Social cognition and theory of mind
Social cognition refers to interpretation of socially relevant signals to understand intentions, mental and emotional states of others. These signals may be verbal, or transmitted through prosody, gaze and expressions of the face and body (Adolphs, 2001; Grandjean et al., 2005; Beer and Ochsner, 2006; Frith and Frith, 2012; Pavlova, 2012). Theory of mind (ToM) is an important concept in social cognition, meaning inference and representation of others' beliefs and intentions, (Frith and Frith, 2005). Lesion and imaging studies have contributed to improve knowledge of the correlates of social cognition in the cerebral cortex, that mainly involve the orbitofrontal and anterior cingular cortices as well as the temporo-parietal junction (Happe et al., 1996; Anderson et al., 1999; Gallagher et al., 2000; Bird et al., 2004; Samson et al., 2004; Baird et al., 2006; Shamay-Tsoory et al., 2009; Barbey et al., 2014). More recently, behavioral data in patients with affection of the cerebellum and brain imaging studies have suggested cerebellar involvement in social cognition.
Socio-cognitive deficits in patients with focal cerebellar lesions
Lesion data on specifically altered social cognition after cerebellar damage is still sparse and heterogeneous. Patients with left lateral but not medial cerebellar tumors including Crus I exhibited deficits in perceiving human motion (Sokolov et al., 2010; Figure 1B). On the other hand, in 15 patients tested 1–5 weeks after cerebellar stroke, no significant overall impairments were found in perception of emotions from prosody and photographs of faces (Adamaszek et al., 2014). However, sub-analyses revealed difficulties in selecting the facial expression matching a specific emotion, naming the emotional expression of prosody that may or may not correspond to the semantic content, and matching faces to prosody with similar emotional expression. Furthermore, absent late positive event-related potential on electroencephalography (EEG) during processing of emotional face expressions in patients with cerebellar stroke indicated the network for interpretation of emotional information may be altered after cerebellar damage (Adamaszek et al., 2015). A patient with massive bilateral ponto-cerebellar ischemia was impaired on the Reading the Mind in the Eyes Test (RMET), along with other ToM deficits (Roldan Gerschcovich et al., 2011). A larger scale study on 57 patients with various types of cerebellar damage (degeneration, hemorrhage, ischemia, and tumors; confined to the cerebellum in 26 patients) reported deficient performance on the RMET and impaired emotional regulation (Hoche et al., 2016).
