- 1JSS Medical College and Hospital, JSS Academy of Higher Education and Research, Mysuru, India
- 2Division of Nanoscience and Technology, School of Life Sciences, JSS Academy of Higher Education and Research, Mysuru, India
- 3Division of Infectious Disease & Global Medicine, University of Florida, Gainesville, FL, United States
This case report highlights the management of recurrent urinary tract infections (UTIs) caused by multidrug-resistant (MDR) Pseudomonas aeruginosa in a post-renal transplant patient. Despite the challenges posed by antibiotic resistance, the patient was successfully treated with an extended infusion of meropenem, underscoring the efficacy of this approach in such difficult cases. The patient's recurrent infections required multiple hospitalizations and adjustments in treatment protocols, including the use of alternative antibiotics like fosfomycin and tailored immunosuppressive management to control both infection and rejection. This case is noteworthy for demonstrating the successful management of recurrent UTIs in the immunocompromised patient population, providing valuable insights into the treatment strategies that can be employed in similar clinical scenarios.
Introduction
Infections are a significant issue in kidney transplant recipients (KTRs) (1). Over 80% of patients experience a bacterial infection within the first year after transplantation. Immunosuppressive therapy, which is essential to prevent acute and chronic rejection, increases the risk of infectious complications (2). In the first month post-transplant, these infections are often related to surgical complications and include wound infections, urinary tract infections (UTIs), pneumonia, and sepsis (3). The most common pathogens are multidrug-resistant (MDR) bacteria, especially Gram-negative rods (4). Late infections, typically occurring in the following five months, are caused by opportunistic bacteria, highlighting the serious consequences of immunosuppression. “UTIs are the most frequent infection after kidney transplantation and may lead to bloodstream infections”, which are reported to worsen graft function and reduce patient survival (5).
The incidence of UTIs after hospital discharge is over 90% in renal transplant recipients. The insertion of a double J catheter and Foley catheter are major risk factors for UTIs shortly after kidney transplantation (6). Additionally, an extended period of hemodialysis before transplantation and prolonged bladder catheterization increases the risk of UTIs (7). The timing and pattern of infections are influenced by the selection of immunosuppressive agents as well as the choice and duration of prophylactic antimicrobial therapy (8).
UTIs after kidney transplantation are typically caused by Gram-negative pathogens, with Escherichia coli, Klebsiella pneumoniae, Proteus species, and Pseudomonas aeruginosa being the most commonly isolated (9). UTIs can become more severe when caused by P. aeruginosa, especially MDR strains. P. aeruginosa is a significant uropathogen responsible for complicated UTIs, which can lead to fatal sepsis in older patients or immunocompromised individuals with deteriorating general conditions, such as those with diabetes or undergoing steroid or anticancer chemotherapy treatments.
P. aeruginosa is a common cause of severe healthcare-associated invasive infections, particularly pneumonia, bloodstream infections (BSIs), and complicated UTIs (cUTIs) (10). “The World Health Organization (WHO) has identified carbapenem-resistant P. aeruginosa (CRPA) as one of the priority pathogens for research and the development of new antibiotics” (11). Infections with P. aeruginosa that have limited treatment options are frequently reported in Intensive Care Units (ICUs) and long-term acute care hospitals, likely due to the extensive use of antimicrobials, which promotes the selection of this microorganism (12). “P. aeruginosa is among the top six pathogens responsible for deaths associated with antimicrobial resistance, alongside third-generation cephalosporin-resistant E.coli, K.pneumoniae, and Methicillin-resistant Staphylococcus aureus (MRSA)” (13, 14).
Several molecular mechanisms; intrinsic, acquired, and adaptive contribute to the antimicrobial resistance of P. aeruginosa. In a single clinical isolate, multiple resistance mechanisms can often coexist (10). Although each mechanism is associated with a specific class of antibiotics, these mechanisms collectively mediate varying levels of resistance across different antibiotic classes (10). Key contributors to the MDR phenotypes of P. aeruginosa isolates include a deficiency of outer membrane porins, overproduction of active efflux pumps, AmpC β-lactamase, extended-spectrum β-lactamases (ESBL), and Carbapenamase, particularly mettalo-β-lactamase (MBL) (15). Here, we describe an MDR P. aeruginosa isolated in renal transplant recipients with recurrent UTI treated with prolonged infusion of meropenem and intravenous fosfomycin. Written consent was obtained from the patient's family member to present this case.
