- 1Department of Agricultural Sciences, University of Naples Federico II, Portici, Italy
- 2Department of Veterinary Medicine and Animal Production, University of Naples Federico II, Naples, Italy
Probiotics are considered as the twenty-first century panpharmacon due to their competent remedial power to cure from gastrointestinal dysbiosis, systematic metabolic diseases, and genetic impairments up to complicated neurodegenerative disorders. They paved the way for an innovative managing of various severe diseases through palatable food products. The probiotics’ role as a “bio-therapy” increased their significance in food and medicine due to many competitive advantages over traditional treatment therapies. Their prophylactic and therapeutic potential has been assessed through hundreds of preclinical and clinical studies. In addition, the food industry employs probiotics as functional and nutraceutical ingredients to enhance the added value of food product in terms of increased health benefits. However, regardless of promising health-boosting effects, the probiotics’ efficacy still needs an in-depth understanding of systematic mechanisms and factors supporting the healthy actions.
Introduction
The probiotic concept was first introduced by the Nobel Prize, Ilya Ilyich Mechnikov in 1908 during his study about yogurt-derived health-boosting effects on Bulgarian peasants (Kareb and Aïder, 2019). The word probiotic originates from the Greek word “pro-bios,” which means “for life.” In 2001, the Food and Agriculture Organization of the United Nations (FAO) and the Health Organization (WHO) provided a collaborative and comprehensive probiotics’ definition as “Live microorganisms, which when administered in adequate amounts; exert a beneficial effect on the host’s health.” However, a slight modification in probiotics definition is accomplished by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as “Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). Probiotics mostly belong to Gram-positive bacteria, having natural habitat in the human’s gut. Probiotics may alleviate gastrointestinal dysbiosis, lower serum cholesterol, ameliorate cancer, and prevent allergic and autoimmune disorders (Hajavi et al., 2019).
Lactobacillus, Bifidobacterium, Streptococcus, Saccharomyces, Bacillus, and Enterococcus are the most popular probiotics genus sources, and their application as probiotics are extensive in fermented food products, non-fermented food items, as well as functional and nutraceutical dietary supplements (Di Cerbo et al., 2016). Because of the various health claims, probiotics-based food currently occupies a considerable number of market shelves. As a matter of fact, nearly 2,000 clinical research studies regarding various prophylactic and therapeutic health benefits related to probiotics have been currently published. In addition, outstanding pace was observed toward exploring various molecular mechanisms by which probiotics interact with host and exert their impact on the target cells. However, there is still a gap when translating the several recognized probiotics’ health benefits mechanisms into target therapies with specific probiotics (Kleerebezem et al., 2019).
The probiotics’ efficiency may be compromised by several factors: an adequate delivery system is one of them. Food processing and storage conditions (water activity, temperature, pH, oxidation, osmotic pressure, etc.), coupled with the survival through the hostile gastrointestinal tract (GIT) environment (acidic pH, bile salts, digestive enzymes, etc.) and the ability to colonize the gut, are the major challenges (Li et al., 2019a). However, microencapsulation seems to be a highly productive and holistic approach to overcome this problem (Feng et al., 2020).
Probiotics functionality is mainly based on bacterial interaction with host gut microbiota through a number of actions including (Sharma, 2019):
• enhancing the intestinal barrier function by regulating proinflammatory cytokines and chemokines’ profile;
• improving the intestinal barrier selectivity through higher mucin, immunoglobulin A (IgA), and defensins production;
• increasing the production of vitamins, minerals, short-chain fatty acids (SCFAs), and growth regulators to strengthen the intestinal epithelium layer;
• promoting antiangiogenic factors, cytokines (IL-2 and IL-12), and antioxidants;
• reducing the intestinal pH;
• increasing anti-inflammatory molecules formation to enhance the immune system;
• modifying the intestinal microbiota;
• regulating mechanisms of apoptosis and cell differentiation;
• retarding tyrosine kinase and other deleterious pathways.
Recent preclinical and clinical trials highlighted the significance of probiotics’ intake in the prevention and treatment of gastrointestinal diseases [salmonellosis, traveler’s diarrhea, antibiotic-associated diarrhea (AAD), acute diarrhea, Helicobacter pylori gastroenteritis, irritable bowel syndrome (IBS), inflammatory bowel diseases (IBDs), hyperlipidemia and hypercholesterolemia (Kim et al., 2017), cancer (Vivarelli et al., 2019), lactose intolerance (LI) (Vitellio et al., 2019), autoimmune diseases (Dwivedi et al., 2016), as well as bone (Nath et al., 2018), neurodegenerative (Li W. et al., 2020), and metabolic disorders (León et al., 2019)].
Health-Boosting Spectrum of Probiotics
Lactose Intolerance
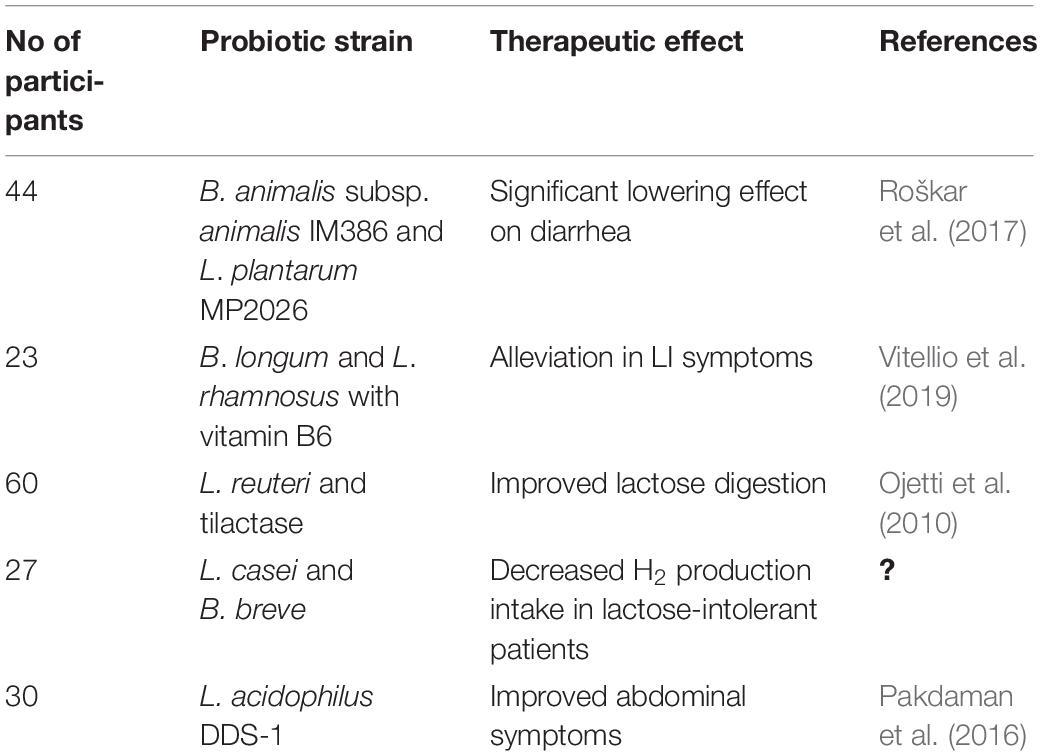
Lactose intolerance is a genetically determined β-galactosidase deficiency resulting in the inability to hydrolyze lactose into the monosaccharides glucose and galactose. Unabsorbed lactose is then metabolized by colonic bacteria (Oak and Jha, 2019). Lactase activity is age dependent, as it declines with age. Symptoms of LI include diarrhea, abdominal discomfort, and flatulence after consumption of milk and dairy products (Syngai et al., 2016). The consumption of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus in yogurt proved to be helpful in alleviating LI, partly because of the higher beta-galactosidase activity (Kechagia et al., 2013). However, a number of studies demonstrated that milk containing Bifidobacterium longum and Lactobacillus acidophilus can be easily tolerated by LI individuals (Vonk et al., 2012). Gingold-Belfer et al. (2020) carried out a pilot study to assess the efficacy of probiotics with β-galactosidase activity on symptoms of lactose malabsorption by means of hydrogen breadth test (LHBT). Eight female patients with positive LHBT were included in the study and treated for 6 weeks with probiotics. According to outcomes, in more than 50% of cases, symptoms’ severity or frequency decreased, as even revealed by the LBHT normalization. Furthermore, the use of a novel formulation, containing B. longum BB536 and Lactobacillus rhamnosus HN001 plus vitamin B6, in 23 LI subjects with persistent symptoms proved to be useful in alleviating the condition through a positive modulation of the gut microbial composition (Vitellio et al., 2019). Similarly, the administration of two new probiotic strains, i.e., Bifidobacterium animalis subsp. animalis IM386 and Lactobacillus plantarum MP2026 to 44 LI subjects for 6 weeks, significantly lowered the diarrhea and flatulence symptoms (Roškar et al., 2017) (Table 1).
Gastrointestinal Tract Disorders
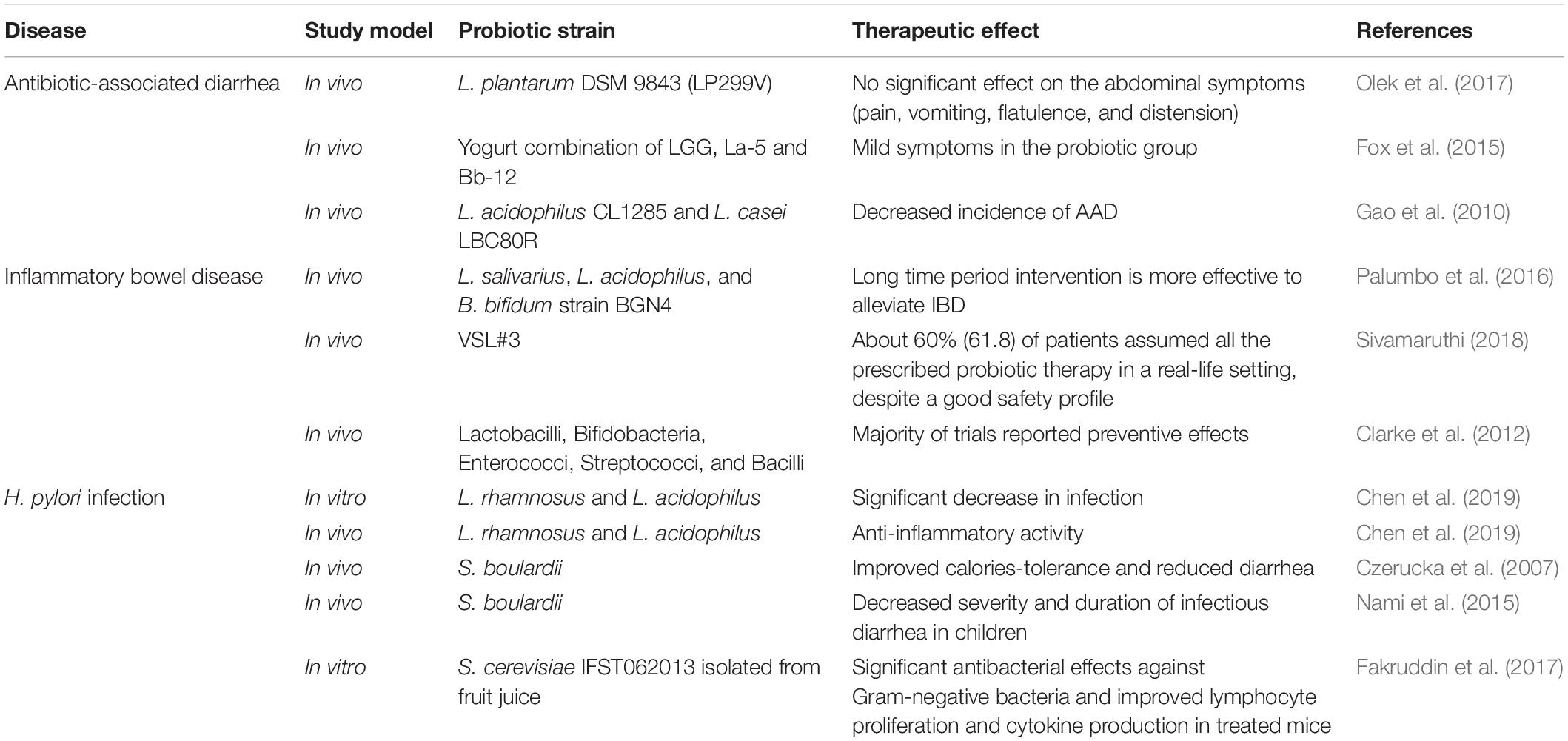
Probiotics have been shown to promote health benefits in many diseases related to an unbalanced GIT microbiota. Scientific evidences about probiotics’ health benefits in alleviating the symptoms of several GIT diseases are well documented (Ritchie and Romanuk, 2012). This section summarizes the main GIT diseases for which probiotics’ restoration of the microbial balance proved to be useful (Table 2).
