- 1Institute of Medical Psychology and Behavioral Immunobiology, University Hospital Essen, Essen, Germany
- 2Department of Internal Medicine VI, University Hospital Tuebingen, Tuebingen, Germany
Translational research aiming to elucidate mediators and moderators of placebo and nocebo effects is highly relevant. This experimental study tested effects of a brief progressive muscle relaxation (PMR) exercise, designed to alter psychobiological stress parameters, on the magnitude of placebo and nocebo effects in a standardized psychosocial treatment context. In 120 healthy volunteers (60 men, 60 women), pain expectation, pain intensity, and pain unpleasantness in response to individually-calibrated rectal distensions were measured with visual analog scales during a baseline. Participants were then randomized to exercise PMR (relaxation group: N = 60) or a simple task (control group: N = 60), prior to receiving positive (placebo), negative (nocebo) or neutral suggestions regarding an intravenous administration that was in reality saline in all groups. Identical distensions were repeated (test). State anxiety, salivary cortisol, heart rate, and blood pressure were assessed repeatedly. Data were analyzed using analysis of covariance, planned Bonferroni-corrected group comparisons, as well as exploratory correlational and mediation analyses. Treatment suggestions induced group-specific changes in pain expectation, with significantly reduced expectation in placebo and increased expectation in nocebo groups. PMR had no discernable effect on pain expectation, state anxiety or cortisol, but led to significantly lower heart rate and systolic blood pressure. Relaxation significantly interacted with positive treatment suggestions, which only induced placebo analgesia in relaxed participants. No effects of negative suggestions were found in planned group comparisons, irrespective of relaxation. Exploratory correlation and mediation analyses revealed that pain expectation was a mediator to explain the association between treatment suggestions and pain-related outcomes. Clearly, visceral pain modulation is complex and involves many cognitive, emotional, and possibly neurobiological factors that remain to be fully understood. Our findings suggest that a brief relaxation exercise may facilitate the induction of placebo analgesia by positive when compared to neutral treatment suggestions. They underscore the contribution of relaxation and stress as psychobiological states within the psychosocial treatment context—factors which clearly deserve more attention in translational studies aiming to maximize positive expectancy effects in clinical settings.
Introduction
Although placebo research spans many medical disciplines, the pain field continues to drive conceptual, mechanistic, and clinical advances in placebo knowledge, providing fruitful opportunities of forward- and backward translation. Placebo analgesia constitutes one of the most fascinating and impressive examples of such translational research. Laboratory and preclinical studies in healthy populations and in patients with chronic pain conditions have elucidated the psychological and neurobiological mechanisms underlying placebo and nocebo effects (1, 2). The clinical potential offered by a transfer of this knowledge into treatment settings has been recognized within the pain field (3, 4) and beyond (5). This is underscored by trials supporting the efficacy of placebo interventions in patients with chronic low back pain (6, 7) and chronic visceral pain (8, 9). Facilitating placebo while minimizing nocebo effects may contribute to refining treatment approaches to provide patients with improved and more personalized patient care (10, 11). Toward this end, translational research aiming to optimize the efficacy of placebo interventions is highly relevant. In the context of chronic visceral pain and related gastrointestinal symptoms, the potential of placebo knowledge has been recognized but is far from fulfilled (12–14).
Various aspects of the psychosocial treatment context, including the setting (15), nature of the intervention, as well as the quality and quantity of patient-provider interactions (9, 16, 17), shape treatment expectations and thereby the presence and magnitude of placebo effects. Optimizing the psychosocial treatment context has the potential to improve the efficacy of placebo treatment, and to maximize the benefits of placebo-elements that are an inherent part of therapeutic interventions, including pharmacological treatments (4, 18). Interestingly, two laboratory studies in healthy volunteers support the idea that placebo analgesia can be enhanced with specific pharmacological interventions, i.e., the administration of vasopressin and oxytocin, respectively (19, 20). Whether behavioral approaches that target stress-related psychobiological factors are capable of facilitating placebo analgesia has not been tested. Herein, we explore for the first time the modulatory effects of a brief behavioral intervention, i.e., progressive muscle relaxation (PMR), on placebo and nocebo effects in a clinically-relevant model of visceral pain. The rationale was inspired by evidence supporting that enhanced stress [e.g., increased state anxiety (21–24), subjective stress levels (25–28), experimentally-induced fear (29), acute psychosocial stress (30)] moderates placebo and/or nocebo effects. As part of a larger experimental study (30), we herein implemented PMR aiming to test effects of reduced stress-related psychobiological factors on the magnitude of placebo and nocebo effects induced by treatment suggestions. Building on our earlier experimental studies on placebo/nocebo effects in the context of visceral pain (22, 30–33), we specifically aimed to test whether a brief relaxation exercise, carried out immediately prior to the delivery of deceptive positive (placebo), deceptive negative (nocebo), or truthful neutral (control) treatment suggestions, can facilitate placebo analgesia or reduce nocebo hyperalgesia in an established and clinically-relevant model of visceral pain in healthy volunteers. To explore if the effects of relaxation or treatment suggestions on outcomes were mediated by stress markers or expectations, we conducted correlational and mediation analyses.
Materials and Methods
Participants
Healthy adults were recruited by local advertisements seeking volunteers for an experimental study on the modulation of visceral pain perception. We herein report on a total of N = 120 healthy volunteers (60 men, 60 women) who were randomized to a brief relaxation exercise or a control task on the experimental study day just prior to undergoing an established placebo/nocebo paradigm (see below, study design). Note that this study was conducted as part of a larger trial which also included an additional N = 60 volunteers who were randomized to a psychosocial stress protocol (data on the psychosocial stress and control groups have been reported in Roderigo et al. (30). Recruitment and screening procedures were accomplished with a total of N = 219 participants originally interested in the study. Reasons for non-participation were lack of interest, exclusion based on criteria specified below, and a high pain threshold that was above the herein applied safety cut-off for distensions at 55 mmHg. The study was conducted at Essen University Hospital with data collection between January 2015 and June 2016. The study protocol was approved by the local ethics committee (protocol number 13-5565-BO, approval date: August 28, 2013). All volunteers gave informed written consent in accordance with the Declaration of Helsinki and were paid for their participation.
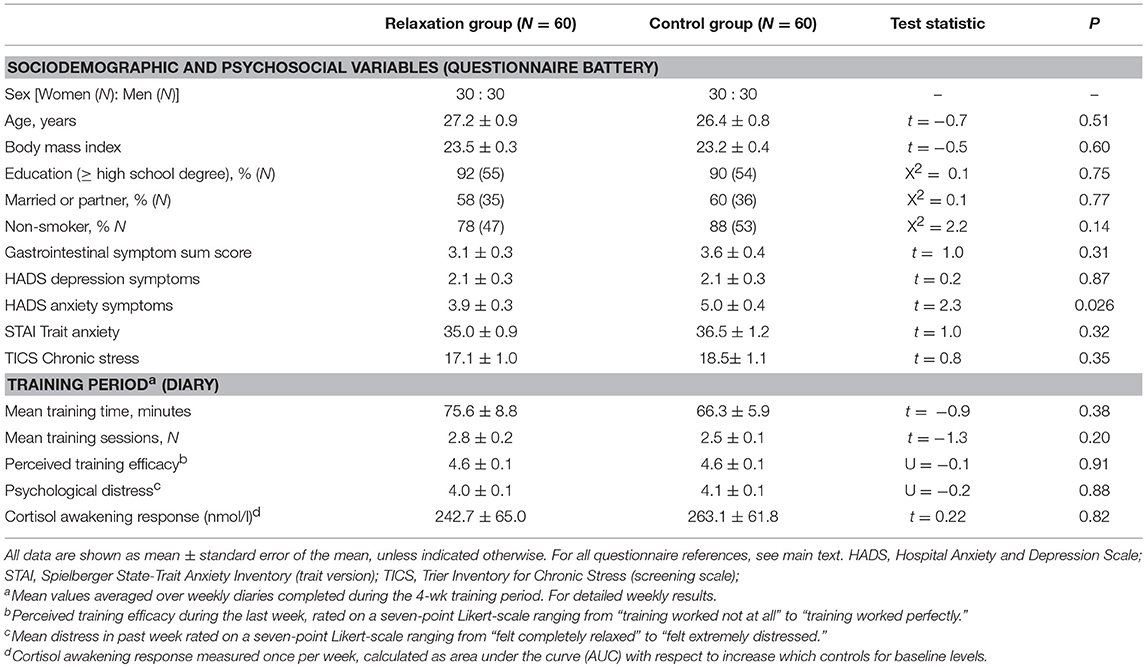
Exclusion criteria included age <18 or >65 years, a body mass index (BMI) <18 or >30, any known medical or psychological conditions, current medication use (except thyroid medication, occasional over-the-counter drugs for minor allergies, benign headaches, etc.), current anxiety or depression symptoms above the published cut-off values on the Hospital Anxiety and Depression Scale (HADS) (34), current gastrointestinal (GI) symptoms suggestive of an undiagnosed GI condition (35), peri-anal tissue damage (e.g., painful hemorrhoids or fissures which may interfere with rectal balloon placement), and prior participation in any of our previous placebo studies. In an effort to reduce possible variability related to fluctuations of hormones across the female menstrual cycle, only women on hormonal contraceptives were recruited. All participants completed a comprehensive questionnaire battery, as detailed in Roderigo et al. (30). We herein characterized groups using the HADS (34) for symptoms of anxiety and depression, the trait version of the STAI (36) for trait anxiety, the TICS (screening scale) (37) for chronic perceived stress, and sum scores from a gastrointestinal (GI) symptom questionnaire (35) to assess frequency and severity of common upper and lower GI symptoms. Note that previous experience with any type of relaxation technique, including progressive muscle relaxation (PMR) was not an inclusion or exclusion criterion, however, it was required that volunteers were willing to complete a home-based PMR training program as part of the study, as detailed below.
