- Advanced Lung Disease and Transplant Program, Inova Heart and Vascular Institute, Inova Fairfax Hospital, Falls Church, VA, United States
Allograft dysfunction is a major limitation of survival in organ transplant recipients including those who have received lung transplantation. Early detection of allograft dysfunction is thus crucial to improve outcomes in these patients. However, there are several causes of allograft dysfunction with allograft infection and rejection being the two important causes. It is often difficult to distinguish between those causes as the presentation can be similar. Allograft rejection, especially antibody-mediated rejection (AMR) and chronic lung allograft dysfunction (CLAD) are often identified too late where progression has already occurred. Biomarkers like anti-HLA antibodies including donor-specific antibodies (DSA), donor-derived cell-free DNA (dd-cfDNA), immune cell function (ICF) assays and next-generation sequencing for microorganisms allow for early identification of allograft dysfunction as well as differentiate rejection from other processes such as infection. This in turn allows for early intervention and, ideally, improved long-term allograft outcomes. Greater evidence exists for these biomarkers in other solid organ transplantations including kidney and heart transplantation, but application to lung transplant recipients is increasing and seems equally promising. In this review, we evaluate existing evidence for using these biomarkers and share our center practice in utilizing a combination of these biomarkers post-transplantation to assess for allograft dysfunction.
Introduction
Lung transplantation is a life-saving therapy for many individuals with end-stage lung disease. However, the median survival after lung transplantation is only 6.5 years based on the cohort of transplant recipients from 1990 to 2015, far lower than other solid organ transplants including kidney and heart transplantation (1). Allograft dysfunction is a major cause of morbidity and mortality in lung transplant recipients (1). Between 30 days and one year after the transplant, graft failure accounts for 22.7% of deaths (1). Beyond one year, graft failure accounts for 40% of deaths (1). Historically, monitoring for rejection has been primarily accomplished via pulmonary function testing and clinical evaluation. Surveillance bronchoscopies with bronchoalveolar lavage (BAL) and transbronchial biopsies may be performed; however, there is no clear evidence that surveillance bronchoscopy is better for detecting acute rejection in comparison to clinically indicated bronchoscopy (2). In recent years, several biomarkers have become available for the evaluation of allograft function with mounting evidence, even in the absence of clinical features of rejection. These biomarkers can be utilized to quantify the net state of immunosuppression in patients and may help to describe the risk of allograft dysfunction.
Many biomarkers have been developed and evaluated in solid organ transplant recipients. These biomarkers include anti-human leukocyte antibodies (HLA) including those directed against donor organs and thus termed donor-specific antibodies (DSA), non-HLA antibodies, Torque tenovirus (TTV) testing, gene expression profiling (GEP), donor-derived cell-free deoxyribonucleic acid (dd-cfDNA), cell immune monitoring assays, and micro ribonucleic acid (RNA), among others (3–5). Some molecular monitoring tools, including DSA, dd-cfDNA, immune cell function (ICF) assays, TTV testing, tissue transcriptomics, microRNA evaluation from blood and BAL samples, exosomes, and methylation markers, have varying degrees in evidence in lung transplant recipients (6). Biomarkers with the most robust evidence are discussed below.
Donor-specific antibodies (DSA)
Acute antibody-mediated rejection (AMR) in lung transplantation is defined by the presence of four criteria, namely allograft dysfunction, the presence of DSAs, characteristics histopathologic findings, and deposition of complement factor 4d (C4d) on the capillary endothelium, in addition to ruling out other etiologies of graft dysfunction (7). Peripheral immunologic cells express HLA antigens, which are subdivided into Class I (HLA-A, B, C) and Class II (HLA-DR, DP, DQ) based on their structure and function (7). C4d detection can be performed using two immunopathologic assays-immunofluorescence and immunohistochemistry.
DSAs are central to the development of AMR through multiple mechanisms (Figure 1). These molecules can lead to complement activation through the classical pathway and cause an increase in immune response, endothelial cell necrosis and, ultimately, graft damage (8). Interaction between these HLAs and non-HLA antigens on cell surface as well as antibody-mediated pathway activation can lead to natural killer cell activation, further leading to graft damage (8). Leukocyte recruitment and endothelial activation also contribute to AMR (8). Measurement of DSAs has historically been performed utilizing donor-derived peripheral T-lymphocytes as surrogates to detect complement-dependent activations (9, 10). Advances in laboratory techniques now allow detection of DSA through flow cytometry independent of complement fixation (8, 9). Most recently, a bead-based immunoassay platform allows for enhanced sensitivity and specificity in the detection of these antibodies (8, 11). Measurement of circulating HLA antibody titer is important in the surveillance of graft function, especially in combination with evaluation for clinical dysfunction, histologic evidence of AMR, and C4d detection (7). While studies have attempted to categorize patients with AMR based on DSA positivity (12), controversy remains on how to utilize DSA as a sole marker of rejection, especially in clinically stable patients (7).
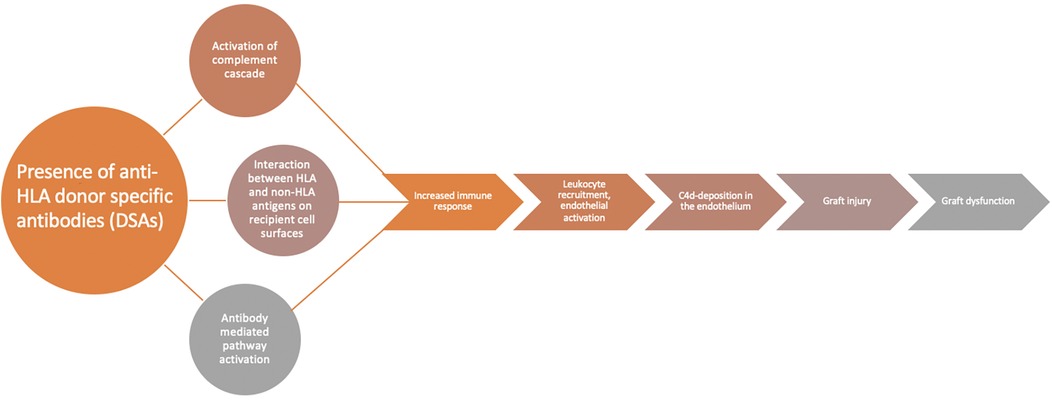
Figure 1. DSAs and AMR. Flow diagram demonstrating the role of DSAs in the development of AMR. The presence of DSAs leads to activation of complement cascade, the interaction between HLA and non-HLA antigens, and antibody-mediated activation which all lead to increased immune response, leukocyte recruitment, endothelial activation, and ultimately result in CD4 deposits in the allograft, graft injury, and graft dysfunction. Adapted with permission from “Diagnostic criteria for AMR” and “Mechanism of AMR” by Shourjo Chakravorty, Shambhu Aryal Adam Cochrane, and Steven D. Nathan licensed under CC-BY 4.0.
