1 Introduction
This contribution is to recognize the scientific contribution of Paul Siegel and explore the report from Siegel and Honaker (2025) on sexual dimorphism of chicken. The present discussion explores the putative physiological mechanisms for the consistency of sexual dimorphism in growth rate across 67 generations. Paul Siegel has been conducting research on chicken growth and its genetic control for over 60 years. He is one of the people who established that the growth rate of chickens is highly heritable with the heritability of growth calculated as 0.39 from a study by Siegel (1962) and 0.41 from 176 reports as 0.41 (Siegel, 1962). Recently, he published a paper on the effects of selection for growth over 67 generations (one generation per year) focusing on the effects on sexually dimorphism in growth (Siegel and Honaker, 2025). Birds were selected for either high growth or slow growth (specifically body weight at 8 weeks old). Breeding employed 4 dams for each sire with matings of full- and half-sibs being avoided. Sexually dimorphism of growth was stable over 67 generations selected for growth. This indicates that there is a strong selection constraint for sexually dimorphism of growth and/or that it is a canalized genetic response. Sexually dimorphism of growth was also markedly greater (2.17-fold in the high growth line and 2.51-fold in the slow growth line) at 8-weeks old compared to 4-weeks old (Table 1).
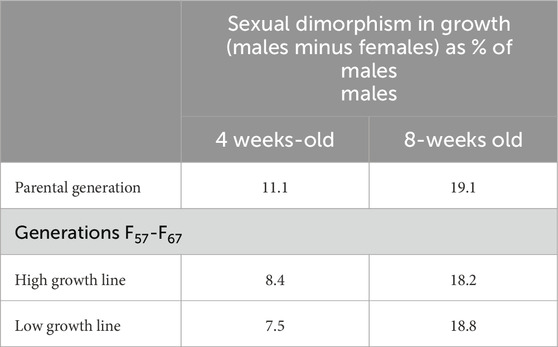
Table 1. Comparison of sexual dimorphism in 4- and 8 – week-old chickens selected for 8-week-old body weight for 67 generations (calculated from data in Siegel and Honaker, 2025).
Experimentally, growth is measured as either weight or height/length at one or several time points or the delta increase in weight or height/length (average daily gain or ADG) or expressed as parameters in an equation for growth such as the Gompertz equation. In livestock and poultry growth is most frequently expressed as weight or weight gain. In contrast, human growth is assessed as height (e.g., Gasser et al., 2009). while studies in reptiles employ length; the latter being the distance between snout–vent length in reptiles (e.g., Cox and John-Alder, 2007). Siegel and Honaker (2025) employed body weight at 8 weeks old as their parameters of growth.
While growth is a change in weight or height/length, a confounding conceptual issue is that there can be sexual dimorphism in mature weight or height/length at sexual maturity or when epiphyseal plates fuse. It is noted that either adult males or females can be larger even in closely related species. For instance, there is opposite sexual adult size dimorphism in lizards (Sceloporus virgatus: male < female; Sceloporus jarrovii: male > females) (Cox and John-Alder, 2007).
2 Sexual dimorphism and growth
It is reasonable to assume that there has been tremendous selection pressure for animals to have the optimal size/weight and growth profile (delta size per unit time) for a specific environment. The corollary is that there will be optimal size/weight together with growth for the food available and other environmental considerations such as predators, temperature, and water availability.
In humans, sex differences in height are only small until puberty (reviewed: Gasser et al., 2009). Similarly, there is greater sexual dimorphism in body weight at 8- compared to 4–weeks of age (Table 1) (Siegel and Honaker, 2025).
3 Genetic basis of sexual dimorphism and growth
Sexual dimorphism of growth may have a simple genetic basis. In eutherian mammals, females have two X chromosomes and, consequently, two sets of genes. While one X chromosome is inactivated, some genes escape inactivation and there can be gene dosing (reviewed; Moeser et al., 2022). In birds having ZZ (males) and ZW (females), there is dosage with the Z chromosome gene, Z chromosome gene Doublesex and Mab-3-Related Transcription factor 1 (DMRT1) (Ioannidis et al., 2021; Li et al., 2025; reviewed: Zhang et al., 2023).