Study Design and Procedures
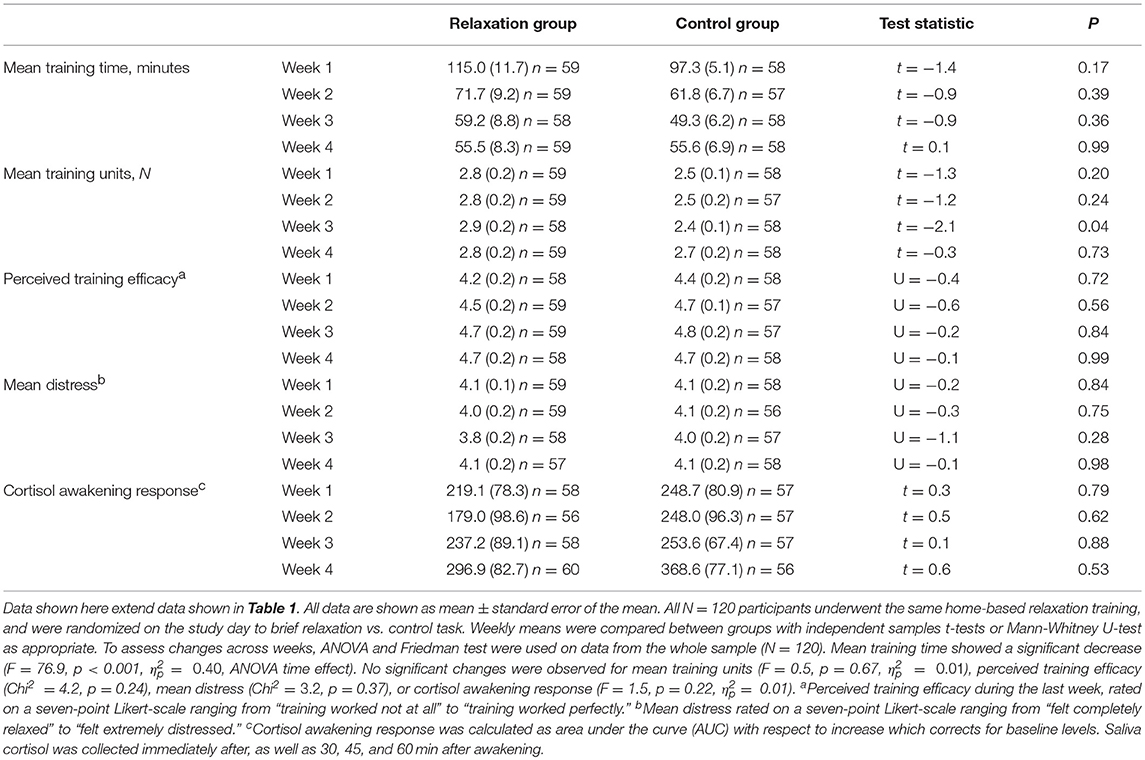
During a 4-week period preceding the experimental study day, volunteers were instructed to complete a home-based, standardized training program in progressive muscle relaxation (PMR). This was done to achieve a large enough sample of individuals capable of completing a short relaxation exercise on the day of the study. In order to achieve proper blinding and randomization, all 180 participants underwent the training program. To do so, we selected a commercially available training manual that consisted of an illustrated book with an audio CD that contained guided training sessions. Note that the same audio-guided training CD was used by participants randomized to the brief relaxation group on the study day. Every volunteer—irrespective of possible prior experience with the PMR or other relaxation techniques—was instructed to start the training in the first week with two sessions of a long program that lasted ~40 min. Thereafter, participants could choose between the long version and a shorter 15-min. version in the remaining training weeks, but were required to practice at least twice per week. Participants recorded their practice in a training log, and at the end of the week (i.e., on Sundays) completed a standardized questionnaire assessing the number of training sessions (N), training duration (in minutes), perceived training efficacy (7-point Likert-scale ranging from “training worked not at all” to “training worked perfectly”), psychological distress (7-point Likert-scale ranging from “felt completely relaxed” to “felt extremely distressed”) and various bodily symptoms (not reported here) for the past week. Together with each weekly questionnaire, participants collected morning saliva samples for analysis of the cortisol awakening response (CAR). In case of non-compliance (i.e., on average <2 training sessions per week) participants were encouraged to continue practicing for up to two additional weeks before the study day was scheduled. Note that questionnaire data and CAR were not acquired to verify the efficacy of PMR training (which is impossible given the absence of a control group that did not undergo training) but rather to provide sample characteristics for comparisons of groups that on the study day were randomized to brief relaxation exercise vs. a control task.
On the experimental study day, rectal sensory and pain thresholds were initially determined with a pressure-controlled barostat system (modified ISOBAR 3 device, G & J Electronics, Ontario, Canada), using well-established methodology [e.g., (22, 30–33, 38). During a BASELINE, each participant received a series of painful rectal distensions titrated individually to rectal threshold (6 distensions, duration each 30 s; pauses in-between 30 s). Participants were then randomized to relaxation (practice relaxation using the 15-min. audio-CD program, N = 60) or control intervention (engage in an easy cognitive activity, e.g., crosswords, reading a magazine, N = 60) while stratifying for sex. Immediately afterwards, participants were randomized to positive (placebo), negative (nocebo), or neutral treatment suggestions (details on suggestions below). This resulted in a total of 2 (relaxation, control) x 3 (positive, negative, neutral suggestions) experimental groups consisting of N = 20 participants per group. The series of rectal distensions using the same individualized pressures as during BASELINE was then repeated (TEST).
Treatment Suggestions and Blinding
We herein implemented previously used methodology to induce placebo and nocebo effects in this visceral pain model [e.g., (30, 33); for recent discussions of methodology aspects, see (13, 14)]. In this paradigm, deceptive or truthful treatment suggestions are delivered in combination with an i.v. administration that in reality contains saline. In placebo groups, volunteers receive positive treatment suggestions regarding pain relief induced by a spasmolytic drug (i.e., Butylscopolaminiumbromid). In nocebo groups, negative suggestions regarding increased pain sensitivity due to administration of an opioid antagonist (i.e., Naloxone) are delivered. In control groups, truthful information about saline are provided. These control groups (herein referred to as “neutral” groups to distinguish from the relaxation vs. control intervention group terminology) are an essential part of the study design as they allow a differentiation and separate analyses of placebo and nocebo effects, respectively, as well as controlling for effects of time (e.g., habituation), etc.
