- 1ICAR – Central Institute of Fisheries Education, Mumbai, Maharashtra, India
- 2College of Fisheries, Bihar Animal Sciences University, Bihar, India
- 3College of Fisheries, Maharana Pratap University of Agriculture & Technology, Udaipur Rajasthan, India
- 4ICAR – Central Inland Fisheries Research Institute, Barrackpore, Kolkata, West Bengal, India
The onset of diseases, combined with the reliance on therapeutics such as chemicals and antibiotics, as well as the alarming rise of antibiotic-resistant pathogen strains, postulates the urgent exploration of alternatives to antibiotics in semi-intensive and intensive aquaculture systems. In response to this critical need, a sixty-day feeding trial was conducted to evaluate the dietary effect of khejri seed extract (KSE) on the growth performance and immunological responses of rohu (Labeo rohita) fingerlings. Following this trial, a fifteen-day challenge study assessed the rohu fingerlings' resistance against Aeromonas hydrophilia. Four experimental diets were supplemented with different levels of khejri seed extract (KSE) viz. 0.0, 2.5, 5.0, and 7.5 g KSE kg−1 of diet and designated as C, KSE0.25, KSE0.50, and KSE0.75, respectively. The supplementation of dietary KSE led to significant improvements in growth performance and protein utilization in rohu fingerlings (P < 0.05). The activity of digestive (except amylase) and metabolic enzymes (except lactate dehydrogenase in muscle) activities varied significantly (P < 0.05). For the enzymes superoxide dismutase (SOD) and catalase (CAT), the observed trend was control > KSE0.25 > KSE0.50 > KSE0.75 in both pre- and post-challenge studies. Total serum protein showed significant variations (P < 0.05) in both pre- and post-challenge studies, with albumin and globulin concentrations being significantly higher in the KSE0.50 group compared to control during the post-challenge phase. Moreover, KSE supplementation significantly enhanced the respiratory burst activity and haematological parameters (except total leukocyte count in the pre-challenge and serum glucose levels in pre- and post-challenge study) of rohu fingerlings (P < 0.05). Following A. hydrophilia challenge, fish mortality significantly decreased with higher dietary KSE levels (P < 0.05), reaching the lowest mortality in the KSE0.50 group. These results suggest that supplementing the diet with 5.0 g kg−1 khejri seed extract can effectively enhance growth, improve feed conversion, boost immunity, and increase survival against A. hydrophila infection in rohu fingerlings.
1 Introduction
Aquaculture, one of the fastest-growing sectors in animal food-producing industries, plays a pivotal role in ensuring global nutritional and livelihood security. Fish production increased significantly, rising from 125 to 179 million tons during 2001–2020, while per capita fish consumption ascended from 16 to 20.5 kg during the same period (Food and Agriculture Organization, 2022). In recent years, there has been a shift toward intensified feed-based aquaculture and the diversification of fish species to enhance production. Meanwhile, there has been a decrease in extensive aquaculture practices, declining from 43.9 to 30.5% from 2000 to 2020, respectively (Food and Agriculture Organization, 2022).
The intensive aquaculture practices expose fish to environmental stress, and increases their vulnerability to various pathogens, including viruses, bacteria, fungi, and parasites (Wang et al., 2015). These disease outbreaks can lead to high mortality rates and reduced meat quality, causing significant economic losses for producers. In fact, pathogens can results in hatchery losses reaching up to 20% (Shinn et al., 2015). While, bacterial infections are a major concern in fish farming, accounting for nearly 50% of production losses (Ibrahim et al., 2014; Leung and Bates, 2013).
Carps are the predominantly cultured fish species in Asia, especially in India (Tejaswini et al., 2024) where they represent the largest share (51.60%) of global finfish production (Food and Agriculture Organization, 2024). Rohu (Labeo rohita) is among the most widely cultured species, with production steadily increasing (Food and Agriculture Organization, 2024). In 2020-2021, rohu and catla accounted for a substantial 6.03 million tons, constituting 12.30% of global production (Food and Agriculture Organization, 2022). Among freshwater aquaculture, Aeromonas hydrophilia is a prevalent bacterial pathogen, causing significant harm to carp production (Giri et al., 2013). The rampant use of antibiotics and chemotherapeutics in intensive aquaculture has rightfully drawn criticism, due to concern over antibiotic-resistant bacterial strains and harmful accumulation of antibiotic residues in fish tissues. Following the European Union's ban on sub-therapeutic antibiotics in 2003, research has aggressively pursued alternative, environmentally sustainable methods to improve fish health without jeopardizing safety for consumers and the environment (Ringø and Song, 2016; Hoseinifar et al., 2020). Researchers are now actively exploring a variety of substitutes, including probiotics, immuno-stimulants, and herbal products (Giri et al., 2015; Reverter et al., 2017; Shiu et al., 2016).
Moreover, there is a growing commitment to leveraging indigenous technological knowledge for effective fish health management, as highlighted by the Food and Agriculture Organization (FAO) (Food and Agriculture Organization, 2002). Herbal immunostimulants offer a wide range of beneficial effects and serve as viable alternatives to chemicals or antibiotics in aquaculture practices (Van Doan et al., 2019) and are being recognized as eco-friendly and cost-effective (Elumalai et al., 2020). Several studies have documented the immunostimulatory effects of herbal extracts from plants like Catharanthus roseus, Rosmarinus officinale, and Eclipta alba in fishes (Christybapita et al., 2007; Divyagnaneswari et al., 2007; Xie et al., 2008). Similarly, it had been reported that dietary supplementation of lemon peel extract positively impacted feed intake, growth performance, and digestive-metabolic enzyme activities in L. rohita fingerlings at low temperatures (Tejaswini et al., 2024). Likewise, Sundararajan et al. (2024) also observed the growth-promoting and immunostimulant properties of dietary papaya peel extract (PPE) in L. rohita fingerlings.
Prosopis cineraria, commonly referred to as the golden tree or wonder tree. It belongs to the Fabaceae family, is widely distributed in arid and semi-arid regions globally (Rai et al., 2021; Yadav et al., 2022). In India, it holds special significance as the state tree in Rajasthan and Telangana, and it is also the national tree of the United Arab Emirates (Afifi, 2018; Krishnan and Jindal, 2015). While P. cineraria is the only native species in the Indian subcontinent, along with other species such as P. julifora, P. pallida, P. glandulosa, P. chilensis, P. tamarugo, and P. alba are cultivated (Bhansali, 2010; Kalia et al., 2014). Beyond its nutritional value, Prosopis spp. is well-known for its medicinal properties like antiviral, antibacterial, antifungal, anthelmintic, and anticancer properties, effectively treating various diseases without causing adverse side effects (Preeti et al., 2017; Kuchana et al., 2014; Vaza and Bhalerao, 2018). The wide-ranging benefits of Prosopis spp. such as analgesic, antipyretic, hypoglycaemic, antioxidant, and hypocholesterolemic stem from various phytochemicals found throughout its pods, stems, roots, leaves, and seeds. These include alkaloids, saponins, tannins, flavonoids, flavonols, total phenols, vitamins, fatty acids, and free amino acids (Garg and Mittal, 2013; Garg et al., 2020; Malik et al., 2013; Yadav et al., 2018). Furthermore, the Prosopis extract have demonstrated antibiotic properties comparable to broad-spectrum antibiotics, effectively combating the issue of multidrug resistance (Khan et al., 2010). The focus of contemporary aquaculture nutrition research is decisively aimed at developing innovative products that enhance fish immune systems without compromising the health of fish, environment, or human safety. Despite the known benefits of Prosopis spp., limited research has explored the potential of P. cineraria seed extract in aquaculture nutrition. In this context, this study aims to evaluate the effects of dietary khejri (P. cineraria) seed extract (KSE) on growth, immune responses, and disease resistance in L. rohita fingerlings.
2 Materials and methods
2.1 Site, collection, and preparation of khejri seed flour
A feeding trial, hematological analysis, and challenge study against A. hydrophilia were conducted at the wet laboratory, ARSU-Aquaculture Research and Seed Unit, under the MPUAT-Maharana Pratap University of Agriculture and Technology, Udaipur, Rajasthan, India-313001. Biochemical and enzymatic analysis were performed at the laboratories of the Divisions of Aquaculture and FNBP-Fish Nutrition, Biochemistry & Physiology, ICAR-CIFE, Mumbai, Maharashtra, India-400061. Khejri (P. cineraria) seeds were obtained from Jaipur (Latitude: 26.8400°N and Longitude: 75.7964°E), Rajasthan, India. They were cleaned, oven-dried at 45°C, ground, and sieved (mesh size 60; 250 μm particle size). The powdered seeds were then stored in labeled airtight polythene bags at room temperature.
2.2 Preparation of khejri seed extract
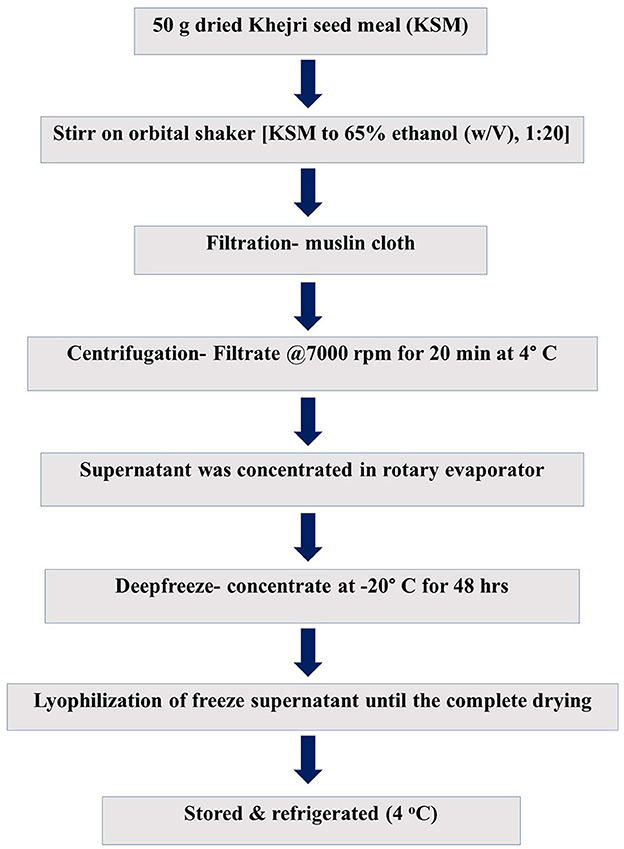
Ethanolic extract from khejri seed meal was prepared by following the methodology of Sundararajan et al. (2024) and illustrated in Figure 1.
