- 1School of Agriculture and Science, University of KwaZulu-Natal, Pietermaritzburg, South Africa
- 2Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa
- 3Plant Health and Protection, Agricultural Research Council, Hilton, South Africa
Chromolaena odorata, a weed of neotropical origin, remains insufficiently controlled by biological means in South Africa. The stem-galling fly Polymorphomyia basilica was introduced as a potential agent and previously shown to be largely host-specific under no-choice conditions. This study conducted multichoice and no-choice trials to test five nontarget plant species previously selected by P. basilica in no-choice trials, and to assess the fly’s preference and performance on various C. odorata phenotypes, including the southern African biotype (SAB) and the Asian/West African biotype (AWAB). Survival and development rates of P. basilica were highest on C. odorata (SAB) and Ageratum conyzoides. Only a few galls produced adult flies on Stomatanthes africanus and Campuloclinium macrocephalum, and these adults showed low longevity. P. basilica displayed a strong preference for and high performance on C. odorata (SAB) in both trial types, with over 90% of progeny surviving to adulthood. Many larvae also developed successfully on the Taiwan 129/130 (AWAB) and Jamaican 117 phenotypes, whereas development was poorer on other phenotypes. Although the cause of variation among phenotypes remains unclear, the results indicate that P. basilica is a suitable biocontrol agent for C. odorata in South Africa and can sustain populations on the AWAB biotype where it is invasive.
Introduction
Interactions between plants and herbivorous insects have been widely studied due to their significant implications for agriculture and invasive species management (Naranjo et al., 2015; Giron et al., 2018). Herbivorous insects can drastically alter the growth and reproductive capacity of their host plants, often reducing overall vigour (Dube et al., 2019). In particular, insects with highly specific host ranges are excellent candidates for biological control programs targeting invasive alien plants (Dube et al., 2019). Classical biological control involves introducing these host-specific natural enemies from the native range of an invasive plant to reduce its populations in the introduced range (Uyi and Igbinosa, 2013; Schwarzländer et al., 2018).
Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) is among the most problematic invasive alien plants in South Africa and worldwide (Gautier, 1992). It is a semi-woody perennial shrub native to the Americas. The biotype invading southern Africa (SAB) exhibits morphological and genetic differences from C. odorata invading other parts of the Old World humid tropics and subtropics, referred to as the Asian/West African biotype (AWAB) (Paterson and Zachariades, 2013; Uyi et al., 2017). The SAB has a Jamaican/Cuban origin (Zachariades et al., 1999; Paterson and Zachariades, 2013; Shao et al., 2018), whereas the AWAB likely originates from Trinidad and Tobago (Yu et al., 2014; Shao et al., 2018).
Biological control efforts against C. odorata in South Africa began in 1988 (Zachariades et al., 2007). Initially, the performance of some control agents was suboptimal due to phenotypic and genetic differences between the SAB and other C. odorata biotypes (Yu et al., 2014; Zachariades, 2021). While two agents, viz. Calycomyza eupatorivora Spencer (Diptera: Agromyzidae) and Pareuchaetes insulata Walker (Lepidoptera: Erebidae: Arctiinae), have established and are now widely distributed, they remain largely restricted to more moist habitats (Zachariades et al., 2016, 2021). Consequently, C. odorata remains a persistent threat in South Africa, prompting the consideration of additional biocontrol agents. This includes the stem-galling fly Polymorphomyia basilica Snow (Diptera: Tephritidae), which was imported from Jamaica in 2012 (Dube et al., 2020).
Gall-inducing insects such as P. basilica are considered promising biocontrol agents because of their typically narrow host ranges and their adverse effects on the growth and reproduction of host plants (e.g., Hoffmann et al., 2002; Aigbedion-Atalor et al., 2019). Preliminary host-specificity tests indicated that P. basilica is highly specific to C. odorata, although a few other plant species supported limited feeding and development under no-choice conditions (Dube et al., 2020).
Adult progeny were obtained from Campuloclinium macrocephalum (Less.) DC., Ageratina riparia (Regel) R.M. King and H. Rob., Stomatanthes africanus (Oliv. and Hiern) R.M. King and H. Rob., Ageratum conyzoides L., and Felicia amelloides (L.) Voss, whereas galls were initiated, but larvae did not complete development on Adenostemma viscosum J.R. Forst. and G. Forst (Dube et al., 2020). Except for F. amelloides, all these species belong to the same asteraceous tribe (Eupatorieae) as C. odorata. The development of galls and production of adult progeny on F. amelloides was of particular concern, as it belongs to the tribe Astereae, which is more distantly related. The complete development of P. basilica on S. africanus was also worrisome, since this species is indigenous and has a restricted range in South Africa. Consequently, these five plant species were selected for further testing.
The current study, therefore, focused on the preference (determined by adult oviposition, i.e., the number of galled shoots) and performance (larval survival and development times) of P. basilica on the five asteraceous plants selected during earlier adult no-choice trials. Additionally, the study aimed to examine the specificity of P. basilica on several phenotypes of C. odorata from both its native range and other parts of its invasive range. The objective was to assess its suitability and safety for release as a biological control agent in South Africa, as well as its potential for the biological control of AWAB C. odorata.
Materials and methods
Rearing insects and growing plants
Methods for rearing P. basilica and conducting trials on C. odorata were adopted from Mahlobo et al. (2023). Trials were carried out in the glasshouse and quarantine laboratory at the Agricultural Research Council, Plant Health and Protection (ARC-PHP), Cedara, KwaZulu-Natal Province, South Africa. The facilities were maintained at 25°C ± 3°C with a relative humidity of 40% to 70%. Six standard insect cages (0.5 m × 0.5 m × 0.9 m, with steel frames and gauze panels), each with a transparent plastic curtain covering the entrance, were used as breeding cages.
As described in Mahlobo et al. (2023), four potted SAB C. odorata plants were placed in each cage, along with 10 pairs of P. basilica and a small container of enzymatic yeast hydrolase mixed with sugar at a ratio of 1:3. This nutrient mixture supported ovule development and increased female fecundity. Females oviposited in C. odorata shoot tips, inducing gall formation. After 2 weeks, plants with galls were transferred to a large walk-in culturing cage (2 m × 4 m × 2 m) for further larval development and pupation. Eclosing adults were captured in glass vials and used for further culturing and experiments. These methods were considered efficient and have been successfully applied in previous studies (see Wang et al., 2018; Dube et al., 2020).
Plants used were selected from the C. odorata “international collection”, which includes specimens from both the native and adventive ranges of the species. The collection is maintained at ARC-PHP, Cedara. In November 2019, plants were propagated from C. odorata shoot-tip cuttings of SAB (collected from the field in South Africa), four Jamaican varieties (JA 109, 112, 114, and 117), and other phenotypes, including one Venezuelan (VE 126), one Brazilian (BR 27), one Taiwanese (TW 129/130) (representing AWAB), and one Floridian (USA) (FL 108). Campuloclinium macrocephalum was also included, as earlier no-choice trials suggested that P. basilica might develop on this species, indicating its potential as a biocontrol agent for this weed (Dube et al., 2020).
Shoot-tip cuttings were rooted with Seradix® No. 1 in a heated mist bed at the University of KwaZulu-Natal, Agricultural Campus, Pietermaritzburg, and later transferred into labelled 18 cm diameter pots (1:1 river sand and Gromor™ potting medium). Plants were watered daily and fertilised using a fertigation dripper system or Osmocote™ for about three months before use in the trials. Prior to trials, pots were placed in a half-filled water bath (0.6 m × 0.4 m × 0.35 m) for 3 h to eliminate ants, and then spray-washed with water to remove spiders. Plants were then moved to the quarantine glasshouse for the trials.
Multichoice trials using adults on asteraceous plants
Propagation of plants used in these trials followed the methods described above. Trial procedures were adapted from previous multichoice tests on C. connexa (McFadyen et al., 2003; Day et al., 2016). Six species were used, viz. C. odorata, S. africanus, A. riparia, C. macrocephalum, A. conyzoides, and F. amelloides. These species supported some development in earlier trials (Dube et al., 2020). Stomatanthes africanus plants were collected from the field in Mpumalanga (December 2019 and February 2020) and used as soon as they produced new shoots due to propagation difficulty.