In order to achieve proper blinding and a randomization to treatment suggestions on the study day, all volunteers received deceptive information about all possible drug treatments during recruitment and informed consent, including detailed information about typical clinical uses, pharmacodynamics, and possible side effects. Blinding of the study team interacting with volunteers on the study day was accomplished as follows: The clinical psychologist responsible for recruitment and conducting the study protocol (relaxation, control) was blinded to subsequent treatment information, the physician who delivered treatment information was blinded to prior relaxation vs. control intervention, the female study nurse was fully blinded throughout the study day.
Pain-Related Measures
Primary outcome measures were overall perceived visceral pain intensity and pain unpleasantness, quantified with visual analogs scales (VAS, 0−100 mm, ends defined as none—very much). In addition, expected pain intensity was quantified with a VAS (0−100 mm, ends defined as none—very much) prior to BASELINE and TEST, respectively.
Additional Measures
State anxiety (STAI-S), salivary cortisol concentrations (see below), heart rate (Task Force Monitor, CNSystems Medizintechnik AG, Graz, Austria), and blood pressure were assessed repeatedly and are herein presented for a baseline (prior to first randomization to relaxation vs. control intervention), after treatment suggestions, and after the TEST series of distensions. Note that we chose not to additionally assess these stress-related measures in-between the intervention and delivery of treatment suggestions given concerns that this may disrupt or interfere with effects of relaxation on the subsequent experimental procedures.
Saliva samples were collected using Salivettes (Sarstedt, Nümbrecht, Germany). To assess the cortisol awakening response (CAR) during the 4-week home PMR training period, participants collected samples once per week immediately after awakening and 30, 45, 45, and 60 min. afterwards and stored the samples in their freezers until bringing them to the laboratory on the study day. All saliva samples, including all samples collected on the study day, were centrifuged (2,000 rpm, 2 min, 4°C) and stored at −20°C. Salivary cortisol concentrations were measured using a commercially available enzyme-linked immunosorbent assay (ELISA; IBL International, Hamburg, Germany) according to the manufacturer's protocol. Intra- and interassay variances were 4.8 and 5.9%, respectively. The detection limit was 0.138 nmol/l. The CAR was calculated as area under the curve (AUC) with respect to increase, which corrects for baseline levels, according to published recommendations (39).
Statistical Analyses
All statistical analyses were conducted using SPSS version 22.0 (IBM Corporation, Armonk, NY). Power analysis using G-Power (http://www.gpower.hhu.de/) indicated that a total sample size of N = 120 has a sufficient statistical power of 1-β = 0.96 to detect large effects (f = 0.40, α = 0.05) for ANOVA interaction effects. The groups were characterized and compared with respect to sociodemographic, psychological, and clinical characteristics using Chi-Square Tests, t-tests, or Mann-Whitney-U-tests where appropriate.
Effects of the relaxation vs. control on stress markers were tested with repeated measures analysis of covariance (ANCOVA) with time as repeated factor and two between factors, namely intervention (relaxation, control) and treatment suggestions (positive, negative, neutral). Note that the factor “treatment suggestions” was included as a group factor in this analysis to test for possible interactions between the intervention and treatment suggestions on stress markers. Post hoc tests were conducted as Bonferroni-corrected ANCOVA (for comparisons between groups) or Bonferroni-corrected paired t-tests (for changes across time points within one group).
To address effects of relaxation and treatment suggestions on changes in pain expectation, pain intensity, and pain unpleasantness from BASELINE to TEST, repeated measures ANCOVAs were computed with the repeated factor time and two group factors (intervention; treatment suggestions). Bonferroni-corrected planned comparisons of pre-specified group means were accomplished with univariate ANCOVAs testing differences between positive and neutral treatment suggestion groups (for placebo effects) and between negative and neutral treatment suggestion groups (for nocebo effects). In all ANCOVAs, Greenhouse-Geisser correction was applied if the sphericity assumption was violated (based on results of Mauchly test), and HADS anxiety scores were included as a covariate, given a small but significant group difference between the relaxation and control groups (see results, Table 1).
To explore if the effects of relaxation or treatment suggestions on outcomes were mediated by stress markers or expectations, we conducted correlational and mediation analyses. Correlations were computed as Pearson's r. Mediation analyses were conducted using the PROCESS SPSS macro provided by A.F. Hayes (version 2.12.2, downloaded from http://www.processmacro.org/download.html). Bootstrapping with 10,000 samples was used to determine 95% confidence intervals (CIs) to test for statistical significance.
In case of missing data (e.g., due to technical problems), data from this participant for all time points for the affected variable were omitted from analyses. Missing data for each variable are indicated in the result section. All results are reported as mean ± standard error of the mean (SEM) unless indicated otherwise. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Participants
Volunteers randomized to practice brief relaxation (N = 60) or control (N = 60) did not differ with respect to sociodemographic variables or psychosocial questionnaire scores (Table 1, upper section). As per exclusion criteria, mean HADS scores were within the normal range and below the clinically-relevant cut-offs. Nevertheless, mean HADS anxiety score was significantly higher in the control group (p = 0.026), and was therefore included as a covariate in subsequent analyses. No significant differences were observed in trait anxiety assessed with the STAI. This is however not unusual given that the HADS measures clinical symptoms of anxiety, while STAI scores primarily reflect non-clinical anxiety. The groups were comparable with respect to all measures collected during the 4-week PMR training phase (Table 1, lower section), including training intensity, frequency, perceived training efficacy, psychological distress, and the CAR (for weekly means, see Table 2). Rectal thresholds, assessed on the study day prior to first randomization, were comparable between groups (sensory threshold: 14.8 ± 0.7 mmHg relaxation group, 15.0 ± 0.7 mmHg control group, t = −0.2, p = 0.87; pain threshold: 36.6 ± 1.3 mmHg relaxation group, 35.9 ± 1.9 mmHg control group, t = −0.5, p = 0.65).
