- 1Department of Medicine, Queen's University, Kingston, ON, Canada
- 2Respiratory Research Unit, Bispebjerg University Hospital, Copenhagen, Denmark
- 3Research Group for Rehabilitation in Internal Disorders, Respiratory Rehabilitation and Respiratory Division, Department of Rehabilitation Sciences, University Hospital Leuven, KU Leuven, Leuven, Belgium
Cardiopulmonary exercise testing (CPET) has traditionally included ventilatory and metabolic measurements alongside electrocardiographic characterization; however, research increasingly acknowledges the utility of also measuring inspiratory neural drive (IND) through its surrogate measure of diaphragmatic electromyography (EMGdi). While true IND also encompasses the activation of non-diaphragmatic respiratory muscles, the current review focuses on diaphragmatic measurements, providing information about additional inspiratory muscle groups for context where appropriate. Evaluation of IND provides mechanistic insight into the origins of dyspnea and exercise limitation across pathologies; yields valuable information reflecting the integration of diverse mechanical, chemical, locomotor, and metabolic afferent signals; and can help assess the efficacy of therapeutic interventions. Further, IND measurement during the physiologic stress of exercise is uniquely poised to reveal the underpinnings of physiologic limitations masked during resting and unloaded breathing, with important information provided not only at peak exercise, but throughout exercise protocols. As our understanding of IND presentation across varying conditions continues to grow and methods for its measurement become more accessible, the translation of these principles into clinical settings is a logical next step in facilitating appropriate and nuanced management tailored to each individual's unique physiology. This review provides an overview of the current state of understanding of IND measurement during CPET: its origins, known patterns of behavior and links with dyspnea in health and major respiratory diseases, and the possibility of expanding this approach to applications beyond exercise.
Introduction
Measuring diaphragmatic electromyography (EMGdi) as a surrogate of inspiratory neural drive (IND) has a tradition extending over 100 years. Its ability to reveal the mechanistic underpinnings of exercise limitation and dyspnea during cardiopulmonary exercise testing (CPET) has popularized its use in research; however, IND is rarely measured in non-research clinical settings. With aims of familiarizing a broad audience with the fundamental principles of IND measurement and its presentation in health and respiratory disease, this review outlines the valuable insights provided by IND measurement during the physiologic stressor of exercise, what these reveal beyond standard testing approaches, and emerging areas of interest in applying IND in diverse research settings. It also reflects on current barriers to the clinical adoption of IND assessment and how these might be overcome.
Fundamentals of Ind Measurement
Muscles of Inspiration
The inspiratory muscles fall into two categories: primary (i.e., diaphragm, external intercostal, scalene, and parasternal internal intercostal muscles) and accessory (e.g., sternocleidomastoid, pectoralis minor, etc.) (1, 2). The diaphragm is the foremost driver of inspiration at rest and during exercise, accounting for ~2/3 of lung volume change (3, 4). The scalene and external intercostal muscles show lesser activation during healthy quiet breathing but play an increasingly important role in loaded, high-volume, or distressed breathing patterns (5, 6), while the parasternal internal intercostal muscles are active during resting eupneic breathing, assisting with upper thoracic expansion as well as stabilizing the thorax to the effects of diaphragmatic movement (7, 8). The accessory muscles contribute to inspiration in conditions with higher ventilatory requirements or where breathing pattern is altered (e.g., more rapid) as a result of impaired respiratory mechanics (1). Diaphragmatic IND is the focus of this review. While not discussed herein, the expiratory muscles (i.e., abdominal muscles and internal intercostals) also play an active role in forced exhalations and in supporting the increased ventilation of exercise (9, 10). This is especially critical in conditions of gas trapping, where expiratory recruitment supports subsequent inspiration through elevation of the diaphragm at end-expiration (11, 12).
It is worth noting that rather than being a singular entity, as implied by the nomenclature, the diaphragm consists of two distinct regions: the costal diaphragm, apposing the ribs, and the crural diaphragm, the electrically active region of which is located medially and forms the esophageal hiatus (13, 14). Whereas, the costal diaphragm is involved in the displacement of both abdominal contents and the ribcage, the crural diaphragm displaces abdominal contents only in its caudal, inspiratory descent (13). Thus, the crural diaphragm has a lesser role in thoracic expansion and force generation than the costal diaphragm.
History of Neural Drive Measurement
EMG measurement via intramuscular needle electrodes has been used to investigate ventilatory mechanisms since the early 20th century (15–19). These earliest observations in dogs and rabbits demonstrated the direct link between phrenic nerve activity and diaphragmatic activation: namely, that action potentials of the phrenic nerve result in electrical activation of the diaphragm (20). Later work ultimately determined the origin of this phrenic activity to be ventilatory drive from the respiratory medulla (21–23). However, the invasive nature and contamination of intramuscular EMGdi with adjacent intercostal muscle activation and breathing movement artifact limited the uptake of this approach in human populations (24). This spurred the development of less invasive techniques using either surface electrodes to measure costal or parasternal EMGdi (25–28) or nasally inserted esophageal catheters to measure crural EMGdi (29–33). Although appealingly non-invasive and relatively easy to use, surface measurements can underestimate EMG activity (vs. esophageal recordings), be contaminated by the electrical activity of neighboring accessory muscles (34–36), or be vulnerable to position and limb muscle mobilization (37, 38). By contrast, esophageal measurements of crural EMGdi are relatively robust, but more technically demanding and potentially uncomfortable for patients. However, the authors' own experiences using this technology, as well as the documented experiences of others, support esophageal catheters being well-tolerated by most patients when skillfully utilized (39).
Contemporary catheter designs build off of earlier designs that utilized a single electrode pair (40, 41). These were prone to artifactual changes in EMG activity due to the relative movement of the diaphragm during breathing as compared with the fixed catheter electrode. Current designs employ multiple electrode arrays arranged as overlapping pairs, which help with positioning the electrodes across the electrically active region (EAR) of the crural diaphragm via cross-correlation analysis as well as compensate for movement of the EAR relative to the electrode during breathing (42–44). [For a more detailed review of esophageal EMGdi measurement, please refer to Luo et al. (45)].
Recent findings suggest that while crural and costal diaphragmatic activation is similar at rest, costal activation (measured by intramuscular recording) increases disproportionately to crural activation when ventilation increases either voluntarily or involuntarily (46–48). This differs from earlier studies that measured costal activity via surface EMG and found parallel increases in costal and crural activity during increase ventilation; however, this difference in findings may be attributable to the greater contamination of surface costal EMGdi with intercostal and abdominal muscle activity (14–16). Thus, while there is significant methodological appeal in the robustness of relatively non-invasive esophageal measurements, it is worth considering that crural recordings may not fully represent IND to the diaphragm, especially during increased ventilatory demand. Parasternal intercostal surface EMG has also gained recent attention as a potential alternative to esophageal crural measurements of IND (49, 50), with emerging data showing strong congruence in baseline activation and profiles of increasing activation in response to increasing IND between surface parasternal intercostal and esophageal crural measurements (28, 50–53).
Contemporary Approaches to Measuring Neural Drive
Modern IND assessment increasingly combines multipair esophageal EMGdi with invasive (esophageal/gastric manometry) or non-invasive (please see accompanying review by (54): “Non-invasive evaluation of dynamic respiratory mechanics”) measurement of respiratory mechanics (45, 55). EMGdi now routinely replaces traditional IND estimates during CPET, such as minute ventilation (VE), esophageal (Pes), transdiaphragmatic (Pdi), or mouth occlusion pressures (37). While these are influenced by obesity (56) or disease-altered respiratory mechanics (57–61), measuring the initiating contractile signal rather than resulting mechanical response provides a more direct assessment of IND. Measuring centrally originating IND with the resulting mechanical (e.g., Pdi) or ventilatory [VE, tidal volume/vital capacity (VT/VC), or VT/VCpred] response of the system additionally enables direct investigation of neuromechanical and neuroventilatory coupling or dissociation, respectively (55, 62). Whereas coupling is used to refer to the efficiency with which the electrical signal is converted into a mechanical or ventilatory response, dissociation refers to EMGdi not translating into a mechanical or ventilatory response as efficiently as in health. While there is some variation in how EMGdi is reported alongside mechanical or ventilatory outcomes between authors, in the present work, these are represented by the commonly employed EMGdi:Pdi and EMGdi:VT/VCpred, respectively, unless stated otherwise.
Although modern esophageal EMGdi is relatively robust to movement artifact or neighboring muscle activity, two technical notes are warranted. (1) While crural EMGdi necessarily contains electrocardiographic artifact, its regularity and distinct profile allows for ready isolation (visual or computational) from the surrounding respiratory signal (45, 63). (2) Between-individual (or within-individual, when measured during different sessions) differences in electrode: muscle fiber orientation, impedance, muscle blood flow, and distance (or amount of tissue) between electrode and muscle surface necessitate signal standardization (64). As per the values reported in this review, this is typically achieved by presenting EMGdi as a percentage of maximum voluntary activation (EMGdi%max) obtained during inspiratory capacity (IC) or sniff maneuvers (65, 66). Such maximum maneuvers show strong between-visit reliability (67); however, it is worth mentioning evidence that EMGdi%max may most appropriately be used to normalize for between-group differences, while normalization to ECG R-wave amplitude or to resting tidal EMGdi may be more reliable when investigating intra-individual, inter-visit differences (68).
