- Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Ministry of Education, Department of Surgical Oncology and General Surgery, The First Affiliated Hospital of China Medical University, Shenyang, China
The prognosis of stage IV gastric cancer (GC) is poor, with palliative chemotherapy remaining the main therapeutic option. Studies increasingly indicate that patients with unresectable stage IV GC, who undergo gastrectomy with radical intention after responding to several regimens of combined chemotherapy, can achieve good survival outcomes. Thus, surgery aiming at radical resection for unresectable stage IV GC after combined chemotherapy has received increasing attention in recent years. This novel therapeutic strategy was defined as conversion surgery in patients with unresectable stage IV GC and it can associate with significant improved survival when R0 resection can be achieved. Despite the recent advances in conversion surgery for patients with unresectable stage IV GC, selection criteria for combination chemotherapy regimens, indications for conversion surgery, optimal timing to surgery, and postoperative chemotherapy all remain controversial. This article reviews the current state of conversion surgery for unresectable stage IV GC.
Introduction
Despite early screening and improved intensive therapy, gastric cancer (GC) remains to be the fifth most common cancer and third most common cause of cancer-related deaths worldwide, leading to increased health care burden (1, 2). The prognosis for patients with stage IV gastric cancer is poor, and palliative chemotherapy remains the main therapeutic approach for this cohort (3, 4). Despite recent developments in chemotherapy, the median overall survival (OS) of stage IV GC patients remains to be 9–11 months (5, 6). For unresectable stage IV GC patients, only surgeries to relieve symptoms, such as a palliative resection or bypass operations, are commonly considered (7–9). The possibility of additional survival benefits achieved by chemotherapy following palliative surgery has been controversial. Recently, the REGATTA trial randomized 175 stage IV GC patients with a single incurable factor (liver metastases, peritoneal metastases, or para-aortic lymph node metastases) to chemotherapy alone or to initial gastrectomy followed by chemotherapy, but the surgery-first approach failed to show improvements in survival (10). Even with the greater advances achieved by adding a targeted monoclonal antibody to conventional chemotherapy (11–13), the prognosis for unresectable stage IV GC is still unsatisfactory. Thus, novel therapeutic strategies are required for treating unresectable stage IV GC patients.
Several combined S-1 based chemotherapy regimens may allow for conversion of initially unresectable GC to resectable cancer in clinical trials, and additional surgery following this chemotherapy was associated with long-term survival in selected patients (14–16). These advances raise new clinical issues in the treatment of unresectable stage IV GC patients. In some patients, incurable factors apparently disappear or are well-controlled during chemotherapy. For such patients, surgery with curable intent may be possible. Previous reviews investigating the effects of surgery for unresectable stage IV GC patients after chemotherapy also indicate that surgery with curable intention after chemotherapy is associated with prolonged survival in selected patients with a single incurable factor (17, 18), such as liver metastases, peritoneal metastases, or para-aortic lymph node metastases (19, 20). Thus, “conversion surgery” is defined as a surgical treatment aiming at a curable intention after tumors initially deemed technically or oncologically unresectable or only marginally resectable respond to chemotherapy (21, 22). Notably, conversion surgery refers to a radical cure and is different from palliative surgery or other terms concerning surgical resection for advanced incurable tumors such as “salvage,” “adjuvant,” or “secondary” surgery (23–27). Furthermore, there is no consensus on a clear border between curative surgery scheduled after neoadjuvant chemotherapy and conversion surgery (28, 29). Neoadjuvant chemotherapy can be used for initially resectable advanced GC to reduce tumor size and eradicate micrometastases to improve survival (30, 31), whereas chemotherapy for conversion surgery is used for unresectable advanced GC patients (18, 21).
Developments in conversion surgery have improved life expectancy in patients with incurable advanced GC, attracting increasing attention to conversion surgery for unresectable advanced GC in recent years (18, 32–34). However, selection criteria for combination chemotherapy regimens, indications for conversion surgery, optimal time to surgery, and postoperative chemotherapy all remain controversial. Therefore, we summarize the current state of the field regarding conversion surgery for stage IV GC in this review.
Search Strategy and Selection Criteria
We searched PubMed for articles published in English from 1997 to 2019 using the search terms “gastric cancer”, “conversion therapy,” “conversion surgery,” “stage IV gastric cancer,” “incurable advanced GC,” and “unresectable advanced GC.” No other parameters were applied. Case reports were excluded. Ultimately, 20 articles were included (shown in Table 1).
Conversion Surgery of Peritoneal Dissemination
Peritoneal metastases (PM), or peritoneal carcinomatosis, is the most common type of metastasis in stage IV GC with poor prognosis (38, 50, 51). Although GC patients with PM undergo combined intensive chemotherapy, the prognosis for this cohort was still unsatisfactory due to their relative resistance to systemic chemotherapy and low drug delivery into the abdominal cavity (35, 36). Developments in S-1 based chemotherapeutic regimens (S-1 plus cisplatin, SP; docetaxel plus cisplatin and S-1, DCS) for advanced GC patients (52–55) resulted in improved overall survival (OS) rate for advanced GC patients with PM. Thus, these advances in chemotherapy are expected to improve survival in unresectable stage IV GC patients with PM. A phase II trial of preoperative S-1 plus cisplatin (SP, oral S-1 plus intravenous cisplatin) chemotherapy, followed by gastrectomy with curable intention in unresectable stage IV GC patients with PM, showed a high response rate to SP with a longer OS over chemotherapy alone. Although most of the eligible patients in this trial had PM, R0 resection was still achieved in 51% of patients after preoperative SP chemotherapy, suggesting that controlling peritoneal dissemination is extremely important in conversion surgery for this cohort (15). Similarly, a trial of SP induction chemotherapy, followed by curative resection in unresectable stage IV GC patients with PM, showed a good response to SP chemotherapy followed by R0 resection with a high median survival time (MST) relative to chemotherapy alone (39). Despite advances in intravenous chemotherapy for unresectable stage IV GC patients with PM (22, 38–40, 55), drug delivery into the abdominal cavity remained low and sustained intraperitineal concentrations were still relatively poor with limited controlled efficacy (56).