Plants with 15–20 actively growing shoot tips were arranged in two rows within the walk-in cage. Plant positions in the cage were randomised using www.random.org/integers/ to minimise spatial bias. For each trial, a vial containing five pairs of fertile P. basilica adults was placed at the centre of the cage. Two individuals of each plant species and a control were exposed to adults for 4 days (Monday to Friday). At 24-h intervals, P. basilica positions were recorded (on plants, cage floors, or walls), plant positions were re-randomised, and any missing or dead adults were replaced to maintain five pairs. On Fridays, adults were recorded, and the plants were transferred to another culture cage. They were inspected on Mondays, Wednesdays, and Fridays for indicators of P. basilica performance, including oviposition, gall formation, larval development, and adult eclosion.
The width and length of galls from which adults had eclosed were measured, and developmental times were recorded. Each trial was replicated five times. Due to limited space, galls from A. conyzoides and C. macrocephalum were removed and placed in labelled Petri dishes once pupation was confirmed (via visible “windows” on the galls). Resulting adults from all the species were used in continuation tests (Dube et al., 2020).
Continuation test
Emerging adults from the test species were used to assess F1 viability, longevity, and fecundity (measured by the number of galls formed by the F1 adults). Adults were tested on the same species from which they had emerged. Up to four pairs of P. basilica were introduced per plant. Plants were replaced after gall formation or if ants were present. Trials were replicated three times, and each trial was terminated 2 weeks after the P. basilica pre-oviposition period or when all adults had died. Replication was not possible for C. macrocephalum and S. africanus because F1 adults died shortly after emergence, preventing gender synchronisation.
All adults were frozen and weighed, and excess adults from C. odorata were returned to the culture. Unfortunately, a Technomyrmex pallipes Smith (Hymenoptera: Formicidae) ant infestation occurred during the trial, causing premature adult mortality, which was recorded.
Risk analysis
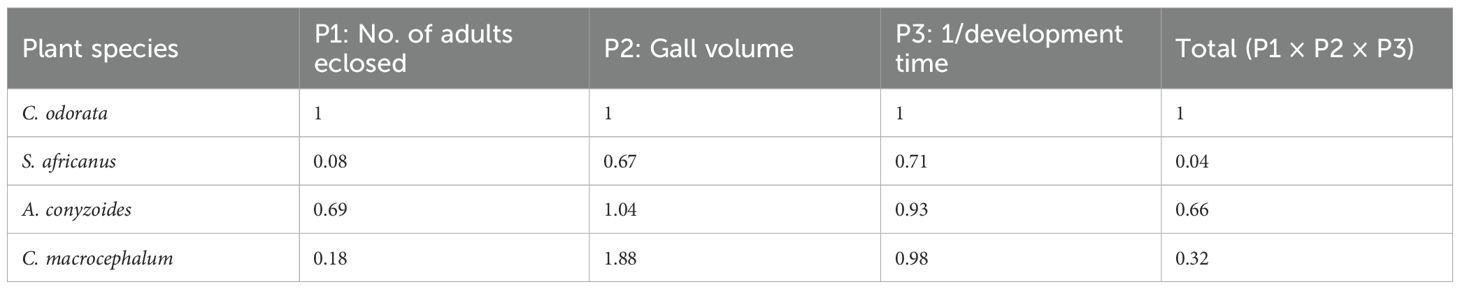
A risk analysis (sensu Wan and Harris, 1997; as applied by Olckers, 2000 and McConnachie, 2015) was conducted using data from the multichoice trials for three parameters: survival to adulthood, gall size, and development rate. Survival was defined as the number of adults that eclosed per species, and gall size was measured as volume. Development rate was assessed by summing the median larval and pupal development times (in days). As shorter development indicates higher fitness, the inverse of total development times (1/number of days from egg to adults) was calculated for each plant species. Values for C. odorata were standardised to one, and those of the other test plants were expressed as proportions (e.g., two adults from a test plant vs. four from C. odorata = 0.5). These proportional values were then multiplied to obtain a single, final comparative measure of risk.
Multichoice and no trials on C. odorata phenotypes and C. macrocephalum
In February 2020, eclosed P. basilica adults were collected from the culturing walk-in cage and introduced into two standard breeding cages containing four SAB plants and enzymatic yeast hydrolase for 1 week to ensure sexual maturity (Dube et al., 2020). Subsequent trials tested the propagated C. odorata phenotypes and C. macrocephalum, each with 12–20 growing shoots. In each trial, two plants from each accession and C. macrocephalum were randomly placed in a walk-in cage (1.2 m × 2.2 m × 1.9 m), along with 10 pairs of P. basilica adults, for 4 days (Monday to Friday). Plants were watered daily, and their positions were rotated. Missing or dead adults were recorded and replaced to maintain 10 pairs. Each trial was replicated five times.
Campuloclinium macrocephalum was included in these trials because adult progeny had emerged from this species during earlier host-range tests (Dube et al., 2020). Since C. macrocephalum is invasive in South Africa, it was hypothesised that P. basilica could serve as a biological control agent for this species.
On Fridays, plants were transferred to a separate 2 m × 4 m × 2 m walk-in cage for larval development and inspected from day three. The number of galls per phenotype was recorded. However, due to the coronavirus disease (COVID-19; Nidovirales: Coronaviridae) lockdown (27 March–16 April 2020), detailed development data could not be collected. After the lockdown, plants were inspected three times per week for survival to adulthood, which was assessed by the number of galls showing a “window” for pupation and galls with an open window for adult eclosion.
Gall lengths were measured, including those with open windows, which indicated adult emergence. The remaining galls with windows were removed from the plants and placed in labelled Petri dishes to track survival to eclosion. Adults in the walk-in cage were captured and transferred to the general culture maintenance walk-in cage.
In no-choice trials, two plants from each phenotype and two C. macrocephalum plants were placed separately in breeding cages, each provided with enzymatic yeast hydrolase and three pairs of P. basilica for 4 days (Monday to Friday). Missing or dead adults were replaced daily. Plants were then transferred to a walk-in cage for larval development and inspected for gall formation. Development times were recorded daily, except on weekends. Upon pupation, plants were covered with gauze sleeves (0.95 m × 0.4 m) to capture adults. Upon emergence, gall dimensions (width and length) were measured, adults were frozen and weighed (using an electronic balance), and their wing length was measured (using an ocular micrometre on a dissecting microscope). Wing length and mass of flies are typically correlated, allowing estimation of body size (Navarro-Campos et al., 2011). The trials were replicated three times.
Data analysis
In both multichoice and no-choice trials, female preference (and viability in the continuation tests) was measured by the number of galls developing on each plant, including SAB C. odorata, other phenotypes, and the asteraceous plants used. Offspring performance was determined by tracking the survival of the P. basilica across life stages, from egg to pupation and adult eclosion, along with developmental time. Gall size was represented as volume, calculated by assuming the gall to be cylindrically shaped, using V = πr2h, where r was the width/2 and h was the length of the gall.
Differences in the number of P. basilica galls formed, galls that reached pupation, and galls with adult eclosion were analysed using a Generalised Linear Model (GLM) with a negative binomial distribution and a log-link function. Gall size, adult weights, and wing lengths were analysed using a Generalised Linear Model with a Gaussian distribution and identity link function. C. odorata was set as the reference category for comparisons among non-C. odorata asteraceous plants, and SAB was the reference category when testing differences between C. odorata phenotypes and C. macrocephalum. All analyses were performed in Python using the statsmodels package (Seabold and Perktold, 2010). The normality of residuals was assessed using the Shapiro–Wilk test and by visually inspecting Q–Q plots. Data were also checked for clamping by examining residual vs. fitted value plots.
Results
The number of P. basilica pairs used was sufficient, and most adults survived throughout the trial period. Healthy, newly eclosed adults emerged from most galls.
Multichoice trials using adults on asteraceous plants
Compared to the other asteraceous plant species, C. odorata had the highest number of galls laid on it (coefficient = − 1.741; p < 0.001). Ageratum conyzoides (coefficient = − 0.018; p = 0.971) and C. macrocephalum (coefficient = − 0.305; p = 0.534) had slightly lower numbers of galls, but these did not differ significantly from C. odorata (Figure 1). In contrast, S. africanus had a significantly lower number of galls (coefficient = − 1.401; p = 0.009), and no galls were recorded on A. riparia and F. amelloides (Figure 1). These species (A. riparia and F. amelloides) were excluded from all analyses.