Neural Drive in the Evaluation of the Breathless Patient
The Spectrum of Normal
In healthy adults, resting tidal EMGdi represents only 7–10% of maximum voluntary activation (39, 69). However, this range belies variations. Resting EMGdi can double to 22%max in obesity, for example, due to increased ventilatory load and effort (Pes) (70). Healthy aging's impact on baseline IND is also an important consideration, especially when assessing individuals with chronic respiratory diseases. This is particularly relevant considering the strong relationship between IND and dyspnea (71, 72), i.e., the “subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” (73). Unlike the VE:dyspnea relationship, which is limited when respiratory mechanics are impaired, EMGdi%max robustly correlates with dyspnea in health and across disease severity (50, 74). Dyspnea is thought to reflect awareness of the mismatch that results when increased IND does not or cannot result in an adequate mechanical or ventilatory response (75). While not present during resting tidal breathing in health, the stressor of exercise or pathophysiologic processes of disease typically provoke sensations of dyspnea (76).
Aging induces emphysema-like changes in the lung (increased pulmonary compliance) while decreasing chest wall compliance (77, 78). Aging additionally reduces inspiratory muscle strength, decreases diffusing capacity, decreases the proportion of Type II muscle fibers in the diaphragm, and decreases the number of phrenic motoneurons (79–81). Investigation into whether these changes translate into altered IND found that resting crural EMGdi was 40% greater in individuals > 51 years than those <50 years (39); however, these findings standardized EMGdi to maximum voluntary activation, which may be reduced (e.g., inability to achieve—or motivation to perform—truly maximal maneuvers) (82, 83). Recent work specifically investigating motor unit discharge rate (monopolar needle recording of costal diaphragm) found no changes across age groups at rest, despite neurogenic changes in motor unit potential area and discharge time that may become more relevant at higher ventilation (84). Interestingly, despite known sex differences in pulmonary structure [smaller lungs, narrower airways (85)] and function [increased resistive work of breathing and greater propensity for expiratory flow limitation and exercise-induced hypoxemia (86)], resting EMGdi does not vary between age-matched healthy males and females (87, 88).
Healthy Responses to Exercise
Two common exercise protocols that are used to study IND are constant work rate (i.e., constant load; CWR), where a constant submaximal output is maintained, and incremental (ICR), where work rate increases in stepwise fashion at predetermined time intervals. The ability of ICR protocols to interrogate the IND profile to the boundaries of maximal exercise capacity offers unique advantages over CWR protocols, including continually increasing IND in concert with continually increasing dyspnea from rest to symptom limitation. This is in contrast with CWR protocols, where IND initially increases before maintaining a submaximal plateau until end exercise (Figure 1A). EMGdi activation during exercise typically plateaus at submaximal values <80%max, with some variability reported between studies and populations (55, 69, 89, 90). This begs the question: is this submaximal activation appropriate for the required output or reflective of central inhibition (69, 91, 92)? The maintenance of maximal voluntary IND as achieved through IC maneuver throughout various exercise protocols suggest that the former interpretation of task-appropriate IND is true, rather than neural inhibition (69).
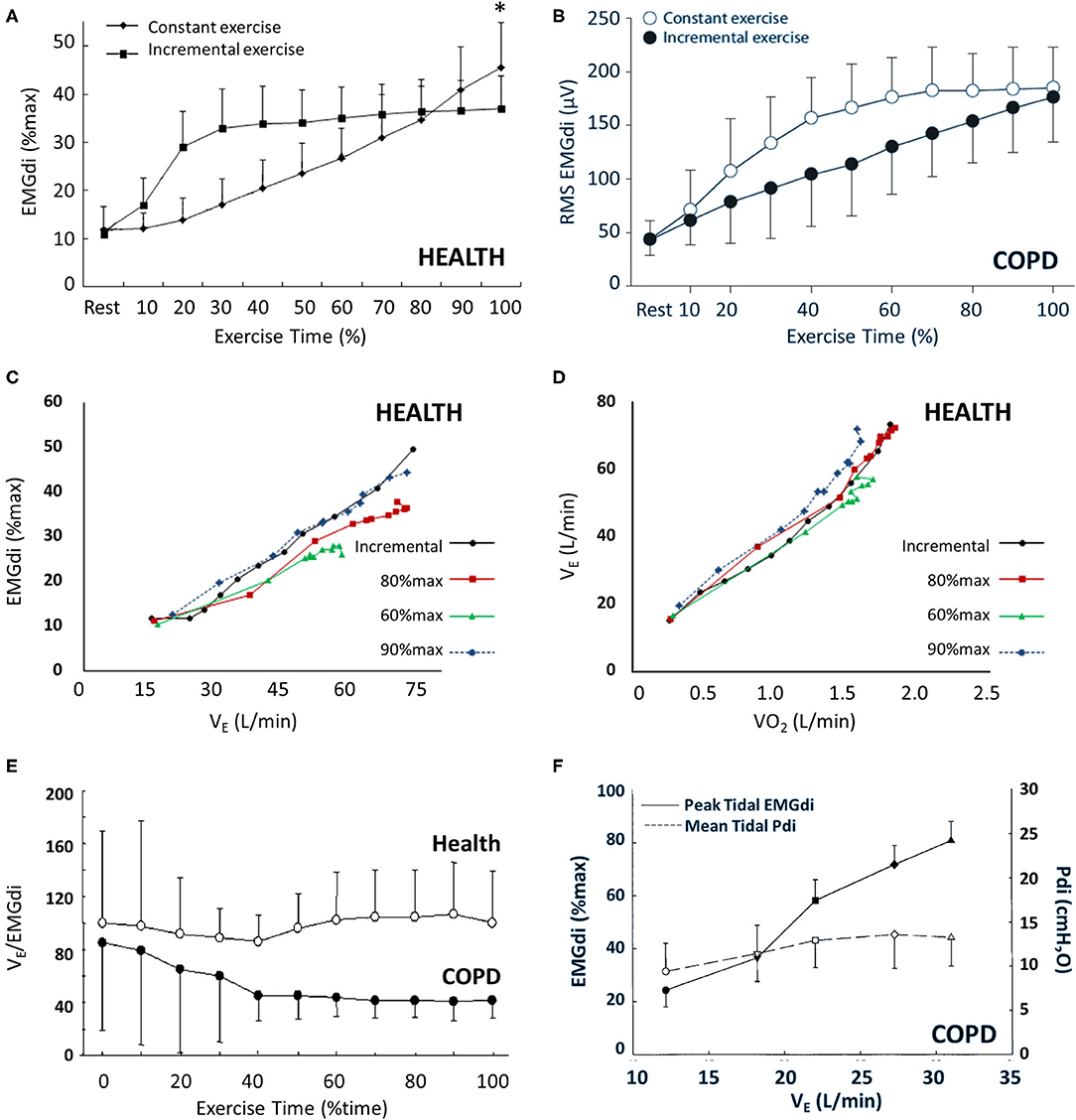
Figure 1. EMGdi behavior during incremental (ICR) and constant work rate (CWR) exercise in health and COPD. Panel (A) shows the gradual increase in EMGdi (%max) associated with ICR and the rapid increase in EMGdi (%max) and subsequent plateau associated with CWR exercise in health (*p < 0.05). A similar pattern of behavior is seen in COPD (B). The relationship between EMGdi and VE (C) and between VE and VO2 (D) is maintained regardless of exercise type (ICR vs. CWR) or intensity (CWR at 60, 80, or 90% of maximum work rate). While VE/EMGdi is maintained in health during CWR (E), and Pdi/EMGdi is maintained in COPD (F), there is uncoupling of VE and EMGdi in COPD during exercise (E,F). Panels (A), (C), and (D) were adapted from (69); panel (B) was adapted from (57); panel (E) was adapted from (89); and panel (F) was adapted from (90). Panels (A), (C), and (D) are reprinted from Resp Physiol Neurobiol, 189(1), Zhang D, Gong H, Lu G, Guo H, Li R, Zhong N, et al. Respiratory motor output during an inspiratory capacity maneuver is preserved despite submaximal exercise, 87–92, Copyright 2013, with permission from Elsevier. Panel (B) is reprinted from Respiration 81(4), Luo YM, Li RF, Jolley C, Wu HD, Steier J, Moxham J, et al., Neural respiratory drive in patients with COPD during exercise tests. 294–301, Copyright 2011, with permission from S. Karger AG, Basel. Panel (E) is reprinted from Chest, 138(6), Qin YY, Steier J, Jolley C, Moxham J, Zhong NS, Luo YM. Efficiency of neural drive during exercise in patients with COPD and healthy subjects, 1309–1315, Copyright 2010, with permission from Elsevier. Panel (F) is adapted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society. All rights reserved. Cite: Sinderby C, Spahija J, Beck J, Kaminski D, Yan S, Comtois N, et al. (2001) Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163(7):1637–41. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. Readers are encouraged to read the entire article for the correct context at https://doi.org/10.1164/ajrccm.163.7.2007033. The authors, editors, and The American Thoracic Society are not responsible for errors or omissions in adaptations.
