- 1Department of Medicine, Geisel School of Medicine, Hanover, NH, United States
- 2Department of Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States
- 3Department of Psychiatry, University of Pennsylvania, Philadelphia, PA, United States
Background: Motion sickness drugs can improve symptoms but also cause drowsiness and reduce performance as side effects. We assessed whether the psychomotor vigilance task (PVT) could provide an objective performance measure when motion sickness occurs and when drugs are used to prevent motion sickness.
Methods: Data were from a previously published placebo-controlled study of chlorpheniramine (C) or chlorpheniramine plus ephedrine (CE). Participants did the PVT before drug/placebo, after drug/placebo, and after provocative motion in an off-vertical axis rotation chair. Eighteen individuals were randomized to receive one of six different orders of placebo, C, or CE. Data were analyzed using linear mixed effect models and repeated measures ANOVAs.
Results: Mean and median response speeds were significantly reduced after chair rides for the placebo condition indicating the PVT was sensitive to motion sickness effects. C and CE both improved motion sickness symptoms but response speeds post motion with C were significantly worse than CE post ride measures.
Conclusion: Ephedrine given with C negated the response speed effects from C alone and enabled subjects to sustain vigilance after drug treatment. The PVT offered an objective assessment of the effects of both motion sickness and effects of motion sickness treatment on attention and vigilance.
Introduction
Motion sickness can be a significant problem in operational environments. Stressful motion in ships, vehicles, and spacecraft can lead to troublesome motion sickness symptoms in individuals who are not adapted to the environment. In some cases, this can be life-threatening. Vomiting due to motion sickness while on a spacewalk, for example, could obscure vision, lead to aspiration, and damage space suit systems (Buckey, 2006). A key factor in motion sickness treatment, however, is that the treatment should not be worse than the condition itself. If an individual takes a motion sickness medication that produces sedation and reduces the ability to pay attention and maintain vigilance, this could also have operational effects that might be more severe than motion sickness. This means that in the evaluation of motion sickness remedies, important considerations are how much does the drug affect attention and vigilance, and does the medication improve or further impair behavioral performance.
The psychomotor vigilance task (PVT) provides reliable and objective measures of behavioral alertness (Basner and Dinges, 2011; Basner et al., 2021). The standard PVT measures sustained or vigilant attention by recording reaction times to visual (or auditory) stimuli presented at random inter-stimulus intervals. Reaction time to stimuli has been used since the late 19th century in sleep deprivation research because it is an objective measure of alertness without the confounding effects of aptitude and learning (Basner and Dinges, 2011). The PVT has been used to measure sustained attention and reaction time objectively in many operational environments. The study data from the previous study and analyzed here (Buckey et al., 2007), used a 10-min version of the test where individuals attended to a counter on a computer screen and pressed a button as soon as the counter started (Dinges and Powell, 1985). The inter-stimulus interval varied randomly from 2–10 s, and a reaction time >100 milliseconds was considered valid (reaction times <100 ms were considered “false starts”). Although the core measure in the PVT is reaction time, other measures from the test also provide key information including the top 10% fastest reaction times, the slowest 10% reaction times, lapses (i.e., omissions), and the response speed (reciprocal of the reaction time). In previous studies, response speed was one of the first PVT outcomes found to be sensitive to alertness and is the primary measure used in this study (Basner and Dinges, 2011; Basner et al., 2011; Basner et al., 2021).
The PVT was administered to participants in a placebo-controlled, double-blind, study of motion sickness medications. The study used placebo (P), 12 mg chlorpheniramine (C), and 12 mg chlorpheniramine plus 50 mg ephedrine compound (CE) (also abbreviated as Chlorphedra) (Buckey et al., 2007). This previous study provided a unique opportunity to assess the effects of motion sickness on PVT reaction time results used to evaluate the sedative side effects of C and the improvement in those effects with the CE compound. Nonetheless, the previous analysis did not specifically assess whether the PVT was sensitive to motion sickness. Therefore, the goal of this analysis was to determine the test’s sensitivity to motion sickness effects and evaluate whether the addition of an alertness-enhancing drug (ephedrine) provided objective evidence of improved PVT performance using a more widely accepted response speed measure.
