- 1Institute of Sustainable Industries & Liveable Cities, College of Engineering and Science, Victoria University, Melbourne, VIC, Australia
- 2Environmental Science Discipline, Khulna University, Khulna, Bangladesh
Weed control through allelopathic plants is a promising approach that may minimize many of negative consequences of synthetic herbicides. We have studied potential of Chrysanthemoides monilifera subsp. monilifera (boneseed) leaf extract for controlling growth of Lolium rigidum (annual ryegrass) in wheat (Triticum aestivum) fields. Both pre-and post-emergent ryegrass-control experiments were conducted in greenhouse using field soil. Treatments such as boneseed leaf extracts (5 and 10% for pre-emergent and 10 and 20% for post-emergent experiments) alone or as a mixture combined with different strength (¼ and ½ strength) of pre-emergent (boxer gold) and post-emergent (hussar OD) herbicides were applied on pre- and post-emergent ryegrass and wheat. The findings revealed that none of the boneseed leaf extracts alone or as mixture had significant inhibitory impact on pre-emergent ryegrass compared with herbicide alone. Although we observed significant inhibitory impacts on post-emergent ryegrass with boneseed leaf extracts alone (10 and 20%) compared with control, they were negligible compared to full strength herbicides. Mixtures had significant inhibitory impact on post-emergent ryegrass compared with herbicide alone with same doses and impact increased with herbicide concentration. Despite the greater impacts by higher herbicides concentration alone, findings suggest the use of mixture of ¼-strength herbicide and 10% boneseed leaf extract was able to control ryegrass successfully than the herbicide alone without adverse impacts on wheat. This study suggests that use of boneseed leaf extract mixed with lower doses of post-emergent herbicides may be effective in controlling ryegrass with concomitant reductions in expenses and ecological health risks linked with the practice of synthetic herbicides.
Introduction
Weeds cause massive losses in crop production and threaten native species and forest ecosystems worldwide despite only comprising ~0.1% of the planet's flora (Singh et al., 2003). Australia is one of the top 10 wheat-producing countries in the world. The control of weeds in wheat fields in Australia, in particular Lolium rigidum (ryegrass) control, is becoming a challenge, as they are growing resistant to synthetic herbicides (Preston et al., 1999; Llewellyn and Powles, 2009; Seal et al., 2010). Infestation of ryegrass at 200 plants/m2 resulted huge yield loss (20–50%) in wheat that costs about $AU100–250/ha (Lemerle et al., 1995). Resistance by ryegrass to multiple synthetic herbicides including diclofop-methyl, clethodim, chlorosulfuron, sulfometuron, glyphosate, etc. were identified in wheat belts in Australia (Preston et al., 1999; Llewellyn and Powles, 2009; Owen and Powles, 2010). This problem is not unique to Australia, with mesosulfuron-resistant ryegrass also identified in wheat fields in the USA (Ellis et al., 2008). Recent studies using Boxer gold (Prosulfocarb 800 g/L + S-Metolachlor 120 g/L) at 2,300 g a.i ha−1 and Hussar OD (Iodosulfuron-methyl-sodium 100 g/L) 2 g a.i. ha−1 showed success in controlling pre-emergent and post-emergent ryegrass in wheat fields, respectively, though resistance has been reported for post-emergent (Ruchs, 2008; Hashem and Borger, 2014; Mahmood et al., 2016).
However, voices were being raised worldwide against the use of synthetic herbicides due to their potential threat to the plant community structure, environmental impacts and potential health hazards (Dayan et al., 1999; Fuhlendorf et al., 2009; Davis et al., 2013). In addition, evidence suggest that herbicides may also reduce the nutritional value of crops (Saghir and Bhatti, 1970). To exacerbate the issue, a number of herbicide-resistant weeds have emerged that again threaten agricultural productivity (Holt and Lebaron, 1990). The environmental and health concern of using synthetic herbicides has led to the impetus for agronomists and environmental scientists to investigate alternative strategies. Thus, searching for alternative, natural herbicides in controlling ryegrass in wheat field is of great interest.
Natural herbicides produced from allelochemicals tend to be more environmentally benign but their commercialization is challenging due to high segregation and processing costs (Duke et al., 2000, 2002; Bhowmik and Inderjit, 2003). Direct use of aqueous extracts of allelopathic plants in controlling weeds has been trialed as a suitable substitute technique for ecological and economic weed management (Cheema et al., 2003b; Javaid et al., 2006; Anjum and Bajwa, 2007; Haig et al., 2009; Meksawat and Pornprom, 2010; Omezzine et al., 2011). The mixture of allelopathic plant extracts with lower amount of herbicides was successfully used (Iqbal et al., 2009; Jabran et al., 2010) in controlling weeds to minimize uses of herbicides. The success in controlling other weed species in wheat fields using allelopathic plant extracts (Jamil et al., 2009) supports the strategy of using such extracts in controlling ryegrass. In addition, Haig et al. (2005) also explored alternate control strategy of ryegrass by using herbicidal potential of plant though the study was limited to bioassay in petri dishes only whereas field study is essential to draw more robust conclusion.
Chrysanthemoides monilifera subsp. monilifera (boneseed) has been considered as a noxious weed with a possession of national significance in Australia. Allelopathic phytotoxicity of boneseed through leaching (Harun et al., 2014), litter decomposition (Harun et al., 2015c), root exudates and volatilization (Harun et al., 2015a) have been observed. We have also recently identified specific phytotoxic phenolic compounds in boneseed tissues (Harun et al., 2015c) but that was absent in ashes after burning (Harun et al., 2015b). In the search for cheaper and more environmentally benign natural herbicides, the suitability of boneseed aqueous extracts needs to be investigated.
Hence, as a continuation of our previous research in studying the chemo-ecological properties of boneseed, we aimed to evaluate the herbicidal potential of boneseed aqueous extract in controlling both pre-emergent and post-emergent ryegrass in wheat fields.
Materials and Methods
Sample Collection and Processing
In August 2013, boneseed leaves (at early flowering stage) and soil samples from boneseed unoccupied area were collected from You Yangs Regional Park, Victoria (37° 59′ 44′′ S, 144° 24′ 39′′ E), sealed in plastic bags and immediately transported to the laboratory. Boneseed leaves and soil were dried in air at room temperature to constant weights. Dried leaves were processed to prepare desired concentration of aqueous extracts following the same procedures that we published earlier (Harun et al., 2014). The supernatant was passed through a 0.22 μm filter before storage at −80°C. To control for possible extraneous effects, the pH of the extracts was neutralized using 1N NaOH solution (Fu and Viraraghavan, 2002). After drying, the soils were passed through a 0.5 mm mesh sieve before storage in sealed plastic bags at room temperature.