Ventilation and dyspnea parallel EMGdi during exercise: all three increase with exercise time and intensity [Figures 1C,D; 2A–C; (57, 69)]. Neuroventilatory and neuromechanical relationships (EMGdi relative to VE or Pdi) are maintained throughout exercise in health (69, 89, 90). This is especially relevant in the context of healthy aging, which is accompanied by decreased ventilatory efficiency (i.e., increased VE/VCO2) and increased ventilatory demand (80). These changes are thought to occur as a result of increased physiologic dead space, i.e., ventilation–perfusion (V/Q) inequalities (93, 94), decreased PaCO2 setpoint (95–97), increased anatomic dead space (95), and greater likelihood of terminal airway closure at higher closing volumes (98). Exertional dyspnea also increases alongside loss of static muscle strength in aging, and older females report greater dyspnea than older males for a given absolute VE (99). While you will recall the lack of sex differences in healthy resting EMGdi, exercise protocol type seems to influence the occurrence of sex-specific exercise responses in young adults. Specifically, while EMGdi does not vary between healthy young males and females during CWR protocols performed at the same relative intensity (87), females have higher EMGdi%max and dyspnea for given absolute workloads during ICR exercise (88). This likely reflects the higher ventilation (as a fraction of maximum ventilatory capacity) required to sustain a given absolute work rate in females vs. males (85, 88, 100, 101).
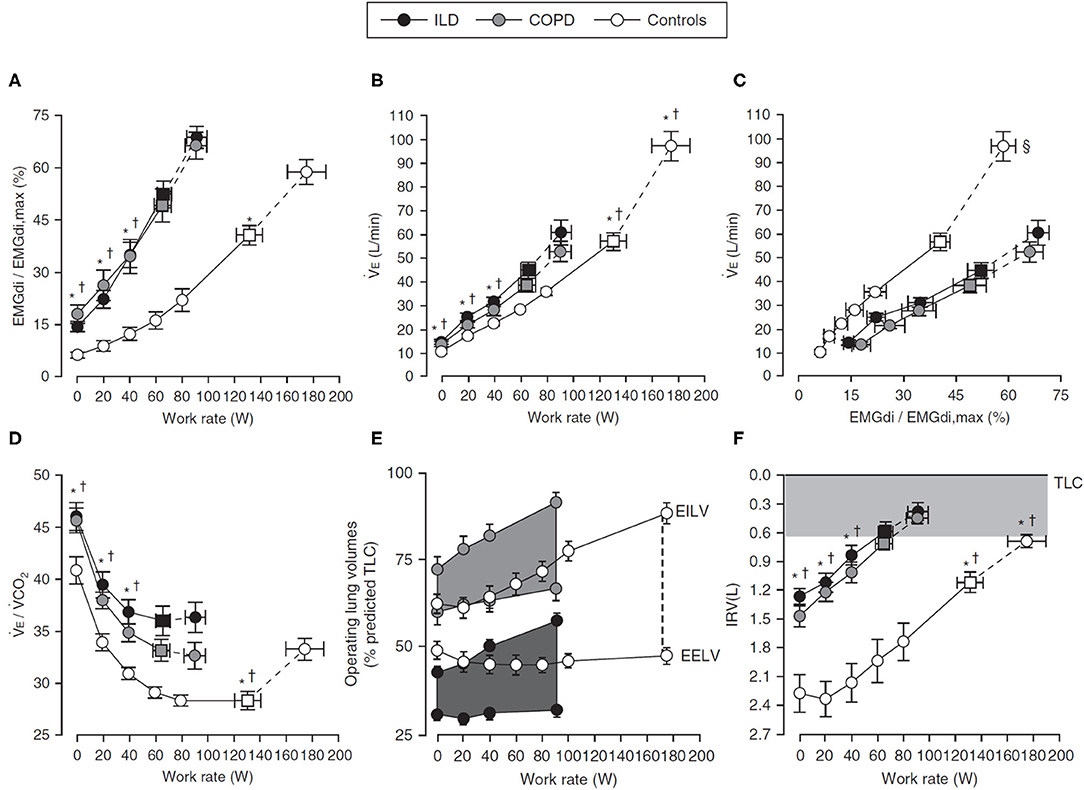
Figure 2. Response to incremental cycle exercise in health (Controls), COPD, and ILD. Values are mean ± SEM, and squares represent VT-VE inflection points. *p < 0.05 (ILD vs. Control); †p < 0.05 (COPD vs. Control); ‡p < 0.05 (COPD vs. ILD); §p < 0.05 for for differences in VE/(EMGdi/EMGdi,max) slopes between patient groups and control participants. Panel (A) shows IND as EMGdi (%max) increasing throughout ICR, panel (B) shows the associated ventilatory response (VE), and panel (C) shows the coupling of EMGdi with VE. Respiratory efficiency is decreased (D) (i.e., VE/VCO2 increased) in respiratory disease relative to Control, in part due to significant ventilatory constraints occurring alongside dynamic hyperinflation [(E), VT expansion during exercise and earlier attainment of inspiratory reserve volume threshold, (F)]. Figure adapted from (55). Figure is adapted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society. All rights reserved. Faisal A, Alghamdi BJ, Ciavaglia CE, Elbehairy AF, Webb KA, Ora J, et al. (2016) Common Mechanisms of Dyspnea in Chronic Interstitial and Obstructive Lung Disorders. Am J Respir Crit Care Med, 193(3):299–309. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. Readers are encouraged to read the entire article for the correct context at https://doi.org/10.1164/rccm.201504-0841OC. The authors, editors, and The American Thoracic Society are not responsible for errors or omissions in adaptations.
Neural Drive and Dyspnea Are Elevated in Respiratory Disease
Many respiratory conditions with diverse underlying pathological mechanisms result in elevated resting tidal IND and dyspnea. In chronic obstructive pulmonary disease (COPD), resting IND is increased 2-fold (EMGdi%max >20%) vs. age-matched health (39, 90). Similar magnitudes of increase are seen in interstitial lung disease (ILD) (55, 102) and cystic fibrosis (49). This is linked to pathophysiologic alterations in mechanical and chemical factors (103) and can already appear in very early disease, as detailed in the accompanying review by (104): “Dyspnea and Exercise Limitation in COPD: the value of CPET.”
In obstructive disease, mechanically characterized by increased compliance, gas trapping, hyperinflation, and reduced IC, IND correlates with the severity of airflow limitation (decreased forced expired volume in 1 s; FEV1) and degree of hyperinflation (39, 49), due to reduced pressure-generating ability of the diaphragm (105). Mechanical impairment causing increased IND is experimentally supported by acutely increased EMGdi alongside loss of FEV1 post-histamine bronchoprovocation challenge in asthmatic children (106). IND is also increased in restrictive diseases like ILD, where decreased compliance and low lung volumes decrease IC. Thus, in both obstructive and restrictive conditions, IND typically increases alongside increasing mechanical impairment (39). Such situations of increased diaphragmatic loading or impairment also increase recruitment of non-diaphragmatic inspiratory muscles (5, 6).
Increased IND can reflect underlying mechanical impairment, but how chemical impairment (e.g., gas exchange abnormalities) might also be reflected is of increasing interest. For example, it has been demonstrated that increased physiologic dead space (i.e., V/Q mismatch), necessitating increased VE, reducing ventilatory efficiency, and ultimately resulting in earlier attainment of mechanical constraints, contributes to the increased IND observed in disease (107, 108). This is experimentally supported by increased IND during dead space loading (109) or acute increases in PaCO2 in health, with the EMGdi-PCO2 relationship suggested as an index of chemosensitivity (68). Data suggest that IND also increases linearly with increasing CO2 during rebreathing in COPD (110); however, the impact of chronic hypercapnia on IND and CO2 responsiveness in respiratory disease is equivocal. While some groups report blunted CO2 responsiveness in hypercapnic COPD (111), others report increased IND in hypercapnic COPD with equivalent mechanical impairment to normocapnic COPD (112). These differences may arise from methodological or group differences (acute CO2 exposure vs. chronic hypercapnia; degree of mechanical impairment; analysis of the EMG signal through integration, moving average, or peak) and highlight the need for further studies to clarify the role of chronic hypercapnia, increased physiologic dead space, and diffusion impairment on IND. Increased IND secondary to hypercapnia is likely attributable to a combination of chemosensory inputs, resultant ventilatory changes and the mechanical limitations they precipitate, and afferent signals from mechanically overloaded inspiratory muscles (113). Finally, patients with hypercapnic COPD tend to also experience chronic hypoxia, which may further contribute to IND via chemo-afferent pathways (114, 115) and through diaphragmatic fatigue (116).
Diaphragmatic Responses to Exercise in Respiratory Disease
Despite different pathophysiologic underpinnings, there is interesting similarity in the diaphragmatic and ventilatory responses to exercise seen in obstructive and restrictive diseases, both of which are exaggerated compared with health [Figures 2A–C; see also (117)]. As in health, baseline IND increases with increasing exercise and metabolic CO2 output in respiratory disease (57, 89, 90), either to a plateau in CWR protocols or until end exercise is achieved in ICR protocols [Figure 1B; (57, 89)], but the relative IND is elevated for an absolute work rate vs. health. Further, whereas EMGdi is maintained relative to VE throughout CWR exercise in health, both VE and Pdi gradually decline relative to EMGdi throughout exercise in COPD [Figures 1E,F; (89, 90)], indicative of a declining efficiency of IND during exertion in this population. A similar pattern is seen in the neuromechanical and neuroventilatory dissociation of EMGdi/Pdi and EMGdi%max:VT/VCpred during ICR, with persistently increasing IND in the face of earlier constraints in increasing Pdi or VT.
