- 1Maternal and Fetal Health Research Centre, Division of Developmental Biology and Medicine, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom
- 2Saint Mary’s Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
- 3Manchester Academic Health Science Centre, Manchester, United Kingdom
Introduction: This study aimed to investigate the acute effects of statins on maternal and fetoplacental vascular reactivity in vessels from pregnancies affected by pre-eclampsia (PE), a leading cause of maternal and fetal morbidity and mortality. Statins have been proposed as a candidate therapy due to their pleiotropic effects but evidence of statins’ ability to ameliorate the observed endothelial dysfunction in PE is lacking.
Methods: Human chorionic plate arteries (CPAs) and omental arteries (OAs) from normal and PE pregnancies were mounted on a wire myograph. Contraction was assessed with KPSS and the thromboxane mimetic U46619. Arteries were incubated for 2 h with 1 µM or 10 µM pravastatin, pitavastatin or simvastatin (pitavastatin only in OAs). U46619 dose–response curves were repeated or dose-response curves with NO-donor SNP or endothelium-dependent bradykinin (BK) performed following U46619 pre-constriction.
Results: CPAs from normal and PE pregnancies showed similar responses following exposure to the vasoconstrictive agent U46619 and the relaxatory agent SNP. Short-term exposure to pravastatin, simvastatin and pitavastatin did not cause detrimental effects on CPA reactivity. Acute exposure of OAs from PE pregnancies to pitavastatin (1 µM) did not reduce U46619-mediated contraction or enhance BK-mediated relaxation of vessels although in this study ex vivo endothelial function of OAs from PE pregnancies was not different to those in normotensive pregnancy pre incubation.
Discussion: In conclusion, this study did not demonstrate an effect on vascular reactivity of maternal systemic or fetoplacental arteries following acute treatment of statins. Future studies investigating the effect of longer-term statin exposure on maternal and fetoplacental vascular reactivity may help towards treatment strategies for vascular dysfunction in PE-affected patients.
1 Introduction
Preeclampsia (PE) is defined by hypertension at ≥ 20 weeks gestation and proteinuria or evidence of placental, renal, hepatic, or hematological dysfunction (Khan et al., 2020). PE affects 4%–5% of pregnancies and is a major contributor to maternal and fetal morbidity and mortality (Phipps et al., 2019), including increased risk of preterm birth and fetal growth restriction (FGR) (Haddad et al., 2004; Madazli et al., 2014; Rezk et al., 2015). Additionally, women diagnosed with PE have an increased risk of cardiovascular disease (CVD) later in life (Kuklina et al., 2009; Chen et al., 2014; Breetveld et al., 2014; Wu et al., 2017; Leon et al., 2019; Ormesher et al., 2022). Meta-analyses indicate an estimated 3.7-fold increased risk for hypertension and 1.8-fold for stroke in women previously diagnosed with preeclampsia (Bellamy et al., 2007).
PE is associated with placental dysfunction and placental ischemia (Bakrania et al., 2020; Chappell et al., 2021), and severe early-onset cases of PE are linked to inadequate spiral artery remodeling (Labarrere et al., 2017; Staff et al., 2020). In addition, PE is associated with an angiogenic imbalance in the maternal circulation (Ahmad and Ahmed, 2004; Phipps et al., 2019; Kluivers et al., 2023), ultimately resulting in maternal endothelial dysfunction (Maynard et al., 2003; Levine et al., 2004; Possomato-Vieira and Khalil, 2016; Tomimatsu et al., 2019; McElwain et al., 2020).
There are no effective treatments for PE. Clinical management following diagnosis currently focuses on the use of antihypertensive medications such as labetalol and nifedipine (NICE, 2023) and increased fetal monitoring. The only current treatment option for clinicians is delivery, often preterm, of the fetus and placenta. While this intervention halts the clinical progression of PE, there remains an urgent need for effective maternal treatment as an alternative to preterm birth (Berzan et al., 2014; Rana et al., 2019; Maayeh and Costantine, 2020).
Several drugs have been suggested for the treatment of PE, including statins, which are HMG-CoA inhibitors primarily used to reduce cholesterol levels (Phipps et al., 2019). Statin use in pregnancy was previously contraindicated, but progress has been made now that the FDA has requested removal of the “Pregnancy Category X” label for statins (US Food and Drug Administration, 2024). This was mainly due to previous results from systematic reviews and cohort studies linking increased rates of fetal abnormalities with statin exposure (Bateman et al., 2015; Edison and Muenke, 2004; Pollack et al., 2005; Taguchi et al., 2008; Winterfield et al., 2013). Although these studies are limited in their size and scope, current guidance does not recommend statin use during pregnancy.
Several clinical and preclinical studies using pravastatin have been performed, demonstrating lower rates of preeclampsia and improvement in the sFlt-1/PlGF ratio, yet showing little or no definitive evidence of benefit. Many of the clinical trials were underpowered (Ahmed, 2011; Brownfoot et al., 2016; Costantine et al., 2016; Costantine et al., 2021; Döbert et al., 2021; Mendoza et al., 2021). These studies have shown no evidence of detrimental effects of statins on the placenta, but there are limited in vivo data (Zarek et al.,2013).
Currently, there is a lack of knowledge on the effect of statins on vascular reactivity in either the maternal systemic arteries or the placental arteries. Various statins have been explored to assess the biological plausibility of statins for treatment in preeclampsia. These include lovastatin (Minsker et al., 1983), simvastatin (Cudmore et al., 2007; Rossoni et al., 2011), and pravastatin (Ahmed et al., 2010; Costantine et al., 2011; Kumasawa et al., 2010), although Rossoni and colleagues only investigated the acute effects of simvastatin on isolated mesenteric resistance arteries in male Wistar rats. Three statins were chosen for the current study: pravastatin, pitavastatin, and simvastatin. These were used to assess chorionic plate artery (CPA) reactivity, while pitavastatin alone was used for omental arteries (OAs) from pregnancies complicated by PE.
The different metabolic states of these three statins made them useful for comparison of pleiotropic effects on vascular function. Pitavastatin has high bioavailability in the bloodstream due to its avoidance of the first-pass metabolism route in the liver. Pitavastatin has never been used in pregnancy studies previously and is therefore an exciting therapeutic candidate to explore. In the current study, OAs and CPAs were used as models of the maternal systemic and fetoplacental vasculature, respectively. CPAs are resistance arteries, and they, along with other fetoplacental small arteries, play an important role in regulating vascular tone in the fetoplacental circulation (Mulvany and Aalkjaer, 1990).
We thus hypothesized that short-term (2 h) exposure to statins would improve the vascular reactivity of omental arteries from pregnancies complicated by preeclampsia and have no detrimental effects on fetoplacental CPA function. The aims of the study were 1) to assess whether vascular reactivity of OAs or CPAs was altered in PE versus normal pregnancies and 2) to assess whether short-term statin exposure affects vascular function in OAs and CPAs from both uncomplicated pregnancies and those complicated by PE.
2 Materials and methods
2.1 Ethical approval
All work herein was carried out following Research Ethics Committee approval (REC number 15/NW/0829). Studies were carried out in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all women prior to the collection of placental and omental samples.
2.2 Inclusion/exclusion criteria
Placental samples were taken from women with a normal pregnancy (normal defined as an uncomplicated pregnancy birthing ≥37 weeks with an appropriately grown fetus; N = 21) or a pregnancy complicated by PE (PE as defined by ISSHP guidelines (Brown et al., 2018; Duhig et al., 2019); N = 14). Following a Cesarean section, omental samples were taken from women with a normal pregnancy (N = 34) or a pregnancy complicated by PE (N = 9).
To control for women in the obese category, a BMI cut-off of <35 kg/m2 was used, and to control for women of advanced maternal age (aged 35 years and above), a cut-off of <40 years was utilized for uncomplicated pregnancies. Normotensive women were excluded if they had a BMI ≥35 kg/m2 or age ≥40 years, or they presented with maternal diabetes, multiple pregnancy, hyperthyroidism, or had other existing medical conditions or complications of pregnancy. Women with PE were excluded if they presented with diabetes, hyperthyroidism, multiple pregnancy, or if they had sexually transmitted diseases. Samples were considered “normal” and included if the individualized birthweight ratio (IBR) was between the 10th and the 100th centiles. IBR was calculated using the UK BulkCentileCalculator GROW 8.04 (Perinatal, 2019). Large-for-gestational age fetuses (>90th centile (Pasupathy et al., 2012)) were included where maternal characteristics were normal.
Unless otherwise stated, all chemicals and statins were obtained from Sigma–Aldrich (Poole, Dorset, United Kingdom) or BDH (Poole, Dorset, United Kingdom).
2.3 Sample collection
Omental biopsies ∼2 × 2 cm in size were excised from the greater omentum in the abdomen of women at the time of Cesarean section from normal pregnancies. Following collection, fat and scar tissue were removed, and OAs (138–521 µM) were isolated from the omental biopsy and placed directly into ice-cold physiologic salt solution (PSS). Resistance arteries were identified and mounted under a microscope onto a DMT multi-chamber 620 wire myograph using small dissecting scissors and forceps. For placentae, biopsies were taken and placed into ice-cold PSS, and small chorionic plate arteries (CPAs) were identified under the microscope and dissected free from surrounding connective tissue (112–501 µM) within 20 min of delivery, before being mounted onto a wire myograph.
2.4 Wire myography
Short (∼2 mm long) sections of omental arteries (OAs) and CPAs were mounted onto a Multi Myograph System 620M (Wareing et al., 2002). Initially, the bath contained 6 mL of PSS (in mmol 1–1; 127.76 NaCl, 25 NaHCO3, 4.69 KCl, 2.4 MgSO4, 1.6 CaCl2, 1.18 KH2PO4, 6.05 glucose, 0.034 EDTA; pH 7.4), warmed to 37°C, and gassed with 20% air/5% CO2 (OA) and 5% air/5% CO2 (CPA). Mounted OAs were normalized to 0.9L13.3 kPa while CPAs were normalized to 0.9L5.1 kPa following the method described in detail elsewhere (Wareing et al., 2002).
