Abstract
This article reviews nasal structure and function in the light of intranasal pharmacotherapy. The nose provides an accessible, fast route for local treatment of nose and sinus diseases, with lower doses than are necessary systemically and few adverse effects. It can also be used for other medications as it has sufficient surface area protected from local damage by mucociliary clearance, absence of digestive enzymes, responsive blood flow, and provides a rapid route to the central nervous system.
Introduction
Medicines are usually given orally or systemically by injection: intramuscular or intravenous. Indeed when patients are asked about their drug history the use of inhalers or sprays is often inadvertently omitted, unless specifically requested. However, other routes not only exist, but can prove more effective in placing a drug accurately, often using smaller doses. One such is the intranasal route, now brought to prominence by SARS-CoV2, which uses it to invade the body.
The nose, even though obvious, “it's as plain as the nose on your face” is an English expression, is often disregarded by non-otorhinolaryngologists. However, it has much to recommend it: as an organ for conditioning inspired air, for immune defense, for hosting smell receptors and for application of therapy. The leading role of the epithelium in respiratory diseases such as Allergic Rhinitis (AR) and Chronic Rhinosinusitis with Nasal Polyps (CRSwNPs) has become apparent in recent years and the ability to interact with it by direct application of molecules, rather than allowing them to reach it via the circulation, having been absorbed via the gut or injected into the system, seems sensible.
Part 1 of this review article involves nasal pharmacotherapy. It begins with a consideration of nasal structure and function, including the nature of the pseudostratified columnar ciliated respiratory epithelium. It is important to understand nasal anatomy, histology, innervation, and blood supply in order to assess the nasal cavity as a route for a particular drug. Necessary factors are a large surface area for absorption and high blood flow for transport. Factors which might interfere with drug absorption are vasoconstriction secondary to stimulation of the adrenergic nerves or irritation stimulating the 5th nerve and causing the 7th to respond by increased glandular mucus secretion, washing away the therapeutic product into the nasopharynx, where it is swallowed. Nasal pH and the lipophilicity of a drug are also relevant.
The article continues with various intranasal therapies, varying from those used locally to treat respiratory diseases, to those countering entirely other problems, such as diabetes insipidus. Cheap and simple measures such as nasal saline or lysine aspirin can prove prophylactic, therapeutic or both. Unfortunately the use of nasal adrenalin for rescue in anaphylaxis was considered too commercially sensitive for inclusion in this paper.
Prevention of COVID-19 infection by copper—containing face masks has just emerged as an idea, doubtless other intranasal approaches will follow. The nose, overlooked for so long, is finally becoming prominent.
Part 2 will follow with a consideration of nasal immunology and immunologically—based nasal therapeutics.
Nasal Structure and Function
Nasal Anatomy
The nasal cavity is a midline airway passage of some 15 ml in volume and 14 cm in length in the adult, extending from the nares anteriorly to the post- nasal space. Approximately cylindrical, it is divided by the nasal septum into two nostrils. Above it are sinuses: frontal, ethmoid, and sphenoid, from front to back; maxillary sinuses are present on each side. Its surface area is ~160 cm2, but if microvilli are included this rises to nearly 10 m.2
Nasal Histology
The vestibular entrance to the nose is lined internally by a squamous epithelial layer. The lining changes to a pseudostratified columnar epithelium of respiratory type, bearing cilia and with numerous glands of serous and mucinous type, after the first 1–2 cm (1). This lines most of the respiratory tract, including the sinuses, down to the alveoli.
Epithelial Cells (Cilia)
The epithelium functions as a physical block on the entry of pathogens into the deeper tissues. The movement of their cilia occurs in the deeper sol layer of the nasal mucus, with a stiff- armed forward stroke followed by a limp backward one. This pushes the mucus, including the upper gel layer into which particles entering the nose become contained, backwards in the direction of the nasopharynx. These substances are then usually swallowed. This phenomenon is termed mucociliary clearance (2). Intranasal drugs therefore have a short absorption window before being cleared to the throat and swallowed. This clearance mechanism means that corticosteroids applied locally do not cause atrophy, unlike dermal application, provided septal deposition is avoided. Unlike the oral cavity and gut lumen there is no regular secretion of digestive enzymes capable of disrupting peptides into the nasal cavity, though peptidases may be released from epithelial cells upon stimulation by allergen.
The epithelium also plays a role in regulating inflammation by the secretion of cytokines (3).
Figure 1 shows the lateral nasal wall and the direction of mucus movement.
Figure 1
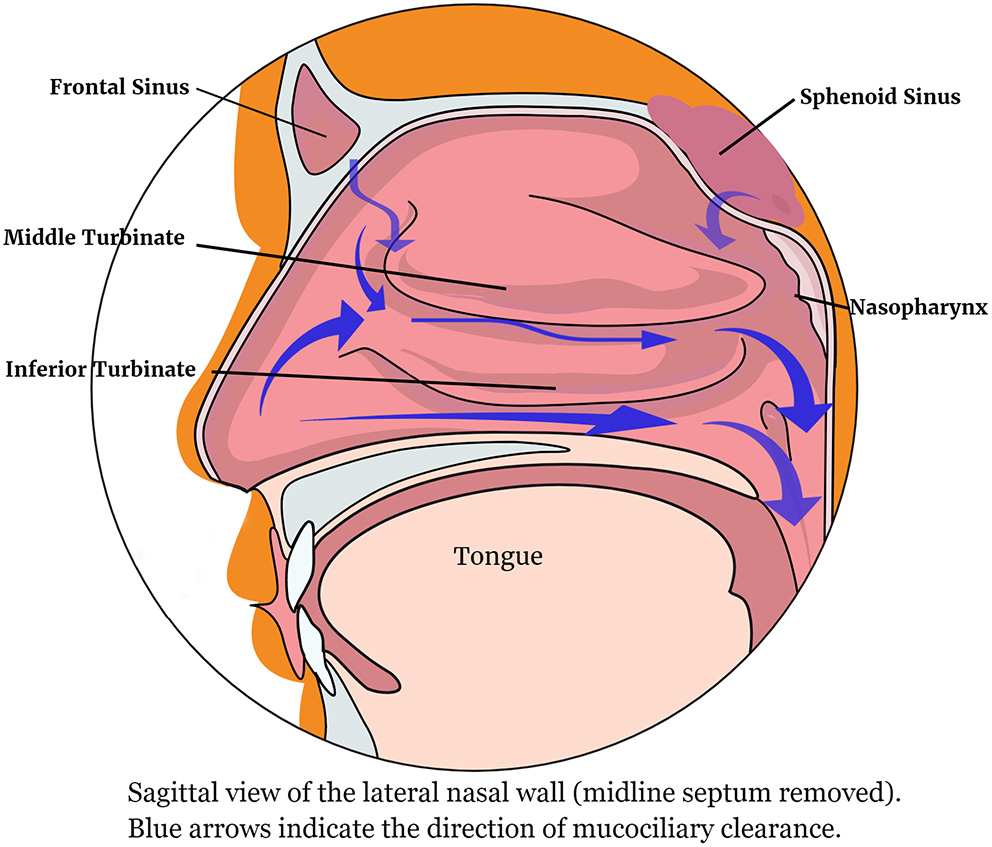
The lateral nasal wall showing the direction of mucociliary clearance.
Endothelium (Sub-epithelial Blood Vessels)
The nasal lining has an abundant vascular supply via capillaries lined by endothelial cells. The endothelial layer is of minimal thickness, so that heat can be rapidly transferred to inhaled air. Encircling the endothelium is a smooth muscle layer, which acts to narrow or widen the vasculature. This regulatory action on vessel diameters is a key feature of inflammation (3).
Mucous Glands
Small serous glands, similar to salivary glands are scattered in the front part of the nose. They secrete watery fluid, sometimes visible as droplets in cold conditions.
Seromucous glands secreting more proteineous secretions are located in the lamina propria elsewhere in the nasal cavity. They deposit mucus onto the external surface of the epithelium. The secreted mucus can immobilize external matter and helps to conserve the integrity of the physical barrier. Parasympathetic nervous impulses result in more mucus being synthesized and excreted. The mucus contains lysozyme and immunoglobulin A, which help to attack potentially invasive microbial organisms (3, 4).
Physiology of the Nose
The nasal cavity has a variety of roles, notably respiratory, olfactory, immunological, and the conditioning of air before entry into the lower respiratory tract. The cavity offers a very extensive, humid surface area which is optimal for adjusting the temperature and humidity of inhaled air prior to its passage toward the oxygen exchanging pulmonary surfaces. Mucus secreted by the nasal lining stops external matter from damaging the epithelial layer, especially in the course of an inflammatory response. The nose is the only human organ where olfaction occurs and depends on specialized sensory neurones that form part of the olfactory nerve.
Nasal Cycle
There is a continuously operating nasal cycle whereby the two sides of the cavity alternate between congestion and decongestion (3). In adults without health problems, the total resistance to airflow offered by the nose remains fairly constant, although there is an alternating pattern of one side of the nasal interior offering a greater level of resistance to airflow, whilst the other remains fully patent, followed by the inverse (5, 6). This pattern of flow restriction is referred to as the nasal cycle. It is produced by alternating changes in blood flow to the turbinates and the tubercle of the septum. In healthy individuals, this cycle occurs without the person noticing it, since there is no net alteration in how much airflow through the nose can take place. Likewise, the moisture content of inhaled air passing to the lungs does not vary (7). The hypothalamus contains the pacemaker area controlling the nasal cycle (8).
Vascular and Lymphatic Supply
The blood supply to the nasal cavity is extensive, involving six arterial branches, forming a good route for drug administration. There are two main sources of vascular supply to the nose: the internal and external carotid arteries. The former gives rise to the ophthalmic artery and its branches, the anterior ethmoid artery and the posterior ethmoid artery. From the latter arise the sphenopalatine, greater palatine, superior labial, and angular arteries.
The posterior and inferior portions of the interior aspect of the lateral nasal wall receive arterial blood from the sphenopalatine artery, whilst its superior portion is supplied by the ethmoid arteries, both anterior and superior. The septum of the nose receives a vascular supply from these same three arteries. This supply is augmented in the anterior portion by the superior labial artery and in the posterior portion the greater palatine artery makes its contribution. Little's area (also referred to as the Kiesselbach plexus) is an area situated in the most anterior and inferior third of the septum and is where most epistaxes occur. Here the principal arteries providing vascular supply to the nose all anastomose.
The nasal venous network has a similar layout to that of the arteries. The arterial blood flow into the nasal region is overabsorbed into the nasal veins, with the excess draining into the lymphatics, forming a good route for vaccine delivery. The veins do not possess valves and thus communicate directly with the cavernous sinus. In this way, they may render it easy for pathogens and drugs to disseminate within the cranium. Although the nose enjoys a rich vascular supply, smokers suffer from impaired recovery following surgery to the nose.
The lymph vessels originate in the outer layers of the mucosa with drainage from the posterior nasal cavity toward the retropharyngeal nodes and from the anterior cavity to the superior deep cervical or submandibular nodes.
The nasal mucosa contains a network fenestrated veins beneath the mucous membrane. These may provide some humidifying fluid. The epithelium also possesses a network of vascular erectile tissue, which is also cavernous and well-developed over the lower conchae and septum as shown in Figure 2, thus providing a good absorption route. Adrenergic vasoconstriction will decrease the rate of absorption, cholinergic vasodilatation may increase it, thus altering drug penetration.
Figure 2
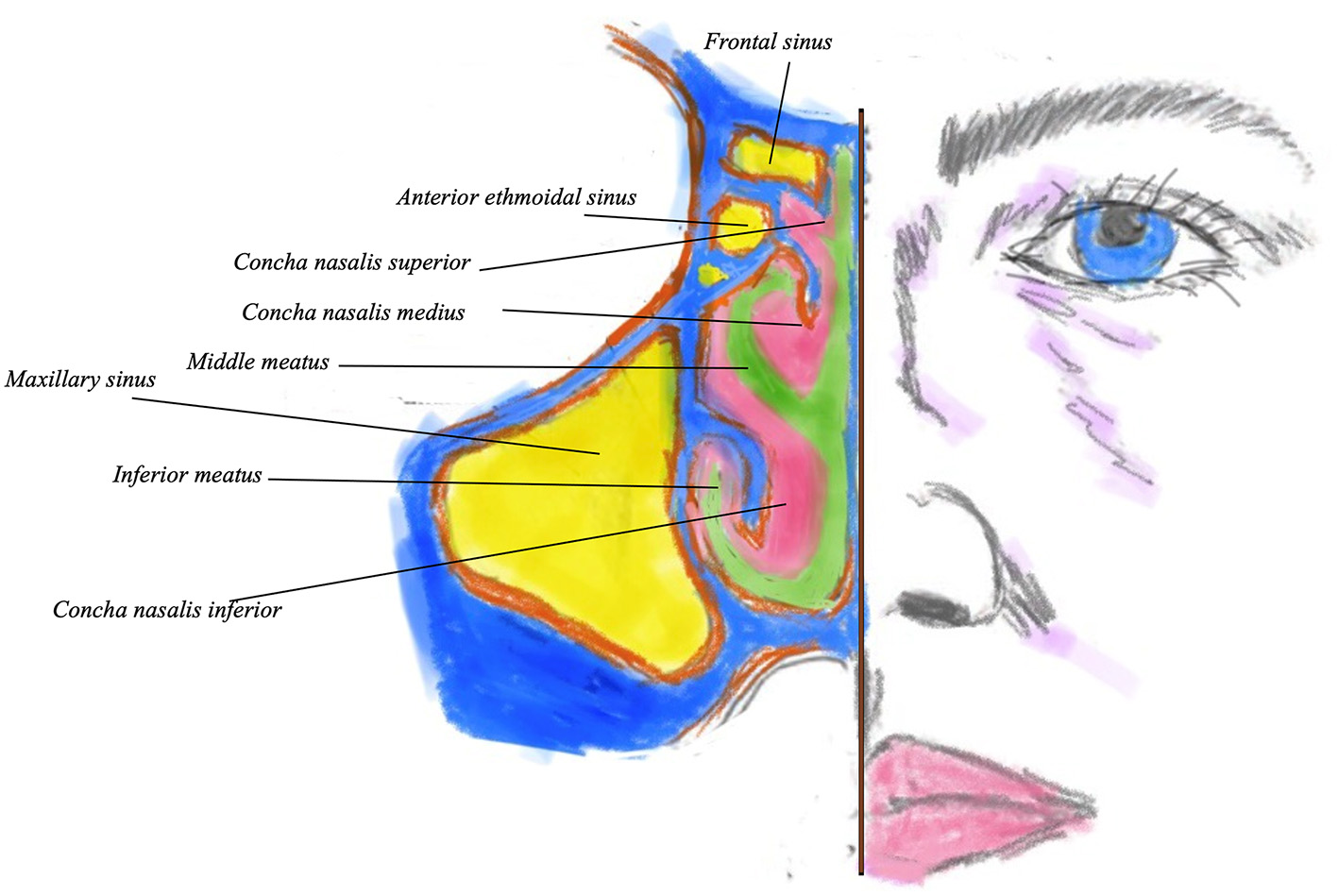
Coronal view of the nostril showing the sinuses (in yellow) turbinates and meati. Bony turbinate structure is in blue, with pink denoting the overlying mucosa. Green indicates the nasal airway.
