- 1Department of Biological Sciences, University of Manitoba, Winnipeg, MB, Canada
- 2Northwest Atlantic Fisheries Centre, Fisheries and Oceans Canada, St. John’s, NL, Canada
Identifying natal origins of animals is key to determining the relative productivity of natal habitats, dispersal of individuals among local populations (i.e., connectivity), and, ultimately, metapopulation dynamics. As marine fish larvae have a high potential for dispersal, natural tags such as otolith chemistry are often used to determine natal origins. Trace elements may be incorporated into embryonic otoliths while larvae are developing in the egg, resulting in chemical signatures in the pre-hatch region of the otolith that reflect the natal habitat. Our goal was to determine whether the natal origins of 1- to 3-day-old larval capelin (Mallotus villosus), a key forage fish in many northern marine ecosystems, could be determined using otolith chemistry. We sampled larvae from five Newfoundland regions (i.e., embayments: Placentia Bay, St. Mary’s Bay, Witless Bay, Trinity Bay, and Notre Dame Bay) during July–August 2019 to quantify regional differences in otolith chemistry. Additionally, eggs/larvae were field-reared within two regions over multiple years (Notre Dame Bay: 2014, 2015, 2018, and 2019; Trinity Bay: 2018 and 2019) to quantify interannual variation in region-specific otolith chemistry. Multielemental otolith signatures (i.e., Mg, Mn, Zn, Sr, and Ba), as determined by laser ablation inductively coupled plasma–mass spectrometry (LA ICP–MS), differed significantly among regions, with individuals classified into their natal region with 78% success (region-specific success: 68–100%). Classification success into natal region remained high (67–76%) despite interannual variation in otolith trace element concentrations within regions. Characterizing region-specific otolith chemical signatures that reflect natal origins of capelin larvae is the first step in determining the productivity and relative contributions of different regions of coastal Newfoundland to capelin recruitment.
Introduction
Natal origins of animals are used to evaluate relative productivity of natal habitats and determine dispersal and connectivity of individuals among local populations and metapopulations (Cowen et al., 2007). Natal origin can be difficult to determine for marine species due to their high potential dispersal rates, which are typically associated with the planktonic duration of the larval stage (Bohonak, 1999). However, there is growing evidence that some larvae are retained and recruited locally (e.g., Bradbury et al., 2008; Stanley et al., 2013). To determine the natal origins of fish larvae, tagging or marking is required. Natural tags have determined natal origins of fish larvae, providing important information on local retention (e.g., Jones et al., 1999; Swearer et al., 1999) and connectivity (e.g., Davoren and Halden, 2014). Trace elements in the surrounding aquatic environment are incorporated into the calcium carbonate structure of fish otoliths as individuals grow, allowing chronological reconstruction of environmental histories including natal origin (Campana, 1999; Elsdon and Gillanders, 2003). Some essential elements that are required for growth [e.g., magnesium (Mg) and manganese (Mn)] are actively incorporated into the otolith, while nonessential elements [e.g., strontium (Sr) and barium (Ba)] are inadvertently incorporated during calcium uptake due to their chemical similarity to calcium (Ca) (Crichton, 2008; Loewen et al., 2016). Although ambient water chemistry primarily influences trace element incorporation into the otoliths, other extrinsic factors including salinity and temperature also influence trace element incorporation rates (reviewed in Loewen et al., 2016).
Capelin (Mallotus villosus) is a small, short-lived (3–6 years) circumpolar marine forage fish species in the sub-Arctic (Carscadden and Vilhjálmsson, 2002). Capelin is also a commercially fished species, with Newfoundland and Labrador representing one of three economically important capelin fisheries along with Iceland and the Barents Sea (Carscadden et al., 2013). Capelin occupies a vital position in marine food webs as it is one of a few species that transfer energy from lower trophic levels (i.e. zooplankton) to upper-trophic-level predators, including whales, seabirds, and large piscivorous fish species, such as Atlantic cod (Gadus morhua; Carscadden and Vilhjálmsson, 2002). Capelin is primarily distributed offshore on the Newfoundland shelf and undergoes annual migrations into coastal embayments during June and July (Carscadden et al., 2013) to spawn at both beach (intertidal) and deepwater (subtidal, 15–40 m) sites (Crook et al., 2017). Post-spawning mortality in capelin is high, with higher proportions of dead males than females observed at spawning beaches (Templeman, 1948). Fertilized capelin eggs adhere to sediment at spawning sites, where they incubate until hatch and larvae are dispersed to offshore nursery habitats. Tagging (Nakashima, 1992) and genetic studies (Kenchington et al., 2015) suggest that capelin from the southeast and eastern coast of Newfoundland [Northwest Atlantic Fisheries Organization (NAFO) Divisions 2J3KL] represent one stock/management unit (Carscadden et al., 2013). The importance of specific embayments to stock productivity is unknown.
Trace elements are incorporated into embryonic otoliths (i.e. pre-hatch region) of capelin while larvae are developing in the egg (Loeppky et al., 2018), resulting in chemical signatures in the embryonic otolith that reflect the natal habitat (Davoren et al., 2015; Loeppky et al., 2018; Loeppky and Davoren, 2018). Furthermore, previous work revealed divergent otolith chemical signatures from 1- to 3-day-old larvae incubated within nearby (<20 km) beach and deepwater sites in coastal Newfoundland (Davoren et al., 2015; Loeppky and Davoren, 2018). As ambient water chemistry and salinity did not differ between these sites throughout the incubation period (2–4 weeks), habitat-specific otolith elemental concentrations appeared to result from divergent temperatures in the two habitats. This hypothesis was supported by laboratory and field experiments where treatments that varied by 4°C resulted in high classification success (73–88%) of larvae into their natal habitat (beach or deepwater; Loeppky and Davoren, 2018). Lazartigues et al. (2016) also successfully classified capelin larvae from different hatching sites within the St. Lawrence Estuary primarily based on otolith concentrations of Ba and Mg, with the highest misclassification rate (17%) occurring between nearby relative and more distant sites. Although these regional comparisons have not been conducted on capelin in coastal Newfoundland, a recent study found that juvenile cod could be successfully classified into coastal regions of Newfoundland, with success rates increasing with geographic scale (i.e., sites, bays, and coastal regions; Stanley et al., 2016). Together, these studies suggest that the natal origin of capelin larvae can be identified from geographically distant (>100 km) coastal regions of Newfoundland using otolith chemistry.
The objectives of this study were to investigate whether otolith chemical signatures of larval capelin differ among regions (i.e., embayments) of Newfoundland, Canada, and among years within regions. Region-specific otolith chemical signatures in the embryonic otolith prior to larvae dispersing offshore could be used to identify natal origins of capelin larvae. Therefore, this study will evaluate the potential use of chemical signatures in adult capelin otoliths to address knowledge gaps related to connectivity and productivity, such as the relative contribution of different regions of coastal Newfoundland to capelin recruitment.
Materials and Methods
Field Sampling
For regional comparisons of otolith chemistry, preemergent larvae (3–6 mm, yolk sac present) within sediments at spawning beaches were opportunistically collected during July and August 2019 in Placentia Bay (St. Brides Beach), St. Mary’s Bay (Branch Beach), Witless Bay (Burnt Cove Beach), and Notre Dame Bay (Mussel Shells Beach; Figure 1 and Table 1). Preemergent larvae were collected by scooping beach sediment with adherent eggs in late developmental stages (stages V–VII; Fridgeirsson, 1976) into plastic bags, and then the bags were filled with seawater to separate eggs and sediment from preemergent larvae. The seawater was then poured over a 0.270-mm sieve, and preemergent capelin larvae were retained and preserved in 95% ethanol. Although we did not aim to examine mechanisms underlying otolith chemical variation, one water sample was collected at each site either on the same day or up to 17 days prior to the collection of preemergent larvae to characterize the ambient water chemistry during egg/larval development (Table 1). Multiple water samples were not collected because previous studies in coastal Newfoundland found negligible variation in ambient water chemistry from samples collected during capelin egg incubation (∼10 days apart) and across years (Davoren et al., 2015; Loeppky and Davoren, 2018). Water samples were collected in a 60-ml disposable syringe, filtered using a 0.45-μm filter into a plastic sample bottle and preserved with 3 ml of 1:3 ultrapure nitric acid (HNO3)/deionized water. Field-reared larvae (see below) from Trinity Bay (Bellevue Beach) were also included in regional comparisons.