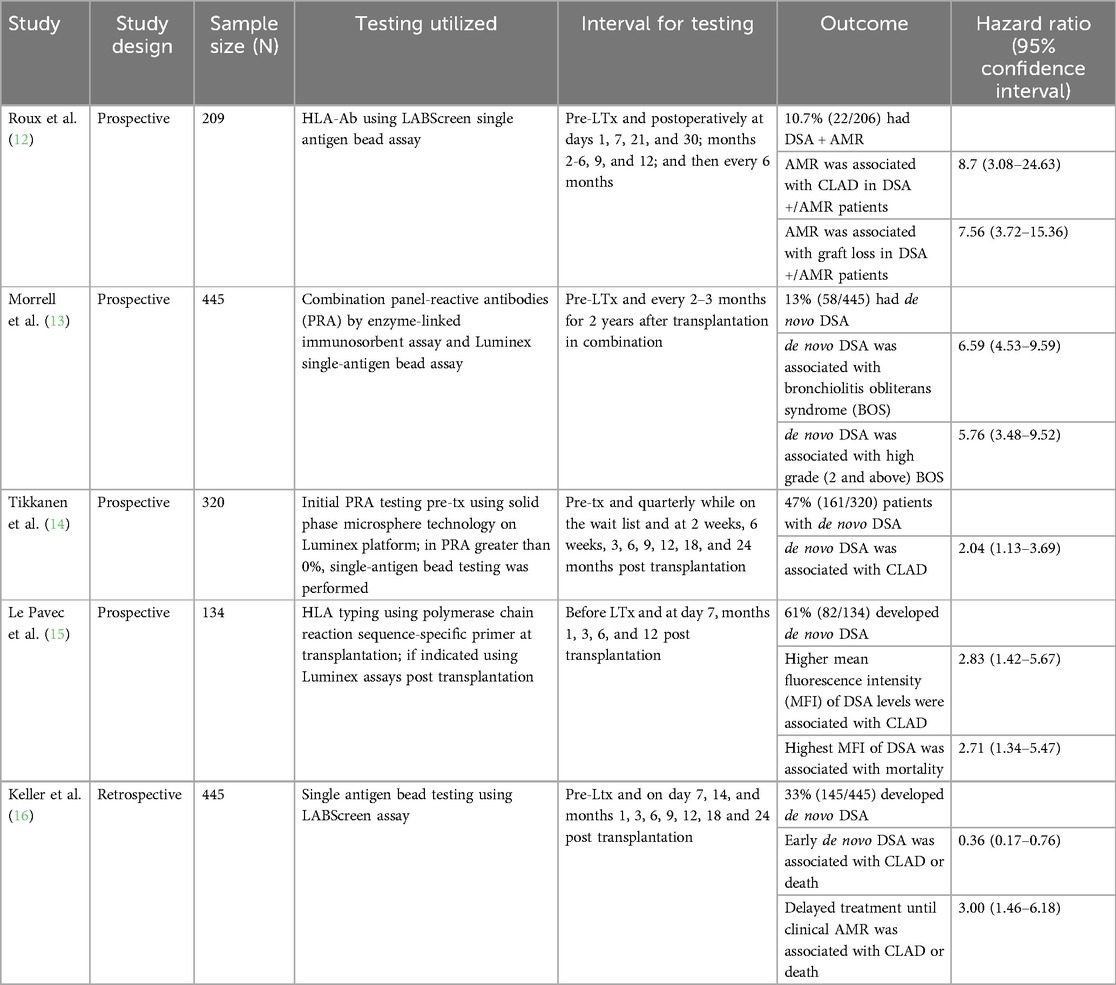
Donor-specific anti-HLA antibodies contribute to the development of antibody-mediated rejection (AMR) in lung transplant recipients (7, 12) (Table 1), though the assays used to measure DSAs and the intervals at which DSAs are monitored vary from study to study. DSAs have also been associated with CLAD (12–15), especially development of de novo HLA-DQ DSA (14). Development of de novo DSA has been reported to occur in 13%–61% of lung transplant recipients (13–15). The development of clinical AMR is often a late phenomenon, and poor outcomes of AMR despite treatment is probably due to delayed intervention. As such, preemptive treatment of de novo DSA may have a role in lung transplant recipients. In a study of 445 patients, 145 of whom developed de novo DSA after transplantation (including re-do transplantation), early treatment of de novo DSA was associated with a decreased risk of CLAD or death (HR: 0.36, p < 0.01) (16). Deferring treatment until the patient clinically developed AMR was associated with an increased risk of CLAD or death (HR: 3.00, p < 0.01) (16). Early treatment was defined as preemptive antibody-directed therapy (including intravenous immune globulin, rituximab, plasma exchange, proteasome inhibitor and/or steroids) based only on the positive DSA (16). In fact, treating molecular AMR as defined by DSAs with elevation in other biomarkers like the dd-cfDNA may improve outcomes, and this is an area of active research. Studies of DSAs in lung transplant recipients vary widely in terms of lack of standardization of DSA testing, including difference in intervals at which DSAs are routinely monitored, particularly longer term. The interpretation of histologic AMR varies by institution, and sampling bias during transbronchial biopsy may limit the identification of AMR or C4d positivity on tissue samples. Generally, data is derived from retrospective or cohort studies, and randomized clinical trials are not available to guide assessment or treatment approaches.
DSA characteristics may also provide prognostic value. Patients with AMR have increased frequency of anti-HLA DQ-specific DSA and increased sum mean fluorescence intensity compared to patients without AMR (17). Persistent DSA and DQ-specific DSAs are associated with shorter time to chronic lung allograft dysfunction (CLAD) and decreased CLAD-free survival (18). Patients who developed C1q + DSAs also had shorter time to CLAD, and those with multiple DSAs had decreased CLAD-free survival (18). Further, HLA mismatch may have prognostic value in the development of primary graft dysfunction and the severity. Using high-resolution HLA matching, a study in 59 lung transplant recipients found that as the number of HLA antigen mismatch increased, including allele-level mismatch, the severity of primary graft dysfunction also increased (19). Higher HLA-DQ mismatch grade was significantly associated with severe primary graft dysfunction (19). In a retrospective cohort analysis of 128 lung transplant patients, HLA compatibility scores were calculated for B-cell epitopes, T-cell epitopes, and missing self-induced NK cell activation (20). Higher HLA compatibility scores for B-cell and T-cell epitopes were associated with more rapidly developing anti-HLA-DQ antibodies, and the HLA compatibility score of B-cell epitopes for HLA-DQ was significantly associated with worse survival (20).
Non-HLA antibodies
Non-HLA antibodies have also been associated with increased risk of allograft dysfunction. These are alloantibodies directed against polymorphic antigens that vary between the donor and the recipient and autoantibodies (21). These self-antigens can develop against various receptors including collagen V, angiotensin type 1 receptor, and endothelin type A receptor (21). The exposure to these self-antigens is associated ultimately with loss of peripheral tolerance, which may lead to allograft dysfunction and rejection (21). In lung transplant patients with AMR without DSAs, non-HLA-positive antibodies are significantly higher than in patients without AMR (22). Non-HLA antibodies are also associated with an increased risk of CLAD (23), and the presence of DSA and non-HLA antibodies concurrently increases the CLAD risk further (23). In other solid organ transplantation, non-HLA antibody detection is typically performed through cell-based crossmatching assays or antigen detection methods such as enzyme-linked immunosorbent assay use, but genome-wide analyses and protein microarrays are also available (24). Nevertheless, screening for non-HLA antibodies is still not routinely performed in clinical practice at this time, and further efforts must be made to understand the impact of non-HLA antibodies in larger cohorts and standardize approach to testing (24).
