Abstract
Background:
Kidney perfusion is a tool that allows organs to be assessed before transplantation. After brain death (BD), hormonal dysfunction can compromise graft quality. Hormonal treatment of donors has shown positive outcomes, and treatment during ex vivo perfusion may be advantageous. The combination of 17β-estradiol (E2) and methylprednisolone (MP) has been shown to modulate inflammation in donors. Therefore, this study aims to evaluate treatment with E2 and MP during isolated perfusion of kidneys in brain-dead male and female rats.
Methods:
Female and male Wistar rats were submitted to BD and maintained for 4 h. In the same animal, the right kidney [RK—no isolated perfusion of kidney (IPK)] was removed and stored, while the left kidney (LK—with IPK) had the ureter and the renal artery cannulated and flushed with 5 ml of cold saline. The LK was then taken directly to the IPK system for 90 min. Experimental groups were performed in both male and female: IPK (without treatment) and IPK + Treat (MP and E2 added to the perfusate). Perfusion was performed with a constant pressure of 100 mmHg, using William's Medium E supplemented with HEPES, creatinine, and albumin as perfusate. Perfusate and urine were collected, and flow measurements were recorded. After IPK, the LK was stored.
Results:
IL-6 was reduced in all perfused groups, regardless of treatment. In female IPK + Treat, there was a reduction in perfusion flow, followed by reduced creatinine clearance and Na+ excretion. No difference was observed in males in regards to treatment.
Conclusion:
The combined treatment of E2 and MP during isolated kidney perfusion compromised kidney function in females. In males, no detrimental effects were observed. These results show a sex-dependent action of the proposed treatment.
Background
In kidney transplantation, the majority of donations are derived from brain-dead patients (1). Brain death (BD) results from cranial trauma or hemorrhagic stroke that leads to an excessive increase in intracranial pressure, culminating in herniation of the brainstem and subsequent ischemia of the brain. This process triggers a hemodynamic imbalance via the release of catecholamines, resulting in systemic inflammation and hormonal dysfunction (2).
Our group has previously shown sex differences in an experimental model of BD, with females presenting a more exacerbated inflammatory response to BD induction (3). Additionally, a study by Armstrong-Jr et al. (4) has shown that sex differences persist during ex vivo perfusion of kidneys. The use of a perfusion system is a tool to assess and treat organs in a controlled and isolated environment before transplantation, and several therapeutic options have been explored to improve graft quality (5–7). Compared with methylprednisolone (MP) administration alone, the combination of 17β-estradiol (E2) and MP improved donor hemodynamics and modulated leukocyte infiltration into the lungs (8) and kidneys (unpublished data) in an experimental model of BD in females. These results point to a positive outcome of MP and E2 administration in the donor and in an isolated perfusion system in different organs. Therefore, we aimed to investigate how the association of MP and E2 could affect kidneys from male and female rats after a period of BD using a model of isolated perfusion of kidneys (IPK).
Methods
Animals
Female and male Wistar rats (8–12 weeks old) from Envigo (the Netherlands) were maintained at 23 ± 2°C, with a 12 h light and dark cycle and food and water ad libitum. The animals were cared for in accordance with the Principles of Laboratory Animal Care (NIH Publication No. 86-23, revised 1985) and the Dutch Law on Experimental Animals Care. This study was approved by the Institutional Animal Care and Use Committee of the University of Groningen (IvD number: 171245-01-004).
The animals were allocated into groups: male and female naive, control animals that did not undergo any surgical procedure (n = 4); male and female BD, animals that underwent BD induction without IPK (n = 8); male and female IPK, animals that underwent BD, followed by IPK without treatment (n = 8); and male and female IPK + Treat, animals that underwent BD, followed by IPK with the combined treatment of 17β-estradiol and methylprednisolone (n = 8).
Estrous cycle identification
The female animals were selected by estral cycle phase, identifying the higher estradiol period (estrous and proestrous). Vaginal lavage was performed with a Pasteur pipette filled with 10 μl of saline solution (0.9% NaCl). The cellular profile was identified by optical microscopy observation.
Brain death induction
All animals were placed in a closed chamber, anesthetized with 5% isoflurane, and maintained with 2% isoflurane during the surgical procedure. The temperature was maintained at 37°C via a heating mat. The jugular vein was cannulated for fluid administration and influx of vasoactive drugs for hemodynamic stabilization; the carotid artery was used for blood pressure measurements. The animals were connected to a small animal ventilator after tracheostomy (Harvard Apparatus, model 683, USA) and kept at a frequency of 70 breaths/min and a tidal volume of 10 ml/kg. For BD induction, a Fogarty 4F catheter was inserted into the intracranial space and slowly inflated for 30 min. BD was confirmed by bilateral mydriasis and apnea. After BD confirmation, anesthesia was stopped, and fluid administration was initiated (2 ml/h) until the end of the experiment. Blood samples were collected at the beginning and the end of the 4 h period. After 4 h, the animals were exsanguinated, and a whole-body flush (maximum pressure of 30 mmHg) was performed with 40 ml of cold saline. Blood, urine, and organs were collected. The left kidney (LK) was prepared for perfusion, while the right kidney was immediately snap-frozen. For the LK, a nephrectomy was performed. The ureter and the renal artery were cannulated, and the kidney was then flushed with 5 ml of cold saline solution and directly transferred to the isolated kidney perfusion (IPK) system.
