- 1Graduate School of Pharmaceutical Sciences, Nihon Pharmaceutical University, Saitama, Japan
- 2Faculty of Pharmaceutical Sciences, Nihon Pharmaceutical University, Saitama, Japan
Blood oxygen saturation (SpO2) is a widely used oxygenation index in clinical and physiological settings. However, recent phenomena, such as asymptomatic hypoxia in COVID-19 and the superior performance of athletes in high-altitude conditions under hypoxia, have highlighted the significant variability in individual tolerance to blood oxygen saturation. Age, health status, disease, and hypoxic adaptation influence it. This brief review introduces the concept of the SpO2 switch as a dynamic. We also proposed a physiological compensatory response of SpO2 switch to SpO2 criticality that triggers compensatory responses, including ventilatory, autonomic, cardiovascular, and metabolic adaptations. Furthermore, individuals can exhibit markedly different responses to hypoxia at the same SpO2 value. It reflects a “threshold switch mechanism” driven by an individual’s internal physiological settings. This suggests that the SpO2 value demonstrates the onset of hypoxia symptoms and reacts to the body’s difference in compensatory capacity. This reconceptualisation shifts the focus from static thresholds to dynamic response analysis, offering new perspectives for precision health, mountain medicine, and personalised risk assessment of hypoxia.
1 Introduction
Oxygen saturation (SpO2) is a key indicator to assess respiratory and cardiovascular function (Swartz et al., 2020). Oxygen is essential for aerobic metabolism and maintaining cellular homeostasis (Trayhurn, 2019). The central respiratory control centers dynamically adjust breathing patterns and frequency in response to changes in arterial carbon dioxide (CO2) and oxygen concentrations (Urfy and Suarez, 2014). The nervous system is critical in voluntary and involuntary respiratory regulation (Cherniack, 1990; Health, 2022). Traditionally, SpO2 levels below 94% have been considered clinically alarming. However, during the COVID-19 pandemic, the phenomenon of “silent hypoxemia”—in which patients exhibit significant hypoxemia without overt symptoms—challenged traditional understandings of hypoxia and exposed limitations in current monitoring and critical care strategies (Dhont et al., 2020; Simonson et al., 2021; Bartlett et al., 2020; Yang et al., 2020).
Similarly, elite athletes and people living at high altitudes have excellent tolerance to low blood oxygen saturation (SpO2) levels. Systemic hypoxic stress increases as air pressure decreases with increasing altitude (Barnes and Kilding, 2015; Green, 2000). Hypoxic training has been used for a long time to enhance aerobic capacity by promoting adaptation to reduced oxygen availability (Sinex and Chapman, 2015). Since the outstanding performance of athletes from East African countries at the 1968 Mexico Olympics, altitude training has become a cornerstone of endurance training (Daniels, 1979; Jackson and Balke, 1971), Although hypoxic exposure can stimulate erythropoiesis, mitochondrial efficiency, and ventilatory responses, it can also impair performance in certain conditions (Sinex and Chapman, 2015).
There is growing interest in individual differences in hypoxic tolerance. Factors such as age, sex, genotype, history of altitude exposure, and ethnic background contribute to individual susceptibility to altitude-related illnesses, including acute mountain sickness (AMS), high altitude pulmonary edema (HAPE), and high-altitude cerebral edema (HACE) (Beall, 2014; Villafuerte and Corante, 2016). These differences are critical in designing altitude training programs and predicting adaptive responses (McLean et al., 2013).
Hypoxia is caused by a mismatch between oxygen supply and tissue metabolic demand (Maltepe and Saugstad, 2009). Of note, intense exercise under normoxic conditions also produces hypoxia-like responses due to the dramatic increase in oxygen demand (Radak et al., 2013). These responses span cognitive, visual, emotional, motor, and autonomic domains, and are influenced by physiological status, stress reactivity, exposure duration, and altitude, resulting in substantial interindividual variability (Asshauer, 2006). Although molecular biomarkers for predicting hypoxia tolerance have been explored, no reliable pre-exposure markers have been validated in humans or animal models (Dzhalilova and Makarova, 2020). Furthermore, ventilatory parameters such as tidal volume or respiratory rate may not fully capture the core drivers of respiration (Mortola, 2019).
These observations prompt reevaluating how SpO2 thresholds function and why individual tolerance varies. In this context, we introduced the concept of SpO2 dependence as a physiological switch that describes how changes in metabolic and ventilatory compensation shape individual hypoxic responses. This “switch” is a threshold-triggered response mechanism, indicating that SpO2 tolerance is not static, but can be dynamically adjusted and hierarchically trained.
Notably, even at similar or similar SpO2, individuals exhibit significant variability in their responses to hypoxia symptoms. Some people rapidly experience symptoms like dizziness and dyspnea, while others experience little to no symptoms. This phenomenon suggests that there may be an adjustable physiological threshold or “switch mechanism” that determines when to initiate the hypoxic compensatory response.
2 Individual differences in SpO2 tolerance
Individual tolerance to SpO2 varies significantly and is influenced by multiple factors, including age, physical condition, chronic diseases, genetics, and ethnic background.
• Age Factor
In healthy adults, resting SpO2 remains between 97% and 99%, with values below (Ceylan et al., 2016; Collins et al., 2015). SpO2 tends to decline with aging. Studies have shown that the mean arterial oxygen partial pressure (PaO2) in people over 80 years of age is approximately 66 mmHg, corresponding to an SpO2 of approximately 90%–92% (Malmberg et al., 1987; Sorbini et al., 1968; Cerveri et al., 1995; Madan, 2017).
• Chronic Disease Factors
Resting SpO2 values in patients with chronic diseases, including diabetes (Laursen et al., 2022), chronic cough (Sumanto and Ningtyas, 2022), chronic obstructive pulmonary disease (COPD) (Furian et al., 2018), and COVID-19 infection (Dhont et al., 2020; Simonson et al., 2021; Fuglebjerg et al., 2020), often range from 88% to 92%.