Figure 1
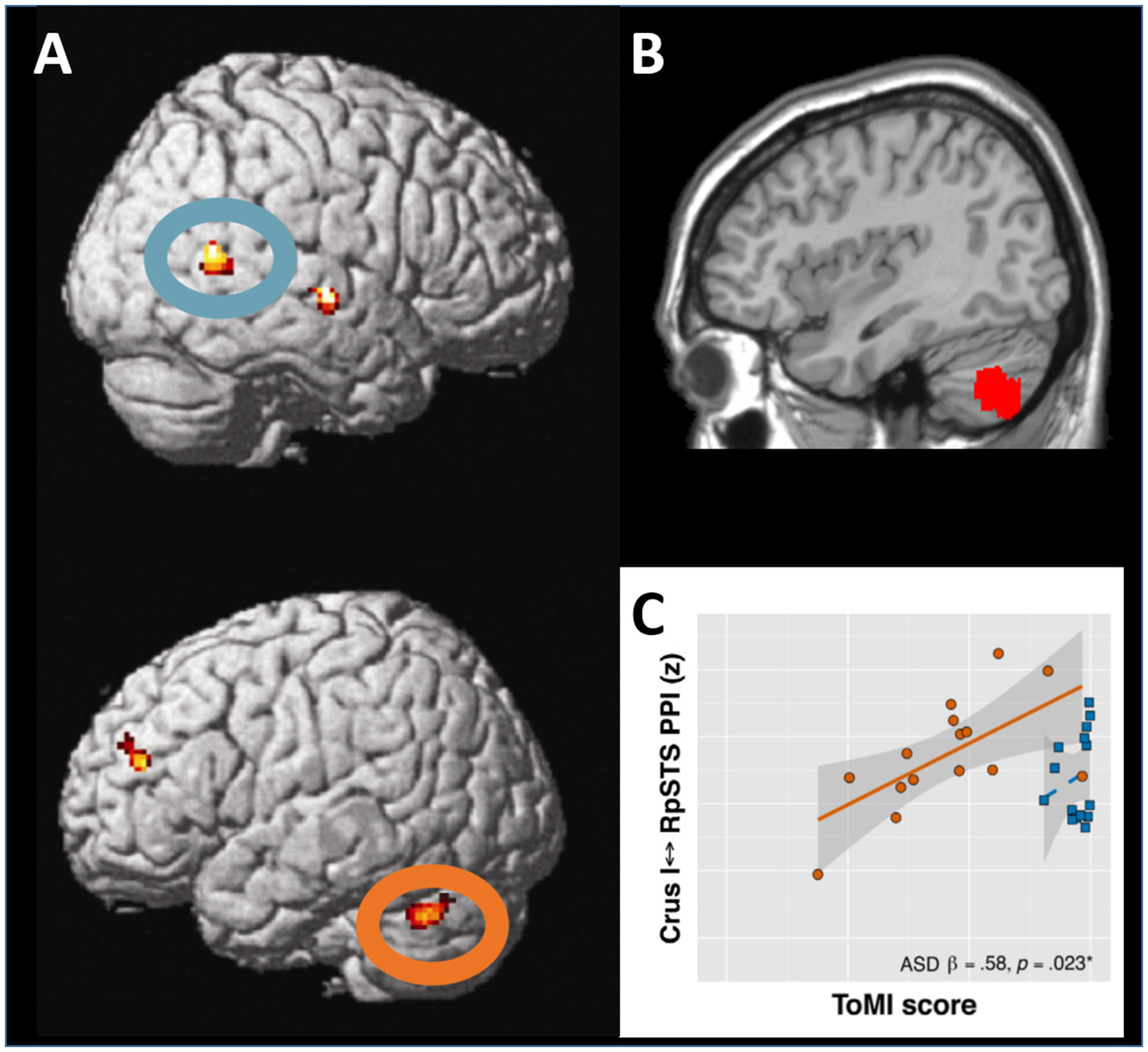
Interaction between the lateral posterior cerebellum and the superior temporal sulcus in social cognition. (A) During visual perception of body motion, the right superior temporal sulcus (upper panel, blue oval) and the left lateral cerebellar lobule Crus I (lower panel, orange oval) show functional and effective connectivity. Adapted from Sokolov et al. (2012), with permission from Elsevier. (B) When corresponding regions in the left lateral posterior cerebellar cortex are affected by tumors, patients exhibit significant deficits in perception of body motion. Adapted from Sokolov et al. (2010), with permission from Oxford University Press. (C) In adolescents with autism spectrum disorders, theory of mind capacities correlate with the strength of effective connectivity between the cerebellar lobule Crus I and the superior temporal sulcus. Adapted from Jack and Morris (2014), with permission from Elsevier.
Evidence from cerebellar degeneration
Some evidence on cerebellar involvement in social cognition has been found in patients with spinocerebellar ataxia (SCA), although the diffuse (also extracerebellar) affection limits inference on topography. Altered recognition of complex facial emotions related to social interaction (such as flirtatiousness or arrogance) but largely preserved basic emotion discrimination (such as happiness or sadness) were found in 20 patients with various SCA types (D'Agata et al., 2011). A patient with cerebellar atrophy exhibited deficient RMET performance (Parente et al., 2013). Absence of deficits in mental state attribution but altered attribution of emotions to a character of a short story were reported in eight patients with SCA types 1, 2, and 7 (Sokolovsky et al., 2010), with the opposite picture in 15 patients with SCA 3 and 6 (Garrard et al., 2008). This dissociation could reflect distinct pathophysiology in SCA subtypes. ToM deterioration was found with SCA progression (Moriarty et al., 2016). Deficient representation of mental states was also seen in six patients with superficial siderosis, a condition with diffuse hemosiderin depots in superficial layers throughout the brain, but mainly affecting the brainstem and cerebellum (van Harskamp et al., 2005). When interpreting a social situation in a short story, both SCA and superficial siderosis patients tend to employ explicit physical explanations instead of more implicit social abstraction, with social abstraction being the function that most frequently elicits cerebellar activation in healthy subjects (Van Overwalle et al., 2014).