Since intraperitoneal (IP) delivery of chemotherapy can attain higher drug exposure in the peritoneal cavity with reduced systemic toxicity (57), intraperitoneal administration of paclitaxel can provide sustained high local concentrations to increase its antitumor effects in GC patients with PM (58). Although promising results have been achieved for combination chemotherapy of IP paclitaxel with S-1 for patients with unresectable GC and PM, yielding a MST of 17.6 months and a 1-year OS of 77.1%(59), salvage gastrectomy on advanced GC patients with PM after disappearance or apparent shrinkage of PM yielded a MST of 26.4 months and a 1-year OS of 82%(60), indicating that conversion surgery may be considered in the cohort with a favorable response after IP paclitaxel plus systemic chemotherapy (50). A single-arm phase II study of conversion surgery following eight cycles of IP paclitaxel with systemic oxaliplatin and capecitabine (XELOX) in unresectable GC patients with PM and/or positive peritoneal washing cytology showed that six patients who underwent conversion gastrectomy, after a favorable response rate to combined XELOX and IP paclitaxel experienced a MST of 21.6 months, compared to patients receiving systemic chemotherapy alone in other trials who had MST of 3.1–10.6 months (50). Additionally, a recent meta-analysis indicated that there are survival benefits associated with hyperthermic intraperitoneal chemotherapy (HIPEC), delivering a high drug concentration for advanced GC patients with PM involvement compared with systemic chemotherapy alone (61). A cohort study of conversion surgery after HIPEC, plus chemotherapy in a small cohort of stage IV GC patients with PM, showed good long-term outcomes, suggesting that combination HIPEC may represent a useful and feasible technique to improve survival in GC patients with PM undergoing conversion surgery (47). In a GIRCG retrospective cohort study, 23 unresectable stage IV GC patients with PM received conversion gastrectomy after HIPEC plus chemotherapy, with a conversion rate of 60.5%(48).
A phase III trial was conducted (PHOENIX-GC), with the IP arm showing a better response in the amount of ascites and a high negative conversion rate of 78% for peritoneal cytology, further supporting the clinical benefit of the IP regimen for advanced GC with PM. However, OS was not significantly affected, indicating that further studies might be necessary to explore favorable candidate selection and new therapeutic strategies for intraperitoneal therapy (62). On the other hand, cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CRS+HIPEC) has been applied in GC with PM (63, 64). Although a large retrospective study showed that long-term survival could only be achieved in GC patients with limited PM, it is still expected to explore in unresectable GC patients with advanced PM (63). Therefore, additional trials involving various combinations of therapeutic options for GC patients with PM including cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CRS+HIPEC), neoadjuvant intraperitoneal and systemic chemotherapy (NIPS), and neoadjuvant laparoscopic hyperthermic intraperitoneal chemoperfusion (NLHIPEC) are still needed to explore their feasibility and efficacy for conversion.
Conversion Surgery of Liver Metastases
Stage IV GC patients present with various metastatic sites, and the liver is one of the most common sites of synchronous and metachronous GC metastases through the hematogenous pathway (65, 66). For unresectable advanced GC patients with liver metastases (LM), conversion surgery options encompass surgical therapies including liver resection, radiofrequency ablation (RFA), or microwave coagulation therapy (MCT) combined with systemic chemotherapy. Han et al. retrospectively reviewed clinicopathological data for surgery aiming at curative resection in GC patients with LM who responded well to induction chemotherapy. Of these, five GC patients with LM underwent radical gastric resection plus liver metastectomy after Docetaxel-Cisplatin-5-FU (DCF) chemotherapy, with a R0 resection rate of 100% (37). A retrospective trial conducted by Kinoshita et al. included 18 stage IV GC patients with LM receiving DCS chemotherapy. Among them, 11 underwent conversion gastrectomy (including 5 liver metastectomy) after DCS chemotherapy, with a MST of 18.9 months and a 3-year OS rate of 40.4%, whereas the MST was 15.6 months and 3-year OS rate was 27.5% for the 7 patients who did not achieve conversion surgery (40). Following this, a multi-institutional retrospective study conducted by Sato et al. included 29 GC patients with LM, among whom six underwent conversion surgery after DCS chemotherapy. Importantly, among the six patients with liver metastases, two underwent partial hepatectomies with a complete pathological response and two were treated with RFA, and after chemotherapy the metastatic lesions completely disappeared in two cases. Interestingly, DCS treatment led to conversion therapy in these patients with synchronous unresectable LM, and this cohort had good prognosis with a MTS of 22 months compared with chemotherapy alone (41). Additionally, Yamaguchi et al. reported that 20 stage IV GC patients with LM underwent conversion surgery plus liver metastasectomy after chemotherapy with a conversion rate of 21.5% (20/93), and suggested that metastasectomy along with primary tumor resection might be feasible for this population, provided that the metastases respond well to chemotherapy (46). Similarly, Beom et al. reported that three stage IV GC patients with LM who received radical gastrectomy plus hepatectomy after a better response (CR/PR, complete response/partial response) to chemotherapy had a good MST of 49.2 months compared with other types of distant metastasis with a MST of 13.6 months (32). Furthermore, Li et al. reported that stage IV GC patients with LM saw remarkable survival benefit from simultaneous liver resection or RFA after a good response to chemotherapy relative to chemotherapy alone, which may relate to their nearly tumor-free status after simultaneous surgery or RFA of LMs (49).