• Fitness and Training Status
Well-trained athletes typically have a delayed and smaller physiological response to decreased SpO2. During intense exercise, individuals often maintain elevated SpO2 levels (Rojas-Camayo et al., 2018; Eroglu et al., 2018; Martín-Escudero et al., 2021). Furthermore, individuals who engage in long-term high-altitude training, even with low resting SpO2, demonstrate high efficiency of their cardiopulmonary and oxygen transport systems (Rojas-Camayo et al., 2018).
• Ethnic and social factors
Ethnic differences may influence the clinical assessment and treatment strategies for hypoxemia. For example, oxygen therapy regimens in intensive care units vary across ethnic groups, and pulse oximetry may underestimate hypoxemia in patients with darker skin (Giovanelli et al., 2023; Sjoding et al., 2020; Fawzy et al., 2022). Furthermore, genetic background (such as high-altitude acclimatization; (Beall, 2007; Nishimura et al., 2022), access to healthcare, and socioeconomic status (Shi et al., 2022) also influence the diagnosis and prognosis of hypoxemia.
In summary, the triggering of hypoxic symptoms depends not only on the absolute SpO2 value but also on the individualised “SpO2 threshold switch.” In other words, even at the same blood oxygen concentration, different individuals may exhibit completely different symptomatic responses or no symptoms due to different threshold settings.
3 Physiological mechanisms of hypoxic compensation
When the body senses hypoxia, it initiates a series of compensatory mechanisms to maintain oxygen homeostasis, including increased respiratory rate, heart rate, sympathetic nerve activity, and redistribution of blood flow to vital organs (Grimminger et al., 2017). These responses are mainly mediated by chemoreceptors, especially those in the carotid arteries and aortic bodies, which can sense the decrease in arterial blood oxygen and trigger downstream physiological pathways (Prabhakar et al., 2015; Prabhakar and Semenza, 2015; Heymans and Heymans, 1927).
The autonomic nervous system (ANS) plays a central role in hypoxic adaptation. Increased sympathetic nervous system activity enhances cardiac output and pulmonary ventilation, while parasympathetic nervous system activity is typically suppressed to support the acute stress response (Hainsworth et al., 2007; Schagatay et al., 2000). Respiratory centres within the brainstem are highly sensitive to hypoxia and rapidly initiate a hypoxic ventilatory response (HVR) to increase ventilation and partially compensate for decreased blood oxygen levels (Pamenter and Powell, 2016). Prolonged hypoxia can cause a shift in baseline autonomic function, and individual differences in this response are closely related to genetic background, physical status, age, and sex (Puri et al., 2021). Previous studies have shown that exercise training can help improve autonomic stability, enhancing hypoxic tolerance (Calbet et al., 2003).
Acute hypoxia causes a decrease in arterial oxygen content, affecting multiple physiological functions. Under moderate hypoxic conditions, peripheral muscles are prone to fatigue and inhibit motor output through sensory afferent centres to reduce energy expenditure and maintain physiological stability. This is also one of the core assumptions of the “perception-limited fatigue theory” (Amann et al., 2006; Amann et al., 2007; Gandevia, 2001). Under more severe hypoxic conditions, even if muscles have not reached maximal fatigue, the body will actively reduce exercise output to avoid systemic instability (Fulco et al., 1994).
Under constant perceived exertion (RPE) conditions, exercise intensity and duration decrease significantly as ambient oxygen concentration decreases. This phenomenon is closely associated with a rapid decrease in SpO2 and a premature increase in respiratory rate, indicating that SpO2 levels and respiratory compensation are important physiological signals regulating perceived exertion (Jeffries et al., 2019). Exercise-induced hypoxemia still significantly limits aerobic capacity (Faoro et al., 2017). Low baseline SpO2 at rest is a significant risk factor for severe exercise-induced desaturation (EID) (Gao et al., 2025).
There is also significant inter-individual variability in ventilatory responses to intense exercise, which is difficult to predict using resting hypoxic or hypercapnic stimulation tests. Previous literature has generally suggested that trained endurance athletes exhibit blunted chemoreceptor responsiveness, but this phenomenon is highly heterogeneous and may be related to baseline SpO2 (Dempsey and Wagner, 1999).
At high altitude, the decrease in ambient oxygen partial pressure with increasing altitude naturally causes SpO2 to decrease. Despite this, most healthy adults can acclimate within hours to days, maintaining arterial oxygen saturation (SaO2) within the functional range of 80%–90% (Shaw et al., 2021). In contrast, elderly individuals exhibit blunted respiratory and cardiovascular responses to hypoxia and hypercapnia, suggesting that their oxygen dependence may increase (Kronenberg and Drage, 1973). Elderly individuals and those with chronic medical conditions are more affected by hypoxia-related symptoms and complications (Albert and Swenson, 2014; Chapman, 2013; Havalko et al., 2022).
Notably, an individual’s physiological response to hypoxia is highly related to their resting SpO2 level. Studies have shown that non-pharmacological interventions such as acupuncture may help improve hypoxemia-related symptoms by lowering SpO2 levels (Sumanto and Ningtyas, 2022). Intermittent hypoxia (IH) training is a non-pharmacological method for preventing and treating hypoxia in patients with various diseases and healthy adults (Serebrovskaya and Xi, 2016; Verges et al., 2015).
The extent and duration of the decrease in SpO2 at low oxygen doses (F(IO)2) can reflect an individual’s compensatory capacity. SpO2 levels remain stable in tolerant individuals, whereas SpO2 decreases rapidly and recovers slowly in dependent individuals, suggesting increased oxygen sensitivity (Peltonen et al., 1999). Furthermore, patients undergoing obesity surgery experienced elevated cardiopulmonary parameters and decreased SpO2 after a 6-min walk (Shrivastava, 2025). A study of sprinters undergoing high-intensity intermittent hypoxic training demonstrated that higher mean SpO2 levels were associated with improved performance, highlighting how changes in SpO2 influence training responses (Takei et al., 2025).
