- Department of Urology, Institute of Urology, National Clinical Research Center for Geriatrics and Center of Biomedical Big Data, West China Hospital of Sichuan University, Chengdu, China
Background: Primary signet-ring cell carcinoma (SRCC) is a rare variation of adenocarcinoma. Although SRCC of the urinary bladder is highly malignant, it is often neglected due to its rarity.
Materials and Methods: We used the national Surveillance, Epidemiology, and End Results (SEER) database (2004–2016) to compare SRCC with urothelial carcinoma (UC) and investigated the prognostic values of the clinicopathological characteristics and survival outcomes in SRCC of the urinary bladder. Multivariable Cox proportional hazard model, subgroup analyses, and propensity score matching (PSM) were used.
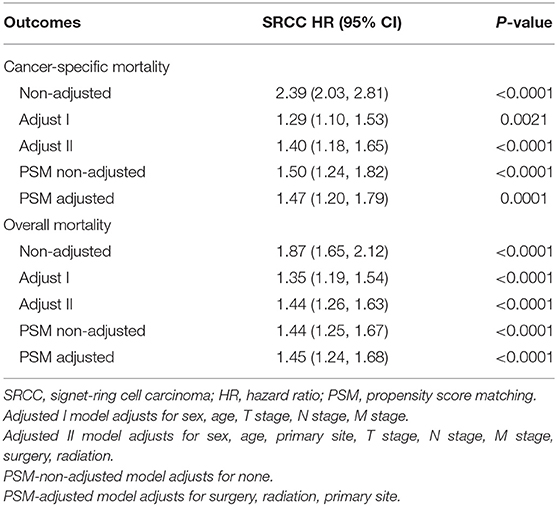
Results: In all, 318 patients with SRCC and 57,444 patients with UC were enrolled. Compared with those with UC, patients with SRCC were younger at diagnosis (P < 0.001) and had higher rates of muscle invasive disease (P < 0.001), lymph node metastasis (P < 0.001), and distal metastasis (P < 0.001), as well as higher-grade tumors (P = 0.004). A Cox proportional hazard regression analysis showed that the SRCC group was associated with significantly higher risks of overall mortality (OM) compared with the UC group [hazard ratios (HR) = 1.44, 95% confidence intervals (95% CI) = 1.26–1.63, P < 0.0001]. Patients with SRCC also had a higher risk of cancer-specific mortality (CSM; HR = 1.40, 95% CI = 1.18–1.65, P < 0.0001). After PSM, the SRCC group also experienced higher risks of OM (HR = 1.45, 95% CI = 1.24–1.68, P < 0.0001) and CSM (HR = 1.47, 95% CI = 1.20–1.79, P = 0.0001) compared with the UC group. In the subgroup analyses, no significant interactions were observed in sex, age, N stage, M stage, and lymph nodes removed in terms of both OM and CSM.
Conclusion: The prognosis of SRCC is poorer than that of UC, even after adjustment for baseline demographic and clinicopathological characteristic as well as cancer treatment. SRCC is an independent prognostic factor for patients with urinary bladder cancer.
Introduction
Bladder cancer is one of the most common tumors and is a significant cause of tumor-related death worldwide (1). Its worldwide age-standardized incidence rate (per 100,000 person/years) is 9.0 for men and 2.2 for women (2). Although etiological factors have been identified for bladder cancer, most have not been used successfully to reduce its prevalence (3). The most common pathological type is urothelial carcinoma (UC); thus, treatments for bladder cancer primarily focus on UC. Among the bladder cancer types, primary signet ring cell carcinoma (SRCC) is a rare variation of adenocarcinoma, as it accounts for ~0.24% of all bladder malignancies (4). Compared with UC, SRCC tends to present with high-grade disease according to several studies. As a result, the presence of signet ring cells is often associated with a worse prognosis (5).
