- 1School of Athletic Training, Guangzhou Sport University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Human Sports Performance Science, Guangzhou Sport University, Guangzhou, Guangdong, China
- 3Department of Physical Education, Guangzhou Sport University, Guangzhou, China
- 4Faculty of Health Sciences and Sports, Macao Polytechnic University, Macao, China
- 5Badminton Technical and Tactical Analysis and Diagnostic Laboratory, Guangzhou Sport University, Guangzhou, China
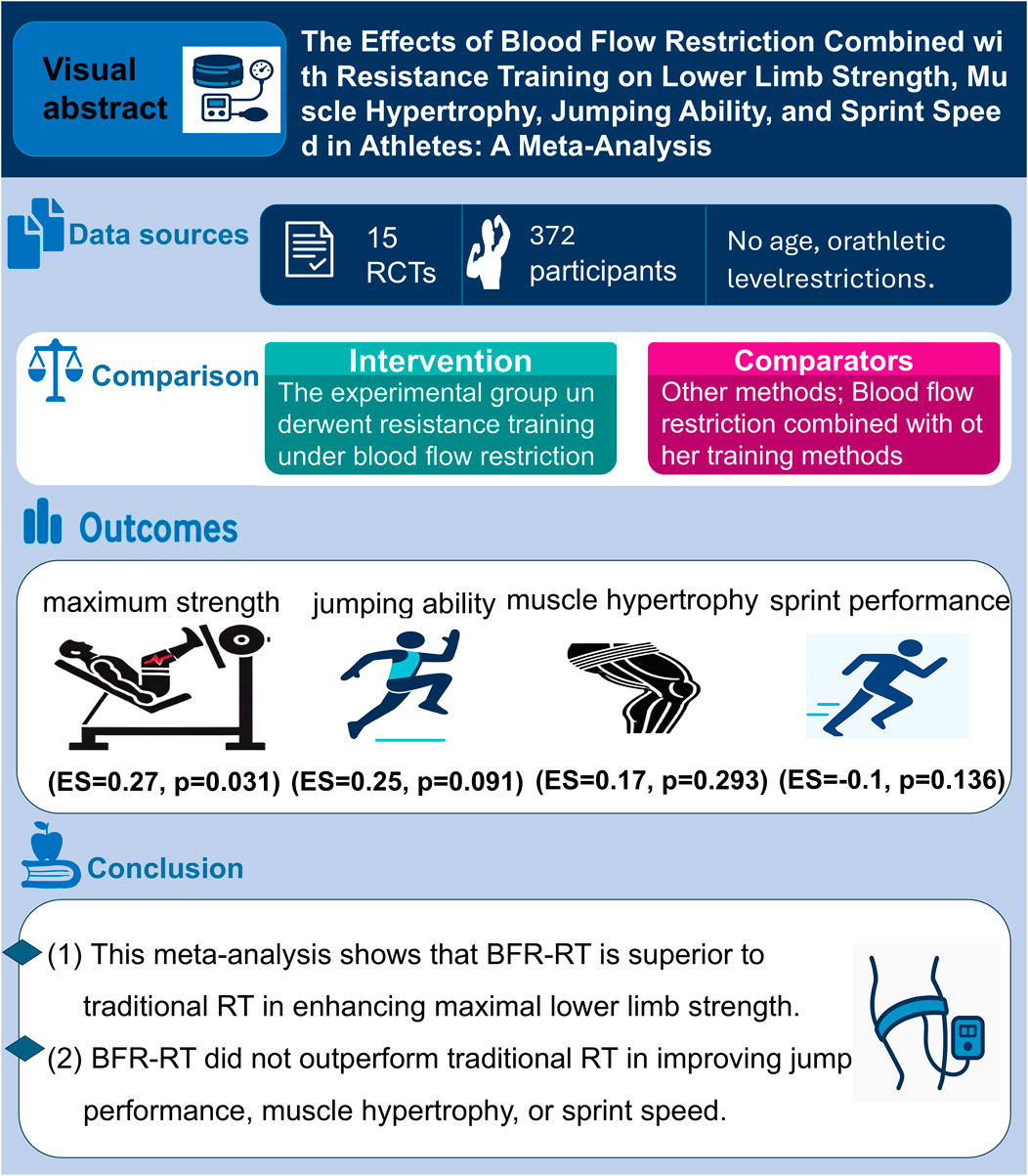
Objective: This meta-analysis aims to evaluate the comparative effects of blood flow restriction resistance training (BFR-RT) versus traditional resistance training (RT) on lower limb muscle hypertrophy, maximal strength, jumping ability, and sprint performance in athletes.
Methods: A comprehensive search was conducted across PubMed, Web of Science, the Cochrane Library, Embase and SPORTDiscus databases. This search identified 181 studies, of which 15 met the inclusion criteria. The quality of the studies was assessed using the Cochrane risk-of-bias tool, and data were analyzed using StataMP 17.0.
Results: The analysis revealed that BFR-RT significantly enhanced lower limb maximal strength (ES = 0.27, 95% CI: 0.03–0.52, p = 0.031, I2 = 25%), demonstrating its effectiveness in improving strength. However, no significant differences were observed between BFR-RT and RT for lower limb muscle hypertrophy (ES = 0.17, 95% CI: -0.15–0.50, p = 0.293, I2 = 0%), jumping ability (ES = 0.25, 95% CI: -0.04 to 0.54, p = 0.091, I2 = 0%), or sprint performance (ES = −0.1, 95% CI: 0.39–0.19, p = 0.136, I2 = 0%).
Conclusion: The findings suggest that while BFR-RT is effective in improving maximal strength, it does not offer additional benefits over traditional RT in terms of muscle hypertrophy, jumping ability, or sprint performance.
1 Introduction
Blood Flow Restriction Training (BFRT) is a technique that uses a cuff or tourniquet applied to the proximal limb to partially occlude blood flow during exercise (Anderson et al., 2019; Sato, 2005). By restricting venous return while allowing partial arterial inflow, BFRT creates a localized hypoxic environment that intensifies fatigue and stimulates muscular and metabolic adaptations even at low training loads (Yang et al., 2024; The Occlusion Cuff ®, 2025). This method has become increasingly widespread in both sports performance and rehabilitation contexts, as clinicians and coaches integrate BFRT to promote muscle growth, preserve function, and facilitate recovery in post-operative and athletic populations (Loenneke et al., 2015; Saraf et al., 2022). Notably, combining low-intensity exercise with vascular occlusion enables athletes to achieve gains in muscle size and strength typically associated with high-load resistance training (HL-RT), but with substantially reduced mechanical stress on joints and connective tissues (Yang et al., 2024; Lorenz et al., 2021).
Among its reported benefits, BFRT combined with resistance exercise (BFR-RT) has demonstrated considerable efficacy in eliciting hypertrophy and strength improvements comparable to conventional heavy lifting (Yang et al., 2024; Chang et al., 2024). Research indicates that BFR-RT activates similar molecular pathways, including mTOR signaling and myokine release, thereby driving protein synthesis and muscle adaptation (Yang et al., 2024). Because BFR-RT typically employs loads of only 20%–40% of one-repetition maximum (1RM), it offers a lower-risk training strategy for both healthy athletes and those recovering from injury (Lorenz et al., 2021). Additionally, BFRT has been linked to improvements in aerobic capacity and endurance performance, with studies showing greater increases in VO2max and time-to-exhaustion when low-load exercise is performed under vascular restriction compared to non-occluded training (Cognetti et al., 2022). From a clinical standpoint, BFRT is also used to attenuate muscle atrophy during periods of immobilization or rehabilitation (Saraf et al., 2022). Lorenz et al. and others have highlighted that BFRT enables patients to maintain strength and muscle mass when heavy loading is not yet feasible, effectively bridging early and later rehabilitation stages (Lorenz et al., 2021).
In athletic settings, BFR-RT has attracted attention as a tool to support or complement high-load training. For example, meta-analyses and controlled trials report that BFR-RT can improve explosive capabilities, such as jump height and sprint performance, and contribute to better overall conditioning (Yang et al., 2024; Wang et al., 2023). Its advantages include the use of much lighter loads (20%–40% 1RM with high repetitions) compared to traditional strength training protocols (70%–90% 1RM), which can reduce cumulative joint stress and the risk of overuse injury (Weaver et al., 2024). Despite the reduced mechanical tension, BFRT tends to induce comparable hypertrophy and strength gains while potentially allowing more frequent training sessions and less delayed-onset muscle soreness (Lorenz et al., 2021; Rodríguez et al., 2024).
