- 1Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States
- 2Harvard Medical School, Boston, MA, United States
- 3Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States
Introduction: Catheter-related bloodstream infections (CRBSI) incidence is well-studied in general hemodialysis patients. There is a lack of data on CRBSI rates specifically in solid organ transplant (SOT) recipients requiring hemodialysis. This study aims to investigate CRBSI incidence in this population at a single center.
Methods: This retrospective, single-center cohort study at Massachusetts General Hospital (MGH) investigated CRBSI incidence in non-kidney SOT (i.e., heart, lung, liver) who required hemodialysis via a tunneled dialysis catheter (TDC). Data was collected from January 2016 to October 2024, with patients followed for up to two years post-transplant or until death/end of study.
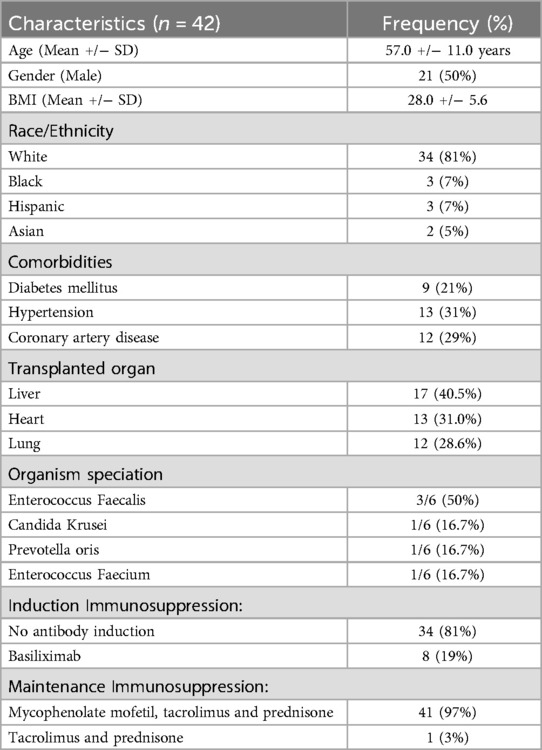
Results: 42 individuals met the study's inclusion criteria. The mean age of this cohort was 57 years, 50% were male, and 81% were White. The group consisted of 17 liver transplant recipients (40.5%), 13 heart transplant recipients (31.0%), and 12 lung transplant recipients (28.6%). Among the 12 lung transplant recipients, 8 received basiliximab induction, and 4 received no antibody induction therapy. 97% of the patients received mycophenolate mofetil, tacrolimus, and prednisone, while 3% received steroid-free maintenance. The median follow-up was 51.5 days (interquartile range 16–233). During this period, six individuals developed CRBSI, resulting in an incidence rate of 0.86 infections per 1,000 catheter-days. No deaths were attributed to CRBSI.
Conclusions: Our findings suggest that intense immunosuppression in the setting of SOT is not associated with an increased risk of CRBSI in patients with renal failure utilizing TDC especially when a consistent and standardized protocol for the access and care of these catheters is utilized.
Introduction
In 2023, the United States performed a total of 46,629 organ transplants, with an anticipated increase in the number of transplants completed during 2024 (1); among those, 18,223 patients underwent liver, lung, or heart transplant. Dialysis-dependent kidney failure, whether acute or chronic is a well known complication of non-kidney (liver, lung, or heart) solid organ transplantation (SOT) (2–4). Furthermore, hemodialysis carries a significant risk of infection due to the patients' compromised immune systems and repeated access to the bloodstream (5, 6). Infections account for a substantial proportion of post-transplant mortality, ranging from 13% to 16% in kidney and heart recipients, up to 21% in lung recipients and approximately 50% within the first year after liver transplantation (7).
Among the various hemodialysis vascular access types, central venous catheters (CVCs) are associated with the highest infection risk (8, 9). Catheter-related bloodstream infection (CRBSI) is a serious complication associated with CVC use in patients on hemodialysis (10) and is associated with increased hospitalization and mortality rates. While the incidence rate of CRBSI in the general hemodialysis population is well-documented (8, 10–12), there are no published data on CRBSI incidence in solid organ transplant recipients with renal failure (acute or chronic) requiring hemodialysis and it is unknown if the intense immunosuppressed status post SOT is associated with an increase risk for CRBSI.
