- 1School of Agriculture, Yunnan University, Chenggong District, Kunming, China
- 2Yunnan ICL YTH Phosphate Research and Technology Center Co. Ltd., Kunming, China
Introduction: Hemp (Cannabis sativa L.) has gained worldwide attention for its emerging role as a valuable medicinal source, particularly for cannabidiol (CBD) extraction. Yunnan province is currently the only region in China where hemp grown for CBD production is legalized; yet information on optimal agronomic practices for maximizing CBD output in this region remains scarce.
Methods: In the present study, field experiments were conducted in Yunan over two consecutive growing seasons (2019 and 2020) to evaluate CBD productivity and variations in CBD content between female and male plants, as well as among branches along the stem, using a local dioecious hemp cultivar, Yunma #7. Plants were grown under five treatments: a control (CK) with NPK fertilization; additional calcium magnesium phosphate (CK+CMP); and additional boron applied either as powdered boron at the basal stage (CK+CMP+PB1), powdered boron at the budding stage (CK+CMP+PB2), or liquid sugar-alcohol-chelated boron at the budding stage (CK+CMP+LB2).
Results: The average inflorescence yield reached 4 Mg ha−1, with female plants producing 2.4 Mg ha−1. Inflorescence CBD content averaged approximately 1% (w/w), being 15% higher in female than male plants, and 30% higher in upper than lower inflorescences. Mg and B fertilization showed no statistically significant effects on inflorescence yield and CBD content, the average CBD yield across all fertilization treatments was 20.1 kg ha−1.
Discussion: The study underscores the potential for increasing CBD content through breeding and optimizing harvest methods that distinguish between female and male plants, and separate upper and lower inflorescences.
1 Introduction
Hemp (Cannabis sativa L.) is a multifaceted crop traditionally cultivated for fiber and grain, but it has emerged as a valuable medicinal resource in recent years. To date, more than 100 cannabinoids have been identified in hemp, with tetrahydrocannabinol (THC) and cannabidiol (CBD) being the most abundant. In many countries, hemp varieties containing THC under the threshold of 0.3% (w/w) are classified as industrial hemp to distinguish from marijuana (Bertoli et al., 2010; Cherney and Small, 2016; Hartsel et al., 2016; Fiorini et al., 2019). THC is known for its psychoactive effects, while CBD is non-psychoactive and valued as a potential therapeutic agent due to its anti-inflammatory, antioxidant, and neuroprotective properties (Hill et al., 2012; Mechoulam et al., 2007; ElSohly and Gul, 2014; Aizpurua-Olaizola et al., 2016; Bonaccorso et al., 2019). From medicines to cosmetics, a wide range of CBD-containing products can be found on the market (Pisanti et al., 2017; Li et al., 2021).
Yunnan was the first province in China to legalize the hemp industry, with the Provisional Regulation on Industrial Hemp announced in 2003 and updated in 2010 (Zhao et al., 2021). It is currently the only region in China that licenses the planting and processing of hemp for CBD production. The first high-purity CBD production line in Yunnan was established in 2014 by Hankang (Yunnan) Biotechnology Limited Company. Since then, the hemp industry for CBD production in Yunnan has been rapidly expanding, making Yunnan the largest producer of CBD in China (Zhao et al., 2021). By 2020, over 13,000 hectares of hemp were grown in open fields for CBD production, and nearly 160 companies registered for CBD-related businesses in Yunnan, covering cultivation, extraction, and the manufacturing of CBD products (Zhao et al., 2021). However, there is a lack of research focused on cultivation methods specifically designed for CBD production. To support the thriving CBD industry, it is therefore necessary to explore the optimal production strategies aimed at enhancing hemp productivity specifically for CBD production in this region.
Hemp includes both dioecious and monoecious cultivars. Although a few monoecious cultivars have been developed in recent years in the Yunnan region, they have not yet been registered with the Department of Drug Control Bureau of Public Security; therefore, all currently legalized cultivars in Yunnan are dioecious. Dioecious hemp cultivars produce male and female plants, with male plants senescing shortly after pollination, while the senescence of female plants is influenced by fertilization status. Unfertilized female plants continue developing new leaves and pistils for longer period than fertilized plants (Chandra et al., 2020). To optimize CBD production, farmers in Yunnan usually harvest male plants at the budding stage to prevent pollination, thereby extending female plants growth period and increase inflorescence yield. During harvest, farmers manually cut off branches and air-dry them either in the field or under shelter. Air-dried branches of the whole canopy of both male and female plants are pooled and processed to collect inflorescences (i.e., a mixture of fan leaves, sugar leaves, and flowers), which are sold to CBD extraction factories. However, such harvesting strategy may result in heterogeneous raw materials for CBD extraction, as several studies on indoor-grown cannabis plants have shown that female inflorescences contain higher CBD content than male inflorescences, and CBD content increased with plant height in the canopy (Bernstein et al., 2019; Stack et al., 2023). Despite this, information is scarce on field-grown hemp plants regarding the variation in CBD content between male and female plants, or along the canopy height, even though field-grown plants experience greater competition for light and nutrients than those grown in controlled environments (Tang et al., 2017).
Although hemp generally requires relatively low inputs of fertilizers (Struik et al., 2000; Amaducci et al., 2015), careful management of both macronutrients and micronutrients is essential. In Yunnan, if hemp is grown for CBD, the seedlings are transplanted between April and May, with approximately 300 kg ha−1 of compound fertilizer (mainly containing N, P2O5, and K2O) applied as basal fertilizer and around 150 kg ha−1 of urea applied in one or two splits during the cultivation period (Zhang et al., 2023). Such management recognized the importance of macronutrients for crop growth and yield production, but often overlooks the requirement of secondary macronutrients and micronutrients, such as magnesium (Mg) and boron (B). This is despite frequent reports of Mg or/and B deficiencies in major crops and the common use of Mg and B fertilizers in Yunnan (Zhang et al., 2010; Xu. et al., 2014). Mg is a key component of chlorophyll and is essential for protein biosynthesis (Gerendás and Führs, 2013), while B is crucial for cell wall synthesis, as well as the synthesis, transport and metabolism of carbohydrates, and plays an important role in determining crop yield and secondary metabolite production (O’Neill et al., 2004). In medical cannabis, Mg deficiency impaired photosynthetic capacity and caused nutrient uptake disorder, resulting in reduced biomass (Cockson et al., 2019). Prolonged B deficiency in hemp caused distorted leaf growth, necrosis of growing tips and roots, and reduced biomass (Morad and Bernstein, 2023). Furthermore, applying Mg or/and B fertilizers at different developmental stages could induce the differentiation of female flowers on jatropha curcas plant (Xu and Wang, 2011), and promote the vegetative and reproductive growth of tobacco (Feng et al., 2010). Our previous study in sand cultivation system indicated that B and Mg deficiencies in hemp resulted in growth retardation and a decrease in CBD content in leaves (Ou et al., 2022), whereas there is a lack of information on the response of field-grown hemp plants to Mg and B fertilizers in this region.
In the present study, we hypothesized that field-grown hemp plants would exhibit similar patterns to those observed in indoor cultivation, with differences in inflorescence CBD content between male and female, as well as across canopy heights (Bernstein et al., 2019; Stack et al., 2023). These differences may support harvesting practices that involve separating female from male plants, and distinguishing upper from lower inflorescences, thereby improving CBD quality. Additionally, we further hypothesized that applying additional Mg and B fertilizers could increase the inflorescence CBD content and yield of field-grown hemp plants in Yunnan. Hence, field trials were conducted over two consecutive years to (1) evaluate the extent of differences in inflorescence CBD content between male and female plants, and across different canopy heights under field conditions; and (2) investigate the effect of Mg and B fertilizers on CBD content in inflorescences under field conditions. These results could provide novel information to develop strategies for optimizing CBD production in the open field.
2 Materials and methods
2.1 Description of the experimental site and soil properties
Two-year (2019 and 2020) field trials were carried out at the experimental field of Hanshengfeng Industrial Hemp Cultivation Co., LTD. (25°52′N, 103°40′E, 2040 m above sea level), located in Yunnan, China. The trials were conducted in two different fields within close proximity (less than 1 km apart) to avoid potential carryover effects of fertilizers applied in the previous year on subsequent plant growth. The preceding crop was maize in both years. The soil content of total organic carbon, hydrolyzable N, Exchangeable Mg, and available B was 32.2 g kg−1, 150 mg kg−1, 96 mg kg−1, 0.38 mg kg−1, respectively, in 2019; and 33.6 g kg−1, 143 mg kg−1, 432 mg kg−1, 0.20 mg kg−1, respectively, in 2020 (Table 1). During the experimental period, daily average air temperatures ranged between 9.3°C and 25.8°C in 2019, and between 7.3°C and 25.4°C in 2020, total rainfall was 988 mm and 532 mm in 2019 and 2020, respectively (Figure 1).