Nevertheless, the precise impact of BFRT on performance-related outcomes remains subject to debate. Some studies have reported that low-load BFR-RT produces smaller increases in strength compared to traditional high-load protocols. For example, a recent meta-analysis by Chang et al. (2024) observed that while muscle hypertrophy did not differ significantly between BFR-RT and HL-RT, strength gains were modestly inferior in BFR-RT groups (Chang et al., 2024). Similarly, trials involving semi-professional soccer players found no additional improvements in sprinting or jumping ability when BFR-RT was added to regular training (Scott et al., 2017). These results suggest that in already well-trained athletes, the translation of BFR-induced adaptations to sport-specific performance may be inconsistent. In contrast, other systematic reviews and meta-analyses have highlighted substantial benefits of BFR-RT, reporting significant improvements in strength, power, speed, and endurance relative to conventional training (Wang et al., 2023). Such discrepancies likely arise from variations in training volume, frequency, cuff pressure, exercise selection, and the training status of participants.
While previous reviews have examined the effects of BFRT across diverse populations and training modalities—including aerobic BFR, passive BFR, and mixed interventions (Yang et al., 2024; Rui et al., 2023)—few studies have specifically focused on the impact of combining BFRT with resistance training in athletes. Given that BFR-RT has become the most common and practically applied form of BFRT in sports settings, there is an urgent need to clarify its relative effects on key performance indicators essential for athletic development, including lower-limb strength, muscle hypertrophy, jumping performance, and sprint speed. This study aims to concentrate on resistance training and further elucidate the influence of BFR-RT on athletic performance. Through a meta-analysis, the study will systematically evaluate the effects of BFR-RT on performance outcomes, synthesize the existing body of evidence, and determine its actual effectiveness.
2 Research methods
2.1 Search strategy
The literature search was conducted across the PubMed, Web of Science, the Cochrane Library, Embase and SPORTDiscus databases using the following keywords: “Blood flow Restriction,” “Kaatsu,” “Ischemic Training,” “BFRT Therapy,” “BFRT Therapies,” “BFRT,” “Blood Flow Restriction Training,” “Blood Flow Restriction Exercise,” “resistance training,” “resistance exercise,” “Resistance,” “Strength Training,” “Strengthening Programs,” “Weight Bearing Exercise Program,” “players,” “sportsman,” “athlete,” “sports person,” and “sportswomen.” The search timeframe covered all publications from the inception of each database until June 29, 2025. The detailed search strategy for each database is provided in Supplementary Appendix 1.
To ensure the accuracy of the literature search, two researchers independently verified the search terms. In cases where there was disagreement between the two researchers (B.D and R. Y) regarding the selection of search terms, a third researcher (G.L) made the final decision.
2.2 Study selection
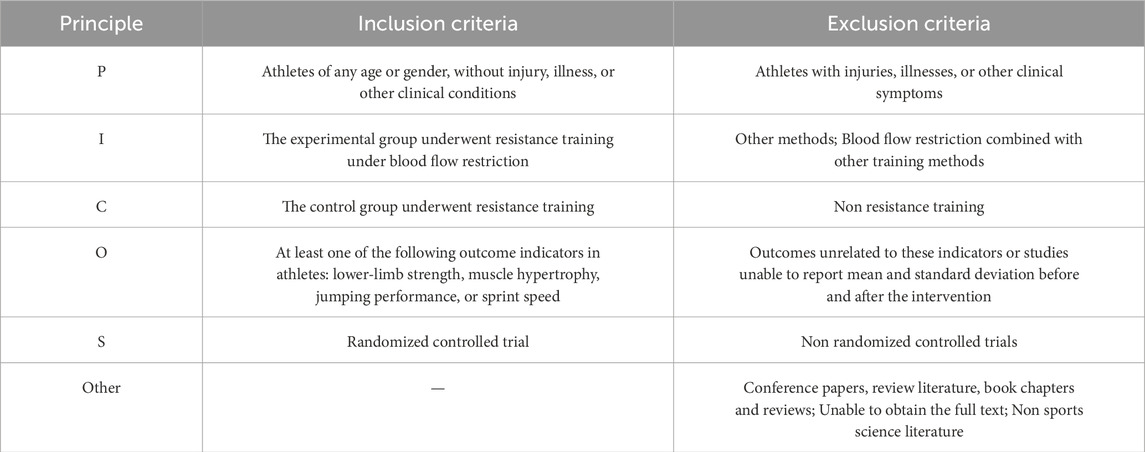
This meta-analysis was conducted in accordance with the guidelines of the Cochrane Collaboration (Cumpston et al., 2019) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). The study protocol was registered with the International Platform of Registered Systematic Review and Meta-Analysis Protocols (INPLASY202480005). The Population, Intervention, Comparator, Outcomes, and Study Design (PICOS) criteria were used to define the inclusion and exclusion criteria for this meta-analysis, as outlined in Table 1.
The retrieved records from each database were imported into EndNote X9 software for duplicate removal. Two researchers independently conducted an initial screening of the titles and abstracts to identify potentially relevant studies. Following this, both researchers thoroughly reviewed the full texts and assessed the studies based on the population, intervention, comparator, outcomes, and study design criteria to determine their eligibility according to the inclusion and exclusion standards. The study selection process was conducted independently by two researchers. In cases of disagreement, a third researcher (G.L) was consulted, and any conflicts were resolved through discussion until a consensus was reached. Inter-rater agreement for study eligibility assessment based on full-text review was substantial (Almost Perfect, Cohen’s kappa = 0.85).
2.3 Data extraction
Upon completing the search, detailed information was collected from the eligible articles. Two researchers (B.D and R. Y) independently extracted the data into a Microsoft Excel spreadsheet. The extracted data included the article title, publication year, author names, characteristics of the subjects (age, sample size of the experimental and control groups), training protocols (intervention duration, intervention methods, training frequency, training load, BFR-RT duration, cuff location, width, pressure, and intervention methods in both the experimental and control groups), outcome measures, and authors’ conclusions. If discrepancies arose in the data extracted by the two researchers, a third researcher (G.L) would extract the data and confirm the final version. Pre- and post-intervention data were recorded as mean ± standard deviation in the Excel spreadsheet, If the full text or specific data from the study were not accessible, the corresponding author was contacted to obtain the relevant information. Inter-rater reliability for study selection was calculated using Cohen’s kappa statistic (Almost Perfect, Cohen’s kappa = 0.83).
2.4 Risk of Bias Assessment
The quality of all included studies was assessed using the Cochrane Risk of Bias Tool. The evaluation criteria included “Low risk,” “Unclear risk,” and “High risk,” and the final results served as the basis for assessing the quality of the included studies. The quality assessment for all studies included in this meta-analysis was independently conducted by two researchers (B.D and R. Y). Inter-rater reliability for the Risk of Bias Assessment was also calculated using Cohen’s kappa statistic (Substantial, Cohen’s Kappa = 0.72).
2.5 Data analysis
All statistical analyses were performed using StataMP 17.0. As all variables were continuous, outcomes were reported as means and standard deviations. Standardized mean differences (SMDs) were calculated to estimate effect sizes, and forest plots were generated accordingly. A random-effects model was applied to compute pooled effect sizes across studies, regardless of heterogeneity, to account for potential clinical and methodological differences among included studies (Borenstein et al., 2021; Chandler et al., 2019). Heterogeneity was assessed using the I2 statistic, with values interpreted as follows: <25% indicating low heterogeneity, 25%–75% moderate, and >75% substantial heterogeneity (Van Tulder et al., 2003). When I2 exceeded 75%, sensitivity analyses were conducted to assess the robustness of the pooled results (Higgins et al., 2003). The magnitude of effect sizes was interpreted according to the scale proposed by Hopkins et al. 2009: <0.2 = trivial; 0.2–0.6 = small; 0.6–1.2 = moderate; 1.2–2.0 = large; 2.0–4.0 = very large; and >4.0 = extremely large. To assess potential publication bias, a funnel plot was constructed to visually inspect asymmetry in the distribution of study effect sizes. In addition, Egger’s regression test was performed to statistically evaluate the presence of publication bias (Sedgwick and Marston, 2015). A p-value of <0.05 was considered statistically significant.
3 Results
3.1 Literature search results
A total of 181 articles were retrieved from the databases, and an additional four articles were identified through manual search of the references in related studies. After removing duplicates, an initial screening was conducted based on the titles and abstracts. Full texts were then reviewed and re-screened, excluding studies that did not meet the inclusion criteria. Ultimately, 15 studies were included in the meta-analysis. The detailed process is illustrated in Figure 1.