Stress Markers
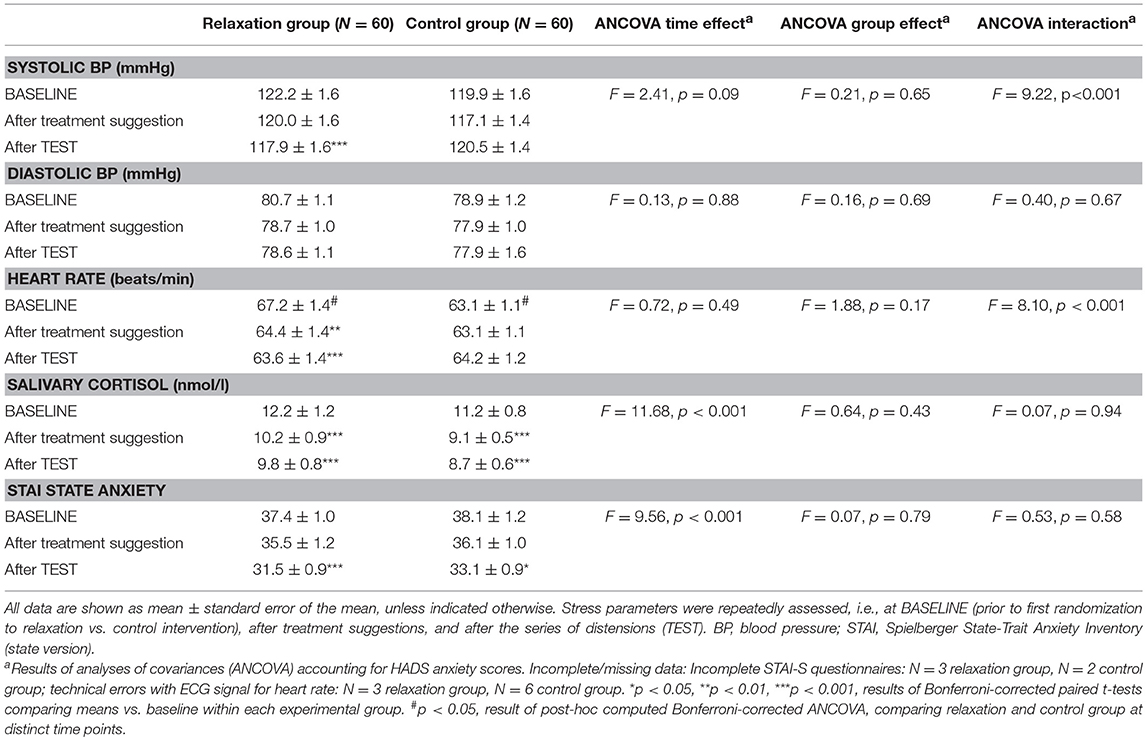
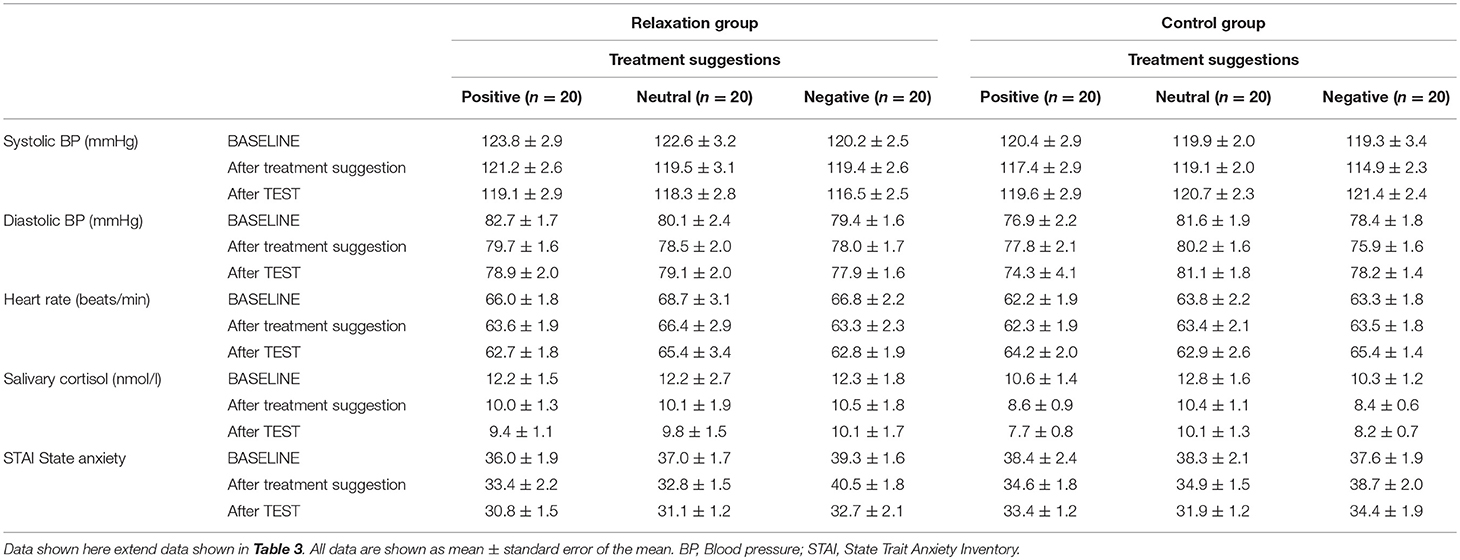
The ANCOVA computed to test effects of the brief relaxation (N = 60) vs. control intervention (N = 60) on stress markers (see Table 3; for group means per treatment suggestion group, see Table 4) revealed significant group × time interactions for systolic blood pressure (F = 9.22, p < 0.001, ηp2 = 0.08) and heart rate (F = 8.10, p < 0.001, ηp2 = 0.07), which decreased significantly in the relaxation but not in the control group. Salivary cortisol and state anxiety showed significant decreases over time (salivary cortisol: F = 11.68, p < 0.001,ηp2 = 0.09; state anxiety scores: F = 9.56, p < 0.001, ηp2 = 0.08), however, without evidence of significant group × time interactions (salivary cortisol: F = 0.07, p = 0.86, ηp2 = 0.01; state anxiety scores: F = 0.53, p = 0.59, ηp2 = 0.01). No significant effects were observed for diastolic blood pressure.
Pain-Related Measures
Expected pain intensity (Figure 1A) was reduced by positive and increased by negative treatment suggestions (F = 8.84, p < 0.001, ηp2 = 0.14, ANCOVA main effect of treatment information; F = 32.25, p < 0.001, ηp2 = 0.37, ANCOVA interaction effect of time × treatment information). Pain expectation was not affected by relaxation, as indicated by the absence of significant main or interaction effects.
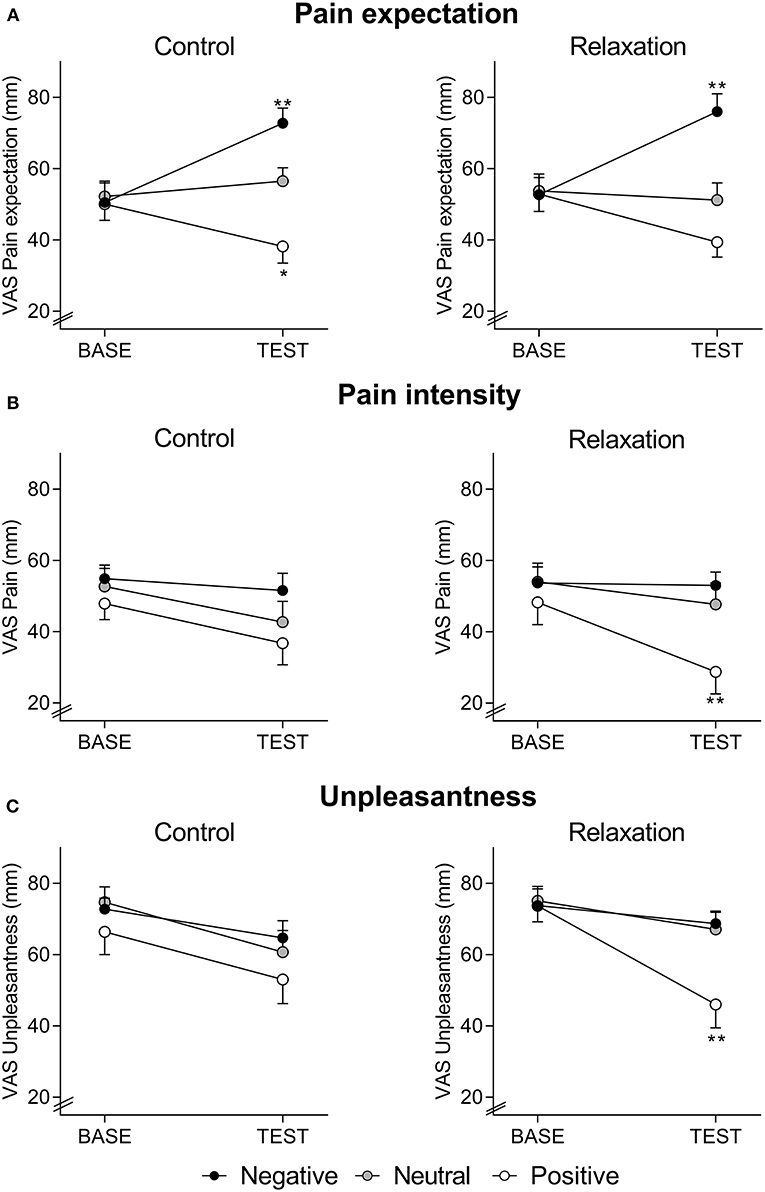
Figure 1. Expected pain intensity (A), perceived pain intensity (B), and perceived pain unpleasantness (C), assessed with visual analog scale (VAS, 0–100 mm) at BASELINE and TEST, in groups receiving positive, neutral, or negative treatment information after relaxation (right panels) or control (left panels). Note that pain expectation was assessed before, whereas perceived pain intensity and unpleasantness were assessed after the series of distensions during BASELINE and TEST, respectively. For ANCOVA results, please see text. *p < 0.05; **p < 0.01 results of planned comparisons with Bonferroni-correction at TEST (for exact p-values, see text) comparing groups with positive information to groups with neutral information (to test for placebo effects) and groups with negative information to groups with neutral information (to test for nocebo effects) after either relaxation or control.