Antibiotic-Associated Diarrhea
Antibiotic-associated diarrhea is a condition characterized by disturbance in endogenous GIT microbiota following antimicrobial therapies. Heavy antibiotic treatments may cause mild or severe episodes of diarrhea by suppressing the normal microflora and by stimulating the overgrowth of opportunistic or pathogenic strains. AAD related to the overgrowth of Clostridium difficile is also termed as C. difficile-associated diarrhea (Sanchez et al., 2017). However, the use of Leuconostoc cremoris and of Bacillus, Bifidobacterium, Lactobacillus, Lactococcus, Saccharomyces, or Streptococcus spp.—individually or in combination—has been proved to have a protective effect in the treatment of AAD (Syngai et al., 2016). Alberda et al. (2018) demonstrated that the administration of Lactobacillus casei drink in an intensive care unit provided a noticeable preventative effect for AAD as well as C. difficile-associated diarrhea. According to a recent meta-analysis, in people treated with probiotics, AAD appeared in 8.0% of the cases, against the 17.7% of the control group. Data from 17 studies, for a total of 3,631 patients, demonstrated that use of probiotics can reduce the risk of AAD by 51% (Blaabjerg et al., 2017). More recently, Esposito et al. (2018) evaluated the efficacy of Lactobacillus rhamnosus (LGG), AAD in children with hypospadias repair. In the randomized controlled trial (RCT), L. rhamnosus GG associated with antibiotics significantly reduced the incidence and the duration of postoperative AAD and the incidence of postoperative complications, such as urethral fistula and foreskin dehiscence.
Traveler’s Diarrhea
According to epidemiological data, 20–60% of people suffers from traveler’s diarrhea around the world, usually following transferring from industrialized to developing countries. This condition is associated with the passage of three or more unformed stools per day coupled with symptoms such as abdominal pain or cramps. Bacteria are the most common cause in 60–85% of cases, and the usually pointed out pathogen is Escherichia coli followed by Campylobacter jejuni, Shigella, and Salmonella spp. (Giddings et al., 2016). According to an adaptive meta-analysis based on double-blind RCTs, probiotics exhibited a statistically significant efficacy in the prevention of traveler’s diarrhea (Bae, 2018). Sniffen et al. (2018) noted that it is important to properly select the probiotic strain(s), matching with the targeted disease and condition. In detail, Saccharomyces boulardii CNCM I-745 was found to be more beneficial on bacterial diarrhea, while L. rhamnosus GG (LGG) showed beneficial effects against viral and idiopathic diarrhea (Sniffen et al., 2018).
Inflammatory Bowel Diseases
Inflammatory bowel disease is a chronic, multifactorial disorder caused by inflammation of the GIT that may induce severe watery and bloody diarrhea accompanied by abdominal pain. IBD is a broader term and includes ulcerative colitis (UC), Crohn’s disease (CD), and pouchitis affecting both colon and small intestine (Derwa et al., 2017). Genetic and environmental factors, dysregulation of immune system, intestinal dysbiosis, and oxidative stresses are reported as key factors for the IBD onset (Moeinian et al., 2013). Probiotics have been proven to exert an actual influence toward both the GIT microbiota and the immune system (Di Cerbo et al., 2016). The multiple strains formulation VSL#3—eight probiotic strains plus E. coli Nissle 1917—proved to be effective in the prevention of IBD (Jia K. et al., 2018). In a double−blind, placebo−controlled study with 107 patients, the BIO−25 capsules (a combination of 11 probiotic strains belonging to Lactobacillus, Bifidobacterium, Streptococcus, and Lactococcus spp. genera) administration twice daily for 8 weeks did not result in significant fecal microbial changes. Response to therapy was associated with a reduction in Bilophila and increased baseline proportions of bacterial genera such as Faecalibacterium and Ruminococcus spp. (Hod et al., 2018). Evidence collected with reference to CD do not recommend probiotics; indeed, systematic surveys are scarce and mostly refer to the pediatric age. On the other hand, for UC treatment, some clinical trials support efficacy, especially when multistrain probiotics are used (Guandalini and Sansotta, 2019). Probiotics may exert therapeutic effects toward IBD condition by affecting the composition of the microbial ecosystem, by competing for nutrients and adhesion sites, via cell–cell communication, and by producing antimicrobial substances. In addition, probiotics interact with the host immune system through bacterial products, cell wall components, or DNA (Jonkers et al., 2012). Until now, no considerable adverse effect of the probiotic intervention on IBD patients has been reported except one UC patient who suffered from mild dry cough in the case of B. longum 536 supplementation (Sivamaruthi, 2018).
Irritable Bowel Syndrome
Irritable bowel syndrome is a chronic, heterogeneous condition often characterized by abdominal pain, bloating, and altered bowel habits (constipation, diarrhea, or alternation of both) (Barbara et al., 2016). The main pathophysiological factors leading to IBS are psychological (stress and emotional status), social (upbringing and support system), and biological (gut motility and visceral sensitivity), which interact in a complex way to exacerbate the symptoms (Lacy et al., 2016). Breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea and other IBS symptoms in two independent RCTs (Kim et al., 2018; Shin et al., 2018). Dale et al. (2019), in a recent meta-analysis, reported that multistrain probiotic supplementation is more effective as compared to single strain for IBS symptoms alleviation. Similar findings were also mentioned in a further recent systematic meta-analysis, even if, according to the authors, no definitive conclusion can be derived (Ford et al., 2018).
Helicobacter pylori Infection
Helicobacter pylori is a widespread Gram-negative and spiral-shaped pathogen. It is the major causative agent of chronic gastritis and peptic ulcer, and it is a risk factor for gastric malignancies (Patel et al., 2014). Probiotic strains may reduce the rate of H. pylori by 5–10%, and it was found successful in the remission of the associated side effects (Alvi et al., 2016). The results of a double-blind placebo-controlled study suggested that S. boulardii and Lactobacillus johnsonii La1 can decrease H. pylori loads but are not able to induce a complete eradication of the pathogen (Homan and Orel, 2015). Furthermore, S. boulardii has been proven to improve the eradication rate if coupled to the standard H. pylori eradication therapeutic protocol. Further studies are needed in the case of Lactobacillus reuteri, as good results might be achieved (Homan and Orel, 2015). Different studies suggested that H. pylori eradication rate may be improved by a pretreatment with bifidobacterial-containing yogurt for 4 weeks (Sheu et al., 2006). Additionally, a network meta-analysis from 140 studies concluded that the eradication rate in the control (standard therapy alone) and in the probiotics-supplemented group was 70.5 and 84.1%, respectively. By contrast, adverse events rates were 30.1 and 14.4%, respectively (Wang et al., 2017). There is a robust evidence on supporting the use of probiotics in conjunction with antibiotics for the treatment of H. pylori infection. Nevertheless, optimal probiotic strain, dose, and treatment length are yet to be determined (Boltin, 2016).
Disorders of Body Districts Other Than GIT
Respiratory Tract Infections
Respiratory tract infection (RTI) is a frequently occurring disease in humans, and a large range of etiological agents challenge the development of efficient therapies. Recent researches suggest that probiotics may decrease the risk or the duration of RTI symptoms. However, the antiviral mechanisms of probiotics are not clear yet (Lehtoranta et al., 2014).
Strain-specific effects of probiotics against RTI still have inadequate supporting data. This is evident by Laursen and Hojsak (2018) meta-analysis on 15 RCTs in which LGG and B. animalis subsp. lactis BB-12 were orally administered to daycare children. LGG significantly reduced RTI, while BB-12 did not show any noticeable results. Further findings about BB-12 probiotic strain efficacy for RTI treatments in children reported no considerable preventive effect (Hojsak et al., 2016). However, Rautava et al. (2008) reported that BB-12 combined with LGG substantially reduced the RTI recurrences in children. In another RCT open-label clinical study, yogurt added with probiotic Lactobacillus paracasei N1115, orally administered for 12 weeks to middle- and older-aged people, proved to be effective to mitigate RTI through increasing T cells (CD3+), which modulate the native immune system (Pu et al., 2017). By contrast, a meta-analysis of 21 RCTs reported insufficient evidence to support the therapeutic effect of probiotics against RTI, except for probiotic L. casei LCA (Amaral et al., 2017).
Urinary Tract Infections
Urinary tract infection (UTI) is more common among women compared to men and frequently tend to recur. E. coli, Proteus, Klebsiella, and Staphylococcus spp. are intestinal uropathogens generally considered as causative agents. The repletion of vaginal lactobacilli might be beneficial, as depletion can lead toward higher UTI risk (Fontana et al., 2013). It has been observed that high levels of vaginal colonization with probiotic Lactobacillus crispatus are associated with a significant reduction in recurrent UTI (Stapleton et al., 2011). A meta-analysis comparing the occurrence of recurrent UTI in 294 adult female patients across five studies showed no statistically significant difference in the risk for recurrent UTI in patients receiving probiotic lactobacilli versus controls. However, upon ineffective strains removal, a statistically significant decrease was found in recurrent UTI patients treated with probiotic lactobacilli (Grin et al., 2013). Wolff et al. (2019) conducted a double-blinded RCT to evaluate if oral probiotics could induce changes in the uropathogens/Lactobacillus (U/L) ratio among healthy premenopausal young women. Oral administration of L. rhamnosus GR-1 and L. reuteri RC-14 induced no considerable changes in U/L ratio in healthy female individuals. Sadeghi-Bojd et al. (2020) conducted RCTs among healthy children who recovered from first febrile UTI. Oral intervention with a probiotic mixture of L. acidophilus, L. rhamnosus, Bifidobacterium bifidum, and Bifidobacterium lactis was given for 18 months. Results indicated that E. coli and Klebsiella pneumoniae were the two core agents responsible for UTI recurrence in children, while probiotics reduced the UTI recurrence risk. Similar findings were also reported by Koradia et al. (2019) in a double-blind, placebo-controlled pilot efficacy trial based on Bio-Kult Pro-Cyan (a combination of cranberry extract and two lactobacilli strains) administration against recurrence of UTI in premenopausal mature women. A 26-week intervention illustrated a significant preventive action of Bio-Kult Pro-Cyan in UTI recurrence risk.
Chronic Kidney Disease
Chronic kidney disease (CKD) is a lethal global syndrome, also called “silent disease,” characterized by progressive reduction in renal functions, kidney infection commencement, and premature death. Incidence of diabetes mellitus, obesity, and hypertension largely contributed to the prevalence of CKD (Fagundes et al., 2018). Wang et al. (2015) reported prophylactic and therapeutic effects of Lactobacillus and Bifidobacterium spp. in peritoneal dialysis patients. Probiotics oral administration for 6 months considerably reduced the serum endotoxins and proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-5, while it improved anti-inflammatory cytokine IL-10 activity. Considerable variations in the structure and functionality of gut microbiota between humans and animals with severe CKD were reported. The most evident effect is the abatement in the Bifidobacterium/Enterobacteriaceae ratio. In addition, many investigations asserted that CKD is associated with lower counts of Bifidobacterium and Lactobacillus spp., coupled with Enterobacteriaceae prevalence in the intestine (Sircana et al., 2019).
Guida et al. (2017) investigated the symbiotic effect of probiotics on plasma p-cresol concentration (uremia toxin) in kidney transplant recipients through an in vivo randomized trial. A 1 month trial demonstrated that probiotics could reduce the uremia toxin concentrations. Jia L. et al. (2018) carried out a meta-analysis of randomized controlled interventions in order to evaluate the efficacy of probiotic supplementations in adult CKD patients. Probiotics therapy could decrease p-cresyl sulfate, increase IL-6 levels, and improve the gut barrier function. However, blood urea nitrogen, hemoglobin, and serum creatinine concentrations remained constant, independently by the sample size and the poor number of studies. Similarly, another RCT-based meta-analysis of probiotics in CKD control revealed more promising results to manage urea levels in non-dialysis CKD patients. On the other hand, no significant change in glomerular filtration rate, uric acid, serum creatinine, and C-reactive protein concentrations were found (Tao et al., 2019).
Bacterial Vaginosis
Bacterial vaginosis (BV) is the predominant vaginal infection mainly occurring in early age female individuals, in which continuous off-white irregular vaginal discharge is the main symptom. Downfall growth of Lactobacillus species and surging overgrowth of vaginal anaerobic Gram-negative bacteria, i.e., Gardnerella vaginalis, lead to imbalance in the vaginal microecology (Masoudi et al., 2016). The pathological severity of BV is associated with elevated risks of preterm delivery, abrupt abortion, pelvic inflammation, and endometritis. G. vaginalis is the main pathogen producing polymicrobial biofilm, which acts as a binding agent against vaginal epithelial cells (Sabbatini et al., 2018).
Lactobacillus, Bifidobacterium, and Saccharomyces spp. have the potential to interfere with the pathological pattern of BV. Sabbatini et al. (2018) demonstrated the inhibitory response of probiotic S. cerevisiae against G. vaginalis infection in in vivo trials. Such preventive effects were based on different actions, such as amelioration of inflammatory cytokines, retard sialidase enzyme activity, and reduced G. vaginalis binding capacity to vaginal epithelial cells. In addition, in a recent test on mice, probiotic Clostridium butyricum WZ001 strain not only suppressed the growth of G. vaginalis and decreased inflammatory cytokines but also improved the lactobacilli growth to normalize the vaginal microecological balance (Zhou et al., 2019). A meta-analysis of probiotics-based RCTs for VB treatment reported that currently combo treatments strategies, i.e., probiotics plus antibiotics, are more effective than single applications of both. Moreover, probiotics are useful and effective against BV infection, but efficacy is not clear due to quality and heterogeneity of clinical trials (Li et al., 2019b).