In summary, when the body senses hypoxia, it triggers a compensatory response through chemoreceptors, including increased respiratory and heart rates, sympathetic activity, and redistribution of blood flow to maintain oxygen homeostasis. The intensity of this response is influenced by genetics, age, physical fitness, and health status.
4 Regulation and adaptation of the SpO2 switch
Aerobic capacity—the ability to sustain prolonged exercise under normoxic conditions—is a key determinant of endurance performance (Feng et al., 2023; Girard et al., 2020). The brain and skeletal muscle have different oxygen requirements, and physiological or pathological states can alter tissue sensitivity to oxygen supply (Kulkarni et al., 2007). Although well-trained individuals typically have a low resting heart rate, they can still exhibit a pronounced heart rate response to hypoxic or high-intensity exercise (Goorakani et al., 2020; Patel and Zwibel, 2024).
Among various exercise training methods, interventions such as intermittent hypoxic training (IHT), breath-hold diving, and paced breathing exercises have significantly improved tolerance to low SpO2. These exercises can enhance autonomic balance (Rybnikova et al., 2022), ventilatory efficiency and metabolic regulation, oxygen transport and utilisation (Park et al., 2018; Park et al., 2018), and even exert neuroprotective effects (Rybnikova et al., 2022).
Intermittent hypoxia (IH) training, with the development and widespread use of equipment that induces systemic or localised hypoxia, has recently seen considerable research on related training methods. Methods such as “hypoxic living-hyperoxic training” have gained widespread popularity and become effective and efficient training methods for various professional athletes (Millet et al., 2010; Girard et al., 2017; Girard et al., 2020).
Well-trained freedivers can maintain a 1:1 apnea-to-repnea ratio while stationary without experiencing progressive hypoxia, and their physiological responses adapt with repeated pauses (Mulder et al., 2025). Furthermore, elite divers can tolerate prolonged apnea with minimal anaerobic metabolic burden (Drviš et al., 2025), suggesting that training strengthens the ability to regulate the SpO2 switch and prolongs tolerance. We believe this is due to the regulation of the SpO2 switch, resulting in adaptation after training.
In this study, arterial oxygen saturation was measured in healthy subjects and patients with chronic heart failure during spontaneous breathing, at 15, 6, and 3 breaths per minute, at rest, and during exercise (Bernardi et al., 1998). These exercises help maintain calmness and physiological stability under low oxygen pressure, supporting that spontaneous respiratory regulation can enhance autonomic function (Jerath, 2016). Even brief, conscious control of breathing rate and depth is considered a health-promoting strategy, similar to the mechanisms of altitude acclimatisation. In hypoxic emergencies, these techniques may help delay the onset of severe hypoxemia (Miles, 1964).
Acute hypoxia increases cardiac output and sympathetic drive to maintain oxygen delivery to vital organs (Heinonen et al., 2016; Fox et al., 2006). In severe COVID-19, the concurrent decrease in oxygen saturation and increased heart rate are associated with autonomic dysfunction or enhanced baroreflex sensitivity (Swenson and Hardin, 2023). Interestingly, despite metabolic changes under hypoxia, VO2 during fatigue was similar across normoxia, hypoxia, and hyperoxia, suggesting oxygen availability may not limit short-to moderate-duration exercise (Adams and Welch, 1980).
In summary, the best way to explain the varying manifestations of symptoms at the same SpO2 level is to view SpO2 as a dynamic physiological switch. Its individualised critical threshold (SpO2-CR) determines when compensatory responses are initiated.
5 Discussion
5.1 SpO2 switch: critical response range and regulation of hypoxia tolerance
SpO2 is commonly used to quantify oxygen transport status. However, recent studies suggest that a decrease in SpO2 can trigger a series of physiological compensatory responses, potentially acting as a “switch.” For example, high-altitude studies have shown that men with higher BMIs are more susceptible to hypoxemia during winter mountaineering (Vignati et al., 2021), and BMI is negatively correlated with SpO2 (Ceylan et al., 2016; Gupta et al., 2014). Obese subjects also have worse altitude sickness scores and nighttime SpO2 at a simulated altitude of 3,658 m (Ri-Li et al., 2003), reflecting limited respiratory acclimatisation and hypoxia tolerance (Caravedo et al., 2022). Furthermore, exercise testing has shown that a significant decrease in SpO2 shortens exercise time and reduces performance (Jeffries et al., 2019). Some non-pharmacological interventions, such as acupuncture, can also adjust SpO2 levels and alleviate hypoxia-related symptoms (Sumanto and Ningtyas, 2022).
In addition to high-altitude exposure, SpO2 during exercise also exhibits intensity-dependent characteristics. Cycling exercise studies showed that SpO2 after anaerobic exercise decreased significantly compared to before and after warm-up (Tahhan et al., 2018). Henslin Harris et al. (2013) and Campbell et al. (2009) noted that the decrease increased with increasing exercise intensity (Campbell et al., 2009; Henslin Harris et al., 2013); however, no significant changes were observed during warm-up or low-to-moderate-intensity aerobic exercise, SpO2 usually remains close to resting levels (Rowell et al., 1964). This may be because the respiratory and circulatory systems can maintain stability, keeping SpO2 close to resting levels (Tahhan et al., 2018).
Nikooie et al. (2009) used SpO2 to measure the anaerobic threshold (AT) noninvasively. They found that when exercise intensity reaches AT, SpO2 drops sharply and is highly correlated with the lactate threshold (LT), reaching its lowest point at maximal oxygen uptake (VO2max). Similar phenomena are observed in different types of exercise: for example, a rapid drop in SpO2 during the high-intensity phase can be observed in both short-distance, high-intensity anaerobic sprints (100 m) and medium- and long-distance aerobic events (400 m and 800 m).