2.3 Experimental design and fish rearing
Three hundred fingerlings of rohu (Labeorohita) were acclimated for 15 days in well-oxygenated 3000 L FRP tanks at the Aquaculture Research and Seed Unit, (MPUAT, Udaipur, India. Twelve fiber reinforced plastic (FRP) rectangular tanks (500 L; 1.0 * 1.0 * 0.50 m) were used as experimental units. FRP tanks were first cleaned and disinfected with potassium permanganate (KMnO4), thoroughly rinsed with water, and then sun-dried. Subsequently, each tank was filled with 350 L of chlorine-free borewell water, and continuous aeration was maintained throughout the trial. Nylon nets were placed over all tanks to prevent fish from escaping. One hundred twenty L. rohita fingerlings (mean weight 7.23 ± 0.07 g) were randomly distributed among twelve experimental tanks. The fish were fed to satiation twice daily (at 9:30 am and 5:30 pm). Uneaten feed and fecal matter were siphoned daily, with an equal volume of clean groundwater replacing the removed water. Sampling was conducted every 2 weeks to monitor the growth and health of the fish. The physico-chemical parameters, including water temperature, pH, alkalinity, dissolved oxygen, ammonia, nitrite, and nitrate, were routinely monitored following APHA (American Public Health Association, 2005) guidelines. The pH ranged from 7.71 to 8.09, and the water temperature varied between 23.25 and 26.10°C. Alkalinity and dissolved oxygen levels ranged from 93.75 to 104.05 mg L−1 and 5.78 to 6.53 mg L−1, respectively. Ammonia, nitrite, and nitrate levels were between 0.01 and 0.04 mg L−1, 0.01 and 0.06 mg L−1, and 0.14 and 0.35 mg L−1, respectively. No traces of free carbon dioxide were detected in any experimental tank.
2.4 Formulation and preparation of experimental diets
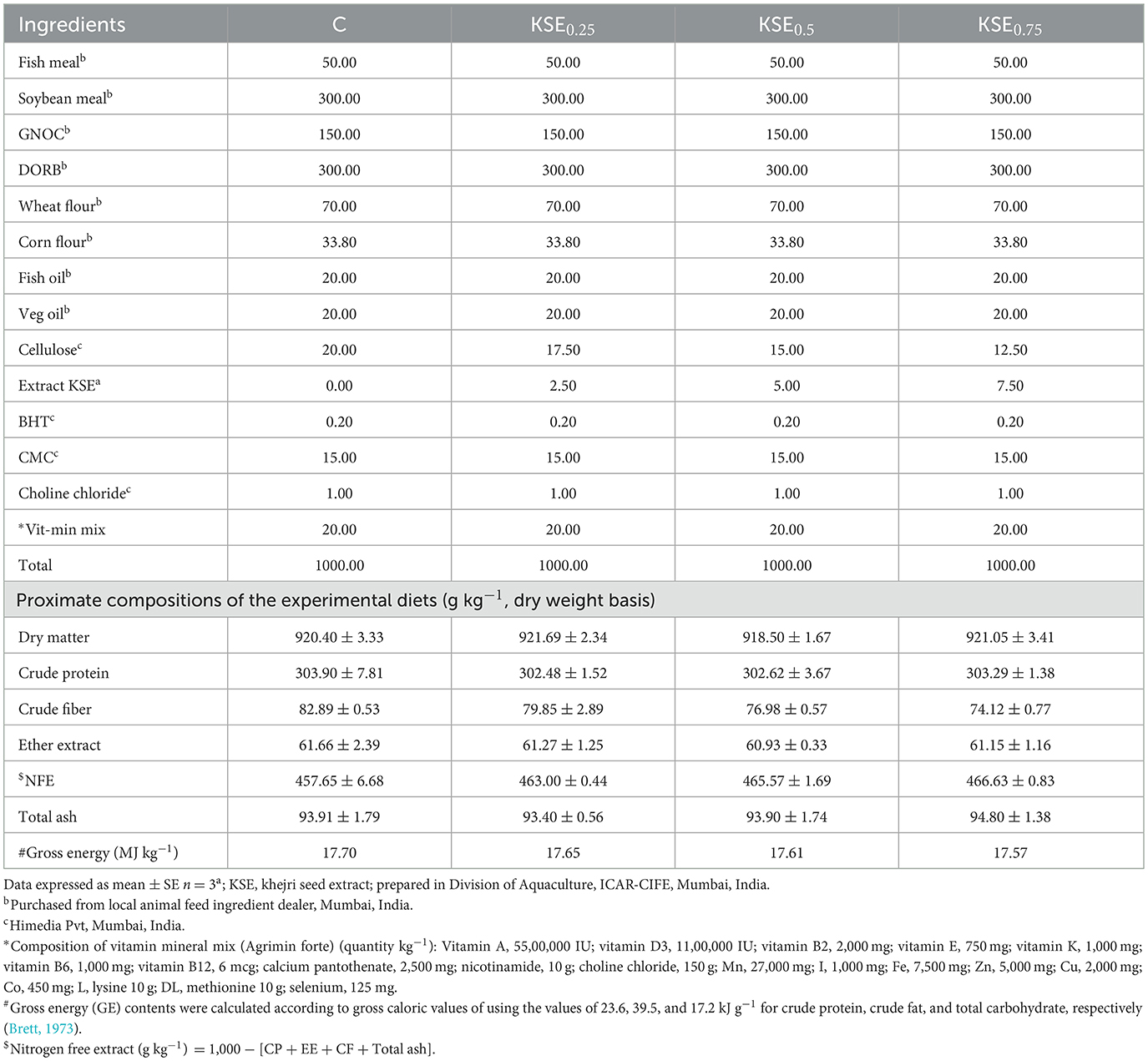
Four iso-nitrogenous (303.07 g crude protein kg−1) and iso-caloric (17.63 MJ gross energy kg−1) experimental diets were supplemented with graded levels of khejri seed extract (KSE) at 0, 2.5, 5, and 7.5 g kg−1 of diet, denoted as C, KSE0.25, KSE0.50, and KSE0.75, respectively (Table 1). We weighed the dietary ingredients, mixed thoroughly, and moist-steamed for 30 min. The mixer was cooled and mixed thoroughly with oils & feed additives (butylated hydroxyl toluene, choline chloride, vitamin-mineral premix, and extract. The mixed was pressurized through a 2- 2.5 mm die in mechanical pelletizer, air-dried and stored in well-labeled container at room temperature.
2.5 Proximate composition
Method of Association of Official Analytical Chemists (A. O. A. C. Animal feed, 1995) followed to analyze composition of fish body and experimental diets. For moisture content, pre-weighed samples were subjected to oven-drying at 105°C. The crude protein content was assessed by measuring crude nitrogen through the Kjeldahl method, which involved digestion with concentrated sulphuric acid (H2SO4), distillation using 40% (w/V) sodium hydroxide (NaOH) in a semi-automatic distillation unit (KES 08L E, Pelican, Chennai, India), followed by titration with N/10 hydrochloric acid using a SI Analytics TitroLine 5000 titrator (Xylem Analytics, Weilheim, Germany). The ether extract was determined via solvent extraction, employing diethyl ether at temperature between 80–180°C using a Socsplus-SCS-08-AS apparatus (Pelican Equipment, Chennai, India). The ash content of experimental diets and carcass tissue was quantified by incinerating moisture-free samples at 550°C for 6 h in a muffle furnace (Muffle Furnace 1400c, Faridabad, Haryana, India). To determine crude fiber in fish feed, the method begins with an initial acid digestion using 1.25% H2SO4 (v/V) followed by alkali digestion with 1.25% NaOH (w/V) and incineration in a muffle furnace. The nitrogen-free extract of diet was calculated by subtracting the other components of proximate composition (crude protein, ether extract, crude fire and ash) from 100. Lastly, the gross energy (GE) content of the feed was assessed using a Parr bomb calorimeter (Model 1241, Fifty-Third Street, Moline, IL).
2.6 Growth and nutrient utilization parameters
Nutrient utilization, growth, conversion, survival and body indices were calculated by using the following formula (Jayant et al., 2020; Yadav et al., 2022).
i. Weight gain = FW - IW
ii. Weight gain percentage = FW - IW*100/IW
iii. Specific growth rate (%/day) = Ln (FW) – Ln (IW)*100/Experiment days
iv. Feed conversion ratio = FC (DW)/BWG (WW)
v. Feed efficiency ratio = BWG (WW)/FC (DW)
vi. Protein efficiency = NWG (WW)/Protein diet
vii. Hepatosomatic index (%) = LW(g)*100/WF(g)
viii. Viscero-somatic index (%) = VW(g) (g)*100/WF(g)
ix. Survival (%) = Total no. of fishes catch*100/Total no. of fishes stocked.
Where, FW: Final weight; IW: Initial weight; DW: Dry weight; WW: Wet weight; FC: Feed consumed; BWG: Body weight gain; NWG: Net weight gain; LW: Liver weight; VW: Viscera weight; WF: Weight of Fish.
2.7 Biochemical and enzymes assay of fish tissue samples
2.7.1 Sampling
The overall biomass of each tankor triplicate was recorded to calculate the growth parameters. Fish were starved for 24 h for the gut emptying. Three fish from each treatment group were randomly selected to assess the whole-body composition. They were then sedated with clove oil (50–60 μl L−1). Further, blood was withdrawn, transferred to 1 ml Eppendorf tube coated with a thin layer of EDTA and shaken to avoid haemolysis. Similarly, blood was collected from the sedated fish, transferred to 1 ml Eppendorf tubes, and allowed to clot at room temperature. The samples were then centrifuged at 3,500 rpm for 10 min at 4°C. The resulting yellow straw-colored serum was collected and stored at −20°C. Three fish from each treatment were dissected out to collect intestine, gill and liver. The tissue was ground in a 0.25 M sucrose solution (1:20 w/v) and centrifuged to obtain 0.5% tissue homogenates, which were then stored at −20°C for enzyme assay. The protein content in the tissue was measured utilizing the Bradford method (Bradford, 1976).