Nervous Supply
The extensive sensory nervous supply to the nose is provided by the initial two branches of the fifth cranial nerve (1): the ophthalmic and maxillary divisions. The latter includes the anterior superior alveolar nerve, which is important in sneezing. The 5th (trigeminal) nerve is responsible for sensing pain and irritation following nasal administration, but it is the 7th (facial) nerve which contains motor fibers and responds to such irritation by stimulating facial movements and glandular secretion.
The 1st cranial (olfactory) nerve is the only site where the central nervous system is directly expressed on the mucosal surface and is hence in contact with the external world. This gives a route for central nervous system (CNS) access for drugs, but also for pathogens.
Parasympathetic Innervation
Parasympathetic innervation occurs via the greater superficial petrosal branch of the facial nerve. This branch combines with the deep petrosal nerve (carrying sympathetic fibers). The deep petrosal nerve emerges from the carotid plexus. Together they make up the vidian nerve within the pterygoid canal. The vidian nerve passes through the pterygopalatine ganglion, but the sympathetic fibers do not make any synaptic connections in the ganglion. Then the vidian nerve joins with fibers from the maxillary division of the fifth cranial nerve to supply the lacrimal gland, the nasal glands and the palate (1).
Osteology
There are twin nasal bones, the superior aspects of which articulate with the frontal bone. The nasal bones articulate with the lacrimal bones on their superolateral aspect, whilst on their inferolateral aspect they articulate with the maxilla on its ascending process. In a posterior and superior direction, the osseous septum of the nose is formed by the ethmoidal perpendicular plate. The septum is thinner centrally and often bent to one side or the other (septal deviation), which may interfere with drug delivery (9). In the posterior and inferior direction there is the vomer, which contributes a portion of the choanae, leading into the nasopharynx. The bony nasal floor is formed by the premaxilla and the palate.
Situated on the lateral walls of the nasal cavity are the three conchae (superior, middle, and inferior), providing the osseous support to the turbinates, projections into the lateral wall which promote turbulent airflow, enabling particle deposition and also act as radiators, warming the inspired air. The medial wall of the maxillary sinus is situated laterally to the turbinates.
Below each turbinate there are apertures, the meatuses, named after the turbinate immediately superior to them. For example, the middle meatus into which most sinuses ventilate and drain is just below the middle turbinate. The conchae and meatus increase nasal surface area, allowing significant absorption of medications (10) (Figure 2).
Viewed from the internal aspect of the nasal cavity, the roof consists of the ethmoidal cribriform plate. Behind and below the roof and angled posteriorly lies the bony face of the sphenoid sinus (1).
Paranasal Sinuses
These develop and enlarge after birth; it is not until some 3–7 years of age that the ethmoid and sphenoid sinuses are of significant size. The frontal sinuses develop last, not reaching full size until adolescence.
The sinuses in human beings exist as four pairs, each of which is lined by epithelial cells of the pseudostratified columnar type. The maxillary sinuses located within the maxilla and inferior to the orbit are the biggest. The frontal sinuses are within the frontal bone and are found above the orbit. The ethmoid sinuses consist of a number of separate pneumatized sacs within the ethmoid bone in between the nasal cavity and the orbit. They are divided into anterior and posterior groups, with differing drainage. The anterior ethmoids drain into the middle meatus via the ethmoid infundibulum; the posterior ethmoids sinuses drain into the superior meatus via the sphenoethmoidal recess. The sphenoid sinuses are inside the sphenoid bone (8).
It is an unresolved issue as to precisely what functions the paranasal sinuses perform, but they appear to accomplish the following (8):
-
They help to reduce skull weight
-
They allow the voice to have a more resonant quality
-
They help to absorb the impact of a blow to the face
-
They protect against abrupt changes in the temperature of the nasal cavity and thereby prevent injury to some structures that are sensitive to heat or cold
-
They condition air by adding moisture and warming it before it passes to the lungs
-
They perform an immune defensive function via the formation of nitric oxide.
Nasal Saline
Probably the oldest and by far the most frequently used nasal treatment is that of saline. Almost all nasal morbidities [e.g. allergic rhinitis (AR), chronic rhinosinusitis (CRS), infectious rhinitis etc.] are characterized by increased nasal secretions and/or congestion and are empirically countered by patients with nasal douches. Moreover, the concept of nasal irrigation (NI) is supported by many physicians, usually as an add-on to pharmacological treatment. For diseases that follow a chronic course, and therefore need chronic treatment, concerns about drug usage are raised and non-pharmacological approaches are preferred (11, 12). This is of importance in pediatric and elderly populations where parents/caregivers are often skeptical or unwilling regarding protracted pharmacological treatments and adherence is low (13).
Methods
There are many ways to perform NI: sprays, pumps, squeezy bottles, even plain syringes have been used, following various protocols. Moreover, the device may deliver high or low volume of saline, isotonic or hypertonic. NI are widely used and accepted, being included in therapeutic algorithms for AR and CRS (14, 15).
Mode of Action
This, though not fully delineated, seems to be multiple. First, it humidifies and moisturizes the nasal mucosa and hypertonic saline may reduce mucosal edema. Second, it removes particles, allergens, air pollutants leading to less interaction with the mucosa and, probably, less inflammation. Third, saline seems to make the mucus thinner and more easily expelled and, in turn, mucociliary clearance is improved. Of importance, the release of inflammatory mediators such as histamine, prostaglandins and leukotrienes is reduced and/or receptors, such as ICAM-1 that are used for viral entry to the epithelium (16) are down-regulated. It seems, therefore, that apart from the “mechanical” mode of action, NI may exert immunological effects. Finally, in a post-operative setting, the removal of thick crusts, clotted blood and debris may result in faster wound healing.
Tonicity
The first key issue that needs to be addressed is whether hypertonic solutions (i.e., > 0.9% in sodium chloride) are better than normal (iso-osmotic) saline. Such studies as are available favor the former. Mucociliary clearance is increased (17–19) and clinical studies in children with both allergic rhinitis and chronic sinusitis showed the superiority of hypertonic solutions (20–22); systematic reviews confirm these results (23, 24). In adults with CRS, the advantage of hypertonic solutions albeit probable, is less evident (25–27). Tonicities above 3% may both decrease mucociliary clearance and open tight junctions thus increasing epithelial permeability (18, 28). Post-operatively, where there is no inflammatory/allergic background, the main goal is the removal of crusts and debris. In this setting, high osmolarity seems to be of minor importance (29) whereas the volume of the NI is more crucial (30); nevertheless there are contradictory results (31).
Saline in Allergic Rhinitis
The efficacy of NI in children and adults with AR has been well-studied. Even though these studies are characterized by large heterogeneity, they all point to increased efficacy, either as add-on to pharmacological treatment (antihistamines and/or nasal steroids) or alone, compared to no intervention at all (21, 32, 33). Indicatively, the study of 220 children (aged 5–9 years old) with AR showed the superiority of hypertonic saline (2.7%) compared to normal saline (and even more compared to no intervention) regarding nasal symptoms and turbinate swelling and/or adenoidal hypertrophy. Moreover, NI resulted in reduced antihistamine use, especially in the hypertonic NI group (21). Similarly, 44 children (5–14 years old) with seasonal AR were prescribed hypertonic NI (or not) as add on to antihistamine treatment. The active group had significantly better rhinoconjunctivitis score and less drug usage (32). Recently, 76 children and adolescents (6–18 years old) with seasonal or perennial AR, used NI with a sea-water solution supplemented with algal extracts as an add on to regular treatment. The active group showed significantly improved AR symptom control as judged by CARAT questionnaires, better combined symptom and medication scores using the MASK Allergy Diary (a mobile application designed by the ARIA group) and reduced drug usage (33). Meta-analyses also suggest that NI have no adverse events, lead to less drug usage and can be used as add-on treatment for AR (34, 35).
Saline in Chronic Rhinosinusitis
In CRS, NI have also proven useful (22, 25, 26, 36, 37). Thirty children (3–16 years old) with rhinosinusitis were treated with either hypertonic (3.5%) or normal saline. The first group improved significantly in cough and nasal secretion/post nasal drip score, as well as radiology score, while the normal saline group showed significant improvement only in the post nasal drip score (22). Nasal patency and mucociliary clearance was studied in 80 adult patients with CRS. Both hypertonic and normal saline improved subjective symptoms (i.e., stuffiness and obstruction) and mucociliary clearance (greater effect with hypertonic saline). Nasal patency was increased with normal saline (25). Finally, a randomized control trial of 76 adult patients with chronic sinonasal symptoms showed significantly improved scores [Rhinosinusitis Disability Index (RSDI) and Single-Item Sinus-Symptom Severity Assessment (SIA)] and reduced use of medication, such as antibiotics, for patients with daily hypertonic saline NI (37). Large volume intervention is more efficacious compared to low volume NI (38).
Pregnancy Rhinitis
Hormonal changes during pregnancy alter AR symptoms and approximately one third of pregnant women observe increased morbidity. There also exists a hormonally—induced rhinitis of pregnancy. However, especially during the first trimester, physicians are reluctant to prescribe drugs, let alone in increased doses. NI is not expected to harm the fetus. Hypertonic saline (3% NaCl) was studied in 45 pregnant women, followed for 6 weeks: the active group had significantly better rhinitis score and less antihistamine use after the first week. Similarly, nasal resistance, albeit similar on week 1, was significantly decreased on weeks 3 and 6. No adverse events were reported and therefore, NI seem to be invaluable for the treatment of rhinitis of pregnant women (39).
The Common Cold
The role of NI for the therapy of acute upper respiratory infections (URTI- common cold) is not clearly established. Early studies showed no difference in the use of NI for URTI (40, 41), while a large pediatric study of 401 children indicated faster resolution of some nasal symptoms for those that used NI (42). A subsequent Cochrane review acknowledges possible benefits of NI; this, however, is based on small studies with bias risk (43). Similarly, the most recent EPOS guidelines suggest that NI possibly has benefits for relieving symptoms, mainly in children, and could be a therapeutic option (4). Moreover, NI could be used as prophylaxis for prevention of frequent URTI in both children and adults (42, 44).
Adverse Events
NI does not pose a risk for major adverse events. Epistaxis, nasal and/or aural burning or irritation and middle ear effusions occasionally occur especially with large volume, high pressure, hypertonic solutions. Sodium loading could be problematical in those with concomitant renal or cardiac problems if the solution is swallowed, so advice should be given to spit it out once it reaches the post nasal space. In general NI benefit far outweighs risk.
Conclusion
NI (especially with hypertonic saline) is a useful add-on to pharmacological treatments and can be used alone in pregnant women, small children and those with mild disease. Further studies are needed to delineate NI use in terms of underlying pathology, volume, tonicity, delivery method, supplementary extracts, or minerals.
Allergic Rhinitis
The therapeutic mainstays of Allergic Rhinitis (AR) are antihistamines and corticosteroids, both can be given intranasally, with minimal adverse events, since lower doses can be used. This is particularly important when the severe adverse effects of oral corticosteroid use are considered (45). Figure 3 shows the EUFOREA treatment algorithm for AR.