2.5 Contraction/relaxation responses for omental arteries/CPAs
Following equilibration, contractile viability was assessed with 2x high potassium PSS (KPSS; 120 mM KCl, equimolar substitution for NaCl) exposures with 10–20-min intervals in between. This was followed by concentration–response constriction curves to U46619 for OA/CPA (incremental doses of 10–10–10–5.7 M; 2 min intervals). Vessels (OAs) were washed to baseline tension with PSS and then incubated for 2 h with either 1 µM or 10 µM pitavastatin, 1 µM simvastatin, or 1 µM or 10 µM pravastatin (CPAs), with an appropriate control (1 µM DMSO or water) running in parallel. Following incubation, constriction to U46619/relaxation to bradykinin (BK; 10–10–10–5 M) was performed for OAs, and constriction to U44619/relaxation to sodium nitroprusside [SNP; (10–10–10–5 M)] was performed for CPAs. U44619-mediated contraction and basal tone pre- and post-statin were assessed to confirm if any statin-related effects were observed.
2.6 Statistical analyses
The Kolmogorov–Smirnov test was used to assess whether the data fitted a Gaussian distribution. This is used for datasets where only one comparison was made; for example, basal tone pre- and post-statin incubation and KPSS contraction pre- and post-statin incubation. Demographics were compared using the Mann–Whitney U test and Fisher’s exact test for categorical data, that is, smoking, parity, and sex of fetus, while the chi-squared test was used for ethnicity. U46619-induced constriction and SNP/bradykinin-induced relaxation of OAs and CPAs from women with normal and pathological pregnancies post-statin incubation were assessed using repeated-measures two-way ANOVA. Data are expressed as mean ± S.E.M, median ± SD, or median (range) depending on the outcome of the normality tests. The number of observations (n = arteries from N patients) is given in parentheses. Statistical significance was considered to be p < 0.05.
3 Results
3.1 Patient characteristics
Patient demographics for women donating placental and/or omental samples from NP and PE pregnancies are shown in Tables 1, 2. Large-for-gestational age (LGA) fetuses (>90th centile (Pasupathy et al., 2012) were included where maternal characteristics were normal. There were 7/34 LGA fetuses in the normotensive omental group only. Demographic clinical details for the two groups are shown in Tables 1, 2 (samples for placental and omental analyses are from different patients). Blood pressure was taken at booking (approximately 12 weeks of gestation) for both normotensive women and women who ultimately were diagnosed with PE; however, maximal blood pressure near delivery was collected for the PE group only. As expected, maximal recorded systolic (p < 0.01) and diastolic blood pressures (p < 0.05) were significantly higher in the PE group than in NP women (Mann–Whitney U test). Gestational age at delivery (p < 0.0001), birthweight (p < 0.0001), and IBR (p < 0.01, omental samples only) were significantly lower in the PE group than the NP group (Mann–Whitney U test). Furthermore, for women donating placental samples, a significant difference was seen with regard to parity (p < 0.0001) and a lower BW:PW ratio (p < 0.05) in the PE group. Smoking status, parity, and sex of the fetus were also different (Fisher’s exact test). Maternal age, BMI, and gravidity were not different between groups in those individuals donating omental and placental samples, while for omental samples, ethnicity was also significantly different between groups (p < 0.001).
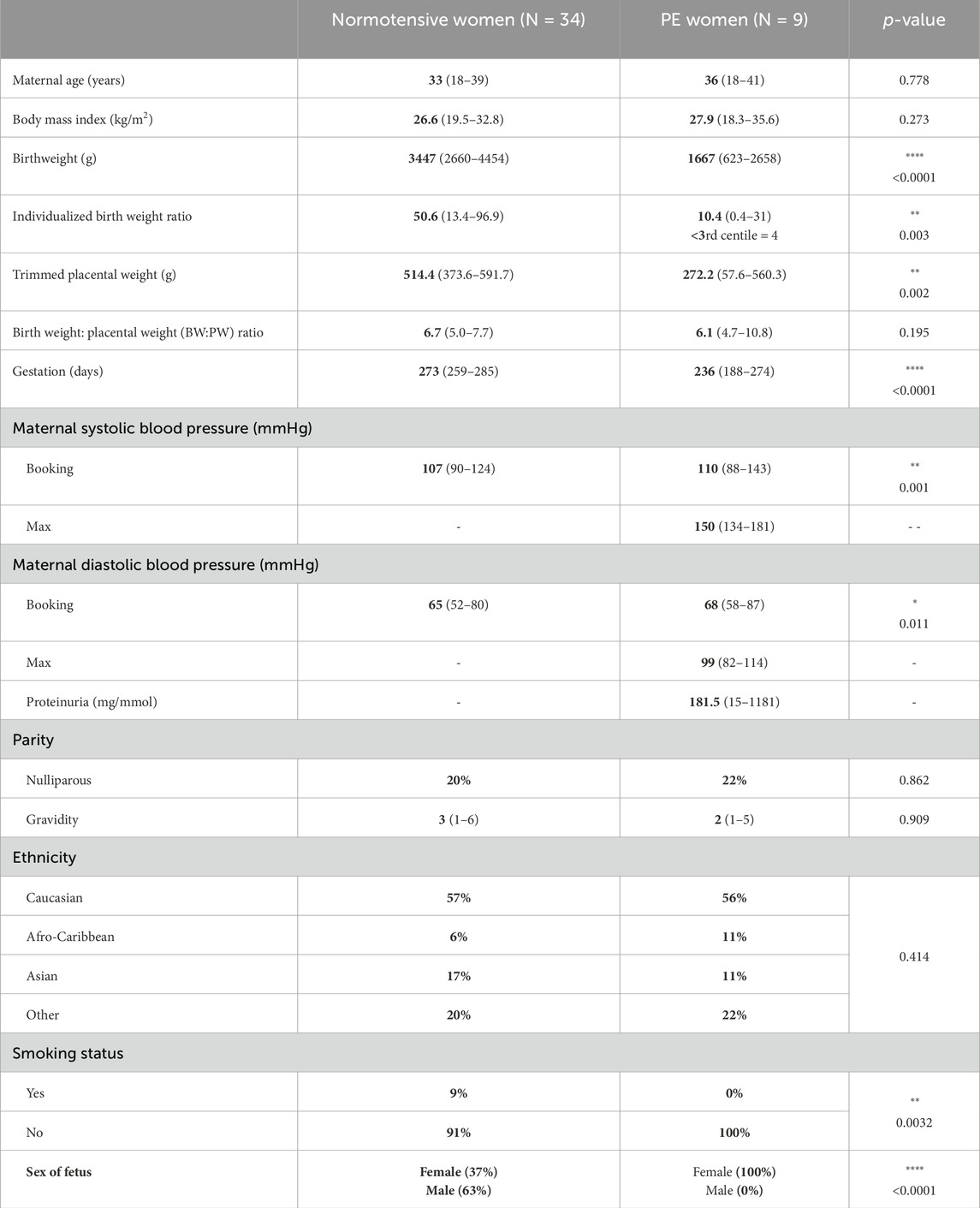
Table 1. The maternal and pregnancy characteristics of women who agreed to the recruitment of omental samples are summarized below. For quantitative non-parametric data, information is presented as median and range. Statistical tests used for numerical data (Mann–Whitney U test) and categorical data (Fisher’s exact test or chi-squared test).
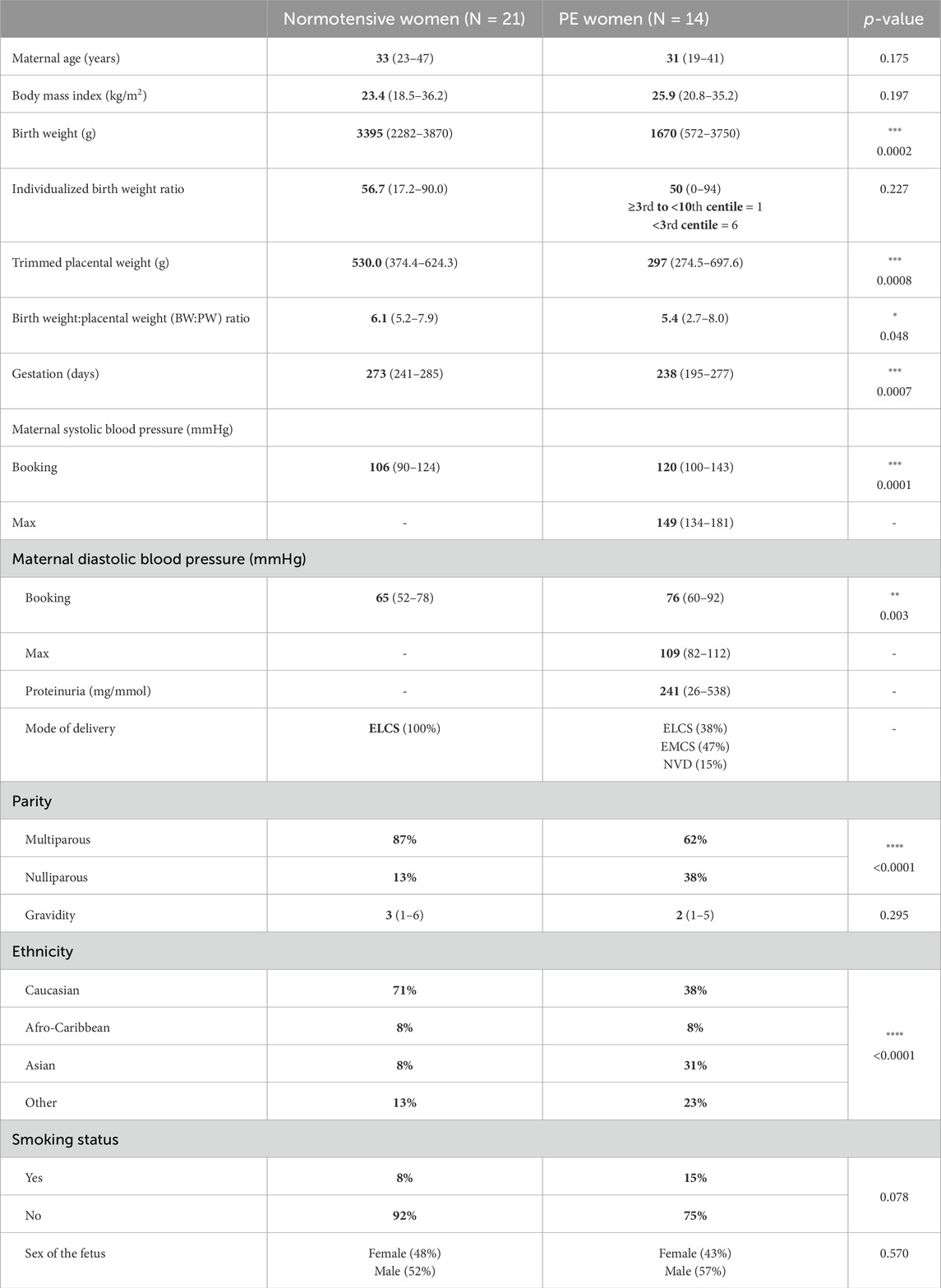
Table 2. The maternal and pregnancy characteristics of women who agreed to the recruitment of placental samples are summarized below. For quantitative nonparametric data, information is presented as median and range. Statistical tests used for numerical data (Mann–Whitney U test) and categorical data (Fisher’s exact test or chi-squared test).