Case presentation
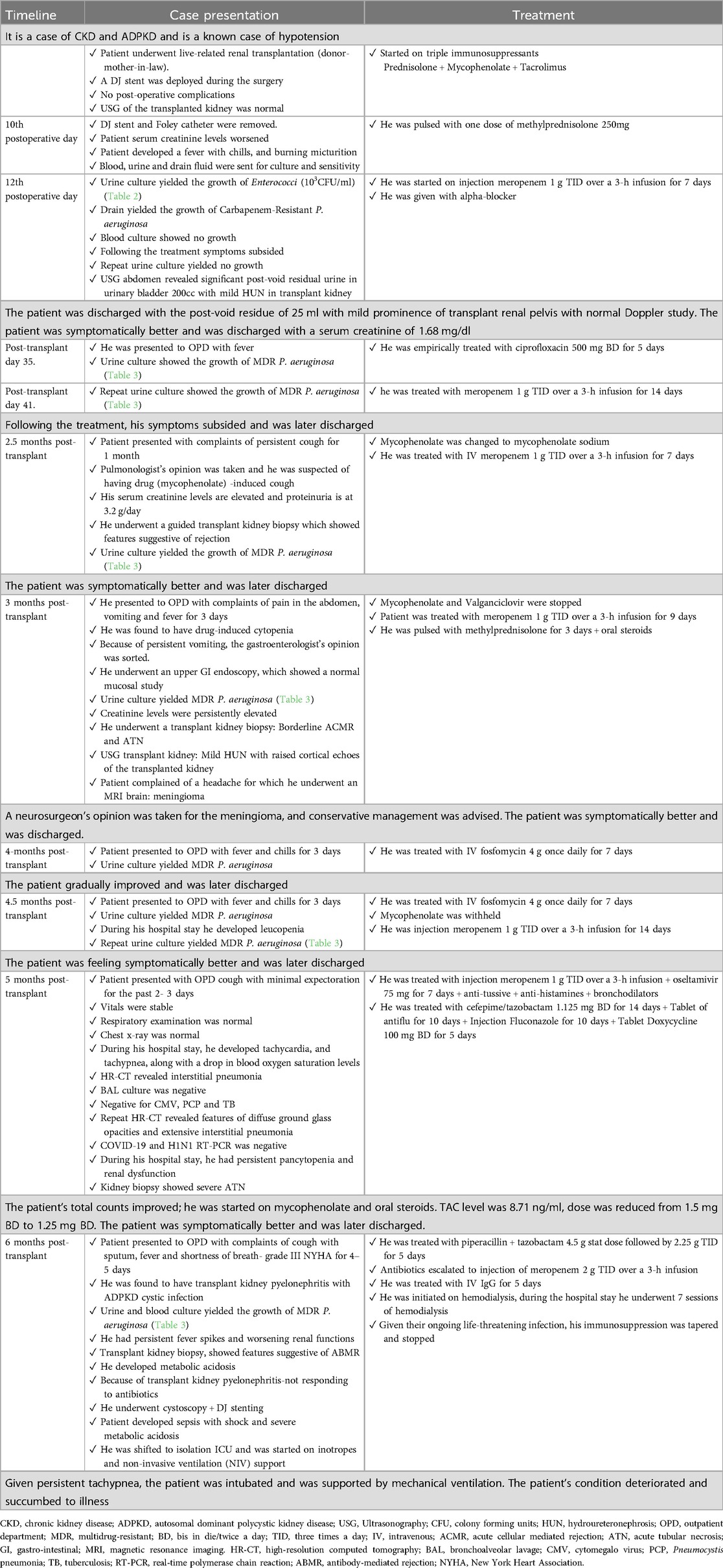
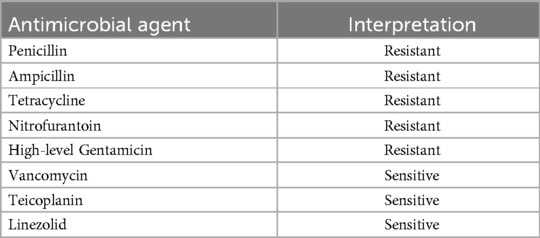
A 40-year-old male patient with a known history of hypertension, chronic kidney disease and autosomal dominant polycystic kidney disease (ADPKD) underwent live-related renal transplantation (donor-mother-in-law). A DJ stent was deployed during the surgery. Post-operatively, he was started on triple immunosuppressants (prednisolone + mycophenolate + tacrolimus). He had no post-operative complications. During the hospital stay, ultrasound sonography (USG) of the transplanted kidney was normal. His DJ stent and Foley catheter were removed on postoperative day 10 (refer to Table 1 for the treatment timeline). He was planned for discharge, but due to worsening serum creatinine levels, he was pulsed with one dose of methylprednisolone 250 mg. Following this, he developed a fever with chills and burning micturition. Blood, urine and drain fluid were sent for culture and sensitivity. Urine culture showed the growth of Enterococci (103 CFU/ml). The antimicrobial susceptibility pattern of Enterococci is given in Table 2. Drain culture showed the growth of CRPA (refer to Table 3 for antimicrobial susceptibility results), and the blood culture showed no