2.7.2 Digestive enzymes assays
Amylase, Lipase and protease activities were assayed as per Rick and Stegbauer (1974), Cherry and Crandall (1932) and Drapeau (1974), respectively.
2.7.3 Metabolic enzymes assays
Malate dehydrogenase (MDH) activity was evaluated following the protocol outlined by Ochoa (1955) and lactate dehydrogenase (LDH) as detailed by Wróblewski and Ladue (1955), respectively. Alanine aminotransferase (ALT) activity in various tissues of L. rohita was determined following the procedure specified by Wootton (1964). Similarly, aspartate aminotransferase (AST) activity was assessed using the same methodology as for ALT activity. Superoxide dismutase (SOD) and Catalase (CAT) activities were measured following the protocol detailed by Misra and Fridovich (1972) and Takahara et al. (1960), respectively.
2.7.4 Biochemical, and haemato-immunological parameters
Red blood cell (Nos. × 106 mm−3), White blood cell (Nos. × 103 mm−3) counts and hemoglobin (g dL−1) were measured as described by Blaxhall and Daisley (1973) and Drabkin and Austin (1932), respectively. Serum protein levels and albumin levels were determined using the biuret and bromocresol green binding method by Reinhold (1953) and Doumas et al. (1971), respectively. Nitro blue tetrazolium (NBT) assay and serum glucose levels were determined using the method described by Stasiack and Bauman (1996) and Nelson (1944), respectively.
2.8 Challenge study
In the study, four fish from each replicate (twelve fish each treatment) were randomly selected at the conclusion of a 60-day feeding trial. A virulent strain of A. hydrophilia (MTCC-12301) was sourced from the Microbial Type Culture Collection (MTCC) at the Institute of Microbial Technology (IMTECH) in Chandigarh, India. The bacteria were grown on nutrient agar and incubated at 37°C for 24 h. After incubation, the bacterial cells were collected by centrifugation at 4,000 rpm for 10 min. The resulting pellet was washed twice with phosphate-buffered saline (PBS) and then resuspended in PBS to achieve a turbidity equivalent to a 5 McFarland standard (108 CFU ml−1).
The pathogenic bacterial strain was grown in Brain Heart Infusion (BHI) broth and incubated at 30°C for 28–30 h in a BOD incubator. Following incubation, the bacterial cells were collected by centrifugation at 3,000 rpm for 10 min, washed twice with sterile PBS, and diluted to a concentration of 108 cells ml−1. Serial dilutions were performed to achieve a final concentration of 107 cells ml−1. Each fish was intraperitoneally injected with 100 μL of the bacterial suspension and challenged for 15 days. During this period, mortality and pathological symptoms were closely monitored. Three fish from each replicate were sampled, anesthetized, and sacrificed for blood and tissue collection. The exact sampling and tissue homogenization procedures described previously were followed. Enzyme activities, along with biochemical and hematological analyses, were performed on the blood/serum as well as liver and gill tissue samples using the same methods. Mortality was recorded daily for 15 days, and relative percentage survival (RPS) was calculated following the methods of Amend (1981). The RPS was determined using the following formula:
Where, MT (%), mortality of KSE fed group (%); M (%), mortality of control (%).
2.9 Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) in IBM-SPSS Statistics 22.0. Duncan's multiple range test (DMRT) was applied to identify significant differences between means, with a significance level set at p < 0.05. All data are presented as mean ± standard error of the mean. To assess significant changes between pre- and post-challenge conditions in L. rohita fingerlings, a paired t-test was utilized at a 5% level of significance.
3 Results
3.1 Proximate analysis of the experimental diets
The proximate composition of the experimental diets included dry matter (918.50 ± 1.67 to 921.69 ± 2.34 g kg−1), crude protein (302.48 ± 1.52 to 303.90 ± 7.81 g kg−1), ether extract (74.12 ± 0.77 to 82.89 ± 0.53 g kg−1), crude fiber (60.93 ± 0.33 to 61.66 ± 2.39 g kg−1), nitrogen-free extract (457.65 ± 6.68 to 466.63 ± 0.83 g kg−1), and total ash contents (93.40 ± 0.56 to 94.80 ± 1.38 g kg−1), respectively (Table 1).
3.2 Growth parameters
Significant differences (P < 0.05) were observed in body weight gain (WG), weight gain (%), and specific growth rate (SGR) among the various treatment groups (Table 2). The highest growth rates were recorded in the KSE0.50 fed group (P < 0.05), while the growth rates in the C, KSE0.25, and KSE0.75 groups were similar (P > 0.05). The mean values of FCR, FER, and PER differed significantly (P < 0.05) among the various dietary treatments. The highest FER was observed in KSE0.50, while it was significantly lower in C, KSE0.25, and KSE0.75 (P < 0.05) whereas, FCR followed a negative trend with FER, and growth rates (P < 0.05). Highest PER and ANPU was observed in KSE0.25 andKSE0.50 fed groups whereas the lowest ANPU was found in C and KSE0.75 fed groups, (P < 0.05).
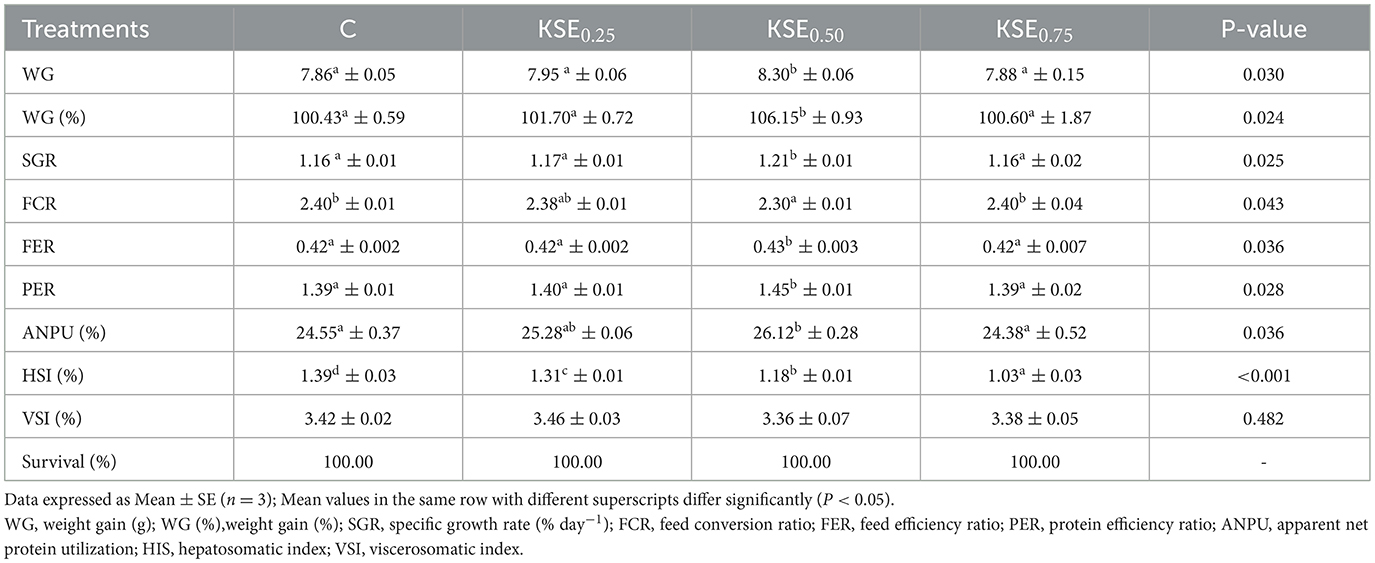
Table 2. Growth performance, nutrient utilization, body indices, and survival (%) of L. rohita fingerlings fed with different experimental diets.
3.2.1 Body indices and survival (%)
The Hepatosomatic Index (HSI) values of rohu fingerlings were significantly different (P < 0.05) among the various treatment groups (Table 2). HSI was decreased linearly with dietary KPE inclusion (P < 0.05). Dietary KSE supplementation did not alter the VSI values in rohu, L. rohita fingerlings (P > 0.05). There was no mortality observed between control and dietary KSE fed groups (P > 0.05).
3.3 Digestive enzymes activities
There was no significant difference (P > 0.05) in the amylase activities observed in rohu fingerlings fed with control and KSE-based experimental diets (Table 3). However, significant variations were noted in protease and lipase activities in L. rohita fingerlings in response to dietary KSE (P < 0.05). The higher protease activities were found in KSE0.50 whereas, the lowest value was found in C. KSE fed fish exhibited significantly higher lipase activities (P < 0.05) than control. However, lipase activities in KSE fed groups did not differ (P > 0.05).
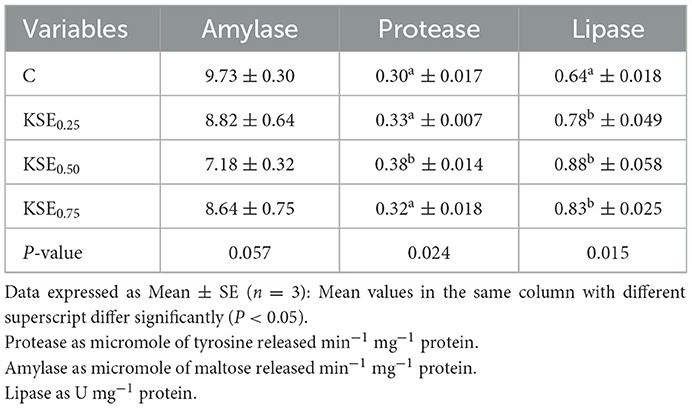
Table 3. Digestive enzymes activities of L. rohitafingerlings fed with fed with different experimental diets.