Figure 3
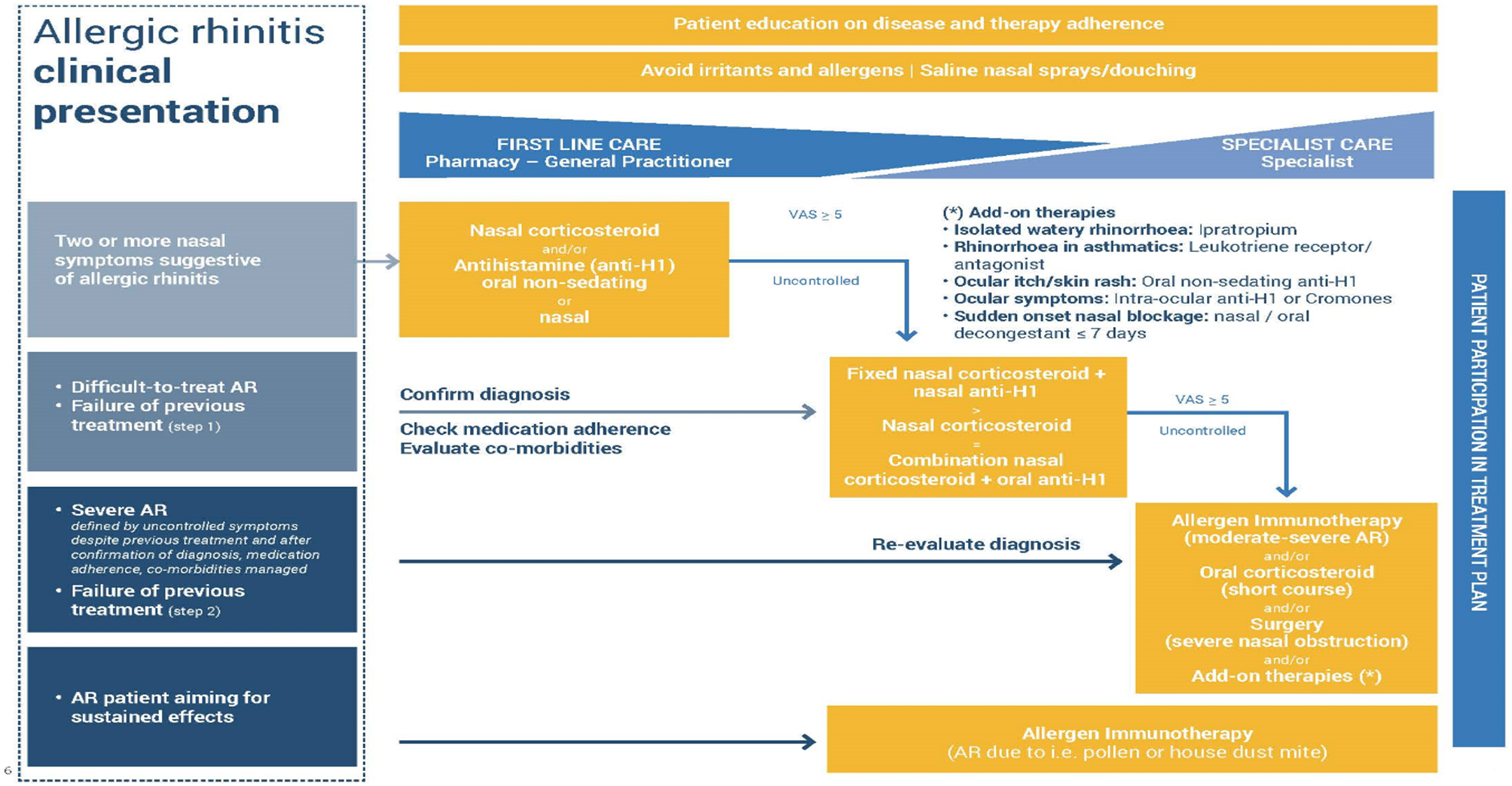
Treatment algorithm for AR as proposed by EUFOREA, taking into account the reality of patient phenotypes and existing international guidelines. EUFOREA treatment algorithm for Allergic Rhinitis (with permission from EUFOREA). The patient should be involved and educated regarding treatment, which starts with allergen and irritant avoidance, plus nasal saline. Further therapies are used as indicated, depending on disease severity and responsiveness to treatment. Failure to control AR should lead to revisiting the diagnosis, the major symptoms, disease extent, and other factors such as patient concordance.
Intranasal Antihistamines
Histamine acts in the early phase of allergic responses through H1 receptors (46). Antihistamines are mostly inverse agonists, stabilizing the receptor in an inactive conformation. The H1 receptor is widely distributed throughout the body (besides upper and lower airways in smooth muscle, heart, adrenal medulla, sensory nerves, central nervous system, and others (47) and are G-protein coupled transmembrane receptors that transduce extracellular signals through G proteins to intracellular second messenger systems (48) and may be considered a “cellular switcher,” functioning in equilibrium between two active or inactive conformation states.
Azelastine hydrochloride, levocabastine, and olopatadine hydrochloride are the mostly used intranasal antihistamine (INAH) spray formulations in Europe and the US. The pharmacological profile and clinical efficacy of these drugs have been extensively reviewed elsewhere (49–54). INAH are classified as inverse agonists, as they do not antagonize the binding of histamine, but instead bind to different sites on the receptor (55, 56). Binding of antihistamines to the histamine receptor stabilizes the receptor in the inactive state thereby reducing the intrinsic activity of the receptor in response to histamine (46, 49).
They are classed as second-generation antihistamines with high affinities for the H1 receptor and little affinity for the H2 receptor (57, 58) and typically have a fast onset of action (15 to 30 min) (57, 59, 60) with effects lasting up to 12 h (58, 61). In comparison to oral antihistamines, INAH are more effective at reducing symptoms of itching, rhinorrhoea and sneezing, but less effective at ocular symptoms (62, 63) and have variable effects on nasal congestion (64, 65).
Besides histamine, other mediators released from various immune cells are responsible for amplifying and maintaining inflammation and symptoms. There is some evidence that specific antihistamines including INAH can exert anti-allergic effects beyond inhibiting the action of histamine, including actions on arachidonic acid pathway mediators such as leukotrienes, thromboxanes, inflammatory cells, and mediators (66–70). The mechanisms behind this action have not been fully elucidated but may involve interference with calcium ion channels (50, 54, 71, 72).
The major adverse effect in trials is a bitter taste with azelastine, experienced as severe by a subset (around 10%) of subjects, probably genetic supertasters. It can be mitigated to an extent by correct technique of use as indicated in the manufacturer's advice sheet. The sedating effects of oral azelastine are avoided in the majority of nasal users, since the nasal dose is around one twentieth of the oral one (50).
Intranasal Corticosteroids (INS)
Topical intranasal corticosteroids (INS) are considered the single most effective treatment for AR and suppress most allergic inflammatory reactions (73). INS have been demonstrated to be more effective for relieving nasal symptoms of AR than oral and intranasal antihistamines (74, 75), especially for nasal congestion (76) and are particularly useful for improving ocular symptoms in AR patients (77, 78). INS also reduce bronchial hyperreactivity (79), as with ocular effects suggesting an effect on neurally—mediated distant symptoms via control of local inflammation and mediator release. Not all INS are equally effective (80).
Beclamethasone was the first steroid to be effectively modified for use in a pressurized INS spray in 1972 (81) and 8 compounds for intranasal application have been approved for AR in Europe and USA including triamcinolone acetonide, budesonide, ciclesonide, mometasone furoate, flunisolide, beclomethasone dipropionate, fluticasone propionate, and fluticasone furoate (73, 82).
Glucocorticosteroids diffuse across cell membranes, therefore lipophilicity is an important property, where they bind to the cytoplasmic glucocorticoid receptor (GR) (primary mechanism) (73, 83). On binding of the GR with the corticosteroid ligand, the heat shock proteins dissociate, allowing the GC-GR complex to translocate into the nucleus or interact with transcription factors in the cytoplasm (84). The anti-inflammatory effects are the result of modifications to gene transcription occurring via transactivation or transrepression. In the transactivation pathway, the activated GC-GR complex migrates to the nucleus where it binds as a dimer to the promotor region of palindromic DNA sequences termed Glucocorticoid Response Elements (GRE) (85). Interaction between the activated GR complex and GRE promotes an increase in the transcription of anti-inflammatory genes and of genes encoding proteins that have inhibitory effects on transcription of inflammatory and immune genes (86). The main anti-inflammatory effects of GCs occur via the suppression of multiple genes that encode inflammatory proteins, a process known as trans-repression (87, 88). INS have been shown to inhibit cytokine production in a range of different cell types. Epithelial generated cytokines act as chemoattractants and recruit effector cells such as eosinophils, basophils, and T cells to the nasal mucosa. Fluticasone propionate or fluticasone furoate significantly reduced levels of GM-CSF, IL-6, and IL-8 in stimulated nasal epithelial cells (89–91). Moreover, fluticasone propionate inhibited the release of IL-4, IL-6, IL-8, and TNF-a at an IC50 of <1 nM (92) in stimulated murine mast cells and to significantly reduce IL-4 and IL-5 levels from stimulated peripheral blood CD4C T cells (93). Different classes of steroid drugs (94) induce a different degree of cytokine inhibition with mometasone furoate being the most potent inhibitor of IL-1, IL-6, and TNF-a production among five different ones (mometasone furoate, hydrocortisone, betamethasone, dexamethasone, and beclomethasone).
Corticosteroids may inhibit the maturation of mast cells via regulating the expression of anti- or pro-apoptotic molecules in mast cell progenitors. Glucocorticoid facilitates apoptosis of eosinophils (95, 96) and reduces the numbers of immune cells, production of Th2 cytokines and chemokines and the release of inflammatory mediators in nasal mucosal samples, mostly they seem to actively target Th2 related cytokines (GM-CSF, IL-6, IL-4, IL-5, IL-10, and IL-13) involved in perpetuating the allergic response, in contrast to Th1 cytokines (IFN-g, IL-2) where no effect of steroid treatment was observed (87).
Again the nasal route involves microgram doses, rather than the milligram ones necessary for oral effectiveness. However, INS vary considerably in their systemic bioavailability and the least bioavailable ones: fluticasone propionate, fluticasone furoate and mometasone furoate should be used in children and when long term use is advisable (15). Unlike topical dermal use, there is no local atrophy from properly—applied INS, probably because of the continual movement of any applied drug by mucociliary clearance. Correct application of the spray onto the lateral wall of the nose, with different directions if two squirts are used, should be taught to every person for whom INS are prescribed or to whom they are sold over the counter. Avoidance of the nasal septum, less well-provided with ciliary action, reduces the chance of epistaxis or the extremely rare complication of septal atrophy (15).
Combination Therapy
Recently sprays containing both INS and intranasal antihistamine (fluticasone propionate and azelastine hydrochloride; mometasone furoate with olopatadine) have been formulated and tested in AR patients. Both are more effective on symptom reduction compared to either single molecule alone (97). The low pH of the second combination may cause nasal discomfort. Combining INS with intranasal decongestant is slightly more effective than INS alone and does not appear to cause rhinitis medicamentosa (97).
Intranasal Decongestants
Catecholamines (e.g., phenylephrine) or imidazolines (e.g., oxymetazoline) serve as active agents of intranasal decongestants usually classed as vasoconstrictor sympathomimetic agents (98). Their decongestion effects exert through direct and indirect activation of postsynaptic a1 and a2 adrenergic receptors on smooth muscles lining nasal capacitance vessels). On activation of these receptors, the smooth muscle contraction constricts blood vessels and thus reduces nasal tissue edema (98–100) followed by rapid reduction of nasal congestion (98, 99) without effect on other symptoms of AR [such as nasal itching, rhinorrhea, and sneezing (63, 100, 101). Prolonged or repeated use of decongestants (>3–5 days) may lead rebound swelling and congestion (101, 102) known as rhinitis medicamentosa. Septal atrophy may result from repeated septal application of such sprays and can also occur with the use of intranasal cocaine (103).
Intranasal Anticholinergics
Intranasal anticholinergic agents (INAA) such as ipratropium bromide can lead to the reduction of rhinorrhoea in AR (104–106) by blocking parasympathetic pathways in the nose that release acetylcholine. Acetylcholine acts on muscarinic receptors on nasal mucus glands to induce hypersecretion (104, 107, 108). Ipratropium bromide is a cholinergic receptor antagonist that blocks the interaction of acetylcholine on muscarinic receptors to inhibit release of watery secretions from mucous glands (104, 107), but has no effect on symptoms of sneezing or nasal congestion or inflammatory responses (104, 109, 110). Side effects include the predictable dry mouth and constipation.
Intranasal Cromones
Cromones are considered mildly effective in relieving symptoms of nasal itching, rhinorrhoea and sneezing, without affecting nasal congestion (83, 101). Their duration of action is short, requiring frequent dosing (up to four times per day) (101, 111).
Both cromoglicic acid, a derivative of chromone-2-carboxylic acid and nedocromil sodium, a pyranoquinolone, are available as intranasal formulations. The exact mechanism of action of chromones is unknown, although several theories have been postulated. Chromones are thought to exert their anti-inflammatory effects by preventing the release of histamine, tryptase and leukotrienes from mast cells following binding of IgE antibodies to the Fc+RI receptor and crosslinking with allergenic peptides (108, 111, 112). Chromones also have reported effects on eosinophils involved in the allergic response (113), but had no significant effect on basophils (114).
Non-Allergic Rhinitis (NAR)
When no allergic or other cause is found for nasal symptoms the diagnosis by exclusion of non-allergic rhinitis is made. This exists in two main forms- with and without eosinophilic inflammation. The former can be treated similarly to AR with saline, antihistamines, intranasal corticosteroids, alone or in combination. The latter is neurogenic and may respond to anti-cholinergics, such as ipratropium, or to capsaicin, an extract from chili peppers which reduces overexpression of a cation channel, TRPV1, in the nasal lining. Capsaicin(8-methyl-N-vanillyl-6-nonenamide) is a natural irritant which initially excites neurones, but then has a long refractory period, during which those neurons are unresponsive, not only to capsaicin, but to a variety of stimuli (115). Capsaicin desensitization performed correctly, is safe and effective for reducing NAR symptoms (number needed to treat = 4; 95% confidence interval [CI], 1 to 22) for several months (116). There is insufficient evidence to compare the effectiveness of capsaicin to other topical or systemic medications.
Acute Rhinosinusitis (ARS)
This is an inflammatory disease affecting the nose and paranasal sinuses with duration up to 12 weeks. Usually initiated by viral infection (common cold) it can be prolonged (post-viral) and, in a few subjects it is complicated by bacterial infection. Nasal saline, decongestants and ipratropium bromide can be used in the common cold; for post viral symptoms INS may help reduce symptoms in adults, but there is a paucity of evidence for any intranasal treatment when bacterial superinfection occurs (117).
Chronic Rhinosinusitis
Chronic rhinosinusitis (CRS) is a symptomatic inflammatory disease of the nasal and paranasal mucosa lasting more than 12 weeks (118). CRS has polypoid (CRSwNP) and non-polypoid (CRSsNP) subforms (118). Asthma is a prolonged bronchial inflammatory disease with an increased and variable tendency for bronchial contraction (119). Both CRS and asthma are significant health problems; the prevalence of each is ~10% (120), and the prevalence of co-morbid asthma and CRS is ~50% (118). The impact of CRS on the quality of life is significant, analogous to diabetes mellitus (118), and it leads to remarkable costs (121). The main treatment of both CRS and asthma is topically administered corticosteroids and nasal saline douching. Use of corticosteroid locally applied into the maxillary sinus via an indwelling tube was found effective in a group pf HDM sensitive subjects with CRS unresponsive to sinus surgery (122). Corticosteroid- eluting sinus implants reduce polyp size and the need for sinus surgery and are considered an option by EPOS (118). Application using corticosteroids in the nasal douche is now a popular treatment option, but there is no firm evidence for it being more or less effective than when the two are used separately (118).
