- 1Department of Space Studies, American Public University System, Charles Town, WV, United States
- 2Department of Space Studies, University of North Dakota, Grand Forks, ND, United States
Introduction: A key element for sustainable off-world habitation is the ability to grow food through in-situ resource utilization (ISRU). Growth substrates are required to overcome the challenges of ISRU in the space environment, including the use of regolith. Biofertilizers such as algae are a promising avenue for supporting plant growth with ISRU; algae can potentially mitigate the lack of nutrients, alkalinity, heavy-metal contamination, poor water-carrying capacity, and presence of perchlorates in regolith as well as increase plant growth at elevated levels of atmospheric CO2. The blue-green cyanobacterium Arthrospira platensis is an ionizing radiation resistant strain with high temperature tolerance and nutritional properties. It has been used successfully as a bio-fertilizer in heavy metal contaminated, highly alkaline terrestrial soils
Methods: Our research is a large-scale investigation of the efficacy of spirulina to enhance the growth of Raphanus sativus (Organic Daikon radish) microgreens using lunar and Martian regolith simulants. We present a study of growth for a wide range of regolith simulant-soil mixtures as a function of fertilizer concentration and the level of environmental CO2.
Results: Spirulina boosts growth for radish microgreens significantly; Martian regolith simulant with 0.6% spirulina under elevated CO2 yielded the best results. Water-only groups declined after the initial growth phase; spirulina fertilized groups maintained steady growth rates over the full growing period. Regolith simulant type influenced biomass more than height, with the best growth found in Martian rather than lunar regolith simulant. No difference was found between the different regolith simulant-to-soil mixtures.
Discussion: This research advances the application of ISRU to enhance self-sufficient, sustainable space exploration and resource use. In this research, spirulina fertilization compensated for the non-nutritive properties of regolith, supporting plant growth without the addition of terrestrial soils. This work suggests that spirulina can serve as an effective biofertilizer in soil farming practices using ISRU as a potential means of supporting off-world habitation.
1 Introduction
The success of long-term space exploration and planetary settlement hinges on achieving self-sufficiency through effective in-situ resource utilization (ISRU). A critical component of this challenge is sustaining plant-based life support systems under stressors relevant to space farming, including partial gravity, radiation exposure, and native substrates. Crops must withstand elevated cosmic radiation, adapt to reduced or microgravity environments, ranging from 0.17 g on the Moon, 0.38 g on Mars, to microgravity aboard orbital stations or during interplanetary transit (NASA, 2021), and grow in native regolith, which presents both chemical and structural limitations as a growth substrate within closed artificial habitats. Each of these challenges must be addressed to develop self-sustaining systems capable of meeting astronauts’ nutritional needs (De Pascale et al., 2021; Tang et al., 2021; Zabel et al., 2016). This study presents a comprehensive evaluation of spirulina as a biofertilizer for microgreen cultivation, with a specific focus on plant growth in regolith simulants and elevated CO2 environments.
Regolith, the loose layer of fragmented rock and dust covering the surface of planetary bodies such as the Moon and Mars, poses significant challenges for plant cultivation. Unlike terrestrial soil, it lacks a natural biome and is deficient in essential macronutrients such as nitrogen, as well as key micronutrients including copper, zinc, and boron (Eichler et al., 2021; Duri et al., 2022). In addition, both lunar and Martian regolith lack water-carrying capacity (Wamelink et al., 2014). The presence of perchlorates in Martian regolith is an additional challenge as it has been shown to inhibit plant growth even with nutritional supplementation (Eichler et al., 2021).
Several groups have studied plant growth in lunar and Martian regolith simulants. Their findings show that growth is possible when nutrient supplementation is provided, particularly for simulants with lower pH levels (Eichler et al., 2021; Chinnannan et al., 2023). However, growth rates tend to be slower, and plants often exhibit signs of phenotypic stress (Paul et al., 2022), including a decline in telomere dynamics and a loss of sustainable genome integrity (Barcenilla et al., 2024). These results confirm the importance of nutrient enhancement to support ISRU plant production in space environments. While progress has been made in understanding and overcoming the challenges associated with plant growth using lunar and Martian regolith simulants, much remains to be done to develop a fully functioning ISRU plant production model for off-world environments (Fackrell et al., 2024; Meinen et al., 2018).
Space habitats often maintain elevated CO2 concentrations (700–1,000 ppm) to promote crop productivity (Zabel et al., 2022; NASA, 2022), and pockets of CO2 which exceed those limits have been reported on the International Space Station (ISS) (Georgescu et al., 2020). In terrestrial studies, elevated levels of CO2 have shown increased plant growth and leaf production as well as reduced water usage (Prior et al., 2011). This is particularly true for dicots, where it has been shown that leaves emerge earlier and more abundantly, within a similar size range, when grown in high CO2 conditions (Morison and Lawlor, 1999). Plants grown under elevated CO2 concentrations often exhibit increased root length and diameter; however, they are also associated with altered branching patterns in both shoots and roots which may impair the plant’s ability to compete for resources both below and above ground (Pritchard et al., 1999). Above a certain threshold, high amounts of CO2 are toxic to plants, impacting both plant growth and overall health (CO2 meter, 2023). Elevated levels of CO2 have been shown to reduce transpiration and produce plants which have lower nutritional content with smaller percentages of nitrogen, zinc, iron, and protein (Loladze, 2002; Myers et al., 2014; Morison and Lawlor, 1999). One of the goals of this study is to evaluate the interaction between the concentration of spirulina fertilization and the CO2 enrichment on plant health.
Short-term plant growth studies on the space shuttle missions, Skylab, and the ISS have successfully grown seedlings and young plants in microgravity (Porterfield et al., 2003; Johnson et al., 2021). Research on the growth of seedlings in Petri dishes indicate that weightlessness does not change the plant development framework but the nutritional storage in plant cells is altered due to stressors inherent in the space environment (Kordyum and Hasenstein, 2021). More recent studies report no significant difference in photosynthesis rates for wheat grown on the ISS (Monje et al., 2005); however, a 25% reduction in the CO2-saturated photosynthetic rate of wheat plants grown on the space shuttle Discovery compared to ground controls has been noted (Tripathy et al., 1996). Analysis of gene transcription from the Advanced Plant Habitat Experiment 2 (APHE2) directly comparing growth of radish plants, indicates that plants grown on the ISS showed signs of stress, but only very small changes in plant development over time (Massa et al., 2016). Lettuce grown on the ISS in VEGGIE chambers demonstrates shorter maturation times compared to ground controls and differs in macro- and micro-nutrient content as well as in the levels of total phenolics (Khodadad et al., 2020). On-orbit tests of substrate-less hydroponic systems show promise to support plant growth as well (Poulet et al., 2022).
Substantial progress has been made in the knowledge base supporting the incorporation of vascular plants in space environments to supplement astronaut diets. Many outstanding questions remain, however, including further quantitative characterization of plant response to stressors in the space environment and the subsequent effect on photosynthetic rate, overall growth, and nutritional content for a wide range of plant species. ISS-based studies inform plant responses to microgravity during interplanetary travel. In contrast, regolith simulant experiments address substrate and nutrient limitations relevant to lunar and Martian greenhouses under partial gravity. Both approaches are complementary but distinct.
This study investigates the potential of Arthrospira platensis, a blue-green cyanobacteria recently reclassified as Limnospira platensis, as a bioremediation additive to support healthy plant growth under conditions of elevated environmental CO2 and growth in lunar and Martian regolith simulants. Spirulina is part of the Microcoleaceae family, known for its nutritional properties and rapid growth (Fais et al., 2022). Microcoleaceae have been shown to improve soil fertility and support healthy plant growth (Singh et al., 2016; Shariatmadari et al., 2013; Rai et al., 2019). In terrestrial soils, they contribute to soil aggregation and stability (Rashid et al., 2016), increase the water-holding capacity and nutrient content of degraded soils (Singh et al., 2016), and enhance salt tolerance, disease resistance, and polysaccharide production (Singh, 2014).
Under terrestrial conditions, plants fertilized with spirulina exhibit increased biomass (Singh, 2014), enhanced photosynthetic pigment content, improved growth and yield rates (Shedeed et al., 2022), greater productivity including marketable yield and nitrogen fixation (Geries and Elsadany, 2021), and elevated micronutrient content (Anitha et al., 2016). For radish plants in particular, the application of spirulina leads to increased root length, wet mass, and chlorophyll content (Godlewska et al., 2019). In stressed soils, spirulina treatments have led to increases in yield production, improved photosynthetic rates, and decreased oxidative as well as DNA damage (Taha et al., 2023).
These studies suggest that spirulina holds promising potential as an agent for growth remediation in space agriculture. Prior research demonstrates spirulina’s capacity to support plant growth in degraded soils, suggesting its potential as a candidate for testing in extraterrestrial regolith simulants. This body of work forms the theoretical foundation for the present study and supports the methodological approach. The current work evaluates growth in a range of soil-to-regolith simulant mixtures and hypothesizes that spirulina fertilization can enhance plant growth in a dose-dependent manner across both lunar and Martian regolith simulants and CO2 levels, with the strongest response expected for Martian regolith simulant due to increased levels of phosphorus and iron and under elevated-CO2 greenhouse conditions due to increased carbon assimilation.
2 Experimental setup and methods
2.1 Materials
To simulate a controlled growth environment for biological productivity studies in support of NASA’s closed-loop life support initiatives, an indoor plant cultivation system was developed using commercial-grade components. The core of the setup was a Mars Hydro 4’×4’×6′8″ growth chamber constructed from heavy-duty 1680D canvas fabric and lined with 98% reflective Mylar, reducing light loss and improving photon utilization. The tent’s construction is sealed to prevent light leaks and minimize gas exchange with the surrounding atmosphere, with leakage measured at <5% of chamber volume per 24 h. Its steel frame supports up to 155 lbs, enabling vertical integration of lighting and environmental control devices.
Lighting was provided by a Mars Hydro TSW2000 LED array, featuring a full-spectrum output that included key wavelength bands at 660–665 nm, 730–740 nm, 3000–3200K, and 6000–6500K. The system outputs a total PPF of 776 μmol/s with a Photosynthetic Photon Efficacy (PPE) of 2.6 μmol/J and covers a core canopy area of 4 ft × 4 ft at lower intensities; two levels were used with commercial off the shelf powder-coated industrial steel shelving. Peak molar production across the mid-range spectrum was centered between 630 nm and 660 nm, critical for chlorophyll-A absorption, and a 16-h light/8-h dark cycle at a wavelength of 640 nm with 160 μmol/m2/s, or 30 W/m2. Each level had its own lighting; the lights were hung 29 cm above the top of the pods.
The growth chambers used in this study were maintained at distinct CO2 concentrations: ambient (400 ppm) and elevated (1,200 ppm). A CO2 control unit (Autopilot APC8200) was integrated to maintain elevated CO2 levels with precision within a range of 1,100–1300 ppm in the elevated chamber, avoiding CO2 stagnation using a mini circulation fan placed at canopy level. Temperature and humidity were continuously monitored using a Temp Stick IoT sensor (Ideal Sciences, model TS-01), with values held at approximately 26.7 °C and 75% RH, respectively. Elevated CO2 levels were monitored and controlled with an Autopilot APC8200 CO2 controller. This combination enabled optimal vapor pressure deficit (VPD) for accelerated growth. Ventilation was achieved through a 6-inch inline duct fan and carbon filter system, maintaining negative pressure and odor control while ensuring consistent airflow. The duct fan provided ∼180–200 CFM, corresponding to ∼0.25–0.35 m/s at canopy level. A supplementary mini fan introduced on day 25 increased canopy airflow to ∼0.15 m/s.
Raphanus sativus var. longipinnatus, an organic, non-GMO, heirloom Daikon radish microgreen, was selected due to its rapid life cycle (30–40 days to maturity), high nutrient density, and adaptability to constrained environments (Vandenbrink et al., 2019). The seeds were obtained from True Leaf Market Seed Company. The seeds were stored in the light-protective seed pouches in a dark, climate-controlled laboratory. Prior to planting, the seeds were cleaned with rubbing alcohol (<2 min) and then inspected under a microscope to ensure fidelity of seed coat. Seeds with an average size of 1 mm were selected for the study.