Although there is good prognosis for multiple conversion options in stage IV GC patients with LM, a multi-institutional retrospective study of conversion surgery after DCS chemotherapy in GC patients with LM showed a recurrence rate was 50% (41). Furthermore, previous studies of incurable GC patients with LM undergoing liver resection or RFA without preoperative therapy found recurrence rates up to 63.6–91.0% (65–67). Therefore, postoperative chemotherapy should be accompanied by cautious follow up (37). Despite promising indications for conversion surgery in unresectable GC patients with LM, the potential benefits of surgical resection and best treatment regimens in this cohort remain to be determined by further prospective studies and randomized controlled trials.
Conversion Surgery of Lymph Node Metastases
GC patients with extensive lymph node metastases, including para-aortic lymph node (PAN) metastases or bulky nodes around the hepatic, splenic, or celiac arteries, are often considered to be unresectable and have poor prognosis (68). However, an adequate lymphadenectomy during surgical treatment is crucial for GC treatment, especially for unresectable GC patients with PAN metastases who undergo combined chemotherapy. A study conducted by Park et al. followed outcomes of GC patients with isolated PAN metastases following palliative chemotherapy, finding a 3-year OS of only 13.1% (69). Even when GC patients with PAN metastases can undergo gastrectomy, these patients still had poor survival outcomes, with a 3-year OS of 5% (68). Therefore, a preoperative chemotherapy approach has been recommended as a treatment strategy for GC patients with PAN metastases. Alternatively, a randomized controlled trial of JCOG9501 indicated that D2 lymphadenectomy plus preventative PAN dissection (PAND) does not improve survival rate in patients with curable GC compared with D2 lymphadenectomy alone (70), however cases with macroscopic PAN metastases at surgery were excluded from analysis, leading to a low incidence of metastatic PAN in patients with PAND. Therefore, the prognostic efficacy of PAND after chemotherapy for GC patients with PAN metastases is still unclear (71). Thus, further studies are necessary to clarify the importance of PAND after induction chemotherapy.
Two phase II trials (JCOG0001and JCOG0405) were conducted to evaluate the safety and efficacy of gastrectomy with D2 lymphadenectomy plus PAND for GC patients with PAD metastases after preoperative combined chemotherapy. In JCOG001 and JCOG0405, GC patients with PAD metastases who received two or three cycles of irinotecan and cisplatinor cisplatin and S-1 chemotherapy, followed by gastrectomy with D2 lymphadenectomy plus PAND yielded a 3-year survival of 27.0 and 58.5%, respectively (72, 73). Therefore, combined chemotherapy followed by gastrectomy with D2 lymphadenectomy plus PAND are considered as safe and effective treatments for GC patients with PAD metastases. Since S-1 based chemotherapy was indicated to improve outcomes for advanced unresectable GC patients with PAD metastases (53, 54, 74), recent trials have seen encouraging outcomes for conversion gastrectomy with D2 lymphadenectomy plus PAND after chemotherapy in stage IV GC patients with PAN metastases. Saito et al. reported that unresectable stage IV GC patients with PAN metastases, who underwent radical gastrectomy with D2 lymphadenectomy plus PAND after induction CS chemotherapy, yielded a conversion surgery rate of 25.0% (4/16) (39). Additionally, a multi-institutional retrospective study of unresectable advanced GC patients with PAN metastases who underwent radical gastrectomy with D2 lymphadenectomy plus PAND after DCS chemotherapy showed a good conversion surgery rate of 33.3% (9/27) and a good median OS of 47.8 months, over the median OS of 15.7 months for chemotherapy alone (41). Furthermore, a retrospective study of stage IV GC patients with PAN metastases undergoing conversion surgery with D2 lymphadenectomy plus PAND after DCS chemotherapy showed a high conversion surgery rate of 73.9% (17/23) and a good 3-year OS over chemotherapy alone (72.9 vs. 15.2%) (40).
Although, unresectable stage IV GC patients with PAN metastases, receiving induction chemotherapy followed by conversion surgery with D2 lymphadenectomy plus PAND, have achieved better conversion resection rates and survival outcomes compare to chemotherapy alone, the prognosis for this cohort is still unsatisfactory. Based on the promising outcomes of radiotherapy combined with chemotherapy for locally advanced GC patients with lymph node metastases (75–77), preoperative radiotherapy may improve the long-survival of unresectable stage IV GC patients with PAN metastases. Therefore, further research must identify optimal preoperative multimodal treatments of radiotherapy combined with chemotherapy for this cohort and further explore its feasibility and efficacy in the near future.
