- 1Department of Anesthesia and Perioperative Medicine, Zhengzhou University People’s Hospital and Henan Provincial People’s Hospital, Zhengzhou, Henan, China
- 2Xinxiang Medical University, Xinxiang, Henan, China
- 3Zhengzhou University, Zhengzhou, Henan, China
Introduction: Perioperative stroke is a rare but severe complication that significantly impacts postoperative recovery and survival. This study aimed to develop a machine learning-based predictive model for perioperative stroke risk in patients undergoing noncardiac, nonvascular, and nonneurosurgical procedures.
Methods: This retrospective cohort study was conducted using electronic medical records from 106,328 patients at Henan Provincial People’s Hospital, with data from 2,986 patients analyzed. Nine machine learning models were developed to predict perioperative stroke risk, incorporating key variables such as age, history of stroke, comorbidities, surgical factors, and intraoperative data. The models’ performance was evaluated using standard metrics, including area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, specificity, and F1 score.
Results: Among the nine models, the gradient boosting machine (GBM) demonstrated the best performance. In the training set, GBM achieved an AUC of 0.966 (95% CI: 0.957–0.975), with accuracy, sensitivity, specificity, and an F1 score of 90.4%, 90.4%, 81.8%, and 79.0%, respectively. In the validation set, the model maintained strong performance, with an AUC of 0.936 (95% CI: 0.917–0.954), accuracy of 82.6%, sensitivity of 88.8%, specificity of 81.0%, and an F1 score of 67.1%. In comparison, other models, such as logistic regression, support vector machine (SVM), and neural networks, exhibited lower AUC and less favorable performance metrics. Overall, GBM outperformed all models, demonstrating the best balance across accuracy, sensitivity, specificity, and F1 score.
Conclusion: The GBM model demonstrated strong predictive performance and generalizability for perioperative stroke risk in noncardiac, nonvascular, and nonneurosurgical patients. The integration of this model into a real-time clinical decision support system enhances clinical decision-making by enabling the early identification of high-risk patients and facilitating personalized interventions.
1 Introduction
Perioperative stroke is a rare yet severe postoperative complication characterized by the sudden onset of neurological impairment during surgery. This condition significantly affects recovery times and long-term survival outcomes (Mashour et al., 2011). Perioperative stroke results from the interplay of preoperative comorbidities, surgical risks, intraoperative hemodynamic fluctuations, and individual physiological factors (Vlisides and Mashour, 2016). Given the serious clinical consequences, improving preoperative risk assessment methods is crucial.
Traditional risk assessment methods, such as static scoring systems (e.g., CHA2DS2-VASc) (Tasbulak and Sahin, 2022), rely heavily on manually selected variables, including age and hypertension. Although these systems serve as useful tools for initial risk stratification, they have notable limitations. Their linear assumptions fail to account for nonlinear interactions, such as the combined effect of carotid stenosis (>50%) and intraoperative hypotension on stroke risk (Lengyel et al., 2024). Moreover, these methods overlook dynamic intraoperative factors, like fluctuations in the neutrophil-to-lymphocyte ratio, which have been linked to postoperative thrombotic events. These shortcomings undermine their generalizability across different surgical populations, as demonstrated by a study where traditional models failed to predict strokes in cancer patients with hypercoagulable states (Al Mouslmani et al., 2025). Furthermore, the manual selection of features can result in the exclusion of important predictive factors, such as intraoperative glycemic variability, diminishing the model’s robustness.
In contrast, machine learning (ML) offers a promising alternative by processing high-dimensional data and revealing complex, nonlinear relationships. Recent studies have highlighted its superior performance in predicting perioperative strokes (Pfitzner et al., 2021; Abraham et al., 2023). For instance, deep learning models integrating fibrinogen levels and carotid plaque morphology have demonstrated high sensitivity in predicting embolic strokes, outperforming traditional logistic regression models (Jamthikar et al., 2021; Patel et al., 2024). Additionally, reinforcement learning algorithms, which utilize real-time hemodynamic data, have successfully reduced stroke incidence in vascular surgery patients by adapting blood pressure management strategies. However, despite these advancements, research remains fragmented, with limited exploration of cross-specialty applicability and integration of multi-omics data. As a result, perioperative stroke prediction remains inadequately addressed.
This study used real-world clinical data from a large-scale database to develop predictive models for perioperative stroke using various machine learning algorithms. By integrating preoperative assessments, surgical variables, and perioperative events, we aimed to create an early warning system that enhances clinical decision-making and facilitates precision interventions (Kwun et al., 2025).
2 Methods
2.1 Data source
This retrospective cohort study analyzed electronic medical records of patients treated at Henan Provincial People’s Hospital between November 2014 and June 2021. The database contained comprehensive, high-quality demographic and clinical data, including patient characteristics, comorbidities, and laboratory results, forming a solid basis for analysis. The hospital’s Institutional Review Board approved the study protocol (Approval No. 2021-157), and all procedures complied with the Declaration of Helsinki. The ethics committee waived the need for informed consent due to the use of anonymized data and adherence to ethical standards.
2.1.1 Inclusion criteria
1. Age ≥18 years, undergoing noncardiac, nonvascular, and nonneurosurgical procedures under general anesthesia.
2. American Society of Anesthesiologists (ASA) physical status classification I–III.
3. Availability of complete perioperative clinical and laboratory data.
4. At least one postoperative follow-up within 30 days.
2.1.2 Exclusion criteria
1. Patients undergoing cardiac, major vascular, or neurosurgical procedures.
2. Patients admitted preoperatively to neurology or neurological intensive care units (neuro-ICUs).
3. Patients with ASA classification IV–V or classified as critically ill.
4. Patients with missing or incomplete clinical/laboratory data.
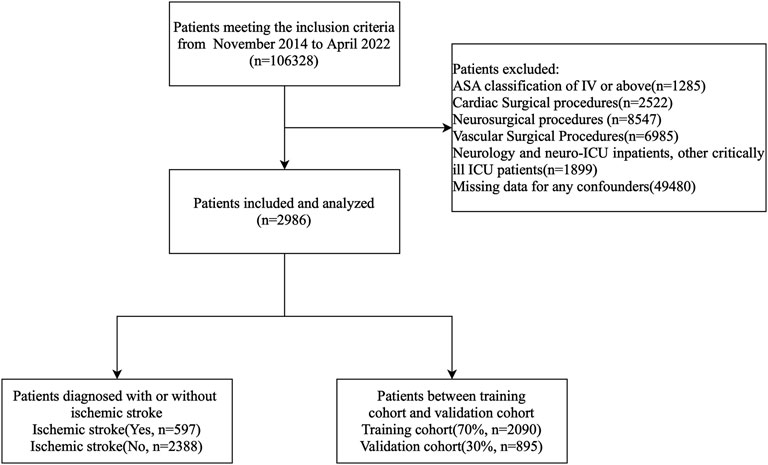
Of the 106,328 patients who underwent noncardiac, nonvascular, and nonneurosurgical surgeries, only those meeting the inclusion criteria were enrolled. A flowchart of the study is presented in Figure 1.
2.2 Data collection
2.2.1 Definition of perioperative stroke
The primary outcome was perioperative ischemic stroke, defined as a clinically confirmed cerebral infarction occurring within 30 days postoperatively. Diagnosis was confirmed through imaging (CT/MRI) and neurologist evaluation (Mashour et al., 2011). Diagnostic details were extracted from the electronic medical records (Furie et al., 2011).
