- 1Department of Electrical, Computer, and Energy Engineering, College of Engineering and Applied Science, University of Colorado Boulder, Boulder, CO, United States
- 2Ann and H.J. Smead Department of Aerospace Engineering Sciences, College of Engineering and Applied Science, University of Colorado Boulder, Boulder, CO, United States
Life on Earth evolved and exists within the geomagnetic field which currently ranges from approximately 25–65 µT. Voyages beyond Earth’s magnetosphere expose astronauts to the unique conditions of deep space, characterized by significantly reduced magnetic fields ranging from 2 to 8 nT. This review examines the growing body of evidence concerning the biological impacts of hypomagnetic and altered magnetic fields on humans and other organisms, highlighting the implications for long-duration spaceflight and space mission. Research using human cell cultures and mammalian models indicates that exposure to varying magnetic field conditions, including hypomagnetic fields (HMF), can induce diverse biological effects. These include changes in cellular proliferation, nervous system function, oxidative stress reactive oxygen species levels, and DNA integrity, with outcomes often dependent on specific field intensity, frequency, and length of exposures. Furthermore, HMF exposure has been shown to affect bacterial behavior and the human microbiome, potentially altering antibiotic resistance and increasing risks of infection, given the compromised immune function astronauts may experience in space. Considering these biological impacts on the wellbeing of astronauts on long-term space mission, providing artificial magnetic fields onboard spacecraft is proposed as a critical strategy to mitigate HMF effects, support astronaut health, and enhance the feasibility and safety of future deep space missions.
1 Hypomagnetic field is a key stressor for spaceflight
The geomagnetic field (GMF) supports life on Earth by providing a relatively stable magnetic environment with an average surface intensity of approximately 45 μT. Various organisms including birds, fish, sea turtles, insects, and bacteria rely on GMF environments in their daily lives with respect to navigation, circadian balance, and biochemical regulation. In addition, the GMF forms the magnetosphere which is useful for deflecting charged solar and cosmic particles to protect the biosphere from harmful radiation—an essential prerequisite for the flourishing of life. Nearly every organism on Earth has adapted to life under exposure to the GMF and rely on it as a persistent environmental factor to maintain their naturally functioning physiology.
A hypomagnetic or hypo-geomagnetic field (HMF) is commonly defined as a magnetic field with intensity below 5 μT. Such conditions are exceedingly rare on Earth but become increasingly relevant when exploring extraterrestrial environments. With the advancement of human spaceflight and ongoing programs such as NASA’s Artemis mission, which aims to return humans to the Moon and prepare for Mars exploration, it is imperative to understand the biological consequences of spaceflight stressors. Among these, HMF exposure has historically received less attention compared with microgravity or ionizing radiation, yet it may play an equally important role in shaping astronaut health and performance during long-duration missions.
Spaceflight exposes biological systems to multiple concurrent stressors. Microgravity alters musculoskeletal and cardiovascular physiology, radiation increases cancer risk and damages to DNA, and isolation and circadian disruption affect neurocognitive performance (Demontis et al., 2017). In this context, HMF conditions represent an additional stressor that could act synergistically with these factors. Evidence from cell culture and limited human data suggests that HMF exposure influences fundamental biological processes, including metabolism, oxidative stress response, proliferation, differentiation, immune regulation, and neural function (Sarimov et al., 2023; Tian et al., 2024). Importantly, many of these responses overlap with or exacerbate the well-documented effects of microgravity, raising questions about additive or combined risks during extended missions.
While astronauts aboard the International Space Station (ISS) remain largely within Earth’s magnetosphere and experience only small and negligible reductions in geomagnetic exposure, missions beyond low Earth orbit—including lunar surface stays, with crustal field intensity ranging from 1–100 nT (nT), and deep-space transit to Mars, where background field can be as low as 1 nT in magnitude—will place crews in sustained HMF conditions (Stevenson, 2003). These exposures will likely be measured in weeks to months, at intensities 10,000 times lower than terrestrial GMF levels. The health implications of such prolonged exposure remain uncertain, underscoring the need for systematic study.