Future Work and Perspectives
Selecting Stage IV GC Patients That Can Benefit From Conversion Surgery
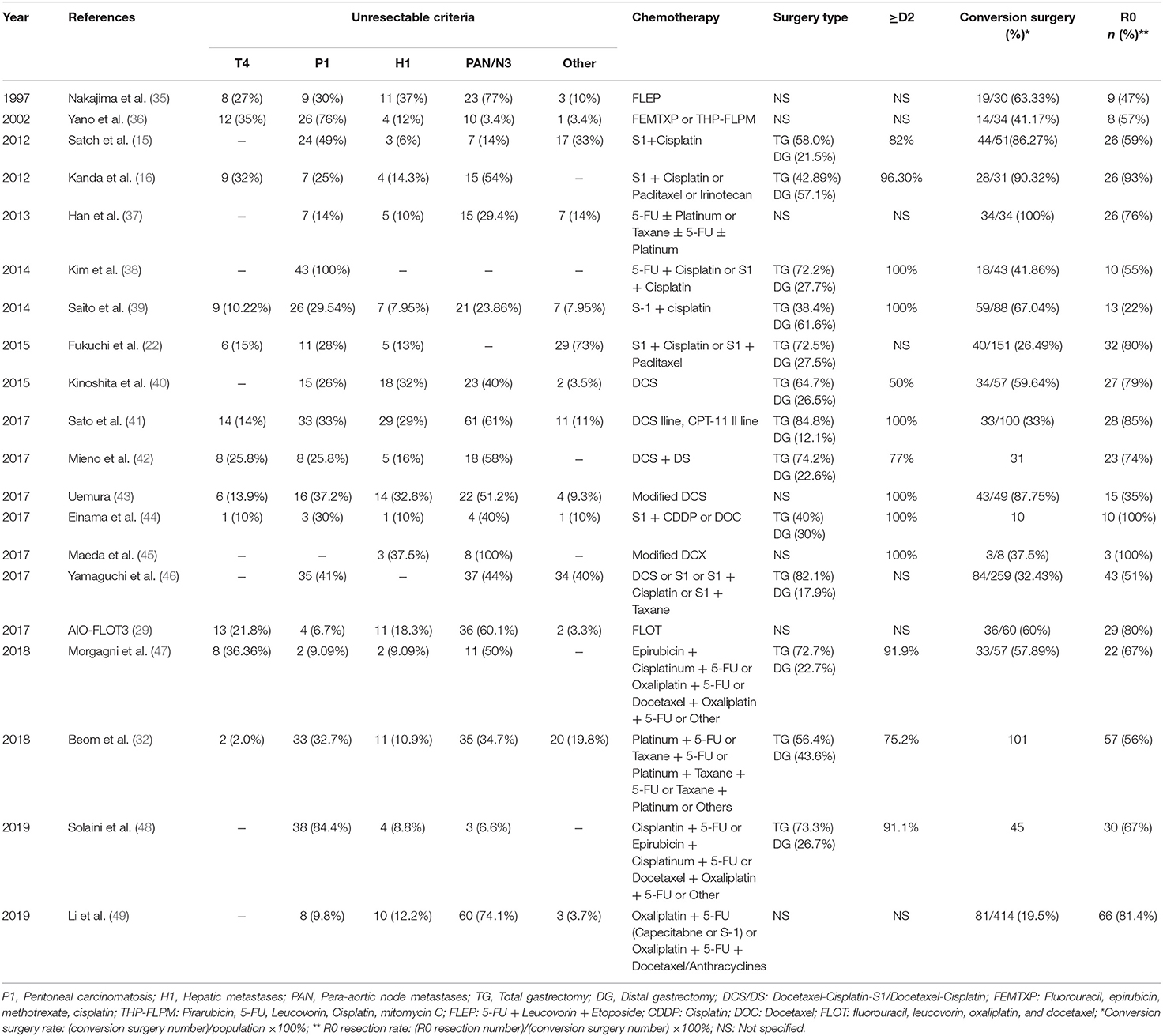
It is extremely important to identify stage IV GC patients that can benefit from conversion surgery. Although palliative gastrectomy followed by chemotherapy showed no survival benefit for these patients, compared with chemotherapy alone in the REGATTA trial (10), this trial helped oncological surgeons to select eligibility criteria for surgery in unresectable advanced GC. Further studies also indicated that unresectable stage IV GC patients with a single incurable factor (liver metastases, peritoneal metastases, or para-aortic lymph node metastases) receiving combined chemotherapy followed by conversion surgery have achieved high R0 resection rates and good prognosis (32, 41–43, 46–48). Thus, the number of metastatic sites may be an important indicator for obtaining down-staging by chemotherapy and a good prognosis after conversion surgery. Additionally, rates of relatively severe postoperative complications between 24.2 and 40% (35, 40, 41, 48) make stringent selection of unresectable stage IV GC patients who may benefit from conversion surgery increasingly necessary. Criteria for conversion surgery included: no sign of organ failure, age between 20 and 80 years, Eastern Cooperative Oncology Group scale performance status 0–2, and one single incurable factor (41, 45). Moreover, modern diagnostic tools, such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography CT (PET-CT), upper gastrointestinal tract endoscopy, and ultrasonography, may help determine clinical staging before undertaking surgical intervention for GC patients (41, 42, 44). Additionally, staging laparoscopy with or without peritoneal lavage also plays an important role in order to confirm whether the peritoneal deposits disappeared completely or whether positive cytology turned negative (50). Despite recent trials suggesting that candidates for conversion surgery were those for whom R0 resection could be obtained following response to chemotherapy (22, 41), new categories of classification were proposed by Yoshida et al. based on the absence or presence of macroscopic peritoneal dissemination (17) (shown in Figure 1). Optimal recommended indications for conversion surgery include marginally resectable metastasis, some incurable and unresectable except certain circumstances of local palliation needs, and a few non-curable metastasis patients with GC. Based on this novel classification, promising results of conversion surgery in unresectable stage IV GC patients have been achieved in three cohort studies (46–48). However, as it is sometimes extremely difficult to determine between marginally resectable or unresectable tumors, it is controversial whether the Yoshida classification can be used as a definite standard (18). Thus, adequate selection of stage IV GC patients for conversion surgery is an important upcoming task for surgical oncologists.
Figure 1. Biological categories for conversion surgery introduced by Yoshida et al. (17).
Selecting the Best Timing for Conversion Surgery
Optimally, surgery is performed when the tumor has decreased most in size in response to chemotherapy and before chemotherapy resistance allows it to grow again (17, 22, 78). This literature review found interval times between chemotherapy and surgery ranging from 4 to 391 days (Table 2). Yoshida et al. estimated the optimal operation opportunity to be after a CR or PR response is determined following chemotherapy (17), with a mean interval time for resection after chemotherapy of approximately 126 days (Table 2). However, a randomized phase II study (COMPASS trial) by Yoshikawa et al. reported that 2–6 weeks after completion of neoadjuvant chemotherapy might be adequate (79). This is consistent with results from many studies listed in Table 2. Thus, there are currently two perspectives for optimal surgery time among surgical oncologists: (1) patients who have achieved the indications of surgical treatment after definitive chemotherapy should have conversion surgery performed, or (2) the chemotherapy duration could be extended to 6 months or even 1 year. After the disease condition is stable, conversion surgery could then be carried out, possibly increasing patient benefits and safety. Both views are reasonable, however whether one is superior remains to be further explored with additional evidence needed.