Topical antifungals and topical antibiotics have been trialed in CRS, without significant benefit, except perhaps in special cases such as tobramycin in cystic fibrosis (118).
However, there is one relatively common CRS subtype in which local nasal therapy, other than saline and corticosteroids, may prove effective.
Nasal Acetylsalicylic Acid Desensitization in Non-steroidal Anti-inflammatory Drug-Exacerbated Respiratory Disease (N-ERD)
Patients with non-steroidal anti-inflammatory drug (NSAID)—exacerbated respiratory disease (N-ERD) have co-morbid asthma, CRS, and NSAID intolerance often with severe disease forms. They are prone to difficult symptoms and recurrent acute exacerbations despite adequate treatment by local or systemic corticosteroids, nasal saline lavages, antibiotics and sinus surgery. Acetylsalicylic acid (ASA, aspirin) treatment after desensitization (ATAD) may be beneficial. Oral ATAD has been shown to improve the quality of life and sino-nasal symptom scores in patients with N-ERD. However, if ASA is not taken regularly, ATAD is associated with a risk of severe anaphylactoid reactions.
Despite active treatment, some 10–20% of CRS/asthma patients have severe disease, purulent exacerbations and impaired productivity (118, 123, 124). Up to 70% of the uncontrolled cases have Type 2 inflammation, nasal polyps (NP), and/or N-ERD (125–129). The triad of co-morbid CRSwNP, asthma, and N-ERD has previously been called Samter's triad (130). The prevalence of N-ERD is 10–16% in hospital-level CRSwNP patients (131, 132). If endoscopic sinus surgery (ESS) combined with appropriate medical treatment fails, additional therapies including ATAD can be considered to treat N-ERD (118, 133).
Oral ATAD has Level of evidence 1a, although the placebo-controlled studies have had relatively small sample sizes (118). Since ATAD has side- and adverse effects including gastritis, gastrointestinal ulcerations and bleedings, attempts have been made to reduce the risk of side effects. Nasal ATAD (nATAD) tends to have fewer side effects than peroral ATAD both in diagnosis and in therapy (134). The use of nATAD is not suggested (level of evidence 1b-) in the current the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS 2020), since it lacks sufficient evidence for treatment of CRSwNP patients with N-ERD, but further double- blind studies are recommended (118). Here, we will review the nATAD literature, and communicate our own experience regarding its use.
Non-steroidal Anti-inflammatory Drug (NSAID) Exacerbated Respiratory Disease (N-ERD)
N-ERD is an inflammatory airway disease usually consisting of a triad of hypersensitivity to NSAIDs, asthma and CRSwNP (130–133). Patients with N-ERD have severe eosinophilic hyperplastic inflammation and fibrotic tissue remodeling in both their paranasal sinuses and lower airways (130, 135, 136). The age of onset for N-ERD is usually around 30 years, it is slightly more common in females (137, 138).
About 9% of asthmatics have N-ERD, the asthma of N-ERD patients tends to be moderate to severe (133). Compared to other asthmatics, N-ERD patients are more likely to need high dose inhaled corticosteroid treatment or steroid bursts (133, 135), and their asthma is more likely to be uncontrolled and to lead to asthma related healthcare visits, hospitalizations, and intubations (135, 137).
CRSwNP treatment of N-ERD patients consists of saline irrigations, nasal steroids, antileukotrienes, oral steroids, oral antimicrobials (139), and endoscopic sinus surgery (ESS) if conservative treatment is not sufficient (118, 135). The need for recurrent sinus surgeries is common in N-ERD patients (140, 141). ATAD (133), and/or biological agents are also considered if other treatments are insufficient (118).
In N-ERD patients, NSAIDs cause exacerbation of respiratory tract symptoms, provoking nasal congestion, rhinitis and obstruction of the lower airways, usually within 45–60 min of administration, urticaria, dyspepsia, and angioedema can also occur (142). The pathomechanisms behind this are not fully understood; it has been suggested that the hypersensitivity to NSAIDs is not caused by an allergic, immunoglobin E (IgE)—based mechanism, but rather by abnormal metabolism of the lipoxygenase (LO) and cyclooxygenase (COX) pathways (136, 143). Three forms of COX enzyme exist, one of these is COX-1. ASA and its other cross reacting NSAIDs inhibit COX-1, leading to decrease in COX-1 products, including prostaglandins. In N-ERD patients, ingestion of NSAIDs leads to an imbalance in the products of these pathways (143) (Figure 4).
Figure 4
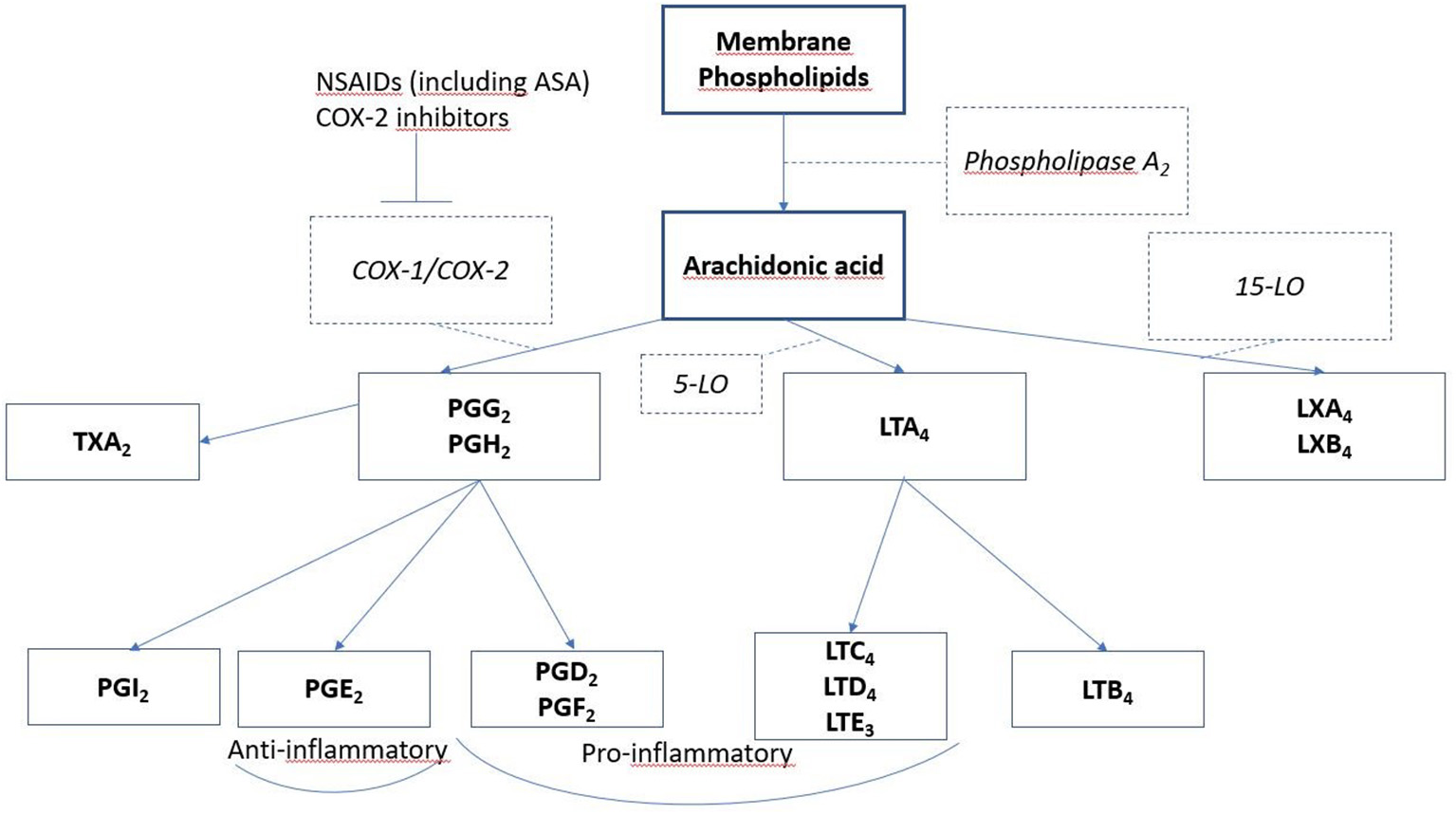
Lipid mediators involved in N-ERD. Arachidonic acid is released from degranulating cells (mast cells and eosinophils) and is metabolized by several routes to form prostaglandins, leukotrienes, and lipoxins. Inhibitors of cyclooxygenase 1, such as aspirin and NSAIDS, block this pathway, reducing bronchoprotective PGE2 and allowing increased pro- inflammatory leukotriene and lipoxin formation.
Ideally, N-ERD is diagnosed with a NSAID-challenge test. However, if a patient with confirmed asthma and CRSwNP has had multiple reactions with respiratory symptoms within 2 h after two different NSAID ingestions, this history is sufficient for N-ERD diagnosis (133). In unclear cases, for research purposes, or to evaluate for the provocation dose of ASA in oral desensitization, challenge tests are needed (133). The following contraindications for ASA challenge, ASA desensitization (AD), and ASA treatment after desensitization (ATAD) must be appreciated: prior anaphylactic/anaphylactoid reaction(s) due to NSAIDs, gastrointestinal bleeding, renal failure, uncontrolled asthma (Forced expiratory volume in one second [FEV1] <70% of the predicted value), ongoing respiratory tract infection or asthma exacerbation, current treatment with β-blocker, or pregnancy (133).
The initial use of nasal lysine aspirin (in the USA where this is unavailable, ketorolac is used) for challenge, coupled with sensitive upper airway measurements, means that highly sensitive subjects can be identified at a low dose without causing an asthma exacerbation. A negative nasal challenge necessitates oral challenge with larger doses until 300 mg has been tolerated (144).
Acetylsalicylic Acid (ASA)
ASA, also known as aspirin, is a very commonly used drug worldwide (145). It has been used to reduce pain, fever, inflammation, and lately mostly as prevention for cardiovascular diseases, but it may also have other preventive effects (146). The history of acetylsalicylic acid began over 3,500 years ago, when salicylate- containing willow bark was used to treat pain by ancient Sumerians and Egyptians (145). Aspirin was synthesized by Bayer company's chemist Felix Hoffmann in 1897 (145). The molecular formula of ASA is C9H8O4 (146) (Figure 5A).
Figure 5
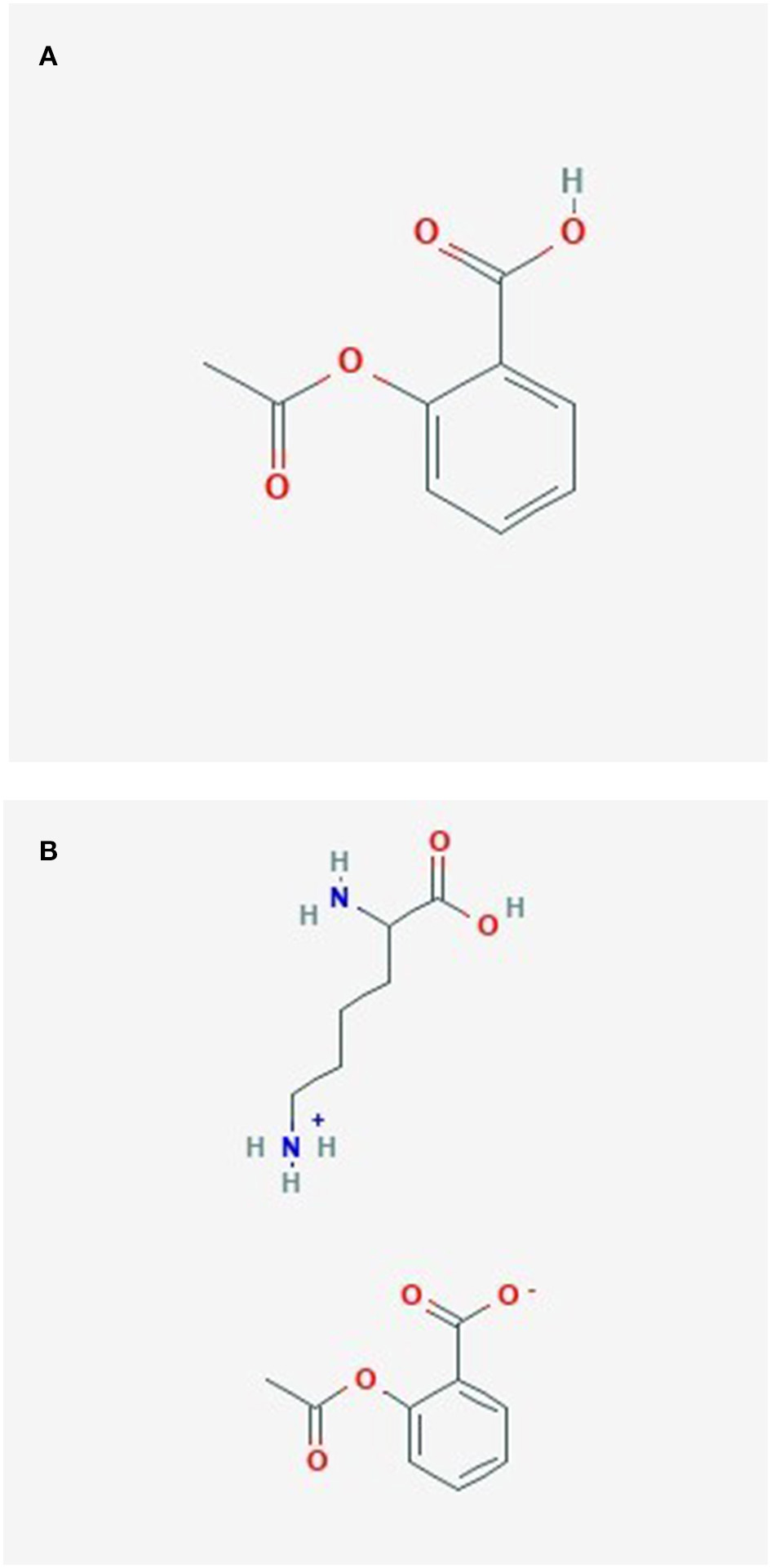
(A) Acetylsalicylate. (B) Lysine acetylsalicylate.
