- Department of Chemistry and Chemical Engineering, Jinzhong University, Jinzhong, Shanxi, China
A phytotoxicity-based toxicity identification evaluation (TIE) approach was employed to identify toxic substances in river water, focusing on the Taiyuan section of the Fen River in China as a case study. Four vegetable seed species—amaranth, oilseed rape, cabbage, and lettuce—were selected for toxicity screening. An analysis of variance was performed on the germination index (GI%) of these seeds exposed to nine initial water samples from the Fen River. This analysis revealed that the sampling site with the lowest GI% was S6. Consequently, samples from the S6 site were used for subsequent TIE treatments, which included aeration, EDTA chelation, reduction with oxidizing agents, filtration, solid-phase extraction using C18, and pH adjustment. As a result, EDTA and its related treatment significantly improved seed viability across nearly all species, underscoring the role of cationic metals in contributing to toxicity. Correspondingly, 10 metals in the initial water samples were analyzed using inductively coupled plasma mass spectrometry. Linear correlations were observed between the concentrations of metals (cobalt, chromium, nickel, and lead) and the phytotoxicity observed in the four vegetable species, with correlation coefficients exceeding 0.5 (p < 0.01). Finally, Canonical correlation analysis suggested that exposure to lead in riverine water was most likely associated with the phytotoxic effects observed in oilseed rape and cabbage, contributing to overall toxicity. In conclusion, lead was confirmed as the primary toxicant responsible for the observed phytotoxic effects in oilseed rape and cabbage in the Fen River. Although metal concentrations remained below the Environmental Quality Standards for Surface Water thresholds, the identified ecological risks underscore the need for enhanced monitoring and management. The phytotoxicity-based TIE approach proved effective in identifying specific toxicants, providing valuable insights for water quality management and ecological risk assessment.
1 Introduction
River contamination represented a significant global environmental concern, threatening the health of aquatic ecosystems. The principal sources of this pollution included agricultural runoff, wastewater treatment facility discharges, and industrial wastewater. River water functioned as a complex system containing a variety of pollutants, including pesticides (Ramage et al., 2025), pharmaceuticals (Rodrigues et al., 2024), heavy metals (Wang et al., 2025), personal care products (Hawash et al., 2023), microplastics (Priya et al., 2025), halogenated flame retardants (Olaniyan et al., 2023), estrogens (Waniek et al., 2025), per-and polyfluoroalkyl substances (Kali et al., 2025), and other emerging contaminants (Picinini-Zambelli et al., 2025). These pollutants had the potential to disrupt species abundance (Muhie et al., 2025), alter biome composition (Malla et al., 2025), and compromise the ecological functionality of aquatic ecosystems (Jiang et al., 2025). The environmental risk posed by these pollutants in water bodies may be underestimated by conventional chemical monitoring and biological toxicity testing alone (Yan et al., 2025).
The toxicity identification evaluation (TIE) technique, which integrated organism toxicity testing with chemical manipulations, proved effective in identifying causative toxicants in complex environmental samples (USEPA, 1991, 1993a, 1993b). It successfully characterized toxicity in various contexts, including sediments (Ho and Burgess, 2013; Ke et al., 2015), seafood wastewater (Tsipa et al., 2023), shale gas wastewater (Zhou et al., 2024), pharmaceutical wastewater (Chen et al., 2025), and excess sludge (Nobili et al., 2024). Within the TIE framework, various test organisms were employed, with Daphnia magna, fish, and plants being the most commonly used. Furthermore, terrestrial plant seeds demonstrated their ability to assess phytotoxicity in environmental media, offering advantages such as sensitivity, simplicity, rapidity, low cost, and minimal equipment requirements. However, seed germination was infrequently utilized in TIE procedures for riverine water, despite its application in identifying toxicity in landfill leachate (Budi et al., 2016) and toxic industrial effluents (Ra et al., 2016).
The Fen River, recognized as the largest river in Shanxi Province and the second-largest tributary of the Yellow River in China, experienced notable improvements in water quality in recent years. However, prior research revealed concerning levels of pollutants, including heavy metals (Li et al., 2023), estrogens (Liu et al., 2017), nitrates (Xiao et al., 2022), and polyfluoroalkyl substances (Zhou et al., 2019). Despite improvements in chemical quality, the ecological factors contributing to phytotoxicity in the Fen River remained unclear, as conventional chemical analysis failed to account for plant-relevant exposure pathways. Identifying the primary causes of biotoxicity was essential for establishing a scientific foundation for effective water management in the Fen River. Based on the technical route outlined in Figure 1, it was hypothesized that the observed phytotoxicity during seed germination resulted from volatile pollutants, cationic metals, oxidants, particulate matter, or nonpolar organic compounds. This study aimed to identify key toxicants in riverine water using a phytotoxicity-based TIE approach, following an assessment of initial water toxicity on seed germination, with the Taiyuan section of the Fen River serving as a case study.
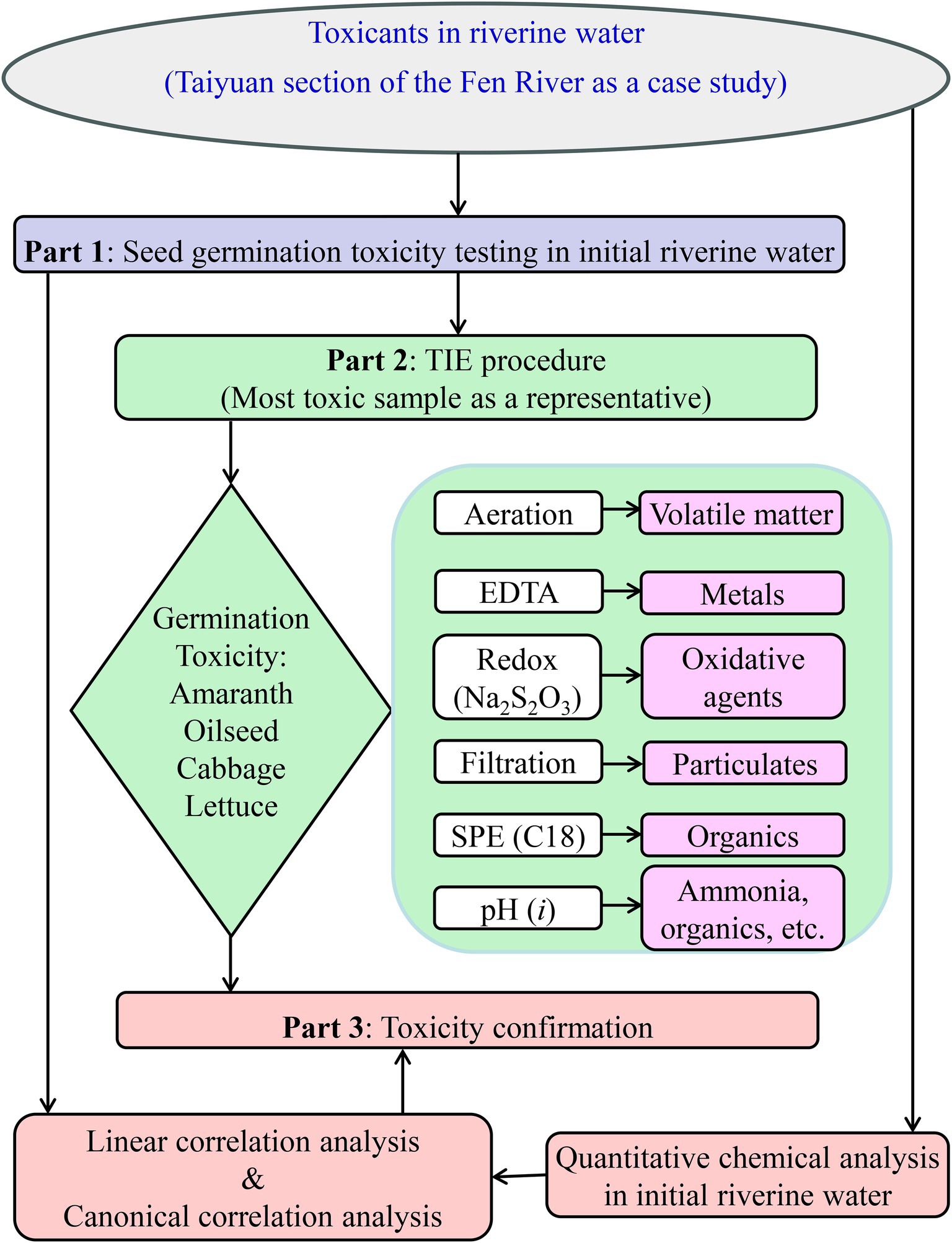
Figure 1. Scheme of toxicity identification evaluation based on seed germination toxicity in riverine water.