The higher ventilatory requirements of exercise stress the physiologic tolerances of the respiratory system, exposing underlying impairments. For example, in COPD, baseline CO2 retention occurring due to ventilation–perfusion mismatch at rest is further exaggerated during exercise by the inability of the mechanically disadvantaged system to meet the increased metabolic demands of exercise (Figures 2C,D) (118). This, in turn, further increases IND and ventilation. When paired with a rapid, shallow breathing pattern increasing dead space, and underlying expiratory flow limitation leading to dynamic hyperinflation and encroachment of tidal volume on critical inspiratory reserve (Figure 2E) (119, 120), early cessation of exercise and a higher symptom burden for a given work rate ensue (75). In ILD, low diffusing capacity and low pulmonary compliance result in increased ventilatory drive and a rapid, shallow breathing pattern due to limited VT expansion, ultimately also leading to premature termination of exercise and exaggerated dyspnea (55).
The exercise limitations observed in obstructive and restrictive disease are due to an inadequate mechanical response to the higher IND, with the lower IC of both populations limiting VT expansion and causing earlier attainment of the lowest critical inspiratory reserve volume, IRV, and a reliance on increases in breathing frequency to increase VE [Figures 2E,F; (90, 119–121)]. Whether resulting from the hyperinflation-disadvantaged length–tension relationships of the diaphragm (122, 123) and impaired ability to generate inspiratory pressure in situations of increased inspiratory flow (41, 124, 125) in COPD or due to low compliance and low operating lung volumes in ILD, mechanical impairments prevent the efficient translation of drive into ventilatory response. Thus, in both obstructive and restrictive disease, the slope of the relationship between EMGdi%max and work rate is increased relative to health, as is the slope of the dyspnea: work rate relationship (55).
Using Exercise to Reveal Impairments Hidden at Rest
The utility of CPET as an adjunct to resting pulmonary function testing is further highlighted by respiratory conditions with normal or relatively preserved resting IND, such as exercise-induced laryngeal obstruction (EILO), which presents primarily in young individuals during high-intensity exercise (126). Here, normal resting IND becomes progressively augmented relative to health at increasing work rates, reflecting increasing inspiratory resistive work of breathing, with a significantly elevated IND approaching end exercise (127). Thus, in contrast to the possible beneficial effects of exercise-associated laryngeal closure associated with obstructive pulmonary conditions (128), the laryngeal closure observed in EILO causes both mechanical impairment and increased IND (127). Interestingly, individuals with EILO and those with obstructive pulmonary disease report “unsatisfied inspiration” at high work rates, a convergence of symptoms despite markedly different underlying pathophysiological mechanism contributing to each group's increased IND (117, 127).
IND measurement and CPET are particularly valuable in smokers at risk of COPD and individuals with mild COPD. Despite relatively preserved resting spirometry, subtle decreases in diffusive capacity, increases in dead space, and changes in pulmonary mechanics translate into increased IND at rest, helping to explain the symptoms experienced by these individuals despite relatively preserved lung function (108, 129). These resting differences are exaggerated throughout exercise, with decreased exercise endurance, increased IND, and increased dyspnea in smokers-at-risk and mild COPD vs. health (108, 129). This increased dyspnea has recently been linked to ventilatory inefficiency causing premature mechanical constraint, with individuals with DLCO lower than the lower limit of normal (LLN) experiencing a higher ventilatory requirement and thus greater dyspnea and exercise intolerance than patients with DLCO > LLN despite equivalent spirometry (130). This topic is covered in greater detail in the accompanying review by (104). “Dyspnea and Exercise Limitation in Mild COPD: the value of CPET.”
New Frontiers For Neural Drive Measurement
Evaluating Responses to Interventions
In addition to providing insight into the mechanisms of exercise intolerance, IND measurement enables a more detailed mechanistic assessment of pharmacotherapeutic and other interventions. For example, bronchodilator-based improvements in neuromechanical coupling mirroring improvements in dyspnea during exercise challenges are documented in COPD (131, 132), while respiratory system unloading (i.e., helium unloading) independent of airway tone is similarly associated with improved indices of neuromuscular output (133, 134). Other interventions, such as supplemental O2 therapy or opiates, are specifically targeted at decreasing IND rather than altering respiratory mechanics (135, 136). Thus, the measurement of EMGdi in research settings can provide valuable information about IND, ultimately helping to better inform clinical approaches targeted at improving exercise performance and/or dyspnea. A possible application would include the measurement of EMGdi alongside respiratory mechanics (e.g., as outlined in the accompanying review by (54) “Non-invasive evaluation of dynamic respiratory mechanics”) to help evaluate pulmonary rehabilitation interventions targeting sarcopenia or the deconditioning of aging or chronic respiratory disease.
One application where this approach has been increasingly applied is in the evaluation of improvements in dyspnea and reductions in IND following inspiratory muscle training (IMT), proposed to occur due to improved neuromechanical coupling (137). As different IMT protocols have been assessed in diverse populations, these studies have yielded equivocal results. This includes no improvements in IND despite improvements in dyspnea and maximum inspiratory pressure when used by healthy young adults (138) or improved (decreased) IND despite maintained VE and breathing pattern in COPD with baseline inspiratory muscle weakness (137). Differences in IMT study outcomes may also in part be due to the preferential recruitment of accessory muscles of inspiration during different IMT approaches and resulting breathing patterns (51, 138). Use of EMGdi measurement during IMT performed with inspiratory threshold training has shown this approach to generate better diaphragmatic recruitment and activation than IMT performed using inspiratory resistive devices in severe COPD with inspiratory muscle weakness (74, 139), while focused instruction outlining diaphragmatic breathing strategies similarly improves diaphragmatic activation during IMT in health (140). Pursed-lip breathing, a commonly employed intervention linked with improved symptoms of dyspnea and resulting in deeper and slower breathing patterns, has also been associated with reduced diaphragmatic recruitment and increased engagement of accessory muscles in advanced COPD (141). These types of targeted investigations may help optimize future rehabilitation approaches (142), and further investigation is needed to clarify those results attributable to training protocol vs. those linked directly to between-population differences.
Applying IND Measurement in Non-CPET Settings
Emerging interest lies in the measurement of IND within novel areas of research. Two with promise are sleep and acute exacerbations of COPD. IND measurement can successfully differentiate periods of central vs. obstructive sleep apnea (143), while continuous monitoring of overnight EMGdi shows greater decreases in IND in the transition from wakefulness to non-rapid eye movement (NREM) and REM sleep in COPD vs. health, possibly holding clues to the nocturnal hypoventilation commonly observed in COPD (144). More recent work has shown the benefits of nocturnal bronchodilator therapy in improving overnight IND and respiratory mechanics (145). IND monitoring has also generated interest as a possible means of predicting recovery from acute exacerbations, with failure of acutely increased parasternal EMGdi to return to baseline conditions after hospitalization for exacerbation strongly correlated with failure to experience subjective improvements in dyspnea (Borg), lack of clinical improvement, and likelihood of readmission (146).
Overcoming Barriers to Clinical Adoption
The integration of IND measurement into clinical settings has historically been limited by the cost of one-time use electrodes, the relative invasiveness and complexity of crural measurement approaches, challenges in standardizing measurements between visits or between individuals, and the significant technical complexities and time requirements associated with existing manual analysis approaches (39, 70). Advances in surface assessment of parasternal EMG hold significant promise for overcoming the technical barriers and patient burden associated with esophageal catheter use. This has already been successfully employed in diverse and vulnerable populations, including pediatric asthma (147), and may form the foundation of more routine adoption of IND assessment in clinical practice. The reporting of normalized values, regardless of approach, also helps to account for possible differences in signal detection between testing sessions (64).
Addressing concerns surrounding complex and time-consuming analysis approaches, significant computational advances now enable semi-automated analyses of crural EMGdi (63) as well as novel approaches to IND assessment via diaphragmatic signal entropy (148, 149), significantly improving analysis speed and consistency. Further, there is promise in the fully automated, real-time integration of IND information to inform mechanical ventilation approaches through EMGdi-based or non-invasive Neurally Adjusted Ventilatory Assist (150–152). The ongoing refinement of these approaches provides fertile ground for a more seamless integration of IND measurement into standard care. A final requirement for the translation of IND from research to clinical laboratories is the establishment of normative resting and exercise values of EMGdi in both sexes across age groups. Until such values are available, the use of age- and sex-matched comparator populations is essential in the investigation of disease.
Conclusions
As far back as 1929, the Lancet submitted a “plea for a careful clinical study of the diaphragm in chest disease” (153). In the century that has followed, significant progress has been made in elucidating not only the structure, but increasingly the function, of our primary pump muscle. The foundation that has been laid surrounding the utility of EMGdi as a marker of IND and its associated sequelae of dyspnea and exercise limitation is now well-positioned for translation into clinical practice. The ability of IND to reflect alterations in ventilatory load and capacity holds significant promise for its possible use as a global marker of disease severity and ventilatory dysfunction, as well as a useful target for monitoring the success of therapeutic interventions.