Lysine Acetylsalicylate (LAS)
The molecular formula of Lysine acetylsalicylate (Aspirin Lysine salt, Aspirin DL-Lysine, DL-Lysine-acetylsalicylate) is C15H22N2O6, its component compounds are DL-Lysine and ASA (147) (Figure 5B). Lysine acetylsalicylate (LAS) is soluble, it is the only genuinely soluble aspirin preparation (148) and was developed for intravenous administration to treat pain (148, 149).
ASA Treatment After Desensitization (ATAD) in N-ERD Patients
Due to the severity of symptoms in N-ERD, there has been an interest to improve its treatment, one of these developments is ATAD. Since the ASA intolerance of N-ERD patients is not due to IgE-mediated allergy, ATAD is not comparable to allergen desensitization in IgE confirmed allergic diseases. The aim of ATAD is to reduce polyp growth and decrease (upper) airway symptoms. ATAD is considered in N-ERD patients with insufficient response to pharmacological treatment, high recurrence of NPs leading to recurrent surgeries, insufficient control of asthma symptoms with standard medications, need to reduce corticosteroid dose, or in patients who need ASA or NSAID treatment (133, 150).
Our real-world follow-up study showed high discontinuation rates of peroral ATAD with a lack of effect on revision sinus surgery rates, prescribed antibiotics and oral corticosteroid courses (151). The latest EPOS 2020, however, concludes that peroral ATAD improves the quality of life and total nasal symptom scores in patients with N-ERD (118, 147, 152–155).
AD can be performed in an outpatient setting as an extension of ASA challenge, with ATAD continuing straight after the challenge by gradually increasing ASA doses (133, 144, 150). ATAD is usually performed with peroral ASA, with the effective dose varying between 300 and 1,300 mg daily (133, 147, 155–161). Intranasal ASA has been used in both ASA challenge and ATAD (133, 134). There is some evidence, that the surgical removal of NPs, or ESS may be beneficial prior to ATAD (133, 136, 144, 158–162).
Nasal Lysine Aspirin Challenge in N-ERD Patients
Although thus far not a routine part of clinical diagnostics, nasal challenge test with Lysine aspirin (LAS) was introduced for N-ERD assessment in the 1990s (163). The LAS doses, duration of observation period, and criteria for positivity have varied in different studies. One study group performed nasal ASA challenge tests (ASA-NCT) for 51 patients with N-ERD, confirmed by oral ASA challenge (163). The study did not report systemic reactions, including bronchospasm. They concluded that ASA-NCT is highly specific (95.7%) and sensitive (86.7%), that the nasal test is simple, safe, and quick for N-ERD diagnostics, but that negative results do not exclude possible ASA intolerance (163). Another study group performed ASA-NCT with relatively little side effects and showed positive result in 100 of 131 patients with severe CRSwNP and asthma (164). This study concluded that provided patients are carefully chosen and monitored, ASA-NCT is suitable for day-case practice (164).
Nasal Lysine Aspirin Treatment After Desensitization in N-ERD Patients
Nasal Lysine Aspirin treatment after desensitization (nATAD) has been used to treat N-ERD patients, but it has not been in wide clinical use. In the one existing double blind placebo controlled trial by Parikh and Scadding, 22 subjects with ASA sensitive nasal polyposis were enrolled, they were randomized to receive either 16 mg of topical LAS or placebo every 48 h for 6 months before cross-over. Only 11 study subjects completed the study, and no clinical benefit could be demonstrated (165) (Table 1). However, a reduction in the characteristically elevated levels of cysLT1 receptors was seen (169) and confirmed in a further study which also showed that this phenomenon did not occur in aspirin tolerant subjects (170).
Table 1
| References | Study method | Study participants | Dose | Outcome measures | Study results |
|---|---|---|---|---|---|
| Patriarca et al. (166) | Prospective, non-randomized controls | 20 patients with N-ERD and CRSwNP/43 patients with CRSwNP 191 control patients | 2 mg (ASA equivalent) per week | NP relapse | NP relapse rate decreased in LAS group |
| Nucera et al. (167) | Prospective, non-randomized controls | (1) 28 (N-ERD+CRSwNP)/ out of 76 patients. (2) 14 (N-ER+CRSwNP)/out of 49 patients Control group 191 CRSwNP patients |
4 mg (ASA equivalent) 6 times per week | Recurrence of NP (in CT and clinical control) | Recurrence of NPs reduced in LAS group |
| Parikh and Scadding (165) | Double blind placebo controlled cross-over trial | 22 ASA intolerant patients (of these 19 had CRSwNP), 11 completed the study | 16 mg (ASA equivalent) every 48 h for 6 months before cross-over | Nasal and pulmonary symptom scores ARM PEF rate PNIF | No significant differences between the groups But cysLT1 receptors reduced |
| Ogata et al. (168) | Prospective, open n of 1 study | 13 | 54 mg LAS [ASA equivalent 37.8 mg (53)] per day | NP volume NIPF, nNO, eNO, PEFR | NP volume reduced, NIPF, and nNO improved |
| Howe et al. (134) | Audit | 105 AERD + LAS treatment/out of 121 patients with AERD | 75–100 mg ASA equivalent per day | Subjective symptom evaluation + VAS PNIF Exhaled + nasal NO Olfaction Spirometry Asthma questionnaire | Symptom improvement Reduced airway inflammation Improvement of olfaction Improvement of asthma outcomes |
Trials of intranasal lysine aspirin in nasal polyposis.
Nasal Lysine aspirin (LAS) treatment, N-ERD (Non-steroidal anti-inflammatory drug exacerbated respiratory disease) patients with CRSwNP (chronic rhinosinusitis with nasal polyposis). N-ERD, Non-steroidal anti-inflammatory drug exacerbated respiratory disease; AERD, aspirin exacerbated respiratory disease; LAS, Lysine aspirin; CRSwNP, chronic rhinosinusitis with nasal polyps; NP, nasal polyps; ASA, aspirin; CT, computed tomography; ARM, acoustic rhinometry; PEF, peak expiratory flow; PNIF, Nasal inspiratory peak flow; VAS, visual analog scale; NO, nitric oxide.
Prospective, non-randomized studies have shown clinical benefits in LAS treated NP patients (166–168, 171) (Table 1). In an n of 1 study 13 N-ERD subjects who were uncontrolled on standard therapy were studied for 3 months and then for a further 3 months with the addition of nasal lysine aspirin, gradually increased to 54 mg daily (168). Significant improvement was seen in nasal inspiratory peak flow rate, p = 0.014) and nasal nitric oxide levels rose significantly (in both sides, p = 0.028), suggesting opening up of the nasal airway and sinus orifices. Exhaled nitric oxide and peak expiratory flow did not change. Compared with the preceding 3 months, adding intranasal lysine-aspirin had an effect on decreasing nasal polyp volume (right side, p = 0.031; left side, p = 0.016) (168).
Howe et al. (134) performed a non-controlled audit study including 105 N-ERD patients with intranasal LAS in gradually increasing doses following positive LAS challenge. Symptoms improved/stabilized in over 70% subjects at 3 and 12 months, and nasal inspiratory peak flow, olfaction, exhaled and nasal nitric oxide levels were also improved significantly. Asthma outcomes, including use of oral corticosteroids, exacerbations and emergency visits were all reduced in 22 subjects taking lysine aspirin over a year, compared to 20 challenge- positive subjects who ceased using it. Gastrointestinal side effects occurred in 3.8%, which is lower than those reported for oral ASA therapy (145). LAS has also been studied in treatment of patients with CRSwNP but no ASA intolerance. A prospective study involving 20 patients with CRSwNP but no ASA intolerance, receiving 2,000 μg LAS in one nostril and saline in one nostril, showed that polyp recurrence tended to be milder on the LAS treated side. However, a double blind, placebo- controlled trial was negative in aspirin- tolerant nasal polyp patients (172).
Finnish Experience With Nasal Lysine Aspirin Desensitization in N-ERD Patients
Seven nasal aspirin challenge- positive subjects [at 10 mg (n = 2) and 20 mg, n = 5] were given nasal ASA-desensitization (nAD) according to an earlier published method (134, 173), at the Department of Otorhinolaryngology—Head and Neck Surgery of Helsinki University Hospital.
Six of the seven patients discontinued the nAD (mean duration of the desensitization 19.2 days, range 7–39 days). The known reasons for the discontinuation were severe abdominal pain in three in one accompanied by an asthma exacerbation, and exacerbation of nasal blockage in two. Fortunately, the side effects were transient when nAD was discontinued.
Only one patient continued nAD. Although the dose was low (50 mg) the patient felt symptom relief. Uveitis developed 2 months after onset of nAD, however, according to the ophthalmologist it was not caused by the nAD.
It is possible that the Finnish population does not tolerate ATAD as well as other populations (151), certainly the high reports of gastrointestinal symptoms are concerning. However, it is necessary to warn patients that the desensitization process takes time and that usually they will be worse before their condition improves. Gradual updosing is necessary, with reduction of the dose when adverse events become problematical, as in allergen immunotherapy, although the mechanism of effect of ATAD appears more likely to relate to exhaustion of mediator- bearing cells and to receptor downregulation.
Conclusions
Nasal acetylsalicylic acid treatment after desensitization is a treatment option for N-ERD. The evidence of its benefits for N-ERD patients is not yet convincing, further randomized double-blind placebo-controlled studies are needed. According to the literature, nasal acetylsalicylic acid treatment after desensitization causes fewer side effects than oral ATAD, our own limited experience, however, contradicts this.
Intranasal Drugs for Diseases Outside the Nose
While the intranasal administration of drugs for the treatment of nasal diseases is well-established, intranasal drug delivery is increasingly recognized as being a useful and reliable alternative to oral and parenteral application of drugs for systemic diseases and the nasal mucosa has seriously emerged as a therapeutically viable route for systemic drug delivery. In particular nasal delivery seems to be able to circumvent the blood-brain barrier allowing direct drug delivery in the biophase of central nervous system-active compounds. Also, pharmacologically active compounds with poor stability in gastrointestinal fluids, poor intestinal absorption or unfavorable gastrointestinal and hepatic pre-systemic metabolism are of interest.
Peptide drugs (hormone replacement) treatments in different diseases appear to provide good indications under these circumstances. Different peptide hormones are available as nasal sprays, e.g., authorized products exist for estradiol steroid substitution of estradiol (Aerodiol®) (174, 175) and Gonadorelin hormone for undescended testicle (Kryptocur®) (176).
For the treatment of diabetes insipidus, the peptide analog desmopressin is available for both, nasal and oral administration with a given bioavailability of the commercial tablet of 0.1% and of 3–5% for the nasal spray. It can also be used for nocturnal enuresis in children and in multiple sclerosis. Recently the desmopressin spray was withdrawn from use for mild hemophilia and von Willebrand's disease because of higher than specified dosage1. Too much desmopressin can cause sodium levels in the blood to drop sufficiently to result in seizures, coma, and death.
Syntocinon nasal spray containing oxytocin is used to increase duration and strength of contractions during labor and has been investigated for some psychiatric conditions such as anorexia nervosa, autism, anxiety disorders, schizophrenia and alcohol deprivation (177).
Gonadotropin-Releasing-Hormone (GnRH) analogs such as Nafarelin (Synarel®) and busurelin are used for the treatment of endometriosis, precocious puberty, anovulatory infertility, hypogonadotropic, and cryptorchidism (178).
Further authorized products exist for nicotine withdrawal for smoking cessation (Nicotrol NS®) (179).
Future Uses of the Intranasal Route
Anti- viral molecules are under investigation for the reduction of COVID-19 transmission2.
Since there is some evidence for an intranasal, virally—mediated etiology for some neurodegenerative conditions (Parkinson's and Alzheimer's diseases) it may eventually be possible to prevent these by prophylactic use of a non-toxic intranasal antiviral.
Intranasal adrenalin is under trial for urgent anaphylaxis therapy. It will be necessary to block the nasal mucosa from giving a vasoconstrictive response to the applied adrenalin.
The united airways concept, wherein the nose and lower airways react as one unit to stimuli (180, 181) has led to some attempts to treat both areas via the nose, rather than using both nasal spray and inhaler. As yet this sensible concept has not proved successful.
Immunologically active intranasal preparations will be considered in Part 2.
Conclusion
The nose provides a useful route for therapy of airways diseases and also for other conditions such as CNS and endocrine disorders. Its accessibility, simplicity of use, good blood flow and protective epithelium should allow it to be investigated further as an alternative to systemic administration.
Statements
Author contributions
CC and NB contributed the section on nasal structure and function and Figures 1, 2. DM and NP contributed the saline section. LK contributed intranasal drugs. AL-H, MH, and ST-S contributed the section on lysine aspirin. GS conceived the idea of the paper, edited the contributions, and wrote the Abstract, Introduction, and Conclusion. All authors contributed to the article and approved the submitted version.
Conflict of interest
AL-H reports receiving funding from Orion Research Foundation, outside of this submitted work. GS reports fees and consultancies from ALK, Mylan, Bayer, GSK outside the submitted work. ST-S reports a grant of GSK and consultancies for ERT, Novartis, Sanofi Pharma and Roche outside the submitted work. LK reports grants and personal fees from Allergopharma, grants and personal fees from MEDA/Mylan, personal fees from HAL Allergie, grants from ALK Abelló, grants and personal fees from LETI Pharma, grants from Stallergenes, grants from Quintiles, grants and personal fees from Sanofi, grants from ASIT biotech, grants from Lofarma, personal fees from Allergy Therapeut., grants from AstraZeneca, grants from GSK, grants from Inmunotk, personal fees from Cassella med, outside the submitted work; and Membership of AeDA, DGHNO, Deutsche Akademie für Allergologie und klinische Immunologie, HNO-BV, GPA, EAACI. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1.^ Available online at: https://hemophilianewstoday.com/2020/10/05/recall-on-ferrings-intranasal-desmopressin-therapies-to-affect-availability-into-2021/
2.^ Available online at: https://www.itv.com/news/central/2020-11-16/new-face-mask-that-kills-coronavirus-could-be-available-by-december-says-nottingham-scientist-nottingham-trent-university-dr-gareth-cave?utm_source=upday&utm_medium=referral
References
1.
