Abstract
Objective: Oxygenation impairment is a common complication of acute aortic syndrome (AAS) patients after surgical repair. The aim of this study is to identify the relationship between body mass index (BMI) and the risk of postoperative oxygenation impairment in AAS patients.
Methods: A total of 227 consecutive patients who were diagnosed as AAS and underwent surgical repair were recruited. They were divided into two groups based on the postoperative oxygenation impairment (non-oxygenation impairment group and oxygenation impairment group). Logistic regression was conducted to evaluate the association between BMI and the risk of oxygenation impairment after surgery. Dose-response curve and subgroup analysis were used to test the reliability of the results of regression analysis. A meta-analysis was then performed to further confirm these results using Pubmed, Embase, and Web of Science databases.
Results: For the retrospective study, a significant association was observed after adjusting for a series of variables. BMI was significantly correlated with postoperative oxygenation impairment in patients with AAS (OR, 95% CI, P: 1.27, 1.17–1.46, 0.001). Compared with the normal weight group (18.5 kg/m2 ≤ BMI <23.0 kg/m2), patients with excessive BMI were at a higher risk of oxygenation impairment for the overweight group (23.0 kg/m2 ≤ BMI <25 kg/m2) and obesity group (BMI ≥25 kg/m2) (OR, 95% CI, P: 4.96, 1.62–15.15, 0.005; 9.51, 3.06–29.57, <0.001). The dose-response curve showed that the risk of oxygenation impairment after surgery increased with the increased BMI. Besides, subgroup analysis showed that AAS patients who have an excess weight with a TNF-α ≥ 8.1 pg/ml carried an excess risk of postoperative oxygenation impairment. For the meta-analysis, the pooled result also indicated that AAS patients with high BMI had a significantly increased risk of oxygenation impairment after surgery (OR, 95% CI, P: 1.40, 1.18–1.66, 0.001).
Conclusion: Excessive BMI was an independent risk factor for AAS with postoperative oxygenation impairment.
Introduction
Acute aortic syndrome (AAS) is a challenging medical emergency, which can be fatal and requires immediate surgical intervention. In recent years, despite increased advances in surgical techniques, the in-hospital mortality of the AAS remains above 25% due to a variety of postoperative complications (Trimarchi et al., 2005; Lee et al., 2018). One of the major complications is oxygenation impairment (Möller et al., 2021). Studies have shown that as many as 51% of AAS patients are complicated with oxygenation impairment after surgical repair (Nakajima et al., 2006). These patients often have a prolonged duration of mechanical ventilation, prolonged intensive care unit (ICU) stay, and increased incidence of hospital-acquired pneumonia, leading to higher hospital costs and poorer clinical prognosis (Zhao et al., 2021). Therefore, risk stratification is of great significance for postoperative oxygenation impairment in AAS patients.
Overweight and obesity are becoming relevant medical and socio-economic problems in contemporary society and have been recognized as among the most significant risk factors for cardiovascular diseases (Peitz et al., 2014; De Santo et al., 2018). Studies have revealed that an increased risk of postoperative oxygenation impairment occurred in acute aortic dissection (AD) patients with excessive body mass index (BMI) due to their disproportionately higher burden of comorbidities and underlying structural or functional changes (e.g., pulmonary vascular endothelial barrier disruption or dysfunction) in the lungs of these patients (Shen et al., 2018; Zhao et al., 2021). It is also reported that obese AD patients with postoperative oxygenation impairment exhibited a higher level of inflammation and oxidative stress, which contribute to the development of postoperative oxygenation impairment (Li et al., 2020). Moreover, excessive BMI is related to oxygenation impairment in ICU or postoperative patients (Filsoufi et al., 2008; De Jong et al., 2020). These studies demonstrate the potential value of excessive BMI for risk evaluation in AAS patients. However, other studies have shown that there is no association between excess weight and increased risk of oxygenation impairment, such as hypoxemia after Sun’s procedure (Wang KH et al., 2013; Gao et al., 2019), which makes the subject still questionable. Herein, we conducted a retrospective study to determine the relationship between BMI and postoperative oxygenation impairment in AAS patients. We then performed a meta-analysis to confirm or otherwise our findings. Our study aims to clarify these inconsistent findings, and provide new clinical evidence for risk stratification and further guidance in AAS patients with postoperative oxygenation impairment.
Materials and methods
Study population
After obtaining approval from the ethics committee of Xiangya Hospital (approval number 202101003), AAS patients who underwent surgery and were admitted to the Department of Cardiovascular Surgery at Xiangya Hospital from December 2018 to December 2020 were all retrospectively examined. The diagnosis was confirmed by aortic computed tomography angiography (CTA). The definition of AAS is AD with a patent false lumen and intramural hematoma (IMH) within 2 weeks of presentation. The exclusion criteria are listed as follows: 1) patients who had postoperative complications that may influence oxygenation indices including cardiogenic pulmonary edema, atelectasis, pneumothorax, and so on; 2) patients who only received medical therapy or refused surgical repair; 3) patients who died during operation or within 24 h after surgery; and 4) patients who had missing data more than 50% variables.
Data collection and variables definition
The preoperative, intraoperative, and postoperative data were collected, including patients’ demography (age, gender, BMI, current smoking, etc.), vital signs (blood pressure, heart rate, etc.), comorbidities (hypertension, hyperlipemia, Marfan syndrome, etc.), perioperative laboratory tests and imaging examinations (white blood cell count, blood gas analysis, echocardiography etc.), operative details (operation time, cross-clamp time, plasma transfusion, etc.), and clinical outcomes (complications, mechanical ventilation time, ICU stay, etc.). Postoperative oxygenation impairment was defined as an arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) ratio ≤200 with positive end-expiratory pressure (PEEP) ≥ 5 cm H2O at around 12 h (patients with a temporary, solvable conditions such as patient-ventilator conflict resulting in temporary hypo-oxygenation were reevaluated with another blood gas) after transferring patients to the ICU after surgery, which was also reported in other studies (Figueiredo et al., 2008; Ranieri et al., 2012; Yousefshahi et al., 2019). In addition, extubation ventilator was excluded from the study included that the patient was responsive and cooperative, PaO2 ≥ 80 mm Hg and FiO2 ≤ 0.4, breathing rate <30 times/min, and a stable hemodynamic state. All patients were divided into two groups according to the postoperative oxygenation impairment. In the stratified analysis, these patients were further subdivided into three groups based on the Asian criteria of BMI stratification: normal weight (18.5 kg/m2 ≤ BMI <23.0 kg/m2), overweight (23.0 kg/m2 ≤ BMI <25.0 kg/m2), and obesity (BMI ≥25 kg/m2) (Cui et al., 2018; Kim et al., 2021).
Surgical procedure
All operations were performed by the same surgical team. The femoral artery or the axillary artery was selected as the site of cannulation. Briefly, for Stanford type A AAS, the aortic root procedure, entailing Bentall or David surgery was done according to the lesions of the aortic root. Besides, based on the length of the dissection, the ascending aorta, the ascending aorta with the proximal hemiarch, or the ascending aorta and the total arch were replaced or a stented elephant graft was released in the descending aorta. For most of the complicated Stanford type B AAS, thoracic endovascular aortic repair (TEVAR) was performed. Open surgical repair was performed in some situations, such as lower extremities artery disease, severe tortuosity of the iliac arteries, a sharp angulation of the aortic arch, and the absence of a proximal landing zone for the stent graft.
Retrieval strategy, literature selection and quality assessment
The systematic literature search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., 2009). The protocol was registered at the international prospective register of systematic reviews (PROSPERO) (ID number: CRD42022300844). Literature retrieval was conducted in PubMed, Embase, and Web of Science databases up to 1 January 2022. The main search terms were: acute aortic syndrome (including AD, IMH) AND postoperative AND oxygenation impairment (including acute respiratory distress syndrome, acute lung injury, hypoxemia) AND body mass index (including obesity).
Studies were included if they satisfied: 1) studies reported that the patients with AAS were diagnosed by contrast-enhanced computed tomography (CT) of the aorta and complicated with postoperative oxygenation impairment; 2) BMI was measured; 3) studies explored the relationship between BMI or excess weight (such as obesity) and oxygenation impairment after surgery; and 4) availability of an odds ratio (OR) or relative risk (RR) or hazard ratio (HR) and 95% Confidence Interval (CI). The exclusion criteria were: 1) articles of abstracts, letters, case reports, reviews, meta-analysis, and animal research type; 2) duplicated publications; and 3) articles with insufficient information. The clinical outcome of our analysis was limited to AAS with postoperative oxygenation impairment by any of these definitions.
Data extraction
Two investigators (Chiyuan Zhang and Hui Bai) performed literature searches and reviewed the potential studies independently, and a third investigator (Yanfeng Zhang) was consulted to solve any disagreements. The following items from each study were recorded: first author’s surname, publication year, country, ethnicity, study size, sex, number of cases, diagnostic criteria of oxygenation impairment, BMI, OR/RR/HR value with the corresponding 95% CI and adjustment factors in the multivariable analysis. The quality of the included studies was assessed by the Newcastle-Ottawa Scale (NOS) score (Weis et al., 2019), and a score ≥6 was assigned as high-quality studies.