The plants were grown in mixtures of Martian (MGS-1S, batch code 002-02–001-0621, 2022) or lunar (LMS-1, batch code 002-07-001-0621, 2022) regolith simulants, manufactured by Space Resources Technologies (Oviedo, FL, USA) and a commercially available bonsai soil (Bonsai Jack® Universal Mix, Part #111, Bonsai Jack LLC, Tampa, FL, USA; see Long-Fox and Britt (2023) for specific information and composition list for the regolith simulants and Supplementary Appendix A for a comparison of the macro/micronutrients for the simulants and a composition list for the soil). Bonsai soil was included only as a control comparison. ISRU scenarios would not involve importing soil, and future work will focus solely on regolith simulants. Particles larger than 2 mm were removed from the bonsai soil prior to creating the soil mixtures. Trays were randomized within chambers and rotated frequently to mitigate position effects. Detailed information on the biochemical properties of the soil can be found in Supplementary Appendix A.
Plant pods were created with 5 g of regolith simulant-soil mixture inside a lining of SuperMoss Orchid and Nursery grade sphagnum moss (global trade ID: 00759834223252). Sphagnum moss was used to stabilize water retention. While it improved hydration, it means plants were grown in mixed substrates rather than pure simulants. This limitation is acknowledged. The moss was completely natural with no exposure to pesticides or preservatives. Pre-treatment by the manufacturer included manual harvesting (no automated or heavy machinery was used) by permit on regulated federal lands, washing to remove dust and small particles, and drying.
2.2 Plant groups
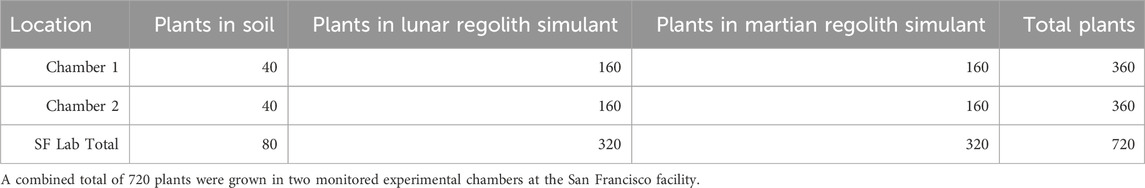
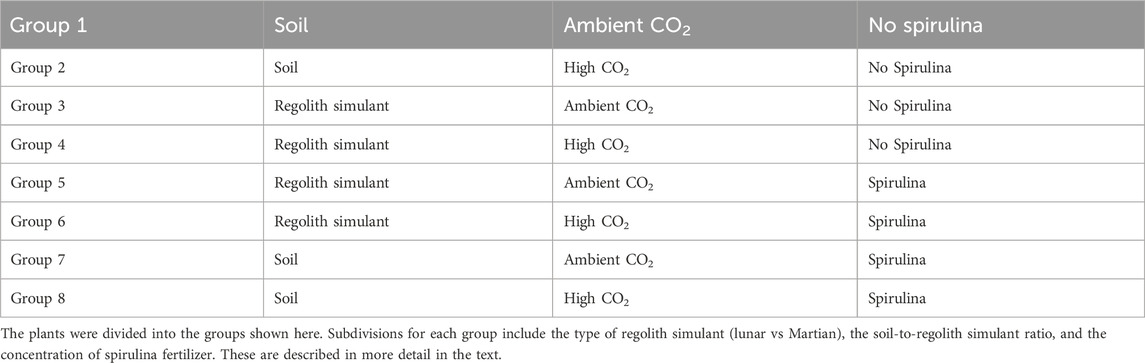
In total 720 plants were grown and analyzed as part of this study, with 360 plants grown in each tent (see Table 1). Each “group” consisted of 10 replicates and represented a different combination of variables: type of regolith simulant; soil-to-regolith simulant ratio; level of environment CO2; and spirulina concentration, as shown in Table 2. The design included 72 total treatment groups (n = 720).
The soil-to-regolith simulant ratios used were 0/100, 85/15, 90/10, 95/5, 100/0 percent by mass ratios for each type of regolith simulant. These ratios were chosen to evaluate potential improvement in plant growth from incorporating soil into the regolith simulant while minimizing the amount of terrestrial material used. Each treatment group consisted of 10 biological replicates. Statistical analyses included repeated measures ANOVA, two-way ANOVA, and MANOVA, with Tukey HSD post hoc tests applied. This sample size provides sufficient statistical power consistent with standards in plant biology.
The plants were watered daily using reverse osmosis (RO) deionized (DI) water to maintain adequate soil moisture levels. Control groups received only water, while experimental groups 5-8 were additionally fertilized every other day with an aqueous solution of spirulina at concentrations of 0.2%, 0.6%, and 1.4% (by mass). The concentrations were selected based on prior trials indicating that 0.6% offers a balance of solubility and nutrient delivery, while 1.4% provides upper-bound enrichment without visible phytotoxicity. spirulina was obtained as a raw sample, prior to any processing except standard drying procedures. Spirulina solutions were freshly prepared prior to each application to minimize nutrient degradation. The trays were separated by type of regolith simulant and spirulina concentration to avoid any contamination due to excess solution pooling in the tray liners; the pods were randomized within each tray. The experimental design followed a full-factorial structure: 2 CO2 concentrations × 3 spirulina treatments × 2 regolith simulant types × 5 soil-to-regolith simulant ratios, plus controls, totaling 72 treatment groups (10 plants per group).
Plants were grown for 40 + days in the chambers, encapsulating a complete ‘seed to flower’ cycle. Specifically, the water-only and the 1.4% spirulina groups were harvested at day 44; the 0.2% and 0.6% spirulina groups were harvested at day 64. The difference in growth time was due to lifecycle completion but may limit direct comparisons for final biomass, leaf count, root development. Lifecycle completion times were found to be dependent on the level of CO2 and fertilization.
2.3 Soil conditions
Soil conditions, including both soil temperature and soil moisture levels were measured daily for each plant. Soil pH was also measured for each soil mixture and compared with the initial values for the raw materials. Baseline nutrient content of the soil and regolith simulants was assumed based on manufacturer specifications; however, chemical profiling was not independently verified and may contribute to growth differences.
2.4 Confounds
A supplementary fan system was introduced to the high CO2 growth chamber on day 25 to improve air circulation after early yellowing was observed. The supplementary fan increased local canopy airflow from ∼0.05 m/s to ∼0.15 m/s, reducing CO2 stagnation and improving leaf color. While intended to stabilize airflow, this change introduces a potential confounding variable for late-stage leaf color and health metrics.
Humidity levels were high (95%–100% RH) for a period of 6 days starting on day 33; dehumidifiers were installed and the system stabilized by day 39. The high levels of humidity represent an additional stressor for the plants and may have influenced plant color and health. Both interventions (fan and dehumidifier) were applied only to the high-CO2 chamber, not to the ambient CO2 chamber.
The use of sphagnum moss as a liner for the plant pods is another potential confound. The moss was chosen for its potential to aid in maintaining consistent hydration for root systems and substrate matrices without over-saturating or introducing complex capillary behaviors often triggered by potting soils. Sphagnum moss acts as a self-regulating medium, delivering moisture when needed while maintaining airflow and oxygen availability around the roots or test specimens. Its nitrogen content was 0.4% as measured by the manufacturer; this minimal nitrogen load suggests that the moss did not significantly contribute to or interfere with nutrient assays or growth observations. (See Supplementary Appendix A for a record of chemical composition for the moss and Supplementary Appendix B for a complete description of its use as a biologically inert, high-capacity water absorber.) Importantly the consistent use of sphagnum moss in all plant groups allows for the establishment of a baseline against which the relative effects of each variable may be compared.
The data was analyzed quantitatively for vertical plant height, plant volume, and leaf count and color, assessed according to the standards in the Pantone Munsell Plant Tissue Color Book. Final biomass was obtained by drying harvested microgreens at 60 °C for up to 36 h and recording wet and dry weights for the plant and root system. Root length was measured, and root development—including color and formation of root nodules or caps—was analyzed. Qualitative observations regarding the health and well-being of the plants were also recorded. Both quantitative measurements and qualitative observations were compared as a function of soil-to-regolith simulant mixture for each type of regolith simulant used. Data were also compared based on the concentration of the spirulina solution each plant group received and for growth at ambient vs. elevated levels of CO2.
3 Results
3.1 Vertical plant height
3.1.1 Effect of spirulina treatment
The plants which received the spirulina treatment show a factor of two or more increased growth compared to the water-only groups at ambient levels of CO2; they also demonstrate sustained growth throughout the study (see Figure 1 (lunar), Figure 2 (Martian)). A two-sample t-test confirms the significance of the results for all concentrations of spirulina treatment: 0.2% spirulina: t (6) = 11.22, p < 0.001 (lunar); t (7) = 25.67, p < 0.001 (Martian); 0.6% spirulina: t (8) = 19.24, p < 0.001 (lunar); t (7) = 31.77, p < 0.001 (Martian); 1.4% spirulina: t (8) = 21.46, p < 0.001 (lunar); t (7) = 23.98, p < 0.001 (Martian). The plateau occurs earlier for the water-only groups (around day 14) than for the spirulina-fertilized groups (around day 20 for all concentrations of spirulina tested). The water-only groups show a declining trend after day 24 (slope = −0.051 (lunar); −0.10 (Martian)), while the groups treated with the spirulina show either stable (0.2% spirulina solution; slope = 0.047 (lunar); 0.080 (Martian)) or slightly increasing trends (0.6% and 1.2% spirulina solutions; slope = 0.11 (lunar); 0.20 (Martian)) throughout the entire growth period. The groups which received the treatments with the highest spirulina concentration showed the highest rate of increase during the periods of maintained growth (the plateau period).
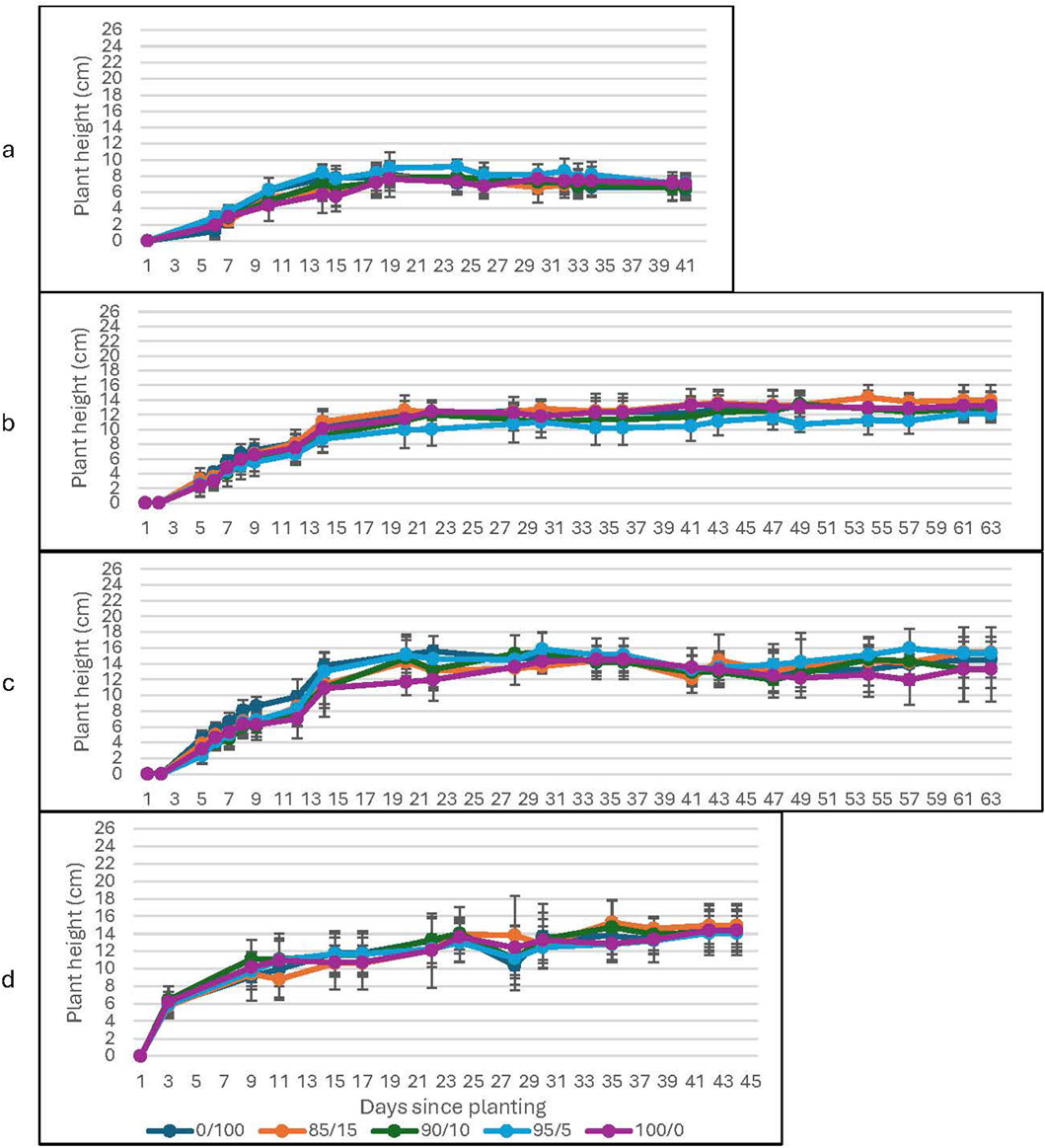
Figure 1. Plant Height as a function of soil to lunar regolith simulant mixture at ambient CO2. The graphs correspond to growth with (a) water-only; (b) 0.2% Spirulina; (c) 0.6% Spirulina; and (d) 1.4% Spirulina. Error bars represent ±1 standard deviation from the mean of 10 replicates per group.