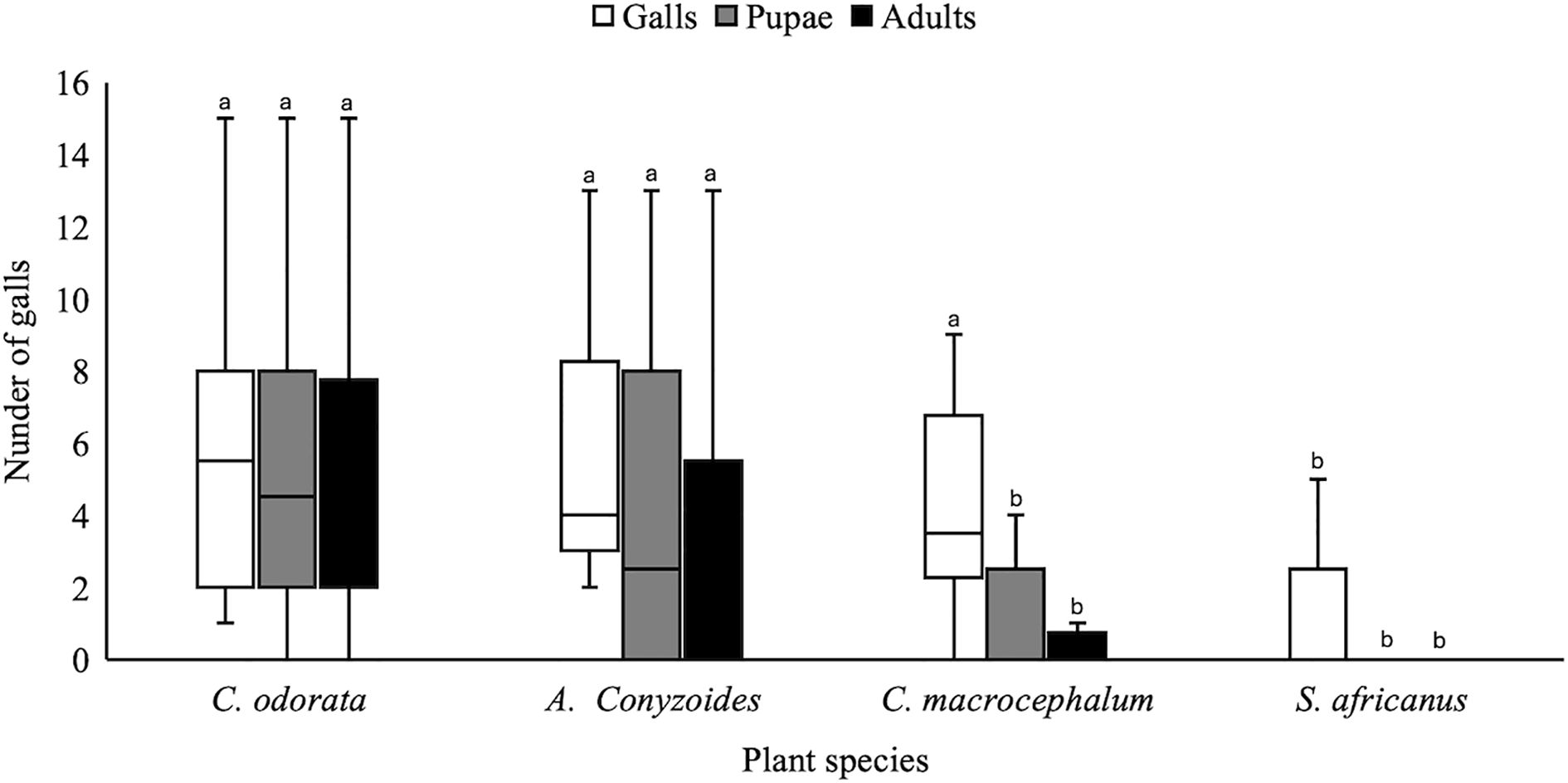
Figure 1. Gall parameters of Polymorphomyia basilica from multichoice trials on asteraceous plants. Different lowecase letters denote a significant difference between C. odorata (SAB) and the other asteraceae/ phenotypes.
High pupation rates were recorded on C. odorata (coefficient = 1.668; p ≤ 0.001), followed by A. conyzoides (coefficient = − 0.233; p = 0.637), which did not differ significantly from C. odorata. In contrast, both C. macrocephalum (coefficient = − 1.668; p = 0.003) and S. africanus (coefficient = − 2.279; p ≤ 0.001) had a significantly low number of galls reaching pupation (Figure 1).
The highest number of galls with adults eclosed was recorded on C. odorata (coefficient = 1.629; p < 0.001), followed by A. conyzoides (coefficient = − 0.377; p = 0.450), which did not significantly differ from C. odorata. In contrast, a significantly low number of adults eclosed from the galls laid on C. macrocephalum (coefficient = − 1.735; p = 0.003) and S. africanus (coefficient = − 2.546; p < 0.001) (Figure 1). Notably, adults from C. macrocephalum and S. africanus were found dead on the first day after eclosion (see continuation tests below).
Campuloclinium macrocephalum (coefficient = 54.963; p < 0.001) developed significantly larger galls compared to C. odorata (coefficient = 76.351; p < 0.001). The galls on A. conyzoides (coefficient = − 1.476; p = 0.869) did not differ significantly from those on C. odorata. Meanwhile, S. africanus (coefficient = − 26.509; p = 0.160) had the smallest galls, although this difference was also not statistically significant compared to C. odorata (Figure 2). Larval development was fastest on C. odorata, followed by A. conyzoides, then C. macrocephalum. Polymorphomyia basilica took the longest to develop on S. africanus (Table 1).
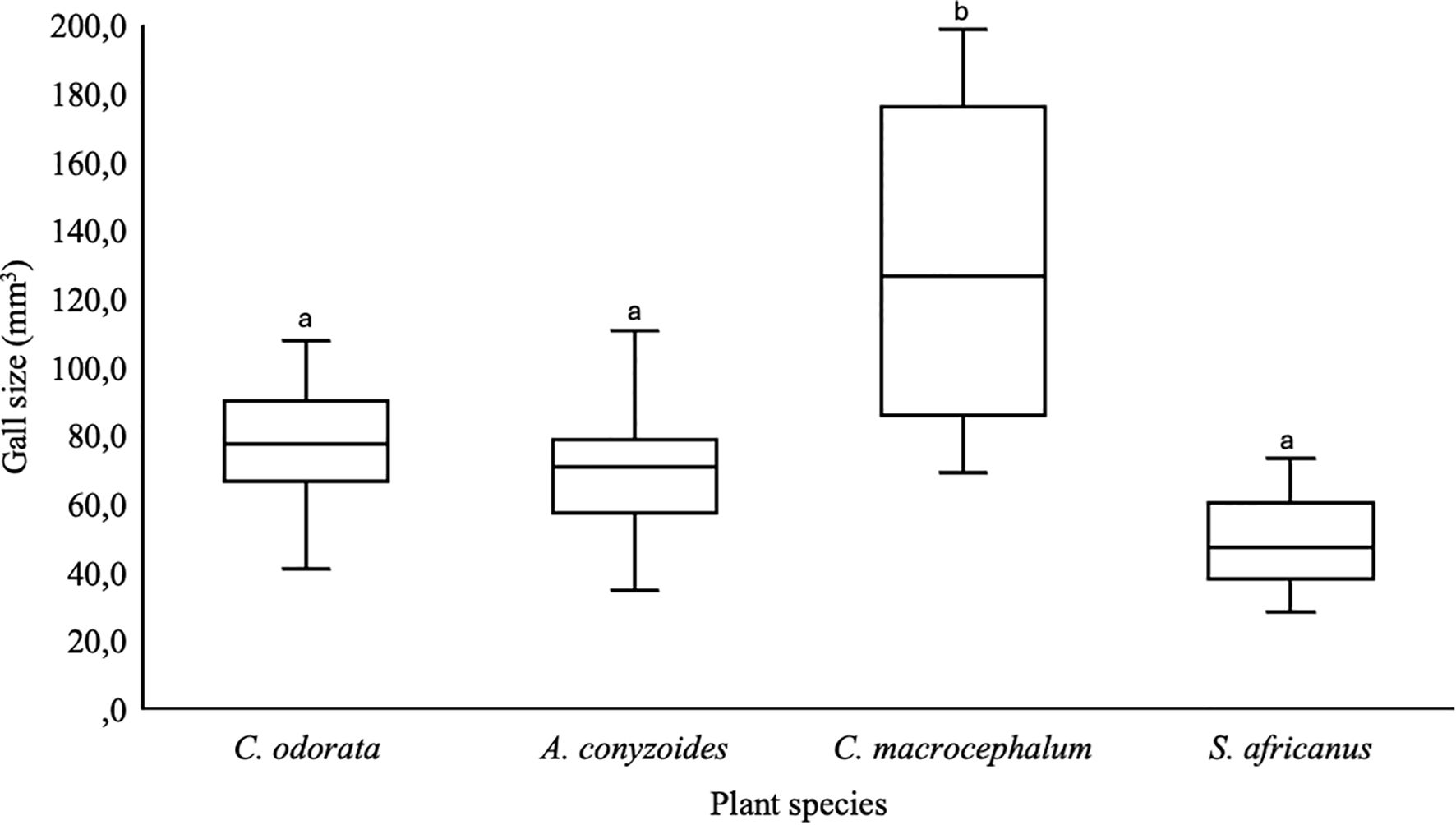
Figure 2. Gall size of Polymorphomyia basilica on asteraceous plants. Different lowecase letters denote a significant difference between C. odorata (SAB) and the other asteraceae/ phenotypes.