This review examines current evidence on HMF effects with a focus on human cell culture systems and microbial systems relevant to human health. We explore how HMF impacts metabolism, neurocognition, skeletal health, immune and microbiome regulation, and how these effects may interact with other spaceflight stressors. Furthermore, we aim to clarify the mechanisms by which HMF influences physiology, highlight parallels with microgravity and radiation, and identify key knowledge gaps. Such insights are essential for developing countermeasures to safeguard astronaut health during future lunar and Martian missions.
2 Effects of hypomagnetic field on human cell lines and body
Recent studies have broadened our understanding of the influence of HMFs on human biology, with mechanistic investigations primarily conducted in vitro. Frequently examined cell models include SH-SY5Y neuroblastoma cells for neuronal function, embryonic stem cells for developmental plasticity, fibroblasts as a model for structural and connective tissue function, and cancer-derived lines such as HT-1080 fibrosarcoma and breast cancer cells for investigating altered proliferative and metabolic states. In contrast, whole-body studies of HMF effects in humans remain scarce. Practical challenges, such as the difficulty of generating prolonged hypomagnetic environments outside of specialized facilities and the small number of human subjects available for controlled exposure, have limited the scope and statistical power of such investigations (Markin et al., 2023; Kashirina et al., 2023).
At the cellular level, HMF exposure has been shown to alter proliferation, differentiation, and other gene expression pathways, elevate and suppress oxidative stress responses, and even reorganize cytoskeletal structures (Sarimov et al., 2023; Tian et al., 2024). Importantly, these responses are not uniform across different human cell types, as summarized in Figure 1. Instead, the magnitude, direction, and sensitivity of changes appear to depend strongly on baseline cellular phenotype and metabolic state (Sarimov et al., 2023; Tian et al., 2024). This variability underscores the importance of studying multiple human-derived models in parallel, as different tissues may experience distinct vulnerabilities during prolonged exposure to reduced magnetic environments such as those encountered in deep-space missions. To better understand the cellular basis of these effects, the following sections review findings from specific human cell models, highlighting how neuronal, developmental, connective tissue, and cancer-derived lines each reveal distinct pathways of magnetic field sensitivity.
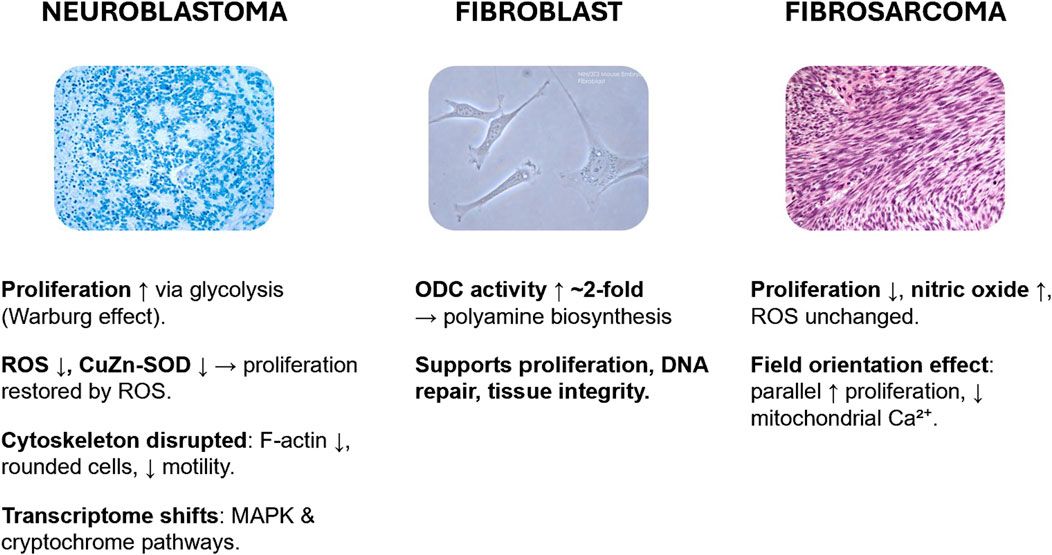
Figure 1. HMF effects on human cell lines. In SH-SY5Y neuroblastoma cells, HMF increases proliferation via glycolytic reprogramming, reduces ROS and antioxidant activity, disrupts cytoskeleton, and alters MAPK/cryptochrome signaling. In dermal fibroblasts, HMF elevates ornithine decarboxylase activity, enhancing polyamine metabolism with implications for proliferation and tissue repair. In HT-1080 fibrosarcoma cells, HMF reduces proliferation, elevates nitric oxide, and produces orientation-dependent responses in growth and mitochondrial calcium. Images used from SubtleGuest (2007) and Nephron (2009).