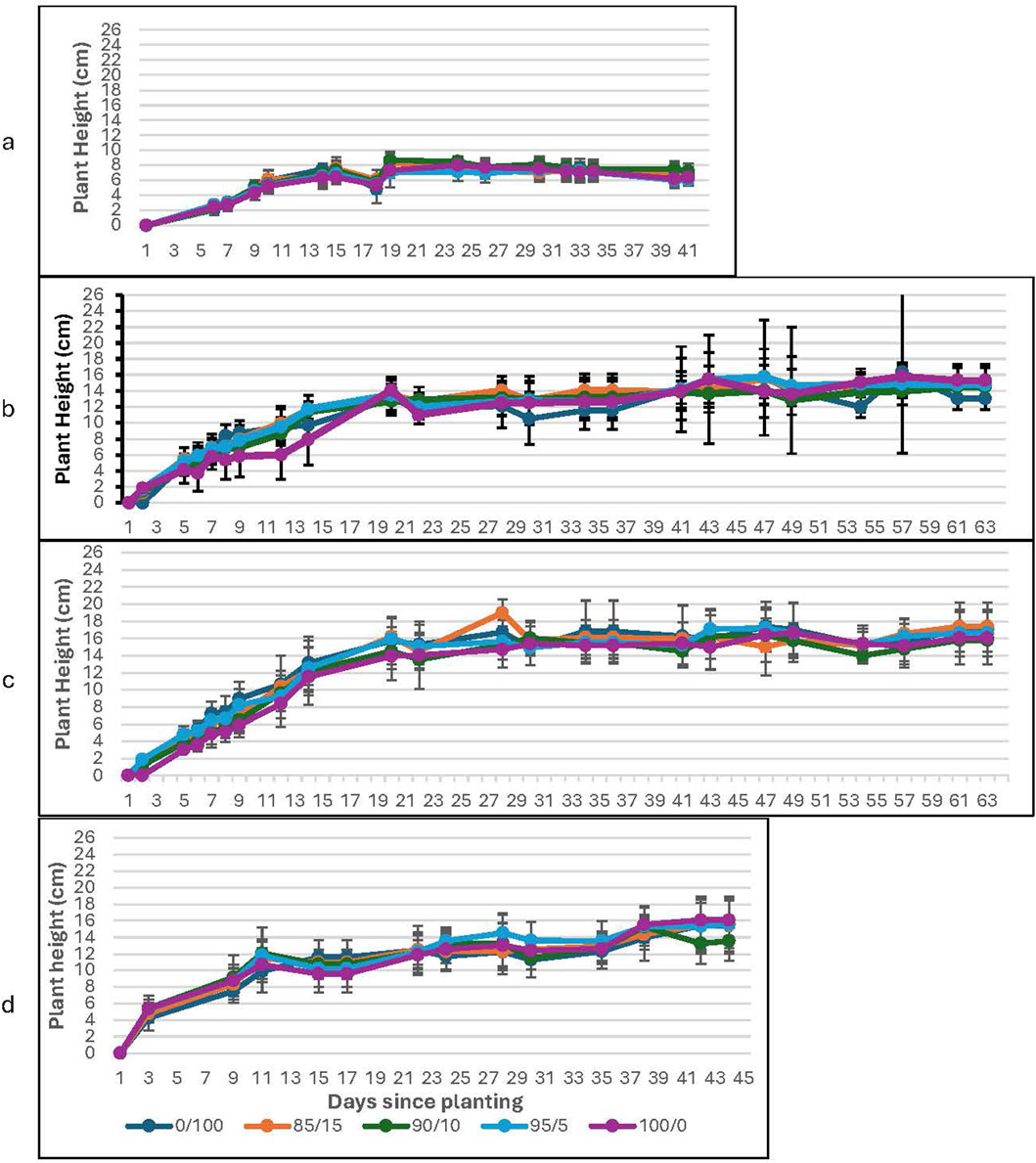
Figure 2. Plant Height as a function of soil to Martian regolith simulant mixture at ambient CO2. The graphs correspond to growth with (a) water-only; (b) 0.2% Spirulina; (c) 0.6% Spirulina; and (d) 1.4% Spirulina. Error bars represent ± 1 standard deviation from the mean of 10 replicates per group.
Similar trends are seen for plants grown under elevated concentrations of CO2 (see Figure 3 (lunar) and Figure 4 (Martian)); significance is confirmed by a two-sample t-test for all conditions: 0.2% spirulina: t (4) = 12.43, p < 0.001 (lunar); t (6) = 20.91, p < 0.001 (Martian); 0.6% spirulina: t (4) = 13.84, p < 0.001 (lunar); t (6) = 17.08, p < 0.001 (Martian); 1.4% spirulina: t (5) = 27.42, p < 0.001 (lunar); t (8) = 23.41, p < 0.001 (Martian). One notable difference is the strong declining trend after day 19 for the water-only groups (slope = −0.075 (lunar); −0.066 (Martian)). In general, overall growth is seen to increase as the concentration of spirulina treatment increases. Compared with the plant groups grown at ambient levels of CO2, the plants grown at high levels of CO2 exhibit slightly higher levels of growth; this is seen in the water-only groups as well as the spirulina-fertilized groups. The water-only plants grown in Martian regolith simulant at high levels of CO2 plateau earlier than any other group but show less decline in the sustained growth phase than the other water-only runs, including the lunar regolith simulant water-only runs.
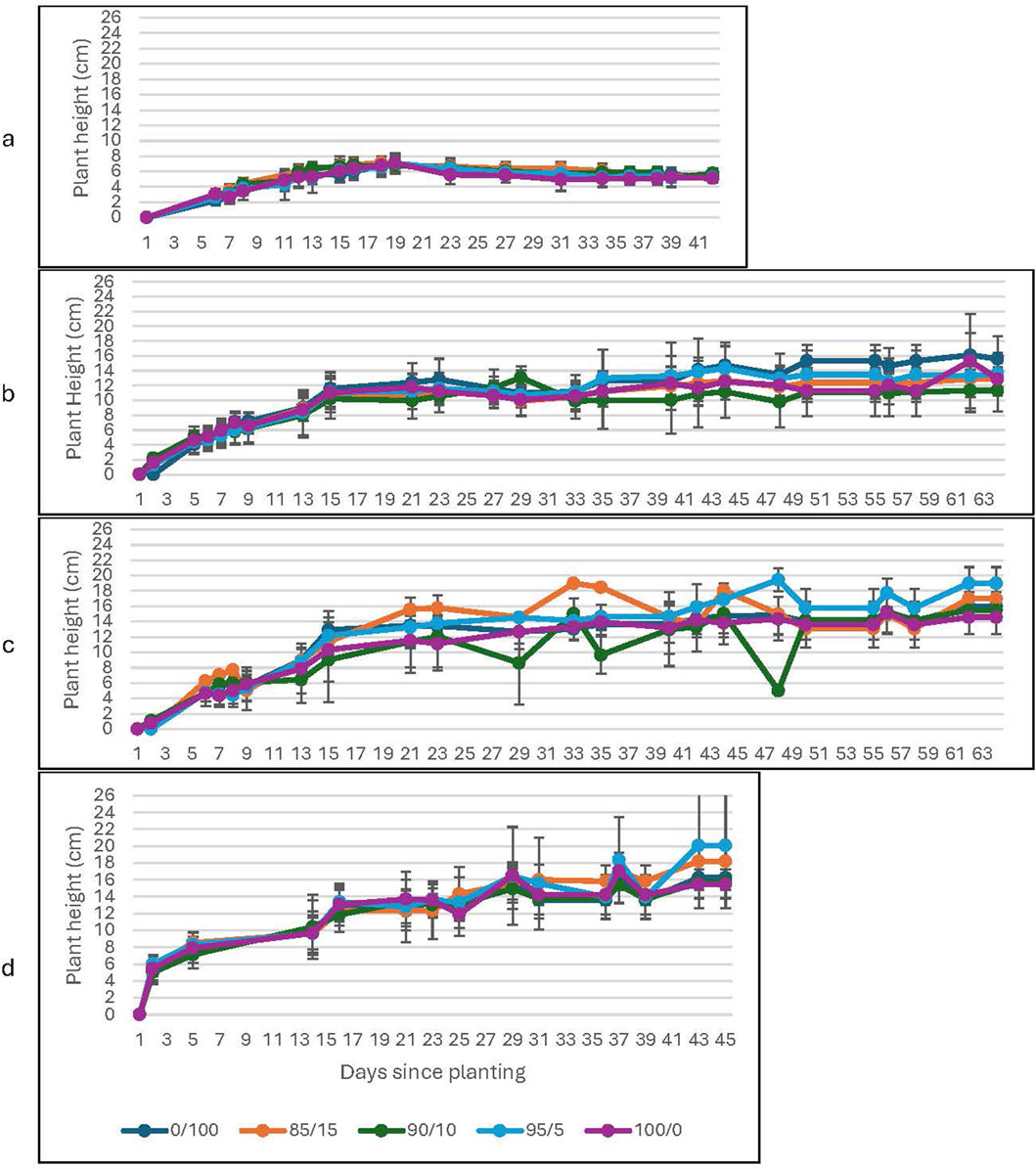
Figure 3. Plant Height as a function of soil to lunar regolith simulant mixture at high CO2. The graphs correspond to growth with (a) water-only; (b) 0.2% Spirulina; (c) 0.6% Spirulina; and (d) 1.4% Spirulina. Error bars represent ±1 standard deviation from the mean of 10 replicates per group.
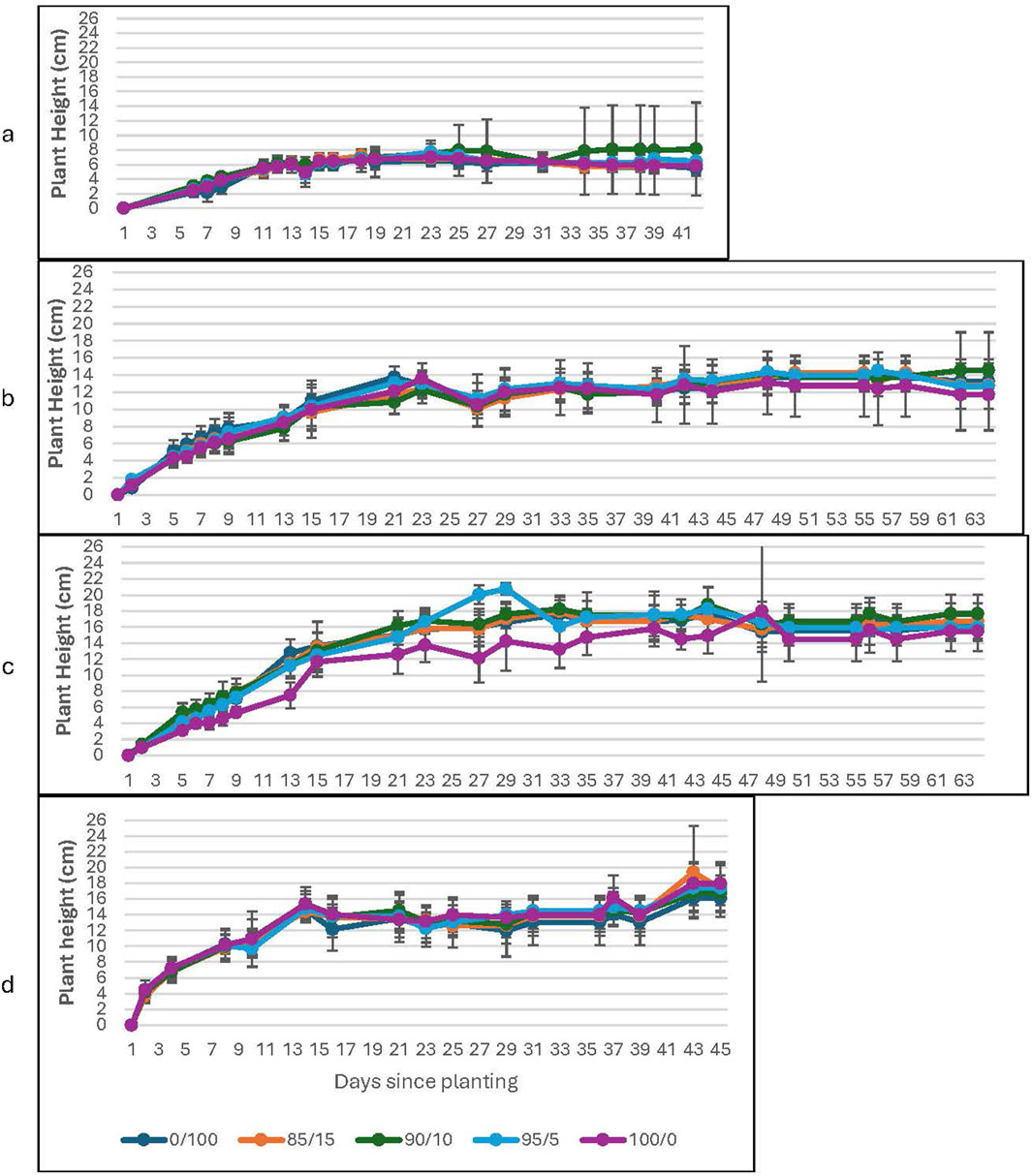
Figure 4. Plant Height as a function of soil to Martian regolith simulant mixture at high CO2. The graphs correspond to growth with (a) water-only; (b) 0.2% Spirulina; (c) 0.6% Spirulina; and (d) 1.4% Spirulina. Error bars represent ±1 standard deviation from the mean of 10 replicates per group.