2.2.2 Clinical and perioperative parameters
The analysis included clinical data from 2,986 patients who underwent noncardiac, nonvascular, and nonneurosurgical procedures. Comprehensive demographic, clinical, imaging, and laboratory parameters were systematically collected from the preoperative period through 7 days postoperatively.
2.2.3 The key variables included the following
1. Demographics: Age, sex, height, weight, and BMI.
2. Surgical Parameters: ASA classification, procedure type and duration, anesthesia duration and method, urine output, and blood loss.
3. Comorbidities: Smoking, alcohol use, ascites, hypertension, diabetes, cardiac disorders, COPD, renal disease, and cerebrovascular disease.
4. Laboratory Tests: Complete blood count; creatinine, albumin, and liver enzyme levels; and coagulation profiles.
5. Preoperative Medications: Antihypertensives, anticoagulants, and antiplatelet agents, including documentation of discontinuation timelines.
6. Intraoperative Monitoring: Blood pressure, heart rate, temperature, bispectral index (BIS), and end-tidal CO2, recorded at 5-min intervals.
7. Intraoperative Medications: Inhalational anesthetics, diuretics, and anticoagulants.
8. Intraoperative Fluids/Transfusions: Colloids, crystalloids, and blood products.
9. Vasopressors: Ephedrine and phenylephrine.
10. Postoperative Medications: Statins, anticoagulants, and antiplatelet agents (dosage and timing recorded) administered within 7 days to prevent secondary cerebrovascular events.
2.3 Data preprocessing
The data preprocessing and model development for this study involved feature selection, multiple imputation, and standardization (Bellini et al., 2022). Variables were initially excluded based on two criteria: (1) missing values exceeding 20% (Austin et al., 2021) and (2) lack of established associations with perioperative stroke, as supported by the literature and clinical expertise (Mashour et al., 2011). After screening, 22 variables were retained: emergency surgery, angina, valvular heart disease, stroke history, tumor history, intraoperative mean arterial pressure ≤75 mmHg, preoperative use of metoprolol/diuretics/insulin, intraoperative use of remifentanil, age, surgical duration, preoperative red blood cell/lymphocyte/basophil counts, mean corpuscular volume, hematocrit, total protein, activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen, and succinylated gelatine administration (Bijker et al., 2012).
Residual missing values were imputed using the R-based MICE (Multiple Imputation by Chained Equations) package (Ren et al., 2023). After imputation, all variables were Z-score normalized to standardize data scales and minimize overfitting.
2.4 Propensity score matching (PSM)
To minimize selection bias and create a balanced control group, propensity score matching (PSM) was performed. Propensity scores were estimated using key demographic and clinical variables, including age, sex, comorbidities (e.g., hypertension, diabetes, stroke history), and other factors known to influence perioperative stroke risk. These variables were chosen based on their clinical relevance, established associations with stroke risk in the literature, and expert consensus.
The matching procedure utilized the nearest neighbor matching algorithm with a 1:4 ratio, where each patient in the stroke group was matched with four controls from the non-stroke group based on the closest propensity score. No caliper was applied, although using one could have improved match quality by restricting matches to those with more similar propensity scores.
After matching, the balance between the stroke and non-stroke groups was assessed using standardized mean differences (SMDs). Covariates with an SMD of less than 0.1 were considered well-balanced. This approach aimed to reduce confounding and ensure comparability between the two groups.
The final cohort included 597 stroke patients and 2,388 non-stroke controls. Given the balanced group proportions, no oversampling was needed to avoid prediction bias. The data were randomly split into training (70%) and validation (30%) sets. LASSO regression identified 11 key predictors, which were validated through 10-fold cross-validation (Staartjes et al., 2022) (Figure 2). These predictors included age, stroke history, succinylated gelatine, preoperative APTT, hematocrit, basophil count, total protein, hypotension, fibrinogen, surgical duration, and preoperative insulin.
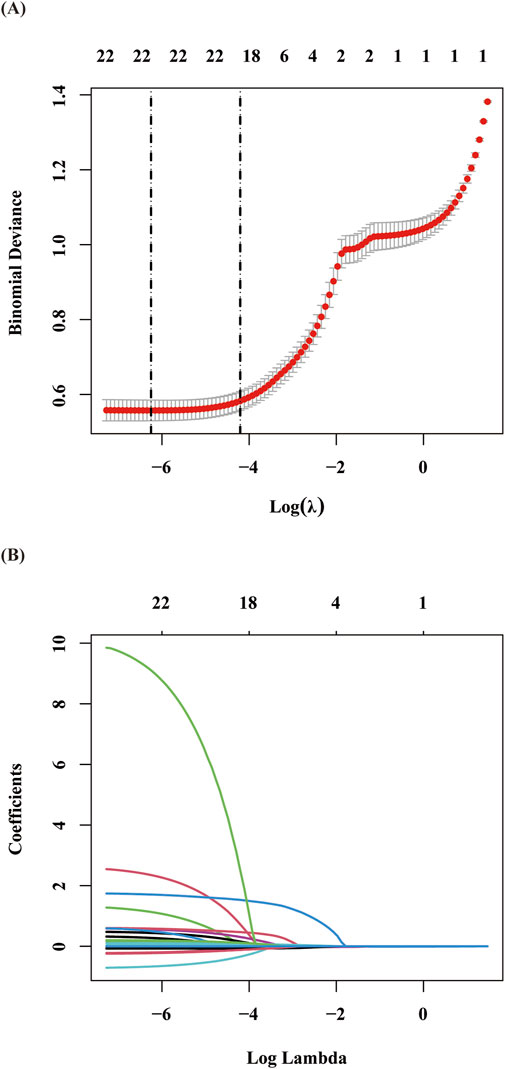
Figure 2. Presentation of the results of the LASSO regression analysis: (A) Variable selection in the LASSO regression model: The left dashed vertical line indicates the optimal lambda value (lambda⋅min) corresponding to the minimum cross-validated error, while the right dashed vertical line represents the largest lambda value within one standard error of the optimal value (lambda.1se). (B) Coefficient shrinkage patterns of the LASSO regression model: The trajectories illustrate the coefficient paths for all candidate variables across decreasing lambda values, demonstrating the feature selection process through progressive regularization.
2.5 Machine learning model development
A total of nine machine learning algorithms were developed to predict perioperative stroke risk: logistic regression (LR), support vector machine (SVM), gradient boosting machine (GBM), neural network, extreme gradient boosting (XGBoost), k-nearest neighbors (KNN), adaptive boosting (AdaBoost), light gradient boosting machine (LightGBM), and categorical boosting (CatBoost) (Lundberg et al., 2020). The hyperparameters were optimized using grid search, and model robustness was enhanced through 10-fold cross-validation and resampling techniques (Collins et al., 2015).
Model performance was evaluated in three key areas: discrimination, calibration, and clinical utility. Discriminatory power was assessed by the area under the receiver operating characteristic curve (AUC). Calibration was examined using calibration plots and Brier scores. Decision curve analysis (DCA) was used to evaluate the net clinical benefit across varying decision thresholds (Steyerberg and Vergouwe, 2014). Additional performance metrics, including accuracy, sensitivity, specificity, precision, and F1 score, were derived from confusion matrices.