Seeds and Herbicides Collection
Triticum aestivum (SF adagio variety) and annual ryegrass seeds were sourced from AGF seeds and Stephen Pasture Seeds Pty Ltd, Victoria, respectively. Seeds were stored inside sealed plastic containers at room temperature (20°C) until use. Boxer gold and Hussar OD as pre-emergent and post-emergent herbicides were collected from Agrisolutions Australia Pty Ltd, Queensland and Bayer Crop Science Pty Ltd, Victoria, respectively. The herbicides were preserved in pre-packed sealed containers at room temperature (20°C) prior to use.
Pre-emergent Ryegrass Control Experiment
Prior to the experimental set-up in August 2013, the germination rate of ryegrass was assessed in a growth chamber at 15/5°C (day/night) temperature and 9 h daylight. Suitable plastic pots (1L) were filled with 500 g field soil for evaluating the potential of boneseed leaf extract in controlling pre-emergent ryegrass. Boxer gold (Prosulfocarb 800 g/L + S-Metolachlor 120 g/L) herbicide was considered for pre-emergent experiment. Seven treatments were assessed along with a control (water only): Treatment 1) 5% boneseed leaf extract; Treatment 2) 10% boneseed leaf extract; Treatment 3) ¼ strength boxer gold herbicide; Treatment 4) ¼ strength herbicide in 10% extract; Treatment 5) ½ strength herbicide; Treatment 6) ½ strength herbicide in 10% extract; and Treatment 7) full strength boxer gold herbicide (2,500 mL/70 L water/ha) (Ruchs, 2008; Hashem and Borger, 2014). The concentration of boneseed leaf extract was decided based on our previous study (Harun et al., 2014) in which 5% leaf extract demonstrated tremendous inhibition to the test species. The soil was saturated with 225 mL dH2O with a total of 40 pots consisting of 5 replicates of each treatment being maintained in a greenhouse in a completely randomized design (CRD). The position of the pots was changed randomly on every other day. After 2 days, 20 ryegrass seeds were sown into each pot at 0.25 cm depth at equidistant spacing. On the same day, the appropriate treatment (herbicide/boneseed leaf extract/mixture or water) were sprayed at a rate of 70 L/ha using 500 mL triggered sprayer (model number PB-009, Oates, Australia). Equal quantities of water were applied regularly to moisten the soil to a depth of 3 cm starting from the next day of spraying. Other undesired species were removed from all pots weekly. At 19 days after sowing, the number of ryegrass germinations were counted and parameters including shoot and root length and weight, chlorophyll (a, b and total) and free proline content of ryegrass were measured. Three (03) randomly selected ryegrass seedlings were considered for the calculation of average biometric parameters. At 20 days after sowing, soil (of each treatment) from 1 cm depth was collected for measuring dehydrogenase activity (DHA). The same procedures were followed for wheat with the exception of the number and time of seeds sowed, specifically 15 wheat seeds were sown in each pot after 3 days of applying the treatment.
Chlorophyll content were measured by following the method of Inskeep and Bloom (1985). Free proline and soil DHA were measured following the method of Bates et al. (1973) and Gu et al. (2009), respectively with slight modification as mentioned in literature (Harun et al., 2015a).
Post-emergent Ryegrass Control Experiment
Hussar OD (Iodosulfuron-methyl-sodium 100 g/L) herbicide was considered for post-emergent experiment. Seven treatments consisting of: treatment 1) 10% boneseed leaf extract; treatment 2) 20% extract; treatment 3) ¼ strength herbicide (Hussar OD); treatment 4) ¼ strength herbicide in 10% extract; treatment 5) ½ strength herbicide; treatment 6) ½ strength herbicide in 10% extract; and treatment 7) full strength herbicide of recommended dosage (75 mL/65 L water/ha) (Bayer Crop Science Pty Ltd, 2008) were assessed along with a control (dH2O) during September 2013. The concentrations of boneseed leaf extract were increased to 10 and 20% as we found negligible herbicidal impact of lower doses of boneseed leaf extract (alone) for pre-emergent experiments. The pots were prepared following a similar method to the pre-emergent experiment, with the exception that 850 g soil was used in this experiment. After 2 days of moistening the soil (with 337.5 mL water/pot), 25 ryegrass seeds were sown in each pot. Nine days later, the seedlings were thinned to 15 per pot. Water was added to the pots to keep them moist and unwanted plants were removed regularly. After 18 days of sowing, the experimental treatment (herbicide/boneseed leaf extract/mixture/dH2O) was sprayed at a rate of 65 L water/ha. Plants were collected from the pots after 3, 21, and 42 days of spraying to measure the same parameters as those investigated in the pre-emergent experiments (above). After harvesting ryegrass on day 3 and day 21 the number of seedlings was thinned to 12 and 5 in each pot, respectively. Soil samples were also collected to measure DHA.
The same procedures were followed for assessing post-emergent wheat, with a few exceptions: 15 seeds were sown and seedlings were thinned to 9 after 10 days of sowing. The experimental treatments were sprayed after 4 weeks of sowing. Data were collected after 3 and 23 days of spraying. After the first data collection on day 3, the number of seedlings was thinned to 6 in each pot.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS 21.0. All data were presented as mean ± standard error (SE). The impact of herbicide, boneseed leaf extracts and mixtures on both ryegrass and wheat (pre-emergent and post-emergent) were evaluated using one-way ANOVA followed by post-hoc Dunnett's test. The difference between the impact shown by herbicide alone and mixture of herbicide and boneseed leaf aqueous extract was evaluated using independent T-Test (2-tailed). Significant differences between the means were determined at a 5% level of probability (p ≤ 0.05). Linear regression was adopted to express the relationship among different parameters in both pre-emergent and post-emergent experiments.
Results
Pre-emergent Ryegrass Control
The boneseed leaf extracts alone (Treatment 1 and 2) had no impact on germination of ryegrass while Treatment 3 and 4 significantly (p = 0.000) inhibited the germination of ryegrass, compared with control (Table 1). No germination has been observed at treatment 5, 6, and 7 because those treatments might be more effective to control weed. The boneseed leaf extracts alone slightly stimulated the shoot length and weight compared with control while Treatment 3 and 4 significantly (p = 0.000) inhibited shoot length and weight. Compared with control, Treatment 1 had negligibly stimulatory impact on root length and weight but treatment 2 inhibited the same parameters of ryegrass by 13 and 16%, respectively. Compared with the impact on shoot, the treatment 3 and 4 had more inhibitory impact on root length and weight, and the impact was significant (p = 0.000) (Table 1).
