Abstract
Aims: The study aimed to assess the association of hyperlipidemia and the risk of death in the aneurysm population, focusing on age, gender, and aneurysm location differences.
Methods: All patients’ data on this retrospective cohort study were obtained from the Medical Information Mart for Intensive Care (MIMIC-III) database, and the baseline characteristics and laboratory parameters of all patients were collected. The COX regression model was established to explore the association of hyperlipidemia and the risk of death for patients with aneurysms. More importantly, subgroup analyses based on the age, gender, and aneurysm location differences were performed.
Results: A total of 1,645 eligible patients were enrolled in this study. These patients were divided into the survival group (n = 1,098) and the death group (n = 547), with a total mortality rate of approximately 33.25%. The result displayed that hyperlipidemia was associated with a decreased death risk in aneurysm patients. In addition, we also found that hyperlipidemia was associated with a lower death risk of abdominal aortic aneurysm and thoracic aortic arch aneurysm among aneurysm patients aged ≥60 years; hyperlipidemia was only a protective factor for the death risk of male patients diagnosed with abdominal aortic aneurysm. For female patients diagnosed with abdominal aortic aneurysm and thoracic aortic arch aneurysm, hyperlipidemia was associated with a decreased death risk.
Conclusion: The relationship of hyperlipidemia, hypercholesterolemia, and the risk of death for patients diagnosed with aneurysms was significantly associated with age, gender, and aneurysm location.
Introduction
An aneurysm is a persistent dilation of the vascular wall caused by a lesion or damage of an artery wall, which is commonly an asymptomatic disease but could cause death because of artery ruptures (Schmitz-Rixen et al., 2016; Liu et al., 2020). It is estimated that there are around 200,000 aneurysm-related deaths worldwide each year (Liu et al., 2020). Nowadays, several literature reports have proposed that aneurysm is one of the typical gender-related aorta diseases (Sweeting et al., 2012; Deery et al., 2017; Boese et al., 2018). In the study by Boese and colleagues, they pointed out that abdominal aortic aneurysms were more likely to occur in men, but women were at a greater risk of rupture and had a worse prognosis; moreover, women who were diagnosed with abdominal aortic aneurysms commonly were older than men (Boese et al., 2018). Understanding gender and age differences of aneurysm would help make more accurate prognosis methods for patients.
Hyperlipidemia is a pathological condition, in which the lipid concentration exceeds normal levels due to the disorder of lipid metabolism in the human body (He and Ye, 2020). Several clinical studies have indicated that hyperlipidemia could affect heart function by promoting the development of atherosclerosis and increasing the risk of non-ischemic heart failure and coronary heart disease, which has been proven to be associated with an increased risk of cardiovascular disease (El-Tantawy and Temraz, 2019; Yao et al., 2020). Nevertheless, to the best of our knowledge, a number of studies have pointed out that hyperlipidemia appears to have a protective effect on the death of patients with aneurysms. There were few studies that comprehensively evaluated the relationship between hyperlipidemia and death due to aneurysms among patients of different ages and genders so far (Cheng et al., 2019; Huber et al., 2019). Understanding the effect of hyperlipidemia on death in aneurysm patients of different ages or genders plays an important role in improving the prognosis of different populations.
Herein, the aims of the present study are to investigate the association of hyperlipidemia and death in the population with aneurysms, focusing on age, gender, and aneurysm location differences.
Methods
Data sources and study design
All data on this retrospective cohort study were obtained from the Medical Information Mart for Intensive Care (MIMIC-III) database (version 1.4), which is a large, single-center, freely available database and contains information related to 53,423 patients admitted to the intensive care unit of a large tertiary hospital between 2001 and 2012 (Johnson et al., 2016; Long et al., 2021).
A total of 1,793 patients diagnosed with aneurysms were extracted from the MIMIC-III database. We excluded some patients who had ruptured aneurysms or had abnormal age records. Due to the public availability of the MIMIC-III database, all patients’ private information has been anonymized and does not require approval from the local ethics committee.
Data collection
We collected the baseline characteristics and laboratory parameters of all patients (Cheng et al., 2019; Jeon-Slaughter et al., 2019), including gender, age, the history of diseases [chronic obstructive pulmonary disease (COPD), lung cancer, atrial fibrillation (AF), liver cirrhosis, congestive heart failure, heart disease, diabetes mellitus, respiratory failure, hyperlipidemia, renal failure, cancer, hypertension, and hypercholesterolemia], respiratory rate, temperature, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), oxygen saturation (SpO2 %), white blood cell count (WBC, 103/uL), red blood cell count (RBC, 103/uL), sodium (mEq/L), potassium (mEq/L), calcium (mg/dL), platelets (PLT, k/uL), international normalized ratio (INR), mean corpuscular volume (MCV, 10 g/L), glucose (mg/dL), creatinine (mg/dL), blood urea nitrogen (BUN, mg/dL), bicarbonate (mEq/L), hematocrit (%), mean corpuscular hemoglobin concentration (MCHC, %), red cell distribution width (RDW, %), simplified acute physiology score II (SAPSII), sequential organ failure assessment (Sofa), and the history of medication use, such as atorvastatin, imipenem–cilastatin, lovastatin, nystatin, pravastatin, simvastatin, statins, and other lipid-lowering drugs (ezetimibe, cholestyramine, colestipol, colesevelam, ciprofibrate, fenofibrate, gemfibrozil, omega-3, and niacin).