3.4 Whole body composition
The moisture, crude protein, and total ash contents did not show significant differences (P > 0.05) among the various experimental groups (Table 4). The crude lipid value was significantly higher (P < 0.05) in the C (4.39 ± 0.03), and a lower value was found in the KSE0.75 (3.97 ± 0.01). The crude lipid contents were found in decreasing trend from C (control) to KSE0.75 (P < 0.05).

Table 4. Whole body composition of L. rohitafingerlings fed with fed with different experimental diets (% wet weight basis).
3.5 Metabolic enzymes activities
3.5.1 Lactate dehydrogenase (LDH) and malate dehydrogenase (MDH)
Dietary KSE inclusion significantly lowered (P < 0.05) the LDH activity in the liver of rohu, L. rohita fingerlings (Table 5). Maximum and minimum hepatic LDH activities were observed in C and KSE0.75 fed groups, respectively. However, LDH activity in muscle did not differ (P > 0.05) among the different treatments. The MDH activity in the liver and muscle of L. rohita fingerlings was significantly influenced by dietary KSE inclusion (P < 0.05). MDH activities in these tissues exhibited a negative linear relationship with the level of dietary KSE inclusion (P < 0.05).
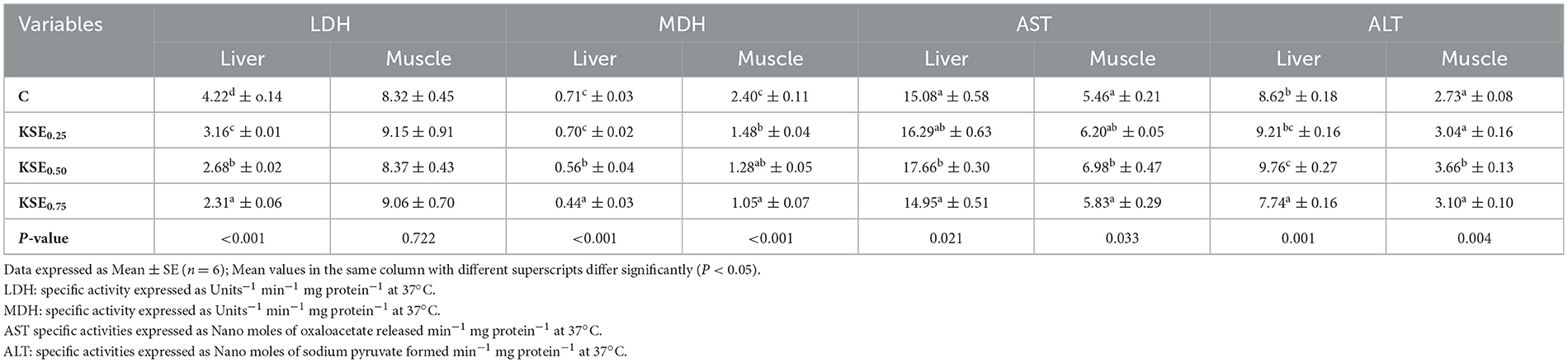
Table 5. Metabolic enzymes activities of L. rohita fingerlings fed with different experimental diets.
3.5.2 Aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
The AST and ALT activities in the liver and muscle differed significantly (P < 0.05) among the various treatment groups (Table 5). The highest AST and ALT activities were observed in the KSE0.50 fed groups, showing a linear relationship with dietary KSE inclusion (P < 0.05).
3.6 Challenge study
3.6.1 Survival
The challenge study results demonstrated that including KSE in the diet offers improved protection for the fish against A. hydrophila. The relative percentage survival (RPS) and cumulative survival percentage of rohu fingerlings of the different experimental groups were presented in Figures 2, 3. Mortalities significantly decreased (P < 0.05) in fish fed a diet containing extract (KSE0.50) compared to the control. The highest RPS (%) was observed in fish fed with 5 g kg−1 khejri seed extract (KSE).
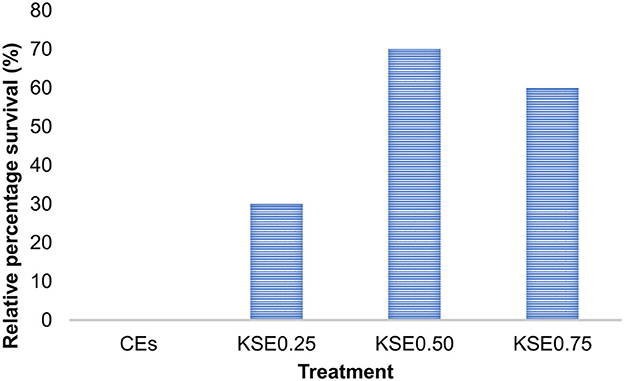
Figure 2. Relative percentage survival of rohu, L. rohita fingerlings challenged with A. hydrophila for 15 days.
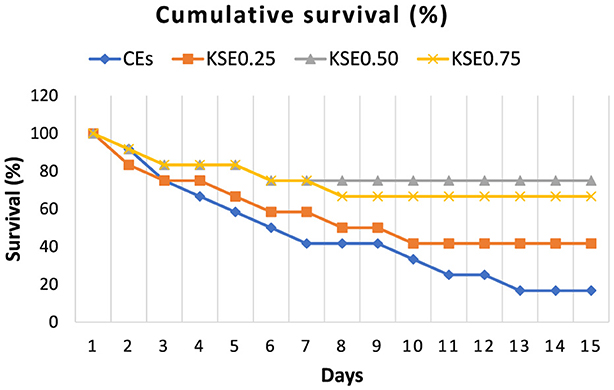
Figure 3. Cumulative survival percentage of L. rohita fingerlings challenged with A. hydrophila (0 to 15 days).
3.7 Anti-oxidative enzymes activities
3.7.1 Superoxide dismutase (SOD) and catalase (CAT) activities
SOD and CAT activities in the liver and gills of fish showed significant differences (P < 0.05) between the control (C) and KSE-fed groups in both pre- and post-challenge studies (Table 6). The SOD and CAT activities in the liver and gills of L. rohita fingerlings exhibited the following pattern: C > KSE0.25 > KSE0.50 > KSE0.75. Statistical analysis (paired t-test) indicated a significant increase in SOD and CAT activities in the liver and gills of rohu fingerlings during post-challenge study. But, KSE fed groups exhibited lower SOD and CAT activities than control group.
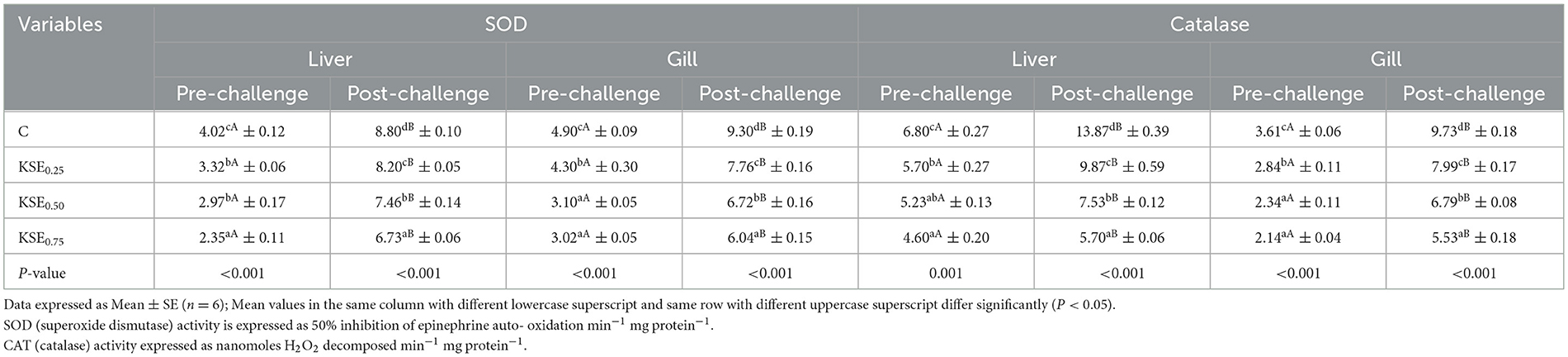
Table 6. Oxidative stress enzymes activities of L. rohitafingerlings during pre and post challenges conditions.
3.7.2 Serum protein biochemistry
The serum protein indices, including total protein, albumin, globulin contents, and the A/G ratio of L. rohita fingerlings, are presented in Table 7. Dietary KSE inclusions significantly enhanced (P < 0.05) the serum total protein contents in rohu fingerlings compared to control (C) during the pre-challenge study. It was maximum in KSE0.50 (3.43 ± 0.06) fed groups while minimum in C and KSE0.50 fed groups. Albumin, globulin contents, and A/G ratio were found to be non-significant in a pre-challenge study (P > 0.05). Statistical analysis using paired t-tests revealed a significant increase in total protein and globulin contents, along with a decrease in the A/G ratio of fish during the post-challenge study (P < 0.05). The highest levels of total protein, albumin, and globulin were observed in the KSE0.50 and KSE0.75 fed groups (P < 0.05). However, the A/G ratio was higher in the treatment groups compared to the control during the post-challenge study.

Table 7. Serum protein biochemistry of rohu, L. rohita fingerlings during the pre and post challenged conditions.
3.8 Haemato-immunological parameters
3.8.1 Nitro blue tetrazolium (NBT) and glucose contents
The NBT activity was significantly increased (P < 0.05) in the groups fed with KSE, both before and after the challenge study (Table 8). Statistical analysis of paired t-test revealed that NBT activity was significantly enhanced during the post-challenge study. Maximum NBT activity was observed in KSE0.50 and KSE0.75 fed groups during the pre- and post-challenge study. Dietary inclusion of KSE significantly lowered the serum glucose content in L. rohita fingerlings (P < 0.05) in both pre-and post-challenge studies. Statistical analysis of paired t-test revealed that serum glucose content was significantly increased during the post-challenge study. Maximum glucose content was noticed in C (control) during pre- and post-challenge studies, and minimum in KSE0.50 &KSE0.75 during pre-challenge studies, andKSE0.75 fed groups during post-challenge studies, respectively.