Androgenetic Alopecia
Under normal conditions, 50–100 hairs fall from humans daily. However, if hair fall is >100 hairs/day, then it is a symptom of alopecia disorders. Hair loss is a multifactorial disease induced by hormonal disturbance, psychological instability (dementia, depression, and stress), poor nutrition, dandruff issue, and chemotherapy (Choi et al., 2015). 5-α reductase inhibitors are commonly used to cope with this disorder, particularly in men. However, various preclinical and clinical interventions model studies pointed out a potential therapeutic role of probiotic supplementations against androgenetic alopecia by improving peripheral blood circulation (Poller et al., 2017).
In in vivo trial, Song et al. (2012) reported that L. rhamnosus may exert a growth-promoting effect on hairs. Fermented essences prepared by using backryeoncho (Opuntia ficus indica var. saboten) extract and L. rhamnosus showed significant results in increasing hairs’ depth and number in mice without any side effect on body weight and food intake. Recently, Park et al. (2020) investigated the consequence of probiotic-enriched kimchi and cheonggukjang (a traditional Korean fermented soybean product), namely, two fermented vegetable products, on androgenetic alopecia. In this clinical study, 4 months probiotics-enriched foods interventions significantly enhanced hair counts and hair thickness, through improving blood flow without any intestinal side effect, such as diarrhea.
Dental Caries
Streptococcus mutants is the microorganism mainly contributing to dental caries. Gradual oral biofilm development is supported by different oral pathogenic bacteria, with S. mutants assisting to the proliferation by exopolysaccharides secretion (Lee and Kim, 2014). Lactobacillus and Bifidobacterium spp. are core probiotics promoting teeth demineralization with ultimate rise in dental plaque acidity. However, regular intake of probiotics readily reduces the S. mutants proliferation (Hasslöf and Stecksén-Blicks, 2020). LGG has been used for inhibiting oral colonization by cariogenic pathogens, thus reducing tooth decay incidence in children (Twetman and Keller, 2012).
According to López-López et al. (2017), oral inhabitants more actively influence oral health status, as compared to commensal gut microbiota. This is evident from Streptococcus dentisani activity in healthy dental plaque subjects. Such bacterium suppresses the proliferation of several oral pathogens, including Streptococcus sobrinus and S. mutans, by the production of bacteriocins. In Chile, an RCT conducted on 2- to 3-year-old children for 10 months demonstrated that a prolonged and regular intake of milk supplemented with probiotic L. rhamnosus SP1 significantly decreases dental caries development in preschool children (Rodríguez et al., 2016). Likewise, a 90-day-long supplementation of probiotic Streptococcus salivarius M18 to children provided promising outcomes against reduction in dental caries due to the strain’s ability to produce bacteriocins. Moreover, such probiotic strain synthesizes dextranase and urease enzymes that offset dental plaque and saliva acidity through colonization to oral mucosa (Di Pierro et al., 2015). Stensson et al. (2014) assessed that the regular and long-time intake of L. reuteri ATCC 55730 may prevent dental caries and gingivitis in children during the first year of life.
Osteoporosis
Osteoporosis is a complicated metabolic bone disorder in which poor bone mass, deteriorated bone tissues, and higher bone porosity lead to loss of the bone density and strength (Behera et al., 2020). Bone remodeling is a dynamic and natural process to cope with the bone damages. Osteoporosis can also occur due to bone remodeling imbalance (Collins et al., 2018). Gut microbiota play a key role in the development of osteoporosis, as the gut microbiota imbalance, particularly the increase in firmicutes, proteobacteria, and bacteroidetes populations, is one of the main causes of osteoporosis. This systematic imbalance is caused by different possible syndromes, including IBD, short bowel syndrome, systematic inflammation, hyperthyroidism, oxidative stress, abnormal somatostatin profile, non-alcoholic live fatty disease, celiac disorder, and diabetes mellitus (Nath et al., 2018). Certain drugs, including protein kinase inhibitors, heparin (anticoagulants), glucocorticoids, phenobarbital, cyclosporine, thiazolidinedione, cinacalcet, rifampicin, and doxorubicin, are also negatively associated with osteoporosis. Such drugs induce calcium, phosphorus, and vitamin D malabsorption (Aggarwal et al., 2019). Liu et al. (2019) highlighted the promising role of LGG against tenofovir-induced mandibular bone loss in in vivo studies. In detail, LGG inhibited the inflammation of mandible-derived mesenchymal stem cells and osteogenesis.
Nath et al. (2018) demonstrated the effective symbiotic application of lactose-based prebiotics and probiotics to retard osteoporosis by improving mineral absorption flux through the intestinal epithelium. Furthermore, conversion of insoluble inorganic salts into soluble forms, protection of intestinal mineral absorption sites, triggering of the modulation of calcium-binding proteins, and minimization of the interaction of minerals with phytic acids are the main actions reported (Nath et al., 2018). A comparative in vivo study conducted to evaluate the efficacy of five different probiotic strains (L. acidophilus, L. reuteri, L. casei, B. longum, and B. coagulans) against ovariectomized medicated bone loss indicated that probiotics efficacy is mainly strain specific, and an overall higher effectiveness was recorded for L. acidophilus and L. casei strains. Both probiotics were associated with high bone marrow concentration, bone mineral density, and bone area (global, femur, spine, and tibia) (Montazeri-Najafabady et al., 2019). Similar findings were reported by Collins et al. (2018), too: LGG and VSL3# suppressed inflammation biomarkers, i.e., TNF-α, IL-17, and receptor activator of nuclear factor kappa-B ligand (RANKL).
Hand, Foot, and Mouth Disease
Hand, foot, and mouth disease (HFMD) is a viral infection, common within children. Enterovirus A, i.e., Coxsackievirus-A16 (CV-A16) and enterovirus-A71 (EV-A71), are responsible for such disease. The main symptoms include oral ulcers, fever, and vesicle lesions on the hands, feet, and thigh (Xu et al., 2019). Pathologically, HFMD is considered as mild, even if it can sporadically trigger to myocarditis, meningitis, pulmonary edema, encephalitis, and lethal central nervous system problems, like acute flaccid paralysis (Zhou et al., 2016). HFMD virus is vulnerable under acidic conditions. Fu et al. (2015) identified effective HFMD antiviral activity of kombucha due to its symbiotic probiotics composition, i.e., Saccharomyces pastorianus and Acetobacter xylinum, which exhibit very high acidic activity. In vitro and in vivo intervention studies based on kombucha administration proposed its usage as potential anti-HFMD medicine. Zhu et al. (2017) evaluated the effectiveness of “Golden Bifid,” an intestinal probiotic formulation, against severe HFMD in children. Oral administration of Golden Bifid for 2 weeks to 63 severe HFMD children patients expressed impressive results. With the modulation of gut immunity, anti-inflammatory profile (IL-13, IL-4, and IL-10), and gut barrier function, intestinal probiotics can provide an efficient bio-therapy in severe HFMD.
Immunity Disorders
Alleviation of Food Allergy Symptoms
In the last few decades, incidence of allergic diseases progressively increased throughout the world, with higher prevalence in western countries (Fishbein and Fuleihan, 2012). The development of oral tolerance requires association with microbes. As a matter of fact, a poor gut biodiversity during the first months of life has been associated with the development of atopic eczema. Actually, lower population levels of lactobacilli and bifidobacteria have been reported in the GIT of infants who later developed allergies (Kuitunen, 2013). Allergic diseases are disorders mainly characterized by an excessive Th2 immune response and by an unnecessary inflammatory activation of the innate immune cells. The increased incidence of allergic responses to several food products is supposed to be the result of a relative decrease in the microbial induction to the intestinal immune system during infancy and childhood (Castellazzi et al., 2013). Probiotics may improve allergies-associated symptoms by pro-T helper type 1 activation after bacterial contact. However, for optimizing results, it is necessary to define probiotic types, administration time, individual microbiota, and diet (Hajavi et al., 2019).
A study on oral immunotherapy to peanuts highlighted that participants treated with LGG were desensitized compared to the control group. In detail, the probiotics’ therapeutic effect was focused on T regulatory cells promotion (Tang et al., 2015). According to a meta-analysis encompassing 17 studies, probiotics may significantly lower the risk of infantile eczema, thus suggesting a new potential indication for probiotic use in pregnancy and infancy (Zuccotti et al., 2015). Recently, L. rhamnosus Lr-0601 proved to be able to modulate the balance of Th1/Th2 and Treg/Th17 in ovalbumin-sensitized mice (Song et al., 2020). In mouse model with FA induced by ovalbumin, Li N. et al. (2020) observed an abnormal gut bacterial composition, accompanied by increased immunoglobulin G, immunoglobulin E, and interleukin-4/interferon-gamma. According to the authors, Bifidobacterium breve M-16V may alter the gut microbiota, thus alleviating the allergy symptoms by IL-33/ST2 signaling.
Autoimmune Diseases
Autoimmune diseases are related to loss of the immune system’s resilience that leads to pathological immune responses. Autoimmune subjects have higher genetic biasness toward autoreactivity. Type-1 diabetes (T1D), multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) are the most common autoimmune disorders (Davidson and Diamond, 2010). Various autoimmune diseases have higher tendency toward female sex; some animal studies demonstrate the effects of the intestinal metabolism on hormonal status and of gender bias in autoimmune diseases. Gut microbiota can be a core factor to regulate immune responses and autoimmune disorders by different strategies (Yousefi et al., 2019). RA affects body joints by causing cartilages erosion, bones, and eventually joint deformities. According to a recent RCT meta-analysis on the therapeutic effect of probiotics on RA, probiotics can suppress proinflammatory cytokine IL-6—a RA biomarker—but the specific probiotic’s clinical impact is still unknown (Mohammed et al., 2017). Daily oral administration of probiotic capsules (L. acidophilus, L. casei, and B. bifidum) in 30 RA subjects for 8 weeks significantly reduced insulin levels, concentrations of C-reactive protein, and the disease activity score of 28 joints (Zamani et al., 2016).
Multiple sclerosis is a chronic neurological disorder that impairs the central nervous system through swelling and neural tissue damage. Probiotics may play a key role in the rehabilitation of microbial and immune balance in MS subjects (Sales-Campos et al., 2019). Clinical studies focused on MS treatment with probiotics are comparatively fewer compared to those on other autoimmune disorders. The intervention with probiotic capsules (L. acidophilus, L. casei, B. bifidum, and Lactobacillus fermentum) to 60 MS patients decreased inflammatory markers such as C-reactive protein and plasma nitric oxide metabolites. Unexpectedly, this probiotic mixture also lowered blood insulin levels, β-cell function, and total high-density lipoproteins ratio (Kouchaki et al., 2017).
T1D is related to the destruction of pancreatic b-cells, i.e., the core insulin producers. This lack of insulin production directly influences blood glucose levels (Sales-Campos et al., 2019). In one of the comprehensive cohort study entitled “The Environmental Determinants of Diabetes in the Young (TEDDY),” 7,473 Finland children from Germany, Sweden, and the United States, aged 4–10 years old and with high genetic risk of T1D, were subjected to probiotic supplementation. Results showed that the probiotic intake may decrease the occurrence of islet autoimmunity (Uusitalo et al., 2016).
The prevalence of renal and cardiovascular disease in patients with SLE is higher than in the general population. Despite strong evidence on the beneficial effects of probiotics in the development of autoimmunity in SLE, the preventive effects of probiotics in renal and cardiovascular disease in humans have not been established yet (de la Visitación et al., 2019).
