- 1General Nutraceutical Technology LLC, Elmsford, NY, United States
- 2Department of Pathology, Microbiology & Immunology, New York Medical College, Valhalla, NY, United States
- 3Guangdong Hospital Department of Integrated Traditional Chinese and Western Medicine, Guangzhou University of Chinese Medicine, Foshan, China
- 4Department of Chinese Medicine, Henan University of Chinese Medicine, Zhengzhou, China
- 5Department of Biological Engineering, Utah State University, Logan, UT, United States
- 6Department of Otolaryngology, New York Medical College, Valhalla, NY, United States
Rationale: IgE plays an important pathologic role in most, if not all, allergic conditions. We previously showed that ASHMI (anti-asthma herbal medicine intervention) suppressed IgE production in murine models of asthma and in asthma subjects. However, the active compounds in ASHMI responsible for the IgE suppression are still unknown.
Objective: We sought to identify the compound(s) in ASHMI that are responsible for IgE inhibition as well as investigate the mechanisms by which the identified compound(s) decreases IgE production.
Methods: The compounds in Sophorae Flavescentis were separated using Column chromatography and preparative-HPLC. The separated compounds were identified using LC-MS and 1H-NMR. U266 cells, an IgE-producing plasma cell line, were cultured with various concentrations of identified compounds. The levels of IgE production by the U266 cell were measured by ELISA. Trypan blue exclusion was used to determine the cell viability. The gene expression of XBP-1 and IgE-heavy chain was determined by RT-PCR.
Results: A single compound identified as formononetin was isolated from Sophorae Flavescentis. Formononetin significantly and dose dependently decreased the IgE production in U266 cells across a concentration range of 2–20 µg/ml (p < 0.05–0.001 vs. untreated cells) with an IC50 value of 3.43 μg/ml. There was no cytotoxicity at any tested concentration. Formononetin significantly decreased XBP-1, and IgE-heavy chain gene expression compared with untreated cells (p < 0.001).
Conclusion: Formononetin decreased IgE production in human B cell line U266 cells in a dose-dependent fashion through the regulation of XBP-1 ER transcription. Formononetin may be a potential therapy for allergic asthma and other IgE-mediated diseases.
Introduction
There has been a steady increase in the prevalence of allergic diseases around the world. Allergy is now the 6th leading cause of chronic illnesses in the United States, with more than 50 million people suffering from the disease yearly (1). Globally about 300 million people suffer from asthma while about 30 million have allergies to some type of food (2). Most allergic diseases are IgE mediated. IgE is the central molecule that has been studied to be responsible for degranulating mast cells and basophils. It is one of the immunoglobulin isotypes, with the lowest abundance in the serum of normal individuals at a minute concentration of 50–200 ng/ml (3). Concentration of IgE in allergic individuals has been shown to increase to 10-fold that of normal serum levels (4). In order to trigger an allergic reaction, the ingested or inhaled allergen will bind to IgE which is bound to the FcεRI high affinity IgE receptors present on the surface of mast cells or basophils. This causes the crosslinking of the receptors and downstream degranulation to release the pharmacologic mediators responsible for clinical symptoms seen in allergic reactions (5).
The therapies approved for use in treatment of allergic diseases or that are currently undergoing clinical trials mostly function by binding circulating IgE; they do not directly suppress the production of IgE from plasma cells. Omalizumab (6), Ligelizumab (7), and MEDI4212 (8) are monoclonal antibodies that have been approved or are undergoing clinical trials, they function by neutralizing serum IgE. Quilizumab (9), Bsc-IgE/CD3 (10), and XmAb7195 (11) are monoclonal antibodies that have undergone clinical trials to treat IgE-mediated diseases by targeting IgE+ B cells leading to the decrease in the number of these cells, which, in turn, leads to a decrease in the production of serum IgE. Suplatast tosilate inhibits the production of IL-4 and IL-5 in Th2 cells, which causes a decrease in the synthesis of IgE, It has been used in the treatment of allergic asthma (12–16). Most of the current therapies used in treatment of IgE function by binding to circulating IgE, depleting IgE+ B cells or suppressing the production of Th2 cytokines. However, they are expensive, are associated with adverse reactions and do not offer long term protection.