Thus far, although SRCC of the urinary bladder is highly malignant, it is often neglected due to its rarity. Therefore, this study comprehensively compared SRCC with UC, which represents the most common pathological subtype of urinary bladder cancer (6). We used the national Surveillance, Epidemiology, and End Results (SEER) database (2004–2016) to investigate the prognostic values of the clinicopathological characteristics and survival outcomes in SRCC of the urinary bladder.
Materials and Methods
Data Resource and Study Population
Adult patients (≥18 years of age) who were registered from 2004 to 2016 in the SEER database were selected. The primary cancer site was restricted to the urinary bladder (C67) according to the International Classification of Disease for Oncology, Third Edition. Patients were included only if the histology was SRCC (ICD-O-3 8490/3) or UC. UC included ICD-O-3 8120/3 (transitional cell carcinoma), ICD-O-3 8122/3 (transitional cell carcinoma, spindle cell), and ICD-O-3 8131/3 (transitional cell carcinoma, micropapillary). The diagnosis was confirmed by positive histology and was their first or only cancer diagnosis (first positive indicator of malignancy). Patients without histological diagnosis and survival data were excluded.
End Points
The main end points were overall mortality (OM) and cancer-specific mortality (CSM) according to data in the SEER database. OM refers to deaths from any cause, while CSM is defined as death from SRCC or UC according to the recorded cause of death. Survival time was the duration from initial diagnosis to death from any cause or to the last follow-up.
Statistical Analysis
Baseline characteristics were assessed to determine whether there were significant differences in the distribution of the study population. Two-sample t-tests, Pearson's chi-square tests, and Fisher's exact tests were performed for continuous variables and categorical variables, as appropriate. Kruskal–Wallis H tests were used for non-Gaussian distributions. Continuous variables were presented as the mean ± SD. For age at diagnosis and survival (in months), medians and interquartile ranges were also reported. Categorical variables were shown as frequencies and their proportions. The OM and CSM of each histological subtype were compared using unadjusted Kaplan–Meier curves and the log-rank test.
