiation or chemoradiation regimens for cancers of the head and neck or lung.Support Care Cancer23433–439. 10.1007/s00520-014-2395-9
65
SougiannisA. T.VanderVeenB. N.DavisJ. M.FanD.MurphyE. A. (2021). Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience.Am. J. Physiol. Gastrointest. Liver Physiol.320G712–G719. 10.1152/ajpgi.00380.2020
66
SteimbachL.BorgmannA. V.GomarG. G.HoffmannL. V.RutckeviskiR.de AndradeD. P.et al (2020). Fungal beta-glucans as adjuvants for treating cancer patients - A systematic review of clinical trials.Clin. Nutr.403104–3113. 10.1016/j.clnu.2020.11.029
67
TangC.DingR.SunJ.LiuJ.KanJ.JinC. (2019). The impacts of natural polysaccharides on intestinal microbiota and immune responses - a review.Food Funct.102290–2312. 10.1039/c8fo01946k
68
Van WeeldenG.BobińskiM.OkłaK.Van WeeldenW. J.RomanoA.PijnenborgJ. M. A. (2019). Fucoidan structure and activity in relation to anti-cancer mechanisms.Mar. Drugs17:32. 10.3390/md17010032
69
WangC.YangS.GaoL.WangL.CaoL. (2018). Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice.Food Funct.92695–2704. 10.1039/c7fo01886j
70
WangH.LiuY. M.QiZ. M.WangS. Y.LiuS. X.LiX.et al (2013). An overview on natural polysaccharides with antioxidant properties.Curr. Med. Chem.202899–2913. 10.2174/0929867311320230006
71
WangY. Z.ZhangJ.ZhaoY. L.LiT.ShenT.LiJ. Q.et al (2013). Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: a review.J. Ethnopharmacol.147265–276. 10.1016/j.jep.2013.03.027
72
WardillH. R.BowenJ. M.Al-DasooqiN.SultaniM.BatemanE.StansboroughR.et al (2013). Irinotecan disrupts tight junction proteins within the gut : implications for chemotherapy-induced gut toxicity.Cancer Biol. Ther.15236–244. 10.4161/cbt.27222
73
WardillH. R.TissingW. J. E.KissowH.StringerA. M. (2019). Animal models of mucositis: critical tools for advancing pathobiological understanding and identifying therapeutic targets.Curr. Opin. Support. Palliat. Care13119–133. 10.1097/SPC.0000000000000421
74
XiangD.GuoY.ZhangJ.GaoJ.LuH.ZhuS.et al (2010). Interleukin-1 receptor antagonist attenuates cyclophosphamide-induced mucositis in a murine model.Cancer Chemother. Pharmacol.671445–1453. 10.1007/s00280-010-1439-1
75
XuS. Y.HuangX.CheongK. L. (2017). Recent advances in marine Algae polysaccharides: isolation, structure, and activities.Mar. Drugs15:388. 10.3390/md15120388
76
YadavH.KarthikeyanC. (2019). Natural polysaccharides: Structural features and properties. Department of Pharmacy, Indira Gandhi National Tribal University, Amarkantak, India. Sabyasachi Maiti, Sougata Jana.Sawston, UK: Woodhead Publishing.
77
YangJ.LiuK. X.QuJ. M.WangX. D. (2013). The changes induced by cyclophosphamide in intestinal barrier and microflora in mice.Eur. J. Pharmacol.714120–124. 10.1016/j.ejphar.2013.06.006
78
YazbeckR.LindsayR. J.GeierM. S.ButlerR. N.HowarthG. S. (2019). Prebiotics fructo-, galacto-, and mannan-oligosaccharide do not protect against 5-Fluorouracil-induced intestinal mucositis in rats.J. Nutr.1492164–2173. 10.1093/jn/nxz192
79
YeungC. Y.ChanW. T.JiangC. B.ChengM. L.LiuC. Y.ChangS. W.et al (2015). Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model.PLoS One10:e0138746. 10.1371/journal.pone.0138746
80
YingM.ZhengB.YuQ.HouK.WangH.ZhaoM.et al (2020). Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice.Food Chem. Toxicol.138:111244. 10.1016/j.fct.2020.111244
81
ZhangL.KoyyalamudiS. R.JeongS. C.ReddyN.BaileyT.LongvahT. (2013). Immunomodulatory activities of polysaccharides isolated from Taxillus chinensis and Uncaria rhyncophylla.Carbohydr. Polym.981458–1465. 10.1016/j.carbpol.2013.07.060
82
ZhangL.ZhangW.WangQ.WangD.DongD.MuH.et al (2015). Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots.Int. J. Biol. Macromol.72975–983. 10.1016/j.ijbiomac.2014.09.056
83
ZhangQ. Y.WangF. X.JiaK. K.KongL. D. (2018). Natural product interventions for chemotherapy and radiotherapy-induced side effects.Front. Pharmacol.9:1253. 10.3389/fphar.2018.01253
84
ZhaoY.YanB.WangZ.LiM.ZhaoW. (2020). Natural polysaccharides with immunomodulatory activities.Mini Rev. Med. Chem.2096–106. 10.2174/1389557519666190913151632
85
ZhaoY.ZhengY.WangJ.MaS.YuY.WhiteW. L.et al (2018). Fucoidan extracted from undaria pinnatifida: source for Nutraceuticals/Functional Foods.Mar. Drugs16:321. 10.3390/md16090321
86
ZuoT.CaoL.LiX.ZhangQ.XueC.TangQ. (2015a). The squid ink polysaccharides protect tight junctions and adherens junctions from chemotherapeutic injury in the small intestinal epithelium of mice.Nutr. Cancer67364–371. 10.1080/01635581.2015.989369
87
ZuoT.CaoL.SunX.LiX.WuJ.LuS.et al (2014a). Dietary squid ink polysaccharide could enhance SIgA secretion in chemo7therapeutic mice.Food Funct.53189–3196. 10.1039/c4fo00569d
88
ZuoT.CaoL.XueC.TangQ. J. (2015b). Dietary squid ink polysaccharide induces goblet cells to protect small intestine from chemotherapy induced injury.Food Funct.6981–986. 10.1039/c4fo01191k
89
ZuoT.LiX.ChangY.DuanG.YuL.ZhengR.et al (2014b). Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice.Food Funct.6415–422. 10.1039/c4fo00567h
Summary
Keywords
anticancer therapy, intestinal mucositis, natural polysaccharide, mucosal inflammation, intestinal inflammation and injury
Citation
Sauruk da Silva K, Carla da Silveira B, Bueno LR, Malaquias da Silva LC, da Silva Fonseca L, Fernandes ES and Maria-Ferreira D (2021) Beneficial Effects of Polysaccharides on the Epithelial Barrier Function in Intestinal Mucositis. Front. Physiol. 12:714846. doi: 10.3389/fphys.2021.714846
Received
26 May 2021
Accepted
30 June 2021
Published
22 July 2021
Volume
12 - 2021
Edited by
Pavel Strnad, University Hospital RWTH Aachen, Germany
Reviewed by
Michael Meir, University Hospital Würzburg, Germany; Michael Thomsen, The University of Sydney, Australia
Updates
Copyright
© 2021 Sauruk da Silva, Carla da Silveira, Bueno, Malaquias da Silva, da Silva Fonseca, Fernandes and Maria-Ferreira.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Maria-Ferreira, daniele.ferreira@pelepequenoprincipe.org.br; danielemariaferreira@gmail.com
This article was submitted to Gastrointestinal Sciences, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.