Statistical analysis
In the retrospective analysis, data are reported as mean ± standard deviation for normally distributed continuous variables, as median and interquartile range (IQR) for non-normally distributed continuous variables, and as number and percentage for categorical variables. For quantitative data, Student t test or One-way ANOVA test were conducted for normally distributed values and Mann-Whitney U test was applied for non-normally distributed values. For qualitative data, Chi-squared test was performed for categorical values. Risk factors for the AAS with postoperative oxygenation impairment in association with the preoperative, intraoperative, and postoperative variables were analyzed by using univariate and multivariate logistic regression models, and a dose-response relationship curve was used to assess the relationship between BMI and postoperative oxygenation impairment in AAS patients. In addition, stratified analysis was conducted to evaluate different subgroups, including gender, age, Stanford classification, current smoking, CAD, hypertension, diabetes, and so on.
In the meta-analysis, the combined OR and 95% CI were used to identify the relationship between BMI and the risk of AAS with oxygenation impairment after surgery. Cochran’s Q test and I2 statistics were utilized to evaluate the heterogeneity of studies included, and a random effect model was applied on the condition that p < 0.1 or I2 ≥ 50%. Leave-one-out sensitivity analysis and subgroup analysis based on diagnostic criteria of oxygenation impairment, age, sample size, and different forms of BMI were further performed to explain the source of heterogeneity. Besides, funnel plot and Begg’s and Egger’s test were conducted to assess publication bias.
STATA 12.0 and R 4.0.3. Were used for statistical analysis and p < 0.05 (two-sided) was considered as statistical significance.
Results
Patients’ clinical characteristics
Overall, 245 patients with AAS were initially identified from the electronic healthcare records from Xiangya hospital. Among them, 18 patients were excluded owing to the postoperative cardiogenic pulmonary edema (n = 1), atelectasis (n = 3), pneumothorax (n = 2) and large pleural effusion (n = 2), death during operation or within 24 h after surgery (n = 6), and missing data for more than 50% variables (n = 4). Thus, a total of 227 patients with AAS were finally included in this present study (Supplementary Figure S1). The mean age of all patients was 52.19 ± 12.98 years, and 78.0% of them were male (177/227). The incidence of postoperative oxygenation impairment was 48.0% (n = 109), and patients were initially divided into two groups (the non-oxygenation impairment group and the oxygenation impairment group) based on it. Among the preoperative characteristics, male (OR, 95% CI, P: 2.1, 1.09–4.05, 0.026), BMI (OR, 95% CI, P: 1.24, 1.13–1.36, <0.001), obesity (OR, 95% CI, P: 4.42, 2.33–8.39, <0.001), hypertension (OR, 95% CI, P: 1.86, 1.04–3.33, 0.038), fatty liver (OR, 95% CI, P: 1.91, 1.02–3.60, <0.044), heart rate (OR, 95% CI, P: 1.02, 1.01–1.04, 0.010), hemoglobin (Hb) (OR, 95% CI, P: 1.02, 1.01–1.04, 0.004), and AD (OR, 95% CI, P: 7.58, 1.68–34.20, 0.008) were found to be significant risk factors for the occurrence of postoperative oxygenation impairment (Table 1). Among the intraoperative and postoperative characteristics, none of the intraoperative variables showed a predictive value (all p > 0.05) but postoperative mechanical ventilation time (OR, 95% CI, P: 1.01, 1.00–1.01, 0.032), pneumonia (OR, 95% CI, P: 1.83, 1.04–3.21, 0.036), and acute kidney injury (AKI) (OR, 95% CI, P: 2.56, 1.10–5.93, 0.029) were found to be related to AAS with postoperative oxygenation impairment (Table 2).
TABLE 1
| Non-oxygenation impairment (n = 118) | Oxygenation impairment (n = 109) | Or (95% CI) | p Values | |
|---|---|---|---|---|
| Age (years) | 53.00 (43.00–62.00) | 52.00 (43.00–59.00) | 1.00 (0.98–1.02) | 0.629 |
| Sex, male (%) | 85 (72.0) | 92 (84.4) | 2.10 (1.09–4.05) | 0.026 |
| BMI (kg/m2) | 23.88 ± 3.23 | 26.08 ± 3.15 | 1.24 (1.13–1.36) | <0.001 |
| BMI (%) | ||||
| 18.5 ≤ BMI<23 (n = 73) | 53 (44.9) | 20 (18.3) | References | — |
| 23 ≤ BMI<25, (n = 42) | 23 (19.5) | 19 (17.4) | 2.19 (0.99–4.85) | 0.054 |
| BMI ≥25, (n = 112) | 42 (35.6) | 70 (64.2) | 4.42 (2.33–8.39) | <0.001 |
| Current smoking, n (%) | 59 (50.0) | 59 (54.1) | 1.18 (0.70–1.99) | 0.534 |
| Time from symptom onset to surgery (h) | 25.39 (13.05–79.48) | 20.67 (10.00–41.64) | 1.00 (1.00–1.00) | 0.308 |
| Medical history | ||||
| Hypertension, n (%) | 76 (64.4) | 84 (77.1) | 1.86 (1.04–3.33) | 0.038 |
| CAD, n (%) | 12 (10.2) | 8 (7.3) | 0.70 (0.28–1.78) | 0.454 |
| Hyperlipemia, n (%) | 5 (4.2) | 3 (2.8) | 0.64 (0.15–2.74) | 0.547 |
| Diabetes mellitus, n (%) | 3 (2.5) | 1 (0.9) | 0.36 (0.04–3.46) | 0.373 |
| Fatty liver, n (%) | 27 (31.0) | 37 (46.3) | 1.91 (1.02–3.60) | 0.044 |
| Marfan syndrome, n (%) | 2 (1.7) | 1 (0.9) | 0.54 (0.05–6.01) | 0.614 |
| COPD, n (%) | 2 (1.7) | 2 (1.8) | 1.08 (0.15–7.83) | 0.936 |
| Chronic liver disease, n (%) | 3 (2.5) | 6 (5.5) | 2.23 (0.55–9.16) | 0.265 |
| Chronic kidney disease, n (%) | 5 (4.2) | 1 (0.9) | 0.21 (0.02–1.82) | 0.156 |
| Preoperative oxygenation impairment, n (%) | 29 (24.6) | 31 (28.4) | 1.22 (0.68–2.20) | 0.510 |
| Vital signs on admission | ||||
| Respiratory rate (/min) | 20.00 (20.00–20.00) | 20.00 (18.00–20.00) | 1.02 (0.90–1.15) | 0.768 |
| SBP (mmHg) | 140.32 ± 27.71 | 144.83 ± 33.60 | 1.01 (1.00–1.01) | 0.270 |
| DBP (mmHg) | 69.00 (62.00–79.00) | 74.00 (64.00–86.00) | 1.01 (1.00–1.03) | 0.082 |
| Heart rate (/min) | 74.00 (65.00–88.00) | 82.00 (70.00–96.50) | 1.02 (1.01–1.04) | 0.010 |
| Preoperative laboratory data | ||||
| Hb (g/L) | 127.50 (113.00–136.00) | 133.00 (122.50–145.00) | 1.02 (1.01–1.04) | 0.004 |
| WBC (×109/L) | 9.85 (6.80–13.30) | 11.10 (8.35–14.25) | 1.03 (0.98–1.09) | 0.279 |
| PLT (×109/L) | 174.00 (135.50–241.25) | 172.00 (118.00–212.50) | 1.00 (0.99–1.00) | 0.158 |
| D-dimer (μg/ml) | 1.52 (0.62–2.67) | 1.40 (0.61–2.74) | 0.98 (0.91–1.06) | 0.624 |
| FDP (mg/L) | 15.50 (5.95–32.83) | 13.55 (6.95–29.30) | 1.00 (0.99–1.01) | 0.704 |
| Cr (μmol/L) | 88.05 (72.58–108,30) | 96.40 (82.55–116.15) | 1.00 (1.00–1.00) | 0.402 |
| CRP (mg/L) | 51.25 (7.39–90.08) | 35.20 (9.15–107.70) | 1.00 (0.99–1.01) | 0.992 |
| Preoperative ultrasound and CT findings | ||||
| Stanford classification | ||||
| Type A, n (%) | 79 (67.5) | 78 (71.6) | References | — |
| Type B, n (%) | 38 (32.5) | 31 (28.4) | 0.83 (0.47–1.46) | 0.510 |
| Aortic dissection, n (%) | 97 (87.4) | 105 (98.1) | 7.58 (1.68–34.20) | 0.008 |
| Intramural hematoma, n (%) | 21 (17.8) | 14 (13.1) | 0.65 (0.31–1.35) | 0.243 |
| LVEDD (mm) | 50.00 (45.00–55.00) | 51.00 (47.00–54.00) | 1.00 (0.96–1.04) | 0.901 |
| LVEF (%) | 60.00 (56.00–66.00) | 60.00 (56.00–65.00) | 0.98 (0.95–1.02) | 0.337 |
| Aortic regurgitation, n (%) | 92 (84.4) | 84 (84.8) | 1.04 (0.49–2.20) | 0.929 |
| Pleural effusion, n (%) | 39 (40.2) | 40 (43.5) | 1.14 (0.64–2.04) | 0.649 |
| Medications on admission | ||||
| Vasodilators, n (%) | 73 (61.9) | 80 (73.4) | 1.70 (0.97–3.00) | 0.065 |
The preoperative characteristics of patients with AAS.