For perceived pain intensity (Figure 1B), there was a significant effect of treatment suggestions (F = 4.38, p = 0.015 ηp2 = 0.07, time x suggestion interaction; F = 3.70, p = 0.028 ηp2 = 0.06, main effect of suggestion), but no main effect of the intervention (F = 0.01, p = 0.98 ηp2 = 0.01, time x intervention interaction; F = 0.31, p = 0.58 ηp2 = 0.01, main effect of intervention) and no interaction effect (F = 1.29, p = 0.29 ηp2 = 0.02, time × suggestion × intervention interaction). Planned comparisons of group means revealed significantly reduced perceived pain intensity at TEST due to positive compared to neutral suggestions in the relaxation groups (F = 8.04, p = 0.008, ηp2 = 0.19), while a similar placebo effect was not observed in the control groups (F = 0.44, p = 0.51, ηp2 = 0.01). Nocebo effects, tested by comparing groups with negative vs. neutral treatment suggestions, were not observed in either intervention group (relaxation: F = 0.3, p = 0.57, ηp2 = 0.01; control: F = 1.9, p = 0.17, ηp2 = 0.05).
For pain unpleasantness (Figure 1C), a significant interaction between intervention, treatment suggestions, and time (F = 3.53, p = 0.032, ηp2 = 0.06), as well as a significant effect of treatment suggestions (F = 4.41, p = 0.014 ηp2 = 0.07, time × suggestion interaction; F = 3.21, p = 0.044 ηp2 = 0.05, main effect of treatment suggestion) emerged, while effects of the intervention were not significant (F = 0.82, p = 0.37, ηp2 = 0.01, time × intervention interaction; F = 0.37, p = 0.54 ηp2 = 0.01, main effect of intervention). Planned comparisons of group means revealed significantly reduced unpleasantness at TEST in response to positive when compared to neutral suggestions (F = 7.8, p = 0.008, ηp2 = 0.18) in relaxation groups, but not in control groups (F = 0.9, p = 0.34, ηp2 = 0.02). No significant effects of negative suggestions were observed (relaxation groups: F = 0.02, p = 0.88, ηp2 = 0.01; control groups: F = 0.63, p = 0.43, ηp2 = 0.02).
Exploratory Correlational and Mediation Analyses
To explore the role of pain expectation, we conducted correlational and mediation analyses both in the whole sample and in groups with positive suggestions (placebo groups) and negative suggestions (nocebo groups). In the whole sample of N = 120, pain expectation was significantly associated with both perceived pain intensity (r = 0.58, p < 0.001) and pain unpleasantness (r = 0.38, p < 0.001). In addition, pain expectation correlated with state anxiety (r = 0.25, p = 0.007), but not with other stress markers. No significant correlations between any other stress marker and pain outcomes were found (all p > 0.05, data not shown).
Within placebo groups (N = 40), pain expectation was positively correlated with perceived pain intensity (r = 0.54, p < 0.001, Figure 2A) and unpleasantness (r = 0.32, p = 0.047, Figure 2B). To explore if pain expectation mediated effects of positive treatment suggestions, we conducted mediation analyses on data from placebo and neutral suggestion groups (N = 80) after ensuring that positive associations remained significant in multiple regression analyses including treatment suggestions in addition to pain expectation as independent variables (data not shown). We found an indirect effect of pain expectation which mediated the association between treatment suggestions and pain intensity (Figure 3A) as well as unpleasantness (Figure 3B).
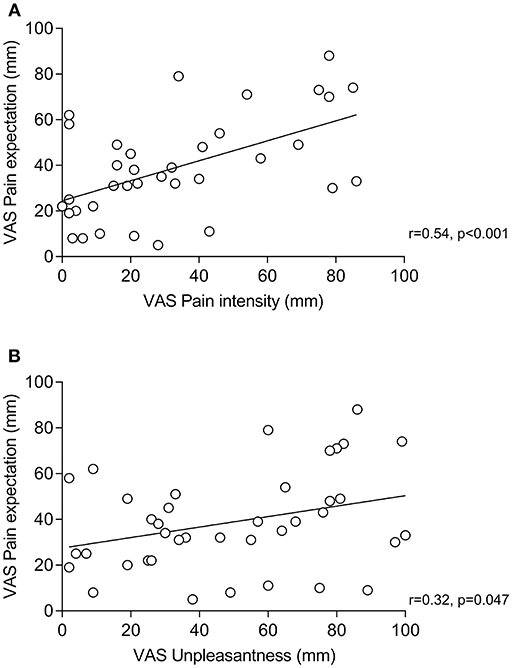
Figure 2. Correlations (Pearson's r) between pain expectation and perceived pain intensity (A) and pain expectation and perceived pain unpleasantness (B) within groups receiving positive treatment suggestions (i.e., placebo groups).
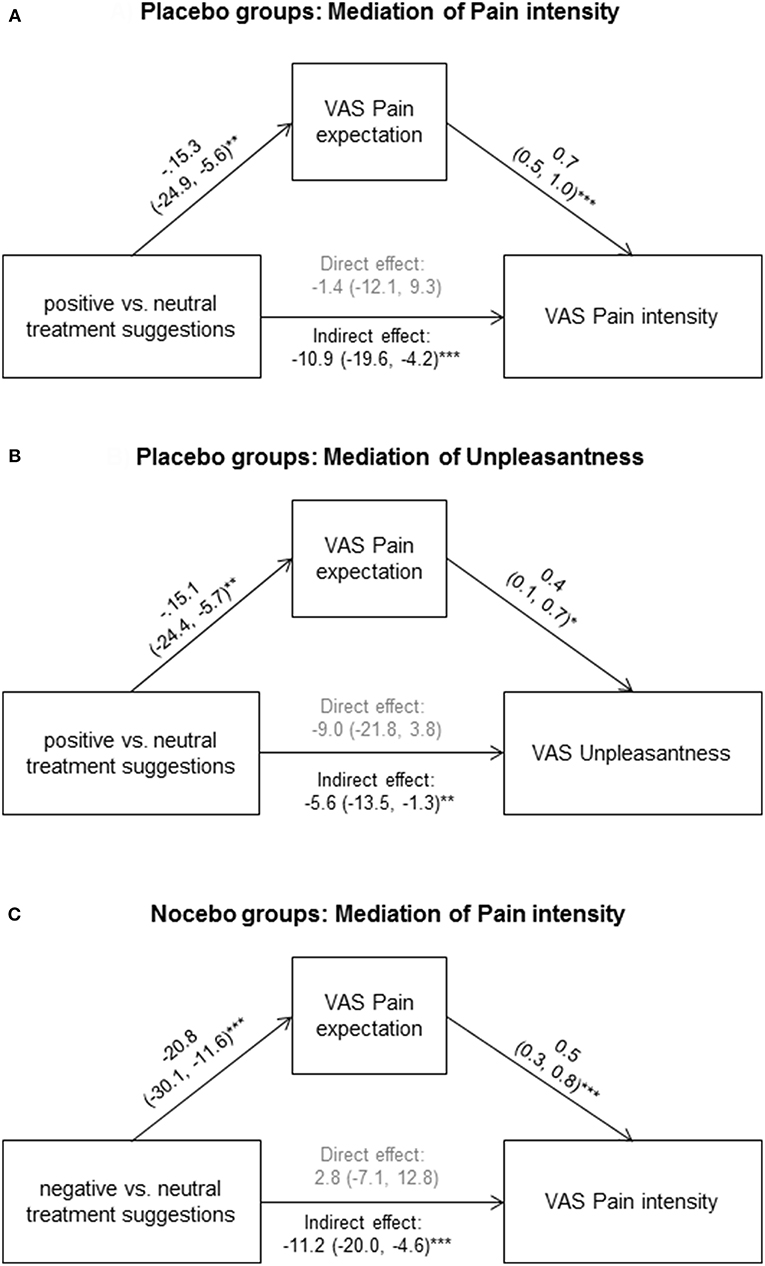
Figure 3. To explore if pain expectation mediated effects of positive treatment suggestions, we conducted mediation analyses on data from placebo and neutral suggestion groups for pain intensity (A) and unpleasantness (B), as well as on data from nocebo and neutral suggestion groups for pain intensity (C). Standardized coefficients with 95% CIs are shown. *p < 0.05; **p < 0.01; ***p < 0.001.