3.2 Assessment of vascular reactivity
The mean diameter of CPAs was not statistically different prior to treatment with statins between normal and PE pregnancies, and diameters were 314 µm [218–485] and 272 µm [173–414], respectively (p = 0.087). The mean diameter of OAs was also not statistically different prior to treatment with statins between normal and PE pregnancies; diameters were 324 µm [173–536] and 333 µm [239–466], respectively (p = 0.781).
3.3 Omental arteries
3.3.1 Assessment of vascular reactivity in normal and PE pregnancies
There was no significant difference in U46619-induced contraction between the NP and PE groups when expressed as tension (kPa) (Figure 1A, p = 0.557), or when U46619 data were expressed as %KPSS = high potassium physiological saline solution (Figure 1B, p = 0.188). When assessing endothelial function pre-incubation, there was significantly less relaxation to bradykinin in OAs from PE versus normal pregnancies (Figure 1D, p = 0.037). Following the 2-h incubation with DMSO, relaxation of OAs to BK was not different between normal or PE OAs (Figure 1C, p = 0.974). A high concentration of U46619 (10–5 M) was used to pre-constrict vessels prior to relaxation.
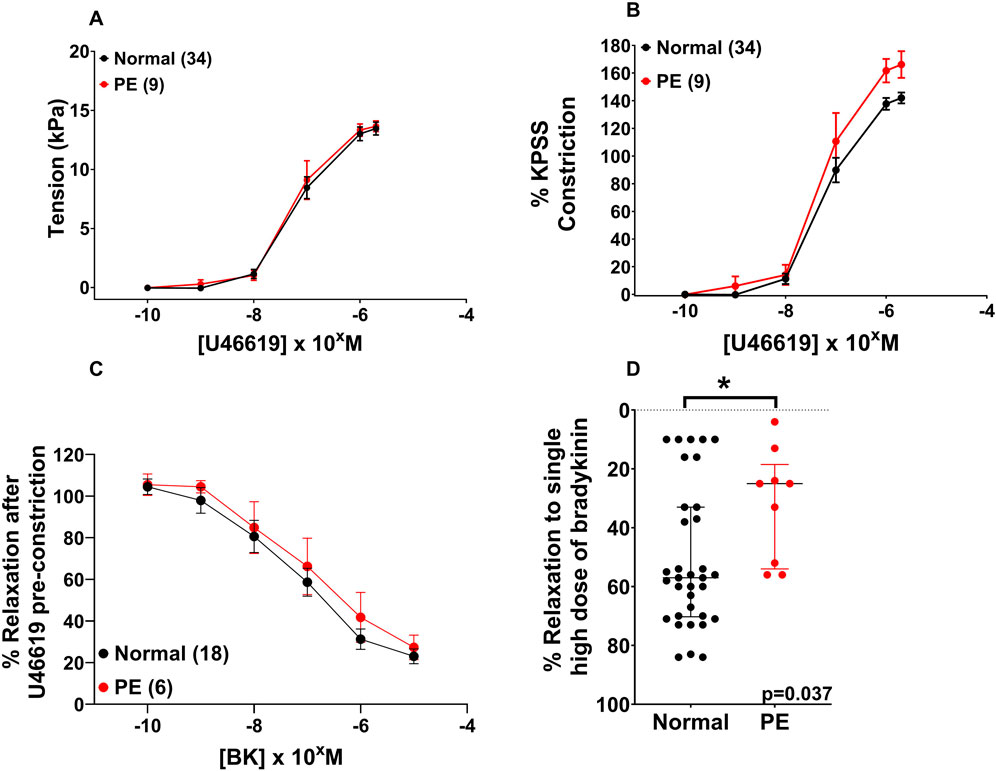
Figure 1. Assessment of vascular reactivity in OAs from normal and PE pregnancies. Assessment of U46619-induced contraction expressed as tension (A) or as %KPSS (B). Relaxation to bradykinin post-incubation (C). Compared to the endothelial function pre-incubation, there was a significant change in endothelial function between the NP and PE groups after relaxation with bradykinin (D). Replicate vessels from the same pregnancy were averaged for each sample. Graphs A and B show the combined pre-incubation U46619 data for normal pregnancy (N = 34) from the pitavastatin (N = 18) and pravastatin (N = 16) experiments. Data are expressed as mean ± SEM; two-way ANOVA (A–C) and as median, IQR with Mann–Whitney test for graph (D).
3.3.2 Effect of statins on vasoconstriction of omental small arteries
Following a 2-h incubation with 1 µM pitavastatin, there was no significant effect on the contraction of OAs to U46619 from normal pregnancies (Figure 2A: DMSO versus pitavastatin, p = 0.980) or from PE pregnancies (Figure 2B: DMSO versus pitavastatin, p = 0.796).
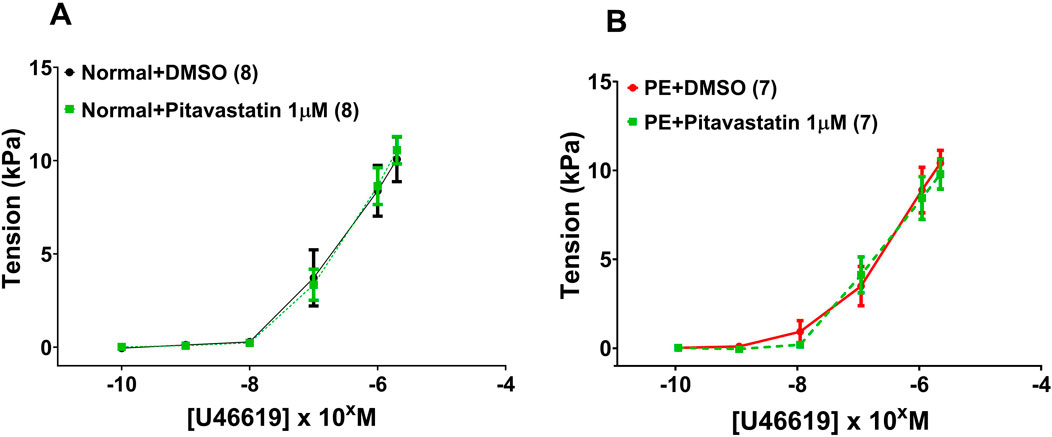
Figure 2. Effect of 2-h incubation with 1 µM pitavastatin on the contraction of OAs from normal pregnancies. Assessment of U46619-induced contraction expressed as tension (kPa) in normal pregnancy (A) and PE pregnancy (B). There was no significant effect on the contraction of OAs. U46619 dose–response curves were compared using two-way ANOVA. Data are expressed as mean ± SEM; number of omental biopsies in parentheses.
3.3.3 Effect of statins on vasodilatation of omental small arteries
Following a 2-h incubation with 1 µM pitavastatin, there was no significant effect on relaxation of OAs to BK in vessels from normal pregnancies (Figure 3A: DMSO versus pitavastatin, p = 0.690) or from PE pregnancies (Figure 3B: DMSO versus pitavastatin, p = 0.963).
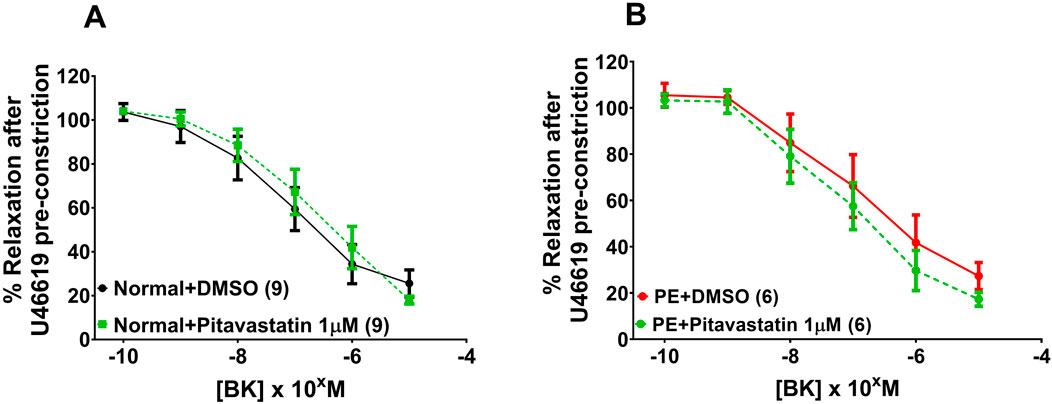
Figure 3. Effect of 2-h statin incubation on relaxation of OAs from normal pregnancies. Following a 2-hr incubation with pitavastatin at 1 µM, there was no significant effect on relaxation of OAs to BK in vessels from normal pregnancies (A) or from PE pregnancies (B). BK dose–response curves were compared using two-way ANOVA. Data are expressed as mean ± SEM; number of omental biopsies in parentheses.
3.4 Chorionic plate arteries
3.4.1 Assessment of CPA vascular reactivity in normal and PE pregnancies
Figure 4 includes all vessels prior to exposure to statin or DMSO. The endothelial-independent SNP dose–response curves shown are for DMSO controls from normal pregnancies and DMSO controls from PE pregnancies (post-incubation). A SNP dose–response curve was not conducted in PSS alone (pre-incubation). Contraction to U46619 expressed as tension (kPa) (Figure 4A, p = 0.700) and %KPSS (Figure 4B, p = 0.930), as well as relaxation of chorionic plate arteries to SNP (Figure 4C, p = 0.623) was not different between CPAs from normal versus PE pregnancies. When DMSO and water controls were separated to assess any differences between them, there was no significant difference in CPA relaxation to SNP between DMSO and water controls from normal pregnancies (p = 0.814) or PE pregnancies (p = 0.909).