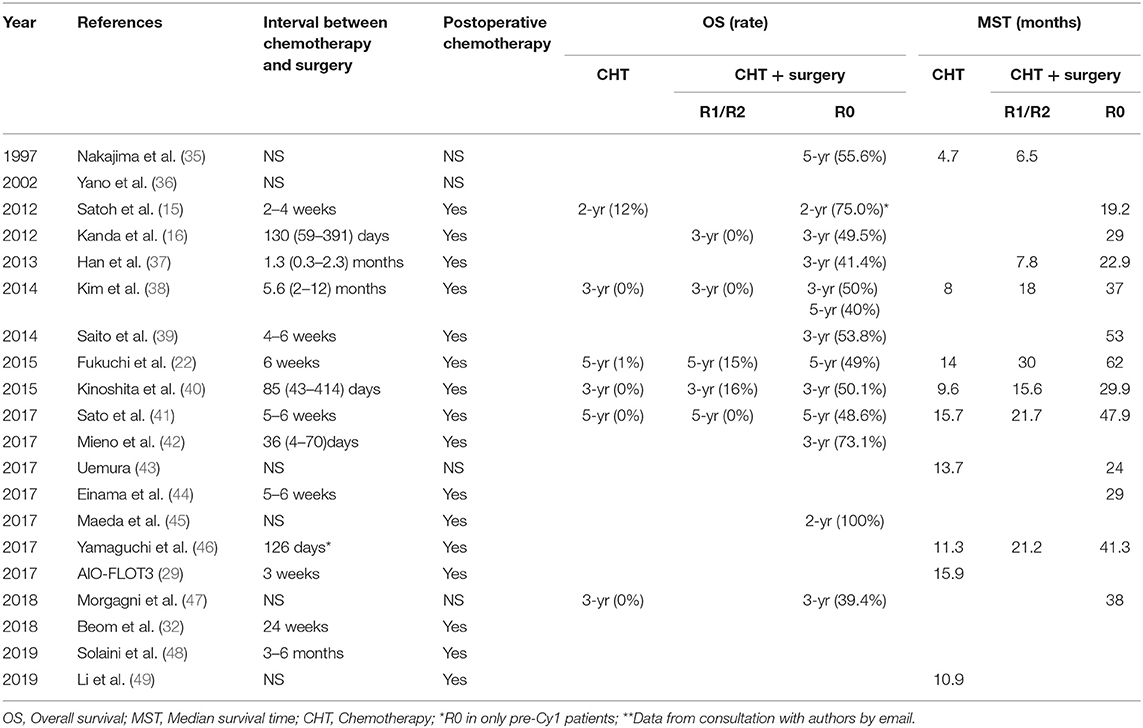
Table 2. Time of interval to surgery, postoperative chemotherapy, overall survival, and median survival time.
Selecting Preoperative Drug Therapeutic Strategies to Achieve Conversion
Based on good response to S-1/cisplatin (SP)(52), S-1/docetaxel (DS) (80), capecitabine plus cisplatin (XP) with or without trastuzumab (11), S-1 plus irinotecan (IRI-S) (81) and S-1 plus docetaxel, cisplatin (DCS) (68), and cisplatin/paclitaxel (82) in advanced unresectable GC, preoperative S-1 based chemotherapies are considered as main therapeutic options for conversion therapy. Additionally, clinical targeted drugs have been developing quickly, especially in the fields of lung cancer, breast cancer and soft tissue tumor. For GC, the ToGA study gives hope that HER2 positive advanced GC patients undergoing chemotherapy combined with trastuzumab can significantly prolong their OS compared with chemotherapy alone, and this regimen has become a standard treatment for HER2 positive advanced GC patients (11, 56). Additionally, ramucirumab, an anti-angiogenesis drug, has been well-verified in clinical practice for treating advanced GC (12, 13), indicating that targeted drugs for conversion surgery in unresectable stage IV GC patients may serve as promising therapeutic options to improve clinical outcomes. Furthermore, combination immunotherapy for conversion therapy in unresectable advanced colorectal cancer or inoperable advanced lung cancer has achieved broad prospects with a good rate of conversion or high rate of R0 resection (83, 84). Although several clinical trials examining PD-1/PD-L blockade combination treatments in advanced GC were identified (NCT01848834, NCT01928394, NCT02335411, NCT02340975), studies of immunotherapy for conversion surgery in unresectable stage IV GC are still scarce. Thus, combination immunotherapy for conversion surgery in unresectable stage IV GC is expected to prolong survival of this cohort with a high rate of conversion, and further studies are necessary to determine its feasibility and safety.
In conclusion, conversion surgery for unresectable stage IV gastric cancer was associated with longer survival over chemotherapy alone. GC patients with a single incurable factor who experienced a favorable response to combination chemotherapy achieved better survival outcomes than those with multiple metastatic organs. Additionally, patients undergoing R0 resection had better prognosis than those with R1 or R2 resection. Common definitions remain to be clarified regarding the selection of initial combination chemotherapy, the timing of conversion surgery, and indications for postoperative chemotherapy. Additional trials are imperative to address these important issues and to confirm their feasibility and validity to further improve the prognosis of unresectable stage IV GC patients.
Author Contributions
FZ played a major role in writing the manuscript. XH and PG provided important feedback and helped in editing the manuscript. YS, ZWa, and PG participated in studies selection. CZ, ZG, JS, and ZWu contributed to the literature search. All authors have approved the final version of the manuscript.