Chang EW . Nasal anatomy. In: MeyersAD editors. Medscape (2015). Available online at: https://emedicine.medscape.com/article/835134-overview#a7 (accessed August 3, 2020).
2.
Masuda N Mantani Y Yoshitomi C Yuasa H Nishida M Arai M et al . Immunohistochemical study on the secretory host defense system with lysozyme and secretory phospholipase A2 throughout rat respiratory tract. J Vet Med Sci. (2018) 80:323–32. 10.1292/jvms.17-0503
3.
Freeman SC Karp DA Kahwaji CI . Physiology, Nasal. Treasure Island, FL: StatPearls Publishing (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK526086/ (accessed August 3, 2020).
4.
Sahin-Yilmaz A Naclerio RM . Anatomy and physiology of the upper airway. Proc Am Thorac Soc. (2011) 8:31–9. 10.1513/pats.201007-050RN
5.
Lang C Grutzenmacher S Mlynski B Plontke S Mlynski G . Investigating the nasal cycle using endoscopy, rhinoresistometry, and acoustic rhinometry. Laryngoscope. (2003) 113:284–9. 10.1097/00005537-200302000-00016
6.
Lindemann J Leiacker R Rettinger G Keck T . The relationship between water vapour saturation of inhaled air and nasal patency. Eur Respir J. (2003) 21:313–6. 10.1183/09031936.03.00061103
7.
Mirza N Kroger H Doty RL . Influence of age on the ‘nasal cycle’. Laryngoscope. (1997) 107:62–6. 10.1097/00005537-199701000-00014
8.
Cappello ZJ Minutello K Dublin AB . Anatomy, Head and Neck, Nose Paranasal Sinuses. Treasure Island, FL: StatPearls Publishing (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK499826/ (accessed August 3, 2020).
9.
Gray L . Deviated nasal septum. III. Its influence on the physiology and disease of the nose and ears. J Laryngol Otol. (1967) 81:953–86. 10.1017/S0022215100067943
10.
Gizurarson S . The relevance of nasal physiology to the design of drug absorption studies. Adv Drug Deliv Rev. (1993) 11:329–4710.1016/0169-409X(93)90015-V
11.
Passalacqua G Bousquet PJ Carlsen KH Kemp J Lockey RF Niggemann B et al . ARIA update: I–systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. (2006) 117:1054–62. 10.1016/j.jaci.2005.12.1308
12.
Qiu J Grine K . Complementary and alternative treatment for allergic conditions. Prim Care. (2016) 43:519–26. 10.1016/j.pop.2016.04.012
13.
Baiardini I Novakova S Mihaicuta S Oguzulgen IK Canonica GW . Adherence to treatment in allergic respiratory diseases. Expert Rev Respir Med. (2019) 13:53–62. 10.1080/17476348.2019.1554438
14.
Fokkens WJ Lund VJ Hopkins C Hellings PW Kern R Reitsma S et al . Executive summary of EPOS 2020 including integrated care pathways. Rhinology. (2020) 58:82–111. 10.4193/Rhin20.601
15.
Scadding GK Kariyawasam HH Scadding G Mirakian R Buckley RJ Dixon T et al . BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis. Clin Exp Allergy. (2017) 47:856–89. 10.1111/cea.12953
16.
Di Lorenzo G Drago A Esposito Pellitteri M Candore G Colombo A Gervasi F et al . Measurement of inflammatory mediators of mast cells and eosinophils in native nasal lavage fluid in nasal polyposis. Int Arch Allergy Immunol. (2001) 125:164–75. 10.1159/000053811
17.
Talbot AR Herr TM Parsons DS . Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope. (1997) 107:500–3. 10.1097/00005537-199704000-00013
18.
Homer JJ Dowley AC Condon L El-Jassar P Sood S . The effect of hypertonicity on nasal mucociliary clearance. Clin Otolaryngol Allied Sci. (2000) 25:558–60. 10.1046/j.1365-2273.2000.00420.x
19.
Greiff L Andersson M Wollmer P Persson CGA . Hypertonic saline increases secretory and exudative responsiveness of human nasal airway in vivo. Eur Respir J. (2003) 21:308–12. 10.1183/09031936.03.00290303
20.
Keojampa BK Nguyen MH Ryan MW . Effects of buffered saline solution on nasal mucociliary clearance and nasal airway patency. Otolaryngol Head Neck Surg. (2004) 131:679–82. 10.1016/j.otohns.2004.05.026
21.
Marchisio P Varricchio A Baggi E Bianchini S Capasso ME Torretta S et al . Hypertonic saline is more effective than normal saline in seasonal allergic rhinitis in children. Int J Immunopathol Pharmacol. (2012) 25:721–30. 10.1177/039463201202500318
22.
Shoseyov D Bibi H Shai P Shoseyov N Shazberg G Hurvitz H . Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. J Allergy Clin Immunol. (1998) 101:602–5. 10.1016/S0091-6749(98)70166-6
23.
Kanjanawasee D Seresirikachorn K Chitsuthipakorn W Snidvongs K . Hypertonic saline versus isotonic saline nasal irrigation: systematic review and meta-analysis. Am J Rhinol Allergy. (2018) 32:269–79. 10.1177/1945892418773566
24.
Li CL Lin HC Lin CY Hsu TF . Effectiveness of hypertonic saline nasal irrigation for alleviating allergic rhinitis in children: a systematic review and meta-analysis. J Clin Med. (2019) 8:64. 10.3390/jcm8010064
25.
Hauptman G Ryan MW . The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. (2007) 137:815–21. 10.1016/j.otohns.2007.07.034
26.
Bachmann G Hommel G Michel O . Effectof irrigation of the nose with isotonic salt solution onadult patients with chronic paranasal sinus disease. Eur Arch Otorhinolaryngol. (2000) 257:537–41. 10.1007/s004050000271
27.
Casale M Moffa A Cassano M Carinci F Lopez MA Trecca EMC et al . Saline nasal irrigations for chronic rhinosinusitis: from everyday practice to evidence-based medicine. An update. Int J Immunopathol Pharmacol. (2018) 32:1–6. 10.1177/2058738418802676
28.
Nilsson H Dragomir A Ahlander A Johannesson M Roomans GM . Effects of hyperosmotic stress on cultured airway epithelial cells. Cell Tissue Res. (2007) 330:257–69. 10.1007/s00441-007-0482-7
29.
Chen XZ Feng SY Chang LH Lai XP Chen XH Li X et al . The effects of nasal irrigation with various solutions after endoscopic sinus surgery: systematic review and meta-analysis. J Laryngol Otol. (2018) 132:673–9. 10.1017/S0022215118000919
30.
Salib RJ Talpallikar S Uppal S Nair SB . A prospective randomised single-blinded clinical trial comparing the efficacy and tolerability of the nasal douching products Sterimar™ and Sinus Rinse™ following functional endoscopic sinus surgery. Clin Otolaryngol. (2013) 38:297–305. 10.1111/coa.12132
31.
Süslü N Bajin MD Süslü AE Ogretmenoglu O . Effects of buffered 2.3%, buffered 0.9%, and non-buffered 0.9% irrigation solutions on nasal mucosa after septoplasty. Eur Arch Otorhinolaryngol. (2009) 266:685–9. 10.1007/s00405-008-0807-5
32.
Garavello W Di Berardino F Romagnoli M Sambataro G Gaini RM . Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. Int Arch Allergy Immunol. (2005) 137:310–4. 10.1159/000086462
33.
Mitsias DI Dimou MV Lakoumentas J Alevizopoulos K Sousa-Pinto B Fonseca JA et al . Effect of nasal irrigation on allergic rhinitis control in children; complementarity between CARAT and MASK outcomes. Clin Transl Allergy. (2020) 10:9. 10.1186/s13601-020-00313-2
34.
Head K Snidvongs K Glew S Scadding G Schilder AG Philpott C et al . Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. (2018) 6:CD012597. 10.1002/14651858.CD012597.pub2
35.
Hermelingmeier KE Weber RK Hellmich M Heubach CP Mösges R . Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. (2012) 26:e119–25. 10.2500/ajra.2012.26.3787
36.
Ottaviano G Marioni G Staffieri C Giacomelli L Marchese-Ragona R Bertolin A et al . Effects of sulfurous, salty, bromic, iodic thermal water nasal irrigations in nonallergic chronic rhinosinusitis: a prospective, randomized, double-blind, clinical, and cytological study. Am J Otolaryngol. (2011) 32:235–9. 10.1016/j.amjoto.2010.02.004
37.
Rabago D Zgierska A Mundt M Barrett B Bobula J Maberry R . Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. J Fam Pract. (2002) 51:1049–55.
38.
Chong LY Head K Hopkins C Philpott C Glew S Scadding G et al . Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. (2016) 4:CD011995. 10.1002/14651858.CD011995.pub2
39.
Garavello W Somigliana E Acaia B Gaini L Pignataro L Gaini RM . Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol. (2010) 151:137–41. 10.1159/000236003
40.
Adam P Stiffman M Blake RL . A clinical trial of hypertonic saline nasal spray in subjects with the common cold or rhinitis. Arch Fam Med. (1998) 7:39–43. 10.1001/archfami.7.1.39
41.
Bollag U Albrecht E Wingert W . Medicated versus saline nose drops in the management of upper respiratory infection. Helv Paediatr Acta. (1984) 39:341–5.
42.
Slapak I Skoupá J Strnad P Horník P . Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children. Arch Otolaryngol Head Neck Surg. (2008) 134:67–74. 10.1001/archoto.2007.19
43.
King D Mitchell B Williams CP Spurling GKP . Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. (2015) 4:CD006821. 10.1002/14651858.CD006821.pub3
44.
Tano L Tano K . A daily nasal spray with saline prevents symptoms of rhinitis. Acta Otolaryngol. (2004) 124:1059–62. 10.1080/00016480410017657
45.
Hox V Lourijsen E Jordens A Aasbjerg K Agache I Alobid et al . Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. (2020) 10:1. 10.1186/s13601-020-00343-w
46.
Leurs R Church MK Taglialatela M . H1-antihistamines: inverse agonism, anti inflammatory actions and cardiac effects. Clin Exp Allergy. (2002) 32:489–98. 10.1046/j.0954-7894.2002.01314.x
47.
Mahdy AM Webster NR . Histamine and antihistamines. Anaesth Intens Care Med. (2011) 12:324–9. 10.1016/j.mpaic.2011.04.012
48.
Simons FER Simons KJ . Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. (2011) 128:1139–50.e1134. 10.1016/j.jaci.2011.09.005
49.
Sharif NA Xu SX Yanni JM . Olopatadine (AL-4943A): ligand binding and functional studies on a novel, long acting H1-selective histamine antagonist and anti-allergic agent for use in allergic conjunctivitis. J Ocular Pharmacol Ther. (1996) 12:401–7. 10.1089/jop.1996.12.401
50.
Bernstein JA . Azelastine hydrochloride: a review of pharmacology, pharmacokinetics, clinical efficacy and tolerability. Curr Med Res Opin. (2007) 23:2441–52. 10.1185/030079907X226302
51.
Horak F . Effectiveness of twice daily azelastine nasal spray in patients with seasonal allergic rhinitis. Ther Clin Risk Manage. (2008) 4:1009–22. 10.2147/TCRM.S3229
52.
Berger WE . Pharmacokinetic characteristics and safety and tolerability of a reformulated azelastine hydrochloride nasal spray in patients with chronic rhinitis. Expert Opin Drug Metab Toxicol. (2009) 5:91–102. 10.1517/17425250802670474
53.
Horbal JM Bernstein JA . Azelastine HCl: a review of the old and new formulations. Clin Med Insights Ther. (2010) 2:CMT.S3865. 10.4137/CMT.S3865
54.
Kaliner MA Oppenheimer J Farrar JR . Comprehensive review of olopatadine: the molecule and its clinical entities. Allergy Asthma Proc. (2010) 31:112–19. 10.2500/aap.2010.31.3317
55.
Wieland K Laak AM Smit MJ Kühne R Timmerman H Leurs R . Mutational analysis of the antagonist-binding site of the histamine H(1) receptor. J Biol Chem. (1999) 274:29994–30000. 10.1074/jbc.274.42.29994
56.
Gillard M Van Der Perren C Moguilevsky N Massingham R Chatelain P . Binding characteristics of cetirizine and levocetirizine to human H(1) histamine receptors: contribution of Lys(191) and Thr(194). Mol Pharmacol. (2001) 61:391–9. 10.1124/mol.61.2.391
57.
Patel P D'Andrea C Sacks HJ . Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamber. Am J Rhinol. (2007) 21:499–503. 10.2500/ajr.2007.21.3058
58.
Greiff L Andersson M Svensson C Persson CG . Topical azelastine has a 12-hour duration of action as assessed by histamine challenge-induced exudation of alpha 2-macroglobulin into human nasal airways. Clin Exp Allergy. (1997) 27:438–44. 10.1111/j.1365-2222.1997.tb00730.x
59.
Horak F Zieglmayer UP Zieglmayer R Kavina A Marschall K Munzel U et al . Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. (2006) 22:151–7. 10.1185/030079906X80305
60.
Patel D Garadi R Brubaker M Conroy JP Kaji Y Crenshaw K et al . Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc. (2007) 28:592–9. 10.2500/aap2007.28.3033
61.
Patel P Roland PS Marple BF Benninger PJ Margalias H Brubaker M et al . An assessment of the onset and duration of action of olopatadine nasal spray. Otolaryngol Head Neck Surg. (2007) 137:918–24. 10.1016/j.otohns.2007.08.005
62.