3.2 Study characteristics
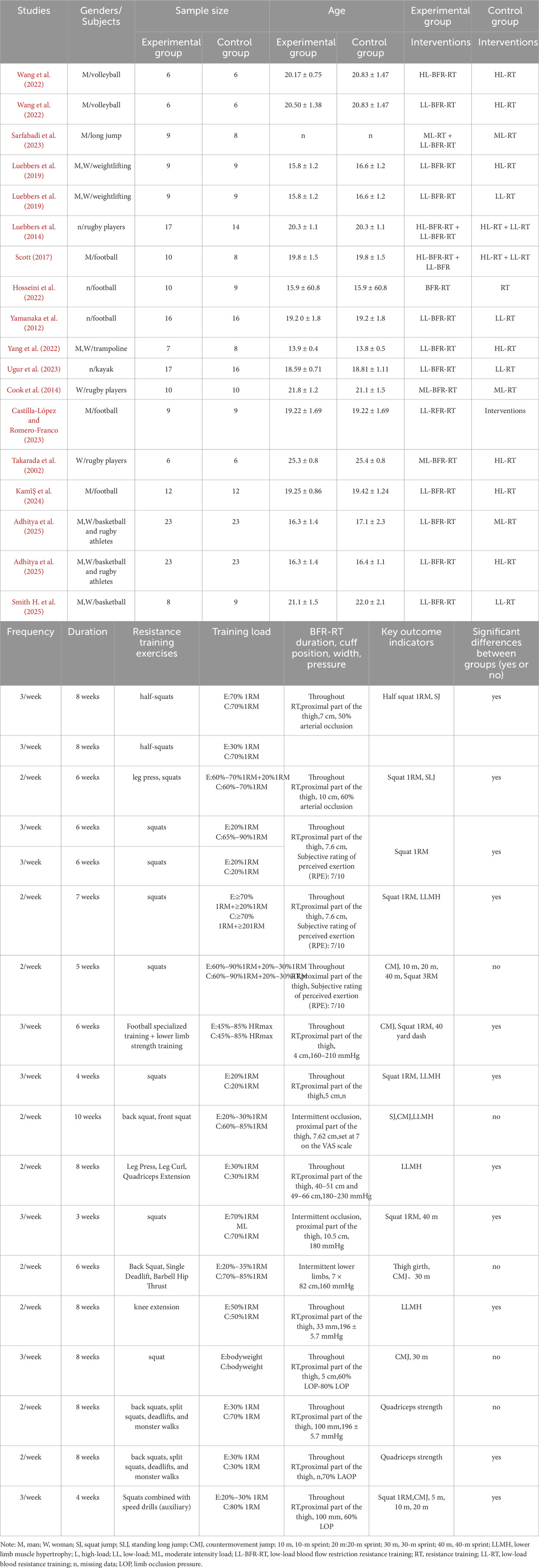
A total of 15 studies were included in this meta-analysis, encompassing 372 participants, all of whom were athletes. The experimental group, which underwent BFR-RT, included 185 participants, while the control group, which performed traditional RT, included 205 participants. The majority of the interventions were conducted at a frequency of 2–3 sessions per week, with intervention durations ranging from 3 to 10 weeks. The types of athletes included in the studies were volleyball players (Wang et al., 2022), long jumpers (Hopkins et al. 2009), weightlifters (Van Tulder et al., 2003), rugby players (Jessee et al., 2018; Geng et al., 2024; Vergara et al., 2024; Adhitya et al., 2025), football players (Wang et al., 2022; Garber et al., 2011; Sarfabadi et al., 2023; Luebbers et al., 2019; Kami̇Ş et al., 2024), netball athletes (Manimmanakorn et al., 2013), trampoline athletes (Yang et al., 2022), Basketball players (Adhitya et al., 2025; Smith H. et al., 2025), and canoeists (Ugur et al., 2023). Detailed characteristics of the included studies are presented in Table 2.
3.3 Risk of Bias Assessment
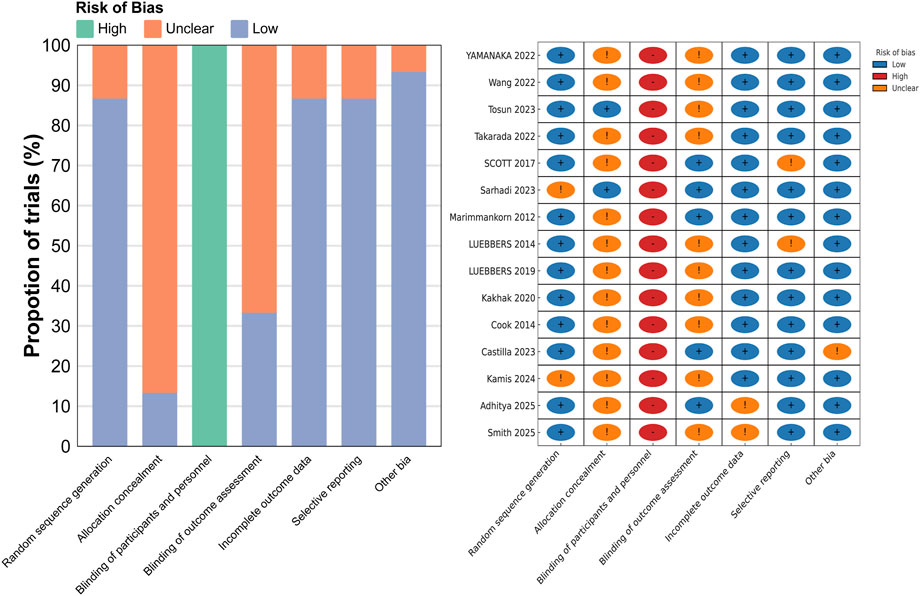
The quality assessment of the 15 included studies was conducted based on the Cochrane Risk of Bias Tool criteria, and the results are presented in Figure 2. All the included studies were randomized controlled trials. Regarding allocation concealment, 13 studies were assessed as having unclear risk, while only 2 studies were considered to have a low risk. Due to the nature of the interventions, all studies were rated as high risk for blinding of participants and personnel, as blinding was not feasible. For blinding of outcome assessment, 10 studies were assessed as having unclear risk, and 5 studies were rated as low risk. In terms of incomplete outcome data, 2 studies were assessed as having unclear risk, and 13 studies were rated as low risk. Regarding selective reporting, 2 studies were assessed as having unclear risk, while the remaining 13 studies were considered to have low risk. Regarding other biases, 1 study was assessed as having unclear risk, while the remaining studies were considered to have low risk.
3.4 Meta-analysis results
3.4.1 Lower limb muscle hypertrophy
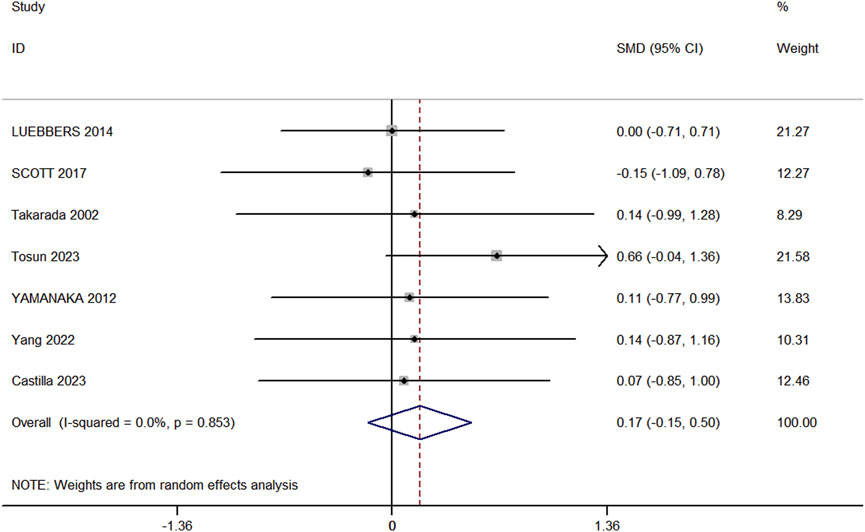
Seven studies (comprising 7 experimental and 7 control groups, totaling 147 participants) were included in the analysis comparing the effects of RT and BFR-RT on lower limb muscle hypertrophy (Figure 3). The heterogeneity test yielded an I2 of 0% and a p-value of 0.853, indicating no significant heterogeneity. The meta-analysis showed a pooled effect size (ES) of 0.17 (95% CI: 0.15 to 0.50, p = 0.293), suggesting no statistically significant difference between the groups.