Within nocebo groups (N = 40), pain expectation was significantly associated pain intensity (r = 0.53, p = 0.03), but not with unpleasantness (r = 0.25, p = 0.11). The former association remained significant in multiple regression analyses including treatment suggestions in addition to pain expectation as independent variables (data not shown). To explore if pain expectation mediated effects of negative treatment suggestions, we conducted mediation analysis for pain intensity on data from nocebo and neutral suggestion groups (N = 80). We found an indirect effect of pain expectation which mediated the association between treatment suggestions and pain intensity (Figure 3C).
We conducted additional mediation analyses to explore if putative effects of relaxation vs. control on pain intensity or unpleasantness could be explained by pain expectation. In separate analyses within the placebo, nocebo, and control groups, we did not find evidence of direct or indirect effects of relaxation on pain outcomes (data not shown). This is in line with (1) the absence of significant effects of relaxation on expectations and (2) the non-significant correlations between stress markers and outcome variables.
Discussion
This is the first study testing whether a behavioral intervention aimed at reducing acute stress parameters affects the response to positive and/or negative treatment suggestions in a clinically-relevant model of visceral pain. Our findings suggest that a brief relaxation exercise may facilitate the induction of placebo analgesia by positive when compared to neutral treatment suggestions. These findings extend evidence that placebo analgesia can be boosted with pharmacological interventions (19, 20). There are clearly many facets surrounding the psychosocial treatment context that ultimately determine the presence and magnitude of expectancy effects. Our results support the contribution of relaxation and stress as psychobiological states within the psychosocial treatment context—factors which clearly deserve more attention in translational studies aiming to maximize positive expectancy effects in clinical settings.
Healthy volunteers were randomized to a brief muscle relaxation exercise or a control task just prior to randomly receiving deceptive positive, deceptive negative, or truthful neutral treatment suggestions regarding an intravenous infusion that was in reality saline in all groups. These treatment suggestions induced group-specific changes in pain expectation, with reduced pain expectation in groups receiving positive suggestions of pain relief (i.e., placebo groups) and increased pain expectation in groups receiving negative suggestions of enhanced pain sensitivity (i.e., nocebo groups). While the relaxation exercise had no discernable effect on pain expectation, relaxation significantly interacted with positive treatment suggestions. Planned comparisons of group means showed significantly reduced pain intensity and lower pain unpleasantness after positive compared to neutral treatment suggestions only in the relaxation groups. In other words, positive treatment suggestions only induced placebo analgesia in relaxed participants, which is partly in line with our hypothesis assuming a facilitated placebo effect. On the other hand, relaxation had no discernable effect on groups receiving negative suggestions. Since no nocebo effects were observed in either relaxation or control group, we could not confirm our hypothesis that relaxation may reduce nocebo hyperalgesia.
We chose a brief relaxation exercise as behavioral intervention with the intention to acutely reduce stress parameters within a highly standardized psychosocial treatment context. This approach was conceptually and methodologically based on our earlier brain imaging work on the role of emotional context in visceral pain processing (38). It complements placebo/nocebo studies in the broader pain field aiming to discern effects of acute stress, state anxiety or fear (23, 25, 26, 28–30, 40) on placebo analgesia or nocebo hyperalgesia. In order to verify the efficacy of the intervention and to gain insight into possible mechanisms, we assessed several relevant stress markers reflecting different biopsychological aspects of stress. Brief relaxation significantly reduced systolic blood pressure and heart rate, supporting effects on the autonomic nervous system (ANS). On the other hand, no effects on state anxiety or cortisol concentrations were found. This could indicate that measures of ANS function (herein: heart rate and blood pressure) are more sensitive or responsive to short-term effects of PMR, at least in healthy individuals. However, it should be noted that cortisol and state anxiety significantly decreased in both groups, and that these measures could not be assessed immediately after the relaxation exercise for methodological considerations. Hence, effects on state anxiety or cortisol could be difficult to detect given reductions in both groups and may have been missed herein. Nevertheless, the ANS is increasingly appreciated in the context of pain modulation [e.g., (41)], especially in acute and chronic visceral pain as a key component of the brain axis (42–50). Within the placebo field, the ANS has been proposed as a primary mediator of peripheral placebo effects in conditioning models (51, 52). Placebo analgesia evokes complex effects within the cardiovascular system, including changes in heart rate and blood pressure (25, 53). Blood pressure and stress were found to mediate hyperalgesia after nocebo suggestions (27), and a recent study supports a role of autonomic arousal in the persistence of nocebo hyperalgesia (54). Interestingly, the same study (54) found no correlation between either self-reported anxiety or autonomic arousal and placebo analgesia/nocebo hyperalgesia. We also explored these relationships in our dataset, and found no correlations between placebo effects and stress markers. In fact, pain expectation was the only mediator we could identify to explain the association between treatment suggestions and pain-related outcomes. These results call for caution with respect to any speculation about stress-related mechanisms and underscore the need to further study possible moderators of placebo analgesia, especially emotional factors that have been proposed to play a role in placebo analgesia (55, 56). Clearly, visceral pain modulation is complex and involves many cognitive, emotional, and possibly neurobiological factors that remain to be fully understood.
This study has strengths and limitations. Strengths include the clinically-relevant visceral pain model, blinding procedures, the combination of different psychobiological measures for traits and states, and the inclusion of groups receiving positive, negative or neutral treatment suggestions within one study. The full factorial within-between study design goes beyond correlational approaches aiming to identify psychological mediators and moderators of placebo and nocebo effects. At the same time, final group Ns are relatively small, posing limitations of statistical power, and risk of Type II error. This may for example explain why post hoc testing revealed a statistically significant reduction in pain expectation induced by positive vs. neutral suggestions only in the control but not in the relaxation group. Further, for reasons of feasibility and cost effectiveness, data from the control group were also used in Roderigo et al. (30), and there was also no additional control group that did not undergo prior relaxation training for feasibility reasons and to ensure blinding and randomization on the study day. We therefore cannot assess possible effects of prior relaxation training on measures obtained on the study day. While the absence of the brief PMR vs. control exercise effects on pain-related outcomes on the study day may be interpreted as evidence supporting a lack of relaxation effects on visceral pain, this would in our view be premature. First, we could not ascertain whether regular PMR exercise of 4 weeks did in fact induce changes in variables relevant to chronic stress. To do so was not our intention since this was not a treatment study but rather herein implemented in order to teach a sufficiently large number of study volunteers to perform PMR on the study day, aiming to realize a study design with proper randomization and blinding. We recruited a tightly-screened, healthy population of young individuals with comparatively low levels of chronic stress or stress-related symptom burden. Hence, our findings likely do not transfer to other populations at risk for stress-related health conditions or even patients with chronic pain, and should not be viewed as evidence for or against the potential clinical use of relaxation techniques in patients. In irritable bowel syndrome, for example, a recent meta-analysis (57) showed a clinical benefit of relaxation methods, and an older, more comprehensive Cochrane review (58) on relaxation therapy and stress management revealed medium effect sizes for symptom severity after 2–3 months, but inconsistent longer-term findings (after 6–12) months with regard to abdominal pain and quality of life. The lack of control group without prior relaxation training further limits our ability to test the possibility that the absence of nocebo effects could be explained by effect(s) of previous relaxation training. There are other methodological considerations regarding the absence of nocebo effects herein: Given clear effects of negative suggestions on expected pain intensity, we would argue that the nocebo manipulation did not “fail” per se. This is supported by positive correlations between pain expectation and intensity and to a smaller extent pain with unpleasantness, supporting the connection between negative pain-related expectations and ratings. Whether, negative expectations are more tightly “linked” with intensity than unpleasantness requires further study. Nocebo effects in visceral pain models have thus far not been studied outside of our group, and they may be more difficult to reliably elicit in the laboratory setting than placebo effects. It is conceivable that they can more effectively be induced in healthy individuals under conditions of heightened stress or arousal, e.g., in the scanner setting (33) that is per se stressful (59) or after acute psychosocial stress as shown in a separate arm of this study (30). Our nocebo paradigm relied exclusively on treatment suggestions, and the study was only powered to detect large effects. Combining suggestions with a learning experience (i.e., a preconditioning procedure consisting of the surreptitious increase/decrease of pain intensity prior to suggestions) may be more efficacious and enhance effect size (13, 22). Finally, our approach to utilize truthful information regarding i.v. administration of saline as a control (i.e., groups with “neutral suggestions”) is essential to properly quantify placebo/nocebo effects and distinguish them from other effects, like habituation, sensitization, order effects, etc. At the same time, these “neutral” groups are not untreated and hence by definition not free of treatment-related expectations. This may also reduce the magnitude of expectancy effects when their detection essentially relies on group comparisons [for more detailed methodological considerations, see (13)].