Outcomes
The primary outcome of our study was death. Hyperlipidemia was diagnosed in terms of the Chinese guidelines for the management of dyslipidemia in adults (Chen et al., 2020); hypercholesterolemia was defined as the condition where patients were treated with antihyperlipidemic agents or had a total cholesterol level ≥220 mg/dL (Kang et al., 2017). Aneurysms included abdominal aortic aneurysms, thoracic aortic arch aneurysms, cerebral aneurysms, and others [including aneurysm of the artery of the lower extremity, aneurysm of the artery of the neck, aneurysm of the artery of the upper extremity, aneurysm of coronary vessels, aneurysm of the heart (wall), aneurysm of the iliac artery, aneurysm of other specified arteries, aneurysm of other visceral arteries, aneurysm of the pulmonary artery, aneurysm of the renal artery, aneurysm of the subclavian artery, aortic aneurysm of unspecified sites without mention of rupture, other aneurysms of the heart, and thoracoabdominal aneurysm without mention of rupture] in the study. The starting date of follow-up was the date of the patient’s admission. All patients were followed for 10 years.
Statistical analysis
The measurement data on the normal distribution were described by mean ± standard deviation (mean ± SD), and comparison between groups was performed by an independent sample t-test. The measurement data on the non-normal distribution used the median and quartile spacing [M (Q1, Q3)], and the Mann–Whitney U rank-sum test was adopted to compare the two groups. The categorical data were conducted by the number of cases and composition ratio n (%), and were compared by the chi-squared or Fisher’s exact test. Several variables with many missing data were deleted (more than 10%). We interpolated the missing data by using R mice, and the sensitivity analysis of data after interpolation is shown in Supplementary Table S1.
First, we conducted a descriptive statistical analysis by univariate difference analysis. Then, univariate COX analysis was carried out to explore the confounding factors. Next, a COX regression model was established with hyperlipidemia and hypercholesterolemia as independent variables and death within 10 years as the dependent variable. Model 1 was regarded as unadjusted. Model 2 adjusted the age, COPD, lung cancer, AF, liver cirrhosis, congestive heart failure, heart disease, diabetes mellitus, respiratory failure, and cancer. Model 3 adjusted several covariates that included age, respiratory rate, heart rate, DBP, MAP, SpO2, WBC, sodium, potassium, PLT, INR, MCV, glucose, creatinine, BUN, MCHC, RDW, SAPSII, Sofa, imipenem–cilastatin, COPD, lung cancer, AF, liver cirrhosis, congestive heart failure, heart disease, diabetes mellitus, respiratory failure, and cancer. More importantly, we assessed the relationships between hyperlipidemia, hypercholesterolemia, and death in patients with aneurysms based on the age, gender, and type of aneurysms. We adopted restricted cubic spline (RCS) curves to assess the dose-response relationship between age and death risk. The hazard ratio (HR) and 95% confidence interval (CI) were calculated in the study. p < 0.05 was considered statistically significant.
Results
Baseline characteristics
After excluding patients who had ruptured aneurysms (n = 87) and had abnormal age records (n = 61), a total of 1,645 eligible patients were enrolled in this study, with an average age of 67.17 ± 14.20 years old. These patients were divided into the survival group (n = 1,098) and the death group (n = 547), with a total mortality rate of approximately 33.25%. There were 968 men and 677 women, and the incidence of male and female death was approximately 32.64% and 34.12%, respectively. Detailed baseline data on all eligible patients are shown in Table 1.
TABLE 1
| Variable | Total (n = 1,645) | Survival group (n = 1,098) | Death group (n = 547) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 968 (58.84) | 652 (59.38) | 316 (57.77) |
| Female | 677 (41.16) | 446 (40.62) | 231 (42.23) |
| Age, mean ± SD | 67.17 ± 14.20 | 63.75 ± 14.16 | 74.04 ± 11.54 |
| COPD, n (%) | |||
| No | 1,381 (83.95) | 978 (89.07) | 403 (73.67) |
| Yes | 264 (16.05) | 120 (10.93) | 144 (26.33) |
| Lung cancer, n (%) | |||
| No | 1,638 (99.57) | 1,097 (99.91) | 541 (98.90) |
| Yes | 7 (0.43) | 1 (0.09) | 6 (1.10) |
| AF, n (%) | |||