Of particular interest are the plants grown in 100% regolith simulants (see Figures 5 (lunar), Figure 6 (Martian)). For these plant groups, the water-only groups demonstrate significantly lower levels of growth during both the initial growth phase and the plateau period compared with plants which received the spirulina treatment (t (46) = 6.76, p < 0.001 (lunar); t (36) = 4.96, p < 0.001 (Martian) at ambient CO2; t (46) = 10.05, p < 0.001 (lunar); t (47) = 9.03, p < 0.001 (Martian) at high CO2). The water-only plants show a clear decline during the plateau period, starting at day 31 (lunar) and 25 (Martian) for the plants grown with ambient levels of CO2 and between days 19 (lunar) and 23 (Martian) for the plants grown at high levels of CO2. Plant growth increases with increasing concentration of spirulina solution; this increase is most noticeable for plants grown under conditions of high CO2 and for plants grown in Martian regolith simulant compared to lunar. The 1.4% spirulina solution supports the highest growth rates initially, but plateaus at the level of the 0.6% solution.
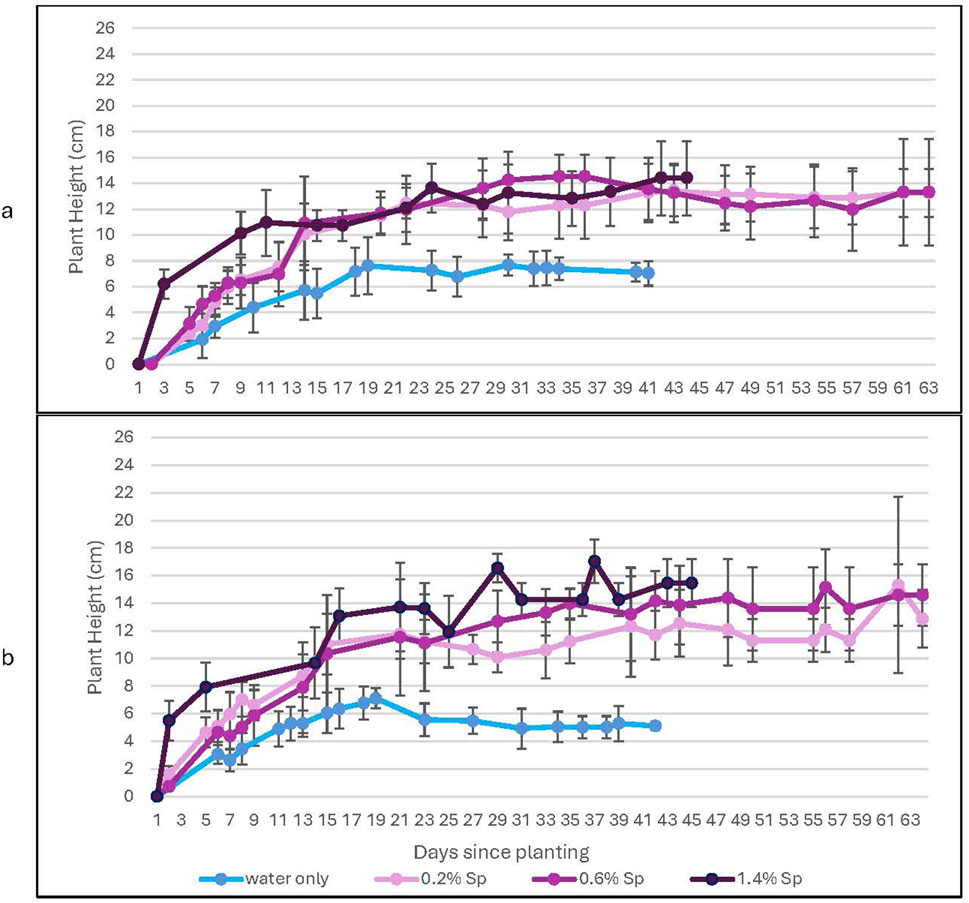
Figure 5. A comparison of plant height with and without the spirulina treatment for growth in 100% lunar regolith simulant. This figure shows a comparison of plants treated with varying concentrations of Spirulina for (a) ambient levels of CO2 and (b) high levels of CO2. Error bars represent ± 1 standard deviation from the mean of 10 replicates per group.
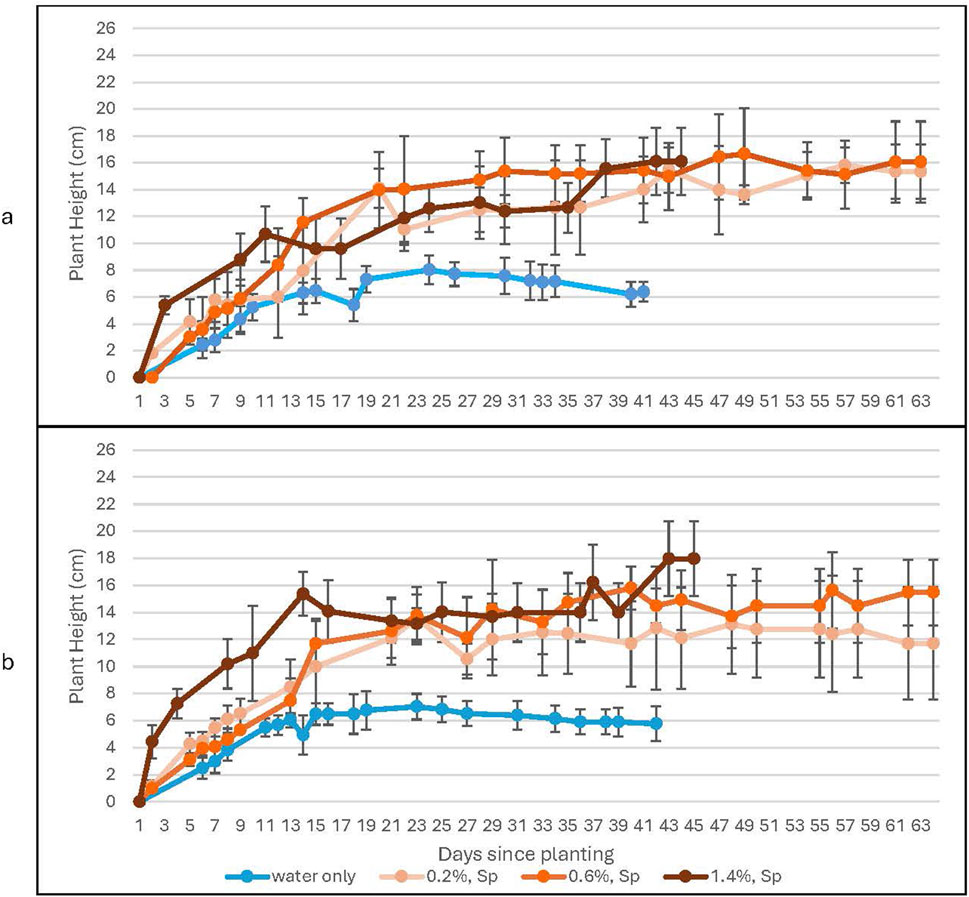
Figure 6. A comparison of plant height with and without the spirulina treatment for growth in 100% Martian regolith simulant. This figure shows a comparison of plants treated with varying concentrations of Spirulina for (a) ambient levels of CO2 and (b) high levels of CO2. Error bars represent ± 1 standard deviation from the mean of 10 replicates per group.
3.1.2 Growth in regolith simulant-soil mixtures
Groups using 100% regolith simulant (without bonsai soil) were tested independently and compared with the bonsai soil/regolith simulant mixtures, which were used to model early-stage ISRU augmentation scenarios and/or minimal Earth soil supplementation. No significant difference in growth (including both growth timeframe and overall growth) was noted between simulant–soil mixtures and pure simulant groups for any of the experimental conditions; this was true for both lunar and Martian regolith simulant (see Figures 1–4). Each group of plants grown in regolith simulant shows an initial high rate of growth, which plateaus around 15–20 days after planting and afterwards maintains a small but positive growth rate for the remainder of the experiment.
3.1.3 Effect of environmental CO2
No significant difference is noted between growth under ambient vs. high CO2 for both regolith simulants (see Figures 7–9); both the initial growth rates and the sustained growth during the plateau period are similar regardless of CO2 profile. Growth in ambient CO2 is slightly higher during the plateau period than in elevated CO2 for all plant groups except those which received the 1.4% spirulina treatment, where the trend is reversed (Figure 7). Differences are seen between plants fertilized with the spirulina solutions and those which were not, with increasing growth occurring for increasing concentrations of spirulina, although there is little difference between the 0.6% and 1.4% spirulina groups.
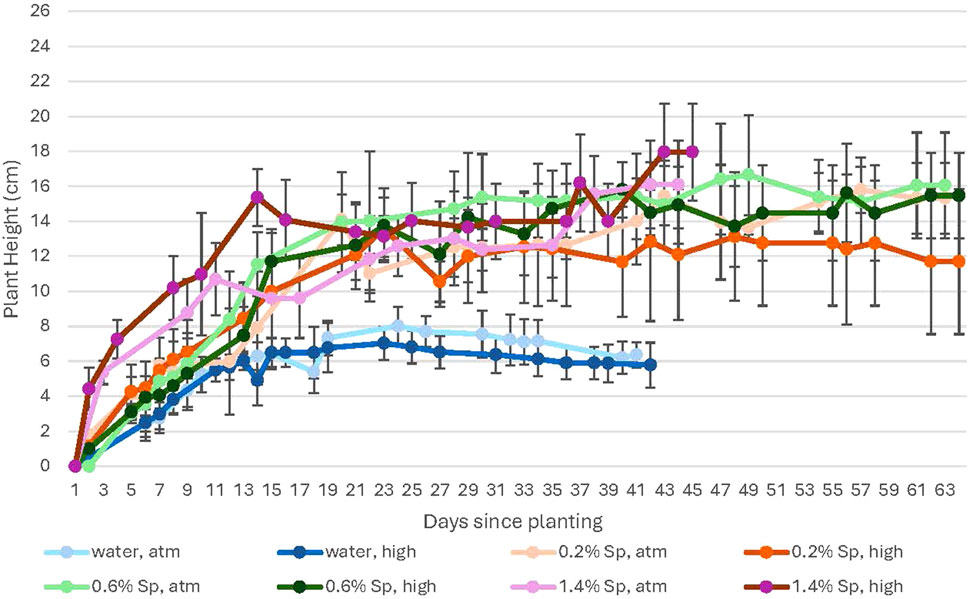
Figure 7. A comparison of plant height in 100% Martian regolith simulant for ambient vs. high levels of CO2. In this figure, the colors represent the treatment received: water-only (blue); 0.2% (orange); 0.6% (green); and 1.4% (purple). Darker shades indicate growth with high CO2 while lighter shades show growth at ambient CO2. Error bars represent ± 1 standard deviation from the mean of 10 replicates per group.