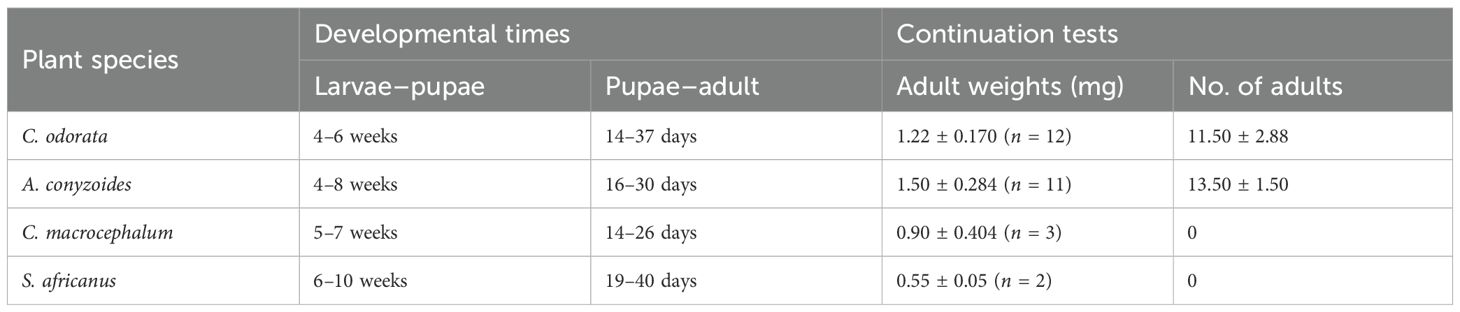
Table 1. Developmental time and survival of Polymorphomyia basilica in multichoice trials with asteraceous plants.
Continuation tests
A few adults in these trials were found dead prematurely. Although T. pallipes was recorded in some cages across all plant species, it is generally not aggressive, so it is unclear whether the ants contributed to the observed P. basilica mortalities. For example, five adults were found dead in the C. odorata cage the day after the trial was set up. Similarly, on two separate occasions, seven adults were found dead in the A. conyzoides cage on the second day after setup. Additionally, one adult was found dead in the C. macrocephalum cage.
In the S. africanus cage, two adults were missing on two occasions, and ants were present. However, ants were also observed in the C. odorata and A. conyzoides cages on other occasions, yet the adults remained alive and continued laying viable offspring until the trial was terminated.
Most P. basilica adults ex C. odorata survived for more than 2 weeks after the pre-oviposition period and oviposited, with an average of 11.5 galls forming on C. odorata. Adults ex A. conyzoides also survived and oviposited, producing an average of 13.5 galls on this species (Table 1). No galls were recorded on C. macrocephalum or S. africanus (Table 1). Adults from C. macrocephalum were either dead or missing, and those from S. africanus died soon after the trials were set up, despite the absence of ants in the cages.
There was no significant difference in the weights of F1 adults from C. odorata (coefficient = 1.217; p < 0.001) and A. conyzoides (coefficient = 0.283; p = 0.360). Adults from C. macrocephalum (coefficient = − 0.317; p = 0.0508) and S. africanus (coefficient = − 0.717; p = 0.034) weighed less, but only adults from S. africanus differed significantly from C. odorata (Table 1).
Risk analysis
This analysis indicated that, compared to C. odorata, A. conyzoides posed the highest utilisation risk, followed by C. macrocephalum, while S. africanus had the lowest risk (Table 2).
Multichoice trial on C. odorata phenotypes and C. macrocephalum
Galls of P. basilica were observed on all C. odorata phenotypes used in the trial, as well as on C. macrocephalum. The highest number of galls per plant was recorded on the SAB phenotype (coefficient = 2.272; p < 0.001), followed by the JA 117 (coefficient = − 0.203; p = 0.625) phenotype (Figure 3). Only the JA 109 (coefficient = − 0.991; p = 0.042) and JA 114 (coefficient = − 1.174; p = 0.017) phenotypes had a significantly lower number of galls compared to the SAB (Figure 4). The number of galls on the TW129/130 phenotype (coefficient = 0.586; p = 0.221) and on C. macrocephalum (coefficient = − 0.791; p = 0.102) was lower but did not differ significantly from the galls on SAB (Figure 4).
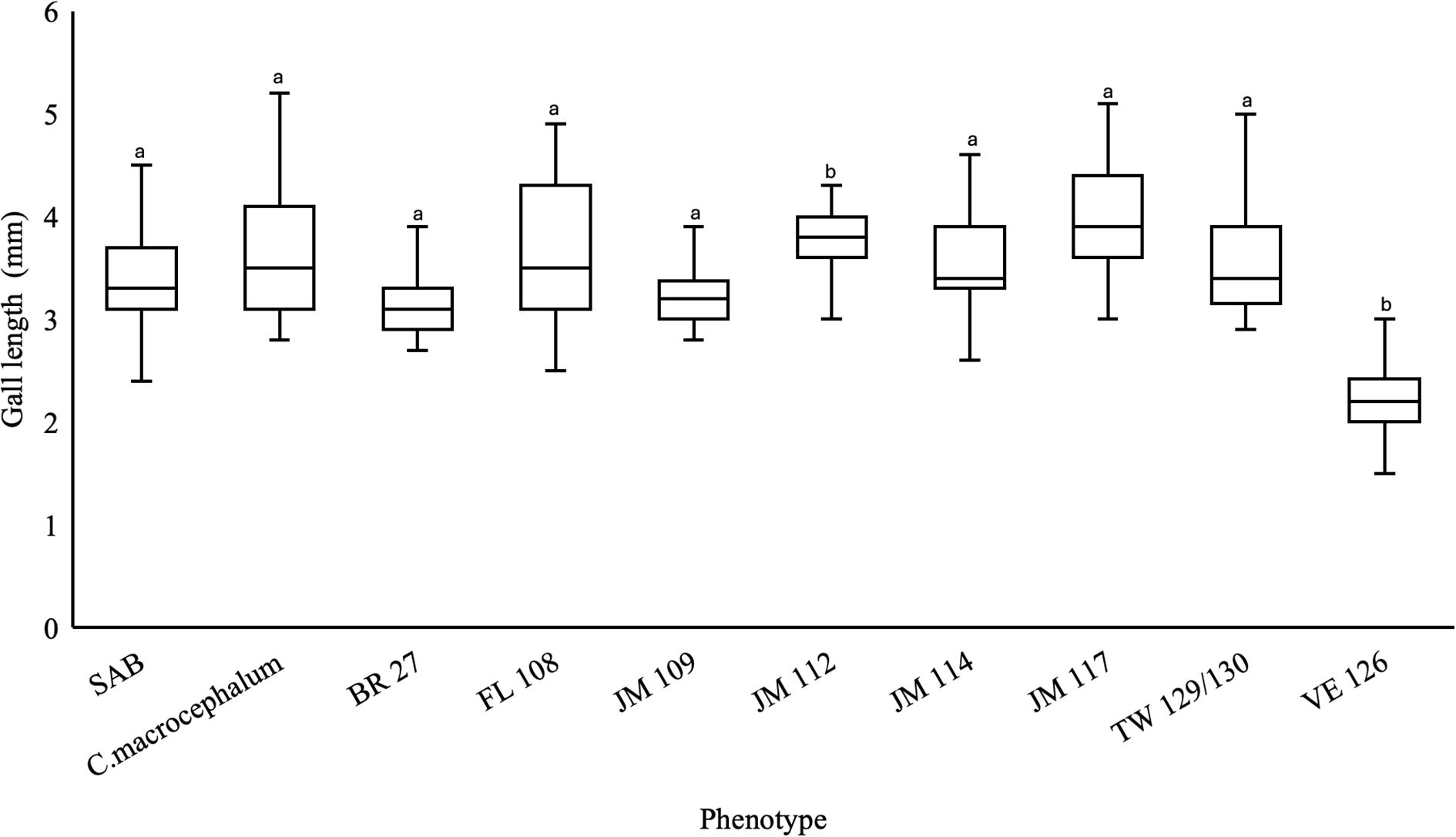
Figure 3. Gall length of Polymorphomyia basilica in multichoice trials with C. odorata phenotypes and C. macrocephalum. Different lowecase letters denote a significant difference between C. odorata (SAB) and the other asteraceae/ phenotypes.