Data are presented as mean ± SD, n (%), or medians (interquartile ranges). p values for univariate logistic regression. AAS, acute aortic syndrome; OR, odds ratio; CI, confidence interval; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; FDP, fibrinogen degradation products; Cr, creatinine; CRP, C reactive protein; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction.
TABLE 2
| Non-oxygenation impairment (n = 118) | Oxygenation impairment (n = 109) | OR (95% CI) | p Values | |
|---|---|---|---|---|
| Intraoperative data | ||||
| Aortic root replacement, n (%) | 65 (55.1) | 69 (63.3) | 1.41 (0.83–2.40) | 0.209 |
| Hemiarch replacement, n (%) | 9 (7.6) | 10 (9.2) | 1.22 (0.48–3.13) | 0.674 |
| Total arch replacement, n (%) | 61 (51.7) | 67 (61.5) | 1.49 (0.88–2.53) | 0.139 |
| Stented elephant trunk, n (%) | 58 (49.2) | 64 (58.7) | 1.47 (0.87–2.49) | 0.149 |
| Bentall operation, n (%) | 23 (19.5) | 14 (12.8) | 0.61 (0.30–1.25) | 0.178 |
| David operation, n (%) | 2 (1.7) | 0 (0.0) | — | — |
| TEVAR, n (%) | 34 (28.8) | 26 (23.9) | 0.77 (0.43–1.40) | 0.398 |
| CABG operation, n (%) | 6 (5.1) | 3 (2.8) | 0.53 (0.13–2.17) | 0.375 |
| CPB time (h) | 3.43 (2.82–4.07) | 3.43 (2.77–4.39) | 1.04 (0.87–1.24) | 0.694 |
| Cross-clamp time (h) | 1.64 (1.22–2.44) | 1.52 (1.21–2.08) | 0.95 (0.71–1.28) | 0.751 |
| Circulatory arrest time (min) | 30.00 (21.00–36.00) | 30.00 (22.00–40.00) | 1.00 (0.98–1.01) | 0.538 |
| Operation time (h) | 6.33 (2.04–7.69) | 6.75 (4.92–8.42) | 1.07 (0.99–1.15) | 0.082 |
| Nasopharyngeal temperature (°C) | 25.20 (24.35–26.50) | 25.00 (24.30–27.00) | 0.98 (0.89–1.07) | 0.604 |
| RBC transfusion (u) | 4.00 (2.00–6.00) | 4.00 (2.00–5.50) | 1.01 (0.95–1.09) | 0.680 |
| PLT transfusion (u) | 1.00 (1.00–2.00) | 1.00 (1.00–2.00) | 1.00 (0.99–1.01) | 0.703 |
| Plasma transfusion (ml) | 550.00 (350.00–650.00) | 500.00 (380.00–600.00) | 1.00 (1.00–1.00) | 0.755 |
| Postoperative data | ||||
| CRP (mg/L) | 136.00 (85.05–176.25) | 124.29 (78.20–199.00) | 1.00 (1.00–1.00) | 0.885 |
| Mechanical ventilation time (h) | 37.40 (16.47–81.22) | 89.61 (36.45–138.38) | 1.01 (1.00–1.01) | 0.032 |
| Tracheotomy, n (%) | 113 (95.8) | 109 (100.0) | 4.78 (0.55–41.57) | 0.156 |
| Pneumonia, n (%) | 71 (60.2) | 80 (73.4) | 1.83 (1.04–3.21) | 0.036 |
| Cerebral infarction, n (%) | 5 (4.2) | 6 (5.5) | 1.32 (0.39–4.44) | 0.658 |
| Delirium, n (%) | 39 (33.1) | 44 (40.4) | 1.37 (0.80–2.36) | 0.253 |
| AKI, n (%) | 9 (7.6) | 19 (17.4) | 2.56 (1.10–5.93) | 0.029 |
| Renal replacement therapy, n (%) | 6 (5.1) | 8 (7.3) | 1.48 (0.50–4.41) | 0.483 |
| ICU stay (days) | 5.00 (3.75–8.00) | 6.00 (4.00–9.00) | 1.01 (0.96–1.05) | 0.762 |
| Hospital stay (days) | 12.00 (9.00–18.00) | 13.00 (9.50–18.00) | 1.00 (0.97–1.03) | 0.913 |
| In hospital death, n (%) | 7 (5.9) | 12 (11.0) | 1.96 (0.74–5.18) | 0.174 |
The intraoperative and postoperative characteristics of patients with AAS.
Data are presented as mean ± SD, n (%), or medians (interquartile ranges). p values for univariate logistic regression. AAS, acute aortic syndrome; OR, odds ratio; CI, confidence interval; TEVAR, thoracic endovascular aortic repair; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; RBC, red blood cell; PLT, platelet; CRP, C reactive protein; AKI, acute kidney injury; ICU, intensive care unit.
Stratified analysis was then conducted according to BMI, which is presented in Supplementary Table S1 and Supplementary Table S2. The preoperative data showed that a fatty liver, preoperative oxygenation impairment, and a higher white blood cell (WBC) were more common in the obesity group (all p < 0.05), and the proportion of male and hypertension, and diastolic blood pressure (DBP) were elevated in the excessive BMI group (both overweight and obesity group) (all p < 0.05) (Supplementary Table S1). Besides, the intraoperative and postoperative data indicated that mechanical ventilation time was increased in these groups (p < 0.05), and the proportion of Bentall and coronary artery bypass grafting (CABG) operation had a negative correlation with BMI, while postoperative oxygenation impairment has a positive correlation with BMI (all p < 0.05) (Supplementary Table S2).
The association between BMI and AAS with postoperative oxygenation impairment
To determine the relationship between BMI and AAS with postoperative oxygenation impairment, a multivariate logistic regression analysis was further performed. After adjusting to sex, hypertension, fatty liver, hyperlipemia, diabetes mellitus, heart rate, Hb, preoperative oxygenation impairment, aortic dissection, mechanical ventilation time, pneumonia, and AKI, BMI remained significantly correlated with postoperative oxygenation impairment in patients with AAS (OR, 95% CI, P: 1.27, 1–1.46, 0.001) and excess weight. Both overweight and obesity were independent risk factors (OR, 95% CI, P: 4.96, 1.62–15.15, 0.005; 9.51, 3.06–29.57, <0.001), which are presented in Table 3. Our study then explored the non-linear correlation between BMI and AAS with postoperative oxygenation impairment. The dose-response relationship analysis suggests that the risk of oxygenation impairment after surgery increased with the increased BMI, which is presented in Figure 1. To determine the development of its effect sizes, interaction analysis was also performed and all patients were divided into different subgroups according to sex, age, current drinking, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), systolic blood pressure (SBP), preoperative WBC, and so on. As shown in Table 4, there was no interaction in most strata (P for interaction = 0.057–0.904), and only AAS patients with excessive BMI who had a tumor necrosis factor-α (TNF-α) ≥ 8.1 pg/ml had an excess risk of postoperative oxygenation impairment (p = 0.027).
TABLE 3
| OR (95% CI) | p Values | |
|---|---|---|
| Sex, male (%) | 1.14 (0.41–3.16) | 0.809 |
| BMI (kg/m2) | 1.27 (1.10–1.46) | 0.00 |
| BMI (n, %) | ||
| 18.5 ≤ BMI< 23 | References | — |
| 23 ≤ BMI <25 | 4.96 (1.62–15.15) | 0.005 |
| BMI ≥25 | 9.51 (3.06–29.57) | <0.001 |
| Hypertension, n (%) | 0.63 (0.27–1.44) | 0.271 |
| Hyperlipemia, n (%) | 0.18 (0.03–1.35) | 0.096 |
| Diabetes mellitus, n (%) | 0.56 (0.02–15.44) | 0.730 |
| Fatty liver, n (%) | 1.18 (0.48–2.88) | 0.725 |
| Preoperative oxygenation impairment, n (%) | 0.52 (0.22–1.28) | 0.155 |
| Heart rate (/min) | 1.01 (0.99–1.03) | 0.475 |
| Hb (g/L) | 1.02 (1.00–1.04) | 0.055 |
| Aortic dissection, n (%) | 11.62 (1.07–126.55) | 0.052 |
| Mechanical ventilation time (h) | 1.00 (1.00–1.00) | 0.605 |
| Pneumonia, n (%) | 1.54 (0.68–3.47) | 0.302 |
| AKI, n (%) | 2.13 (0.69–6.56) | 0.187 |
Multivariate logistic regression analysis for AAS with postoperative oxygenation impairment.