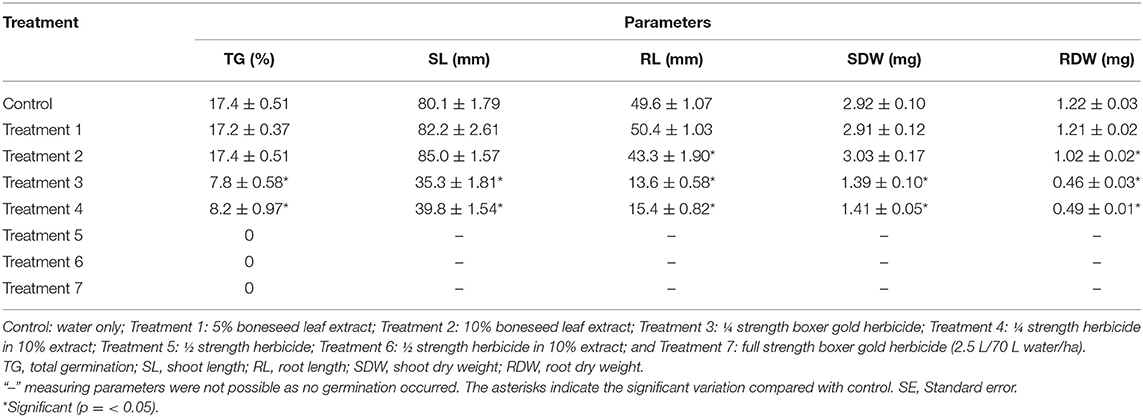
Table 1. Effects of boneseed extract, herbicide, and mixtures on pre-emergent ryegrass. Data was collected at 19 days after sowing and presented as average ± SE.
Boneseed leaf extracts alone had no/negligible impact on chlorophyll content of ryegrass while treatment 3 and 4 significantly inhibited chlorophyll a (p = 0.000), chlorophyll b (p ≤ 0.05) and total chlorophyll (p = 0.000) content (Figure 1). The impact of boneseed leaf extracts alone on the free proline content of ryegrass was not significant but treatment 3 and 4 tremendously increased proline content in ryegrass by 293 and 252% compared with control (Figure 1). Neither of the treatments had significant impact on soil DHA though treatment 1, 2 and 4 had slight stimulatory impacts while treatment 3 had slightly inhibitory impact (Figure 1). Resilient relationships (r = 0.93) between total chlorophyll content and shoot length, and chlorophyll content and root length (r = 0.95) of ryegrass were found (Supplementary Figure 1). Proline content of ryegrass leaf exhibited a resilient negative correlation with both shoot (r = −0.96) and root (r = −0.92) length (Figure 2). None of the treatments had significant impacts on wheat except for full strength herbicide that significantly destroyed chlorophyll content and amplified proline content in wheat (Supplementary Table 1).
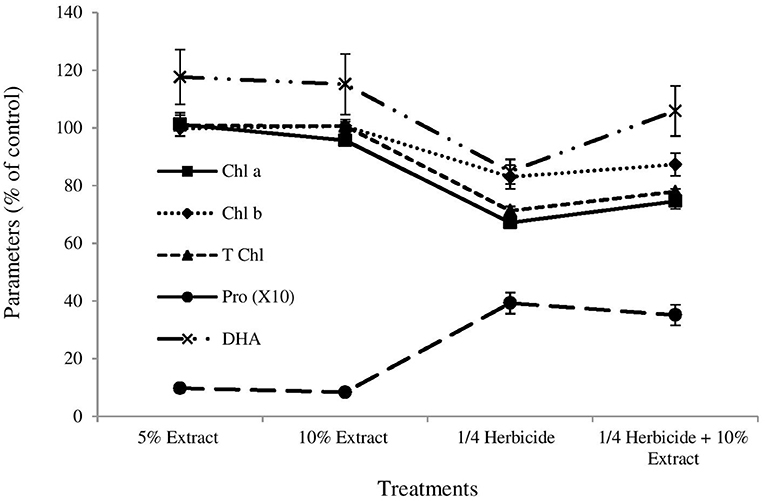
Figure 1. Effects of boneseed extracts, herbicide and mixture on pre-emergent ryegrass. The x-axis denotes the treatment types (5% aqueous extract of boneseed leaf, 10% extract, ¼ strength herbicide, and mixture of ¼ strength herbicide with 10% extract). Values on y-axis denote the impact (% of control) on chlorophyll a (chl a) (solid line with square marker), chlorophyll b (chl b) (round dot line with diamond marker), total chlorophyll (T chl) (square dot line with triangular marker) and proline (pro) (long dash line with circle marker) content of ryegrass, and soil dehydrogenase activities (DHA) (long dash dot dot line with cross marker). The error bar indicates the standard error.
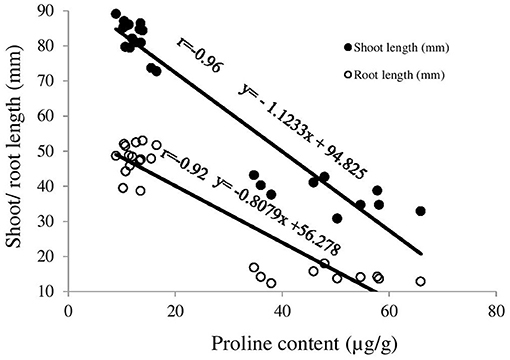
Figure 2. Correlation between proline content and shoot/root length of ryegrass (pre-emergent). The x-axis denotes proline content of leaf (μg/g) and y-axis denotes shoot and root length (mm) of ryegrass. The trend line with filled circle marker represents correlation between proline content and shoot length while trend line with non-filled circle marker represents correlation between proline content and root length.
Post-emergent Ryegrass Control
No ryegrass died after 3 days of spraying herbicide/boneseed leaf extracts/mixtures. Compared with control, none of the treatments had significant impact on the shoot length and weight of ryegrass with the exception of the impact of full strength herbicide that inhibited them significantly (p = 0.005) after 3 days of spraying (Table 2). Root length and weight of ryegrass was not affected significantly by any of the treatments compared with control though most of the experiments slightly inhibited them (Table 2). After 3 days of spraying, boneseed leaf extracts alone had no significant impact on the chlorophyll a, chlorophyll b and total chlorophyll content of ryesgrass compared with control (Figure 3). Treatments 4–7 inhibited the chlorophyll a and total chorophyll content of ryegrass significantly (p ≤ 0.05) compared with control while chlorophyll b content was inhibited significantly (p ≤ 0.05) by the treatments 5–6 (Figure 3). All the treatments except 1 and 2 (boneseed leaf extracts alone) increased the free proline content of ryegrass significantly (p ≤ 0.001) compared with control (Figure 3). None of the treatments had significantly inhibitory impacts on the soil DHA except for treatment 6 and 7 that significantly (p ≤ 0.05) inhibited it compared with control (Figure 3).
