- 1Department of Psychiatry, Beitou Branch, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 2Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan
- 3Department of Psychiatry, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 4Department of Psychiatry, Taipei Veterans General Hospital, Taipei, Taiwan
- 5School of Medicine, National Yang-Ming University, Taipei, Taiwan
- 6Institute of Brain Science, National Yang-Ming University, Taipei, Taiwan
- 7Division of Interdisciplinary Medicine and Biotechnology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
- 8Department of Psychiatry, Chiayi Branch, Taichung Veterans General Hospital, Chiayi, Taiwan
The chronic autoimmune disease myasthenia gravis (MG) is characterized by fluctuating muscle weakness, which can lead to a large amount of stress in the patient. The current investigation plans to assess the risk of depressive disorders in MG patients. A retrospective cohort study of patients ageing 20 years and older and also newly diagnosed with MG between January 1, 2000, and December 31, 2008, was conducted from the National Health Insurance Research Database (NHIRD) in Taiwan. Observations of all 349 MG patients and 1,396 control individuals were made until a diagnosis of a depressive disorder by a psychiatrist, until death, or until December 31, 2013. A range of comorbidities were found, such as coronary artery disease, hypertension, diabetes mellitus, and dyslipidemia, with cerebrovascular disease being reported more frequently in MG patients in comparison with control subjects. After adjustment of patients’ sex, age, urbanization, comorbidities, and monthly income, results indicated that MG individuals are 1.94 times more at risk (95% confidence interval [CI], 1.15–3.27, P = 0.014) of developing depressive disorders than are controls. This showed an increased risk in the development of depressive disorders in people with MG. Thus, depressive symptoms in MG patients should be regularly assessed.
Introduction
Myasthenia gravis (MG), one of the chronic immune diseases, is described with symptoms of unpredictable fluctuating weakness and fatigue in neck muscles, eye muscles, limb muscles, swallowing, chewing, breathing, and talking throughout the day by having an impact on the skeletal neuromuscular junction. Symptoms are usually lighter during the morning hours, worsen as the day progresses and also with exertion, but improve with rest (1).
The concurrent reduction in skeletal muscle contractions in the pathophysiology of MG is due to the antibodies directed to nicotinic acetylcholine receptors and other proteins, changing the motor circuits (2). MG has a disability prevalence of 6/100,000 (3). In a previous study using the National Health Insurance Research Database (NHIRD) to investigate the epidemiology of myasthenia gravis in Taiwan, the average annual incidence was 2.1/100,000. The prevalence increased steadily during the study period from 8.4/100,000 in 2000 to 14.0/100,000 in 2007 (4). MG is a life-long and remitting/relapsing disease with an unpredictable course (3). The cholinergic system plays a key role in memory impairment and sleep disorders as described in MG (5, 6). On the other hand, continued treatment and chronic symptoms may lead to significant restraint and diminution of health-related quality of life (7–10).
Depressive disorder has a lifetime prevalence of 7% to 17% in the general population (11, 12). In a study using NHIRD to investigate the epidemiology of depressive disorder in Taiwan, the annual incidence of depressive disorder was 3.91/1,000 (13). The fact that frequency of depression increases significantly in chronically ill individuals, a well-known significant limitation in physical functioning, was also associated with depression. Other authors have found that depression has significant impacts on the course and outcome of medical illnesses (14), including increased ambulatory visits (15), more somatic complaints (16), and reduced motivation for self-care (17, 18). It may be difficult for depression patients to get used to the aversive manifestations of chronic medical illness. With control of the severity of physical illnesses, around 50% increase of the medical costs for chronic illnesses is still associated with depression. Therefore, clinicians should pay particular attention to depression in patients with chronic medical illnesses.
Brain functions determining behavioral changes such as suicidal behaviors and depressive disorders may be affected by MG (19). In previous studies in Taiwan using NHIRD, patients with neurological disorders such as cluster headache (5.6 times) (20), Parkinson disease (4.1 times) (21), and Tourette syndrome (4.9 times) (22) are found to have a higher risk of developing depression during the follow-up period. Furthermore, psychological reactions may be predicted in patients diagnosed with MG as this disease is attenuating, chronic, and life-threatening with unpredictable progression (23). Just like patients with other chronic diseases, MG patients may possess a wide range of social and psychological disabilities that may be more critical than defective physical functions (24–26). Often, psychiatric consequences such as depressive and anxiety disorders are the result of chronic inflammatory disease conditions (3, 23, 27–29). In our previous studies using NHIRD, for example, the incidence rate of depressive disorder was higher in patients with ankylosing spondylitis (5.48 per 1,000 person-years) than in control patients (3.29 per 1,000 person-years) (28). With regard to MG, 74 patients with MG examined by Magni et al. revealed that 22% had adjustment disorder with mixed emotional features and depressed mood and that 14% matched the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III) criteria for an affective disorder (30). Considerations regarding careful psychiatric/psychological evaluation may look to the temporal association between depressive disorders and MG. However, none of the studies investigating the association between MG and the subsequent risk of depressive disorders had used large databases for such research. Therefore, we used the Taiwan National Health Insurance Research Database (NHIRD) to assess whether MG patients are at a higher risk for developing depressive disorders. We also performed a Cox proportional-hazards regression model to identify risk factors that predicted depressive disorders in the MG patients.