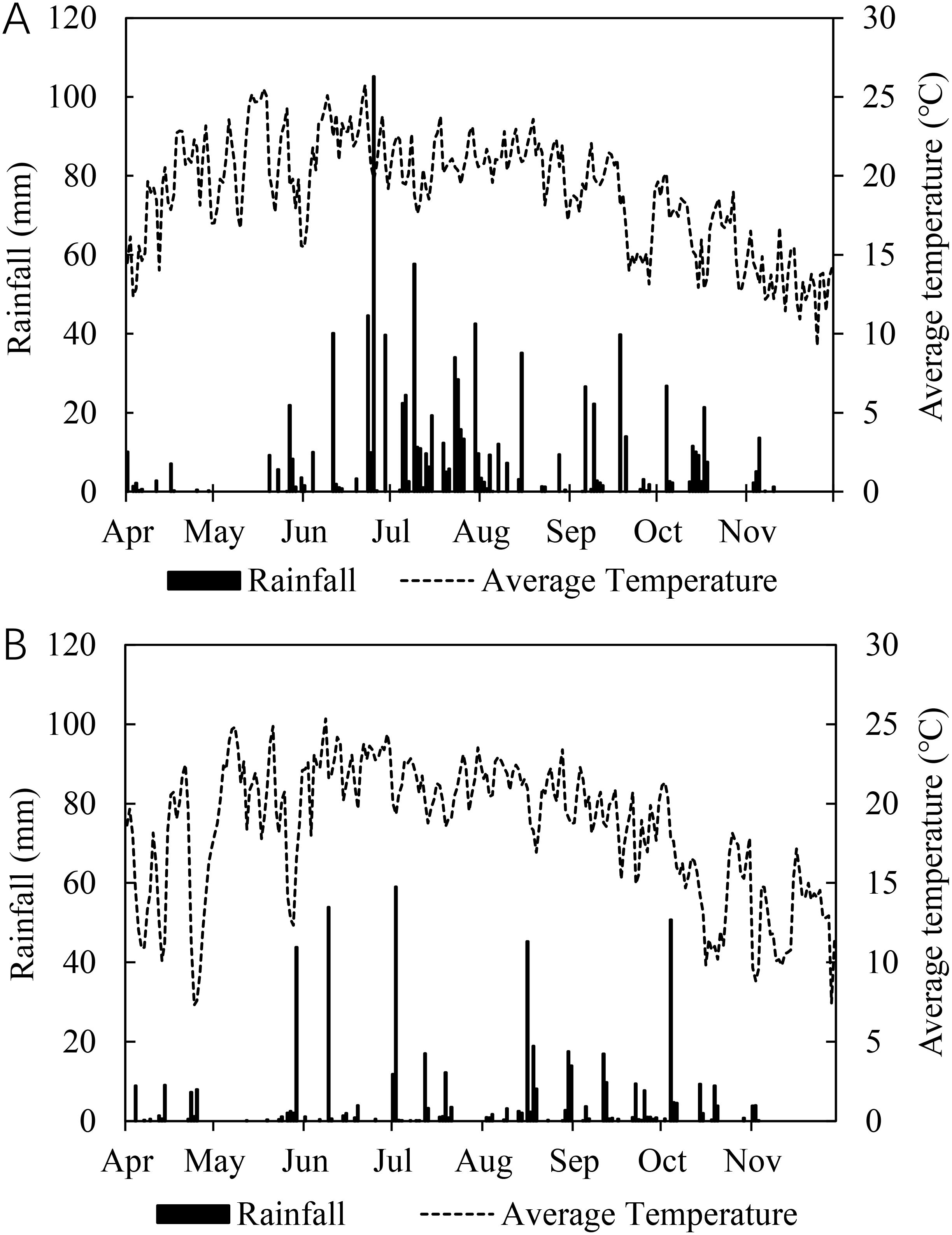
Figure 1. Daily average air temperature and rainfall during the experimental period in 2019 (A) and 2020 (B).
Soil samples were collected from a depth of 0–25 cm using a five-point sampling method before transplanting. pH value was measured using a digital pH meter (PHS-3E, Shanghai Leici, China). Total organic carbon (TOC) was determined using a rapid dichromate oxidation-titration technique, where soil samples were oxidized with a mixture of potassium dichromate and concentrated sulfuric acid at 170°C to 180°C, followed by titration with iron (II) sulfate (Ciavatta et al., 1991). Alkali-hydrolysable nitrogen (N), available phosphorus (P), and available potassium (K) in soil samples were determined using the alkali N-proliferation method (40°C, 24h) (Cornfield, 1960), the Bray and Kurtz I method (Bray and Kurtz, 1945), and neutral ammonium acetate extraction followed by flame photometry (Simonis, 1996), respectively. Exchangeable magnesium (Mg) was determined using Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, ICPA 7400, Thermo Fisher Scientific, USA) after being extracted by ammonium acetate solution (Ministry of Agriculture of the PRC, 2006a). Available boron (B) was determined using Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, ICPA 7400, Thermo Fisher Scientific, USA) after being extracted by magnesium sulfate solution (Ministry of Agriculture of the PRC, 2006b).
2.2 Plant material and trial arrangement
A locally bred dioecious cultivar, Yunma #7, was cultivated in both experimental years. Yunma #7 is a hemp cultivar bred for fiber and CBD production by Yunnan Academy of Agricultural Sciences, China, in 2014. After being released, this cultivar was predominantly cultivated for CBD production in the Yunnan region. Seeds of Yunma #7 were sown in nursery bags on April 25 and May 11, respectively, in 2019 and 2020 (Table 2). Seedlings, approximately 10–15 cm in height, were transplanted into the experimental field in ridges at a hill spacing of 1 m, with 3–5 seedlings per hill. The ridges were 1.2 m apart and covered with black plastic film. One month after transplanting, hand-thinning was carried out to retain 2 healthy plants per hill where 3 or more seedling were present. In hills with 1–2 healthy plants, all the plants were retained.