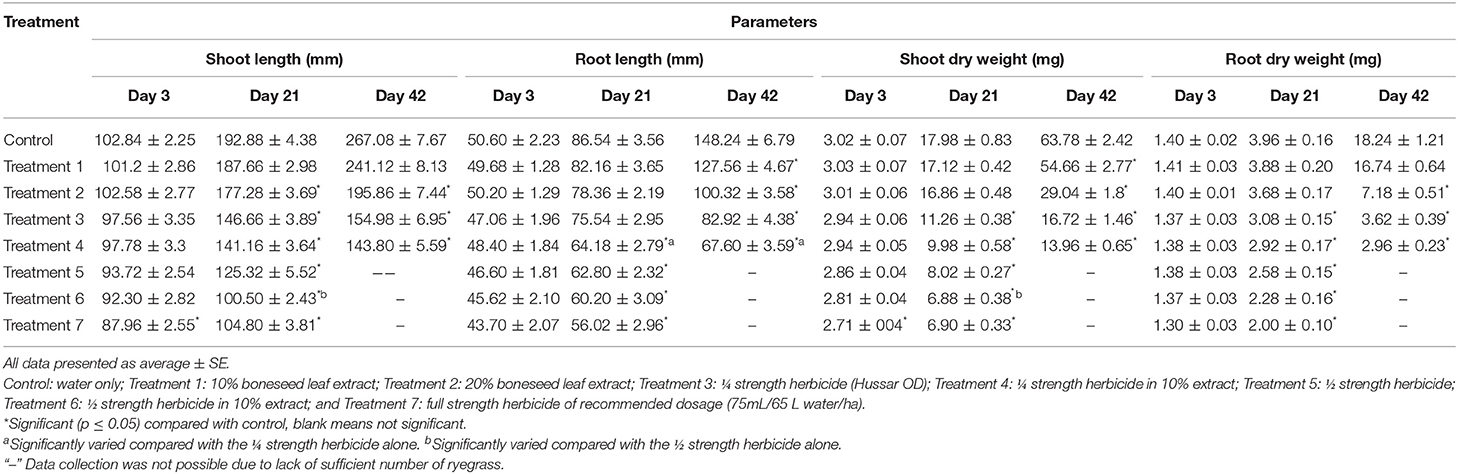
Table 2. Effects of boneseed extract, herbicide, and mixtures on post-emergent ryegrass. Data was collected after 3, 21, and 42 days of spraying.
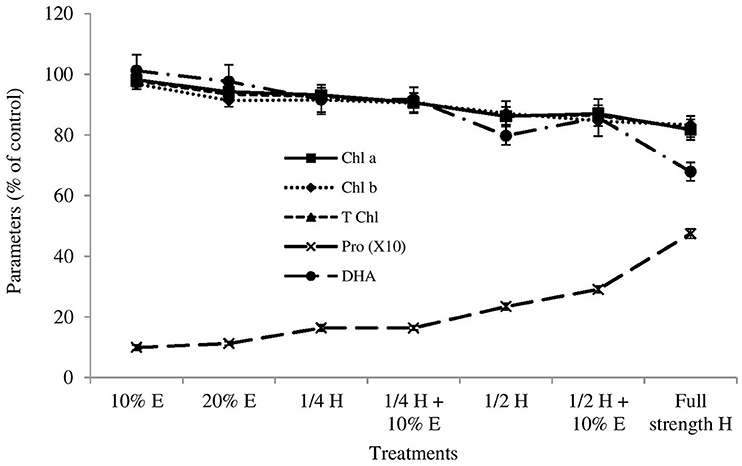
Figure 3. Effects of boneseed extracts, herbicide and mixture on post-emergent ryegrass (3 days after spray). The x-axis denotes the treatment types (10% aqueous extract of boneseed leaf, 20% extract, ¼ strength herbicide, mixture of ¼ strength herbicide with 10% extract, ½ strength herbicide, mixture of ½ strength herbicide with 10% extract and full-strength herbicide). Values on y-axis denote the impact (% of control) on chlorophyll a (chl a) (solid line with square marker), chlorophyll b (chl b) (round dot line with diamond marker), total chlorophyll (T chl) (square dot line with triangular marker) and proline (pro) (long dash line with circle marker) content of ryegrass, and soil dehydrogenase activities (DHA) (long dash dot dot line with cross marker). The error bar indicates the standard error.
The 8, 15, 32, 37, and 38% of the ryegrasses died after 21 days of spraying at treatment 3, 4, 5, 6, and 7, respectively, but no ryegrasses died by the application of 10 and 20% boneseed leaf extracts (data not shown). After 21 days of spraying, treatment 1 did not exhibit significant impact on shoot lentgh of ryegrass compared with control while all other treatments significantly (p ≤ 0.05) inhibited the shoot length of ryegrass (Table 2). All the treatments significantly (p = 0.000) inhibited shoot weight of ryegrass except for the boneseed leaf extracts alone (treatment 1 and 2). The root length and weight of ryegrass were significantly (p ≤ 0.05) inhibited at the treatment 3–7 compared with control after 21 days of spraying (Table 2). All the treatments significantly (p = 0.000) inhibited chlorophyll a, chlorophyll b and total chlorophyll compared with control (Figure 4). The above treatments greatly increased free proline content in ryegrass and that were significant (p ≤ 0.05) at all the treatments with the exception of treatment 1. All the treatments significantly (p = 0.000) inhibited chlorophyll a, chlorophyll b and total chlorophyll compared with control (Figure 4). Although treatment 1 and 2 stimulated soil DHA by 15 and 29% compared with control after 21 days of spraying, all other treatments inhibited soil DHA and that were significant for treatments 3, 5, 6, and 7 (Figure 4). The 28, 84, 88, 100, and 100% of ryegrasses were died after 42 days of spraying at treatment 3–7, respectively, but no ryegrass died when sprayed with 10% or 20% extracts (data not shown). After 42 days of spraying all the tretaments where ryegrass existed in necessary number for calculaing parameters (treatments 2–4) had significant (p = 0.000) inhibitory impacts on shoot and root length, and weight compared with control. However, the inhibition by treatment 1 was significant for shoot weight and root length (Table 2) only. Treatments 1–4 significantly (p ≤ 0.005) inhibited chlorophyll a, chlorophyll b and total chlorophyll compared with control (data not shown).
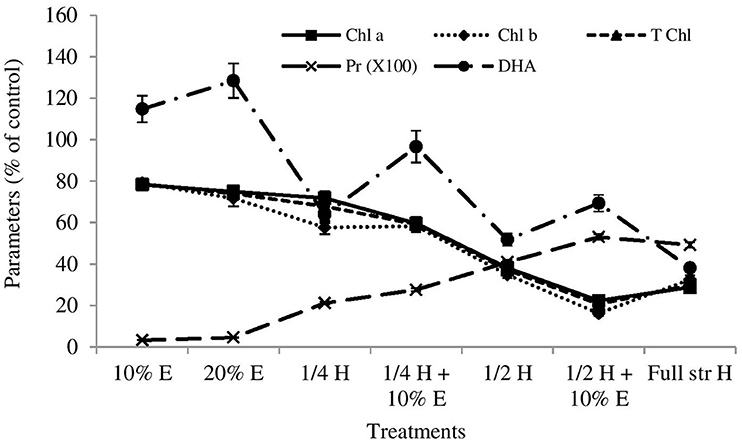
Figure 4. Effects of boneseed extracts, herbicide and mixture on post-emergent ryegrass (21 days after spray). The x-axis denotes the treatment types (10% aqueous extract of boneseed leaf, 20% extract, ¼ strength herbicide, mixture of ¼ strength herbicide with 10% extract, ½ strength herbicide, mixture of ½ strength herbicide with 10% extract and full-strength herbicide). Values on y-axis denote the impact (% of control) on chlorophyll a (chl a) (solid line with square marker), chlorophyll b (chl b) (round dot line with diamond marker), total chlorophyll (T chl) (square dot line with triangular marker) and proline (pro) (long dash line with circle marker) content of ryegrass, and soil dehydrogenase activities (DHA) (long dash dot dot line with cross marker). The error bar indicates the standard error.