ASHMI, Antiasthma Simplified Herbal Medicine Intervention, is a non-steroidal Chinese herbal formula with components; Ganoderma lucidum (GL), Sophorae flavescens (SF), and Glycyrrhiza uralensis (GU). ASHMI is effective in the treatment of Allergic Asthma, with clinical trials demonstrating acceptable levels of safety and tolerability (17, 18). Previous studies on chronic asthma murine model showed that ASHMI significantly reduced the Th2 cytokine levels and the OVA specific IgE levels vs. Sham group (19, 20) with sophorae flavescentis and ganoderma lucidum identified as the main components of ASHMI responsible for the Th2 cytokine suppression (21, 22). Compounds isoliquiritigenin, 7,4’ dihydroxyflavone (7, 4’-DHF), and liquiritigenin, isolated from g. uralensis were identified as the active compounds that are responsible for the suppression of the Th2 cytokines IL-4 and IL-5. Among these compounds, 7, 4’-DHF showed the highest potency on Th2 cytokine suppression (23). However, IgE inhibitory compounds from ASHMI remained to be identified. In this study, we sought to isolate and identify the bioactive components from ASHMI formula, which has IgE inhibitory effect in vitro, and investigate the mechanisms behind it.
Material and methods
Material and herbal products
RPMI 1640 and penicillin-streptomycin were purchased from Mediatech Inc (Manassas, VA), Fetal Bovine Serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). 2-Mercaptoethanol (2-ME), sodium pyruvate, [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich (St Louis, MO). Acetonitrile, dichloromethane, ethylacetate, methanol, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA).
ASHMI product is a dried aqueous extract of SF, GU, and GL, in capsule form (17). ASHMI and the water extract of all three herbal medicines were manufactured in a GMP-certified facility, the Sino-Lion Pharmaceutical Company in Weifang, China (17). All the herbs used for manufacture originated from China. The raw herbs used meet acceptable quality criteria as defined by the Pharmacopoeia of People's Republic of China (24). Briefly, the raw herbs were cut into small pieces and soaked in water for 60 min, then boiled for 2 h. The procedure was repeated twice, and the decoctions were collected and concentrated under vacuum (18).
Fractionation of radix Sophora flavescens and identification of active compounds
Dichloromethane extract of SF was performed utilizing liquid-liquid extraction method. Briefly, 50 g of SF provided by Sino-lion Pharmaceutical Company (P.R. China), was dissolved into 4 L water, and mixed with 3 L of CH2Cl2 in liquid-liquid extractor. 24 h later, the non-polar fraction was collected and dried on a rotary evaporator under low pressure. Methanol (300 ml) was used to re-dissolve the dried residues. The mixture was centrifuged at 2,500 rpm for 30 min and further separated with an AutoPurification system coupled with UV/Vis Detector (Waters, MA). Four fractions were collected based on the major peaks presented in the analytic HPLC chromatogram data.
Analysis of licorice flavonoids by liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance spectroscopy (NMR)
Compounds characterization was done using a Waters 2695 HPLC system (Waters Corporation, Milford, MA) coupled to a Waters Micromass LCT Premier mass spectrometer. Samples were separated on an analytical Column: Zorbax® SB C18 (5.0 µm, 4.6 × 150 mm) (Agilent Technologies, Santa Clara, CA) with 0.1% formic acid as mobile phase A and acetonitrile with 0.1% formic acid as mobile phase B. A gradient program was used as follows: 0–75 min, linear change from A–B (98:2, v/v) to A–B (52:48, v/v). A 15 min equilibration time was used before each HPLC run. The flow rate was set at 1 ml/min. The UV spectra were recorded between 200 and 460 nm. The detection wavelength was set at 254 nm, as previously described (25).
Molecular weight was identified using a Time-of-Flight mass spectrometer (LC-MS/TOF, Micromass LCT Premier, Waters Corporation) in both electrospray (ESI) positive and negative mode. 10 μl of sample was analyzed under the same gradient condition described above. The parameters were set as: Capillary Voltage: 3,200 v; Cone Voltage: 15 v; Aperture I: 25 v; Desolvation Temperature: 300°C; Source Temperature: 110°C; Desolvation gas: 500 L/h; Nebulizing gas: 40 L/h; Ionization mode: Electrospray; Positive Ion Acquisition Range: m/z 50–1,000. The results were collected and analyzed by Empower and Masslynx software (25).
1H NMR spectra were obtained on a JOEL instrument at 300 MHz using DMSO-d6 as the solvent (25).