Figure 4. Gall parameters of Polymorphomyia basilica in multichoice trials with C. odorata phenotypes and C. macrocephalum. Different lowecase letters denote a significant difference between C. odorata (SAB) and the other asteraceae/ phenotypes.
On average, over half of the galls formed across the different phenotypes developed pupation windows. Galls on the SAB phenotype had the highest rate, at approximately 94% (coefficient = 2.208; p < 0.001). Similarly, galls on C. macrocephalum (coefficient = − 0.822; p = 0.091) and the TW129/130 (coefficient = − 0.727; p = 0.133) also showed high pupation rates, at approximately 91% and 92%, respectively. However, five C. odorata phenotypes, viz. BR 27 (coefficient = − 0.985; p = 0.045), FL 108 (coefficient = − 1.556; p = 0.002), JA 109 (coefficient = − 1.252; p = 0.012), JA 114 (coefficient = − 1.253; p = 0.012), and VE 126 (coefficient = − 1.109; p = 0.025) differed significantly from the SAB (Figure 4). Only 45% of the larvae in galls on FL 108 proceeded to pupation, compared to the 94% pupation observed on the SAB.
Compared to the SAB, the number of adults emerging from the galls was lower in all phenotypes. Only the TW 129/130 (coefficient = − 0.922; p = 0.061) and the JA 117 (coefficient = − 0.612; p = 0.206) phenotypes did not differ significantly from the SAB (coefficient = 2.112; p < 0.001) (Figure 3). The number of adults emerging from galls on C. macrocephalum (coefficient = − 1.200; p = 0.017) was significantly lower than that from the SAB. Less than 55% of the galls on C. macrocephalum produced adults, and most of those adults were found dead or moving slowly in the Petri dishes. The C. odorata phenotypes from Venezuela (VE 126), the USA (FL 108), and JA 109 were the least preferred hosts, with only 14.2% (coefficient = − 2.339; p < 0.001), 21.4% (coefficient = − 2.222; p = 0.014), and 41.7% (coefficient = − 2.222; p < 0.001) of galls producing adults, respectively (Figure 4).
Galls of Polymorphomyia basilica formed on the VE 126, BR 27, and JA 109 phenotypes were shorter than those on SAB (coefficient = 3.381; p < 0.001); however, only the galls on VE 126 were significantly shorter than those on SAB (coefficient = − 1.147; p < 0.001). In contrast, galls on other C. odorata phenotypes were longer, with only JA 112 (coefficient = 0.445; p < 0.001) and JA 117 (coefficient = 0.631; p < 0.001) differing significantly from SAB. Galls on C. macrocephalum were also significantly longer (coefficient = 0.3064; p = 0.008) (Figure 3).
No-choice trials on C. odorata phenotypes and C. macrocephalum
The no-choice trial showed a trend similar to the multichoice trials regarding P. basilica preference. The SAB phenotype had the highest number of galls (coefficient = 2.708; p < 0.001), followed by JA 117 (coefficient = − 0.693; p = 0.252) and TW 129/130 (coefficient = − 0.762; p = 0.209). Significantly fewer galls were recorded on FL 108 (coefficient = − 1.667; p = 0.009), JA 112 (coefficient = − 1.408; p = 0.024), and VE 126 (coefficient = − 1.935; p = 0.003) (Figure 5). The difference in the number of galls formed between the SAB and C. macrocephalum was not significant (coefficient = − 1.204; p = 0.051) (Figure 5).

Figure 5. Gall parameters of Polymorphomyia basilica in no-choice trials with C. odorata phenotypes and C. macrocephalum. Different lowecase letters denote a significant difference between C. odorata (SAB) and the other asteraceae/ phenotypes.
Compared to the SAB (coefficient = 2.663; p < 0.001), only JA 117 (coefficient = − 0.741; p = 0.223) and TW 129/130 (coefficient = − 0.791; p = 0.194) had a similar number of galls in which larvae reached pupation (Figure 5). On C. macrocephalum (coefficient = − 2.257; p = 0.001), only 33% of the galls formed showed pupation windows, compared to 94.3% on the SAB. All other C. odorata phenotypes had a significantly lower number of galls developing pupation windows.
Regarding adult emergence, no phenotype had a similar number of adults emerging from the galls as the SAB (coefficient = 2.651; p < 0.001). Adult flies eclosed from 90% of the galls on the SAB, followed by 57.1% on JA 117 (coefficient = − 1.265; p = 0.042) and 42.5% on TW 129/130 (coefficient = − 1.804; p = 0.005), although these phenotypes differed significantly from the SAB. In all other C. odorata phenotypes and on C. macrocephalum (coefficient = − 2.833; p < 0.001), fewer than 40% of the galls produced adults (Figure 5).
Galls formed on C. odorata from Brazil (BR 27) (coefficient = − 37.046; p = 0.016) were significantly smaller than those formed on the SAB (coefficient = 82.471; p < 0.001). In contrast, galls on TW 129/130 (coefficient = 20.241; p = 0.017), JA 117 (coefficient = 28.600; p < 0.001), and FL 108 (coefficient = 44.516; p = 0.007) were significantly larger (Figure 6a). The size of galls laid on C. macrocephalum (coefficient = 1.6282; p = 0.921) was similar to that on the SAB.

Figure 6. Gall size (a), adult weights (b), and wing length (c) of Polymorphomyia basilica in a no-choice trial with C. odorata phenotypes and C. macrocephalum. Different lowecase letters denote a significant difference between C. odorata (SAB) and the other asteraceae/phenotypes.
Larval development was fastest on the TW 129/130 phenotype. On average, P. basilica completed development in approximately 51 days on the TW 129/130 phenotype, compared to ~ 66 days on the SAB. In contrast, galls on all other C. odorata phenotypes and on C. macrocephalum took longer than the SAB to progress through the observed life stages and complete development. Galls on the SAB began developing pupation windows as early as 24 days after trial setup, with some adults emerging 7 days later. Galls on phenotypes such as BR 27 and VE 126 only began showing signs of pupation around 40 days posttermination. Similarly, galls on C. macrocephalum also took longer than those on the SAB to reach pupation and complete development (Table 3). Additionally, adults emerging from C. macrocephalum were found dead or moving slowly on the gauze sleeve.