2.5.1 The optimal parameters for each model are as follows
1. Logistic Regression (LR): C = 10, penalty = l2, solver = saga
2. Support Vector Machine (SVM): sigma = 0.001, C = 0.09
3. Gradient Boosting Machine (GBM): n.trees = 100, interaction.depth = 5, shrinkage = 0.1, n.minobsinnode = 30
4. Multi-Layer Perceptron (MLP): size = 6, decay = 0.6
5. XGBoost (XGB): nrounds = 10, max_depth = 3, eta = 0.001, gamma = 0.5, colsample_bytree = 0.5, min_child_weight = 1, subsample = 0.6
6. K-Nearest Neighbors (KNN): kmax = 12, distance = 1, kernel = optimal
7. AdaBoost: mfinal = 2, maxdepth = 2, coeflearn = “Zhu”
8. LightGBM: objective = binary, metric = auc, learning_rate = 1.0, num_threads = 2
9. CatBoost: iterations = 100, eval_metric = AUC, learning_rate = 0.03, random_seed = 123
2.6 Model interpretation and visualization
To enhance model interpretability and clinical transparency, SHapley Additive exPlanations (SHAP) analysis was conducted to assess the contribution of each feature to individual predictions. A higher SHAP value indicates a greater influence of the variable on stroke risk estimation (Lundberg et al., 2020).
2.7 Statistical analysis
Continuous variables are presented as medians with interquartile ranges and compared using either t-tests or Mann–Whitney U tests, depending on their distribution. Categorical variables are reported as frequencies (percentages) and analyzed with the chi-square test or Fisher’s exact test. Statistical significance was set at P < 0.05. All analyses were performed using R version 4.3.0. Model performance metrics included accuracy, sensitivity, precision, specificity, F1 score, and AUC.
3 Results
3.1 Baseline patient characteristics
As shown in Figure 1, a total of 597 patients with confirmed perioperative stroke were included in the stroke group. To minimize selection bias and ensure a balanced control group, propensity score matching (PSM) was performed using the nearest neighbor matching algorithm at a 1:4 ratio. This was based on key demographic and clinical variables, including age, sex, and comorbidities, which were selected based on clinical relevance and previous studies linking them to perioperative stroke risk. This matching resulted in 2,388 controls for comparative analysis.
Balance between the stroke and non-stroke groups was assessed using standardized mean differences (SMDs). Covariates with an SMD of less than 0.1 were considered well-balanced after matching. The data were then divided into model development (n = 2,090, 70%) and validation (n = 895, 30%) sets using stratified random sampling. Table 1 presents the baseline characteristics of stroke and non-stroke patients, while Table 2 outlines the intergroup differences between the training and validation sets.
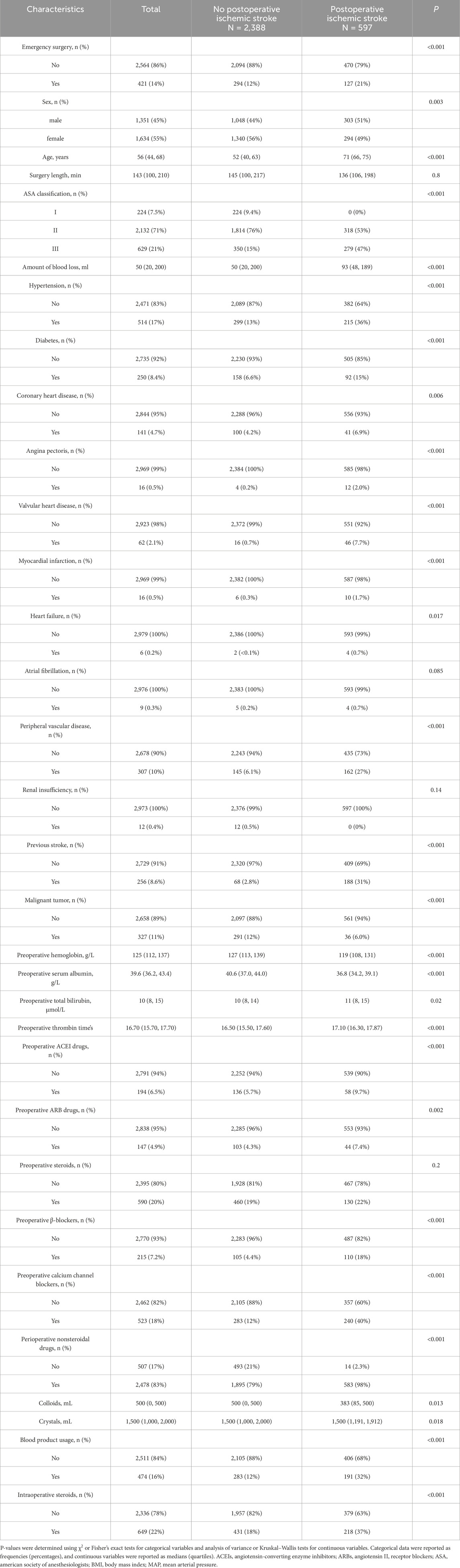
Table 1. Baseline demographic and clinical characteristics of included patients diagnosed with or without ischemic stroke.
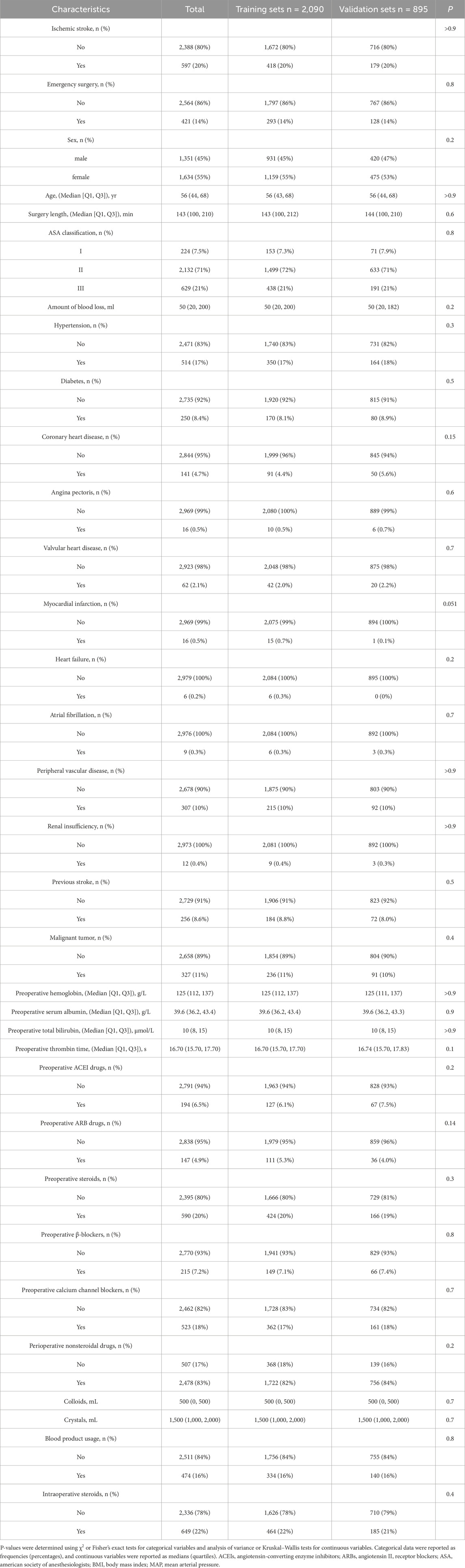
Table 2. Baseline demographic and clinical characteristics of included patients between training and validation sets.