The strong positive correlations between chlorophyll content and shoot length (r = 0.93) and chlorophyll content and root length (r = 0.83) were profound (Supplementary Figure 2). There was a very strong negative correlation between proline content and shoot length (r = −0.96), and proline content and root length (r = −0.83) of ryegrass (Figure 5). In case of post-emergent wheat, none of the mentioned parameters were inhibited significantly by the treatments (Supplementary Table 2) with the exception for the proline content that was increased significantly compared with control by the treatment 7 after 3 days of spraying, and by the treatment 3, 5, 6, and 7 after 21 days of spraying (Supplementary Table 2).
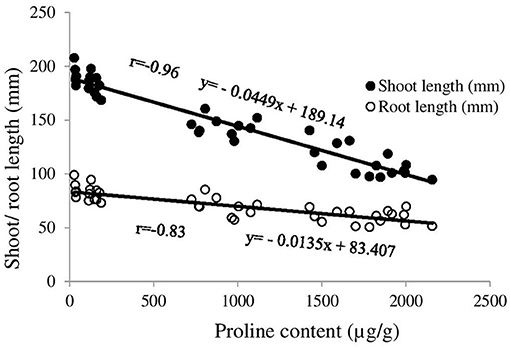
Figure 5. Correlation between proline content and shoot/root length of ryegrass (post-emergent). The x-axis denotes proline content of leaf (μg/g) and y-axis denotes shoot and root length (mm) of ryegrass. The trend line with filled circle marker represents correlation between proline content and shoot length while trend line with non-fill circle marker represents correlation between proline content and root length.
Discussion
As a continuation of our previous study on boneseed that identified four phenolic compounds (catechin, p-coumaric acid, ferulic acid, and phloridzin) in boneseed organs and litter (Al Harun et al., 2015) this study investigated the impact of boneseed leaf extract in controlling ryegrass minimizing herbicidal load to the ecosystem. Previously, we found high concentration of ferulic acid compared with other allelochemicals that is a well-known for herbicidal potential (Putnam, 1988; Reigosa et al., 2001; Durán-Serantes et al., 2002). The evidence of high concentration of total phenolics in boneseed when compared to other allelopathic species (about 3 times more than both Pueraria montana and Phragmites australis) (Rashid et al., 2010; Uddin et al., 2012) indicates the boneseed allelopathy as herbicidal potential.
Boneseed leaf extract alone had negligible herbicidal impacts on the germination and growth parameters of ryegrass in both pre-emergent and post-emergent experiment, however, as a mixture with lower concentration of herbicides had a substantial impact on ryegrass. To our knowledge, this is the first study of assessing the use of a plant extract in controlling pre-emergent weeds, although there have many studies investigating the control of post-emergent weeds (Cheema and Khaliq, 2000; Farooq et al., 2011). The non-significant impact of the boneseed leaf extracts alone to pre-emergent ryegrass length and biomass compared with herbicide alone indicates that boneseed leaf extract at the tested concentrations was not effective in controlling ryegrass. We did not conduct a pre-emergent experiment with more highly concentrated extracts (e.g., 20% or more) which may inhibit the germination, growth and biochemical parameters of ryegrass as has been found in the post-emergent experiment in this study and in the literature on other species (Javaid et al., 2006). However, as an interesting aside, we found that boxer gold at half of the recommended concentration was effective at preventing germination in the conditions investigated during this study.
Post-emergent treatments had substantial impacts in controlling ryegrass compared with pre-emergent treatment. We considered only one spray of the treatment in the current study, however, spraying the treatment twice at a specific time intervals may control ryegrass growth more effectively, as addressed in other studies (Cheema et al., 2003a). Similar techniques of using plant extract and herbicide mixtures have been successfully adopted in other studies to reduce herbicide use and herbicide-related hazards (Cheema and Khaliq, 2000; Chon et al., 2003; Anjum and Bajwa, 2007; Farooq et al., 2011). However, there is also evidence to support the use of aqueous extracts of plants alone in successfully controlling weeds (Haig et al., 2009). Over the longer period (42 days after spraying) the observed significant increase in herbicidal activity on ryegrass by the mixture of herbicide and boneseed leaf extract compared with herbicide (at the same concentration) alone, suggests that boneseed leaf extract is useful in controlling ryegrass when used as a mixture with diluted herbicides. Furthermore, the mixture with reduced doses of herbicide concentration was almost as effective as the full-strength herbicide in controlling the ryegrass. Increasing concentration of boneseed leaf extract demonstrated higher inhibitory impacts on weed revealing the allelopathic potential of boneseed leaf extract as herbicidal use. Further, the boneseed leaf extract together with herbicide showed substantial impact in post-emergent ryegrass control which might be due to the presence of high concentration of ferulic acid in boneseed leaf extract that is well-known allelochemicals and have herbicidal potential (Putnam, 1988; Reigosa et al., 2001; Durán-Serantes et al., 2002). It is important to note that our experiment was limited to the greenhouse with lower doses of extracts and does not consider the weed-crop competition that exists in the field. Anjum and Bajwa (2007) have addressed the potential of concentrated sunflower leaf extracts (80–100%) in controlling Rumex, overcoming weed-crop competition despite the result that the extracts did not kill 100% of the weeds. In addition to the significant impact showed by the boneseed extracts compared with control, the significant inhibition by the mixture to biometric and biochemical parameters (e.g., shoot, root, chlorophyll) compared with herbicide alone may reveal how the boneseed leaf extract enhances the effectiveness of the herbicide in retarding the growth of ryegrass. Although the mixture of ½ strength herbicide in 10% extract had similar impacts to full strength herbicide in ryegrass control and the mixture of ¼ strength herbicide in 10% extract had slightly less impact, our findings suggest the use of a mixture of ¼ strength herbicide and 10% extract as it created no stress condition in wheat (in terms of proline content) as created by full strength herbicide and the ½ strength mixture. However, field studies are critical to draw more rigorous conclusions and to more authentically demonstrate the impact of the tested weed control strategies on wheat yield as there is evidence that in the case of sorghum aqueous extracts their application reduced weed biomass with a simultaneous increase in crop yield (Cheema and Khaliq, 2000) in field conditions. Though this study has not found any significant impact on soil DHA for pre-emergent experiments but it was significantly decreased at few of the post emergent experiments. Soil dehydrogenase activity (DHA), an indicator of soil quality and microbial activity is substantially reduced by pesticides and other pollutants as mentioned in literature (Xie et al., 2009; Tejada et al., 2010). Dehydrogenases play a significant role in the biological oxidation of organic matter by transferring hydrogen from organic matter to inorganic acceptors (Zhang et al., 2010).