Metabolic Disorders
Anticancer Effects
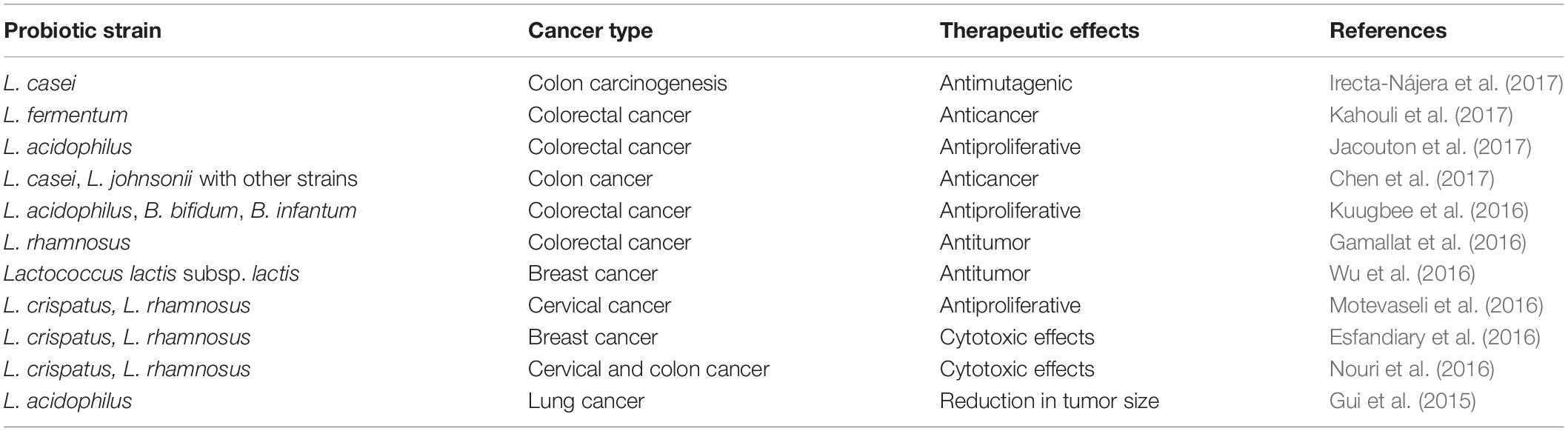
Cancer is the second cause of death among non-communicable chronic diseases. It results in the loss of natural apoptotic cell cycle due to spontaneous accumulation of various mutagens during DNA replication process (Tomasetti and Vogelstein, 2015). Currently, cancer treatments are based on chemotherapy, radiotherapy, and immunotherapy. However, such therapeutic treatments expose cancer patients to more than one severe health side effects. Clinical and preclinical studies have been carried on in order to evaluate the efficacy of probiotics administration to manage cancer by improving the intestinal eubiosis (Vivarelli et al., 2019) (Table 3). Probiotics’ anticancer activity lies on the stabilization of intestinal microflora, strengthening of the GIT mucosal barrier, enhancing of the anti-inflammatory response, and on delaying cancer cells proliferation and metastasis development (Eslami et al., 2019). Even if probiotics are generally recognized as safe, there is still a potential risk for immune-compromised cancer patients to suffer from possible infections (Vivarelli et al., 2019).
LGG is currently one of the most widely investigated strains for improving oncology-therapy-based side effects. LGG’s antiproliferative or antimetastatic effects have been extensively evaluated through in vitro models of breast, hepatic, ovary, and colorectal cancer. Besides this, LGG modulates the immune system by promoting the inhibition of tumor cells’ outgrowth (Behzadi et al., 2017; Mendes et al., 2018). Inflammation provokes cancer growth through various inflammatory-cell-based pathways in epithelial cells. The anti-inflammatory potential of probiotics can counteract cancer development under various discrete pathways, such as increased secretion of immunoglobulin A (IgA) levels, regulation of cyclooxygenase-2, and modulation of nuclear factor kappa B (NF-kB) and interferon-gamma (IFN-γ) (Shamekhi et al., 2020). Furthermore, the suppression of tumorigenesis by probiotics may be related to SCFA production, gut pathogen inhibition, intestinal enzyme activation, secondary bile acid reduction, and potential carcinogenic molecule trapping (Chong, 2014). Recently, Lagier et al. (2019) identified 11 human microbiota-based probiotic strains having the potential of triggering anticancer and anti-infectious immunity. Moreover, a specific mechanism of cecal and colonic mucus colonization and modulation of mucosal CD103+ dendritic cells seems to be involved.
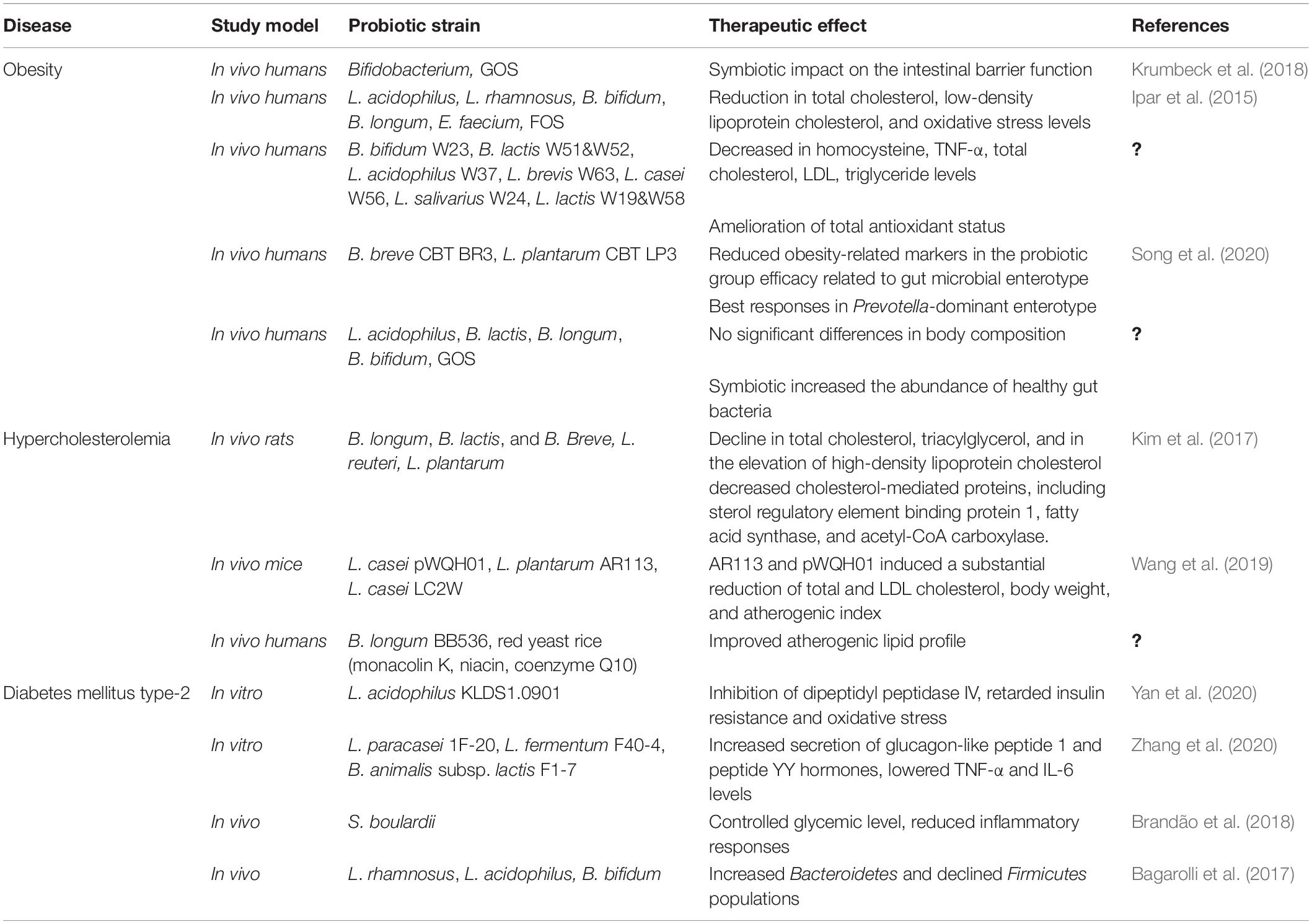
Obesity
Obesity is considered as global epidemic resulting in abnormal or excessive fat accumulation that may be harmful for human health. Its etiology has been mainly associated with an energy imbalance along with other factors including genetic, environmental, hormonal, and infectious agents (Mirmiran et al., 2015). Gut microbiota imbalance directly influences metabolic mechanisms with ultimate results in several metabolic disorders, including obesity (León et al., 2019). Bifidobacterium and Lactobacillus spp. have been associated with improving barrier integrity. Krumbeck et al. (2018) described the symbiotic impact of galacto-oligosaccharides and Bifidobacterium strains on the intestinal barrier function improvement, but no synergistic effects were reported. By contrast, Ipar et al. (2015) demonstrated the symbiotic effect of L. acidophilus, L. rhamnosus, B. bifidum, B. longum, E. faecium, plus fructo-oligosaccharides in obese children (Table 4). After 4 weeks, probiotics-treated children experienced a significant reduction in total cholesterol, low-density lipoprotein cholesterol, and oxidative stress levels. According to Kobyliak et al. (2018), a combination of probiotics and omega-3-fatty acids proved to be effective in counteracting obesity in an in vivo trial. A recent investigation demonstrated that the antiobesity impact of probiotics is strain specific (Brusaferro et al., 2018). Various in vitro and in vivo studies demonstrated that probiotics, specifically L. casei, L. rhamnosus, L. gasseri, L. plantarum, and Bifidobacterium, i.e., B. longum, B. breve, and B. animalis, are widely associated with weight and fat mass index reduction (Ejtahed et al., 2019). With regard to weight control, the most promising next-generation probiotics will probably be Akkermansia muciniphila strains. This mucin-degrading Gram-negative bacterium, represents from 3 to 5% of the microbial community in healthy individuals and is inversely correlated with body weight in both humans and rodents (Mazloom et al., 2019).
Hypercholesterolemia
Hypercholesterolemia is a metabolic syndrome mediated by abnormal serum and cellular cholesterol level (Kumar et al., 2012). Cholesterol is an important biological waxy and fat-like compound associated with lipoproteins. High serum cholesterol levels may have a direct role in the onset of many severe health disorders, i.e., coronary heart diseases, diabetes type II, hypertension, and atherosclerosis (Chien et al., 2010). Furthermore, hypercholesterolemia is considered predisposing to non-alcoholic fatty liver diseases (Wang et al., 2019). Recently, the probiotics, especially lactobacilli and bifidobacteria, is regarded as a novel therapeutic approach against hypercholesterolemia (Pavloviæ et al., 2012). Within probiotics’ cholesterol lowering mechanisms, bile salt hydrolase (BSH) enzyme is considered the most significant. In the enterohepatic circulation, BSH enzyme deconjugates the bile salts that lead to hydroxylation of conjugated glycodeoxycholic and taurodeoxycholic acids. This metabolic mechanism results in the release of glyco- and tauro-bile acids (Anandharaj et al., 2020). Besides BSH activity, 3-hydroxy 3 methylglutaryl-CoA reductase, 7-α- and 27-α-hydroxylases, and Niemann–Pick C1-like 1 protein are the proposed pathways, but a thorough understanding is still missing (Hassan et al., 2019).
Kim et al. (2017) investigated the efficacy of a probiotic mixture of Bifidobacterium (B. longum, B. lactis, and B. breve) and Lactobacillus (L. reuteri and L. plantarum) strains in hypercholesteremic rats. Results showed a considerable decline in total cholesterol and triacylglycerol and in the elevation of high-density lipoprotein cholesterol levels. Furthermore, in liver, probiotics decreased cholesterol-mediated proteins, including sterol regulatory element binding protein 1, fatty acid synthase, and acetyl-CoA carboxylase (Table 4). Similarly, Wang et al. (2019) compared in vivo the BSH activity of L. casei pWQH01, L. plantarum AR113, and L. casei LC2W. Strains AR113 and pWQH01 induced a substantial reduction in the total serum cholesterol, body weight, low-density lipoprotein cholesterol levels, and atherogenic index. The group treated with probiotic strain LC2W showed unsatisfactory results. According to a recent meta-analysis investigation, probiotic yogurt consumption induces a substantial reduction in total and low-density cholesterol levels among patients characterized by mild to moderate hypercholesterolemia. On the other hand, probiotic yogurt did not exert any valuable impact on high-density lipoprotein cholesterol levels (Pourrajab et al., 2020).
Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is the fourth most common non-transmissible disease. Frequent weight loss, high urination, thirst, and poor foot lesions healing power are the most common symptoms, which may later lead to complications, such as coronary heart diseases, obesity, retinopathy, sexual weakness, kidney failures, and atherosclerosis (Sun J. et al., 2020). T2DM has multifactor roots, including physiological, psychological, genetic, and environmental factors (Ardeshirlarijani et al., 2019). Recent studies reported that the gut microbiota plays a crucial role in the occurrence and the management of T2DM (Vallianou et al., 2018). Probiotics can regulate insulin secretion and intestine hormonal secretion. Mainly glucagon-like peptide 1 and peptide YY can reduce fat accumulation in liver cells through L cells modulation (Will et al., 2017). Moreover, probiotics’ therapeutic potential against T2DM is based on the improvement of the gut barrier function, the increase in insulin sensitivity and critins levels, the reduction in serum lipopolysaccharides levels, as well as the oxidative and endoplasmic reticulum stress amelioration. L. acidophilus KLDS1.0901 is a novel promising antidiabetic probiotic strain, as demonstrated by in vitro dipeptidyl peptidase IV inhibitory activity along with increased blood glucose level balance and retarded insulin resistance and oxidative stress (Yan et al., 2020).