The multivariable Cox proportional hazard model was used to calculate hazard ratios (HR) and their 95% confidence intervals (95% CI) stratified by histological types. The following covariates were adjusted: sex, age at diagnosis, treatment modality (surgery and radiation), and TNM stage. Subgroup analyses were performed by multivariate regression analysis. Sex, primary site, TNM stage, and lymph nodes removed were adjusted in the Cox model. Tests to determine interactions were also used in the subgroup analyses. Propensity score matching (PSM) was used to further adjust the model for potential baseline confounding factors. All analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA).
Results
Baseline Characteristics of the Study Population
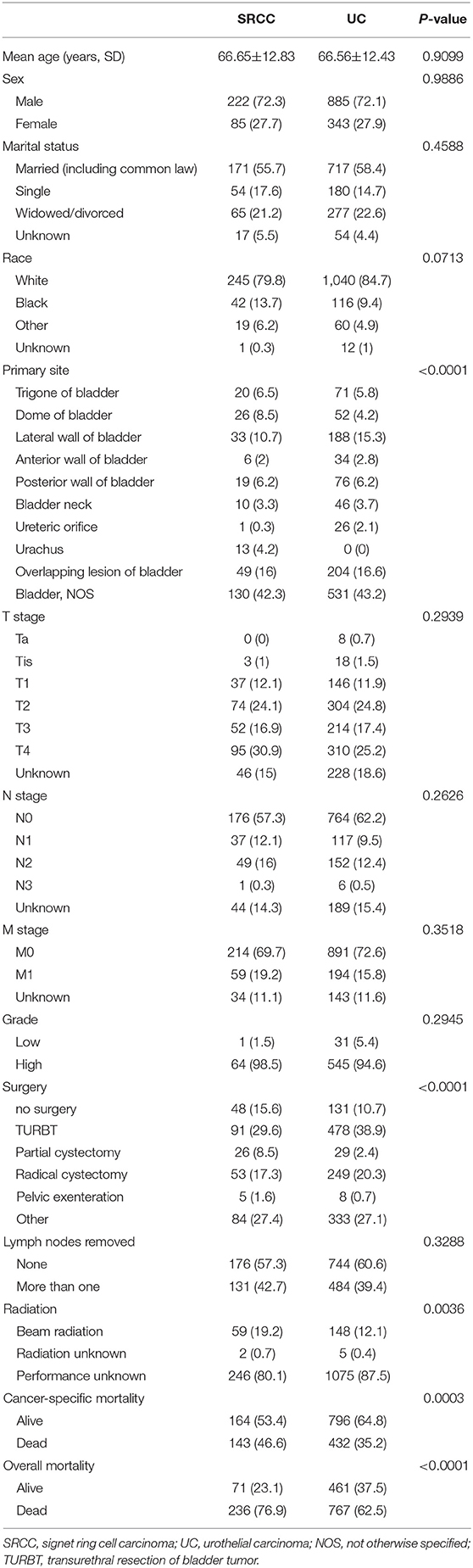
In all, 57,762 patients, including 57,444 patients with UC and 318 patients with SRCC, were identified in the SEER database from 2004 to 2016. Table 1 includes the baseline characteristics of these patients. SRCC patients were younger at diagnosis compared with UC patients (SRCC 66.77 ± 12.74 vs. UC 72.62 ± 11.53, P < 0.001). The majority of patients were males in both the UC (75.39%) and SRCC (72.96%) groups, but no difference was found in the proportion of males or females between the two groups (P = 0.315). The SRCC group presented with a more advanced stage than the UC group, as shown by a higher rate of muscle invasive disease (71.38 vs. 45.18%, P < 0.001), lymph node metastasis (27.99 vs. 8.57%, P < 0.001), and distal metastasis (19.50% vs. 7.12%, P < 0.001). Higher-grade disease was more common in the SRCC group (98.57 vs. 88.86%, P = 0.004). The SRCC group had a lower rate of surgery than the UC group (83.65 vs. 91.32%, P < 0.001), whereas lymph nodes were more likely to be removed in the SRCC group than in the UC group (42.14 vs. 20.80%, P < 0.001). Moreover, when the type of radiation therapy was known, external beam radiation was more frequently used in the SRCC group than in the UC group (19.18 vs. 9.76%, P < 0.001).
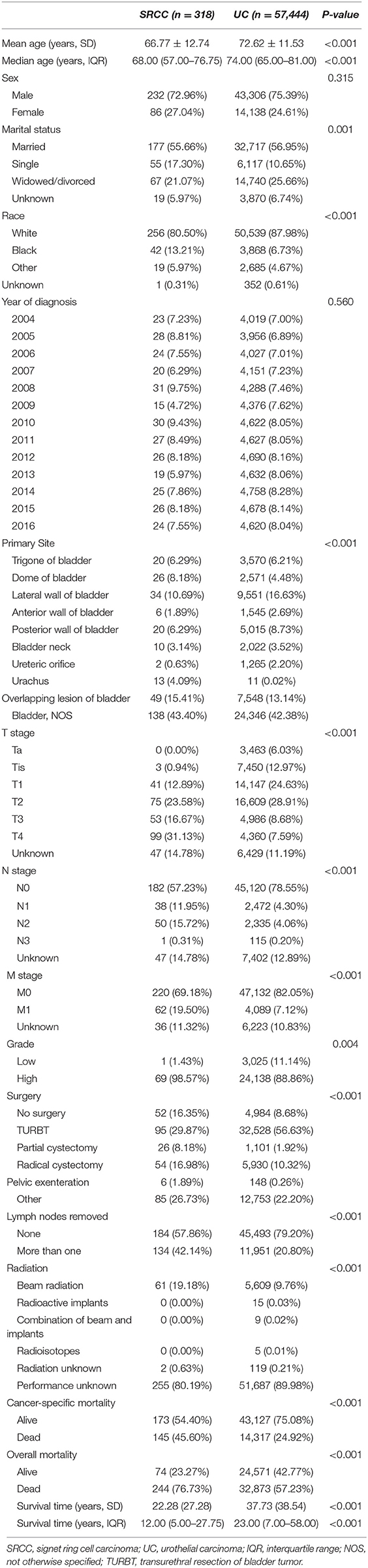
Table 1. Baseline demographic and clinicopathologic characteristics of patients with SRCC compared to UC.