Author Contributions
ND contributed to planning and drafting of the submission. ND, EW, and DL all contributed to editing of the article and approved the submitted version.
Funding
ND was supported by a Canadian Institutes of Health Research Banting Postdoctoral Fellowship and Queen's University Spear Endowment Fund. DL was supported by Research Foundation Flanders: G0A4516N–ZKC9570–C22/15/035.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Roussos C, Macklem PT. The respiratory muscles. N Engl J Med. (1982) 307:786–97. doi: 10.1056/NEJM198209233071304
2. Campbell EJ. The role of the scalene and sternomastoid muscles in breathing in normal subjects; an electromyographic study. J Anat. (1955) 89:378–86.
3. Mead J, Loring SH. Analysis of volume displacement and length changes of the diaphragm during breathing. J Appl Physiol. (1982) 53:750–5. doi: 10.1152/jappl.1982.53.3.750
4. Wade OL. Movements of the thoracic cage and diaphragm in respiration. J Physiol. (1954) 124:193–212. doi: 10.1113/jphysiol.1954.sp005099
5. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. (2003) 168:10–48. doi: 10.1164/rccm.2206020
6. Tobin MJ. Why physiology is critical to the practice of medicine: a 40-year personal perspective. Clin Chest Med. (2019) 40:243–57. doi: 10.1016/j.ccm.2019.02.012
7. De Troyer A, Sampson MG. Activation of the parasternal intercostals during breathing efforts in human subjects. J Appl Physiol. (1982) 52:524–9. doi: 10.1152/jappl.1982.52.1.286-s
8. Han JN, Gayan-Ramirez G, Dekhuijzen R, Decramer M. Respiratory function of the rib cage muscles. Eur Respir J. (1993) 6:722–8.
9. Henke KG, Sharratt M, Pegelow D, Dempsey JA. Regulation of end-expiratory lung volume during exercise. J ApplPhysiol. (1988) 64:135–46. doi: 10.1152/jappl.1988.64.1.135
10. Loring SH, Mead J. Abdominal muscle use during quiet breathing and hyperpnea in uninformed subjects. J Appl Physiol. (1982) 52:700–4. doi: 10.1152/jappl.1982.52.3.700
11. Wolfson DA, Strohl KP, Dimarco AF, Altose MD. Effects of an increase in end-expiratory volume on the pattern of thoracoabdominal movement. Respir Physiol. (1983) 53:273–83. doi: 10.1016/0034-5687(83)90119-6
12. Martin JG, De Troyer A. The behaviour of the abdominal muscles during inspiratory mechanical loading. Respir Physiol. (1982) 50:63–73. doi: 10.1016/0034-5687(82)90007-X
13. De Troyer A, Sampson M, Sigrist S, Macklem PT. The diaphragm: two muscles. Science. (1981) 213:237–8. doi: 10.1126/science.7244632
14. Sharshar T, Hopkinson NS, Ross ET, Jonville S, Dayer MJ, Nickol AH, et al. Motor control of the costal and crural diaphragm–insights from transcranial magnetic stimulation in man. Respir Physiol Neurobiol. (2005) 146:5–19. doi: 10.1016/j.resp.2004.10.010
15. Tokizane T, Kawamata K, Tokizane H. Electromyographic studies on the human respiratory muscles; studies on the activity pattern of neuromuscular units. Jpn J Physiol. (1952) 2:232–47. doi: 10.2170/jjphysiol.2.232
16. Rossier PH, Nieporent HJ, Pipberger H, Kalin R. [Electromyographical studies of respiratory muscle function in normal test persons]. Z Gesamte Exp Med. (1956) 127:39–52. doi: 10.1007/BF02053581
17. Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibres. J Physiol. (1929) 67:9–151. doi: 10.1113/jphysiol.1929.sp002557
18. Dittler R. Über die Innervation des zwerchfelles als beispiel einer tonischen innervation. Arch Ges Physiol Menschen Tiere. (1909) 130:400–43. doi: 10.1007/BF01686342
19. Dittler R. Über die Aktionsströme des Nervus phrenicus bei natürlicher Innervation. Arch Ges Physiol Menschen Tiere. (1910) 131:581–8. doi: 10.1007/BF01679856
20. Dittler R, Garten S. Die zeitliche folge der aktionsströme in phrenicus und zwerchfell bei der natürlichen innervation. Zeitsch Biol. (1912) 58:420–50.
21. Duffin J. A model of respiratory rhythm generation. Neuroreport. (1991) 2:623–6. doi: 10.1097/00001756-199110000-00018
22. Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. (1992) 2:788–93. doi: 10.1016/0959-4388(92)90135-8
23. Berger AJ, Dick TE, Jodkowski JS, Viana F. Phrenic motoneurons: decscending inputs, electrical properties and recruitment. In: Lahiri S, Forster RE, Davies RO, Pack AI, editors. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. Oxford: Oxford University Press (1989). p. 343–50.
24. Hodges PW, Gandevia SC. Pitfalls of intramuscular electromyographic recordings from the human costal diaphragm. Clin Neurophysiol. (2000) 111:1420–4. doi: 10.1016/S1388-2457(00)00341-2
25. Tusiewicz K, Moldofsky H, Bryan AC, Bryan MH. Mechanics of the rib cage and diaphragm during sleep. J Appl Physiol. (1977) 43:600–2. doi: 10.1152/jappl.1977.43.4.600
26. Prechtl HF, van Eykern LA, O'Brien MJ. Respiratory muscle EMG in newborns: a non-intrusive method. Early Hum Dev. (1977) 1:265–83. doi: 10.1016/0378-3782(77)90040-8
27. Lansing R, Savelle J. Chest surface recording of diaphragm potentials in man. Electroencephalogr Clin Neurophysiol. (1989) 72:59–68. doi: 10.1016/0013-4694(89)90031-X
28. Lin L, Guan L, Wu W, Chen R. Correlation of surface respiratory electromyography with esophageal diaphragm electromyography. Respir Physiol Neurobiol. (2019) 259:45–52. doi: 10.1016/j.resp.2018.07.004
29. Petit JM, Milic-Emili G, Delhez L. [Examination of the electrical activity of the diaphragm by the esophageal approach in the normal man]. J Physiol. (1960) 52:190–1.
30. Hixon TJ, Siebens AA, Minifie FD. An EMG electrode for the diaphragm. J Acoust Soc Am. (1969) 46:1588–90. doi: 10.1121/1.1911912
31. Gandevia SC, McKenzie DK. Human diaphragmatic EMG: changes with lung volume and posture during supramaximal phrenic stimulation. J Appl Physiol. (1986) 60:1420–8. doi: 10.1152/jappl.1986.60.4.1420
32. Luo YM, Polkey MI, Johnson LC, Lyall RA, Harris ML, Green M, et al. Diaphragm EMG measured by cervical magnetic and electrical phrenic nerve stimulation. J Appl Physiol. (1998) 85:2089–99. doi: 10.1152/jappl.1998.85.6.2089
33. Macklem PT, Roussos C, Gross D, Ladd H, Riley E, Grassino A, et al. Diaphragmatic fatigue [proceedings]. Am Rev Respir Dis. (1979) 119(2 Pt 2):93.
34. Repko KD, Greene JG, Enderle JD. A comparison of esophageal and chest electrodes in studying the diaphragmatic EMG. Biomed Sci Instrum. (1989) 25:89–91.