Zhang et al. (2020) screened the in vitro efficacy of three strains (L. paracasei 1F-20, L. fermentum F40-4, and B. animalis subsp. lactis F1-7) to understand the mechanism of insulin resistance. All probiotic strains well survived and harmonized with the gastrointestinal environment, inducing genetic modification for increasing the secretion of glucagon-like peptide 1 and peptide YY hormones, reducing the fat accumulation in liver cells, improving the glucose-uptake level, and retarding the inflammatory response through lowering the TNF-α and IL-6 levels. In vivo probiotic intervention study with yeast S. boulardii provided promising results in controlling the glycemic level and by reducing inflammatory responses and cardiovascular disorders (Brandão et al., 2018). In another in vivo study, probiotics L. rhamnosus, L. acidophilus, and B. Bifidum positively influenced the Bacteroidetes population, while Firmicutes population declined. Furthermore, a high-caloric diet was proven to have a negative influence on the gut microbiota by lowering the glucose tolerance and by increasing inflammation and intestinal permeability (Bagarolli et al., 2017). Kesika et al. (2019) explained that the overall efficacy of RCTs is not clear, due to some limitations, which include sample size, preparation of supplements, monitoring of intervention, sampling strategies, inconsistency in measurements, and questionnaire biasness. Authors stressed that further well-designed studies are required to understand the link between the role of the microbiome, the interaction of probiotics with the host microbiota, and the glycemic control.
Neurodegenerative Disorders
Mental illness is referred as cognitive, emotional, and behavioral impairment. The central nervous system and brain mainly coordinate each other for normal cognitive functions. However, any abnormality in this coordination results in neurodegenerative disorders such as schizophrenia, epilepsy, dementia, autism, Parkinson’s disease (PD), and Alzheimer’s disease (AD). Inflammation and oxidation are two fundamental factors that trigger neurodegeneration through retarding normal physiological functions (Westfall et al., 2017). Currently, no direct medication is available for dementia, but medical treatments can delay this disorder (Li W. et al., 2020).
Parkinson’s Disease
Parkinson’s disease is the second most common complicated neurodegenerative disorder, and it occurs due to the dysfunction of the motor system that triggers impairment of dopaminergic neurons in the substantia nigra. The main symptomatic features of PD are postural variability and muscle stiffness. However, non-motor manifestations were also identified, including gastrointestinal dysbiosis, olfactory impairment, and emotional and sensorial instability (Gazerani, 2019). Levodopa is the most commonly employed drug against PD; however, this medication has certain limitations toward gastrointestinal dysbiosis and dopaminergic neuron degeneration. These side effects have promoted the interest around other potential bio-based therapies, such as gene therapy, cellular therapy, and deep brain modulation. Recently, GIT is considered as spotlight owing to its central coordination in the development of PD (Gazerani, 2019). PD patients expressed higher intestinal barrier permeability as well as accumulation of gut α-synuclein due to increasing inflammation and oxidative stress in the gut (Forsyth et al., 2011).
Preclinical and clinical intervention studies toward beneficial aspects of probiotics are yet in the narrow spectrum. Inflammation, peripheral immunomodulation, and oxidation by reactive oxygen species are vital pathological attributes of PD. Magistrelli et al. (2019) conducted an in vitro intervention study by using Bifidobacterium and Lactobacillus probiotics strains on isolated peripheral blood mononuclear cells obtained from PD patients. Promising and effective results were reported through reduced oxidative stress and inflammation, along with an improved gut barrier function. Moreover, a sufficient decline in the growth of gut pathogenic microorganisms, i.e., E. coli and K. pneumonia without restricting levodopa levels were observed. Most recently, Hsieh et al. (2020) in vivo evaluated the neuroprotective efficacy of daily and long-term consumption of probiotics on dopaminergic neurons. Results showed that regular administration of probiotics substantially decreased the motor impairments in gait pattern, balance function, and motor coordination. Probiotics can protect dopamine neurons and prevent motor dysfunction. Analogously, the novel formulation SLAB51, a combination of eight different strains of Lactobacillus, Bifidobacterium, and Streptococcus species, was able to counteract the detrimental effect of 6-hydroxydopamine in vitro and in vivo models of PD (Castelli et al., 2020).
Alzheimer’s Disease
Alzheimer’s disease is the most prevalent neurodegenerative form of dementia characterized by a gradual decline in memory, thinking, and reasoning response. The exact pathological mechanism of AD has not been confirmed yet, but there are several factors involved in the systematic pathogenesis. These include increased intestine barrier permeability, higher proinflammatory cytokines production, and impaired mitochondrial functionality, leading to excessive amounts of reactive oxygen species and oxidation (Ton et al., 2020).
Extensive scientific researches indicate that gut microbiota play a crucial role in AD neurodegenerative mechanism. Neuroactive compounds like dopamine, melatonin, serotonin, and γ-aminobutyric acid (GABA) are produced by gut microbiota (Szczechowiak et al., 2019). However, certain gut bacteria synthesize detrimental lipopolysaccharides and amyloid peptides, inducing inflammation in AD patients. Instability in gut-barrier function also contributes to neuroinflammation through contact of gut microbiota with lymphoid tissues (Jiang et al., 2017). Recently, in vivo studies pointed out the positive influence of oral bacterio-therapy on gut microbiota modification, which indirectly improves the neural functionality. This systematic mechanism is based on the genetic configuration enhancement that is further responsible for inflammation and neural dysfunction (Sochocka et al., 2019).
The most prevalent hallmark of AD is cognitive deficits that could be improved through the administration of lactobacilli and bifidobacteria species, as evidenced in a recent in vivo study (Mancuso and Santangelo, 2018). However, Sun Z. et al. (2020) carried out a comprehensive in vivo investigation by using probiotic C. butyricum to retard neuroinflammation. The probiotic intervention considerably averted cognitive impairment, neurodegeneration, microglia stimulation, Aβ accumulation, and production of tumor biomarkers (TNF-α and IL-1β). The modulations of gut microbiota and metabolic butyrate are also key phenomena observed in this study. In terms of clinical trials, Ton et al. (2020) assessed the efficacy of probiotic-based kefir intervention for 3 months in AD patients having cognitive impairments. The main biomarkers were oxidative stress levels, cytokine levels, cognitive impairment, and blood cell damage. Results showed satisfactory improvement in memory and basic cognitive functions of AD patients through the reduction in inflammation, oxidation and cell damage.
Abraham et al. (2019) proposed a combo strategy of exercise and probiotics supplementation, i.e., B. longum, L. acidophilus lysates, vitamins, and omega 3 fatty acids against AD. In such in vivo study, cognitive deficit reduction was related to gut microbiota regulation, improved butyrogenesis, and decreased beta-amyloid plaques level. Cerebral glucose uptake and metabolism impairment proved to be strongly associated with the pathogenesis of AD. This mechanism has been recently highlighted in mice. The oral administration of SLAB51 induced a gut microbiota prompt modification, with reference to energy metabolism and glycolysis cycle through modulation of glucose transporters and insulin growth factor-1 receptor β. Amelioration of cognitive impairment was supported by the modulation of the proteolytic cycle, reduction in β amyloid plaques, and increase in concentrations of gut microbiota neuroprotective hormones (Abraham et al., 2019).
Conclusion
In the last two decades, extensive preclinical and clinical therapeutic studies focused on the use of probiotic strains to counteract several diseases were carried out. However, many lacking points still linger on, thus making such claims a question mark (Zucko et al., 2020). Food industry seems to be following shallow approaches instead of a holistic one regarding probiotics’ clinical efficiency. In addition, in the latest investigations, variations in results seem to be a consequence of negligence of systematic factors related to probiotics treatment protocols. Probiotics’ safety seems to be a limiting factor in most of the researches. This could be due to the widespread perception of probiotics as health-promoting agents, and this contradiction has a direct influence on probiotics’ efficacy (Zucko et al., 2020).
The probiotics’ main therapeutic potential site is believed to be the GIT, and various gastrointestinal dysbiosis are subjected to probiotics treatment. The meta-analysis of Derwa et al. (2017) mentioned uncertain probiotics’ efficacy against CD and UC, i.e., both forms of IBD. LGG and B. animalis subsp. lactis BB-12 are two of the most recognized probiotics. However, studies presently available about them are still insufficient to satisfy the confining European health claims legislation in spite of having a potential as health promoters (Flach et al., 2018). Probiotics’ interaction with host gut microbiota is another point concerning efficacy treatment. Probiotics’ beneficial aspects could be more discernable if antagonistic mechanisms against pathogenic species and growth-promoting interaction with beneficial species of gut microbiota are deeply explored (Hu et al., 2017).
Author Contributions
MS carried on the bibliographic research. MS and NM wrote the article. MA did corrections of the manuscript. All authors thoroughly discussed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abraham, D., Feher, J., Scuderi, G. L., Szabo, D., Dobolyi, A., Cservenak, M., et al. (2019). Exercise and probiotics attenuate the development of Alzheimer’ disease in transgenic mice: role of microbiome. Exp. Gerontol. 115, 122–131. doi: 10.1016/j.exger.2018.12.005
Aggarwal, A., Sharma, M., Maisnam, I., Ghosh, S., Aggarwal, S., Bhattacharya, S., et al. (2019). Drug-induced bone disorders: a systematic review. Indian J. Rheumatol. 14(Suppl. S1), 44–51.
Alberda, C., Marcushamer, S., Hewer, T., Journault, N., and Kutsogiannis, D. (2018). Feasibility of a Lactobacillus casei drink in the intensive care unit for prevention of antibiotic associated diarrhea and Clostridium difficile. Nutrients 10:539. doi: 10.3390/nu10050539
Almeida, C. C., Lorena, S. L. S., Pavan, C. R., Akasaka, H. M. I., and Mesquita, M. A. (2012). Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 27, 247–251. doi: 10.1177/0884533612440289
Alvi, S., Javeed, A., Akhtar, B., Sharif, A., and Akhtar, M. F. (2016). Probiotics for cure of Helicobacter pylori infection: a review. Int. J. Food Properties 20, 2215–2222. doi: 10.1080/10942912.2016.1233432
Amaral, M. A., Guedes, G. H. B. F., Epifanio, M., Wagner, M. B., Jones, M. H., and Mattiello, R. (2017). Network meta-analysis of probiotics to prevent respiratory infections in children and adolescents. Pediatr. Pulmonol. 52, 833–843. doi: 10.1002/ppul.23643
Anandharaj, M., Sivasankari, B., and Rani, R. P. (2020). Corrigendum to “effects of probiotics, prebiotics, and synbiotics on hypercholesterolemia: a review”. Chin. J. Biol. 2014, 1–7. doi: 10.1155/2020/8236703
Ardeshirlarijani, E., Tabatabaei-Malazy, O., Mohseni, S., Qorbani, M., Larijani, B., and Jalili, R. B. (2019). Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. Daru 27, 827–837. doi: 10.1007/s40199-019-00302-2
Bae, J. M. (2018). Prophylactic efficacy of probiotics on travelers’ diarrhea: an adaptive meta-analysis of randomized controlled trials. Epidemiol. Health 40:e2018043. doi: 10.4178/epih.e2018043
Bagarolli, R. A., Tobar, N., Oliveira, A. G., Araújo, T. G., Carvalho, B. M., Rocha, G. Z., et al. (2017). Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 50, 16–25. doi: 10.1016/j.jnutbio.2017.08.006
Barbara, G., Cremon, C., Annese, V., Basilisco, G., Bazzoli, F., Bellini, M., et al. (2016). Randomised controlled trial of mesalazine in IBS. Gut 65, 82–90.
Behera, J., Ison, J., Tyagi, S. C., and Tyagi, N. (2020). The role of gut microbiota in bone homeostasis. Bone 135:115317. doi: 10.1016/j.bone.2020.115317
Behzadi, E., Hosseini, H. M., and Fooladi, A. A. I. (2017). The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 110, 1–6. doi: 10.1016/j.micpath.2017.06.016
Blaabjerg, S., Artzi, D. M., and Aabenhus, R. (2017). Probiotics for the prevention of antibiotic-associated diarrhea in outpatients-a systematic review and meta-analysis. Antibiotics 6:21. doi: 10.3390/antibiotics6040021
Boltin, D. (2016). Probiotics in Helicobacter pylori-induced peptic ulcer disease. Best Pract. Res. Clin. Gastroenterol. 30, 99–109. doi: 10.1016/j.bpg.2015.12.003
Brandão, A. B., de Abreu, I. C., Aimbire, F., Higa, E. M., Casali, A., Ferreira, F. G., et al. (2018). Saccharomyces boulardii attenuates autonomic cardiovascular dysfunction and modulates inflammatory cytokines in diabetic mice. Diabetes 67(Suppl. 1):2365-PUB.
Brusaferro, A., Cozzali, R., Orabona, C., Biscarini, A., Farinelli, E., Cavalli, E., et al. (2018). Is it time to use probiotics to prevent or treat obesity? Nutrients 10:1613. doi: 10.3390/nu10111613
Castellazzi, A. M., Valsecchi, C., Caimmi, S., Licari, A., Marseglia, A., Leoni, M. C., et al. (2013). Probiotics and food allergy. Ital. J. Pediatr. 39:47.