Funding
This work was supported by grants from the National Key R&D Program of China (MOST-2016YFC1303200, MOST-2016YFC1303202, MOST-2017YFC0908300, and MOST-2017YFC0908305), and the guidance plan of the Natural Science Foundation of Liaoning Province of China (20180550582).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CRS, cytoreductive surgery; CR, complete response; EIPL, extensive intraoperative peritoneal lavage; GC, gastric cancer; HIPEC, hyperthermic intraperitoneal chemotherapy; IP, intraperitoneal; LM, liver metastases; MST, median survival time; NAC, neoadjuvant chemotherapy; NLHIPEC, neoadjuvant laparoscopic hyperthermic intraperitoneal chemoperfusion; NIPS, neoadjuvant intraperitoneal and systemic chemotherapy; OS, overall survival; PAN, para-aortic lymph node; PM, Peritoneal metastases; PR, partial response; RR, response rate.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2018) 68:394–24. doi: 10.3322/caac.21492
2. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. (2018) 4:1553–68. doi: 10.1200/JCO.2018.36.15_suppl.1568
3. Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. (2011) 14:97–100. doi: 10.1007/s10120-011-0040-6
4. Dittmar Y, Rauchfuss F, Goetz M, Jandt K, Scheuerlein H, Heise M, et al. Non-curative gastric resection for patients with stage 4 gastric cancer–a single center experience and current review of literature. Langenbeck's Arch Surg. (2012) 397:745–53. doi: 10.1007/s00423-012-0902-3
5. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. (2006) 24:4991–7. doi: 10.1200/JCO.2006.06.8429
6. Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. (2009) 10:1063–9. doi: 10.1016/S1470-2045(09)70259-1
7. Lordick F, Siewert JR. Recent advances in multimodal treatment for gastric cancer: a review. Gastric Cancer. (2005) 8:78–85. doi: 10.1007/s10120-005-0321-z
8. Mahar AL, Coburn NG, Karanicolas PJ, Viola R, Helyer LK. Effective palliation and quality of life outcomes in studies of surgery for advanced, non-curative gastric cancer: a systematic review. Gastric Cancer. (2012) 15 (Suppl. 1):S138–45. doi: 10.1007/s10120-011-0070-0
9. Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. (2013) 14:e535–47. doi: 10.1016/S1470-2045(13)70436-4
10. Fujitani K, Yang H-K, Mizusawa J, Kim Y-W, Terashima M, Han S-U, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. (2016) 17:309–18. doi: 10.1016/S1470-2045(15)00553-7
11. Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
12. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. (2014) 15:1224–35. doi: 10.1016/S1470-2045(14)70420-6
13. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. (2014) 383:31–9. doi: 10.1016/S0140-6736(13)61719-5
14. Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K, et al. Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer Res. (2015) 35:401–6.
15. Satoh S, Okabe H, Teramukai S, Hasegawa S, Ozaki N, Ueda S, et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. (2012) 15:61–9. doi: 10.1007/s10120-011-0066-9
16. Kanda T, Yajima K, Kosugi S, Ishikawa T, Ajioka Y, Hatakeyama K. Gastrectomy as a secondary surgery for stage IV gastric cancer patients who underwent S-1-based chemotherapy: a multi-institute retrospective study. Gastric Cancer. (2012) 15:235–44. doi: 10.1007/s10120-011-0100-y
17. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19:329–38. doi: 10.1007/s10120-015-0575-z
18. Zurleni T, Gjoni E, Altomare M, Rausei S. Conversion surgery for gastric cancer patients: a review. World J Gastrointestinal Oncol. (2018) 10:398–409. doi: 10.4251/wjgo.v10.i11.398
19. Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol. (2004) 34:654–9. doi: 10.1093/jjco/hyh120
20. Lee JH, Paik YH, Lee JS, Song HJ, Ryu KW, Kim CG, et al. Candidates for curative resection in advanced gastric cancer patients who had equivocal para-aortic lymph node metastasis on computed tomographic scan. Ann Surg Oncol. (2006) 13:1163–7. doi: 10.1245/s10434-006-9002-3
21. Terashima M. Conversion therapy for gastric cancer: who can make conversion as successful as Goromaru? Gastric Cancer. (2016) 19:685–6. doi: 10.1007/s10120-016-0609-1
22. Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. (2015) 22:3618–24. doi: 10.1245/s10434-015-4422-6
23. Muto O, Kotanagi H. Palliative resection for stage IV gastric cancer. J Clin Oncol. (2011) 29(4 Suppl):148. doi: 10.1200/jco.2011.29.4_suppl.148
24. Muto O, Kotanagi H. The efficacy of gastrectomy plus chemotherapy for stage IV gastric cancer. J Clin Oncol. (2012) 30(4 Suppl):132. doi: 10.1200/jco.2012.30.4_suppl.132
25. Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. (2013) 13:577. doi: 10.1186/1471-2407-13-577
26. Markar S, Gronnier C, Duhamel A, Pasquer A, Théreaux J, Rieu MCd, et al. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol. (2015) 33:3866–73. doi: 10.1200/JCO.2014.59.9092
27. Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, et al. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. (2010) 1:743–7. doi: 10.3892/ol_00000130
28. Ramos M, Pereira MA, Charruf AZ, Dias AR, Castria TB, Barchi LC, et al. Conversion therapy for gastric cancer: expanding the treatment possibilities. Arquivos Brasileiros de Cirurgia Digestiva. (2019) 32:e1435. doi: 10.1590/0102-672020190001e1435
29. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 Trial. JAMA Oncol. (2017) 3:1237–44. doi: 10.1001/jamaoncol.2017.0515