These changes in SpO2 are not simply due to insufficient oxygen supply but result from coordinated regulation between the central and peripheral systems. This leads us to propose the “SpO2-CR switch” hypothesis: baseline SpO2 remains stable. When exercise intensity approaches VO2max, SpO2 drops to an individualised nadir, but does not deviate significantly from baseline. This “switch” may trigger the hypoxic response, determining the body’s compensation pattern under high load (see Figure 1).
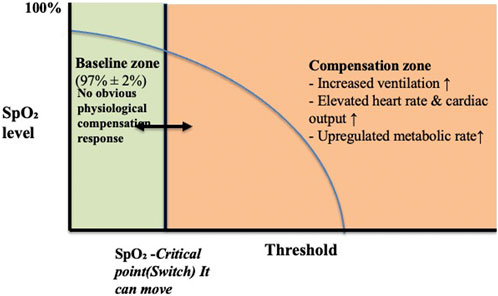
Figure 1. Physiological responses to declining SpO2: activation of the SpO2-CR switch. Note: When SpO2 remains within the normal range (approximately 97% ± 2%), the body is in a stable “baseline zone.” As SpO2 slowly decreases and approaches the individual’s critical point (But this varies from person to person), the SpO2-CR switch is triggered, entering the “compensatory zone.” Central and peripheral regulatory systems work together within this zone, including increased ventilation, heart rate, cardiac output, and metabolic rate.
Furthermore, the hypoxic threshold may vary among individuals. Modulating this threshold “switch” through medication, acupuncture, or other non-pharmacological approaches may further optimise hypoxia-related physiological responses and athletic performance.
These responses aim to maintain tissue oxygen delivery and exercise performance in hypoxic environments. When SpO2 rises and exceeds the critical point, the switch “resets,” and physiological functions gradually return to baseline levels.
It is important to note that the “baseline zone” and “critical point” are not fixed values but can be adjusted through training, environmental adaptation, and even pharmacological or non-pharmacological interventions. Training adaptation can lower baseline SpO2 levels or delay the triggering of the critical point, thereby improving hypoxic tolerance and exercise performance.
Based on this, we propose the concept of the SpO2 switch and critical range as individualised indicators for inducing compensatory responses. Its core components include:
1. Baseline SpO2: The average SpO2 range of an individual’s stable SpO2 at rest and normal pressure.
2. Critical Range (SpO2-CR): A certain drop below the baseline value is considered a threshold that may trigger a response.
3. Switch Activation: When SpO2 enters the critical range, compensatory mechanisms such as increased respiratory and heart rates, sympathetic nerve activation, and blood flow redistribution are triggered.
4. Trainability: Interventions such as breathing training, endurance exercise, high-altitude exposure, or acupuncture can adjust baseline and critical ranges to improve hypoxia tolerance.
This concept can be applied to athletic performance monitoring, chronic disease management, and altitude acclimatisation assessment. Future research could explore its feasibility as a clinical predictive and training indicator.
6 Future research directions and clinical applications
SpO2 should not be understood simply as a passive reflection of oxygen delivery but as a dynamic physiological switch that controls the body’s compensatory response to hypoxic stress. This switch influences the individualised SpO2 critical threshold (SpO2-CR). Below this threshold, the body initiates a series of adaptive mechanisms, including increased ventilation, increased heart rate, sympathetic nervous system activation, and redistribution of blood to vital organs. This switch-like behaviour of SpO2 has important implications for understanding exercise tolerance, fatigue, and resilience under both hypoxic and non-hypoxic conditions. It is expected to be a comprehensive physiological indicator encompassing multiple fields, including altitude acclimatisation, physiological monitoring, exercise training, and critical care.
Although previous research has explored the significance of SpO2 in clinical and environmental physiology, its regulation, modelling, and systematic validation remain limited.
Future research should explore various interventions to modulate the SpO2 switch. Breathing training, structured exercise in hypoxic conditions, and high-altitude exposure may help lower the critical threshold and enhance hypoxic tolerance. Furthermore, previous studies have shown preliminary efficacy in modulating SpO2 responses, particularly in individuals with irregular blood pressure or chronic respiratory symptoms, warranting further investigation as a non-pharmacological intervention. Pharmacological modulation of the SpO2 switch response also represents an emerging area, promising therapies to enhance oxygen utilisation or prevent hypoxic injury.
This approach could be applied to high-altitude travel, aviation medicine, geriatric care, sports training, and rehabilitation medicine to develop personalised health management and risk prevention strategies. Integrating genetic, epigenetic, and environmental exposure profiles can help better understand the cross-scale mechanistic integration of individual differences in hypoxic adaptation.
7 Conclusion
Blood oxygen saturation (SpO2) should not be viewed solely as a passive indicator of oxygen delivery. Instead, it acts as an active physiological switch, regulating the body’s compensatory response to hypoxic stress. This conceptual model redefines the SpO2 switch as a dynamic and trainable trait, determined by an individual’s baseline level and a critical threshold (SpO2-CR). When SpO2 levels fall below this personalized threshold, a series of compensatory mechanisms are activated to maintain physiological and functional stability.
This conceptual model redefines SpO2 tolerance as a dynamic and adjustable trait, offering new perspectives for preventive medicine and precision health. Moving beyond a static threshold model and toward a personalized SpO2 response model can enhance early intervention, optimize training outcomes, and improve human adaptability and resilience to various physiological and environmental challenges.
In summary, even at the same or similar SpO2 percentages, significant differences exist between individuals in their physiological and symptomatic responses to hypoxia. This variability reflects the individualized SpO2 switching mechanism, whose critical threshold (SpO2-CR) determines when to initiate respiratory and circulatory compensatory responses.