3.2 Performance evaluation of the nine models
Nine machine learning models were developed to predict stroke risk in noncardiac, nonvascular, and nonneurosurgical patients. Figure 3 illustrates the model performance metrics, including receiver operating characteristic (ROC) curves, calibration plots, and decision curve analysis (DCA), for both the training and validation sets.
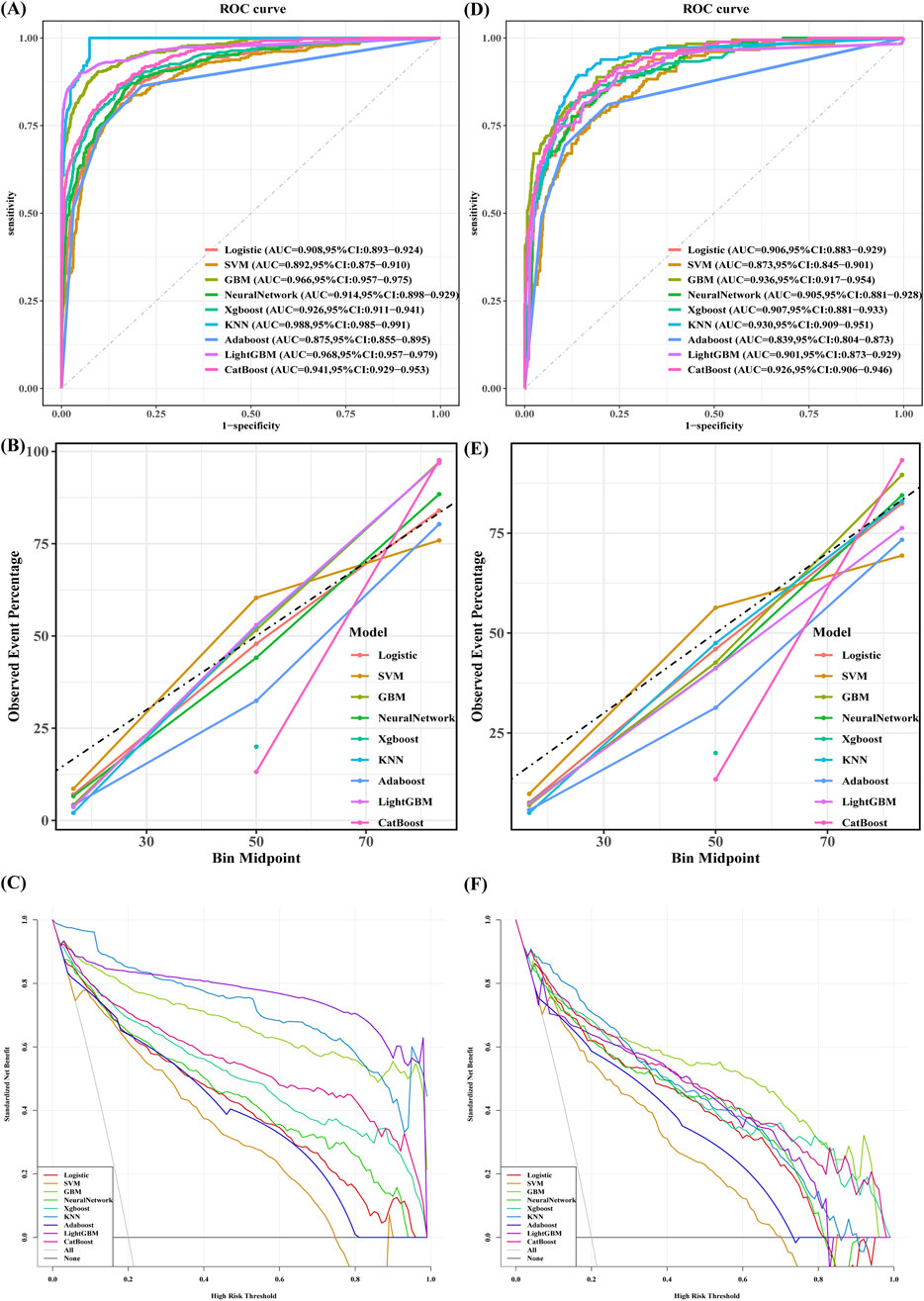
Figure 3. The performance and comparison of nine different predictive models: (A) ROC Curves for the Training sets; (B) Calibration Curve for the Training sets; (C) Decision Curve Analysis for the Training sets; (D) ROC Curves for the Validation sets; (E) Calibration Curve for the Validation sets; (F) Decision Curve Analysis for the Validation sets.
The gradient boosting machine (GBM) model demonstrated the best overall predictive performance. In the training set, GBM achieved an AUC of 0.966 (95% CI: 0.957–0.975), with an accuracy of 90.4%, sensitivity of 90.4%, precision of 70.1%, specificity of 81.8%, and an F1 score of 79.0%. Its performance remained robust on the validation set, with an AUC of 0.936 (95% CI: 0.917–0.954), accuracy of 82.6%, sensitivity of 88.8%, precision of 53.9%, specificity of 81.0%, and an F1 score of 67.1%.
In contrast, the k-nearest neighbors (KNN) algorithm achieved the highest training AUC of 0.988 (Figure 3A), but its performance was less generalizable due to overfitting, as reflected in its lower performance on the validation set. Specifically, KNN had an accuracy of 86.6%, sensitivity of 89.4%, specificity of 85.9%, precision of 61.3%, an F1 score of 72.7%, and an AUC of 0.930.
Similarly, CatBoost demonstrated competitive performance with a validation AUC of 0.926 (Figure 3D). Its accuracy was 85.3%, sensitivity 84.4%, specificity 85.5%, precision 59.2%, F1 score 69.6%, and AUC 0.926.
Although KNN showed the highest training AUC, GBM was ultimately selected as the optimal model due to its consistent performance across both the training and validation sets. GBM outperformed KNN on the validation set, achieving a higher AUC and F1 score, and providing a better balance of sensitivity, specificity, and precision. Despite CatBoost showing competitive results, particularly in sensitivity and specificity, it was still outperformed by GBM in accuracy, sensitivity, and AUC.
In conclusion, GBM demonstrated superior overall performance, consistently outperforming KNN, CatBoost, and other models, including logistic regression, neural networks, XGBoost, and LightGBM (Table 3). Consequently, it was selected as the optimal model for predicting perioperative stroke risk.
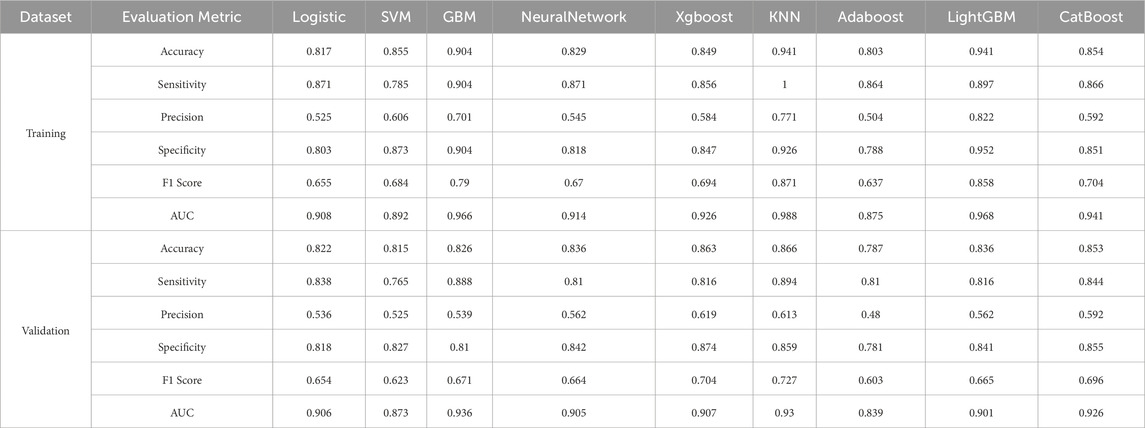
Table 3. Performance metrics of various machine learning models for predicting perioperative ischemic stroke risk in patients across training and validation sets.