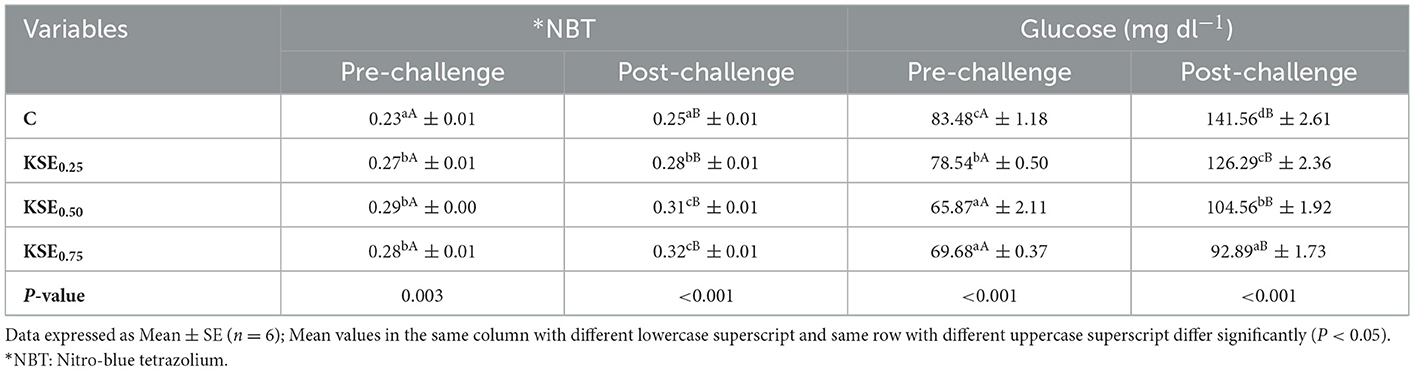
Table 8. Respiratory burst activity (NBT) and glucose content of rohu, L. rohita fingerlings during the pre and post challenged conditions.
3.8.2 Hematological parameters
Dietary inclusion of KSE significantly affected the hemoglobin (Hb) content and total erythrocyte count (TEC) in L. rohita fingerlings in a pre-challenge study (Table 9). Maximum Hb content and TEC count were observed in the KSE0.50 fed group and the minimum in the C fed group. Statistical analysis of paired t-tests stated an enhancement in Hb content and TEC count during the post-challenge study, with the highest values in KSE0.50 and KSE0.75 fed group (P < 0.05). However, dietary KSE inclusion did not significantly influence TLC (P > 0.05). TLC count was significantly higher in treatment groups than control-fed group during the post-challenge study (P < 0.05). The highest TLC count was observed in fish fed with 5 g kg−1and 7.5 g kg−1 KSE-supplemented diet.

Table 9. Hematological parameters of L. rohita fingerlings during pre and post challenged conditions.
4 Discussion
The use of natural immunostimulants in aquaculture could be an effective approach for preventing and managing fish diseases. As these substances offer environmentally friendly solutions, provide a sustainable option that diverge from chemotherapy, and contribute to a conducive environment within the host organism thereby offering eco-friendly preventive measures. These extracts have demonstrated several benefits including improved growth, enhanced feed utilization, stress reduction, bolstered immune function, and heightened metabolic activity in animals (Tejaswini et al., 2024; Rashidian et al., 2020; Sundararajan et al., 2024). Prosopis spp. are recognized for containing bioactive compounds such as alkaloids, flavonoids, terpenes, and phenolic compounds (Gurib-Fakim, 2006). The aim of this study was to investigate the effect of khejri seed extract as an antioxidant on the growth, immunity and disease of L. rohita against A. hydrophilia.
The supplementation of khejri seed extract (KSE) to the diet of L. rohita had a significant impact on various growth rates and protein utilization indices. Dietary KSE supplementation improved the growth (WG, WG%, SGR) and nutrient utilization (FCR, PER, FER, and ANPU) parameters. It suggests that bioactive compounds present in KSE have growth-promoting effects. Earlier study of Mahdavi et al. (2013) suggested that aloe vera extract can be used as a growth promoter, appetite stimulant, tonic, and immune-stimulant in fish. Similarly, growth-promoting effects have been demonstrated in different fishes fed with different plant-based extracts viz. Apium graveolens leaf extract in L. chrysophekadion (Sutthi et al., 2020), shatavari herb feed in L. rajasthanics (Keer et al., 2020), and lemon peel extract in L. rohita (Tejaswini et al., 2024). Hepatosomatic index (HSI) showed significant differences in response to diet, consistent with the findings of Syed et al. (2022) showed that Nile tilapia feeding groups fed different levels of aloe vera extract had lower HSI compared to the control group. However, there was no significant difference in visceral body measurements between the control and other treatment groups. Furthermore, no mortality was observed in L. rohita fingerlings throughout the entire feeding trial, indicating that there was not any toxicity from KSE and results are in agreement with finding of Adeshina et al. (2019) in Clarias gariepinus fed with Eugenia caryophyllata bud extract.
Digestive enzymes play a vital role in breaking down the nutrients, which are then metabolized to either assimilate nutrients or meet energy demands (Furne et al., 2005). Nutrients from the feed are broken down in the fish gut with the help of digestive enzymes like lipase, protease, and amylase (Nopitawati and Jusadi, 2015). The inclusion of herbal preparations in feed can enhance the release of digestive fluids and promote intestinal motility, potentially leading to increased feed intake and growth in fish (Pu et al., 2017). In this study, a significant increase in digestive enzyme activity (protease and lipase) was observed in groups fed with KSE compared to the control group, while amylase activity did not differ between the groups. This suggests that supplementation with 5 g kg−1 KSE promoted the secretion of protease and lipase, thereby enhancing nutrient digestion and absorption, ultimately leading to improved growth in L. rohita. It had been reported that phyto-additives may enhance gut health by promoting the growth of beneficial microbes, which in turn could modulate the activity of digestive enzymes in fish (Hashemi and Davoodi, 2011). These findings are supported by Bilen et al. (2018), who reported a significant improvement in lipase and protease activities in Cyprinus carpio fed with Anethum graveolens and Lepidium sativum.
Moisture, protein (CP) and total ash (TA) contents did not differ in control and KSE-fed groups. It indicates that KSE did not have a significant impact on the biochemical constituents of L. rohita. Similar findings have been documented in previous studies. For example, Yousefi et al. (2019) observed that supplementation with rosemary leaf in Cyprinus carpio did not significantly affect whole-body composition. Likewise, Keer et al. (2020) reported that feeding L. rajasthanics with shatavari extract had no notable impact on whole-body composition. However, a decrease in crude lipid content was observed with dietary KSE levels. It infers that bio-active compounds in KSE might have hypolipidemic properties. Previous studies also witnessed the hypolipidemic and hypercholesterolemic properties of khejri bean in mice (Agarwal and Chauhan, 1988) and pod extract in rabbits (Ram et al., 2020), respectively.
In our study, fish fed with 2.5 and 5.0 g KSE kg−1 diet exhibited higher AST and ALT activities compared to the control. This finding aligns with Abalaka et al. (2011), who reported enhanced ALT and AST activities in Clarias gariepinus when fed with aqueous and ethanolic pod extracts from Parkia biglobasa. Moreover, fish on diets supplemented with KSE extract exhibited reduced LDH activity in both muscle and liver compared to the control group. The reduced LDH activity in the treatment groups indicates a stress-mitigating effect of dietary KSE plant extracts in L. rohita. A similar decreasing trend in LDH activity with herbal plant extract supplementation was also observed by Kazeem et al. (2017). Malate dehydrogenase (MDH), an enzyme involved in the TCA cycle that catalyzes the conversion of malate to oxaloacetate and vice versa (Hemre et al., 2002), showed decreased activity in our study with dietary KSE supplementation compared to the control. Likewise, Garg et al. (2019) found lower LDH and MDH activity in L. rohita fingerlings fed Houttuynia cordata leaf extract, supporting the stress-mitigating role of phytochemicals present in KSE.
In this study, groups fed with khejri seed extract (KSE) had lower activities of antioxidant enzymes (SOD and CAT) compared to the control group during both pre- and post-challenge studies. This suggests the seed extract may effectively scavenge or neutralize free radicals produced by oxidative stress caused by pathogenic organisms. The phenolic compounds found in khejri seed extract likely play a significant role in these scavenging, corroborating the findings of Henciya et al. (2017) and Chaudhary et al. (2018). Additionally, Fawole et al. (2018) also reported similar results indicating that ethanolic leaf extracts from Psidium guajava and Mangifera indica reduced SOD and CAT activities in rohu.
In this study, it was clearly demonstrated that rohu fingerlings fed dietary KSE exhibited a significant enhancement in serum total protein levels. However, the albumin and globulin contents remained consistent across both control and treatment groups. Notably, infected fish consuming KSE showed substantial increases in serum total protein, albumin, globulin contents, and higher albumin-globulin ratio. This rise in serum total protein and globulin levels indicates an augmentation in immunoglobulin concentration, particularly within the gamma fractions of globulin. These findings support the assertion that bioactive compound in KSE enhance the non-specific immune system and substantially improve the overall health of infected fish. Moreover, similar effects were observed by Das et al. (2015) with extracts from Ocimum sanctum (Tulsi), which significantly elevated total immunoglobulin levels in plasma alongside serum total protein, globulin, total erythrocyte count (TEC), total leukocyte count (TLC), hemoglobin, superoxide anion production, and lysozyme activity, all in comparison to the control group. Additionally, Sutthi et al. (2020) also observed marked increases in serum total protein, albumin, and globulin levels in Labeo chrysophekadion fed with ethanolic leaf extract of Apium graveolens, which further aligns with the findings of Fawole et al. (2016).
Dietary supplementation of KSE positively affected NBT activity in both pre- and post-challenge studies. The enhanced NBT activity confirms increased phagocyte activity, which h is essential for effectively eliminating bacterial pathogens (Sahu et al., 2007). Fish fed with 5.0 and 7.5 g KSE kg−1 diet exhibited maximum NBT activities, aligning with Musthafa et al. (2018)' findings in Nile tilapia that utilized Mucuna pruriens seed meal extract. Furthermore, our study confirmed that KSE supplementation led to a reduction in glucose levels in the fish in both pre and post-challenge studies, indicating a lower stress response (Citarasu et al., 2006). This finding is supported by Heydari et al. (2018), who observed lower glucose levels in diabetic rats fed with hydroalcoholic fruit extract of Prosopis farcta. The anti-diabetic properties of P. farcta extract are in line with the findings of Soni et al. (2018).