Overall, the clinical evidence is still far from being convincing or complete. Most importantly, it remains uncertain whether cerebellar damage in humans causes substantial, specific and persistent impairment in social cognition. In terms of specificity, although executive functions are closely associated with ToM (Aboulafia-Brakha et al., 2011), connectivity analyses suggest most cerebellar modules activated during social cognition may be involved in specific socio-cognitive rather than executive networks (Van Overwalle et al., 2015a). Brain imaging may therefore shed some light on functional organization of potential cerebro-cerebellar networks for social cognition.
Cerebellar activation and connectivity patterns in social cognition
A meta-analysis of over 350 functional magnetic resonance imaging (fMRI) studies reported consistent cerebellar activations during different tasks on social cognition, including observation of human motion, mentalizing about intentions in social interactions, inference on personality traits and abstraction (Van Overwalle et al., 2014). The left lateral cerebellum was activated (Gobbini et al., 2007; Jack et al., 2011; Sokolov et al., 2012) and communicated with the right posterior superior temporal sulcus (STS; Jack et al., 2011; Sokolov et al., 2012; Jack and Pelphrey, 2015) during observation of others' actions and social interactions represented by geometric shapes. Similar patterns of cerebellar activation were reported when watching a movie (Nguyen et al., 2017). Indeed, a functional and structural loop appears to connect the cerebellar lobule Crus I with the STS (Sokolov et al., 2012, 2014; Figure 1A). Meta-analytic connectivity data in healthy subjects also indicated that interactions between Crus I and temporo-parietal junction (TPJ) may be of importance for social cognition (Van Overwalle et al., 2015b). A study in 103 children with subacute traumatic brain injury showed that reduced gray matter volumes in the cerebellum, STS and TPJ are related to poorer cognitive ToM function (Ryan et al., 2017). Neuropsychiatric conditions with altered social cognition often involve cerebellar affection and brain imaging in these patients is therefore thought to provide additional insights on cerebellar interplay with the network for social cognition.
Neuropsychiatry: social cognition and cerebellar connectivity
In adults with autism spectrum disorders (ASD), reduced eye contact was related to the volumes of bilateral Crus I and cerebellar vermis (Laidi et al., 2015). ASD individuals exhibited lower resting-state functional connectivity between the left cerebellar lobule Crus II and right TPJ adjacent to the STS (Igelstrom et al., 2017), and altered information flow from the left dentate nucleus to right cortical regions involved in social cognition (Olivito et al., 2017). Furthermore, alterations of effective connectivity between the STS and the cerebellar lobule Crus I were linked to social impairment in patients with ASD (Jack and Morris, 2014; Jack et al., 2017; Figure 1C).
In schizophrenia, the evidence is less numerous and converging. Lower right cerebellar activity was found during mental state attribution (Andreasen et al., 2008), but stronger albeit delayed activity was seen in the left cerebellum when observing social-like interactions between geometric shapes (Pedersen et al., 2012). Inference on approachability from faces led to stronger activation in bilateral posterior cerebellum and left TPJ in adults with schizotypal personality disorder as compared to those with ASD and controls (Stanfield et al., 2017). Disrupted microstructure of cerebro-cerebellar pathways (Kanaan et al., 2009) and of intracerebellar white matter, particularly in lobule Crus II (Kim et al., 2014) has also been shown in schizophrenia. Analyses of cerebro-cerebellar resting-state functional connectivity in schizophrenia are somewhat incongruent (Guo et al., 2015; Shinn et al., 2015) and specific cerebro-cerebellar communication during social cognition has not been reported. The available data may agree with the view on schizophrenia as pathophysiologically and clinically heterogeneous disease (Ross et al., 2006), and underline the need for further research.