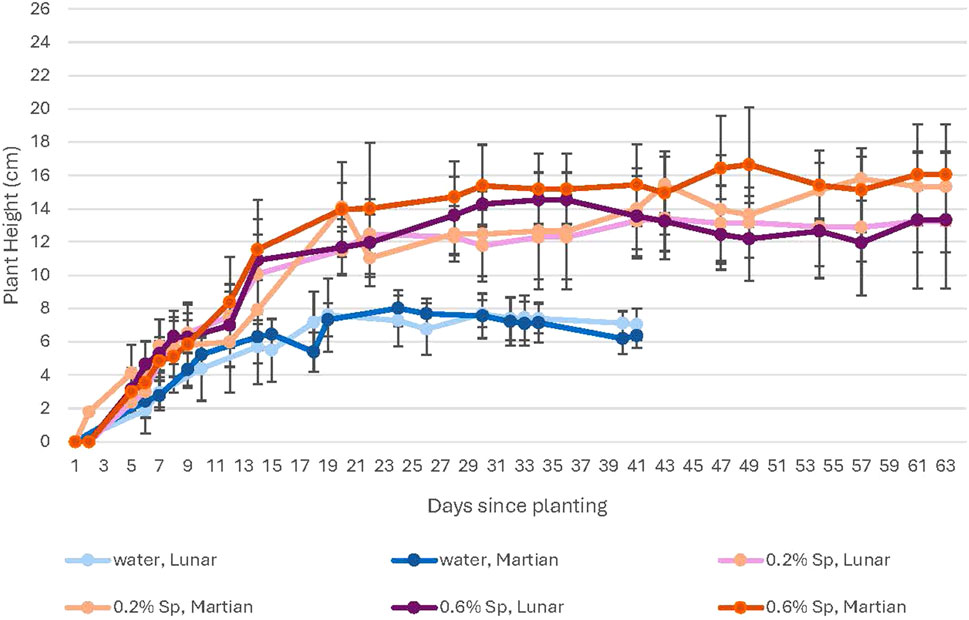
Figure 8. A comparison of plant growth for in 100% lunar vs. Martian regolith simulant mixtures at ambient levels of CO2. This figure compares growth in lunar (purple) and Martian (orange) regolith simulant. Growth is generally lower in lunar regolith simulant. Error bars represent ±1 standard deviation from the mean of 10 replicates per group.
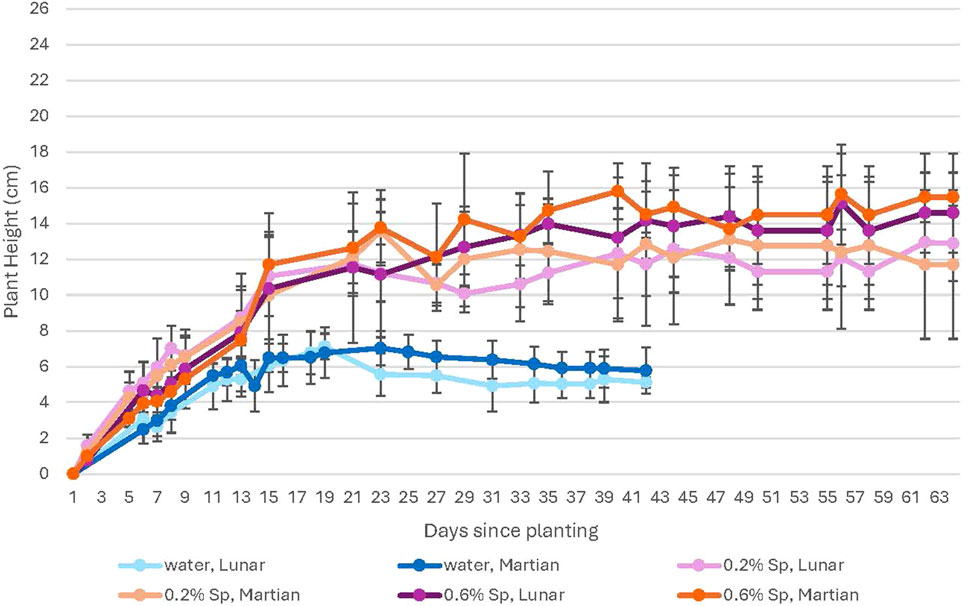
Figure 9. A comparison of plant growth for 100% lunar vs. Martian regolith simulant mixtures at high levels of CO2. This figure compares growth in lunar (purple) and Martian (orange) regolith simulant. Growth in Martian regolith simulant is slightly higher than in lunar for all conditions. Error bars represent ± 1 standard deviation from the mean of 10 replicates per group.
For the water-only groups, plants grown in Martian regolith simulant at elevated CO2 show higher growth rates compared to those in lunar regolith simulant during the plateau period, although plants in both types of regolith simulants show a clear decline during this period (Figure 9). No difference is noted for the water-only plants grown at ambient CO2 (Figure 8). For the spirulina-treated plants, higher growth rates are seen for the plants grown in Martian regolith simulant for all spirulina concentrations at ambient CO2; there is no noticeable difference between growth in lunar and Martian regolith simulants for any concentration of spirulina at high CO2.
Statistical analysis confirms the results described above. A repeated measures ANOVA confirms the significant differences in growth rate as a function of spirulina dosage (F (2,24) = 12.31, p < 0.001). A two-way ANOVA reveals a significant main effect of spirulina dosage (F (2,36) = 18.02, p < 0.001); a significant main effect of CO2 (F (1,36) = 9.84, p = 0.003); and a significant interaction effect (F (2,36) = 4.65, p = 0.016). MANOVA results also confirm the combined effects of CO2, regolith simulant, and spirulina on plant growth (Wilks’ Λ = 0.72, F = 3.09, p = 0.002).
Post-hoc comparisons using the Turkey HSD test confirm that the water-only groups are statistically different (p < 0.01) than all of the groups which received the spirulina treatment for both types of regolith simulant and at both ambient and high levels of CO2. Significance is also seen between the 0.2% solution and the 0.6% spirulina groups (p < 0.01) for all conditions and between the 0.2% and 1.4% spirulina groups for both types of regolith simulant at high concentrations of CO2 (p < 0.01). At ambient levels of CO2, the 0.2% spirulina group differs significantly (p < 0.05) from the 1.4% spirulina group for lunar regolith simulant; no significant difference is found between the 0.2% and 1.4% solutions for the Martian regolith simulant mixtures. The 0.6% and 1.4% spirulina groups differ significantly (p < 0.01) for both lunar and Martian regolith simulant mixtures for the high CO2 runs, but do not differ significantly for the ambient CO2 runs.
A principal component analysis was performed to compare final plant height distributions for 0.2%, 0.6%, and 1.4% spirulina feed treatments across both regolith simulant types (see Figure 10). Plants grown in Martian regolith simulant with 0.6% spirulina exhibit the strongest median growth, while those which received the 1.4% spirulina treatment show diminished performance in both regolith simulant types. Growth in lunar regolith simulant with 0.2% spirulina produces extreme outliers, suggesting dose-response variability.
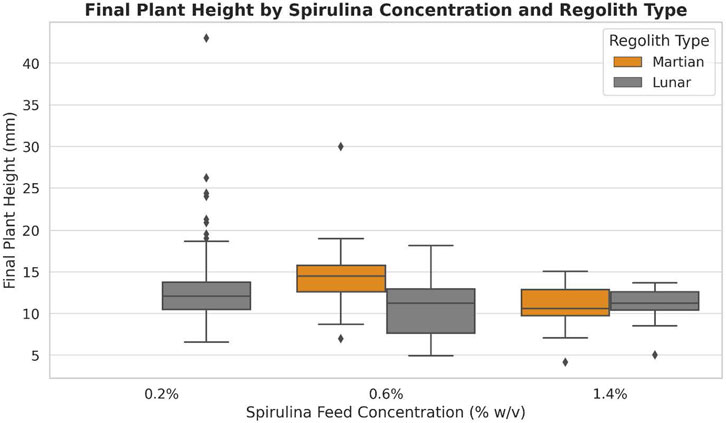
Figure 10. Principal component analysis of plant height across spirulina concentration and regolith simulant type. This figure shows the results of a principal component analysis of plant height during the plateau period. Results for plants grown in Martian regolith simulant are shown in orange; those for plants grown in lunar regolith simulant are shown in grey. Results were evaluated across three different spirulina concentrations. The highest median growth is seen for Martian regolith simulant with 0.6% spirulina. A few outliers are noted, most prominently observed for growth in lunar regolith simulant at 0.2% spirulina.
3.1.4 Average plateau height
Analysis of the average plateau height highlights the difference between water-only vs. spirulina-treated plant groups (ref. Figure 11). Growth is a factor of two higher for all groups which received any concentration of spirulina treatment compared to the water-only groups. For the water-only groups and those which received the 0.2% concentration of spirulina, slightly higher growth is maintained for the groups grown under ambient CO2 concentrations compared to high CO2; this trend reverses for the groups which received 0.6% and 1.4% spirulina solutions. For all plants growth in the Martian regolith simulant mixtures is similar or slightly higher than in lunar regolith simulant mixtures.
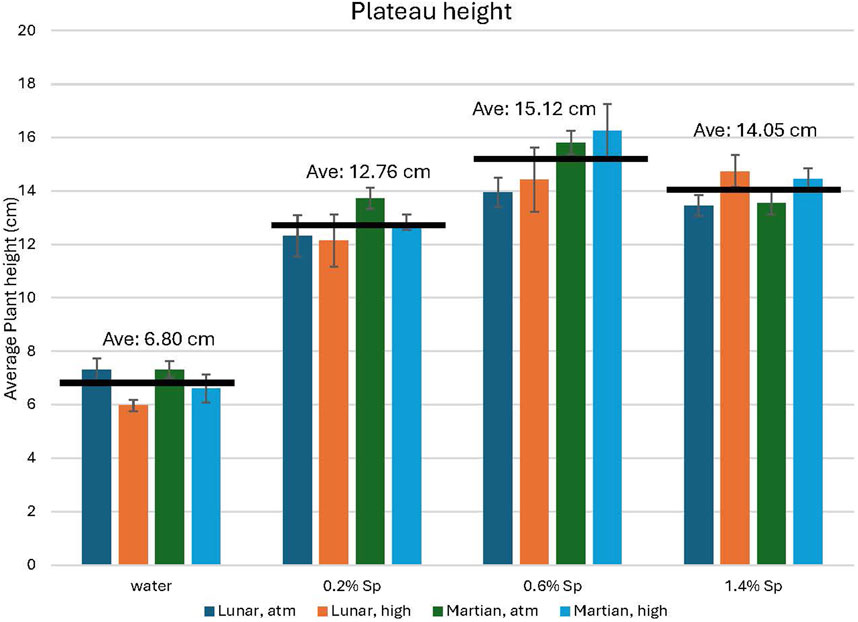
Figure 11. Average height during the sustained growth (plateau) period for all plants. The black bar indicates the average height for each treatment group. Significant differences are seen between those plants which received the Spirulina treatment and those which did not. Error bars represent ± 1 standard deviation from the mean of 10 replicates per group.
A single-factor ANOVA statical analysis indicates significant differences in average plateau height for plants grown in lunar regolith simulant at both ambient (F (3,16) = 119.5, p < 0.001) and high (F (3,16) = 95.68, p < 0.001) levels of CO2. Likewise, the AVOVA tests for plants grown in Martian regolith simulant at both ambient (F (3,16) = 210.4, p < 0.001) and elevated (F (3,16) = 346.5, p < 0.001) levels of CO2 show significance. The ANOVA tests confirm that the variance is between groups, not within groups; this supports the statement that there is no significant difference observed for the different soil-to-regolith simulant mixtures. The between group significance is indicative of the difference between the groups which did vs. did not receive the spirulina treatment (see Figure 11).
3.2 Plant volume
Plant volume was calculated as a cylindrical volume based on the vertical plant height and tip-to-tip leaf distance. Plant volume gives a holistic measure of general plant growth, complementing the plant height data described above. Plants grown in Martian regolith simulant show similar trends but with volumes reduced by 15% for plants grown in ambient CO2; plant volume increases by 35%–40% for groups grown in elevated CO2 (Figure 12). A strong increasing growth trend is noted for the plants which received the spirulina treatment (Figure 13). Further study is needed to determine if the increasing trend continues beyond day 20; the height data discussed above suggests it may. The water-only plants show a bell curve-like growth profile; the volume measurements clearly demonstrate the decline in overall plant growth over time (see Figure 13).