2 Materials and methods
2.1 Sample collection
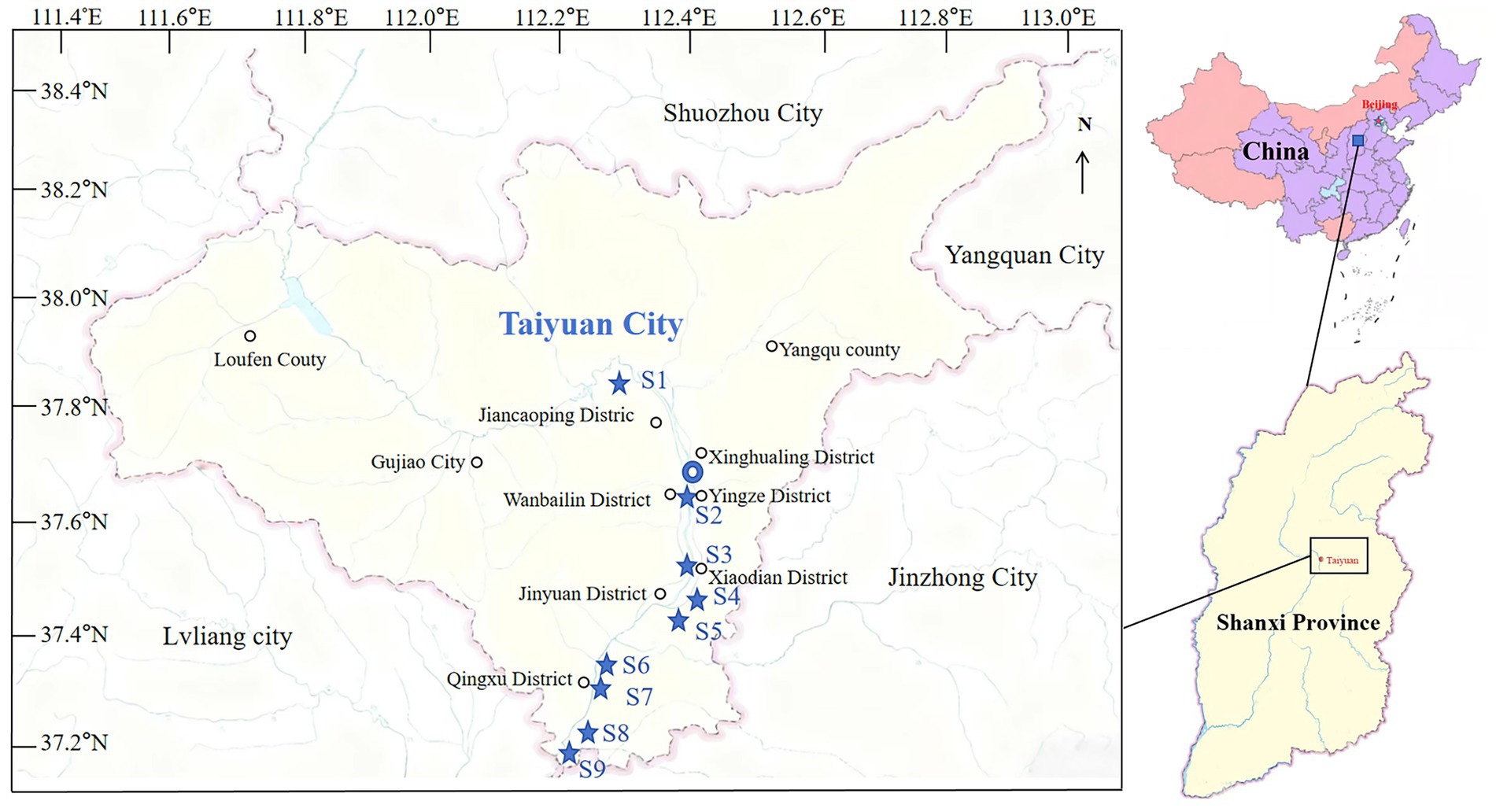
In September 2024, nine water samples were collected from the upper water column at a depth of 0.5 meters in the Taiyuan section of the Fen River in China, as illustrated in Figure 2. Seed toxicity tests were performed within 1 day of sample collection. For heavy metal analysis, the samples were acidified, stored at 4°C, shielded from light, and tested within 3 days.
2.2 Chemical analysis
The concentrations of 10 metals—cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), vanadium (V), and zinc (Zn)—were analyzed using an inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7,700) with internal calibration, as presented in Supplementary Table S1. Additional details regarding the metal analysis are available in the Supplementary material.
2.3 Toxicity test for seeds
The seeds used in this study included amaranth (Amaranth tricolor L.), oilseed rape (Brassica napus L.), cabbage (Brassica campestris L.), and lettuce (Lactuca sativa L.), all obtained from Shanxi Dafeng Seed Industry Co., Ltd. in China. These species were chosen for their rapid germination rates, sensitivity to pollutants, and significance as common vegetable crops in the region. Phytotoxicity tests were conducted in accordance with the agricultural seed testing guidelines established in China (GB/T3543.4–2025) (CSAMR, 2025). In brief, 50 seeds from each species were germinated on filter paper within a clean 10 cm-diameter petri dish, each containing 10 mL of treatment water. The Petri dishes were incubated in the dark at 25°C. After 5 days of exposure, seed germination was evaluated by measuring primary root length. Only seeds with a primary root length of at least 3 mm were considered successfully germinated. All tests were independently replicated three times. Following the recommendation from the literature (Zucconi et al., 1985), the germination index (GI) was calculated using the following formula:
Where G represents the number of seeds germinated in the test, Gc denotes the number of seeds germinated in the control, RL indicates the mean root length in the test, and RLc signifies the mean root length in the control.