Corren J Storms W Bernstein J Berger W Nayak A Sacks H . Effectiveness of azelastine nasal spray compared with oral cetirizine in patients with seasonal allergic rhinitis. Clin Ther. (2005) 27:543–53. 10.1016/j.clinthera.2005.04.012
63.
Bousquet J Khaltaev N Cruz AA Denburg J Fokkens WJ Togias A et al . Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN * and AllerGen **). Allergy. (2008) 63:8–160. 10.1111/j.1398-9995.2007.01620.x
64.
Golden SJ Craig TJ . Efficacy and safety of azelastine nasal spray for the treatment of allergic rhinitis. J Am Osteopathic Assoc. (1999) 99:S7–12. 10.7556/jaoa.1999.99.7.S7
65.
Chand N Pillar J Nolan K Diamantis W Sofia D . Inhibition of allergic and Nonallergic leukotriene C4 formation and histamine secretion by azelastine: implication for its mechanism of action. Int Arch Allergy Immunol. (1989) 90:67–70. 10.1159/000235002
66.
Hamasaki Y Shafigeh M Yamamoto S Sato R Zaitu M Muro E et al . Inhibition of leukotriene synthesis by azelastine. Ann Allergy Asthma Immunol. (1996) 76:469–75. 10.1016/S1081-1206(10)63465-5
67.
Hide I Toriu N Nuibe T Inoue A Hide M Yamamoto S et al . Suppression of TNF-alpha secretion by azelastine in a rat mast (RBL-2H3) cell line: evidence for differential regulation of TNF-alpha release, transcription, and degranulation. J Immunol. (1997) 159:2932–40.
68.
Yoneda K Yamamoto T Ueta E Osaki T . Suppression by azelastine hydrochloride of NF-kappa B activation involved in generation of cytokines and nitric oxide. Jap J Pharmacol. (1997) 73:145–53. 10.1254/jjp.60.145
69.
Matsuo S Takayama S . Influence of the anti-allergic agent, azelastine, on tumor necrosis factor-alpha (TNF-alpha) secretion from cultured mouse mast cells. In Vivo. (1998) 12:481–4.
70.
Kaise T Akamatsu Y Ohmori K Ishii A Karasawa A . Effects of olopatadine hydrochloride on the release of thromboxane B2 and histamine from nasal mucosa after antigen–antibody reaction in guinea pigs. Allergol Int. (2001) 50:337–41. 10.1046/j.1440-1592.2001.00236.x
71.
Norman PS . A rational approach to desensitization. J Allergy. (1969) 44:129–45. 10.1016/0021-8707(69)90137-3
72.
Letari O Miozzo A Folco G Belloni PA Sala A Rovati GE et al . Effects of loratadine on cytosolic Ca2+ levels and leukotriene release: novel mechanisms of action independent of the anti-histamine activity. Eur J Pharmacol. (1994) 266:219–27. 10.1016/0922-4106(94)90130-9
73.
Bousquet J Van Cauwenberge P Khaltaev N . Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. (2001) 108:S147–334. 10.1067/mai.2001.118891
74.
Weiner JM Abramson MJ Puy RM . Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. (1998) 317:1624.
75.
Yáñez A Rodrigo GJ . Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. (2002) 89:479–84. 10.1016/S1081-1206(10)62085-6
76.
Berger WE Nayak AS Staudinger HW . Mometasone furoate improves congestion in patients with moderate-to-severe seasonal allergic rhinitis. Ann Pharmacother. (2005) 39:1984–9. 10.1345/aph.1G202
77.
DeWester J Philpot EE Westlund RE Cook CK Rickard KA . The efficacy of intranasal fluticasone propionate in the relief of ocular symptoms associated with seasonal allergic rhinitis. Allergy Asthma Proc. (2003) 24:331–7.
78.
Anolik R Nathan RA Schenkel E Danzig MR Gates D Varghese S et al . Intranasal mometasone furoate alleviates the ocular symptoms associated with seasonal allergic rhinitis: results of a post hoc analysis. Int Arch Allergy Immunol. (2008) 147:323–30. 10.1159/000144040
79.
Shaaban R Zureik M Soussan D Antó JM Heinrich J Janson C et al . Allergic rhinitis and onset of bronchial hyperresponsiveness. a population-based study. Am J Respir Crit Care Med. (2007) 176:659–66. 10.1164/rccm.200703-427OC
80.
Keith PK Scadding GK . Are intranasal corticosteroids all equally consistent in managing ocular symptoms of seasonal allergic rhinitis?Curr Med Res Opin. (2009) 25:2021–41. 10.1185/03007990903094106
81.
Brown HM Storey G George WH . Beclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthma. Br Med J. (1972) 1:585–90. 10.1136/bmj.1.5800.585
82.
Derendorf H Meltzer EO . Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. (2008) 63:1292–300. 10.1111/j.1398-9995.2008.01750.x
83.
Barnes PJ . New directions in allergic diseases: mechanism-based anti-inflammatory therapies. J Allergy Clin Immunol. (2000) 106(Pt. 1):5–16. 10.1067/mai.2000.107930
84.
Okano M . Mechanisms and clinical implications of glucocorticosteroids in the treatment of allergic rhinitis. Clin Exp Immunol. (2009) 158:164–73. 10.1111/j.1365-2249.2009.04010.x
85.
Barnes PJ . How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. (2006) 148:245–54. 10.1038/sj.bjp.0706736
86.
Uva L Miguel D Pinheiro C Antunes J Cruz D Ferreira J et al . Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol. (2012) 2012:561018. 10.1155/2012/561018
87.
Klimek L Bachert C . Aktuelle Aspekte der nasalen Glukokortikosteroidtherapie. HNO. (2000) 48:544–55. 10.1007/s001060050614
88.
Coutinho AE Chapman KE . The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. (2011) 335:2–13. 10.1016/j.mce.2010.04.005
89.
Ohnishi M Takizawa R Ohkubo K Yokosima K Okuda M Yagi T . Fluticasone propionate reduced the production of GM-CSF, IL-6 and IL-8 generated from cultured nasal epithelial cells. Allergy. (1994) 43:441–7.
90.
Smith SJ Piliponsky AM Rosenhead F Elchalal U Nagler A Levi-Schaffer F . Dexamethasone inhibits maturation, cytokine production and Fc epsilon RI expression of human cord blood-derived mast cells. Clin Exp Allergy. (2002) 32:906–13. 10.1046/j.1365-2745.2002.01418.x
91.
Mullol J Pujols L Alobid I Pérez-Gonzalez M Fuentes M de Borja Callejas F et al . Fluticasone furoate inhibits cytokine secretion from nasal epithelial cells and reduces eosinophil survival in an in vitro model of eosinophilic inflammation. Int Arch Allergy Immunol. (2014) 163:225–33. 10.1159/000358489
92.
Fuller R Johnson M Bye A . Fluticasone propionate–an update on preclinical and clinical experience. Respir Med. (1995) 89:3–18. 10.1016/0954-6111(95)90259-7
93.
Umland SP Nahrebne DK Razac S Beavis A Pennline KJ Egan RW et al . The inhibitory effects of topically active glucocorticoids on IL-4, IL-5, and interferon-gamma production by cultured primary CD4+ T cells. J Allergy Clin Immunol. (1997) 100:511–9. 10.1016/S0091-6749(97)70144-1
94.
Schafer T Schnoor M Wagenmann M Klimek L Bachert C . Therapeutic Index (TIX) for intranasal corticosteroids in the treatment of allergic rhinitis. Rhinology. (2011) 49:272–80. 10.4193/Rhin10.170
95.
Meagher LC Cousin JM Seckl JR Haslett C . Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunology. (1996) 156:4422–8.
96.
Stellato C Atsuta J Bickel CA Schleimer RP . An in vitro comparison of commonly used topical glucocorticoid preparations. J Allergy Clin Immunol. (1999) 104(Pt. 1):623–9. 10.1016/S0091-6749(99)70334-9
97.
Greiwe JC Bernstein JA . Combination therapy in allergic rhinitis: what works and what does not work. Am J Rhinol Allergy. (2016) 30:391–610.2500/ajra.2016.30.4391
98.
Greiner AN Meltzer EO . Overview of the treatment of allergic rhinitis and nonallergic rhinopathy. Proc Am Thoracic Soc. (2011) 8:121–31. 10.1513/pats.201004-033RN
99.
Kushnir NM . The role of decongestants, cromolyn, guafenesin, saline washes, capsaicin, leukotriene antagonists, and other treatments on rhinitis. Immunol Allergy Clin N Am. (2011) 31:601–17. 10.1016/j.iac.2011.05.008
100.
Klimek L Mullol J Hellings P Gevaert P Mösges R Fokkens W . Recent pharmacological developments in the treatment of perennial and persistent allergic rhinitis. Expert Opin Pharmacother. (2016) 17:657–69. 10.1517/14656566.2016.1145661
101.
Gentile DA Bartholow A Skoner D . Allergic rhinitis. In: LeungD editor. Pediatric Allergy: Principles Practice.3rd ed.London; New York, NY: Elsevier (2015). p. 210–18.
102.
Scadding GK . Rhinitis medicamentosa. Clin Exp Allergy. (1995) 25:391–4. 10.1111/j.1365-2222.1995.tb01068.x
103.
Sapmaz E Toplu Y Somuk BT . A new classification for septal perforation and effects of treatment methods on quality of life. Braz J Otorhinolaryngol. (2019) 85:716–23. 10.1016/j.bjorl.2018.06.003
104.
Kaiser HB Findlay SR Georgitis JW Grossman J Ratner PH Tinkelman DG et al . The anticholinergic agent, ipratropium bromide, is useful in the treatment of rhinorrhea associated with perennial allergic rhinitis. Allergy Asthma Proc. (1997) 19:23–9. 10.2500/108854198778557962
105.
van Cauwenberge P Bachert C Passalacqua G Bousquet J Canonica GW Durham SR et al . Consensus statement on the treatment of allergic rhinitis. Eur Acad Allergol Clin Immunol Allergy. (2000) 55:116–34. 10.1034/j.1398-9995.2000.00526.x
106.
Tran NP Vickery J Blaiss MS . Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. (2011) 3:148–56. 10.4168/aair.2011.3.3.148
107.
Quraishi SA Davies MJ Craig TJ . Inflammatory responses in allergic rhinitis: traditional approaches and novel treatment strategies. J Am Osteopathic Assoc. (2004) 104(Suppl. 5):S7–15.
108.
Ridolo E Montagni M Melli V Braido F Incorvaia C Canonica GW . Pharmacotherapy of allergic rhinitis: current options and future perspectives. Expert Opin Pharmacother. (2014) 15:73–83. 10.1517/14656566.2014.860445
109.
Borum P Mygind N Schultz Larsen F . Intranasal ipratropium: a new treatment for perennial rhinitis. Clin Otolaryngol Allied Sci. (1979) 4:407–11. 10.1111/j.1365-2273.1979.tb01773.x
110.
Meltzer EO Orgel HA Bronsky EA Findlay SR Georgitis JW Grossman J et al . Ipratropium bromide aqueous nasal spray for patients with perennial allergic rhinitis: a study of its effect on their symptoms, quality of life, and nasal cytology. J Allergy Clin Immunol. (1992) 90:242–9. 10.1016/0091-6749(92)90078-G
111.
Leung KB Flint KC Brostoff J Hudspith BN Johnson NM Lau HY et al . Effects of sodium cromoglycate and nedocromil sodium on histamine secretion from human lung mast cells. Thorax. (1988) 43:756–61. 10.1136/thx.43.10.756
112.
Shichijo M Inagaki N Nakai N Kimata M Nakahata T Serizawa I et al . The effects of anti-asthma drugs on mediator release from cultured human mast cells. Clin Exp Allergy. (1998) 28:1228–36. 10.1046/j.1365-2222.1998.00394.x
113.
Spry CJF Kumaraswami V Tai PC . The effect of nedocromil sodium on secretion from human eosinophils. Eur J Respir Dis. (1986) 69:241–3.
114.
Orgel HA Meltzer EO Kemp JP Ostrom NK Welch MJ . Comparison of intranasal cromolyn sodium, 4%, and oral terfenadine for allergic rhinitis: symptoms, nasal cytology, nasal ciliary clearance, and rhinomanometry. Ann Allergy. (1991) 66:237–44.
115.
Van Gerven L Alpizar YA Wouters MM Hox V Hauben E Jorissen M et al . Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J Allergy Clin Immunol. (2014) 133:1332–39. 10.1016/j.jaci.2013.08.026
116.
Gevorgyan A Segboer C Gorissen R van Drunen CM Fokkens W . Capsaicin for non-allergic rhinitis. Cochrane Database Syst Rev. (2015) CD010591. 10.1002/14651858.CD010591.pub2
117.
Jaume F Valls-Mateus M Mullol J . Common cold and acute rhinosinusitis: up-to-date management in 2020. Curr Allergy Asthma Rep. (2020) 20:28. 10.1007/s11882-020-00917-5
118.
Fokkens WJ Lund VJ Hopkins C Hellings PW Kern R Reitsma S et al . European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. (2020) 58(Suppl. 29):1–464. 10.4193/Rhin20.600
119.
Reddel HK Bateman ED Becker A Boulet LP Cruz AA Drazen JM et al . A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. (2015) 46:622–39. 10.1183/13993003.00853-2015
120.
Hastan D Fokkens WJ Bachert C Boulet LP Cruz AA Drazen JM et al . Chronic rhinosinusitis in Europe–an underestimated disease. A GA2LEN study. Allergy. (2011) 66:1216–23. 10.1111/j.1398-9995.2011.02646.x
121.
Lourijsen ES Fokkens WJ Reitsma S . Direct and indirect costs of adult patients with chronic rhinosinusitis with nasal polyps. Rhinology. (2020) 58:213–7. 10.4193/Rhin19.468
122.
Lavigne F Cameron L Renzi PM Planet JF Christodoulopoulos P Lamkioued B et al . Intrasinus administration of topical budesonide to allergic patients with chronic rhinosinusitis following surgery. Laryngoscope. (2002) 112:858–64. 10.1097/00005537-200205000-00015
123.