Inhibition of photosynthesis in target species has been identified as one of the modes of action induced by phytotoxic allelochemicals (Einhellig et al., 1993; Einhellig, 1995). Although we did not directly identify the allelopathic impact on photosynthesis, the decreasing chlorophyll level in ryegrass exposed to boneseed leaf extracts and mixtures may reduce photosynthesis and ultimately retard plant growth as suggested in another study (Singh et al., 2002). Evidence also supports the direct impact of allelochemicals on photosynthesis of target species (Reigosa et al., 2001; Wang et al., 2013). Fluorescence, another important plant parameters in denoting the impact of herbicide on target species (Christensen et al., 2003) has not been studied at this paper but would be worthy in future study. The strong positive correlation between chlorophyll content and shoot/root length both in pre-emergent and post-emergent experiments suggest that inhibition of chlorophyll content is one of the mechanisms that might retard growth of ryegrass. Although we didn't study other modes of action of allelopathic plant extracts on this occasion, several studies, including our previous study on boneseed, suggested excessive reactive oxygen species (ROS) production that enhances lipid peroxidation and electrolyte leakage and damages macromolecules like proteins, nucleic acids, etc., may be an important mechanism underpinning the phytotoxic nature of the allelochemicals (Weir et al., 2004; Batish et al., 2006; Harun et al., 2014).
Along with the significant increase in proline content in ryegrass treated with boneseed leaf extract compared with control, the large increase in storage of proline in ryegrass exposed to mixture compared with herbicide alone, again, supports the impact of boneseed leaf extract in pushing ryegrass into a stressed condition with a more substantial impact in post-emergent experiment (30–55 times more proline at treatment 3–7 compared with control). Furthermore, the strong negative correlation between proline content and shoot/root length both in pre-emergent and post-emergent experiments suggest that proline content is a decent measure of stress intensity in ryegrass. The excessive proline storage in test species due to application of both commercial herbicides and allelochemicals has also been reported earlier (Reigosa et al., 2001).
Overall, the study found that boneseed leaf extract as a mixture in lower doses of herbicides had potential role in controlling ryegrass with a comparatively stronger role for post-emergent ryegrass control that might enhance sustainable agriculture while minimizing health and environmental impacts. Though the herbicidal treatments had not significant impact on biometric parameters of wheat but the decrease of chlorophyll level and increase of proline level in wheat is still an important concern in identifying whether the herbicides have any phytotoxicity on wheat grains. Hence, field studies are important to investigate the impact of suggested mixtures in that environment and on crop yield to draw more rigorous conclusions regarding the herbicidal potential of boneseed.
Conclusions
This study suggests that boneseed leaf extract was more effective in controlling post-emergent ryegrass compared with pre-emergent ryegrass. The use of mixture of boneseed leaf extract and herbicide had more impact that is substantial on post-emergent ryegrass control compared with boneseed leaf extract or herbicide alone. The inhibition was profound in terms of shoot & root length and weight, chlorophyll content and soil DHA. The strong positive correlation between chlorophyll content and shoot/root length both in pre-emergent and post-emergent experiments suggests that inhibition of chlorophyll content is one of the mechanisms that retarded the growth of ryegrass. Furthermore, the strong negative correlation between proline content and shoot/root length both in pre-emergent and post-emergent experiments suggest that proline content represents a good measure of stress intensity in ryegrass. In particular, the use of ¼-strength herbicide in 10% boneseed leaf extract appears to be promising as it showed levels of ryegrass inhibition only slightly below that of full-strength herbicide, while appearing to produce less stress in the wheat crop. This study builds our understanding of the use of boneseed leaf extract with lower doses of herbicides in controlling ryegrass that might enhance sustainable agriculture while minimizing health and environmental impacts.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author Contributions
MH, MU, and RR conceptualized and organized the idea. MH and MU conducted the experiments. MH wrote the manuscript. MU, RR, and JJ reviewed, edited, and corrected the manuscript to its final form. All authors approved the submitted version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Parks Victoria for permitting us to collect boneseed. Our warm thanks to all technical staff and colleagues that supported the field works and laboratory experiments. We acknowledge AGF seeds and Stephen Pasture Seeds Pty Ltd., Victoria for supplying wheat seeds and ryegrass seeds, respectively. Thanks to Syngenta Crop protection Pty Limited, NSW and Bayer Crop Science Pty Ltd., Victoria for providing Boxer gold and Hussar OD herbicides, respectively. The authors are grateful to Australian Government for the international post graduate research scholarship (IPRS) to the first author to conduct this research during his PhD program.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2021.785845/full#supplementary-material
Supplementary Figure 1. Correlation between total chlorophyll content and shoot/root length of ryegrass (pre-emergent). The x-axis denotes total chlorophyll content of leaf (μg/mg) and y-axis denotes shoot and root length (mm) of ryegrass. The trend line with filled circle marker represent correlation between chlorophyll content and shoot length while trend line with non-filled circle marker represents correlation between chlorophyll content and root length.
Supplementary Figure 2. Correlation between total chlorophyll content and shoot/root length of ryegrass (post-emergent). The x-axis denotes total chlorophyll content of leaf (μg/mg) and y-axis denotes shoot and root length (mm) of ryegrass. The trend line with filled circle marker represents correlation between chlorophyll content and shoot length while trend line with non-filled circle marker represents correlation between chlorophyll content and root length.
Supplementary Table 1. Effects of boneseed extract, herbicide and mixture on wheat in pre-emergent ryegrass control experiment.
Supplementary Table 2. Effects of boneseed extract, herbicide and mixture on wheat in post-emergent ryegrass control experiment.
References
Al Harun, M. A. Y., Johnson, J., Uddin, M. N., and Robinson, R. W. (2015). Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed). PLoS ONE 10:e0139992. doi: 10.1371/journal.pone.0139992
Anjum, T., and Bajwa, R. (2007). Field appraisal of herbicide potential of sunflower leaf extract against Rumex dentatus. Field Crops Res. 100, 139–142. doi: 10.1016/j.fcr.2006.06.001
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Batish, D., Singh, H., Setia, N., Kaur, S., and Kohli, R. (2006). 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol. Biochem. 44, 819–827. doi: 10.1016/j.plaphy.2006.10.014
Bhowmik, P. C., and Inderjit (2003). Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 22, 661–671. doi: 10.1016/S0261-2194(02)00242-9
Cheema, Z., Jaffer, I., and Khaliq, A. (2003a). Reducing isoproturon dose in combination with sorgaab for weed control in wheat. Pak. J. Weed Sci. Res. 9, 153–160.