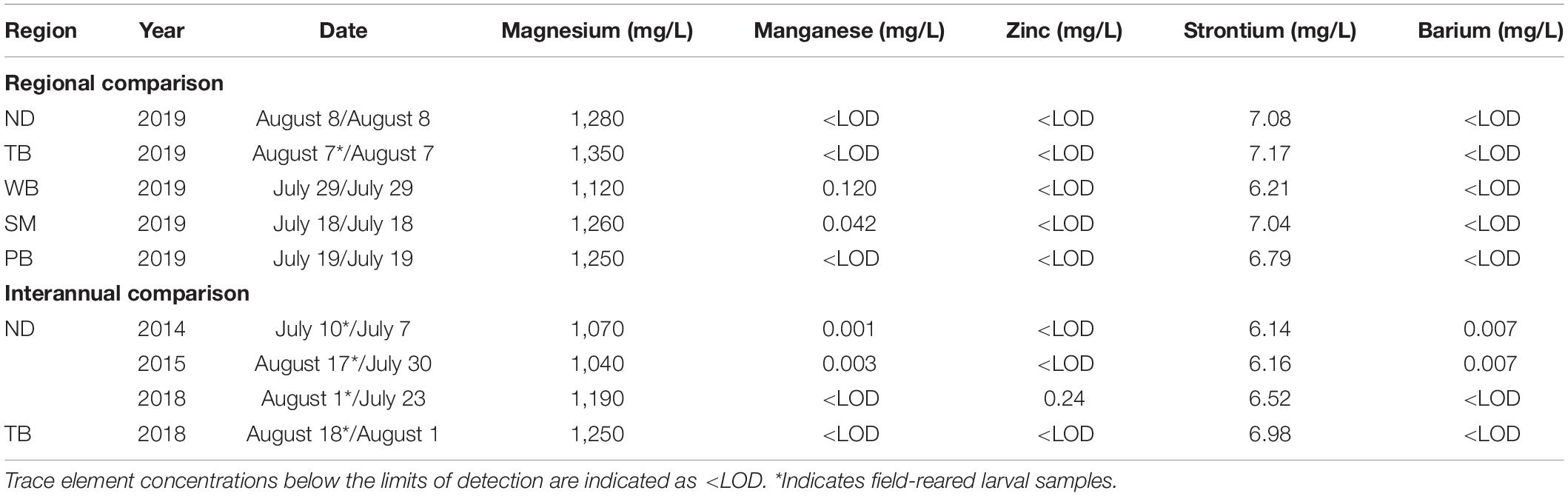
Table 1. Regions where capelin larvae and water chemistry were sampled from Notre Dame Bay (ND), Trinity Bay (TB), Witless Bay (WB), St. Mary’s Bay (SM), and Placentia Bay (PB), with the dates of larvae/water sampling, along with water trace element concentrations from one water sample per region.
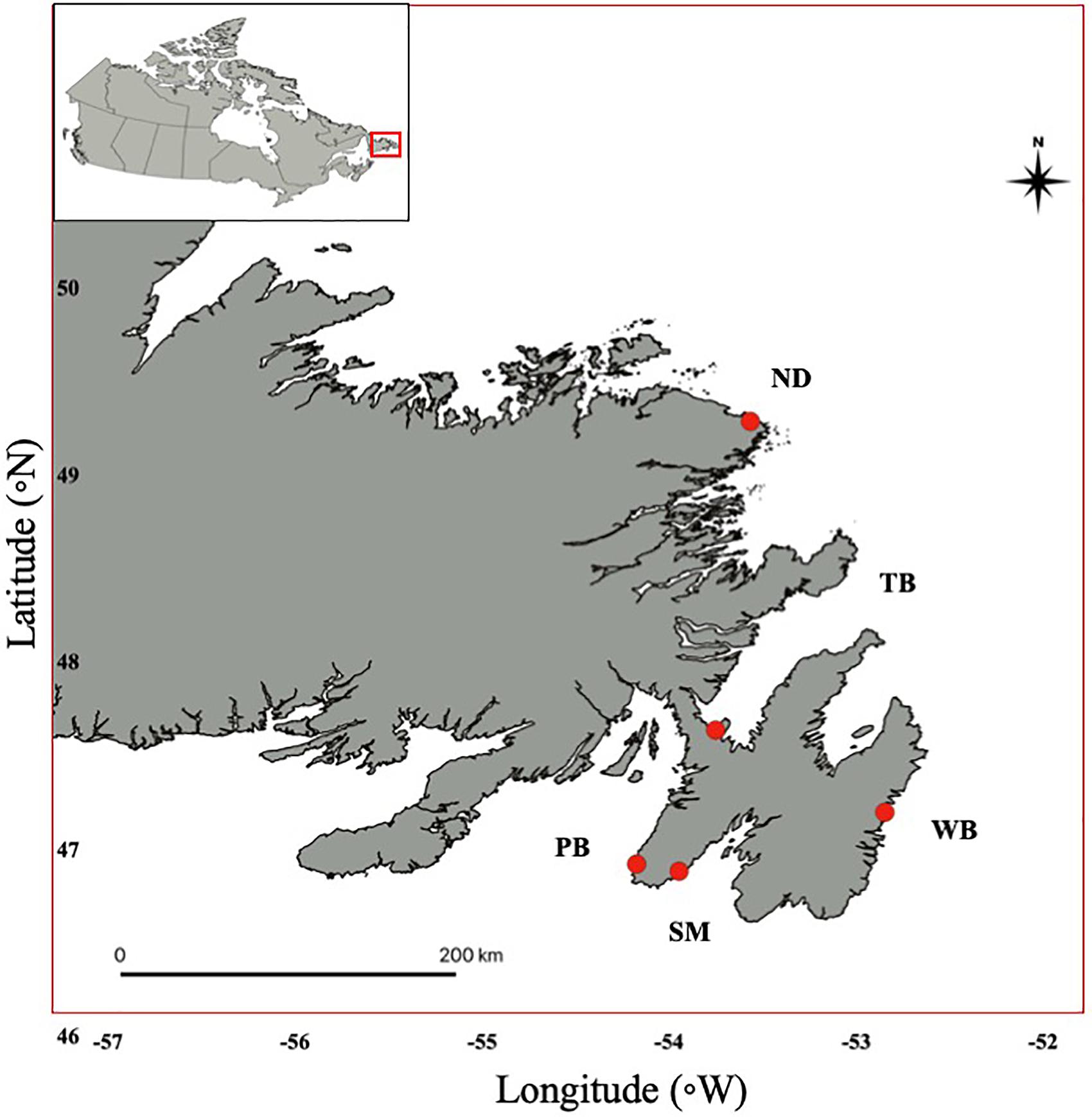
Figure 1. Map of Newfoundland, Canada, indicating locations where capelin larvae were sampled in five coastal regions: Notre Dame Bay (ND; Mussel Shells Beach; 49.2856, -53.5673), Trinity Bay (TB; Bellevue Beach; 47.6338, -53.7519), Witless Bay (WB; Burnt Cove Beach; 47.1965, -52.8486), St. Mary’s Bay (SM; Branch Beach; 46.8824, -53.9470), and Placentia Bay (PB; St. Brides Beach; 46.9204, -54.1748).
To determine whether interannual variation in otolith chemistry could influence classification success of larvae into their natal regions, capelin larvae (1–3 days old) were field-reared within two bays across multiple years (Notre Dame Bay: 2014, 2015, 2018, and 2019; Trinity Bay: 2018 and 2019; Figure 1 and Table 1) by placing naturally fertilized eggs in early developmental stages (stages I and II; Fridgeirsson, 1976) from spawning beaches in incubation canisters, which were moored near the beach in ∼1 m of water (see details in Davoren et al., 2015; Loeppky and Davoren, 2018). In brief, the incubation canisters consisted of 20-ml plastic vials (n = 10 per site) perforated with holes and covered in 0.28-mm mesh. The canisters were retrieved close to the hatching date, which was estimated using water temperature measurements taken at waist height from the beach using a YSI Pro30 every 48 h (Nakashima and Wheeler, 2002; Penton et al., 2012). Upon retrieval, canister contents were poured over a 0.270-mm sieve, and capelin larvae (3–6 mm, yolk sac present) were retained and preserved with 95% ethanol. One water sample was collected per site during field rearing using the same method as above (Table 1).
Lab Processing
Each larva was placed in a drop of deionized water on a clean glass slide (similar to Loeppky and Davoren, 2018), and one sagittal otolith was removed using tungsten needles (Roboz) under a dissecting microscope (Olympus SZX7) with a polarizing lens (Olympus SZX-PO). Otoliths were left to dry for 30 s before being mounted on a 1-cm2 square grid (5 × 5) microscope slide using double-sided tape (ScotchTM), following Loeppky and Davoren (2018). Individual otoliths were centered in each square and photographed for easier detection during processing. Concentrations of six trace elements (i.e., 25Mg, 43Ca, 55Mn, 66Zn, 88Sr, and 137Ba) were quantified using laser ablation inductively coupled plasma–mass spectrometry (LA ICP–MS; Perkin-Elmer DRC II, Loeppky and Davoren, 2018). Laser ablation inductively coupled plasma–mass spectrometry analyses were conducted at the Department of Geological Sciences, University of Manitoba, using a Thermo Finnigan Element 2 ICP–MS coupled to a Merchantek LUV 213 neodymium : yttrium aluminum garnet (Nd-YAG) laser. A Ca standard (MACS-3) was used as an external standard to calibrate the LA ICP–MS and was ablated in triplicate before and after ablation of all otoliths on each microscope slide (25 otoliths per slide). Otoliths were ablated using a spot technique (beam size = 40 μm) to ensure ablation of the entire otolith (diameter ∼30 μm). As the beam size was slightly larger than the circular otoliths, the tape was tested for any background chemical signatures, and none were found. Laser parameters were 56% output (3.3 J/cm2), 2-Hz repetition rate, and a dwell time of 40 s. Background trace element concentrations were measured for 40 s prior to ablating each otolith for 40 s followed by a 60-s washout, whereby the laser stopped firing but detection continued to remove any remaining material before ablating the next otolith to avoid contamination.
