- 1Department of Biomedical Engineering, Northwestern University, Chicago, IL, United States
- 2Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
Introduction: Islet transplantation offers a potential curative treatment for patients with type 1 diabetes (T1D). To make this therapy widely available, a stable supply chain of human islets is essential. Developing techniques like cryopreservation and culture for long-term islet storage, or islet banking, with minimal functional loss would strengthen this supply chain. This study provides a systematic review of the current methods for long-term human islet storage.
Methods: A search strategy and query were developed according to the PICO framework. We included studies published on PubMed, Embase, and Web of Science from inception until August 2024.
Results: 6,945 studies were screened with 47 meeting criteria for full text extraction. The primary outcomes recorded were measures of islet viability and glucose stimulated insulin secretion. Optimization of culture parameters such as temperature, medium selection, and scaffolds can extend islet viability and function.
Discussion: Recent studies on human islet cryopreservation report promising results for long-term storage; however, the field remains underexplored. Several cytoprotective supplements with potential utility across both culture and cryopreservation conditions have also been reviewed. Although long-term islet storage has been a critical focus since the advent of the Edmonton protocol, the literature lacks the rigor needed to drive clinical translation. Notably, we observe substantial variability in experimental design and reported outcomes, which complicates meaningful comparison between interventions.
1 Introduction
In June 2023, the Food and Drug Administration approved Lantidra, the first allogeneic pancreatic islet therapy, for treating patients with type 1 diabetes (T1D) experiencing severe hypoglycemia (1). While patients receiving Lantidra must undergo immunosuppressive therapy, this approval signals a potential future where islet transplantation could become a curative option for all T1D patients. However, two major obstacles must be overcome to realize this future fully: the need for immunosuppression and the limited supply of islets. Here, we focus on the challenge of islet shortage. Current potential sources of islets include human, xenogeneic, and stem cell-derived islets. Each of these options presents unique challenges. Immunosuppressive protocols have yet to be optimized to enable clinical xenogeneic islet transplants. Stem cell-derived islets, while promising, also carry risks, including the potential for teratoma formation (2). At this point in time, human islets are the most suitable for transplant. However, the current supply of human islets cannot meet the demand of all existing and newly diagnosed patients.
Approximately 7,000 pancreases are donated each year in the United States (3). The timing and geographical constraints of deceased donor transplantations limit this number. With 64,000 newly diagnosed cases of T1D every year (4), this supply of pancreata is not enough for curative treatment of new T1D patients, much less the existing population of 2 million. In addition, it is unclear whether each pancreas would supply the recommended 5,000 islet equivalents (IEQ)/kg for insulin independence in a patient (5). Islet isolation after pancreas harvesting leads to a 15%–50% reduction in islet mass and function (6). Further loss of islet viability occurs during transplantation and engraftment. If islets could be stored for extended periods, the geographic pool of viable recipients could be expanded, and islets could be banked to build a sufficient supply of necessary IEQs for each patient. However, the clinical standard for islet preservation only makes them viable for transplantation for a few days after isolation. Possible solutions to long-term storage include optimized culture conditions and cryopreservation. Islet culture occurs in an enriched medium at physiologic temperatures (37°C) (7). Islets die quickly in culture due to inadequate oxygen delivery to the center of the cell clusters (8). Cryopreservation involves freezing islets to ultra-low temperatures (−196°C) using liquid nitrogen (9). Ultra-low temperatures drastically reduce the biological and chemical activity of cells, limiting energy consumption and cell death (10). Optimization of both methods is measured by islet death and the loss of islet function. In this systematic review, the current state of long-term human islet storage, via culture and cryopreservation is summarized. In addition, cytoprotective supplements, such as antioxidants and oxygen carriers, and in vivo experimentation with stored human islets are reviewed.
2 Methods
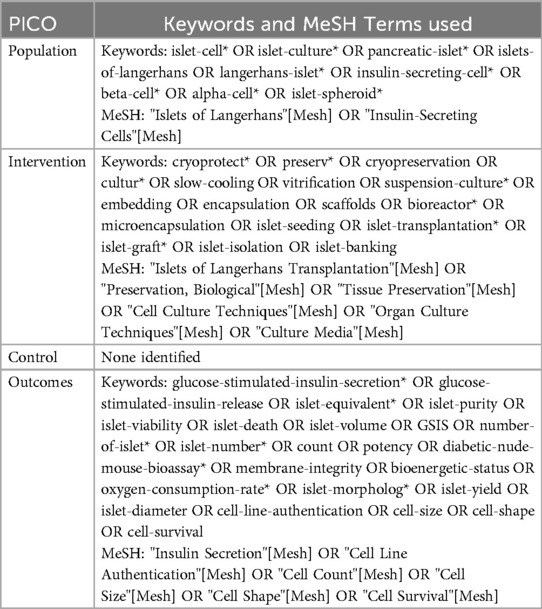
The PRISMA 2020 guidelines and PICO framework were utilized to develop this systematic review (11). The PICO or population, intervention, control, outcome framework is a widely used approach to boolean query of scientific databases (12). Specifying key terms for each component of PICO ensures accurate knowledge representation of a research question that will capture all available studies that are related (13). A PICO framework search query was developed focused on the research question “What are the best techniques for ex vivo human islet cell preservation as measured by islet viability and glucose sensitive insulin secretion?” was developed in coordination with Northwestern University Galter Library Systematic Review Services. The population was identified as adult human islets, intervention was identified as islet preservation by cryopreservation or culture, a control was defined as freshly isolated human islets but was not used in the search, and outcome was identified as glucose-stimulated insulin release (GSIS) or islet viability. The search was limited to studies using human islets only to maximize the clinical relevance of this review as non-human islet models have significantly different architecture and biochemistry (14, 15). The full PICO-based query is reported in Table 1. This query was used to extract studies from PubMed, Embase, and Web of Science.
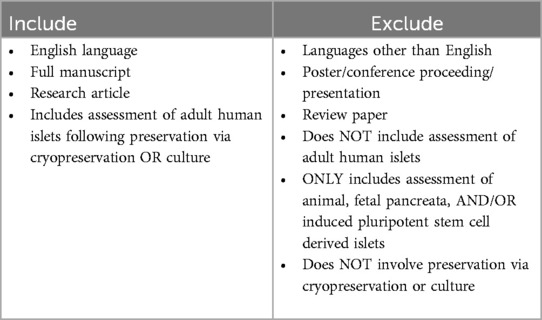
Deduplication and screening of query results was carried out using the Rayyan platform (16). Query records were deduplicated by manual review of text with exact Title, Author, and Year matches by ARC. Inclusion and exclusion criteria specified in Table 2 were used by ARC and JAB to screen abstracts. All possible inclusions were reviewed again by ARC. Conflicts were resolved via discussion between ARC and JAB.
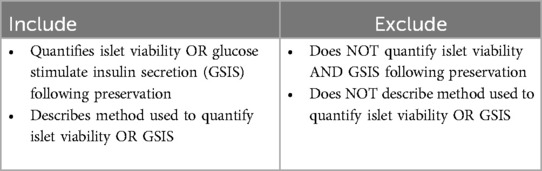
Full text retrieval and extraction were performed by ARC and JC. Eligibility of the full text was evaluated based on the criteria in Table 3. Alongside measurements of viability and GSIS, methods and associated storage time and temperature were summarized for each study and associated treatment groups. Due to lack of standardized measures of islet viability and GSIS, units were collected for each study.
3 Results
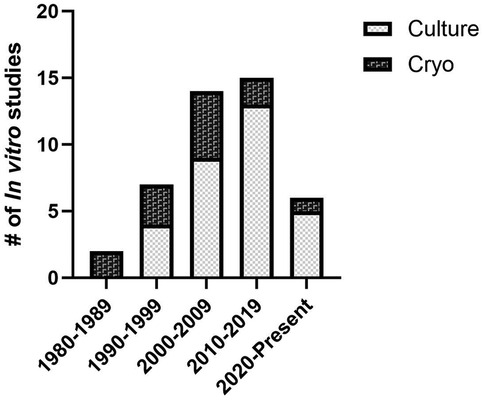
A total of 47 studies were included in the systematic review. Of these studies 66% involved only in vitro assessment, 6% involved only in vivo assessment, and 28% involved both in vitro and in vivo methods of assessment (Figure 1). Two general methods of preservation were utilized: culture (> 0°C) and cryopreservation (< 0°C) (Figure 2). Approximately 66% of studies used culture and 33% used cryopreservation (Figure 1).