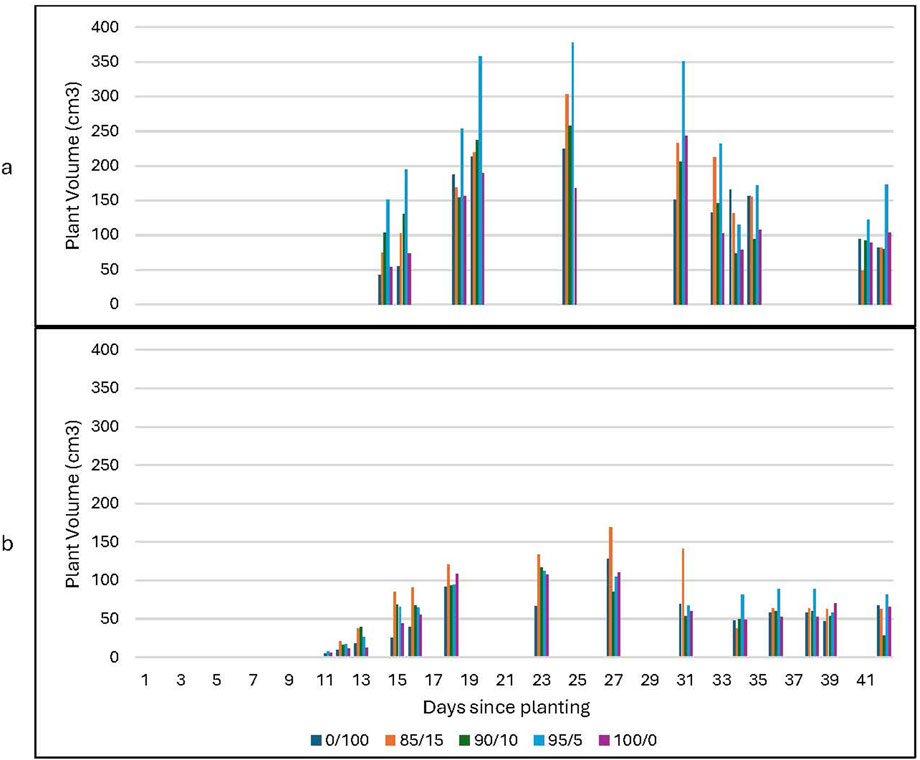
Figure 12. Plant volume at ambient and high levels of CO2 for the water-only plants. Plant volume is shown for: (a) lunar regolith simulant at ambient CO2; (b) lunar regolith simulant at high CO2. The data includes plants grown in all soil mixtures.
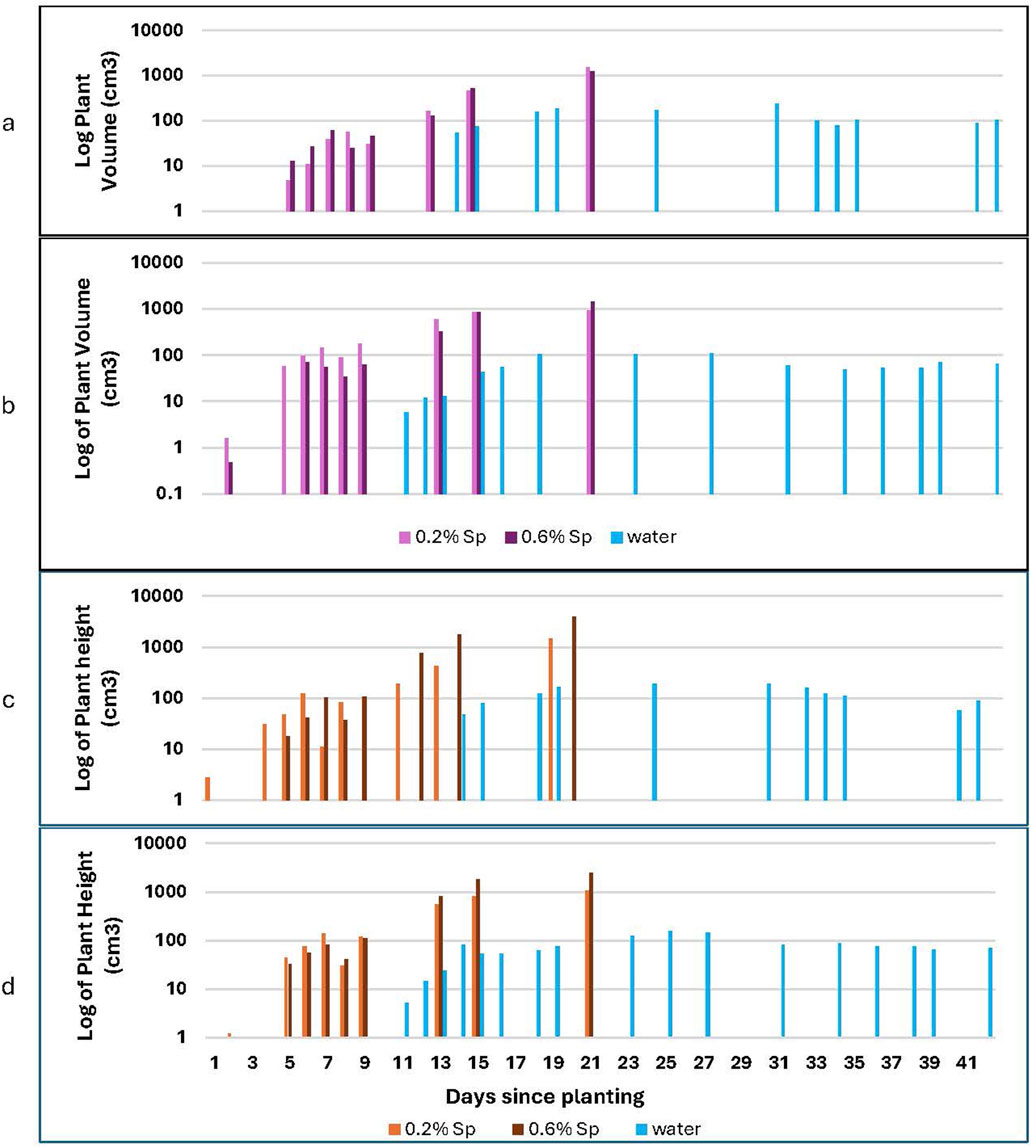
Figure 13. Plant volume for both 100% lunar and Martian regolith simulant. This graph compares plant volume for lunar (a,b) and Martian (c,d) regolith simulant at both ambient (a,c) and high (b,d) CO2 as a function of the amount of Spirulina treatment received.
3.3 Leaf metrics
Leaf color was assessed using the standard Munsell color palette as a measure of general plant health over the growing period (see Supplementary Appendix C). Leaf colors for the plants in soil correspond to “green/yellow” on the Munsell standards; those in lunar regolith simulant average ‘5gy 5/6’; and those in Martian regolith simulant best match ‘7.5gy 4/4’ over the 60-day growth periods. A general yellowing as a function of time is noted in the table for all runs, although the yellowing is most noticeable for plants grown in lunar regolith simulant compared to Martian. Plants which received lower concentrations of spirulina treatment tend to be yellower and less healthy. In addition, plants grown at ambient levels of CO2 show more yellowing with time while plants grown at high levels of CO2 show more yellowing initially but then become gradually greener. This last trend may be due in part to the implementation of a more effective fan system within the high CO2 tents which was implemented at approximately 25 days of growth.
Plants in lunar regolith simulant develop comparable numbers of true leaves to those grown in Martian regolith simulant. Leaf counts show a direct dependence on the concentration of spirulina treatment. Plants fertilized with 0.2% spirulina grew 1.4 times more leaves; those fertilized with the 0.6% solution resulted in 1.5 times more leaves than the controls.
3.4 Harvest data
Wet and dry weights were measured post-harvest for the spirulina-treated plants. The plants grown in lunar regolith simulant yield larger weights than their counterparts grown in Martian regolith simulant for both wet and dry weights at ambient levels of CO2, although the difference is only significant for the plants which received the 0.6% spirulina concentration (t (14) = 1.76, p = 0.034). The trend reverses for plants grown at high levels of CO2, although the differences between plants grown in lunar and Martian regolith simulant are not significant (see Figure 14). For the high CO2 runs, there is a significant difference at the 99th percentile between plants fertilized with the 0.2% spirulina solution compared to 0.6% for both lunar (t (7) = 1.89, p < 0.001) and Martian (t (9) = 1.83, p = 0.004) regolith simulant. The data for the 1.4% spirulina plants grown in lunar regolith simulant at ambient CO2 does not fit the trends shown but represents only 44 days of growth compared with 64 days for the other runs.
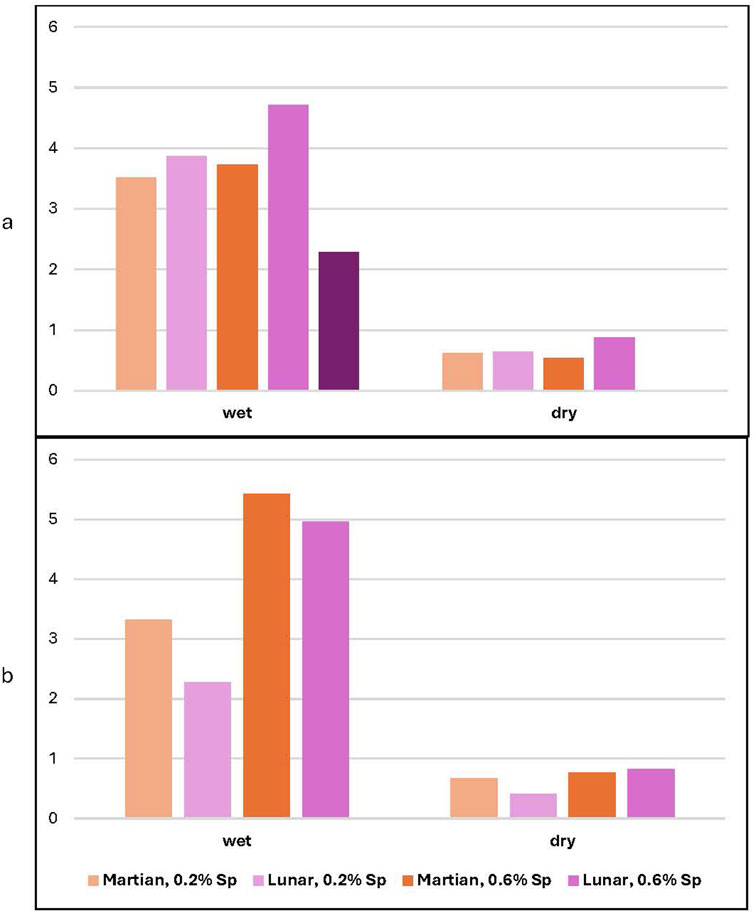
Figure 14. Final biomass (wet and dry) for lunar and Martian regolith simulant. Average wet and dry weights for plants grown in ambient (a) and high (b) CO2.
Under elevated CO2 conditions, the mean dry biomass of radish microgreens increases from 1.02 g in the control group to 1.45 g for the 3 g spirulina group and peaks at 1.73 g for the 7 g spirulina group. In ambient CO2 conditions, the corresponding values are slightly lower: 0.94 g (control), 1.26 g (3 g), and 1.45 g (7 g). The variability within each group is low, as indicated by standard deviations ranging from ±0.08 g to ±0.15 g; the effect size is greater under elevated CO2.
3.5 Qualitative comparison
In general, the R. Sativus plants grew in all soil-regolith simulant mixtures, exhibiting expected growth profiles over time. Each group demonstrated high growth rates initially as the cotyledon emerged; the rates plateaued as the true leaves emerged but were still generally positive. The plants sprouted 2–3 days after planting, but showed signs of stress over time, including leaf yellowing, faster initial growth rates, light-seeking behaviors, and lack of flowering/seed production. These effects were more pronounced for the water-only groups and for the elevated CO2 groups. The plants grown in Martian regolith simulant under elevated CO2, which received the 0.6% spirulina treatment had a general appearance of greater health: their stems and leaves were observed to be thicker, stronger, and healthier. Overall, growth in lunar regolith simulant was generally comparable to growth in Martian regolith simulant, but plant health metrics, as evidenced by leaf count, leaf color, and visual inspection of the plants, were better for plants grown in Martian regolith simulant in high CO2 environments.