2.1 SH-SY5Y human neuroblastoma
Exposure of human neuroblastoma SH-SY5Y cells to HMF induces a range of metabolic, proliferative, structural, and transcriptomic changes that together highlight the cellular sensitivity of neuronal models to altered magnetic environments. One of the most consistent findings is an increase in cell proliferation, which has been closely associated with a metabolic shift toward anaerobic glycolysis, reminiscent of the Warburg effect. This is evidenced by elevated glucose consumption and enhanced lactate dehydrogenase (LDH) activity, with the proliferative response strongly dependent on glucose availability, suggesting that HMF-driven metabolic reprogramming is substrate-sensitive (Wang et al., 2022).
The redox state within the cell appears to be a critical mediator of HMF responses as multiple redox molecules are direct downstream byproducts of glycolysis pathways within the cell. HMF exposure reduces intracellular levels of reactive oxygen species (ROS), including hydrogen peroxide and superoxide anion, which coincides with a decrease in activity and accelerated denaturation of the key antioxidant enzyme CuZn-superoxide dismutase (CuZn-SOD). Importantly, restoring ROS levels with exogenous hydrogen peroxide reverses the proliferation-promoting effect, underscoring that altered redox balance is central to the HMF-induced phenotype (Zhang et al., 2017).
Structural changes are also evident, particularly in the cytoskeleton. HMF disrupts actin assembly, leading to reduced F-actin content, fewer cellular processes, and a more rounded morphology. These alterations diminish cell adhesion and migration, in line with transcriptional downregulation of genes linked to cytoskeletal dynamics and motility (Mo et al., 2016). Such changes may have broader implications for neuronal development, synaptic connectivity, and migration under altered geomagnetic conditions.
At the transcriptional level, large-scale transcriptome profiling has identified over 2,400 differentially expressed genes following HMF exposure. These genes cluster in pathways associated with macromolecule localization, protein transport, RNA processing, and brain function. In particular, the MAPK pathway and cryptochrome signaling have been implicated as potential early mediators of the cellular response to magnetic field depletion, linking HMF effects to core processes of neuronal signaling and circadian regulation (Mo et al., 2014).
2.2 Human dermal fibroblast
HMF exposure has been shown to modulate fundamental enzymatic activity in human fibroblasts, a non-cancerous model relevant to connective tissue function. Notably, HMF significantly increases the activity of ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis, producing approximately a two-fold elevation at key field strengths. ODC plays a critical role in polyamine-mediated cell proliferation, DNA stabilization, and repair, indicating that HMF may directly influence cellular growth dynamics in non-neuronal, non-malignant human cells (Bajtoš et al., 2024).
This finding suggests that even cells typically considered quiescent or non-transformed retain sensitivity to magnetic cues at the metabolic level. Polyamine production, via ODC upregulation, could enhance fibroblast proliferation and influence wound healing or tissue integrity under reduced geomagnetic conditions. However, the underlying processes connecting HMF exposure to ODC activation remain unclear.
2.3 HT-1080 human fibrosarcoma
In the context of long-term space missions, including lunar and Martian colonization, the study of cancer cell models under HMF conditions provides important insights into how altered magnetic environments may influence disease biology. HT-1080 fibrosarcoma cells, widely used as an experimental model for connective tissue cancer, have recently been examined to assess their responses to magnetic field depletion.
Exposure to HMF has been shown to reduce cellular growth rates in HT-1080 cultures, while selectively altering redox balance. In particular, cellular nitric oxide levels increase significantly, whereas other ROS remain largely unchanged relative to cultures maintained under normal geomagnetic field conditions (Gurhan et al., 2021). These findings suggest that HMF influences signaling pathways linked to nitric oxide metabolism more strongly than general oxidative stress.