Author contributions
EY: Investigation, Writing – review and editing, Writing – original draft. H-YC: Writing – original draft, Writing – review and editing, Supervision, Investigation, Formal Analysis, Validation, Visualization, Conceptualization. F-SC: Writing – review and editing, Investigation, Supervision, Writing – original draft, Project administration, Data curation, Methodology, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams R. P., Welch H. G. (1980). Oxygen uptake, acid-base status, and performance with varied inspired oxygen fractions. J. Appl. Physiol. Respir. Environ. Exerc Physiol. 49, 863–868. doi:10.1152/jappl.1980.49.5.863
Albert T. J., Swenson E. R. (2014). Peripheral chemoreceptor responsiveness and hypoxic pulmonary vasoconstriction in humans. High. Alt. Med. Biol. 15, 15–20. doi:10.1089/ham.2013.1072
Amann M., Romer L. M., Pegelow D. F., Jacques A. J., Hess C. J., Dempsey J. A. (2006). Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J. Appl. Physiol. 101, 119–127. doi:10.1152/japplphysiol.01596.2005
Amann M., Romer L. M., Subudhi A. W., Pegelow D. F., Dempsey J. A. (2007). Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J. Physiol. 581, 389–403. doi:10.1113/jphysiol.2007.129700
Barnes K. R., Kilding A. E. (2015). Strategies to improve running economy. Sports Med. 45, 37–56. doi:10.1007/s40279-014-0246-y
Bartlett R. H., Ogino M. T., Brodie D., Mcmullan D. M., Lorusso R., Maclaren G., et al. (2020). Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 66, 472–474. doi:10.1097/MAT.0000000000001173
Beall C. M. (2007). Two routes Funct. Adapt. Tibetan Andean high-altitude Nativ. Proceedings of the National Academy of Sciences. 104, 8655–8660. doi:10.1073/pnas.0701985104
Beall C. M. (2014). Adaptation to high altitude: phenotypes and genotypes. Annu. Rev. Anthropol. 43, 251–272. doi:10.1146/annurev-anthro-102313-030000
Bernardi L., Spadacini G., Bellwon J., Hajric R., Roskamm H., Frey A. W. (1998). Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet 351, 1308–1311. doi:10.1016/S0140-6736(97)10341-5
Calbet J. A., Boushel R., Rådegran G., Søndergaard H., Wagner P. D., Saltin B. (2003). Determinants of maximal oxygen uptake in severe acute hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R291–R303. doi:10.1152/ajpregu.00155.2002
Campbell A., Minniti C. P., Nouraie M., Arteta M., Rana S., Onyekwere O., et al. (2009). Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br. J. Haematol. 147, 352–359. doi:10.1111/j.1365-2141.2009.07854.x
Caravedo M. A., Mozo K., Morales M. L., Smiley H., Stuart J., Tilley D. H., et al. (2022). Risk factors for acute Mountain sickness in travellers to Cusco, Peru: coca leaves, obesity and sex. J. travel Med. 29. doi:10.1093/jtm/taab102
Cerveri I., Zoia M. C., Fanfulla F., Spagnolatti L., Berrayah L., Grassi M., et al. (1995). Reference values of arterial oxygen tension in the middle-aged and elderly. Am. J. Respir. Crit. Care Med. 152, 934–941. doi:10.1164/ajrccm.152.3.7663806
Ceylan B., Khorshid L., Güneş Ü. Y., Zaybak A. (2016). Evaluation of oxygen saturation values in different body positions in healthy individuals. J. Clin. Nurs. 25, 1095–1100. doi:10.1111/jocn.13189
Chapman R. F. (2013). The individual response to training and competition at altitude. Br. J. Sports Med. 47 (Suppl. 1), i40–i44. doi:10.1136/bjsports-2013-092837
Cherniack N. S. (1990). The central nervous System and respiratory muscle coordination. CHEST 97, 52S–57S. doi:10.1378/chest.97.3_supplement.52s-a
Collins J. A., Rudenski A., Gibson J., Howard L., O'Driscoll R. (2015). Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe (Sheff) 11, 194–201. doi:10.1183/20734735.001415
Daniels J. (1979). Altitude and athletic training and performance. Am. J. sports Med. 7, 371–373. doi:10.1177/036354657900700617
Dempsey J. A., Wagner P. D. (1999). Exercise-induced arterial hypoxemia. J. Appl. Physiology 87, 1997–2006. doi:10.1152/jappl.1999.87.6.1997
Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B. N. (2020). The pathophysiology of 'happy' hypoxemia in COVID-19. Respir. Res. 21, 198. doi:10.1186/s12931-020-01462-5
Drviš I., Vrdoljak D., Dujić G., Foretić N., Dujić Ž. (2025). Aerobic and anaerobic metabolism during monofin swimming in trained breath-hold divers. J. Funct. Morphol. Kinesiol 10, 218. doi:10.3390/jfmk10020218
Dzhalilova D., Makarova O. (2020). Differences in tolerance to hypoxia: physiological, biochemical, and molecular-biological characteristics. Biomedicines 8, 428. doi:10.3390/biomedicines8100428
Eroglu H., Okyaz B., Türkçapar Ü. (2018). The effect of acute aerobical exercise on arterial blood oxygen saturation of athletes. J. Educ. Train. Stud. 6, 74–79. doi:10.11114/jets.v6i9a.3562
Faoro V., Deboeck G., Vicenzi M., Gaston A.-F., Simaga B., Doucende G., et al. (2017). Pulmonary vascular function and aerobic exercise capacity at moderate altitude. Med. and Sci. Sports and Exerc. 49, 2131–2138. doi:10.1249/MSS.0000000000001320
Fawzy A., Wu T. D., Wang K., Robinson M. L., Farha J., Bradke A., et al. (2022). Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med. 182, 730–738. doi:10.1001/jamainternmed.2022.1906
Feng X., Zhao L., Chen Y., Wang Z., Lu H., Wang C. (2023). Optimal type and dose of hypoxic training for improving maximal aerobic capacity in athletes: a systematic review and Bayesian model-based network meta-analysis. Front. Physiology 14, 1223037. doi:10.3389/fphys.2023.1223037
Fox W. C., Watson R., Lockette W. (2006). Acute hypoxemia increases cardiovascular baroreceptor sensitivity in humans. Am. J. Hypertens. 19, 958–963. doi:10.1016/j.amjhyper.2006.02.005
Fuglebjerg N. J. U., Jensen T. O., Hoyer N., Ryrsø C. K., Lindegaard B., Harboe Z. B. (2020). Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int. J. Infect. Dis. 99, 100–101. doi:10.1016/j.ijid.2020.07.014
Fulco C. S., Rock P. B., Trad L. A., Rose M. S., Forte V. A., Young P. M., et al. (1994). Effect of caffeine on submaximal exercise performance at altitude. Aviat. Space Environ. Med. 65, 539–545.