Integrating cognitive with cellular neuroscience
In summary, converging evidence from behavioral studies in patients with cerebellar damage and neuroimaging in typically developing individuals and those with neuropsychiatric conditions suggests the posterior lateral cerebellar lobules Crus I and II may be involved in the social cognition networks. Correlations between cerebellar activation or connectivity and social impairment provide further insights. However, intrinsic limitations of both lesion studies and brain imaging call for translational research assessing causality and specificity.
This is where the increasingly pursued “from bedside to bench and back to bedside” approach comes into play. Besides its unrivaled potential for fundamental discovery, the causality afforded by cellular neuroscience is very helpful to evaluate hypotheses. With its rather regular and well-known architecture and connectivity, the cerebellum represents a particularly useful blueprint for translational efforts in neuroscience. Insights on cellular mechanisms are indispensable for valid models of large-scale networks and pathophysiology, and vice versa. In a cerebellum previously conceptualized as rather uniform, rodent electrophysiology has already unveiled different rules for synaptic plasticity (Wadiche and Jahr, 2005; Zhou et al., 2014; Suvrathan et al., 2016), and recently demonstrated some granule cells encode non-sensorimotor predictions and their unexpected violations (Wagner et al., 2017). These data offered novel perspectives on how the cerebellum may be equipped to contribute to diverse cognitive processes, but assessing higher cognition in animals and particularly rodents with a truly translational potential poses a significant challenge. Nonetheless, initial promising steps have been undertaken over the past year.
Promising translational approaches
Patients with schizophrenia exhibit impaired interval timing performances and reduced EEG delta frequency in the medial frontal cortex. In Long-Evans rats, delta band coherence was found between deep cerebellar nuclei and contralateral medial frontal cortex, and muscimol-mediated inactivation of their deep cerebellar nuclei neurons altered interval timing performance. Most importantly, in rats with pharmacologically inactivated medial frontal dopamine receptors D1, delta range optogenetic stimulation on thalamic terminals of deep cerebellar nuclei axons specifically improved interval timing and function of the medial frontal cortex (Parker et al., 2017). Another translational study directly looked at social behavior: children with ASD as well as healthy adults who underwent transcranial direct current stimulation over the right cerebellar lobule Crus I exhibited increased functional connectivity between right Crus I and contralateral inferior parietal cortex. Similar alterations in structural connectivity were found in mice with Purkinje cell dysfunction due to a tuberous sclerosis complex mutation, showing ASD-like behavior. In normal mice, chemogenetic inhibition of right Crus I Purkinje cells resulted in both increased parietal single cell firing rates (potentially through disinhibition of excitatory deep cerebellar nuclei efference) as a measure of cerebello-parietal connectivity and reduced preference for social novelty as a marker of social behavior, not explained by sensorimotor or visual deficits. Furthermore, chemogenetic activation of right Crus I Purkinje cells in the mutant mice reduced left parietal firing rates and specifically improved social interaction (Stoodley et al., 2017). These data suggest cerebro-cerebellar connectivity and resulting pro-social behavior may be restored through stimulation of Crus I.
Mechanisms for cerebellar contribution to social cognition
Translational research of this kind, bridging different species and modalities may substantially contribute to understanding the mechanisms and eloquence of cerebellar regions' involvement in social cognition. As to the mechanisms, because of the similar cytoarchitecture across the cerebellum, it has been repeatedly suggested its operations for motor control and coordination may also apply to cognition (Wolpert et al., 1998; Ito, 2008; Sokolov et al., 2017). These operations are believed to involve outcome prediction based on forward models and signaling deviations from these outcomes (prediction errors) to the cerebral cortex, a hypothesis supported by recent electrophysiology data on non-sensorimotor expectations in rodents (Wagner et al., 2017) and concepts of ASD as a prediction deficit with prominent cerebellar pathology (Sinha et al., 2014). As anticipation, adaptation and learning appear indispensable for successful social behavior, extending these core cerebellar functional roles from sensorimotor models to those of social perception and behavior would seem reasonable, although several issues remain to be carefully considered and explored (Sokolov et al., 2017).