growth. He was initiated on injection meropenem 1 g TID (Three times a day) over a 3-h infusion for 7 days, following which his symptoms subsided. Repeat urine culture showed no growth. Repeat USG abdomen showed significant post-void residual urine in the urinary bladder, 200cc, with mild hydroureteronephrosis (HUN) in the transplant kidney, following which an alpha-blocker was added to the patient. At the time of discharge, repeat USG (Ultrasonography) showed a post-void residue of 25 ml with mild prominence of transplant renal pelvis with normal Doppler study. The patient was symptomatically better and was discharged with a serum creatinine of 1.68 mg/dl.
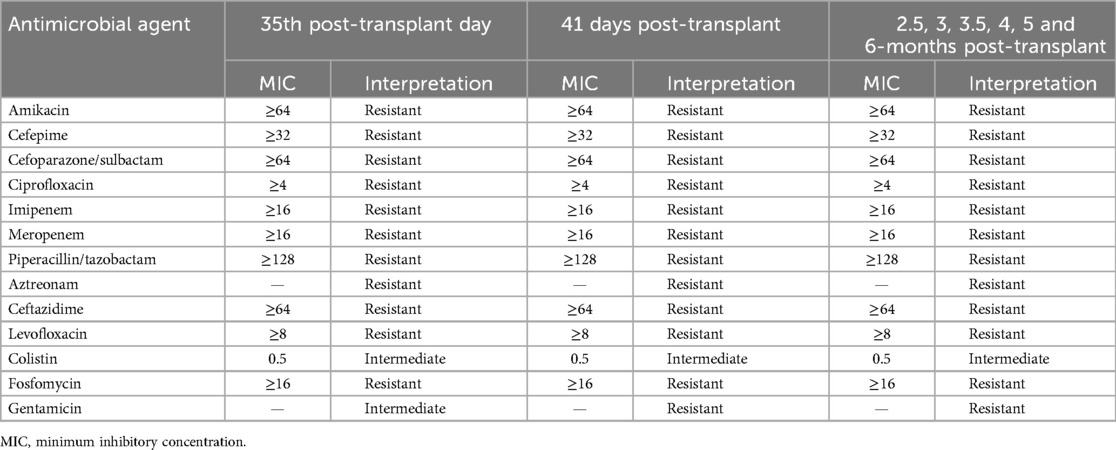
On the 35th post-transplant day, the patient visited for follow-up on an outpatient department basis, urine culture showed the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results) for which he was empirically treated with ciprofloxacin 500 mg BD (Bis in Die/Twice a day) for 5 days. A repeat urine culture after one week still showed the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results), for which he was treated with meropenem 1 g TID over a 3-h infusion for 14 days, following which his symptoms subsided and he was later discharged.