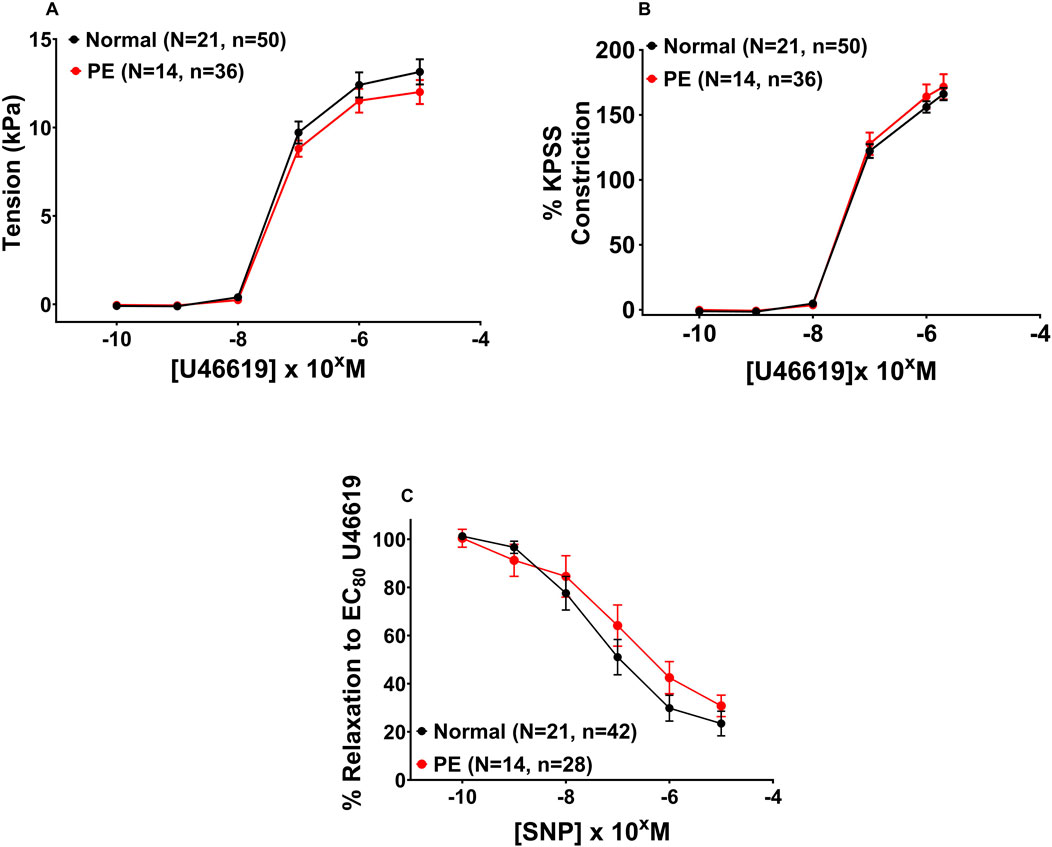
Figure 4. Assessment of vascular reactivity in CPAs from normal and PE pregnancies. Assessment of U46619-induced contraction expressed as tension (A) or as %KPSS (B). Relaxation to SNP (C). There was no significant difference between CPAs from normal versus PE pregnancies. Graph A shows the combined pre-incubation U46619 data for normal pregnancy (N = 21) and PE (N = 14) from pitavastatin, pravastatin, and simvastatin experiments (at all concentrations), while Graph B shows post-incubation CPA data with control groups from PE and normal pregnancy. Replicate vessels from the same pregnancy were averaged for each group. Data are expressed as mean ± SEM, two-way ANOVA.
3.5 Chorionic plate arteries from normal pregnancies
3.5.1 Effect of statins on vasoconstriction of chorionic plate arteries (1 μM and 10 µM)
Following 2-h incubation with either 1 µM pitavastatin (Figure 5A, p = 0.924) or 10 µM pitavastatin (Figure 5C, p = 0.990), CPA contraction to U46619 was not significantly different between the pitavastatin-exposed group versus controls. When U46619 contraction curves were expressed as %KPSS with 1 µM pitavastatin (Figure 5B, p = 0.912), there was again no significant difference between pitavastatin-exposed CPAs and controls, but 10 µM pitavastatin attenuated the contraction of CPAs (Figure 5D, p = 0.026). There was no difference in contraction to U46619 in the pravastatin and simvastatin groups (Supplementary Figure S4).
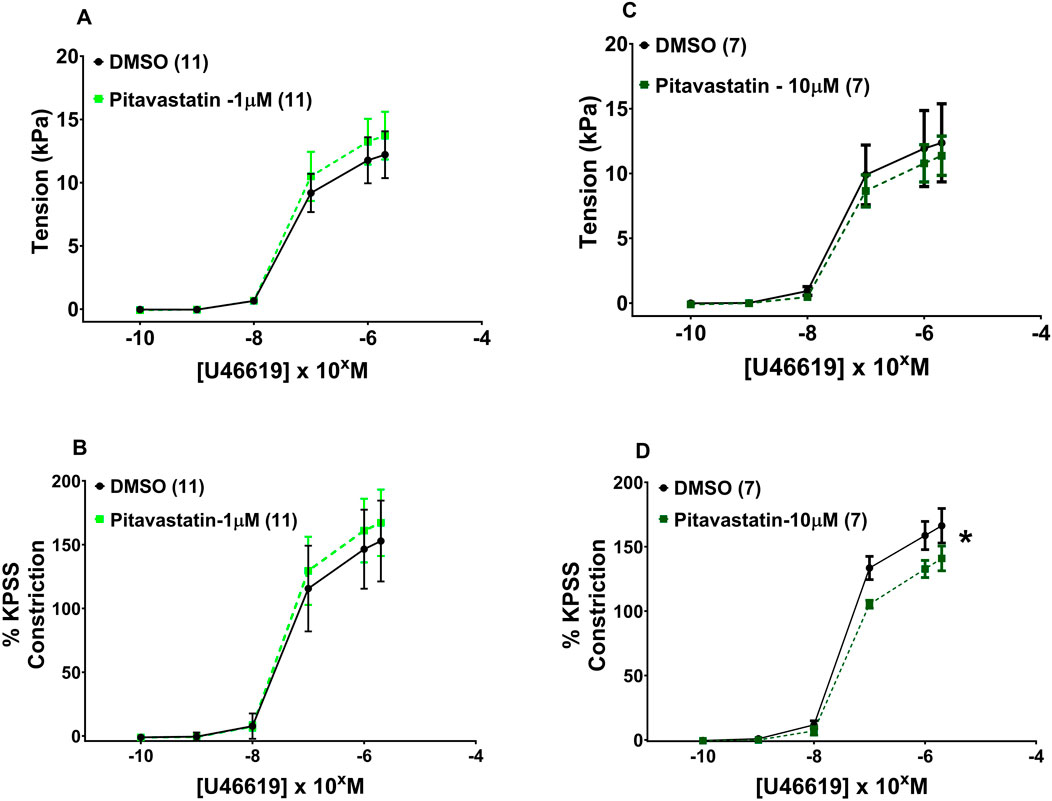
Figure 5. Effect of 2-h statin incubation on the contraction of CPAs from normal pregnancies. Assessment of U46619-induced contraction with either 1 µM pitavastatin (A, B) or 10 µM pitavastatin (C). There was no significant difference between the statin-exposed and control groups. Only when expressed as % KPSS was a significant decrease in contraction seen with 10 µM pitavastatin (D); p < 0.05. U46619 dose–response curves were compared using two-way ANOVA. Data are expressed as mean ± SEM; number of placentas in parentheses.
3.5.2 Effect of statins on vasodilatation of chorionic plate arteries (1 μM and 10 µM)
Following a 2-h incubation with either 1 µM pitavastatin (Figure 6A, p = 0.086) or 10 µM pitavastatin (Figure 6B, p = 0.147), overall CPA relaxation to SNP was not significantly different between the pitavastatin-exposed group versus controls (Figure 6A, p = 0.086), although a trend was seen with 1 µM pitavastatin (p = 0.086). There was no difference in vasodilation to SNP in the pravastatin and simvastatin groups (Supplementary Figure S5).
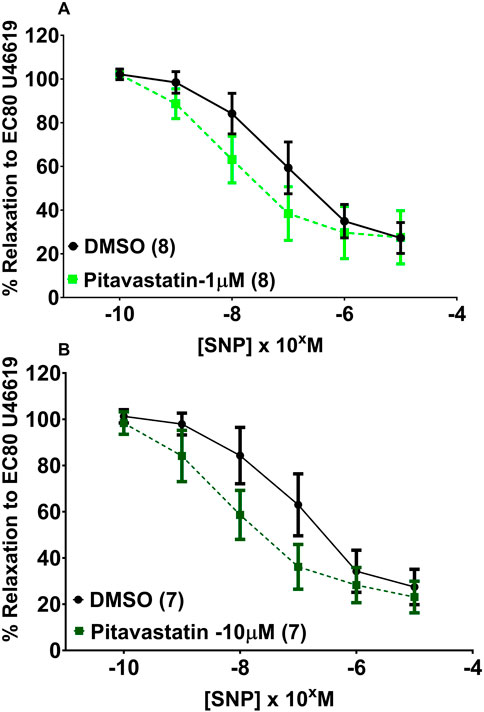
Figure 6. Effect of 2-h pitavastatin incubation on relaxation of CPAs from normal pregnancies. Assessment of CPA relaxation to SNP with either pitavastatin at 1 µM (A) or 10 µM pitavastatin (B). No significant effect was seen between the statin-exposed and control groups; p > 0.05. SNP dose–response curves were compared using two-way ANOVA with Sidak’s post hoc test, where applicable. Data are expressed as mean ± SEM; number of placentas in parentheses.