Together, our data provide further evidence that psychological states may alter how individuals respond to treatment suggestions. They complement recent conceptual developments on how bodily symptoms are experienced (60), especially interoceptive symptoms (61) which are demonstrably particularly salient and unpleasant when compared to exteroceptive, somatic stimuli even at matched intensities (62). Inter-individual variability in the presence and magnitude of placebo and nocebo effects is likely not only moderated by individual traits, characteristics of the treatment, and patient-provider interactions, but also by the psychological state in which treatment expectations are formed. Our findings call for more research to unravel how psychological states and their neurobiological correlates contribute to inter-individual variability in expectancy effects on symptom perception. Further, these experimental data acquired in a clinically-relevant pain model pave the way toward translation into clinical populations implementing behavioral interventions that target patients' expectancies and (also) consider psychobiological states. Indeed, placebo and nocebo effects for interoceptive, visceral symptoms are relevant to the treatment of the large group of patients with functional gastrointestinal disorders like IBS (12), but studies are needed to test whether findings from healthy volunteers can be transferred to patients. The role of the psychobiological stress systems in the pathophysiology of these clinical conditions is undisputable, as is the importance of pain or symptom-related cognitive and emotional factors (12, 42, 63, 64). If indeed these very same systems (or one of these) impacts how treatment expectations are processed, the implications are broad both for clinical practice and treatment trials. Indeed, placebo research has impressively demonstrated the clinical potential offered by psychological interventions (1, 2, 11), especially in the context of pain (1, 4). Effort to transfer knowledge from mechanistic work to clinical routine (65) are built on evidence that placebo analgesia engages similar neurobiological mechanisms as those responsible for the efficacy of pharmacological analgesic treatment (11, 66), and effectively enhances the “pure” pharmacological effect of analgesics in experimental but also in clinical settings (1, 2, 4, 18). Together, these findings pave the way for future studies. Our findings provide a small, additional “piece of the puzzle,” at minimum supporting that the recent statement “Implementation of successful treatment requires effective communication skills to improve patient acceptance, adherence and to optimize the patient provider relationship.” (67) may need amendment to incorporate additional aspects of the psychosocial treatment context, including individual treatment expectations and psychobiological states.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
SB and SE: planning of the study and acquisition of funding. TR and SB: conducting the study. TR, SB, SE, and PE: data analysis and interpretation. SE, SB, and PE: drafting of the manuscript. All authors: revision of the manuscript for critical intellectual content and approval of the final draft submitted. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Funded by a research grant (EL 236/8-2) from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG (FOR 1328). The DFG had no role in the study design, collection, analysis, interpretation of the data, or in the writing of the report.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express their gratitude to Dr. M. Schöls and M. Hetkamp for their support during data acquisition.
References
1. Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. Pain. (2013) 154:511–4. doi: 10.1016/j.pain.2013.02.002
2. Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev. (2015) 67:697–730. doi: 10.1124/pr.114.009423
3. Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain. (2016) 157:1590–8. doi: 10.1097/j.pain.0000000000000566
4. Klinger R, Colloca L, Bingel U, Flor H. Placebo analgesia: clinical applications. Pain. (2014) 155:1055–8. doi: 10.1016/j.pain.2013.12.007
5. Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. (2014) 84:623–37. doi: 10.1016/j.neuron.2014.10.023
6. Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. (2016) 157:2766–72. doi: 10.1097/j.pain.0000000000000700
7. Xiang Y, He JY, Li R. Appropriateness of sham or placebo acupuncture for randomized controlled trials of acupuncture for nonspecific low back pain: a systematic review and meta-analysis. J Pain Res. (2017) 11:83–94. doi: 10.2147/JPR.S152743
8. Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE. (2010) 5:e15591. doi: 10.1371/journal.pone.0015591
9. Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. (2008) 336:999–1003. doi: 10.1136/bmj.39524.439618.25
10. Bingel U, Placebo Competence Team. Avoiding nocebo effects to optimize treatment outcome. JAMA. (2014) 312:693–4. doi: 10.1001/jama.2014.8342
11. Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. (2013) 12:191–204. doi: 10.1038/nrd3923
12. Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. (2015) 12:472–85. doi: 10.1038/nrgastro.2015.117
13. Elsenbruch S, Labrenz F. Nocebo effects and experimental models in visceral pain. In “Neurobiology of the Placebo Effect Part I”. Int Rev Neurobiol. (2018) 138:285–306. doi: 10.1016/bs.irn.2018.01.010
14. Enck P, Chae Y, Elsenbruch S. Novel designs and paradigms to study the placebo response in gastroenterology. Curr Opin Pharmacol. (2017) 37:72–9. doi: 10.1016/j.coph.2017.10.003
15. Ulrich RS. View through a window may influence recovery from surgery. Science. (1984) 224:420–1. doi: 10.1126/science.6143402
16. He X, Sun Q, Stetler C. Warm communication style strengthens expectations and increases perceived improvement. Health Commun. (2018) 33:939–45. doi: 10.1080/10410236.2017.1322482
17. Suarez-Almazor ME, Looney C, Liu Y, Cox V, Pietz K, Marcus DM, et al. A randomized controlled trial of acupuncture for osteoarthritis of the knee: effects of patient-provider communication. Arthritis Care Res. (2010) 62:1229–36. doi: 10.1002/acr.20225
18. Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. (2011) 3:70ra14. doi: 10.1126/scitranslmed.3001244
19. Colloca L, Pine DS, Ernst M, Miller FG, Grillon C. Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol Psychiatry. (2016) 79:794–802. doi: 10.1016/j.biopsych.2015.07.019
20. Kessner S, Sprenger C, Wrobel N, Wiech K, Bingel U. Effect of oxytocin on placebo analgesia: a randomized study. JAMA. (2013) 310:1733–5. doi: 10.1001/jama.2013.277446
21. Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. (2007) 20:435–9. doi: 10.1097/ACO.0b013e3282b972fb
22. Elsenbruch S, Schmid J, Bäsler M, Cesko E, Schedlowski M, Benson S. How positive and negative expectations shape the experience of visceral pain: an experimental pilot study in healthy women. Neurogastroenterol Motil. (2012) 24:914–e460. doi: 10.1111/j.1365-2982.2012.01950.x
23. Van Den Houte M, Van Oudenhove L, Bogaerts K, Van Diest I, Van den Bergh O. Endogenous pain modulation: association with resting heart rate variability and negative affectivity. Pain Med. (2018) 19:1587–96. doi: 10.1093/pm/pnx165
24. Woo KY. Unravelling nocebo effect: the mediating effect of anxiety between anticipation and pain at wound dressing change. J Clin Nurs. (2015) 24:1975–84. doi: 10.1111/jocn.12858
25. Aslaksen PM, Flaten MA. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosom Med. (2008) 70:811–8. doi: 10.1097/PSY.0b013e31818105ed
26. Aslaksen PM, Lyby PS. Fear of pain potentiates nocebo hyperalgesia. J Pain Res. (2015) 8:703–710. doi: 10.2147/JPR.S91923
27. Aslaksen PM, Zwarg ML, Eilertsen HI, Gorecka MM, Bjørkedal E. Opposite effects of the same drug: reversal of topical analgesia by nocebo information. Pain. (2015) 156:39–46. doi: 10.1016/j.pain.0000000000000004
28. Bjørkedal E, Flaten MA. Expectations of increased and decreased pain explain the effect of conditioned pain modulation in females. J Pain Res. (2012) 5:289–300. doi: 10.2147/JPR.S33559
29. Lyby PS, Forsberg JT, Asli O, Flaten MA. Induced fear reduces the effectiveness of a placebo intervention on pain. Pain. (2012) 153:1114–21. doi: 10.1016/j.pain.2012.02.042
30. Roderigo T, Benson S, Schöls M, Hetkamp M, Schedlowski M, Enck P, et al. Effects of acute psychological stress on placebo and nocebo responses in a clinically relevant model of visceroception. Pain. (2017) 158:1489–98. doi: 10.1097/j.pain.0000000000000940
31. Elsenbruch S, Kotsis V, Benson S, Rosenberger C, Reidick D, Schedlowski M, et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain. (2012) 153:382–90. doi: 10.1016/j.pain.2011.10.036
32. Schmid J, Langhorst J, Gaß F, Theysohn N, Benson S, Engler H, et al. Placebo analgesia in patients with functional and organic abdominal pain: a fMRI study in IBS, UC and healthy volunteers. Gut. (2015) 64:418–27. doi: 10.1136/gutjnl-2013-306648
33. Schmid J, Theysohn N, Gaß F, Benson S, Gramsch C, Forsting M, et al. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain. (2013) 154:2372–80. doi: 10.1016/j.pain.2013.07.013
34. Herrmann-Lingen C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale (HADS) - Deutsche Version (2. Auflage). Bern: Hans Huber (2005).