Survival Analyses
According to the survival analyses, the landmark OM of the SRCC population was higher than that of the UC population (P < 0.0001) (Figure 1). When the landmark was set to 5 years (60 months), survival probability of the SRCC group also declined more quickly according to the OM analyses but was not obviously different from the SRCC group when the follow-up period was longer than 5 years (Supplementary Figure 1). The SRCC group also had a lower survival probability according to the CSM analyses (P < 0.0001) (Figure 1).
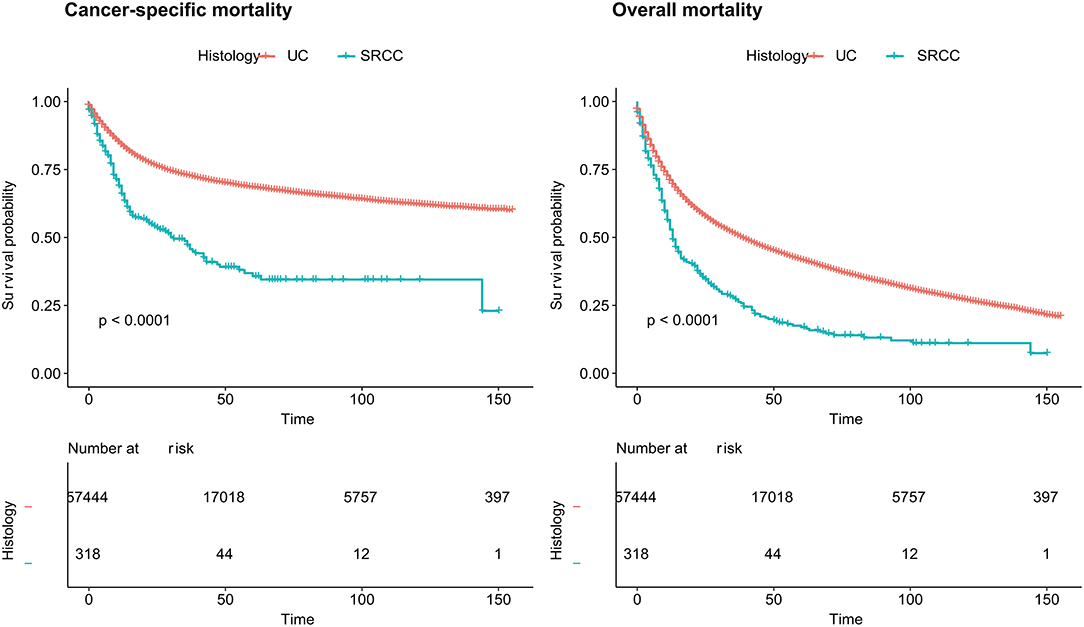
Figure 1. Cancer-specific mortality and overall mortality of patients with SRCC and UC, respectively. SRCC, primary signet ring cell carcinoma; UC, urothelial carcinoma.
The non-adjusted and two adjusted models are presented in Table 2. After adjustments for age, sex, TNM stage, primary tumor site, and treatment method, multivariable Cox proportional hazard regression showed that the SRCC group had a significantly higher risk of OM compared with patients with UC (HR = 1.44, 95% CI = 1.26–1.63, P < 0.0001). The SRCC group also had a higher risk of CSM (HR = 1.40, 95% CI = 1.18–1.65, P < 0.0001). To minimize selection bias, PSM was performed for baseline factors and treatments (Table 3). Most baseline factors were matched except for primary tumor site, surgery, and radiation. Furthermore, we made an extra adjustment to analyze the mismatched baseline factors. Compared with the UC group, the SRCC group faced higher risks of OM (HR = 1.45, 95% CI = 1.24–1.68, P < 0.0001) and CSM (HR = 1.47, 95% CI = 1.20–1.79, P = 0.0001) (Table 2).