Interestingly, the orientation of the static magnetic field relative to the cell layer exerts an additional layer of control. When field lines are aligned parallel to the culture plane, HT-1080 cells exhibit enhanced proliferation and reduced mitochondrial calcium concentrations compared to perpendicular or oblique orientations (45°) at the same field strength (Gurhan et al., 2021). This highlights not only the sensitivity of tumor cells to magnetic field intensity but also to vector orientation, pointing to anisotropic cellular responses to magnetic environments.
3 Effects of hypomagnetic fields on microorganism and microbiome
The human body is host to trillions of microorganisms and relies heavily on the microbiomes in the intestines, skin, and airways to maintain overall health. Both microgravity and HMF significantly influence microbial physiology, bacterial growth rates, motility, and metabolism which indicates the potential for bodily disfunction under such conditions (Sarimov et al., 2023; Tian et al., 2024). Therefore, it is crucial to preserve the natural function of microbiomes on human spaceflight missions to reduce antibiotic resistance, preserve microbial physiology, and maintain magnetic and structural sensing capabilities to ensure immune health in astronauts.
Research on microbiome exposure to HMF remains largely unexplored with an underwhelming body of work reported in the literature. However, microorganisms like Pseudomonas, E. coli, and Enterobacter, all commonly found in soil, water, and gut environments, exhibit significantly altered responses to common antibiotic medications. Depending on the specimen and medication, exposure to HMF below 500 nT, and as low as 40 nT, can either increase or decrease the minimum inhibitory concentration (MIC) of antibiotic required to prevent bacterial growth by as much as 90% (Poiata et al., 2003; Creanga et al., 2004).
Structural changes such as maximum relative viscosity in E. coli can also be affected by HMF, where fields at 30, 60, and 80 nT decreased viscosity by 18% while fields at 45, 70, and 95 nT increased viscosity by the same amount (Binhi et al., 2001). Magnetic sensing in magnetotactic microorganisms, such as Magnetospirillium magneticum, can also be affected by HMFs. These microbes have evolved unique properties allowing for sensitivity to magnetic field environments. When exposed to HMF below 500 nT, these bacteria exhibited both downregulation and upregulation of genes related to production of magnetite nanoparticles needed for production of magnetosomes which was accompanied by an overall decrease in the size of magnetosomes (Wang et al., 2008).
More recently, microbial viability and abundance was investigated on various surfaces of the China Space Station (CSS) following a low-earth orbit of about 6 months. In total, 103 types of bacteria were found on board with dominant bacteria strains such as Pseudomonas, Stenotrophomonas, Bacillus, and Staphylococcus. Three billion colony-forming units of culturable bacteria were found per 100 square centimeters with an average viability of 65%, showing that various types of microorganisms can persist for long periods of time in HMF conditions. It is important to note that just over half of the microorganisms collected from the CSS have human origins. This provides insight into how microbiome ecosystems are generated in HMF conditions and the potential hazards associated with astronaut health in space (Zhang et al., 2025).
Microgravity and HMF environments clearly influence microbial systems but the effect of these changes within the human body remains largely unknown. Further research on HMF interactions with microbiomes is required to fully understand how to properly treat sickness, limit changes in gene expression, and maintain natural microorganism health in astronauts during deep-space missions.
4 Implications for human spaceflight and deep-space mission
4.1 Skeletal fragility and space-induced osteoporosis
Skeletal fragility has long been recognized as a central health risk for astronauts, traditionally attributed to microgravity-induced unloading of the musculoskeletal system. However, the severity of bone loss observed in spaceflight often exceeds Earth-based analogues such as disuse osteoporosis, suggesting that additional mechanisms contribute to skeletal decline (Vico et al., 2000; LeBlanc et al., 2007). Emerging evidence indicates that HMFs exacerbate microgravity-induced bone loss and impair post-mission bone recovery (Yang et al., 2018; Xue et al., 2019). Rodent models exposed to HMF conditions exhibit reduced bone mineral density, thinner trabeculae, and decreased biomechanical strength compared with controls maintained under geomagnetic fields (Yang et al., 2018; Xue et al., 2019). Mechanistic studies further suggest that HMF exposure is associated with iron overload and altered redox balance, which compromise bone microstructure and reduce resistance to mechanical stress (Yang et al., 2018; Xue et al., 2019). These findings suggest that the absence of geomagnetic cues not only accelerates skeletal degeneration in space but also undermines the potential for recovery after returning to Earth.