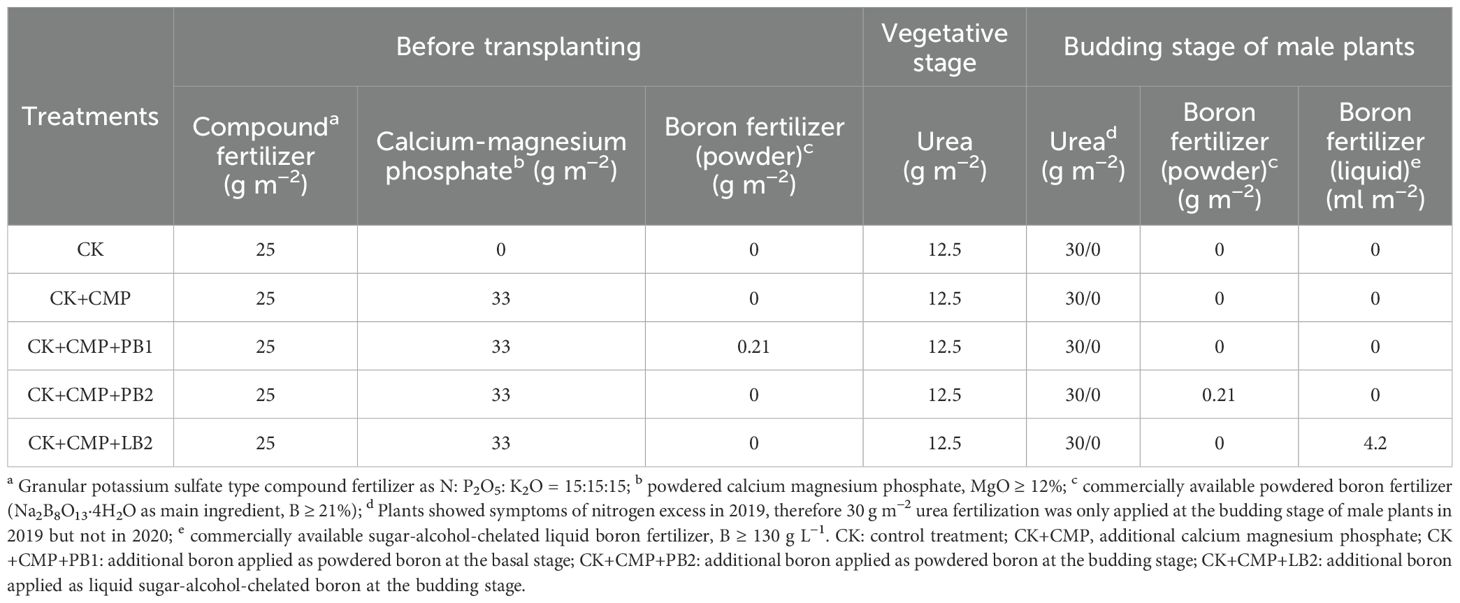
The effects of magnesium (Mg) and boron (B) fertilizers on hemp growth were evaluated in five treatments: CK, CK+CMP, CK+CMP+PB1, CK+CMP+PB2, and CK+CMP+LB2 (Table 3). For the control treatment (CK), 25 g m−2 granular potassium sulfate type compound fertilizer (N: P2O5: K2O = 15:15:15) was applied as basal fertilizer in both experimental years; in addition, 12.5 g m−2 granular urea (N≥46%) was top-dressed at the onset of linear growth period (when plants reached a height of around 60 cm) and 30 g m−2 granular urea was applied at the budding stage of male plants. However, plants grown in 2019 showed symptoms of nitrogen excess (a number of broken and senesced branches at the lower canopy), therefore urea application at the budding stage of male plants was not conducted in 2020. On the basis of CK, 33 g m−2 of powdered calcium magnesium phosphate (MgO ≥ 12%) was applied as basal fertilizer for CK+CMP, CK+CMP+PB1, CK+CMP+PB2 and CK+CMP+LB2, while 0.21 g m−2 of commercially available powdered boron fertilizer (Na2B8O13·4H2O as main ingredient, B ≥ 21%) was applied in the form of powder as basal fertilizer for CK+CMP+PB1, 0.21 g m−2 of commercially available powdered boron fertilizer was dissolved in 500 mL water and applied at the budding stage of male plants for CK+CMP+PB2, 4.2 mL m−2 of commercially available sugar-alcohol-chelated liquid boron fertilizer (B ≥ 130 g L−1) was applied at the budding stage of male plants for CK+CMP+LB2. The experiment was conducted in a completely randomized block design with four replicates per treatment. The area of each plot was 5 × 6 m2, with a planting density of 25 hills per plot. No irrigation was applied after transplanting.
2.3 Data collection
Harvest was carried out for each plot independently and manually following local farming practices. Male plants were harvested at multiple times as soon as their inflorescence buds became visible, ranging from August to September (Table 2). The harvested plants were separated into stems and inflorescences. Inflorescences comprising sugar leaves (secondary leaves emerging from buds), fan leaves (primary leaves developed during the vegetative phase), and flowers. Yield of stems and inflorescences were determined after air-dried under shelter. Subsamples of approximately 500 g air-dried inflorescence (ca. 8% of moisture) were collected from each plot in 2019 to determine the content of CBD.
Female plants were harvested at the onset of seed filling, 143 and 169 days after transplanting in 2019 and 2020, respectively. During the harvest, one representative branch at the top, middle, and bottom of the main stem was selected from each plant in the plot. The uppermost 10 cm of each selected branch was sampled and pooled by canopy position within each plot for CBD content analysis. Subsequently, all plants in the plot were cut from the shoot base. Stem diameter at 20 cm from the base and stem height were measured. Then, the plants were separated into stems and inflorescences, and their respective yields were determined after air-dried under shelter. Subsamples of approximately 500 g air-dried inflorescence were collected to determine the contents of CBD, Mg, and B. In total, four replicates per treatment were assigned according to the plots for CBD analysis, and samples collected from the four replicate plots were pooled for Mg and B content analysis in the inflorescences.
CBD content of inflorescence was determined using a high-performance liquid chromatography method with UV detection (UltiMate TM 3000, Thermo Fisher Scientific, USA), according to the Chinese agricultural standard NY/T 3252.1-2018 (Industrial hemp seed – Part 1: Definition of industrial hemp variety) with modifications (Ministry of Agriculture and Rural Affairs of PR, 2018). Briefly, approximately 0.2 g dry samples were oven-heated at 150°C for 10 min to facilitate decarboxylation. The oven-heated samples were then extracted in 10 mL of methanol in an ultrasonic bath for 30 min at room temperature. After allowing the mixture to stand for at least 90 min, about 1.5 mL supernatant solution was filtered through a 0.45 µm syringe filter (Biosharp BS-PES- 45, Labgic Technology Co., Ltd., Beijing, China) into HPLC vials and injected into the HPLC system (UltiMate TM 3000, Thermo Fisher Scientific, USA). The injection volume was 10 µL, and the total run time was 20 min. Chromatographic separation was carried out on a Syncronis C18 chromatographic column (250 × 4.6 mm, 5 μm, Thermo Fisher Scientific Inc. Bellefonte, USA). The mobile phase consisted of acetonitrile and H2O at a volume ratio of 85:15. The flow rate was set at 1 mL/min and the column temperature was maintained at 30°C. Detection was performed at a wavelength of 220 nm. Standard CBD was purchased from Sigma-Aldrich (Saint Louis, MO, USA). Quantification was performed using calibration curves ranging from 5 to 2,000 µg·mL−1. Each sample was analyzed in triplicate, and the mean values were used as replicates for further analysis.
Mg and B contents of inflorescence were determined for each treatment after mixing the samples collected from four replicate plots, because of limited analytical power. The contents were determined using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES), according to the Chinese national standard GB 5009.268-2016 (National food safety standard – determination of multi-elements in foods) (National Health and Family Planning Commission of the PRC, 2016). Briefly, inflorescences were oven-dried at 80 °C to constant weight and ground to powder. 1 g of the powder was weighed and microwave digested with 10 mL of concentrated HNO3 for 1 h using a microwave digestion system (CEM MARS 6, manufactured by CEM Corp., USA). After cooling to room temperature, the digested samples were heated in an ultrasonic water bath at 100°C for 30 min to ensure ultrasonic degassing, and then diluted with ultrapure water to a final volume of 50 mL. The obtained solution was used to determine Mg and B contents using ICP-OES (ICPA 6300, Thermo Fisher Scientific, USA).
2.4 Statistical analysis
Data analysis was conducted using SPSS v.26 (SPSS Inc., Chicago, IL). Three-way ANOVA was used to assess the effect of fertilization treatment × plant gender × experiment year on plant density, male-to-female ratio, plant height, stem diameter, stem yield, and inflorescence yield, and the effect of fertilization treatment × branch position × experiment year on CBD content of female inflorescences. If interaction was significant between two variables, one-way ANOVA was used to assess the interaction effect after combining the two variables into one. Two-way ANOVA was used to assess the effect of fertilization treatment × plant gender on inflorescence CBD content and yield in 2019. Multiple comparison was carried out using the Tukey HSD test if a significant effect was detected (p < 0.05). Pearson correlation was performed to assess the relationship between CBD and Mg contents and between CBD and B contents.
3 Results
3.1 Morphology and biomass yield
Data collected from each plot were treated as one replicate, resulting in a total of four replicates used to evaluate the differences in morphological traits and biomass yield between female and male plants, as well as the effect of fertilization treatments on these parameters. The plant height and stem diameter ranged from 278~422 cm and 29.8~45.9 mm in 2019, respectively, and from 201~314 cm and 22.9~37.5 mm in 2020, respectively. About half of the plants in the plots were female (Table 4). Across fertilization treatments and years, female plants were on average 20% taller and 16% thicker than male plants. Mg or B fertilizer treatments did not induce any significant changes in plant height and stem diameter (p > 0.05).