Dd-cfDNA
dd-cfDNA is a noninvasive measure of nucleosomes that are released into the bloodstream as cfDNA, which occur during graft injury, regardless of the underlying cause (25). These dd-cfDNA are markers of cell apoptosis and necrosis (26). Quantification of dd-cfDNA analyzes single-nucleotide polymorphisms to distinguish between donor and recipient molecules (27). Once the single-nucleotide polymorphisms are identified as related to the donor or recipient, the amount of cfDNA that exists is amplified with whole genome sequencing, quantitative polymerase chain reaction, or targeted sequencing, and typically, the percent dd-cfDNA over total cfDNA is reported, though some assays may report the absolute amount of dd-cfDNA as well (26). A retrospective case series (28) and prospective observational cohort study (29) of kidney transplant recipients suggests that absolute quantification of dd-cfDNA is better in discriminating biopsy-proven rejection compared to relative dd-cfDNA levels. However, a larger, prospective, biopsy-matched study of 367 kidney transplant patients suggests that using a combination of dd-cfDNA fraction (percent of total cfDNA) and quantity (genomic copies/ml), rather than either alone, is better to detect rejection, with a 73.5% sensitivity and 80.8% specificity (30). Use of dd-cfDNA in other solid organ transplantation has more evidence, particularly in kidney transplantation. In kidney transplantation, the European Society of Organ Transplantation recommends dd-cfDNA measurement in patients with allograft dysfunction to exclude rejection, particularly AMR, with a moderate strength based on a moderate quality of evidence (31). In heart transplantation, the 2023 International Society of Heart and Lung Transplant guidelines for the care of heart transplant recipients include the use of GEP (AlloMap® test), a plasma-based test for 11 genes associated with immune activation and inflammation (5), for surveillance of rejection (4).
Natural progression of dd-cfDNA
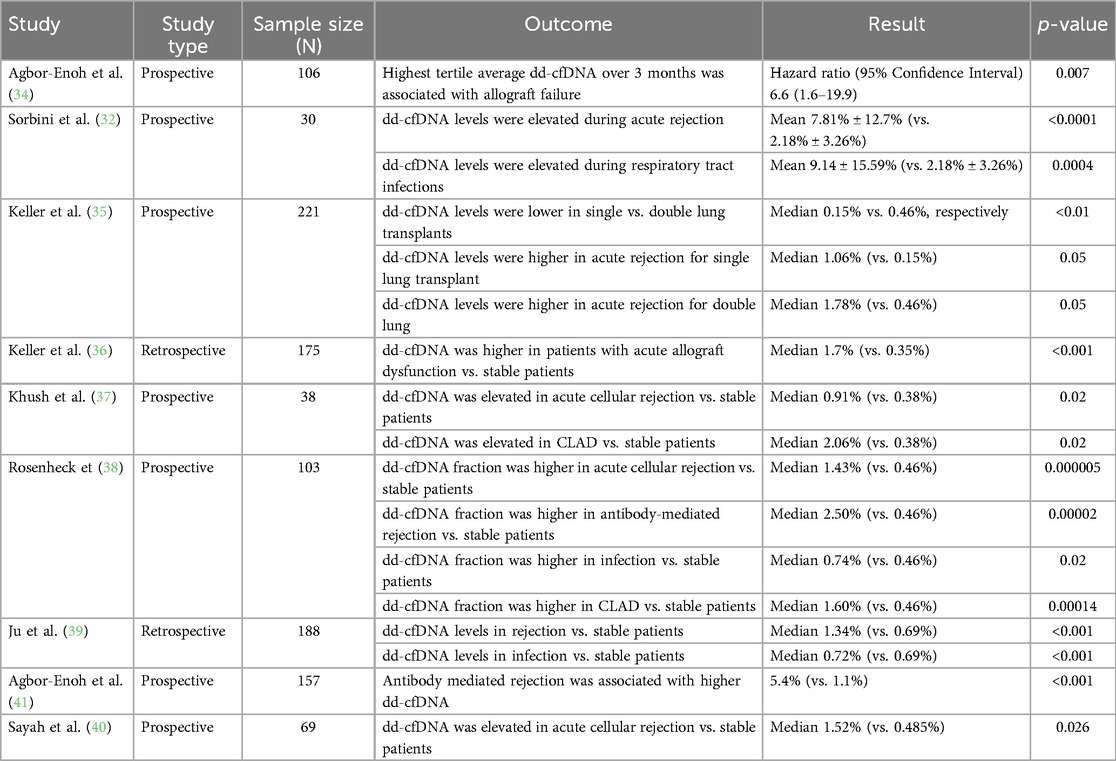
The natural progression of dd-cfDNA is a stepwise decay over time in stable lung transplant recipients (32, 33). These levels are higher than those observed in heart or kidney transplant recipients. During the first two weeks after transplantation, dd-cfDNA levels increase due to ischemic-reperfusion injury (median 6.36%) (32). These levels stabilize thereafter but increase again during acute rejection (7.81%) and respiratory infections (9.14%) (32). Average dd-cfDNA levels vary over the first three months post-transplantation and follow three patterns of decay, divided into tertiles (34). Immediately after the transplant, all groups had high dd-cfDNA (34). Subjects in the lowest tertile had a rapid decline to a low level within one month of transplantation (34). Those in the middle tertile had a slow decline initially but, by three months, reached a stable level comparable to the lowest tertile (34). Subjects in the higher tertile showed a slower decay with persistent elevations in dd-cfDNA levels compared to the lower and middle tertile groups (34). An average dd-cfDNA increase of 1% was associated with a 1.4-fold increased risk of allograft failure (34). Those with higher tertile levels had a 6.6-fold increased risk of developing allograft failure compared to those in the low tertile, with a median time to developing allograft failure of 25 months compared to 45 months in the low tertile (34).
Several studies suggest lower baseline dd-cfDNA levels in single-lung recipients compared to double-lung recipients (32, 35). Dd-cfDNA level in one cohort of stable patients was 2.8% in single-lung recipients, 6.2% in double-lung recipients, and 13.3% in combined heart-lung recipients (32). In another cohort, dd-cfDNA levels were 0.15% in single-lung recipients compared to 0.46% in double-lung recipients (35). Discrepancies between the two fractions reported in these two studies stem from a difference in the method by which dd-cfDNA is measured in the varying assays. In acute rejection too, median dd-cfDNA levels vary, with median levels of 1.06% in single-lung recipients and 1.78% in double-lung recipients (35). The optimal thresholds of dd-cfDNA for the detection of acute rejection was 0.54% in single lung transplant and 1.1% in double-lung transplant recipients based on one study of 220 patients (35). The heterogeneity in the dd-cfDNA assays and reporting presents a challenge to the interpretation of the literature. However, in general, clinicians can expect a higher dd-cfDNA value in double-lung transplant recipients compared to single-lung recipients, though the difference in relative or absolute assay values is unclear.