Castelli, V., d’Angelo, M., Lombardi, F., Alfonsetti, M., Antonosante, A., Catanesi, M., et al. (2020). Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 12, 4641–4659. doi: 10.18632/aging.102927
Chen, Y. H., Tsai, W. H., Wu, H. Y., Chen, C. Y., Yeh, W. L., Chen, Y. H., et al. (2019). Probiotic Lactobacillus spp. act against Helicobacter pylori-induced inflammation. J. Clin. Med. 8:90. doi: 10.3390/jcm8010090
Chen, Z. Y., Hsieh, Y. M., Huang, C. C., and Tsai, C. C. (2017). Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules 22:107. doi: 10.3390/molecules22010107
Chien, Y. L., Wu, L. Y., Lee, T. C., and Hwang, L. S. (2010). Cholesterol-lowering effect of phytosterol-containing lactic-fermented milk powder in hamsters. Food Chem. 119, 1121–1126. doi: 10.1016/j.foodchem.2009.08.023
Choi, J. C., Uyama, H., Lee, C. H., and Sung, M. H. (2015). In vivo hair growth promotion effects of ultra-high molecular weight poly-γ-glutamic acid from Bacillus subtilis (Chungkookjang). J Microbiol. Biotechnol. 25, 407–412. doi: 10.4014/jmb.1411.11076
Chong, E. S. L. (2014). A potential role of probiotics in colorectal cancer prevention: review of possible mechanisms of action. World J. Microbiol. Biotechnol. 30, 351–374. doi: 10.1007/s11274-013-1499-6
Clarke, G., Cryan, J. F., Dinan, T. G., and Quigley, E. M. (2012). Review article: probiotics for the treatment of irritable bowel syndrome - focus on lactic acid bacteria. Aliment. Pharmacol. Ther. 35, 403–413. doi: 10.1111/j.1365-2036.2011.04965.x
Collins, F. L., Rios-Arce, N. D., Schepper, J. D., Parameswaran, N., and McCabe, L. R. (2018). “The potential of probiotics as a therapy for osteoporosis,” in Bugs as Drugs, eds R. A. Britton and P. D. Cani (Hoboken, NJ: John Wiley & Sons), 213–233. doi: 10.1128/microbiolspec.bad-0015-2016
Czerucka, D., Piche, T., and Rampal, P. (2007). Review article: yeast as probiotics – Saccharomyces boulardii. Aliment. Pharmacol. Ther. 26, 767–778. doi: 10.1111/j.1365-2036.2007.03442.x
Dale, H. F., Rasmussen, S. H., Asiller, Ö. Ö., and Lied, G. A. (2019). Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients 11:2048. doi: 10.3390/nu11092048
Davidson, A., and Diamond, B. (2010). Activated basophils give lupus a booster shot. Nat. med. 16, 635–636. doi: 10.1038/nm0610-635
de la Visitación, N., Robles-Vera, I., Toral, M., and Duarte, J. (2019). Protective effects of probiotic consumption in cardiovascular disease in systemic lupus erythematosus. Nutrients 11:2676. doi: 10.3390/nu11112676
Derwa, Y., Gracie, D. J., Hamlin, P. J., and Ford, A. C. (2017). Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 46, 389–400. doi: 10.1111/apt.14203
Di Cerbo, A., Palmieri, B., Aponte, M., Morales-Medina, J. C., and Iannitti, T. (2016). Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 69, 187–203. doi: 10.1136/jclinpath-2015-202976
Di Pierro, F., Zanvit, A., Nobili, P., Risso, P., and Fornaini, C. (2015). Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 7, 107–113. doi: 10.2147/ccide.s93066
Dwivedi, M., Kumar, P., Laddha, N. C., and Kemp, E. H. (2016). Induction of regulatory T cells: a role for probiotics and prebiotics to suppress autoimmunity. Autoimmun. Rev. 15, 379–392. doi: 10.1016/j.autrev.2016.01.002
Ejtahed, H. S., Angoorani, P., Soroush, A. R., Atlasi, R., Hasani-Ranjbar, S., Mortazavian, A. M., et al. (2019). Probiotics supplementation for the obesity management; a systematic review of animal studies and clinical trials. J. Funct. Foods 52, 228–242. doi: 10.1016/j.jff.2018.10.039
Esfandiary, A., Taherian-Esfahani, Z., Abedin-Do, A., Mirfakhraie, R., Shirzad, M., Ghafouri-Fard, S., et al. (2016). Lactobacilli modulate hypoxia-inducible factor (HIF)-1 regulatory pathway in triple negative breast cancer cell line. Cell J. 18, 237–244.
Eslami, M., Yousefi, B., Kokhaei, P., Hemati, M., Nejad, Z. R., Arabkari, V., et al. (2019). Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 234, 17127–17143. doi: 10.1002/jcp.28473
Esposito, C., Roberti, A., Turrà, F., Cerulo, M., Severino, G., Settimi, A., et al. (2018). Frequency of antibiotic-associated diarrhea and related complications in pediatric patients who underwent hypospadias repair: a comparative study using probiotics vs placebo. Probiotics Antimicrob. Proteins 10, 323–328. doi: 10.1007/s12602-017-9324-4
Fagundes, R. A. B., Soder, T. F., Grokoski, K. C., Benetti, F., and Mendes, R. H. (2018). Probiotics in the treatment of chronic kidney disease: a systematic review. J. Bras. Nefrol. 40, 278–286.
Fakruddin, M., Hossain, M. N., and Ahmed, M. M. (2017). Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 17:64. doi: 10.1186/s12906-017-1591-9
Feng, K., Huang, R. M., Wu, R. Q., Wei, Y. S., Zong, M. H., Linhardt, R. J., et al. (2020). A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 310:125977. doi: 10.1016/j.foodchem.2019.125977
Fishbein, A. B., and Fuleihan, R. L. (2012). The hygiene hypothesis revisited: does exposure to infectious agents protect us from allergy? Curr. Opin. Pediatr. 24, 98–102. doi: 10.1097/mop.0b013e32834ee57c
Flach, J., van der Waal, M. B., Kardinaal, A. F. M., Schloesser, J., Ruijschop, R. M. A. J., and Claassen, E. (2018). Probiotic research priorities for the healthy adult population: a review on the health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12. Cogent Food Agric. 4:1452839.
Fontana, L., Bermudez-Brito, M., Plaza-Diaz, J., Munoz-Quezada, S., and Gil, A. (2013). Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 109, S35–S50.
Ford, A. C., Harris, L. A., Lacy, B. E., Quigley, E. M., and Moayyedi, P. (2018). Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 48, 1044–1060. doi: 10.1111/apt.15001
Forsyth, C. B., Shannon, K. M., Kordower, J. H., Voigt, R. M., Shaikh, M., Jaglin, J. A., et al. (2011). Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6:e28032. doi: 10.1371/journal.pone.0028032
Fox, M. J., Ahuja, K. D., Robertson, I. K., Ball, M. J., and Eri, R. D. (2015). Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open 5:e006474. doi: 10.1136/bmjopen-2014-006474
Fu, N., Wu, J., Lv, L., He, J., and Jiang, S. (2015). Anti-foot-and-mouth disease virus effects of Chinese herbal kombucha in vivo. Braz. J. Microbiol. 46, 1245–1255. doi: 10.1590/s1517-838246420140701
Gamallat, Y., Meyiah, A., Kuugbee, E. D., Hago, A. M., Chiwala, G., Awadasseid, A., et al. (2016). Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. 83, 536–541. doi: 10.1016/j.biopha.2016.07.001
Gao, X. W., Mubasher, M., Fang, C. Y., Reifer, C., and Miller, L. E. (2010). Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am. J. Gastroenterol. 105, 1636–1641. doi: 10.1038/ajg.2010.11
Giddings, S. L., Stevens, A. M., and Leung, D. T. (2016). Traveler’s Diarrhea. Med. Clin. North Am. 100, 317–330.
Gingold-Belfer, R., Levy, S., Layfer, O., Pakanaev, L., Niv, Y., Dickman, R., et al. (2020). Use of a novel probiotic formulation to alleviate lactose intolerance symptoms-a pilot study. Probiotics Antimicrob. Proteins 12, 112–118. doi: 10.1007/s12602-018-9507-7
Grin, P. M., Kowalewska, P. M., Alhazzan, W., and Fox-Robichaud, A. E. (2013). Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can. J. Urol. 20, 6607–6614.
Guandalini, S., and Sansotta, N. (2019). Probiotics in the treatment of inflammatory bowel disease. Adv. Exp. Med. Biol. 1125, 101–107.
Gui, Q. F., Lu, H. F., Zhang, C. X., Xu, Z. R., and Yang, Y. H. (2015). Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 14, 5642–5651. doi: 10.4238/2015.may.25.16
Guida, B., Cataldi, M., Memoli, A., Trio, R., di Maro, M., Grumetto, L., et al. (2017). Effect of a short-course treatment with synbiotics on plasma p-cresol concentration in kidney transplant recipients. J. Am. Coll. Nutr. 36, 586–591. doi: 10.1080/07315724.2017.1334602
Hajavi, J., Esmaeili, S. A., Varasteh, A. R., Vazini, H., Atabati, H., Mardani, F., et al. (2019). The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 234, 2386–2398. doi: 10.1002/jcp.27263
Hassan, A., Din, A. U., Zhu, Y., Zhang, K., Li, T., Wang, Y., et al. (2019). Updates in understanding the hypocholesterolemia effect of probiotics on atherosclerosis. Appl. Microbiol. Biotechnol. 103, 5993–6006. doi: 10.1007/s00253-019-09927-4
Hasslöf, P., and Stecksén-Blicks, C. (2020). “Probiotic bacteria and dental caries,” in The Impact of Nutrition and Diet on Oral Health, eds F. V. Zohoori and R. M. Duckworth (Berlin: Karger Publishers), 99–107. doi: 10.1159/000455377
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hod, K., Dekel, R., Aviv Cohen, N., Sperber, A., Ron, Y., Boaz, M., et al. (2018). The effect of a multispecies probiotic on microbiota composition in a clinical trial of patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 30:e13456. doi: 10.1111/nmo.13456
Hojsak, I., Paviæ, A. M., Kos, T., Dumanèiæ, J., and Kolaèek, S. (2016). Bifidobacterium animalis subsp. lactis in prevention of common infections in healthy children attending day care centers – randomized, double blind, placebo-controlled study. Clin. Nutr. 35, 587–591. doi: 10.1016/j.clnu.2015.05.004
Homan, M., and Orel, R. (2015). Are probiotics useful in Helicobacter pylori eradication? World J. Gastroenterol. 21, 10644–10653.
Hsieh, T. H., Kuo, C. W., Hsieh, K. H., Shieh, M. J., Peng, C. W., Chen, Y. C., et al. (2020). Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease. Brain Sci. 10, 206. doi: 10.3390/brainsci10040206
Hu, S., Wang, L., and Jiang, Z. (2017). Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept. Lett. 24, 382–387. doi: 10.2174/0929866524666170223143615
Ipar, N., Aydogdu, S. D., Yildirim, G. K., Inal, M., Gies, I., Vandenplas, Y., et al. (2015). Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children. Benef. Microbes 6, 775–781. doi: 10.3920/bm2015.0011
Irecta-Nájera, C. A., del Rosario Huizar-López, M., Casas-Solís, J., Castro-Félix, P., and Santerre, A. (2017). Protective effect of Lactobacillus casei on DMH-induced colon carcinogenesis in mice. Probiotics Antimicrob. Proteins 9, 163–171. doi: 10.1007/s12602-017-9253-2
Jacouton, E., Chain, F., Sokol, H., Langella, P., and Bermudez-Humaran, L. G. (2017). Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front. Immunol. 8:1553. doi: 10.3389/fimmu.2017.01553
Jia, K., Tong, X., Wang, R., and Song, X. (2018). The clinical effects of probiotics for inflammatory bowel disease: a meta-analysis. Medicine 97:e13792. doi: 10.1097/md.0000000000013792
Jia, L., Jia, Q., Yang, J., Jia, R., and Zhang, H. (2018). Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Press. Res. 43, 1623–1635. doi: 10.1159/000494677
Jiang, C., Li, G., Huang, P., Liu, Z., and Zhao, B. (2017). The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 58, 1–15.
Jonkers, D., Penders, J., Masclee, A., and Pierik, M. (2012). Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs 72, 803–823. doi: 10.2165/11632710-000000000-00000
Kahouli, I., Malhotra, M., Westfall, S., Alaoui-Jamali, M. A., and Prakash, S. (2017). Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc Min/+ mouse model. Appl. Microbiol. Biotechnol. 101, 1999–2019. doi: 10.1007/s00253-016-7885-x
Kareb, O., and Aïder, M. (2019). Whey and its derivatives for probiotics, prebiotics, synbiotics, and functional foods: a critical review. Probiotics Antimicrob. Proteins 11, 348–369. doi: 10.1007/s12602-018-9427-6
Kechagia, M., Basoulis, D., Konstantopoulou, S., Dimitriadi, D., Gyftopoulou, K., Skarmoutsou, N., et al. (2013). Health benefits of probiotics: a review. ISRN Nutr. 2013:481651.
Kesika, P., Sivamaruthi, B. S., and Chaiyasut, C. (2019). Do probiotics improve the health status of individuals with diabetes mellitus? a review on outcomes of clinical trials. Biomed Res. Int. 2019:1531567.