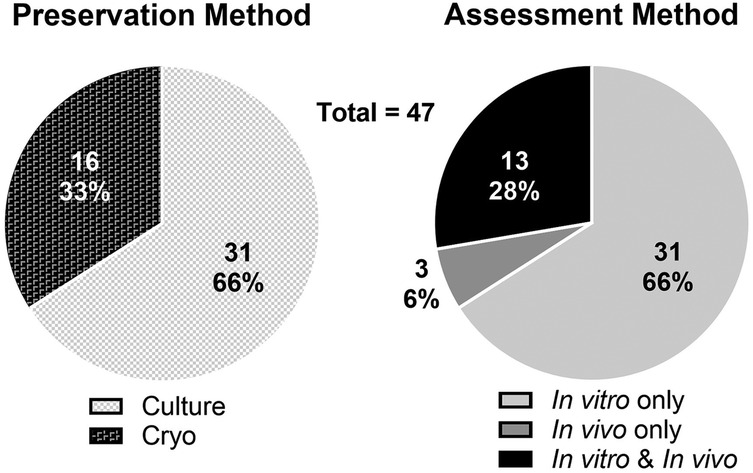
Figure 1. Distribution of preservation methods (culture or cryopreservation; left) and assessment methods (in vivo and/or in vitro experimentation; right) of the 47 reviewed studies.
3.1 Islet culture
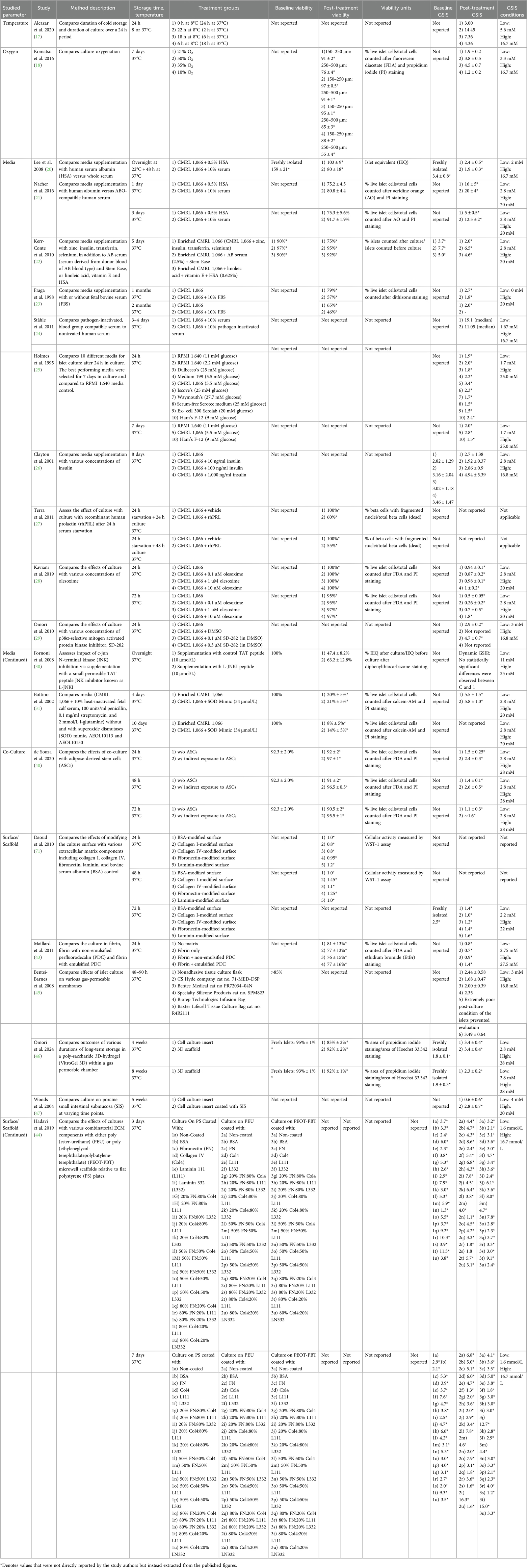
Islet culture studies were categorized by manipulation of temperature, oxygen conditions, media composition, use of scaffolds or alternative culture surfaces and co-culture. Most studies (21 of 31 studies) involved manipulation of a single factor (Table 4). Several other studies manipulated multiple factors (10 of 31 studies; Table 5).
3.1.1 Temperature
Cold cell culture has been associated with prolonged cell viability, as metabolic processes slow down, thereby reducing protein degradation. Alcazar et al. 2020 focused their investigation on the duration of cold culture (8°C) over a 24-hour period and the resulting effects on islet function (17). A longer cold storage period was associated with a higher dynamic GSIS index.
3.1.2 Oxygen
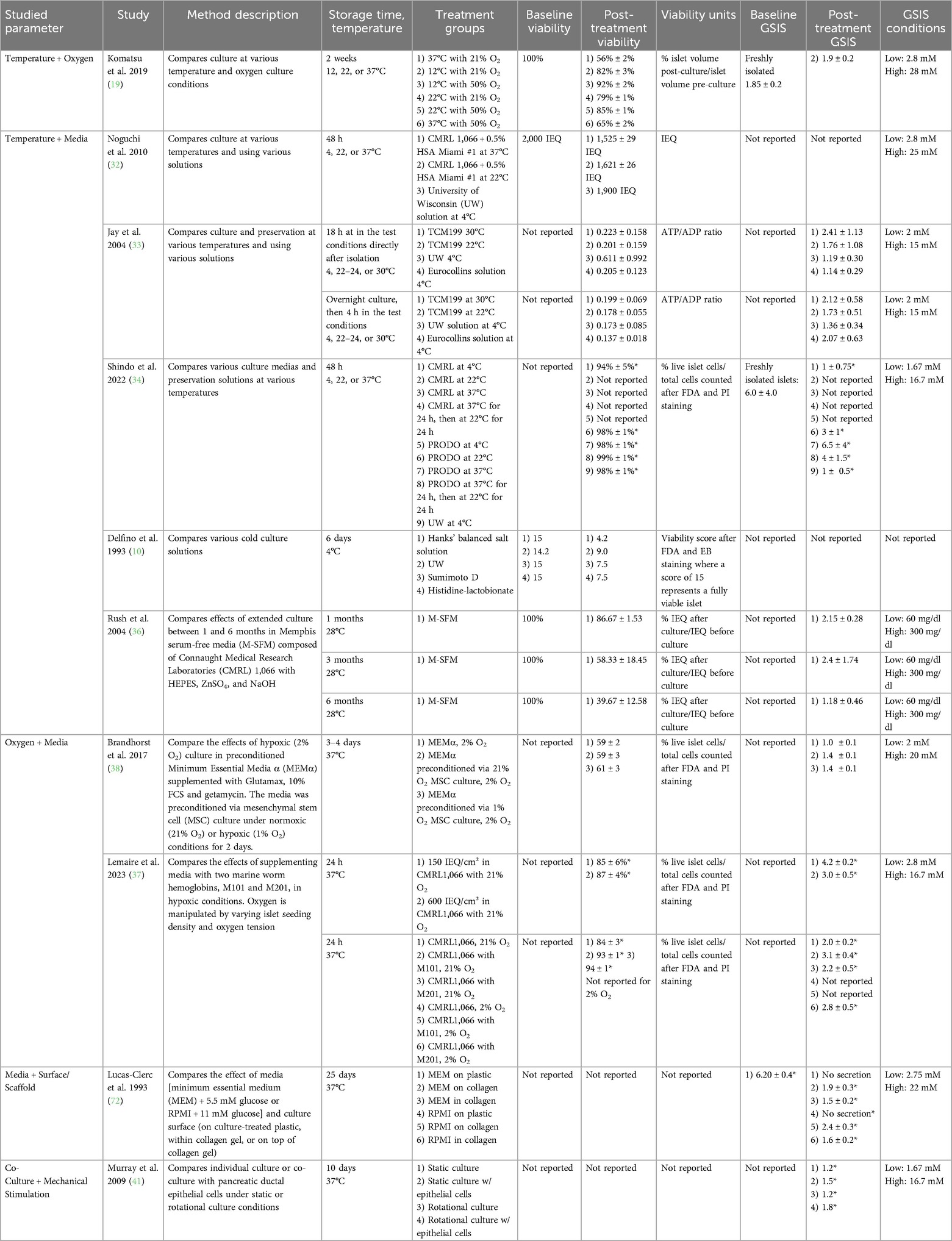
Another critical factor for islet viability and function is oxygenation. Komatsu et al. 2016 studied varied oxygen tensions (10%, 21%, 35%, 50%) over a 7-day culture period at 37°C, concluding that hyperoxia (35%, 50%) helps maintain islet volume and GSIS (18). A further study builds on this work by investigating the combined effects of optimizing temperature and oxygen conditions in islet cultures. Via a 2-week islet culture, Komatsu et al. 2019 explored several temperatures (12°C, 22°C, 37°C) combined with oxygenation adjustments (21%, 50%) on a 2-week culture (19). The most effective combination, 12°C with 50% oxygenation, was not statistically significantly different from freshly isolated islets in terms of viability or GSIS (19).