| No | 1,061 (64.50) | 778 (70.86) | 283 (51.74) |
| Yes | 584 (35.50) | 320 (29.14) | 264 (48.26) |
| Liver cirrhosis, n (%) | |||
| No | 1,595 (96.96) | 1,074 (97.81) | 521 (95.25) |
| Yes | 50 (3.04) | 24 (2.19) | 26 (4.75) |
| Congestive heart failure, n (%) | |||
| No | 1,207 (73.37) | 902 (82.15) | 305 (55.76) |
| Yes | 438 (26.63) | 196 (17.85) | 242 (44.24) |
| Heart disease, n (%) | |||
| No | 1,473 (89.54) | 1,012 (92.17) | 461 (84.28) |
| Yes | 172 (10.46) | 86 (7.83) | 86 (15.72) |
| Diabetes mellitus, n (%) | |||
| No | 1,380 (83.89) | 953 (86.79) | 427 (78.06) |
| Yes | 265 (16.11) | 145 (13.21) | 120 (21.94) |
| Respiratory failure, n (%) | |||
| No | 1,384 (84.13) | 1,001 (91.17) | 383 (70.02) |
| Yes | 261 (15.87) | 97 (8.83) | 164 (29.98) |
| Hyperlipidemia, n (%) | |||
| No | 978 (59.45) | 613 (55.83) | 365 (66.73) |
| Yes | 667 (40.55) | 485 (44.17) | 182 (33.27) |
| Renal failure, n (%) | |||
| No | 1,271 (77.26) | 950 (86.52) | 321 (58.68) |
| Yes | 374 (22.74) | 148 (13.48) | 226 (41.32) |
| Cancer, n (%) | |||
| No | 1,364 (82.92) | 953 (86.79) | 411 (75.14) |
| Yes | 281 (17.08) | 145 (13.21) | 136 (24.86) |
| Respiratory rate, mean ± SD | 16.37 ± 5.16 | 15.57 ± 4.68 | 17.98 ± 5.70 |
| Temperature, °C, mean ± SD | 36.21 ± 2.16 | 36.15 ± 2.06 | 36.32 ± 2.33 |
| Heart rate, mean ± SD | 81.19 ± 16.43 | 79.49 ± 15.02 | 84.60 ± 18.50 |
| SBP, mean ± SD | 126.22 ± 24.30 | 125.61 ± 22.55 | 127.43 ± 27.46 |
| DBP, mean ± SD | 64.48 ± 14.97 | 65.14 ± 14.28 | 63.17 ± 16.18 |
| MAP, mean ± SD | 83.92 ± 17.16 | 84.56 ± 16.19 | 82.63 ± 18.91 |
| SpO2, %, mean ± SD | 97.80 ± 3.88 | 98.19 ± 3.41 | 97.02 ± 4.58 |
| WBC (103/uL), M (Q1, Q3) | 9.30 (7.10, 12.50) | 9.20 (6.90, 12.30) | 9.80 (7.30, 13.40) |
| RBC (103/uL), mean ± SD | 3.82 ± 0.75 | 3.82 ± 0.76 | 3.81 ± 0.74 |
| Sodium (mEq/L), mean ± SD | 139.18 ± 3.71 | 139.48 ± 3.51 | 138.59 ± 4.03 |
| Potassium (mEq/L), mean ± SD | 4.21 ± 0.67 | 4.16 ± 0.58 | 4.32 ± 0.82 |
| Calcium (mg/dL), mean ± SD | 8.53 ± 0.77 | 8.52 ± 0.72 | 8.54 ± 0.86 |
| PLT (k/uL), M (Q1, Q3) | 206.00 (153.00, 266.00) | 203.00 (151.00, 262.00) | 218.00 (156.00, 278.00) |
| INR, M (Q1, Q3) | 1.20 (1.10, 1.40) | 1.20 (1.10, 1.40) | 1.20 (1.10, 1.40) |
| MCV, 10 g/L, mean ± SD | 89.57 ± 6.46 | 89.02 ± 5.91 | 90.68 ± 7.32 |
| Glucose (mg/dL), M (Q1, Q3) | 121.00 (102.00, 146.00) | 120.00 (102.00, 142.00) | 123.00 (103.00, 157.00) |
| Creatinine (mg/dL), M (Q1, Q3) | 0.90 (0.70, 1.20) | 0.90 (0.70, 1.10) | 1.10 (0.80, 1.60) |
| BUN (mg/dL), M (Q1, Q3) | 17.00 (13.00, 24.00) | 16.00 (12.00, 21.00) | 22.00 (16.00, 34.00) |
| Bicarbonate (mEq/L), mean ± SD | 24.78 ± 3.83 | 24.74 ± 3.34 | 24.86 ± 4.67 |
| Hematocrit, %, mean ± SD | 34.06 ± 6.51 | 33.92 ± 6.62 | 34.33 ± 6.26 |
| MCHC, %, mean ± SD | 34.04 ± 1.43 | 34.24 ± 1.34 | 33.63 ± 1.51 |
| RDW, %, mean ± SD | 14.39 ± 1.74 | 14.04 ± 1.40 | 15.08 ± 2.11 |
| SAPSII, M (Q1, Q3) | 32.00 (24.00, 40.00) | 29.00 (21.00, 37.00) | 37.00 (30.00, 44.00) |
| Sofa, M (Q1, Q3) | 4.00 (2.00, 8.00) | 4.00 (2.00, 7.00) | 5.00 (3.00, 8.00) |
| Hypertension, n (%) | |||
| No | 575 (34.95) | 387 (35.25) | 188 (34.37) |
| Yes | 1,070 (65.05) | 711 (64.75) | 359 (65.63) |
| Hypercholesterolemia, n (%) | |||
| No | 1,391 (84.56) | 912 (83.06) | 479 (87.57) |
| Yes | 254 (15.44) | 186 (16.94) | 68 (12.43) |
| Atorvastatin, n (%) | |||
| No | 1,157 (70.33) | 785 (71.49) | 372 (68.01) |
| Yes | 488 (29.67) | 313 (28.51) | 175 (31.99) |
| Imipenem–cilastatin, n (%) | |||
| No | 1,638 (99.57) | 1,096 (99.82) | 542 (99.09) |
| Yes | 7 (0.43) | 2 (0.18) | 5 (0.91) |
| Lovastatin, n (%) | |||
| No | 1,642 (99.82) | 1,096 (99.82) | 546 (99.82) |
| Yes | 3 (0.18) | 2 (0.18) | 1 (0.18) |
| Nystatin, n (%) | |||
| No | 1,642 (99.82) | 1,097 (99.91) | 545 (99.63) |
| Yes | 3 (0.18) | 1 (0.09) | 2 (0.37) |
| Pravastatin, n (%) | |||
| No | 1,593 (96.84) | 1,064 (96.90) | 529 (96.71) |
| Yes | 52 (3.16) | 34 (3.10) | 18 (3.29) |
| Simvastatin, n (%) | |||
| No | 1,320 (80.24) | 870 (79.23) | 450 (82.27) |
| Yes | 325 (19.76) | 228 (20.77) | 97 (17.73) |
| Statins, n (%) | |||
| No | 817 (49.67) | 551 (50.18) | 266 (48.63) |
| Yes | 828 (50.33) | 547 (49.82) | 281 (51.37) |
| Other lipid-lowering drugs, n (%) | |||
| No | 1,594 (96.90) | 1,061 (96.63) | 533 (97.44) |
| Yes | 51 (3.10) | 37 (3.37) | 14 (2.56) |
Baseline characteristics of all included participants.