Furian M., Flueck D., Latshang T. D., Scheiwiller P. M., Segitz S. D., Mueller-Mottet S., et al. (2018). Exercise performance and symptoms in lowlanders with COPD ascending to moderate altitude: randomized trial. Int. J. Chronic Obstr. Pulm. Dis. 13, 3529–3538. doi:10.2147/COPD.S173039
Gandevia S. C. (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 81, 1725–1789. doi:10.1152/physrev.2001.81.4.1725
Gao B., Wang S., Zhao L., Liao H., Qumu S., Wang P., et al. (2025). Characteristics and quality of life of patients with COPD with different degrees of exercise-induced desaturation on six-minute walk Test. Int. J. Chron. Obstruct Pulmon Dis. 20, 2381–2391. doi:10.2147/COPD.S513089
Giovanelli L., Malacarne M., Pagani M., Biolo G., Mekjavic I. B., Bernardelli G., et al. (2023). Moderate aerobic exercise reduces the detrimental effects of hypoxia on cardiac autonomic control in healthy volunteers. J. Pers. Med. 13, 585. doi:10.3390/jpm13040585
Girard O., Brocherie F., Millet G. P. (2017). Effects of Altitude/Hypoxia on Single- and multiple-sprint performance: a comprehensive review. Sports Med. 47, 1931–1949. doi:10.1007/s40279-017-0733-z
Girard O., Brocherie F., Goods P. S., Millet G. P. (2020). An updated panorama of “living low-training high” altitude/hypoxic methods. Front. Sports Act. Living 2, 26. doi:10.3389/fspor.2020.00026
Goorakani Y., Sedigh Rahimabadi M., Dehghan A., Kazemi M., Chijan M. R., Bijani M., et al. (2020). Correlation of resting heart rate with anthropometric factors and serum biomarkers in a population-based study: Fasa PERSIAN cohort study. BMC Cardiovasc. Disord. 20, 319. doi:10.1186/s12872-020-01594-y
Green H. J. (2000). Altitude acclimatization, training and performance. J. Sci. Med. Sport 3, 299–312. doi:10.1016/s1440-2440(00)80039-0
Grimminger J., Richter M., Tello K., Sommer N., Gall H., Ghofrani H. A. (2017). Thin air resulting in high pressure: mountain sickness and hypoxia-induced pulmonary hypertension. Can. Respir. J. 2017, 8381653. doi:10.1155/2017/8381653
Gupta S. S., Gothi D., Narula G., Sircar J. (2014). Correlation of BMI and oxygen saturation in stable COPD in Northern India. Lung India 31, 29–34. doi:10.4103/0970-2113.125891
Hainsworth R., Drinkhill M. J., Rivera-Chira M. (2007). The autonomic nervous system at high altitude. Clin. Auton. Res. 17, 13–19. doi:10.1007/s10286-006-0395-7
Havalko A., Asanov E., Shatilo V. (2022). Response of some indicators of the respiratory system to dosed hypoxia in elderly people with impaired glucose tolerance. Ageing Longev. 3, 27–31. doi:10.47855/10.47855/jal9020-2022-1-4
Health N. I. O. (2022). How the lungs work. How your body controls breathing. National Institutes of Health. Available online at: https://www.nhlbi.nih.gov/health/lungs/body-controls-breathing (Accessed July 15, 2025).
Heinonen I. H., Boushel R., Kalliokoski K. K. (2016). The circulatory and metabolic responses to hypoxia in humans - with special reference to adipose tissue physiology and obesity. Front. Endocrinol. (Lausanne) 7, 116. doi:10.3389/fendo.2016.00116
Henslin Harris K. B., Foster C., De Koning J. J., Dodge C., Wright G. A., Porcari J. P. (2013). Rapidity of response to hypoxic conditions during exercise. Int. J. Sports Physiol. Perform. 8, 330–335. doi:10.1123/ijspp.8.3.330
Heymans J., Heymans C. (1927). Sur les modifications directes et sur la régulation réflexe de l’activité du centre respiratoire de la tête isolée du chien. Arch. Int. Pharmacodyn. Ther. 33, 273–372.
Jackson R., Balke B. (1971). Training at altitude for performance at sea level. Schweiz. Z. fur Sportmed. Suppl, 19–27.