dd-cfDNA for determining presence of rejection
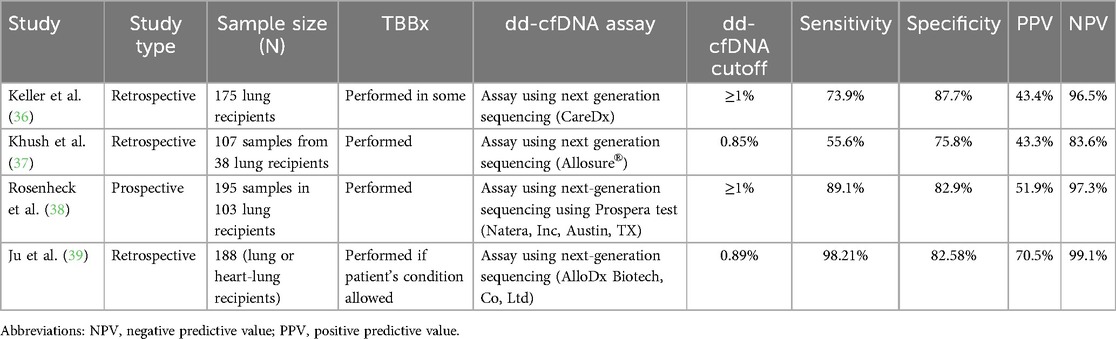
While the use of biomarker data to identify rejection in transplant recipients is promising, the evidence for the use of dd-cfDNA as a plasma biomarker of graft injury in lung transplantation is limited to retrospective and prospective cohort studies (Table 2). The most robust data comes from two cohorts, the single center Genome Transplant Dynamics study and the multicenter Genomic Research Alliance for Transplant (GRAfT) study of dd-cfDNA (34). Dd-cfDNA is inversely correlated with forced expiratory volume at one second (FEV1) (R = −0.26) (32). The level of dd-cfDNA that indicates graft injury varies from study to study in the literature, as do the sensitivities, specificities, positive predictive values, and negative predictive values (Table 3). Overall, most studies in the literature of lung transplantation recipients suggest an optimal dd-cfDNA cut-off varying between 0.85% and 1% (36–38, 39). When using a cut-off of ≥1% dd-cfDNA, sensitivities vary from 59.9 to 89.1%, specificities from 82.9 to 87.7%, positive predictive values of 43.4%–51.9%, and negative predictive values of 91.0%–96.5% (36, 38). Based on a study of 38 lung transplant recipients, using a threshold dd-cfDNA percent of 0.85% was associated with a sensitivity of 55.6%, specificity of 75.8%, a positive predictive value of 43.4%, and a negative predictive value of 83.6% to detect any allograft injury (37). The heterogeneity in these studies is important to note. The assays utilized to measure dd-cfDNA in these studies were varied, and it is difficult to know the comparison between assays. Further, not all cohorts performed transbronchial biopsies to confirm a diagnosis of ACR. Thus, an incorrect clinical diagnosis of ACR or a false negative biopsy may introduce bias.
The mean baseline dd-cfDNA levels in stable patients also vary. In general, evidence suggests that dd-cfDNA levels are higher in acute rejection compared to stable patients without rejection. In a retrospective study of 188 lung and heart-lung transplant recipients, patients with rejection had higher levels of dd-cfDNA (median level 1.34%) compared with stable patients (median level 0.69%, p < 0.001) (39). A multicenter, retrospective study of 175 patients similarly found that dd-cfDNA levels were higher in patients with acute lung allograft dysfunction, defined as a composite of acute rejection and infection, with a median level of 1.7% compared with a median level of 0.35% in stable patients, p < 0.001 (36). In a study utilizing archived biorepository plasma samples, dd-cfDNA levels were elevated (1.06%) in the aggregate cohort of rejection [ACR—including A1 ACR, AMR, and bronchiolitis obliterans syndrome (BOS)], compared to dd-cfDNA levels in stable patients (0.38%) (37). Dd-cfDNA levels in the subtypes of acute rejection are inconsistent in the literature. A biorepository study of 69 lung transplant patients identified dd-cfDNA of 1.52% in patients with ACR compared to 0.485% in stable patents, though the diagnosis of ACR in these patients was predominantly A2B0 and A1B2r rejection, so higher grade rejection is not represented in this data (40). Prospectively collected data from 195 samples in 103 patients evaluated dd-cfDNA levels in four clinical-pathologic diagnoses of rejection (ACR, AMR, CLAD/neutrophil responsive allograft dysfunction, isolated lymphocytic bronchiolitis, and infection) and demonstrated statistically significant differences in median dd-cfDNA fraction among the groups (38). Patients identified to have ACR, AMR, or infection had higher median dd-cfDNA levels compared to stable patients (38). The median dd-cfDNA level was higher for ACR (1.43%), AMR (2.50%), infection (0.74%), and CLAD/neutrophil-responsive allograft dysfunction (1.6%) compared to stable patients (38). Of note, in this study, ACR and AMR were both classified as acute rejection (38). A study of the GRAfT and GTD cohorts utilizing 2016 International Society of Heart and Lung Transplantation (ISHLT) consensus criteria to adjudicate AMR and the ISHLT histopathologic criteria to adjudicate found that levels of dd-cfDNA are higher in patients with AMR (5.4%) compared with ACR (1.1%) (41). Dd-cfDNA was similarly found to be elevated with AMR, with median levels of 1.34%, compared with stable patients (0.38%), in a study utilizing 107 archived biorepository plasma samples from 38 patients, though this small sample size study did not reach statistical significance (37). Dd-cfDNA was able to identify AMR a median of 2.8 months before a clinical diagnosis of AMR was possible (41). In CLAD, too, data suggests elevated dd-cfDNA, with a median dd-cfDNA of 2.06% (37).
dd-cfDNA and allograft infection
Differentiating infection from stable patients or acute rejection using dd-cfDNA is also difficult and adds uncertainty when utilizing dd-cfDNA in post-lung transplant monitoring. There was no statistically significant difference in dd-cfDNA levels in patients with allograft infection (median 0.39%) compared to stable patients in one study of 38 patients (37). A retrospective review of 188 lung transplant recipients attempted to utilize a combination of dd-cfDNA and next-generation sequencing for pathogen detection to differentiate between rejection and infection in patients who presented with new onset pulmonary complication. An elevated dd-cfDNA, combined with negative next-generation sequencing for pathogen results, was strongly indicative of rejection, with a sensitivity of 98.21%, specificity of 94.7%, positive predictive value of 88.7%, and negative predictive value of 99.2% (39). In contrast, elevated dd-cfDNA alone without next-generation sequencing for pathogen detection had a 98.21% sensitivity, 82.58% specificity, 70.5% positive predictive value, and 99.1% negative predictive value for diagnosing rejection (39). Patients with rejection had higher levels of dd-cfDNA, with a median level of 1.34%, compared to those with infection (median 0.72%, p < 0.001) (39). In this cohort, dd-cfDNA levels were significantly increased during infection compared to in stable patients (median 0.69%, p < 0.001) (39). Patients with CMV infection had significantly higher levels of dd-cfDNA compared to those with no infection (p < 0.001) (39).