3.1.3 Media composition
Twelve studies investigated islet culture medium composition alone. An additional 9 studies focused on the impact of media in combination with another factor, such as temperature, oxygen or scaffold.
Connaught Medical Research Laboratories 1,066 medium (CMRL 1,066) has been widely used in pre-transplantation islet culture studies due to its ability to inhibit β-cell depolarization, preserve cellular function, and enhance glucose responsiveness (7). Lee et al. 2008 and Nacher et al. 2016 both compared CMRL 1,066 islet culture media supplemented with 10% human serum vs. 0.5% human albumin (20, 21). While both groups cultured the human islets for 3 days at 37°C, these studies provided conflicting evidence. Lee et al. 2008 concluded that albumin is superior to human serum (20), while Nacher et al. 2016 reported that human serum more effectively preserves islet viability and GSIS (21). Kerr-Conte et al. reported that 2.5% human serum was superior to 0.625% albumin for both 1 and 5 day culture (22). For long-term storage, Fraga et al. 1998 found that serum-free islet culture led to better viability and function as compared to culture supplemented with 10% FBS (23). Ståhle et al. found that pathogen-inactivation of serum did not influence islet outcomes. Discrepancies between the investigations may have resulted from differences in other conditions, such as culture temperature, in addition to methodology for assessing islet viability and GSIS (24).
Insulin and glucose concentrations in culture also affect islet function. Holmes et al. 1995 cultured islets for 1 week in media formulations with various glucose concentrations ranging from 2.2 to 27.7 mM (25). Holmes and colleagues found that CMRL 1,066 supplemented with 5 mM (90 mg/dl) glucose yields the highest GSIS after both 24 hours and 7 days in culture (25). Variability between isolations prevented Clayton et al. 2001 from making conclusions regarding the effects of insulin concentration in culture medium on islet viability and function (26).
Other studies utilized media additives that have been shown to mitigate cellular apoptosis [e.g., human recombinant prolactin (rhPRL), olesoxime] (27, 28), inhibit proinflammatory cytokine production [e.g., p38α-selective mitogen activated protein kinase inhibitor SD-282 (29), c-Jun N-terminal kinase inhibitor L-JNKI (30)], or break down toxic superoxide radicals [e.g., superoxide dismutase (SOD) mimics] (31).
Five studies combine alterations in temperature and media. A commonality among many of the studies was to assess culture in various mediums at 22°C and 37°C and compare to cold culture in various organ preservation solutions at 4°C (32–35). There was not a consensus regarding the optimal temperature for islet preservation. For 4°C storage, all four studies showed that University of Wisconsin (UW) solution, commonly used for solid organ flushing and cold storage, was associated with the best outcomes. Other studies fixed temperature and assessed alternative solutions. For example, Rush et al. 2004 cultured islets in serum-free media at 28°C for 6 months and demonstrated marginal viability and function (36).
A single study assessed both oxygen and media supplementation (37). Marine worm hemoglobins M101 and M201 were evaluated as a supplement to human islet culture at normoxic and hypoxic conditions due to its associated anti-inflammatory and antioxidant properties. Moreover, these hemoglobins were investigated as oxygen carriers due to their high oxygen-binding capacity, which may help mitigate the hypoxic conditions commonly encountered during pre-transplant islet storage. Oxygen conditions were manipulated either by modifying islet seeding density or oxygen tension. In both normoxic and hypoxic conditions, the marine worm hemoglobin improved islet viability and glucose stimulation index (GSI)—a ratio reflecting insulin secretion at high vs. low glucose derived from the GSIS assay—compared to islets cultured in unsupplemented media.
Brandhorst et al. 2017 cultured islets under hypoxic conditions (2% oxygen) in mesenchymal stem cell (MSC) preconditioned medium under normoxic (21% oxygen) or hypoxic (1% oxygen) conditions (38). MSCs are multipotent stromal cells derived from connective tissues, with immunomodulatory and regenerative properties, including the secretion of anti-inflammatory proteins and growth factors that may prevent β-cell apoptosis and support islet cell survival and function (39). The preconditioned media improved GSI relative to the control. No difference in GSI was observed between the preconditioned media from MSCs cultured under normoxic or hypoxic conditions.
3.1.4 Co-culture
Additionally, co-culturing islets with other cell types has shown promise in enhancing islet health and reducing cellular stress. Stem cells or epithelial cells have been reported to generate a supportive microenvironment for islets (38, 40, 41). After 72 hour culture, islets cocultured with indirect contact to adipose-derived stem cells were 95.2 ± 1% viable with GSIS of 1.6 compared to viability 90.5 ± 2% with GSIS 1.1 ± 0.3 without coculture (40). While pancreatic ductal cell co-culture had some preservative effect on islet GSIS after 10 days in culture relative to islets cultured alone, significance was only observed when cultured in a rotational system (41).
3.1.5 Culture surfaces and scaffolds
Seven studies utilized modified culture surfaces or scaffolds in efforts to improve viability by enhancing engraftment and oxygen delivery. Most of these studies (4 of 6) focused on creating culture surfaces that mimic the native extracellular matrix (ECM). Daoud et al. 2010 and Maillard et al. 2011 assessed ECM-component scaffolds and fibrin matrices with perfluorodecalin (PDC) (42, 43). Daoud's study utilized a poly(lactide-co-glycolide) acid (PGLA) scaffold embedded with collagen I gel, fibronectin, and collagen IV. By optimizing pore size, after 10 days in culture, islets showed GSIS on par with freshly isolated islets (42). Maillard's work found that fibrin with emulsified PDC decreased hypoxia and improved GSIS after 24 hours in culture (43).
Hadavi et al. 2019 found that functionalization of a scaffold with ECM components was more important than the choice of material for the scaffold. Both Hadavi et al. 2019 and Daoud et al. 2011 found that displaying a combination of ECM components (as compared to a single component) was critical to preserve islet viability and function long term (42, 44).
Two studies focused on investigating gas-permeable membranes as alternatives to a traditional culture flask (45, 46). Bentsi-Barnes et al. 2008 investigated a variety of commercial membranes and found that after 48 hours of culture, the Baxter Lifecell Tissue culture bag most effectively preserved GSIS (45). When cultured on other gas-permeable membrane products, islets did not survive or showed functional decline inferior to non-adherent tissue culture flasks (45). Omori et al. 2024 found that human islets cultured on poly-saccharide 3D-hydrogel (VitroGel 3D) within a gas permeable chamber had enhanced viability after 4 weeks in culture, but no difference in GSI compared to islets cultured in suspension (46).
In contrast, Woods et al. 2004 explored using porcine small intestinal submucosa as a substrate for functional islet recovery (47). After 5 weeks in culture, islets on small intestinal submucosa had a GSI of 2.8 ± 0.7 compared to 0.6 ± 0.6 for control islets.