Data reduction was performed in Igor Pro graphing software with Iolite version 3.71 by first removing spurious spikes (>3 SD), indicating surface-level contamination in trace element concentrations. Calcium (43Ca) in counts per second (CPS) was then used to standardize trace element concentrations (40.04 wt.%) to account for changes in sample volume. Mean concentrations and standard error (ppm ± SE) of otolith trace elements were calculated by averaging each trace element concentration over the period the otolith was ablated, which was indicated by high and relatively stable 43Ca CPS. As the otolith center, or primordial, region had Mn concentrations that were 6–10 times higher than those of the outer otolith region, similar to previous findings (Lazartigues et al., 2014), and more likely represent maternal investment instead of the natal environment (Loeppky et al., 2018), mean Mn concentrations were calculated excluding the higher concentrations in the center, similar to those of DiMaria et al. (2010). Mn concentrations were averaged starting from the edge of the otolith, which was visually indicated in Igor Pro by high and relatively stable Ca concentrations, and ending just before Mn concentrations spiked, indicating the center otolith region. External precision estimates (%RSD) and limits of detection (LODs) were as follows: 43Ca = 2.1%, 0 μg/g; 25Mg = 1.1%, 28.6 μg/g; 55Mn = 0.88%, 3.7 μg/g; 66Zn = 1.0%, 9.5 μg/g; 88Sr = 1.2%, 84.2 μg/g; and 137Ba = 0.82%, 9.0 μg/g. Laser ablation inductively coupled plasma–mass spectrometry recoveries were as follows: 25Mg = 2.02%; 55Mn = 8.71%; 66Zn = 13.6%; 88Sr = 2.16%; and 137Ba = 8.66%.
Water samples were analyzed for trace element concentrations at ALS Environmental Laboratories (Burnaby, BC). Samples were diluted 10×, and major elements (e.g., Ca) were quantified by ICP–optical emission spectroscopy (OES) (Thermo iCAP 6500), while trace elements (e.g., Mg, Mn, Sr, and Ba) were quantified by solution-based ICP–MS following EPA Method 6010B (see Davoren et al., 2015). To correct for drift and matrix effects, 6Li, 45Sc, 74Ge, 115In, and 175Lu were used as internal standards. Laboratory control spikes were used in all water sample analyses, and external standards for spike recovery were 43Ca (101.6%), 25Mg (99.6%), 55Mn (99.4%), 88Sr (101.8%), and 137Ba (98.8%).
Statistical Analysis
Individuals with mean trace element concentrations of >2 SD from the mean of all larvae within a region were considered outliers and excluded from statistical analyses. These individuals were considered outliers based on previous studies on larval capelin otolith microchemistry (Loeppky et al., 2018; Loeppky and Davoren, 2018) that found abnormal trace element concentrations resulted from the laser not ablating the entire otolith or the otolith exploding prematurely. For regional comparisons, four to six otoliths per region were removed as outliers (24 total), resulting in a total of 72 otoliths used in analyses (8–19 per region; Table 2). For interannual comparisons, two to eight otoliths per year were removed as outliers (26 total), resulting in 113 otoliths (9–24 per region per year) used in analyses. All otolith trace element concentrations were log-transformed to meet the underlying assumptions of parametric statistics. For regional comparisons, a principal component analysis (PCA) was used to explore which otolith trace element concentrations (i.e., Mg, Mn, Zn, Sr, and Ba) were important in explaining the variation in the dataset without considering a priori groupings. To determine whether larvae could be correctly classified into their natal regions based on otolith chemical signatures, all five otolith trace element concentrations were used in a backward stepwise discriminant function analysis (DFA), and based on a p to enter of 0.05 and a p to exit of 0.25 (Davoren and Halden, 2014), all elements were retained by the model. Therefore, all five trace elements were included in a quadratic discriminant function analysis (QDFA). One-way ANOVAs were then used to determine whether each trace element concentration in otoliths differed among regions. When otolith concentrations of a trace element differed significantly among regions, post hoc Tukey’s HSD tests were used to determine which regions differed from each other.
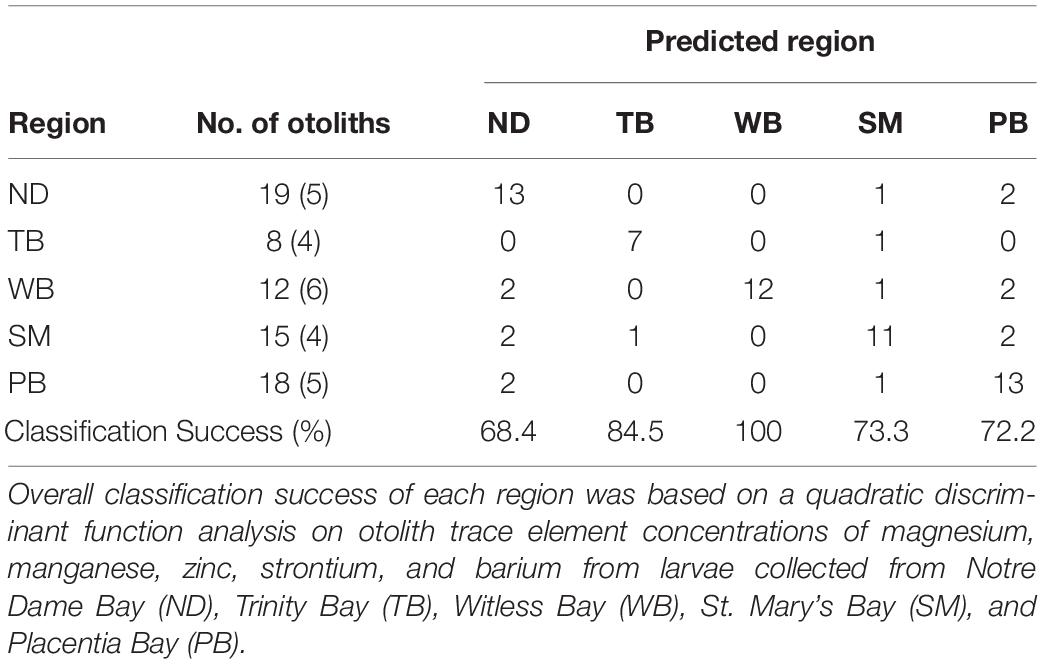
Table 2. The number of larval otoliths included in analysis per region (number of outliers discarded from analysis in parentheses), as well as the number of otoliths classified into each region (“predicted region,” columns).
For interannual comparisons (Notre Dame Bay: 2014, 2015, 2018, and 2019; Trinity Bay: 2018 and 2019), MANOVAs and ANOVAs were used to test whether otolith chemistry differed among years within the two regions. If otolith chemistry differed among years within a region, the QDFA for regional comparisons was rerun, each time substituting the 2019 data with another year to determine if the regional classification rate varied as a result of this interannual variability. For the interannual comparison, Mn was excluded from the analysis as mean Mn concentrations were averaged across the entire embryonic otolith for 2014 and 2015 in Notre Dame Bay (see Loeppky and Davoren, 2018), but the center region was excluded from averages in 2018 and 2019. All statistical analyses were performed in JMP Pro (version 14.1), with α = 0.05 and means reported as ± SE.
Results
Otolith chemistry differed for some trace elements between field-reared larvae in incubation canisters and preemergent larvae collected from sediments in Notre Dame Bay during 2019, with significant differences in Zn (t38 = 3.24, p = 0.0025), Mn (t42 = 2.504, p = 0.0163), and Ba (t41 = 2.023, p = 0.0496). Consequently, otoliths from preemergent larvae were used in all analyses for regional comparisons, with the exception of Trinity Bay where only field-reared larvae were available for 2019. For interannual comparisons, only field-reared larvae from incubation canisters were used in the analyses.