2.4 TIE procedure
The TIE procedure was conducted with the recommendations of the United States Environmental Protection Agency (USEPA, 1991). The evaluation included several treatments: aeration, ethylenediaminetetraacetic acid (EDTA) chelation, oxidant reduction, filtration, solid phase extraction with C18, and pH adjustment. These methods allowed for the tentative classification of toxic substances into the following categories: volatile pollutants, cationic metals, oxidants, particulate matter, non-polar organics, and pH-sensitive chemicals. (1) The water used for toxicity testing was lightly aerated at a controlled rate of 4 L/min, ensuring optimal conditions with an air pump. (2) EDTA was added through dilution, achieving a final concentration of 30 mg/L for the four seeds. (3) In the oxidant reduction test, sodium thiosulfate (Na2S2O3) was carefully introduced into the solution at a concentration of 0.1 g/L for four seeds, effectively reducing oxidants. (4) Samples were filtered using a 0.45 μm Whatman GF/C filter membrane to eliminate harmful particles. (5) Solid phase extraction (SPE) was carried out with a C18 column (2000 mg/12 mL, Shanghai Titan Scientific Co., Ltd.). (6) During the pH adjustment tests, the initial pH of the water was accurately modified to pH 3 with hydrochloric acid (HCl) or adjusted to pH 11 with sodium hydroxide (NaOH), facilitating thorough assessments of pH-sensitive chemicals.
2.5 Statistical analysis
Data on biological effects were reported as the mean ± standard deviation from three independent experiments. A one-way analysis of variance (ANOVA) was employed to identify statistically significant differences, and the Tukey Test was utilized for multiple comparisons. Canonical correlation analysis was performed using SPSS 26.0 software.
3 Results and discussion
3.1 Toxicity screening
Four vegetable seeds were chosen for toxicity tests of the initial water due to the relative simplicity and sensitivity of seed toxicity screening. The GI% was analyzed using one-way ANOVA for multiple comparisons. As shown in Supplementary Table S2 and Table 1, no statistically significant differences were found among items labelled with the same letter within the same column. Conversely, statistically significant differences were observed between items labelled with different letters, suggesting that they could be classified into distinct categories. Among the evaluated categories, seed viability consistently ranked lowest for amaranth (C), oilseed rape (E), cabbage (C), and lettuce (D) at site S6. Consequently, a sample from site S6, located downstream along the Fen River, was selected for further TIE studies.
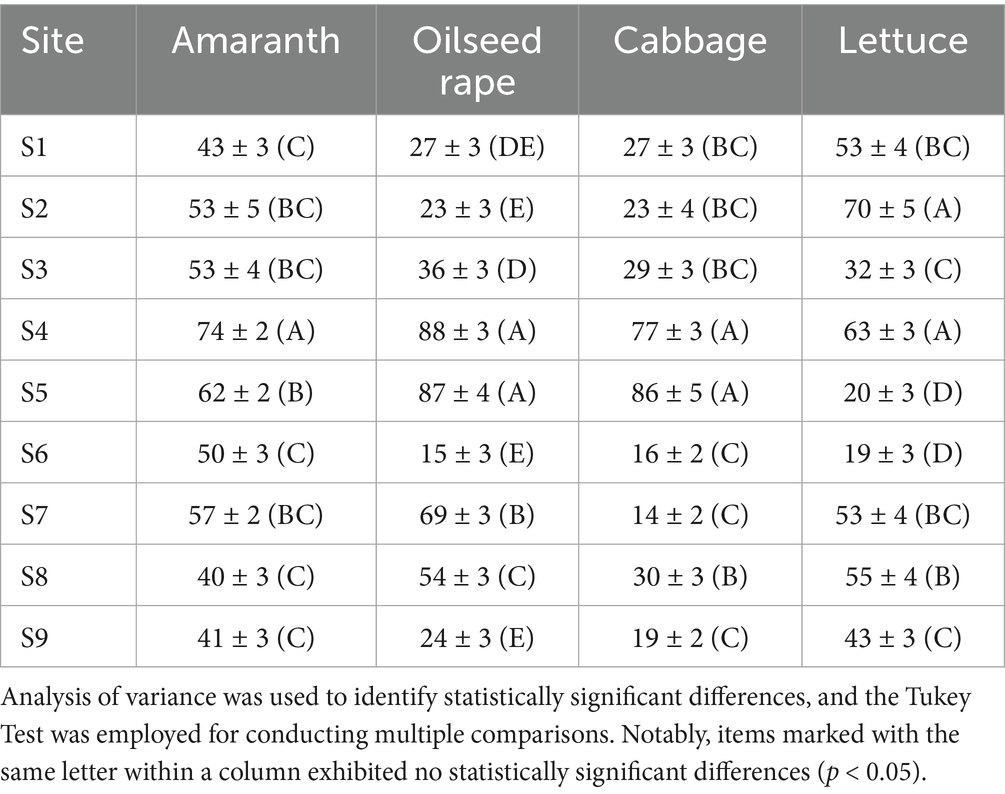
Table 1. The analysis of variance and multiple comparisons for the (GI%) in the Taiyuan section of the Fen River in China (mean ± SD, n = 3).
3.2 Toxicity characterization in TIE
To statistically differentiate among the various TIE treatments, a one-way ANOVA was performed on the mean GI% across treatments. As shown in Supplementary Table S3 and Figure 3, significant differences were primarily observed in the EDTA and redox treatments compared with raw water, except for amaranth, which showed statistically significant differences not only in the EDTA and Redox treatments but also in the pH adjustment.