Early experimentation by Lucas-Clerc et al. 1993 assessed both culture surface and media composition. Islets cultured on plastic were compared to those cultured in or on collagen gel. Additionally, MEM + 5.5 mM glucose was compared to Roswell Park Memorial Institute 1640 Medium (RPMI) + 11 mM glucose. RPMI is rich in amino acids, vitamins, glucose, salts, and a bicarbonate buffer that are biochemically necessary for cell survival. After 17 days in culture, islets cultured on plastic had no secretion response to glucose stimulation, while those cultured in or on collagen gel retained some responsiveness (GSI: 1.50–2.40). Islets cultured on collagen retained function in a superior manner (GSI: 1.90–2.40) to those cultured in the collagen (GSI: 1.50–1.60). RPMI + 11 mM glucose (GSI: 1.60–2.40) was found to be superior to MEM + 5.5 mM glucose (GSI: 1.50–1.90) for both islets cultured in and on collagen (40).
A comprehensive summary of all reviewed papers on islet culture is provided in Table 4 (Single Factor) and 4 (Multiple Factors).
3.2 Cryopreservation
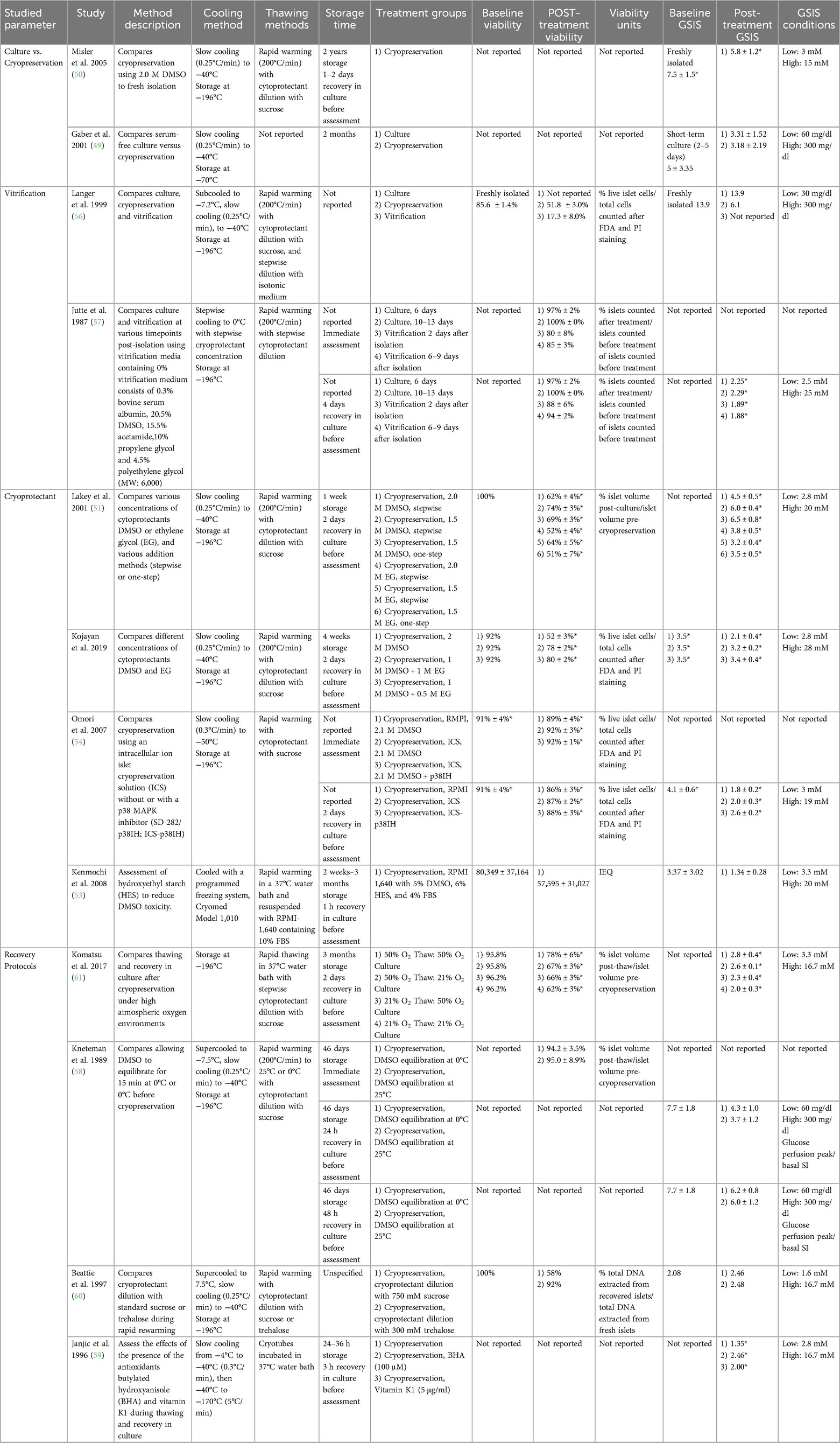
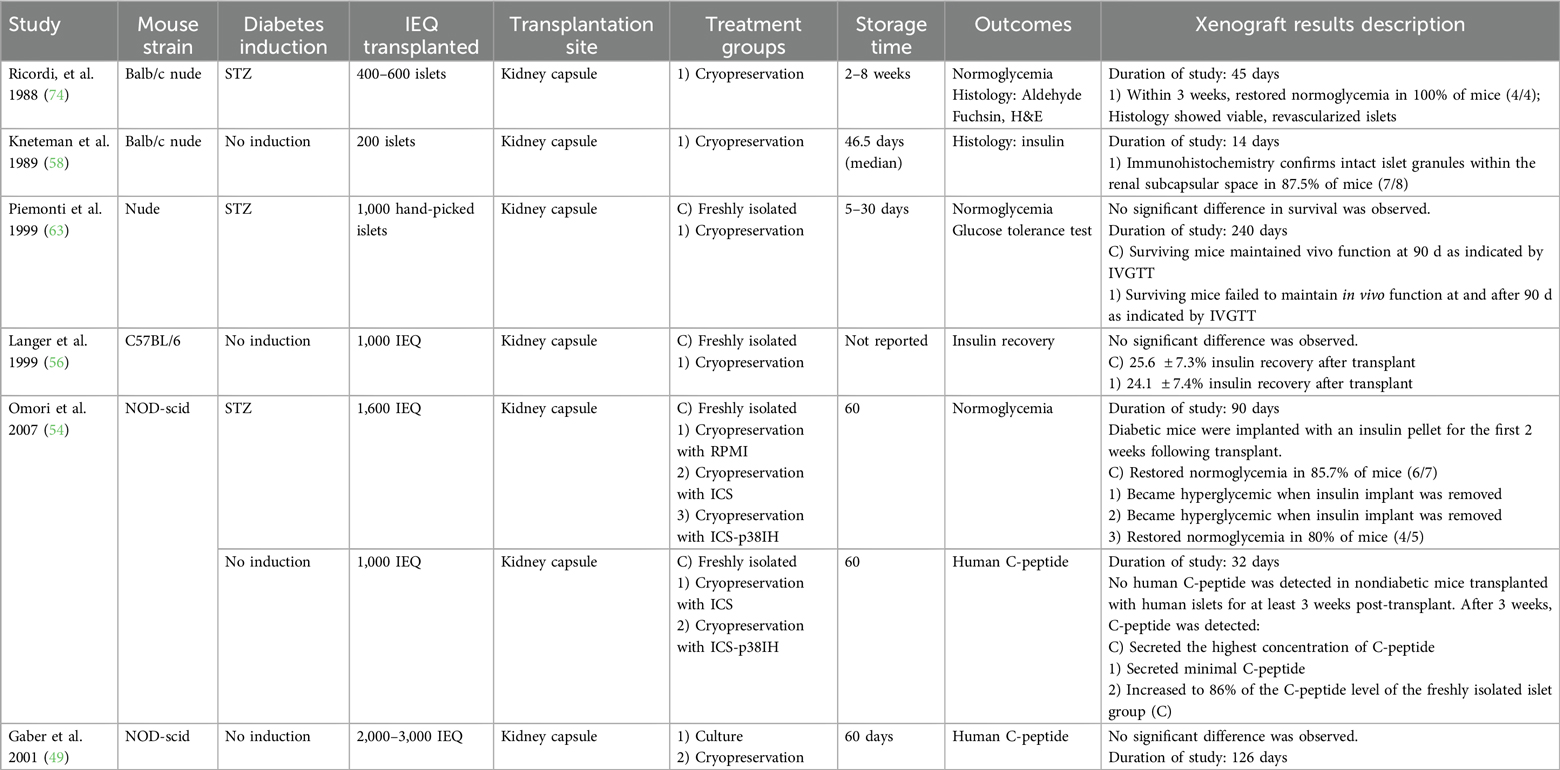
Cryopreservation is a promising alternative strategy for islet preservation, in which cells are frozen to −196°C in order to arrest cellular metabolism. When frozen, water no longer solvates solutes, creating an increasingly concentrated solution that causes cell injury via osmotic dehydration (48). Cryoprotectant selection is critical to mitigating damage to islets during the cryopreservation process. Cryoprotectant prevents ice crystal formation from damaging cells by permeabilizing the cell membrane. However, cell membrane permeabilization can also be toxic, impairing functional recovery. Herein, 13 studies utilizing cryopreservation to preserve islets were analyzed (Tables 6, 7). While islet (1–3 months) culture outcomes are superior at early timepoints (49), Misler et al. 2005 found that islets could be preserved via cryopreservation using dimethyl sulfoxide (DMSO) for 2 years. After 1 or 2 days of recovery in culture, insulin secretion and single-cell action potential were not statistically significantly different from fresh islets (50).