3.6 Chorionic plate arteries from PE pregnancies
3.6.1 Effect of statins on vasoconstriction of chorionic plate arteries in PE (1 μM and 10 µM)
Following a 2-h incubation with 1 µM pitavastatin (Figure 7A, p = 0.971) and 10 µM pitavastatin (Figure 7C, p = 0.245), CPA contraction to U46619 was not significantly different between the pitavastatin-exposed group versus controls. When U46619 contraction curves were expressed as %KPSS, with 1 µM pitavastatin (Figure 7B, p = 0.891), CPA contraction to U46619 was not significantly different between the pitavastatin-exposed group versus controls. However, with 10 µM pitavastatin, there was a decreased contractile response (Figure 7D, p = 0.0003). There was no difference in vasoconstriction to U46619 in the pravastatin and simvastatin groups (Supplementary Figure S8).
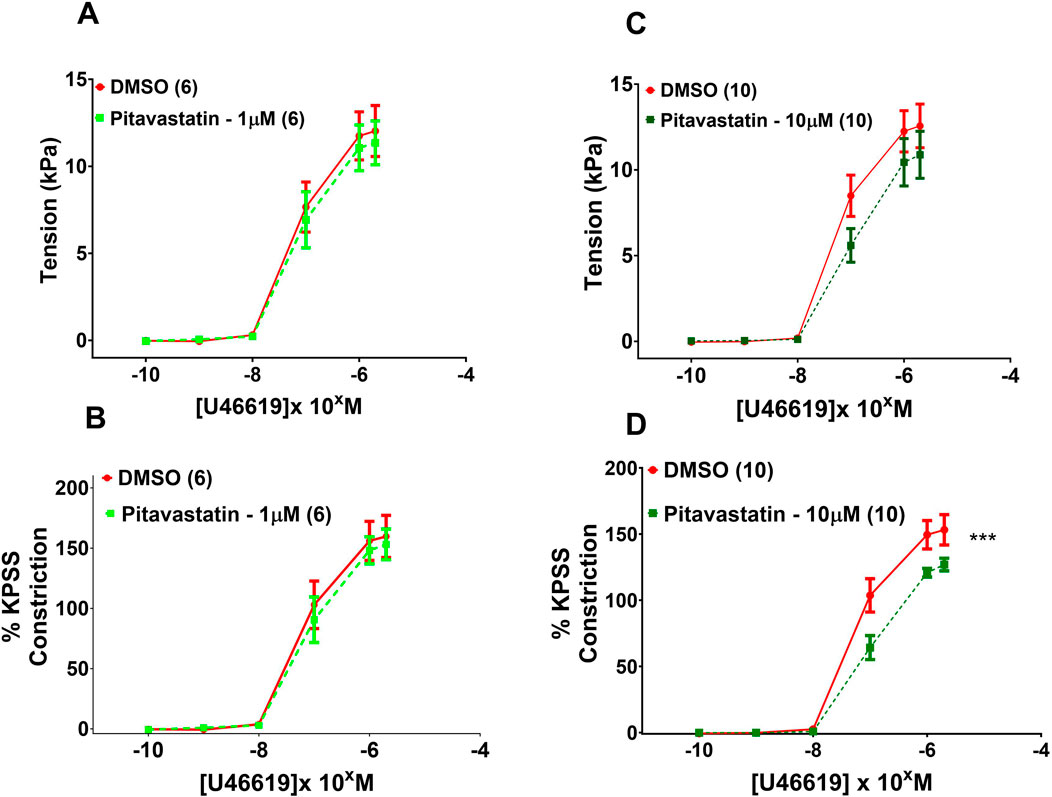
Figure 7. Effect of 2-h statin incubation on contraction of CPAs from PE pregnancies. Assessment of U46619-induced contraction with either 1 µM pitavastatin (A,B) or 10 µM pitavastatin (C). There was no significant effect when expressed as tension (kPa) or as % KPSS; p > 0.05. However, a significant effect was seen for 10 µM pitavastatin (D) when data were expressed as % KPSS; p < 0.05. U46619 dose–response curves were compared using two-way ANOVA with Sidak’s post hoc test, where applicable. Data are expressed as mean ± SEM; number of placentas in parentheses.***p < 0.001.
3.6.2 Effect of statins on vasodilatation of chorionic plate arteries in PE (1 µM)
Following a 2-h incubation with either 1 µM pitavastatin (Figure 8A, p = 0.961) or 10 µM pitavastatin (Figure 8B, p = 0.195), CPA relaxation to SNP was not significantly different between the pitavastatin-exposed group versus controls. There was no difference in CPA relaxation to SNP in the pravastatin and simvastatin groups (Supplementary Figure S9).
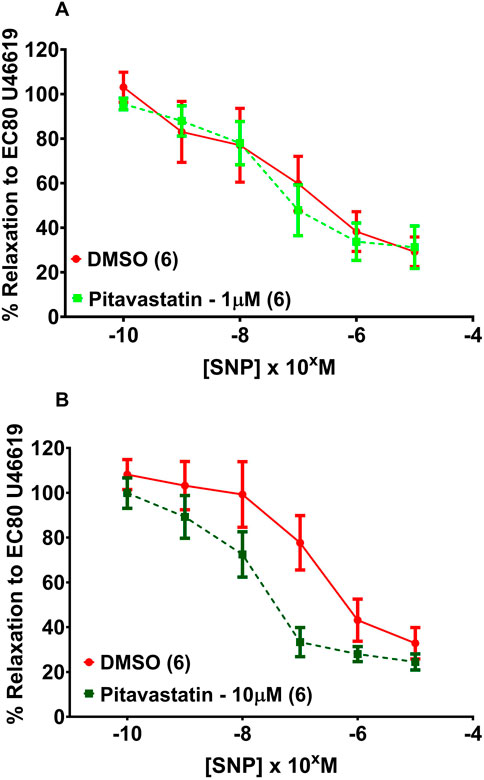
Figure 8. Effect of 2 hr statin incubation on relaxation of CPAs from PE pregnancies. Assessment of CPA relaxation to SNP with either 1 µM pitavastatin (A) or 10 µM pitavastatin (B), there was no significant effect on relaxation between statin-exposed and control groups; p > 0.05. SNP dose response curves were compared using two-way ANOVA. Data are expressed as mean ± SEM; number of placentas in parenthesis.
4 Discussion
The current study demonstrated evidence of endothelial dysfunction in OAs from pregnancies with PE, but short-term pitavastatin exposure (at 1 and 10 µM) had no effect on vascular reactivity in these vessels. Although there was no difference in vasorelaxation or vasoconstriction in CPAs between PE and normal pregnancies, there was no evidence of statins having detrimental effects on vascular reactivity in CPAs, and in fact, 10 µM pitavastatin attenuated U46619-mediated contraction in both PE and normal pregnancies. Whilst these data suggest that statins do not show any deleterious effects on the fetoplacental vasculature, the data also do not support pitavastatin being a potential drug treatment to improve maternal vascular function in preeclampsia.
4.1 Vascular reactivity of omental arteries in normal and PE pregnancies
OAs from women with PE did not show increased U46619-induced contraction compared to OAs from normotensive women. Multiple studies using different vasoconstrictive agents have shown that OAs from PE women showed increased contractile response compared to normotensive women (Aalkjaer et al., 1985; Mishra et al., 2011; Pascoal et al., 1998; Wareing et al., 2006b). Like the data in the current study, other studies have demonstrated no difference in contraction compared to normotensive women (Belfort et al., 1996; Knock and Poston, 1996; Vedernikov et al., 1995; Walsh, 1985; Wolff et al., 1996; Vedernikov et al., 1999). In the present study, thromboxane receptor expression, agonist affinity, and signal transduction post-receptor activation may not have been enhanced, resulting in no difference in contraction between normotensive and PE OAs. The selected testing method could also contribute to differences as some studies used pressure myography, while the current study used wire myography. Pre-incubation, endothelial function appeared to be significantly reduced in OAs from PE pregnancies compared to normotensive pregnancies following a single dose of bradykinin. However, because a control BK relaxation curve was not performed with PSS, we could not demonstrate differences in endothelium-dependent relaxation at different BK doses. The pitavastatin control, DMSO, at a dose of 0.1%, was able to cause maximal relaxation of vessels from normotensive and PE pregnancies, potentially masking endothelial dysfunction. However, the numbers are relatively small to be certain of this outcome. Other studies in different vascular beds have demonstrated attenuated endothelium-dependent relaxation to different vasodilatory agents in vessels from hypertensive pregnancies compared to normotensive pregnancies (Ashworth et al., 1997; Knock and Poston, 1996; Kublickiene et al., 1997; Mccarthy et al., 1994a).
4.2 Vascular reactivity of omental arteries following pitavastatin exposure in normal and PE pregnancies
Pitavastatin was used because it is a novel statin with high bioavailability in the bloodstream and has never been used in pregnancy studies. The doses used in this study are suprapharmacological versus those previously measured in human serum. For pitavastatin: 1 µM is 4× higher than a 4-mg dose in non-pregnant women, and 10 µM is 40× higher (Luo et al., 2015). Independent of their cholesterol-lowering ability, statins have been shown to increase the bioavailability of NO through mechanisms including, but not limited to, raised expression of eNOS and activation of the eNOS enzyme, as well as decreasing oxidative stress (Takemoto and Liao, 2001). In male Wistar rats, when the aorta and superior mesenteric arteries were pre-constricted with EC80 noradrenaline, the direct application of simvastatin produced relaxation in a concentration-dependent manner in both the presence and absence of a functional endothelium. These effects were attributed to the release of both NO and vasodilator products from cyclooxygenase by a mechanism sensitive to O2 scavengers, superoxide dismutase (Alvarez De Sotomayor et al., 2000). It was suggested that this relaxation is associated with both Ca2+ release from intracellular stores and blockade of extracellular Ca2+ entry (Alvarez De Sotomayor et al., 2001). Finally, Mukai et al. demonstrated that, following a 2 h incubation with the hydrophobic cerivastatin (1 μM), ACh-induced endothelium-dependent relaxation was enhanced in the rat aorta via the PI3 kinase/Akt pathway, and they also demonstrated endothelium-independent relaxation via Kv channel-mediated smooth muscle hyperpolarizations (dose-dependent: 1–100 μM). Direct application of fluvastatin (a moderately hydrophilic statin) was also used in this study and was able to stimulate relaxation of vascular smooth muscle in the aorta and mesenteric arteries in a dose-dependent manner (1–300 μM); vessels were pre-constricted with prostaglandin F2 alpha (Mukai et al., 2003).