Normothermic isolated perfused kidney setup
For the IPK, pressure-controlled normothermic machine perfusion was performed via a roller pump (Ismatec ISM404, Switzerland). The pressure was constantly set at 100 mmHg, and a pressure sensor (Edwards Lifesciences, USA) was placed close to the renal artery. For the perfusion solution, 100 ml of William's medium E supplemented with 30 mmol/L HEPES, 7 mmol/L creatinine, and 50 g/L albumin (Sigma-Aldrich, USA) was used. Carbogen (95% O2, 5% CO2) was used to oxygenate the perfusate at a flow rate of 0.5 L/min. Perfusate gas analyses were performed at the initial and final points of IPK, and the values were maintained at approximately 525 mmHg. A water bath and heat exchanger were used to heat the solution. In the first 30 min of perfusion, temperature was slowly increased (1°C/2 min) during the rewarming phase. After that, the temperature was kept at 37°C. Flow measurements were recorded during perfusion.
Perfusate and ultrafiltrate (urine) samples were collected at T15, T30, T60, and T90 (Tmin). After 90 min, the LK was stored for further analysis.
Treatment
For treatment, 40 mg of methylprednisolone and 5 μg/ml of 17β-estradiol were added to the IPK perfusion solutions.
Biochemical analysis
lactate dehydrogenase (LDH) was measured in the IPK perfusate. Creatinine and Na+ levels were determined in the IPK perfusate and ultrafiltrate. Measurements were performed in accordance with the Clinical Laboratory, University Medical Center Groningen, following standard biochemical methods.
Creatinine clearance and Na excretion (FENa+)
The creatinine and Na+ concentrations in the ultrafiltrate and perfusate obtained via biochemical analysis were used to estimate creatinine clearance and Na+ excretion via the following calculations:
IL-6 quantification
IL-6 was measured in kidney homogenate and IPK perfusate. Quantifications were performed via Duo Set commercial ELISA kits (R&D Systems, USA) in accordance with the manufacturer's specifications.
Gene expression
Total RNA was extracted from kidney tissue via TRIzol reagent (Invitrogen, USA). The yield of extracted RNA was analyzed with a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, USA), and the quality was assessed via RNA electrophoresis. The extracted RNA was reverse transcribed with random primers at 37°C for 50 min. Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted with specific primers (Table 1). SYBR Green (Applied Biosystems, the Netherlands) and a QuantStudio 7 Flex qPCR machine (Applied Biosystems, the Netherlands), which was configured with 1 cycle of 10 min at 95°C and 40 consecutive cycles of 15 s at 95°C and 1 min at 60°C. CT values were corrected for β-actin levels.
Table 1
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| β-Actin | GGAAATCGTGCGTGACATTAAA | GCGGCAGTGGCCATCTC |
| IL-6 | CCAACTTCCAATGCTCTCCTAATG | TTCAAGTGCTTTCAAGAGTTGGAT |
| KIM-1 | AGAGAGAGCAGGACACAGGCTTT | ACCCGTGGTAGTCCCAAACA |
RT-PCR primers.
RT-PCR, real-time polymerase chain reaction; β-actin, beta-actin; IL-6, interleukin 6; KIM-1, kidney injury marker 1.
Immunohistochemistry
For the immunohistochemical analyses, the right and left kidneys were placed in formalin and subsequently paraffin-embedded. Slices (3 mm) were prepared and placed on glass slides. Deparaffinization was performed via xylene and ethanol. Antigen retrieval was performed via the use of an ethylenediaminetetraacetic acid (EDTA) (pH 8) solution. The slides were incubated in this solution for 3 h at 60°C. Later, endogenous peroxidase was blocked with H2O2 (2%) for 15 min. Non-specific blockage was performed with TBST-BSA (2%) solution for 1 h at 37°C. Antibodies against myeloperoxidase (MPO), inducible nitric oxide synthase (iNOS), kidney injury molecule-1 (KIM-1), endothelial nitric oxide synthase (eNOS), and endothelin-1 (ET-1) (Table 2) were incubated with the samples overnight at 4°C. The sections were then incubated with secondary HRP-conjugated antibodies (1:200, BA1054; Boster Biological Technology, USA) for 2 h at 37°C. A peroxidase substrate was subsequently used. Hematoxylin was used for counterstaining. For the slide analyses and image acquisition, NIS-Elements BD (Nikon, Japan) software was used.
Table 2
| Antibody | Dilution and code |
|---|---|
| MPO | 1:50, PA1054; Boster Biological Technology, USA |
| iNOS | 1:100, AB3523; Abcam, UK |
| KIM-1 | 1:100, LS-C312791; LSBio, USA |
| eNOS | 1:100, AO1604-2; Boster Biological Technology, USA |
| Endothelin-1 | 1:200, LS-B13907; LSBio, USA |
Primary antibodies.
MPO, myeloperoxidase; iNOS, inducible nitric oxide synthase; KIM-1, kidney injury marker 1; eNOS, endothelial nitric oxide synthase.
Morphological analysis
Kidney morphology was analyzed via periodic acid–Schiff (PAS) staining of paraffin-embedded kidney sections (5 μm). The scoring system used is presented in Table 3.