At 2.5 months post-transplant, he presented with complaints of a persistent cough for 1 month. Because of lower respiratory tract infection (LRTI), a pulmonologist's opinion was taken, and it was suspected of having drug (mycophenolate) -induced cough, given that mycophenolate was changed to mycophenolate sodium. He had persistently elevated creatinine levels and proteinuria at 3.2 g/day. Because of this, he underwent a guided transplant kidney biopsy, which showed features suggestive of rejection. Given plenty of pus cells in the urine routine, a urine culture was sent, which showed the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results). He was treated with IV (Intravenous) antibiotic meropenem 1 g TID over a 3-h infusion for 7 days. The patient was symptomatically better and was later discharged.
At 3 months post-transplant, he presented with complaints of abdominal pain and fever for 3 days. On evaluation, he was found to have drug-induced cytopenia. Given this, tablet mofiran and tablet valren were withheld. Because of persistent vomiting, a gastroenterology opinion was taken, and he underwent an upper GI endoscopy, which showed a normal mucosal study. A urine culture was sent, which showed the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results), for which, after discussion with a consulting microbiologist, the patient was started on meropenem 1 g TID over a 3-h infusion for 9 days. The patient had persistent rising creatinine levels, for which he was pulsed with methylprednisolone for 3 days, and oral steroids were restarted. Later, he underwent a kidney transplant biopsy, which revealed borderline acute cellular-mediated rejection (ACMR) and acute tubular necrosis (ATN). USG transplant kidney showed features of mild HUN with raised cortical echoes of the transplanted kidney, for which a urologist's opinion was taken and advice was followed. The patient complained of a headache, for which a neurologist's opinion was taken, and the patient underwent a magnetic resonance imaging (MRI) of the brain with contrast, which revealed meningioma, for which the neurosurgeon advised conservative management. The patient was symptomatically better and was discharged.
At 4 months post-transplant, he presented with complaints of fever with chills for 3 days. Urine culture showed the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results), the patient was admitted and was started on IV antibiotic fosfomycin 4 g once daily for 7 days. The patient gradually improved and was later discharged.
At 4.5 months post-transplant, the patient presented with complaints of fever with chills for 3 days. On evaluation, he was found to have a UTI. The urine culture showed the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results), and the patient was treated with an injection of 4 g of fosfomycin once daily for 12 days. During the hospital stay patient developed leucopenia, for which mycophenolate mofityl was withheld. A repeat urine culture was sent, which still showed the growth of MDR P. aeruginosa, sensitive to meropenem. He was then treated with an injection of meropenem 1 g TID over a 3-h infusion for 14 days. The patient was feeling symptomatically better and was later discharged.
At 5 months post-transplant, he presented with complaints of cough with minimal expectoration for the past 2–3 days, whitish colour sputum-not blood stained, non-foul smelling, nasal stuffiness and common cold for 2 days with no other complaints. On initial evaluation, the patient's vitals were stable, the respiratory examination was normal, the chest x-ray was normal, and he was treated with injection meropenem 1 g TID over a 3-h infusion and oseltamivir 75 mg for 7 days with antitussive, antihistamines and bronchodilators. But the patient later started developing tachycardia, tachypnea, along with a drop in blood oxygen saturation levels (on room air-SPO2 level: 83%, with 4 L of 02-SPO2 levels: 99%). High-resolution computed tomography (HRCT) chest was done, and was diagnosed with interstitial pneumonia. Bronchoscopy was done, and bronchoalveolar lavage (BAL) culture was sent, which was negative, even for cytomegalovirus syndrome (CMV), pneumocystis pneumonia (PCP) and tuberculosis (TB). Because of a persistent cough, a pulmonologist's opinion was taken, and repeat HRCT was done, which showed features of diffuse ground glass opacities and extensive interstitial pneumonia. Because of this, the sample was sent for COVID-19 and H1N1 RT-PCR, which were negative. During the hospital stay patient had persistent pancytopenia and renal dysfunction. The patient underwent a transplant kidney biopsy, which showed severe ATN. During the hospital stay, he was treated with IV antibiotics (injection of actamase 1.125 mg BD for 14 days, tablet antiflu for 10 days, injection of fluconazole for 10 days and tablet doxycycline 100 mg BD for 5 days). The patient's total counts improved; hence, he was started on mycophenolate mofitil and oral steroids. TAC level was 8.71 ng/ml, dose was reduced from 1.5 mg BD to 1.25 mg BD. The patient was symptomatically better and was later discharged.