Cheema, Z., and Khaliq, A. (2000). Use of sorghum allelopathic properties to control weeds in irrigated wheat in a semi arid region of Punjab. Agric. Ecosyst. Environ. 79, 105–112. doi: 10.1016/S0167-8809(99)00140-1
Cheema, Z., Khaliq, A., and Mubeen, M. (2003b). Response of wheat and winter weeds to foliar application of different plant water extracts of sorghum (S. bicolor). Pak. J. Weed Sci. Res 9, 89–97.
Chon, S. U., Kim, Y. M., and Lee, J. C. (2003). Herbicidal potential and quantification of causative allelochemicals from several Compositae weeds. Weed Res. 43, 444–450. doi: 10.1046/j.0043-1737.2003.00361.x
Christensen, M. G., Teicher, H. B., and Streibig, J. C. (2003). Linking fluorescence induction curve and biomass in herbicide screening. Pest Manage. Sci. Formerly Pesticide Sci. 59, 1303–1310. doi: 10.1002/ps.763
Davis, A. M., Thorburn, P. J., Lewis, S. E., Bainbridge, Z. T., Attard, S. J., Milla, R., et al. (2013). Environmental impacts of irrigated sugarcane production: herbicide run-off dynamics from farms and associated drainage systems. Agric. Ecosyst. Environ. 180, 123–135. doi: 10.1016/j.agee.2011.06.019
Dayan, F., Romagni, J., Tellez, M., Romando, A., and Duke, S. (1999). Managing weeds with natural products. Pesticide Outlook 10, 185–188.
Duke, S., Dayan, F., Romagni, J., and Rimando, A. (2000). Natural products as sources of herbicides: current status and future trends. Weed Res. 40, 99–111. doi: 10.1046/j.1365-3180.2000.00161.x
Duke, S., Scheffler, B., Dayan, F., Reigosa, M., and Pedrol, N. (2002). “Allelochemicals as herbicides,” in Allelopathy: From Molecules to Ecosystems, eds M. Reigosa and N. Pedrol (Enfield, CT: Science Publishers, Inc.), 183–195.
Durán-Serantes, B., González, L., and Reigosa, M. J. (2002). Comparative physiological effects of three allelochemicals and two herbicides on Dactylis glomerata. Acta Physiol. Plantarum 24, 385–392. doi: 10.1007/s11738-002-0034-4
Einhellig, F. A. (1995). Mechanism of Action of Allelochemicals in Allelopathy. Washington, DC: ACS Publications.
Einhellig, F. A., Rasmussen, J. A., Hejl, A. M., and Souza, I. F. (1993). Effects of root exudate sorgoleone on photosynthesis. J. Chem. Ecol. 19, 369–375. doi: 10.1007/BF00993702
Ellis, A. T., Morgan, G. D., and Mueller, T. C. (2008). Mesosulfuron-resistant Italian ryegrass (Lolium multiflorum) biotype from Texas. Weed Technol. 22, 431–434. doi: 10.1614/WT-08-032.1
Farooq, M., Jabran, K., Cheema, Z. A., Wahid, A., and Siddique, K. H. M. (2011). The role of allelopathy in agricultural pest management. Pest Manag. Sci. 67, 493–506. doi: 10.1002/ps.2091
Fu, Y., and Viraraghavan, T. (2002). Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Advances in Environmental Research 7, 239–247. doi: 10.1016/S1093-0191(01)00123-X
Fuhlendorf, S. D., Engle, D. M., O'meilia, C. M., Weir, J. R., and Cummings, D. C. (2009). Does herbicide weed control increase livestock production on non-equilibrium rangeland? Agric. Ecosyst. Environ. 132, 1–6. doi: 10.1016/j.agee.2009.02.015
Gu, Y., Wang, P., and Kong, C. (2009). Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 45, 436–441. doi: 10.1016/j.ejsobi.2009.06.003
Haig, T., Pratley, J., An, M., Haig, T., and Hildebrand, S. (2005). “Using allelopathy to search for new natural herbicides from plants,” in Proceedings of the 4th World Congress on Allelopathy (Wagga, NSW: Charles Sturt University), 565–568.
Haig, T. J., Seal, A. N., Pratley, J. E., An, M., and Wu, H. (2009). Lavender as a source of novel plant compounds for the development of a natural herbicide. J. Chem. Ecol. 35, 1129–1136. doi: 10.1007/s10886-009-9689-2
Harun, M. A. Y. A., Johnson, J., and Robinson, R. W. (2015a). The contribution of volatilization and exudation to the allelopathic phytotoxicity of invasive Chrysanthemoides monilifera subsp. monilifera (boneseed). Biol. Invasions. 17, 3609–3624. doi: 10.1007/s10530-015-0983-3
Harun, M. A. Y. A., Johnson, J., and Robinson, R. W. (2015b). Do phytotoxic allelochemicals remain in ashes after burning Chrysanthemoides monilifera subsp. monilifera (boneseed)?. J. Environ. Sci. 44, 109–119. doi: 10.1016/j.jes.2015.09.020
Harun, M. A. Y. A., Johnson, J., Uddin, M. N., and Robinson, R. W. (2015c). The effects of temperature on decomposition and allelopathic phytotoxicity of boneseed litter. Journal of Environmental Sciences 33, 1–11. doi: 10.1016/j.jes.2014.12.017
Harun, M. A. Y. A., Robinson, R. W., Johnson, J., and Uddin, M. N. (2014). Allelopathic potential of Chrysanthemoides monilifera subsp. monilifera (boneseed): a novel weapon in the invasion processes. South Afr. J. Bot. 93, 157–166. doi: 10.1016/j.sajb.2014.04.008
Hashem, A., and Borger, C. (2014). Lime Effects on the Control of Annual Ryegrass and Wild Radish in Low pH Soils. Northam and Merredin, WA: Department of Agriculture and Food.
Holt, J. S., and Lebaron, H. M. (1990). Significance and distribution of herbicide resistance. Weed Technol. 4, 141–149. doi: 10.1017/S0890037X00025148
Inskeep, W. P., and Bloom, P. R. (1985). Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol. 77, 483–485. doi: 10.1104/pp.77.2.483
Iqbal, J., Cheema, Z., and Mushtaq, M. N. (2009). Allelopathic crop water extracts reduce the herbicide dose for weed control in cotton (Gossypium hirsutum). Int. J. Agric. Biol. 11, 360–366.
Jabran, K., Cheema, Z., Farooq, M., and Hussain, M. (2010). Lower doses of pendimethalin mixed with allelopathic crop water extracts for weed management in canola (Brassica napus). Int. J. Agric. Biol. 12, 335–340.