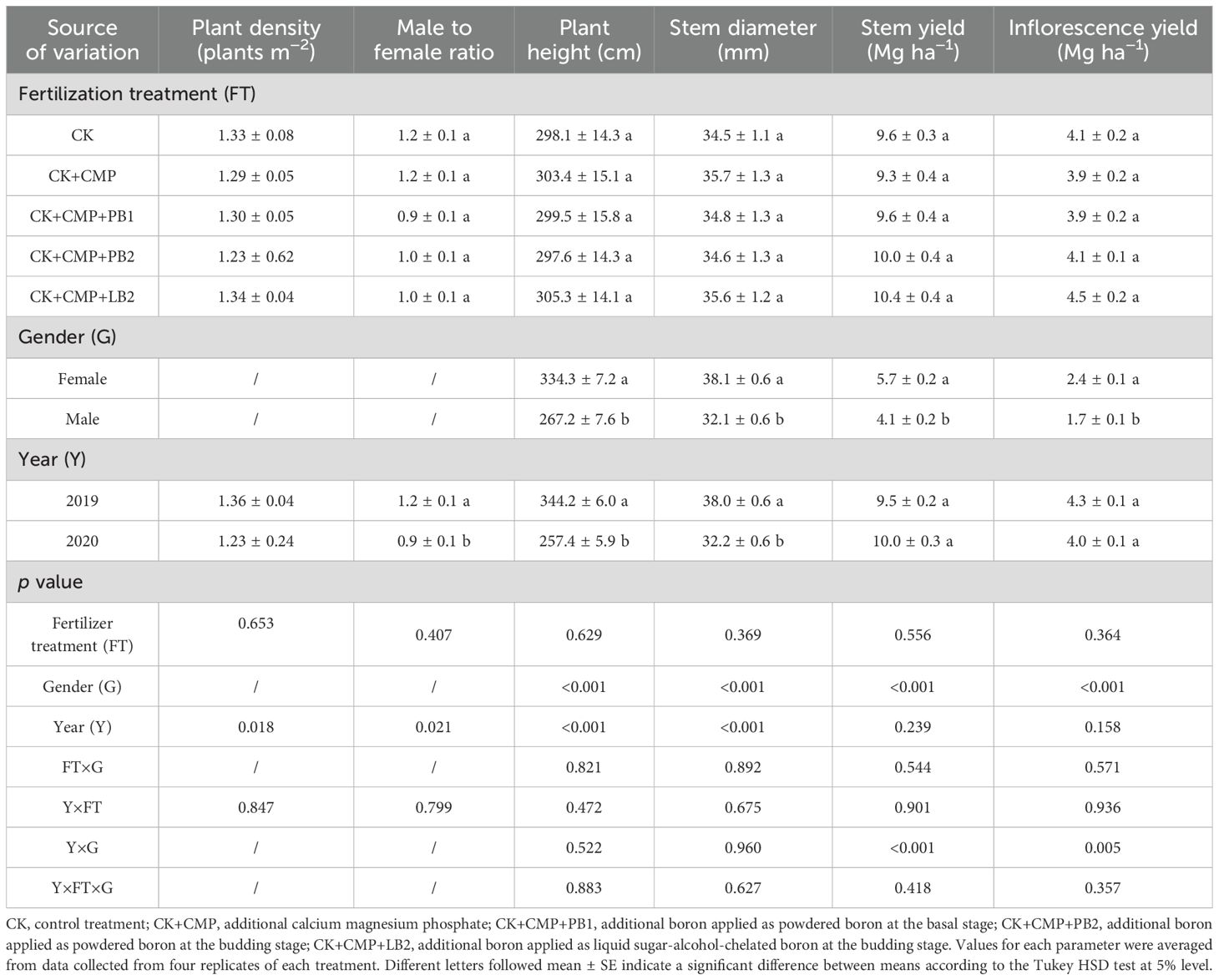
Table 4. Effects of fertilizer treatment, gender, and year and their interaction on plant height, stem diameter, stem yield, and inflorescence yield.
Inflorescence yield ranged from 3.3 Mg ha−1 to 5.4 Mg ha−1. A significant interaction between year and plant gender was observed for inflorescence yield (p < 0.01; Table 4; Figure 2). In 2019, the average inflorescence yields of female plants was 2.6 Mg ha−1, which was 39% higher than that of male plants (p < 0.01). In 2020, female plants yielded an average of 2.2 Mg ha−1, only 12% higher than that of male plants (p = 0.02). Fertilization treatments had no significant effect on inflorescence yield in both experimental years (p > 0.05).
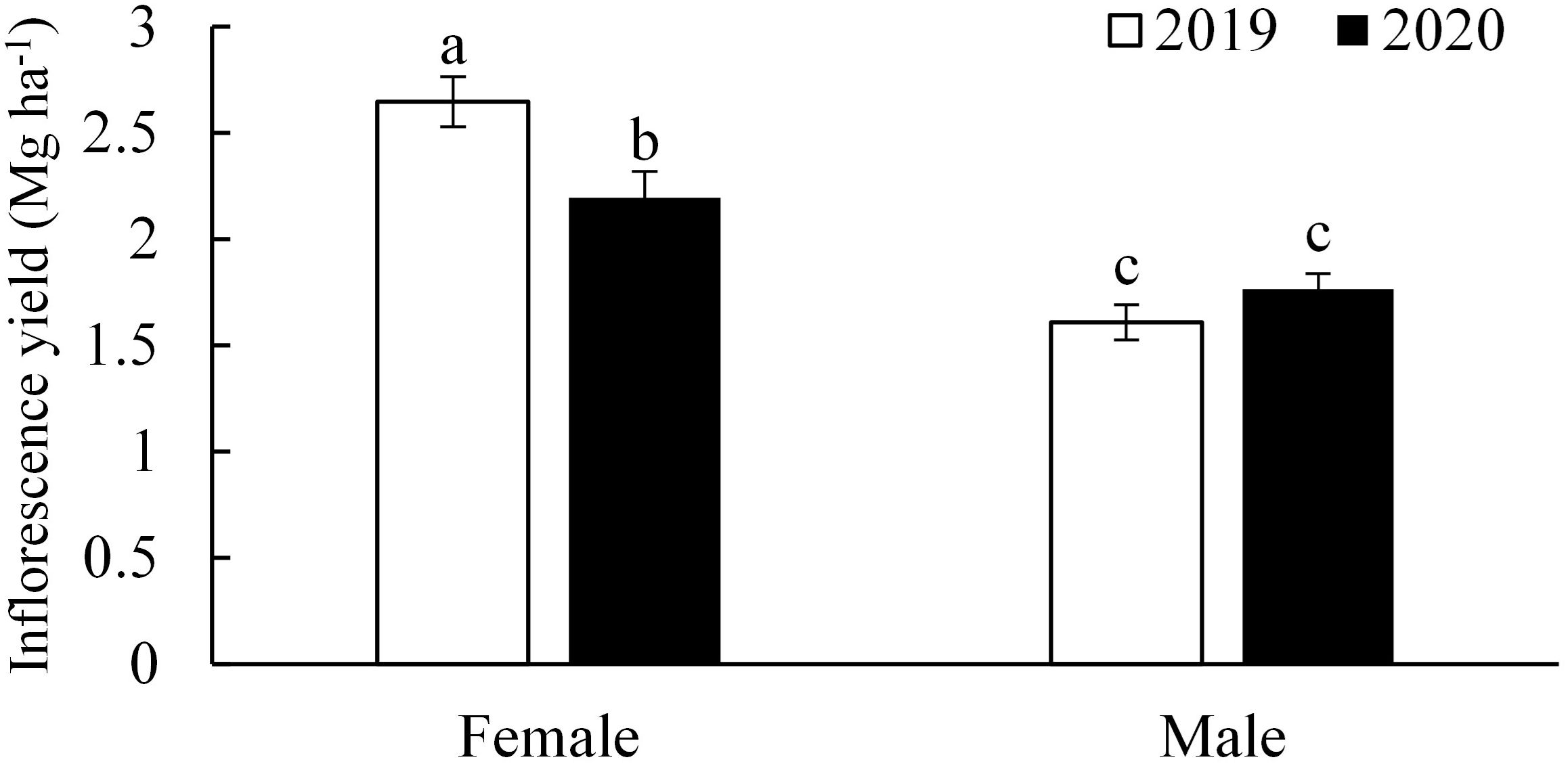
Figure 2. The difference in inflorescence yields between female and male plants in two experiment years. Values were averaged from data collected from four replicates of each treatment. Different letters above the bars indicate significant differences between means according to the Tukey HSD test at p = 5%.
3.2 Cannabidiol content and yield
Each plot’s data were considered as a single replicate, with a total of four replicates used to assess the differences in cannabidiol content and yield between female and male plants, as well as the variation in CBD across different plant heights. Female plants yielded higher CBD than male plants (Figure 3). Across fertilization treatments in 2019, the CBD content in female inflorescences ranged from 0.69% (w/w) to 0.76%, resulting in CBD yield ranging from 16.15 kg ha−1 to 22.16 kg ha−1; while the CBD content in male inflorescences ranged from 0.55% to 0.66%, resulted in CBD yield ranging from 8.03 kg ha−1 to 11.85 kg ha−1, respectively. Overall, the CBD content and yield in female inflorescences were higher than those in male inflorescences by 15% (p = 0.010) and 48% (p < 0.001), respectively.