IgE producing human U266 myeloma and cell culture
The human U266 myeloma cell line, purchased from ATCC (American Type Culture Collection; Rockville, MD) is widely used in the allergy research to screen the effects of compounds on B cell IgE production (26). Cells were cultured at 37°C under 5% CO2 in complete media containing RPMI 1640 medium supplemented, 10% FBS, 1 mM sodium pyruvate, 1 × 10−5 M β-ME and 0.5% penicillin-streptomycin. Cells were grown at an initial concentration of 2 × 105 cells/ml. The cells were treated with different concentrations of ASHMI, individual herb extracts, and purified compounds after 6 days culture. Supernatants were harvested, and IgE levels were determined by using an ELISA Kit (Mabtech Inc, OH). In some experiments, U266 cells were treated with active compound for 3 days. Cells were then subjected to western or qPCR analyses.
IgG producing ARH-77 cell culture
ARH-77 cell line, purchased from ATCC (American Type Culture Collection; Rockville, MD) to screen the effects of compound formononetin on B cell IgG production (27). Cells were cultured at 37°C under 5% CO2 in complete media containing RPMI 1640 medium supplemented, 10% FBS, 1 mM sodium pyruvate, 1 × 10−5 M β-ME and 0.5% penicillin-streptomycin. Cells were grown at an initial concentration of 2 × 105 cells/ml. The cell culture was incubated with test compound at concentration of 20, 10, 5, 2.5 (μg/ml) on day 0, supernatants were harvested after 3 days culture, and IgG levels were determined by using an ELISA Kit (Mabtech Inc, OH).
Human U266 and ARH-77 cell viability via trypan blue exclusion
Cell viability was evaluated using a trypan blue exclusion as previously described. Briefly, a 10 µl of cells suspension from each culture was mixed with equal volume of trypan blue dye (23). The mixture was loaded into a hemocytometer and cells were counted under a microscope. The percentage of viable cells was calculated as follows: Viable cells (%) = (total number of viable cells)/(total number of cells) × 100.
CCK-8 assay
Cell cytotoxicity was evaluated using cell counting kit-8 according to manufacturer's instruction, and previously published including ours (28, 29). We cultured U266 cells as described 2.4 and incubated with test compound formononetin at concentrations of 20, 10, 5 and 2.5 (μg/ml) for 3 days in a 96 well plate. 10 μl of CCK-8 was added to each well, after 10 min of incubation at 37°C, the optical density was read using a microplate reader at 450 nm.
Real time polymerase chain reaction (RT-PCR)
Quantitative real time PCR was used to detect the expression of Xbp-1 and (IgE-H). U266 (1.5 × 106 cells/ml) were co-incubated with formononetin (10 µg/ml) at 37°C under 5% CO2 for 3 days. Cells were then harvested, and total RNA was isolated using Trizol reagent (Gibco BRL, Rockville, MD) following the manufacturer's instructions. The RNA concentrations were then quantified by triplicate optical density (OD) readings (Bio-Rad SmartSpect 3000; Bio-Rad, Hercules, CA). The reverse transcription was performed to get the cDNA using RevertAid RT Reverse Transcription Kit (ThermoFisher, Waltham, MA) as described by the manufacturer's instructions. Pre-chill sterile, thin-walled tubes and reaction tubes on ice, pre-chill nuclease-free water. Combine the RNA (up to 1 µg) and the cDNA primer in nuclease-free water for a final volume of 5 µl per RT reaction on ice. Place the tube into preheated 70°C heat block for 5 min immediately chill the tube on ice for 5 min spin each tube to collect all condensate. Prepare the reverse transcription reaction mix ImProm-II™ 5X Reaction Buffer, MgCl2, dNTP, Recombinant RNasin® Ribonuclease Inhibitor, ImProm-II™ Reverse Transcriptase with nuclease-Free Water to a final volume of 15 μl. Add 15 µl of revese transcription reaction mix to each reaction tube containing the 5 µl RNA and primer mix. Final volume is 20 µl. Use PCR to finish the RT reaction at 25°C for 5 min, 42°C for 60 min, 70°C for 15 min. The RT-PCR amplification was performed using Maxima™ SYBR Green qPCR Master Mix (2X) kit (ThermoFisher, Waltham, MA) with epsilon germline primers or GAPDH primers. Primers were synthesized by Sigma-Aldrich Corporation (St. Louis, MO), see Supplementary Table S1 for the primer list.