35. Sharp JT, Hammond MD, Aranda AU, Rocha RD. Comparison of diaphragm EMG centroid frequencies: esophageal versus chest surface leads. Am Rev Respir Dis. (1993) 147:764–7. doi: 10.1164/ajrccm/147.3.764
36. Sinderby C, Friberg S, Comtois N, Grassino A. Chest wall muscle cross talk in canine costal diaphragm electromyogram. J Appl Physiol. (1996) 81:2312–27. doi: 10.1152/jappl.1996.81.5.2312
37. Ramsook AH, Mitchell RA, Bell T, Calli S, Kennedy C, Lehmann J, et al. Is parasternal intercostal EMG an accurate surrogate of respiratory neural drive and biomarker of dyspnea during cycle exercise testing? Respir Physiol Neurobiol. (2017) 242:40–4. doi: 10.1016/j.resp.2017.03.003
38. Hudson AL, Butler JE. Assessment of 'neural respiratory drive' from the parasternal intercostal muscles. Respir Physiol Neurobiol. (2018) 252–253:16–7. doi: 10.1016/j.resp.2017.11.003
39. Jolley CJ, Luo YM, Steier J, Reilly C, Seymour J, Lunt A, et al. Neural respiratory drive in healthy subjects and in COPD. Eur Respir J. (2009) 33:289–97. doi: 10.1183/09031936.00093408
40. Petit JM, Milic-Emili G, Delhez L. Role of the diaphragm in breathing in conscious normal man: an electromyographic study. J Appl Physiol. (1960) 15:1101–6. doi: 10.1152/jappl.1960.15.6.1101
41. Agostoni E, Sant'Ambrogio G, Del Portillo Carrasco H. Electromyography of the diaphragm in man and transdiaphragmatic pressure. J Appl Physiol. (1960) 15:1093–7. doi: 10.1152/jappl.1960.15.6.1093
42. Beck J, Sinderby C, Weinberg J, Grassino A. Effects of muscle-to-electrode distance on the human diaphragm electromyogram. J Appl Physiol. (1995) 79:975–85. doi: 10.1152/jappl.1995.79.3.975
43. Beck J, Sinderby C, Lindstrom L, Grassino A. Influence of bipolar esophageal electrode positioning on measurements of human crural diaphragm electromyogram. J Appl Physiol. (1996) 81:1434–49. doi: 10.1152/jappl.1996.81.3.1434
44. Luo YM, Lyall RA, Harris ML, Hawkins P, Hart N, Polkey MI, et al. Effect of lung volume on the oesophageal diaphragm EMG assessed by magnetic phrenic nerve stimulation. Eur Respir J. (2000) 15:1033–8. doi: 10.1034/j.1399-3003.2000.01510.x
45. Luo YM, Moxham J, Polkey MI. Diaphragm electromyography using an oesophageal catheter: current concepts. Clin Sci. (2008) 115:233–44. doi: 10.1042/CS20070348
46. Tagliabue G, Ji M, Suneby Jagers JV, Zuege DJ, Kortbeek JB, Easton PA. Distinct neural-mechanical efficiency of costal and crural diaphragm during hypercapnia. Respir Physiol Neurobiol. (2019) 268:103247. doi: 10.1016/j.resp.2019.06.004
47. Ikegami T, Ji M, Fujimura N, Suneby Jagers JV, Kieser TM, Easton PA. Costal and crural diaphragm function during sustained hypoxia in awake canines. J Appl Physiol. (2019) 126:1117–28. doi: 10.1152/japplphysiol.00242.2018
48. Nguyen DAT, Amirjani N, McCaughey EJ, Gandevia SC, Butler JE, Hudson AL. Differential activation of the human costal and crural diaphragm during voluntary and involuntary breaths. J Appl Physiol. (2020) 128:1262–70. doi: 10.1152/japplphysiol.00790.2019
49. Reilly CC, Ward K, Jolley CJ, Lunt AC, Steier J, Elston C, et al. Neural respiratory drive, pulmonary mechanics and breathlessness in patients with cystic fibrosis. Thorax. (2011) 66:240–6. doi: 10.1136/thx.2010.142646
50. Wu W, Guan L, Li X, Lin L, Guo B, Yang Y, et al. Correlation and compatibility between surface respiratory electromyography and transesophageal diaphragmatic electromyography measurements during treadmill exercise in stable patients with COPD. Int J Chron Obstruct Pulmon Dis. (2017) 12:3273–80. doi: 10.2147/COPD.S148980
51. Rodrigues A, Louvaris Z, Dacha S, Janssens W, Pitta F, Vogiatizis I, et al. Differences in respiratory muscle responses to hyperpnea or loaded breathing in COPD. Med Sci Sports Exerc. (2019) 52:1126–34. doi: 10.1249/MSS.0000000000002222
52. Nguyen DAT, Amirjani N, McCaughey EJ, Gandevia SC, Butler JE, Hudson AL. Electromyographic activity in the human costal and crural diaphragm during voluntary and involuntary breaths. In:16th International Conference of Sleep and Breathing. Tampere (2019).
53. Amirjani N, Hudson AL, Butler JE, Gandevia SC. Comparison of EMG in teh costal and crural diaphragm with increments in tidal volume. In: American Thoracic Society. Denver (2011). doi: 10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A5287
54. Milne KM, Domnik NJ, Phillips DB, James MD, Vincent SG, Neder JA. Evaluation of dynamic respiratory mechanical abnormalities during conventional CPET. Front Med. (2020). doi: 10.3760/cma.j.issn.0366-6999.2012.20.006
55. Faisal A, Alghamdi BJ, Ciavaglia CE, Elbehairy AF, Webb KA, Ora J, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med. (2016) 193:299–309. doi: 10.1164/rccm.201504-0841OC
56. Ora J, Laveneziana P, Wadell K, Preston M, Webb KA, O'Donnell DE. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol. (2011) 111:10–9. doi: 10.1152/japplphysiol.01131.2010
57. Luo YM, Li RF, Jolley C, Wu HD, Steier J, Moxham J, et al. Neural respiratory drive in patients with COPD during exercise tests. Respiration. (2011) 81:294–301. doi: 10.1159/000317136
58. Hamnegard CH, Polkey MI, Kyroussis D, Mills GH, Green M, Bake B, et al. Maximum rate of change in oesophageal pressure assessed from unoccluded breaths: an option where mouth occlusion pressure is impractical. Eur Respir J. (1998) 12:693–7. doi: 10.1183/09031936.98.12030693
59. Polkey MI, Hamnegard CH, Hughes PD, Rafferty GF, Green M, Moxham J. Influence of acute lung volume change on contractile properties of human diaphragm. J Appl Physiol. (1998) 85:1322–8. doi: 10.1152/jappl.1998.85.4.1322
60. Ninane V, Rypens F, Yernault JC, De Troyer A. Abdominal muscle use during breathing in patients with chronic airflow obstruction. Am Rev Respir Dis. (1992) 146:16–21. doi: 10.1164/ajrccm/146.1.16
61. Xiao SC, Lu YR, Guo HX, Qiu ZH, Luo YM. Effect of expiratory load on neural inspiratory drive. Chin Med J. (2012) 125:3629–34.
62. Jolley CJ, Luo YM, Steier J, Rafferty GF, Polkey MI, Moxham J. Neural respiratory drive and breathlessness in COPD. Eur Respir J. (2015) 45:355–64. doi: 10.1183/09031936.00063014
63. Dacha S, Janssens L, Rodrigues A, Louvaris Z, Janssens L, Gosselink R, et al. Comparison between manual and (semi-)automated analyses of esophageal diaphragm electromyography during endurance cycling in patients with COPD. Front Physiol. (2019) 10:885. doi: 10.3389/fphys.2019.00885
64. Halaki M, Ginn K. Normalization of EMG signals: to normalize or not to normalize and what to normalize to? In: Naik GR, editor. Computational Intelligence in Electromyography Analysis: A Perspective on Current Applications and Future Challenges. London: IntechOpen Ltd. (2012). doi: 10.5772/49957
65. Sinderby C, Beck J, Spahija J, Weinberg J, Grassino A. Voluntary activation of the human diaphragm in health and disease. J Appl Physiol. (1998) 85:2146–58. doi: 10.1152/jappl.1998.85.6.2146
66. Ramsook AH, Schaeffer MR, Syed N, Wilkie SS, Guenette JA. Which respiratory maneuvers generate the greatest electromyography activity of the inspiratory muscles? Am J Respir Crit Care Med. (2015) 191:A2682.
67. Mendonca CT, Schaeffer MR, Riley P, Jensen D. Physiological mechanisms of dyspnea during exercise with external thoracic restriction: role of increased neural respiratory drive. J Appl Physiol. (2014) 116:570–81. doi: 10.1152/japplphysiol.00950.2013
68. Singh B, Panizza JA, Finucane KE. Diaphragm electromyogram root mean square response to hypercapnia and its intersubject and day-to-day variation. J Appl Physiol. (2005) 98:274–81. doi: 10.1152/japplphysiol.01380.2003
69. Zhang D, Gong H, Lu G, Guo H, Li R, Zhong N, et al. Respiratory motor output during an inspiratory capacity maneuver is preserved despite submaximal exercise. Respir Physiol Neurobiol. (2013) 189:87–92. doi: 10.1016/j.resp.2013.07.008
70. Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. (2009) 64:719–25. doi: 10.1136/thx.2008.109728
71. O'Donnell DE, Milne KM, Vincent SG, Neder JA. Unraveling the causes of unexplained dyspnea: the value of exercise testing. Clin Chest Med. (2019) 40:471–99. doi: 10.1016/j.ccm.2019.02.014
72. Sinderby C, Spahija J, Beck J. Changes in respiratory effort sensation over time are linked to the frequency content of diaphragm electrical activity. Am J Respir Crit Care Med. (2001) 163:905–10. doi: 10.1164/ajrccm.163.4.2005121
73. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. (2012) 185:435–52. doi: 10.1164/rccm.201111-2042ST
74. Wu W, Guan L, Zhang X, Li X, Yang Y, Guo B, et al. Effects of two types of equal-intensity inspiratory muscle training in stable patients with chronic obstructive pulmonary disease: a randomised controlled trial. Respir Med. (2017) 132:84–91. doi: 10.1016/j.rmed.2017.10.001
75. O'Donnell DE, Milne KM, James MD, de Torres JP, Neder JA. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. (2019) 37:41–60. doi: 10.1007/s12325-019-01128-9
76. Jensen D, Ofir D, O'Donnell DE. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir Physiol Neurobiol. (2009) 167:87–100. doi: 10.1016/j.resp.2009.01.011
77. Veldhuizen RAW, McCaig LA, Pape C, Gill SE. The effects of aging and exercise on lung mechanics, surfactant and alveolar macrophages. Exp Lung Res. (2019) 45:113–22. doi: 10.1080/01902148.2019.1605633
78. Estenne M, Yernault JC, De Troyer A. Rib cage and diaphragm-abdomen compliance in humans: effects of age and posture. J Appl Physiol. (1985) 59:1842–8. doi: 10.1152/jappl.1985.59.6.1842
79. Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. (1969) 99:696–702.