Methods
The PVT performance data analyzed here came from a double blind, placebo-controlled study which compared P and C to CE (Buckey et al., 2007). The details of the study, including drug administration and motion sickness induction, have been published previously (Buckey et al., 2004; Buckey et al., 2007) and are summarized briefly below. All procedures were reviewed and approved by the Committee for the Protection of Human Subjects at Geisel School of Medicine at Dartmouth (study protocol #14230).
Subjects
Eighteen subjects participated in the study, nine women and nine men, with a mean age of 29 years (SD 5.8), mean height of 173 cm (SD 9.6), mean weight of 66.9 kg (SD 15.3), and mean BMI of 23.4 (SD 2.4). To determine eligibility, each subject underwent a neurovestibular functional assessment (Romberg test, extraocular eye movement assessment, tandem walk, single leg stand) and a baseline ECG to ensure normal cardiac function at the time of the study. Subjects were excluded if they had any history of neuro-otological disease (e.g., vertigo, Meniere’s disease, labyrinthine dysfunction), cardiac disease, hypertension, diabetes, or prostate disease. Each subject had normal blood pressure, pulse, audiogram, complete blood count, and underwent a comprehensive metabolic panel. No subjects were taking herbal preparations or medications, neither over the counter nor prescribed. Reason and Brand’s motion sickness questionnaire (MSQ) was administered to each subject to confirm that only subjects who believed themselves to be susceptible to motion sickness would continue with the study (Reason and Brand, 1975). The subjects had a mean MSQ of 51.8 (SD 29.3). Normative MSQ scores provided by Golding et al. show an average score of 45.5 (SD 37.6) in a similar age group (mean age 26.6 years - SD 7.3) of 147 subjects (Golding, 1998). For the study, the Dartmouth Hitchcock Medical Center Investigational Pharmacy formulated doses of chlorpheniramine (12 mg) (C), a mixture of chlorpheniramine (12 mg) and ephedrine (50 mg) (CE), and a placebo (lactose) (P). Subjects were randomly assigned to one of six possible orderings for administration of these formulations, and neither subjects nor investigators were informed of which formulation the subject was receiving at the time of each experiment.
Equipment
Motion sickness was induced by a Ritter ENT off-axis vertical rotation chair (OVRC), modified specifically for these studies, similar to the Stille chair modifications described in Graybiel and Miller (1970). The chair was tilted to 15° off vertical and accelerated to 17.5 revolutions per minute at a rate of 5° per second. Chair deceleration was accomplished at the same rate as acceleration. Subjects were secured in the chair with a seat belt, and their heads were immobilized with a strap to the headrest of the chair to reduce subject fatigue. Subjects’ eyes were covered with opaque goggles (Buckey et al., 2007).
Procedure
A familiarization ride in the chair was done at least 1 week before the study rides to allow the participants to experience motion sickness symptoms. They were instructed not to ride to the point of vomiting. Motion sickness severity was assessed in three ways. For one, subjects used Bock and Oman’s ratio scaling method (Bock and Oman, 1982) in a similar way to Stott et al. (1989). They rode in the chair until they either experienced severe nausea or felt so ill that they needed to stop. The participants were asked to rate the peak discomfort during the familiarization ride as “10”, remember this level, and rate other levels of discomfort relative to it (i.e., discomfort which was half as severe would be a “5”).
The second method involved symptom recording. Before the familiarization run participants were told about the symptoms that might arise (drowsiness, epigastric awareness, etc.). Each minute during the rotation, they were queried about the symptoms. The answers were recorded on a log similar to Miller and Graybiel’s scoring sheet (Miller and Graybiel, 1970), which is a variant of the Pensacola Diagnostic Rating Scale. Symptoms were assigned point values and from these, five severity levels were determined (frank sickness, severe malaise, moderate malaise (a), moderate malaise (b), moderate malaise, slight malaise). The third technique was recording the total number of minutes the subject rode in the chair before stopping.