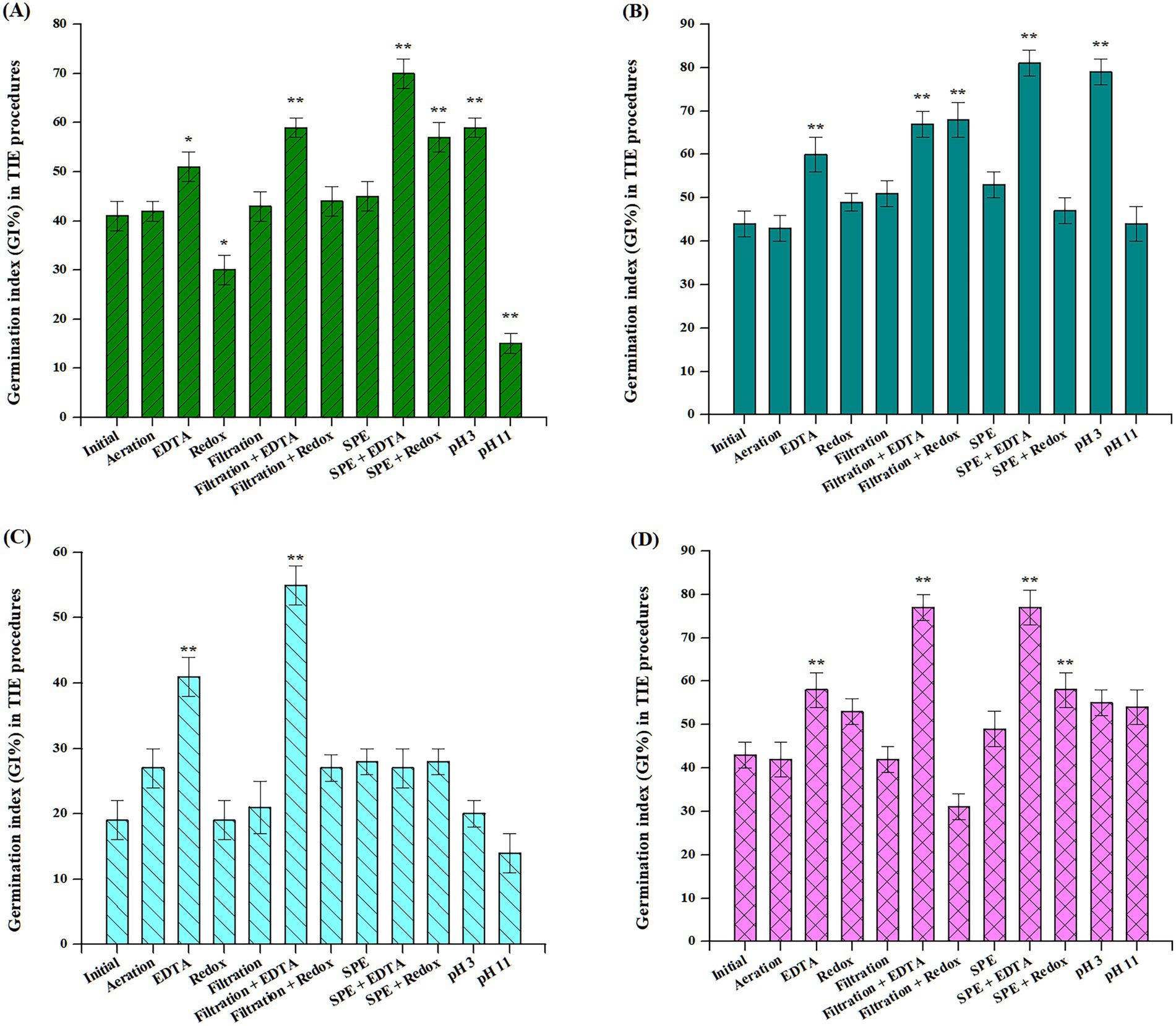
Figure 3. Analysis of variance for the germination index (GI%) among TIE treatment groups. (A): amaranth, (B): oilseed rape, (C): cabbage, (D): lettuce. Significant differences between the test and initial water were determined by a one-way analysis of variance and indicated by *p < 0.05 and **p < 0.01, respectively. Error bars represent standard deviation (n = 3).
Analysis of variance was used to identify statistically significant differences, and the Tukey Test was employed for conducting multiple comparisons. Notably, items marked with the same letter within a column exhibited no statistically significant differences (p < 0.05).
Regarding EDTA, the treatments included EDTA chelation, filtration combined with EDTA treatment, and SPE combined with EDTA treatment. Firstly, seed viability significantly improved (n = 3, p < 0.01) after EDTA treatment in oilseed rape, cabbage, and lettuce compared to the initial water treatment. Amaranth also showed a significant difference under EDTA chelation (n = 3, p < 0.05). Secondly, for filtration combined with EDTA treatment, seed viability significantly improved (n = 3, p < 0.01) across all four plant species compared to the initial water treatment. Thirdly, for the SPE combined with EDTA treatment, significant differences were observed in amaranth, oilseed rape, and lettuce (n = 3, p < 0.01). However, cabbage did not show a statistically significant difference (n = 3, p > 0.05). These reductions in toxicity suggested that the presence of certain metals in the water was consistent with previous studies linking heavy metals to reduced phytotoxicity (Sperdouli, 2022). EDTA is a potent chelator that forms stable complexes with cations such as aluminium, barium, cadmium, cobalt, copper, iron, lead, manganese, nickel, strontium, and zinc. This process reduces metal bioavailability and alleviates phytotoxicity. Conversely, arsenic and mercury exhibit relatively weak chelation, while the formation of complexes such as selenides, chromates, and hydrochromates proved more challenging (USEPA, 1991).
Regarding the redox treatments, the options included redox treatment alone, filtration combined with redox treatment, and SPE combined with redox treatment. Firstly, only amaranth showed a significant difference (n = 3, p < 0.05) with the redox treatment, while oilseed rape exhibited a highly significant difference with the filtration combined with redox treatment (n = 3, p < 0.01). Additionally, the SPE combined with redox treatments demonstrated the substantial differences (n = 3, p < 0.01) for amaranth, cabbage, and lettuce, with oilseed rape being the exception (n = 3, p > 0.05). The reduction in toxicity associated with redox treatments indicated the presence of toxic oxidizers, such as chlorine, certain electrophilic organics, or specific cationic metals (USEPA, 1991). Chlorine, functioning as an oxidant and biocide, oxidizes sodium thiosulfate to form sodium chloride and sodium tetrathionate, both of which are non-toxic to the samples. Furthermore, electrophilic organic compounds were neutralized by sodium thiosulfate, thereby reducing their toxicity. The observed reduction in toxicity may also be attributed to the formation of metal complexes with thiosulfate anions, similar to the EDTA chelation process, which is designed to decrease toxicity (Hockett and Mount, 1996).
In summary, the seeds of the four plant species exhibited varying degrees of restorable toxicity, primarily due to treatments with EDTA and Redox agents. This evidence suggested that cationic metals and certain electrophilic organic compounds significantly contributed to phytotoxicity in the Taiyuan section of the Fen River in China. Consequently, further identification of TIE was conducted, focusing on the metals present in the riverine water. The identification of the electrophilic organics will be carried out in future research.
3.3 Toxicity identification in TIE
To confirm the potent candidates, the levels of metals in the Taiyuan section of the Fen River were measured using ICP-MS. The concentrations of 10 metals were detailed in Supplementary Table S4, with their descriptive statistics summarized in Table 2. The detection rates for these metals were 100% in the water samples. The mean concentrations of heavy metals were ranked in the following order: Mo > Zn > Ni > Mn > Cu > Pb > Cr > V > Co > Cd. Mo was the most abundant metal, with a mean of 8.94 ± 5.67 μg∙L−1, while Cd exhibited the lowest mean concentration at 0.016 ± 0.01 μg∙L−1. Furthermore, the maximum concentrations of heavy metals in all samples remained below the Chinese Environmental Quality Standard for Surface Water (GB 3838–2002) (MEPC, 2002).