Adjusted to sex, hypertension, fatty liver, hyperlipemia, diabetes mellitus, heart rate, Hb, preoperative oxygenation impairment, aortic dissection, mechanical ventilation time, pneumonia and AKI. AAS, acute aortic syndrome; OR, odds ratio; CI, confidence interval; BMI, body mass index; Hb, hemoglobin; AKI, acute kidney injury.
FIGURE 1
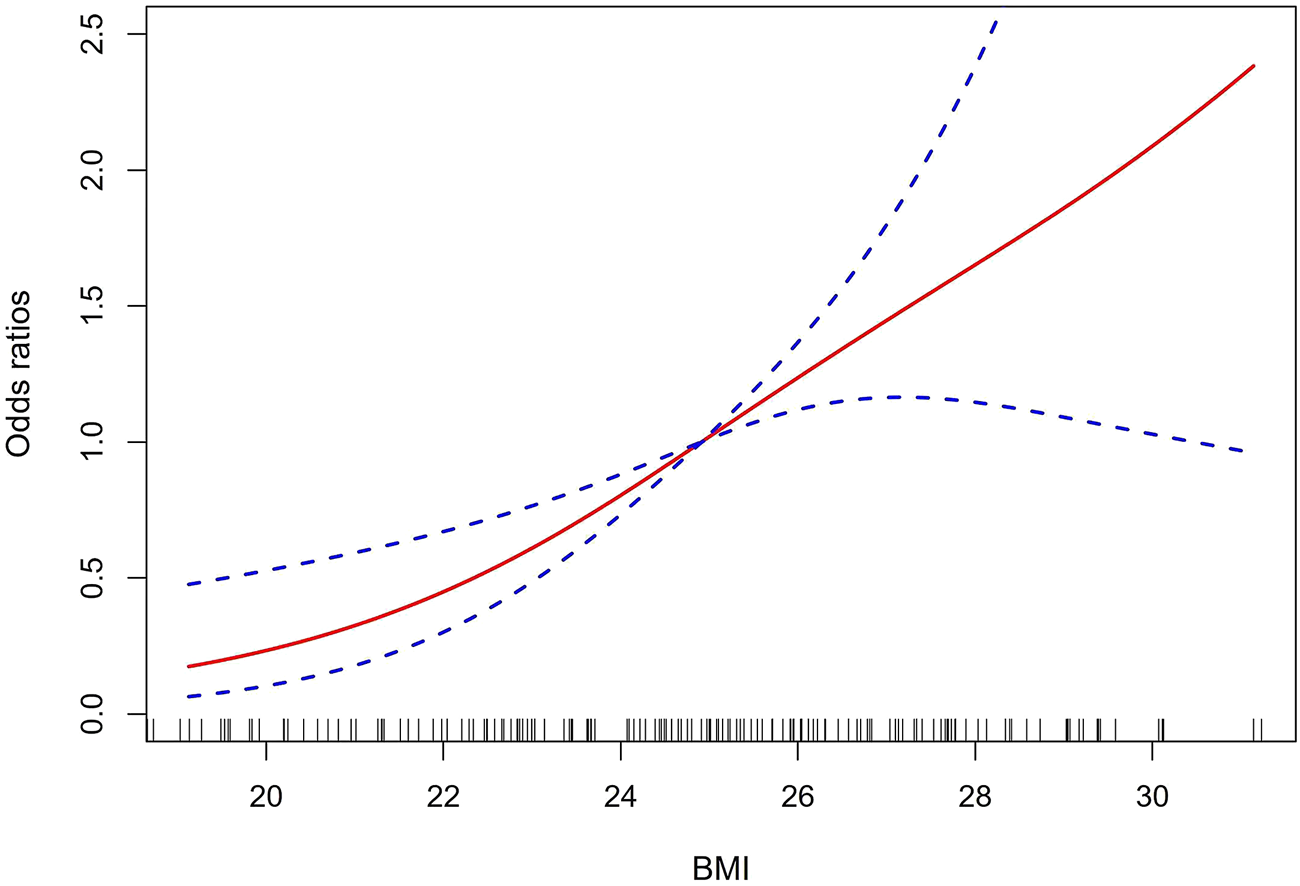
The dose-response relationship analysis of BMI and AAS with postoperative oxygenation impairment. The solid red line and the dashed-blue line represent the estimated OR and 95% CI, respectively. BMI, body mass index; AAS, acute aortic syndrome; OR, odds ratio; CI, confidence interval.
TABLE 4
| No. of patients | BMI levels (kg/m2) | P For interaction | |||
|---|---|---|---|---|---|
| <23 | 23–25 | ≥25 | |||
| Sex | 0.482 | ||||
| Male | 177 | ref | 3.86 (1.51–9.86) | 4.30 (1.96–9.44) | |
| Female | 50 | ref | 1.50 (0.22–10.36) | 2.00 (0.55–7.24) | |
| Age, years | 0.500 | ||||
| <60 | 165 | ref | 4.63 (1.72–12.47) | 4.63 (1.99–10.75) | |
| ≥60 | 62 | ref | 1.43 (0.27–7.44) | 2.56 (0.84–7.83) | |
| Stanford classification | 0.743 | ||||
| Type A | 157 | ref | 3.96 (1.46–10.75) | 3.68 (1.68–8.06) | |
| Type B | 69 | ref | 2.40 (0.57–10.04) | 3.84 (1.14–12.95) | |
| AD | 0.539 | ||||
| No | 16 | ref | — | 3.33 (0.36–30.70) | |
| Yes | 202 | ref | 3.39 (1.43–8.05) | 4.12 (2.02–8.39) | |
| IMH | 0.326 | ||||
| No | 183 | ref | 3.30 (1.32–8.27) | 4.59 (2.15–9.79) | |
| Yes | 35 | ref | 8.75 (0.74–103.82) | 2.19 (0.47–10.21) | |
| Current smoking | 0.198 | ||||
| No | 109 | ref | 2.45 (0.84–7.21) | 2.05 (0.85–4.90) | |
| Yes | 118 | ref | 6.14 (1.58–23.79) | 8.61 (2.71–27.39) | |
| Current drinking | 0.274 | ||||
| No | 171 | ref | 4.48 (1.76–11.43) | 3.52 (1.68–7.38) | |
| Yes | 56 | ref | 1.33 (0.22–7.98) | 3.62 (0.81–16.15) | |
| Fatty liver | 0.500 | ||||
| No | 103 | ref | 3.90 (1.34–11.34) | 4.80 (1.70–13.59) | |
| Yes | 64 | ref | 15.00 (1.03–218.30) | 6.91 (0.75–63.53) | |
| Hypertension | 0.884 | ||||
| No | 67 | ref | 2.25 (0.44–11.48) | 3.41 (1.07–10.86) | |
| Yes | 160 | ref | 3.25 (1.18–8.96) | 3.37 (1.42–7.99) | |
| CAD | 0.533 | ||||
| No | 207 | ref | 3.10 (1.34–7.14) | 3.25 (1.65–6.38) | |
| Yes | 20 | ref | — | — | |
| Diabetes | 0.564 | ||||
| No | 223 | ref | 3.24 (1.42–7.38) | 3.54 (1.83–6.85) | |
| Yes | 4 | ref | — | — | |
| COPD | 0.261 | ||||
| No | 223 | ref | 3.61 (1.58–8.26) | 3.90 (2.00–7.62) | |
| Yes | 4 | ref | — | — | |
| SBP, mmHg | 0.057 | ||||
| <140 | 104 | ref | 4.14 (1.35–12.66) | 2.07 (0.83–5.17) | |
| ≥140 | 123 | ref | 2.69 (0.76–9.59) | 6.55 (2.41–17.83) | |
| DBP, mmHg | 0.658 | ||||
| <90 | 192 | ref | 3.77 (1.57–9.05) | 3.65 (1.78–7.51) | |
| ≥90 | 35 | ref | 1.33 (0.14–12.82) | 3.25 (0.63–16.79) | |
| Preoperative WBC, 109/L | 0.209 | ||||
| <9.5 | 93 | ref | 1.89 (0.59–6.07) | 1.89 (0.72–5.00) | |
| ≥9.5 | 134 | ref | 5.57 (1.74–17.86) | 5.95 (2.31–15.27) | |
| Preoperative D-dimer, μg/mL | 0.385 | ||||
| <0.5 | 42 | ref | 1.14 (0.15–8.59) | 2.55 (0.52–12.37) | |
| ≥0.5 | 184 | ref | 4.27 (1.71–10.66) | 3.99 (1.93–8.25) | |
| Preoperative FDP, mg/L | 0.904 | ||||
| <5 | 36 | ref | 3.00 (0.42–21.30) | 3.00 (0.58–15.61) | |
| ≥5 | 184 | ref | 3.50 (1.41–8.68)_ | 4.02 (1.91–8.44) | |
| Preoperative Cr, μmol/L | 0.349 | ||||
| <111 | 168 | ref | 2.54 (0.96–6.70) | 4.01 (1.89–8.52) | |
| ≥111 | 59 | ref | 5.40 (1.12–26.04) | 2.80 (0.73–10.76) | |
| Preoperative PaO2/FiO2 ratio | 0.592 | ||||
| ≤200 | 60 | ref | 2.46 (1.27–6.63) | 5.32 (1.75–8.74) | |
| >200 | 167 | ref | 2.19 (0.95–5.06) | 4.78 (2.28–10.02) | |
| Preoperative ESR, mm/h | 0.616 | ||||
| <20 | 65 | ref | 7.71 (1.25–47.75) | 4.91 (1.57–15.31) | |