Jeffries O., Patterson S. D., Waldron M. (2019). The effect of severe and moderate hypoxia on exercise at a fixed level of perceived exertion. Eur. J. Appl. Physiol. 119, 1213–1224. doi:10.1007/s00421-019-04111-y
Jerath R. (2016). Physiology of long pranayamic breathing: neural respiratory elements May provide a mechanism that explains how slow deep breathing shifts the autonomic nervous System. J. Yoga and Phys. Ther. 6. doi:10.4172/2157-7595.1000252
Kronenberg R. S., Drage C. W. (1973). Attenuation of the ventilatory and heart rate responses to hypoxia and Hypercapnia with aging in normal men. J. Clin. Investigation 52, 1812–1819. doi:10.1172/JCI107363
Kulkarni A. C., Kuppusamy P., Parinandi N. (2007). Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxidants and redox Signal. 9, 1717–1730. doi:10.1089/ars.2007.1724
Laursen J. C., Jepsen R., Bruun-Rasmussen N. E., Frimodt-Møller M., Jørgensen M. E., Rossing P., et al. (2022). Blood oxygen saturation is lower in persons with pre-diabetes and screen-detected diabetes compared with non-diabetic individuals: a population-based study of the Lolland-Falster Health Study cohort. Front Epidemiol. 2, 1022342. doi:10.3389/fepid.2022.1022342
Madan A. (2017). Correlation between the levels of SpO(2)and PaO(2). Lung India 34, 307–308. doi:10.4103/lungindia.lungindia_106_17
Malmberg P., Hedenstrom H., Fridriksson H. V. (1987). Reference values for gas exchange during exercise in healthy nonsmoking and smoking men. Bull. Eur. Physiopathol. Respir. 23, 131–138.
Maltepe E., Saugstad O. D. (2009). Oxygen in health and disease: regulation of oxygen homeostasis-clinical implications. Pediatr. Res. 65, 261–268. doi:10.1203/PDR.0b013e31818fc83f
Martín-Escudero P., Cabanas A. M., Fuentes-Ferrer M., Galindo-Canales M. (2021). Oxygen saturation behavior by Pulse oximetry in female athletes: breaking myths. Biosensors 11, 391. doi:10.3390/bios11100391
Mclean B. D., Buttifant D., Gore C. J., White K., Kemp J. (2013). Year-to-year variability in haemoglobin mass response to two altitude training camps. Br. J. sports Med. 47, i51–i58. doi:10.1136/bjsports-2013-092744
Miles W. R. (1964). Oxygen consumption during three yoga-type breathing patterns. J. Appl. Physiology 19, 75–82. doi:10.1152/jappl.1964.19.1.75
Millet G. P., Roels B., Schmitt L., Woorons X., Richalet J. P. (2010). Combining hypoxic methods for peak performance. Sports Med. 40, 1–25. doi:10.2165/11317920-000000000-00000
Mortola J. P. (2019). How to breathe? Respiratory mechanics and breathing pattern. Respir. Physiol. Neurobiol. 261, 48–54. doi:10.1016/j.resp.2018.12.005
Mulder E. R., Bouten J., Holmström P. K., Schagatay E. K. (2025). Progressive changes of oxygenation, diving response, and involuntary breathing movements during repeated apneas. Respir. Physiol. Neurobiol. 336, 104455. doi:10.1016/j.resp.2025.104455
Nikooie R., Gharakhanlo R., Rajabi H., Bahraminegad M., Ghafari A. (2009). Noninvasive determination of anaerobic threshold by monitoring the %SpO2 changes and respiratory gas exchange. J. Strength and Cond. Res. 23, 2107–2113. doi:10.1519/JSC.0b013e3181b73bc2
Nishimura T., Arima H., Koirala S., Ito H., Yamamoto T. (2022). Individual variations and sex differences in hemodynamics and percutaneous arterial oxygen saturation (SpO2) in Tibetan highlanders of tsarang in the Mustang district of Nepal. J. Physiological Anthropol. 41, 9. doi:10.1186/s40101-022-00282-4
Pamenter M. E., Powell F. L. (2016). Time domains of the hypoxic ventilatory response and their molecular basis. Compr. Physiol. 6, 1345–1385. doi:10.1002/cphy.c150026
Park H. Y., Jung W. S., Kim J., Hwang H., Lim K. (2018). Efficacy of intermittent hypoxic training on hemodynamic function and exercise performance in competitive swimmers. J. Exerc Nutr. Biochem. 22, 32–38. doi:10.20463/jenb.2018.0028
Patel P. N. H. M., Zwibel H. (2024). Exercise physiology. Treasure Island (FL): StatPearls Publishing.
Peltonen J. E., Leppävuori A. P., Kyrö K. P., Mäkelä P., Rusko H. K. (1999). Arterial haemoglobin oxygen saturation is affected by F(I)O2 at submaximal running velocities in elite athletes. Scand. J. Med. Sci. Sports 9, 265–271. doi:10.1111/j.1600-0838.1999.tb00244.x
Prabhakar N. R., Semenza G. L. (2015). Oxygen sensing and homeostasis. Physiology 30, 340–348. doi:10.1152/physiol.00022.2015
Prabhakar N. R., Peng Y. J., Kumar G. K., Nanduri J. (2015). Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr. Physiol. 5, 561–577. doi:10.1002/cphy.c140039
Puri S., Panza G., Mateika J. H. (2021). A comprehensive review of respiratory, autonomic and cardiovascular responses to intermittent hypoxia in humans. Exp. Neurol. 341, 113709. doi:10.1016/j.expneurol.2021.113709
Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. (2013). Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal 18, 1208–1246. doi:10.1089/ars.2011.4498
Ri-Li G., Chase P. J., Witkowski S., Wyrick B. L., Stone J. A., Levine B. D., et al. (2003). Obesity: associations with acute mountain sickness. Ann. Intern. Med. 139, 253–257. doi:10.7326/0003-4819-139-4-200308190-00007
Rojas-Camayo J., Mejia C. R., Callacondo D., Dawson J. A., Posso M., Galvan C. A., et al. (2018). Reference values for oxygen saturation from sea level to the highest human habitation in the Andes in acclimatised persons. Thorax 73, 776–778. doi:10.1136/thoraxjnl-2017-210598