Subgroup Analyses
The results of the subgroup analyses are shown in Figure 2. After adjusting for potential covariates, the tests for interaction were not statistically significant for sex, age, N stage, M stage, and lymph nodes removed in both OM and CSM. The results also indicated that patients with SRCC had a worse prognosis out of all the groups.
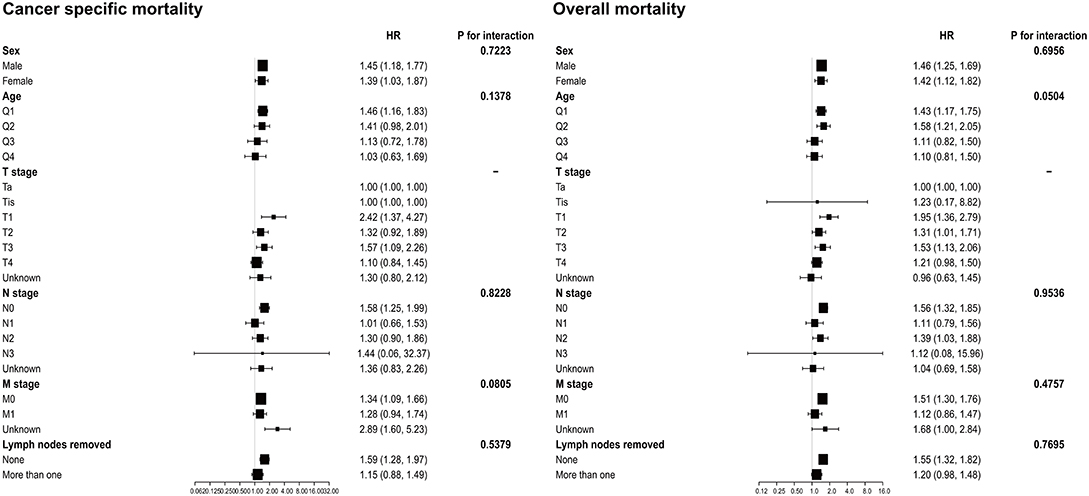
Figure 2. Subgroup analysis for interaction between SRCC and potential covariates in both overall mortality and cancer-specific mortality. SRCC, primary signet ring cell carcinoma.
Discussion
In this study, we aimed to investigate the prognostic values of the clinicopathological characteristics and survival outcomes in SRCC of the urinary bladder. Given that UC accounts for ~95% of bladder cancers, SRCC and UC were compared in records from the SEER database under specified inclusion criteria (6). SRCC and UC had differing effects on patients' OM, especially for the 5-year survival status (P < 0.0001). Moreover, patients with SRCC had a higher risk of OM (HR = 1.44, 95% CI = 1.26–1.63, P < 0.0001) and CSM (HR = 1.40, 95% CI = 1.18–1.65, P < 0.0001). The results indicated that SRCC could be an independent prognostic factor for patients with urinary bladder cancer. Furthermore, no interactions were found between sex, age, N stage, M stage, and lymph nodes removed and these two histological subtypes that would influence patient survival.