At least 1 week after a familiarization ride in the chair, subjects began the drug administration sequence. Each subject rode in the chair three times, with one ride for each phase of the sequence depending on the study arm (P, C, CE). Each trial was no closer than 1 week apart to reduce stimulus habituation. Before taking the study drug, the subject was given the Neuro-1 neurobehavioral assessment battery developed by Dinges, Neri, and Wyatt, which includes a psychomotor vigilance task (PVT), probed memory recall task, serial-addition task, and visual analog scales describing sleepiness and cognitive/emotional state (Dinges et al., 1997). This established baseline neurobehavioral performance (PREDRUG). The subject then took the drug assigned to them for that day. Three hours later, the neurobehavioral assessment battery was repeated to record performance at maximum drug effect (POSTDRUG).
For the study chair rides, the participants rode in the chair until they either experienced severe nausea or felt so ill that they wanted the ride to stop, but not to the point of vomiting. Once stopped, the subjects were asked to rate their discomfort relative to the level- “10” baseline set by the familiarization ride. Participants rated their experience of various symptoms of motion sickness that they were previously briefed on using five levels of severity. The number of minutes that each subject spent in the chair was recorded. Finally, the subjects completed the neurobehavioral assessment battery a third time to record performance after the chair ride with the drug/placebo (POSTRIDE).
Statistical analysis
The primary PVT outcome was response speed. Response speed was one of the first PVT outcomes found to be sensitive to alertness and so is a better outcome measure than the reaction times used in the original analysis. Also, the slowest and fastest 10% response times were analyzed, which had not been done previously. Two analysis approaches were used. Firstly, to assess the differences between the nine conditions (three drugs with three timepoints) a repeated measures ANOVA was used with post hoc comparisons using a Tukey correction. Secondly, to examine the effects of chlorphedra (CE), data were also analyzed using a linear mixed effect model with the CE and POSTRIDE conditions as the baseline. The primary outcome was response speed with drug (P, C, CE), time relative to drug administration and chair ride (PREDRUG, POSTDRUG, POSTRIDE) and order of study (first, second, third) as fixed effects. Subjects were considered as random effects. Group means were compared using an ANOVA on the linear mixed effect model output. Coefficients from the model were compared and examined for interaction effects using F tests. For example, the coefficients for PREDRUG compared to POSTRIDE and C compared to CE were examined for interaction effects to determine if the difference between PREDRUG and POSTRIDE changed differently for C and CE. Statistical analyses were done using MATLAB 2022b (Mathworks, Natick, MA) with a significance threshold of p = 0.05.
Results
As reported previously, chair time increased significantly from placebo (6.6 min) for both C (10.3 min) and CE (10.2 min) indicating relief of motion sickness compared to placebo. The increase in chair time did not differ between C and CE (Buckey et al., 2007).
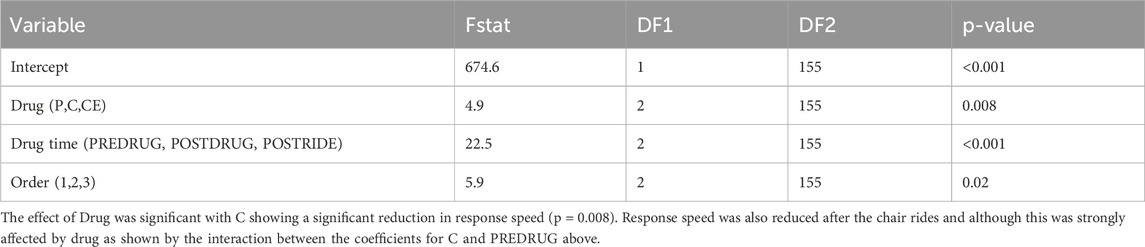
The isolated effects of motion sickness without any drug administration were assessed by comparing baseline PVT response speeds measured before placebo administration (P-PREDRUG) with PVT response speeds measured after the post-placebo chair ride (P-POSTRIDE), as shown in Table 1 (p < 0.001). An ANOVA on the timepoints (PREDRUG, POSTDRUG, and POSTRIDE) shows a highly significant effect of timepoint (p < 0.001) with POSTRIDE having the slowest PVT response speeds (Table 3; Figure 1). Significant differences for the other PVT measures (slowest 10%, fastest 10%) across timepoints are also included in Table 1 (all p < 0.02).