Other lipid-lowering drugs: ezetimibe, cholestyramine, colestipol, colesevelam, ciprofibrate, fenofibrate, gemfibrozil, omega-3, and niacin; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SpO2, oxygen saturation; WBC, white blood cell count; RBC, red blood cell count; PLT, platelet; INR, international normalized ratio; MCV, mean corpuscular volume; BUN, blood urea nitrogen; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; SAPSII, simplified acute physiology score II; Sofa, sequential organ failure assessment.
Assessment of confounding factors by univariate Cox regression analysis
We performed a univariate Cox regression to analyze the possible confounding factors related to death in patients diagnosed with aneurysms. Supplementary Table S2 indicates that age, COPD, lung cancer, AF, liver cirrhosis, congestive heart failure, heart disease, diabetes mellitus, respiratory failure, renal failure, cancer, respiratory rate, heart rate, DBP, MAP, SpO2, WBC, sodium, potassium, PLT, INR, MCV, glucose, creatinine, BUN, MCHC, RDW, SAPSII, Sofa, and imipenem–cilastatin might be associated with the risk of death for patients with aneurysms (p < 0.05).
The relationship between hyperlipidemia, hypercholesterolemia, and death risk of aneurysm patients
The effects of hyperlipidemia on the death risk of aneurysm patients are presented in Table 2. Model 1 displayed that hyperlipidemia could decrease the death risk of aneurysm patients (HR = 0.69, 95% CI: 0.58–0.83, p < 0.001), with a similar result in models 2 (HR = 0.51, 95% CI: 0.42–0.61, p < 0.001) and Model 3 (HR = 0.52, 95% CI: 0.43–0.62, p < 0.001). In addition, we found that hypercholesterolemia was also associated a reduced risk of aneurysm patients’ death in Model 1 and Model 2. Nevertheless, after adjusting covariates, Model 3 demonstrated that there was no statistically significant difference between hypercholesterolemia and death (HR = 0.77, 95% CI: 0.60–1.01, p = 0.055).
TABLE 2
| Variables | Sample size | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |||||
| Non-hyperlipidemia | 978 | Reference | Reference | Reference | |||
| Hyperlipidemia | 667 | 0.69 (0.58–0.83) | <0.001 | 0.51 (0.42–0.61) | <0.001 | 0.52 (0.43–0.62) | <0.001 |
| Non-hypercholesterolemia | 1,391 | Reference | Reference | Reference | |||
| Hypercholesterolemia | 254 | 0.73 (0.57–0.94) | 0.016 | 0.69 (0.53–0.88) | 0.004 | 0.77 (0.60–1.01) | 0.055 |
Relationship between hyperlipidemia, hypercholesterolemia, and the death risk of aneurysm patients.
HR: hazard ratio; CI: confidence interval.
Model 1: unadjusted.
Model 2: adjusted age, chronic obstructive pulmonary disease (COPD), lung cancer, atrial fibrillation (AF), liver cirrhosis, congestive heart failure, heart disease, diabetes mellitus, respiratory failure, and cancer.
Model 3: adjusted age, respiratory rate, heart rate, diastolic blood pressure, mean arterial pressure, oxygen saturation, white blood count, sodium, potassium, platelets, international normalized ratio, mean corpuscular volume, glucose, creatinine, blood urea nitrogen, mean corpuscular hemoglobin concentration, red cell distribution width, simplified acute physiology score II, sequential organ failure assessment, imipenem–cilastatin; COPD, lung cancer; AF, liver cirrhosis, congestive heart failure, heart disease, diabetes mellitus, respiratory failure, and cancer.