Western blotting
We used western blotting analysis to measure the protein U266 cells at 3.0 × 106 cells/ml were cultured with formononetin at a concentration of 10 (µg/ml) for 3 days. After the culture time point the cells were harvested, centrifuged at 3,000 rpm for 5 min and the supernatant discarded. The cell suspension was washed with PBS and centrifuged at 3,000 rpm for 5 min. Then the cell lysis was done for protein extraction using 100–200 µl of RIPA lysis buffer, with intermittent vigorous vortexing and incubating on ice for about 1 h. After completion of lysis, the tubes were spined in centrifuge at 14,000 rpm for 20 min at 4 degrees Celsius. The supernatant was collected and transferred to another tube to measure the protein concentration. Electrophoresis was done at 90 V for 15 min followed by 100–110 V for the remaining time and transferred to a PVDF membrane. The membrane was incubated with primary antibodies of the XBP-1, β-actin overnight. The next day, the membrane was washed with PBST and incubated with secondary antibody for 2 h and after the membrane was washed with PBST 4 times before adding the chemiluminescence substrate and imaging. The primary antibodies of XBP-1 and β-actin were ordered from Cell Signaling (Danvers, MA, USA) and were used at a 1:1,000 dilution, the secondary antibody was used at a 1:10,000 dilution.
Statistics
All statistical analyses were performed using Sigma Stat 3.5 (Systat Software Inc., Chicago, IL) or GraphPad Prism (version 9, GraphPad Software, Inc, San Diego, CA). One way ANOVA (analysis of variance) was performed followed by Bonferroni correction for all pairwise comparisons. For skewed data, the differences between the groups were performed by one way ANOVA on rank followed by Donne's method for all pairwise comparisons. p-values were determined by a two-sided calculation. p value ≤ 0.05 was considered as statistically significant.
Results
ASHMI formula and its herbal constituents inhibited IgE production in a dose dependent manner
To evaluate the inhibitory effect of ASHMI formula on IgE production, human myeloma cell line U266 cells were cultured with ASHMI at different concentrations (0, 31, 62, 125, 250 and 500 µg/ml) for 6 days. After the culture time point, the cells were harvested, supernatant was collected and using ELISA IgE concentration was measured. Results showed that ASHMI formula significantly inhibited IgE production in a dose-dependent manner (Figure 1A, p < 0.05; p < 0.001) with an IC50 value of 49.76 µg/ml (Figure 1B). Trypan blue exclusion analysis showed that ASHMI did not affect cell viability at the concentrations tested (Figure 1C). Sophora favescens, Glycyrrhiza uralensis, and Ganoderma lucidum (three herb components of ASHMI) were cultured with U266 cells at a concentration of 250 µg/ml each. All three herbs significantly inhibited the production of IgE in U266 cells. Of the three herbs Sophora flavescens had the highest inhibitory effect on IgE production when compared to the others Glycyrrhiza uralensis and Ganoderma lucidum. The IgE inhibition percentage of Sophora flavescens is 96.54% ± 3.01%, while Glycyrrhiza uralensis and Ganoderma lucidum were 33.90% ± 5.63% and 33.35% ± 8.20% respectively (Figure 1C, p < 0.001). Further experiment showed that Sophora flavescens dose-dependently inhibited IgE production (Figure 1D, p < 0.01) with an IC50 values of 43.13 µg/ml (Figure 1F) without any significant toxicity (Figure 1E).