Prognostic and clinical use of dd-cfDNA
Extreme elevations of dd-cfDNA may provide prognostic value. A multicenter prospective cohort study of 328 lung transplant recipients evaluated dd-cfDNA and found that extreme molecular injury, defined as extreme elevation in dd-cfDNA in the upper quartile range (≥5%) of all patients with acute rejection after 45 days post-transplant, was associated with increased risk of severe CLAD or death (HR: 2.78, p = 0.012) (42). The time at which there was first evidence of this extreme molecular injury was a significant predictor of the likelihood of CLAD or death (AUC = 0.856) (42).
The use of dd-cfDNA may reduce the need for routine bronchoscopies. and one study suggested an 82.1% reduction in bronchoscopies performed compared to expected (36).
Based on the existing body of evidence, the 2024 European Society for Organ Transplantation Consensus Statement weakly recommends the use of dd-cfDNA to diagnose clinical and subclinical acute rejection compared to standard diagnostic methods, based on a low level of evidence (43). The group also weakly recommends the use of dd-cfDNA to diagnose infection of and as a reliable marker to stratify prognosis for CLAD, based on very low levels of evidence (43). Notably, groups recommend weakly against the use of dd-cfDNA as a therapeutic marker to monitor treatment response for acute rejection or infection, based on very low evidence (43). Further evidence is still needed to identify whether fraction or absolute dd-cfDNA levels are more relevant in lung transplant recipients (43).
Next-generation sequencing for pathogens
Plasma microbial cell-free DNA sequencing uses next-generation sequencing to detect microbial cfDNA in the bloodstream that may not be identified by culture-based methods (44). The use of such next-generation sequencing tests for pathogens has been shown to increase diagnostic yield in immunocompromised patients (specifically, patients with hematologic malignancy who underwent hematopoietic cell transplantation (44). These tests have not been specifically validated in lung transplant recipient populations. Moreover, while the next-generation sequencing library is created and compared to an existing library of over 1,000 pathogens, this test has the potential to identify organisms that may not be pathogenic (5), and results must be interpreted within the clinical context.
Markers of immunity
The immune cell function (ICF) assay measures the adenosine triphosphate levels that are released by CD4+ T cells (45). This assay can be used to evaluate the cellular immune response in lung transplant recipients and potentially identify patients who may be at increased risk of developing rejection or infection (45). Median ICF values have been noted to be significantly different in cytomegalovirus (CMV) disease, viral infections, and bacterial infections compared with stable patients (46). ICF assay levels are lower in infected lung transplant recipients compared to non-infected patients (47). In retrospective studies of ICF assay in heart transplant recipients, levels <300 ng/ml identified patients at risk of CMV infection but noted that ICF assay levels were not significantly different in fungal or bacterial infections (48). TTV testing involves monitoring of TTV levels, which are small single-stranded DNA viruses that are ubiquitous in the majority of humans (49). Levels of viral replication are known to correlate with immune response (49, 50). TTV levels increase in response to immunosuppressive therapy, suggesting increased viral replication with the reduction of immunocompetence, and higher levels of posttransplant TTV are associated with increased microbial infections (49). Monitoring of Epstein–Barr virus (EBV) DNA loads has also been utilized as a measure of immunosuppression in lung transplant recipients and may help guide adjustments in immunosuppression (51).
Discussion
The use of routine bronchoscopy at specified intervals after luntr transplantation has been the standard of care for the identification of subclinical rejection. The availability of biomarkers holds significant promise for the future, though the literature remains largely retrospective, prospectively collected on biorepository samples, or with sample sizes. In general, there is consistentcy in the literature that supports the use of dd-cfDNA, combined with clinical evaluation and monitoring of other biomarkers, such as DSAs, for identification of rejection. However, the exact values of dd-cfDNA which indicate rejection, differentiation of the etiology of dd-cfDNA elevation, and differentiation of infection from acute rejection from chronic rejection, relies largely on the clinical context. Future research is needed to evaluate the diagnostic accuracy of dd-cfDNA in diagnosing acute rejection compared with the current gold standard of transbronchial biopsy. Additionally, the role of newer biomarkers such as non-HLA antigens and TTV in clinical practice is yet to be determine, and further real-world application is needed.
Our current practice
As noted above, evidence for the use of biomarkers in lung transplantation recipients is still accumulating, and there are no clear guidelines yet. However, we are gathering more experience through the use of biomarkers in clinical practice. We share our current approach, summarized in Figure 2. It should be noted that the use of the biomarkers is often individualized and requires careful review of clinical, spirometric, radiologic, and functional data.
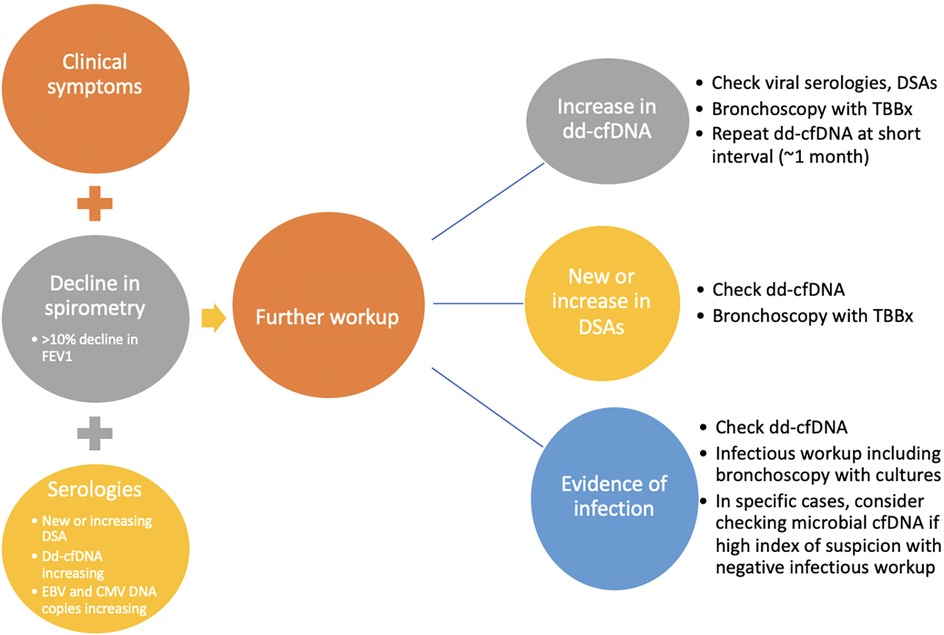
Figure 2. Approach to evaluation for allograft dysfunction. Allograft function is evaluated routinely by monitoring clinical symptoms, spirometry, and serologies. Evidence of clinical symptoms of rejection or infection, decline in spirometry (specifically decline in FEV1 > 10%, and evidence of serologic changes including new or increasing DSAs, increasing dd-cfDNA, or increasing EBV or CMV DNA copies prompts further investigation. Further investigation includes more inclusive infectious workup, bronchoscopy with transbronchial biopsy (TBBx), repeat of dd-cfDNA at a short interval, checking of immune cell function (ICF) assay, and consideration for evaluation of microbial cfDNA, in the right clinical context. Adapted with permission from “Overview of the potential genetic and epigenetic approaches associated with the analysis of cell free DNA” by Michael B. Keller, Temesgen E. Andargie and Sean Agbor-Enoh, licensed under CC BY 4.0.