At 6 months posttransplant, the patient presented with complaints of cough with sputum, fever and shortness of breath- grade III NYHA for 4–5 days. On evaluation, he was found to have transplant kidney pyelonephritis with ADPKD cystic infection, given which he was started on piperacillin + tazobactam 4.5 g stat dose followed by 2.25 g TID for 5 days. Later, because of urine and blood culture showing the growth of MDR P. aeruginosa (refer to Table 3 for antimicrobial susceptibility results), antibiotics were escalated to intravenous meropenem 2 g TID over a 3-h infusion. However patient had persistent fever spikes and worsening renal function, he underwent a transplant kidney biopsy, which showed features suggestive of antibody-mediated rejection (ABMR). The patient was treated with IV Ig G for 5 days [given ongoing urinary sepsis and cystic infection, plasma exchange (PLEX) was not possible]. However, the patient had worsening renal function and developed metabolic acidosis, hence, he was initiated on hemodialysis. During the hospital stay, he underwent 7 sessions of hemodialysis. Given the ongoing life-threatening infection, his immunosuppression was tapered and stopped (the risk of tapering immunosuppressants and graft dysfunction was explained to the attendees and the risk consent was taken and agreed to for the same). Because of a transplant kidney, pyelonephritis (not responding to IV antibiotics), a urology opinion was taken, and he underwent cystoscopy + DJ stenting. However, the patient developed sepsis with shock and severe metabolic acidosis, because of which he was shifted to the isolation ICU and was started on inotropes and non-invasive ventilation (NIV) support. Given persistent tachypnea, the patient was intubated and was supported by mechanical ventilation. The patient's condition deteriorated and succumbed to illness.
Discussion
KTRs are predisposed to UTIs due to a combination of immunosuppression and urinary tract structural modifications such as urethral catheters, double-J stents, or ureteral anastomosis. Because of increased infection susceptibility and consequences associated with immunosuppressive medication, any symptomatic UTI in these patients, whether it affects the lower or upper urinary tract, is classified as complicated (7).
P. aeruginosa is especially alarming in patients with complicated UTIs because it can cause high rates of treatment failure and major consequences like relapse and antibiotic resistance (16). These individuals frequently carry MDR bacteria, which makes treatment challenging. P. aeruginosa, which has a high inherent resistance, is associated with poor prognoses and has spread globally, hampering treatment efforts due to a paucity of effective medicines (17).
According to multicenter cohort research by Gomila et al., younger individuals are more prone to have MDR P. aeruginosa than older patients. This was most likely because younger patients are more likely to receive strong antibiotic, surgical treatments and are more frequently admitted to intensive care units, which increases the chance of acquiring MDR strains. The study also discovered that procedures involving the urinary tract were a significant risk factor for MDR P. aeruginosa, and patients with this pathogen had a higher readmission rate (18).
The Centers for Disease Control and Prevention (CDC) reported 32,600 cases of MDR P. aeruginosa infections in hospitalized patients in the United States in 2017, with 2,700 deaths (12). The Infectious Disease Society of America (IDSA) 2023 guidelines recommend ceftolozane-tazobactam, ceftazidime-avibactam, imipenem-cilastatin-relebactum, and cefiderocol as preferable treatments for pyelonephritis and complicated UTIs caused by MDR P. aeruginosa (19). Similarly, the ICMR 2022 guidelines advocate combination therapy for severe infections caused by CRPA, which is only sensitive in vitro to polymyxins, aminoglycosides, or fosfomycin (20).
The most frequently indicated antibiotics for treating MDR Gram-negative bacteria are carbapenems (e.g., meropenem), colistin, fosfomycin, tigecycline, and aminoglycosides. Carbapenems, including imipenem and meropenem, are the preferred treatments for ESBL infections (20). Following intravenous infusion, carbapenems diffuse broadly into multiple bodily fluids, increasing their potency against a wide range of infections (21).