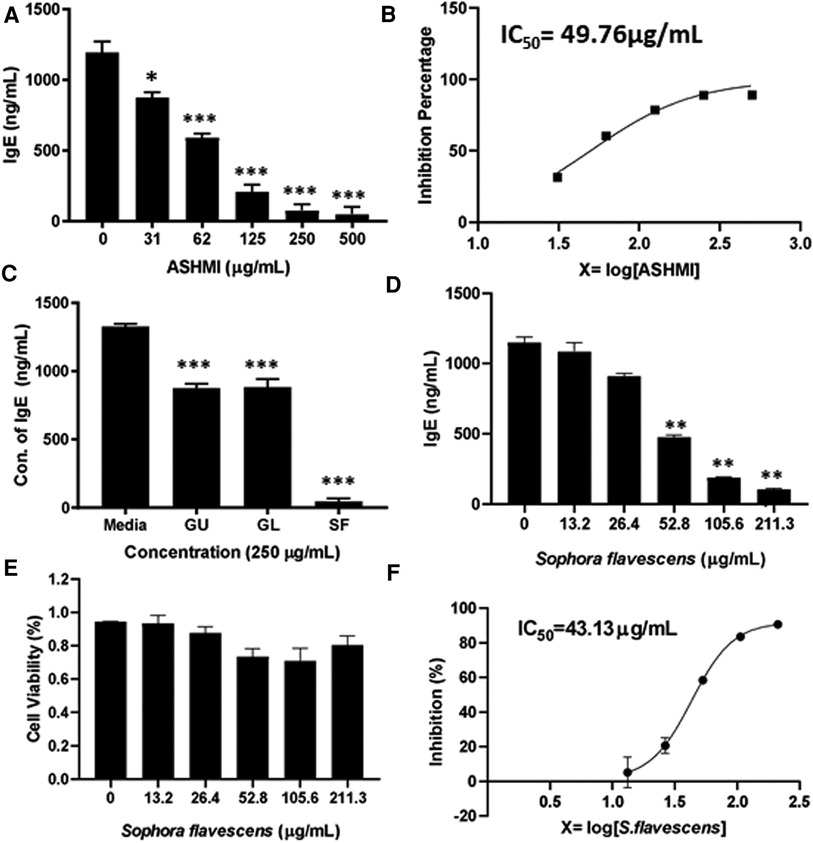
Figure 1. Dose-dependent effect of ASHMI formula and its herbal constituents on IgE production in U266 cells. (A) Inhibitory effect of ASHMI formula on IgE production by U266 cells. (B) Percentage of IgE inhibition vs. log [ASHMI concentration] curve. (C) Inhibitory effect of individual herbal constituents of ASHMI on IgE production at 250 µg/ml. (D) Inhibitory effect of Sophora flavescens on IgE production in vitro. (E) Cell viability of Sophora flavescens on U266 cells. (F) Percentage of IgE inhibition vs. log [Sophora flavescens concentration] curve. U266 cells (2 × 105 cells/ml) were cultured with ASHMI and individual herbal constituents at concentrations as indicated for 6 days. The supematants were harvested and IgE levels were measured by ELISA. Results were expressed as the mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001. Data represents triplicate experiments.
Sophora flavescens dichloromethane extract and its sub fractions inhibit IgE production in U266 cells
The process of isolating the active compounds in Sophora flavescens is shown in (Figure 2A). Organic solvent dichloromethane was used to isolate extract from Sophora flavescens. The extraction was collected and named SF-D. SF-D was further separated using Waters Auto-Purification system. Four subfractions were collected using auto-purification system based on the polarity (Figure 2B). Active compounds were isolated through a bioactivity guided fractionation process. The dichloromethane extract of Sophora flavescens, SF-D, was tested for its inhibitory effect on IgE production. The result showed that SF-D inhibits IgE production in a dose dependent fashion (Figure 3A, p < 0.001) with an IC50 value of 1.612 µg/ml (Figure 3C) without significant cytotoxicity (Figure 3B). The four sub-fractions of SF-D were also tested for their inhibitory effect on IgE. Results showed that all four subfractions significantly inhibited IgE production, with an inhibition percentage of; SF-DA −10.93% ± 3.17%; SF-DB −97.37% ± 0.03%; SF-DC −97.30% ± 0.25%; and SF-DD −94.37% ± 0.1% (Figure 3D, p < 0.01; p < 0.001). We next focused on SF-DB for further study because this fraction contains two major peaks, in addition to its potency of IgE suppression. SF-DC fraction showed one major peak and several small peaks and had very low yield therefore it would be difficult to isolate the pure compound from SF-DC. SF-DD was the last collection of the fraction process (i.e., it was collected as the washout) that contains many small peaks; therefore, it would be difficult to isolate pure compounds. Prior to further isolation and purification of SF-DB compounds, we tested SF-DB dose dependent effect on IgE production. It was shown that SF-DB dose-dependently inhibited the IgE production in a non-toxicity manner (Figure 3E, p < 0.05; Figure 3F, p < 0.01, Figure 3F). Further separation process was performed using auto purification system and one compound was purified. The purity of this compound was >96% as determined by analytical HPLC.