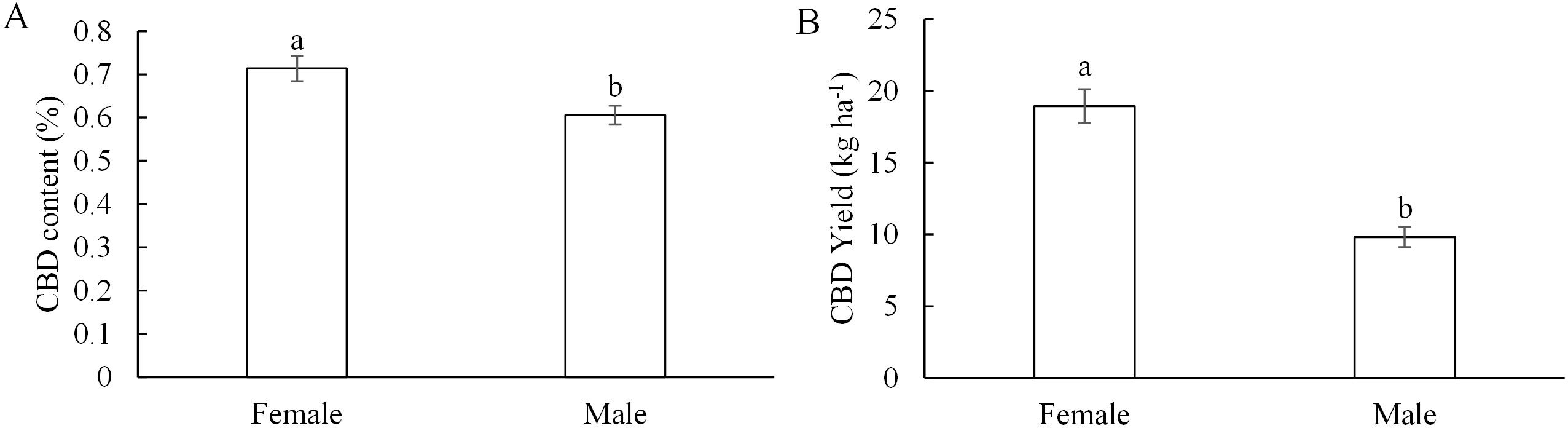
Figure 3. Difference in CBD content (A) and yield (B) between female and male plants in 2019. Values were averaged from data collected from four replicates of each treatment. Different letters above the bars indicate significant differences between means according to the Tukey HSD test at p = 5%.
In female inflorescence, the content of CBD varied significantly along plant height in both experimental years (p < 0.001, Figure 4). Across fertilization treatments, the CBD content in the inflorescence of the top branch was 0.95% and 1.28% in 2019 and 2020, respectively. In comparison, the CBD content in the inflorescence at the middle and bottom parts was lower than those of top inflorescence by 18.8%/13.7% and 31.7%/29.7% in 2019/2020, respectively.
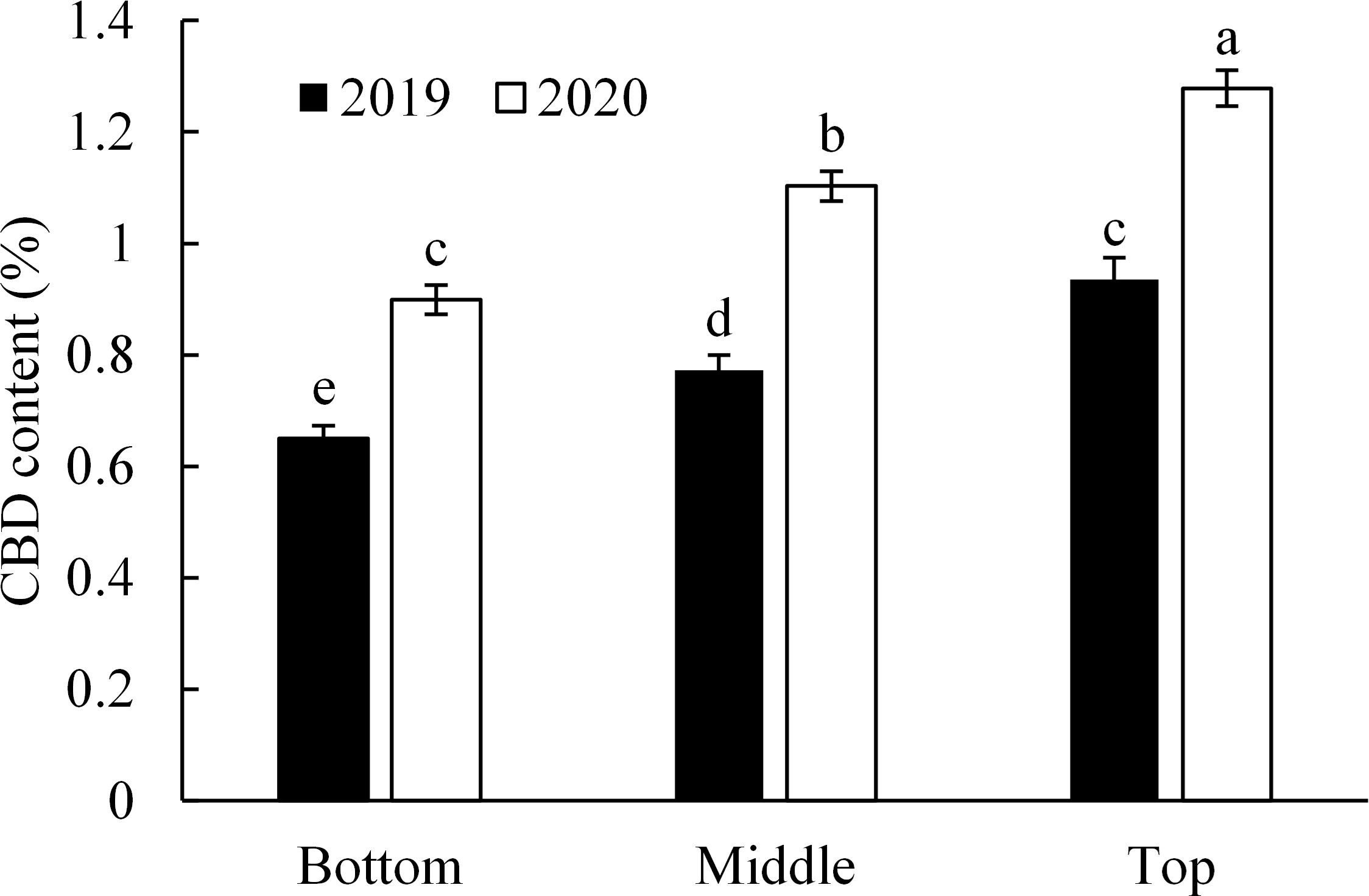
Figure 4. CBD content in inflorescences taken from the branches at the top, middle, and bottom part of the plant. Values represent the average of four replicates per treatment. Different letters indicate significant differences between means according to the Tukey HSD test at 5%.
The Mg and B contents in inflorescence were determined by mixing the samples collected from four replicate plots of each treatment. In the control (CK) plot, Mg content in female inflorescences was 3926 mg kg−1 in 2019 and 7935 mg kg−1 in 2020 (Table 5). Application of Mg fertilizer resulted in an increase in Mg content in female inflorescences by 8.1%~13.5% and -6.1%~1.6% in 2019 and 2020, respectively. B content in female inflorescences of the CK plot in 2019 and 2020 were 2.33 mg kg−1 and 2.96 mg kg−1, respectively. Application of B fertilizers resulted in an increase in B content in female inflorescences by 5%~120% and -6%~166% in 2019 and 2020, respectively. However, no significant difference in CBD content was detected among the treatments of different Mg and B fertilizers (Figure 5), with the average CBD yield across all fertilization treatments being 20.1 kg ha−1. Pearson correlation was also nonsignificant between CBD and Mg contents and between CBD and B contents (Table 6).