As is standard practice in many transplant programs, DSA evaluation is performed routinely before, during, and after the transplantation period. Our practice is to monitor dd-cf-DNA monthly for the first year and quarterly thereafter. We measure DSAs at month 1 and then every 3 months for the first year and if negative, every 6 months thereafter; patients with known DSAs get them assessed every 3 months. Extreme changes in DSA or trends toward elevation provide a clue to the presence of AMR, oftentimes before clinical evidence. Routine use of dd-cfDNA beginning one month after transplantation allows for early identification of ACR or acute rejection, or it may coincide with clinical manifestations and culture evidence of infection which requires treatment. We do not routinely employ one value as a dd-cfDNA cutoff for rejection. Rather, the trend in dd-cfDNA allows for heightened suspicion of rejection. Our practice is to perform bronchoscopy with transbronchial biopsy at one month and three months post transplantation, with additional bronchoscopies and transbronchial biopsies performed based on changes in dd-cfDNA, DSAs, spirometry, or clinical symptoms. In patients with clinical evidence of infection, such as recurrent fevers, increased secretions on bronchoscopy, etc., we selectively send next-generation microbial cf-DNA sequencing, though we do not use this solely to diagnose infections in the vast majority of our patients. Routine monitoring of ICF assay and EBV DNA copies after transplantation also allows for early detection of reduction in immune cell function and consideration for increased risks for certain infections, particularly CMV or other viral infections. While the ICF assay may be an older test, we utilize the test during management of immune suppression medications post-transplantation and while weaning immune suppression. Our approach can be illustrated in an example case of a 42-year-old man who underwent bilateral lung transplantation for cystic fibrosis about 13 months prior to presentation. Routine DSA monitoring remained negative. Dd-cfDNA was also being monitored every 2–3 months and were consistently between 0.40 and 0.44% (Figure 3). The patient was initially on triple immunosuppression with tacrolimus, prednisone, and mycophenolate mofetil during this time. Due to an anal pap smear demonstrating atypical cells, the mycophenolate dose was reduced. The patient's routine ICF assay values, which were 518 ng/ml ATP and 674 ng/ml ATP (consistent with high immune cell response), decreased to 277 ng/ml ATP (moderate immune cell response). Routine follow up of dd-cfDNA about one month after mycophenolate dose reduction demonstrated an increase to 1.4%. The patient underwent transbronchial biopsy, and pathologic review revealed A2B0 rejection. The patient received intravenous steroids, and repeat dd-cfDNA subsequently demonstrated reduction to 0.51%, closer to baseline, and the patient did not require a repeat biopsy to confirm resolution. Utilizing a combination of biomarkers, the diagnosis of ACR was suggested prior to any clinical evidence of rejection. Thus, on a case-by-case basis, we can combine biomarkers with a careful history, physical examination, and routine pulmonary function testing to monitor for acute rejection in the post-lung transplantation patient.
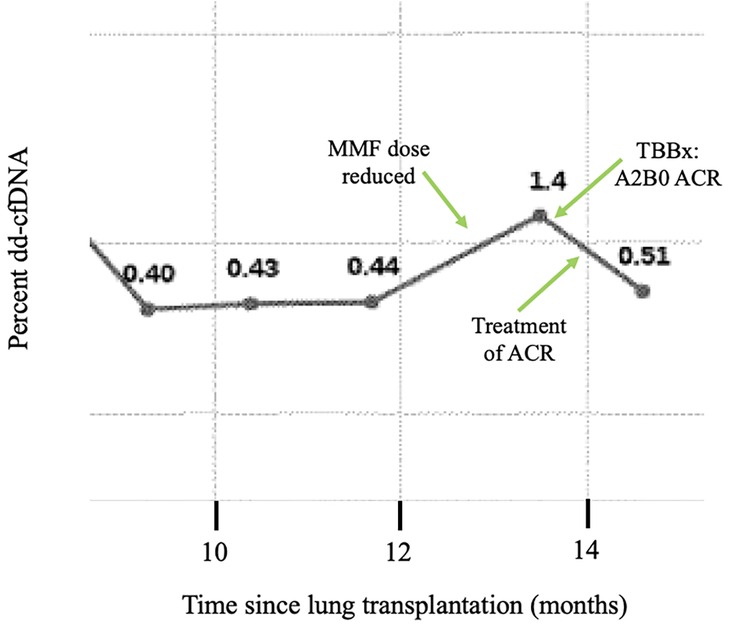
Figure 3. Example case: trends of dd-cfDNA in ACR. The dd-cfDNA percent is shown across time in a 42-year-old bilateral lung transplant recipient for cystic fibrosis. The patient had stable dd-cfDNA until around 12 months post-transplantation. Due to a positive anal pap smear, his mycophenylate mofetil dose was reduced. Around month 13, routine dd-cfDNA demonstrated increase compared to prior. A transbronchial biopsy demonstrated A2B0 acute cellular rejection, and the patient was treated with steroids. Subsequently, repeat dd-cfDNA declined. Abbreviations: ACR, acute cellular rejection; MMF, mycophenylate mofetil.
Conclusion
Allograft dysfunction remains a large contributor to morbidity and mortality in lung transplant recipients. Current methods of monitoring for rejection rely on clinical presentation of symptoms or decline in pulmonary function tests which may not be present, or which may present later in the course of rejection. Biomarkers have the potential to allow for early and less invasive diagnosis of rejection, but further studies are required to fully elucidate their exact application in this patient population. Methods to differentiate between rejection and infection are required. Utilization of current biomarkers, namely DSA, dd-cfDNA, measures of immune suppression, and sometimes plasma microbial cell-free DNA next-generation sequencing, in combination with clinical evaluation and histology assessment, may help in the diagnosis of allograft dysfunction including different forms of rejection as well as infection.
Author contributions
ZK: Conceptualization, Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing. SA: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACR, acute cellular rejection; AMR, antibody-mediated rejection; BAL, bronchoalveolar lavage; C4d, complement factor 4d; CLAD, chronic lung allograft dysfunction; CMV, cytomegalovirus; dd-cfDNA, donor-derived cell-free DNA; DNA, deoxyribonucleic acid; DSA, donor-specific antibodies; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GEP, gene expression profiling; HR, Hazard ratio; HLA, human leukocyte antigens; ICF, immune cell function; ISHLT, International Society of Heart and Lung Transplantation; RNA, ribonucleic acid; TTV, Torque tenovirus.