Subgroup analysis was performed based on age, gender, and aneurysm location
We could find that there was a non-linear relationship between age and death in the RCS graph (Figure 1), and the relationship was statistically significant (p = 0.001). Interestingly, there was a protective trend for patients aged <60 years and a risk trend for patients aged ≥60 years. Herein, we discussed the relationship between hyperlipidemia, hypercholesterolemia, and the death risk of different aneurysm locations based on patients of different ages. As shown in Table 3, hyperlipidemia was associated with a lower death risk of abdominal aortic aneurysm, thoracic aortic arch aneurysm, and others among aneurysm patients aged ≥60 years. However, there was no statistically significant difference between hyperlipidemia and death of different aneurysm locations in patients aged <60 years. Simultaneously, the association of hypercholesterolemia and the death risk of different aneurysm locations is also analyzed in Table 3. The result indicated that hypercholesterolemia could decrease the death risk of other aneurysm locations in patients aged ≥60 years, while hypercholesterolemia was a risk factor for patients aged <60 years. Similarly, we performed the subgroup analysis based on gender and the aneurysm location. Table 4 implies that hyperlipidemia was only a protective factor for the death risk of male patients diagnosed with abdominal aortic aneurysms. For female patients diagnosed with abdominal aortic aneurysms and thoracic aortic arch aneurysms, hyperlipidemia was still associated with a decreased death risk.
FIGURE 1
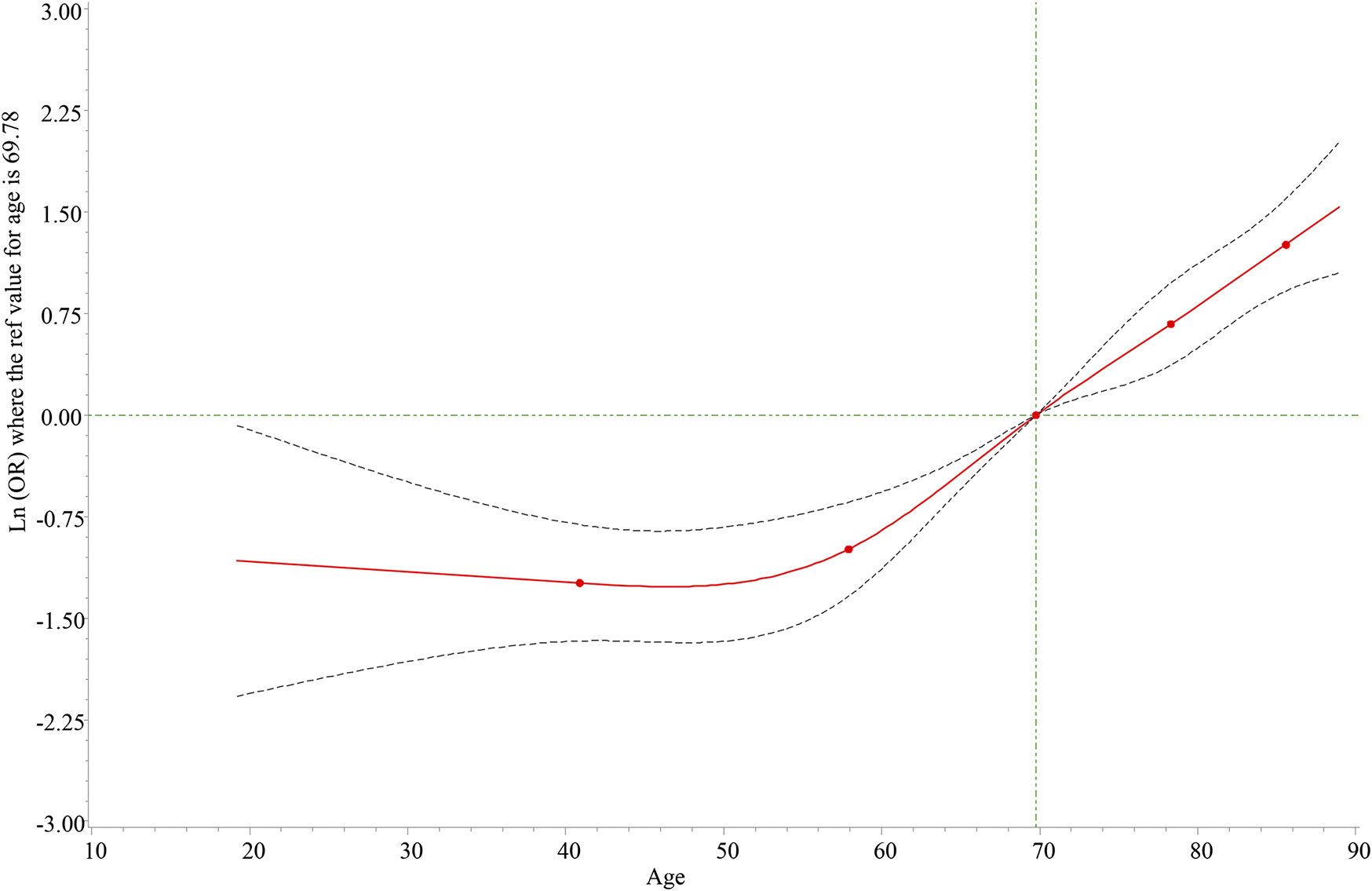
Dose-response relationship between age and death risk. The solid line represents the odds ratios, and the dotted line represents the 95% confidence interval.