4.2 Neurocognitive function
In addition to musculoskeletal health, neurocognitive function is increasingly recognized as a vulnerable target of hypomagnetic exposure. HMF conditions can impair neurogenesis and are consistently associated with elevated oxidative stress and altered circadian gene expression in neuronal and stem cell models (Blaber et al., 2015; Zhang et al., 2021; Luo et al., 2022). For astronauts, these effects are especially concerning given the intense and tightly scheduled operational demands of long-duration missions. Cognitive performance, memory retention, and rapid decision-making under stress are mission critical. Furthermore, circadian rhythm disruption, potentially exacerbated by both HMF and altered light–dark cycles in orbit, can further contributes to sleep loss and mood disturbances. Such impairments not only reduce individual performance but also compromise group cohesion and teamwork, directly affecting the execution of highly technical and cooperative tasks essential for spacecraft operations and planetary exploration.
4.3 Immune and metabolic systems
The immune and metabolic systems represent another axis of vulnerability to HMF conditions. Changes in microbial health, motility, and growth rates due to microgravity conditions have critical implications for infection risk in closed spacecraft environments and for the stability of bioregenerative life support systems. Increased virulence of opportunistic pathogens, together with the reactivation of latent viruses such as herpesviruses observed in astronauts, pose a heightened risk during long-duration missions (Rooney et al., 2019; Acres et al., 2021; Mehta et al., 2022). The combined stress of microgravity, radiation, and HMF may therefore synergistically weaken host defenses while enhancing microbial aggressiveness, creating a dual challenge to astronaut health and mission sustainability.
5 Mitigation strategies and future directions
5.1 Restoration of the geomagnetic field around space habitats
Among the proposed strategies, as summarized in Figure 2, the restoration or correction of a geomagnetic field within spacecraft or planetary habitats represents the most direct approach to mitigating HMF effects. In principle, artificial generation of a stable magnetic field provides the simplest conceptual solution, re-establishing environmental cues that human biology and microbial ecosystems have evolved under.
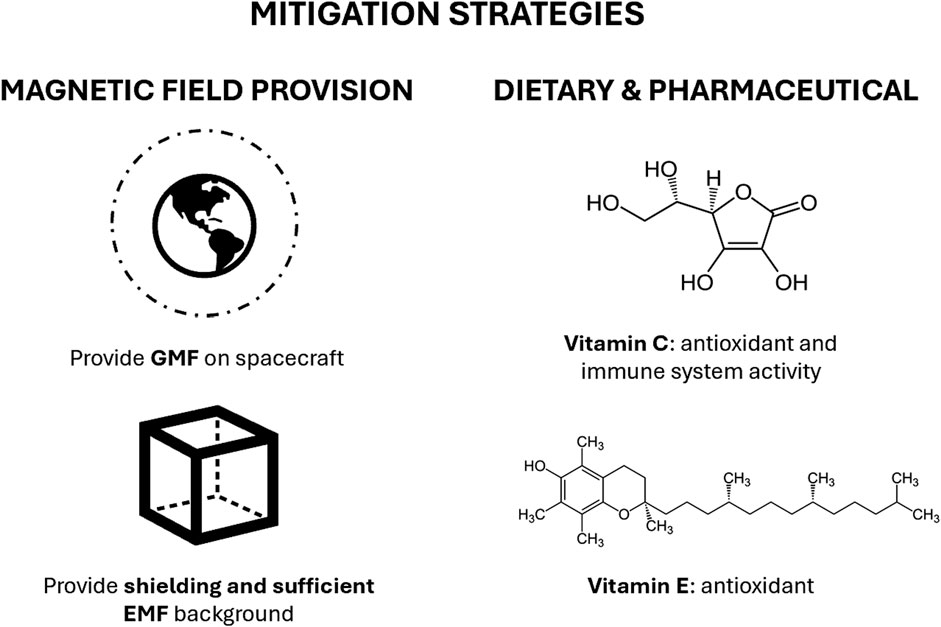
Figure 2. Mitigation strategies for hypomagnetic field (HMF) effects in spaceflight. Restoration of a geomagnetic-like field within spacecraft or planetary habitats offers the most direct approach but is limited by mass, power, and volume constraints, as well as the need to define minimum exposure parameters and avoid interference with instrumentation. Complementary pharmaceutical and nutritional countermeasures—such as antioxidant supplementation, dietary adjustments, or pharmacological modulation of mitochondrial and redox pathways—may help buffer oxidative stress.