Method
Data Sources
Instituted in 1995, the NHI program is a mandatory health insurance program offering comprehensive medical care coverage, including outpatient, inpatient, emergency, and traditional Chinese medicine to all residents of Taiwan; the coverage rate is as high as 99% (31). The NHIRD contains comprehensive information regarding clinical visits, with prescription details and diagnostic codes based on the International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM). Confidentiality of the NHIRD is maintained according to directives of the Bureau of the NHI, and NHIRD itself is managed by the National Health Research Institutes (NHRI). Longitudinal Health Insurance Database 2000 (LHID 2000), which is a subset of NHIRD created by NHRI, is the data source for our study. LHID 2000 contains all original claim data of 1,000,000 beneficiaries that were randomly sampled from the year 2000 Registry for Beneficiaries of the NHIRD, where registration data of everyone who was a beneficiary of the National Health Insurance program during the period of January 1, 2000, to January 1, 2001, were drawn for random sampling. There are approximately 23.75 million individuals in this registry. All the registration and claim data of these 1,000,000 individuals collected by the National Health Insurance program constitute the LHID 2000. The NHRI of Taiwan reported that there were no significant differences in gender distribution, age distribution, or average insured payroll-related amount between the patients in the LHID and those in the original NHIRD (National Health Insurance Research Database, Taiwan; http://nhird.nhri.org.tw/en/index.htm).
Ethics Statement
This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (VGHIRB No.: 2018-07-016AC). As the NHI dataset contains only de-identified secondary data for research purposes, and a formal written waiver for the need for consent was issued by the Institutional Review Board of Taipei Veterans General Hospital, written consents were not obtained.
Study Population
We selected patients aged 20 years and older who were newly diagnosed with MG between January 1, 2000, and December 31, 2008, to carry out a retrospective cohort study. MG was defined as ICD-9-CM code: 358.0. Patients diagnosed with depressive disorders were excluded (ICD-9-CM codes: 296.2X-296.3X, 300.4, and 311.X) before enrollment. For each MG patient included in the final cohort, four age-, sex-, and enrolment-date-matched control patients who were not diagnosed with MG or depressive disorder were randomly selected from the LHID 2000. The random assignment procedures were performed by SAS statistical software and were based on the random numbers that were generated from the uniform distribution.
Insurance premiums, calculated according to the beneficiaries’ total income, were used to estimate monthly income. Monthly income was divided into no income, low income [monthly income < 20,000 New Taiwan Dollar (NTD)], median income (20,000 NTD ≤ monthly income < 40,000 NTD), and high income (monthly income ≥ 40,000 NTD). Urbanization was categorized into three groups: urban, suburban, and rural. Urbanization and monthly income levels were used to represent socioeconomic status. Furthermore, the preexisting comorbidities at the date of enrolment, including hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, congestive heart failure, hyperthyroidism, hypothyroidism, and cerebrovascular disease, were identified in both cohorts.
All MG and control patients were observed until a diagnosis of a depressive disorder by a psychiatrist, until death, or until December 31, 2013. The primary clinical outcome assessed was psychiatrist-diagnosed depressive disorder.
Statistical Analyses
Demographic differences between MG and control patients were examined using independent t-tests and chi-squared tests. Calculation of the incidence rate (per 1,000 person-years) of newly diagnosed depressive disorders in MG and control individuals was made. To investigate potential surveillance bias, subgroups were stratified according to the duration since MG diagnosis.
Variables that predicted depressive disorder in MG and control patients as well as in MG patients only were identified using Cox proportional-hazards regression model. Covariates in the univariate model included control variables such as age; sex; common comorbidities, including hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, congestive heart failure, hyperthyroidism, hypothyroidism, and cerebrovascular disease; urbanization; and monthly income. Factors that demonstrated a moderately significant statistical relationship in the univariate analysis (P < 0.1) were entered by forward selection in a multivariate Cox proportional-hazards regression model (32).
Data were extracted and computed using the Perl programming language (version 5.12.2). The Microsoft SQL Server 2005 (Microsoft Corp., Redmond, WA, USA) was used for data linkage, processing, and control sampling. IBM SPSS (version 19.0 for Windows; IBM Corp., New York, NY, USA) and SAS statistical software (version 9.2; SAS Institute Inc., Cary, NC, USA) were used to perform all statistical analyses. Comparisons resulting in a P value of less than 0.05 were considered to indicate a statistically significant relationship.