Given that SRCC was a rare variant, most recent studies were still case reports (7–9). The results of this study support previous studies based on case series and the SEER database before 2004 (10–12). Previous studies suggested that SRCC patients have worse cancer-specific survival than those with UC despite aggressive surgical therapy (10). Compared with the study by Wang et al., the distribution of patient characteristics in this study was similar, but this study included more males with SRCC (75.39%, 232/318 vs. 66%, 68/103). In terms of treatment, our study population had a higher rate of radiation therapy compared with the study by Wang et al. (19.18 vs. 15.5%). In addition, lymph nodes were more frequently removed in the SRCC group than in the UC group (42.14 vs. 20.80%, P < 0.001). For TNM stage, higher rates of muscle invasive disease, lymph node metastasis, and distal metastasis of SRCC were confirmed, which could cause a poor prognosis of the SRCC group. Moreover, subgroup analyses indicated that SRCC primarily led to poor prognosis of patients in all subgroups regardless of the age, N stage, M stage, or lymph nodes removed. Interestingly, for the lymph-node-removed subgroup, no difference was observed in either CSM or OM between the SRCC and UC groups. This indicated that removal of lymph nodes might not influence the prognosis of patients with SRCC. To further explore the value of lymph node removal in SRCC, a future study should collect more details regarding the numbers of lymph nodes removed and the number of positive nodes to conduct a stratified analysis.
The majority of SRCC cases in this study were muscle invasive bladder cancer (MIBC), while most bladder cancers do not involve the muscle wall of the bladder (13). SRCC is highly malignant, dedifferentiated, and aggressive, but its mechanism is not yet clear. Fukui Y assumed that an abnormally activated ErbB2/ErbB3 complex can enhance cell growth and lead to the loss of adherence and tight junctions (14). Thomas et al. analyzed immunohistochemical markers in the signet ring component of bladder adenocarcinomas and suggested that component was associated with invasion of the surrounding tissue and lymphatic metastasis (15). Loss of E-cadherin and MUC4 is important for SRCC malignancy (16, 17). The proportion of the SRCC component is associated with advanced-stage disease and worse outcomes. Its invasion pattern could spread laterally and widely without protruding through the surface (18). Cystoscopy might only reveal edematous, bullous, or erythematous mucosa (19). Therefore, rapid infiltration of the submucosa and a lack of obvious mucosal lesions in the early stage could lead to inappropriate staging under cystoscopy without full-thickness biopsy and allow SRCC more time to progress.
The most common surgery in the SRCC group in this study was transurethral resection of bladder tumor (95/318), and the second was radical cystectomy (54/318). However, according to the National Comprehensive Cancer Network guidelines, MIBC without metastasis should be evaluated for radical cystectomy (20). Given that this study enrolled 318 patients with SRCC, their treatments were relatively conservative and might be responsible for their worse prognosis. Other possible explanations were put forward to explain why fewer patients than expected may have undergone surgery. Some older patients might have had a poorer health condition and could not undergo surgery. For patients with metastasis, surgery might not be the standard treatment, and consequently, they may be considered for palliative therapy instead of radical cystectomy. Patients with MIBC were usually treated with systemic chemotherapy, cystectomy, or radiotherapy, and those whose cancer is too advanced to be cured may receive radiotherapy and systemic chemotherapy. However, the SEER database does not provide information on chemotherapy, which was an obvious weakness of our study. Although some studies found that SRCC was resistant to systematic chemotherapy based on small sample sizes (18), some recent studies reported that adjuvant chemotherapy may be a potential component of effective treatment (21, 22). Checkpoint-inhibitor drugs and genomic research offer hope to improve the treatment of bladder cancer (23). We suggest that once SRCC components are found by biopsy, an advanced combined treatment should be considered, but multicenter clinical trials are needed to establish a better therapeutic protocol for this rare but aggressive cancer.
Our study also had some strengths. First, we enrolled 318 patients with SRCC of the urinary bladder who presented from 2004 to 2016 and, thus, had sufficient samples to make more exact and multiform analyses. Subgroup analyses and PSM were used to analyze potential confounding factors. Second, we updated the clinicopathological characteristics and survival outcomes of SRCC based on recent data. This study also had some limitations. Selective bias may exist, which is inevitable for clinical observational studies even when PSM is used. Moreover, treatment regimens were classified into two major categories as surgery and radiotherapy, but chemotherapy and new therapies such as checkpoint-inhibitor drugs, which may lead to different outcomes, were not included in the SEER database.