TABLE 3
| Aneurysm locations | Variable | Sample size | <60 years | P | Sample size | ≥60 years | P |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||||
| Abdominal aortic aneurysm | Non-hyperlipidemia | 40 | Reference | 471 | Reference | ||
| Hyperlipidemia | N/A | 0.48 (0.36–0.65) | <0.001 | ||||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | N/A | 0.75 (0.49–1.15) | 0.191 | ||||
| Thoracic aortic arch aneurysm | Non-hyperlipidemia | 151 | Reference | 280 | Reference | ||
| Hyperlipidemia | 7.95 (0.91–69.51) | 0.061 | 0.57 (0.33–0.98) | 0.042 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 0.83 (0.06–12.63) | 0.894 | 0.82 (0.41–1.66) | 0.583 | |||
| Cerebral aneurysm | Non-hyperlipidemia | 172 | Reference | 142 | Reference | ||
| Hyperlipidemia | 0.81 (0.15–4.38) | 0.802 | 0.33 (0.11–1.02) | 0.054 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 3.94 (0.30–52.60) | 0.299 | 0.48 (0.11–2.00) | 0.310 | |||
| Others | Non-hyperlipidemia | 104 | Reference | 285 | Reference | ||
| Hyperlipidemia | 2.55 (0.16–41.06) | 0.510 | 0.46 (0.30–0.70) | <0.001 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 632.62 (9.73–41126.69) | 0.003 | 0.55 (0.31–0.96) | 0.036 | |||
Subgroup analysis was performed based on the age and aneurysm location.
HR: hazard ratio; CI: confidence interval; NA: the sample size was insufficient to fit.
Others: include aneurysm of the artery of the lower extremity, aneurysm of the artery of the neck, aneurysm of the artery of the upper extremity, aneurysm of coronary vessels, aneurysm of the heart (wall), aneurysm of the iliac artery, aneurysm of other specified arteries, aneurysm of other visceral arteries, aneurysm of the pulmonary artery, aneurysm of the renal artery, aneurysm of the subclavian artery, aortic aneurysm of the unspecified site without a mention of rupture, other aneurysm of the heart, and thoracoabdominal aneurysm without a mention of rupture.
TABLE 4
| Aneurysm location | Variable | Sample size | Male | P | Sample size | Female | P |
|---|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | ||||||
| Abdominal aortic aneurysm | Non-hyperlipidemia | 358 | Reference | 153 | Reference | ||
| Hyperlipidemia | 0.40 (0.27–0.58) | <0.001 | 0.48 (0.27–0.87) | 0.015 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 0.83 (0.58–1.44) | 0.504 | 0.78 (0.36–1.70) | 0.535 | |||
| Thoracic aortic arch aneurysm | Non-hyperlipidemia | 281 | Reference | 150 | Reference | ||
| Hyperlipidemia | 1.18 (0.61–2.25) | 0.626 | 0.19 (0.06–0.61) | 0.006 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 1.49 (0.67–3.33) | 0.331 | 0.35 (0.07–1.83) | 0.214 | |||
| Cerebral aneurysm | Non-hyperlipidemia | 99 | Reference | 215 | Reference | ||
| Hyperlipidemia | 0.23 (0.02–3.11) | 0.266 | 0.63 (0.23–1.78) | 0.385 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 0.51 (0.03–7.89) | 0.629 | 0.64 (0.18–2.27) | 0.485 | |||
| Others | Non-hyperlipidemia | 230 | Reference | 159 | Reference | ||
| Hyperlipidemia | 0.65 (0.38–1.12) | 0.121 | 0.40 (0.22–0.76) | 0.005 | |||
| Non-hypercholesterolemia | Reference | Reference | |||||
| Hypercholesterolemia | 0.91 (0.48–1.75) | 0.787 | 0.50 (0.18–1.36) | 0.174 | |||
Subgroup analysis was performed based on the gender and aneurysm location.
HR: hazard ratio; CI: confidence interval; Others: include aneurysm of the artery of the lower extremity, aneurysm of the artery of the neck, aneurysm of the artery of the upper extremity, aneurysm of coronary vessels, aneurysm of the heart (wall), aneurysm of the iliac artery, aneurysm of other specified arteries, aneurysm of other visceral arteries, aneurysm of the pulmonary artery, aneurysm of the renal artery, aneurysm of the subclavian artery, aortic aneurysm of the unspecified site without a mention of rupture, other aneurysm of the heart, and thoracoabdominal aneurysm without a mention of rupture.
Discussion
Aneurysms are generally asymptomatic and are not diagnosed until a serious complication occurs, such as aortic rupture or dissection (Pinard et al., 2019). It is reported that there were nearly 9,000 deaths annually caused by aneurysms, and ruptured abdominal aortic aneurysms have been the 13th leading cause of death in the United States, which posed a health crisis and economic burden for most families (Sen et al., 2021). Nowadays, deaths from aneurysms have attracted widespread attention. Several studies have also proposed that these deaths can be prevented if the patients at risk could be identified and through appropriate aneurysm management (Yiu and Cheng, 2016; Algra et al., 2019). In this retrospective cohort study, we reported the relationships between hyperlipidemia, hypercholesterolemia, and death risk in patients diagnosed with aneurysms, and expounded that hyperlipidemia could decrease the death risk. More importantly, we explored the association of hyperlipidemia, hypercholesterolemia, and death based on the aneurysm location, age, and gender differences.