References
1. Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, et al. The registry of the international society for heart and lung transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. (2017) 36(10):1047–59. doi: 10.1016/j.healun.2017.07.016
2. Fricke K, Sievi NA, Schmidt FP, Schuurmans MM, Kohler M. Efficacy of surveillance bronchoscopy versus clinically indicated bronchoscopy for detection of acute lung transplant rejection: a systematic review and meta-analysis. ERJ Open Res. (2024) 10(5):00404-2024. doi: 10.1183/23120541.00404-2024
3. Kobashigawa J, Hall S, Shah P, Fine B, Halloran P, Jackson AM, et al. The evolving use of biomarkers in heart transplantation: consensus of an expert panel. Am J Transplant. (2023) 23(6):727–35. doi: 10.1016/j.ajt.2023.02.025
4. Velleca A, Shullo MA, Dhital K, Azeka E, Colvin M, DePasquale E, et al. The international society for heart and lung transplantation (ISHLT) guidelines for the care of heart transplant recipients. J Heart Lung Transplant. (2023) 42(5):e1–141. doi: 10.1016/j.healun.2022.10.015
5. Huang AL, Hendren N, Carter S, Larsen C, Garg S, La Hoz R, et al. Biomarker-based assessment for infectious risk before and after heart transplantation. Curr Heart Fail Rep. (2022) 19(4):236–46. doi: 10.1007/s11897-022-00556-z
6. Pradère P, Zajacova A, Bos S, Le Pavec J, Fisher A. Molecular monitoring of lung allograft health: is it ready for routine clinical use? Eur Respir Rev. (2023) 32(170):230125. doi: 10.1183/16000617.0125-2023
7. Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: a consensus report of the international society for heart and lung transplantation. J Heart Lung Transplant. (2016) 35(4):397–406. doi: 10.1016/j.healun.2016.01.1223
8. Chakravorty S, Aryal S, Cochrane A, Nathan SD. Antibody-mediated rejection: mechanisms, pathology, and therapeutics. Curr Pulmonol Rep. (2024) 13:173–82. doi: 10.1007/s13665-024-00349-w
9. Maguire O, Tario JD Jr, Shanahan TC, Wallace PK, Minderman H. Flow cytometry and solid organ transplantation: a perfect match. Immunol Invest. (2014) 43(8):756–74. doi: 10.3109/08820139.2014.910022
10. Scornik JC. Detection of alloantibodies by flow cytometry: relevance to clinical transplantation. Cytometry. (1995) 22(4):259–63. doi: 10.1002/cyto.990220402
11. Tait BD, Hudson F, Cantwell L, Brewin G, Holdsworth R, Bennett G, et al. Review article: luminex technology for HLA antibody detection in organ transplantation. Nephrology. (2009) 14(2):247–54. doi: 10.1111/j.1440-1797.2008.01074.x
12. Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Hamid AM, Picard C, et al. Antibody-mediated rejection in lung transplantation: clinical outcomes and donor-specific antibody characteristics. Am J Transplant. (2016) 16(4):1216–28. doi: 10.1111/ajt.13589
13. Morrell MR, Pilewski JM, Gries CJ, Pipeling MR, Crespo MM, Ensor CR, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. (2014) 33(12):1288–94. doi: 10.1016/j.healun.2014.07.018
14. Tikkanen JM, Singer LG, Kim SJ, Li Y, Binnie M, Chaparro C, et al. De novo DQ donor-specific antibodies are associated with chronic lung allograft dysfunction after lung transplantation. Am J Respir Crit Care Med. (2016) 194(5):596–606. doi: 10.1164/rccm.201509-1857OC
15. Le Pavec J, Suberbielle C, Lamrani L, Feuillet S, Savale L, Dorfmüller P, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. (2016) 35(9):1067–77. doi: 10.1016/j.healun.2016.05.020
16. Keller M, Yang S, Ponor L, Bon A, Cochrane A, Philogene M, et al. Preemptive treatment of de novo donor-specific antibodies in lung transplant patients reduces subsequent risk of chronic lung allograft dysfunction or death. Am J Transplant. (2023) 23(4):559–64. doi: 10.1016/j.ajt.2022.12.019
17. Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Picard C, Grenet D, et al. Characteristics of donor-specific antibodies associated with antibody-mediated rejection in lung transplantation. Front Med (Lausanne). (2017) 4:155. doi: 10.3389/fmed.2017.00155
18. Iasella CJ, Ensor CR, Marrari M, Mangiola M, Xu Q, Nolley E, et al. Donor-specific antibody characteristics, including persistence and complement-binding capacity, increase risk for chronic lung allograft dysfunction. J Heart Lung Transplant. (2020) 39(12):1417–25. doi: 10.1016/j.healun.2020.09.003
19. Zhang J, Liu D, Zhang C, Zhou M, Lv J, Wang H, et al. The value of high-resolution HLA in the perioperative period of non-sensitized lung transplant recipients. Ann Transl Med. (2020) 8(3):37. doi: 10.21037/atm.2019.10.45
20. Daniëls L, Beeckmans H, Zajacova A, Kerckhof P, Bos S, Naesens M, et al. The clinical significance of HLA compatibility scores in lung transplantation. Transpl Int. (2025) 37:13484. doi: 10.3389/ti.2024.13484
21. Hachem RR. The impact of non-HLA antibodies on outcomes after lung transplantation and implications for therapeutic approaches. Hum Immunol. (2019) 80(8):583–7. doi: 10.1016/j.humimm.2019.04.008
22. Comins-Boo A, Mora-Fernández VM, Padrón-Aunceame P, Toriello-Suárez M, González-López E, Roa-Bautista A, et al. Non-HLA antibodies and the risk of antibody-mediated rejection without donor-specific anti-HLA antibodies after lung transplantation. Transplant Proc. (2025) 57(1):73–6. doi: 10.1016/j.transproceed.2024.11.031
23. Xu Q, Elrefaei M, Taupin J-L, Hitchman KMK, Hiho S, Gareau AJ, et al. Chronic lung allograft dysfunction is associated with an increased number of non-HLA antibodies. J Heart Lung Transplant. (2024) 43(4):663–72. doi: 10.1016/j.healun.2023.12.007
24. Lammerts RGM, Altulea D, Hepkema BG, Sanders J-S, Born JVD, Berger SP. Antigen and cell-based assays for the detection of non-HLA antibodies. Front Immunol. (2022) 13:864671. doi: 10.3389/fimmu.2022.864671
25. Khush KK, Patel J, Pinney S, Kao A, Alharethi R, DePasquale E, et al. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: a prospective multicenter study. Am J Transplant. (2019) 19(10):2889–99. doi: 10.1111/ajt.15339
26. Keller MB, Andargie TE, Agbor-Enoh S. Biomarkers in the management of the lung transplant allograft: a focus on donor-derived cell-free DNA. OBM Transplantat. (2023) 07(02):1. doi: 10.21926/obm.transplant.2302190
27. Oellerich M, Budde K, Osmanodja B, Bornemann-Kolatzki K, Beck J, Schütz E, et al. Donor-derived cell-free DNA as a diagnostic tool in transplantation. Front Genet. (2022) 13:1031894. doi: 10.3389/fgene.2022.1031894
28. Osmanodja B, Akifova A, Budde K, Choi M, Oellerich M, Schütz E, et al. Absolute or relative quantification of donor-derived cell-free DNA in kidney transplant recipients: case series. Transplant Direct. (2021) 7(11):e778. doi: 10.1097/TXD.0000000000001237