The present study did not show an additional effect of pitavastatin on contraction or relaxation in OAs from normal or pathological pregnancies. An important issue to highlight is that OAs from the pathological pregnancies achieved a relaxation equivalent to vessels from normotensive pregnancies, and therefore, there was no apparent pathology to correct. The use of DMSO may have contributed to this, which could have altered the endothelial function of OAs post-incubation. Furthermore, different doses of statins were used in other studies, and there were differences in experimental design, as direct application of statins in the form of a dose-response curve was not performed in the present study. The use of different statin concentrations in the studies on omental arteries, as per studies in chorionic plate arteries, would have been insightful. Additionally, the current study focused upon resistance vessels in a closed system in the absence of flow, and hence, 2 h may not have been sufficient time to observe an effect on the endothelium. It is also possible that some of the statin pleiotropic effects, such as eNOS activation or alleviation of inflammation or oxidative stress, failed to occur within the timeline of these studies. Clinically, in a high-risk subpopulation, women may be given statins prophylactically during pregnancy; thus, maternal vessels may be exposed to statins despite the absence of disease. Thus, this study suggests that we did not see any detrimental effects of pitavastatin on the vascular reactivity of vessels from normal pregnancies. Clinically, this is an important finding, but care should be taken in extrapolating these findings to the clinic, given the ex vivo protocol and short-term statin exposure in this study.
4.3 Vascular reactivity of chorionic plate arteries in normal and PE pregnancies
In the limited ex vivo studies of statins on human placenta, statins reduced IGF-1-mediated trophoblast proliferation, leading to caution being expressed about their use in pregnancy, particularly in the first trimester (Forbes and Westwood, 2008; Forbes et al., 2015; Kenis et al., 2005). Additionally, Nanovskaya et al. carried out dual perfusion of a placental lobule with 50 ng/mL pravastatin (based upon achieved serum concentrations following a 40-mg dose) and showed that pravastatin was transferred to the fetal circulation. However, pravastatin was also transferred back to the maternal circulation, hypothesized to be via the action of the efflux transporters, MRP2 and BCRP, located on the microvillous membrane (MVM) of the transporting epithelium of the placenta, the syncytiotrophoblast (Nanovskaya et al., 2013), likely limiting the concentrations of statins reached within the fetal circulation.
In CPAs from normal pregnancy, contraction and endothelium-independent relaxation were not significantly different compared to PE. Functional studies have demonstrated conflicting results regarding the aberrant dysfunction of CPAs from women with PE compared to CPAs from normotensive women. Studies have shown no difference in contraction (Ong et al., 2002), reduced maximal contraction (Wareing and Baker, 2004), and significantly greater contraction when comparing normal and PE placentas (Benoit et al., 2007; Bertrand et al., 1993).
The data in this study do not support the current literature suggesting that CPAs from PE pregnancies are less responsive to SNP or show altered response to U46619. DMSO has been suggested to cause the endothelium to release NO to trigger cGMP production in the smooth muscle to promote relaxation and decrease Ca2+ sensitivity, partially through inhibiting Rho-kinase to attenuate constriction. However, we saw no evidence of this in the current study compared to water (vehicle). Other potential confounders that could have affected the results in the current study include the presence of smokers in the PE group, as smoking is associated with the production of carbon monoxide (CO) (Karumanchi and Levine, 2010; Wei et al., 2015). Furthermore, factors such as the effects of analgesia (Samanta et al., 2018) and the use of anti-coagulants (Atallah et al., 2017), such as aspirin and heparin, could alter vascular responses measured by wire myography.
4.4 Vascular reactivity of chorionic plate arteries following pitavastatin exposure in normal and PE pregnancies
The levels of vasodilators and vasoconstrictors in the maternal and placental circulation are regulated to ensure a homeostatic balance of placental vascular function. From a safety perspective, it is crucial that statins do not negatively affect this differential vascular response of the placenta as this could affect any adaptive responses the placenta may try to initiate in response to stimuli or stresses (Myatt, 1992; Wang and Zhao, 2010). Balan et al., who utilized perfused normal placental cotyledons and placental explants, demonstrated that feto-placental vascular tone or vasoconstriction in response to the vasoconstrictor angiotensin-II was unaltered following exposure to pravastatin from the maternal circulation (Balan et al., 2017). A pilot randomized controlled trial investigating the safety and pharmacokinetics of 10 mg pravastatin in high-risk PE women also showed no difference in infant birthweight between the pravastatin and placebo groups (Costantine et al., 2016).
The current study failed to demonstrate a beneficial effect of statins on the relaxation of CPAs from normal or PE pregnancies; however, 10 µM pitavastatin was able to attenuate U46619 contraction in CPAs from both normal and PE pregnancies. This suggests that pitavastatin may target the thromboxane A2 pathway to diminish its contractile effects, via antagonism of the thromboxane A2 receptor and/or thromboxane synthase in the endothelium (Kontogiorgis and Hadjipavlou-Litina, 2010). Alternatively, pitavastatin could diminish the anti-inflammatory effects of U46619 by blocking the ERK pathway and reducing expression of U46619-stimulated IL-1ß, IL-6, iNOS expression, and IL-1ß (Yang et al., 2016). Impaired vascular function in PE CPAs was not demonstrated, and the addition of statins did not have a biologically significant effect on vascular function; however, it was encouraging to see that no acute detrimental effect on vascular function occurred.
Limitations of the present study include the choice of statin concentration used in the ex vivo acute study. A suprapharmacological concentration was chosen because, for ex vivo myography experiments, a higher dose than those typically achieved in vivo is generally needed to result in similar effects. Furthermore, the endothelin-1 pathway was not explored directly. In addition, due to the relative lack of differences in the present study, extensive mechanistic studies were not performed, but future studies could include assessments of whether statins altered eNOS signaling/activation and levels of oxidative stress/damage. These may also help explain the lack of any effects of statins on omental artery function in the current study. Finally, it is important to acknowledge that several women in the PE group were prescribed aspirin and antihypertensive medication at various points within their pregnancy, and this may have contributed to altered vascular functional responses ex vivo.
5 Conclusion
Taking into account the data from this study using pravastatin, pitavastatin, and simvastatin, statins did not negatively affect vascular function in placental vessels, suggesting that these drugs are unlikely to have a large vasoactive effect within the placenta at physiological doses. The outcome of the in vitro data does not provide convincing evidence for pitavastatin as an optimal treatment to modify endothelial function in PE, but there are limitations to this study, such as the acute exposure time, as detailed above. Mechanistic studies, such as increased exploration of statins’ pleiotropic effects, are essential to providing a greater understanding of the potential clinical applications of statins in PE. This study focused on short-term effects of statins on vascular function but investigating the effects of longer-term statin exposure in pregnancy will be important to demonstrate its potential as a therapy in PE. This requires appropriate preclinical in vivo models to provide the necessary safety and mechanistic data to underpin further clinical trials. Overall, these data give greater confidence that, if statins are shown to be beneficial in the clinical treatment/management of PE, the doses used, at least in the short term, are unlikely to be detrimental to fetoplacental vascular function. While we failed to see a positive effect of pitavastatin on maternal omental artery function, this does not mean that other statins will not show beneficial effects. The future studies suggested above will provide improved mechanistic understanding of the validity of statin use in individuals with preeclampsia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee, University of Manchester. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CA: data curation, formal analysis, methodology, visualization, and writing – original draft. JM: conceptualization, formal analysis, supervision, and writing – review and editing. MW: conceptualization, supervision, and writing – review and editing. MD: formal analysis, supervision, and writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank our research midwives, Jessica Morecroft and Christine Hughes (who recruited and collected data from participants), and the women and staff of St. Mary’s Hospital, Manchester, United Kingdom, for participating in the study. This work was funded by Tommy’s the Baby Charity.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1575128/full#supplementary-material
References
Aalkjaer C., Danielsen H., Johannesen P., Pedersen E. B., Rasmussen A., Mulvany M. J. (1985). Abnormal vascular function and morphology in pre-eclampsia: a study of isolated resistance vessels. Clin. Sci. (Lond) 69 (4), 477–482. doi:10.1042/cs0690477
Ahmad S., Ahmed A. (2004). Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circulation Res. 95 (9), 884–891. doi:10.1161/01.RES.0000147365.86159.f5
Ahmed A. (2011). 'New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thromb. Res. 127 (Suppl. 3), S72–S75. doi:10.1016/S0049-3848(11)70020-2
Ahmed A., Singh J., Khan Y., Seshan S. V., Girardi G. (2010). A new mouse model to explore therapies for preeclampsia. PLoS One 5, e13663. doi:10.1371/journal.pone.0013663
Alvarez De Sotomayor M., Herrera M. D., Marhuenda E., Andriantsitohaina R. (2000). Characterization of endothelial factors involved in the vasodilatory effect of simvastatin in aorta and small mesenteric artery of the rat. Br. J. Pharmacol. 131 (6), 1179–1187. doi:10.1038/sj.bjp.0703668
Alvarez de Sotomayor M., Perez-Guerrero C., Herrera M. D., Marhuenda E. (2001). Effect of simvastatin on vascular smooth muscle responsiveness: involvement of Ca(2+) homeostasis. Eur. J. Pharmacol. 415 (2-3), 217–224. doi:10.1016/s0014-2999(01)00819-6
Ashworth J. R., Warren A. Y., Baker P. N., Johnson I. R. (1997). 'Loss of endotheliumdependent relaxation in myometrial resistance arteries in pre-eclampsia. Br. J. Obstet. Gynaecol. 104 (10), 1152–1158. doi:10.1111/j.1471-0528.1997.tb10939.x
Atallah A., Lecarpentier E., Goffinet F., Doret-Dion M., Gaucherand P., Tsatsaris V. (2017). Aspirin for prevention of preeclampsia. Drugs 77 (17), 1819–1831. doi:10.1007/s40265-017-0823-0
Bakrania B. A., Spradley F. T., Drummond H. A., LaMarca B., Ryan M. J., Granger J. P. (2020). Preeclampsia: linking placental ischemia with maternal endothelial and vascular dysfunction. Compr. Physiol. 11 (1), 1315–1349. [online]. doi:10.1002/cphy.c200008