This study aims to address this knowledge gap by examining the incidence of CRBSI post-organ transplant at our center and comparing our findings to national rates.
Materials and methods
Study design
This retrospective observational single-center cohort study at Massachusetts General Hospital (MGH) examined the incidence of catheter-related bloodstream infections (CRBSI) in non-kidney solid organ transplant (SOT) recipients (heart, lung, liver) requiring intermittent hemodialysis or continuous renal replacement therapy via a tunneled dialysis catheter (TDC). The study period spanned January 1, 2016—October 31, 2024, a nearly 9 year duration, with patients followed for up to 2 years, death, or study end. The study adhered to STROBE guidelines and was approved by the Massachusetts General Brigham IRB (2025P000242) with a waiver of informed consent.
Data source and study population
Patients were identified through EMR reports of TDC insertions in SOT recipients, with manual chart review confirming eligibility. Inclusion criteria: (1) age ≥18 years, (2) history of non-kidney SOT, and (3) renal failure requiring dialysis via TDC. We excluded kidney transplant recipients only but not silmutaneous liver and kidney (SLK) recipients where patients were not dialysis dependent prior to the SLK, to focus on non-renal SOT recipients, where the risks and management of renal failure requiring dialysis are distinct. Kidney transplant recipients typically have pre-established nephrology care and dialysis plans, unlike non-kidney recipients. Further, most kidney transplant patient who present for a kidney transplant have already established dialysis access and the dialysis needs after transplant are often limited to 1–2 weeks in the case of delayed kidney graft function. Data were entered into a secure web application.
Data collection & outcomes
The primary outcome was CRBSI, defined as a bloodstream infection attributed to TDC use, confirmed by two nephrologists and an infectious disease consultant. Collected variables included demographics, comorbidities, transplant type, immunosuppression, and dialysis access details.
MGH CVC access protocol
MGH Dialysis Nurses follow a strict protocol to prevent CRBSIs, using See-Luer caps and sterile techniques for accessing TDCs. The protocol includes:
• Before access: Hand hygiene, sterile gloves, and a sterile field. Ports are disinfected with alcohol pads (15 s), then See-Luer caps before flushing with saline.
• Post-session: Catheters are flushed with saline + anticoagulant, disinfected, and sealed with See-Luer caps. Used catheters are labeled appropriately.
Statistical analysis
Continuous variables are summarized as mean (±SD) or median (IQR), categorical as counts (percentages). CRBSI incidence was calculated as total events per catheter-days, defined as TDC duration from placement to removal, death, or study end. Given the small sample size and descriptive nature of the data, we focused on incidence rates (per 1,000 catheter-days) rather than inferential statistics.
Results
Study population
During the study period from January 1st, 2016 to October 31st, 2024, 42 individuals met our study's inclusion. Table 1 shows the characteristics of the cohort. The mean age was 57 years, 50% were male, and 81% were White. The cohort included 17 liver transplant recipients (40.5%), 13 heart transplant recipients (31.0%), and 12 lung transplant recipients (28.6%).
Immunosuppressive regimen
None of the heart or liver transplant recipients received antibody induction therapy. Among the 12 lung transplant recipients, 8 received basiliximab induction and 4 received no antibody induction therapy. With regards to maintenance immunosuppression 97% of the patients received maintenance immunosuppression with mycophenolate mofeti, tacrolimus and prednisone, while 3% received steroid-free maintenance. 5 patients experienced transplant rejection. 2 of these were liver transplant recipients, both of whom were treated with steroid pulse therapy. The remaining 3 patients were lung transplant recipients; 1 of these received basiliximab, and the other 2 received steroid pulse therapy.
Acute kidney injury
Among the 42 individuals meeting inclusion criteria, identified causes of renal dysfunction included ischemic acute tubular necrosis (i-ATN) in 21 individuals (50%), per nephrology consultation, hepatorenal syndrome in 14 (34%), cardiorenal syndrome in 5 (12%), delayed graft function (DGF) in 1 (2%), and calcineurin inhibitor-associated acute kidney injury (CNI-AKI) in 1 (2%). At the time of dialysis initiation, all transplanted organs (heart, liver, or lung) were functional and renal failure was not due to primary graft dysfunction.