Rowell L. B., Taylor H. L., Wang Y., Carlson W. S. (1964). Saturation of arterial blood with oxygen during maximal exercise. J. Appl. Physiology 19, 284–286. doi:10.1152/jappl.1964.19.2.284
Rybnikova E. A., Nalivaeva N. N., Zenko M. Y., Baranova K. A. (2022). Intermittent hypoxic training as an effective tool for increasing the adaptive potential, endurance and working capacity of the brain. Front. Neurosci. 16, 941740. doi:10.3389/fnins.2022.941740
Schagatay E., Van Kampen M., Emanuelsson S., Holm B. (2000). Effects of physical and apnea training on apneic time and the diving response in humans. Eur. J. Appl. Physiol. 82, 161–169. doi:10.1007/s004210050668
Serebrovskaya T. V., XI L. (2016). Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: practical analysis on methods and equipment. Exp. Biol. Med. 241, 1708–1723. doi:10.1177/1535370216657614
Shaw D. M., Cabre G., Gant N. (2021). Hypoxic Hypoxia and brain function in military aviation: basic physiology and applied perspectives. Front. physiology 12, 665821. doi:10.3389/fphys.2021.665821
Shi C., Goodall M., Dumville J. C., Hill J., Norman G., Hamer O., et al. (2022). The accuracy of Pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: a systematic review and meta-analysis. BMC Med. 20, 267. doi:10.1186/s12916-022-02452-8
Shrivastava S. (2025). Physiological characteristics of surgical patients with obesity in response to the 6-Min walk Test. Int. J. Life Sci. Biotechnol. Pharma Res. 14 (2), 398–403. doi:10.69605/ijlbpr_14.2.2025.75
Simonson T. S., Baker T. L., Banzett R. B., Bishop T., Dempsey J. A., Feldman J. L., et al. (2021). Silent hypoxaemia in COVID-19 patients. J. Physiol. 599, 1057–1065. doi:10.1113/JP280769
Sinex J. A., Chapman R. F. (2015). Hypoxic training methods for improving endurance exercise performance. J. Sport Health Sci. 4, 325–332. doi:10.1016/j.jshs.2015.07.005
Sjoding M. W., Dickson R. P., Iwashyna T. J., Gay S. E., Valley T. S. (2020). Racial bias in Pulse Oximetry measurement. N. Engl. J. Med. 383, 2477–2478. doi:10.1056/NEJMc2029240
Sorbini C. A., Grassi V., Solinas E., Muiesan G. (1968). Arterial oxygen tension in relation to age in healthy subjects. Respiration 25, 3–13. doi:10.1159/000192549
Sumanto S., Ningtyas L. A. W. (2022). The effect of acupuncture therapy on blood oxygen saturation in patients with blood pressure disorders and coughs in elderly health post, Surakarta. J. Epidemiol. Public Health 7 (3), 304–310. doi:10.26911/jepublichealth.2022.07.03.03
Swartz H. M., Flood A. B., Schaner P. E., Halpern H., Williams B. B., Pogue B. W., et al. (2020). How best to interpret measures of levels of oxygen in tissues to make them effective clinical tools for care of patients with cancer and other oxygen-dependent pathologies. Physiol. Rep. 8, e14541. doi:10.14814/phy2.14541
Swenson K. E., Hardin C. C. (2023). Pathophysiology of hypoxemia in COVID-19 lung disease. Clin. Chest Med. 44, 239–248. doi:10.1016/j.ccm.2022.11.007
Tahhan A. M. A. A., Özdal M., Vural M., Pancar Z. (2018). Influence of aerobic and anaerobic exercise on oxygen saturation. European Journal of Physical Education and Sport Science.
Takei N., Muraki R., Girard O., Hatta H. (2025). Inter-individual variability in performance benefits from repeated sprint training in hypoxia and associated training parameters. Front. Sports Act. Living 7, 1524437–2025. doi:10.3389/fspor.2025.1524437
Trayhurn P. (2019). Oxygen-A critical, but overlooked, nutrient. Front. Nutr. 6, 10. doi:10.3389/fnut.2019.00010
Urfy M. Z., Suarez J. I. (2014). “Chapter 17 - breathing and the nervous system,” in Handbook of clinical neurology. Editors J. BILLER, and J. M. FERRO (Elsevier).
Verges S., Chacaroun S., Godin-Ribuot D., Baillieul S. (2015). Hypoxic conditioning as a new therapeutic modality. Front. Pediatr. 3, 58. doi:10.3389/fped.2015.00058
Vignati C., Mapelli M., Nusca B., Bonomi A., Salvioni E., Mattavelli I., et al. (2021). A breathtaking lift: sex and body mass index differences in cardiopulmonary response in a large cohort of unselected subjects with acute exposure to high altitude. High. Alt. Med. Biol. 22, 379–385. doi:10.1089/ham.2021.0039
Villafuerte F. C., Corante N. (2016). Chronic Mountain sickness: clinical aspects, etiology, management, and treatment. High. Alt. Med. Biol. 17, 61–69. doi:10.1089/ham.2016.0031
Keywords: SpO2 switch, physiological switch, hypoxia adaptation, autonomic nervous system regulation, threshold response, intermittent hypoxia training, SpO2 criticality
Citation: Yuri E, Chung H-Y and Chen F-S (2025) Reframing SpO2 tolerance as a physiological switch: implications for hypoxic adaptation and exercise regulation. Front. Physiol. 16:1667238. doi: 10.3389/fphys.2025.1667238
Received: 16 July 2025; Accepted: 26 August 2025;
Published: 03 September 2025.
Edited by:
Elisabetta Salvioni, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Massimo Mapelli, Monzino Cardiology Center (IRCCS), ItalyAlessandro G. M. Pisano, Italian AirForce Institute of Aerospace Medicine, Italy
Copyright © 2025 Yuri, Chung and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enomoto Yuri, MjQ3MDAxQHltLm5pY2hpeWFrdS5hYy5qcA==; Hui-Yu Chung, aGFubmFoY2h1bmc3NzA2MjhAZ21haWwuY29t
 Enomoto Yuri1*
Enomoto Yuri1* Hui-Yu Chung
Hui-Yu Chung