Balan A., Szaingurten-Solodkin I., Swissa S. S., Feinshtein V., Huleihel M., Holcberg G., et al. (2017). The effects of pravastatin on the normal human placenta: lessons from ex-vivo models. PLoS One 12 (2), e0172174. doi:10.1371/journal.pone.0172174
Bateman B. T., Hernandez-Diaz S., Fischer M. A., Seely E. W., Ecker J. L., Franklin J. M., et al. (2015). Statins and congenital malformations: cohort study. Bmj 350, h1035. doi:10.1136/bmj.h1035
Belfort M. A., Saade G. R., Suresh M., Kramer W., Vedernikov Y. P. (1996). Effects of selected vasoconstrictor agonists on isolated omental artery from premenopausal nonpregnant women and from normal and preeclamptic pregnant women. Am. J. Obstet. Gynecol. 174 (2), 687–693. doi:10.1016/s0002-9378(96)70451-9
Bellamy L., Casas J.-P., Hingorani A. D., Williams D. J. (2007). Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335 (7627), 974. [online]. doi:10.1136/bmj.39335.385301.BE
Benoit C., Zavecz J., Wang Y. (2007). Vasoreactivity of chorionic plate arteries in response to vasoconstrictors produced by preeclamptic placentas. Placenta 28 (5-6), 498–504. doi:10.1016/j.placenta.2006.09.002
Bertrand C., Duperron L., St-Louis J. (1993). 'Umbilical and placental vessels: modifications of their mechanical properties in preeclampsia. Am. J. Obstet. Gynecol. 168 (5), 1537–1546. doi:10.1016/s0002-9378(11)90795-9
Berzan E., Doyle R., Brown C. M. (2014). Treatment of preeclampsia: current approach and future perspectives. Curr. Hypertens. Rep. 16 (9), 473. doi:10.1007/s11906-014-0473-5
Breetveld N., Ghossein-Doha C., van Kuijk S., van Dijk A., van der Vlugt M., Heidema W., et al. (2014). Cardiovascular disease risk is only elevated in hypertensive, formerly preeclamptic women. BJOG Int. J. Obstetrics and Gynaecol. 122 (8), 1092–1100. doi:10.1111/1471-0528.13057
Brown M. A., Magee L. A., Kenny L. C., Karumanchi S. A., McCarthy F. P., Saito S., et al. (2018). Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72 (1), 24–43. doi:10.1161/HYPERTENSIONAHA.117.10803
Brownfoot F. C., Hastie R., Hannan N. J., Cannon P., Tuohey L., Parry L. J., et al. (2016). 'Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 214 (3), 356.e1–356.e15. doi:10.1016/j.ajog.2015.12.019
Chappell L. C., Cluver C. A., Kingdom J., Tong S. (2021). Pre-eclampsia. Lancet 398 (10297), 341–354. doi:10.1016/s0140-6736(20)32335-7
Chen C. W., Jaffe I. Z., Karumanchi S. A. (2014). 'Pre-eclampsia and cardiovascular disease. Cardiovasc Res. 101 (4), 579–586. doi:10.1093/cvr/cvu018
Costantine M. M., Cleary K., Hebert M. F., Ahmed M. S., Brown L. M., Ren Z., et al. (2016). 'Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am. J. Obstetrics Gynecol. 214 (6), 720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
Costantine M. M., Tamayo E., Lu F., Bytautiene E., Longo M., Hankins G. D., et al. (2011). Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms-like tyrosine kinase-1-induced preeclampsia. Obstet. Anesth. Dig. 31 (3), 166–167. doi:10.1097/01.aoa.0000400311.47926.46
Costantine M. M., West H., Wisner K. L., Caritis S., Clark S., Venkataramanan R., et al. (2021). A randomized pilot clinical trial of pravastatin versus placebo in pregnant patients at high risk of preeclampsia. Am. J. Obstetrics Gynecol. 225 (6), 666.e1–666.e15. doi:10.1016/j.ajog.2021.05.018
Cudmore M., Ahmad S., Al-Ani B., Fujisawa T., Coxall H., Chudasama K., et al. (2007). Negative regulation of soluble flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115 (13), 1789–1797. doi:10.1161/circulationaha.106.660134
Döbert M., Varouxaki A. N., Mu A. C., Syngelaki A., Ciobanu A., Akolekar R., et al. (2021). Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation 144 (9), 670–679. [online]. doi:10.1161/CIRCULATIONAHA.121.053963
Duhig K. E., Myers J., Seed P. T., Sparkes J., Lowe J., Hunter R. M., et al. (2019). 'Placental growth factor testing to assess women with suspected preeclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 393 (10183), 1807–1818. doi:10.1016/S0140-6736(18)33212-4
Edison R. J., Muenke M. (2004). Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am. J. Med. Genet. A 131 (3), 287–298. doi:10.1002/ajmg.a.30386
Forbes K., Shah V. K., Siddals K., Gibson J. M., Aplin J. D., Westwood M. (2015). Statins inhibit insulin-like growth factor action in first trimester placenta by altering insulin-like growth factor 1 receptor glycosylation. Mol. Hum. Reprod. 21 (1), 105–114. doi:10.1093/molehr/gau093
Forbes K., Westwood M. (2008). The IGF axis and placental function. a mini review. Horm. Res. 69 (3), 129–137. doi:10.1159/000112585
Haddad B., Deis S., Goffinet F., Paniel B. J., Cabrol D., Sibaı̈ B. M. (2004). Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am. J. Obstetrics Gynecol. 190 (6), 1590–1597. doi:10.1016/j.ajog.2004.03.050
Karumanchi S. A., Levine R. J. (2010). How does smoking reduce the risk of preeclampsia? Hypertension 55 (5), 1100–1101. doi:10.1161/HYPERTENSIONAHA.109.148973
Kenis I., Tartakover-Matalon S., Cherepnin N., Drucker L., Fishman A., Pomeranz M., et al. (2005). Simvastatin has deleterious effects on human first trimester placental explants. Hum. Reprod. 20 (10), 2866–2872. doi:10.1093/humrep/dei120
Khan N., Andrade W., De Castro H., Wright A., Wright D., Nicolaides K. H. (2020). Impact of new definitions of pre-eclampsia on incidence and performance of first-trimester screening. Ultrasound Obstetrics and Gynecol. 55 (1), 50–57. doi:10.1002/uog.21867
Kluivers A. C. M., Biesbroek A., Visser W., Saleh L., Russcher H., Danser A. H. J., et al. (2023). Angiogenic imbalance in pre-eclampsia and fetal growth restriction: enhanced soluble fms-like tyrosine kinase-1 binding or diminished production of placental growth factor? Ultrasound Obstetrics and Gynecol. 61 (4), 466–473. doi:10.1002/uog.26088
Knock G. A., Poston L. (1996). Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am. J. Obstet. Gynecol. 175 (6), 1668–1674. doi:10.1016/s0002-9378(96)70123-0
Kontogiorgis C., Hadjipavlou-Litina D. (2010). Thromboxane synthase inhibitors and thromboxane A2 receptor antagonists: a quantitative structure activity relationships (QSARs) analysis. Curr. Med. Chem. 17 (28), 3162–3214. doi:10.2174/092986710792231978
Kublickiene K. R., Kublickas M., Lindblom B., Lunell N. O., Nisell H. (1997). A comparison of myogenic and endothelial properties of myometrial and omental resistance vessels in late pregnancy. Am. J. Obstet. Gynecol. 176 (3), 560–566. doi:10.1016/s0002-9378(97)70548-9
Kuklina E. V., Ayala C., Callaghan W. M. (2009). Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol. 113 (6), 1299–1306. doi:10.1097/AOG.0b013e3181a45b25
Kumasawa K., Ikawa M., Kidoya H., Hasuwa H., Saito-Fujita T., Morioka Y., et al. (2010). Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl. Acad. Sci. 108 (4), 1451–1455. doi:10.1073/pnas.1011293108
Labarrere C. A., DiCarlo H. L., Bammerlin E., Hardin J. W., Kim Y. M., Chaemsaithong P., et al. (2017). Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am. J. Obstetrics Gynecol. 216 (3), 287.e1–287.e16. doi:10.1016/j.ajog.2016.12.029
Leon L. J., McCarthy F. P., Direk K., Gonzalez-Izquierdo A., Prieto-Merino D., Casas J. P., et al. (2019). Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a caliber study. Circulation 140 (13), 1050–1060. doi:10.1161/CIRCULATIONAHA.118.038080
Levine R. J., Maynard S. E., Qian C., Lim K.-H., England L. J., Yu K. F., et al. (2004). Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350 (7), 672–683. doi:10.1056/NEJMoa031884
Luo Z., Zhang Y., Gu J., Feng P., Wang Y. (2015). 'Pharmacokinetic properties of single- and multiple-dose pitavastatin calcium tablets in healthy Chinese volunteers. Curr. Ther. Res. Clin. Exp. 77, 52–57. doi:10.1016/j.curtheres.2015.02.001
Maayeh M., Costantine M. M. (2020). Prevention of preeclampsia. Seminars Fetal Neonatal Med. 25 (5), 101123. doi:10.1016/j.siny.2020.101123
Madazli R., Yuksel M. A., Imamoglu M., Tuten A., Oncul M., Aydin B., et al. (2014). Comparison of clinical and perinatal outcomes in early- and late-onset preeclampsia. Archives Gynecol. Obstetrics 290 (1), 53–57. doi:10.1007/s00404-014-3176-x
Maynard S. E., Min J.-Y., Merchan J., Lim K.-H., Li J., Mondal S., et al. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investigation 111 (5), 649–658. doi:10.1172/jci17189
McCarthy A. L., Taylor P., Graves J., Raju S. K., Poston L. (1994a). 'Endothelium-dependent relaxation of human resistance arteries in pregnancy. Am. J. Obstet. Gynecol. 171 (5), 1309–1315. doi:10.1016/0002-9378(94)90153-8
McElwain C. J., Tuboly E., McCarthy F. P., McCarthy C. M. (2020). Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front. Endocrinol. 11, 655. doi:10.3389/fendo.2020.00655
Mendoza M., Ferrer-Oliveras R., Bonacina E., Garcia-Manau P., Rodo C., Carreras E., et al. (2021). Evaluating the effect of pravastatin in early-onset fetal growth restriction: a nonrandomized and historically controlled pilot study. Am. J. Perinatology 38 (14), 1472–1479. doi:10.1055/s-0040-1713651
Minsker D. H., MacDonald J. S., Robertson R. T., Bokelman D. L. (1983). Mevalonate supplementation in pregnant rats suppresses the teratogenicity of mevinolinic acid, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase. Birth Defects Res. 28 (3), 449–456. doi:10.1002/tera.1420280316
Mishra N., Nugent W. H., Mahavadi S., Walsh S. W. (2011). Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension 58 (5), 867–873. doi:10.1161/HYPERTENSIONAHA.111.176602
Mukai Y., Shimokawa H., Matoba T., Hiroki J., Kunihiro I., Fujiki T., et al. (2003). Acute vasodilator effects of HMG-CoA reductase inhibitors: involvement of PI3- kinase/Akt pathway and Kv channels. J. Cardiovasc Pharmacol. 42 (1), 118–124. doi:10.1097/00005344-200307000-00018
Mulvany M. J., Aalkjaer C. (1990). Structure and function of small arteries. Physiol. Rev. 70 (4), 921–961. doi:10.1152/physrev.1990.70.4.921
Myatt L. (1992). Control of vascular resistance in the human placenta. Placenta 13 (4), 329–341. doi:10.1016/0143-4004(92)90057-z
Nanovskaya T. N., Patrikeeva S. L., Paul J., Costantine M. M., Hankins G. D., Ahmed M. S. (2013). Transplacental transfer and distribution of pravastatin. Am. J. Obstet. Gynecol. 209 (4), 373.e1–373.e3735. doi:10.1016/j.ajog.2013.05.038
NICE (2023). Recommendations | Hypertension in pregnancy: Diagnosis and Management | Guidance | NICE. Available at: https://www.nice.org.uk/guidance/ng133/chapter/Recommendations#management-of-pre-eclampsia.pdf
Ong S. S., Crocker I. P., Warren A. Y., Baker P. N. (2002). 'Functional characteristics of chorionic plate placental arteries from normal pregnant women and women with preeclampsia. Hypertens. Pregnancy 21 (3), 175–183. doi:10.1081/PRG-120015844
Ormesher L., Higson S., Luckie M., Roberts S. A., Glossop H., Trafford A., et al. (2022). Postnatal cardiovascular morbidity following preterm pre-eclampsia: an observational study. Pregnancy Hypertens. 30, 68–81. doi:10.1016/j.preghy.2022.08.007
Pascoal I. F., Lindheimer M. D., Nalbantian-Brandt C., Umans J. G. (1998). 'Preeclampsia selectively impairs endothelium-dependent relaxation and leads to oscillatory activity in small omental arteries. J. Clin. Investig. 101 (2), 464–470. doi:10.1172/JCI557
Pasupathy D., McCowan L. M. E., Poston L., Kenny L. C., Dekker G. A., North R. A., et al. (2012). Perinatal outcomes in large infants using customised birthweight centiles and conventional measures of high birthweight. Paediatr. Perinat. Epidemiol. 26 (6), 543–552. doi:10.1111/ppe.12002
Perinatal I. (2019). Fetal growth. Available online at: https://www.perinatal.org.uk/FetalGrowth/fetalgrowth.aspx.