Incidence rate of CRBSI
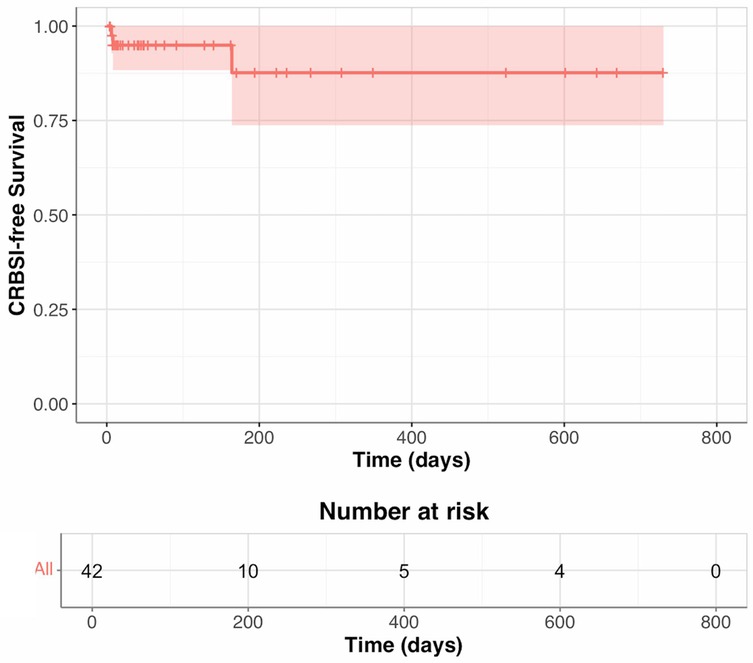
The median follow-up was 51.5 days (IQR 16–233). During follow-up, six individuals developed CRBSI. The incidence rate of CRBSIs in the cohort was 0.86 infections per 1,000 catheter-days. There were no deaths due to CRBSI.
CRBSI microbiology
During the first 90 days of follow-up, two patients developed CRBSIs: one by Candida krusei and the other by Prevotella oris. Between 90 and 179 days, there was one CRBSI due to Enterococcus faecalis. Between 180 and 365 days, three CRBSIs occurred: two Enterococcus faecalis infections and one Enterococcus faecium infection. From days 366 to 730, no CRBSIs occurred (Table 2; Figure 1).
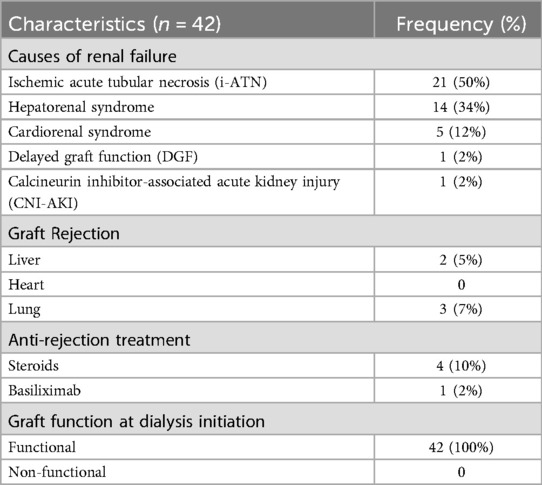
Table 2. Causes of renal failure, acute rejection episodes, and graft function at dialysis initiation.
Discussion
CVC use is prevalent in US ICUs, with an estimated 15 million days of CVC exposure reported annually, which includes the total number of days CVC exposure among all patients in a selected population over a time period (13). CRBSIs are a significant concern, independently increasing hospital costs and length of stay (14, 15). Mortality rates associated with CRBSIs have declined, from 7.9% in 1997 to 5.9% in 2008 according to the National Hospital Discharge Survey (16). However, a 10-year cohort study reported 17.4% of patients with CRBSIs died within 30 days, with a population attributable mortality rate of 18.2% (17). The overall cost of these infections remains substantial, both in terms of morbidity and resource utilization.