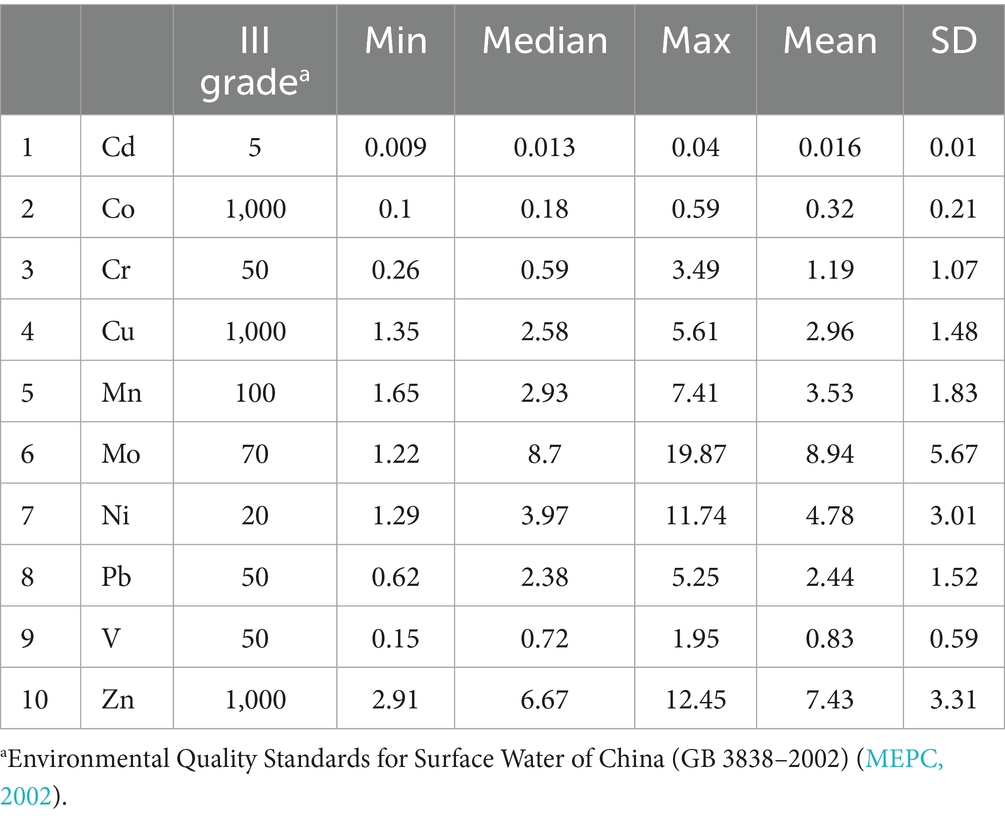
Table 2. Statistical description of metal concentrations in 2024 from the Taiyuan section of the Fen River in China (n = 9, μg·L−1).
Water quality benchmarks are essential not only for physicochemical monitoring but also for ecological risk assessment. Until now, only three water quality ecological criteria have been issued in China in 2020 for ammonia nitrogen (CMEE, 2020a), cadmium (CMEE, 2020b), and phenol (CMEE, 2020c). However, the technical guideline for deriving water quality criteria of freshwater organisms (HJ 831–2022) (CMEE, 2022) was issued on March 10, 2022. The release of this guideline is of great significance for establishing a comprehensive water quality criteria system, effectively controlling toxic and harmful pollutants, and protecting aquatic biodiversity and the integrity of water ecosystems.
3.4 Toxicity confirmation in TIE
3.4.1 Linear correlation analysis between phytotoxicity and the concentrations of metals
Phytotoxicity was first confirmed by evaluating nine initial water samples from the Fen River. Subsequently, a linear correlation analysis was performed to investigate the relationship between the GI% of seeds converted to probit values and the logarithmic values of metal concentrations in the corresponding water samples. The correlation coefficients are presented in Table 3. The toxicity of plant seeds exhibited strong correlations with the concentrations of Co, Cr, Ni, and Pb, with correlation coefficients exceeding 0.5 (p < 0.01). Consequently, these four metals were selected for a canonical correlation analysis to confirm their toxic effects further.
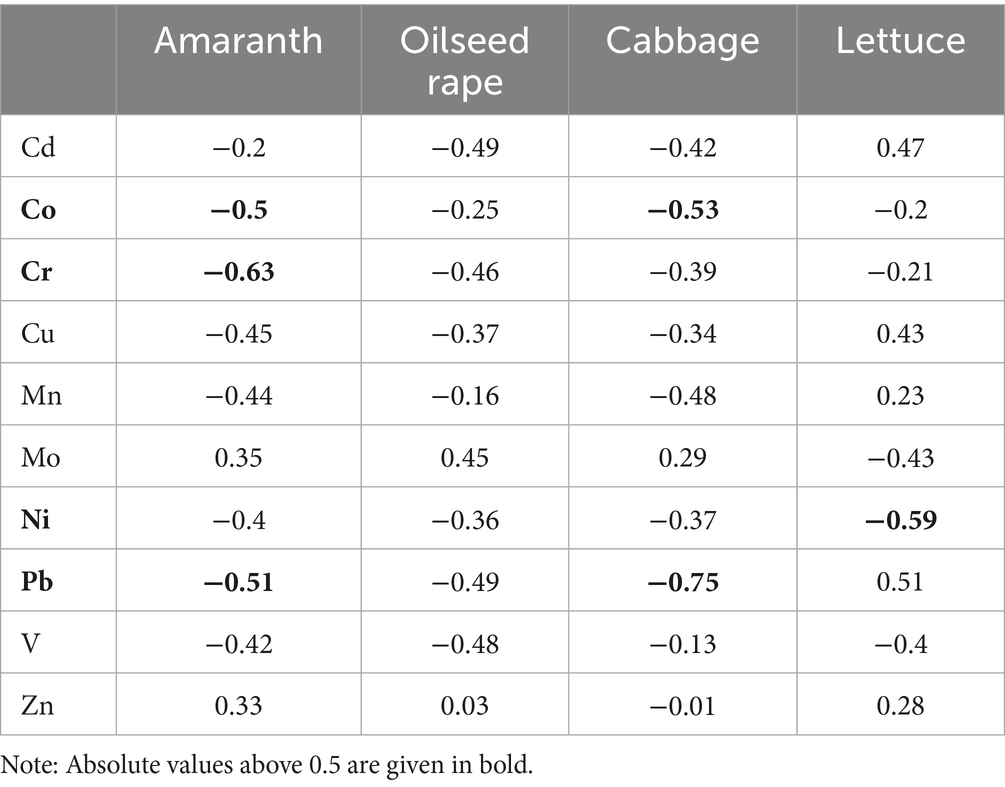
Table 3. The linear correlation coefficients between the GI% converted to probit and the log values of the metal concentrations in water.
3.4.2 Canonical correlation analysis between phytotoxicity and candidate metals
A canonical correlation analysis was conducted to confirm the relationship between phytotoxicity and the candidate metal concentration, based on the results of the preceding linear correlation analysis. One set of variables (X1–X4) represented phytotoxicity, which included the GI% of amaranth, oilseed rape, cabbage, and lettuce. The other set of variables (Y1–Y4) consisted of the logarithmic values of the concentrations of Co, Cr, Ni, and Pb in the water. As a result, the analysis yielded four pairs of canonical correlations; however, only the first pair [Corr (U1, V1)] exhibited a significant correlation, as indicated in Table 4, with a canonical coefficient (ρ1) of 0.99 (p < 0.01) and an explained variance ratio of 22.9%. The initial variables (Xi and Yi) with canonical loadings exceeding 0.35 (in absolute value) suggested a meaningful interpretation between U1 and V1. The canonical variables to phytotoxicity effects (Xi) were notably higher for oilseed rape (0.651) and cabbage (0.695). In contrast, the variables representing metal concentrations (Yi) showed a strong negative correlation with Pb concentration (canonical loading: −0.422), indicating its likely role in the observed phytotoxicity. Contributions from the remaining toxicity responses and toxicants were minimal. Consequently, this analysis identified Pb as the primary toxic metal influencing the phytotoxicity effects observed in oilseed rape and cabbage in water from the Taiyuan section of the Fen River in China.