Leaf color, a qualitative measure of photosynthesis, showed a strong variation with the amount of fertilization, with plants that received only water or lower concentrations of spirulina treatment producing leaves that were more yellow-green compared to the deep greens found in the plants cultivated with higher spirulina concentrations. Leaf size and general appearance also improved when higher concentrations of spirulina solution were used.
The root systems of the plants were analyzed using an OMAX Lab Grade Microscope at × 40 and × 100 magnification. Root systems showed substantial growth and were generally healthy. Roots were white in color and firm, including root nodes and caps. Full root penetration was noted for all soil mixtures1.
3.6 Qualitative analysis of root cap morphology and hair density
Across samples, root caps were well-formed and structurally intact, with no evidence of abrasion or necrosis. Root hairs were prolific, present in the thousands, along the primary taproots and showed dense, uniform distribution. This was particularly notable in specimens fertilized with 0.6% and 1.4% spirulina concentrations, where hair density was highest and overall morphology was consistent with healthy nutrient absorption structures.
Live video microscopy confirmed active cellular respiration localized at the root tips, visible as oscillatory cytoplasmic streaming within the cap cells, confirming metabolic activity. All observations were consistent across multiple imaging sessions conducted during this study. Preliminary assessments of aboveground biomass and below-ground radish formation further support the qualitative root observations; plants in the midrange spirulina treatments exhibited the most vigorous shoot and fruit development1.
4 Discussion
4.1 Efficacy of spirulina treatment
Growth was achieved for all plant groups. The initial high rate of growth demonstrated reflects sustainment from the seed, while the growth during the plateau period represents the plant’s ability to thrive based on nutrition absorbed from the soil and fertilization. The water-only control plants showed significant signs of distress due to the non-nutritive growing conditions. These plants struggled, but did grow, reaching the lowest plant heights and leaf counts. They showed the most yellowing and the fewest seeds produced. In contrast, the plants that received the spirulina treatment experienced more growth and greater plant health. These results clearly suggest the efficacy of spirulina as a biofertilizer capable of supporting plant growth in regolith simulant and high CO2 environments. In each case, the plants that received the spirulina treatment showed larger growth metrics and healthier leaves than those that only received water, and growth was sustained well beyond the point where nutrition from the seed was exhausted. Flowers were observed in approximately 20%–30% of the plants. Seed formation was rare, but present, with three pods produced in the water-only, high-CO2 tent, and several seeds observed in high-CO2 spirulina groups during the July/August run. All seed formation occurred under high-CO2 conditions. This reflects a typical stress response in plants, where early flowering and limited seed production occur as a survival mechanism under severe stress. These results indicate that while vegetative growth was improved with spirulina supplementation, reproductive capacity remained constrained, consistent with challenges reported in space crop trials.
4.2 Impact of spirulina concentration
The most significant differences in all plant metrics, both qualitative and quantitative, resulted from variations in the amount of spirulina fertilizer used for the treatment. All concentrations of spirulina solutions resulted in healthier plants and higher growth rates. This was especially true for the two higher concentrations of spirulina, which resulted in substantial differences compared to the controls. In these results, the 0.6% concentration yielded the largest gains in plant growth metrics, suggesting that optimum growth can be achieved with a mid-range concentration.
Spirulina significantly enhanced plant biomass and height, especially when paired with elevated CO2 levels. This synergy is attributed to complementary physiological and biochemical mechanisms. Elevated CO2 increases photosynthetic activity and biomass accumulation by boosting the Calvin cycle’s efficiency and reducing photorespiration (Zabel et al., 2022). Simultaneously, spirulina delivers essential macro- and micronutrients such as nitrogen, iron, magnesium, and potassium, all of which are typically scarce in Martian and lunar regolith simulants (Jones et al., 2016). Furthermore, spirulina supplements deliver nutrients and bioactive compounds through aqueous extraction, making phytohormones, antioxidants, and enzymes available to plants, improving root growth, stress tolerance, and nutrient uptake (Henard et al., 2021). This occurs via release from spirulina biomass rather than active culture.
4.3 Growth in regolith simulant
Importantly, no major differences in root development or health resulted from the addition of bonsai soil to the regolith simulant, suggesting that the increased water holding capacity of the sphagnum moss was sufficient to alleviate compression and compacting behaviors in both lunar and Martian regolith simulants used. This suggests that the augmentation of regolith simulants with spirulina is sufficient may alleviate the need to transport terrestrial soil for space agriculture endeavors.
The use of lunar and Martian regolith simulants yielded similar height and volume results for the plants; while plants were somewhat healthier when grown in the Martian regolith simulant, the difference was not large. Notably, root systems grown in 100% Martian regolith simulant exhibited an opaquer appearance and lacked the greenish translucence observed in the bonsai-soil control group. Despite this, plants grown in 100% regolith simulant with consistent moisture maintained performed well and developed nearly pristine root caps. A slight increase in branching and lateral extension was observed in treatments with minimal additions of bonsai soil, suggesting that even small quantities of organic matrix enhance soil-root interface dynamics.
As an indication of both plant health and chlorophyll production, the measured leaf color indicated the lunar plants were slightly more stressed compared with the Martian; the plants grown in Martian registered at a healthier level on the color scale. This difference may be attributed to the higher nitrogen content in the Martian regolith simulant.
The main differences in plant growth metrics are likely due to the water-carrying capacities and compaction of the regolith simulant; the former is higher for the Martian regolith simulant used in this study, which the latter is higher for the lunar regolith simulant used. Plants grown in lunar regolith simulant mixtures also required more frequent watering and larger amounts of water in order to maintain the same soil moisture levels as the Martian regolith simulant mixtures. The application of spirulina, which enhances water-holding and nutrient-binding, accentuated differences between lunar and Martian simulants. Martian simulants retain water more effectively, while lunar simulants are more compact, leading to divergent responses. In addition, the seeds needed to be planted a little higher in these samples to offset the higher compaction of lunar regolith simulant.
4.4 Growth in high CO2
The concentration of environmental CO2 produced differences in the color data for plant groups. Very high CO2 environments can be stressful for plants, especially without adequate circulation, which is difficult to achieve in growth chambers. Significant yellowing occurred for plants grown under the highest CO2 levels, indicative of diminished transpiration (Morison and Lawlor, 1999). The addition of two small fans alleviated the issue and allowed the plants to maintain photosynthetic rates as indicated by leaf color. The high CO2 plants developed more quickly than expected from terrestrial timetables; this is likely a response to the environmental stressors compounding the limited nutrient availability in the soil mixtures. While the spirulina fertilizer supplied sufficient nutrition to sustain plant growth over the growing cycle, it did not fully compensate for the environmental stressors. These results align with plant growth studies on the ISS (where environmental CO2 levels are elevated compared to atmospheric values on Earth) described earlier in the paper.
4.5 Limitations
This study utilized regolith simulant from Space Resources Technologies (532 S Econ Circle. Oviedo, Florida 32765, US; https://spaceresourcetech.com/). The accuracy with which manufactured simulants mimic actual lunar and Martian regolith has been called into question (Eichler et al., 2021). This is a known and unavoidable limitation of the study due to the lack of Martian samples and the limited availability of lunar samples.
The study was limited to short-cycle microgreens. Crops with longer lifecycles may exhibit different responses. The lab notes indicate a stressed plant after 45–55 days.
While CO2 was controlled, other atmospheric variables (e.g., ethylene accumulation) were not.
5 Key Takeaways
Spirulina boosts growth for radish microgreens significantly; Martian regolith simulant with 0.6% spirulina under elevated CO2 yielded the best results. Water-only groups declined after the initial growth phase; spirulina fertilized groups maintained steady growth rates over the full growing period. Regolith simulant type influenced biomass more than height, with the best growth found in Martian rather than lunar regolith simulant. No difference was found between the different regolith simulant-to-soil mixtures, suggesting that spirulina fertilization can compensate for the non-nutritive properties of regolith, supporting plant growth without the addition of terrestrial soils.
6 Conclusion
This project demonstrates the potential of spirulina supplementation to enhance the growth of radish microgreens in lunar and Martian regolith simulants under both elevated and ambient CO2 conditions. Application of aqueous spirulina solution led to healthier plant growth in both regolith simulants under different levels of environmental CO2 as evidenced by plant height, plant volume, and leaf count and colour, over a full seed-to-flowering cycle, with limited seed formation only in the high-CO2 tents. Harvest wet and dry weights and root measurements also support this trend. This study suggests that spirulina could play an important role in supporting ISRU in cultivation efforts by eliminating the need to transport terrestrial soils to support healthy plant growth. The strongest growth trends and healthiest plants were those in the Martian regolith simulation as opposed to lunar; this has important implications for human exploration beyond cislunar space.
As a cyanobacterium, spirulina could have future implications for rhizosphere dynamics and microbial symbiosis, especially in Martian regolith simulant; this is an important area for future research in bioregenerative systems. Additionally, terrestrial studies have confirmed the efficacy of spirulina in supporting the growth of a variety of legumes, tomato, and lettuce varieties (Shedeed et al., 2022; Bhowmik et al., 2010; Taha et al., 2023; Anitha et al., 2016; Akgul and Akgul, 2019), suggesting that the results reported here may have applicability beyond radish microgreens. Finally, current research in space agriculture spans a range of systems, including both aquaponic and soil-based initiatives. This work suggests that spirulina can serve as an effective biofertilizer in soil farming practices using ISRU as a potential means of supporting off-world habitation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
KM: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft. DT: Conceptualization, Investigation, Methodology, Supervision, Writing – review and editing. GC: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by NASA under proposal number 23-2023 R3-0040 issued through the Established Program to Stimulate Competitive Research (EPSCoR) Rapid Research Response (R3) funding opportunity #NNX23EXPLORA through the West Virginia Space Grant Consortium.
Conflict of interest
The spirulina required for this study was provided through a collaboration with Cyanotech Corporation (73-4460 Queen Ka'ahumanu Hwy #102, Kailua-Kona, HI 96740, US; https://www.cyanotech.com/).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frspt.2025.1651978/full#supplementary-material
SUPPLEMENTARY TABLE 1 | Appendix C: Plant Color: Munsell color heat map of the plant groups as a function of time for the first 30 days of growth.
SUPPLEMENTARY DATA SHEET 1 | Data Repository: The complete dataset for the project.
SUPPLEMENTARY DATA SHEET 2 | Appendix A: Composition information for the materials, including the regolith simulants, bonsai soil, and sphagnum moss.
SUPPLEMENTARY DATA SHEET 3 | Appendix B: Sphagnum Moss as a Moisture Manager with Minimal Nutrient Contribution: A Literature Review.
Footnotes
1San Francisco Daily Lab Notes. (2023–2024). Root microscopy and spirulina field trial observations. NASA Radish Growth Trial Log, NASA Grant #NNX23EXPLORA. Unpublished internal laboratory record, Space 4 All Research Facility, San Francisco, CA.
References
Akgul, F., and Akgul, R. (2019). Proceedings: 3rd international Conference on Food and Agricultural Economics: the effect of Spirulina Platensis (Gomont) Geitler extracts on seed germination of Lactuca Sativa L.
Anitha, L., Sai Bramari, G., and Kalpana, P. (2016). Effect of supplementation of Spirulina platensis to enhance the zinc status in plants of Amaranthus gangeticus, Phaseolus aureus and tomato. Adv. Biosci. Biotechnol. 7 (06), 289–299. http://dx.doi.org/10.4236/abb.2016.76027
Astolfi, C., Sosa, L., Mazzeo, L., Morbelli, M., Larcher, L., Iturriaga, L., et al. (2024). Chemical and antioxidant differences between Sphagnum moss and peat: a comparative multi-technique analysis from Tierra del Fuego. PLOS ONE 19 (1), e0294724. doi:10.1371/journal.pone.0294724
Barcenilla, B. B., Kundel, I., Hall, E., Hilty, N., Ulianich, P., Cook, J., et al. (2024). Telomere dynamics and oxidative stress in Arabidopsis grown in lunar regolith simulant. Front. Plant Sci. 15, 1351613. doi:10.3389/fpls.2024.1351613
Bhowmik, D., Dubey, J., and Mehra, S. (2010). Evaluating potential of Spirulina as innoculant for pulses. Acad. J. Plant Sci. 3 (4), 161–164.