Conclusions and outlook
Taken together, preliminary but converging lesion and imaging data suggest social cognition may recruit loops connecting the lateral cerebellar lobules Crus I and II with medial frontal and temporo-parietal areas. Conclusions on eloquence of the cerebellum for social cognition would benefit from sufficiently powered studies in patients with rather homogeneous lesion size, topography and etiology at defined time-points after disease onset. In addition, consideration of ecological validity appears helpful when assessing impact on everyday social function (Henry et al., 2015).
Of note, the relative lack of convincing lesion data may also be accounted for by compensatory mechanisms. As hypothesized for other functions (Andreasen et al., 1998; Schmahmann, 1998), prediction and anticipation afforded by the cerebellum may facilitate speedy, adaptive social cognition and behavior—yet, the remainder of the network may be able to sufficiently compensate for acquired cerebellar lesions (Sokolov et al., 2017). This compensatory potential may be limited in congenital or early developmental damage to the cerebellum, including neuropsychiatric disease and concussion (Wang et al., 2014; Ryan et al., 2017), or concomitant changes in affect and emotional control potentially interfering with social behavior, such as in the cerebellar cognitive and affective syndrome (Schmahmann and Sherman, 1998), for which a scale has been recently introduced (Hoche et al., 2017). Translational approaches will further deepen our understanding of functional and compensatory mechanisms, as well as causality with respect to cerebellar involvement in social cognition.
Statements
Author contributions
AS conceptualized and wrote the manuscript.
Acknowledgments
This publication was supported by the Baasch-Medicus Foundation, the Leenaards Foundation and the Wellcome Trust (Principal fellowship 088130/Z/09/Z). I would like to thank Marina Pavlova for valuable advice, and Paola Giunti and Arron Cook for helpful discussion of their data on spinocerebellar ataxia.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Aboulafia-Brakha T. Christe B. Martory M. D. Annoni J. M. (2011). Theory of mind tasks and executive functions: a systematic review of group studies in neurology. J. Neuropsychol.5, 39–55. 10.1348/174866410X533660
2
Adamaszek M. D'Agata F. Kirkby K. C. Trenner M. U. Sehm B. Steele C. J. et al . (2014). Impairment of emotional facial expression and prosody discrimination due to ischemic cerebellar lesions. Cerebellum13, 338–345. 10.1007/s12311-013-0537-0
3
Adamaszek M. Kirkby K. C. D'Agata F. Olbrich S. Langner S. Steele C. et al . (2015). Neural correlates of impaired emotional face recognition in cerebellar lesions. Brain Res.1613, 1–12. 10.1016/j.brainres.2015.01.027
4
Adolphs R. (2001). The neurobiology of social cognition. Curr. Opin. Neurobiol.11, 231–239. 10.1016/S0959-4388(00)00202-6
5
Anderson S. W. Bechara A. Damasio H. Tranel D. Damasio A. R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci.2, 1032–1037. 10.1038/14833
6
Andreasen N. C. Calarge C. A. O'Leary D. S. (2008). Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr. Bull.34, 708–719. 10.1093/schbul/sbn034
7
Andreasen N. C. Paradiso S. O'Leary D. S. (1998). “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry?Schizophr. Bull.24, 203–218. 10.1093/oxfordjournals.schbul.a033321
8
Baird A. Dewar B. K. Critchley H. Dolan R. Shallice T. Cipolotti L. (2006). Social and emotional functions in three patients with medial frontal lobe damage including the anterior cingulate cortex. Cogn. Neuropsychiatry11, 369–388. 10.1080/13546800444000245