80. Faisal A, Webb KA, Guenette JA, Jensen D, Neder JA, O'Donnell DE. Effect of age-related ventilatory inefficiency on respiratory sensation during exercise. Respir Physiol Neurobiol. (2015) 205:129–39. doi: 10.1016/j.resp.2014.10.017
81. Fogarty MJ, Omar TS, Zhan WZ, Mantilla CB, Sieck GC. Phrenic motor neuron loss in aged rats. J Neurophysiol. (2018) 119:1852–62. doi: 10.1152/jn.00868.2017
82. Newell SZ, McKenzie DK, Gandevia SC. Inspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitation. Thorax. (1989) 44:903–12. doi: 10.1136/thx.44.11.903
83. Allen GM, McKenzie DK, Gandevia SC, Bass S. Reduced voluntary drive to breathe in asthmatic subjects. Respir Physiol. (1993) 93:29–40. doi: 10.1016/0034-5687(93)90065-I
84. Nguyen DAT, Lewis RHC, Gandevia SC, Butler JE, Hudson AL. Discharge properties of human diaphragm motor units with ageing. J Physiol. (2019) 597:5079–92. doi: 10.1113/JP278498
85. Harms CA, Rosenkranz S. Sex differences in pulmonary function during exercise. Med Sci Sports Exerc. (2008) 40:664–8. doi: 10.1249/MSS.0b013e3181621325
86. Guenette JA, Sheel AW. Exercise-induced arterial hypoxaemia in active young women. Appl Physiol Nutr Metab. (2007) 32:1263–73. doi: 10.1139/H07-122
87. Mitchell RA, Schaeffer MR, Ramsook AH, Wilkie SS, Guenette JA. Sex differences in respiratory muscle activation patterns during high-intensity exercise in healthy humans. Respir Physiol Neurobiol. (2018) 247:57–60. doi: 10.1016/j.resp.2017.09.002
88. Schaeffer MR, Mendonca CT, Levangie MC, Andersen RE, Taivassalo T, Jensen D. Physiological mechanisms of sex differences in exertional dyspnoea: role of neural respiratory motor drive. Exp Physiol. (2014) 99:427–41. doi: 10.1113/expphysiol.2013.074880
89. Qin YY, Steier J, Jolley C, Moxham J, Zhong NS, Luo YM. Efficiency of neural drive during exercise in patients with COPD and healthy subjects. Chest. (2010) 138:1309–15. doi: 10.1378/chest.09-2824
90. Sinderby C, Spahija J, Beck J, Kaminski D, Yan S, Comtois N, et al. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2001) 163:1637–41. doi: 10.1164/ajrccm.163.7.2007033
91. Ferguson GT. Effects of oxygenation and hypercapnia on diaphragmatic function and central drive during respiratory failure. J Appl Physiol. (1995) 78:1764–71. doi: 10.1152/jappl.1995.78.5.1764
92. Ferguson GT. Respiratory failure due to altered central drive during inspiratory loading in rabbits. Respir Physiol. (1995) 99:75–87. doi: 10.1016/0034-5687(94)00073-9
93. Wagner PD, Laravuso RB, Uhl RR, West JB. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. J Clin Invest. (1974) 54:54–68. doi: 10.1172/JCI107750
94. Mummery HJ, Stolp BW, de LDG, Doar PO, Natoli MJ, Boso AE, et al. Effects of age and exercise on physiological dead space during simulated dives at 2.8 ATA. J Appl Physiol. (2003) 94:507–17. doi: 10.1152/japplphysiol.00367.2002
95. Habedank D, Reindl I, Vietzke G, Bauer U, Sperfeld A, Glaser S, et al. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur J Appl Physiol Occup Physiol. (1998) 77:421–6. doi: 10.1007/s004210050354
96. Neder JA, Nery LE, Peres C, Whipp BJ. Reference values for dynamic responses to incremental cycle ergometry in males and females aged 20 to 80. Am J Respir Crit Care Med. (2001) 164(8 Pt 1):1481–6. doi: 10.1164/ajrccm.164.8.2103007
97. Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. (2002) 166:1443–8. doi: 10.1164/rccm.2202033
98. Leblanc P, Ruff F, Milic-Emili J. Effects of age and body position on “airway closure” in man. J Appl Physiol. (1970) 28:448–51. doi: 10.1152/jappl.1970.28.4.448
99. Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol. (2008) 104:1583–93. doi: 10.1152/japplphysiol.00079.2008
100. Harms CA. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol. (2006) 151:124–31. doi: 10.1016/j.resp.2005.10.010
101. Kilbride E, McLoughlin P, Gallagher CG, Harty HR. Do gender differences exist in the ventilatory response to progressive exercise in males and females of average fitness? Eur J Appl Physiol. (2003) 89:595–602. doi: 10.1007/s00421-003-0853-z
102. Schaeffer MR, Ryerson CJ, Ramsook AH, Molgat-Seon Y, Wilkie SS, Dhillon SS, et al. Neurophysiological mechanisms of exertional dyspnoea in fibrotic interstitial lung disease. Eur Respir J. (2018) 51:1701726. doi: 10.1183/13993003.01726-2017
103. Reilly CC, Jolley CJ, Ward K, MacBean V, Moxham J, Rafferty GF. Neural respiratory drive measured during inspiratory threshold loading and acute hypercapnia in healthy individuals. Exp Physiol. (2013) 98:1190–8. doi: 10.1113/expphysiol.2012.071415
104. James MD, Milne KM, Phillips DB, Neder JA, O'Donnell DE. Dyspnea and Exercise Limitation in Mild COPD: The Value of CPET. Front Med. (2020). doi: 10.3389/fmed.2020.00442
105. Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (1996) 154:1310–7. doi: 10.1164/ajrccm.154.5.8912741
106. Sprikkelman AB, Van Eykern LA, Lourens MS, Heymans HS, Van Aalderen WM. Respiratory muscle activity in the assessment of bronchial responsiveness in asthmatic children. J Appl Physiol. (1998) 84:897–901. doi: 10.1152/jappl.1998.84.3.897
107. Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest. (1977) 59:203–16. doi: 10.1172/JCI108630
108. Elbehairy AF, Ciavaglia CE, Webb KA, Guenette JA, Jensen D, Mourad SM, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. (2015) 191:1384–94. doi: 10.1164/rccm.201501-0157OC
109. Jensen D, O'Donnell DE, Li R, Luo YM. Effects of dead space loading on neuro-muscular and neuro-ventilatory coupling of the respiratory system during exercise in healthy adults: implications for dyspnea and exercise tolerance. RespirPhysiol Neurobiol. (2011) 179:219–26. doi: 10.1016/j.resp.2011.08.009
110. Luo YM, Moxham J. Measurement of neural respiratory drive in patients with COPD. Respir Physiol Neurobiol. (2005) 146:165–74. doi: 10.1016/j.resp.2004.12.014
111. Lourenco RV, Miranda JM. Drive and performance of the ventilatory apparatus in chronic obstructive lung disease. N Engl J Med. (1968) 279:53–9. doi: 10.1056/NEJM196807112790201
112. Gorini M, Spinelli A, Ginanni R, Duranti R, Gigliotti F, Scano G. Neural respiratory drive and neuromuscular coupling in patients with chronic obstructive pulmonary disease (COPD). Chest. (1990) 98:1179–86. doi: 10.1378/chest.98.5.1179
113. Roussos C, Moxham J. Respiratory muscle fatigue. In: Roussos C, Macklem PT, editors. The Thorax (Part B). New York, NY: Marcel Dekker (1985). p. 829–70.