3.4.2 Lower limb maximal strength
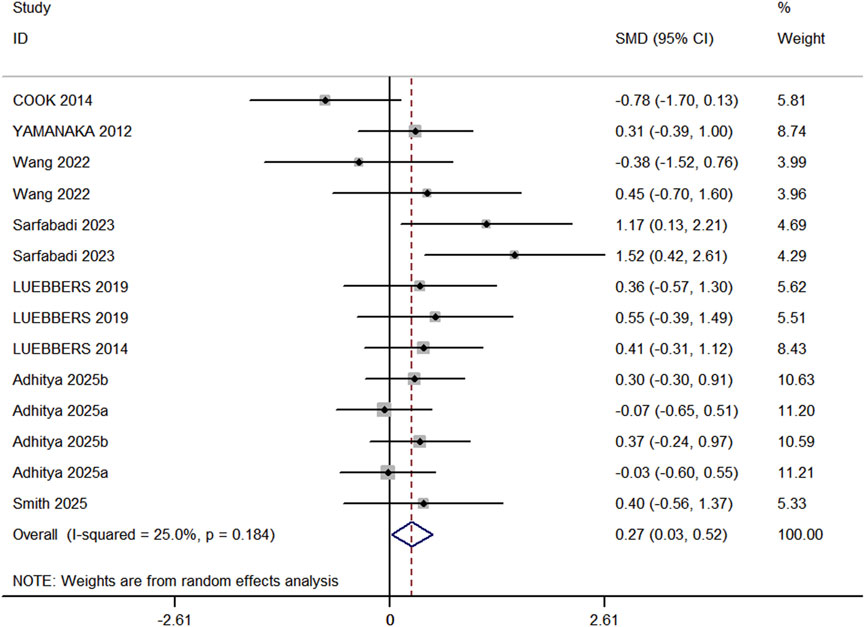
In the included studies, 8 studies (comprising 11 experimental groups and 14 control groups, with a total of 248 participants) compared the effects of RT and BFR-RT on lower limb athletic performance in athletes (Figure 4). The heterogeneity test revealed an I2 value of 25% and a P value of 0.184, indicating low heterogeneity. The meta-analysis results showed a pooled effect size (ES) of 0.27 (95% CI: 0.03–0.52, p = 0.031), indicating a statistically significant difference favoring BFR-RT over RT.
3.4.3 Jump performance
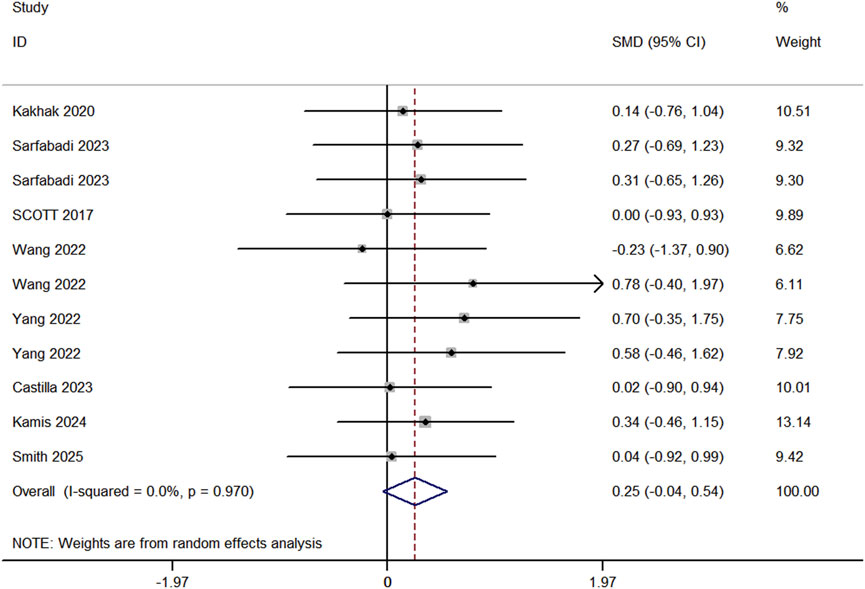
Eight studies (comprising 11 experimental and 10 control groups, with a total of 207 participants) compared the effects of RT and BFR-RT on jump performance in athletes (Figure 5). The heterogeneity test indicated an I2 of 0% and a p-value of 0.970, showing no significant heterogeneity. The meta-analysis resulted in a pooled effect size (ES) of 0.25 (95% CI: 0.04 to 0.54, p = 0.091), with no statistically significant difference between the groups.
3.4.4 Sprint speed
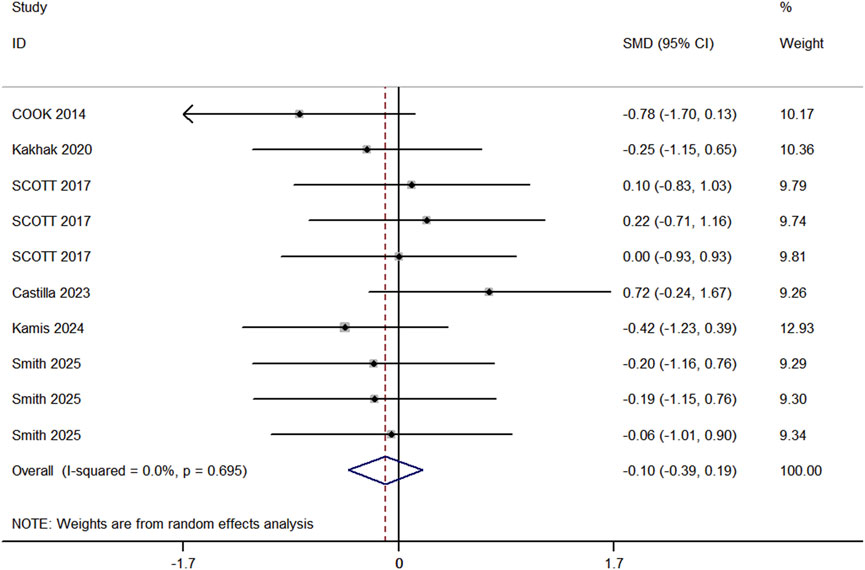
In the included studies, 6 trials compared the sprint performance between RT and BFR-RT groups in athletes (Figure 6), comprising 9 experimental groups, 9 control groups, and a total of 201 participants. The heterogeneity test revealed I2 = 0% and p = 0.695, indicating substantial heterogeneity across the studies. The meta-analysis showed a pooled effect size (ES) of −0.10 (95% CI: 0.39 to 0.19, p = 0.136), with no statistically significant difference between the groups.
3.5 Publication bias
Funnel plots for all outcomes showed approximate symmetry, and Egger’s regression tests revealed no significant publication bias, suggesting a low risk of publication bias (Figure 7).
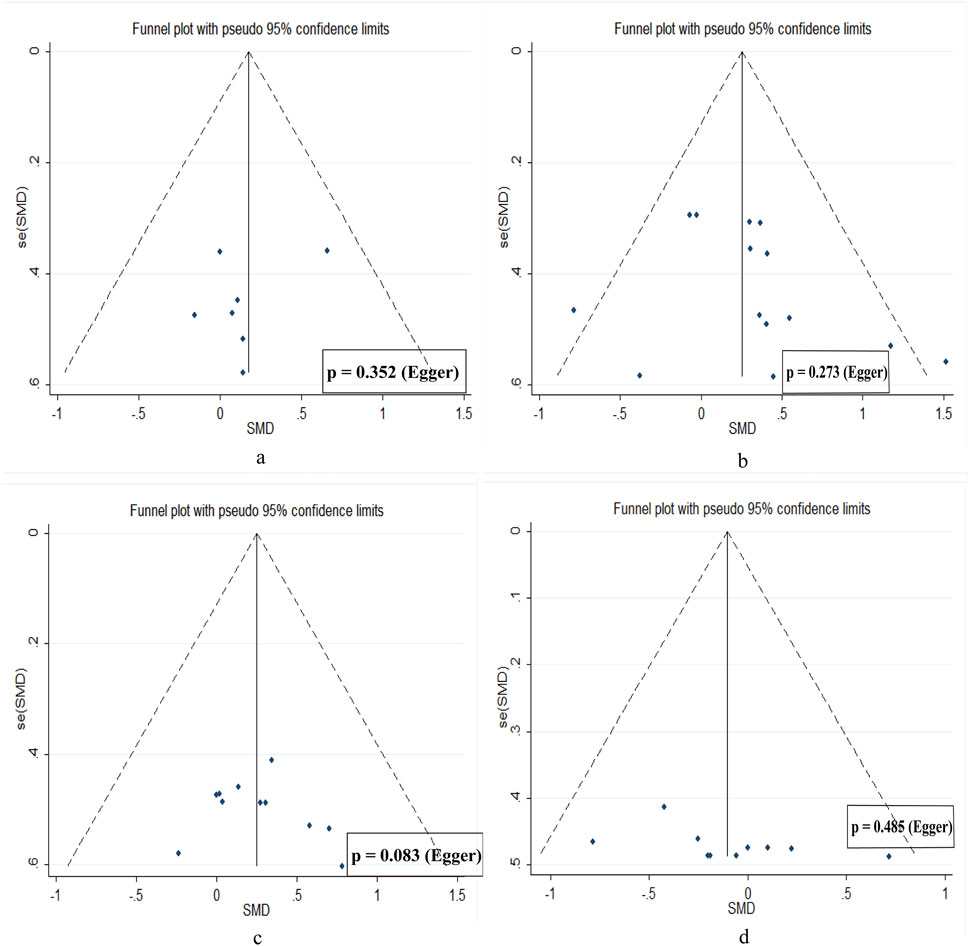
Figure 7. Publication bias diagram. (a) muscle hypertrophy. (b) low limb strength. (c) jump performance. (d) sprint speed.