Zhanel et al. found that intravenous (IV) fosfomycin effectively treated both Gram-negative and Gram-positive infections. The most prevalent Gram-negative infections treated with IV fosfomycin were carbapenem-resistant Enterobacterials and MDR P. aeruginosa. Fosfomycin was primarily used as directed therapy because of resistance to first-line antimicrobial drugs, clinical failure of previous antimicrobial therapy, or side effects associated with previous antimicrobial medications. Despite its primary usage as a salvage treatment, IV fosfomycin was associated with rather good microbiological and clinical success rates (22).
Studies have suggested that fosfomycin has been associated with the treatment of MDR bacteria such as MDR P. aeruginosa, ESBL-producing Enterobacteriaceae, carbapenem-resistant Klebsiella pneumoniae (CRKP), vancomycin-resistant Enterococcus faecium (VRE), and MRSA. Another observational research by Zhanel et al. comprised 59 hospitalized patients who were given intravenous fosfomycin. Two patients were treated for complicated UTI caused by MDR P. aeruginosa, and both had clinical and microbiological cures (23).
“Due to the high level of intrinsic and acquired resistance among Pseudomonal isolates, higher doses or extended infusions of beta-lactams may be required to ensure early achievement of target concentrations and maximize the duration of drug concentration required to exceed the organism's minimum inhibitory concentration (MIC) in severe infections” (24). The IDSA and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines propose greater doses for resistant and severe illnesses compared to susceptible and mild infections (24). A Monte Carlo simulation analysis concluded that complicated P. aeruginosa infections should be treated with a 2 g extended infusion of meropenem every 8 h rather than the conventional dose of 1 g every 8 h (25). Taccone et al. published a case study of a 70-year-old patient with septic shock caused by extensively drug-resistant P. aeruginosa who was treated with an extended infusion of meropenem (2 g every 8 h) and had favourable outcomes (26).
Zhao et al. conducted a study to assess the pharmacokinetics and pharmacodynamics of meropenem in critically ill patients and to determine if prolonged injection time improves meropenem therapy. “Prolonged infusion duration was advantageous when the MIC is ≤4 mg/L, but not for patients with drug-resistant or severe infections (MIC > 4 mg/L) who require a greater therapeutic goal. Their findings imply that prolonged injection duration may not always improve the success of antimicrobial therapy” (25).
Paul et al. conducted two randomized controlled trials (RCTs) comparing intravenous fosfomycin to piperacillin-tazobactam and meropenem. The piperacillin-tazobactam trial included patients with complicated UTIs or acute pyelonephritis, whereas the meropenem trial included patients with bacteremia. Both studies found no significant difference in clinical or microbiological cure rates between intravenous fosfomycin and the comparator drugs, meropenem and piperacillin-tazobactam (27).
Despite initial stability, the patient developed recurrent MDR infections, mycophenolate drug-induced adverse effects, and chronic renal impairment. Managing these issues necessitated several hospital stays, changes in the dosage of immunosuppressive medicine, and intensive antibiotic treatments. Unfortunately, despite multiple interventions, the patient's conditions deteriorated succumbing to illness (Lower respiratory tract infection, DTR-Pseudomonas infection of the urinary tract and graft rejection).
Conclusion
This case study highlights the crucial need for close monitoring, early and appropriate antibiotic use in sufficient doses for sufficient duration and comprehensive management techniques to effectively combat reoccurring MDR infections in transplant patients. Though the standard guidelines for treatment for DTR-pseudomonas infection were followed, the patient succumbed due to multifactorial issues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethical Committee JSS Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SS: Data curation, Methodology, Writing – original draft, Writing – review & editing. MNS: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – review & editing. MS: Conceptualization, Data curation, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. YM: Data curation, Methodology, Writing – review & editing. RR: Data curation, Investigation, Methodology, Writing – review & editing. AS: Data curation, Writing – review & editing. VD: Data curation, Methodology, Supervision, Writing – review & editing. GK: Validation, Writing – review & editing. GM: Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by JSS AHER, Mysuru.