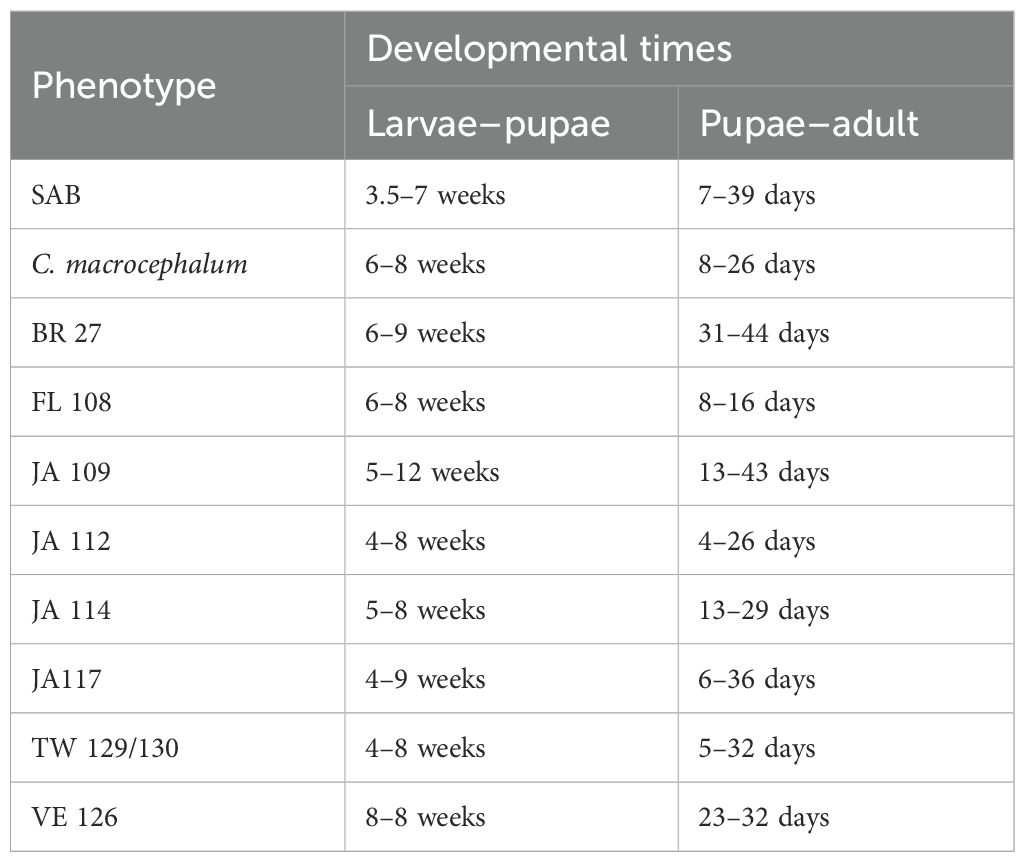
Table 3. Developmental times of Polymorphomyia basilica in no-choice trials with C. odorata phenotypes and C. macrocephalum.
P. basilica adults that eclosed from the JA 109 phenotype (coefficient = − 0.459; p = 0.030) weighed significantly less than those emerging from galls on the SAB (coefficient = 1.439; p < 0.001). Adults eclosing from all other C. odorata morphotypes and C. macrocephalum (− 0.359; p = 0.295) weighed less but did not differ significantly from those emerging on the SAB (Figure 6b).
Polymorphomyia basilica adults emerging from the BR 27 (coefficient = − 0.539; p < 0.001), JM 109 (coefficient = − 0.158; p = 0.0220), and VE 126 (coefficient = − 0.396; p < 0.001) phenotypes were significantly smaller in wing length than those that emerged from the SAB (coefficient = 3.099; p < 0.001). Conversely, significantly larger adults emerged from the JA 112 phenotype (coefficient = 0.185; p = 0.004). Adults emerging from C. macrocephalum (coefficient = − 0.172; p = 0.124) did not differ significantly from those emerging from the SAB (Figure 6c).
Discussion
This study examined the ability of A. conyzoides, C. macrocephalum, S. africanus, A. riparia, and F. amelloides to support the development of P. basilica, compared to its target weed, C. odorata. Galls developed and adults eclosed on C. odorata, A. conyzoides, C. macrocephalum, and S. africanus, but no galls formed on A. riparia and F. amelloides. Felicia amelloides is phylogenetically more distantly related (tribe Astereae) to C. odorata than the other four test species, which all belong to the Eupatorieae tribe along with C. odorata (Panero and Crozier, 2016). Among the Eupatorieae species tested, A. riparia was the only one on which galls did not develop.
As previously shown by Dube et al. (2020), oviposition and gall formation on A. conyzoides, C. macrocephalum, and S. africanus may be attributed to the presence of pyrrolizidine alkaloids, which are common in most Eupatorieae and to which the herbivores of C. odorata are adapted (Hartmann, 2009). Additionally, several biological control agents of C. odorata have been observed selecting A. conyzoides during host-specificity tests or in the field (Kluge and Caldwell, 1993; Dube et al., 2017). These include Conotrachelus reticulatus (Coleoptera: Curculionidae) (Delgado et al., 2014), Dichrorampha odorata (Lepidoptera: Tortricidae) (Dube et al., 2017), as well as Pareuchaetes insulata and Pareuchaetes pseudoinsulata Rego Barros (Walker) (Lepidoptera: Arctiidae) (de Chenon et al., 2002, Zachariades et al., 2011).
The survival and development rates of P. basilica were highest on C. odorata and A. conyzoides, lower on C. macrocephalum, and lowest on S. africanus. This aligns with the results of previous no-choice trials, where P. basilica survival was minimal on S. africanus, and adults did not live more than 1 day after eclosion from the plants (Dube et al., 2020). These findings indicate that S. africanus and C. macrocephalum are less preferred hosts. Continuation tests supported multichoice results, as adult progeny from S. africanus and C. macrocephalum died on the first day after emergence. In contrast, A. conyzoides and C. odorata successfully supported the complete development of P. basilica, even when ants were present.
Risk analysis indicated that, compared to C. odorata, only A. conyzoides is a reasonably suitable host, while C. macrocephalum and S. africanus are much less suitable. Since A. conyzoides and C. macrocephalum are native to the Americas and invasive in South Africa, their attack by P. basilica is of no concern. Stomatanthes africanus presents minimal risk. Additionally, it occurs in high-altitude, colder grasslands in Mpumalanga and Limpopo provinces (Retief, 2002), with no overlap in distribution with C. odorata.
This study also examined the oviposition preference and offspring performance of P. basilica across different phenotypes of C. odorata and C. macrocephalum. In the laboratory, P. basilica preferred and performed best on the SAB C. odorata, which showed the highest oviposition rates, fastest development, and lowest mortality in both multi- and no-choice trials. P. basilica also performed reasonably well on the JA 117 phenotype, which closely resembles the SAB. For some parameters, including the number of galls formed, pupation rates, and developmental times, P. basilica performed well on the TW 129/130 = AWAB phenotype.
In contrast, low oviposition, high gall mortality, and slow development rates were observed in the other phenotypes from Jamaica and other regions of the native range of C. odorata. While C. odorata from Florida and the South American mainland have been shown to differ genetically from the SAB, no genetic differences were detected between the SAB and Jamaican plants (Paterson and Zachariades, 2013; Shao et al., 2018).
The observed increase in preference and performance of P. basilica on the SAB, compared to most Jamaican phenotypes, could be explained by the Evolution of Increased Competitive Ability (EICA) hypothesis. The EICA hypothesis proposes that when plants are introduced to new areas without natural enemies, they often reallocate resources from defence to growth and development (Blossey and Notzold, 1995; Handley et al., 2008). Consequently, these plants may become more susceptible to their natural enemies upon reintroduction (Handley et al., 2008). Dube (2019) found some support for this in SAB plants collected from field sites in South Africa with and without prior exposure to P. insulata—some plant growth and reproductive metrics were lower in plants with exposure to the moth, indicating that SAB may have regained some of its defence mechanisms since the moth’s introduction. Alternatively, the laboratory colony of P. basilica may have evolved since its introduction in 2012—as it has been continuously reared on SAB since then. It may have adapted to it, resulting in higher preference and performance metrics than those observed on other C. odorata phenotypes.
Polymorphomyia basilica formed galls on all the C. odorata phenotypes tested, indicating that these lie within the fly’s physiological host range. However, host selection for oviposition may be influenced by encounter rate, which provides limited information about the suitability of a host plant (Singer, 1986). Insects tend to be more discriminating when the likelihood of encountering a previously preferred host is high (Singer, 1986; van Driesche and Reardon, 2004). In the current study, this is reflected by the lower average number of galls formed per plant on the other phenotypes during multichoice trials compared to the means observed in no-choice trials.
The results showed that P. basilica larvae exhibited differences in performance across the various C. odorata phenotypes and C. macrocephalum. Phenotypes that enhanced offspring survival, such as SAB, JA 117, and TW 129/130, were preferred, and larval growth rates were faster on these plants. In contrast, P. basilica larvae experienced higher mortality and slower development on C. odorata phenotypes from Brazil and Venezuela, making these phenotypes less suitable. Similarly, C. macrocephalum proved to be a poor host for P. basilica, with larvae taking longer to develop and adults exhibiting low survival rates.