29. Oellerich M, Shipkova M, Asendorf T, Walson PD, Schauerte V, Mettenmeyer N, et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: results from a prospective observational study. Am J Transplant. (2019) 19(11):3087–99. doi: 10.1111/ajt.15416
30. Halloran PF, Reeve J, Madill-Thomsen KS, Kaur N, Ahmed E, Cantos C, et al. Combining donor-derived cell-free DNA fraction and quantity to detect kidney transplant rejection using molecular diagnoses and histology as confirmation. Transplantation. (2022) 106(12):2435–42. doi: 10.1097/TP.0000000000004212
31. Park S, Sellares J, Tinel C, Anglicheau D, Bestard O, Friedewald JJ. European society of organ transplantation consensus statement on testing for non-invasive diagnosis of kidney allograft rejection. Transpl Int. (2024) 36:12115. doi: 10.3389/ti.2023.12115
32. Sorbini M, Togliatto G, Mioli F, Simonato E, Marro M, Cappuccio M, et al. Validation of a simple, rapid, and cost-effective method for acute rejection monitoring in lung transplant recipients. Transpl Int. (2022) 35:10546. doi: 10.3389/ti.2022.10546
33. De Vlaminck I, Martin L, Kertesz M, Patel K, Kowarsky M, Strehl C, et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci U S A. (2015) 112(43):13336–41. doi: 10.1073/pnas.1517494112
34. Agbor-Enoh S, Wang Y, Tunc I, Jang MK, Davis A, De Vlaminck I, et al. Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine. (2019) 40:541–53. doi: 10.1016/j.ebiom.2018.12.029
35. Keller MB, Meda R, Fu S, Yu K, Jang MK, Charya A, et al. Comparison of donor-derived cell-free DNA between single versus double lung transplant recipients. Am J Transplant. (2022) 22(10):2451–7. doi: 10.1111/ajt.17039
36. Keller M, Sun J, Mutebi C, Shah P, Levine D, Aryal S, et al. Donor-derived cell-free DNA as a composite marker of acute lung allograft dysfunction in clinical care. J Heart Lung Transplant. (2022) 41(4):458–66. doi: 10.1016/j.healun.2021.12.009
37. Khush KK, De Vlaminck I, Luikart H, Ross DJ, Nicolls MR. Donor-derived, cell-free DNA levels by next-generation targeted sequencing are elevated in allograft rejection after lung transplantation. ERJ Open Res. (2021) 7(1):00462-2020. doi: 10.1183/23120541.00462-2020
38. Rosenheck JP, Ross DJ, Botros M, Wong A, Sternberg J, Chen Y-A, et al. Clinical validation of a plasma donor-derived cell-free DNA assay to detect allograft rejection and injury in lung transplant. Transplant Direct. (2022) 8(4):e1317. doi: 10.1097/TXD.0000000000001317
39. Ju C, Wang L, Xu P, Wang X, Xiang D, Xu Y, et al. Differentiation between lung allograft rejection and infection using donor-derived cell-free DNA and pathogen detection by metagenomic next-generation sequencing. Heliyon. (2023) 9(11):e22274. doi: 10.1016/j.heliyon.2023.e22274
40. Sayah D, Weigt SS, Ramsey A, Ardehali A, Golden J, Ross D. Plasma donor-derived cell-free DNA levels are increased during acute cellular rejection after lung transplant: pilot data. Transplant Direct. (2020) 6(10):e608. doi: 10.1097/TXD.0000000000001063
41. Agbor-Enoh S, Jackson AM, Tunc I, Berry GJ, Cochrane A, Grimm D, et al. Late manifestation of alloantibody-associated injury and clinical pulmonary antibody-mediated rejection: evidence from cell-free DNA analysis. J Heart Lung Transplant. (2018) 37(7):925–32. doi: 10.1016/j.healun.2018.01.1305
42. Keller MB, Newman D, Alnababteh M, Ponor L, Shah P, Mathew J, et al. Extreme elevations of donor-derived cell-free DNA increases the risk of chronic lung allograft dysfunction and death, even without clinical manifestations of disease. J Heart Lung Transplant. (2024) 43(9):1374–82. doi: 10.1016/j.healun.2024.04.064
43. Nikolova A, Agbor-Enoh S, Bos S, Crespo-Leiro M, Ensminger S, Jimenez-Blanco M, Minervini A, et al. European society for organ transplantation (ESOT) consensus statement on the use of non-invasive biomarkers for cardiothoracic transplant rejection surveillance. Transpl Int. (2024) 37:12445. doi: 10.3389/ti.2024.12445
44. Bergin SP, Chemaly RF, Dadwal SS, Hill JA, Lee YJ, Haidar G, et al. Plasma microbial cell-free DNA sequencing in immunocompromised patients with pneumonia: a prospective observational study. Clin Infect Dis. (2024) 78(3):775–84. doi: 10.1093/cid/ciad599
45. Shino MY, Weigt SS, Saggar R, Elashoff D, Derhovanessian A, Gregson AL, et al. Usefulness of immune monitoring in lung transplantation using adenosine triphosphate production in activated lymphocytes. J Heart Lung Transplant. (2012) 31(9):996–1002. doi: 10.1016/j.healun.2012.05.012
46. Husain S, Raza K, Pilewski JM, Zaldonis D, Crespo M, Toyoda Y, et al. Experience with immune monitoring in lung transplant recipients: correlation of low immune function with infection. Transplantation. (2009) 87(12):1852–7. doi: 10.1097/TP.0b013e3181a75ad2
47. Bhorade SM, Janata K, Vigneswaran WT, Alex CG, Garrity ER. Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant. (2008) 27(9):990–4. doi: 10.1016/j.healun.2008.06.005
48. Khumri TM, St Clair K, Lawhorn SL, Magalski A, Stevens TL, Borkon AM, et al. 467: low immune cell function assay value predicts increased risk for cytomegaloviral, but not other opportunistic infections in heart transplant patients: a mid America experience. J Heart Lung Transplant. (2010) 29(2):S153–4. doi: 10.1016/j.healun.2009.11.483
49. Jaksch P, Kundi M, Görzer I, Muraközy G, Lambers C, Benazzo A, et al. Torque teno virus as a novel biomarker targeting the efficacy of immunosuppression after lung transplantation. J Infect Dis. (2018) 218(12):1922–8. doi: 10.1093/infdis/jiy452
50. Svorcova M, Hubacek P, Zajacova A, Vaculova M, Pozniak J, Kolarik J, et al. Torque teno virus level as predictor of acute rejection in lung transplant recipients. J Heart Lung Transplant. (2024) 43(4):S320. doi: 10.1016/j.healun.2024.02.478
Keywords: cell-free DNA, antibody-mediated rejection, acute cellular rejection, chronic lung allograft dysfunction, lung transplant
Citation: Kattih Z and Aryal S (2025) Using a combination of biomarkers to monitor allograft dysfunction in lung transplant recipients. Front. Transplant. 4:1574898. doi: 10.3389/frtra.2025.1574898
Received: 11 February 2025; Accepted: 3 April 2025;
Published: 8 May 2025.
Edited by:
Reut Hod Dvorai, Upstate Medical University, United StatesReviewed by:
Qingyong Xu, University of Pittsburgh, United StatesAylin Akifova, Charité University Medicine Berlin, Germany
Purav Shah, Emory University, United States
Copyright: © 2025 Kattih and Aryal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shambhu Aryal, U2hhbWJodS5hcnlhbEBpbm92YS5vcmc=
 Zein Kattih
Zein Kattih Shambhu Aryal
Shambhu Aryal