| ≥20 | 80 | ref | 3.21 (0.83–12.47) | 3.12 (1.03–9.46) | |
| Preoperative CRP, mg/l | 0.272 | ||||
| <8 | 23 | ref | 1.13 (0.08–16.31) | 1.69 (0.25–11.34) | |
| ≥8 | 70 | ref | 12.80 (2.02–81.12) | 10.35 (2.09–51.30) | |
| Preoperative IL-1β, pg/ml | 0.672 | ||||
| <5.0 | 43 | ref | 2.89 (0.33–25.70) | 3.00 (0.66–13.66) | |
| ≥5.0 | 50 | ref | 4.50 (0.82–24.57) | 6.00 (1.46–24.69) | |
| Preoperative TNF-α, pg/ml | 0.788 | ||||
| <8.1 | 34 | ref | 4.67 (0.53–40.89) | 2.80 (0.45–17.38) | |
| ≥8.1 | 49 | ref | 4.53 (0.75–27.39) | 5.10 (1.34–19.47) | |
| Preoperative IL-6, pg/ml | 0.078 | ||||
| <5.0 | 3 | ref | — | — | |
| ≥5.0 | 90 | ref | 3.57 (0.93–13.66) | 4.70 (1.68–13.15) | |
| Preoperative IL-10, pg/ml | 0.763 | ||||
| <9.1 | 58 | ref | 5.63 (1.02–30.90) | 4.18 (1.12–15.65) | |
| ≥9.1 | 34 | ref | 2.00 (0.24–16.61) | 3.43 (0.65–18.22) | |
| Aortic regurgitation | 0.349 | ||||
| No | 32 | ref | 6.00 (0.58–61.82) | 5.20 (0.71–37.90) | |
| Yes | 176 | ref | 1.02 (0.42–2.48) | 1.77 (0.90–3.44) | |
| Pleural effusion | 0.373 | ||||
| No | 110 | ref | 2.92 (0.83–10.27) | 4.33 (1.69–11.08) | |
| Yes | 79 | ref | 3.82 (1.05–13.91) | 2.06 (0.69–6.15) | |
| Postoperative ESR, mm/h | 0.511 | ||||
| <20 | 15 | ref | 2.50 (0.10–62.51) | 1.25 (0.12–13.24) | |
| ≥20 | 69 | ref | 6.25 (1.15–34.12) | 6.25 (1.78–22.01) | |
| Postoperative CRP, mg/l | — | ||||
| <8 | 1 | ref | — | — | |
| ≥8 | 77 | ref | 5.25 (1.13–24.42) | 4.09 (1.18–14.15) | |
| Postoperative IL-1β, pg/ml | 0.072 | ||||
| <5.0 | 33 | ref | 1.00 (0.13–8.00) | 1.33 (0.27–6.50) | |
| ≥5.0 | 37 | ref | — | 6.67 (1.16–38.25) | |
| Postoperative TNF-α, pg/ml | 0.027 | ||||
| <8.1 | 14 | ref | 0.67 (0.03–18.06) | 0.11 (0.01–1.78) | |
| ≥8.1 | 50 | ref | 12.50 (1.60–97.65) | 6.36 (1.46–27.67) | |
| Postoperative IL-6, pg/ml | — | ||||
| <5.0 | 0 | ref | — | — | |
| ≥5.0 | 70 | ref | 4.50 (0.94–21.56) | 3.00 (0.97–9.30) | |
| Postoperative IL-10, pg/ml | 0.658 | ||||
| <5.9 | 14 | ref | 6.00 (0.22–162.53) | 1.50 (0.09–25.39) | |
| ≥5.9 | 56 | ref | 3.20 (0.48–21.17) | 3.43 (0.99–11.85) | |
Subgroup analysis and interaction analysis of the relationship between BMI and AAS with postoperative oxygenation impairment.
BMI, body mass index; AAS, acute aortic syndrome; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; AD, aortic dissection; IMH, intramural hematoma; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; FDP, fibrinogen degradation products; Cr, creatinine; ESR, erythrocyte sedimentation rate; CRP, C reactive protein; IL-1β, Interleukin-1β; TNF-α, Tumor necrosis factor-α; IL-6, Interleukin-6; IL-10, Interleukin-10.
Moreover, the mean PaO2/FiO2 ratio was observed in the normal weight, overweight, and obesity groups during the perioperative period (Figure 2). Figure 2 indicates that AAS patients with increased BMI values were related to a lower PaO2/FiO2 ratio on admission and at 0, 6, 12, and 24 h after surgery (p < 0.05). These trends did not change over time (p < 0.05).
FIGURE 2
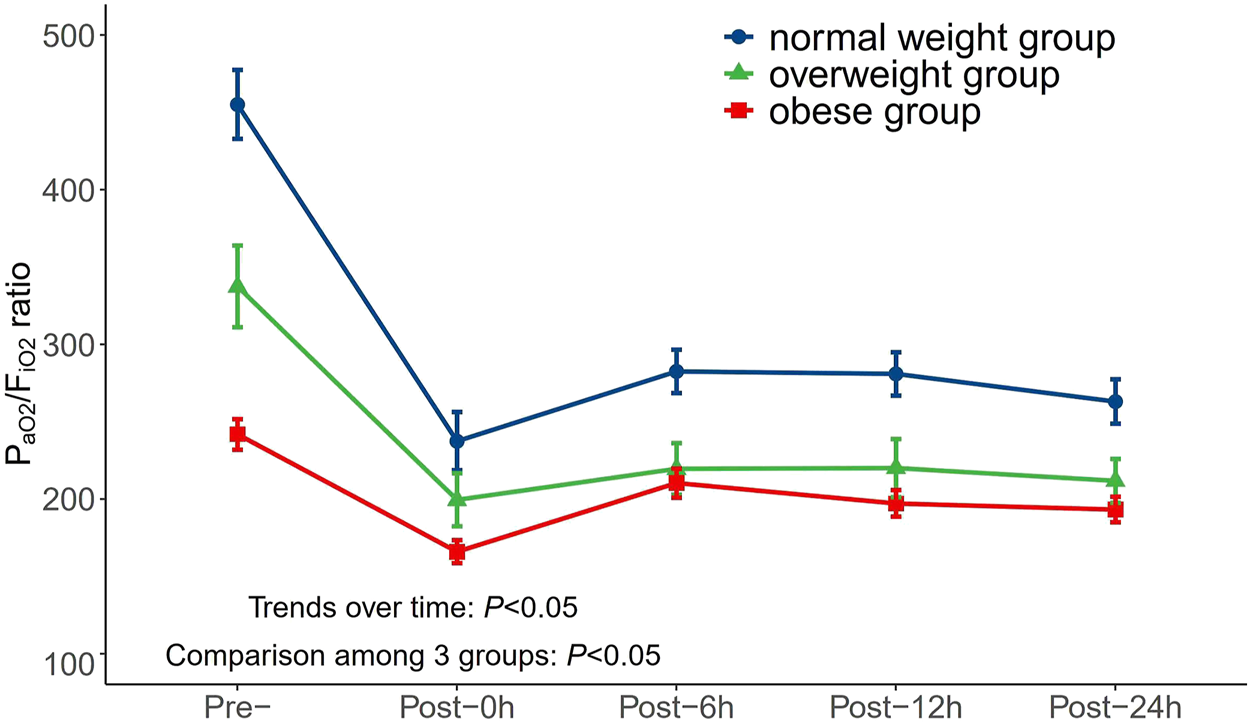
Changes of PaO2/FiO2 ratio in different BMI groups over time during perioperative period. PaO2/FiO2, arterial oxygen tension/inspiratory oxygen fraction; BMI, body mass index.
Literature search results and studies’ characteristics of meta-analysis
Based on this retrieval strategy, 66 articles were initially identified, of which 23 were excluded due to duplication. A list of 14 articles was then selected for further full-text review after screening the titles or abstracts. Finally, eight articles were included in the quantitative analysis by removing six articles due to the lack of OR value or other necessary data. The literature screening flow diagram is shown in Supplementary Figure S2.