Our study stated that hyperlipidemia was a protective factor against death among patients diagnosed with aneurysms, which was supported by previous research studies (Cheng et al., 2019; Huber et al., 2019). A meta-analysis also suggested that hyperlipidemia may significantly decrease the risk of cerebral aneurysm rupture, and the benefits appear to be independent of statin therapy (Cheng et al., 2019). This may be attributed to the direct vascular effects of hyperlipidemia on the aortic wall, thereby impeding the progression of an aneurysm (Zhang et al., 2015; Huang et al., 2018). In addition, some studies also showed that hyperlipidemia’s protective effect may be related to statin therapy (Salata et al., 2018; Xiong et al., 2022). However, the specific protective mechanism remains unclear and needs to be further explored. In the study by Huang, et al., they found that males were associated with a higher risk in the onset and progression of abdominal aortic aneurysm, and men had a higher risk of death from rupture and vasodilation than women after surgery (Huang et al., 2016). In addition, Mahaney and co-workers also found that the morbidity and mortality of aneurysm patients during surgery and hospitalization increased with age (Mahaney et al., 2014). These findings suggest that gender and age differences need to be considered when investigating the relation of hyperlipidemia and hypercholesterolemia, and the risk of death in patients with aneurysms. Interestingly, in the present study, we found different relationships between hyperlipidemia, hypercholesterolemia, and death based on the aneurysm location, age, and gender. To the best of our knowledge, no relevant research has reported this result to date.
Specifically speaking, our study showed that hyperlipidemia was advantageous for the prognosis for patients with abdominal aortic aneurysms aged ≥60 years or female patients with thoracic aortic arch aneurysms aged ≥60 years. Although most studies have reported a favorable effect of hyperlipidemia on the prognosis of aneurysm patients, this study further demonstrated that the relationship between hyperlipidemia and the risk of death for patients diagnosed with aneurysms was significantly associated with age, gender, and aneurysm location. Noteworthily, in terms of the relationship between hypercholesterolemia and risk of death for patients with aneurysms, we found that there was no statistically significant difference, and the reason might be associated with the relatively small sample size. Although the aforementioned guesses may support our findings, we still lack direct experimental evidence. Our conclusion needs to be confirmed by more related studies.
As far as we know, this was the first detailed cohort study regarding the association between hyperlipidemia, hypercholesterolemia, and death based on the aneurysm location, age, and gender. We believed the findings could provide an early warning for clinicians to consider the difference in age, gender, and aneurysm location in assessing the death risk of aneurysms. However, our study was inevitably linked with some limitations. First, there was a relatively small sample size in the study, which might have limited the statistical power, but our study has a long enough follow-up period in investigating the association. Second, due to all data being derived from the MIMIC-III database, we did not collect data related to the surgical treatment, which might also be responsible for the different associations between hyperlipidemia, hypercholesterolemia, and the risk of death in aneurysm populations (Huber et al., 2019). Third, due to the retrospective nature of this study, potential subjective biases may occur during data collection. More studies are needed to explore the association.
Conclusion
In conclusion, our study indicated that the relationship between hyperlipidemia, hypercholesterolemia, and the risk of death for patients diagnosed with aneurysms was significantly associated with age, gender, and aneurysm location, which implied that future research practices and guidelines need to consider the difference in age, gender, and aneurysm location in assessing and treating aneurysms.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found in the MIMIC-III database, https://mimic.physionet.org/iii/.
Ethics statement
Ethical approval was not provided for this study on human participants because of the public availability of the MIMIC-III database. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DD and YP designed the study. DD wrote the manuscript. YY and GJ collected, analyzed, and interpreted the data. YP critically reviewed, edited, and approved the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1081395/full#supplementary-material
References
1
Algra A. M. Lindgren A. Vergouwen M. D. I. Greving J. P. van der Schaaf I. C. van Doormaal T. P. C. et al (2019). Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: A systematic review and meta-analysis. JAMA Neurol.76 (3), 282–293. 10.1001/jamaneurol.2018.4165
2
Boese A. C. Chang L. Yin K. J. Chen Y. E. Lee J. P. Hamblin M. H. (2018). Sex differences in abdominal aortic aneurysms. Am. J. Physiol. Heart Circ. Physiol.314 (6), H1137–h1152. 10.1152/ajpheart.00519.2017
3
Chen H. Chen Y. Wu W. Chen Z. Cai Z. Chen Z. et al (2020). Prolonged hyperlipidemia exposure increases the risk of arterial stiffness in young adults: A cross-sectional study in a cohort of Chinese. BMC Public Health20 (1), 1091. 10.1186/s12889-020-09211-5