This is the first report to describe the incidence rate of CRBSI in non-kidney solid organ transplant recipients, an intensely immunosuppressed population. CRBSIs are a significant concern for patients with tunneled dialysis catheters (TDCs). The incidence rate of CRBSIs varies widely in studies of the general hemodialysis population, ranging from 0.5 to 5.5 episodes per 1,000 catheter-days, likely influenced by factors such as patient population, catheter care practices, and duration of catheter use (12). Higher rates are typically observed in settings with suboptimal infection control protocols or among patient groups with greater comorbidities. Several risk factors have been identified, including prolonged catheter dwell time, underlying conditions such as diabetes mellitus and immunosuppression, and inadequate catheter care practices (18). The Centers for Disease Control and Prevention (CDC) recommend maintaining CRBSI rates below 1.0 episode per 1,000 catheter-days in facilities adhering to best practices (19). However, achieving these benchmarks often depends on the implementation of strict aseptic techniques, regular staff education, and preventive measurements.
In our cohort of intensely immunosuppressed patients after solid organ transplantation, the incidence rate was 0.86 infections per 1,000 catheter-days. This rate is comparable to CRBSI rates reported in general hemodialysis patients with TDCs who are not SOT recipients. Our findings suggest that intense immunosuppression in the setting of solid organ transplantation is not associated with an increased risk of CRBSI in patients with renal failure utilizing TDCs especially when a consistent and standardized protocol for the access and care of these catheters is utilized.
The study provides needed insight about the safety of TDCs in immunosuppressed hosts with renal failure. Recent solid organ transplant recipients with acute kidney injury requiring dialysis are often not readily amenable for dialysis access surgery or kidney transplants and as such TDCs are a needed bridge. However, there are important limitations to consider. First, the retrospective cohort observational design only allows us to make associations and is susceptible to confounding factors. Second, the single-center design, the moderate sample size, and the inability to run inferential statistitics may affect the generalizability of our estimate for the CRBSI incidence rate. Lastly, the comparison of incidence rates between studies depends on the assumption of person-time equivalence, which assumes that the risk of the outcome during any person-time period is equivalent to the risk during any other person-time period, and that assumption may or may not be true during follow-up. This limitation could be overcome in the future, utilizing post transplant national registries mandating the reporting of this specific complication in SOT recipients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This retrospective study involving human participants received approval from the Massachusetts General Brigham IRB and adhered to local legislation and institutional guidelines. The ethics committee/institutional review board waived the requirement for written informed consent from participants or their legal guardians/next of kin due to the study's retrospective nature.
Author contributions
CE: Writing – original draft, Methodology. AA: Writing – review & editing, Data curation. KS: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Detailed Description of Organ Donation Statistics | organdonor.gov. Organdonor.gov. Published October 17, 2024. Available at: https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig2 (Accessed May 6, 2025).
2. Welz F, Schoenrath F, Friedrich A, Wloch A, Stein J, Hennig F, et al. Acute kidney injury after heart transplantation: risk factors and clinical outcomes. J Cardiothorac Vasc Anesth. (2024) 38(5):1150–60. doi: 10.1053/j.jvca.2024.01.024
3. Durand F, Francoz C, Asrani SK, Khemichian S, Pham TA, Sung RS, et al. Acute kidney injury after liver transplantation. Transplantation. (2018) 102(10):1636–1649. doi: 10.1097/TP.0000000000002305
4. Chan EG, Pan G, Clifford S, Hyzny EJ, Furukawa M, Coster JN, et al. Postoperative acute kidney injury and long-term outcomes after lung transplantation. Ann Thorac Surg. (2023) 116(5):1056–62. doi: 10.1016/j.athoracsur.2023.06.016