All of the studies were published between 2006 and 2021, and their characteristics are summarized in Supplementary Table S3. In detail, these studies were case-control studies involving a sum of 1,451 patients with AAS. They were all conducted in China, apart from one study performed in Japan. Six studies defined postoperative oxygenation impairment as PaO2/FiO2 ratio ≤200, while two studies used PaO2/FiO2 ratio ≤100. Besides, according to the NOS criteria, the studies included were of high quality with scores ranging from seven to eight, which is shown in Supplementary Figure S3.
Quantitative synthesis and analysis of meta-analysis
The pooled result revealed that AAS patients with high BMI had a significantly increased risk of postoperative oxygenation impairment (OR, 95% CI, P: 1.40, 1.18–1.66, 0.001) and significant heterogeneity was found among these studies (I2 = 88.0%, p < 0.001) (Figure 3).
FIGURE 3
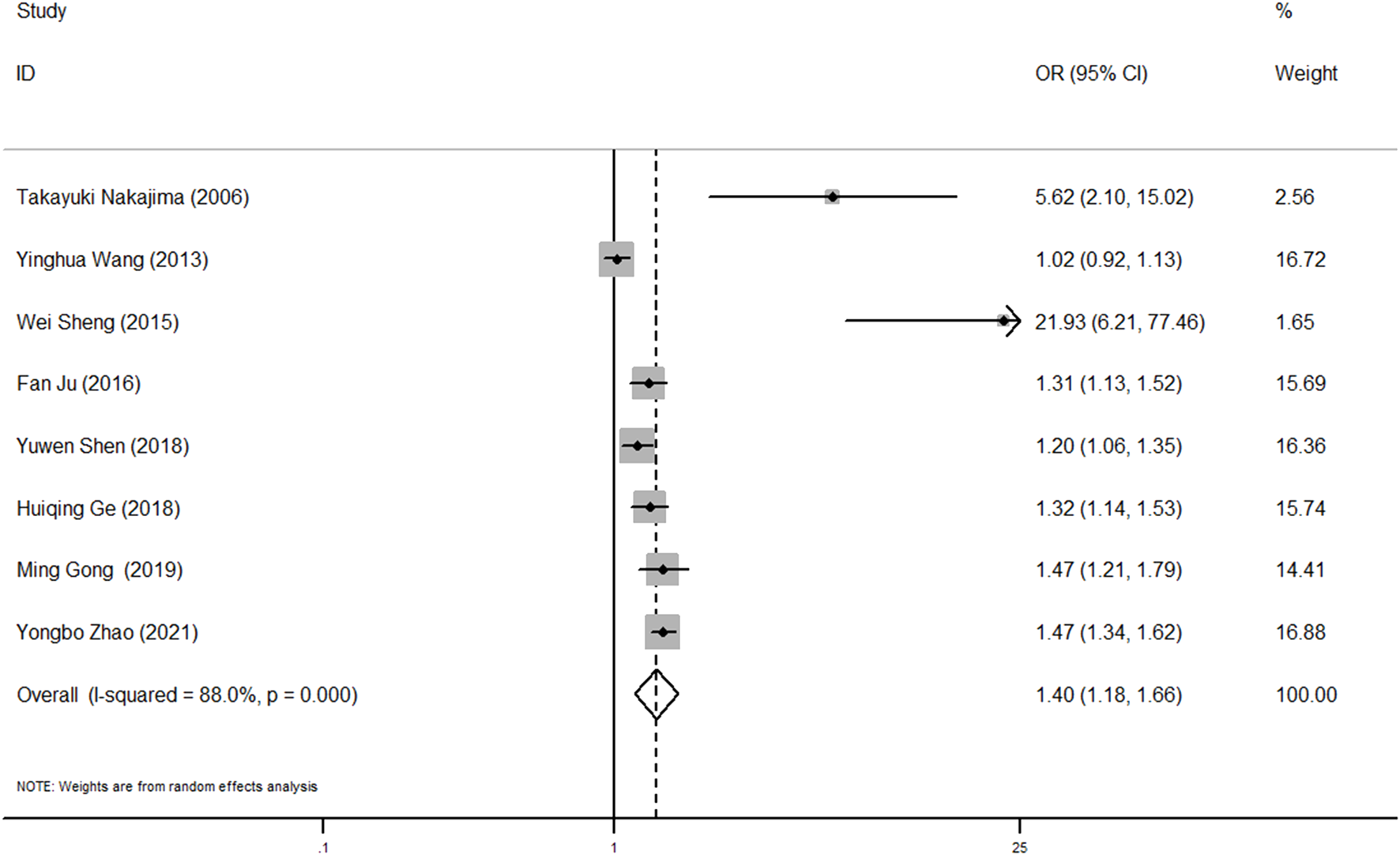
Forest plot of OR of BMI and postoperative oxygenation impairment in AAS patients. OR, odds ratio; BMI, body mass index; AAS, acute aortic syndrome.
To identify the potential source of heterogeneity in these studies, subgroup and sensitivity analyses were conducted. As shown in Supplementary Table S4, the pooled OR values were higher in studies with PaO2/FiO2 ratio ≤100 as diagnostic criteria of postoperative oxygenation impairment, age >50 years, sample size ≤200 or BMI as categorical data. The subgroup results also suggested that there was a consistently significant association between high BMI and increased risk of oxygenation impairment. In addition, the leave-one-out sensitivity analysis indicated that no study had a large significant effect on the main results (Supplementary Figure S4). For the evaluation of publication bias, both Begg’s and Egger’s test were not significant (p = 0.108, 0.060). However, the funnel plot was basically asymmetrical, which remained a potential publication bias (Supplementary Figure S5).
Discussion
In this retrospective study, we enrolled 227 AAS patients and found excess weight to be an independent risk factor of AAS with postoperative oxygenation impairment. Besides, the results of the meta-analysis further confirmed the positive correlation between BMI and the increased risk of oxygenation impairment after surgery in patients with AAS.
AAS often requires emergency surgery owing to its urgent onset, rapid progression, and high mortality. Postoperative oxygenation impairment is one of the most common complications after surgery, and it has an adverse impact on the duration of mechanical ventilation and the length of ICU stay (Liu et al., 2017). Therefore, assessing the risk factors predisposing to this complication is crucial for clinical outcomes. With the increasing incidence of overweight and obese patients, studies have increasingly focused on the relationship between overweight and obesity, and AD patients who have postoperative oxygenation impairment; however, the results are controversial. Shen et al. (2018) performed a retrospective analysis of 169 consecutive patients with Stanford type A AD who underwent a total arch replacement procedure and found that excessive BMI increased the risk of postoperative oxygenation impairment. In addition, Nakajima et al. (2006) demonstrated that BMI ≥25 kg/m2 was an independent risk factor of hypoxemia in acute AD patients after surgery. However, Wang Y et al. (2013) revealed that BMI was related to postoperative hypoxemia in Stanford type A AD patients but not independently after adjusting for clinical variables in multivariate analysis. Similarly, another two studies from China indicated that there was no significant correlation between BMI or excess weight and postoperative hypoxemia following surgical repair of AAS (Liu et al., 2017; Gao et al., 2019). These inconsistent findings are likely to be due to differences in diagnostic criteria for oxygenation impairment, heterogeneity in the study population, and different variables adjustment for risk stratification.
In the present study, we included 227 patients with AAS who underwent surgical treatment and found that the rate of oxygenation impairment after surgery was 48.0%, which is in line with the previous studies (Shen et al., 2018). We stratified all patients based on BMI into three groups (normal weight, overweight, and obese), and a significant positive correlation between BMI and postoperative oxygenation impairment was determined. A multivariate logistic regression analysis showed that excess weight (both overweight and obesity) was an independent risk factor for oxygenation impairment. A dose-response relationship curve and subgroup analysis further confirmed this conclusion. We also compared the mean PaO2/FiO2 ratio among three groups at different perioperative timepoints and found that the group with a higher BMI had a lower PaO2/FiO2 ratio. The variance trend analysis showed no change over time. Moreover, we reviewed eight studies for meta-analysis. Based on the NOS criteria, the included studies were of high quality with a score of seven or eight, which suggests that the results are reliable. The pooled results support the results of our retrospective study and indicate that excessive BMI increased the risk of postoperative oxygenation impairment in AAS patients.