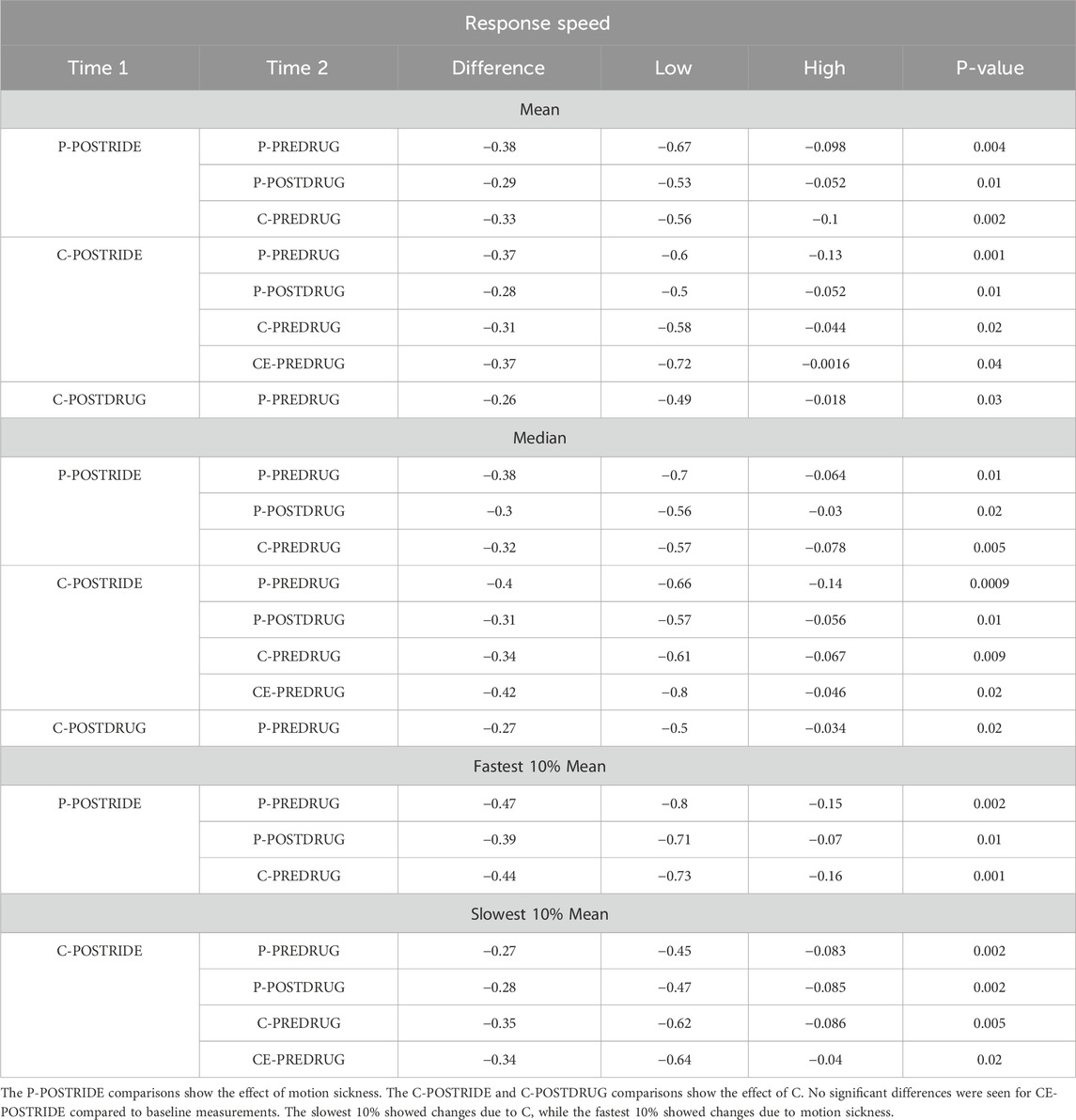
Table 1. Overall results from the PVT at the different study points from the repeated measures ANOVA with Tukey’s Honest Significant Difference procedure.