4 Physiological bases of sexual dimorphism and growth
4.1 Sex steroids
It is frequently assumed that the overall mechanism for sexual dimorphism in growth are sex steroids. Sex steroids promote growth in cattle. Castration reduces growth rate in cattle (e.g., Lee et al., 1990; Marti et al., 2013; Li et al., 2022). Moreover, castration depresses circulating concentrations of growth hormone and thyroid hormones and is followed by shifts in microbial fermentation (Li et al., 2022; Shi et al., 2024). Implanting a mixture of androgens and estrogens (trenbolone acetate and estradiol 17β) in increases growth (average daily gain) in steers while reducing protein turnover and the insulin response to glucose (e.g., Ferguson et al., 2023).
There are markedly differences between the effects of androgens on growth in chickens (negative) and turkeys (positive). Growth is either unaffected or tended to be increased by castration in chickens (Fennell and Scanes, 1992a; Chen et al., 2006; Symeon et al., 2010). It is cautioned that body weight gain reflects the aggregate of growth of multiple tissues some or all of which exhibit sexual dimorphism but of different magnitudes and different directions. For instance, while weights of adipose tissue were increased following castration and decreased by androgen replacement, there was no effect of castration on breast muscle but decreases with androgen at physiological concentrations (Fennell and Scanes, 1992a). Moreover, testosterone depressed ADG with the effect overcome by a peripheral androgen blocker (Fennell et al., 1996). Similarly, in female-larger species of reptiles, testosterone reduces growth but increase growth in male - larger species (Duncan et al., 2020). In turkeys, growth and muscle development are enhanced by exogenous androgens (Fennell and Scanes, 1992b) and castration tends to decrease growth and muscle weight (Pierson et al., 1981; Burke and Edwards, 1994).
4.2 Hypothalamo-pituitary (growth hormone)-insulin-like growth factor axis
Another underlying assumption is the sexual dimorphism is related to growth hormone-insulin-like growth factor. There are sexually dimorphic patterns for growth hormone secretion, for instance, in humans (e.g., Jessup et al., 2003), rats (e.g., Chowen et al., 1996) and chickens where castration is followed by feminization of GH secretion (Pampori and Shapiro, (1994). The physiological mechanism for SSD involves IGF-1. For instance, castration increases hepatic IGF-1 expression in male Sceloporus undulatus while testosterone having no effect (Cox and John-Alder, 2007).
4.3 Hypothalamo-pituitary-adrenocortical (HPA) axis
The HPA axis has been related to sexual dimorphism of growth with SNPs in crhr1 that are associated with rates of growth in yellow catfish (Wang et al., 2024). Moreover, there is sexual dimorphism in the effects of corticosteroid-binding globulin (CBG) on hepatic functioning (Toews et al., 2022).
4.3.1 Immune and gastro-intestinal functioning
The extent to which sexual dimorphism is secondary to other sexual differences such as immune or gastro-intestinal is unclear (reviewed; Moeser et al., 2022). For example, there tends to be a larger immune response to E. coli or sheep red blood cells in young chickens receiving estradiol with this being blocked by estrogen receptor antagonist (Leiner et al., 1996).
5 Discussion
It would be predicted there would be drift in sexual dimorphism over the 67 generations, this was not the case (Table 1) (Siegel and Honaker, 2025). And would suggest that growth and sexual dimorphism are tightly linked. It is speculated that expression of DMRT1 may be, at least partially, responsible for the sexual dimorphism of growth in chickens. What is not known is whether genetic female chickens (ZW) expressing male levels of DMRT1 will grow at male rates or that males with higher levels of DMRT1 expression grow at superior rates. These might be accomplished by selection for DMRT1 expression early in embryonic development or via transgenic approaches.