However, the practical challenges are substantial. Any magnetic shielding or field-generation system must be designed within the strict constraints of spacecraft mass, power consumption, and volume, all of which are at a premium during launch and extended missions. Furthermore, the minimum exposure parameters necessary to achieve biological protection—field strength, spatial uniformity, daily duration of exposure, and threshold timescales—remain poorly defined. Until these parameters are empirically characterized, defining system requirements for spacecraft integration is not possible. Another consideration is the potential for artificial magnetic fields to interfere with sensitive onboard instrumentation and experiments, which places additional requirements on system tunability and shielding.
5.2 Pharmaceutical and nutritional countermeasures
With the limited mass, power, and volume available for space missions, implementation of GMF inside spacecraft and space station environments could be infeasible for specific mission profiles. In parallel with physical countermeasures to assist in mitigating HMF effects, pharmaceutical and nutritional strategies are being considered. Antioxidant supplementation or diet modification may buffer against the oxidative stress consistently observed under HMF conditions, though their application in spaceflight conditions is largely unknown. Targeted pharmacological interventions aimed at preserving mitochondrial function or modulating redox balance may also offer protection, but their efficacy has yet to be systematically validated in space-relevant conditions. Diet-based strategies, such as increasing intake of polyphenols or vitamins C and E, are attractive due to their feasibility, but detailed studies on dose, timing, and long-term safety under altered EMF conditions are lacking. Nevertheless, the efficacy and safety of these countermeasures are largely unexplored with respect to HMF conditions present during spaceflight.
5.3 Future direction and research gaps
Despite progress, major gaps remain in our understanding of mitigation strategies. Continued interdisciplinary research across biomedical and engineering sciences is essential to develop countermeasures against HMF effects, particularly for long-duration missions to other planetary bodies. The following sections outline several key research gaps that need to be prioritized. First and most importantly, the combined influence of multiple space stressors, including microgravity, HMF, ionizing radiation, isolation and confinement effects (ICE), circadian disruption, and psychological stress—must be systematically investigated, as these factors are likely to interact nonlinearly.
Second, the long-term effects of HMF on reproduction remain almost entirely unexplored. This knowledge gap is particularly relevant for both human health and microbial disease dynamics, since altered reproductive cycles in pathogens or commensal organisms could have significant implications for infection risk.
Third, further study of the human and animal microbiome under space-relevant magnetic environments is needed to better understand how altered host–microbe interactions may compromise astronaut health. Finally, the feasibility of engineering large-scale EMF provision systems for habitats, potentially leveraging superconducting coils, modular Helmholtz configurations, or hybrid shielding systems, represents a critical engineering challenge for the future of deep-space exploration. Ultimately, advancing interdisciplinary research at the interface of biomedicine and engineering is essential to overcome these critical knowledge gaps and to engineer the resilient systems needed for humanity’s enduring expansion into space.
Author contributions
ND: Conceptualization, Data curation, Project administration, Visualization, Writing – original draft, Writing – review and editing. JK: Data curation, Writing – original draft, Writing – review and editing. FB: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Ann and H.J. Smead Department of Aerospace Engineering Sciences and the Department of Electrical, Computer, and Energy Engineering at the University of Colorado Boulder, with additional partial support provided by Gene Freeman.