Results
Participant Selection
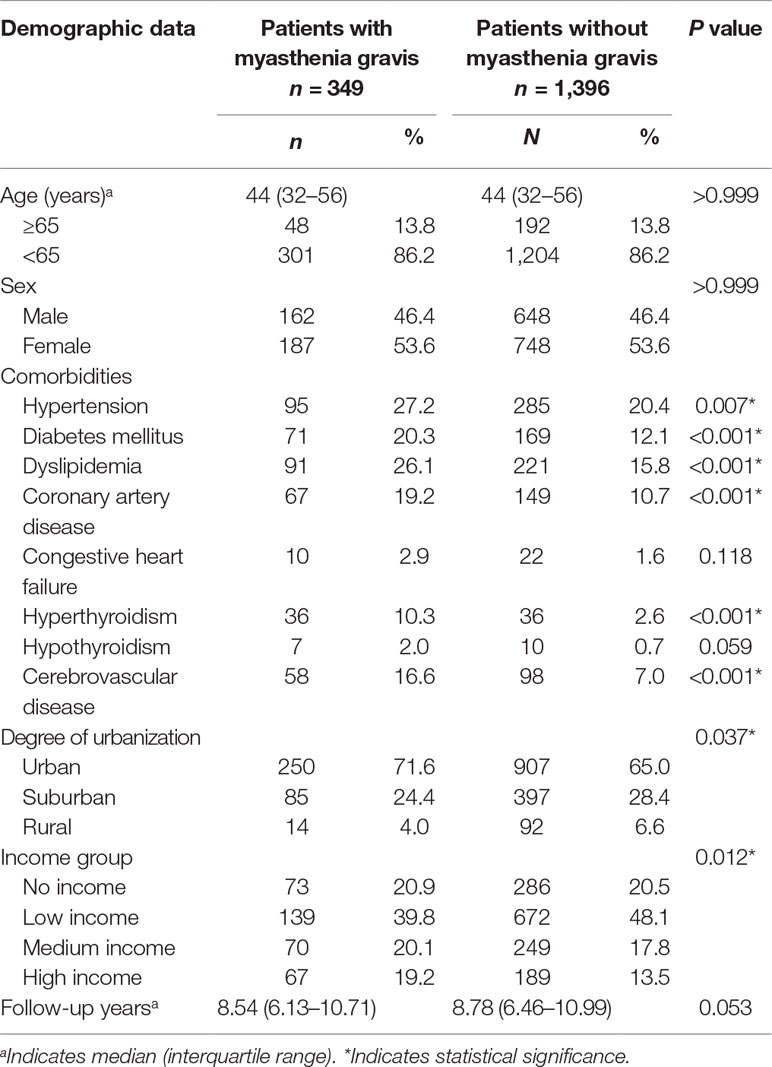
A total of 349 MG patients and 1,396 control individuals without depression were selected; among them, 53.6% were women. The median age at enrollment was 44 years [interquartile range (IQR), 32–56 years], and the median follow-up periods for the MG and control patients were 8.54 (IQR, 6.13–10.71 years) and 8.78 years (IQR, 6.46–10.99 years), respectively. Comorbidities including hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, hyperthyroidism, and cerebrovascular disease were reported more frequently in the MG patients than in the control patients. Table 1 shows the demographic and clinical variables of the MG and control patients.
Incidence Rate of Depression in MG and Control Cohort
During the follow-up period, 22 MG patients (7.60 per 1,000 person-years) and 43 control patients (3.55 per 1,000 person-years) were diagnosed with depressive disorders (Table 2). The incidence risk ratio (IRR) of depressive disorder between the MG and control patients was 2.14 (95% CI, 1.22–3.65). When stratified with the follow-up durations, higher IRR of newly diagnosed depressive disorder remained significantly increased in longer follow-up duration (≥1 year).

Table 2 Number of newly diagnosed depressive disorders between myasthenia gravis and control subjects, which was stratified by follow-up duration.
MG on Risks of Clinical Depression
In MG patients, in comparison with control individuals, the hazard ratio (HR) for developing depressive disorders during the follow-up period was 1.94 times (95% CI, 1.15–3.27, P = 0.014) more likely, after adjustment for age, sex, comorbidities, urbanization, and monthly income (Table 3 and Figure 1).