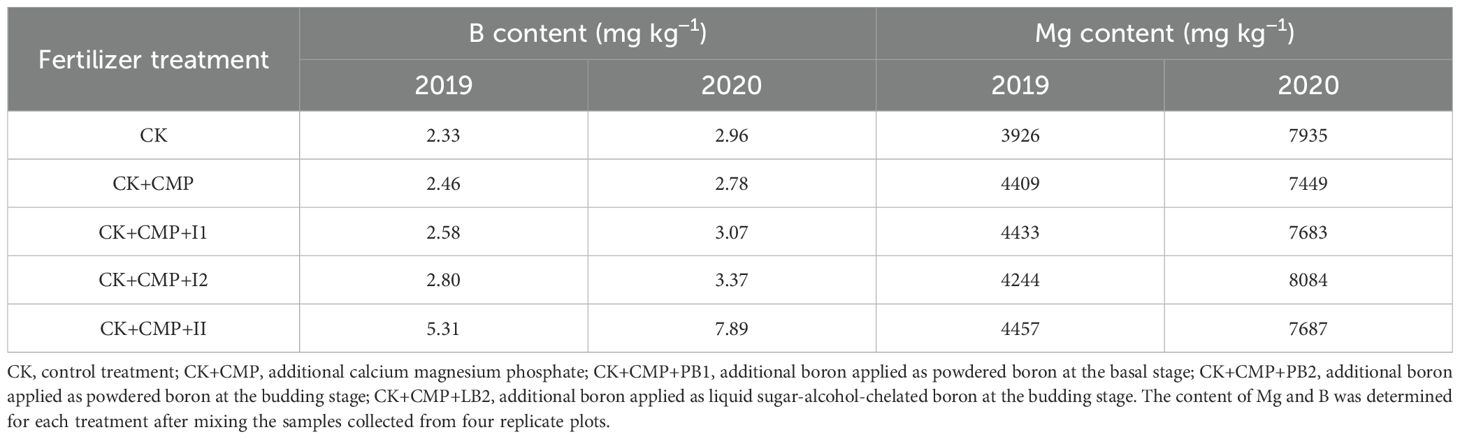
Table 5. The content of Mg and B in female inflorescences of different fertilizer treatments in 2019 and 2020.

Figure 5. CBD content in inflorescences taken from different treatments of Mg and B fertilizers. Treatments include CK (control), CK+CMP (additional calcium magnesium phosphate), CK+CMP+PB1 (powdered boron applied at the basal stage), CK+CMP+PB2 (powdered boron applied at the budding stage), and CK+CMP+LB2 (liquid sugar-alcohol-chelated boron applied at the budding stage). Values represent the average of four replicates per treatment. Different letters indicate significant differences between means according to the Tukey HSD test at 5%.
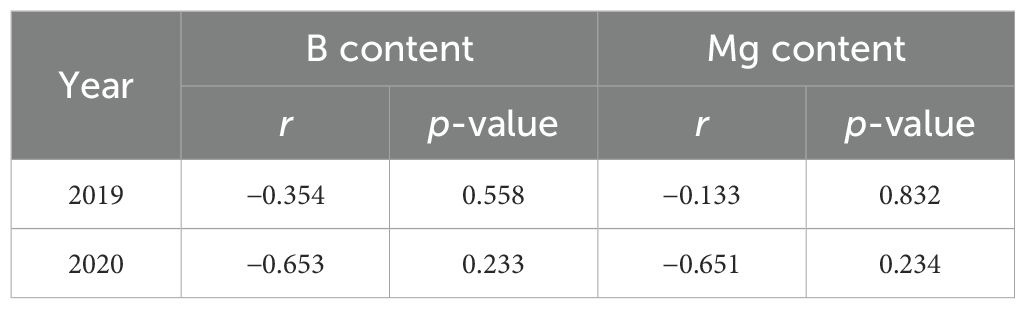
Table 6. Pearson correlation between CBD and Mg contents, and between CBD and B contents in female inflorescence.
4 Discussion
While a number of researchers have assessed indoor hemp cultivation for cannabinoids (Vanhove et al., 2011; Jin et al., 2019), limited information is available regarding the productivity and optimal agronomic practice, particularly in low latitudes. In this study, we addressed this issue by evaluating the yield performance and CBD profile of hemp plants in the open field in Yunnan province, the unique region in China that licenses the planting and processing of hemp for CBD production.
4.1 The yield performance of cannabidiol
To assess the CBD productivity in Yunnan, Yunma #7, a locally bred variety that is well-documented for local adaptation and widespread cultivation in Yunnan, was cultivated following local farming practices. Although the plants in 2019 were taller and thicker compared to 2020 (Table 4), likely influenced by the higher precipitation and urea application in 2019 (Figure 1), Yunma #7 exhibited a similar inflorescence yield in both years, with 2.4 Mg ha−1 for female plants and approximately 4 Mg ha−1 when both male and female plants were considered (Table 4). The inflorescence yields were higher than the value obtained in our previous study on Yunma #1 (a dioecious fiber cultivar) for fiber production but were comparable to the value obtained on Yunma #7 for CBD production in the same region. The yields were also comparable to the reported range of 0.47 Mg ha−1 to 4.71 Mg ha−1 for other hemp varieties in different locations (García-Tejero et al., 2019; Vuerich et al., 2019). However, it is noticeable that the measured CBD content in inflorescence was low (ca. 1%, Figures 3, 4) in comparison with the reported value of 10% or higher (James et al., 2023), which resulted in a relatively low CBD yield (a maximum value of 23.8 kg ha−1). The CBD content measured in the present study was in line with the value reported by the breeder of Yunma #7 and the CBD extract company, which was about 0.7% ~ 0.9% in open field (personal communication). So, such a low CBD content may be a natural characteristic of Yunma #7.
Although CBD content in hemp inflorescence is predominately determined by genotype (Mandolino et al., 2003), crop management and environmental factors could play important roles in CBD content (Trancoso et al., 2022). Variation in CBD content was observed between two experimental years, with a higher CBD content in 2020 than in 2019 (Figure 4). The high CBD content in 2020 could be attributed to scanty rainfall in July and August (Figure 1) and reduced urea application because moderate water shortage and N deficient can stimulate the biosynthesis of secondary metabolites to improve competitive plant abilities in the struggle for vital resources such as water and nutrients (Yadav et al., 2021). Similarly, Calzorari et al. observed that CBD content at full bloom negatively correlated with the sum of rainfall from sowing to full bloom (Calzolari et al., 2017). Saloner et al. reported that the content of cannabidiolic acid (CBDA, the precursor of CBD) decreased by 63% with the increase in N supply from 30 to 320 mg L−1 N (Saloner and Bernstein, 2021).
The low CBD content in hemp inflorescence poses a constraint to a high CBD yield in Yunnan, China. Considering that CBD content in hemp inflorescence could reach 10% or higher (James et al., 2023), increasing CBD content might be a focus for future breeding of cultivation efforts to maximize CBD yield in Yunnan.
4.2 Hemp has gender- and position-specific cannabinoid profile
Diecious hemp plants exhibit sexual dimorphism, as the rate of growth and development are different between male and female hemp plants (Mandolino et al., 2003). In particular, male plants senescence shortly after flowering, rendering a much shorter time for the accumulation of cannabinoids than female plants. Our results supported this by showing that female plants were taller with thicker stems, produced higher yields of stem and inflorescences (Table 4), and had higher CBD content in their inflorescences compared to male plants (Figure 2). Consequently, female plants produced a significantly higher CBD yield than male plants (Figure 2). Given the higher CBD content in female inflorescences, there is a benefit to segregating the inflorescences of male plants from females during harvesting, rather than pooling them together as is commonly practiced by the local farmers in Yunnan. This could increase the consistency in product quality and potentially lead to higher-quality CBD extracts or other cannabinoid-based products. Furthermore, adopting cultivation practices such as using cuttings from female plants (cloning) instead of seeds, or employing hormonal treatments (e.g., ethephon) to feminize male plants, can lead to an increased proportion of female plants in hemp (Trancoso et al., 2022). Nonetheless, the efficacy of these methods needs to be evaluated in further study.