Author contributions
CS: Writing – original draft, Investigation, Resources, Writing – review and editing, Data curation, Project administration, Visualization, Methodology, Supervision, Conceptualization, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The submission seeks to recognize the truly amazing scientific career of Paul Siegel.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Burke W. H., Edwards H. M. (1994). Effect of early castration on body weight, muscle growth, and bone characteristics of male Nicholas strain turkeys. Poult. Sci. 73, 457–463. doi:10.3382/ps.0730457
Chen K. L., Hsieh C. Y., Chiou P. W. S. (2006). Caponization effects on growth performance and lipid metabolism in taiwan country chicken cockerels nchu.edu.tw. J. Anim. Sci. 19, 438–443. doi:10.5713/ajas.2006.438
Chowen J. A., García-Segura L. M., González-Parra S., Argente J. (1996). Sex steroid effects on the development and functioning of the growth hormone axis. Cell. Mol. Neurobiol. 16, 297–310. doi:10.1007/33333333333333BF02088097
Cox R. M., John-Alder H. B. (2007). Growing apart together: the development of contrasting sexual size dimorphisms in sympatric Sceloporus lizards. Herpetol 63, 245–257. doi:10.1655/0018-0831(2007)63[245:gattdo]2.0.co;2
Duncan C. A., Cohick W. S., John-Alder H. B. (2020). Testosterone reduces growth and hepatic IGF-1 mRNA in a female-larger lizard, Sceloporus undulatus: evidence of an evolutionary reversal in growth regulation. Integr. Org. Biol 2, obaa036. doi:10.1093/iob/obaa036
Fennell M. J., Radecki S. V., Proudman J. A., Scanes C. G. (1996). The suppressive effects of testosterone on growth in young chickens appears to be mediated via a peripheral androgen receptor; studies of the anti-androgen ICI 176,334. Poult. Sci. 75, 763–766. doi:10.3382/ps.0750763
Fennell M. J., Scanes C. G. (1992a). Inhibition of growth in chickens by testosterone, 5α-dihydrotestosterone and 19-nortestosterone. Poult. Sci. 71, 357–366. doi:10.3382/ps.0710357
Fennell M. J., Scanes C. G. (1992b). Effects of androgen (testosterone, 5 alpha-dihydrotestosterone, 19-nortestosterone) administration on growth in turkeys. Poult. Sci. 71, 539–547. doi:10.3382/ps.0710539
Ferguson T. D., Loos C. M. M., Vanzant E. S., Urschel K. L., Klotz J. L., McLeod K. R. (2023). Impact of ergot alkaloid and steroidal implant on whole-body protein turnover and expression of mTOR pathway proteins in muscle of cattle. Front. Vet. Sci. 10, 1104361. doi:10.3389/fvets.2023.1104361
Gasser T., Sheehy A., Molinari L., Largo R. H. (2009). Sex dimorphism in growth. Ann. Hum. Biol. 27, 187–197. doi:10.1080/030144600282299
Ioannidis J., Taylor G., Zhao D., Liu L., Idoko-Akoh A., Gong D., et al. (2021). Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. U. S. A. 118, e2020909118. doi:10.1073/pnas.2020909118
Jessup S. K., Dimaraki E. V., Symons K. V., Barkan A. L. (2003). Sexual dimorphism of growth hormone (GH) regulation in humans: endogenous GH-releasing hormone maintains basal GH in women but not in men. J. Clin. Endocrinol. Metab. 88, 4776–4780. doi:10.1210/jc.2003-030246
Lee C. Y., Henricks D. M., Skelley G. C., Grimes L. W. (1990). Growth and hormonal response of intact and castrate male cattle to trenbolone acetate and estradiol. J. Anim. Sci. 68, 2682–2689. doi:10.2527/1990.6892682x