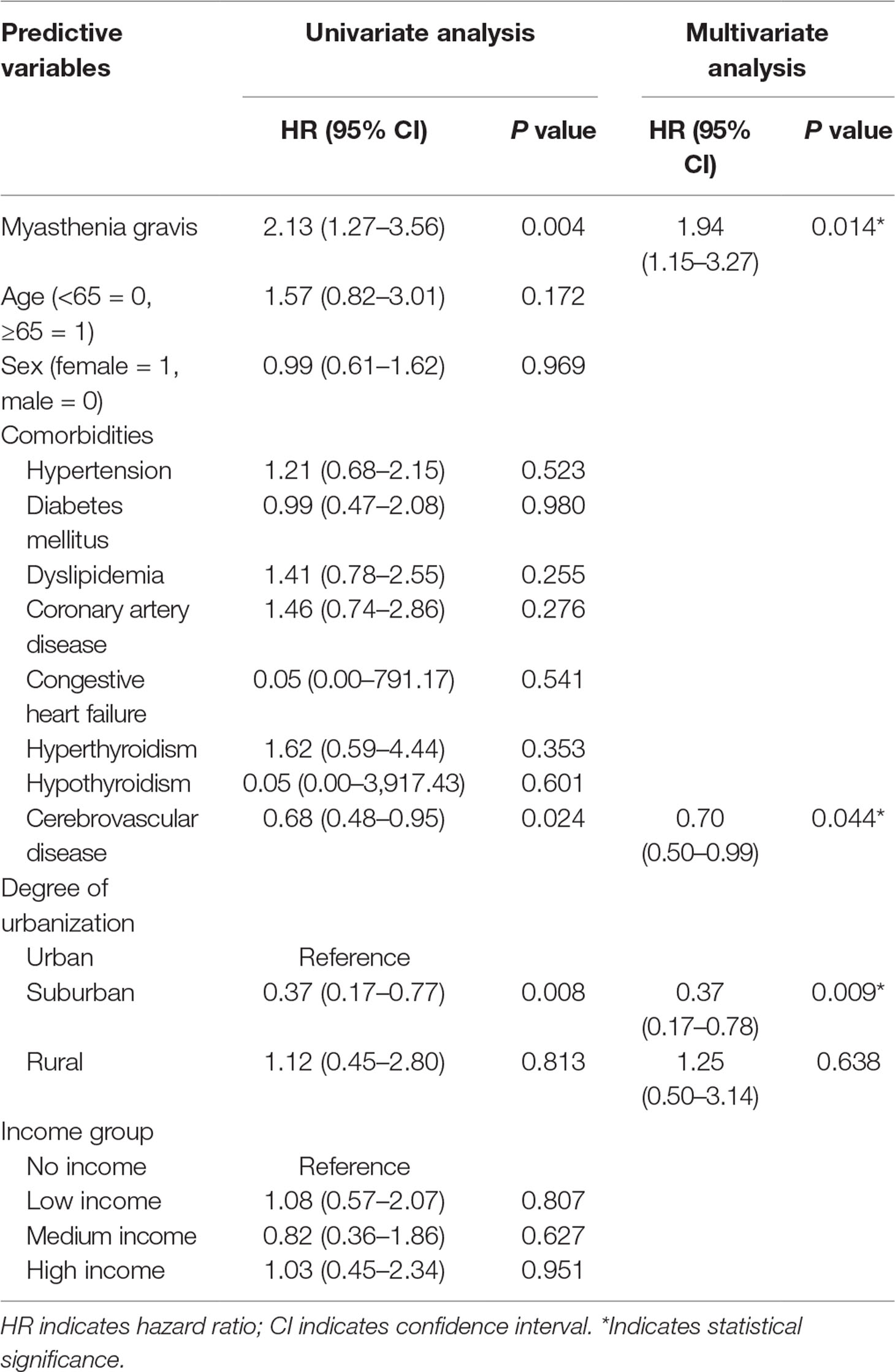
Table 3 Analyses of risk factors for depressive disorder in patients with and without myasthenia gravis.
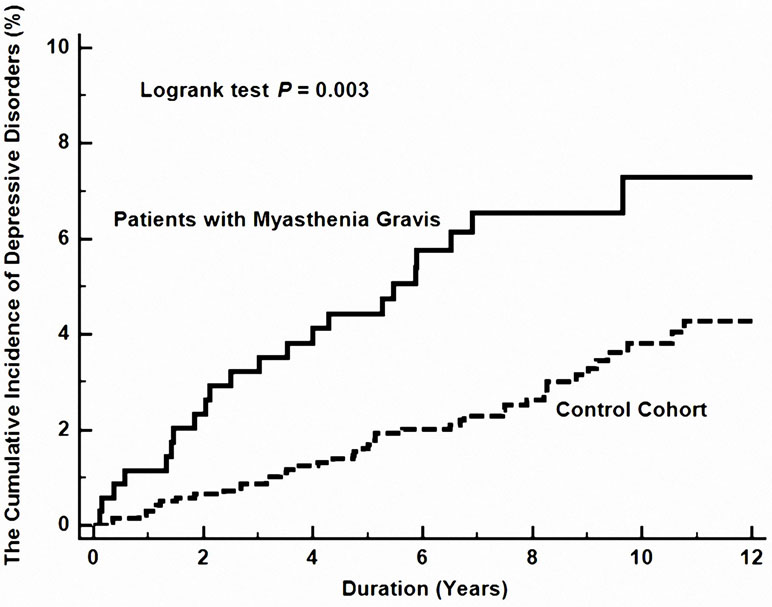
Figure 1 The cumulative risk of depressive disorder between patients with and without myasthenia gravis.