Table 3
| Analyses | Scoring system | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Dilated glomerulus | Normal glomerular space | Moderately enlarged glomerular space | Enlarged glomerular space | Greatly enlarged glomerular space |
| Necrosis of the proximal tubule | – | Partly broken brush border, shedding and blebbing, and normal nucleus | >50% broken brush border, loose cell nuclei, and pyknotic nuclei | More than half of the tubules are necrotic |
| Necrosis of the distal tubule | – | Partly loose cells | >50% loose cells and pyknotic nuclei | – |
Morphological analysis scoring system.
Statistical analysis
The data are expressed as the means ± standard errors of the means (SEMs). The data were analyzed with GraphPad Prism Version 10, and the groups were compared via two-way analysis followed by the post hoc test of the two-stage linear step-up procedure of Benjamin, Krieger, and Yekutieli or three-way ANOVA.
Results
Expression and release of IL-6
IL-6 (Figure 1) was quantified in kidney homogenates (A) and IPK perfusates (B). Gene expression in renal tissue was also evaluated (C). Compared with non-perfused kidneys, all groups presented reduced expression of IL-6 in kidney homogenates after perfusion. For males, the treated group presented even lower concentrations than those in the non-treated group. In females, no difference was observed between the IPK and IPK + Treat groups (A). According to the results of the perfusate analyses, female IPK + Treat mice presented lower release of IL-6 than IPK-treated mice did. No difference was observed in males (B). Finally, gene expression was increased in all groups after IPK, regardless of sex and treatment (C).
Figure 1

Quantification of IL-6 in kidney homogenate (A), IPK perfusate (B), and gene expression in kidney tissue (C). IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results from graphs A and C were analyzed using three-way ANOVA. Results from graph B were analyzed using two-way ANOVA. IL-6, interleukin 6.
Leukocyte infiltrate
The number of infiltrating neutrophils and macrophages was analyzed via quantification of MPO-marked cells and iNOS protein expression (Figure 2). The pre-IPK kidneys of males presented higher MPO levels than did those of females. After IPK, all groups presented similar numbers of infiltrated cells (A). Similarly, in the iNOS analyses, protein expression was increased in males and was reduced after perfusion in all the groups.
Figure 2
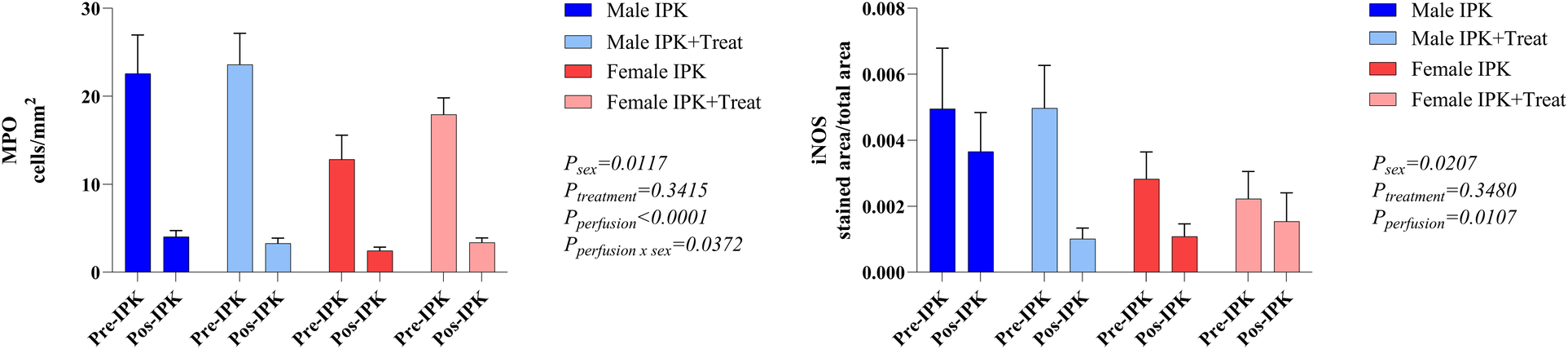
Protein expression of MPO and iNOS in kidney tissue. IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results analyzed using three-way ANOVA. MPO, myeloperoxidase. iNOS, inducible nitric oxide synthase.
Kidney injury marker 1 (KIM-1) expression
There was a reduction in the protein expression of KIM-1 with perfusion. With respect to gene expression, no difference was observed among the groups (Figure 3).
Figure 3

Protein and gene expression of KIM-1 in kidney tissue. IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results analyzed using three-way ANOVA. KIM-1, kidney injury marker 1.
Flow measurement
Perfusion flow was constantly measured during IPK. Females presented with slightly increased flow at the beginning of perfusion. Compared with the control group, the IPK + Treat group presented significantly reduced flow. The male groups presented similar values throughout perfusion (Figure 4).
Figure 4

Flow measurement during perfusion. IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results analyzed using three-way ANOVA.
Modulation of vascular tone
The protein expression of eNOS and endothelin-1 (ET-1) was analyzed in kidney tissue (Figure 5). In females, eNOS expression was reduced in pre-IPK kidneys, and eNOS expression was increased in the IPK + Treat group (A). No difference was observed in the male groups. With respect to ET-1 (B), females also presented reduced expression in pre-IPK kidneys compared with males, and no difference was observed with perfusion or treatment.