Acknowledgments
We greatly appreciate the contributions of Professor Mark Hernandez in supporting us with the equipment and lab space for the operation of the program. We would like to express their sincere gratitude to Gene Freeman for his generous support and encouragement, which contributed to the development of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Check for grammatical errors and provide feedback on writing styles.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acres, J. M., Youngapelian, M. J., and Nadeau, J. (2021). The influence of spaceflight and simulated microgravity on bacterial motility and chemotaxis. npj Microgravity 7 (1), 7. doi:10.1038/s41526-021-00135-x
Bajtoš, M., Kováčik, L., Bajtošová, M., and Kováčiková, L. (2024). Bioimpact of hypomagnetic fields. Lékař a Tech. - Clin. Technol. 54 (1), 12–22. doi:10.14311/CTJ.2024.1.02
Binhi, V. N., Alipov, Y. D., and Belyaev, I. Y. (2001). Effect of static magnetic field on E. coli cells and individual rotations of ion-protein complexes. Bioelectromagnetics 22, 79–86. doi:10.1002/1521-186x(200102)22:2<79::aid-bem1009>3.0.co;2-7
Blaber, E. A., Finkelstein, H., Dvorochkin, N., Sato, K. Y., Yousuf, R., Burns, B. P., et al. (2015). Microgravity reduces the differentiation and regenerative potential of embryonic stem cells. Stem cells Dev. 24 (22), 2605–2621. doi:10.1089/scd.2015.0218
Creanga, D. E., Poiata, A., Morariu, V. V., and Tupu, P. (2004). Zero-magnetic field effect in pathogen bacteria. J. Magnetism Magnetic Mater. 272-276, 2442–2444. doi:10.1016/j.jmmm.2003.12.853
Demontis, G. C., Germani, M. M., Caiani, E. G., Barravecchia, I., Passino, C., and Angelucci, A. (2017). Human pathophysiological adaptations to the space environment. Front. Physiology 8, 547. doi:10.3389/fphys.2017.00547
Gurhan, H., Bruzon, R., Kandala, S., Greenebaum, B., and Barnes, F. (2021). Effects induced by a weak static magnetic field of different intensities on HT-1080 fibrosarcoma cells. Bioelectromagnetics 42 (3), 212–223. doi:10.1002/bem.22332
Kashirina, D. N., Pastushkova, L. K., Brzhozovskiy, A. G., Kononikhin, A. S., Rusanov, V. B., Kukanov, V. Y., et al. (2023). A study on the protein composition of dry blood spots of healthy volunteers in an experiment with hypomagnetic conditions. Hum. Physiol. 49 (1), 77–87. doi:10.1134/s0362119722600369
LeBlanc, A., Schneider, V., Shackelford, L., West, S., Oganov, V., Bakulin, A., et al. (2000). Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. and Neuronal Interact. 1 (2), 157–160.
Luo, Y., Zhan, A., Fan, Y., and Tian, L. (2022). Effects of hypomagnetic field on adult hippocampal neurogenic niche and neurogenesis in mice. Front. Phys. 10, 1075198. doi:10.3389/fphy.2022.1075198
Markin, A. A., Zhuravleva, O. A., Zhuravleva, T. V., Kuzichkin, D. S., Markina, E. A., Polyakov, A. V., et al. (2023). Influence of the hypomagnetic environment on the metabolism and psychophysiological reactions of a healthy human. Hum. Physiol. 49 (6), 84–91. doi:10.31857/s013116462370042x
Mehta, S. K., Szpara, M. L., Rooney, B. V., Diak, D. M., Shipley, M. M., Renner, D. W., et al. (2022). Dermatitis during spaceflight associated with HSV-1 reactivation. Viruses 14 (4), 789. doi:10.3390/v14040789
Mo, W. C., Zhang, Z. J., Wang, D. L., Liu, Y., Bartlett, P. F., and He, R. Q. (2014). Transcriptome profile of human neuroblastoma cells in the hypomagnetic field. Sci. China Life Sci. 57 (3), 448–461. doi:10.1007/s11427-014-4644-z
Mo, W. C., Liu, Y., Bartlett, P. F., He, R. Q., and He, R. Q. (2016). Shielding of the geomagnetic field alters actin assembly and inhibits cell motility in human neuroblastoma cells. Sci. Rep. 6, 22624. doi:10.1038/srep22624
Nephron (2009). Nephron – own work. Available online at: https://commons.wikimedia.org/w/index.php?curid=15375809.