4 Discussion
4.1 The impact of BFR-RT on lower limb muscle hypertrophy
The meta-analysis results indicate that BFR-RT is not an effective method for enhancing lower limb muscle hypertrophy in athletes when compared to RT alone. This finding is consistent with prior systematic reviews by de Ruiter et al. (2007) and Sadamoto et al. (1983), as well as many of the studies included in the present analysis (Scott et al., 2017; Yang et al., 2022; Ugur et al., 2023; Luebbers et al., 2014; Yamanaka et al., 2012), all of which reported similar results.
Individual studies agree. Laurentino et al. (2008) showed that applying an occlusion cuff during 8 weeks of heavy leg extensions did not augment quadriceps CSA gains relative to training without cuffs. Likewise, Dankel et al. (2016) found that blood flow restriction added to high-load biceps curls produced no greater muscle activation or hypertrophy than high-load exercise alone–concluding that BFR “would seem unlikely to induce greater muscle hypertrophy” under those conditions pubmed.ncbi.nlm.nih.gov. Teixeira et al. (2021) reported similar results: after 8 weeks of unilateral knee extensions, all protocols (high-load with BFR during rest, with BFR during lifting, or with no BFR) showed ∼6–7% quadriceps CSA increases with no between-group differencesjournals.lww.com. In sum, adding BFR to an already intense program did not confer any extra muscle size gain.
A likely reason is redundancy of the hypertrophic stimulus. Maximal contractions themselves generate very high intramuscular pressure, effectively occluding blood flow and causing the same hypoxic, metabolite-rich environment that BFR aims to induce. In other words, heavy lifting “per se” creates near-occlusion in the muscle (Teixeira et al., 2021), so supplemental cuff pressure adds little new stress. Moreover, the once-popular hormonal hypothesis has been largely disproven: acute surges of GH, IGF-1, testosterone, etc. from exercise have no clear link to long-term muscle growth (Lixandrão et al., 2018). Recent evidence indicates that systemic hormonal spikes do not predict how much muscle is gained–mechanical and metabolic stress appear to be the true drivers (Lixandrão et al., 2018). Thus, expecting BFR to boost growth via extra hormone release or “metabolic shock” beyond what heavy loads already provide is not supported by current knowledge.
It is worth noting that measurable muscle hypertrophy typically requires several weeks of training. For example, in Teixeira’s study, all groups achieved about 7% muscle growth after 8 weeks (Teixeira et al., 2021). Most RT studies run ≥8–12 weeks to capture muscle growth. Our meta included few very long trials, so we cannot exclude subtle effects over longer periods. Future work should employ longer interventions (e.g., ≥10–12 weeks) to determine if chronic adaptations diverge when BFR is combined with heavy lifting. Until then, the evidence suggests that for trained athletes, BFR + RT offers no hypertrophy advantage over RT alone (Teixeira et al., 2021; Lixandrão et al., 2018). Taken together, these findings suggest that in trained athletes, combining blood flow restriction with resistance training does not produce additional hypertrophic benefits compared to resistance training alone.
4.2 The impact of BFR-RT on lower limb maximal strength
The studies included in this analysis assessed strength by measuring the one-repetition maximum in both full and half squats, with results indicating that BFR-RT has a positive impact on lower limb strength. Additionally, the study by Yasuda et al. (2006) demonstrated that 6 weeks of blood flow restriction resistance training led to an average strength increase of 0.3 kg, further validating the effectiveness of BFR-RT in enhancing lower limb strength. Other studies have also reported significant improvements in muscular strength following BFR-RT (36), reinforcing its applicability in athletic training.
However, not all investigations concur. Gavanda et al. (2020) found no additional improvement in leg-press 1RM after 8 weeks of low-load BFR-RT (20% 1RM, 40% limb-occlusion pressure) compared with volume-matched RT in healthy males. Likewise, Lixandrão et al. (2015) observed only trivial changes in squat strength when cuff pressure was set below 40% LOP, suggesting that sub-threshold pressures may fail to elicit sufficient mechanical and metabolic stress.
Most meta-analyses and studies confirm that BFR-RT significantly enhances lower limb strength (Ugur et al., 2023; Yamanaka et al., 2012; Cook et al., 2014). The primary mechanisms underlying this effect include increased mechanical tension and metabolic stress, both of which are considered key factors in muscle adaptation and strength development (Luebbers et al., 2014; Cook et al., 2014). BFR-RT creates a hypoxic and acidic environment (Yasuda et al., 2006), promoting lactate accumulation, which in turn affects muscle contraction and sustains force output by increasing motor unit recruitment (Yamanaka et al., 2012). Additionally, the accumulation of metabolites and cellular swelling (Jessee et al., 2018; Loenneke et al., 2012) may further enhance training adaptations, while the additional recruitment of type II muscle fibers (Loenneke et al., 2015) is regarded as one of the crucial mechanisms by which BFR-RT improves strength performance. Furthermore, research suggests that BFR-RT can stimulate the secretion of anabolic hormones and accelerate the fatigue process, thereby promoting greater motor unit recruitment and overall strength gains (Yasuda et al., 2014).
From a physiological perspective, the high training frequency and appropriate loading levels in BFR-RT enhance muscle activation and increase motor unit recruitment rates during slow, high-tension contractions (Wang et al., 2022). The application of cuff pressure not only facilitates metabolite accumulation but also influences hormone secretion levels (Takano et al., 2005), thereby amplifying training adaptations. Specifically, BFR-RT elicits strength-type neural adaptations—greater motor-unit recruitment and firing rates under sustained tension—while simultaneously promoting protein synthesis, lactate accumulation, and growth-hormone release (Takano et al., 2005; Takarada et al., 2000). Notably, although BFR-RT is effective in improving strength, its adaptive mechanisms may rely more on neural adaptations rather than muscle hypertrophy (Gabriel et al., 2006).
However, several trials indicate that BFR-RT may function mainly as a complement to high-load RT rather than provide additional strength adaptations—especially when strength is evaluated via 1RM, where it often fails to surpass traditional ≥70% 1RM training (Held et al., 2020). Discrepancies across studies appear to hinge on programme and cuff-application variables. For example, Chen (Chen et al., 2022) employed twice-weekly sessions at 30% 1RM with 40% limb-occlusion pressure (LOP) and reported modest gains, whereas Hosseini Kakhak (Hosseini et al., 2022) used four sessions per week at 40% 1RM with 80% LOP and a 13 cm pneumatic cuff, yielding significantly larger improvements. Such contrasts suggest that load (<30% vs. ≥ 40% 1RM), weekly frequency (≤2 vs. ≥ 3 sessions), cuff width (≤5 cm vs. ≥ 13 cm), and—most critically—relative pressure (<50% vs. 60%–80% LOP) jointly modulate mechanical tension, metabolic stress, and thus strength adaptation. Therefore, future research should systematically manipulate these parameters to define an optimal BFR-RT prescription and to clarify its suitability across diverse athletic populations.
4.3 The impact of BFR-RT on jump performance
The results of the meta-analysis indicate that, compared to RT, BFR-RT is not an effective method for improving lower limb explosiveness in athletes. This finding is not entirely consistent with Xiaolin’s meta-analysis (Wang et al., 2023), which may be due to the inclusion of a “healthy population,” while our study specifically targeted athletes. This difference may have led to contrasting trends in the effects of BFR-RT on lower limb explosiveness.
First, Enhancing lower-limb explosiveness relies on rapid motor-unit firing synchrony, a high rate of force development, and efficient stretch–shortening-cycle behaviour (Enoka, 1997; Moritani, 1993). The BFR-RT interventions included in our meta-analysis mainly used slow-tempo, single-plane resistance exercises and did not incorporate plyometric or ballistic elements—stimuli that are essential for eliciting the explosive-type neural adaptations underlying vertical-jump improvements (Markovic and Mikulic, 2010; Iacono et al., 2016). These findings suggest that the specificity of the movement stimulus—rather than the blood-flow-restriction modality itself—determines whether strength-type gains translate into explosive ability.
Secondly, the impact of BFR-RT on the tendon and tendon-aponeurosis complex is also an important consideration. Existing research indicates that tendon stiffness and elasticity play a critical role in lower limb explosiveness (Maciejewska-Skrendo et al., 2020). Kubo et al.’s study found that, after 12 weeks, LL-BFR-RT did not significantly alter the tensile properties and stiffness of the tendon-aponeurosis complex. Tendon stiffness is believed to be positively correlated with explosive performance in athletes (Kubo et al., 2006; Madarame et al., 2011), especially in actions such as jumping and sprinting, where tendons contribute to force output by storing and releasing elastic energy. Therefore, the lack of significant improvement in lower limb explosiveness in this study may be related to insufficient effects of BFR-RT on tendon characteristics.