Many studies have compared various concentrations of cryoprotectants DMSO and ethylene glycol (EG). Work by Lakey et al. 2001 compared various concentrations (1.5 M and 2.0 M) of DMSO and EG, added to the culture in a stepwise manner or all at once. DMSO yielded greater islet post-thaw recovery as compared to EG. 1.5 M DMSO yielded superior post-cryopreservation viability and GSIS as compared with 2.0 M treatment. No significant difference was observed between stepwise and one-step addition (51). Kojayan et al. 2019 compared 2 M DMSO alone and 1M DMSO plus 0.5 or 1M EG. Results indicated that 1 M DMSO with 0.5 M EG was the most effective (52). Kenmochi et al. 2008 found that the addition of hydroxyethyl starch (HES) could be used to reduce the required concentration of DMSO, thereby reducing associated toxicity (53). Of note, no controls assessments were used in Kenmochi's study.
In addition to combatting cellular damage from ice crystal formation, supplements have been used to inhibit inflammatory processes. Omori et al. 2007 found that supplementation of an intercellular cryopreservation solution with p38 inhibitor SD-282 enhanced post-storage GSIS relative to conventional medium or intracellular during islet cryopreservation (54).
3.2.1 Vitrification
Vitrification is a type of cryopreservation in which freezing occurs more quickly, preventing ice crystals from forming. Vitrification requires direct plunge of cells treated with vitrification solution into −196°C liquid nitrogen. Theoretically, supercooling of the cryoprotective solution solidifies it into a metastable, highly viscous glass phase that limits ice formation, molecular diffusion, and metabolic activity. To achieve vitrification rapid cooling and rewarming occur at a rates of approximately −200°C/min and 250°C/min respectively (55). However, in the studies reviewed herein, vitrification failed to result in superior outcomes with respect to islet viability or function post-storage (56, 57).
3.2.2 Thawing
In addition to the freezing process, islet thawing can also impact islet viability. Kneteman et al. 1989 studied the impact of the rewarming temperature after DMSO cryopreservation (58). Islets were rapidly warmed to 0°C or 25°C. However, no significant difference was observed between the treatment groups. A few years later, Janjic et al. 1996 and Beattie et al. 1997 reported that the addition of agents that combat DMSO toxicity during rewarming improved outcomes for islets (59, 60). Janjic and coauthors demonstrated that the addition of antioxidants butylated hydroxyanisole (BHA) or vitamin K1 during thawing and recovery improved GSI. Beattie et al. showed that substituting the sucrose in cryoprotectant dilution solution with trehalose improved islet viability as measured via extracted DNA, however no difference was observed in GSI (60). Komatsu et al. 2017 exposed islets to high atmospheric oxygen during the thawing process. GSIS was found to be the highest in the treatment group that received the highest oxygen concentration during thawing (50%) and culture (50%) (61).
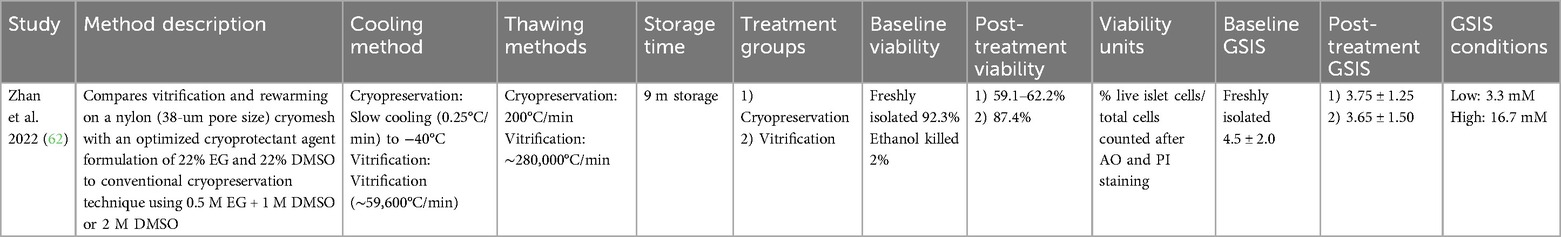
Zhan et al. optimized many of the previously discussed factors impacting cryopreservation (62). This group used vitrification to both quickly freeze and thaw islets on a nylon cryomesh in an optimized cryopreservation solution consisting of 22% DMSO and 22% EG. The optimized techniques enabled islet storage for 9 months with minimal reduction in viability and GSI.
3.3 In vivo experiments
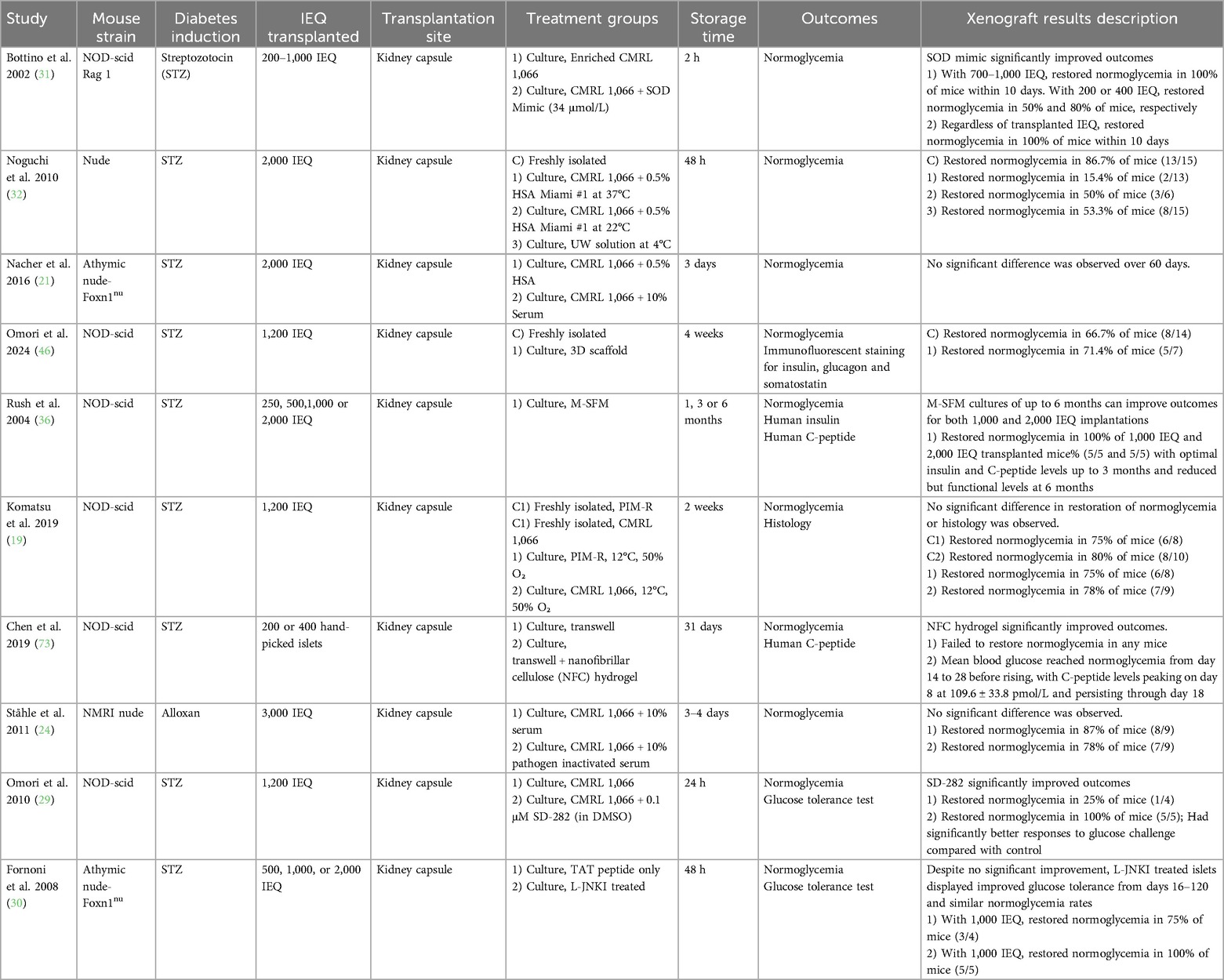
Of the 47 studies included in this systematic review, 13 conducted additional in vivo experiments following in vitro work, while 3 other studies involved only in vivo testing. Seven studies utilized culture storage techniques (Table 8), and 9 studies utilized cryopreservation (Table 9). All these in vivo experiments involved transplanting stored human islets into the renal subcapsular space in an animal model. Immunocompromised mice were used in all studies, except for one, in which immunocompetent C57BL/6 mice were used (56). Most studies utilized nonobese diabetic-severe combined immunodeficiency (NOD-scid). Other studies used Rag1, BALB/C nude, NMRI nude, or athymic nude-Foxn1nu. Two studies reported the use of nude mice without further clarification (32, 63).