30. Kodera Y. Neoadjuvant chemotherapy for gastric adenocarcinoma in Japan. Surg Today. (2017) 47:899–907. doi: 10.1007/s00595-017-1473-2
31. Hu S-B, Liu C-H, Wang X, Dong Y-W, Zhao L, Liu H-F, et al. Pathological evaluation of neoadjuvant chemotherapy in advanced gastric cancer. World J Surg Oncol. (2019) 17:3. doi: 10.1186/s12957-018-1534-z
32. Beom SH, Choi YY, Baek SE, Li SX, Lim JS, Son T, et al. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer. (2018) 18:1116. doi: 10.1186/s12885-018-4998-x
33. Kodera Y. Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg. (2018) 2:339–47. doi: 10.1002/ags3.12191
34. Du R, Hu P, Liu Q, Zhang J. Conversion surgery for unresectable advanced gastric cancer: a systematic review and meta-analysis. Cancer Investig. (2019) 37:16–28. doi: 10.1080/07357907.2018.1551898
35. Nakajima T, Ota K, Ishihara S, Oyama S, Nishi M, Ohashi Y, et al. Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol. (1997) 4:203–8. doi: 10.1007/BF02306611
36. Yano M, Shiozaki H, Inoue M, Tamura S, Doki Y, Yasuda T, et al. Neoadjuvant chemotherapy followed by salvage surgery: effect on survival of patients with primary noncurative gastric cancer. World J Surg. (2002) 26:1155–9. doi: 10.1007/s00268-002-6362-0
37. Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, et al. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol. (2013) 107:511–6. doi: 10.1002/jso.23284
38. Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastr Cancer. (2014) 14:266–70. doi: 10.5230/jgc.2014.14.4.266
39. Saito M, Kiyozaki H, Takata O, Suzuki K, Rikiyama T. Treatment of stage IV gastric cancer with induction chemotherapy using S-1 and cisplatin followed by curative resection in selected patients. World J Surg Oncol. (2014) 12:406. doi: 10.1186/1477-7819-12-406
40. Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. (2015) 41:1354–60. doi: 10.1016/j.ejso.2015.04.021
41. Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. (2017) 20:517–26. doi: 10.1007/s10120-016-0633-1
42. Mieno H, Yamashita K, Hosoda K, Moriya H, Higuchi K, Azuma M, et al. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surgery Today. (2017) 47:1249–58. doi: 10.1007/s00595-017-1512-z
43. Uemura N, Kikuchi S, Sato Y, Ohnuma H, Okamoto K, Miyamoto H, et al. A phase II study of modified docetaxel, cisplatin, and S-1 (mDCS) chemotherapy for unresectable advanced gastric cancer. Cancer Chemotherap Pharmacol. (2017) 80:707–13. doi: 10.1007/s00280-017-3404-8
44. Einama T, Abe H, Shichi S, Matsui H, Kanazawa R, Shibuya K, et al. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol. (2017) 6:163–6. doi: 10.3892/mco.2017.1128
45. Maeda O, Matsuoka A, Miyahara R, Funasaka K, Hirooka Y, Fukaya M, et al. Modified docetaxel, cisplatin and capecitabine for stage IV gastric cancer in Japanese patients: a feasibility study. World J Gastroenterol. (2017) 23:1090–7. doi: 10.3748/wjg.v23.i6.1090
46. Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. (2018) 21:315–23. doi: 10.1007/s10120-017-0738-1
47. Morgagni P, Solaini L, Framarini M, Vittimberga G, Gardini A, Tringali D, et al. Conversion surgery for gastric cancer: a cohort study from a western center. Int J Surg. (2018) 53:360–5. doi: 10.1016/j.ijsu.2018.04.016
48. Solaini L, Ministrini S, Bencivenga M, D'Ignazio A, Marino E, Cipollari C, et al. Conversion gastrectomy for stage IV unresectable gastric cancer: a GIRCG retrospective cohort study. Gastric Cancer. (2019) 22:1285–93. doi: 10.1007/s10120-019-00968-2
49. Li W, Jiang H, Yu Y, Wang Y, Wang Z, Cui Y, et al. Outcomes of gastrectomy following upfront chemotherapy in advanced gastric cancer patients with a single noncurable factor: a cohort study. Cancer Manage Res. (2019) 11:2007–13. doi: 10.2147/CMAR.S192570
50. Chan DY, Syn NL, Yap R, Phua JN, Soh TI, Chee CE, et al. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. are we ready? J Gastrointest Surg. (2017) 21:425–33. doi: 10.1007/s11605-016-3336-3
51. Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. (2014) 134:622–8. doi: 10.1002/ijc.28373
52. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. (2008) 9:215–21. doi: 10.1016/S1470-2045(08)70035-4
53. Nakayama N, Koizumi W, Sasaki T, Higuchi K, Tanabe S, Nishimura K, et al. A multicenter, phase I dose-escalating study of docetaxel, cisplatin and S-1 for advanced gastric cancer (KDOG0601). Oncology. (2008) 75(1–2):1–7. doi: 10.1159/000151613
54. Fushida S, Fujimura T, Oyama K, Yagi Y, Kinoshita J, Ohta T. Feasibility and efficacy of preoperative chemotherapy with docetaxel, cisplatin and S-1 in gastric cancer patients with para-aortic lymph node metastases. Anti-cancer Drugs. (2009) 20:752–6. doi: 10.1097/CAD.0b013e32832ec02b
55. Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, Okubo S, et al. Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemotherap Pharmacol. (2010) 66:721–8. doi: 10.1007/s00280-009-1215-2
56. Shitara K, Ohtsu A. Advances in systemic therapy for metastatic or advanced gastric cancer. J Natl Comp Cancer Netw. (2016) 14:1313–20. doi: 10.6004/jnccn.2016.0138
57. Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol. (1994) 12:970–4. doi: 10.1200/JCO.1994.12.5.970
58. Shitara K. Chemotherapy for advanced gastric cancer: future perspective in Japan. Gastric Cancer. (2017) 20(Suppl 1):102–10. doi: 10.1007/s10120-016-0648-7
59. Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. (2013) 119:3354–8. doi: 10.1002/cncr.28204
60. Kitayama J, Ishigami H, Yamaguchi H, Yamashita H, Emoto S, Kaisaki S, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. (2014) 21:539–46. doi: 10.1245/s10434-013-3208-y
61. Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, et al. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. (2017) 79:1–14. doi: 10.1016/j.ejca.2017.03.030
62. Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III Trial Comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin Plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC Trial. J Clin Oncol. (2018) 36:1922–9. doi: 10.1200/JCO.2018.77.8613
63. Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. (2010) 17:2370–7. doi: 10.1245/s10434-010-1039-7
64. Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev. (2016) 48:42–9. doi: 10.1016/j.ctrv.2016.06.007
65. Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. (2008) 19:1146–53. doi: 10.1093/annonc/mdn026
66. Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. (2007) 95:534–9. doi: 10.1002/jso.20739
67. Guner A, Son T, Cho I, Kwon IG, An JY, Kim H-I, et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer. (2016) 19:951–60. doi: 10.1007/s10120-015-0522-z
68. Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. (2017) 20:322–31. doi: 10.1007/s10120-016-0619-z
69. Park IH, Kim SY, Kim YW, Ryu KW, Lee JH, Lee JS, et al. Clinical characteristics and treatment outcomes of gastric cancer patients with isolated para-aortic lymph node involvement. Cancer Chemotherap Pharmacol. (2011) 67:127–36. doi: 10.1007/s00280-010-1296-y
70. Nomura E, Sasako M, Yamamoto S, Sano T, Tsujinaka T, Kinoshita T, et al. Risk factors for para-aortic lymph node metastasis of gastric cancer from a randomized controlled trial of JCOG9501. Jpn J Clin Oncol. (2007) 37:429–33. doi: 10.1093/jjco/hym067
71. Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, et al. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol. (2010) 36:439–46. doi: 10.1016/j.ejso.2010.03.008
72. Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. (2009) 96:1015–22. doi: 10.1002/bjs.6665
73. Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. (2014) 101:653–60. doi: 10.1002/bjs.9484
74. Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, et al. A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemotherap Pharmacol. (2012) 69:407–13. doi: 10.1007/s00280-011-1701-1
75. Martin-Romano P, Sola JJ, Diaz-Gonzalez JA, Chopitea A, Iragorri Y, Martinez-Regueira F, et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br J Cancer. (2016) 115:655–63. doi: 10.1038/bjc.2016.252
76. Dong HM, Wang Q, Wang WL, Wang G, Li XK, Li GD, et al. A clinical analysis of systemic chemotherapy combined with radiotherapy for advanced gastric cancer. Medicine. (2018) 97:e10786. doi: 10.1097/MD.0000000000010786
77. Ustaalioglu BBO, Bilici A, Tilki M, Surmelioglu A, Erkol B, Figen M, et al. Capecitabine-cisplatin versus 5-fluorouracil/leucovorin in combination with radiotherapy for adjuvant therapy of lymph node positive locally advanced gastric cancer. J Cancer Res Therap. (2018) 14(Supplement):S736–41. doi: 10.4103/0973-1482.183548
78. Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. (2006) 24:2325–31. doi: 10.1200/JCO.2005.05.3439
79. Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol. (2014) 21:213–9. doi: 10.1245/s10434-013-3055-x
80. Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. (2014) 140:319–28. doi: 10.1007/s00432-013-1563-5
81. Kodera Y, Ito S, Mochizuki Y, Ohashi N, Tanaka C, Kobayashi D, et al. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer. (2012) 15:335–7. doi: 10.1007/s10120-012-0156-3
82. Shin SJ, Chun SH, Kim KO, Kim MK, Lee KH, Hyun MS, et al. The efficacy of paclitaxel and cisplatin combination chemotherapy for the treatment of metastatic or recurrent gastric cancer: a multicenter phase II study. Korean J Int Med. (2005) 20:135–40. doi: 10.3904/kjim.2005.20.2.135
83. Nakai T, Okuno K, Kitaguchi H, Ishikawa H, Yamasaki M. Unresectable colorectal liver metastases: the safety and efficacy of conversion therapy using hepatic arterial infusion immunochemotherapy with 5-fluorouracil and polyethylene glycol-interferon alpha-2a. World J Surg. (2013) 37:1919–26. doi: 10.1007/s00268-013-2043-4
Keywords: conversion surgery, conversion therapy, metastatic gastric cancer, unresectable gastric cancer, combined chemotherapy, stage IV gastric cancer
Citation: Zhang F, Huang X, Song Y, Gao P, Zhou C, Guo Z, Shi J, Wu Z and Wang Z (2019) Conversion Surgery for Stage IV Gastric Cancer. Front. Oncol. 9:1158. doi: 10.3389/fonc.2019.01158
Received: 16 August 2019; Accepted: 17 October 2019;
Published: 07 November 2019.
Edited by:
Lin Chen, PLA General Hospital, ChinaReviewed by:
Bin Xiong, Zhongnan Hospital, Wuhan University, ChinaMarcel Verheij, Antoni van Leeuwenhoek Hospital, Netherlands
Copyright © 2019 Zhang, Huang, Song, Gao, Zhou, Guo, Shi, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenning Wang, josieon826@sina.cn
†These authors have contributed equally to this work
 Fei Zhang
Fei Zhang Xuanzhang Huang
Xuanzhang Huang Yongxi Song
Yongxi Song Peng Gao
Peng Gao Cen Zhou
Cen Zhou Zhexu Guo
Zhexu Guo Jinxin Shi
Jinxin Shi Zhonghua Wu
Zhonghua Wu Zhenning Wang
Zhenning Wang