9
Barbey A. K. Colom R. Paul E. J. Chau A. Solomon J. Grafman J. H. (2014). Lesion mapping of social problem solving. Brain137, 2823–2833. 10.1093/brain/awu207
10
Beer J. S. Ochsner K. N. (2006). Social cognition: a multi level analysis. Brain Res.1079, 98–105. 10.1016/j.brainres.2006.01.002
11
Bird C. M. Castelli F. Malik O. Frith U. Husain M. (2004). The impact of extensive medial frontal lobe damage on ‘Theory of Mind’ and cognition. Brain127, 914–928. 10.1093/brain/awh108
12
D'Agata F. Caroppo P. Baudino B. Caglio M. Croce M. Bergui M. et al . (2011). The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum10, 600–610. 10.1007/s12311-011-0276-z
13
Frith C. D. Frith U. (2012). Mechanisms of social cognition. Annu. Rev. Psychol.63, 287–313. 10.1146/annurev-psych-120710-100449
14
Frith C. Frith U. (2005). Theory of mind. Curr. Biol.15, R644–R646. 10.1016/j.cub.2005.08.041
15
Gallagher H. L. Happe F. Brunswick N. Fletcher P. C. Frith U. Frith C. D. (2000). Reading the mind in cartoons and stories: an fMRI study of 'theory of mind' in verbal and nonverbal tasks. Neuropsychologia38, 11–21. 10.1016/S0028-3932(99)00053-6
16
Garrard P. Martin N. H. Giunti P. Cipolotti L. (2008). Cognitive and social cognitive functioning in spinocerebellar ataxia: a preliminary characterization. J. Neurol.255, 398–405. 10.1007/s00415-008-0680-6
17
Gobbini M. I. Koralek A. C. Bryan R. E. Montgomery K. J. Haxby J. V. (2007). Two takes on the social brain: a comparison of theory of mind tasks. J. Cogn. Neurosci.19, 1803–1814. 10.1162/jocn.2007.19.11.1803
18
Grandjean D. Sander D. Pourtois G. Schwartz S. Seghier M. L. Scherer K. R. et al . (2005). The voices of wrath: brain responses to angry prosody in meaningless speech. Nat. Neurosci.8, 145–146. 10.1038/nn1392
19
Guo W. Liu F. Chen J. Wu R. Zhang Z. Yu M. et al . (2015). Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci. Rep.5:17275. 10.1038/srep17275
20
Happe F. Ehlers S. Fletcher P. Frith U. Johansson M. Gillberg C. et al . (1996). 'Theory of mind' in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport8, 197–201. 10.1097/00001756-199612200-00040
21
Henry J. D. Cowan D. G. Lee T. Sachdev P. S. (2015). Recent trends in testing social cognition. Curr. Opin. Psychiatry28, 133–140. 10.1097/YCO.0000000000000139
22
Hoche F. Guell X. Sherman J. C. Vangel M. G. Schmahmann J. D. (2016). Cerebellar contribution to social cognition. Cerebellum15, 732–743. 10.1007/s12311-015-0746-9
23
Hoche F. Guell X. Vangel M. G. Sherman J. C. Schmahmann J. D. (2017). The cerebellar cognitive affective/Schmahmann syndrome scale. Brain141, 248–270. 10.1093/brain/awx317
24
Igelstrom K. M. Webb T. W. Graziano M. S. A. (2017). Functional connectivity between the temporoparietal cortex and cerebellum in Autism spectrum disorder. Cereb. Cortex. 27, 2617–2627. 10.1093/cercor/bhw079.
25
Ito M. (2008). Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci.9, 304–313. 10.1038/nrn2332
26
Jack A. Morris J. P. (2014). Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev. Cogn. Neurosci.10, 77–92. 10.1016/j.dcn.2014.08.001
27
Jack A. Pelphrey K. A. (2015). Neural correlates of animacy attribution include neocerebellum in healthy adults. Cereb. Cortex. 25, 4240–4247. 10.1093/cercor/bhu146
28
Jack A. Englander Z. A. Morris J. P. (2011). Subcortical contributions to effective connectivity in brain networks supporting imitation. Neuropsychologia49, 3689–3698. 10.1016/j.neuropsychologia.2011.09.024
29
Jack A. Keifer C. M. Pelphrey K. A. (2017). Cerebellar contributions to biological motion perception in autism and typical development. Hum. Brain Mapp.38, 1914–1932. 10.1002/hbm.23493
30