Furthermore, in Li et al. (2023) ’s research, BFR-RT was shown to indirectly promote vertical jump height by enhancing muscle metabolic adaptability and motor unit recruitment. However, the results of this study differ from LI et al.’s meta-analysis, which could be due to differences in experimental design, sample characteristics, and research conditions. Li et al. (2023)’s study may have included various types of training methods or sample groups, while our study focused specifically on resistance training, which might have had a different impact on enhancing lower limb explosiveness.
In conclusion, although BFR-RT shows potential for enhancing strength and explosiveness, its effects appear to be no greater than traditional RT. Future research could further explore the effects of BFR-RT under different conditions, such as varying athletic populations, training loads, and cycles, through subgroup analyses, to better understand its role in explosive power training.
4.4 The impact of BFR-RT on sprint performance
The meta-analysis results indicate that BFR-RT does not significantly improve sprint performance in athletes compared to RT alone. This finding is consistent with the majority of studies included in this analysis (Scott et al., 2017; Manimmanakorn et al., 2013; Hosseini et al., 2022; Cook et al., 2014). Research by Fostiak et al. (2022) also found that 30-m sprint performance did not improve following BFR training. The included studies evaluated sprint performance using measures such as 5-m, 10-m, 20-m, 40-m, and 40-yard sprints to comprehensively assess the impact of BFR-RT on sprint performance.
Critical factors influencing sprint performance include reaction time, technique, electromyography (EMG) activity, and neural factors. Reaction time and technique are largely influenced by genetics and training (Mero et al., 1992). In terms of other factors, EMG is often used to assess changes in motor unit recruitment, firing frequency, and synchronization (Wang et al., 2022). However, studies have shown no significant differences in EMG amplitude between BFR-RT and RT (Wang et al., 2022; Moore et al. 2004). Additionally, neural adaptations, such as increased motor unit activation and synchronization, are considered crucial for improving maximal power output (Enoka, 1997; Moritani, 1993; Fujita et al., 2008). RT research indicates that maximal voluntary contraction can increase motor unit activation (Folland and Williams, 2007). However, studies on BFR-RT have not shown significant changes in motor unit activation (Wang et al., 2022; Gabriel et al., 2006; Kubo et al., 2006; Scott et al., 2016), which may explain why BFR-RT does not significantly outperform RT in sprint performance.
It is noteworthy that one study included in our analysis found that while there was no significant difference between LL-BFR-RT and LL-RT in the 10-m sprint, LL-RT outperformed LL-BFR-RT in the 5-m sprint (Manimmanakorn et al., 2013). This suggests that BFR-RT might be more effective than RT for certain short-distance sprints. Systematic reviews by Wortman (Wortman et al., 2021) and research by Mckee et al. (2023) also pointed to the positive effects of BFR-RT on sprint performance. This might be because BFR increases metabolic stimuli during exercise, which more effectively triggers sympathetic nervous system activity, enhancing reaction speed and, consequently, displacement speed (Scott et al., 2016; Kiyohara et al., 2006).
Given these conflicting results, it is possible that BFR-RT’s effects vary with different sprint distances, and differences in BFR settings, training loads, and athlete adaptation levels may lead to varying outcomes. Therefore, more studies are needed to verify the effects of BFR-RT on specific sprint distances in different sports.
5 Practical applications
Our findings indicate that blood-flow-restriction resistance training (BFR-RT) is a reliable method for increasing maximal lower-limb strength. For muscle hypertrophy, explosive power, and sprint speed, BFR-RT performed comparably to traditional high-load resistance training. When size or speed is the foremost objective, programmes centred on conventional high-load work may therefore remain the first choice, with BFR-RT serving as a useful alternative whenever heavy loads are impractical—such as during rehabilitation, in-season maintenance, or deload phases. Ultimately, coaches should match the training tool to the athlete’s goals, phase of training, and tolerance for mechanical stress, integrating BFR-RT alongside traditional methods when it adds logistical or physiological value.
6 Limitations
Despite the systematic integration of existing research results through this meta-analysis and the evaluation of BFR-RT’s impact on athletes’ jump performance, sprint speed, lower limb maximal strength, and muscle hypertrophy, several limitations exist. First, the study only included publications in English, with only 15 studies meeting the inclusion criteria. Additionally, the majority of the studies included had intervention periods of 6–8 weeks, while some performance indicators might require longer durations to show significant effects. Future research should focus on improving sample sizes, study design, and control of external factors to provide more reliable and comprehensive evidence.
7 Conclusion
This meta-analysis summarizes the effects of BFR-RT on athletic performance. The results indicate that BFR-RT is superior to traditional RT in enhancing maximal lower limb strength. However, BFR-RT did not show superior effects over RT in jump performance, and no significant advantage was found in lower limb muscle hypertrophy, particularly in muscle hypertrophy and sprint speed training, where RT demonstrated more pronounced effects. Therefore, for these training objectives, RT should be prioritized.
Author contributions
BD: Writing – original draft, Methodology, Data curation, Supervision, Software, Conceptualization, Writing – review and editing. GL: Software, Writing – review and editing, Formal Analysis, Data curation, Methodology, Conceptualization, Supervision. YS: Writing – review and editing, Supervision, Formal Analysis, Data curation, Methodology, Investigation. DL: Formal Analysis, Writing – review and editing, Methodology, Supervision, Conceptualization. ZG: Software, Writing – review and editing, Visualization, Methodology, Validation, Supervision, Formal Analysis. CL: Validation, Writing – review and editing, Methodology, Data curation, Formal Analysis, Visualization. JS: Resources, Funding acquisition, Visualization, Project administration, Formal Analysis, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the 2022 Science and Technology Innovation Project of the General Administration of Sport of China (22KJCX013): Research on the Scientific Fitness Demonstration Path of Intelligent Physical Training in the New Media Era.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphys.2025.1687793.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1612685/full#supplementary-material
Abbreviations
BFRT, Blood flow restriction training; RTE, test group Resistance training; BFR-RTE, test group combining Blood Flow Restriction with Resistance Training; CMJ, Countermovement jump; 1RM, one-repetition maximum; E, test group; C, control group; SJ, Squat jump; SLJ, Standing long jump; CMJ, Countermovement jump; 10 m, 10-m sprint; 20 m, 20-m sprint; 30 m, 30-m sprint; 40 m, 40-m sprint; HL, high-load; ML, Moderate intensity load; LL, low-load; LL-BFR-RT, low-load blood flow restriction resistance training; LL-MH, Lower limb muscle hypertrophy; n, missing data. W Woman; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; M, Man; W, Woman.
References
Adhitya I., Aryana I., Kurniawati I., Thanaya S., Utama M. (2025). Effect of low-load blood flow restriction training and high-load resistance training on quadriceps strength, dynamic stability, and functional performance. Clin. J. Sport Med. doi:10.1097/JSM.0000000000001330
Anderson A. B., Owens J. G., Patterson S. D., Dickens J. F., LeClere L. E. (2019). Blood flow restriction therapy: from development to applications. Sports Med. Arthrosc. Rev. 27 (3), 119–123. doi:10.1097/JSA.0000000000000240
Borenstein M., Hedges L. V., Higgins J. P., Rothstein H. R. (2021). Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons. Available online at: https://books.google.com/books?hl=zh-CN&lr=&id=pdQnEAAAQBAJ&oi=fnd&pg=PP1&dq=Introduction+to+Meta-Analysis&ots=WFXnyTfNNZ&sig=sfMfVLCcpvWuAQZb6QIat7GIQQ8.
Castilla-López C., Romero-Franco N. (2023). Low-load strength resistance training with blood flow restriction compared with high-load strength resistance training on performance of professional soccer players: a randomized controlled trial. J. Sports Med. Phys. Fit. 63 (11), 1146–1154. doi:10.23736/S0022-4707.23.14974-7
Chandler J., Cumpston M., Li T., Page M. J., Welch V. (2019). Cochrane handbook for systematic reviews of interventions. Hob: Wiley. Available online at: https://dariososafoula.wordpress.com/wp-content/uploads/2017/01/cochrane-handbook-for-systematic-reviews-of-interventions-2019-1.pdf.