Risks Factors for Depression in MG Patients
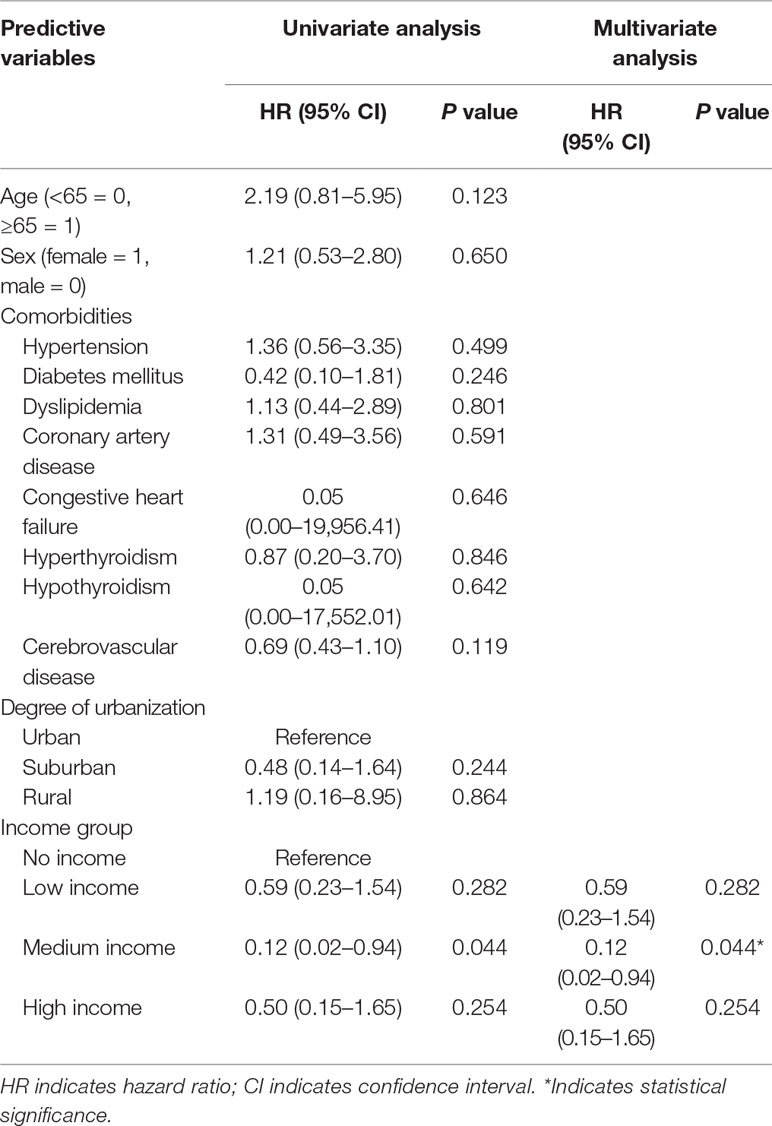
Variables that predicted depressive disorders in MG patients were identified by performing a Cox proportional-hazards regression model. Results showed that only medium monthly income is a favorable prognostic factor for depressive disorders in the MG patients (HR: 0.12; 95% CI, 0.02–0.94, P = 0.044) (Table 4).
Discussion
We analyzed the risk of depressive disorder in MG patients by conducting a nationwide study. Results revealed that in MG patients, in comparison with control individuals, the HR for developing depressive disorders during the follow-up period increased to 1.94 times, after adjustment for age, sex, comorbidities, urbanization, and monthly income. It was also noted that MG patients had higher prevalence of hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, hyperthyroidism, and cerebrovascular disease (Table 1), which are possibly related to the long-term prednisolone use, a first-choice immunosuppressive treatment, of MG patients (33, 34).
Our results revealed that MG might be a risk factor for consequent depressive disorders. Previously, the 74 patients with MG examined by Magni et al. had been found 22% to have adjustment disorder with mixed emotional features and depressed mood (30). In a previous study, depressive symptoms in MG patients were associated with disease severity, dose of oral glucocorticoids, longer duration of illnesses, and muscle weaknesses (35).
The increased risk of depressive disorders in MG patients may be due to various factors. One of the reasons may be that these patients are experiencing chronic and disabling situations such as MG, which result in restrictions in all perspectives of life. A cross-sectional study showed that the more severe the MG in individuals, the higher the likelihood of having depressive symptoms and anxiety symptoms, in comparison with that in individuals with less severe MG (36). Furthermore, treatment options for the disease may also cause psychiatric morbidity. Corticosteroids, which are common immunosuppressive agent, are currently the approved therapy for MG that is intractable to anti-cholinesterase medications (37). Depression and anxiety are associated with corticosteroid use, but corticosteroid use especially tends to induce depressive symptoms in long-term therapy (38, 39). The incidence of adverse effect is directly related to dosage (40). Therefore, dosage regulation, joint therapies, and psychiatric evaluations may be essential in coping with the unfavorable psychiatric effects of corticosteroid use. Also, it has been reported that the cholinergic effects of anticholinesterase agents play a role in the pathophysiology of depression in patients with or without MG (16). In terms of the psychological and social aspects of the individuals, patients with multi-drug therapies or more severe diseases and incapacities may have more emotional disturbances. A previous study demonstrated that stressful life events were associated with anxiety and depression, which developed in patients with MG (36).