Kim, J. Y., Park, Y. J., Lee, H. J., Park, M. Y., and Kwon, O. (2018). Effect of Lactobacillus gasseri BNR17 on irritable bowel syndrome: a randomized, double-blind, placebo-controlled, dose-finding trial. Food Sci. Biotechnol. 27, 853–857. doi: 10.1007/s10068-017-0296-7
Kim, S. J., Park, S. H., Sin, H. S., Jang, S. H., Lee, S. W., Kim, S. Y., et al. (2017). Hypocholesterolemic effects of probiotic mixture on diet-induced hypercholesterolemic rats. Nutrients 9:293. doi: 10.3390/nu9030293
Kleerebezem, M., Binda, S., Bron, P. A., Gross, G., Hill, C., van Hylckama Vlieg, J. E., et al. (2019). Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Curr. Opin. Biotechnol. 56, 55–60. doi: 10.1016/j.copbio.2018.09.007
Kobyliak, N., Falalyeyeva, T., Boyko, N., Tsyryuk, O., Beregova, T., and Ostapchenko, L. (2018). Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res. Clin. Pract. 141, 190–199. doi: 10.1016/j.diabres.2018.05.005
Koradia, P., Kapadia, S., Trivedi, Y., Chanchu, G., and Harper, A. (2019). Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: a controlled pilot study. Expert Rev. Anti Infect. Ther. 17, 733–740. doi: 10.1080/14787210.2019.1664287
Kouchaki, E., Tamtaji, O. R., Salami, M., Bahmani, F., Daneshvar Kakhaki, R., Akbari, E., et al. (2017). Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 36, 1245–1249. doi: 10.1016/j.clnu.2016.08.015
Krumbeck, J. A., Rasmussen, H. E., Hutkins, R. W., Clarke, J., Shawron, K., Keshavarzian, A., et al. (2018). Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6:121.
Kuitunen, M. (2013). Probiotics and prebiotics in preventing food allergy and eczema. Curr. Opin. Allergy Clin. Immunol. 13, 280–286. doi: 10.1097/aci.0b013e328360ed66
Kumar, M., Nagpal, R., Kumar, R., Hemalatha, R., Verma, V., Kumar, A., et al. (2012). Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012:902917.
Kuugbee, E. D., Shang, X., Gamallat, Y., Bamba, D., Awadasseid, A., Suliman, M. A., et al. (2016). Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig. Dis. Sci. 61, 2908–2920.
Lacy, B. E., Mearin, F., Chang, L., Chey, W. D., Lembo, A. J., Simren, M., et al. (2016). Bowel disorders. Gastroenterology 150, 1393–1407.E5.
Lagier, J. C., Million, M., Togo, A. H., Khelaifia, S., and Raoult, D. (2019). Culturomics provide critical prokaryotes strains for anti-Listeria and anti-cancer probiotics. Int. J. Antimicrob. Agents 54, 407–409. doi: 10.1016/j.ijantimicag.2019.05.017
Laursen, R. P., and Hojsak, I. (2018). Probiotics for respiratory tract infections in children attending day care centres—a systematic review. Eur. J. Paediatr. 177, 979–994. doi: 10.1007/s00431-018-3167-1
Lee, S. H., and Kim, Y. J. (2014). A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch. Microbiol. 196, 601–609. doi: 10.1007/s00203-014-0998-7
Lehtoranta, L., Pitkaranta, A., and Korpela, R. (2014). Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1289–1302.
León, S. C., Vergara, P. C., Neira, A. C., Maldonado, R. M., Araneda, C. D., and Zuñiga, M. R. (2019). “Gut microbiota and obesity: prebiotic and probiotic effects,” in Oral Health by Using Probiotic Products. IntechOpen.
Li, C., Bei, T., Niu, Z., Guo, X., Wang, M., Lu, H., et al. (2019a). Adhesion and colonization of the probiotic Lactobacillus rhamnosus labeled by dsred2 in mouse gut. Curr. Microbiol. 76, 896–903. doi: 10.1007/s00284-019-01706-8
Li, C., Wang, T., Li, Y., Zhang, T., Wang, Q., He, J., et al. (2019b). Probiotics for the treatment of women with bacterial vaginosis: a systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 864:172660. doi: 10.1016/j.ejphar.2019.172660
Li, N., Yu, Y., Chen, X., Gao, S., Zhang, Q., and Xu, C. (2020). Bifidobacterium breve M-16V alters the gut microbiota to alleviate OVA-induced food allergy through IL-33/ST2 signal pathway. J. Cell. Physiol. doi: 10.1002/jcp.29751 [Epub ahead of print].
Li, W., Guo, J., Shen, Y., Huang, L., Leng, B., Fan, D., et al. (2020). Probiotics, prebiotics, and synbiotics for the treatment of dementia: protocol for a systematic review. Medicine 99:e18608. doi: 10.1097/md.0000000000018608
Liu, H., Gu, R., Li, W., Xue, J., Cong, Z., Wei, Q., et al. (2019). Probiotics protect against tenofovir-induced mandibular bone loss in mice by rescuing mandible-derived mesenchymal stem cell proliferation and osteogenic differentiation. J. Oral. Rehabil. doi: 10.1111/joor.12840 [Epub ahead of print].
López-López, A., Camelo-Castillo, A., Ferrer, M. D., Simon-Soro, Á., and Mira, A. (2017). Health-associated niche inhabitants as oral probiotics: the case of Streptococcus dentisani. Front. Microbiol. 8:379. doi: 10.3389/fmicb.2017.00379
Magistrelli, L., Amoruso, A., Mogna, L., Cantello, R., Pane, M., and Comi, C. (2019). Probiotics may have beneficial effects in Parkinson’s disease: in vitro evidence. Front. Immunol. 10:969. doi: 10.3389/fimmu.2019.00969
Majewska, K., Kręgielska-Narożna, M., Jakubowski, H., Szulińska, M., and Bogdański, P. (2020). The Multispecies probiotic effectively reduces homocysteine concentration in obese women: a randomized double-blind placebo-controlled study. J. Clin. Medi. 91:998. doi: 10.3390/jcm9040998
Mancuso, C., and Santangelo, R. (2018). Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol. Res. 129, 329–336. doi: 10.1016/j.phrs.2017.12.009
Masoudi, M., Kopaei, M. R., and Miraj, S. (2016). Comparison between the efficacy of metronidazole vaginal gel and Berberis vulgaris (Berberis vulgaris) combined with metronidazole gel alone in the treatment of bacterial vaginosis. Electron. Physician 8, 2818–2827. doi: 10.19082/2818
Mazloom, K., Siddiqi, I., and Covasa, M. (2019). Probiotics: how effective are they in the fight against obesity? Nutrients 11:258. doi: 10.3390/nu11020258
Mendes, M. C. S., Paulino, D. S., Brambilla, S. R., Camargo, J. A., Persinoti, G. F., and Carvalheira, J. B. C. (2018). Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 24, 1995–2008. doi: 10.3748/wjg.v24.i18.1995
Mirmiran, P., Ejtahed, H. S., Bahadoran, Z., Bastan, S., and Azizi, F. (2015). Sugar-sweetened beverage consumption and risk of general and abdominal obesity in Iranian adults: Tehran lipid and glucose study. Iran. J. Public Health 44, 1535–1543.
Moeinian, M., Ghasemi-Niri, S., Mozaffari, S., and Abdollahi, M. (2013). Synergistic effect of probiotics, Butyrate and l-Carnitine in Treatment of IBD. J. Med. Hypotheses Ideas 7, 50–53. doi: 10.1016/j.jmhi.2013.02.003
Mohammed, A. T., Khattab, M., Ahmed, A. M., Turk, T., Sakr, N., Khalil, A. M., et al. (2017). The therapeutic effect of probiotics on rheumatoid arthritis: a systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 36, 2697–2707. doi: 10.1007/s10067-017-3814-3
Montazeri-Najafabady, N., Ghasemi, Y., Dabbaghmanesh, M. H., Talezadeh, P., Koohpeyma, F., and Gholami, A. (2019). Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics Antimicrob. Proteins 11, 1145–1154. doi: 10.1007/s12602-018-9443-6
Motevaseli, E., Azam, R., Akrami, S. M., Mazlomy, M., Saffari, M., Modarressi, M. H., et al. (2016). The Effect of Lactobacillus crispatus and Lactobacillus rhamnosus Culture Supernatants on Expression of Autophagy Genes and HPV E6 and E7 Oncogenes in The HeLa Cell Line. Cell J. 17, 601–607.
Nami, Y., Haghshenas, B., Abdullah, N., Barzegari, A., Radiah, D., Rosli, R., et al. (2015). Probiotics or antibiotics: future challenges in medicine. J. Med. Microbiol. 64, 137–146. doi: 10.1099/jmm.0.078923-0
Nath, A., Molnár, M. A., Csighy, A., Kõszegi, K., Galambos, I., Huszár, K. P., et al. (2018). Biological activities of lactose-based prebiotics and symbiosis with probiotics on controlling osteoporosis, blood-lipid and glucose levels. Medicina 54:98. doi: 10.3390/medicina54060098
Nouri, Z., Karami, F., Neyazi, N., Modarressi, M. H., Karimi, R., Khorramizadeh, M. R., et al. (2016). Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J. 18, 127–134.
Oak, S. J., and Jha, R. (2019). The effects of probiotics in lactose intolerance: a systematic review. Crit. Rev. Food Sci. Nutr. 59, 1675–1683. doi: 10.1080/10408398.2018.1425977
Ojetti, V., Gigante, G., Gabrielli, M., Ainora, M. E., Mannocci, A., Lauritano, E. C., et al. (2010). The effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose intolerant patients: randomized trial. Eur. Rev. Med. Pharmacol. Sci. 14, 163–170.
Olek, A., Woynarowski, M., Ahren, I. L., Kierkus, J., Socha, P., Larsson, N., et al. (2017). Efficacy and Safety of Lactobacillus plantarum DSM 9843 (LP299V) in the prevention of antibiotic-associated gastrointestinal symptoms in children-randomized, double-blind, placebo-controlled study. J. Pediatr. 186, 82–86. doi: 10.1016/j.jpeds.2017.03.047
Pakdaman, M. N., Udani, J. K., Molina, J. P., and Shahani, M. (2016). The effects of the DDS-1 strain of Lactobacillus on symptomatic relief for lactose intolerance - a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 15:56.
Palumbo, V. D., Romeo, M., Marino Gammazza, A., Carini, F., Damiani, P., Damiano, G., et al. (2016). The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 160, 372–377.