Regional Comparisons
The PCA had two components with eigenvalues over 1, explaining cumulatively 60.1% of the variation (PC1: 35.7%; PC2: 24.4%). The otolith trace elements with the highest loadings on PC1 were Mn (0.8764) and Mg (0.8525), and those with the highest loadings on PC2 were Sr (0.7102), Zn (0.5855), and Ba (0.5613). Therefore, PC1 represented variability in most essential elements (i.e., Mn and Mg), while PC2 primarily represented variability in nonessential elements (i.e., Sr and Ba). Capelin larvae could be correctly classified into their natal region based on otolith chemistry (QDFA: Wilk’s λ = 0.172; approximate F20,210 = 7.332, p < 0.0001; Figure 2) with an overall classification success of 78% (region-specific classification success: 68–100%; Table 2). Univariate analyses revealed that all otolith trace element concentrations differed significantly across regions (Mg: F4,84 = 13.67, p < 0.0001; Mn: F4,89 = 22.11, p < 0.0001; Zn: F4,85 = 3.142, p = 0.0184; Sr: F4,86 = 4.817, p = 0.0015; and Ba: F4,85 = 10.19, p < 0.0001), especially between Witless Bay and all other bays (Figure 3). Ambient water chemistry did not appear to vary considerably among regions during 2019, and only Sr and Mg concentrations were consistently above LODs (Table 1). While Sr concentrations in larval otoliths followed similar regional trends as ambient water concentrations, Mg concentrations in otoliths and ambient water did not show similar regional patterns (Table 1 and Figure 3).
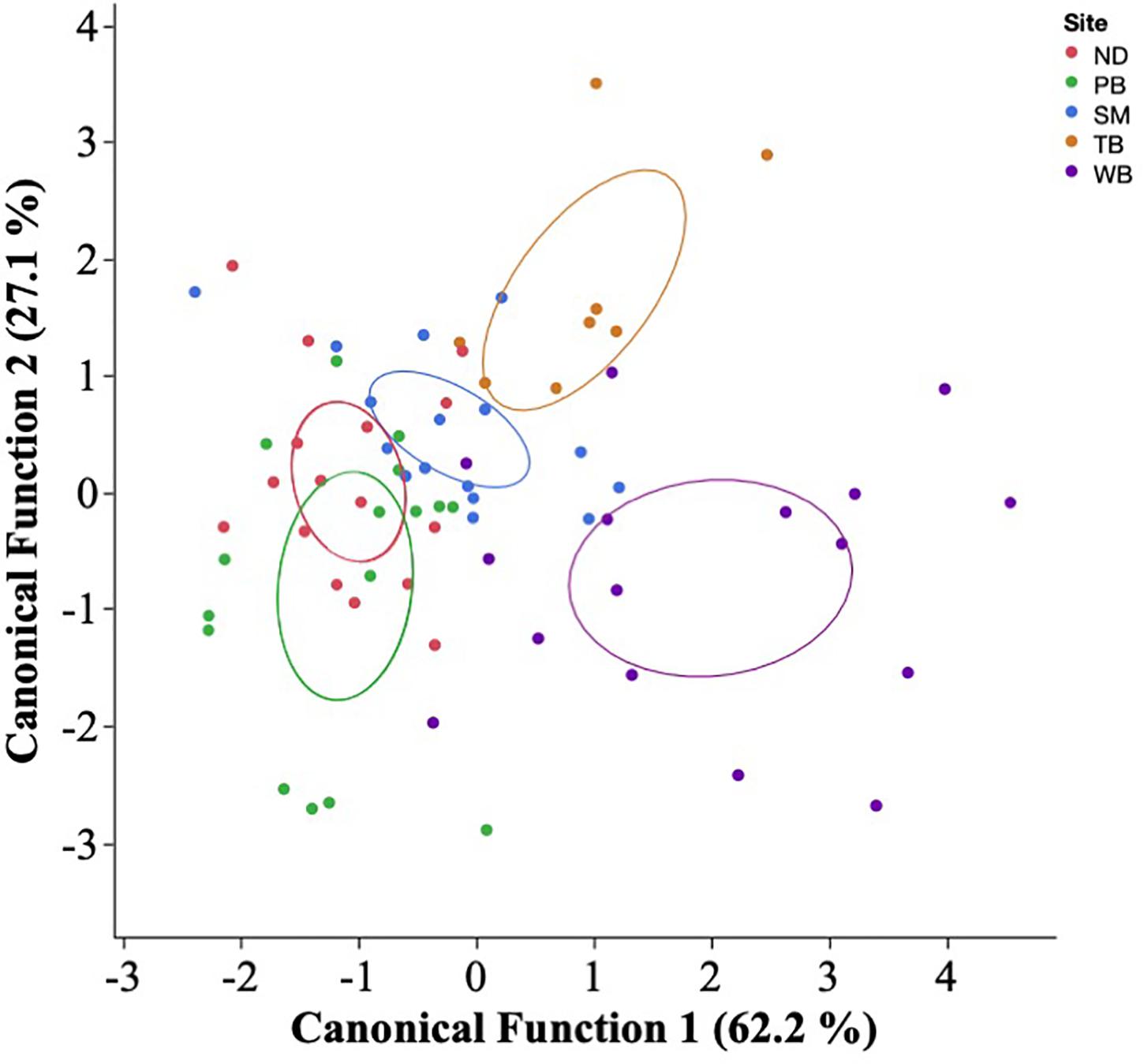
Figure 2. Quadratic discriminant function analysis based on larval capelin otolith trace element concentrations of Mg, Mn, Zn, Sr, and Ba, where each dot represents each individual sampled during July–August 2019 from one of five regions: Notre Dame Bay (ND), Placentia Bay (PB), St. Mary’s Bay (SM), Trinity Bay (TB), and Witless Bay (WB). Ellipses indicate the mean 95% confidence level contours for each bay.
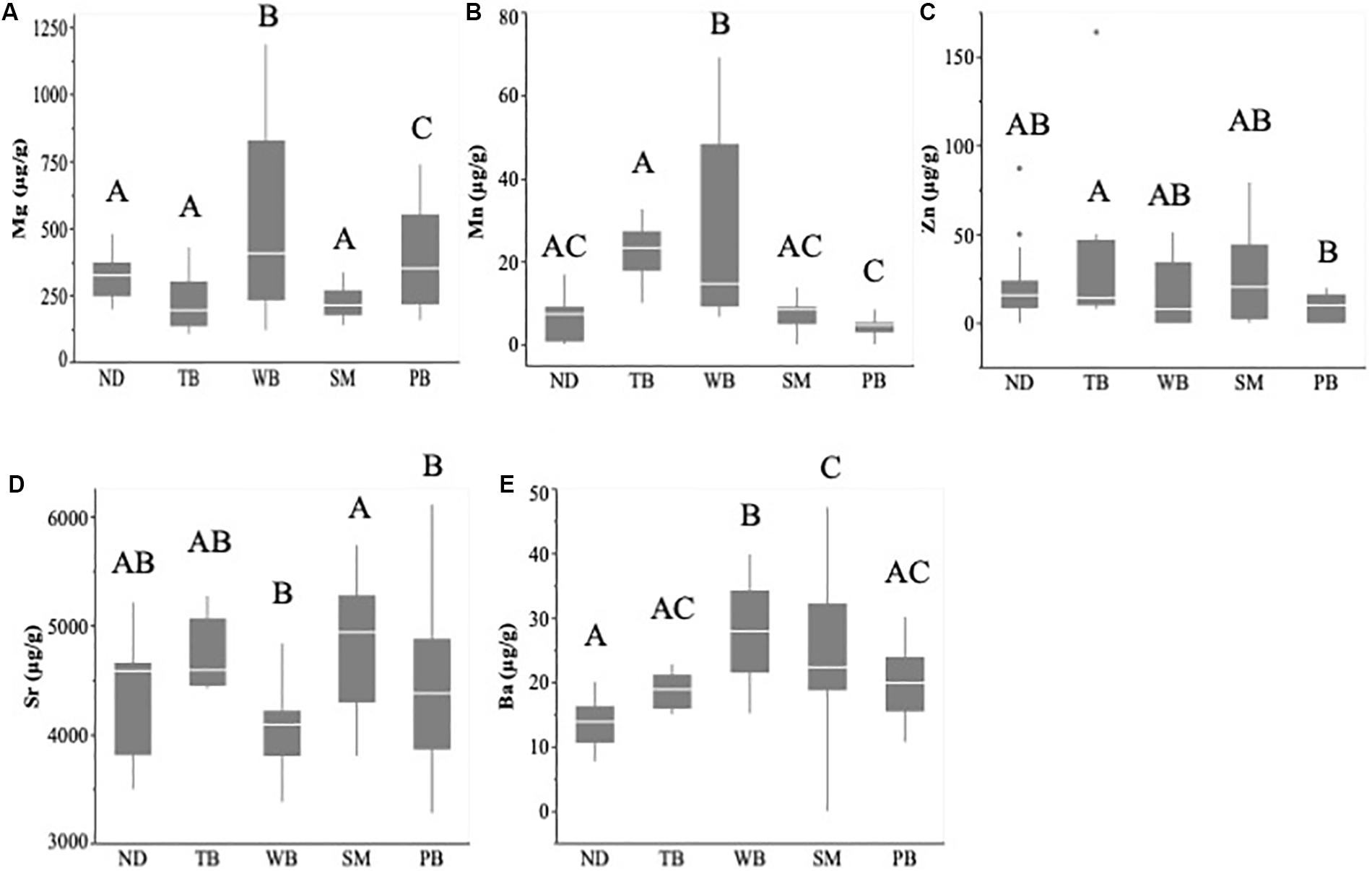
Figure 3. Larval capelin otolith concentrations (μg/g ± SE) of magnesium (A), manganese (B), zinc (C), strontium (D), and barium (E) sampled during July–August 2019 from five coastal regions of Newfoundland: Notre Dame (ND), Trinity (TB), Witless (WB), St. Mary’s (SM), and Placentia (PB). Boxplots show the mean (horizontal bar), 25th percentile (lower bar), 75th percentile (upper bar), and outliers. Significantly different mean otolith concentrations (p < 0.05) are indicated with different letters, while values that are not significantly different (p > 0.05) have the same letters.