Poiata, A., Creanga, D. E., and Morariu, V. V. (2003). Life in zero magnetic field. V. E. coli resistance to antibiotics. Electromagn. Biol. Med. 22 (2,3), 171–182. doi:10.1081/jbc-120024626
Rooney, B. V., Crucian, B. E., Pierson, D. L., Laudenslager, M. L., and Mehta, S. K. (2019). Herpes virus reactivation in astronauts during spaceflight and its application on earth. Front. Microbiol. 10, 16. doi:10.3389/fmicb.2019.00016
Sarimov, R. M., Serov, D. A., and Gudkov, S. V. (2023). Hypomagnetic conditions and their biological action (Review). Biology 12 (12), 1513. doi:10.3390/biology12121513
Stevenson, D. J. (2003). Planetary magnetic fields. Earth Planet. Sci. Lett. 208 (1–2), 1–11. doi:10.1016/s0012-821x(02)01126-3
SubtleGuest (2007). SubtleGuest at English wikipedia. Available online at: https://commons.wikimedia.org/w/index.php?curid=19688924.
Tian, L., Ren, J., Luo, Y., Li, Y., Guo, W., Zhang, B., et al. (2024). Potential health risks of hypomagnetic field for manned deep-space explorations. Natl. Sci. Rev. 11, nwae395. doi:10.1093/nsr/nwae395
Vico, L., Collet, P., Guignandon, A., Lafage-Proust, M. H., Thomas, T., Rehaillia, M., et al. (2000). Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355 (9215), 1607–1611. doi:10.1016/s0140-6736(00)02217-0
Wang, X. K., Ma, Q. F., Jiang, W., Lv, J., Pan, W. D., Song, T., et al. (2008). Effects of hypomagnetic field on magnetosome formation ofMagnetospirillum MagneticumAMB-1. Geomicrobiol. J. 25 (6), 296–303. doi:10.1080/01490450802258295
Wang, G. M., Fu, J. P., Mo, W. C., Zhang, H. T., Liu, Y., and He, R. Q. (2022). Shielded geomagnetic field accelerates glucose consumption in human neuroblastoma cells by promoting anaerobic glycolysis. Biochem. Biophysical Res. Commun. 601, 101–108. doi:10.1016/j.bbrc.2022.01.114
Xue, Y., Yang, J., Luo, J., Ren, L., Shen, Y., Dong, D., et al. (2019). Disorder of iron metabolism inhibits the recovery of unloading-induced bone loss in hypomagnetic field. J. Bone Mineral Res. 34 (12), 2201–2213. doi:10.1002/jbmr.3949
Yang, J., Meng, X., Dong, D., Xue, Y., Chen, X., Wang, S., et al. (2018). Iron overload involved in the enhancement of unloading-induced bone loss by hypomagnetic field. Bone 114, 235–245. doi:10.1016/j.bone.2018.06.012
Zhang, H. T., Zhang, Z. J., Liu, Y., Liu, S. J., He, R. Q., Mo, W. C., et al. (2017). Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase. Protein and Cell 8 (7), 527–537. doi:10.1007/s13238-017-0403-9
Zhang, B., Wang, L., Zhan, A., Wang, M., Tian, L., Guo, W., et al. (2021). Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 12 (1), 1174. doi:10.1038/s41467-021-21468-x
Keywords: hypomagnetic field, human spaceflight, human cell line, microbiome, microorganism, microgravity
Citation: Dang N, Keller J and Barnes F (2025) Biological impacts of hypomagnetic fields in space environment: implications for artificial magnetic field provision in long-duration spaceflight. Front. Space Technol. 6:1704391. doi: 10.3389/frspt.2025.1704391
Received: 12 September 2025; Accepted: 09 October 2025;
Published: 31 October 2025.
Edited by:
Joseph N. Pelton, International Space University, United StatesReviewed by:
Onyinye Nwankwo, University of Michigan, United StatesCopyright © 2025 Dang, Keller and Barnes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nhat Dang, bmhhdC5kYW5nQGNvbG9yYWRvLmVkdQ==
 Nhat Dang
Nhat Dang Jason Keller1
Jason Keller1 Frank Barnes
Frank Barnes