Park, D. W., Lee, H. S., Shim, M. S., Yum, K. J., and Seo, J. T. (2020). Do Kimchi and Cheonggukjang probiotics as a functional food improve androgenetic alopecia? A clinical pilot study. World J. Mens Health 38, 95–102. doi: 10.5534/wjmh.180119
Patel, A., Shah, N., and Prajapati, J. B. (2014). Clinical application of probiotics in the treatment of Helicobacter pylori Infection: a brief review. J Microbiol. Immunol. Infect. 47, 429–437. doi: 10.1016/j.jmii.2013.03.010
Pavloviæ, N., Stankov, K., and Mikov, M. (2012). Probiotics—interactions with bile acids and impact on cholesterol metabolism. Appl. Biochem. Biotechnol. 168, 1880–1895. doi: 10.1007/s12010-012-9904-4
Poller, W. C., Berger, A., Dreger, H., Morgera, S., and Enke-Melzer, K. (2017). Lipoprotein apheresis in patients with peripheral artery disease and lipoprotein (a)-hyperlipoproteinemia: 2-year follow-up of a prospective single center study. Atheroscler. Suppl. 30, 174–179. doi: 10.1016/j.atherosclerosissup.2017.05.007
Pourrajab, B., Fatahi, S., Dehnad, A., Varkaneh, H. K., and Shidfar, F. (2020). The impact of probiotic yogurt consumption on lipid profiles in subjects with mild to moderate hypercholesterolemia: a systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 30, 11–22. doi: 10.1016/j.numecd.2019.10.001
Pu, F., Guo, Y., Li, M., Zhu, H., Wang, S., Shen, X., et al. (2017). Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clin. Interv. Aging 12, 1223–1231. doi: 10.2147/cia.s141518
Rautava, S., Salminen, S., and Isolauri, E. (2008). Specific probiotics in reducing the risk of acute infections in infancy–a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 101, 1722–1726. doi: 10.1017/s0007114508116282
Ritchie, M. L., and Romanuk, T. N. (2012). A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One 7:e34938. doi: 10.1371/journal.pone.0034938
Rodríguez, G., Ruiz, B., Faleiros, S., Vistoso, A., Marró, M. L., Sánchez, J., et al. (2016). Probiotic compared with standard milk for high-caries children: a cluster randomized trial. J. Dent. Res. 95, 402–407. doi: 10.1177/0022034515623935
Roškar, I., Švigelj, K., Štempelj, M., Volfand, J., Štabuc, B., Malovrh, Š., et al. (2017). Effects of a probiotic product containing Bifidobacterium animalis subsp. animalis IM386 and Lactobacillus plantarum MP2026 in lactose intolerant individuals: randomized, placebo-controlled clinical trial. J. Funct. Foods 35, 1–8. doi: 10.1016/j.jff.2017.05.020
Ruscica, M., Pavanello, C., Gandini, S., Macchi, C., Botta, M., Dall’Orto, D., et al. (2019). Nutraceutical approach for the management of cardiovascular risk – a combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: results from a randomized, double-blind, placebo-controlled study. Nutr. J. 18:13. doi: 10.1186/s12937-019-0438-2
Sabbatini, S., Monari, C., Ballet, N., Mosci, P., Decherf, A. C., Pélerin, F., et al. (2018). Saccharomyces cerevisiae–based probiotic as novel anti-microbial agent for therapy of bacterial vaginosis. Virulence 9, 954–966. doi: 10.1080/21505594.2018.1464362
Sadeghi-Bojd, S., Naghshizadian, R., Mazaheri, M., Ghane Sharbaf, F., and Assadi, F. (2020). Efficacy of probiotic prophylaxis after the first febrile urinary tract infection in children with normal urinary tracts. J. Pediatric. Infect. Dis. Soc. 9, 305–310. doi: 10.1093/jpids/piz025
Sales-Campos, H., Soares, S. C., and Oliveira, C. J. F. (2019). An introduction of the role of probiotics in human infections and autoimmune diseases. Crit. Rev. Microbiol. 45, 413–432. doi: 10.1080/1040841x.2019.1621261
Sanchez, B., Delgado, S., Blanco-Miguez, A., Lourenco, A., Gueimonde, M., and Margolles, A. (2017). Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 61:1600240. doi: 10.1002/mnfr.201600240
Sergeev, I. N., Aljutaily, T., Walton, G., and Huarte, E. (2020). Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients 12:222. doi: 10.3390/nu12010222
Shamekhi, S., Lotfi, H., Abdolalizadeh, J., Bonabi, E., and Zarghami, N. (2020). An overview of yeast probiotics as cancer biotherapeutics: possible clinical application in colorectal cancer. Clin. Transl. Oncol. 22, 1227–1239. doi: 10.1007/s12094-019-02270-0
Sharma, A. (2019). “Importance of probiotics in cancer prevention and treatment,” in Recent Developments in Applied Microbiology and Biochemistry, ed. V. Buddolla (San Diego, CA: Elsevier), 33–45. doi: 10.1016/b978-0-12-816328-3.00004-0
Sheu, B. S., Cheng, H. C., Kao, A. W., Wang, S. T., Yang, Y. J., Yang, H. B., et al. (2006). Pretreatment with Lactobacillus- and Bifidobacterium-containing yogurt can improve the efficacy of quadruple therapy in eradicating residual Helicobacter pylori infection after failed triple therapy. Am. J. Clin. Nutr. 83, 864–869. doi: 10.1093/ajcn/83.4.864
Shin, S. P., Choi, Y. M., Kim, W. H., Hong, S. P., Park, J. M., Kim, J., et al. (2018). A double blind, placebo-controlled, randomized clinical trial that breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J. Clin. Biochem. Nutr. 62, 179–186. doi: 10.3164/jcbn.17-73
Sircana, A., De Michieli, F., Parente, R., Framarin, L., Leone, N., Berrutti, M., et al. (2019). Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol. Res. 144, 390–408. doi: 10.1016/j.phrs.2018.01.013
Sivamaruthi, B. (2018). A comprehensive review on clinical outcome of probiotic and synbiotic therapy for inflammatory bowel diseases. Asian Pac. J. Trop. Biomed. 8, 179–186. doi: 10.4103/2221-1691.228000
Sniffen, J. C., McFarland, L. V., Evans, C. T., and Goldstein, E. J. C. (2018). Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One 13:e0209205. doi: 10.1371/journal.pone.0209205
Sochocka, M., Donskow-Łysoniewska, K., Diniz, B. S., Kurpas, D., Brzozowska, E., and Leszek, J. (2019). The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease a- a critical review. Mol. Neurobiol. 56, 1841–1851. doi: 10.1007/s12035-018-1188-4
Song, J., Li, Y., Li, J., Wang, H., Zhang, Y., and Suo, H. (2020). Lactobacillus rhamnosus 2016SWU. 05.0601 regulates immune balance in ovalbumin-sensitized mice by modulating the immune-related transcription factors expression and gut microbiota. J. Sci. Food Agric. doi: 10.1002/jsfa.10554 [Epub ahead of print].
Song, J. H., Lee, J. S., and Choi, H. J. (2012). Hair growth promoting effect of essence manufactured with products fermented by Lactobacillus rhamnosus and Backryeoncho (Opuntia ficus-indica var. sarboten) fruits in mice. Food Sci. Biotechnol. 21, 1101–1104. doi: 10.1007/s10068-012-0143-9
Stapleton, A. E., Au-Yeung, M., Hooton, T. M., Fredricks, D. N., Roberts, P. L., Czaja, C. A., et al. (2011). Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 52, 1212–1217. doi: 10.1093/cid/cir183
Stensson, M., Koch, G., Coric, S., Abrahamsson, T. R., Jenmalm, M. C., Birkhed, D., et al. (2014). Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res. 48, 111–117. doi: 10.1159/000354412
Sun, J., Xu, J., Yang, B., Chen, K., Kong, Y., Fang, N., et al. (2020). Effect of Clostridium butyricum against microglia-mediated neuro inflammation in Alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol. Nutr. Food Res. 64:1900636. doi: 10.1002/mnfr.201900636
Sun, Z., Sun, X., Li, J., Li, Z., Hu, Q., Li, L., et al. (2020). Using probiotics for type 2 diabetes mellitus intervention: advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 60, 670–683. doi: 10.1080/10408398.2018.1547268
Syngai, G. G., Gopi, R., Bharali, R., Dey, S., Lakshmanan, G. A., and Ahmed, G. (2016). Probiotics-the versatile functional food ingredients. J. Food Sci. Technol. 53, 921–933. doi: 10.1007/s13197-015-2011-0
Szczechowiak, K., Diniz, B. S., and Leszek, J. (2019). Diet and Alzheimer’s dementia–Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 184:172743. doi: 10.1016/j.pbb.2019.172743
Tang, M. L., Ponsonby, A. L., Orsini, F., Tey, D., Robinson, M., Su, E. L., et al. (2015). Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J. Allergy Clin. Immunol. 135, 737. doi: 10.1016/j.jaci.2014.11.034
Tao, S., Tao, S., Cheng, Y., Liu, J., Ma, L., and Fu, P. (2019). Effects of probiotic supplements on the progression of chronic kidney disease: a meta-analysis. Nephrology 24, 1122–1130. doi: 10.1111/nep.13549
Tomasetti, C., and Vogelstein, B. (2015). Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81. doi: 10.1126/science.1260825
Ton, A. M. M., Campagnaro, B. P., Alves, G. A., Aires, R., Côco, L. Z., Arpini, C. M., et al. (2020). Oxidative stress and dementia in Alzheimer’s Patients: effects of synbiotic supplementation. Oxid. Med. Cell. Longev. 2020:2638703.
Twetman, S., and Keller, M. K. (2012). Probiotics for caries prevention and control. Adv. Dent. Res. 24, 98–102. doi: 10.1177/0022034512449465
Uusitalo, U., Liu, X., Yang, J., Aronsson, C. A., Hummel, S., Butterworth, M., et al. (2016). Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 170, 20–28. doi: 10.1001/jamapediatrics.2015.2757
Vallianou, N. G., Stratigou, T., and Tsagarakis, S. (2018). Microbiome and diabetes: Where are we now? Diabetes Res. Clin. Pract. 146, 111–118. doi: 10.1016/j.diabres.2018.10.008
Vitellio, P., Celano, G., Bonfrate, L., Gobbetti, M., Portincasa, P., and De Angelis, M. (2019). Effects of Bifidobacterium longum and Lactobacillus rhamnosus on gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms: a Randomised, double-blind, cross-over study. Nutrients 11:886. doi: 10.3390/nu11040886
Vivarelli, S., Salemi, R., Candido, S., Falzone, L., Santagati, M., Stefani, S., et al. (2019). Gut microbiota and cancer: From pathogenesis to therapy. Cancers 11:38. doi: 10.3390/cancers11010038
Vonk, R. J., Reckman, G. A., Harmsen, H. J., and Priebe, M. G. (2012). Probiotics and lactose intolerance. Intech 7, 149–160.
Wang, F., Feng, J., Chen, P., Liu, X., Ma, M., Zhou, R., et al. (2017). Probiotics in Helicobacter pylori eradication therapy: systematic review and network meta-analysis. Clin. Res. Hepatol. Gastroenterol. 41, 466–475.
Wang, G., Huang, W., Xia, Y., Xiong, Z., and Ai, L. (2019). Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. 10, 1684–1695. doi: 10.1039/c8fo02181c
Wang, I.-K., Wu, Y.-Y., Yang, Y.-F., Ting, I.-W., Lin, C.-C., Yen, T.-H., et al. (2015). The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef. Microb. 6, 423–430. doi: 10.3920/BM2014.0088
Westfall, S., Lomis, N., Kahouli, I., Dia, S. Y., Singh, S. P., and Prakash, S. (2017). Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life Sci. 74, 3769–3787. doi: 10.1007/s00018-017-2550-9
Will, S., Hornigold, D. C., Baker, D. J., Coghlan, M. P., Mesquita, M., Trevaskis, J. L., et al. (2017). Gut check on diabesity: leveraging gut mechanisms for the treatment of type 2 diabetes and obesity. Curr. Opin. Pharmacol. 37, 10–15. doi: 10.1016/j.coph.2017.07.010
Wolff, B. J., Price, T. K., Joyce, C. J., Wolfe, A. J., and Mueller, E. R. (2019). Oral probiotics and the female urinary microbiome: a double-blinded randomized placebo-controlled trial. Int. Urol. Nephrol. 51, 2149–2159. doi: 10.1007/s11255-019-02282-3
Wu, Z., Wang, G., Pan, D., Guo, Y., Zeng, X., Sun, Y., et al. (2016). Inflammation-related pro-apoptotic activity of exopolysaccharides isolated from Lactococcus lactis subsp. lactis. Benef. Microbes 7, 761–768. doi: 10.3920/bm2015.0192
Xu, Y., Wu, Y. F., Luo, H. H., Zhang, D. D., Wu, Y., and Hu, P. (2019). Acute kidney injury secondary to severe hand, foot and mouth disease caused by enterovirus-A71: hypertension is a common. J. Trop. Pediatr. 65, 510–513. doi: 10.1093/tropej/fmy070
Yan, F., Li, N., Yue, Y., Wang, C., Zhao, L., Evivie, S. E., et al. (2020). Screening for potential novel probiotics with dipeptidyl peptidase IV-inhibiting activity for type 2 diabetes attenuation in vitro and in vivo. Front. Microbiol. 10:2855. doi: 10.3389/fmicb.2019.02855
Yousefi, B., Eslami, M., Ghasemian, A., Kokhaei, P., and Sadeghnejhad, A. (2019). Probiotics can really cure an autoimmune disease? Gene Rep. 15:100364. doi: 10.1016/j.genrep.2019.100364
Zamani, B., Golkar, H. R., Farshbaf, S., Emadi-Baygi, M., Tajabadi-Ebrahimi, M., Jafari, P., et al. (2016). Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double?blind, placebo?controlled trial. Int. J. rheumatic dis. 19, 869–879. doi: 10.1111/1756-185X.12888
Zhang, Z., Liang, X., Lv, Y., Yi, H., Chen, Y., Bai, L., et al. (2020). Evaluation of probiotics for improving and regulation metabolism relevant to type 2 diabetes in vitro. J. Funct. Foods 64:103664. doi: 10.1016/j.jff.2019.103664
Zhou, Y., Li, J. X., Jin, P. F., Wang, Y. X., and Zhu, F. C. (2016). Enterovirus 71: a whole virion inactivated enterovirus 71 vaccine. Expert Rev. Vaccines 15, 803–813. doi: 10.1080/14760584.2016.1191357
Zhou, Y., Xu, W., Hong, K., Li, H., Zhang, J., Chen, X., et al. (2019). Therapeutic effects of probiotic Clostridium butyricum WZ001 on bacterial vaginosis in mice. J. Appl. Microbiol. 127, 565–575. doi: 10.1111/jam.14329
Zhu, F., Jiang, Z., and Li, H. W. (2017). Intestinal probiotics in relieving clinical symptoms of severe hand, foot, and mouth disease and potential mechanism analysis. Eur. Rev. Med. Pharmacol. Sci. 21, 4214–4218.
Zuccotti, G., Meneghin, F., Aceti, A., Barone, G., Callegari, M. L., Di Mauro, A., et al. (2015). Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy 70, 1356–1371. doi: 10.1111/all.12700
Keywords: probiotics, health beneficial effects, host–microbe interactions, immunomodulation, gut microbiota, dysbiosis
Citation: Aponte M, Murru N and Shoukat M (2020) Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current Perspective. Front. Microbiol. 11:562048. doi: 10.3389/fmicb.2020.562048
Received: 14 May 2020; Accepted: 12 August 2020;
Published: 11 September 2020.
Edited by:
Lisa Solieri, University of Modena and Reggio Emilia, ItalyReviewed by:
Beniamino Palmieri, University Hospital of Modena, ItalyBhagavathi Sundaram Sivamaruthi, Chiang Mai University, Thailand
Copyright © 2020 Aponte, Murru and Shoukat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahtab Shoukat, m.shoukat@studenti.unina.it
 Maria Aponte
Maria Aponte Nicoletta Murru2
Nicoletta Murru2 Mahtab Shoukat
Mahtab Shoukat