Acknowledgments
The authors would like to acknowledge the management of JSS AHER for permitting us to write the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Katenkamp D. CL Berry transplantation pathology a guide for practicing pathologists 1999 Springer Verlag New York and London 222 pages with 94 figures and 31 tables. Exp Toxicol Pathol. (2001) 52(6):522. doi: 10.1016/S0940-2993(01)80009-9
2. Veroux M, Giuffrida G, Corona D, Gagliano M, Scriffignano V, Vizcarra D, et al. Infective complications in renal allograft recipients: epidemiology and outcome. Transplant Proc. (2008) 40(6):1873–6. doi: 10.1016/j.transproceed.2008.05.065
3. Snydman DR. Epidemiology of infections after solid-organ transplantation. Clin Infect Dis. (2001) 33(Suppl 1):S5–8. doi: 10.1086/320897
4. Parasuraman R, Julian K. AST infectious diseases community of practice. Urinary tract infections in solid organ transplantation. Am J Transplant. (2013) 13:327–36. doi: 10.1111/ajt.12124
5. Daskalaki E, Koukoulaki M, Bakalis A, Papastamopoulos V, Belesiotou E, Perivolioti E, et al. Blood stream infections in renal transplant recipients: a single-center study. Transplant Proc. (2014) 46(9):3191–3. doi: 10.1016/j.transproceed.2014.10.033
6. Bonkat G, Rieken M, Siegel FP, Frei R, Steiger J, Gröschl I, et al. Microbial ureteral stent colonization in renal transplant recipients: frequency and influence on the short-time functional outcome. Transpl Infect Dis. (2012) 14(1):57–63. doi: 10.1111/j.1399-3062.2011.00671.x
7. Adamska Z, Karczewski M, Cichańska L, Więckowska B, Małkiewicz T, Mahadea D, et al. Bacterial infections in renal transplant recipients. Transplant Proc. (2015) 47(6):1808–12. doi: 10.1016/j.transproceed.2015.03.046
8. Gołębiewska J, Dębska-Ślizień A, Komarnicka J, Samet A, Rutkowski B. Urinary tract infections in renal transplant recipients. Transplant Proc. (2011) 43(8):2985–90. doi: 10.1016/j.transproceed.2011.07.010
9. Jamil S, Zafar MN, Siddiqui S, Ayub S. Recurrent urinary tract infections in renal transplant recipients: risk factors and outcomes in low-resource settings. Saudi J Kidney Dis Transpl. (2022) 33(6):761–73. doi: 10.4103/1319-2442.390256
10. Losito AR, Raffaelli F, Del Giacomo P, Tumbarello M. New drugs for the treatment of Pseudomonas aeruginosa infections with limited treatment options: a narrative review. Antibiotics. (2022) 11(5):579. doi: 10.3390/antibiotics11050579
11. World Health Organization. tWHO publishes the list of bacteria for which new antibiotics are urgently needed. (2017). Available online at: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (Accessed August 26, 2024).
12. CDC. Multidrug-resistant Pseudomonas aeruginosa | A.R. & Patient Safety Portal. (2019). Available online at: https://arpsp.cdc.gov/profile/antibiotic-resistance/mdr-pseudomonas-aeruginosa (Accessed August 26, 2024).
13. Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399(10325):629–55. doi: 10.1016/S0140-6736(21)02724-0
14. Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis. (2019) 19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4
15. Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. (2017) 7:39. doi: 10.3389/fcimb.2017.00039
16. Wood SJ, Kuzel TM, Shafikhani SH. Pseudomonas aeruginosa: infections, animal modeling, and therapeutics. Cells. (2023) 12(1):199. doi: 10.3390/cells12010199
17. Kothari A, Kherdekar R, Mago V, Uniyal M, Mamgain G, Kalia RB, et al. Age of antibiotic resistance in MDR/XDR clinical pathogen of Pseudomonas aeruginosa. Pharmaceuticals. (2023) 16(9):1230. doi: 10.3390/ph16091230
18. Gomila A, Carratalà J, Eliakim-Raz N, Shaw E, Wiegand I, Vallejo-Torres L, et al. Risk factors and prognosis of complicated urinary tract infections caused by Pseudomonas aeruginosa in hospitalized patients: a retrospective multicenter cohort study. Infect Drug Resist. (2018) 11:2571–81. doi: 10.2147/IDR.S185753
19. Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis. (2024):ciae403. doi: 10.1093/cid/ciae403
20. Indian Council of Medical Research. Treatment Guidelines for Antimicrobial Use in Common Syndromes. 2nd ed. New Delhi: Indian Council of Medical Research (2019). Available online at: https://www.icmr.gov.in/icmrobject/custom_data/pdf/resource-guidelines/Treatment_Guidelines_2019_Final.pdf (Accessed January 1, 2025).