Interannual Comparisons
Capelin larval otolith chemical signatures within Trinity Bay did not differ significantly between 2018 and 2019 (MANOVA: Wilk’s λ = 0.774; approximate F4,26 = 1.897, p = 0.141; Figure 4). In contrast, otolith chemical signatures differed significantly among years (2014, 2015, 2018, and 2019) in Notre Dame Bay (MANOVA: Wilk’s λ = 0.120; approximate F12,199 = 20.46, p < 0.0001). Univariate analyses revealed significant differences in all trace elements across years within Notre Dame Bay (Mg: F3,88 = 38.42, p < 0.0001; Zn: F3,85 = 22.47, p < 0.0001; Sr: F3,87 = 9.68, p < 0.0001; and Ba: F3,86 = 23.76, p < 0.0001; Figure 5). Post hoc tests revealed that while otolith Mg concentrations varied among all years, Zn and Sr were relatively stable across 2014, 2015, and 2018 but differed significantly in 2019, during which Zn was higher and Sr was lower (Figure 5). Otolith Ba concentrations were more similar within samples from consecutive years relative to samples from nonconsecutive years (Figure 5).
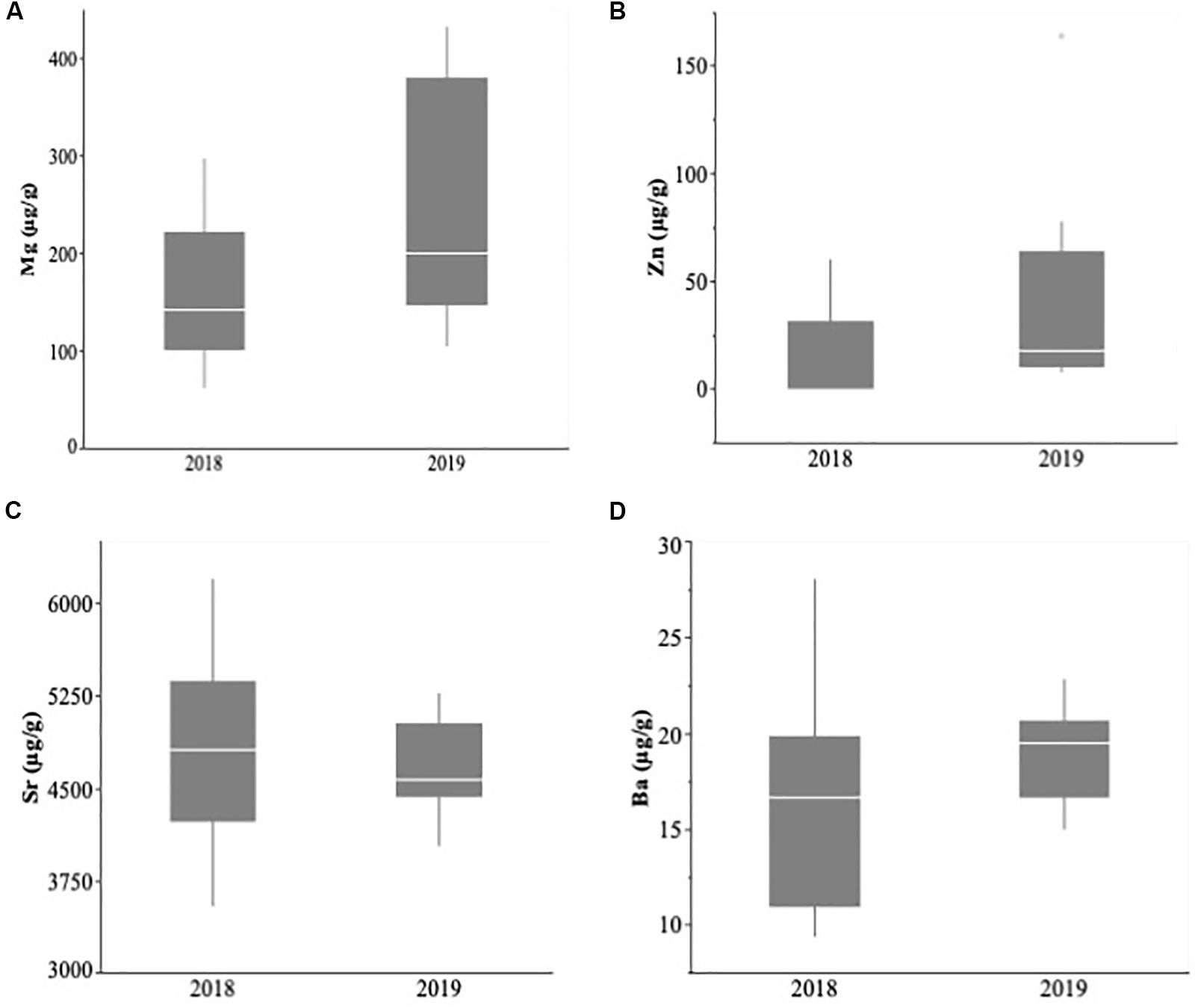
Figure 4. Larval capelin otolith concentrations (μg/g ± SE) of magnesium (A), zinc (B), strontium (C), and barium (D) sampled during July–August 2018 and 2019 in Trinity Bay. Boxplots show the mean (horizontal bar), 25th percentile (lower bar), 75th percentile (upper bar), and outliers.
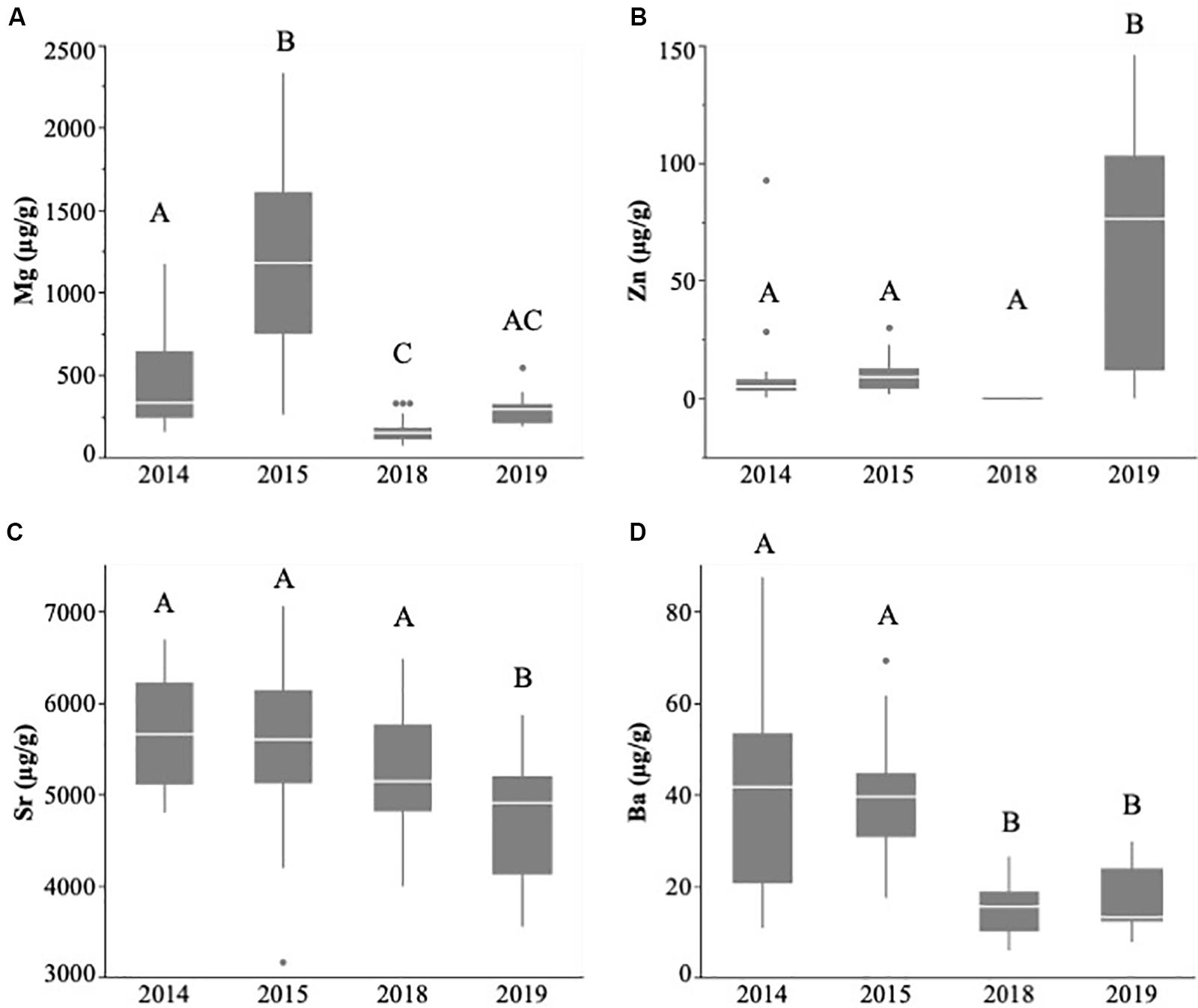
Figure 5. Larval capelin otolith concentrations (μg/g ± SE) of magnesium (A), zinc (B), strontium (C), and barium (D) sampled during July–August 2014, 2015, 2018, and 2019 in Notre Dame Bay. Boxplots show the mean (horizontal bar), 25th percentile (lower bar), 75th percentile (upper bar), and outliers. Significantly different mean otolith concentrations (p < 0.05) are indicated with different letters, while values that are not significantly different (p > 0.05) have the same letters.