The farming practice for CBD cultivation in Yunnan is dominated by undifferentiated harvesting, where the inflorescences of the entire hemp plant are pooled for CBD extraction. However, our study suggested that natural variation in cannabinoid content across different positions in the canopy present in field-grown hemp plants (Figure 4), similar to the variation observed in potted plants (Bernstein et al., 2019; Stack et al., 2023). The content of CBD in the inflorescences notably increased with plant height, with the uppermost inflorescences containing approximately 30% higher CBD content than the bottom ones (Figure 4). This variation in CBD distribution along the plant height may be attributed to factors such as the sharply decreased light intensity and nitrogen content from the top to the bottom of the hemp plant (Tang et al., 2017). While Hawley et al. (2018) reported that supplemental subcanopy lighting could enhance cannabinoid content in lower canopy organs, the practical application of such methods in production fields may present challenges. As the content of CBD in inflorescence increases with an increase in canopy position, applying layered harvesting methods may be beneficial to increase product quality.
4.3 Effects of magnesium and boron fertilization on cannabidiol production
Magnesium (Mg) and Boron (B) have been identified as crucial elements for leaf development and play important roles in photosynthesis and synthesis of secondary metabolites (O’Neill et al., 2004; Gerendás and Führs, 2013). Our previous research in pots demonstrated that deficiencies in Mg or B could result in yield failure and a significant decrease in biomass and CBD content in inflorescence (Ou et al., 2022). However, results in the present study showed that Mg and B fertilizers had nonsignificant effects on biomass yield, CBD content, and CBD yield in field conditions (Table 4; Figure 5).
Mg content in the inflorescence was 3926~4457 mg kg−1 and 7449~7935 mg kg−1 in 2019 and 2020, respectively (Table 5). These values were higher than the value reported by Cockson et al. (2019) (1,200 mg kg−1) when a drug-type hemp showed Mg deficiency symptoms. They were also higher than the values measured in our previous study (900~1,700 mg kg−1) when seedlings of Yunma #7 showed Mg deficiency symptoms (Xu and Wang, 2011). Therefore, the measurement of Mg in the inflorescence supported that all plants in the present study had sufficient Mg. However, it should be noted that measurements of Mg in Cockson et al. (2019) and in our previous study (Ou et al., 2022) were conducted on potted plants which may have different Mg requirement from field grown plants. Interestingly, the Mg content in 2020 was higher than that in 2019 by 102%, probably due to the higher content of exchangeable Mg in the soil in 2020 (Table 1). The variation between experimental years was larger than that caused by Mg fertilization (Table 5). Thus, it can be speculated that applying Mg as basal fertilizer, as practiced in the present study, to increase inflorescence Mg content is ineffective in the soil in Yunnan. B content in the inflorescence was 2.33~5.31 mg kg−1 and 2.78~7.89 mg kg−1 in 2019 and 2020, respectively (Table 5). These values were higher than the values measured in our previous study (i.e., 1.0 mg kg−1) when necrosis symptoms appeared in the growth point of hemp plants, and were comparable to values measured on healthy plants (ranging from 2.3 to 11.3 mg kg−1) (Ou et al., 2022). Therefore, the measurement of B in the inflorescence supported that all plants in the present study had sufficient B. However, it is noteworthy that measurements of B content in our previous study (Ou et al., 2022) were conducted on potted plants. The B requirement of potted plants may differ from field-grown plants.
The lack of response suggests that the application of additional Mg and B fertilizers may not be necessary in the field in Yunnan. However, there was a large variation in the content of Mg and B in the soil (Table 1), further studies are therefore needed to identify the minimum threshold of the content of Mg and B in the soil, below which the intervention with fertilization would necessitate to avoid deficiencies in the plant and production decreases.
5 Conclusion
Field trials of the Yunma #7 hemp cultivar revealed low CBD yield due to limited CBD content. Female plants consistently produced higher CBD content in the inflorescence than males. Additionally, CBD accumulation in female inflorescence varied across canopy positions. To enhance CBD homogeneity in raw material, optimizing harvesting methods for more uniform inflorescence may be necessary. Although previous pot experiments showed the significance of Mg and B in plant growth and CBD production, the present field study found no Mg or B deficiency symptoms, and additional B and Mg fertilization was unnecessary for improving CBD production under open-field conditions. Overall, the findings highlight the need for developing new hemp cultivars with high CBD content and the need to optimize harvesting practices to maximize CBD production and improve product quality in industrial hemp cultivation in Yunnan.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WO: Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing – original draft. JO: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. QZ: Investigation, Methodology, Validation, Writing – review & editing. XY: Investigation, Methodology, Validation, Writing – review & editing. YM: Data curation, Investigation, Methodology, Validation, Writing – review & editing. GD: Conceptualization, Data curation, Formal Analysis, Methodology, Validation, Writing – review & editing. FL: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. YC: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. KT: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Natural Science Foundation of Yunnan province, China under grant number 202201AT070188; China Agriculture Research System of MOF and MARA under grant number CARS-16-E15. Scientific Research Foundation of Yunnan Education department, China under grand number 2023J0007
Acknowledgments
The authors gratefully acknowledge all staff members involved in the field trials. The authors also thank to Institute of Industrial Crops, Yunnan Academy of Agricultural Sciences, China for providing seeds of Yunma #7.
Conflict of interest
Authors YC was/were employed by Yunnan ICL YTH Phosphate Research and Technology Center Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aizpurua-Olaizola O., Soydaner U., Öztürk E., Schibano D., Simsir Y., Navarro P., et al. (2016). Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Natural products 79, 324–331. doi: 10.1021/acs.jnatprod.5b00949
Amaducci S., Scordia D., Liu F. H., Zhang Q., Guo H., Testa G., et al. (2015). Key cultivation techniques for hemp in Europe and China. Ind. Crops Products 68, 2–16. doi: 10.1016/j.indcrop.2014.06.041
Bernstein N., Gorelick J., and Koch S. (2019). Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crops Products 129, 185–194. doi: 10.1016/j.indcrop.2018.11.039
Bertoli A., Tozzi S., Pistelli L., and Angelini L. G. (2010). Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Products 32, 329–337. doi: 10.1016/j.indcrop.2010.05.012
Bonaccorso S., Ricciardi A., Zangani C., Chiappini S., and Schifano F. (2019). Cannabidiol (CBD) use in psychiatric disorders: A systematic review. Neurotoxicology 74, 282–298. doi: 10.1016/j.neuro.2019.08.002
Bray R. H. and Kurtz L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–49. doi: 10.1097/00010694-194501000-00006
Calzolari D., Magagnini G., Lucini L., Grassi G., Appendino G. B., and Amaducci S. (2017). High added-value compounds from Cannabis threshing residues. Ind. Crops Products 108, 558–563. doi: 10.1016/j.indcrop.2017.06.063
Chandra S., Lata H., and ElSohly M. A. (2020). Propagation of Cannabis for clinical research: An approach towards a modern herbal medicinal products development. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00958
Cherney J. H. and Small E. (2016). Industrial hemp in North America: production, politics and potential. Agronomy 6, 58. doi: 10.3390/agronomy6040058
Ciavatta C., Govi M., Antisari L. V., and Sequi P. (1991). Determination of organic carbon in aqueous extracts of soils and fertilizers. Commun. Soil Sci. Plant Anal. 22, 795–807. doi: 10.1080/00103629109368455
Cockson P., Landis H., Smith T., Hicks K., and Whipker B. E. (2019). Characterization of nutrient disorders of Cannabis sativa. Appl. Sci. 9, 4432. doi: 10.3390/app9204432
Cornfield A. H. (1960). Ammonia released on treating soils with N sodium hydroxide as a possible means of predicting the nitrogen-supplying power of soils. Nature 187, 260–261. doi: 10.1038/187260a0
ElSohly M. and Gul W. (2014). “Constituents of Cannabis sativa,” in Handbook of cannabis, vol. 3 . Ed. Pertwee R. G. (Oxford: Oxford University Press), 187–188.
Feng H. L., Liu Y. X., Zheng X., Zhang H. G., and Zeng Y. (2010). Effects of magnesium and boron fertilizers on growth, yield and quality of flue-cured tobacco. Guangxi Agric. Sci. 41, 244–247.
Fiorini D., Molle A., Nabissi M., Santini G., Benelli G., and Maggi F. (2019). Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Products 128, 581–589. doi: 10.1016/j.indcrop.2018.10.045
García-Tejero I. F., Zuazo V. D., Sánchez-Carnenero C., Hernández A., Ferreiro-Vera C., and Casano S. (2019). Seeking suitable agronomical practices for industrial hemp (Cannabis sativa L.) cultivation for biomedical applications. Ind. Crops products 139, 111524. doi: 10.1016/j.indcrop.2019.111524
Gerendás J. and Führs H. (2013). The significance of magnesium for crop quality. Plant Soil 368, 101–128. doi: 10.1007/s11104-012-1555-2
Hartsel J. A., Eades J., and Makriyannis A. (2016). “Cannabis sativa and hemp,” in Nutraceuticals: Efficacy, Safety and Toxicity, 1st ed. Ed. Gupta R. C. (Elsevier Inc, San Diego, CA, USA), 735–754, ISBN: ISBN 978-0-12-802147-7.