In some fruit flies, adult size is an indicator of fitness, and insects tend to be smaller when developing on low-quality host plants (Beukeboom, 2018; Navarro-Campos et al., 2011). This is consistent with the findings of the present study, where P. basilica adults emerging from the BR 27, JA 109, and VE 126 phenotypes were smaller. These phenotypes also had some of the lowest numbers of galls laid and high larval mortality. This supports the “Mother knows best” hypothesis, which posits that females prefer host plants that enhance offspring fitness, resulting in lower gall mortality and shorter developmental time (Santos et al., 2008; Balagawi et al., 2013; de la Masselière et al., 2017). However, this differed for P. basilica on the TW 120/130 phenotype, where many galls survived and adults eclosed in the shortest time, yet the emerging adults had low mass.
The results of this study indicate that host use by herbivorous insects differs from host suitability. Although a positive correlation between preference and performance exists, availability and abundance may influence preference. When insects are introduced into a novel habitat, ecological traps are bound to occur as they try to adapt and apply the Mother Knows Best theory (Hale and Swearer, 2016). The Mother Knows Best hypothesis does not consider ecological shifts that occur over time (García-Robledo and Horvitz, 2012). Therefore, future studies should continue to focus on offspring performance on different host plants (Singer, 1986; Balagawi et al., 2013; Hafsi et al., 2016).
Although this was not tested in the current study, it was apparent that host plants influence the life-history characteristics of the larvae and adults. Plant differences, i.e., physical and chemical composition, result in different attraction levels (Heard and Cox, 2009). The variation in attraction among the phenotypes from Jamaica indicates that these differences may involve more than just physical traits.
Conclusion
It is proposed that P. basilica is sufficiently host-specific for release as a biocontrol agent of C. odorata in South Africa. Although A. conyzoides and C. macrocephalum may experience some oviposition and damage from P. basilica, this is not considered a concern, as both species are declared invaders under NEMBA. Moreover, C. macrocephalum will not support the development or sustain populations of P. basilica in the field.
The risk of releasing P. basilica is minimal compared to its benefits, as demonstrated by its biological performance on C. odorata (Mahlobo et al., 2023). The agent also merits consideration in countries where the AWAB is invasive, since P. basilica showed substantial development on this biotype.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TM: Funding acquisition, Writing – original draft, Investigation, Data curation, Formal analysis, Visualization. ND: Validation, Resources, Writing – review & editing, Conceptualization, Project administration, Supervision, Methodology. CZ: Methodology, Conceptualization, Writing – review & editing, Validation, Supervision, Resources, Project administration. TCM: Project administration, Validation, Supervision, Writing – review & editing, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The DST-NRF Centre of Excellence for Invasion Biology (C·I·B) financially supported the research.
Acknowledgments
The Agricultural Research Council-Plant Health and Protection is thanked for access to the facilities and materials needed to complete these trials. ARC-PHP Cedara staff are also thanked for their assistance in breeding the fly and for watering plants whenever the first author was unavailable.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aigbedion-Atalor P. O., Day M. D., Idemudia I., Wilson D. D., and Paterson I. D. (2019). With or without you: stem-galling of a tephritid fly reduces the vegetative and reproductive performance of the invasive plant Chromolaena odorata (Asteraceae) both alone and in combination with another agent. BioControl 64, 103–114. doi: 10.1007/s10526-018-09917-x
Balagawi S., Drew R. A., and Clarke A. R. (2013). Simultaneous tests of the preference-performance and phylogenetic conservatism hypotheses: is either theory useful? Arthropod-Plant Interact. 7, 299–313. doi: 10.1007/s11829-012-9244-x
Beukeboom L. W. (2018). Size matters in insects–an introduction. Entomol. Experimentalis Applicata 166, 2–3. doi: 10.1111/eea.12646
Blossey B. and Notzold R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J. Ecol. 83, 887–889. doi: 10.2307/2261425
Day M. D., Riding N., and Senaratne K. A. D. W. (2016). The host specificity and climatic suitability of the gall fly Cecidochares connexa (Diptera: Tephritidae), a potential biological control agent for Chromolaena odorata (Asteraceae) in Australia. Biocontrol Sci. Technol. 26, 691–706. doi: 10.1080/09583157.2016.1151477
de Chenon R. D., Sipayung A., and Sudharto P. (2002). “A decade of biological control against Chromolaena odorata at the Indonesian Oil Palm Research Institute in Marihat,” in Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata. Agricultural Research Council-Plant Protection Research Institute, Pretoria, South Africa. Eds. Zachariades C., Muniappan R., and Strathie L. W., 46–52.
de la Masselière M. C., Facon B., Hafsi A., and Duyck. P. F. (2017). Diet breadth modulates preference-performance relationships in a phytophagous insect community. Sci. Rep. 7, 1–9. doi: 10.1038/s41598-017-17231-2
Delgado O. S., Strathie L. W., and Zachariades C. (2014). “Host range of Conotrachelus reticulatus, a potential biological control agent for Chromolaena odorata,” in Proceedings of the XIV International Symposium on Biological Control of Weeds. Eds. Impson F. A. C., Kleinjan C. A., and Hoffman J. H. (Kruger National Park, South Africa), 31–40.
Dube N. (2019). Understanding the fitness, preference and performance of specialist herbivores of the southern African biotype of Chromolaena odorata (Asteraceae), and impacts on phytochemistry and growth rate of the plant. Unpublished PhD Thesis. University of KwaZulu Natal, Pietermaritzburg, South Africa.
Dube N., Uyi O., Zachariades C., Munyai T. C., and Whitwell. M. (2019). Impact of the shoot-boring moth Dichrorampha odorata (Lepidoptera: Tortricidae) on growth and reproductive potential of Chromolaena odorata (Asteraceae) in the laboratory. Biocontrol Sci. Technol. 29, 350–364. doi: 10.1080/09583157.2018.1562038
Dube N., Zachariades C., Munyai T. C., and Uyi O. O. (2017). Laboratory studies on the biology and host range of Dichrorampha odorata (Lepidoptera: Tortricidae), a biological control agent for Chromolaena odorata (Asteraceae). Biocontrol Sci. Technol. 27, 222–236. doi: 10.1080/09583157.2016.1274879
Dube N., Zachariades C., Uyi O., and Munyai T. C. (2020). Life history traits and host suitability of a gall-forming fly, Polymorphomyia basilica (Diptera: Tephritidae), for the biological control of Chromolaena odorata (Asteraceae) in South Africa. Arthropod-plant Interact. 14, 237–250. doi: 10.1007/s11829-019-09731-x
García-Robledo C. and Horvitz C. C. (2012). Parent–offspring conflicts,”optimal bad motherhood” and the “mother knows best” principles in insect herbivores colonizing novel host plants. Ecol. Evol. 2, 1446–1457. doi: 10.1002/ece3.267
Gautier L. (1992). Taxonomy and distribution of a tropical weed: Chromolaena odorata (L.) R. King & H. Robinson. Candollea 47, 645–662.
Giron D., Dubreuil G., Bennett A., Dedeine F., Dicke M., Dyer L. A., et al. (2018). Promises and challenges in Insect-plant interactions. Entomol. Experimentalis Applicata 166, 319–343. doi: 10.1111/eea.12679
Hafsi A., Facon B., Ravigné V., Chiroleu F., Quilici S., Chermiti B., et al. (2016). Host plant range of a fruit fly community (Diptera: Tephritidae): does fruit composition influence larval performance? BMC Ecol. 16, 1–12. doi: 10.1186/s12898-016-0094-8
Hale R. and Swearer S. E. (2016). Ecological traps: current evidence and future directions. Proc. R. Soc. B.: Biol. Sci. 283, 20152647. doi: 10.1098/rspb.2015.2647
Handley R. J., Steinger T., Treier U. A., and Müller-Schärer H. (2008). Testing the evolution of increased competitive ability (EICA) hypothesis in a novel framework. Ecology 89, 407–417. doi: 10.1890/07-0160.1
Hartmann T. (2009). “Pyrrolizidine alkaloids: the successful adoption of a plant chemical defense,” in Tiger moths and woolly bears. Behavior, ecology and evolution of the Arctiidae. Ed. Conner W. E. (Oxford University Press, New York), 55–81.
Heard S. B. and Cox G. H. (2009). Plant module size and attack by the goldenrod spindle-gall moth. Can. Entomol. 141, 406–414. doi: 10.4039/n09-016
Hoffmann J. H., Impson F. A. C., Moran V. C., and Donnelly D. (2002). Biological control of invasive golden wattle trees (Acacia pycnantha) by a gall wasp, Trichilogaster sp.(Hymenoptera: Pteromalidae), in South Africa. Biol. Control 25, 64–73. doi: 10.1016/S1049-9644(02)00039-7
Https://www.random.org/integers/. Available online at: https://www.random.org/integers/ (Accessed 28 January 2021).