To date, the mechanism of postoperative oxygenation impairment in AAS has not been fully elucidated; however, it is currently believed that inflammation plays an important role in this process (Zhou et al., 2021). Several inflammatory reactants can be used as clinical biomarkers. For instance, Gong et al. (2019) reported that Stanford type A AD patients with postoperative oxygenation impairment exhibited a high level of preoperative WBC. Meanwhile, Ge et al.bookmark://bib_Ge_et_al_2018/ (2018) provided further confirmation in their prediction model for postoperative hypoxemia. In addition, increased serum C-reactive protein and D-dimer level were associated with hypoxia after surgery in AD patients, which provided potential clinical biomarkers (Liao et al., 2021). Several studies have shown that some surgical factors (e.g., cardiopulmonary bypass, circulatory arrest, plasma transfusion, etc.) can enlarge the local vascular inflammation and increase the release of inflammatory factors into circulation. Furthermore, augmentative systemic inflammation leads to lung tissue damage and dysfunction in AD patients (Ju et al., 2016; Shen et al., 2018). However, few studies have reported its pathogenesis in obese AAS patients. It is reported that obese AAS patients exhibited an elevated level of Interleukin-1β (IL-1β), Tumor necrosis factor-α (TNF-α) and Interleukin-6 (IL-6) (Wu et al., 2020), while our study has also shown that these patients had a higher preoperative WBC and preoperative and postoperative C reactive protein (CRP) (p = 0.034, 0.253, 0.455), which suggests that they have a more intense level of inflammation and oxidative stress. These inflammatory reactants could be produced by adipose tissue and under the surgical stress can further develop into systemic inflammation through circulation, destroy the pulmonary capillary bed, and cause pulmonary interstitial edema, leading to mismatched ventilation/perfusion distribution and oxygenation impairment (Wu et al., 2016).
AAS patients with high BMI often suggest endocrine disorders and they are environmental exposed daily to several endocrine disrupters at very low doses, which are strictly related to several cardiovascular risk factors including obesity, metabolic disorders, and diabetes (Wang Y et al., 2013; Quagliariello et al., 2019). For instance, Bisphenol A (BPA) is an endocrine disrupter that is commonly used in the manufacturing of plastics and has been shown to stimulate the intracellular calcium accumulation and lipid peroxidation, and increase the production of interleukins, causing pro-oxidative and pro-inflammatory effects on the cardiovascular system (Quagliariello et al., 2019). As a result, these environmental factors can magnify the inflammatory response and expand tissue damage in these patients. Furthermore, excessive BMI adversely affects the physiology of the respiratory system and excess adipose tissue can impair respiratory compliance, increase the respiratory load, and reduce ventilatory drive, which has been treated as a risk factor for hypoxemia after cardiac surgery (Weiss et al., 2000; Santos et al., 2013). The high burden of comorbidities such as subclinical renal injury in overweight or obese patients could be another reason for the increased incidence of postoperative oxygenation impairment (Shi et al., 2020).
Our subgroup analysis revealed that there was no interaction in most strata, confirming the reliability of excessive BMI as an independent risk factor for postoperative oxygenation impairment in AAS patients. However, it has also shown that AAS patients with a high BMI and a TNF-α level ≥8.1 pg/ml had an excess risk of oxygenation impairment after surgery. The reason for this result may be that TNF-α, as an inflammatory factor, participates in the occurrence and development of AAS with postoperative oxygenation impairment, which was mentioned earlier (Wu et al., 2020).
It is believed that excessive BMI has a negative impact on respiratory function after a major cardiac surgery, such as coronary artery bypass grafting (CABG) (Apostolakis et al., 2010; Ranucci et al., 2014). However, there are few reports on the effect of BMI on AAS with postoperative oxygenation impairment, and its role still needs to be confirmed by further studies. Our study focused on patients with AAS, and for the first time explored the relationship between BMI and postoperative oxygenation impairment in them, which has provided potent evidence for risk stratification and further clinical management in such patients. For them, protective intraoperative mechanical ventilation (e.g., an intraoperative high level of PEEP and alveolar recruitment maneuvers) is necessary (Bluth et al., 2019). Besides, nitric oxide inhalation therapy after operation (5–10 ppm, over 24 h) may attenuate postoperative hypoxemia, which has been reported in obese patients with acute type A aortic dissection (Zheng et al., 2022). Due to the important role of inflammation in its pathogenesis, some studies have also shown that perioperative anti-inflammatory therapy, including high-dose ulinastatin (20,000 units/kg), can reduce lung injury and improve the respiratory function by attenuating the elevation of cytokines and polymorphonuclear neutrophil elastase (PMNE) (Xu et al., 2013; Duan et al., 2018). These measures may reduce the risk of AAS with postoperative oxygenation impairment. However, further large-scale research is needed to confirm their efficacy. Finally, proper nutrition and nutraceuticals are also important long-term treatment for these patients (Raynor and Champagne, 2016; Berretta et al., 2020). On the one hand, proper nutrition (e.g., the increased consumption of fruits and vegetables) can promote patients to achieve a state of negative energy balance, which is a key part of weight management (Raynor and Champagne, 2016). On the other hand, some nutraceuticals, particularly ascorbic acid (ASC), by affecting neutrophil migration and apoptosis and stimulating phagocytosis, exert an antioxidant and immunomodulatory effect, which show a good prospect in improving postoperative inflammation in AAS. It is also reported that oral administration of ASC can reduce arterial stiffness and enhance endothelial function (Berretta et al., 2020).
This study has observed some limitations. First, due to its retrospective nature and relatively small sample size, the results in our study may not be universally representative. Second, it is difficult to assess the impact of surgical procedures on the AAS with postoperative oxygenation impairment owing to differences in surgical details. Third, our meta-analysis included case-control studies and significant heterogeneity might have increased the bias of the results. Further prospective studies and an in-depth mechanism exploration will be needed to examine the results of this study.
Conclusion
Our findings show that high BMI patients with AAS are more likely to have oxygenation impairment after surgery and excessive BMI is an independent risk factor for AAS with postoperative oxygenation impairment.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Xiangya Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
GL and QX conceived the study. CZ, HB, YZ, and ZD reviewed publications and extracted the data of eligible studies for the meta-analysis. CZ, LZ, and XC recruited the AAS patients with and without oxygenation impairment and collected their clinical data. ZF, RS, and GZ performed the statistical analysis. CZ wrote the first draft of the paper. All authors revised and commented on the draft and overall responsibility and approved the submitted version.
Funding
This work was supported by two grants from the Hunan Provincial Natural Science Foundation of China (Hunan, China; Grant No. 2021JJ41041 and No. 2022JJ70158).
Acknowledgments
We acknowledge assistance from JW for his review of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.955702/full#supplementary-material
References
1
ApostolakisE. E.KoletsisE. N.BaikoussisN. G.SiminelakisS. N.PapadopoulosG. S. (2010). Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J. Cardiothorac. Surg.5, 1. 10.1186/1749-8090-5-1
2
BerrettaM.QuagliarielloV.MaureaN.Di FranciaR.SharifiS.FacchiniG.et al (2020). Multiple effects of ascorbic acid against chronic diseases: Updated evidence from preclinical and clinical studies. Antioxidants (Basel, Switz).9, 1182. 10.3390/antiox9121182
3
BluthT.Serpa NetoA.SchultzM. J.PelosiP.Gama de AbreuM.BluthT.et al (2019). Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: A randomized clinical trial. Jama321, 2292–2305. 10.1001/jama.2019.7505
4
CuiJ.SunX.LiX.KeM.SunJ.YasmeenN.et al (2018). Association between different indicators of obesity and depression in adults in qingdao, China: A cross-sectional study. Front. Endocrinol.9, 549. 10.3389/fendo.2018.00549
5
De JongA.WriggeH.HedenstiernaG.GattinoniL.ChiumelloD.FratJ. P.et al (2020). How to ventilate obese patients in the ICU. Intensive Care Med.46, 2423–2435. 10.1007/s00134-020-06286-x