Figure 5
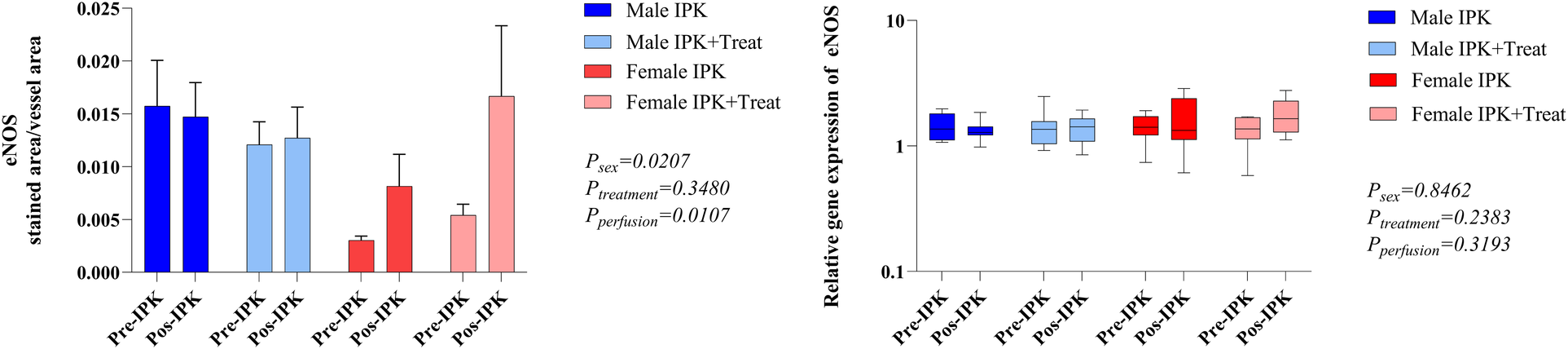
Protein expression of eNOS and endothelin-1 in kidney tissue. IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results analyzed using three-way ANOVA. eNOS, endothelial nitric oxide synthase.
Kidney function
Creatinine clearance was lower in males than in females, and female IPK + Treat presents lower values than female IPK does. No difference was observed over time (A). Fractional excretion of sodium (FENa) in the perfusate revealed greater reabsorption of Na+ in the female IPK + Treat group than in the female IPK group. Both male groups presented similar values at all 90 min (B). Over time, all groups presented a reduction in FENa+ (Figure 6).
Figure 6
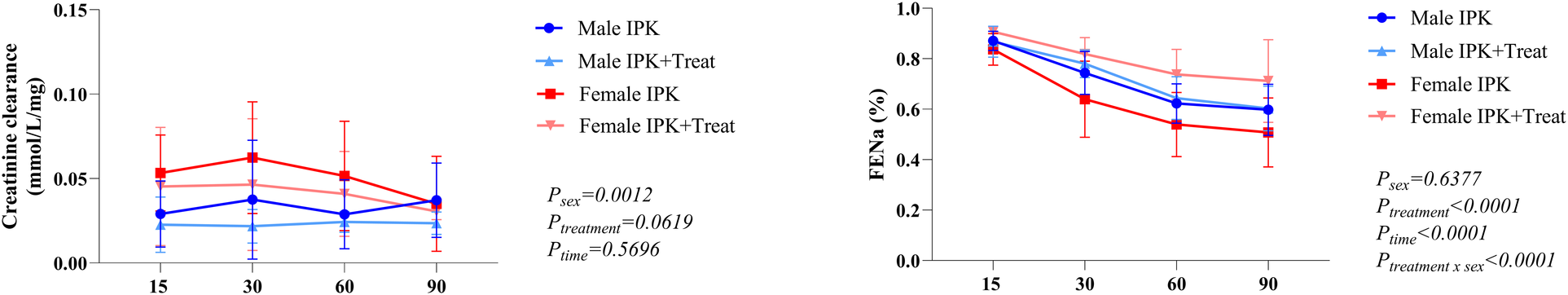
Creatinine clearance and fractional excretion of sodium. IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results analyzed using three-way ANOVA. FENa, fractional excretion of sodium.
Lactate dehydrogenase (LDH) and lactate
LDH was measured in the perfusate at several time points (A). Lactate was quantified at the beginning and end of the perfusion (B). There is an increase in LDH in both male and female IPK + Treat groups compared with IPK. All groups presented an increase in LDH throughout perfusion. Lastly, all groups presented increased lactate from T15 to T90 (Figure 7).
Figure 7
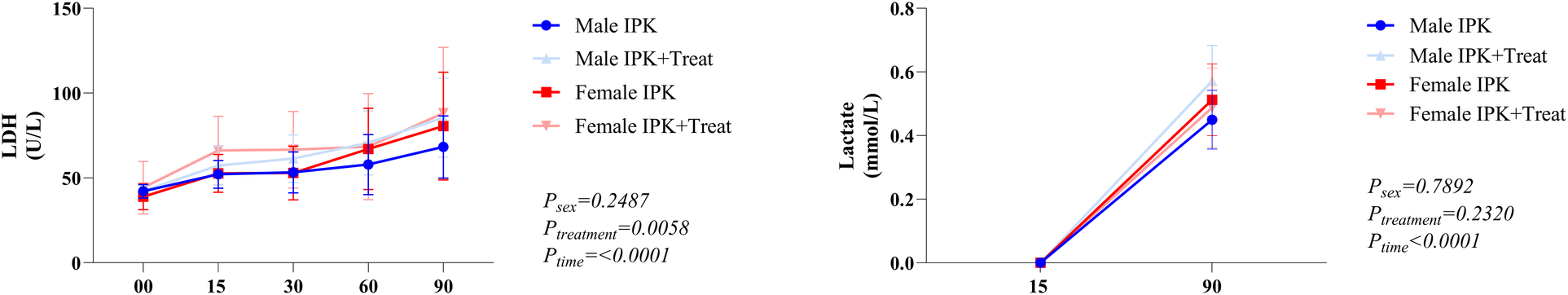
Lactate dehydrogenase and lactate quantification. IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results analyzed using three-way ANOVA. LDH, lactate dehydrogenase.