Jamil, M., Cheema, Z. A., Mushtaq, M. N., Farooq, M., and Cheema, M. A. (2009). Alternative control of wild oat and canary grass in wheat fields by allelopathic plant water extracts. Agron. Sustain. Dev. 29, 475–482. doi: 10.1051/agro/2009007
Javaid, A., Shafique, S., and Bajwa, R. (2006). Effect of aqueous extracts of allelopathic crops on germination and growth of Parthenium hysterophorus L. South Afr. J. Bot. 72, 609–612. doi: 10.1016/j.sajb.2006.04.006
Lemerle, D., Verbeek, B., and Coombes, N. (1995). Losses in grain yield of winter crops from Lolium rigidum competition depend on crop species, cultivar and season. Weed Res. 35, 503–509. doi: 10.1111/j.1365-3180.1995.tb01648.x
Llewellyn, R. S., and Powles, S. B. (2009). High levels of herbicide resistance in rigid ryegrass (Lolium rigidum) in the Wheat Belt of Western Australia. Weed Technol. 15, 242–248. doi: 10.1614/0890-037X(2001)015[0242:HLOHRI]2.0.CO;2
Mahmood, K., Mathiassen, S. K., Kristensen, M., and Kudsk, P. (2016). Multiple herbicide resistance in Lolium multiflorum and identification of conserved regulatory elements of herbicide resistance genes. Front. Plant Sci. 7:1160. doi: 10.3389/fpls.2016.01160
Meksawat, S., and Pornprom, T. (2010). Allelopathic effect of itchgrass (Rottboellia cochinchinensis) on seed germination and plant growth. Weed Biol. Manag. 10, 16–24. doi: 10.1111/j.1445-6664.2010.00362.x
Omezzine, F., Rinez, A., Ladhari, A., Farooq, M., and Haouala, R. (2011). Allelopathic potential of Inula viscosa against crops and weeds. Int. J. Agric. Biol. 13, 841–849.
Owen, M. J., and Powles, S. B. (2010). Glyphosate-resistant rigid ryegrass (Lolium rigidum) populations in the Western Australian grain belt. Weed Technol. 24, 44–49. doi: 10.1614/WT-09-054.1
Preston, C., Roush, R. T., and Powles, S. B. (1999). “Herbicide resistance in weeds of southern Australia: why are we the worst in the world,” in Proceeding of the 12th Australian Weeds Conference (Devonport, TAS: Tasmanian Weed Society), 454–459.
Putnam, A. R. (1988). Allelochemicals from plants as herbicides. Weed Technol. 2, 510–518. doi: 10.1017/S0890037X00032371
Rashid, M. H., Asaeda, T., and Uddin, M. N. (2010). The allelopathic potential of kudzu (Pueraria montana). Weed Sci. 58, 47–55. doi: 10.1614/WS-09-106.1
Reigosa, M., Gonzalez, L., Sanches-Moreiras, A., Duran, B., Puime, D., Fernandez, D., et al. (2001). Comparison of physiological effects of allelochemicals and commercial herbicides. Allelopathy J. 8, 211–220.
Ruchs, C. (2008). “Boxer® Gold, a flexible new pre-emergent herbicide alternative for the control of annual ryegrass (Lolium rigidum) and toad rush (Juncus bufonius L.) in wheat and barley,” in Proceedings of the 16th Australian Weeds Conference (Cairns, QLD: Weed Society of Queensland, Brisbane), 291–293.
Saghir, A., and Bhatti, M. (1970). “The influence of herbicides on the chemical composition of soybean seeds,” in Proceedings of the 10th British Weed Control Conference (Brighton), 384–388.
Seal, A. N., Pratley, J. E., Haig, T. J., An, M., and Wu, H. (2010). Plants with phytotoxic potential: Wollemi pine (Wollemia nobilis). Agric. Ecosyst. Environ. 135, 52–57. doi: 10.1016/j.agee.2009.08.009
Singh, H., Batish, D. R., Kaur, S., Ramezani, H., and Kohli, R. (2002). Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann. Appl. Biol. 141, 111–116. doi: 10.1111/j.1744-7348.2002.tb00202.x
Singh, H., Batish, D. R., and Kohli, R. (2003). Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. CRC Crit. Rev. Plant Sci. 22, 239–311. doi: 10.1080/713610858
Tejada, M., García-Martínez, A. M., Gómez, I., and Parrado, J. (2010). Application of MCPA herbicide on soils amended with biostimulants: short-time effects on soil biological properties. Chemosphere 80, 1088–1094. doi: 10.1016/j.chemosphere.2010.04.074
Uddin, M. N., Caridi, D., and Robinson, R. W. (2012). Phytotoxic evaluation of Phragmites australis: an investigation of aqueous extracts of different organs. Marine Fresh. Res. 63, 777–787. doi: 10.1071/MF12071
Wang, C.-M., Li, T.-C., Jhan, Y.-L., Weng, J.-H., and Chou, C.-H. (2013). The impact of microbial biotransformation of catechin in enhancing the allelopathic effects of Rhododendron formosanum. PLoS ONE 8, e85162. doi: 10.1371/journal.pone.0085162
Weir, T. L., Park, S.-W., and Vivanco, J. M. (2004). Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 7, 472–479. doi: 10.1016/j.pbi.2004.05.007
Xie, W., Zhou, J., Wang, H., Chen, X., Lu, Z., Yu, J., et al. (2009). Short-term effects of copper, cadmium and cypermethrin on dehydrogenase activity and microbial functional diversity in soils after long-term mineral or organic fertilization. Agric. Ecosyst. Environ. 129, 450–456. doi: 10.1016/j.agee.2008.10.021
Keywords: Chrysanthemoides monilifera subsp. monilifera, Lolium rigidum, Triticum aestivum, herbicide, sustainable weed control, allelopathy, pre and post-emergent
Citation: Harun MAYA, Johnson J, Uddin MN and Robinson RW (2021) Allelopathic Effects of Chrysanthemoides monilifera subsp. monilifera on Lolium rigidum in Wheat Field: Implications on the Reduction of Chemical Loads in Agro-Ecosystems. Front. Agron. 3:785845. doi: 10.3389/fagro.2021.785845
Received: 29 September 2021; Accepted: 02 November 2021;
Published: 24 November 2021.
Edited by:
Bhagirath Singh Chauhan, The University of Queensland, AustraliaReviewed by:
Ioannis Roussis, Agricultural University of Athens, GreeceSimerjeet Kaur, Punjab Agricultural University, India
Copyright © 2021 Harun, Johnson, Uddin and Robinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. N. Uddin, bmF6aW0udWRkaW5AdnUuZWR1LmF1
 M. A. Y. A. Harun
M. A. Y. A. Harun Joshua Johnson1
Joshua Johnson1 M. N. Uddin
M. N. Uddin