Kanaan R. A. Borgwardt S. McGuire P. K. Craig M. C. Murphy D. G. Picchioni M. et al . (2009). Microstructural organization of cerebellar tracts in schizophrenia. Biol. Psychiatry66, 1067–1069. 10.1016/j.biopsych.2009.07.028
31
Kim D. J. Kent J. S. Bolbecker A. R. Sporns O. Cheng H. Newman S. D. et al . (2014). Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr. Bull.40, 1216–1226. 10.1093/schbul/sbu059
32
Laidi C. d'Albis M. A. Wessa M. Linke J. Phillips M. L. Delavest M. et al . (2015). Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatr. Scand.131, 223–233. 10.1111/acps.12363
33
Moriarty A. Cook A. Hunt H. Adams M. E. Cipolotti L. Giunti P. (2016). A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J. Rare Dis.11:82. 10.1186/s13023-016-0447-6
34
Nguyen V. T. Sonkusare S. Stadler J. Hu X. Breakspear M. Guo C. C. (2017). Distinct cerebellar contributions to cognitive-perceptual dynamics during natural viewing. Cereb. Cortex27, 5652–5662. 10.1093/cercor/bhw334
35
Olivito G. Clausi S. Laghi F. Tedesco A. M. Baiocco R. Mastropasqua C. et al . (2017). Resting-state functional connectivity changes between dentate nucleus and cortical social brain regions in autism spectrum disorders. Cerebellum16, 283–292. 10.1007/s12311-016-0795-8
36
Parente A. Manfredi V. Tarallo A. Salsano E. Erbetta A. Pareyson D. et al . (2013). Selective theory of mind impairment and cerebellar atrophy: a case report. J. Neurol.260, 2166–2169. 10.1007/s00415-013-6985-0
37
Parker K. L. Kim Y. C. Kelley R. M. Nessler A. J. Chen K. H. Muller-Ewald V. A. et al . (2017). Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol. Psychiatry22, 647–655. 10.1038/mp.2017.50
38
Pavlova M. A. (2012). Biological motion processing as a hallmark of social cognition. Cereb. Cortex22, 981–995. 10.1093/cercor/bhr156
39
Pedersen A. Koelkebeck K. Brandt M. Wee M. Kueppers K. A. Kugel H. et al . (2012). Theory of mind in patients with schizophrenia: is mentalizing delayed?Schizophr. Res.137, 224–229. 10.1016/j.schres.2012.02.022
40
Roldan Gerschcovich E. Cerquetti D. Tenca E. Leiguarda R. (2011). The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase17, 270–275. 10.1080/13554791003730618
41
Ross C. A. Margolis R. L. Reading S. A. Pletnikov M. Coyle J. T. (2006). Neurobiology of schizophrenia. Neuron52, 139–153. 10.1016/j.neuron.2006.09.015
42
Ryan N. P. Catroppa C. Beare R. Silk T. J. Hearps S. J. Beauchamp M. H. et al . (2017). Uncovering the neuroanatomical correlates of cognitive, affective and conative theory of mind in paediatric traumatic brain injury: a neural systems perspective. Soc. Cogn. Affect. Neurosci.12, 1414–1427. 10.1093/scan/nsx066
43
Samson D. Apperly I. A. Chiavarino C. Humphreys G. W. (2004). Left temporoparietal junction is necessary for representing someone else's belief. Nat. Neurosci.7, 499–500. 10.1038/nn1223
44
Schmahmann J. D. (1998). Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn. Sci.2, 362–371. 10.1016/S1364-6613(98)01218-2
45
Schmahmann J. D. Sherman J. C. (1998). The cerebellar cognitive affective syndrome. Brain121, 561–579. 10.1093/brain/121.4.561
46
Shamay-Tsoory S. G. Aharon-Peretz J. Perry D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain132, 617–627. 10.1093/brain/awn279
47
Shinn A. K. Baker J. T. Lewandowski K. E. Ongur D. Cohen B. M. (2015). Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front. Hum. Neurosci.9:134. 10.3389/fnhum.2015.00134
48
Sinha P. Kjelgaard M. M. Gandhi T. K. Tsourides K. Cardinaux A. L. Pantazis D. et al . (2014). Autism as a disorder of prediction. Proc. Natl. Acad. Sci. U.S.A.111, 15220–15225. 10.1073/pnas.1416797111
49