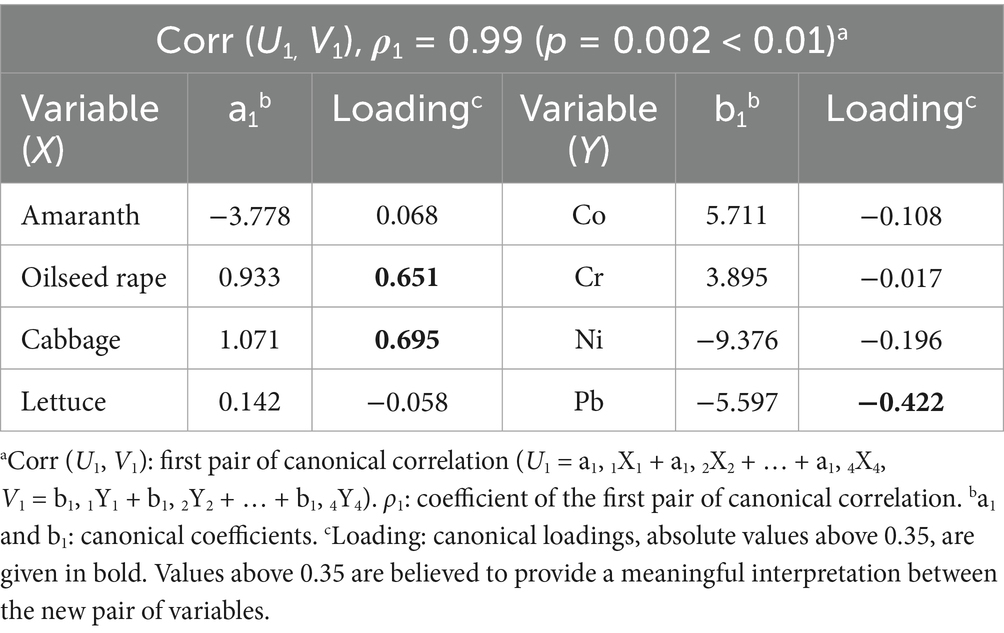
Table 4. Canonical correlation between the two sets of variables [toxicity effects (X1–X4) converted to probit and log values of metal concentrations in water (Y1–Y4)].
In this study, lead (Pb) was identified as the primary toxicant impairing seed germination in oilseed rape and cabbage in the Taiyuan section of the Fen River. A negative correlation between Pb concentrations and the germination index was also observed, which was consistent with findings reported in previous literature (Collin et al., 2022b; Zulfiqar et al., 2019). Furthermore, the findings of this study were consistent with those of the entire Fen River (Li et al., 2023), which highlighted Pb and Cr as the main ecological risk factors. Notably, the Pb concentrations observed in this study were mostly lower than those of the entire Fen River (Li et al., 2023). On the one hand, this difference can be partially explained by the study area’s site in the upper reach of the Fen River, where pollution levels tend to be lower. On the other hand, it also indicated that ecological and environmental remediation strategies in the Fen River basin have achieved some success. As previously reported, concentrations of heavy metals in the water bodies of the Fen River have declined in recent years (Chai et al., 2021).
Although the mean Pb concentration in the Fen River (mean: 4.78 μg·L−1) was below the Class I threshold (50 μg·L−1) established by China’s Environmental Quality Standards for Surface Water (GB 3838–2002) (MEPC, 2002), This finding suggested that current water quality standards may not adequately protect aquatic vegetation from Pb toxicity. It highlights a notable gap between regulatory chemical thresholds and their actual ecological effects. Lead, even at relatively low concentrations, can adversely impact plant growth and development by inhibiting seed germination, root elongation, transpiration, and chlorophyll production, ultimately impacting crop yields (Ashraf et al., 2017). The pervasive presence of Pb in freshwater ecosystems is primarily due to human activities such as mining, fossil fuel combustion, and manufacturing (Adnan et al., 2024), which underscores its persistent environmental threat. Furthermore, when Pb-contaminated water is used for irrigation, lead can accumulate in plants (Collin et al., 2022a). Lead can also enter aquatic organisms and the human body in both inorganic and organic forms through ingestion, inhalation, and dermal contact (Collin et al., 2022b). These concerns have prompted increasingly stringent regulatory measures regarding lead pollution, as evidenced by significant reductions in lead levels in various consumer products (Levin et al., 2021) and the U. S. Environmental Protection Agency’s establishment of a Maximum Contaminant Level Goal of zero for lead in drinking water, acknowledging its potent neurotoxicity even at low concentrations (Waterhouse, 2024). The International Agency for Research on Cancer has classified lead as a Group 2B carcinogen, indicating its possible carcinogenicity to humans (IARC, 2025). Therefore, despite achieving regulatory compliance, the phytotoxicity observed in this study underscores the need for rigorous monitoring and remediation of lead contamination to mitigate potential ecological and human health risks associated with this persistent toxic metal (Kumar et al., 2022).
3.5 Limitations
Firstly, the TIE method employed had certain limitations, primarily its focus on individual classes of toxicants and their contributions to phytotoxicity, while neglecting potential interactions among various toxicants. When two or more heavy metals coexist, they can produce synergistic effects (Hoang et al., 2021), antagonistic effects (Arreguin-Rebolledo et al., 2024), or additive effects (Emenike et al., 2021), resulting in significantly more complex toxic outcomes. Moreover, the interactions between metals and organic pollutants, such as PAHs (Yang et al., 2024), pesticides (Ayala Cabana et al., 2024), and microplastics (Ge et al., 2024), have not been thoroughly investigated. This lack of such a comprehensive analysis may substantially influence the overall toxicity and ecological risk evaluation of these contaminants.
Secondly, the scope for toxicological identification during the confirmation phase of the TIE procedure was limited. While this study successfully identified heavy metals as the primary toxicants, a minor portion of the toxic contribution in the Fen River waters also originated from certain electrophilic organic compounds. Future research should specifically focus on identifying these organic pollutants.
Finally, although the study targeted 10 heavy metals, it did not cover all metallic elements present in the Fen River. Other potentially toxic elements, such as arsenic and mercury, were not investigated due to the mercury memory effect and the interference of arsenic with the ArCl method.