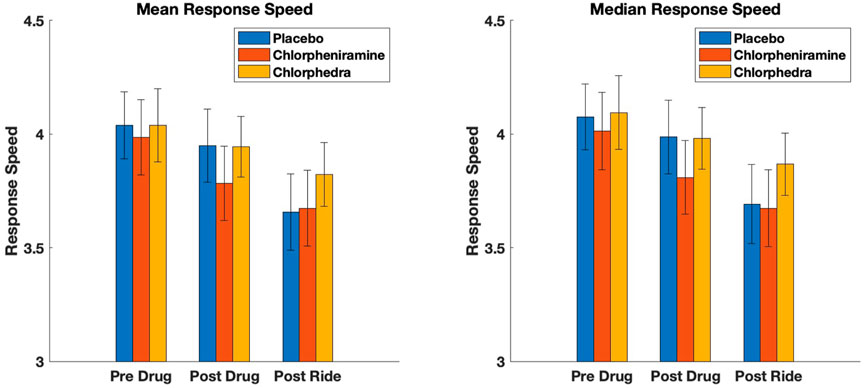
Figure 1. Mean and median response speed results for the three conditions, Placebo, Chlorpheniramine, and Chlorpheniramine + Ephedrine (Chlorphedra). The decrease in response speed with motion sickness post ride for placebo and chlorpheniramine was significant compared to Chlorphedra.
Although C improved tolerance to stressful motion, the linear mixed effect model shows it also reduced PVT response speed significantly (p = 0.005, Table 2; Figure 1) an effect that was not seen with CE (p = 0.78) (i.e., response speed was not reduced with CE). The repeated measures ANOVA shows the individual comparisons, and performance after the chair ride with C (C-POSTRIDE) was significantly worse than all the baseline measures (P-PREDRUG, C-PREDRUG, CE-PREDRUG) as well as the measures after taking CE (CE-POSTDRUG).
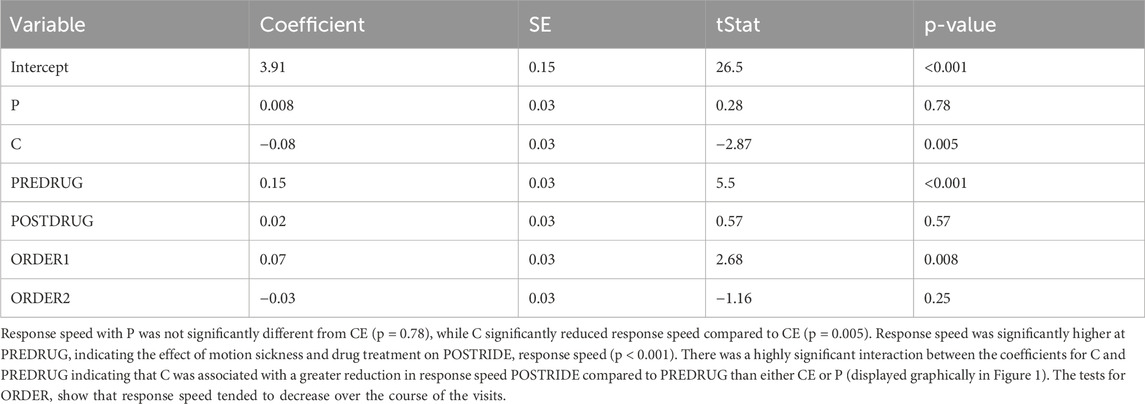
Table 2. Linear mixed effect model results for median response speed with CE, POSTRIDE and ORDER 3 as the baseline conditions.