5. Hörl WH. Neutrophil function and infections in uremia. Am J Kidney Dis. (1999) 33(2):xlv–iii. doi: 10.1016/s0272-6386(99)70294-5
6. Jaber BL. Bacterial infections in hemodialysis patients: pathogenesis and prevention. Kidney Int. (2005) 67(6):2508–19. doi: 10.1111/j.1523-1755.2005.00364.x
7. Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. (2006) 81(12):1645–52. doi: 10.1097/01.tp.0000226071.12562.1a
8. Stevenson KB, Hannah EL, Lowder CA, Adcox MJ, Davidson RL, Mallea MC, et al. Epidemiology of hemodialysis vascular access infections from longitudinal infection surveillance data: predicting the impact of NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. (2002) 39(3):549–55. doi: 10.1053/ajkd.2002.31405
9. Tokars JI, Miller ER, Stein G. New national surveillance system for hemodialysis-associated infections: initial results. Am J Infect Control. (2002) 30(5):288–95. doi: 10.1067/mic.2002.120904
10. Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. (2004) 44(5):779–91. doi: 10.1016/S0272-6386(04)01078-9
11. Jose N, S. M, John K, Prasad R, Jayakumar M. LABSI in hemodialysis– new face to an old foe; a look at current trends and a review of literature. Open Urol Nephrol J. (2022) 15(1). doi: 10.2174/1874303X-v15-e2208180
12. Nguyen DB, Shugart A, Lines C, Shah AB, Edwards J, Pollock D, et al. National Healthcare Safety Network (NHSN) dialysis event surveillance report for 2014. Clin J Am Soc Nephrol. (2017) 12(7):1139–46. doi: 10.2215/CJN.11411116
13. CDC. Updated Recommendations on Chlorhexidine-Impregnated (C-I) Dressings. Infection Control. Published May 10, 2024. Available at: https://www.cdc.gov/infection-control/hcp/c-i-dressings/index.html (Accessed May 6, 2025).
14. Arvaniti K, Lathyris D, Clouva-Molyvdas P, Haidich AB, Mouloudi E, Synnefaki E, et al. Comparison of Oligon catheters and chlorhexidine-impregnated sponges with standard multilumen central venous catheters for prevention of associated colonization and infections in intensive care unit patients: a multicenter, randomized, controlled study. Crit Care Med. (2011) 40(2):420–29. doi: 10.1097/CCM.0b013e31822f0d4b
15. Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med. (2012) 186(12):1272–78. doi: 10.1164/rccm.201206-1038oc
16. Daniels KR, Frei CR. The United States’ progress toward eliminating catheter-related bloodstream infections: incidence, mortality, and hospital length of stay from 1996 to 2008. Am J Infect Control. (2013) 41(2):118–21. doi: 10.1016/j.ajic.2012.02.013
17. Zhong Y, Zhou L, Liu X, Deng L, Wu R, Xia Z, et al. Incidence, risk factors, and attributable mortality of catheter-related bloodstream infections in the intensive care unit after suspected catheters infection: a retrospective 10-year cohort study. Infect Dis Ther. (2021) 10(2):985–99. doi: 10.1007/s40121-021-00429-3
18. Huang H, Chang Q, Zhou Y, Liao L. Risk factors of central catheter bloodstream infections in intensive care units: a systematic review and meta-analysis. PLoS One. (2024) 19(4):e0296723. doi: 10.1371/journal.pone.0296723
19. CDC. Background Information: Strategies for Prevention of Catheter-Related Infections in Adult and Pediatric Patients. Infection Control. Published April 3, 2024. Available at: https://www.cdc.gov/infection-control/hcp/intravascular-catheter-related-infection/prevention-strategies.html (Accessed May 6, 2025).
Keywords: AKI, hemodialysis, organ transplant, CRBSI, TDC
Citation: El Mouhayyar C, Al Jurdi A and Safa K (2025) Incidence of catheter-related bloodstream infection (CRBSI) in immunosuppressed hosts post solid organ transplant (SOT): a single center experience. Front. Transplant. 4:1586035. doi: 10.3389/frtra.2025.1586035
Received: 1 March 2025; Accepted: 29 April 2025;
Published: 14 May 2025.
Edited by:
Osama Ashry Gheith, Mansoura University, EgyptReviewed by:
Medhat Aly, Hamed AlEssa Organ Transplant Center, KuwaitAhmed Denewar, Ministry of Health, Kuwait
Tarek Said Hamed Mahmoud, Jaber Al-Ahmad Armed Forces Hospital, Kuwait
Copyright: © 2025 El Mouhayyar, Al Jurdi and Safa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kassem Safa, a2Fzc2VtLnNhZmFAbWdoLmhhcnZhcmQuZWR1
 Christopher El Mouhayyar
Christopher El Mouhayyar Ayman Al Jurdi
Ayman Al Jurdi Kassem Safa
Kassem Safa