3.3 Model interpretability and visualization
Figure 4A presents the SHAP (SHapley Additive exPlanations) summary plot for GBM, where the X-axis represents SHAP values (higher values indicate stronger contributions to stroke prediction). Feature magnitude is represented by a color gradient, ranging from purple (high values) to yellow (low values).
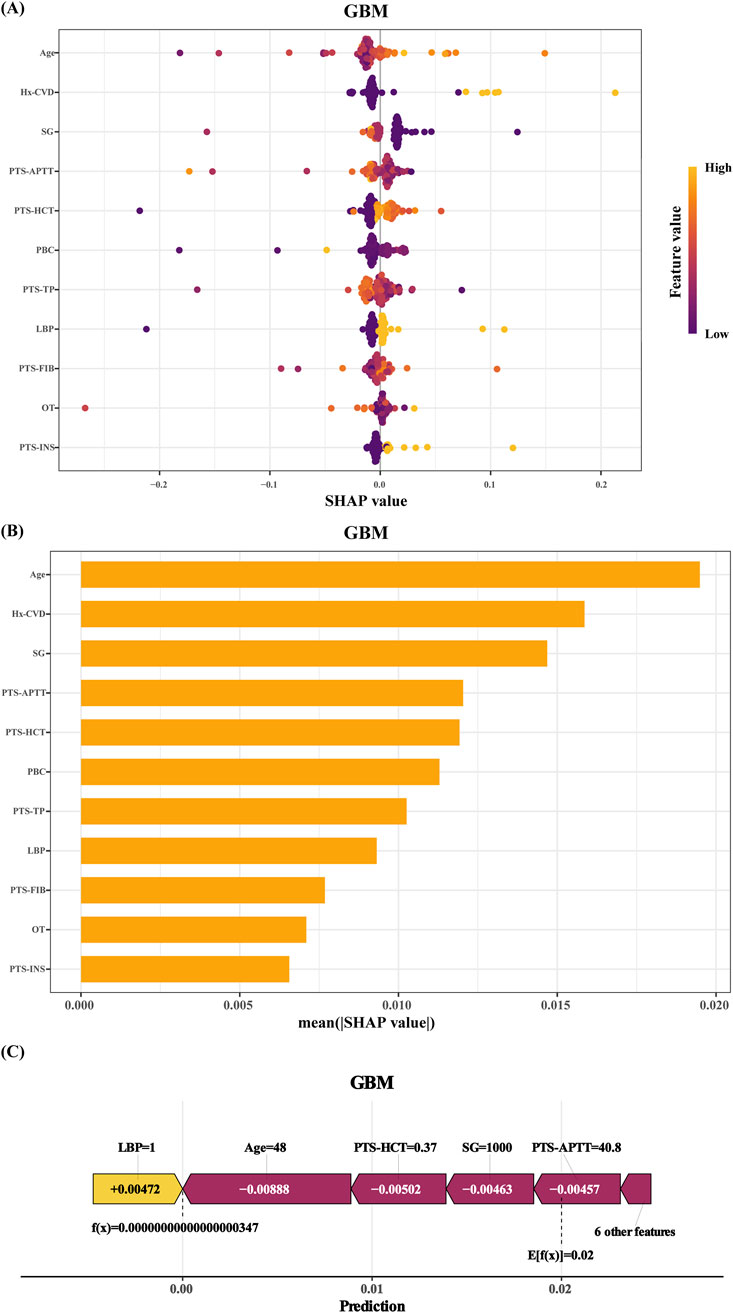
Figure 4. SHAP of the model: (A) Characteristic attributes in SHAP. The abscissa is the SHAP value, and each line denotes a feature. Higher eigenvalues are indicated by purple dots, and lower eigenvalues are indicated by yellow dots; (B) Feature importance ranking of the Gradient Boosting Machine (GBM) model; (C) Interpretability analysis of 1 independent samples. Hx-CVD:a preoperative history of stroke. SG: succinylated gelatin. PTS-APTT: preoperative APTT. PTS-HCT: preoperative hematocrit. PBC: preoperative basophil count. PTS-TP: preoperative total protein. LBP: hypotension. PTS-FIB: preoperative fibrinogen. OT: operative time. PTS-INS: preoperative insulin.
The top four predictors of stroke risk were age, stroke history, succinylated gelatine dosage, and preoperative activated partial thromboplastin time (APTT). Older age, a prior stroke history, higher succinylated gelatine use, and lower preoperative APTT were strongly associated with an increased stroke risk. Other significant factors included reduced haematocrit, lower basophil count, elevated total protein, intraoperative hypotension (mean arterial pressure ≤75 mmHg for ≥5 min), elevated fibrinogen levels, longer surgical duration, and infrequent preoperative insulin use.
These findings were further supported by the feature importance rankings in Figure 4B. The interpretability of the model was also demonstrated through individualized case analyses. Figure 4C shows SHAP force plots for a representative non-stroke patient, highlighting feature-specific contributions to the prediction.
Figures 5A,B complement these statistical findings by presenting the confusion matrix for the GBM model, showing both actual and predicted values. Finally, Figure 6 presents the performance metrics of the GBM model across the 10-fold cross-validation.
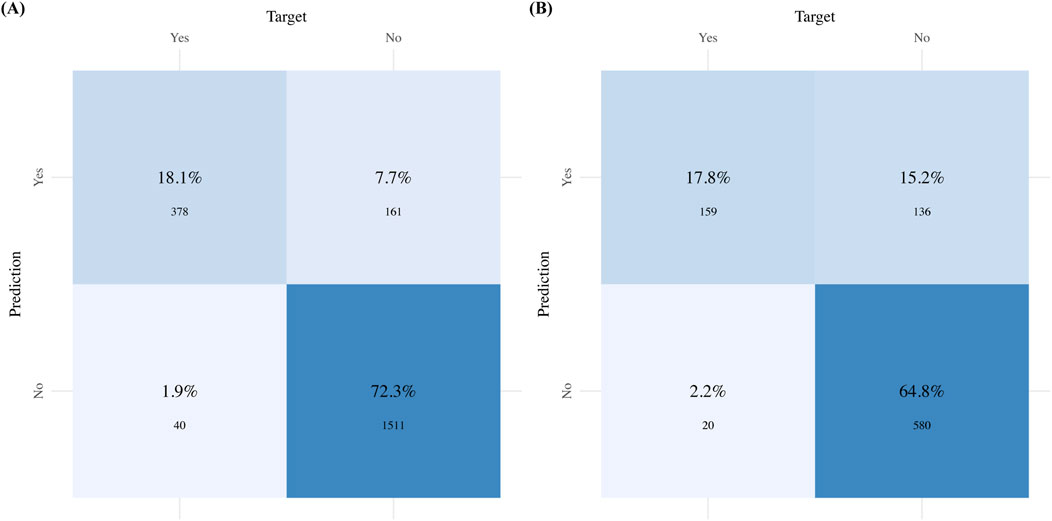
Figure 5. Confusion matrix for the GBM model: (A) Confusion matrix for the Training sets; (B) Confusion matrix for the Validation sets.