Conclusion
The prognosis of SRCC is poorer than that of UC, even after adjustment for baseline demographic and clinicopathological characteristics, as well as cancer treatment. SRCC is an independent prognostic factor for patients with urinary bladder cancer.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data/.
Author Contributions
All authors contributed to the design of the project, data collection, and analysis, and contributed to the final manuscript. All authors have read and approved the final submitted manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2017YFC0908003), National Natural Science Foundation of China (Grant No. 81902578, 81974098), China Postdoctoral Science Foundation (2017M612971), Post-Doctor Research Project, West China Hospital, Sichuan University (2018HXBH085), and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2018C01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully thank Dr. Changzhong Chen, Chi Chen, and Xing-Lin Chen (EmpowerStats X&Y Solutions, Inc, Boston, MA, USA) for providing statistical methodology consultation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00653/full#supplementary-material
Supplementary Figure 1. Five-years cut of overall mortality of patients with primary signet-ring cell carcinoma.
Abbreviations
SRCC, signet ring cell carcinoma; SEER, Surveillance, Epidemiology, and End Results; UC, urothelial carcinoma; PSM, propensity score matching; OM, overall mortality; CSM, cancer-specific mortality; COD, cause of death; IQR, interquartile range; NOS, not otherwise specified; MIBC, muscle invasive bladder cancer.
References
1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. (2017) 71:96–108. doi: 10.1016/j.eururo.2016.06.010
2. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH., Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) -. 2019 Update. Eur Urol. (2019). 76:639–57. doi: 10.1016/j.eururo.2019.08.016
3. Babjuk M. Trends in bladder cancer incidence and mortality: success or disappointment? Eur Urol. (2017) 71:109–10. doi: 10.1016/j.eururo.2016.06.040
4. Torenbeek R, Koot RA, Blomjous CE, De Bruin PC, Newling DW, Meijer CJ. Primary signet-ring cell carcinoma of the urinary bladder. Histopathology. (1996) 28:33–40. doi: 10.1046/j.1365-2559.1996.262303.x
5. Lee WS, Chun HK, Lee WY, Yun SH, Cho YB, Yun HR, et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am J Surg. (2007) 194:294–8. doi: 10.1016/j.amjsurg.2006.12.041
6. Al-Husseini MJ, Kunbaz A, Saad AM, Santos JV, Salahia S, Iqbal M, et al. Trends in the incidence and mortality of transitional cell carcinoma of the bladder for the last four decades in the USA: a SEER-based analysis. BMC Cancer. (2019) 19:46. doi: 10.1186/s12885-019-5267-3
7. Bouhajja L, Farah F, Garbouj N, Rammeh S. Primary signet-ring cell carcinoma of the urinary bladder: A report of two cases. Tunis Med. (2019) 97:167–169.