In this study, the dietary KSE supplementation increased the total erythrocyte count (TEC) and hemoglobin content in rohu fingerlings under both pre- and post-challenge conditions. This enhancement in TEC count and Hb content may contribute to improved oxygen supply during oxidative stress induced by pathogenic infections. While the white blood cell count was not changed during pre-challenge conditions. It was increased in infected fish, possibly indicating enhanced non-specific immunity. This finding is consistent with Amirkhani and Firouzbakhsh (2015), who observed an increase in WBC, Hb, and TEC counts in common carp fingerlings fed with basil (Ocimum basilicum) ethanolic extract compared to the control. Similarly, Kaleeswaran et al. (2011) reported the significant improvements in hematological and biochemical parameters of Catla catla fed with Cynodondactylon ethanolic extract. These results align with the findings of other researchers such as Giri et al. (2017) and Chowdhury et al. (2021).
No mortality was observed in rohu fingerlings during the pre-challenge study, indicating the absence of toxicity from khejri pod extract in fish. Our study demonstrated that supplementation of khejri seed extract enhanced the survival of infected L. rohita fingerlings, and Fish fed with the 5.0 g kg−1 dietary inclusion of khejri seed extract exhibited the highest relative percentage survival. Similarly, various studies have reported improved survival in fish against A. hydrophila with dietary supplementation of different herbal extracts (Basha et al., 2013; Palanikani et al., 2020).
Additionally, the findings from the post-challenge study against A. hydrophila clearly showed that rohu fingerlings fed KSE exhibited enhanced resistance. This was evident through improved innate immune responses, including significant increases in hematological indices (TEC, WBC, and hemoglobin), NBT activity, and serum proteins (total protein, albumin, globulin, and the albumin-globulin ratio) when compared to the control group. Additionally, KSE-fed groups exhibited reduced stress enzyme activities and lower serum glucose levels compared to the control, firmly asserting the efficacy of khejri seed extract in improving fish surivival, health and immunity. Therefore, it can be concluded that the 5.0 g kg−1 KSE dietary supplementation improved the growth performance, feed conversion, digestive enzymes, metabolic enzyme activities, survival, and immune status of L. rohita fingerlings.
5 Conclusion
Dietary khejri seed extract (KSE) significantly influenced the growth performance, nutrient utilization, and body indices of rohu fingerlings. At an inclusion of 5.0 g kg−1, KSE promoted growth without altering digestive enzyme activities. Hematological parameters and certain serum biochemical markers also improve in KSE-fed groups. Post-challenge studies clearly indicate that stress enzyme activities and hematological measures were significantly enhanced compared to the control group. Most importantly, the KSE fed groups consistently achieved the significantly lowest mortality rates in infected L. rohita fingerlings. Therefore, it is unequivocal that ethanolic seed extracts of P. cineraria could serve as powerful growth promoters and herbal immunostimulants, effectively enhancing growth and strengthening non-specific immune responses in carps.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the Corresponding authors.
Ethics statement
The animal study was approved by the ethical procedures for the Animal Care as guided by the Ethical Committee of ICAR-CIFE, Mumbai, India was strictly adhered to conduct the current study. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RY: Investigation, Software, Writing – original draft, Formal analysis. MJ: Data curation, Methodology, Writing – review & editing. NC: Conceptualization, Writing – review & editing. VS: Methodology, Resources, Writing – review & editing. PS: Formal analysis, Writing – review & editing. MO: Formal analysis, Resources, Writing – review & editing. BS: Supervision, Writing – review & editing. DM: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We extend our gratitude to the Director, and Vice Chancellor, ICAR-Central Institute of Fisheries Education (CIFE), Mumbai, India, for the generous financial support and laboratory facilities provided for conducting our research trials. We are also appreciative of the Aquaculture Research and Seed Unit (ARSU) under the Directorate of Research (DoR), MPUAT, Udaipur, India, for their collaboration and the facilities provided for our feeding trials.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abalaka, S. E., Esievo, K. A., and Shoyinka, S. V. (2011). Evaluation of biochemical changes in Clarias gariepinus adults exposed to aqueous and ethanolic extracts of Parkia biglobosa pods. Afr. J. Biotechnol. 10, 234–40. doi: 10.5897/AJB10.331
Adeshina, I., Jenyo-Oni, A., Emikpe, B. O., Ajani, E. K., and Abdel-Tawwab, M. (2019). Stimulatory effect of dietary clove, Eugenia caryophyllata, bud extract on growth performance, nutrient utilization, antioxidant capacity, and tolerance of African catfish, Clarias gariepinus (B.), to Aeromonas hydrophila infection. J. World Aquacult. Soc. 50, 390–405. doi: 10.1111/jwas.12565
Afifi, H. S., and Al-rub, I. A. (2018). “Prosopis cineraria as an unconventional legumes, nutrition and health benefits,” in Legume Seed Nutraceutical Research (London: IntechOpen).
Agarwal, V., and Chauhan, B. M. A. (1988). study on composition and hypolipidemic effect of dietary fibre from some plant foods. Plant Foods Hum. Nutr. 38, 189–97. doi: 10.1007/BF01091723
American Public Health Association (2005). Standard Methods for the Examination of Water and Wastewater, 21 Edn. (Washington DC USA: American Public Health Association), 1220.
Amirkhani, N., and Firouzbakhsh, F. (2015). Protective effects of basil (Ocimum basilicum) ethanolic extract supplementation diets against experimental Aeromonas hydrophila infection in common carp (Cyprinus carpio). Aquacult. Res. 46, 716–24. doi: 10.1111/are.12217
A. O. A. C. Animal feed (1995). Official Methods of Analysis of AOAC International. Arlington, USA: AOAC International, 5–15.
Basha, K. A., Raman, R. P., Prasad, K. P., Kumar, K., Nilavan, E., Kumar, S., et al. (2013). Effect of dietary supplemented andrographolide on growth, non-specific immune parameters and resistance against Aeromonas hydrophilain Labeorohita (Hamilton). Fish Shellfish Immunol. 35, 1433–41. doi: 10.1016/j.fsi.2013.08.005
Bhansali, R. R. (2010). “Biology and multiplication of prosopis species grown in the Thar desert,” Desert Plants, ed. K. Ramawat (Berlin; Heidelberg: Springer), 371–406. doi: 10.1007/978-3-642-02550-1_18
Bilen, S., Özkan, O., Alagöz, K., and Özdemir, K. Y. (2018). Effect of dill (Anethum graveolens) and garden cress (Lepidium sativum) dietary supplementation on growth performance, digestive enzyme activities and immune responses of juvenile common carp (Cyprinus carpio). Aquaculture 495, 611–6. doi: 10.1016/j.aquaculture.2018.06.037
Blaxhall, P. C., and Daisley, K. W. (1973). Routine haematological methods for use with fish blood. J. Fish Biol. 5, 771–81. doi: 10.1111/j.1095-8649.1973.tb04510.x
Bradford, M. M. A. (1976). rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–54. doi: 10.1016/0003-2697(76)90527-3
Brett, J. R. (1973). Energy expenditure of sockeye salmon, Oncorhynchus nerka, during sustained performance. J. Fish. Res. Board Can. 30:1799e1809. doi: 10.1139/f73-290
Chaudhary, K. K., Kumar, G., Varshney, A., Meghvansi, M. K., Ali, S. F., Karthik, K., et al. (2018). Ethnopharmacological and phytopharmaceutical evaluation of Prosopis cineraria: an overview and future prospects. Curr. Drug Metab. 19, 192–214. doi: 10.2174/1389200218666171031125439
Cherry, I. S., and Crandall Jr, L. A. (1932). The specificity of pancreatic lipase: its appearance in the blood after pancreatic injury. Am. J. Physiol. Legacy Content 100, 266–73. doi: 10.1152/ajplegacy.1932.100.2.266
Chowdhury, D. K., Sahu, N. P., Sardar, P., Deo, A. D., Bedekar, M. K., Singha, K. P., et al. (2021). Feeding turmeric in combination with ginger or garlic enhances the digestive enzyme activities, growth and immunity in Labeorohita fingerlings. Anim Feed Sci. Technol. 277:114964. doi: 10.1016/j.anifeedsci.2021.114964
Christybapita, D., Divyagnaneswari, M., and Michael, R. D. (2007). Oral administration of Eclipta alba leaf aqueous extract enhances the non-specific immune responses and disease resistance of Oreochromis mossambicus. Fish Shellfish Immunol. 23, 840–52. doi: 10.1016/j.fsi.2007.03.010
Citarasu, T., Sivaram, V., Immanuel, G., Rout, N., and Murugan, V. (2006). Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol. 21, 372–84. doi: 10.1016/j.fsi.2006.01.002
Das, R., Raman, R. P., Saha, H., and Singh, R. (2015). Effect of Ocimum sanctum Linn. (Tulsi) extract on the immunity and survival of Labeorohita (Hamilton) infected with Aeromonas hydrophila. Aquacult. Res. 46, 1111–21. doi: 10.1111/are.12264
Divyagnaneswari, M., Christybapita, D., and Michael, R. D. (2007). Enhancement of nonspecific immunity and disease resistance in Oreochromis mossambicus by Solanum trilobatum leaf fractions. Fish Shellfish Immunol. 23, 249–59. doi: 10.1016/j.fsi.2006.09.015
Doumas, B. T., Watson, W. A., and Biggs, H. G. (1971). Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 31, 87–96. doi: 10.1016/0009-8981(71)90365-2
Drabkin, D. L., and Austin, J. H. (1932). Spectrophotometric studies: I. Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J. Biol. Chem. 98, 719–33. doi: 10.1016/S0021-9258(18)76122-X
Drapeau, G. (1974). Protease from Staphylococcus aureus, method of enzymology 45b, L. Methods Enzymol. 45, 469–475. doi: 10.1016/S0076-6879(76)45041-3
Elumalai, P., Kurian, A., Lakshmi, S., Faggio, C., Esteban, M. A., Ringø, E., et al. (2020). Herbal immunomodulators in aquaculture. Rev. Fish. Sci. Aquacult. 29, 33–57. doi: 10.1080/23308249.2020.1779651
Fawole, F. J., Sahu, N. P., Pal, A. K., and Ravindran, A. (2016). Haemato-immunological response of Labeorohita (Hamilton) fingerlings fed leaf extracts and challenged by Aeromonas hydrophila. Aquacult. Res. 47, 3788–99. doi: 10.1111/are.12829
Fawole, F. J., Sahu, N. P., Shamna, N., and Adeoye, A. A. (2018). Effect of Psidium guajava and Mangifera indica leaves extracts on growth, antioxidant and metabolic enzymes activities of Labeo rohita fingerlings. J. Assoc. Nigerian Fish. Sci. 1, 40–49. Available online at: https://www.researchgate.net/publication/342717363
Food and Agriculture Organization. (2002). Antibiotics Residue in Aquaculture Products. The State of world fisheries and aquaculture. Rome: FAO, 74–82. Available online at: https://openknowledge.fao.org/handle/20.500.14283/y7300e
Food and Agriculture Organization. (2022). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome: Food and Agriculture Organization, 1–37. doi: 10.4060/cc0461en
Food and Agriculture Organization. (2024). The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action. Rome: Food and Agriculture Organization, 1–264.