Chang H., Zhang J., Yan J., Yang X., Chen B., Zhang J. (2024). Effects of blood flow restriction training on muscle strength and hypertrophy in untrained males: a systematic review and meta-analysis based on a comparison with high-load resistance training. Life (basel Switz.) 14 (11), 1442. doi:10.3390/life14111442
Chen Y. T., Hsieh Y. Y., Ho J. Y., Lin T. Y., Lin J. C. (2022). Running training combined with blood flow restriction increases cardiopulmonary function and muscle strength in endurance athletes. J. Strength Cond. Res. 36 (5), 1228–1237. doi:10.1519/JSC.0000000000003938
Cognetti D. J., Sheean A. J., Owens J. G. (2022). Blood flow restriction therapy and its use for rehabilitation and return to sport: physiology, application, and guidelines for implementation. Arthrosc. Sports Med. Rehabil. 4 (1), e71–e76. doi:10.1016/j.asmr.2021.09.025
Cook C., Kilduff L., Beaven C. (2014). Improving strength and power in trained athletes with 3 weeks of occlusion training. Int. J. Sports Physiol. Perform. 9 (1), 166–172. doi:10.1123/ijspp.2013-0018
Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Db Syst. Rev. 2019 (10), ED000142. doi:10.1002/14651858.ED000142
Dankel S. J., Buckner S. L., Jessee M. B., Mattocks K. T., Mouser J. G., Counts B. R., et al. (2016). Post-exercise blood flow restriction attenuates muscle hypertrophy. Eur. J. Appl. Physiol. 116 (10), 1955–1963. doi:10.1007/s00421-016-3447-2
de Ruiter C. J., Goudsmit J. F., Van Tricht J. A., De Haan A. (2007). The isometric torque at which knee-extensor muscle reoxygenation stops. Med. Sci. Sports Exerc. 39 (3), 443–453. doi:10.1249/mss.0b013e31802dd3cc
Enoka R. M. (1997). Neural adaptations with chronic physical activity. J. Biomech. 30 (5), 447–455. doi:10.1016/s0021-9290(96)00170-4
Folland J. P., Williams A. G. (2007). The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 37 (2), 145–168. doi:10.2165/00007256-200737020-00004
Fostiak K., Bichowska M., Trybulski R., Trabka B., Krzysztofik M., Rolnick N., et al. (2022). Acute effects of ischemic intra-conditioning on 30 m sprint performance. Int. J. Environ. Res. Public Health 19 (19), 12633. doi:10.3390/ijerph191912633
Fujita T., Wf B., Kurita K., Sato Y., Abe T. (2008). Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int. J. KAATSU Train. Res. 4 (1), 1–8. doi:10.3806/ijktr.4.1
Gabriel D. A., Kamen G., Frost G. (2006). Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 36 (2), 133–149. doi:10.2165/00007256-200636020-00004
Garber C. E., Blissmer B., Deschenes M. R., Franklin B. A., Lamonte M. J., Lee I. M., et al. (2011). American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 43 (7), 1334–1359. doi:10.1249/MSS.0b013e318213fefb
Gavanda S., Isenmann E., Schlöder Y., Roth R., Freiwald J., Schiffer T., et al. (2020). Low-intensity blood flow restriction calf muscle training leads to similar functional and structural adaptations than conventional low-load strength training: a randomized controlled trial. PLOS One 15 (6), e0235377. doi:10.1371/journal.pone.0235377
Geng Y., Wu X., Zhang Y., Zhang M. (2024). Potential moderators of the effects of blood flow restriction training on muscle strength and hypertrophy: a meta-analysis based on a comparison with high-load resistance training. Sports Med. - Open 10 (1), 58. doi:10.1186/s40798-024-00719-3
Held S., Behringer M., Donath L. (2020). Low intensity rowing with blood flow restriction over 5 weeks increases V̇O2max in elite rowers: a randomized controlled trial. J. Sci. Med. Sport 23 (3), 304–308. doi:10.1016/j.jsams.2019.10.002
Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hopkins W. G., Marshall S. W., Batterham A. M., Hanin J. (2009). Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 41 (1), 3–13. doi:10.1249/MSS.0b013e31818cb278
Hosseini K. S., Kianigul M., Haghighi A., Nooghabi M., Scott B. (2022). Performing soccer-specific training with blood flow restriction enhances physical capacities in youth soccer players. J. Strength Cond. Res. 36 (7), 1972–1977. doi:10.1519/JSC.0000000000003737
Iacono A. D., Martone D., Padulo J. (2016). Acute effects of drop-jump protocols on explosive performances of elite handball players. J. Strength Cond. Res. 30 (11), 3122–3133. doi:10.1519/JSC.0000000000001393
Jessee M. B., Mattocks K. T., Buckner S. L., Dankel S. J., Mouser J. G., Abe T., et al. (2018). Mechanisms of blood flow restriction: the new testament. Tech. Orthop. 33 (2), 72–79. doi:10.1097/bto.0000000000000252
Kami̇Ş O., Kurt M., Blaisdell R., MacDONALD C., Nobari H. (2024). Effect of bodyweight squat exercise with blood flow restriction on sprint and jump performance in collegiate soccer players. Pamukkale J. Sport Sci. 15 (3), 632–648. doi:10.54141/psbd.1516188
Kiyohara N., Kimura T., Tanaka T., Moritani T. (2006). Enhancement of cardiac autonomic nervous system activity by blood flow restriction in the human leg. Int. J. KAATSU Train. Res. 2 (2), 35–43. doi:10.3806/ijktr.2.35
Kubo K., Komuro T., Ishiguro N., Tsunoda N., Sato Y., Ishii N., et al. (2006). Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. J. Appl. Biomech. 22 (2), 112–119. doi:10.1123/jab.22.2.112
Laurentino G., Ugrinowitsch C., Aihara A., Fernandes A., Parcell A., Ricard M., et al. (2008). Effects of strength training and vascular occlusion. Int. J. Sports Med. 29 (8), 664–667. doi:10.1055/s-2007-989405
Li R., Chee C. S., Kamalden T. F., Ramli A. S., Yang K. (2023). Effects of blood flow restriction training on sports performance in athletes: a systematic review with meta-analysis. J. Sports Med. Phys. Fit. 10, S0022–S4707. doi:10.23736/S0022-4707.23.15220-0
Lixandrão M. E., Ugrinowitsch C., Berton R., Vechin F. C., Conceição M. S., Damas F., et al. (2018). Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 48 (2), 361–378. doi:10.1007/s40279-017-0795-y
Lixandrão M. E., Ugrinowitsch C., Laurentino G., Libardi C. A., Aihara A. Y., Cardoso F. N., et al. (2015). Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur. J. Appl. Physiol. 115 (12), 2471–2480. doi:10.1007/s00421-015-3253-2
Loenneke J. P., Fahs C. A., Rossow L. M., Abe T., Bemben M. G. (2012). The anabolic benefits of venous blood flow restriction training May be induced by muscle cell swelling. Med. Hypotheses 78 (1), 151–154. doi:10.1016/j.mehy.2011.10.014
Loenneke J. P., Kim D., Fahs C. A., Thiebaud R. S., Abe T., Larson R. D., et al. (2015). Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 51 (5), 713–721. doi:10.1002/mus.24448
Lorenz D. S., Bailey L., Wilk K. E., Mangine R. E., Head P., Grindstaff T. L., et al. (2021). Blood flow restriction training. J. Athl. Train. 56 (9), 937–944. doi:10.4085/418-20
Luebbers P., Fry A., Kriley L., Butler M. (2014). The effects of a 7-week practical blood flow restriction program on well-trained collegiate athletes. J. Strength Cond. Res. 28 (8), 2270–2280. doi:10.1519/JSC.0000000000000385
Luebbers P. E., Witte E. V., Oshel J. Q., Butler M. S. (2019). Effects of practical blood flow restriction training on adolescent lower-body strength. J. Strength Cond. Res. 33 (10), 2674–2683. doi:10.1519/JSC.0000000000002302
Maciejewska-Skrendo A., Leźnicka K., Leońska-Duniec A., Wilk M., Filip A., Cięszczyk P., et al. (2020). Genetics of muscle stiffness, muscle elasticity and explosive strength. J. Hum. Kinet. 74, 143–159. doi:10.2478/hukin-2020-0027
Madarame H., Ochi E., Tomioka Y., Nakazato K., Ishii N. (2011). Blood flow-restricted training does not improve jump performance in untrained young men. Acta Physiol. Hung 98 (4), 465–471. doi:10.1556/APhysiol.98.2011.4.10
Manimmanakorn A., Hamlin M. J., Ross J. J., Taylor R., Manimmanakorn N. (2013). Effects of low-load resistance training combined with blood flow restriction or hypoxia on muscle function and performance in netball athletes. J. Sci. Med. Sport 16 (4), 337–342. doi:10.1016/j.jsams.2012.08.009
Markovic G., Mikulic P. (2010). Neuro-musculoskeletal and performance adaptations to lower-extremity plyometric training. Sports Med. 40 (10), 859–895. doi:10.2165/11318370-000000000-00000
Mckee J., Girard O., Peiffer J., Scott B. (2023). Repeated-sprint training with blood flow restriction does not provide additional benefits in repeated-sprint ability. J. Sci. Med. Sport 26, S109–S110. doi:10.1016/j.jsams.2023.08.133
Mero A., Komi P. V., Gregor R. J. (1992). Biomechanics of sprint running: a review. Sports Med. 13 (6), 376–392. doi:10.2165/00007256-199213060-00002
Moher D., Liberati A., Tetzlaff J., Altman D. G.the PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern Med. 151 (4), 264–W64. doi:10.7326/0003-4819-151-4-200908180-00135
Moore D. R., KirstenA B., LeeM S., MartinJ G., DigbyG S., Phillips S. M. (2004). Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur. J. Appl. Physiol. 92 (4–5). doi:10.1007/s00421-004-1072-y
Moritani T. (1993). Neuromuscular adaptations during the acquisition of muscle strength, power and motor tasks. J. Biomech. 26, 95–107. doi:10.1016/0021-9290(93)90082-p
Rodríguez S., Rodríguez-Jaime M. F., León-Prieto C. (2024). Blood flow restriction training: physiological effects, molecular mechanisms, and clinical applications. Crit. Rev. Phys. Rehabil. Med. 36, 13–30. doi:10.1615/critrevphysrehabilmed.2024052624
Rui L., Chee C., Kamalden T., Ramli A., Yang K. (2023). Effects of blood flow restriction training on sports performance in athletes: a systematic review with meta-analysis. J. Sports Med. Phys. Fit. 64 (1), 55–65. doi:10.23736/S0022-4707.23.15220-0
Sadamoto T., Bonde-Petersen F., Suzuki Y. (1983). Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur. J. Appl. Physiol. 51 (3), 395–408. doi:10.1007/BF00429076
Saraf A., Goyal M., Goyal K. (2022). Blood flow restriction training-an overview and implication in new generation physical therapy: a narrative review. J. Lifestyle Med. 12 (2), 63–68. doi:10.15280/jlm.2022.12.2.63
Sarfabadi P., Rizvi M. R., Sharma A., Sami W., Sajid M. R., Arora S., et al. (2023). Elevating athletic performance: maximizing strength and power in long jumpers through combined low-intensity blood flow restriction and high-intensity resistance training. Heliyon 9 (8), e19068. doi:10.1016/j.heliyon.2023.e19068
Sato Y. (2005). The history and future of KAATSU training. Int. J. KAATSU Train. Res. 1 (1), 1–5. doi:10.3806/ijktr.1.1
Scott B. R. (2017). The effects of supplementary low-load blood flow restriction training on morphological and performan. Available online at: https://blog.performancelab16.com/optothoa/2023/02/The_Effects_of_Supplementary_Low_Load_Blood_Flow.12.pdf.