Phipps E. A., Thadhani R., Benzing T., Karumanchi S. A. (2019). 'Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 15 (5), 275–289. doi:10.1038/s41581-019-0119-6
Pollack P., Shields K., Burnett D., Osborne M., Cunningham M., Stepanavage M. (2005). Pregnancy outcomes after maternal exposure to simvastatin and lovastatin. Birth Defects Res. Part A Clin. Mol. Teratol. 73 (11), 888–896. doi:10.1002/bdra.20181
Possomato-Vieira J. S., Khalil R. A. (2016). Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv. Pharmacol. 77, 361–431. doi:10.1016/bs.apha.2016.04.008
Rana S., Lemoine E., Granger J., Karumanchi S. A. (2019). Preeclampsia: pathophysiology, challenges, and perspectives. Circulation Res. 124 (7), 1094–1112. [online]. doi:10.1161/circresaha.118.313276
Rezk M., Gamal A., Emara M. (2015). Maternal and fetal outcome inde novopreeclampsia in comparison to superimposed preeclampsia: a two-year observational study. Hypertens. Pregnancy 34 (2), 137–144. doi:10.3109/10641955.2014.982329
Rossoni L. V., Wareing M., Wenceslau C. F., Al-Abri M., Cobb C., Austin C. (2011). Acute simvastatin increases endothelial nitric oxide synthase phosphorylation via AMP-activated protein kinase and reduces contractility of isolated rat mesenteric resistance arteries. Clin. Sci. 121 (10), 449–458. doi:10.1042/cs20110259
Samanta S., Jain K., Bhardwaj N., Jain V., Singh P. M., Samanta S., et al. (2018). Labor analgesia in parturients of fetal growth restriction having raised umbilical Doppler vascular indices. J. Anaesthesiol. Clin. Pharmacol. 34 (1), 11–17. doi:10.4103/joacp.JOACP_150_16
Staff A. C., Fjeldstad H. E., Fosheim I. K., Moe K., Turowski G., Johnsen G. M., et al. (2020). Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am. J. Obstetrics and Gynecol. 226 (0), S895–S906. [online] 0. doi:10.1016/j.ajog.2020.09.026
Taguchi N., Rubin E. T., Hosokawa A., Choi J., Ying A. Y., Moretti M. E., et al. (2008). Prenatal exposure to HMG-CoA reductase inhibitors: effects on fetal and neonatal outcomes. Reprod. Toxicol. 26 (2), 175–177. doi:10.1016/j.reprotox.2008.06.009
Takemoto M., Liao J. K. (2001). Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler. Thromb. Vasc. Biol. 21 (11), 1712–1719. doi:10.1161/hq1101.098486
Tomimatsu T., Mimura K., Matsuzaki S., Endo M., Kumasawa K., Kimura T. (2019). Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int. J. Mol. Sci. 20 (17), 4246. [online]. doi:10.3390/ijms20174246
US Food and Drug Administration (2024). FDA requests removal of strongest warning against using cholesterol-lowering statins during pregnancy; still advises most pregnant patients should stop taking statins. Available online at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-removal-strongest-warning-against-using-cholesterol-lowering-statins-during-pregnancy (Accessed September 24, 2024).
Vedernikov Y., Saade G. R., Garfield R. E. (1999). Vascular reactivity in preeclampsia. Semin. Perinatol. 23 (1), 34–44. doi:10.1016/s0146-0005(99)80058-5
Vedernikov Y. P., Belfort M. A., Saade G. R., Moise K. J. (1995). Pre-eclampsia does not alter the response to endothelin-1 in human omental artery. J. Cardiovasc Pharmacol. 26 (Suppl. 3), S233–S235. doi:10.1097/00005344-199506263-00070
Walsh S. W. (1985). Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am. J. Obstet. Gynecol. 152 (3), 335–340. doi:10.1016/s0002-9378(85)80223-4
Wang Y., Zhao S. (2010). “Integrated systems physiology: from molecules to function to disease,” in Vascular biology of the placenta. San Rafael (CA): Morgan and Claypool Life Sciences.
Wareing M., Baker P. N. (2004). 'Vasoconstriction of small arteries isolated from the human placental chorionic plate in normal and compromised pregnancy. Hypertens. Pregnancy 23 (3), 237–246. doi:10.1081/PRG-200030297
Wareing M., Crocker I. P., Warren A. Y., Taggart M. J., Baker P. N. (2002). Characterization of small arteries isolated from the human placental chorionic plate. Placenta 23 (5), 400–409. doi:10.1053/plac.2002.0825
Wareing M., Myers J. E., O'Hara M., Kenny L. C., Taggart M. J., Skillern L., et al. (2006b). 'Phosphodiesterase-5 inhibitors and omental and placental small artery function in normal pregnancy and pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 127 (1), 41–49. doi:10.1016/j.ejogrb.2004.06.014
Wei J., Liu C. X., Gong T. T., Wu Q. J., Wu L. (2015). Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget 6 (41), 43667–43678. doi:10.18632/oncotarget.6190
Winterfeld U., Allignol A., Panchaud A., Rothuizen L. E., Merlob P., CuppersMaarschalkerweerd B., et al. (2013). Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. Bjog 120 (4), 463–471. doi:10.1111/1471-0528.12066
Wolff K., Kublickiene K. R., Kublickas M., Lindblom B., Lunell N. O., Nisell H. (1996). Effects of endothelin-1 and the ETA receptor antagonist BQ-123 on resistance arteries from normal pregnant and preeclamptic women. Acta Obstet. Gynecol. Scand. 75 (5), 432–438. doi:10.3109/00016349609033349
Wu P., Haththotuwa R., Kwok C. S., Babu A., Kotronias R. A., Rushton C., et al. (2017). Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circulation Cardiovasc. Qual. Outcomes 10 (2), e003497. doi:10.1161/CIRCOUTCOMES.116.003497
Yang W., Yan A., Zhang T., Shao J., Liu T., Yang X., et al. (2016). Thromboxane A2 receptor stimulation enhances microglial interleukin-1β and NO biosynthesis mediated by the activation of ERK pathway. Front. aging Neurosci. 8, 8. doi:10.3389/fnagi.2016.00008
Zarek J., DeGorter M. K., Lubetsky A., Kim R. B., Laskin C. A., Berger H., et al. (2013). The transfer of pravastatin in the dually perfused human placenta. Placenta 34 (8), 719–721. doi:10.1016/j.placenta.2013.05.002
Keywords: preeclampsia, statins, pregnancy, vascular function, therapeutics, pitavastatin and pravastatin
Citation: Agwu C, Myers J, Wareing M and Dilworth M (2025) Acute effect of statins on vascular reactivity in maternal and placental arteries from pregnancies complicated by preeclampsia. Front. Physiol. 16:1575128. doi: 10.3389/fphys.2025.1575128
Received: 11 February 2025; Accepted: 22 April 2025;
Published: 24 June 2025.
Edited by:
Luciana Venturini Rossoni, University of São Paulo, BrazilReviewed by:
Kedra Wallace, University of Mississippi Medical Center, United StatesIsraelle Freitas, State University of Campinas, Brazil
Copyright © 2025 Agwu, Myers, Wareing and Dilworth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chinedu Agwu, Y2hpbmVkdS5hZ3d1QGJydW5lbC5hYy51aw==
 Chinedu Agwu
Chinedu Agwu Jenny Myers
Jenny Myers Mark Wareing1,2,3
Mark Wareing1,2,3 Mark Dilworth
Mark Dilworth