Furne, M., Hidalgo, M. C., Lopez, A., Garcia-Gallego, M., Morales, A. E., Domezain, A., et al. (2005). Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 250, 391–8. doi: 10.1016/j.aquaculture.2005.05.017
Garg, A., and Mittal, S. K. (2013). Review on Prosopis cineraria: a potential herb of Thar desert. Drug Invent. Today 5, 60–5. doi: 10.1016/j.dit.2013.03.002
Garg, C. K., Sahu, N. P., Shamna, N., Deo, A. D., Fawole, F. J., Kumar, S., et al. (2019). Effect of dietary Houttuynia cordata leaf meal and leaf extract on the growth performance, nutrient utilization and expression of IGF-I gene in Labeorohita. Aquacult. Nutr. 25, 702–11. doi: 10.1111/anu.12891
Garg, D., Chakraborty, S., and Gokhale, J. S. (2020). Optimizing the extraction of protein from Prosopis cineraria seeds using response surface methodology and characterization of seed protein concentrate. LWT. 117:108630. doi: 10.1016/j.lwt.2019.108630
Giri, S. S., Jun, J. W., Sukumaran, V., and Park, S. C. (2017). Evaluation of dietary Hybanthusenneaspermus(Linn F. Muell.) as a growth and haemato-immunological modulator in Labeorohita. Fish Shellfish Immunol. 68, 310–7. doi: 10.1016/j.fsi.2017.07.009
Giri, S. S., Sen, S. S., Chi, C., Kim, H. J., Yun, S., Park, S. C., et al. (2015). Effect of guava leaves on the growth performance and cytokine gene expression of Labeorohita and its susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. 46, 217–24. doi: 10.1016/j.fsi.2015.05.051
Giri, S. S., Sukumaran, V., and Oviya, M. (2013). Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeorohita. Fish Shellfish Immunol. 34, 660–6. doi: 10.1016/j.fsi.2012.12.008
Gurib-Fakim, A. (2006). Medicinal plants: traditions of yesterday and drugs of tomorrow. Mole. Aspects Med. 27, 1–93. doi: 10.1016/j.mam.2005.07.008
Hashemi, S. R., and Davoodi, H. (2011). Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 35, 169–80. doi: 10.1007/s11259-010-9458-2
Hemre, G. I., Mommsen, T. P., and Krogdahl, Å. (2002). Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquacult. Nutr. 8, 175–94. doi: 10.1046/j.1365-2095.2002.00200.x
Henciya, S., Seturaman, P., James, A. R., Tsai, Y. H., Nikam, R., Wu, Y. C., et al. (2017). Biopharmaceutical potentials of prosopis spp. (mimosaceae, leguminosa). J. Food Drug Anal. 25, 187–96. doi: 10.1016/j.jfda.2016.11.001
Heydari, M., Sarir, H., Ghiasi, S. E., and Farhangfar, H. (2018). Effects of Prosopis farcta fruit hydroalcoholic extract on serum concentrations of glucose and lipids in insulin resistance model of rats. Zahedan J. Res. Med. Sci. 20:e13498. doi: 10.5812/zjrms.13498
Hoseinifar, S. H., Shakouri, M., Yousefi, S., Van Doan, H., Shafiei, S., Yousefi, M., et al. (2020). Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. 100, 171–8. doi: 10.1016/j.fsi.2020.02.067
Ibrahim, G. A., Mabrok, M., Alfifi, K. J., Alatawy, M., Al-Otaibi, A. S., Alenzi, A. M., et al. (2014). Pathogenicity, resistance patterns, virulence traits, and resistance genes of re-emerging extensively drug-resistant (XDR) Aeromonas veronii in Oreochromis niloticus. Aquacult. Int. 32, 1–20. doi: 10.1007/s10499-024-01498-0
Jayant, M., Sahu, N. P., Deo, A. D., Gupta, S., Garg, C. K., Valappil, R. K., et al. (2020). Nutritional evaluation of fermented sweet potato leaf meal as a replacer of deoiled rice bran in the diet of Labeorohita fingerlings. J. Exp. Zool. India. 23, 61–70. Available online at: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20203130885
Kaleeswaran, B., Ilavenil, S., and Ravikumar, S. (2011). Dietary supplementation with Cynodondactylon (L.) enhances innate immunity and disease resistance of Indian major carp, Catlacatla (Ham.). Fish Shellfish Immunol. 31, 953–62. doi: 10.1016/j.fsi.2011.08.013
Kalia, R. K., Krishnan, P. R., and Tewari, J. C. (2014). Limitations, progress and prospects of application of biotechnological approaches in improvement of Prosopis: an important genus of the arid regions. Annal Arid Zone. 53, 33–41. Available online at: https://www.researchgate.net/publication/315807948
Kazeem, G. O., Adedayo, F. E., and Thomas, A. O. (2017). Effects of dietary Moringa oleifera extract against Aeromonas hydrophila infection and transportation-induced stress in African catfish Clarias gariepinus (Burchell, 1822) fingerlings. World Appl. Sci. J. 35, 88–95. Available online at: https://idosi.org/wasj/wasj35(1)17/12.pdf
Keer, N. R., Chadha, N. K., Saini, V. P., Ojha, M. L., and Sawant, P. B. (2020). Dietary shatavari, Asparagousracemosus root extract promotes growth, feed conversion and nutrient utilization in Labeorajasthanicus. J. Environ. Biol. 41, 1464–9. doi: 10.22438/jeb/41/6/MRN-1373
Khan, R., Zakir, M., Afaq, S. H., Latif, A., and Khan, A. U. (2010). Activity of solvent extracts of Prosopis spicigera, Zingiber officinale and Trachyspermumammi against multidrug resistant bacterial and fungal strains. J. Infect. Dev. Countries 4, 292–300. doi: 10.3855/jidc.621
Krishnan, P. R., and Jindal, S. K. (2015). Khejri, the king of Indian Thar desert is under phenophase change. Curr. Sci. 108, 1987–1990. Available online at: https://www.jstor.org/stable/24905562
Kuchana, V., Sampathi, S., Pamu, S., and Poosa, M. (2014). Phytochemical screening and antimicrobial activity of roots of Prosopis cineraria. Int. J. Adv. Pharm. Biol. Chem. 3, 502–506. Available online at: http://www.ijapbc.com/files/45-3283.pdf
Leung, T. L., and Bates, A. E. (2013). More rapid and severe disease outbreaks for aquaculture at the tropics: implications for food security. J. Appl. Ecol. 50, 215–22. doi: 10.1111/1365-2644.12017
Mahdavi, M., Hajimoradloo, A., and Ghorbani, R. (2013). Effect of Aloe vera extract on growth parameters of common carp (Cyprinus carpio). World J. Med. Sci. 9, 55–60. Available online at: https://www.idosi.org/wjms/9(1)13/9.pdf
Malik, S., Mann, S., Gupta, D., and Gupta, R. K. (2013). Nutraceutical properties of Prosopis cineraria (L.) druce pods: a component of panchkuta. J. Pharmacogn. Phytochem. 2, 66–73. Available online at: https://www.phytojournal.com/archives/2013/vol2issue2/PartA/10.1.pdf
Misra, H. P., and Fridovich, I. (1972). The univalent reduction of oxygen by reduced flavins and quinones. J. Biol. Chem. 247, 188–92. doi: 10.1016/S0021-9258(19)45773-6
Musthafa, M. S., Asgari, S. M., Kurian, A., Elumalai, P., Ali, A. R., Paray, B. A., et al. (2018). Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 75, 374–80. doi: 10.1016/j.fsi.2018.02.031
Nelson, N. A. (1944). photometric adaptation of the Somogyi method for the determination of glucose. J. biol. Chem. 153, 375–80. doi: 10.1016/S0021-9258(18)71980-7
Nopitawati, T., and Jusadi, D. (2015). Screening of probiotic bacteria candidates from gastrointestinal tract of Pacific white shrimp Litopenaeusvannamei and their effects on the growth performances. Res. J. Microbiol. 10:145. doi: 10.3923/jm.2015.145.157
Ochoa, S. (1955). “Malic enzyme,” in Methods in Enzymology, eds. S. P. Colowick and N. O. Kaplan (New York: Acad Press), 739–753. doi: 10.1016/0076-6879(55)01129-4
Palanikani, R., Chanthini, K. M., Soranam, R., Thanigaivel, A., Karthi, S., Senthil-Nathan, S., et al. (2020). Efficacy of Andrographis paniculata supplements induce a non-specific immune system against the pathogenicity of Aeromonas hydrophila infection in Indian major carp (Labeorohita). Environ. Sci. Pollut. Res. 27, 23420–36. doi: 10.1007/s11356-019-05957-7
Preeti, K., Sharma, R. A., and Kumavat, R. B. (2017). Antimicrobial activity of different parts of Prosopis cineraria. Adv. Biosci. Bioeng. 5, 78–81. doi: 10.11648/j.abb.20170505.11
Pu, H., Li, X., Du, Q., Cui, H., and Xu, Y. (2017). Research progress in the application of Chinese herbal medicines in aquaculture: a review. Engineering 3, 731–7. doi: 10.1016/J.ENG.2017.03.017
Rai, M. K., Shekhawat, J. K., Kataria, V., Phulwaria, M., and Shekhawat, N. S. (2021). Genomic and biotechnological interventions in Prosopis cineraria: current status, challenges and opportunities. Trees 35, 1–3. doi: 10.1007/s00468-020-02073-9