Scott B. R., Loenneke J. P., Slattery K. M., Dascombe B. J. (2016). Blood flow restricted exercise for athletes: a review of available evidence. J. Sci. Med. Sport 19 (5), 360–367. doi:10.1016/j.jsams.2015.04.014
Scott B. R., Peiffer J. J., Goods P. S. R. (2017). The effects of supplementary low-load blood flow restriction training on morphological and performance-based adaptations in team sport athletes. J. Strength Cond. Res. 31 (8), 2147–2154. doi:10.1519/JSC.0000000000001671
Sedgwick P., Marston L. (2015). How to read a funnel plot in a meta-analysis. Bmj, h4718. doi:10.1136/bmj.h4718
Smith H., Bird S., Coskun B., Olsen P., Kavanagh T., Hamlin M. (2025). Effectiveness of blood flow restriction training during a taper phase in basketball players. J. Sports Sci., 1–12. doi:10.1080/02640414.2025.2454712
Takano H., Morita T., Iida H., Asada K. ichi, Kato M., Uno K., et al. (2005). Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur. J. Appl. Physiol. 95 (1), 65–73. doi:10.1007/s00421-005-1389-1
Takarada Y., Nakamura Y., Aruga S., Onda T., Miyazaki S., Ishii N. (2000). Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 88 (1), 61–65. doi:10.1152/jappl.2000.88.1.61
Takarada Y., Sato Y., Ishii N. (2002). Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 86 (4), 308–314. doi:10.1007/s00421-001-0561-5
Teixeira E. L., Ugrinowitsch C., de Salles Painelli V., Silva-Batista C., Aihara A. Y., Cardoso F. N., et al. (2021). Blood flow restriction does not promote additional effects on muscle adaptations when combined with high-load resistance training regardless of blood flow restriction protocol. J. Strength Cond. Res. 35 (5), 1194–1200. doi:10.1519/JSC.0000000000003965
The Occlusion Cuff ® (2025). Blood flow restriction training - a comprehensive overview. Available online at: https://occlusioncuff.com/blogs/news/blood-flow-restriction-training-a-comprehensive-overview.
Ugur T. B., Angin E., Kirmizigil B., Yolcu M. (2023). Blood flow restriction training on physical parameters in elite Male canoe athletes. Med. Baltim. 102 (41), e35252. doi:10.1097/MD.0000000000035252
Van Tulder M., Furlan A., Bombardier C., Bouter L. (2003). Updated method guidelines for systematic reviews in the cochrane collaboration back review group: spine. Spine (Phila. pa. 1976.) 28 (12), 1290–1299. doi:10.1097/01.BRS.0000065484.95996.AF
Vergara I. B., Puig-Diví A., Alonso B. A., Milà-Villarroel R. (2024). Effects of low-load blood flow restriction training in healthy adult tendons: a systematic review and meta-analysis. J. Bodyw. Mov. Ther. 39, 13–23. doi:10.1016/j.jbmt.2023.11.048
Wang J., Fu H., Zhang M., Fan Y. (2022). Effect of leg half-squat training with blood flow restriction under different external loads on strength and vertical jumping performance in well-trained volleyball players. Dose-response 20 (3), 15593258221123673. doi:10.1177/15593258221123673
Wang X., Qin X. M., Ji S., Dong D. (2023). Effects of resistance training with blood flow restriction on explosive power of lower limbs: a systematic review and meta-analysis. J. Hum. Kinet. 89, 259–268. doi:10.5114/jhk/168308
Weaver A. P., Dunkle Z., Giampetruzzi N., Prue J., Pacicca D., Roman D. P. (2024). Blood flow restriction training in the young athlete. HSS Journal® Musculoskelet. J. Hosp. Spec. Surg. 20 (3), 437–443. doi:10.1177/15563316241245700
Wortman R. J., Brown S. M., Savage-Elliott I., Finley Z. J., Mulcahey M. K. (2021). Blood flow restriction training for athletes: a systematic review. Am. J. Sports Med. 49 (7), 1938–1944. doi:10.1177/0363546520964454
Yamanaka T., Farley R. S., Caputo J. L. (2012). Occlusion training increases muscular strength in division IA football players. J. Strength Cond. Res. 26 (9), 2523–2529. doi:10.1519/JSC.0b013e31823f2b0e
Yang K., Chee C. S., Abdul Kahar J., Tengku Kamalden T. F., Li R., Qian S. (2024). Effects of blood flow restriction training on physical fitness among athletes: a systematic review and meta-analysis. Sci. Rep. 14 (1), 16615. doi:10.1038/s41598-024-67181-9
Yang S., Zhang P., Sevilla-Sanchez M., Zhou D., Cao J., He J., et al. (2022). Low-load blood flow restriction squat as conditioning activity within a contrast training sequence in high-level preadolescent trampoline gymnasts. Front. Physiol. 13, 852693. doi:10.3389/fphys.2022.852693
Yasuda T., Fujita T., Miyagi Y., Kubota Y., Sato Y., Nakajima T., et al. (2006). Electromyographic responses of arm and chest muscle during bench press exercise with and without KAATSU. Int. J. KAATSU Train. Res. 2 (1), 15–18. doi:10.3806/ijktr.2.15
Keywords: athletes, resistance training, lower limb strength, blood flow restriction training, athletic performance
Citation: Deng B, Lin G, Shi Y, Li D, Guan Z, Liang C and Sun J (2025) The effects of blood flow restriction combined with resistance training on lower limb strength, muscle hypertrophy, jumping ability, and sprint speed in athletes: a systematic review and meta-analysis. Front. Physiol. 16:1612685. doi: 10.3389/fphys.2025.1612685
Received: 16 April 2025; Accepted: 14 July 2025;
Published: 29 July 2025; Corrected: 01 September 2025.
Edited by:
Dawid Koźlenia, Wroclaw University of Health and Sport Sciences, PolandCopyright © 2025 Deng, Lin, Shi, Li, Guan, Liang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeyun Guan, MzE5Nzg3NTE3M0BxcS5jb20=; Chaoming Liang, MTEwMjZAZ3pzcG9ydC5lZHUuY24=; Jian Sun, c3VuamlhbkBnenNwb3J0LmVkdS5jbg==
†These authors have contributed equally to this work
 Beiwang Deng
Beiwang Deng Gesheng Lin
Gesheng Lin Yuer Shi
Yuer Shi Duanying Li
Duanying Li Zeyun Guan4*
Zeyun Guan4* Jian Sun
Jian Sun