In this study, we found that “medium income” might be a favorable prognostic factor for depressive disorders in the MG patients (Table 3). The marginal significance (P value of 0.044) suggests that the findings might be accidental. Furthermore, Table 1 shows that the MG patients were, on average, significantly richer than the controls, which may also affect the findings. Further study is needed to exclude a spurious association.
The increased risk of depressive disorders in MG patients may be due to surveillance bias. Patients with MG are more likely to visit hospitals, thus leading to an early diagnosis of depressive disorders. To investigate potential surveillance bias, we conducted a subgroup analysis that was stratified according to the duration between the diagnosis of MG and new-onset depressive disorders (Table 2). When patients diagnosed with depressive disorders within 1 year of MG diagnosis were excluded, the incidence risk ratio for the newly diagnosed depressive disorder remained high for the MG cohort, and the ratio was statistically significant. Thus, this result suggests that the increased risk of depressive disorder in MG patients was not caused by surveillance bias.
There is limited published information on the psychological and pharmacological treatments for the psychiatric morbidities associated with MG. The results of the accessible studies may not be utilized as common treatment principles due to methodological limitations or small study population. Supportive and cognitive psychotherapies may be helpful in patients who had neurological disease with subsequent psychiatric symptoms that developed (41, 42). The use of psychotropic medications in MG patients had limited published researches without randomized and placebo-controlled designs. In an open clinical study, fluoxetine had weight-reducing effect found in 13 overweight patients with MG who had long-term corticosteroids treatment, although the study group had no depressed patients (43). In another 12-week open-label, prospective study trial for MG patients with major depression, the use of citalopram showed significant improvement in depression without deterioration in the progression of MG (44). Electroconvulsive therapy is an option for treating resistant affective disorders, including depressive and catatonic episodes. Electroconvulsive therapy is a workable therapeutic option in patients with MG when psychiatric problems secondary to MG or the psychotropic medications show no response (45).
Large sampling size and MG and depressive disorder diagnoses made by specialists were the strengths of our study. In addition, our study design included an unbiased participant selection process. Due to the fact that participation in the NHI was mandatory and all residents of Taiwan could reach health care with low copayments, referral biases were low and follow-up compliance was high.
Certain limitations to our findings should be considered.
1. Due to differences between individual physicians, diagnoses input into the NHI database may be diverse. In order to eliminate this limitation, MG diagnosed by neurologists and depressive disorders diagnosed by psychiatrists where each had at least two consensus diagnoses were included in the current study.
2. Some risk factors for depressive disorders were unable to be extracted from the NHI database (such as drinking habits or smoking, level of education, religious beliefs, exercise habits, and body mass index) and thus could not be included into the evaluation, which may result in a bias.
3. Underlying mechanisms of MG and depressive disorders could not be directly examined and analyzed using population-based retrospective cohort studies.
4. The severity, the subgroup of MG, and the treatment strategy for MG were unknown in our study; and whether these factors influence the risk of developing depressive disorder warrants further study.
The results of our nationwide population-based retrospective cohort study indicated that MG patients possessed a higher risk of developing depressive disorders, with their level of monthly income as a key major factor. This meant that clinicians should pay more attention to MG patients’ psychological evaluation and give suitable psychological care.
Data Availability
The datasets for this study will not be made publicly available because the data that support the findings of this study are available from Taiwan National Health Insurance Research Database (NHIRD). To gain access, interested individuals should contact NHIRD.
Ethics Statement
This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (VGHIRB No.: 2018- 07-016AC). As the NHI dataset contains only de-identified secondary data for research purposes, and a formal written waiver for the need for consent was issued by the Institutional Review Board of Taipei Veterans General Hospital, written consents were not obtained.
Author Contributions
Study conception and design: H-TC, C-CT, and S-JT. Acquisition of data: AY, C-CS, and SJ-T. Analysis and interpretation of data: CC-T, CS-L, TC-Y, L-YH, and C-CS. Drafting of manuscript: H-TC, C-CS, and S-JT. All authors read and approved the final manuscript.
Funding
This work was supported by grant V108C-038 from the Taipei Veterans General Hospital. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Emily Ting for English editing.