6
De SantoL. S.MoscarielloC.ZebeleC. (2018). Implications of obesity in cardiac surgery: Pattern of referral, physiopathology, complications, prognosis. J. Thorac. Dis.10, 4532–4539. 10.21037/jtd.2018.06.104
7
DuanX. Z.XuZ. Y.LuF. L.HanL.TangY. F.TangH.et al (2018). Inflammation is related to preoperative hypoxemia in patients with acute Stanford type A aortic dissection. J. Thorac. Dis.10, 1628–1634. 10.21037/jtd.2018.03.48
8
FigueiredoL. C.AraújoS.AbdalaR. C.AbdalaA.GuedesC. A. (2008). CPAP at 10 cm H2O during cardiopulmonary bypass does not improve postoperative gas exchange. Rev. Bras. Cir. Cardiovasc.23, 209–215. 10.1590/s0102-76382008000200010
9
FilsoufiF.RahmanianP. B.CastilloJ. G.ChikweJ.AdamsD. H. (2008). Logistic risk model predicting postoperative respiratory failure in patients undergoing valve surgery. Eur. J. Cardiothorac. Surg.34, 953–959. 10.1016/j.ejcts.2008.07.061
10
GaoZ.PeiX.HeC.WangY.LuJ.JinM.et al (2019). Oxygenation impairment in patients with acute aortic dissection is associated with disorders of coagulation and fibrinolysis: a prospective observational study. J. Thorac. Dis.11, 1190–1201. 10.21037/jtd.2019.04.32
11
GeH.JiangY.JinQ.WanL.QianX.ZhangZ. (2018). Nomogram for the prediction of postoperative hypoxemia in patients with acute aortic dissection. BMC Anesthesiol.18, 146. 10.1186/s12871-018-0612-7
12
GongM.WuZ.XuS.LiL.WangX.GuanX.et al (2019). Increased risk for the development of postoperative severe hypoxemia in obese women with acute type a aortic dissection. J. Cardiothorac. Surg.14, 81. 10.1186/s13019-019-0888-9
13
JuF.LiuN.PanX. D.QiaoH. Y.LiL.RongT. H.et al (2016). A prediction model for severe postoperative hypoxemia after surgery for Standford type A aortic dissection. Zhonghua yi xue za zhi96, 1001–1006. 10.3760/cma.j.issn.0376-2491.2016.13.004
14
KimB. Y.KangS. M.KangJ. H.KangS. Y.KimK. K.KimK. B.et al (2021). 2020 Korean society for the study of obesity guidelines for the management of obesity in korea. J. Obes. Metabolic Syndrome30, 81–92. 10.7570/jomes21022
15
LeeT. C.KonZ.CheemaF. H.Grau-SepulvedaM. V.EnglumB.KimS.et al (2018). Contemporary management and outcomes of acute type A aortic dissection: An analysis of the STS adult cardiac surgery database. J. Card. Surg.33, 7–18. 10.1111/jocs.13511
16
LiY.JiangH.XuH.LiN.ZhangY.WangG.et al (2020). Impact of a higher body mass index on prolonged intubation in patients undergoing surgery for acute thoracic aortic dissection. Heart Lung Circ.29, 1725–1732. 10.1016/j.hlc.2020.02.008
17
LiaoH.OuS.DongX.LiuJ.XiaoC. (2021). Association of Etoricoxib treatment and incident hypoxia in patients with aortic dissection undergoing endovascular aortic repair. Biomed. Pharmacother. = Biomedecine Pharmacother.139, 111625. 10.1016/j.biopha.2021.111625
18
LiberatiA.AltmanD. G.TetzlaffJ.MulrowC.GøtzscheP. C.IoannidisJ. P.et al (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ Clin. Res. ed)339, b2700. 10.1136/bmj.b2700
19
LiuN.ZhangW.MaW.ShangW.ZhengJ.SunL. (2017). Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact. Cardiovasc. Thorac. Surg.24, 251–256. 10.1093/icvts/ivw272
20
MöllerC. M.EllmauerP. P.ZemanF.BitzingerD.FlörchingerB.GrafB. M.et al (2021). Postoperative acute respiratory dysfunction and the influence of antibiotics after acute type A aortic dissection surgery: A retrospective analysis. PloS One16, e0246724. 10.1371/journal.pone.0246724
21
NakajimaT.KawazoeK.IzumotoH.KataokaT.NiinumaH.ShirahashiN. (2006). Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg. Today36, 680–685. 10.1007/s00595-006-3226-5
22
PeitzG. W.TroyerJ.JonesA. E.ShapiroN. I.NelsonR. D.HernandezJ.et al (2014). Association of body mass index with increased cost of care and length of stay for emergency department patients with chest pain and dyspnea. Circ. Cardiovasc. Qual. Outcomes7, 292–298. 10.1161/CIRCOUTCOMES.113.000702
23
QuagliarielloV.CoppolaC.MitaD. G.PiscopoG.IaffaioliR. V.BottiG.et al (2019). Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol.69, 1–8. 10.1016/j.etap.2019.03.006
24
RanieriV. M.RubenfeldG. D.ThompsonB. T.FergusonN. D.CaldwellE.FanE.et al (2012). Acute respiratory distress syndrome: the berlin definition. Jama307, 2526–2533. 10.1001/jama.2012.5669
25
RanucciM.BallottaA.La RovereM. T.CastelvecchioS.Surgical and Clinical Outcome Research SCORE Group (2014). Postoperative hypoxia and length of intensive care unit stay after cardiac surgery: The underweight paradox?PloS One9, e93992. 10.1371/journal.pone.0093992
26
RaynorH. A.ChampagneC. M. (2016). Position of the Academy of nutrition and dietetics: Interventions for the treatment of overweight and obesity in adults. J. Acad. Nutr. Diet.116, 129–147. 10.1016/j.jand.2015.10.031
27
SantosN. P.MitsunagaR. M.BorgesD. L.Costa MdeA.BaldezT. E.LimaI. M.et al (2013). Factors associated to hypoxemia in patients undergoing coronary artery bypass grafting. Rev. Bras. Cir. Cardiovasc.28, 364–370. 10.5935/1678-9741.20130056
28
ShenY.LiuC.FangC.XiJ.WuS.PangX.et al (2018). Oxygenation impairment after total arch replacement with a stented elephant trunk for type-A dissection. J. Thorac. Cardiovasc. Surg.155, 2267–2274. 10.1016/j.jtcvs.2018.01.085
29
ShiN.LiuK.FanY.YangL.ZhangS.LiX.et al (2020). The association between obesity and risk of acute kidney injury after cardiac surgery. Front. Endocrinol.11, 534294. 10.3389/fendo.2020.534294
30
TrimarchiS.NienaberC. A.RampoldiV.MyrmelT.SuzukiT.MehtaR. H.et al (2005). Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J. Thorac. Cardiovasc. Surg.129, 112–122. 10.1016/j.jtcvs.2004.09.005
31
WangK. H.KaoA. P.ChangC. C.LinT. C.KuoT. C. (2013). Bisphenol A at environmentally relevant doses induces cyclooxygenase-2 expression and promotes invasion of human mesenchymal stem cells derived from uterine myoma tissue. Taiwan. J. Obstet. Gynecol.52, 246–252. 10.1016/j.tjog.2013.04.016
32
WangY.XueS.ZhuH. (2013). Risk factors for postoperative hypoxemia in patients undergoing Stanford A aortic dissection surgery. J. Cardiothorac. Surg.8, 118. 10.1186/1749-8090-8-118
33
WeisS.KesselmeierM.DavisJ. S.MorrisA. M.LeeS.ScheragA.et al (2019). Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect.25, 818–827. 10.1016/j.cmi.2019.03.010
34
WeissY. G.MerinG.KoganovE.RiboA.Oppenheim-EdenA.MedalionB.et al (2000). Postcardiopulmonary bypass hypoxemia: a prospective study on incidence, risk factors, and clinical significance. J. Cardiothorac. Vasc. Anesth.14, 506–513. 10.1053/jcan.2000.9488
35
WuZ.RuanY.ChangJ.LiB.RenW. (2016). Angiotensin II is related to the acute aortic dissection complicated with lung injury through mediating the release of MMP9 from macrophages. Am. J. Transl. Res.8, 1426–1436.
36
WuZ.WangZ.WuH.HuR.RenW.HuZ.et al (2020). Obesity is a risk factor for preoperative hypoxemia in Stanford A acute aortic dissection. Medicine99, e19186. 10.1097/MD.0000000000019186
37
XuC. E.ZouC. W.ZhangM. Y.GuoL. (2013). Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J. Cardiothorac. Vasc. Anesth.27, 479–484. 10.1053/j.jvca.2012.11.001
38
YousefshahiF.SamadiE.PaknejadO.MovafeghA.BarkhordariK.Bastan HaghE.et al (2019). Prevalence and risk factors of hypoxemia after coronary artery bypass grafting: The time to change our conceptions. J. Tehran Heart Cent.14, 74–80.
39
ZhaoY.YueY.WangY.ZhaoW.FengG. (2021). The risk factors for postoperative acute respiratory distress syndrome in Stanford type a acute aortic dissection patients. Am. J. Transl. Res.13, 7318–7326.
40
ZhengP.JiangD.LiuC.WeiX.LiS. (2022). Nitric oxide inhalation therapy attenuates postoperative hypoxemia in obese patients with acute type A aortic dissection. Comput. Math. Methods Med.2022, 9612548. 10.1155/2022/9612548
41
ZhouJ.PanJ.YuY.HuangW.LaiY.LiangW.et al (2021). Independent risk factors of hypoxemia in patients after surgery with acute type A aortic dissection. Ann. Palliat. Med.10, 7388–7397. 10.21037/apm-21-1428
Summary
Keywords
body mass index, overweight, obesity, acute aortic syndrome, postoperative oxygenation impairment
Citation
Zhang C, Bai H, Zhang Y, Deng Z, Zhang L, Chen X, Fu Z, Shi R, Zhang G, Xu Q and Lin G (2022) Impact of body mass index on postoperative oxygenation impairment in patients with acute aortic syndrome. Front. Physiol. 13:955702. doi: 10.3389/fphys.2022.955702
Received
29 May 2022
Accepted
22 July 2022
Published
31 August 2022
Volume
13 - 2022
Edited by
Ilaria Peluso, Council for Agricultural and Economics Research (CREA), Italy
Reviewed by
Shuiyun Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Michael Hofmann, University of Zurich, Switzerland
Robert Jeenchen Chen, Stanford University, United States
Vincenzo Quagliariello, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Nianguo Dong, Huazhong University of Science and Technology, China
Updates
Copyright
© 2022 Zhang, Bai, Zhang, Deng, Zhang, Chen, Fu, Shi, Zhang, Xu and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Xu, elsiexu403@163.com; Guoqiang Lin, 38328513@qq.com
This article was submitted to Vascular Physiology, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.