In most studies, the rodents were rendered diabetic via chemical induction with streptozotocin or alloxan. In 3 studies, diabetes was not induced (49, 56, 58). Between 200 and 3000 IEQ were transplanted. 10 studies involved cultured islets, and 6 studies involved cryopreservation.
In all studies, islets were transplanted to the kidney capsule. Stored islets reversed diabetes in animal models at similar rates to fresh islets in most studies, although islet equivalents were often equal despite greater loss of viable islets in the long-term storage treatment groups. For transplantation studies, the reported measurements varied greatly between studies. Studies reported oral glucose tolerance tests, C-peptide levels, and blood glucose levels at various timepoints and frequencies. Endpoints for sacrifice and islet morphological analysis ranged from 14 days post-transplantation to up to 126 days.
4 Discussion
Experimentation with human islet storage, both via culture and cryopreservation, shows promising results for a future where islets can be banked for effective islet transplantation in as many patients as possible. Lowering culture temperatures, increasing oxygenation, and utilizing ECM-component scaffolds can all improve the viability and function of islets in culture. For cryopreservation, optimization of cryoprotectant concentrations and oxygenation while thawing can reduce islet loss. Culture and cryopreservation supplementation offer further mitigation of the stress-induced damage that islet cells incur.
Study limitations include the heterogeneity of results and methods reported in the reviewed studies. The National Institutes of Health Clinical Islet Transplantation (NIH CIT) consortium established a standard operating procedure for glucose stimulated insulin secretion in 2014 with low glucose concentrations of 2.8 mM and high glucose concentrations of 28 mM (64). Many studies occurred before publication of this SOP and its widespread implementation. While GSIS was a ubiquitous measure of islet function used in the studies reviewed, low and high glucose concentrations used varied widely.
Since the focus of this systematic review was cryopreservation and culture techniques with clinical applicability, the study population was limited to human islets. Many studies relevant in terms of topic were not relevant in terms of population. Human islet preservation remains relatively underexplored compared to experimentation with islet models derived from animals. Advances in scaffolding and reaggregation of cryopreserved human islets with the Insphero 3D InSight Islet Biology Platform may accelerate the study of human islet preservation (65).
This study was limited to cryopreservation and did not explore high subzero methods of preservation such as supercooling, partial freezing, and isochoric subzero. Studies in solid organ preservation using high subzero techniques have shown promise in human liver and rat liver and heart models (66, 67). Another promising approach to addressing the limited supply of freshly isolated human islets that was not explored in this review is utilization of human stem cell derived islets. These clinical trials have investigated the efficacy and safety of autologous and allogeneic mesenchymal stem cell derived islet-like organoids for type 1 and type 2 diabetes therapy (68). Wang et al.'s transplantation of chemically induced pluripotent stem cells into the anterior abdominal rectus sheath of a Type 1 Diabetic patient on preexisting immunosuppression for a liver transplant showed sustained insulin independence, lowered HbA1C, and improved glucose response to oral glucose tolerance test 1-year post transplantation (69). Recently, the VX-880-101 FORWARD study of zimislecel, Vertex Pharmaceuticals’ allogeneic stem cell-derived islet-cell therapy, published promising phase 1–2 study results (70). While the study size is small (n = 14), long-term follow up shows significant sustained decreases in HbA1C, total daily insulin dose, and time out of target glucose range (70–180 mg/dl) (70). At day 365, 10 of 12 participants achieved insulin independence (70).
Zhan et al.'s cryopreservation study highlights that optimizing multiple factors is essential to achieving long-term islet viability and function (62). Success in this complex field also demands a multidisciplinary approach and diverse expertise. Optimization of cryopreservation parameters of human islets remains a relatively underexplored field compared to that of human islet culture. Most studies in this systematic review report on the results of cryopreservation alone or compare cryopreservation to similar length cultures. Extending the possible lifespan of freshly isolated islets is a new opportunity. The ability to stockpile islets for “off the shelf” transplantation would greatly improve the treatment options for patients, especially those outside of Chicago, where Lantidra treatment is currently available. As the market for Lantidra grows, cryopreserved human islets’ impact upon FDA approval will also grow.
Author contributions
AC: Methodology, Data curation, Writing – review & editing, Investigation, Conceptualization, Writing – original draft, Formal analysis. JC: Writing – original draft, Writing – review & editing, Investigation, Visualization, Data curation, Formal analysis, Validation. JB: Writing – review & editing, Funding acquisition, Writing – original draft, Visualization, Conceptualization, Supervision, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by The Northwestern Summer Research Program for Medical Students (NIH NIDDK T35 5T35DK126628-04), in addition to a Strategic Research Agreement (3-SRA-2023-1389-S-B) and Diversifying Diabetes Research Talent in Academia Award (2-SRA-2023-1452-S-B) from BreakthroughT1D (formerly JDRF).
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
JB has financial interests in SNC Therapeutics, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kempler C. FDA approves first cellular therapy to treat patients with type 1 diabetes [press release]. (2023).
2. Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. (2005) 166(6):1781–91. doi: 10.1016/S0002-9440(10)62488-1
3. Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002–18: results from the population-based SEARCH for diabetes in youth study. Lancet Diabetes Endocrinol. (2023) 11(4):242–50. doi: 10.1016/S2213-8587(23)00025-6
4. Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. (2017) 15(1):199. doi: 10.1186/s12916-017-0958-6
5. Walker S, Appari M, Forbes S. Considerations and challenges of islet transplantation and future therapies on the horizon. Am J Physiol Endocrinol Metab. (2022) 322(2):E109–E17. doi: 10.1152/ajpendo.00310.2021
6. Al-Adra DP, Gill RS, Imes S, O'Gorman D, Kin T, Axford SJ, et al. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. (2014) 98(9):1007–12. doi: 10.1097/TP.0000000000000217
7. Murdoch T, McGhee-Wilson D, Shapiro A, Lakey J. Methods of human islet culture for transplantation. Cell Transplant. (2004) 13(6):605–18. doi: 10.3727/000000004783983602
8. Papas KK, Colton CK, Gounarides JS, Roos ES, Jarema MAC, Shapiro MJ, et al. NMR spectroscopy in β cell engineering and islet transplantation. Ann N Y Acad Sci. (2001) 944(1):96–119. doi: 10.1111/j.1749-6632.2001.tb03826.x
9. Rajotte RV. Islet cryopreservation protocols. Ann N Y Acad Sci. (1999) 875(1):200–7. doi: 10.1111/j.1749-6632.1999.tb08504.x
10. Jang TH, Park SC, Yang JH, Kim JY, Seok JH, Park US, et al. Cryopreservation and its clinical applications. Integr Med Res. (2017) 6(1):12–8. doi: 10.1016/j.imr.2016.12.001
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. doi: 10.1136/bmj.n71
12. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. (2007) 7:1–6. doi: 10.1186/1472-6947-7-16
13. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA annual Symposium Proceedings (2006).
14. MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, et al. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. (2011) 286(21):18383–96. doi: 10.1074/jbc.M111.241182
15. Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets. (2009) 1(2):129–36. doi: 10.4161/isl.1.2.9480
16. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:1–10. doi: 10.1186/s13643-016-0384-4
17. Alcazar O, Alvarez A, Ricordi C, Linetsky E, Buchwald P. The effect of recovery warm-up time following cold storage on the dynamic glucose-stimulated insulin secretion of isolated human islets. Cell Transplant. (2020) 29:0963689720908278. doi: 10.1177/0963689720908278
18. Komatsu H, Kang D, Medrano L, Barriga A, Mendez D, Rawson J, et al. Isolated human islets require hyperoxia to maintain islet mass, metabolism, and function. Biochem Biophys Res Commun. (2016) 470(3):534–8. doi: 10.1016/j.bbrc.2016.01.110
19. Komatsu H, Rawson J, Medrano L, Cook CA, Barriga A, Gonzalez N, et al. Optimizing temperature and oxygen supports long-term culture of human islets. Transplantation. (2019) 103(2):299–306. doi: 10.1097/TP.0000000000002280
20. Lee R, Carter J, Szot G, Posselt A, Stock P. Human albumin preserves islet mass and function better than whole serum during pretransplantation islet culture. Transplant Proc. (2008) 40:384–6. doi: 10.1016/j.transproceed.2008.02.016
21. Nacher M, Estil·Les E, Garcia A, Nadal B, Pairó M, Garcia C, et al. Human serum versus human serum albumin supplementation in human islet pretransplantation culture: in vitro and in vivo assessment. Cell Transplant. (2016) 25(2):343–52. doi: 10.3727/096368915X688119
22. Kerr-Conte J, Vandewalle B, Moerman E, Lukowiak B, Gmyr V, Arnalsteen L, et al. Upgrading pretransplant human islet culture technology requires human serum combined with media renewal. Transplantation. (2010) 89(9):1154–60. doi: 10.1097/TP.0b013e3181d154ac
23. Fraga DW, Sabek O, Hathaway DK, Gaber AO. A comparison of media supplement methods for the extended culture of human islet tissue. Transplantation. (1998) 65(8):1060–6. doi: 10.1097/00007890-199804270-00009
24. Ståhle MU, Brandhorst D, Korsgren O, Knutson F. Pathogen inactivation of human serum facilitates its clinical use for islet cell culture and subsequent transplantation. Cell Transplant. (2011) 20(5):775–81. doi: 10.3727/096368910X539056
25. Holmes MA, Clayton HA, Chadwick DR, Bell PR, London NJ, James RF. Functional studies of rat, porcine, and human pancreatic islets cultured in ten commercially available media. Transplantation. (1995) 60(8):854–60. doi: 10.1097/00007890-199510270-00016
26. Clayton H, Turner J, Swift S, James R, Bell P. Supplementation of islet culture medium with insulin may have a beneficial effect on islet secretory function. Pancreas. (2001) 22(1):72–4. doi: 10.1097/00006676-200101000-00013
27. Terra LF, Garay-Malpartida M, Wailemann R, Sogayar MC, Labriola L. Recombinant human prolactin promotes human beta cell survival via inhibition of extrinsic and intrinsic apoptosis pathways. Diabetologia. (2011) 54:1388–97. doi: 10.1007/s00125-011-2102-z
28. Kaviani M, Keshtkar S, Azarpira N, Aghdaei MH, Geramizadeh B, Karimi MH, et al. Cytoprotective effects of olesoxime on isolated human pancreatic islets in order to attenuate apoptotic pathway. Biomed Pharmacother. (2019) 112:108674. doi: 10.1016/j.biopha.2019.108674
29. Omori K, Todorov I, Shintaku J, Rawson J, Al-Abdullah IH, Higgins LS, et al. P38α-Selective mitogen-activated protein kinase inhibitor for improvement of cultured human islet recovery. Pancreas. (2010) 39(4):436–43. doi: 10.1097/MPA.0b013e3181c0dd8f
30. Fornoni A, Pileggi A, Molano R, Sanabria N, Tejada T, Gonzalez-Quintana J, et al. Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia. (2008) 51:298–308. doi: 10.1007/s00125-007-0889-4
31. Bottino R, Balamurugan A, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. (2002) 51(8):2561–7. doi: 10.2337/diabetes.51.8.2561
32. Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, et al. Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation. (2010) 89(1):47–54. doi: 10.1097/TP.0b013e3181be3bf2
33. Jay TR, Paget MB, Heald KA, Downing R. Are organ preservation solutions useful for the storage of isolated human islets? Transplant Proc. (2004) 36(4):1130–2. doi: 10.1016/j.transproceed.2004.04.063
34. Shindo Y, Kalivarathan J, Saravanan PB, Levy MF, Kanak MA. Assessment of culture/preservation conditions of human islets for transplantation. Cell Transplant. (2022) 31:9636897221086966. doi: 10.1177/09636897221086966
35. Delfino VD, Gray DW, Leow CK, Shimizu S, Ferguson DJ, Morris PJ. A comparison of four solutions for cold storage of pancreatic islets. Transplantation. (1993) 56(6):1325–30. doi: 10.1097/00007890-199312000-00007
36. Rush BT, Fraga DW, Kotb MY, Sabek OM, Lo A, Gaber LW, et al. Preservation of human pancreatic islet in vivo function after 6-month culture in serum-free media. Transplantation. (2004) 77(8):1147–54. doi: 10.1097/01.TP.0000116769.94299.F4
37. Lemaire F, Sigrist S, Brassard J, Demini L, Zal F, Jeandidier N, et al. Beneficial effects of two marine oxygen carriers, M101 and M201, on human islet quality in hypoxic culture conditions. Cell Transplant. (2023) 32:09636897231179642. doi: 10.1177/09636897231179642
38. Brandhorst D, Brandhorst H, Acreman S, Schive SW, Bjørnson Scholz H, Johnson PRV. Hypoxia-induced damage in human islets is reduced with the use of mesenchymal stem cell-preconditioned medium. Transplant Proc. (2017) 49(10):2330–2. doi: 10.1016/j.transproceed.2017.11.003
39. Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AJ, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. (2012) 7(5):e38189. doi: 10.1371/journal.pone.0038189
40. de Souza BM, Rodrigues M, de Oliveira FS, da Silva LP, Bouças AP, Portinho CP, et al. Improvement of human pancreatic islet quality after co-culture with human adipose-derived stem cells. Mol Cell Endocrinol. (2020) 505:110729. doi: 10.1016/j.mce.2020.110729
41. Murray H, Paget MB, Bailey CJ, Downing R. Sustained insulin secretory response in human islets co-cultured with pancreatic duct-derived epithelial cells within a rotational cell culture system. Diabetologia. (2009) 52:477–85. doi: 10.1007/s00125-008-1247-x
42. Daoud JT, Petropavlovskaia MS, Patapas JM, Degrandpré CE, DiRaddo RW, Rosenberg L, et al. Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials. (2011) 32(6):1536–42. doi: 10.1016/j.biomaterials.2010.10.036
43. Maillard E, Juszczak MT, Clark A, Hughes SJ, Gray DR, Johnson PR. Perfluorodecalin-enriched fibrin matrix for human islet culture. Biomaterials. (2011) 32(35):9282–9. doi: 10.1016/j.biomaterials.2011.08.044
44. Hadavi E, Leijten J, Engelse M, de Koning E, Jonkheijm P, Karperien M, et al. Microwell scaffolds using collagen-IV and laminin-111 lead to improved insulin secretion of human islets. Tissue Eng Part C Methods. (2019) 25(2):71–81. doi: 10.1089/ten.tec.2018.0336