References
1. Drachman DB. Myasthenia gravis. N Engl J Med (1994) 330:1797–810. doi: 10.1056/NEJM199406233302507
2. Hughes BW, De Casillas MLM, Kaminski HJ. Pathophysiology of myasthenia gravis. Semin Neurol (2004) 24:21–30. doi: 10.1055/s-2004-829585
3. Keesey JC. Does myasthenia gravis affect the brain? J Neurol Sci (1999) 170:77–89. doi: 10.1016/S0022-510X(99)00205-1
4. Lai CH, Tseng HF. Nationwide population-based epidemiological study of myasthenia gravis in Taiwan. Neuroepidemiology (2010) 35:66–71. doi: 10.1159/000311012
5. Tucker DM, Roeltgen DP, Wann PD, Wertheimer RI. Memory dysfunction in myasthenia gravis: evidence for central cholinergic effects. Neurology (1988) 38:1173–7. doi: 10.1212/WNL.38.8.1173
6. Papazian O. Rapid eye movement sleep alterations in myasthenia gravis. Neurology (1976) 26:311–6. doi: 10.1212/WNL.26.4.311
7. Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol (2009) 8:475–490. doi: 10.1016/S1474-4422(09)70063-8
8. Padua L, Evoli A, Aprile I, Caliandro P, Mazza S, Padua R, et al. Health-related quality of life in patients with myasthenia gravis and the relationship between patient-oriented assessment and conventional measurements. Neurol Sci (2001) 22:363–9. doi: 10.1007/s100720100066
9. Mullins LL, Carpentier MY, Paul RH, Sanders DB, Group MS. Disease-specific measure of quality of life for myasthenia gravis. Muscle Nerve (2008) 38:947–56. doi: 10.1002/mus.21016
10. Paul RH, Nash JM, Cohen RA, Gilchrist JM, Goldstein JM. Quality of life and well-being of patients with myasthenia gravis. Muscle Nerve (2001) 24:512–6. doi: 10.1002/mus.1034
11. Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry (1994) 151:979–86. doi: 10.1176/ajp.151.7.979
12. Wells K, Golding J, Burnam M. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry (1988) 145:976. doi: 10.1176/ajp.145.8.976
13. Fife D, Feng Y, Wang MY, Chang CJ, Liu CY, Juang HT, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in Taiwan. Psychiatry Res (2017) 252:277–83. doi: 10.1016/j.psychres.2017.03.006
14. Katon W. The impact of major depression on chronic medical illness. Gen Hosp Psychiatry (1996) 18:215–9. doi: 10.1016/0163-8343(96)00065-5
15. Regier DA, Hirschfeld RM, Goodwin FK, Burke JD, Lazar JB, Judd LL. The NIMH Depression Awareness, Recognition, and Treatment Program: structure, aims, and scientific basis. Am J Psychiatry (1988) 145:1351–7. doi: 10.1176/ajp.145.11.1351
17. Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol (1995) 14:88. doi: 10.1037/0278-6133.14.1.88
18. Surridge D, Erdahl DW, Lawson J, Donald M, Monga T, Bird C, et al. Psychiatric aspects of diabetes mellitus. Br J Psychiatry (1984) 145:269–76. doi: 10.1192/bjp.145.3.269
19. Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin (2006) 24:441–60. doi: 10.1016/j.ncl.2006.03.003
20. Liang JF, Chen YT, Fuh JL, Li SY, Liu CJ, Chen TJ, et al. Cluster headache is associated with an increased risk of depression: a nationwide population-based cohort study. Cephalalgia (2013) 33:182–9. doi: 10.1177/0333102412469738
21. Hsu YT, Liao CC, Chang SN, Yang YW, Tsai CH, Chen TL, et al. Increased risk of depression in patients with Parkinson disease: a nationwide cohort study. Am J Geriatr Psychiatry (2015) 23:934–40. doi: 10.1016/j.jagp.2014.10.011
22. Chou IC, Lin HC, Lin CC, Sung FC, Kao CH. Tourette syndrome and risk of depression: a population-based cohort study in Taiwan. J Dev Behav Pediatr (2013) 34:181–5. doi: 10.1097/DBP.0b013e3182829f2b
23. Kulaksizoglu IB. Mood and anxiety disorders in patients with myasthenia gravis. CNS Drugs (2007) 21:473–81. doi: 10.2165/00023210-200721060-00004
24. Raggi A, Leonardi M, Mantegazza R, Casale S, Fioravanti G. Social support and self-efficacy in patients with myasthenia gravis: a common pathway towards positive health outcomes. Neurol Sci (2010) 31:231–5. doi: 10.1007/s10072-009-0194-8
25. Drulovic J, Pekmezovic T, Matejic B, Mesaros S, Manigoda M, Dujmovic I, et al. Quality of life in patients with multiple sclerosis in Serbia. Acta Neurol Scand (2007) 115:147–52. doi: 10.1111/j.1600-0404.2006.00729.x
26. Peric S, Rakocevic-Stojanovic V, Stevic Z, Basta I, Pavlovic S, Vujanac V, et al. Health-related quality of life in patients with myotonic dystrophy type 1 and amyotrophic lateral sclerosis. Acta Neurol Belg (2010) 110:71.