Morphological analysis of renal tissue
Necrosis of the proximal and distal tubules and glomerular space was quantified via a scoring system (Figure 8). Both proximal and distal necrosis were reduced after perfusion in all groups (A and B). Compared with non-perfused kidneys, all perfused kidneys presented increased glomerular space (C).
Figure 8
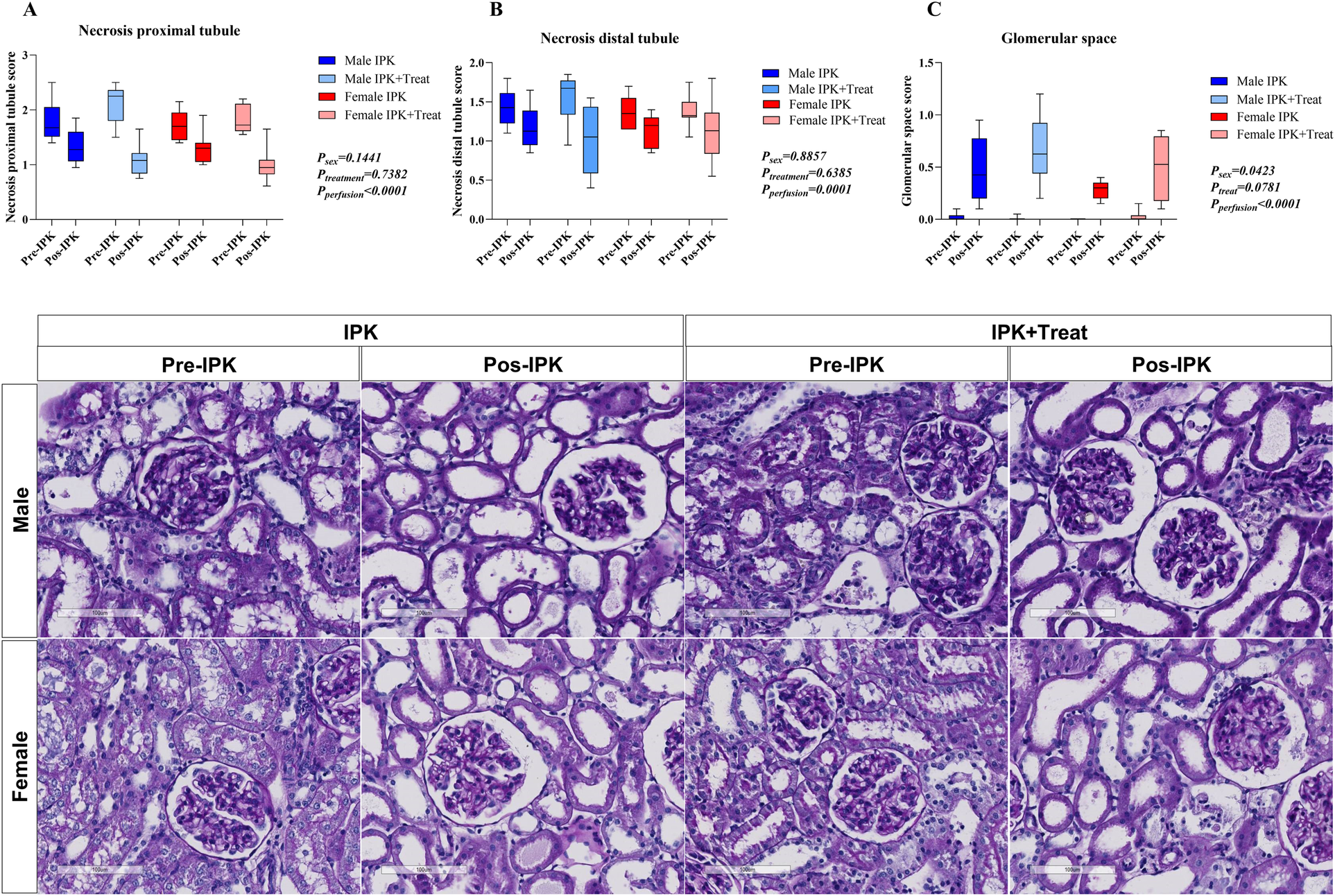
Morphological analysis of kidney tissue. Necrosis of the proximal tubule (A), necrosis of the distal tubule (B), and glomerular space (C). IPK, kidneys that underwent isolated perfusion without treatment; IPK + Treat, kidneys that underwent isolated perfusion with treatment of 17β-estradiol and methylprednisolone. Results are presented as mean and standard error of the mean. Results were analyzed using three-way ANOVA. Representative images are presented (20×).