35. Lacourt TE, Houtveen JH, Doornen LJ, Benson S, Grigoleit JS, Cesko E, et al. Biological and psychological predictors of visceral pain sensitivity in healthy premenopausal women. Eur J Pain. (2014) 18:567–74. doi: 10.1002/j.1532-2149.2013.00397.x
36. Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Goettingen: Beltz (1981).
37. Schulz P, Schlotz W. The Trier Inventory for the Assessment of Chronic Stress (TICS): scale construction, statistical testing, and validation of the scale work overload. Diagnostica. (1999) 45:8–19. doi: 10.1026/0012-1924.45.1.8
38. Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. (2010) 139:1310–9. doi: 10.1053/j.gastro.2010.06.054
39. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. (2003) 28:916–31. doi: 10.1016/S0306-4530(02)00108-7
40. Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. (2005) 115:338–47. doi: 10.1016/j.pain.2005.03.014
41. Fechir M, Breimhorst M, Kritzmann S, Geber C, Schlereth T, Baier B, et al. Naloxone inhibits not only stress-induced analgesia but also sympathetic activation and baroreceptor-reflex sensitivity. Eur J Pain. (2012) 16:82–92. doi: 10.1016/j.ejpain.2011.06.009
42. Boeckxstaens G, Camilleri M, Sifrim D, Houghton LA, Elsenbruch S, Lindberg G, et al. Fundamentals of neurogastroenterology: physiology/motility – sensation. Gastroenterology. (2016) 2016:S0016-5085(16)00221-3. doi: 10.1053/j.gastro.2016.02.030
43. Botha C, Farmer AD, Nilsson M, Brock C, Gavrila AD, Drewes AM, et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut. (2015) 64:611–7. doi: 10.1136/gutjnl-2013-306698
44. Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. (1997) 96:3224–32. doi: 10.1161/01.CIR.96.9.3224
45. Farmer AD, Coen SJ, Kano M, Naqvi H, Paine PA, Scott SM, et al. Psychophysiological responses to visceral and somatic pain in functional chest pain identify clinically relevant pain clusters. Neurogastroenterol Motil. (2014) 26:139–48. doi: 10.1111/nmo.12245
46. Farmer AD, Coen SJ, Kano M, Paine PA, Shwahdi M, Jafari J, et al. Psychophysiological responses to pain identify reproducible human clusters. Pain. (2013) 154:2266–76. doi: 10.1016/j.pain.2013.05.016
47. Frokjaer JB, Bergmann S, Brock C, Madzak A, Farmer AD, Ellrich J, et al. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol Motil. (2016) 28:592–8. doi: 10.1111/nmo.12760
49. Mazurak N, Seredyuk N, Sauer H, Teufel M, Enck P. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neurogastroenterol Motil. (2012) 24:206–16. doi: 10.1111/j.1365-2982.2011.01866.x
50. Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. (2010) 35:653–62. doi: 10.1016/j.psyneuen.2009.10.004
51. Meissner K. Placebo responses on cardiovascular, gastrointestinal, and respiratory organ functions. Handb Exp Pharmacol. (2014) 225:183–203. doi: 10.1007/978-3-662-44519-8_11
52. Ober K, Benson S, Vogelsang M, Bylica A, Günther D, Witzke O, et al. Plasma noradrenaline and state anxiety levels predict placebo response in learned immunosuppression. Clin Pharmacol Ther. (2012) 91:220–6. doi: 10.1038/clpt.2011.214
53. Pollo A, Vighetti S, Rainero I, Benedetti F. Placebo analgesia and the heart. Pain. (2003) 102:125–33. doi: 10.1016/s0304-3959(02)00345-7
54. Colagiuri B, Quinn VF. Autonomic arousal as a mechanism of the persistence of nocebo hyperalgesia. J Pain. (2018) 19:476–86. doi: 10.1016/j.jpain.2017.12.006
55. Flaten MA, Aslaksen PM, Lyby PS, Bjørkedal E. The relation of emotions to placebo responses. Philos Trans R Soc Lond B Biol Sci. (2011) 366:1818–27. doi: 10.1098/rstb.2010.0407
56. Flaten MA. Pain-related negative emotions and placebo analgesia. Handb Exp Pharmacol. (2014) 225:81–96. doi: 10.1007/978-3-662-44519-8_5
57. Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. (2019) 114:21–39. doi: 10.1038/s41395-018-0222-5
58. Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev. (2009) 2009:Cd006442. doi: 10.1002/14651858.CD006442.pub2
59. Vachon-Presseau E, Martel MO, Roy M, Caron E, Albouy G, Marin MF, et al. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. (2013) 33:6826–33. doi: 10.1523/JNEUROSCI.4584-12.2013
60. Van den Bergh O, Witthöft M, Petersen S, Brown RJ. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev. (2017) 74:185–203. doi: 10.1016/j.neubiorev.2017.01.015
61. Petersen S, von Leupoldt A, den Bergh OV. Interoception and the uneasiness of the mind: affect as perceptual style. Front Psychol. (2015) 6:1408. doi: 10.3389/fpsyg.2015.01408
62. Koenen LR, Icenhour A, Forkmann K, Pasler K, Theysohn N, Forsting M, et al. Greater fear of visceral pain contributes to differences between visceral and somatic pain in healthy women. Pain. (2017) 158:1599–608. doi: 10.1097/j.pain.0000000000000924
63. Elsenbruch S, Enck P. The stress concept in gastroenterology: from Selye to today. F1000Res. (2017) 6:2149. doi: 10.12688/f1000research.12435.1
64. Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, Feinle-Bisset C, Holtmann G, et al. Functional dyspepsia. Nat Rev Dis Primers. (2017) 3:17081. doi: 10.1038/nrdp.2017.81
65. Brody H, Miller FG. Lessons from recent research about the placebo effect–from art to science. JAMA. (2011) 306:2612–3. doi: 10.1001/jama.2011.1850
66. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. (2015) 16:403–18. doi: 10.1038/nrn3976
Keywords: placebo effect, nocebo effect, expectation, visceral pain, pain unpleasantness, stress, relaxation
Citation: Elsenbruch S, Roderigo T, Enck P and Benson S (2019) Can a Brief Relaxation Exercise Modulate Placebo or Nocebo Effects in a Visceral Pain Model? Front. Psychiatry 10:144. doi: 10.3389/fpsyt.2019.00144
Received: 22 January 2019; Accepted: 27 February 2019;
Published: 21 March 2019.
Edited by:
Martina De Zwaan, Hannover Medical School, GermanyReviewed by:
Michael Stephan, Hannover Medical School, GermanyGeorgios Paslakis, University of Bamberg, Germany
Copyright © 2019 Elsenbruch, Roderigo, Enck and Benson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sven Benson, c3Zlbi5iZW5zb25AdWstZXNzZW4uZGU=
 Sigrid Elsenbruch
Sigrid Elsenbruch Till Roderigo1
Till Roderigo1 Paul Enck
Paul Enck Sven Benson
Sven Benson