Owing to interannual variation in otolith chemistry within Notre Dame Bay, we reran QDFAs for regional comparison three times, each time replacing the 2019 Notre Dame Bay data with one of the three earlier years. Otolith trace element concentrations remained significantly different among regions regardless of interannual variability in otolith chemical signatures (p < 0.0001), with similar regional classification success (67–76%) relative to the original model using 2019 data only and excluding otolith Mn concentrations (i.e., 66%). The removal of Mn did result in a slightly lower classification success compared to the original model using the 2019 data from all five embayments and all elements (i.e., Mg, Mn, Zn, Sr, and Ba). To further explore whether interannual variation in otolith chemical signatures could influence regional classification success, we used a MANOVA to test if otolith chemical signatures differed between Notre Dame Bay and Trinity Bay in 2018. The MANOVA revealed that otolith trace element concentrations differed significantly between field-reared larvae from Notre Dame Bay and Trinity Bay in 2018 (Wilk’s λ = 0.664; approximate F4,46 = 5.83, p = 0.0007). Ambient water chemistry did not appear to vary considerably among years within regions, and, again, only Sr and Mg concentrations were consistently above LODs (Table 1).
Discussion
Our classification success of capelin larvae into natal embayments (78%) was similar to the classification success (83%) of another study using otolith chemical signatures to examine regional differences of dispersing larval capelin in the Gulf of St. Lawrence (Lazartigues et al., 2016). Classification success of juvenile cod at the broad scale of southern and northern bays of Newfoundland was also similar to this study (75%), although classification success into specific bays was more variable (27–77%; Stanley et al., 2016), possibly due to variable environmental conditions. Classification success also remained consistent among regions in our study despite interannual differences in otolith trace element signatures within one region (Notre Dame Bay). Indeed, interannual variability in otolith chemical signatures had been shown previously for capelin (Davoren et al., 2015; Loeppky et al., 2018) along with a variety of other marine species [e.g., King George whiting, Sillaginodes punctatus, Rogers et al., 2019; juvenile snapper, Pagrus auratus (formerly known as Chrysophyrs auratus), Hamer et al., 2003; and common sole, Solea solea, and Senegalese sole, Solea senegalensis, Tanner et al., 2012]. Despite significant interannual variation in otolith chemistry, larvae in other studies can often still be classified into their natal regions, as was found for King George whiting in southern Australia (82% classification success; Rogers et al., 2019). Overall, these findings suggest that regional differences in otolith chemistry are robust to among-year variation. Otolith chemical differences between Trinity Bay and other regions, however, should be interpreted with caution due to divergent otolith chemistry between field-reared larvae in canisters (subtidal; ∼1 m water; Trinity Bay) relative to preemergent larvae collected within beach spawning sediment (intertidal; all other bays).
Our results support a growing body of evidence that otolith chemistry is a useful tool to identify the natal origin of early life history stages of marine fish (e.g., Vasconcelos et al., 2007; Clarke et al., 2009; Di Franco et al., 2012; Rogers et al., 2019). Indeed, this approach was used successfully for juvenile snapper in southern Australia where chemical signatures in juvenile otoliths demonstrated high connectivity among coastal regions and identified one high-productivity region to target for fisheries management (Hamer et al., 2011). Given that region-specific chemical signatures can be identified in larval capelin otoliths (Lazartigues et al., 2016; this study), the next step is to use these distinct signatures to determine the natal origin of spawning adults by quantifying otolith chemistry in the pre-hatch region. This research will address knowledge gaps regarding embayment-specific productivity of capelin along with connectivity among embayments.
Although the goal of this study was not to investigate the mechanisms underlying region-specific differences in otolith chemistry, we did expect otolith chemical signatures to generally reflect variation in ambient water chemistry, especially for nonessential trace elements (i.e., Sr and Ba; reviewed in Campana, 1999; Loewen et al., 2016). However, this was not the case for most trace elements in this study. For Mg, an essential element, ambient concentrations varied among regions, but this variation was not reflected in otolith concentrations. There was also no trend between ambient and otolith Mn concentrations, another essential element, which may be due to the enrichment of this element in the pre-hatch region of larval otoliths, possibly due to maternal investment (Brophy et al., 2004; Ruttenberg et al., 2005; DiMaria et al., 2010; Lazartigues et al., 2014). For the nonessential element Ba, otolith concentrations also likely reflect maternal investment rather than differences in ambient water chemistry in capelin (Loeppky et al., 2018). Differing maternal investment of otolith Ba concentrations from repeat spawners may explain our finding of more similar concentrations of this element between consecutive years compared to those between nonconsecutive years. Although Sr, another nonessential element, may be maternally invested in other marine species (e.g., salmonids, Volk et al., 2000; Limburg et al., 2001; Zimmerman and Reeves, 2002), this element is known to be incorporated into the pre-hatch region of capelin otoliths during egg incubation and appears to be less maternally derived than Ba (Loeppky et al., 2018). In support, pre-hatch otolith Sr concentrations were higher in regions with higher ambient Sr concentrations in this study. Overall, the inconsistencies between otolith chemistry and most trace element concentrations in ambient water in this study have been found in other studies (Brown and Severin, 2009; Sturrock et al., 2014, 2015; Loewen et al., 2015). While our sampling regime was unable to determine if water chemistry varied throughout incubation, trace element concentrations in ambient water both within and among years were consistent in previous coastal Newfoundland studies (Davoren et al., 2015; Loeppky and Davoren, 2018), suggesting there are other drivers of variability in larval otolith chemical signatures.
Temperature and salinity are known to influence chemical signatures in the embryonic otolith of capelin larvae (Davoren et al., 2015; Loeppky and Davoren, 2018; Loeppky et al., 2018), as also found for other marine species (black bream, Acanthopagrus butcheri, Elsdon and Gillanders, 2005; Izzo et al., 2018; and European plaice, Pleuronectes platessa, Sturrock et al., 2014, 2015) and, thus, may have influenced otolith chemistry differences among regions. Preemergent capelin larvae were likely exposed to region-specific microclimates during incubation as capelin eggs are sticky and adhere to beach sediments for 10+ days before hatching (Frank and Leggett, 1981; Penton et al., 2012). Temperature is known to vary among capelin spawning beaches within a region (Crook et al., 2017), and temperature can indirectly influence otolith chemistry through its impact on somatic growth and transport kinetics, whereby higher temperatures increase larval growth which affects incorporation rates of essential trace elements (e.g., Mg and Mn; Loewen et al., 2016). Indeed, altered otolith biomineralization (e.g., stable isotopes, trace elements, and microstructure) can result from differing growth rates (Limburg et al., 2018; Freshwater et al., 2019), metabolic or energy demands (Chung et al., 2019), and ontogeny (Clarke et al., 2011). In contrast, several lab-based studies found either no effect or a weak effect of growth on both nonessential (i.e., Sr and Ba; Bath et al., 2000; Martin et al., 2004) and essential elements (i.e., Mg and Mn; Martin and Thorrold, 2005). Capelin spawning beaches are frequently associated with varying freshwater inputs (Beirão et al., 2018; Purchase, 2018), influencing the salinity of incubating water, which is likely further influenced by regional variability in summer rainfall. As Sr concentrations are positively related to environmental salinity (Panfili et al., 2015), the otolith concentrations of this trace element have been used as a marker of anadromy in many species (e.g., Arctic char, Salvelinus alpinus, Halden et al., 1995; black bream, Elsdon and Gillanders, 2005; and European bass, Dicentrarchus labrax, Reis-Santos et al., 2013). Salinity is also known to influence the incorporation rates of Sr into the embryonic otoliths of capelin (Loeppky and Davoren, 2018; Loeppky et al., 2018). Overall, microclimate variation during capelin egg incubation likely contributed to divergent otolith chemistry of larvae among regions, among years within a region, and between preemergent larvae within beach spawning sediment (intertidal) relative to field-reared larvae in incubation canisters (subtidal; ∼1 m water).