4 Discussion
This study developed a perioperative stroke risk prediction model for patients undergoing noncardiac, nonvascular, and nonneurosurgical procedures. By integrating multiple machine learning algorithms with a large-scale perioperative database from Henan Provincial People’s Hospital, we identified 16 key factors contributing to perioperative stroke risk prediction (Bilimoria et al., 2013). While traditional perioperative stroke assessment tools, such as the National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), and ABCD2 score, are useful for preliminary risk identification, they have limitations in predicting future stroke incidence (Birman-Deych et al., 2005; Sacco et al., 2013). This study is the first to create a prediction model specifically designed for Chinese surgical patients undergoing noncardiac, nonvascular, and nonneurosurgical procedures. It systematically integrates standardized demographic data, comorbidities, and health-related indicators, providing significant innovation. The model’s strength lies in its ability to reveal complex interactions among high-dimensional variables through machine learning, leading to enhanced predictive accuracy and offering robust evidence for stroke prediction in this surgical cohort (Deo, 2015).
Through LASSO regression analysis, we identified 11 key predictive variables. As shown in Figure 2, advanced age, a history of stroke, increased succinylated gelatine administration, and lower preoperative activated partial thromboplastin time (APTT) were significantly associated with increased stroke risk. Other independent predictors included reduced haematocrit, decreased preoperative basophil count, elevated preoperative total protein, mean arterial pressure ≤75 mmHg for ≥5 min, elevated preoperative fibrinogen levels, prolonged surgical duration, and infrequent preoperative insulin use.
These findings align with existing literature. Advanced age and a prior history of stroke are well-established predictors of perioperative stroke risk (Mashour et al., 2011). Age-related changes such as vascular aging and arteriosclerosis impair cerebral perfusion (Fanning et al., 2024), increasing cerebrovascular fragility and stroke susceptibility. Older patients with a history of cerebrovascular events exhibit significantly higher rates of postoperative stroke recurrence than those without such history (Vlisides and Mashour, 2016). Additionally, the frequent coexistence of chronic comorbidities such as hypertension, diabetes, and atherosclerosis in older populations further exacerbates perioperative stroke risk through shared pathological mechanisms (Sultan et al., 2020). These findings underscore the importance of thorough preoperative risk stratification and tailored interventions for high-risk groups.
Coagulation abnormalities are another critical determinant of perioperative stroke risk. The dose-dependent relationship between succinylated gelatine administration (commonly used in hepatectomy fluid management) and postoperative coagulation dysfunction can predispose individuals to stroke (Kuang et al., 2024). Experimental evidence shows that gelatine inhibits platelet aggregation, particularly at doses exceeding 20 mL/kg (Cai et al., 2021), and induces endothelial cell activation and hypercoagulable states with prolonged administration (Malerba et al., 2017). Similarly, preoperative hypocoagulable profiles (indicated by reduced APTT) and elevated fibrinogen levels serve as markers of prothrombotic tendencies, promoting thrombogenesis and cerebrovascular events (Li et al., 2022; Alemayehu et al., 2024). These findings highlight the importance of preoperative coagulation profiling and evidence-based corrective strategies to mitigate perioperative stroke risk.
Perioperative haemodynamic fluctuations, surgical duration, and insulin management were also identified as key stroke risk factors. Intraoperative hypotension, a major contributor to cerebral hypoperfusion, impairs cerebral blood flow and increases susceptibility to ischemic stroke (Mazzeffi et al., 2021; Wongtangman et al., 2021). Prolonged surgery duration increases exposure to physiological stress, elevating the risk of postoperative complications, including cerebrovascular events (Mazzeffi et al., 2021). Among diabetic patients, those with suboptimal insulin use or poor glycemic control have a significantly higher likelihood of postoperative stroke compared to those with good glycemic control (Eshuis et al., 2011; Sacco et al., 2024). These findings emphasize the need for maintaining haemodynamic stability, optimizing glycemic control, and managing intraoperative blood pressure as essential strategies for reducing stroke risk.
In terms of model performance, the gradient boosting machine (GBM) outperformed other models in the validation cohort, achieving superior AUC values, accuracy, specificity, sensitivity, and F1-score metrics. Decision curve analysis (DCA) demonstrated substantial net clinical benefits for the GBM model across most threshold probabilities, except in highly risk-averse scenarios (Figures 3C,F). Calibration analysis produced Brier scores of 0.05 (95% CI: 0.045–0.057) for the validation sets and 0.072 (95% CI: 0.06–0.085) for the test sets, indicating excellent alignment between predicted probabilities and observed outcomes. A comprehensive evaluation confirmed the robustness and generalizability of the GBM, establishing it as the optimal model. Its computational efficiency, adaptability to complex scenarios, and ability to handle high-dimensional nonlinear relationships affirm its superiority in medical data analytics (Langenberger et al., 2023).
Despite the widespread use of logistic regression in clinical research, its application in perioperative stroke prediction has notable limitations. First, logistic regression’s linearity assumption restricts its predictive power, failing to account for complex interactions and nonlinear associations, thus compromising prediction accuracy (Nusinovici et al., 2020; Song et al., 2023). Second, these models struggle with dynamic data integration, particularly real-time intraoperative monitoring parameters and time-dependent variables (e.g., the interaction between anticoagulant withdrawal duration and surgical timing) (Klug et al., 2024). Additionally, logistic regression models rely heavily on manual feature engineering, risking the omission of critical predictors, such as intraoperative micro-embolic signals or postoperative fluctuations in the neutrophil-to-lymphocyte ratio (NLR) (Zhuang et al., 2021; Oh et al., 2024). Emerging evidence suggests that these dynamic indicators could improve predictive performance by up to 15% (Zhuang et al., 2021).
Moreover, logistic regression is highly sensitive to class imbalance, often requiring remedial techniques like oversampling, which can introduce prediction bias (van den Goorbergh et al., 2022). More importantly, the model lacks the ability to dynamically recalibrate risk, preventing real-time prediction adjustments (Xue et al., 2022). Studies suggest this limitation may lead to the misclassification of up to 38% of high-risk patients (Cooper et al., 2016). These limitations make logistic regression insufficient for modern perioperative stroke prediction in precision medicine (Kim et al., 2023).
This study introduces key advancements in perioperative stroke prediction through the following innovations:
1. Data Integration: We consolidated the perioperative database from Henan Provincial People’s Hospital, integrating preoperative baseline characteristics, intraoperative dynamic monitoring metrics (e.g., haemodynamic fluctuations, heart rate, and oxygen saturation), and postoperative complications. This integration provides comprehensive insights into patients’ physiological status.
2. Cohort Expansion: The analysis included over 10,000 surgical cases, making it the largest cohort in perioperative stroke prediction research. This large-scale approach improves model robustness and generalizability, enhancing clinical applicability.
3. Novel Predictive Framework: We developed a gradient boosting machine (GBM) model capable of processing both structured clinical data and intraoperative continuous monitoring signals. The model showed exceptional discriminative performance (AUC 0.936), outperforming traditional regression models in perioperative stroke prediction.
4. Clinical Decision Support System: A real-time decision support system based on this predictive model was implemented, offering dynamic risk score updates and alerts. Currently being piloted in three tertiary hospitals, the system has received positive feedback for its ability to identify high-risk patients early and facilitate timely interventions.