4
Cheng W. Jia X. Li J. Cheng W. Liu Z. Lin Z. et al (2019). Relationships of statin therapy and hyperlipidemia with the incidence, rupture, postrepair mortality, and all-cause mortality of abdominal aortic aneurysm and cerebral aneurysm: A meta-analysis and systematic review. J. Cardiovasc Pharmacol.73 (4), 232–240. 10.1097/FJC.0000000000000653
5
Deery S. E. Soden P. A. Zettervall S. L. Shean K. E. Bodewes T. C. F. Pothof A. B. et al (2017). Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J. Vasc. Surg.65 (4), 1006–1013. 10.1016/j.jvs.2016.08.100
6
El-Tantawy W. H. Temraz A. (2019). Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem.125 (2), 128–135. 10.1080/13813455.2018.1441315
7
He N. Ye H. (2020). Exercise and hyperlipidemia. Adv. Exp. Med. Biol.1228, 79–90. 10.1007/978-981-15-1792-1_5
8
Huang C. K. Lee S. O. Chang E. Pang H. Chang C. (2016). Androgen receptor (AR) in cardiovascular diseases. J. Endocrinol.229 (1), R1–r16. 10.1530/JOE-15-0518
9
Huang Q. Yang H. Lin Q. Hu M. Meng Y. Qin X. (2018). Effect of statin therapy on survival after abdominal aortic aneurysm repair: A systematic review and meta-analysis. World J. Surg.42 (10), 3443–3450. 10.1007/s00268-018-4586-x
10
Huber T. C. Keefe N. Patrie J. Tracci M. C. Sheeran D. Angle J. F. et al (2019). Predictors of all-cause mortality after endovascular aneurysm repair: Assessing the role of psoas muscle cross-sectional area. J. Vasc. Interv. Radiol.30 (12), 1972–1979. 10.1016/j.jvir.2019.04.032
11
Jeon-Slaughter H. Krishnamoorthi H. Timaran D. Wall A. Ramanan B. Banerjee S. et al (2019). Effects of abdominal aortic aneurysm size on mid- and long-term mortality after endovascular aneurysm repair. J. Endovasc. Ther.26 (2), 231–237. 10.1177/1526602819829901
12
Johnson A. E. Pollard T. J. Shen L. Lehman L. W. Feng M. Ghassemi M. et al (2016). MIMIC-III, a freely accessible critical care database. Sci. Data3, 160035. 10.1038/sdata.2016.35
13
Kang H. Feng X. Zhang B. Guo E. Wang L. Qian Z. et al (2017). The siesta habit is associated with a decreased risk of rupture of intracranial aneurysms. Front. Neurol.8, 451. 10.3389/fneur.2017.00451
14
Liu B. Granville D. J. Golledge J. Kassiri Z. (2020). Pathogenic mechanisms and the potential of drug therapies for aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol.318 (3), H652–h670. 10.1152/ajpheart.00621.2019
15
Long J. Xie X. Xu D. Huang C. Liu Y. Meng X. et al (2021). Association between red blood cell distribution width-to-albumin ratio and prognosis of patients with aortic aneurysms. Int. J. Gen. Med.14, 6287–6294. 10.2147/IJGM.S328035
16
Mahaney K. B. Brown R. D. Jr. Meissner I. Piepgras D. G. Huston J. 3rd Zhang J. et al (2014). Age-related differences in unruptured intracranial aneurysms: 1-year outcomes. J. Neurosurg.121 (5), 1024–1038. 10.3171/2014.6.JNS121179
17
Pinard A. Jones G. T. Milewicz D. M. (2019). Genetics of thoracic and abdominal aortic diseases. Circ. Res.124 (4), 588–606. 10.1161/CIRCRESAHA.118.312436
18
Salata K. Syed M. Hussain M. A. de Mestral C. Greco E. Mamdani M. et al (2018). Statins reduce abdominal aortic aneurysm growth, rupture, and perioperative mortality: A systematic review and meta-analysis. J. Am. Heart Assoc.7 (19), e008657. 10.1161/JAHA.118.008657
19
Schmitz-Rixen T. Keese M. Hakimi M. Peters A. Böckler D. Nelson K. et al (2016). Ruptured abdominal aortic aneurysm-epidemiology, predisposing factors, and biology. Langenbecks Arch. Surg.401 (3), 275–288. 10.1007/s00423-016-1401-8
20
Sen I. Franco-Mesa C. Erben Y. DeMartino R. R. (2021). Abdominal aortic and visceral artery aneurysms. Cardiol. Clin.39 (4), 517–525. 10.1016/j.ccl.2021.06.004
21
Sweeting M. J. Thompson S. G. Brown L. C. Powell J. T. RESCAN collaborators (2012). Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br. J. Surg.99 (5), 655–665. 10.1002/bjs.8707
22
Xiong X. Wu Z. Qin X. Huang Q. Wang X. Qin J. et al (2022). Meta-analysis suggests statins reduce mortality after abdominal aortic aneurysm repair. J. Vasc. Surg.75 (1), 356–362.e4. 10.1016/j.jvs.2021.06.033
23
Yao Y. S. Li T. D. Zeng Z. H. (2020). Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis.19 (1), 23. 10.1186/s12944-019-1171-8
24
Yiu R. S. Cheng S. W. (2016). Natural history and risk factors for rupture of thoracic aortic arch aneurysms. J. Vasc. Surg.63 (5), 1189–1194. 10.1016/j.jvs.2015.12.043
25
Zhang W. Li u. Z. Liu C. (2015). Effect of lipid-modifying therapy on long-term mortality after abdominal aortic aneurysm repair: A systemic review and meta-analysis. World J. Surg.39 (3), 794–801. 10.1007/s00268-014-2858-7
Summary
Keywords
hyperlipidemia, aneurysm death, age, gender, aneurysm location
Citation
Ding D, Yang Y, Jiang G and Peng Y (2023) Relationship between hyperlipidemia and the risk of death in aneurysm: a cohort study on patients of different ages, genders, and aneurysm locations. Front. Physiol. 14:1081395. doi: 10.3389/fphys.2023.1081395
Received
31 October 2022
Accepted
30 May 2023
Published
20 June 2023
Volume
14 - 2023
Edited by
Daniel Bia, Universidad de la República, Uruguay
Reviewed by
Shoudong Guo, Weifang Medical University, China
Lucía Florio, Hospital of Clinics Dr. Manuel Quintela, Uruguay
Dhafer Al-koofee, University of Kufa, Iraq
Updates
Copyright
© 2023 Ding, Yang, Jiang and Peng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhui Peng, yanhuipenghbmu@outlook.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.