Hawley D., Graham T., Stasiak M., and Dixon M. (2018). Improving cannabis bud quality and yield with subcanopy lighting. HortScience 53, 1593–1599. doi: 10.21273/HORTSCI13173-18
Hill A. J., Williams C. M., Whalley B. J., and Stephens G. J. (2012). Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 133, 79–97. doi: 10.1016/j.pharmthera.2011.09.002
James M. S., Vann M. C., Suchoff D. H., McGinnis M., Whipker B. E., Edmisten K. L., et al. (2023). Hemp yield and cannabinoid concentrations under variable nitrogen and potassium fertilizer rates. Crop Sci. 63, 1555–1565. doi: 10.1002/csc2.20966
Jin D., Jin S., and Chen J. (2019). Cannabis indoor growing conditions, management practices, and post-harvest treatment: a review. Am. J. Plant Sci. 10, 925. doi: 10.4236/ajps.2019.106067
Li J., Carvajal R., Bruner L., and Kaminski N. E. (2021). The current understanding of the benefits, safety, and regulation of cannabidiol in consumer products. Food Chem. Toxicol. 157, 112600. doi: 10.1016/j.fct.2021.112600
Mandolino G., Bagatta M., Carboni A., Ranalli P., and de Meijer E. (2003). Qualitative and quantitative aspects of the inheritance of chemical phenotype in Cannabis. J. Ind. Hemp 8, 51–72. doi: 10.1300/J237v08n02_04
Mechoulam R., Peters M., Murillo-Rodriguez E., and Hanuš L. O. (2007). Cannabidiol - recent advances. Chem. Biodivers. 4, 1678–1692. doi: 10.1002/cbdv.200790147
Ministry of Agriculture and Rural Affairs of PR. (2018). Industrial hemp seed - Part 1: Definition of industrial hemp variety, NY/T 3252.1-2018. Available online at: https://www.chinesestandard.net/PDF.aspx/NYT3252.1-2018 (Accessed November 10, 2020).
Ministry of Agriculture of the PRC. (2006a). Soil Testing Part 13: Method for determination of soil exchangeable calcium and magnesium, NY T1121.13-2006. Available online at: https://www.chinesestandard.net/Related.aspx/NYT1121.13-2006 (Accessed June 20, 2020).
Ministry of Agriculture of the PRC. (2006b). Soil Testing Part 8: Method for determination of soil avalible boron, NY T1121.13-2006. Available online at: https://www.chinesestandard.net/Related.aspx/NYT1121.8-2006 (Accessed June 20, 2020).
Morad D. and Bernstein N. (2023). Response of medical cannabis to magnesium (Mg) supply at the vegetative growth phase. Plants 12, 2676. doi: 10.3390/plants12142676
National Health and Family Planning Commission of the PRC. (2016). National food safety standard – Determination of multi-elements in foods, GB 5009.268-2016. Available online at: https://www.chinesestandard.net/Related.aspx/GB5009.268-2016 (Accessed December 15, 2020).
O’Neill M. A., Ishii T., Albersheim P., and Darvill A. G. (2004). Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 55, 109–139. doi: 10.1146/annurev.arplant.55.031903.141750
Ou J., Wang J. Y., Meng Y. Y., Sun J., Du G. H., Yang Y., et al. (2022). Effect of boron and magnesium deficiency on the hemp (Cannabis Sativa L.) growth and cannabidiol concentration. J. Yunan University: Natural Sci. Edition 44, 1314–1320.
Pisanti S., Malfitano A. M., Ciaglia E., Lamberti A., Ranieri R., Cuomo G., et al. (2017). Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 175, 133–150. doi: 10.1016/j.pharmthera.2017.02.041
Saloner A. and Bernstein N. (2021). Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind. Crops Products 167, 113516. doi: 10.1016/j.indcrop.2021.113516
Simonis A. D. (1996). Effects of pH and solvent/soil ratio on extraction of potassium by various extracting solutions compared to neutral molar ammonium acetate. Commun. Soil Sci. Plant Anal. 27, 919–934. doi: 10.1080/00103629609369608
Stack G. M., Carlson C. H., Toth J. A., Philippe G., Crawford J. L., Hansen J. L., et al. (2023). Correlations among morphological and biochemical traits in high-cannabidiol hemp (Cannabis sativa L.). Plant Direct 7, e503. doi: 10.1002/pld3.503
Struik P. C., Amaducci S., Bullard M. J., Stutterheim N. C., Venturi G., and Cromack H. T. H. (2000). Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops products 11, 107–118. doi: 10.1016/S0926-6690(99)00048-
Tang K., Struik P. C., Yin X., Calzolari D., Musio S., Thouminot C., et al. (2017). A comprehensive study of planting density and nitrogen fertilization effect on dual-purpose hemp (Cannabis sativa L.) cultivation. Ind. Crops Products 107, 427–438. doi: 10.1016/j.indcrop.2017.06.033
Trancoso I., de Souza G. A., dos Santos P. R., dos Santos K. D., de Miranda R. M., da Silva A. L., et al. (2022). Cannabis sativa L.: Crop management and abiotic factors that affect phytocannabinoid production. Agronomy 12, 1492. doi: 10.3390/agronomy12071492
Vanhove W., Van Damme P., and Meert N. (2011). Factors determining yield and quality of illicit indoor cannabis (Cannabis spp.) production. Forensic Sci. Int. 212, 158–163. doi: 10.1016/j.forsciint.2011.06.006
Vuerich M., Ferfuia C., Zuliani F., Piani B., Sepulcri A., and Baldini M. (2019). Yield and quality of essential oils in hemp varieties in different environments. Agronomy 9, 356. doi: 10.1016/j.indcrop.2020.11279
Xu. J., Liu G., Zhao X. X., Zhao S. Q., Ren J., and Hu J. F. (2014). Study on the major and trace elements in soil of Yunnan farmland. Chin. Agric. Sci. Bulletin. 30, 150–154.
Xu G. and Wang R. (2011). Sulfur and boron-magnesium-zinc compound fertilizer contribute to the reproductive growth of jatropha curcas l. J. Plant Nutr. 34, 1843–1852. doi: 10.1080/01904167.2011.600411
Yadav B., Jogawat A., Rahman M. S., and Narayan O. P. (2021). Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 23, 101040. doi: 10.1016/j.genrep.2021.101040
Zhang L., Du G. H., Yang Y., Ouyang W. J., Niu J. L., Li T., et al. (2023). Economic benefits and carbon footprint analysis of industrial hemp cultivation for flowers and leaves in Yunnan. Plant Fiber Sci. China 45, 58–65.
Zhang C., Zhou J. H., Yang R. S., Guo H. H., Yang S. Y., Xia K. B., et al. (2010). Distribution of boron in flue-cured tobacco leaf and soil at different altitude and their relationship in Qujing tobacco growing areas of Yunan province. Acta Tabacaria Sinica 16, 48–53.
Keywords: cannabidiol content, dioecy, sex differences, inflorescence position, microelement fertilization
Citation: Ouyang W, Ou J, Zheng Q, Yang X, Meng Y, Du G, Liu F, Chen Y and Tang K (2025) Agronomic evaluation of hemp (Cannabis sativa L.) for cannabidiol production in Yunnan, China. Front. Agron. 7:1539426. doi: 10.3389/fagro.2025.1539426
Received: 04 December 2024; Accepted: 21 April 2025;
Published: 29 May 2025.
Edited by:
Ioannis Roussis, Agricultural University of Athens, GreeceReviewed by:
Amit Anil Shahane, Central Agricultural University, IndiaAndrew D Cartmill, Massey University, New Zealand
Copyright © 2025 Ouyang, Ou, Zheng, Yang, Meng, Du, Liu, Chen and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuewu Chen, eXVld3UuY2hlbkB5cHJ0ZWMuY29t; Kailei Tang, a2FpbGVpLnRhbmdAeW51LmVkdS5jbg==
†These authors have contributed equally to this work
 Wenjing Ouyang1†
Wenjing Ouyang1† Guanghui Du
Guanghui Du Feihu Liu
Feihu Liu Kailei Tang
Kailei Tang