Conclusion
In conclusion, although the mechanisms underlying the observed regional differences in larval otolith chemistry are unclear due to limited sampling of ambient water conditions, our findings support the use of capelin otolith chemical signatures to evaluate the regional productivity and connectivity of this stock. Preemergent (1- to 3-day-old) larval capelin show region-specific otolith chemistry signatures which allowed larvae to be classified into their natal region and provide the basis for future studies to assess the natal origins of juvenile and adult capelin sampled offshore. As a result of the interannual variation in otolith chemistry, capelin recruits will need to be of the same cohort as the larvae sampled for baseline measurements (e.g., Hamer et al., 2011). This will allow for the identification of key coastal regions for larval production that contribute to recruitment (i.e., source and sink regions) and provide information on the scale of dispersal and connectivity among regions. Overall, identifying the natal origins of larval and adult capelin is critical for marine spatial planning and informing stock structure for management of this key forage fish species in the Newfoundland ecosystem.
Data Availability Statement
The datasets generated for this study are available on reasonable request to the corresponding author.
Ethics Statement
We confirm that the research conducted was in adherence with guidelines of the Canadian Council of Animal Care (Protocol: F16-017/1/2/3).
Author Contributions
AT wrote the manuscript and conducted all lab processing. GD and HM acquired funding for the project, conceived of the manuscript, and led the development of the manuscript. All authors were involved in determining appropriate data analyses and manuscript structure and in editing/revising the manuscript.
Funding
Principal funding was provided by the Natural Sciences and Engineering Research Council of Canada Discovery (2019-06290) and Ship Time Grant (486208-2019) to GD, along with a University of Manitoba Faculty of Science Fieldwork Support Program Grant (2019) and a Coastal Restoration Fund grant (funded by WorldWildlife Fund Canada) to GD.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the captain and crew of the Lady Easton for their assistance with fieldwork. Thanks also to B. Squires and the DFO team for sample collection in Trinity Bay and to S. Morrison and L. Bliss for larval collection in Placentia and surrounding bays. Thanks also to A. Loeppky for assistance with lab processing techniques and to P. Yang for laser expertise. Thank you to our two reviewers for their helpful feedback and comments.
References
Bath, G. E., Thorrold, S. R., Jones, C. M., Campana, S. E., McLaren, J. W., and Lam, J. W. H. (2000). Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 64, 1705–1714. doi: 10.1016/S0016-7037(99)00419-6
Beirão, J., Lewis, J. A., Wringe, B. F., and Purchase, C. F. (2018). A novel sperm adaptation to evolutionary constraints on reproduction: pre-ejaculatory sperm activation in the beach spawning capelin (Osmeridae). Ecol. Evol. 8, 2343–2349. doi: 10.1002/ece3.3783
Bradbury, I. R., Laurel, B. J., Robichaud, D., Rose, G. A., Snelgrove, P. V. R., Gregory, R. S., et al. (2008). Discrete spatial dynamics in a marine broadcast spawner: Re-evaluating scales of connectivity and habitat associations in Atlantic cod (Gadus morhua L.) in coastal Newfoundland. Fish. Res. 91, 299–309. doi: 10.1016/j.fishres.2007.12.006
Brophy, D., Jeffries, T. E., and Danilowicz, B. S. (2004). Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins. Mar. Biol. 144, 779–786. doi: 10.1007/s00227-003-1240-3
Brown, R. J., and Severin, K. P. (2009). Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Can. J. Fish. Aquat. Sci. 66, 1790–1808. doi: 10.1139/F09-112
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 188, 263–297. doi: 10.3354/meps188263
Carscadden, J. E., Gjøsæter, H., and Vilhjálmsson, H. (2013). Recruitment in the Barents Sea, Icelandic, and eastern Newfoundland/Labrador capelin (Mallotus villosus) stocks. Prog. Oceanogr. 114, 84–96. doi: 10.1016/j.pocean.2013.05.006
Carscadden, J. E., and Vilhjálmsson, H. (2002). Capelin - What are they good for? Introduction. ICES J. Mar. Sci. 59, 863–869. doi: 10.1006/jmsc.2002.1283
Chung, M. T., Trueman, C. N., Godiksen, J. A., Holmstrup, M. E., and Grønkjær, P. (2019). Field metabolic rates of teleost fishes are recorded in otolith carbonate. Commun. Biol. 2, 1–10. doi: 10.1038/s42003-018-0266-5
Clarke, L. M., Conover, D. O., and Thorrold, S. R. (2011). Population differences in otolith chemistry have a genetic basis in Menidia menidia. Can. J. Fish. Aquat. Sci. 68, 105–114. doi: 10.1139/F10-147
Clarke, L. M., Walther, B. D., Munch, S. B., Thorrold, S. R., and Conover, D. O. (2009). Chemical signatures in the otoliths of a coastal marine fish, Menidia menidia, from the northeastern united states: spatial and temporal differences. Mar. Ecol. Prog. Ser. 384, 261–271. doi: 10.3354/meps07927
Cowen, R. K., Gawarkiewicz, G., Pineda, J., Thorrold, S. R., and Werner, F. E. (2007). Population connectivity in marine systems an overview. Oceanography 20, 14–21.
Crook, K. A., Maxner, E., and Davoren, G. K. (2017). Temperature-based spawning habitat selection by Capelin (Mallotus villosus) in Newfoundland. ICES J. Mar. Sci. 74, 1622–1629. doi: 10.1093/icesjms/fsx023
Davoren, G. K., and Halden, N. M. (2014). Connectivity of capelin (Mallotus villosus) between regions and spawning habitats in Newfoundland inferred from otolith chemistry. Fish. Res. 159, 95–104. doi: 10.1016/j.fishres.2014.05.010
Davoren, G. K., Woloschiniwsky, C. S. A., Halden, N. M., and Wang, F. (2015). Does otolith chemistry indicate the natal habitat of Newfoundland capelin Mallotus villosus? J. Exp. Mar. Bio. Ecol. 464, 88–95. doi: 10.1016/j.jembe.2014.10.025
Di Franco, A., Gillanders, B. M., De Benedetto, G., Pennetta, A., De Leo, G., and Guidetti, P. (2012). Dispersal patterns of coastal fish: implications for designing networks of marine protected areas. PLoS One 7:e13681. doi: 10.1371/journal.pone.0031681
DiMaria, R. A., Miller, J. A., and Hurst, T. P. (2010). Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus macrocephalus. Environ. Biol. Fish. 89, 453–462. doi: 10.1007/s10641-010-9665-2
Elsdon, T. S., and Gillanders, B. M. (2003). Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri. Mar. Ecol. Prog. Ser. 260, 263–272. doi: 10.3354/meps260263
Elsdon, T. S., and Gillanders, B. M. (2005). Consistency of patterns between laboratory experiments and field collected fish in otolith chemistry: an example and applications for salinity reconstructions. Mar. Freshw. Res. 56, 609–617. doi: 10.1071/MF04146
Frank, K. T., and Leggett, W. C. (1981). Prediction of egg development and mortality rates in Capelin (Mallotus villosus) from meteorological, hydrographic, and biological factors. Can. J. Fish. Aquat. Sci. 38, 1327–1338. doi: 10.1139/f81-179
Freshwater, C., Trudel, M., Beacham, T. D., Gauthier, S., Johnson, S. C., Neville, C. E., et al. (2019). Individual variation, population-specific behaviours and stochastic processes shape marine migration phenologies. J. Anim. Ecol. 88, 67–78. doi: 10.1111/1365-2656.12852
Fridgeirsson, E. (1976). Observation on spawning behaviour and embryonic development of the Icelandic capelin. Rit Fiskideildar. 5, 1–35.