114. Sorli J, Grassino A, Lorange G, Milic-Emili J. Control of breathing in patients with chronic obstructive lung disease. Clin Sci Mol Med. (1978) 54:295–304. doi: 10.1042/cs0540295
115. Lee KD, Bishop JM. The reflex hypoxic respiratory drive in patients with chronic bronchitis. Clin Sci Mol Med. (1974) 46:347–56. doi: 10.1042/cs0460347
116. Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol. (1995) 78:82–92. doi: 10.1152/jappl.1995.78.1.82
117. O'Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol. (2009) 167:116–32. doi: 10.1016/j.resp.2009.01.010
118. O'Donnell DE, D'Arsigny C, Fitzpatrick M, Webb KA. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: the role of lung hyperinflation. Am J Respir Crit Care Med. (2002) 166:663–8. doi: 10.1164/rccm.2201003
119. Casaburi R, Rennard SI. Exercise limitation in chronic obstructive pulmonary disease. The O'Donnell threshold. Am J Respir Crit Care Med. (2015) 191:873–5. doi: 10.1164/rccm.201501-0084ED
120. Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortes-Telles A, et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. (2013) 187:1315–23. doi: 10.1164/rccm.201211-1970OC
121. O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. (1993) 148:1351–7. doi: 10.1164/ajrccm/148.5.1351
122. Cassart M, Pettiaux N, Gevenois PA, Paiva M, Estenne M. Effect of chronic hyperinflation on diaphragm length and surface area. Am J Respir Crit Care Med. (1997) 156(2 Pt 1):504–8. doi: 10.1164/ajrccm.156.2.9612089
123. Gauthier AP, Verbanck S, Estenne M, Segebarth C, Macklem PT, Paiva M. Three-dimensional reconstruction of the in vivo human diaphragm shape at different lung volumes. J Appl Physiol. (1994) 76:495–506. doi: 10.1152/jappl.1994.76.2.495
124. Agostoni E, Fenn WO. Velocity of muscle shortening as a limiting factor in respiratory air flow. J Appl Physiol. (1960) 15:349–53. doi: 10.1152/jappl.1960.15.3.349
125. Hyatt RE, Flath RE. Relationship of air flow to pressure during maximal respiratory effort in man. J Appl Physiol. (1966) 21:477–82. doi: 10.1152/jappl.1966.21.2.477
126. Olin JT, Clary MS, Fan EM, Johnston KL, State CM, Strand M, et al. Continuous laryngoscopy quantitates laryngeal behaviour in exercise and recovery. Eur Respir J. (2016) 48:1192–200. doi: 10.1183/13993003.00160-2016
127. Walsted ES, Faisal A, Jolley CJ, Swanton LL, Pavitt MJ, Luo YM, et al. Increased respiratory neural drive and work of breathing in exercise-induced laryngeal obstruction. J Appl Physiol. (2018) 124:356–63. doi: 10.1152/japplphysiol.00691.2017
128. Baz M, Haji GS, Menzies-Gow A, Tanner RJ, Hopkinson NS, Polkey MI, et al. Dynamic laryngeal narrowing during exercise: a mechanism for generating intrinsic PEEP in COPD? Thorax. (2015) 70:251–7. doi: 10.1136/thoraxjnl-2014-205940
129. Elbehairy AF, Guenette JA, Faisal A, Ciavaglia CE, Webb KA, Jensen D, et al. Mechanisms of exertional dyspnoea in symptomatic smokers without COPD. Eur Respir J. (2016) 48:694–705. doi: 10.1183/13993003.00077-2016
130. Elbehairy AF, O'Donnell CD, Abd Elhameed A, Vincent SG, Milne KM, James MD, et al. Low resting diffusion capacity, dyspnea and exercise intolerance in COPD. J Appl Physiol. (2019) 127:1107–16. doi: 10.1152/japplphysiol.00341.2019
131. Qin YY, Li RF, Wu GF, Zhu Z, Liu J, Zhou CZ, et al. Effect of tiotropium on neural respiratory drive during exercise in severe COPD. Pulm Pharmacol Ther. (2015) 30:51–6. doi: 10.1016/j.pupt.2014.11.003
132. O'Donnell DE, Hamilton AL, Webb KA. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol. (2006) 101:1025–35. doi: 10.1152/japplphysiol.01470.2005
133. DeWeese EL, Sullivan TY, Yu PL. Ventilatory and occlusion pressure responses to helium breathing. J Appl Physiol. (1983) 54:1525–31. doi: 10.1152/jappl.1983.54.6.1525
134. Hussain SN, Pardy RL, Dempsey JA. Mechanical impedance as determinant of inspiratory neural drive during exercise in humans. J Appl Physiol. (1985) 59:365–75. doi: 10.1152/jappl.1985.59.2.365
135. Schaeffer MR, Ryerson CJ, Ramsook AH, Molgat-Seon Y, Wilkie SS, Dhillon SS, et al. Effects of hyperoxia on dyspnoea and exercise endurance in fibrotic interstitial lung disease. Eur Respir J. (2017) 49:1602494. doi: 10.1183/13993003.02494-2016
136. Abdallah SJ, Wilkinson-Maitland C, Saad N, Li PZ, Smith BM, Bourbeau J, et al. Effect of morphine on breathlessness and exercise endurance in advanced COPD: a randomised crossover study. Eur Respir J. (2017) 50:1701235. doi: 10.1183/13993003.01235-2017
137. Langer D, Ciavaglia CE, Faisal A, Webb KA, Neder JA, Gosselink R, et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J Appl Physiol. (2018) 125:381–92. doi: 10.1152/japplphysiol.01078.2017
138. Ramsook AH, Molgat-Seon Y, Schaeffer MR, Wilkie SS, Camp PG, Reid WD, et al. Effects of inspiratory muscle training on respiratory muscle electromyography and dyspnea during exercise in healthy men. J Appl Physiol. (2017) 122:1267–75. doi: 10.1152/japplphysiol.00046.2017
139. Wu W, Zhang X, Lin L, Ou Y, Li X, Guan L, et al. Transdiaphragmatic pressure and neural respiratory drive measured during inspiratory muscle training in stable patients with chronic obstructive pulmonary disease. Int J Chron Obst Pulm Dis. (2017) 12:773–81. doi: 10.2147/COPD.S126354
140. Ramsook AH, Koo R, Molgat-Seon Y, Dominelli PB, Syed N, Ryerson CJ, et al. Diaphragm recruitment increases during a bout of targeted inspiratory muscle training. Med Sci Sports Exerc. (2016) 48:1179–86. doi: 10.1249/MSS.0000000000000881
141. Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest. (1992) 101:75–8. doi: 10.1378/chest.101.1.75
142. Langer D, Ciavaglia CE, Neder JA, Webb KA, O'Donnell DE. Lung hyperinflation in chronic obstructive pulmonary disease: mechanisms, clinical implications and treatment. Exp Rev Respir Med. (2014) 8:731–49. doi: 10.1586/17476348.2014.949676
143. Luo YM, Tang J, Jolley C, Steier J, Zhong NS, Moxham J, et al. Distinguishing obstructive from central sleep apnea events: diaphragm electromyogram and esophageal pressure compared. Chest. (2009) 135:1133–41. doi: 10.1378/chest.08-1695
144. Luo YM, He BT, Wu YX, Yuan H, Xu J, Moxham J, et al. Neural respiratory drive and ventilation in patients with chronic obstructive pulmonary disease during sleep. Am J Respir Crit Care Med. (2014) 190:227–9. doi: 10.1164/rccm.201402-0302LE
145. Domnik NJ, James MD, Scheeren RE, Ayoo GA, Taylor SM, Di Luch AT, et al. Deterioration of nighttime respiratory mechanics in chronic obstructive pulmonary disease: impact of bronchodilator therapy. Chest. (2020). doi: 10.1016/j.chest.2020.06.033
146. Murphy PB, Kumar A, Reilly C, Jolley C, Walterspacher S, Fedele F, et al. Neural respiratory drive as a physiological biomarker to monitor change during acute exacerbations of COPD. Thorax. (2011) 66:602–8. doi: 10.1136/thx.2010.151332
147. Maarsingh EJ, van Eykern LA, Sprikkelman AB, Hoekstra MO, van Aalderen WM. Respiratory muscle activity measured with a noninvasive EMG technique: technical aspects and reproducibility. J Appl Physiol. (2000) 88:1955–61. doi: 10.1152/jappl.2000.88.6.1955
148. Lozano-Garcia M, Sarlabous L, Moxham J, Rafferty GF, Torres A, Jolley CJ, et al. Assessment of inspiratory muscle activation using surface diaphragm mechanomyography and crural diaphragm electromyography. Conf Proc IEEE Eng Med Biol Soc. (2018) 2018:3342–5. doi: 10.1109/EMBC.2018.8513046
149. Estrada L, Torres A, Sarlabous L, Jane R. Improvement in neural respiratory drive estimation from diaphragm electromyographic signals using fixed sample entropy. IEEE J Biomed Health Inform. (2016) 20:476–85. doi: 10.1109/JBHI.2015.2398934
150. Karagiannidis C, Strassmann S, Schwarz S, Merten M, Fan E, Beck J, et al. Control of respiratory drive by extracorporeal CO2 removal in acute exacerbation of COPD breathing on non-invasive NAVA. Crit Care. (2019) 23:135. doi: 10.1186/s13054-019-2404-y
151. Beck J, Emeriaud G, Liu Y, Sinderby C. Neurally-adjusted ventilatory assist (NAVA) in children: a systematic review. Minerva Anestesiol. (2016) 82:874–83. doi: 10.1007/978-3-319-21653-9_15
152. Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. (1999) 5:1433–6. doi: 10.1038/71012i
Keywords: inspiratory neural drive, CPET cardiopulmonary exercise testing, diaphragmatic electromyogram EMGdi, respiratory muscles, respiratory disease (RD), chronic obstructive pulmonary disease, diaphragm
Citation: Domnik NJ, Walsted ES and Langer D (2020) Clinical Utility of Measuring Inspiratory Neural Drive During Cardiopulmonary Exercise Testing (CPET). Front. Med. 7:483. doi: 10.3389/fmed.2020.00483
Received: 16 April 2020; Accepted: 16 July 2020;
Published: 18 September 2020.
Edited by:
Pierantonio Laveneziana, INSERM U1158 Neurophysiologie Respiratoire Expérimentale et Clinique, FranceReviewed by:
Jordan A. Guenette, University of British Columbia, CanadaCaroline Jolley, King's College London, United Kingdom
Copyright © 2020 Domnik, Walsted and Langer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolle J. Domnik, bi5qLmRvbW5pa0BxdWVlbnN1LmNh
 Nicolle J. Domnik
Nicolle J. Domnik Emil S. Walsted2
Emil S. Walsted2 Daniel Langer
Daniel Langer