Leitner G., Landsman T., Blum O., Zaltsmann N., Heller E. D. (1996). Effects of gonadal steroids and their antagonists on the humoral immune response of immune-selected broiler chicks. Poult. Sci. 75, 1373–1382. doi:10.3382/ps.0751373
Li J., Zhang X., Wang X., Wang Z., Li X., Zheng J., et al. (2025). Single-nucleus transcriptional and chromatin accessible profiles reveal critical cell types and molecular architecture underlying chicken sex determination. J. Adv. Res. 70, 29–43. doi:10.1016/j.jare.2024.05.007
Li Z., Shi J., Lei Y., Wu J., Zhang R., Zhang X., et al. (2022). Castration alters the cecal microbiota and inhibits growth in Holstein cattle. J. Anim. Sci. 100, skac367. doi:10.1093/jas/skac367
Marti S., Realini C. E., Bach A., Pérez-Juan M., Devant M. (2013). Effect of castration and slaughter age on performance, carcass, and meat quality traits of Holstein calves fed a high-concentrate diet. J. Anim. Sci. 91, 1129–1140. doi:10.2527/jas.2012-5717
Moeser A. J., Roney A., Fardisi M., Thelen K. (2022). Biological sex: an understudied factor driving disease susceptibility in pigs. J. Anim. Sci. 100, skac146. doi:10.1093/jas/skac146
Pampori N. A., Shapiro B. H. (1994). Testicular regulation of sexual dimorphisms in the ultradian profiles of circulating growth hormone in the chicken. Eur. J. Endocrinol. 131, 313–318. doi:10.1530/eje.0.1310313
Pierson F. W., Hester P. Y., Wilson E. K. (1981). The effect of caponization and dietary 17 alpha-methyltestosterone on the incidence of leg abnormalities in turkeys. Poult. Sci. 60, 2144–2149. doi:10.3382/ps.0602144
Shi J., Li Z., Jia L., Ma Y., Huang Y., He P., et al. (2024). Castration alters the ileum microbiota of Holstein bulls and promotes beef flavor compounds. BMC Genomics 25, 426. doi:10.1186/s12864-024-10272-8
Siegel P. B. (1962). Selection for body weight at eight weeks of age. Poult. Sci. 41, 954–962. doi:10.3382/ps.0410954
Siegel P. B., Honaker C. F. (2025). Sexual dimorphism for juvenile body weight in chickens divergently selected for 8-week body weight. Front. Physiol. 15, 1534334. doi:10.3389/fphys.2024.1534334
Symeon G. K., Mantis F., Bizelis I., Kominakis A., Rogdakis E. (2010). Effects of caponization on growth performance, carcass composition, and meat quality of medium growth broilers. Poult. Sci. 89, 1481–1489. doi:10.3382/ps.2009-00411
Toews J. N. C., Philippe T. J., Hill L. A., Dordevic M., Miguelez-Crespo A., Homer N. Z. M., et al. (2022). Corticosteroid-binding globulin (SERPINA6) establishes postpubertal sex differences in rat adrenal development. Endocrinology 163, bqac152. doi:10.1210/endocr/bqac152
Wang Y., Guo W., Gaorui Gong G., Huang P., Mei J. (2024). Phenotypic and genetic analysis of sexual dimorphism in growth, feed intake and feed conversion efficiency in yellow catfish. Aquaculture 586, 740767. doi:10.1016/j.aquaculture.2024.740767
Keywords: sexual dimorphism, chicken, selection, growth, siegel 1
Citation: Scanes CG (2025) General commentary: sexual dimorphism for juvenile body weight in lines of chickens selected for 8-week body weight. Front. Physiol. 16:1607477. doi: 10.3389/fphys.2025.1607477
Received: 07 April 2025; Accepted: 02 June 2025;
Published: 11 June 2025.
Edited by:
Xiquan Zhang, South China Agricultural University, ChinaReviewed by:
Ibrahim F. Rehan, Menoufia University, EgyptCopyright © 2025 Scanes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colin G. Scanes, Y2dzY2FuZXNAaWNsb3VkLmNvbQ==
 Colin G. Scanes
Colin G. Scanes