Chung KF Wenzel SE Brozek JL et al . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73. 10.1183/09031936.00202013
124.
Ilmarinen P Tuomisto LE Niemelä O Kankaanranta H . Prevalence of patients eligible for anti-IL-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. (2019) 7:165–74.e164. 10.1016/j.jaip.2018.05.032
125.
Bakakos A Loukides S Bakakos P . Severe eosinophilic asthma. J Clin Med. (2019) 8:1375. 10.3390/jcm8091375
126.
Amelink M de Groot JC de Nijs SB Lutter R Zwinderman AH Sterk PJ et al . Severe adult-onset asthma: a distinct phenotype. J Allergy Clin Immunol. (2013) 132:336–41. 10.1016/j.jaci.2013.04.052
127.
Fajt ML Wenzel SE . Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. (2015) 135:299–310; quiz 311. 10.1016/j.jaci.2014.12.1871
128.
Rajan JP Wineinger NE Stevenson DD White AA . Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. (2015) 135:676–81.e671. 10.1016/j.jaci.2014.08.020
129.
Canonica GW Malvezzi L Blasi F Paggiaro P Mantero M Senna G et al . Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. (2020) 166:105947. 10.1016/j.rmed.2020.105947
130.
White AAMD Stevenson DDMD . Aspirin-exacerbated respiratory disease. N Engl J Med. (2018) 379:1060–70. 10.1056/NEJMra1712125
131.
Stevens WW Schleimer RP Kern RC . Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. (2016) 4:565–72. 10.1016/j.jaip.2016.04.012
132.
Stevens WW Peters AT Hirsch AG Nordberg CM Schwartz BS Mercer DG et al . Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. (2017) 5:1061–70.e1063. 10.1016/j.jaip.2016.12.027
133.
Kowalski ML Agache I Bavbek S Bakirtas A Blanca M Bochenek G et al . Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. (2018) 74:28–39. 10.1111/all.13599
134.
Howe R Mirakian RM Pillai P Gane S Darby YC Scadding GK . Audit of nasal lysine aspirin therapy in recalcitrant aspirin exacerbated respiratory disease. World Allergy Organ J. (2014) 7:18. 10.1186/1939-4551-7-18
135.
Mascia K Haselkorn T Deniz YM Miller DP Bleecker ER Borish L et al . Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. (2005) 116:970–5. 10.1016/j.jaci.2005.08.035
136.
Roca-Ferrer J Perez-Gonzalez M Garcia-Garcia FJ et al . Low prostaglandin E2 and cyclooxygenase expression in nasal mucosa fibroblasts of aspirin-intolerant asthmatics. Respirology. (2013) 18:711–7. 10.1111/resp.12076
137.
Morales DR Guthrie B Lipworth BJ Jackson C Donnan PT Santiago VH . NSAID-exacerbated respiratory disease: a meta-analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy. (2015) 70:828–35. 10.1111/all.12629
138.
Berges-Gimeno MP Simon RA Stevenson DD . The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. (2002) 89:474–8. 10.1016/S1081-1206(10)62084-4
139.
Oakley GM Christensen JM Sacks R Earls P Harvey RJ . Characteristics of macrolide responders in persistent post-surgical rhinosinusitis. Rhinology. (2018) 56:111–7. 10.4193/Rhin17.049
140.
DeConde AS Mace JC Levy JM Rudmik L Alt JA Smith TL . Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. (2017) 127:550–5. 10.1002/lary.26391
141.
Koskinen A Salo R Huhtala H Myller J Rautiainen M Kääriäinen J et al . Factors affecting revision rate of chronic rhinosinusitis. Laryngosc Investig Otolaryngol. (2016) 1:96–104. 10.1002/lio2.27
142.
Dona I Barrionuevo E Salas M Laguna JJ Agúndez J García-Martín E et al . NSAIDs-hypersensitivity often induces a blended reaction pattern involving multiple organs. Sci Rep. (2018) 8:16710. 10.1038/s41598-018-34668-1
143.
Velazquez JR Teran LM . Aspirin-intolerant asthma: a comprehensive review of biomarkers and pathophysiology. Clin Rev Allergy Immunol. (2013) 45:75–86. 10.1007/s12016-012-8340-0
144.
Lee RU Stevenson DD . Aspirin-exacerbated respiratory disease: evaluation and management. Allergy Asthma Immunol Res. (2011) 3:3–10. 10.4168/aair.2011.3.1.3
145.
Montinari MR Minelli S De Caterina R . The first 3500 years of aspirin history from its roots - a concise summary. Vascul Pharmacol. (2019) 113:1–8. 10.1016/j.vph.2018.10.008
146.
PubChem Compound Summary for CID 2244. Aspirin. Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin (accessed September 3, 2020).
147.
Swierczynska-Krepa M Sanak M Bochenek G Strek P Cmiel A Gielicz A et al . Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J Allergy Clin Immunol. (2014) 134:883–90. 10.1016/j.jaci.2014.02.041
148.
PubChem Compound Summary for CID 44219 Aspirin DL-Lysine. Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin-DL-lysine (accessed September 3, 2020).
149.
Lysine acetylsalicylate. In: AronsonJK editor. Meyler's Side Effects of Drugs. 16th ed. Oxford: Elsevier (2016). p. 706.
150.
Cortellini G Caruso C Romano A . Aspirin challenge and desensitization: how, when and why. Curr Opin Allergy Clin Immunol. (2017) 17:247–54. 10.1097/ACI.0000000000000374
151.
Laulajainen-Hongisto A Turpeinen H Vento SI Numminen J Sahlman J Kauppi P et al . High discontinuation rates of peroral ASA treatment for CRSwNP. A real-world multi-center study of 171 N-ERD patients. J Allergy Clin Immunol Pract. (2020) 8:3565–74. 10.1016/j.jaip.2020.06.063
152.
Mortazavi N Esmaeilzadeh H Abbasinazari M Babaie D Alyasin S Nabavizadeh H et al . Clinical and immunological efficacy of aspirin desensitization in nasal polyp patients with aspirin-exacerbated respiratory disease. Iran J Pharm Res. (2017) 16:1639–47.
153.
Esmaeilzadeh H Nabavi M Aryan Z Arshi S Bemanian MH Fallahpour M et al . Aspirin desensitization for patients with aspirin-exacerbated respiratory disease: a randomized double-blind placebo-controlled trial. Clin Immunol. (2015) 160:349–57. 10.1016/j.clim.2015.05.012
154.
Esmaeilzadeh H Nabavi M Arshi S Hassan Bemanian M Fallahpour M Aryan Z . Clinical and immunological effects of aspirin desensitization in patients with aspirin exacerbated respiratory diseases; a randomized, double blind, placebo controlled trial: 543. J Allergy Clin Immunol. (2015) 135:AB167. 10.1016/j.jaci.2014.12.1483
155.
Fruth K Pogorzelski B Schmidtmann I Springer J Fennan N Fraessdorf N et al . Low-dose aspirin desensitization in individuals with aspirin-exacerbated respiratory disease. Allergy. (2013) 68:659–65. 10.1111/all.12131
156.
Waldram J Walters K Simon R Woessner K Waalen J White A . Safety and outcomes of aspirin desensitization for aspirin-exacerbated respiratory disease: a single-center study. J Allergy Clin Immunol. (2018) 141:250–6. 10.1016/j.jaci.2017.05.006
157.
White AA Stevenson DD . Aspirin desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin N Am. (2013) 33:211–22. 10.1016/j.iac.2012.10.013
158.
Lee JYA Simon RAB Stevenson DDC . Selection of aspirin dosages for aspirin desensitization treatment in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. (2007) 119:157–64. 10.1016/j.jaci.2006.09.011
159.
Rozsasi A Polzehl D Deutschle T Smith E Wiesmiller K Riechelmann H et al . Long-term treatment with aspirin desensitization: a prospective clinical trial comparing 100 and 300 mg aspirin daily. Allergy. (2008) 63:1228–34. 10.1111/j.1398-9995.2008.01658.x
160.
Adappa ND Ranasinghe VJ Trope M Brooks SG Glicksman JT Parasher AK et al . Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. (2018) 8:49–53. 10.1002/alr.22036
161.
Stevenson DD Hankammer MA Mathison DA Christiansen SC Simon RA . Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol. (1996) 98:751–8. 10.1016/S0091-6749(96)70123-9
162.
Tajudeen BA Schwartz JS Bosso JV . The role of aspirin desensitization in the management of aspirin-exacerbated respiratory disease. Curr Opin Otolaryngol Head Neck Surg. (2017) 25:30–4. 10.1097/MOO.0000000000000331
163.
Milewski M Mastalerz L Nizankowska E Szczeklik A . Nasal provocation test with lysine-aspirin for diagnosis of aspirin-sensitive asthma. J Allergy Clin Immunol. (1998) 101:581–6. 10.1016/S0091-6749(98)70163-0
164.
Miller B Mirakian R Gane S Larco J Sannah AA Darby Y et al . Nasal lysine aspirin challenge in the diagnosis of aspirin - exacerbated respiratory disease: asthma and rhinitis. Clin Exp Allergy. (2013) 43:874–80. 10.1111/cea.12110
165.
Parikh AA Scadding GK . Intranasal lysine-aspirin in aspirin-sensitive nasal polyposis: a controlled trial. Laryngoscope. (2005) 115:1385–90. 10.1097/01.MLG.0000166702.38850.1B
166.
Patriarca G Bellioni P Nucera E Schiavino D Papa G Schinco G et al . Intranasal treatment with lysine acetylsalicylate in patients with nasal polyposis. Ann Allergy. (1991) 67:588–92.
167.
Nucera E Schiavino D Milani A Del Ninno M Misuraca C Buonomo A et al . Effects of lysine-acetylsalicylate (LAS) treatment in nasal polyposis: two controlled long term prospective follow up studies. Thorax. (2000) 55(Suppl. 2):S75–8. 10.1136/thorax.55.suppl_2.S75
168.
Ogata N Darby Y Scadding G . Intranasal lysine-aspirin administration decreases polyp volume in patients with aspirin-intolerant asthma. J Laryngol Otol. (2007) 121:1156–60. 10.1017/S0022215107000515
169.
Sousa AR Parikh A Scadding GK Corrigan CJ Lee TH . Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. (2002). 347:1493–9. 10.1056/NEJMoa013508
170.
Corrigan C Mallett K Ying S Roberts D Parikh A Scadding G et al . Expression of the cysteinyl leukotriene receptors cysLT(1) and cysLT(2) in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis. J Allergy Clin Immunol. (2005) 115:316–22. 10.1016/j.jaci.2004.10.051
171.
Scadding GK Hassab M Darby YC Lund VJ Freedman A . Intranasal lysine aspirin in recurrent nasal polyposis. Clin Otolaryngol Allied Sci. (1995) 20:561–3. 10.1111/j.1365-2273.1995.tb01603.x
172.
Parikh A Scadding GK . Topical nasal lysine aspirin in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis with nasal polyposis. Expert Rev Clin Immunol. (2014) 10:657–65. 10.1586/1744666X.2014.901889
173.
Nizankowska-Mogilnicka E Bochenek G Mastalerz L et al . EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. (2007) 62:1111–18. 10.1111/j.1398-9995.2007.01409.x
174.
van den Berg MP Verhoef JC Romeijn SG Merkus FWHM . Uptake of estradiol or progesterone into the CSF following intranasal and intravenous delivery in rats. Eur J Pharm Biopharm. (2004) 58:131–5. 10.1016/j.ejpb.2004.02.010
175.
Wang X Chi N Tang X . Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm. (2008) 70:735–40. 10.1016/j.ejpb.2008.07.005
176.
Arora P Sharma S Garg S . Permeability issues in nasal drug delivery. Drug Discov Today. (2002) 7:967–75. 10.1016/S1359-6446(02)02452-2
177.
Hurlemann R Patin A Onur OA Cohen MX Baumgartner T Metzler S et al . Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. (2010) 30:4999–5007. 10.1523/JNEUROSCI.5538-09.2010
178.
Avrech OM Goldman GA Pinkas H Amit S Neri A Zukerman Z et al . Intranasal nafarelin versus buserelin (short protocol) for controlled ovarian hyperstimulation before in vitro fertilization: a prospective clinical trial. Gynecol Endocrinol. (1996) 10:165–70. 10.3109/09513599609027984
179.
Jung BH Chung BC Chung SJ Lee MH Shim CK . Prolonged delivery of nicotine in rats via nasal administration of proliposomes. J Control Release. (2000) 66:73–9. 10.1016/S0168-3659(99)00258-8
180.
Braunstahl GJ Overbeek SE Kleinjan A Prins JB Hoogsteden HC Fokkens WJ . Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. (2001) 107:469–76. 10.1067/mai.2001.113046
181.
Braunstahl GJ Kleinjan A Overbeek SE Prins JB Hoogsteden HC Fokkens WJ . Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. (2000) 161:2051–7. 10.1164/ajrccm.161.6.9906121
Summary
Keywords
intranasal route, nasal epithelium, mucociliary clearance, allergic rhinitis, chronic rhinosinusitis, lysine aspirin, saline douche, drug delivery
Citation
Cingi C, Bayar Muluk N, Mitsias DI, Papadopoulos NG, Klimek L, Laulajainen-Hongisto A, Hytönen M, Toppila-Salmi SK and Scadding GK (2021) The Nose as a Route for Therapy: Part 1. Pharmacotherapy. Front. Allergy 2:638136. doi: 10.3389/falgy.2021.638136
Received
05 December 2020
Accepted
08 January 2021
Published
22 February 2021
Volume
2 - 2021
Edited by
Jo Rimmer, Monash Health, Australia
Reviewed by
Kachorn Seresirikachorn, Chulalongkorn University, Thailand; Supinda Chusakul, Chulalongkorn University, Thailand
Updates
Copyright
© 2021 Cingi, Bayar Muluk, Mitsias, Papadopoulos, Klimek, Laulajainen-Hongisto, Hytönen, Toppila-Salmi and Scadding.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Glenis Kathleen Scadding g.scadding@ucl.ac.uk
This article was submitted to Rhinology, a section of the journal Frontiers in Allergy
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.