8. Jayarajah U, Fernando DMH, Herath KB, de Silva MVC. Primary signet-ring cell adenocarcinoma of the urinary bladder treated with partial cystectomy: a case report and review of the literature. Case Rep Urol. (2017) 2017:6829692. doi: 10.1155/2017/6829692
9. Lendorf ME, Dohn LH, B AD, Loya AC, Pappot H. An updated review on primary signet-ring cell carcinoma of the urinary bladder and report of a case. Scand J Urol. (2018) 52:87–93. doi: 10.1080/21681805.2017.1418020
10. Wang J, Wang FW, Kessinger A. The impact of signet-ring cell carcinoma histology on bladder cancer outcome. World J Urol. (2012) 30:777–83. doi: 10.1007/s00345-011-0718-8
11. Akamatsu S, Takahashi A, Ito M, Ogura K. Primary signet-ring cell carcinoma of the urinary bladder. Urology. (2010) 75:615–8. doi: 10.1016/j.urology.2009.06.065
12. Grignon DJ, Ro JY, Ayala AG, Johnson DE. Primary signet-ring cell carcinoma of the urinary bladder. Am J Clin Pathol. (1991) 95:13–20. doi: 10.1093/ajcp/95.1.13
13. Bladder cancer: diagnosis and management of bladder cancer: (c) NICE (2015) Bladder cancer: diagnosis and management of bladder cancer. BJU Int. (2017) 120:755–65. doi: 10.1111/bju.14045
14. Fukui Y. Mechanisms behind signet ring cell carcinoma formation. Biochem Biophys Res Commun. (2014) 450:1231–3. doi: 10.1016/j.bbrc.2014.07.025
15. Thomas AA, Stephenson AJ, Campbell SC, Jones JS, Hansel DE. Clinicopathologic features and utility of immunohistochemical markers in signet-ring cell adenocarcinoma of the bladder. Hum Pathol. (2009) 40:108–16. doi: 10.1016/j.humpath.2008.06.022
16. Yokoyama A, Shi BH, Kawai T, Konishi H, Andoh R, Tachikawa H, et al. Muc4 is required for activation of ErbB2 in signet ring carcinoma cell lines. Biochem Biophys Res Commun. (2007) 355:200–3. doi: 10.1016/j.bbrc.2007.01.133
17. Lim MG, Adsay NV, Grignon DJ, Osunkoya AO. E-cadherin expression in plasmacytoid, signet ring cell and micropapillary variants of urothelial carcinoma: comparison with usual-type high-grade urothelial carcinoma. Mod Pathol. (2011) 24:241–7. doi: 10.1038/modpathol.2010.187
18. Wang J, Wang FW. Clinical characteristics and outcomes of patients with primary signet-ring cell carcinoma of the urinary bladder. Urol Int. (2011) 86:453–60. doi: 10.1159/000324263
19. Kinra P, Rashmi SP, Alam A, Singh H, Dash SC. Primary signet cell adenocarcinoma of bladder. Indian J Pathol Microbiol. (2017) 60:584–6. doi: 10.4103/ijpm.ijpm_201_16
20. Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15:1240–67. doi: 10.6004/jnccn.2017.0156
21. Hamakawa T, Kojima Y, Naiki T, Kubota Y, Yasui T, Tozawa K, et al. Long-term survival of a patient with invasive signet-ring cell carcinoma of the urinary bladder managed by combined s-1 and Cisplatin adjuvant chemotherapy. Case Rep Urol. (2013) 2013:915874. doi: 10.1155/2013/915874
22. Ohtaka M, Kawahara T, Kumano Y, Maeda Y, Kondo T, Mochizuki T, et al. Invasive urothelial carcinoma, lymphoma-like/plasmacytoid variant, successfully treated by radical cystectomy with adjuvant chemotherapy: a case report. J Med Case Rep. (2016) 10:48. doi: 10.1186/s13256-016-0806-x
Keywords: signet ring cell, carcinoma, urinary bladder, SEER, prognosis
Citation: Jin D, Qiu S, Jin K, Zhou X, Cao Q, Yang L and Wei Q (2020) Signet-Ring Cell Carcinoma as an Independent Prognostic Factor for Patients With Urinary Bladder Cancer: A Population-Based Study. Front. Oncol. 10:653. doi: 10.3389/fonc.2020.00653
Received: 06 February 2020; Accepted: 08 April 2020;
Published: 15 May 2020.
Edited by:
Marijo Bilusic, National Institutes of Health (NIH), United StatesReviewed by:
Jure Murgic, Sisters of Charity Hospital, CroatiaMatthew Zibelman, Fox Chase Cancer Center, United States
Copyright © 2020 Jin, Qiu, Jin, Zhou, Cao, Yang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wei, d2VpcWlhbmc5MzNAMTI2LmNvbQ==; Lu Yang, d3ljbGVmbHVlQDE2My5jb20=
†These authors have contributed equally to this work
 Di Jin†
Di Jin† Xianghong Zhou
Xianghong Zhou Lu Yang
Lu Yang Qiang Wei
Qiang Wei