PVT response speeds after the chair rides were reduced for both P and C (Table 1), but we found no significant difference between baseline PVT reaction times or response speeds with CE. This supports the conclusion that the ephedrine stimulant offsets the sedative effect of C and allows for greater continued attention. Figure 1 displays the results graphically. Both P and C alone decreased PVT response speed significantly after the chair ride, but this was not seen with CE. There was an effect of order of administration, with PVT response speed decreasing slightly but significantly with the later administrations (p = 0.02) (Table 3).
Discussion
The PVT offers an objective assessment of the effects of both motion sickness itself and the effects of motion sickness treatment on attention and vigilance. Even in the absence of medications, drowsiness is a symptom of motion sickness. The results from the placebo results show that motion sickness reduces PVT performance with a decrease in response speed. Chlorpheniramine was effective in reducing motion sickness symptoms with participants riding longer in the chair and reporting lower levels of motion sickness symptoms (as published previously) (Buckey et al., 2004; Buckey et al., 2007). Despite this, however, PVT reaction time and response speed with C were as impaired POSTRIDE as with the P arm. Although participants reported lower motion sickness symptoms POSTRIDE with C, it did not improve response speed. In fact, PVT performance was worse with C compared to P, indicating that C negatively affected attention and vigilance.
The addition of ephedrine eliminated the impairment in PVT reaction times and attention seen with C. Motion sickness symptoms and chair ride times improved to the same degree in the CE and C conditions, but a significant difference existed in PVT responses. The most significant differences were seen with the POSTRIDE response speed measures as shown in Figure 1. Also, an interesting difference existed between the mean of the fastest and slowest response speeds. The fastest response speeds seemed to be most sensitive to the effects of motion sickness, with the post-ride measurements with placebo (P-POSTRIDE) being slower than the baseline measures. The slow mean measures, by contrast, were significantly slower with chlorpheniramine, suggesting that this measure was most sensitive to the effects of the drug (Table 1).
A limitation to this study is that the PVT was only used to compare two motion sickness remedies. The efficacy of the PVT should be confirmed with other established motion sickness single agent and combination treatments used in operational settings. The PVT should also be verified in a larger cohort of subjects. The error bands observed for each condition in Figure 1 likely reflect individual variability in subjects’ responses to both the drug treatments and the motion sickness stimulus. This variability may arise from natural differences in physiological or perceptual sensitivity among participants, or from inherent heterogeneity within the study cohort. Increasing the sample size in future studies could help reduce this variability.
Treating motion sickness in operational environments like aviation and spaceflight requires attention to the balance between relieving motion sickness symptoms while maintaining peak performance (Stankovic et al., 2019). The analysis presented here shows that the PVT is a valuable test to measure the performance effects of the interaction between motion sickness and its remedies to help select treatments, such as the addition of an alertness-enhancing drug (ephedrine) that are effective but do not degrade performance. The PVT is portable, brief and is without practice effects with repeated administration. The PVT could be useful for evaluating the performance impact of motion sickness remedies for spaceflight operations, astronaut training, cybersickness, and other applications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Dartmouth College Committee for the Protection of Human Subjects. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SG: Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. CN: Investigation, Resources, Validation, Writing – review and editing. PH: Data curation, Investigation, Methodology, Software, Validation, Writing – review and editing. MB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review and editing. JB: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Office of Naval Research (ONR) Grant N00014-00-1-0694 and the National Space Biomedical Research Institute Grant NA00404 supported this work.