Halden, N. M., Babaluk, J. A., Campbell, J. L., and Teesdale, W. J. (1995). Scanning proton microprobe analysis of strontium in an Arctic charr, Salvelinus alpinus, otolith: implications for the interpretation of anadromy. Environ. Biol. Fishes 43, 333–339. doi: 10.1007/BF00001166
Hamer, P. A., Acevedo, S., Jenkins, G. P., and Newman, A. (2011). Connectivity of a large embayment and coastal fishery: spawning aggregations in one bay source local and broad-scale fishery replenishment. J. Fish Biol. 78, 1090–1109. doi: 10.1111/j.1095-8649.2011.02921.x
Hamer, P. A., Jenkins, G. P., and Gillanders, B. M. (2003). Otolith chemistry of juvenile snapper Pagrus auratus in victorian waters: natural chemical tags and their temporal variation. Mar. Ecol. Prog. Ser. 263, 261–273. doi: 10.3354/meps263261
Izzo, C., Reis-Santos, P., and Gillanders, B. M. (2018). Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluation. Fish Fish. 19, 441–454. doi: 10.1111/faf.12264
Jones, G., Milicich, M., Emslie, M., and Lunow, C. (1999). Self-recruitment in a coral reef fish population. Nature 402, 802–804. doi: 10.1038/45538
Kenchington, E. L., Nakashima, B. S., Taggart, C. T., and Hamilton, L. C. (2015). Genetic structure of capelin (Mallotus villosus) in the northwest Atlantic Ocean. PLoS One 10:e0122315. doi: 10.1371/journal.pone.0122315
Lazartigues, A. V., Plourde, S., Dodson, J. J., Morissette, O., Ouellet, P., and Sirois, P. (2016). Determining natal sources of capelin in a boreal marine park using otolith microchemistry. ICES J. Mar. Sci. J. Cons. 73, 2644–2652. doi: 10.1093/icesjms/fsw104
Lazartigues, A. V., Sirois, P., and Savard, D. (2014). LA-ICP-MS analysis of small samples: carbonate reference materials and larval fish otoliths. Geostand. Geoanalytical. Res. 38, 225–240. doi: 10.1111/j.1751-908X.2013.00248.x
Limburg, K. E., Landergren, P., Westin, L., Elfman, M., and Kristiansson, P. (2001). Flexible modes of anadromy in Baltic sea trout: making the most of marginal spawning streams. J. Fish. Biol. 59, 682–695.
Limburg, K. E., Wuenschel, M. J., Hüssy, K., Heimbrand, Y., and Samson, M. (2018). Making the otolith magnesium chemical calendar-clock tick: plausible mechanism and empirical evidence. Rev. Fish. Sci. Aquac. 26, 479–493. doi: 10.1080/23308249.2018.1458817
Loeppky, A. R., and Davoren, G. K. (2018). Temperature and salinity influence the chemistry in the pre-hatch otolith region of capelin, Mallotus villosus, during lab and field egg incubation experiments. J. Exp. Mar. Bio. Ecol. 501, 65–73. doi: 10.1016/j.jembe.2018.01.003
Loeppky, A. R., Purchase, C. F., and Davoren, G. K. (2018). Chemical signatures in embryonic otoliths of capelin (Mallotus villosus) influence of family and environmental conditions. J. Exp. Mar. Bio. Ecol. 498, 25–31.
Loewen, T. N., Carriere, B., Reist, J. D., Halden, N. M., and Anderson, W. G. (2016). Linking physiology and biomineralization processes to ecological inferences on the life history of fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 202, 123–140. doi: 10.1016/j.cbpa.2016.06.017
Loewen, T. N., Reist, J. D., Yang, P., Koleszar, A., Babaluk, J. A., Mochnacz, N., et al. (2015). Discrimination of northern form Dolly Varden Char (Salvelinus malma malma) stocks of the North Slope, Yukon and Northwest Territories, Canada via otolith trace elements and 87Sr/86Sr isotopes. Fish. Res. 170, 116–124. doi: 10.1016/j.fishres.2015.05.025
Martin, G. B., and Thorrold, S. R. (2005). Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus. Mar. Ecol. Prog. Ser. 293, 223–232. doi: 10.3354/meps293223
Martin, G. B., Thorrold, S. R., and Jones, C. M. (2004). Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus). Can. J. Fish. Aquat. Sci. 61, 34–42. doi: 10.1139/f03-143
Nakashima, B. S. (1992). Patterns in coastal migration and stock structure of capelin (Mallotus villosus). Can. J. Fish. Aquat. Sci. 49, 2423–2429. doi: 10.1139/f92-268
Nakashima, B. S., and Wheeler, J. P. (2002). Capelin (Mallotus villosus) spawning behaviour in Newfoundland waters - the interaction between beach and demersal spawning. ICES J. Mar. Sci. 59, 909–916. doi: 10.1006/jmsc.2002.1261
Panfili, J., Darnaude, A. M., Vigliola, L., Jacquart, A., Labonne, M., and Gilles, S. (2015). Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths. J. Exp. Mar. Bio. Ecol. 467, 65–70. doi: 10.1016/j.jembe.2015.03.007
Penton, P. M., Davoren, G. K., Montevecchi, W. A., and Andrews, D. W. (2012). Beach and demersal spawning in capelin (Mallotus villosus) on the northeast Newfoundland coast: egg developmental rates and mortality. Can. J. Zool. 90, 248–256. doi: 10.1139/Z11-132
Purchase, C. F. (2018). Low tolerance of salt water in a marine fish: new and historical evidence for surprising local adaption in the well-studied commercially exploited capelin. Can. J. Fish. Aquat. Sci. 75, 673–681. doi: 10.1139/cjfas-2017-0058
Reis-Santos, P., Tanner, S. E., Elsdon, T. S., Cabral, H. N., and Gillanders, B. M. (2013). Effects of temperature, salinity and water composition on otolith elemental incorporation of Dicentrarchus labrax. J. Exp. Mar. Bio. Ecol. 446, 245–252. doi: 10.1016/j.jembe.2013.05.027
Rogers, T. A., Fowler, A. J., Steer, M. A., and Gillanders, B. M. (2019). Discriminating natal source populations of a temperate marine fish using larval otolith chemistry. Front. Mar. Sci. 6:711. doi: 10.3389/fmars.2019.00711
Ruttenberg, B. I., Hamilton, S. L., Hickford, M. J. H., Paradis, G. L., Sheehy, M. S., Standish, J. D., et al. (2005). Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Mar. Ecol. Prog. Ser. 297, 273–281. doi: 10.3354/meps297273
Stanley, R. R. E., deYoung, B., Snelgrove, P. V. R., and Gregory, R. S. (2013). Factors regulating early life history dispersal of Atlantic cod (Gadus morhua) from coastal Newfoundland. PLoS One 8:75889. doi: 10.1371/journal.pone.0075889
Stanley, R. R. E., Dibacco, C., Thorrold, S. R., Snelgrove, P. V. R., Morris, C. J., Gregory, R. S., et al. (2016). Regional variation in otolith geochemistry of juvenile Atlantic cod (Gadus morhua) in coastal Newfoundland. Can. J. Fish. Aquat. Sci. 73, 1507–1519. doi: 10.1139/cjfas-2015-0353
Sturrock, A. M., Hunter, E., Milton, J. A., Johnson, R. C., Waring, C. P., Trueman, C. N., et al. (2015). Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 6, 806–816. doi: 10.1111/2041-210X.12381
Sturrock, A. M., Trueman, C. N., Milton, J. A., Waring, C. P., Cooper, M. J., and Hunter, E. (2014). Physiological influences can outweigh environmental signals in otolith microchemistry research. Mar. Ecol. Prog. Ser. 500, 245–264. doi: 10.3354/meps10699
Swearer, S. E., Caselle, J. E., Lea, D. W., and Warner, R. R. (1999). Larval retention and recruitment in an island population of a coral-reef fish. Nature 402:799.
Tanner, S. E., Reis-Santos, P., Vasconcelos, R. P., França, S., Thorrold, S. R., and Cabral, H. N. (2012). Otolith geochemistry discriminates among estuarine nursery areas of Solea solea and S. senegalensis over time. Mar. Ecol. Prog. Ser. 452, 193–203. doi: 10.3354/meps09621
Templeman, W. (1948). The life history of the capelin (Mallotus villosus O.F. Müller) in Newfoundland waters. Res. Bull. Newfoundland. Dept. Nat. Res. 17, 1–15.
Vasconcelos, R. P., Reis-Santos, P., Tanner, S., Fonseca, V., Latkoczy, C., Günther, D., et al. (2007). Discriminating estuarine nurseries for five fish species through otolith elemental fingerprints. Mar. Ecol. Prog. Ser. 350, 117–126. doi: 10.3354/meps07109
Volk, E. C., Blakley, A., Schroder, S. L., and Kiehner, S. M. (2000). Otolith chemistry reflects migratory characteristics of Pacific salmonids: using otolith core chemistry to distinguish maternal associations with sea and freshwaters. Fish. Res. 46, 251–266.
Keywords: Mallotus villosus, larvae, laser ablation ICP–MS, natal origin, Newfoundland
Citation: Tripp A, Murphy HM and Davoren GK (2020) Otolith Chemistry Reveals Natal Region of Larval Capelin in Coastal Newfoundland, Canada. Front. Mar. Sci. 7:258. doi: 10.3389/fmars.2020.00258
Received: 16 December 2019; Accepted: 31 March 2020;
Published: 25 May 2020.
Edited by:
Benjamin D. Walther, Texas A&M University Corpus Christi, United StatesReviewed by:
Troy A. Rogers, University of Adelaide, AustraliaJed Ian Macdonald, Pacific Community (SPC), New Caledonia
Copyright © 2020 Tripp, Murphy and Davoren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gail K. Davoren, R2FpbC5EYXZvcmVuQHVtYW5pdG9iYS5jYQ==
 Ashley Tripp
Ashley Tripp Hannah M. Murphy
Hannah M. Murphy Gail K. Davoren
Gail K. Davoren