27. Tseng CC, Hu LY, Liu ME, Yang AC, Shen CC, Tsai SJ. Risk of depressive disorders following sudden sensorineural hearing loss: a nationwide population-based retrospective cohort study. J Affect Disord (2016) 197:94–9. doi: 10.1016/j.jad.2016.03.020
28. Shen CC, Hu LY, Yang AC, Kuo BI, Chiang YY, Tsai SJ. Risk of psychiatric disorders following ankylosing spondylitis: a nationwide population-based retrospective cohort study. J Rheumatol (2016) 43:625–31. doi: 10.3899/jrheum.150388
29. Shen CC, Yang AC, Kuo BI, Tsai SJ. Risk of psychiatric disorders following primary Sjogren syndrome: a nationwide population-based retrospective cohort study. J Rheumatol (2015) 42:1203–8. doi: 10.3899/jrheum.141361
30. Magni G, Micaglio G, Lalli R, Bejato L, Candeago M, Merskey H, et al. Psychiatric disturbances associated with myasthenia gravis. Acta Psychiatr Scand (1988) 77:443–5. doi: 10.1111/j.1600-0447.1988.tb05148.x
31. Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA (2012) 308:1906–14. doi: 10.1001/2012.jama.11975
32. Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med (2011) 364:2208–17. doi: 10.1056/NEJMoa1011600
33. Gilhus N, Nacu A, Andersen J, Owe J. Myasthenia gravis and risks for comorbidity. Eur J Neurol (2015) 22:17–23. doi: 10.1111/ene.12599
34. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol (2015) 14:1023–36. doi: 10.1016/S1474-4422(15)00145-3
35. Suzuki Y, Utsugisawa K, Suzuki S, Nagane Y, Masuda M, Kabasawa C, et al. Factors associated with depressive state in patients with myasthenia gravis: a multicentre cross-sectional study. BMJ open (2011) 1:e000313. doi: 10.1136/bmjopen-2011-000313
36. Aysal F, Karamustafalioğlu O, Özçelik B, Yilmaz M, Karamustafalioğlu N, Yumrukçal H, et al. The relationship of symptoms of anxiety and depression with disease severity and treatment modality in myasthenia gravis: a cross-sectional study. Nöro Psikiyatri Arşivi (2013) 50:295. doi: 10.4274/npa.y5611
37. Skeie G, Apostolski S, Evoli A, Gilhus N, Hart I, Harms L, et al. Guidelines for the treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol (2006) 13:691–9. doi: 10.1111/j.1468-1331.2006.01476.x
38. Brown ES, Suppes T. Mood symptoms during corticosteroid therapy: a review. Harv Rev Psychiatry (1998) 5:239–46. doi: 10.3109/10673229809000307
39. Schmidt LA, Fox NA, Goldberg MC, Smith CC, Schulkin J. Effects of acute prednisone administration on memory, attention and emotion in healthy human adults. Psychoneuroendocrinology (1999) 24:461–483. doi: 10.1016/S0306-4530(99)00007-4
40. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids, Mayo Clinic Proceedings. Amsterdam, Netherlands: Elsevier (2006) p. 1361–1367. doi: 10.4065/81.10.1361
41. Schwartz ML, Cahill R. Psychopathology associated with myasthenia gravis and its treatment by psychotherapeutically oriented group counseling. J Clin Epidemiol (1971) 24:543–52. doi: 10.1016/0021-9681(71)90043-9
42. Kütemeyer M. Symptom changes during the psychotherapy of patients with myasthenia gravis. Psychother Psychosom (1979) 32:279–86. doi: 10.1159/000287397
43. Achiron A, Barak Y, Noy S, Pinhas-Hamiel O. Fluoxetine treatment for weight reduction in steroid-induced obesity: a pilot study in myasthenia gravis patients. Eur Neuropsychopharmacol (1999) 9:111–3. doi: 10.1016/S0924-977X(98)00012-1
44. Kulaksizoglu I, Aldemir D, Parman Y, Degmeer E, Serdaroglu P. Citalopram treatment of depression in myasthenia gravis patients—an open study, European Neuropsychopharmacology. 1000 AE Amsterdam, Netherlands: Elsevier Science BV PO Box 211 (2005) p. S426–S426. doi: 10.1016/S0924-977X(05)80869-7
Keywords: depression, myasthenia gravis, economic condition, comorbidity, retrospective cohort study
Citation: Chu H-T, Tseng C-C, Liang C-S, Yeh T-C, Hu L-Y, Yang AC, Tsai S-J and Shen C-C (2019) Risk of Depressive Disorders Following Myasthenia Gravis: A Nationwide Population-Based Retrospective Cohort Study. Front. Psychiatry 10:481. doi: 10.3389/fpsyt.2019.00481
Received: 04 March 2019; Accepted: 19 June 2019;
Published: 09 July 2019.
Edited by:
Agorastos Agorastos, Aristotle University of Thessaloniki, GreeceReviewed by:
Leonardo Emberti Gialloreti, University of Rome Tor Vergata, ItalyErnestina Santos, Centro Hospitalar do Porto, Portugal
Copyright © 2019 Chu, Tseng, Liang, Yeh, Hu, Yang, Tsai and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shih-Jen Tsai, dHNhaTYxMDkxM0BnbWFpbC5jb20=; Cheng-Che Shen, cHVyZXMxMDAwQHlhaG9vLmNvbS50dw==
 Hsuan-Te Chu
Hsuan-Te Chu Chih-Chieh Tseng
Chih-Chieh Tseng Chih-Sung Liang
Chih-Sung Liang Ta-Chuan Yeh
Ta-Chuan Yeh Li-Yu Hu4,5
Li-Yu Hu4,5 Albert C. Yang
Albert C. Yang Shih-Jen Tsai
Shih-Jen Tsai Cheng-Che Shen
Cheng-Che Shen