Acknowledgments
We thank our dedicated subjects for their commitment to this study. We thank Donna Alvarenga who was instrumental in carrying out these studies. Larry Brown who pointed out to us the benefits that chlorpheniramine offers for the treatment of motion sickness. We would like to thank Wilbur Clark for the design and construction of the rotating chair. The help and assistance of Joe Ronda and James Wyatt in setting up the cognitive testing apparatus was greatly appreciated. We thank Mark Noel for assistance with computing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Basner, M., and Dinges, D. F. (2011). Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 34, 581–591. doi:10.1093/sleep/34.5.581
Basner, M., Mollicone, D., and Dinges, D. F. (2011). Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 69, 949–959. doi:10.1016/j.actaastro.2011.07.015
Basner, M., Moore, T. M., Nasrini, J., Gur, R. C., and Dinges, D. F. (2021). Response speed measurements on the psychomotor vigilance test: how precise is precise enough? Sleep 44, zsaa121. doi:10.1093/sleep/zsaa121
Bock, O. L., and Oman, C. M. (1982). Dynamics of subjective discomfort in motion sickness as measured with a magnitude estimation method. Aviat. Space and Environ. Med. 53, 773–777.
Buckey, J. C. (2006). “Motion sickness in space: prevention and treatment,” in Space physiology (New York: Oxford University Press), 187–206.
Buckey, J. C., Alvarenga, D., Cole, B., and Rigas, J. R. (2004). Chlorpheniramine for motion sickness. J. Vestib. Res. 14, 53–61. doi:10.3233/ves-2004-14106
Buckey, J. C., Alvarenga, D. L., and Mackenzie, T. A. (2007). Chlorpheniramine and ephedrine in combination for motion sickness. J. Vestib. Res. 17, 301–311. doi:10.3233/ves-2007-175-610
Dinges, D. F., Pack, F., Williams, K., Gillen, K. A., Powell, J. W., Ott, G. E., et al. (1997). Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep 20, 267–277.
Dinges, D. F., and Powell, J. W. (1985). Microcomputer analyses of performance on a portable, simple visual rt task during sustained operations. Behav. Res. Methods Instrum. and Comput. 17, 652–655. doi:10.3758/bf03200977
Golding, J. F. (1998). Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res. Bull. 47, 507–516. doi:10.1016/s0361-9230(98)00091-4
Graybiel, A., and Miller, E. F. D. (1970). Off-vertical rotation: a convenient precise means of exposing the passive human subject to a rotating linear acceleration vector. Aerosp. Med. 41, 407–410.
Miller, E. F., and Graybiel, A. (1970). “Perception of the upright and susceptibility to motion sickness as functions of tilt angle and angular velocity in off-vertical rotation,” in Fifth symposium on the role of the vestibular organs in space exploration (NASA), Washington, D.C., 99–103.
Stankovic, A. S., Alvarenga, D. L., Coleman Daniels, V. R., Simmons, R. G., Buckey, J. C., and Putcha, L. (2019). Intranasal scopolamine for motion sickness. Aerosp. Med. Hum. Perform. 90, 917–924. doi:10.3357/amhp.5456.2019
Keywords: chlorpheniramine, ephedrine, motion sickness, psychomotor vigilance task, motion sickness drugs
Citation: Geimer S, Niemczak CE, Howard PT, Basner M and Buckey JC (2025) The psychomotor vigilance task for assessing the effects of motion sickness and its treatment. Front. Space Technol. 6:1591817. doi: 10.3389/frspt.2025.1591817
Received: 11 March 2025; Accepted: 04 June 2025;
Published: 13 June 2025.
Edited by:
Ilaria Cinelli, Aerospace Medical Association, United StatesReviewed by:
T. John Tharakan, Indian Space Research Organisation, IndiaPreeti Sawant, National Board of Examinations in Medical Sciences, India
Copyright © 2025 Geimer, Niemczak, Howard, Basner and Buckey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jay C. Buckey, SmF5LmJ1Y2tleUBkYXJ0bW91dGguZWR1
 Shireen Geimer
Shireen Geimer Christopher E. Niemczak
Christopher E. Niemczak Patrick T. Howard1
Patrick T. Howard1 Mathias Basner
Mathias Basner Jay C. Buckey
Jay C. Buckey