21. Tsala M, Vourli S, Kotsakis S, Daikos GL, Tzouvelekis L, Zerva L, et al. Pharmacokinetic-pharmacodynamic modelling of meropenem against VIM-producing Klebsiella pneumoniae isolates: clinical implications. J Med Microbiol. (2016) 65(3):211–8. doi: 10.1099/jmm.0.000214
22. Pipitone G, Di Bella S, Maraolo AE, Granata G, Gatti M, Principe L, et al. Intravenous fosfomycin for systemic multidrug-resistant pseudomonas aeruginosa infections. Antibiotics. (2023) 12(12):1653. doi: 10.3390/antibiotics12121653
23. Zhanel G, Baxter M, Wong M, Mirzanejad Y, Lee A, Dhami R, et al. Real-life experience with IV fosfomycin in Canada: results from the Canadian LEadership on antimicrobial real-life usage (CLEAR) registry. J Glob Antimicrob Resist. (2023) 33:171–6. doi: 10.1016/j.jgar.2023.03.010
24. Zakhour J, Sharara SL, Hindy JR, Haddad SF, Kanj SS. Antimicrobial treatment of Pseudomonas aeruginosa severe sepsis. Antibiotics. (2022) 11(10):1432. doi: 10.3390/antibiotics11101432
25. Zhao YC, Zou Y, Xiao YW, Wang F, Zhang BK, Xiang DX, et al. Does prolonged infusion time really improve the efficacy of meropenem therapy? A prospective study in critically ill patients. Infect Dis Ther. (2022) 11:201–16. doi: 10.1007/s40121-021-00551-2
26. Taccone FS, Cotton F, Roisin S, Vincent JL, Jacobs F. Optimal meropenem concentrations to treat multidrug-resistant Pseudomonas aeruginosa septic shock. Antimicrob Agents Chemother. (2012) 56(4):2129–31. doi: 10.1128/AAC.06389-11
27. Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European Society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. (2022) 28(4):521–47. doi: 10.1016/j.cmi.2021.11.025
Keywords: Pseudomonas aeruginosa, urinary tract infection, immunocompromised conditions, multidrug resistant organism (MDRO), recurrent hospitalization, recurrent UTIs
Citation: Shettar SR, Sumana MN, Shetty MS, Maheshwarappa YD, Reddy RG, Srinivasan A, Dharan VP, Kalyatanda G and Megha GK (2025) Case Report: Persistent drug-resistant Pseudomonas aeruginosa infection in a young post-kidney transplant patient that proved fatal. Front. Transplant. 4:1500066. doi: 10.3389/frtra.2025.1500066
Received: 22 September 2024; Accepted: 23 June 2025;
Published: 14 July 2025.
Edited by:
Noha A. Hassuna, Minia University, EgyptReviewed by:
Raphaela Bento, Massachusetts General Hospital and Harvard Medical School, United StatesMohammed H. Karrar Alsharif, Prince Sattam Bin Abdulaziz University, Saudi Arabia
Copyright: © 2025 Shettar, Sumana, Shetty, Maheshwarappa, Reddy, Srinivasan, Dharan, Kalyatanda and Megha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahadevaiah Neelambike Sumana, bW5zdW1hbmFAanNzdW5pLmVkdS5pbg==
†These authors have contributed equally to this work and share second authorship
‡These authors have contributed equally to this work and share third authorship
§These authors have contributed equally to this work and share fourth authorship
 Supreeta R. Shettar
Supreeta R. Shettar Mahadevaiah Neelambike Sumana
Mahadevaiah Neelambike Sumana Manjunath S. Shetty1,†
Manjunath S. Shetty1,† Yogeesh D. Maheshwarappa
Yogeesh D. Maheshwarappa Raghukanth G Reddy
Raghukanth G Reddy Asha Srinivasan
Asha Srinivasan Vamshi P Dharan
Vamshi P Dharan Gautam Kalyatanda
Gautam Kalyatanda G. K. Megha
G. K. Megha