Kluge R. P. and Caldwell P. M. (1993). Host specificity of Pareuchaetes insulata (Lep.: Arctiidae), a biological control agent for Chromolaena odorata (Compositae). Entomophaga 38, 451–457. doi: 10.1007/bf02373077
Mahlobo T., Dube N., Zachariades C., and Munyai T. C. (2023). Impact of the gall-inducing fly Polymorphomyia basilica Snow (Diptera: Tephritidae) on the growth and reproduction of Chromolaena odorata (L.) RM King & H. Rob (Asteraceae) in the laboratory. Arthropod-Plant Interact. 17, 639–646. doi: 10.1007/s11829-023-09985-6
McConnachie A. J. (2015). Host range and risk assessment of Zygogramma bicolorata, a defoliating agent released in South Africa for the biological control of Parthenium hysterophorus. Biocontrol Sci. Technol. 25, 975–991. doi: 10.1080/09583157.2015.1023696
McFadyen R. E. C., Desmier de Chenon R., and Sipayung A. (2003). Biology and host specificity of the chromolaena stem gall fly, Cecidochares connexa (Macquart)(Diptera: Tephritidae). Aust. J. Entomol. 42, 294–297. doi: 10.1046/j.1440-6055.2003.00360.x
Naranjo S. E., Ellsworth P. C., and Frisvold G. B. (2015). Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 60, 621–645. doi: 10.1146/annurev-ento-010814-021005
Navarro-Campos C., Martínez-Ferrer M. T., Campos J. M., Fibla J. M., Alcaide J., Bargues L., et al. (2011). The influence of host fruit and temperature on the body size of adult Ceratitis capitata (Diptera: Tephritidae) under laboratory and field conditions. Environ. Entomol. 40, 931–938. doi: 10.1603/en10302
Olckers T. (2000). Biology, host specificity and risk assessment of Gargaphia decoris, the first agent to be released in South Africa for the biological control of the invasive tree. Solanum Mauritianum Biocontrol 45, 373–388. doi: 10.1023/A:1009907209768
Panero J. L. and Crozier B. S. (2016). Macroevolutionary dynamics in the early diversification of Asteraceae. Mol. Phylogenet. Evol. 99, 116–132. doi: 10.1016/j.ympev.2016.03.007
Paterson I. D. and Zachariades C. (2013). ISSRs indicate that Chromolaena odorata invading southern Africa originates in Jamaica or Cuba. Biol. Control 66, 132–139. doi: 10.1016/j.biocontrol.2013.04.005
Retief E. (2002). “The tribe Eupatorieae (Asteraceae) in southern Africa,” in Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata. Eds. Zachariades C., Muniappan R., and Strathie L. W. (ARC-PPRI, Pretoria, South Africa), 81–89.
Santos J. C., Silveira F. A., and Fernandes G. W. (2008). Long term oviposition preference and larval performance of Schizomyia macrocapillata (Diptera: Cecidomyiidae) on larger shoots of its host plant Bauhinia brevipes (Fabaceae). Evol. Ecol. 22, 123–137. doi: 10.1007/s10682-007-9162-z
Schwarzländer M., Hinz H. L., Winston R. L., and Day M. D. (2018). Biological control of weeds: an analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 63, 319–331. doi: 10.1007/s10526-018-9890-8
Seabold S. and Perktold J. (2010). Statsmodels: econometric and statistical modeling with Python. Proceedings of the 9th Python in Science Conference. SciPy 7, 92–96. doi: 10.25080/majora-92bf1922-011
Shao X., Li Q., Lin L., and He T. (2018). On the origin and genetic variability of the two invasive biotypes of Chromolaena odorata. Biol. Invasions 20, 2033–2046. doi: 10.1007/s10530-018-1677-4
Singer M. C. (1986). “The definition and measurement of oviposition preference in plant-feeding insects,” in Insect-Plant Interactions. Springer Series in Experimental Entomology. Eds. Miller J. R. and Miller T. A. (Springer, New York), 65–94. doi: 10.1007/978-1-4612-4910-8_3
Uyi O. O. and Igbinosa I. B. (2013). “The status of Chromolaena odorata and its biocontrol in West Africa,” in Proceedings of the VIII International Workshop on Biological Control and Management of Chromolaena odorata and other Eupatorieae, Nairobi, Kenya, 1–2 November 2010, 86–89 (ARC-PPRI, Pretoria).
Uyi O. O., Uwagiahanor B. I., and Ejomah A. J. (2017). The nocturnal larvae of a specialist folivore prefer Chromolaena odorata (L.) foliage from a sunny environment, but does it matter? Arthropod-Plant Interact. 11, 603–611. doi: 10.1007/s11829-017-9504-x
van Driesche R. G. and Reardon R. (2004). Assessing host ranges for parasitoids and predators used for classical biological control: A guide to best practice (Morgantown, West Virginia, 2004-03: United States Department of Agriculture Forest Health Technology Enterprise Team).
Wan F. H. and Harris P. (1997). Use of risk analysis for screening weed biocontrol agents: Altica carduorum Guer.(Coleoptera: Chrysomelidae) from China as a biocontrol agent of Cirsium arvense (L.) Scop. in North America. Biocontrol Sci. Technol. 7, 299–308. doi: 10.1080/09583159730712
Wang F., Chambi C., Li Z., Huang C., Ma Y., Li C., et al. (2018). Influence of supplemental protein on the life expectancy and reproduction of the Chinese citrus fruit fly, Bactrocera minax (Enderlein)(Tetradacus minax)(Diptera: Tephritidae). J. Insect Sci. 18, 25. doi: 10.1093/jisesa/iey008
Yu X., He T., Zhao J., and Li Q. (2014). Invasion genetics of Chromolaena odorata (Asteraceae): extremely low diversity across Asia. Biol. Invasions 16, 2351–2366. doi: 10.1007/s10530-014-0669-2
Zachariades C., Strathie L., Delgado O., and Retief E. (2007). Pre-release research on biocontrol agents for Chromolaena in South Africa. In Proceedings of the Seventh International Workshop on Biological Control and Management of Chromolaena odorata and Mikania micrantha. Eds. Lai P-Y., Reddy G. V. P., and Munniapan R.. Taiwan: National Pingtung University of Science and Technology (NPUST), pp. 68–80.
Zachariades C., Strathie L. W., Retief E., and Dube N. (2011). Progress towards the biological control of Chromolaena odorata (L.) R.M. King & H.Rob. (Asteraceae) in South Africa. Afr Entomol. 19, 282–302. doi: 10.4001/003.019.0229
Zachariades C. (2021). A catalogue of natural enemies of invasive alien plants in South Africa: classical biological control agents considered, released and established, exotic natural enemies present in the field, and bioherbicides. Afr. Entomol. 29, 1077–1142. doi: 10.4001/003.029.1077
Zachariades C., Strathie-Korrûbel L. W., and Kluge R. L. (1999). “The South African programme in the biological control of Chromolaena odorata (L.) King and Robinson (Asteraceae) using insects,” in Biological Control of Weeds in South Africa, (1990–1998), vol. 1 . Eds. Olckers T. and Hill M. P. (African Entomology Memoir), 89–102.
Keywords: biocontrol, host-range, offspring performance, preference, stem-galling fly
Citation: Mahlobo T, Dube N, Zachariades C and Munyai TC (2025) Preference and performance of Polymorphomyia basilica on different phenotypes of Chromolaena odorata and other Asteraceae in the laboratory. Front. Agron. 7:1699702. doi: 10.3389/fagro.2025.1699702
Received: 05 September 2025; Accepted: 07 October 2025;
Published: 20 October 2025.
Edited by:
Hanwen Wu, NSW Government, AustraliaReviewed by:
Muhammad Nawaz, New South Wales Department of Primary Industries, AustraliaHafiz Muhammad Saqib Mushtaq, Pakistan Agricultural Research Council, Pakistan
Copyright © 2025 Mahlobo, Dube, Zachariades and Munyai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thandeka Mahlobo, VGhhbmRla2F0ZWU5OUBnbWFpbC5jb20=
 Thandeka Mahlobo
Thandeka Mahlobo Nontembeko Dube
Nontembeko Dube Costas Zachariades
Costas Zachariades Thinandavha Caswell Munyai
Thinandavha Caswell Munyai