Discussion
We investigated the effects of E2 and MP on kidney injury during normothermic isolated perfusion to improve graft quality. Previously, Armstrong-Jr et al. (4) highlighted sex differences during perfusion using the same experimental BD model. Their study revealed increased cellular infiltration in males, especially of macrophages, and reduced creatinine clearance compared with females. In our work, we observed a reduction in inflammation in all perfused groups, regardless of treatment and sex, with decreased protein expression of IL-6 in kidney tissues and a reduced presence of leukocytes in the parenchyma. In terms of treatment, female IPK + Treat presented reduced flow compared with female IPK, accompanied by decreased creatinine clearance and increased fractional excretion of sodium, whereas males presented similar renal function results in the treated and non-treated groups.
The first clinical ex vivo kidney perfusion was performed in 2011 to serve as a platform to preserve, assess, and repair kidneys before transplantation (9). Several protocols have been tested to provide a near-physiological environment (10), and studies have linked normothermic kidney perfusion (NKP) to inflammation (11). To date, there is no consensus on the appropriate perfusion conditions and ideal biomarkers for evaluating graft quality. Perfusion solutions usually include red blood cells (RBCs) or even whole blood as oxygen carriers (12), and such compositions can lead to complement activation, even with the use of leukocyte filters or plasma-free perfusate (13). De Beule et al. (14) compared RBCs and whole blood-based solutions during the NKP of ischemic and nonischemic kidneys. Their results point to increased renal damage, with reduced oxygen consumption and increased levels of injury markers in kidneys that underwent warm and cold ischemia periods, with no differences related to perfusate type. These results suggest that increased inflammation during NKP may also be related to reperfusion injury triggered by blood-derived products. In our study, we observed a reduced IL-6 concentration in kidney tissue after perfusion and a reduced presence of infiltrating neutrophils. These findings could be related to the use of RBC-free perfusate, which mitigates the inflammatory response triggered by blood components. No evidence is available on ideal oxygenation during NKP, but most experimental protocols use supraphysiological concentrations, and even though no oxygen carrier was used, our perfusion solution was able to provide pO2 values above 500 mmHg.
Additionally, perfusion flow was evaluated throughout the 90 min of perfusion. In treated females, reduced flow was reflected in lower creatinine clearance rates and increased FENa+. FENa+ is calculated to assess sodium handling by the kidney and is used to evaluate acute kidney injury. Usually, values <1% may suggest prerenal diseases; however, greater reabsorption of Na+ represents an appropriate response to reduced kidney perfusion (15). In a canine model, Dvorak et al. (16) evaluated the administration of several doses of MP during isolated perfusion of kidneys from female dogs and reported that high-dose MP (0.32 mg/ml) led to increased perfusion pressure and reduced perfusion flow. In our study, a similar dose was used (0.40 mg/ml), which could explain the reduction in flow observed in the female IPK + Treat group. In women, pharmacokinetic studies focused on premenopausal kidney transplant recipients have shown that the MP clearance rate is lower in women than in men (17). The same phenomenon is observed in the physiological clearance of MPs (18). However, in the clinic, a standardized dose protocol is used, with no consideration of the age or sex of the patient (19), highlighting the importance of studies focusing on sex differences.
Additionally, activation of the mineralocorticoid receptor (MR) in the kidney by the hormone aldosterone promotes Na+ retention and hypertension. Although MRs present high affinity for aldosterone, other glucocorticoids, such as cortisol, can interact with and activate such receptors (20). Exogenous glucocorticoids were initially considered free of cross-activation of MR; however, Rebuffat et al. (21) reported that dexamethasone was able to occupy MR and promote its translocation to the nucleus. Several studies have shown that female mice and humans present increased expression of endothelial MRs and are at increased risk of vascular damage mediated by MR activation (22–24). In our model, MP administration in females may have activated MR in the renal endothelium, leading to vascular damage. Therefore, we also investigated the modulation of vascular tone by quantifying the protein expression of ET-1 and eNOS in kidney tissue and observed reduced expression of ET-1 in females compared with males. In the literature, studies have shown sex differences in ET-1 expression, with lower levels in females, under both physiological and pathological conditions, and such findings have been associated with higher E2 concentrations (25–27). E2 is also known to stimulate eNOS activation/expression (28, 29), and our group has previously shown that treatment of donors with E2 after BD can increase eNOS (30, 31). Although eNOS is constitutively expressed, several chemical and physical stimuli affect eNOS expression/activity. For example, fluid shear stress generated in vessels during perfusion can activate eNOS mRNA and protein expression (32). Indeed, our results revealed that females presented increased protein expression of eNOS in both perfused groups, especially in the IPK + Treat group.
This study has some limitations. Our protocol uses a small animal model, and correlation to the clinic is limited. Further studies should be conducted in larger animals or discarded human kidneys for a more translational application. Moreover, a transplantation model would also be of interest, aiming to evaluate the effect of the proposed treatment on the recipient.