Chinnannan, K., Somagattu, P., Yammanuru, H., Nimmakayala, P., Chakrabarti, M., and Reddy, U. K. (2023). Effects of Mars global simulant (MGS-1) on growth and physiology of sweet potato: a space model plant. Plants 13 (1), 55. doi:10.3390/plants13010055
CO2 Meter (2023). CO2 Calculator for grow room or indoor greenhouse. Available online at: https://www.co2meter.com/blogs/news/41003521-co2-calculator-for-grow-room-or-indoor-greenhouse#:∼:text=Most%20experts%20agree%20that%201%2C500,will%20produce%20greatly%20improved%20results.&text=of%20CO2%20every%202%20hours%2C%20or%20about%20.&text=We%20recommend%20starting%20at%201%2C000,ppm%20will%20reduce%20plant%20growth.
Connaboy, C., Sinnott, A. M., LaGoy, A. D., Krajewski, K. T., Johnson, C. D., Pepping, G. J., et al. (2020). Cognitive performance during prolonged periods in isolated, confined, and extreme environments. Acta Astronaut. 177, 545–551. doi:10.1016/j.actaastro.2020.08.018
De Pascale, S., Arena, C., Aronne, G., De Micco, V., Pannico, A., Paradiso, R., et al. (2021). Biology and crop production in Space environments: challenges and opportunities. Life Sci. Space Res. 29, 30–37. doi:10.1016/j.lssr.2021.02.005
Duri, L. G., Caporale, A. G., Rouphael, Y., Vingiani, S., Palladino, M., De Pascale, S., et al. (2022). The potential for lunar and martian regolith simulants to sustain plant growth: a multidisciplinary overview. Front. Astronomy Space Sci. 8, 747821. doi:10.3389/fspas.2021.747821
Eichler, A., Hadland, N., Pickett, D., Masaitis, D., Handy, D., Perez, A., et al. (2021). Challenging the agricultural viability of martian regolith simulants. Icarus 354, 114022. doi:10.1016/j.icarus.2020.114022
Fackrell, L. E., Humphrey, S., Loureiro, R., Palmer, A. G., and Long-Fox, J. (2024). Overview and recommendations for research on plants and microbes in regolith-based agriculture. Sustain. Agric. 2 (1), 15. doi:10.1038/s44264-024-00013-5
Fais, G., Manca, A., Bolognesi, F., Borselli, M., Concas, A., Busutti, M., et al. (2022). Wide range applications of spirulina: from earth to space missions. Mar. Drugs 20 (5), 299. doi:10.3390/md20050299
Georgescu, M. R., Meslem, A., and Nastase, I. (2020). Accumulation and spatial distribution of CO2 in the astronaut's crew quarters on the International Space Station. Build. Environ. 185, 107278. doi:10.1016/j.buildenv.2020.107278
Geries, L. S. M., and Elsadany, A. Y. (2021). Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Archives Microbiol. 203 (1), 169–181. doi:10.1007/s00203-020-01991-z
Godlewska, K., Michalak, I., Pacyga, P., Baśladyńska, S., and Chojnacka, K. (2019). Potential applications of cyanobacteria: spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 35, 80–18. doi:10.1007/s11274-019-2653-6
González, A. M., Iturriaga, L., Astolfi, C., Sosa, L., and Castro, S. (2023). Chemical, microstructural and mineralogical characterization of Sphagnum moss vs. peat in Tierra del Fuego. J. Anal. Appl. Pyrolysis 173, 105800. doi:10.1016/j.jaap.2023.105800
Hasenstein, K. H., John, S. P., and Vandenbrink, J. P. (2023). Assessing radish health during space cultivation by gene transcription. Plants 12 (19), 3458. doi:10.3390/plants12193458
Hecht, M. H., Kounaves, S. P., Quinn, R. C., West, S. J., Young, S. M. M., Ming, D. W., et al. (2009). Detection of perchlorate and the soluble chemistry of martian soil at the phoenix lander site. Sci. (80-.) 325, 64–67. doi:10.1126/.science.1172466
Henard, C. A., Smith, H., Knoshaug, E. P., and Mamatha, B. S. (2021). Carotenoid composition of locally found seaweeds of Dakshina Kannada district in India. Algal Res. 53, 102154. doi:10.1016/j.algal.2020.102154
Johnson, C. M., Lucie Poulet, H. B., Bunchek, J., Romeyn, M. W., Spencer, L. E., Dixit, A. R., et al. (2021). Supplemental food production with plants: a step toward bioregenerative life support. Front. Astron. Space Sci. 8, 198. doi:10.3389/fspas.2021.734343
Jones, H. W., Do, S., and Peterson, L. (2016). Mars and Moon agriculture: what we know and what we need. Life Sci. Space Res. 10, 1–12. doi:10.1016/j.lssr.2016.06.003
Khodadad, C. L., Hummerick, M. E., Spencer, L. E., Dixit, A. R., Richards, J. T., Romeyn, M. W., et al. (2020). Microbiological and nutritional analysis of lettuce crops grown on the international space station. Front. Plant Sci. 11, 199. doi:10.3389/fpls.2020.00199
Kordyum, E., and Hasenstein, K. H. (2021). Plant biology for space exploration–building on the past, preparing for the future. Life Sci. space Res. 29, 1–7. doi:10.1016/j.lssr.2021.01.003
Loladze, I. (2002). Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol. and Evol. 17 (10), 457–461. doi:10.1016/s0169-5347(02)02587-9
Long-Fox, J. M., and Britt, D. T. (2023). Characterization of planetary regolith simulants for the research and development of space resource technologies. Front. Space Technol. 4, 1255535. doi:10.3389/frspt.2023.1255535
Manalu, R. R., Nurhayati, M., and Suhendi, A. (2025). Phytochemical screening and antimicrobial potential of Sphagnum spp. from North Sumatra highlands. Indonesian J. Bot. 20 (2), 95–105. doi:10.15408/ijb.v20i2.34567
Massa, G. D., Wheeler, R. M., Morrow, R. C., and Levine, H. G. (2016). Growth chambers on the International Space Station for large plants. VIII Int. Symposium Light Hortic. 1134, 215–222. doi:10.17660/actahortic.2016.1134.29
Meinen, E., Dueck, T., Kempkes, F., and Stanghellini, C. (2018). Growing fresh food on future space missions: environmental conditions and crop management. Sci. Hortic. 235, 270–278. doi:10.1016/j.scienta.2018.03.002
Monje, O., Stutte, G., and Chapman, D. (2005). Microgravity does not alter plant stand gas exchange of wheat at moderate light levels and saturating CO 2 concentration. Planta 222, 336–345. doi:10.1007/s00425-005-1529-1
Morison, J. I. L., and Lawlor, D. W. (1999). Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell and Environ. 22 (6), 659–682. doi:10.1046/j.1365-3040.1999.00443.x
Myers, S. S., Zanobetti, A., Kloog, I., Huybers, P., Leakey, A. D., Bloom, A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510 (7503), 139–142. doi:10.1038/nature13179
NASA (2021). Planetary fact sheet. Greenbelt, Maryland: NASA. Available online at: http://nssdc.gsfc.nasa.gov/planetary/factsheet/.
Navarro-Gonzalez, R., Vargas, E., de la Rosa, J., Raga, A. C., and McKay, C. P. (2010). Reanalysis of the viking results suggests perchlorate and organics at midlatitudes on Mars. J. Geophys. Res. 115, E12010. doi:10.1029/2010JE003599
Paul, A. L., Elardo, S. M., and Ferl, R. (2022). Plants grown in Apollo lunar regolith present stress-associated transcriptomes that inform prospects for lunar exploration. Commun. Biol. 5 (1), 382. doi:10.1038/s42003-022-03334-8
Pérez, C., and González, M. (2022). Sphagnan polysaccharides: structure, function and ecological role in Sphagnum mosses. Plant Biochem. Rep. 11 (3), 87–95. doi:10.1016/j.pbr.2022.05.001
Porterfield, D. M., Neichitailo, G. S., Mashinski, A. L., and Musgrave, M. E. (2003). Spaceflight hardware for conducting plant growth experiments in space: the early years 1960-2000. Adv. Space Res. 31, 183–193. doi:10.1016/s0273-1177(02)00752-4
Poulet, L., Engeling, K., Hatch, T., Stahl-Rommel, S., Velez Justiniano, Y. A., Castro-Wallace, S., et al. (2022). Large-scale crop production for the Moon and Mars: current gaps and future perspectives. Front. Astronomy Space Sci. 8, 733944. doi:10.3389/fspas.2021.733944
Prior, S. A., Runion, G. B., Marble, S. C., Rogers, H. H., Gilliam, C. H., and Torbert, H. A. (2011). A review of elevated atmospheric CO2 effects on plant growth and water relations: implications for horticulture. HortScience 46 (2), 158–162. doi:10.21273/hortsci.46.2.158
Pritchard, S. G., Rogers, H. H., Prior, S. A., and Peterson, C. M. (1999). Elevated CO2 and plant structure: a review. Glob. Change Biol. 5 (7), 807–837. doi:10.1046/j.1365-2486.1999.00268.x
Rai, A. N., Singh, A. K., and Syiem, M. B. (2019). “Plant growth-promoting abilities in Cyanobacteria,” in Cyanobacteria (Academic Press), 459–476.
Rashid, M. I., Mujawar, L. H., Shahzad, T., Almeelbi, T., Ismail, I. M., and Oves, M. (2016). Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 183, 26–41. doi:10.1016/j.micres.2015.11.007
Shariatmadari, Z., Riahi, H., Seyed Hashtroudi, M., Ghassempour, A., and Aghashariatmadary, Z. (2013). Plant growth promoting Cyanobacteria and their distribution in terrestrial habitats of Iran. Soil Sci. plant Nutr. 59 (4), 535–547. doi:10.1080/00380768.2013.782253
Shedeed, Z. A., Gheda, S., Elsanadily, S., Alharbi, K., and Osman, M. E. (2022). Spirulina platensis biofertilization for enhancing growth, photosynthetic capacity and yield of Lupinus luteus. Agriculture 12 (6), 781. doi:10.3390/agriculture12060781
Singh, S. (2014). A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 117 (5), 1221–1244. doi:10.1111/jam.12612
Singh, J. S., Kumar, A., Rai, A. N., and Singh, D. P. (2016). Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 7, 529. doi:10.3389/fmicb.2016.00529
Taha, M. A., Moussa, H. R., and Dessoky, E. S. (2023). The influence of Spirulina platensis on physiological characterization and mitigation of DNA damage in salt-stressed Phaseolus vulgaris L. plants. Egypt. J. Bot. 63 (2), 0–620. doi:10.21608/ejbo.2023.168006.2165
Tang, H., Rising, H. H., Majji, M., and Brown, R. D. (2021). Long-term space nutrition: a scoping review. Nutrients 14 (1), 194. doi:10.3390/nu14010194
Tripathy, B. C., Brown, C. S., Levine, H. G., and Krikorian, A. D. (1996). Growth and photosynthetic responses of wheat plants grown in space. Plant physiol. 110 (3), 801–806. doi:10.1104/pp.110.3.801
Vandenbrink, J. P., Herranz, R., Medina, F. J., and Kiss, J. Z. (2019). Root growth and orientation in space: studies of plant development in microgravity. Plant Biol. 21 (S1), 10–18. doi:10.1111/plb.12918
Wamelink, G. W. W., Frissel, J. Y., Krijnen, W. H. J., Verwoert, M. R., and Goedhart, P. W. (2014). Can plants grow on Mars and the Moon: a growth experiment on Mars and Moon soil simulants. PLoS ONE 9 (8), e103138. doi:10.1371/journal.pone.0103138
Zabel, P., Bamsey, M., Schubert, D., and Tajmar, M. (2016). Review and analysis of over 40 years of space plant growth systems. Life Sci. space Res. 10, 1–16. doi:10.1016/j.lssr.2016.06.004
Keywords: microalgae, spirulina, biofertilization, daikon radish, regolith, space resource utilization, cyanobacteria, plant growth
Citation: Miller KA, Trevino DT and Cauthorn G (2025) Spirulina supported plant growth in regolith simulants and elevated levels of CO2. Front. Space Technol. 6:1651978. doi: 10.3389/frspt.2025.1651978
Received: 23 June 2025; Accepted: 30 September 2025;
Published: 03 November 2025.
Edited by:
Charles Cockell, University of Edinburgh, United KingdomReviewed by:
James A Nabity, University of Colorado Boulder, United StatesAgata Klaudia Zupanska, SETI Institute, United States
Copyright © 2025 Miller, Trevino and Cauthorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristen A. Miller, a3Jpc3Rlbi5taWxsZXI2QG15Y2FtcHVzLmFwdXMuZWR1
 Kristen A. Miller
Kristen A. Miller Dennis Terry Trevino
Dennis Terry Trevino Gilbert Cauthorn
Gilbert Cauthorn