4 Conclusion
This study utilized a TIE method based on seed germination to identify toxicants in the Taiyuan section of the Fen River as a case study. Toxicity screening with four vegetable seeds revealed significant phytotoxicity, particularly at site S6. Subsequent TIE treatments involved aeration, EDTA chelation, oxidant reduction, filtration, solid-phase extraction using a C18 column, and pH adjustment. Notably, lead was identified as the primary toxicant impairing seed germination in oilseed rape and cabbage, even though its concentrations were below regulatory thresholds. This finding underscores a latent ecological risk and demonstrates that the phytotoxicity-based TIE approach serves as a valuable tool for transitioning water-quality assessment from traditional chemical monitoring to more ecologically relevant evaluations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HQ: Writing – original draft. ZL: Methodology, Writing – original draft. HW: Methodology, Writing – original draft. XL: Visualization, Writing – review & editing. LL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Innovation and Entrepreneurship Training Program for College Students in Shanxi Province (20241194) and the project for optimizing and standardizing the water supply and drainage system of the chemical laboratory (2025140711000092).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frwa.2025.1707872/full#supplementary-material
References
Adnan, M., Xiao, B., Ali, M. U., Xiao, P., Zhao, P., Wang, H., et al. (2024). Heavy metals pollution from smelting activities: a threat to soil and groundwater. Ecotoxicol. Environ. Saf. 274:116189. doi: 10.1016/j.ecoenv.2024.116189
Arreguin-Rebolledo, U., Gebara, R. C., Valencia-Castañeda, G., Rico-Martínez, R., Frías-Espericueta, M. G., Longo, E., et al. (2024). Toxicity of binary-metal mixtures (as, cd, cu, Fe, hg, Pb and Zn) in the euryhaline rotifer Proales similis: antagonistic and synergistic effects. Mar. Pollut. Bull. 198:115819. doi: 10.1016/j.marpolbul.2023.115819
Ashraf, U., Kanu, A. S., Deng, Q., Mo, Z., Pan, S., Tian, H., et al. (2017). Lead (Pb) toxicity; physio-biochemical mechanisms, grain yield, quality, and Pb distribution proportions in scented rice. Front. Plant Sci. 8:259. doi: 10.3389/fpls.2017.00259
Ayala Cabana, L., de Santiago-Martín, A., Meffe, R., López-Heras, I., and de Bustamante, I. (2024). Pharmaceutical and trace metal interaction within the water–soil–plant continuum: implications for human and soil health. Toxics 12:457. doi: 10.3390/toxics12070457
Budi, S., Suliasih, B. A., Othman, M. S., Heng, L. Y., and Surif, S. (2016). Toxicity identification evaluation of landfill leachate using fish, prawn and seed plant. Waste Manag. 55, 231–237. doi: 10.1016/j.wasman.2015.09.022
Chai, N. P., Yi, X., Xiao, J., Liu, T., Liu, Y. J., Deng, L., et al. (2021). Spatiotemporal variations, sources, water quality and health risk assessment of trace elements in the Fen River. Sci. Total Environ. 757:11. doi: 10.1016/j.scitotenv.2020.143882
Chen, S., Yi, L., and Chen, Y. (2025). Zebrafish embryos ecotoxicity traceability of pharmaceutical wastewater during simultaneous nitrification-denitrification process. J. Hazard. Mater. 492:138192. doi: 10.1016/j.jhazmat.2025.138192
CMEE (2020a). Water quality standard for freshwater aquatic organisms: Ammonia nitrogen. Available online at: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202004/t20200410_773914.html (accessed March 8, 2025).
CMEE (2020b). Water quality standard for freshwater aquatic organisms: cadmium. Available online at: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202003/t20200303_766970.html (accessed March 8, 2025).
CMEE (2020c). Water quality standard for freshwater aquatic organisms: phenol. Available online at: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202012/t20201224_814675.html (accessed March 8, 2025).
CMEE (2022). Technical guideline for deriving water quality criteria for freshwater organisms (HJ831-2022). Available online at: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/xgbzh/202203/t20220314_971456.shtml (accessed March 8, 2025).
Collin, S., Baskar, A., Geevarghese, D. M., Ali, M. N. V. S., Bahubali, P., Choudhary, R., et al. (2022a). Bioaccumulation of lead (Pb) and its effects in plants: a review. J. Hazard. Mater. Lett. 3:100064. doi: 10.1016/j.hazl.2022.100064
Collin, M. S., Venkatraman, S. K., Vijayakumar, N., Kanimozhi, V., Arbaaz, S. M., Stacey, R. S., et al. (2022b). Bioaccumulation of lead (Pb) and its effects on human: a review. J. Hazard. Mater. Adv. 7:100094. doi: 10.1016/j.hazadv.2022.100094
CSAMR (2025). Rules for agricultural seed testing, Part4: germination test (GB/T 3543.4-2025). Available online at: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=9F73D782EC25D53FA1F55EDB354F81A2 (accessed October 18, 2025).
Emenike, E. C., Iwuozor, K. O., and Anidiobi, S. U. (2021). Heavy metal pollution in aquaculture: sources, impacts and mitigation techniques. Biol. Trace Elem. Res. 200, 4476–4492. doi: 10.1007/s12011-021-03037-x
Ge, J., Jin, P., Xie, S., Beardall, J., Feng, Y., Guo, C., et al. (2024). Micro- and nanoplastics interact with conventional pollutants on microalgae: synthesis through meta-analysis. Environ. Pollut. 342:123127. doi: 10.1016/j.envpol.2023.123127
Hawash, H. B., Moneer, A. A., Galhoum, A. A., Elgarahy, A. M., Mohamed, W. A., Samy, M., et al. (2023). Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic environment, their characteristics, and adopted legislations. J Water Process Eng 52:103490. doi: 10.1016/j.jwpe.2023.103490
Ho, K. T., and Burgess, R. M. (2013). What's causing toxicity in sediments? Results of 20 years of toxicity identification and evaluations. Environ. Toxicol. Chem. 32, 2424–2432. doi: 10.1002/etc.2359
Hoang, H.-G., Lin, C., Chiang, C.-F., Bui, X.-T., Lukkhasorn, W., Bui, T.-P.-T., et al. (2021). The individual and synergistic indexes for assessments of heavy metal contamination in global rivers and risk: a review. Curr. Pollut. Rep. 7, 247–262. doi: 10.1007/s40726-021-00196-2
Hockett, J. R., and Mount, D. R. (1996). Use of metal chelating agents to differentiate among sources of acute aquatic toxicity. Environ. Toxicol. Chem. 15, 1687–1693. doi: 10.1002/etc.5620151006
IARC. (2025). Agents classified by the IARC monographs. Available online at: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed March 8, 2025).
Jiang, T., Sun, T., Guo, Z., Lu, J., Liu, F., and Guan, X. (2025). Effects of ecological water replenishment on microbial denitrification in aquatic environment of infiltration area. J. Hazard. Mater. 494:138688. doi: 10.1016/j.jhazmat.2025.138688
Kali, S. E., Österlund, H., Viklander, M., and Blecken, G.-T. (2025). Stormwater discharges affect PFAS occurrence, concentrations, and spatial distribution in water and bottom sediment of urban streams. Water Res. 271:122973. doi: 10.1016/j.watres.2024.122973
Ke, X., Gao, L. L., Huang, H., and Kumar, S. (2015). Toxicity identification evaluation of sediments in Liaohe River. Mar. Pollut. Bull. 93, 259–265. doi: 10.1016/j.marpolbul.2015.01.020
Kumar, V., Dwivedi, S., and Oh, S. (2022). A critical review on lead removal from industrial wastewater: recent advances and future outlook. J Water Process Eng 45:102518. doi: 10.1016/j.jwpe.2021.102518
Levin, R., Vieira, C. L. Z., Rosenbaum, M. H., Bischoff, K., Mordarski, D. C., and Brown, M. J. (2021). The urban lead (Pb) burden in humans, animals and the natural environment. Environ. Res. 193:110377. doi: 10.1016/j.envres.2020.110377
Li, H., Li, Y., Guo, G., Li, Y., Zhang, R., Feng, C., et al. (2023). Distribution, site-specific water quality criteria, and ecological risk assessment of heavy metals in surface water in Fen River, China. Toxics 11:704. doi: 10.3390/toxics11080704
Liu, X., Shi, J., Bo, T., Meng, Y., Zhan, X., Zhang, M., et al. (2017). Distributions and ecological risk assessment of estrogens and bisphenol a in an arid and semiarid area in Northwest China. Environ. Sci. Pollut. Res. 24, 7216–7225. doi: 10.1007/s11356-017-8434-6
Malla, M. A., Malambule, N., Amoah, I. D., Featherston, J., Ismail, A., Bux, F., et al. (2025). The plastisphere ecology: assessing the impact of different pollution sources on microbial community composition, function and assembly in aquatic ecosystems. Environ. Chem. Ecotoxicol. 7, 75–83. doi: 10.1016/j.enceco.2024.10.010
MEPC. (2002). Environmental quality standards for surface water (GB 3838-2002). Available online at: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/shjzlbz/200206/t20020601_66497.shtml (accessed March 8, 2025).