These innovations collectively improve the accuracy of perioperative stroke prediction and support the clinical implementation of predictive models. The established framework offers a practical solution for optimizing perioperative care.
Although this study represents significant progress, several limitations should be acknowledged. First, the retrospective nature of the analysis, relying on a single data source, may introduce potential selection bias (Oh et al., 2024). Second, the absence of key stroke-related variables such as genetic profiles and socioeconomic factors limits the comprehensiveness of the risk assessment. Finally, as a retrospective cohort study, the lack of prospective follow-up data for disease progression may introduce confounding factors. Future studies should incorporate updated datasets to further refine and validate the model.
Despite these limitations, this research addresses a critical gap in perioperative stroke prediction, using advanced algorithms like the gradient boosting machine (GBM) to enhance predictive accuracy. The web-based prediction platform developed offers considerable clinical potential for rapid high-risk patient identification and personalized interventions (Teng et al., 2024). This tool also empowers patients with self-assessment capabilities, improving disease awareness and management.
5 Conclusion
This study developed a machine learning-based model for perioperative stroke prediction, demonstrating superior accuracy compared to traditional methods. The gradient boosting machine (GBM) model, leveraging a wide range of clinical data, showed robust performance and generalizability. Furthermore, the integration of a real-time decision support system enhances the model’s clinical utility, aiding in early identification of high-risk patients and enabling personalized interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Henan Provincial People’s Hospital’s Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The need for informed consent was waived by the ethics committee because of compliance with ethical standards and the use of anonymized data.
Author contributions
XC: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. XZ: Data curation, Conceptualization, Methodology, Writing – review and editing. RZ: Formal Analysis, Conceptualization, Data curation, Writing – review and editing. YL: Investigation, Software, Data curation, Writing – review and editing. LL: Investigation, Software, Writing – review and editing. JZ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (grant nos. 82071217).
Acknowledgments
We would like to thank Linlin Xu and Tongyan Sun of Hangzhou Le9 Healthcare Technology Co., Ltd., for their assistance in the clinical data extraction during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1624898/full#supplementary-material
References
Abraham J., Bartek B., Meng A., Ryan King C., Xue B., Lu C., et al. (2023). Integrating machine learning predictions for perioperative risk management: towards an empirical design of a flexible-standardized risk assessment tool. J. Biomed. Inf. 137, 104270. doi:10.1016/j.jbi.2022.104270
Alemayehu E., Mohammed O., Belete M. A., Mulatie Z., Debash H., Gedefie A., et al. (2024). Association of prothrombin time, thrombin time and activated partial thromboplastin time levels with preeclampsia: a systematic review and meta-analysis. BMC Pregnancy Childbirth 24 (1), 354. doi:10.1186/s12884-024-06543-7
Al Mouslmani M., Alahmad M. A., Akman Z., Rossi R., Rahman M., Nanna M. G. (2025). CHA(2)DS(2)-VASc score in patients with atrial fibrillation and cancer: a U.S. Nationwide study. Am. J. Cardiol. 249, 59–64. doi:10.1016/j.amjcard.2025.04.025
Austin P. C., White I. R., Lee D. S., van Buuren S. (2021). Missing data in clinical research: a tutorial on multiple imputation. Can. J. Cardiol. 37 (9), 1322–1331. doi:10.1016/j.cjca.2020.11.010
Bellini V., Valente M., Bertorelli G., Pifferi B., Craca M., Mordonini M., et al. (2022). Machine learning in perioperative medicine: a systematic review. J. Anesth. Analg. Crit. Care 2 (1), 2. doi:10.1186/s44158-022-00033-y
Bijker J. B., Persoon S., Peelen L. M., Moons K. G., Kalkman C. J., Kappelle L. J., et al. (2012). Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology 116 (3), 658–664. doi:10.1097/ALN.0b013e3182472320
Bilimoria K. Y., Liu Y., Paruch J. L., Zhou L., Kmiecik T. E., Ko C. Y., et al. (2013). Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J. Am. Coll. Surg. 217 (5), 833–42.e423. doi:10.1016/j.jamcollsurg.2013.07.385
Birman-Deych E., Waterman A. D., Yan Y., Nilasena D. S., Radford M. J., Gage B. F. (2005). Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med. Care 43 (5), 480–485. doi:10.1097/01.mlr.0000160417.39497.a9
Cai Y., Zhang M., Wang Y., Wang N., Zhang L., Zhang K. (2021). Latamoxef-induced coagulation disorders: incidence and risk factors. J. Clin. Pharm. Ther. 46 (5), 1382–1386. doi:10.1111/jcpt.13435
Collins G. S., Reitsma J. B., Altman D. G., Moons K. G. (2015). Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Bmj 350, g7594. doi:10.1136/bmj.g7594
Cooper M., Arhuidese I. J., Obeid T., Hicks C. W., Canner J., Malas M. B. (2016). Perioperative and long-term outcomes after carotid endarterectomy in hemodialysis patients. JAMA Surg. 151 (10), 947–952. doi:10.1001/jamasurg.2016.1504
Deo R. C. (2015). Machine learning in medicine. Circulation 132 (20), 1920–1930. doi:10.1161/circulationaha.115.001593
Eshuis W. J., Hermanides J., van Dalen J. W., van Samkar G., Busch O. R., van Gulik T. M., et al. (2011). Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann. Surg. 253 (4), 739–744. doi:10.1097/SLA.0b013e31820b4bfc
Fanning J. P., Campbell B. C. V., Bulbulia R., Gottesman R. F., Ko S. B., Floyd T. F., et al. (2024). Perioperative stroke. Nat. Rev. Dis. Prim. 10 (1), 3. doi:10.1038/s41572-023-00487-6
Furie K. L., Kasner S. E., Adams R. J., Albers G. W., Bush R. L., Fagan S. C., et al. (2011). Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 42 (1), 227–276. doi:10.1161/STR.0b013e3181f7d043
Jamthikar A. D., Gupta D., Mantella L. E., Saba L., Laird J. R., Johri A. M., et al. (2021). Multiclass machine learning vs. conventional calculators for stroke/CVD risk assessment using carotid plaque predictors with coronary angiography scores as gold standard: a 500 participants study. Int. J. Cardiovasc. Imaging 37 (4), 1171–1187. doi:10.1007/s10554-020-02099-7
Kim R. C., Schick S. E., Muraru R. I., Roch A., Nguyen T. K., Ceppa E. P., et al. (2023). Do weekend discharges impact readmission rate in patients undergoing pancreatic surgery? J. Gastrointest. Surg. 27 (12), 2815–2822. doi:10.1007/s11605-023-05864-w
Klug J., Leclerc G., Dirren E., Carrera E. (2024). Machine learning for early dynamic prediction of functional outcome after stroke. Commun. Med. (Lond) 4 (1), 232. doi:10.1038/s43856-024-00666-w