In conclusion, the data presented in this study show that perfusion alone was able to ameliorate inflammation by reducing the number of infiltrated leukocytes and the expression of IL-6, as well as ameliorating kidney injury by reducing the protein expression of KIM-1 and necrosis of the distal and proximal tubules. Specifically, in males, the use of the proposed treatment had no detrimental effects during perfusion, with the analyzed parameters remaining similar. In females, treatment with E2 and MP had deleterious effects on isolated kidneys, especially by reducing perfusion flow and consequently compromising kidney function. In a parallel study (data not published) using an ex vivo lung perfusion model, the combination of E2 and MP improved lung function in males and modulated inflammation in females, indicating that treatment effectiveness might be organ- and sex-dependent. Subsequent studies should aim to evaluate both male and female subjects, as sex differences may modulate preservation techniques and treatments in positive or negative ways. Thus, personalized protocols could represent the future of graft management.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of the University of Groningen (IACUC-RUG). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MV-d-S: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. RA-J: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. MA: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. LA: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. FR-d-S: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. CC: Data curation, Formal analysis, Funding acquisition, Visualization, Writing – review & editing. PO: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. LM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. AB-F: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. LFPM is a fellow researcher of the CNPq. The author MV-d-S is a scholarship holder by Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP—2020/11211-6 and later 2021/07455-0. The author FR-d-S is a scholarship holder by FAPESP—2021/13020-6. The University of Groningen’s Graduate School of Medical Sciences also provided funding for this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Eurotransplant Annual Report. (2023). Available at:https://www.eurotransplant.org/statistics/annual-report/ (Accessed February 5, 2025).
2.
Westendorp WH Leuvenink HG Ploeg RJ . Brain death induced renal injury. Curr Opin Organ Transpl. (2011) 16:151–6. 10.1097/MOT.0b013e328344a5dc
3.
Breithaupt-Faloppa AC Ferreira SG Kudo GK Armstrong-Jr R Tavares-de-Lima W da Silva LF et al Sex-related differences in lung inflammation after brain death. J Surg Res. (2016) 200(2):714–21. 10.1016/j.jss.2015.09.018
4.
Armstrong-Jr R Ricardo-da-Silva FY Vidal-Dos-Santos M da Anunciação LF Ottens PJ Correia CJ et al Comparison of acute kidney injury following brain death between male and female rats. Clinics (Sao Paulo). (2023) 78:100222. 10.1016/j.clinsp.2023.100222
5.
Maassen H Venema LH Weiss MG Huijink TM Hofker HS Keller AK et al H2S-enriched flush out does not increase donor organ quality in a porcine kidney perfusion model. Antioxidants (Basel). (2023) 12(3):749. 10.3390/antiox12030749
6.
Ogurlu B Pamplona CC Van Tricht IM Hamelink TL Lantinga VA Leuvenink HGD et al Prolonged controlled oxygenated rewarming improves immediate tubular function and energetic recovery of porcine kidneys during normothermic machine perfusion. Transplantation. (2023) 107(3):639–47. 10.1097/TP.0000000000004427
7.
Lohmann S Eijken M Møldrup U Møller BK Hunter J Moers C et al Ex vivo administration of mesenchymal stromal cells in kidney grafts against ischemia-reperfusion injury-effective delivery without kidney function improvement posttransplant. Transplantation. (2021) 105(3):517–28. 10.1097/TP.0000000000003429
8.
Vidal-Dos-Santos M Anunciação LF Armstrong-Jr R Ricardo-da-Silva FY Ramos IYT Correia CJ et al 17β-estradiol and methylprednisolone association as a therapeutic option to modulate lung inflammation in brain-dead female rats. Front Immunol. (2024) 15:1375943. 10.3389/fimmu.2024.1375943
9.
Hosgood S Nicholson M . First in man renal transplantation after ex-vivo normothermic perfusion. Transplantation. (2011) 92:735–8. 10.1097/TP.0b013e31822d4e04
10.
Elliott TR Nicholson ML Hosgood SA . Normothermic kidney perfusion: an overview of protocols and strategies. Am J Transplant. (2021) 21(4):1382–90. 10.1111/ajt.16307
11.
Mellati A Lo Faro L Dumbill R Meertens P Rozenberg K Shaheed S et al Kidney normothermic machine perfusion can be used as a preservation technique and a model of reperfusion to deliver novel therapies and assess inflammation and immune activation. Front Immunol. (2022) 13:850271. 10.3389/fimmu.2022.850271
12.
Hamelink TL Ogurlu B De Beule J Lantinga VA Pool MBF Venema LH et al Renal normothermic machine perfusion: the road toward clinical implementation of a promising pretransplant organ assessment tool. Transplantation. (2022) 106(2):268–79. 10.1097/TP.0000000000003817
13.
de Boer E Sokolova M Jager NM Schjalm C Weiss MG Liavåg OM et al Normothermic machine perfusion reconstitutes porcine kidney tissue metabolism but induces an inflammatory response, which is reduced by complement C5 inhibition. Transpl Int. (2024) 37:13348. 10.3389/ti.2024.13348
14.
De Beule J Keppens D Korf H Jochmans I . Differential cytokine levels during normothermic kidney perfusion with whole blood- or red blood cell-based perfusates—results of a scoping review and experimental study. J Clin Med. (2022) 11:6618. 10.3390/jcm11226618
15.
Okusa MD Chopra TA . Fractional excretion of sodium, urea, and other molecules in acute kidney injury. United States: Wolters Kluwer (2025). Available at:https://www.uptodate.com/contents/fractional-excretion-of-sodium-urea-and-other-molecules-in-acute-kidney-injury# (Accessed February 3, 2025).
16.
Dvorak K Braun W Magnusson M Stowe N Banowsky L . Effect of high doses of methylprednisolone on the isolated, perfused canine kidney. Transplantation. (1976) 21:149–57. 10.1097/00007890-197602000-00010
17.
Tornatore K Gilliland-Johnson K Farooqui M Reed K Venuto R . Pharmacokinetics and pharmacodynamic response of methylprednisolone in premenopausal renal transplant recipients. J Clin Pharmacol. (2004) 44:1003–11. 10.1177/0091270004268130
18.