Muhie, S., Gautam, A., Mylroie, J., Sowe, B., Campbell, R., Perkins, E. J., et al. (2025). Effects of environmental chemical pollutants on microbiome diversity: insights from shotgun metagenomics. Toxics 13:142. doi: 10.3390/toxics13020142
Nobili, S., Masin, C. E., Zalazar, C. S., and Lescano, M. R. (2024). Vermistabilization of excess sludge employing Eisenia fetida: earthworm histopathological alterations and phytotoxicity evaluation. J. Environ. Manag. 368:122174. doi: 10.1016/j.jenvman.2024.122174
Olaniyan, O., Adeniji, A., Semerjian, L., Okoh, A., and Okoh, O. (2023). Global co-occurrence of trace elements and additive legacy brominated flame retardants in aquatic environment: a cause for concern. J. Hazard. Mater. Adv. 11:100337. doi: 10.1016/j.hazadv.2023.100337
Picinini-Zambelli, J., Garcia, A. L. H., and Da Silva, J. (2025). Emerging pollutants in the aquatic environments: a review of genotoxic impacts. Mutat. Res. 795:108519. doi: 10.1016/j.mrrev.2024.108519
Priya, K., Renjith, K., Haddout, S., Azhikodan, G., Yokoyama, K., Chinglenthoiba, C., et al. (2025). Influence of anthropogenic pressures on the microplastic distribution in the riverine-estuarine environment: a source-apportioning approach. J. Contam. Hydrol. 271:104546. doi: 10.1016/j.jconhyd.2025.104546
Ra, J. S., Jeong, T. Y., Lee, S. H., and Kim, S. D. (2016). Application of toxicity identification evaluation procedure to toxic industrial effluent in South Korea. Chemosphere 143, 71–77. doi: 10.1016/j.chemosphere.2015.05.004
Ramage, C. I., Dos Santos, R. A. L., Lisa, Y., Johnson, M. F., and Vane, C. H. (2025). Widespread pesticide pollution in two English river catchments of contrasting land-use: from sediments to fish. Environ. Pollut. 22:126371. doi: 10.1016/j.envpol.2025.126371
Rodrigues, F., Durães, L., Simões, N. E., Pereira, A. M., Silva, L. J., and Feio, M. J. (2024). Pharmaceuticals in urban streams: a review of their detection and effects in the ecosystem. Water Res. 12:122657. doi: 10.1016/j.watres.2024.122657
Sperdouli, I. (2022). Heavy metal toxicity effects on plants. Toxics 10:715. doi: 10.3390/toxics10120715
Tsipa, A., Papalli, M., Christou, A., Pissaridou, P., and Vasquez, M. I. (2023). Ex-situ biological treatment of industrial saline seafood wastewater by salt-tolerant mixed cultures and phytotoxicity evaluation. J. Environ. Chem. Eng. 11:109195. doi: 10.1016/j.jece.2022.109195
USEPA (1991). Methods for aquatic toxicity identification evaluations: Phase I toxicity characterization procedures. EPA/600/6–91/003. Washington, DC: USEPA.
USEPA (1993a). Methods for aquatic toxicity identification evaluations: Phase II toxicity identification procedures. EPA/600/3–88/035. Washington, DC: USEPA.
USEPA (1993b). Methods for aquatic toxicity identification evaluations: Phase III toxicity confirmation procedures. EPA/600/3–88/036. Washington, DC: USEPA.
Wang, J., Zhang, H., Liu, J., Hu, S., Shi, J., Li, X., et al. (2025). Distribution characteristics, source identification and health risk assessment of heavy metals in surface water and groundwater: a case study in mining-affected areas. Front. Water 7:1639009. doi: 10.3389/frwa.2025.1639009
Waniek, J. J., Osterholz, H., and Frazão, H. C. (2025). A global inventory of natural and synthetic estrogens in aquatic systems. Annu. Rev. Mar. Sci. 17, 511–536. doi: 10.1146/annurev-marine-032123-025855
Waterhouse, C. (2024). Lead: the latest federal actions and best practices. Ind. Health L. Rev. 21:355. doi: 10.18060/28408
Xiao, J., Lv, G., Chai, N., Hu, J., and Jin, Z. (2022). Hydrochemistry and source apportionment of boron, sulfate, and nitrate in the Fen River, a typical loess covered area in the eastern Chinese loess plateau. Environ. Res. 206:112570. doi: 10.1016/j.envres.2021.112570
Yan, Z., Jin, X., Feng, C., Leung, K. M., Zhang, X., Lin, Q., et al. (2025). Beyond the single-contaminant paradigm: advancing mixture toxicity and cumulative risk assessment in environmental toxicology. Environ. Sci. Technol. 59, 10711–10714. doi: 10.1021/acs.est.5c05712
Yang, L., Zhang, T., Gao, Y., Li, D., Cui, R., Gu, C., et al. (2024). Quantitative identification of the co-exposure effects of e-waste pollutants on human oxidative stress by explainable machine learning. J. Hazard. Mater. 466:133560. doi: 10.1016/j.jhazmat.2024.133560
Zhou, J., Li, Z., Guo, X., Li, Y., Wu, Z., and Zhu, L. (2019). Evidences for replacing legacy per-and polyfluoroalkyl substances with emerging ones in fen and Wei River basins in central and western China. J. Hazard. Mater. 377, 78–87. doi: 10.1016/j.jhazmat.2019.05.050
Zhou, Z. M., Wu, F., Tong, Y. J., Zhang, S. Q., Li, L., Cheng, F., et al. (2024). Toxicity and chemical characterization of shale gas wastewater discharged to the receiving water: evidence from toxicity identification evaluation. Sci. Total Environ. 912:12. doi: 10.1016/j.scitotenv.2023.169510
Zucconi, F., Monaco, A., Forte, M., and Bertoldi, M.d. (1985). “Phytotoxins during the stabilization of organic matter” in Composting of agricultural and other wastes. ed. J. K. R. Gasser (New York, NY: Elsevier Applied Science Publication), 73–86.
Keywords: toxicity identification evaluation, metals, water, phytotoxicity, river
Citation: Qi H, Liu Z, Wang H, Li X and Li L (2025) Toxicity identification evaluation of riverine water using seed germination: lead toxicity in the Fen River’s Taiyuan section of China. Front. Water. 7:1707872. doi: 10.3389/frwa.2025.1707872
Edited by:
Melida Gutierrez, Missouri State University, United StatesReviewed by:
Md Muzammel Hossain, Jiangsu University, ChinaMariaTeresa Alarcon-Herrera, Centro de Investigación de Materiales Avanzados (CIMAV), Mexico
Copyright © 2025 Qi, Liu, Wang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Li, bGlob25nNDEzNDEzQDE2My5jb20=
 Hongxue Qi
Hongxue Qi Lihong Li
Lihong Li