Kuang L., Lin W., Wang D., Chen B. (2024). Abnormal coagulation after hepatectomy in patients with normal preoperative coagulation function. BMC Surg. 24 (1), 136. doi:10.1186/s12893-024-02406-2
Kwun J. S., Ahn H. B., Kang S. H., Yoo S., Kim S., Song W., et al. (2025). Developing a machine learning model for predicting 30-day major adverse cardiac and cerebrovascular events in patients undergoing noncardiac surgery: retrospective study. J. Med. Internet Res. 27, e66366. doi:10.2196/66366
Langenberger B., Schulte T., Groene O. (2023). The application of machine learning to predict high-cost patients: a performance-comparison of different models using healthcare claims data. PLoS One 18 (1), e0279540. doi:10.1371/journal.pone.0279540
Lengyel B., Magyar-Stang R., Pál H., Debreczeni R., Sándor Á D., Székely A., et al. (2024). Non-invasive tools in perioperative stroke risk assessment for asymptomatic carotid artery stenosis with a focus on the circle of willis. J. Clin. Med. 13 (9), 2487. doi:10.3390/jcm13092487
Li Z., Yu B., Zhang J., Shen J., Wang Y., Qiu G., et al. (2022). Does abnormal preoperative coagulation status lead to more perioperative blood loss in spinal deformity correction surgery? Front. Surg. 9, 841680. doi:10.3389/fsurg.2022.841680
Lundberg S. M., Erion G., Chen H., DeGrave A., Prutkin J. M., Nair B., et al. (2020). From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2 (1), 56–67. doi:10.1038/s42256-019-0138-9
Malerba M., Nardin M., Radaeli A., Montuschi P., Carpagnano G. E., Clini E. (2017). The potential role of endothelial dysfunction and platelet activation in the development of thrombotic risk in COPD patients. Expert Rev. Hematol. 10 (9), 821–832. doi:10.1080/17474086.2017.1353416
Mashour G. A., Shanks A. M., Kheterpal S. (2011). Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology 114 (6), 1289–1296. doi:10.1097/ALN.0b013e318216e7f4
Mazzeffi M., Chow J. H., Anders M., Gibbons M., Okojie U., Feng A., et al. (2021). Intraoperative hypotension and perioperative acute ischemic stroke in patients having major elective non-cardiovascular non-neurological surgery. J. Anesth. 35 (2), 246–253. doi:10.1007/s00540-021-02901-3
Nusinovici S., Tham Y. C., Chak Yan M. Y., Wei Ting D. S., Li J., Sabanayagam C., et al. (2020). Logistic regression was as good as machine learning for predicting major chronic diseases. J. Clin. Epidemiol. 122, 56–69. doi:10.1016/j.jclinepi.2020.03.002
Oh M. Y., Jung Y. M., Kim W. P., Lee H. C., Kim T. K., Ko S. B., et al. (2024). Prediction for perioperative stroke using intraoperative parameters. J. Am. Heart Assoc. 13 (16), e032216. doi:10.1161/jaha.123.032216
Patel R. J., Willie-Permor D., Fan A., Zarrintan S., Malas M. B. (2024). 30-Day risk score for mortality and stroke in patients with carotid artery stenosis using artificial intelligence based carotid plaque morphology. Ann. Vasc. Surg. 109, 63–76. doi:10.1016/j.avsg.2024.05.016
Pfitzner B., Chromik J., Brabender R., Fischer E., Kromer A., Winter A., et al. (2021). Perioperative risk assessment in pancreatic surgery using machine learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2211–2214. doi:10.1109/embc46164.2021.9630897
Ren B., Lipsitz S. R., Weiss R. D., Fitzmaurice G. M. (2023). Multiple imputation for non-monotone missing not at random data using the no self-censoring model. Stat. Methods Med. Res. 32 (10), 1973–1993. doi:10.1177/09622802231188520
Sacco R. L., Kasner S. E., Broderick J. P., Caplan L. R., Connors J. J., Culebras A., et al. (2013). An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44 (7), 2064–2089. doi:10.1161/STR.0b013e318296aeca
Sacco S., Foschi M., Ornello R., De Santis F., Pofi R., Romoli M. (2024). Prevention and treatment of ischaemic and haemorrhagic stroke in people with diabetes mellitus: a focus on glucose control and comorbidities. Diabetologia 67 (7), 1192–1205. doi:10.1007/s00125-024-06146-z
Song Y. X., Yang X. D., Luo Y. G., Ouyang C. L., Yu Y., Ma Y. L., et al. (2023). Comparison of logistic regression and machine learning methods for predicting postoperative delirium in elderly patients: a retrospective study. CNS Neurosci. Ther. 29 (1), 158–167. doi:10.1111/cns.13991
Staartjes V. E., Kernbach J. M., Stumpo V., van Niftrik C. H. B., Serra C., Regli L. (2022). Foundations of feature selection in clinical prediction modeling. Acta Neurochir. Suppl. 134, 51–57. doi:10.1007/978-3-030-85292-4_7
Steyerberg E. W., Vergouwe Y. (2014). Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur. Heart J. 35 (29), 1925–1931. doi:10.1093/eurheartj/ehu207
Sultan I., Bianco V., Kilic A., Jovin T., Jadhav A., Jankowitz B., et al. (2020). Predictors and outcomes of ischemic stroke after cardiac surgery. Ann. Thorac. Surg. 110 (2), 448–456. doi:10.1016/j.athoracsur.2020.02.025
Tasbulak O., Sahin A. (2022). The cha2ds2-VASc score as an early predictor of graft failure after coronary artery bypass surgery. Cureus 14 (3), e22833. doi:10.7759/cureus.22833
Teng X., Han K., Jin W., Ma L., Wei L., Min D., et al. (2024). Development and validation of an early diagnosis model for bone metastasis in non-small cell lung cancer based on serological characteristics of the bone metastasis mechanism. EClinicalMedicine 72, 102617. doi:10.1016/j.eclinm.2024.102617
van den Goorbergh R., van Smeden M., Timmerman D., Van Calster B. (2022). The harm of class imbalance corrections for risk prediction models: illustration and simulation using logistic regression. J. Am. Med. Inf. Assoc. 29 (9), 1525–1534. doi:10.1093/jamia/ocac093
Vlisides P., Mashour G. A. (2016). Perioperative stroke. Can. J. Anaesth. 63 (2), 193–204. doi:10.1007/s12630-015-0494-9
Wongtangman K., Wachtendorf L. J., Blank M., Grabitz S. D., Linhardt F. C., Azimaraghi O., et al. (2021). Effect of intraoperative arterial hypotension on the risk of perioperative stroke after noncardiac surgery: a retrospective multicenter cohort study. Anesth. Analg. 133 (4), 1000–1008. doi:10.1213/ane.0000000000005604
Xue Y., Chen S., Zhang M., Cai X., Zheng J., Wang S., et al. (2022). The prediction models for high-risk population of stroke based on logistic regressive analysis and lightgbm algorithm separately. Iran. J. Public Health 51 (5), 999–1009. doi:10.18502/ijph.v51i5.9415
Keywords: perioperative stroke, machine learning, gradient boosting machine (GBM), noncardiac, nonvascular, and nonneurosurgical procedures, predictive model, clinical decision support
Citation: Cong X, Zou X, Zhu R, Li Y, Liu L and Zhang J (2025) Development and validation of a machine learning-based model for perioperative stroke prediction in noncardiac, nonvascular, and nonneurosurgical patients. Front. Physiol. 16:1624898. doi: 10.3389/fphys.2025.1624898
Received: 10 May 2025; Accepted: 04 June 2025;
Published: 20 June 2025.
Edited by:
Denise Veelo, Amsterdam University Medical Center, NetherlandsCopyright © 2025 Cong, Zou, Zhu, Li, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaqiang Zhang, MTIyMzI0NzI5MUBxcS5jb20=
 Xuhui Cong
Xuhui Cong Xuli Zou1
Xuli Zou1