Ram, H., Jaipal, N., Kumar, P., Charan, J., Kashyap, P., Kumar, S., et al. (2020). Novel phytoconstituents of an aqueous pod extract of Prosopis Cineraria (L.) Druce attenuate atherosclerotic plaque and hypercholesterolemia in rabbits. Lipids Health Dis. 19:6. doi: 10.1186/s12944-020-1188-z
Rashidian, G., Bahrami Gorji, S., Farsani, M. N., Prokić, M. D., and Faggio, C. (2020). The oak (Quercus brantii) acorn as a growth promotor for rainbow trout (Oncorhynchus mykiss): growth performance, body composition, liver enzymes activity and blood biochemical parameters. Na. Product Res. 34, 2413–23. doi: 10.1080/14786419.2018.1538994
Reinhold, J. G. (1953). Total protein, albumin, and globulin. Stand. Methods Clin. Chem, 1, 88–97. doi: 10.1016/B978-0-12-609101-4.50019-8
Reverter, M., Tapissier-Bontemps, N., Sasal, P., and Saulnier, D. (2017). “Use of medicinal plants in aquaculture,” in Diagnosis and Control of Diseases of Fish and Shellfish. Chap. 9, eds. B. Austin and A. Newaj-Fyzul (West Sussex: Willey), 223–261. doi: 10.1002/9781119152125.ch9
Rick, W., and Stegbauer, H. P. (1974). “α-amylase measurement of reducing groups,” in Methods of Enzymatic Analysis, Vol. 2, 2nd Edn. (New York, NY; London: Academic Press), 885–890. doi: 10.1016/B978-0-12-091302-2.50074-8
Ringø, E., and Song, S. K. (2016). Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquacult. Nutr. 22, 4–24. doi: 10.1111/anu.12349
Sahu, S., Das, B. K., Pradhan, J., Mohapatra, B. C., Mishra, B. K., Sarangi, N., et al. (2007). Effect of Magnifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeorohita fingerlings. Fish Shellfish Immunol. 23, 109–18. doi: 10.1016/j.fsi.2006.09.009
Shinn, A. P., Pratoomyot, J., Bron, J. E., Paladini, G., Brooker, E. E., Brooker, A. J., et al. (2015). Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 142, 196–270. doi: 10.1017/S0031182014001437
Shiu, Y. L., Lin, H. L., Chi, C. C., Yeh, S. P., and Liu, C. H. (2016). Effects of hirami lemon, Citrus depressa Hayata, leaf meal in diets on the immune response and disease resistance of juvenile barramundi, Lates calcarifer (bloch), against Aeromonas hydrophila. Fish Shellfish Immunol. 55, 332–8. doi: 10.1016/j.fsi.2016.06.001
Soni, L. K., Dobhal, M. P., Arya, D., Bhagour, K., Parasher, P., Gupta, R. S., et al. (2018). In vitro and in vivo antidiabetic activity of isolated fraction of Prosopis cineraria against streptozotocin-induced experimental diabetes: a mechanistic study. Biomed. Pharmacother. 108, 1015–21. doi: 10.1016/j.biopha.2018.09.099
Stasiack, A. S., and Bauman, C. P. (1996). Netrophil activity as a potential indicator immunomodulator Ecteinascida turbinata extract on Edwardsiellaictaluri infection of channel catfish. J. Aquat. Anim. Health. 7, 141–6. doi: 10.1577/1548-8667(1995)007<0141:EOTIET>2.3.CO;2
Sundararajan, A., Sahu, N. P., Shamna, N., Jayant, M., Sardar, P., Vasanthakumaran, Krishnamenan, N. S., et al. (2024). Dietary papaya peel extract ameliorates the crowding stress, enhances growth and immunity in Labeo rohita fingerlings. Fish Physiol. Biochem. 50, 1047–1064. doi: 10.1007/s10695-024-01317-6
Sutthi, N., Panase, A., Chitmanat, C., Sookying, S., Ratworawong, K., Panase, P., et al. (2020). Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aquacult. Rep. 18:100551. doi: 10.1016/j.aqrep.2020.100551
Syed, R., Masood, Z., Hassan, H. U., Khan, W., Mushtaq, S., Ali, A., et al. (2022). Growth performance, haematological assessment and chemical composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed different levels of Aloe vera extract as feed additives in a closed aquaculture system. Saudi J. Biol. Sci. 29, 296–303. doi: 10.1016/j.sjbs.2021.08.098
Takahara, S., Hamilton, H. B., Neel, J. V., Kobara, T. Y., Ogura, Y., Nishimura, E. T., et al. (1960). Hypocatalasemia: a new genetic carrier state. J. Clin. Invest. 39, 610–9. doi: 10.1172/JCI104075
Tejaswini, K., Deo, A. D., Shamna, N., Jayant, M., Aklakur, M., Annadurai, R., et al. (2024). Effect of flavanone rich lemon peel extract on feed intake and growth of Labeorohita (Hamilton, 1822) fingerlings reared at low temperature recirculatory aquaculture system. Aquaculture 584:740450. doi: 10.1016/j.aquaculture.2023.740450
Van Doan, H., Hoseinifar, S. H., Naraballobh, W., Jaturasitha, S., Tongsiri, S., Chitmanat, C., et al. (2019). Dietary inclusion of orange peels derived pectin and Lactobacillus plantarum for Nile tilapia (Oreochromis niloticus) cultured under indoor biofloc systems. Aquaculture 508, 98–105. doi: 10.1016/j.aquaculture.2019.03.067
Vaza, J. S., and Bhalerao, S. A. (2018). Phytochemistry and pharmacological profile of Prosopis cineraria: a review. Int. J. Sci. Dev. Res. 3, 635–638. Available online at: https://www.ijsdr.org/papers/IJSDR1805095.pdf
Wang, J. L., Meng, X. L., Lu, R. H., Wu, C., Luo, Y. T., Yan, X., et al. (2015). Effects of Rehmanniaglutinosa on growth performance, immunological parameters and disease resistance to Aeromonas hydrophila in common carp (Cyprinus carpio L.). Aquaculture 435, 293–300. doi: 10.1016/j.aquaculture.2014.10.004
Wootton, I. D. P. (1964). Micro-analysis in Medical Biochemistry, 4th Edn.. London: Churchill, 101–103.
Wróblewski, F., and Ladue, J. S. (1955). Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biology Med. 90, 210–3. doi: 10.3181/00379727-90-21985
Xie, J., Liu, B., Zhou, Q., Su, Y., He, Y., Pan, L., et al. (2008). Effects of anthraquinone extract from rhubarb Rheum officinale Bail on the crowding stress response and growth of common carp Cyprinus carpio var. Jian. Aquaculture 281, 5–11. doi: 10.1016/j.aquaculture.2008.03.038
Yadav, E., Singh, D., Yadav, P., and Verma, A. (2018). Antioxidant and anti-inflammatory properties of Prosopis cineraria based phenolic rich ointment in wound healing. Biomed. Pharmacother. 108, 1572–83. doi: 10.1016/j.biopha.2018.09.180
Yadav, R., Chadha, N. K., Saini, V. P., Sawant, P. B., Ojha, M. L., Jayant, M., et al. (2022). Substitution of de-oiled rice bran with Khejri pod meal and groundnut oilcake with Khejri seed meal in the diet of Labeorohita (Hamilton, 1822). Aquacult. Res. 53, 642–56. doi: 10.1111/are.15608
Yousefi, M., Hoseini, S. M., Vatnikov, Y. A., Kulikov, E. V., and Drukovsky, S. G. (2019). Rosemary leaf powder improved growth performance, immune and antioxidant parameters, and crowding stress responses in common carp (Cyprinus carpio) fingerlings. Aquaculture 505, 473–80. doi: 10.1016/j.aquaculture.2019.02.070
Keywords: khejri seed extract, growth indices, digestive-metabolic responses, rohu, Labeo rohita, Aeromonas hydrophilia
Citation: Yadav R, Jayant M, Chadha NK, Saini VP, Sawant PB, Ojha ML, Sharma BK and Meena DK (2025) Khejri seed extract (KSE) enhances the growth, digestive, metabolic, and immunological responses of Labeo rohita fingerlings. Front. Water 7:1552453. doi: 10.3389/frwa.2025.1552453
Received: 28 December 2024; Accepted: 07 April 2025;
Published: 28 April 2025.
Edited by:
Angel Isidro Campa, Centro de Investigación Biológica del Noroeste (CIBNOR), MexicoReviewed by:
Neelesh Kumar, Rani Lakshmi Bai Central Agricultural University, IndiaPrasanta Jana, Birsa Agricultural University, India
Copyright © 2025 Yadav, Jayant, Chadha, Saini, Sawant, Ojha, Sharma and Meena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohitash Yadav, cm9oaXRhc2h5YWRhdjA5M0BnbWFpbC5jb20=; ZHJhcXVheWFkYXYwOTNAZ21haWwuY29t; Narinder Kumar Chadha, bmFyaW5kZXJrY2hhZGhhOTlAZ21haWwuY29t
†ORCID: Rohitash Yadav orcid.org/0000-0002-7280-4070
Narinder Kumar Chadha orcid.org/0000-0002-9703-4543
 Rohitash Yadav
Rohitash Yadav Manish Jayant
Manish Jayant Narinder Kumar Chadha1*†
Narinder Kumar Chadha1*† Ved Prakash Saini
Ved Prakash Saini Paramita Banerjee Sawant
Paramita Banerjee Sawant Dharmendra Kumar Meena
Dharmendra Kumar Meena