Sokolov A. A. Erb M. Gharabaghi A. Grodd W. Tatagiba M. S. Pavlova M. A. (2012). Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus. Neuroimage59, 2824–2830. 10.1016/j.neuroimage.2011.08.039
50
Sokolov A. A. Erb M. Grodd W. Pavlova M. A. (2014). Structural loop between the cerebellum and the superior temporal sulcus: evidence from diffusion tensor imaging. Cereb. Cortex24, 626–632. 10.1093/cercor/bhs346
51
Sokolov A. A. Gharabaghi A. Tatagiba M. S. Pavlova M. (2010). Cerebellar engagement in an action observation network. Cereb. Cortex20, 486–491. 10.1093/cercor/bhp117
52
Sokolov A. A. Miall R. C. Ivry R. B. (2017). The cerebellum: adaptive prediction for movement and cognition. Trends Cogn. Sci.21, 313–332. 10.1016/j.tics.2017.02.005
53
Sokolovsky N. Cook A. Hunt H. Giunti P. Cipolotti L. (2010). A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav. Neurol.23, 17–29. 10.1155/2010/395045
54
Stanfield A. C. Philip R. C. M. Whalley H. Romaniuk L. Hall J. Johnstone E. C. et al . (2017). Dissociation of brain activation in Autism and schizotypal personality disorder during social judgments. Schizophr. Bull.43, 1220–1228. 10.1093/schbul/sbx083
55
Stoodley C. J. D'Mello A. M. Ellegood J. Jakkamsetti V. Liu P. Nebel M. B. et al . (2017). Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci.20, 1744–1751. 10.1038/s41593-017-0004-1
56
Suvrathan A. Payne H. L. Raymond J. L. (2016). Timing rules for synaptic plasticity matched to behavioral function. Neuron92, 959–967. 10.1016/j.neuron.2016.10.022
57
van Harskamp N. J. Rudge P. Cipolotti L. (2005). Cognitive and social impairments in patients with superficial siderosis. Brain128, 1082–1092. 10.1093/brain/awh487
58
Van Overwalle F. Baetens K. Marien P. Vandekerckhove M. (2014). Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage86, 554–572. 10.1016/j.neuroimage.2013.09.033
59
Van Overwalle F. Baetens K. Marien P. Vandekerckhove M. (2015a). Cerebellar areas dedicated to social cognition? A comparison of meta-analytic and connectivity results. Soc. Neurosci.10, 337–344. 10.1080/17470919.2015.1005666
60
Van Overwalle F. D'Aes T. Marien P. (2015b). Social cognition and the cerebellum: a meta-analytic connectivity analysis. Hum. Brain Mapp.36, 5137–5154. 10.1002/hbm.23002
61
Wadiche J. I. Jahr C. E. (2005). Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat. Neurosci.8, 1329–1334. 10.1038/nn1539
62
Wagner M. J. Kim T. H. Savall J. Schnitzer M. J. Luo L. (2017). Cerebellar granule cells encode the expectation of reward. Nature544, 96–100. 10.1038/nature21726
63
Wang S. S. Kloth A. D. Badura A. (2014). The cerebellum, sensitive periods, and autism. Neuron83, 518–532. 10.1016/j.neuron.2014.07.016
64
Wolpert C. Miall R. C. Kawato M. (1998). Internal models in the cerebellum. Trends Cogn. Sci.2, 338–347. 10.1016/S1364-6613(98)01221-2
65
Zhou H. Lin Z. Voges K. Ju C. Gao Z. Bosman L. W. et al . (2014). Cerebellar modules operate at different frequencies. Elife3:e02536. 10.7554/eLife.02536
Summary
Keywords
cerebellum, social cognition, emotion, autism spectrum disorders, spinocerebellar ataxia, stroke, connectivity, theory of mind (ToM)
Citation
Sokolov AA (2018) The Cerebellum in Social Cognition. Front. Cell. Neurosci. 12:145. doi: 10.3389/fncel.2018.00145
Received
17 January 2018
Accepted
14 May 2018
Published
05 June 2018
Volume
12 - 2018
Edited by
Chris I. De Zeeuw, Sophia Children's Hospital, Netherlands
Reviewed by
Stefano F. Cappa, Istituto Universitario di Studi Superiori di Pavia (IUSS), Italy
Updates
Copyright
© 2018 Sokolov.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arseny A. Sokolov arseny.sokolov@chuv.ch
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.