45. Bentsi-Barnes K, Kandeel F, Al-Abdullah IH. Evaluation of human islet-specific functional quality cultured on different gas-permeable membranes. Transplant Proc. (2008) 40:401–2. doi: 10.1016/j.transproceed.2008.01.036
46. Omori K, Qi M, Salgado M, Gonzalez N, Hui LT, Chen K-T, et al. A scalable human islet 3D-culture platform maintains cell mass and function long-term for transplantation. Am J Transplant. (2024) 24(2):177–89. doi: 10.1016/j.ajt.2023.10.001
47. Woods E, Walsh C, Sidner R, Zieger M, Lakey J, Ricordi C, et al. Improved in vitro function of islets using small intestinal submucosa. Transplant Proc. (2004) 36:1175–7. doi: 10.1016/j.transproceed.2004.04.042
48. Elliott GD, Wang S, Fuller BJ. Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. (2017) 76:74–91. doi: 10.1016/j.cryobiol.2017.04.004
49. Gaber AO, Fraga DW, Callicutt CS, Gerling IC, Sabek OM, Kotb MY. Improved in vivo pancreatic islet function after prolonged in vitro islet culture. Transplantation. (2001) 72(11):1730–6. doi: 10.1097/00007890-200112150-00005
50. Misler S, Dickey A, Barnett DW. Maintenance of stimulus-secretion coupling and single beta-cell function in cryopreserved-thawed human islets of langerhans. Pflügers Archiv. (2005) 450:395–404. doi: 10.1007/s00424-005-1401-y
51. Lakey JR, Anderson TJ, Rajotte RV. Novel approaches to cryopreservation of human pancreatic Islets1. Transplantation. (2001) 72(6):1005–11. doi: 10.1097/00007890-200109270-00005
52. Kojayan G, Whaley D, Alexander M, Rodriguez S, Lee S, Lakey JR. Improved cryopreservation yield of pancreatic islets using combination of lower dose permeable cryoprotective agents. Cryobiology. (2019) 88:23–8. doi: 10.1016/j.cryobiol.2019.04.004
53. Kenmochi T, Asano T, Maruyama M, Saigo K, Akutsu N, Iwashita C, et al. Cryopreservation of human pancreatic islets from non-heart-beating donors using hydroxyethyl starch and dimethyl sulfoxide as cryoprotectants. Cell Transplant. (2008) 17(1-2):61–7. doi: 10.3727/000000008783907026
54. Omori K, Valiente L, Orr C, Rawson J, Ferreri K, Todorov I, et al. Improvement of human islet cryopreservation by a p38 MAPK inhibitor. Am J Transplant. (2007) 7(5):1224–32. doi: 10.1111/j.1600-6143.2007.01741.x
55. Sakai A, Engelmann F. Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters. (2007) 28(3):151–72.17898904
56. Langer S, Lau D, Eckhardt T, Jahr H, Brandhorst H, Brandhorst D, et al. Viability and recovery of frozen-thawed human islets and in vivo quality control by xenotransplantation. J Mol Med. (1999) 77:172–4. doi: 10.1007/s001090050330
57. Jutte N, Heyse P, Jansen H, Bruining G, Zeilmaker G. Vitrification of human islets of langerhans. Cryobiology. (1987) 24(5):403–11. doi: 10.1016/0011-2240(87)90043-5
58. Kneteman NM, Alderson D, Scharp DW, Lacy PE. Long-term cryogenic storage of purified adult human islets of langerhans. Diabetes. (1989) 38(3):386–96. doi: 10.2337/diab.38.3.386
59. Janjic D, Andereggen E, Deng S, Bartley C, Buhfer L, Morel P, et al. Improved insulin secretion of cryopreserved human islets by antioxidant treatment. Pancreas. (1996) 13(2):166–72. doi: 10.1097/00006676-199608000-00008
60. Beattie GM, Crowe JH, Lopez AD, Cirulli V, Ricordi C, Hayek A. Trehalose: a cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes. (1997) 46(3):519–23. doi: 10.2337/diab.46.3.519
61. Komatsu H, Barriga A, Medrano L, Omori K, Kandeel F, Mullen Y. Oxygenated thawing and rewarming alleviate rewarming injury of cryopreserved pancreatic islets. Biochem Biophys Res Commun. (2017) 486(3):817–23. doi: 10.1016/j.bbrc.2017.03.134
62. Zhan L, Rao JS, Sethia N, Slama MQ, Han Z, Tobolt D, et al. Pancreatic islet cryopreservation by vitrification achieves high viability, function, recovery and clinical scalability for transplantation. Nat Med. (2022) 28(4):798–808. doi: 10.1038/s41591-022-01718-1
63. Piemonti L, Bertuzzi F, Nano R, Leone BE, Socci C, Pozza G, et al. Effects of cryopreservation on in vitro and in vivo long-term function of human islets1. Transplantation. (1999) 68(5):655–62. doi: 10.1097/00007890-199909150-00011
64. Committee NCCCMCM, Consortium NC. Functional assessment of purified human pancreatic islets: glucose stimulated insulin release by ELISA: a standard operating procedure of the NIH clinical islet transplantation consortium. CellR4–repair, Replacement, Regeneration, Reprogramming. (2014) 2(2):e900.
65. Misun PM, Yesildag B, Forschler F, Neelakandhan A, Rousset N, Biernath A, et al. In vitro platform for studying human insulin release dynamics of single pancreatic islet microtissues at high resolution. Advanced Biosystems. (2020) 4(3):1900291. doi: 10.1002/adbi.201900291
66. Ozgur OS, Namsrai B-E, Pruett TL, Bischof JC, Toner M, Finger EB, et al. Current practice and novel approaches in organ preservation. Frontiers in Transplantation. (2023) 2:1156845. doi: 10.3389/frtra.2023.1156845
67. Pinnelas R, Kobashigawa JA. Ex vivo normothermic perfusion in heart transplantation: a review of the TransMedics®. Organ care system. Future Cardiol. (2022) 18(1):5–15. doi: 10.2217/fca-2021-0030
68. De Klerk E, Hebrok M. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol (Lausanne). (2021) 12:631463. doi: 10.3389/fendo.2021.631463
69. Wang S, Du Y, Zhang B, Meng G, Liu Z, Liew SY, et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell. (2024) 187(22):6152–64.e18. doi: 10.1016/j.cell.2024.09.004
70. Reichman TW, Markmann JF, Odorico J, Witkowski P, Fung JJ, Wijkstrom M, et al. Stem cell–derived, fully differentiated islets for type 1 diabetes. N Engl J Med. (2025). doi: 10.1056/NEJMoa2506549
71. Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. (2010) 31(7):1676–82. doi: 10.1016/j.biomaterials.2009.11.057
72. Lucas-Clerc C, Massart C, Campion J, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. (1993) 94(1):9–20. doi: 10.1016/0303-7207(93)90046-M
73. Chen Y-J, Yamazoe T, Leavens KF, Cardenas-Diaz FL, Georgescu A, Huh D, et al. Iprep is a three-dimensional nanofibrillar cellulose hydrogel platform for long-term ex vivo preservation of human islets. JCI insight. (2019) 4(21). doi: 10.1172/jci.insight.124644
Keywords: type 1 diabetes (T1D), islet transplantation, human islets, islet storage, cryopreservation, culture techniques, islet viability, glucose-stimulated insulin secretion (GSIS)
Citation: Chen AR, Chansky J and Burke JA (2025) Long-term storage, cryopreservation, and culture of isolated human islets: a systematic review. Front. Transplant. 4:1614849. doi: 10.3389/frtra.2025.1614849
Received: 19 April 2025; Accepted: 4 July 2025;
Published: 8 August 2025.
Edited by:
Tatsuya Kin, University of Alberta Hospital, CanadaReviewed by:
Anil Kharga, University of Pennsylvania, United StatesJulie Kerr-Conte, Université de Lille, France
Copyright: © 2025 Chen, Chansky and Burke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacqueline A. Burke, amFjcXVlbGluZS5idXJrZUBub3J0aHdlc3Rlcm4uZWR1
 Austin R. Chen
Austin R. Chen Joshua Chansky1
Joshua Chansky1 Jacqueline A. Burke
Jacqueline A. Burke