Ayyar V DuBois D Nakamura T Almon R Jusko W . Modeling corticosteroid pharmacokinetics and pharmacodynamics, part II: sex differences in methylprednisolone pharmacokinetics and corticosterone suppression. J Pharmacol Exp Ther. (2019) 370:327–36. 10.1124/jpet.119.257527
19.
Tornatore KM Biocevich DM Reed K Tousley K Singh JP Venuto RC . Methylprednisolone pharmacokinetics, cortisol response, and adverse effects in black and white renal transplant recipients. Transplantation. (1995) 59(5):729–36. 10.1097/00007890-199503150-00016
20.
Frey FJ Odermatt A Frey BM . Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. (2004) 13(4):451–8. 10.1097/01.mnh.0000133976.32559.b0
21.
Rebuffat AG Tam S Nawrocki AR Baker ME Frey BM Frey FJ et al The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol Cell Endocrinol. (2004) 214(1-2):27–37. 10.1016/j.mce.2003.11.027
22.
Faulkner JL Belin de Chantemèle EJ . Mineralocorticoid receptor and endothelial dysfunction in hypertension. Curr Hypertens Rep. (2019) 21(10):78. 10.1007/s11906-019-0981-4
23.
Faulkner JL Kennard S Huby AC Antonova G Lu Q Jaffe IZ et al Progesterone predisposes females to obesity-associated leptin-mediated endothelial dysfunction via upregulating endothelial MR (mineralocorticoid receptor) expression. Hypertension. (2019) 74(3):678–86. 10.1161/HYPERTENSIONAHA.119.12802
24.
Faulkner JL Harwood D Kennard S Antonova G Clere N Belin de Chantemèle EJ . Dietary sodium restriction sex-specifically impairs endothelial function via mineralocorticoid receptor-dependent reduction in NO bioavailability in balb/C mice. Am J Physiol Heart Circ Physiol. (2020) 320(1):H211–20. 10.1152/ajpheart.00413.2020
25.
Miyauchi T Yanagisawa M Iida K Ajisaka R Suzuki N Fujino M et al Age- and sex-related variation of plasma endothelin-1 concentration in normal and hypertensive subjects. Am Heart J. (1992) 123(4 Pt 1):1092–3. 10.1016/0002-8703(92)90734-d
26.
Polderman KH Stehouwer CD van Kamp GJ Dekker GA Verheugt FW Gooren LJ . Influence of sex hormones on plasma endothelin levels. Ann Intern Med. (1993) 118(6):429–32. 10.7326/0003-4819-118-6-199303150-00006
27.
Polderman KH Stehouwer CD van Kamp GJ Schalkwijk CG Gooren LJ . Modulation of plasma endothelin levels by the menstrual cycle. Metabolism. (2000) 49(5):648–50. 10.1016/s0026-0495(00)80042-6
28.
Goetz R Thatte H Prabhakar P Cho M Michel T Golan D . Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. (1999) 96(6):2788–93. 10.1073/PNAS.96.6.2788
29.
Musicki B Liu T Strong T Lagoda G Bivalacqua T Burnett A . Post-translational regulation of endothelial nitric oxide synthase (eNOS) by estrogens in the rat vagina. J Sex Med. (2010) 7(5):1768–77. 10.1111/j.1743-6109.2010.01750.x
30.
Armstrong-Jr R Ricardo-da-Silva FY Correia CJ Vidal-Dos-Santos M da Anunciação LF Coutinho E Silva RS et al Treatment with 17β-estradiol protects donor heart against brain death effects in female rat. Transpl Int. (2020) 33(10):1312–21. 10.1111/tri.13687
31.
Vieira RF Breithaupt-Faloppa AC Matsubara BC Rodrigues G Sanches MP Armstrong-Jr R et al 17β-estradiol protects against lung injuries after brain death in male rats. J Heart Lung Transplant. (2018 Nov) 37(11):1381–7. 10.1016/j.healun.2018.06.015
32.
Balligand JL Feron O Dessy C . eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. (2009) 89(2):481–534. 10.1152/physrev.00042.2007
Summary
Keywords
brain death, sex differences, machine perfusion, kidney, rat
Citation
Vidal-dos-Santos M, Armstrong-Jr R, de Assis Ramos MM, da Anunciação LF, Ricardo-da-Silva FY, Correia CdJ, Ottens PJ, Moreira LFP, Leuvenink HGD and Breithaupt-Faloppa AC (2025) Males and females respond differently to treatment during isolated kidney perfusion: combined effects of glucocorticoid and estradiol. Front. Transplant. 4:1595766. doi: 10.3389/frtra.2025.1595766
Received
18 March 2025
Accepted
12 May 2025
Published
30 May 2025
Volume
4 - 2025
Edited by
Sarah Tsou, Brigham and Women's Hospital and Harvard Medical School, United States
Reviewed by
Bulang He, Austin Hospital, Australia
Mukhammad Kayumov, Harvard Medical School, United States
Updates
Copyright
© 2025 Vidal-dos-Santos, Armstrong-Jr, de Assis Ramos, da Anunciação, Ricardo-da-Silva, Correia, Ottens, Moreira, Leuvenink and Breithaupt-Faloppa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Ana Cristina Breithaupt-Faloppa ana.breithaupt@hc.fm.usp.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.