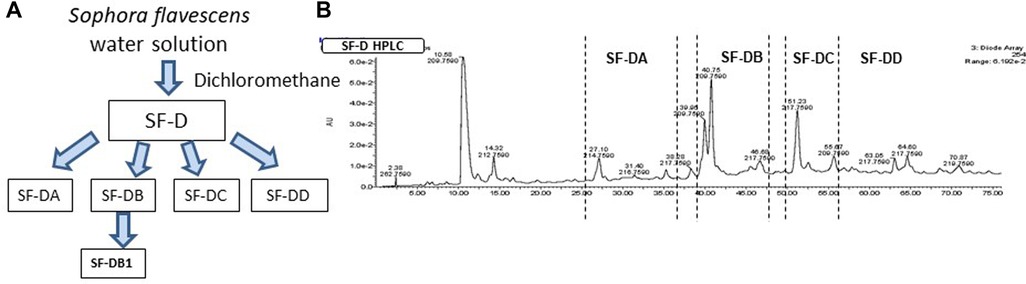
Figure 2. Compound isolation process of Sophora flavescens. (A) Flow chart of isolation and purification process of compounds from Sophora flavescens. (B) HPLC chromatogram of further separation using Auto-Purification system.
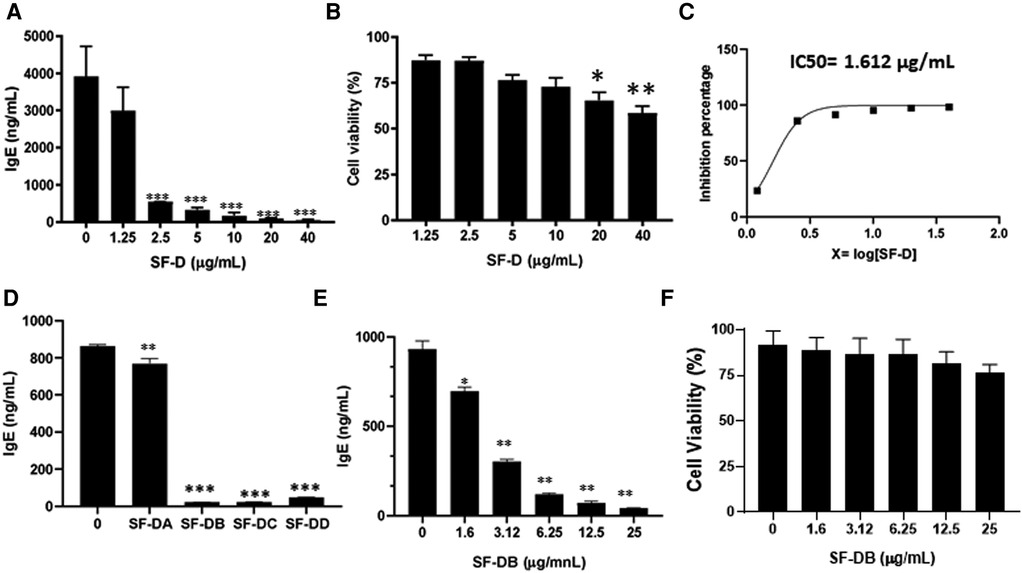
Figure 3. Different fractions of Sophora flavescens on IgE production in U266 cells. (A) Inhibitory effect of SF-D fractions on IgE production by U266 cells. (B) Cell viability of cultured U266 cells with SF-D fractions. (C) Percentage of IgE inhibition vs. log [SF-D concentration] curve. (D) Inhibitory effect of SF-D-sub-fractions, SF-DA, SF-DB, SF-DC, SF-DD on IgE production by U266 cells. (E) Dose-dependent experiment of SF-DB on IgE production by U266 cells. (F) Cell viability of cultured U266 cells with or without SF-DB.*p < 0.05; **p < 0.01; ***p < 0.001. Data represents triplicate experiments.
Highly potent sub-fraction of Sophora flavescens identified as formononetin
The molecular weight of the major compound isolated from fraction SF-DB was determined with LC-MS in both positive and negative mode (Figure 4A). Mass spectra data showed a significant [M + H]+ ion peak at m/z 269 and [M-H]− value of 267, and 1H-NMR spectrum was shown in Supplementary Figure S1) The molecular weight (MW) of this compound was then determined to be 268 g/mol. The 1H NMR data defined the isolated compound is formononetin (Figure 4B); this is consistent with previously reported data of formononetin (30). Thus, based on the LC-MS and 1H-NMR data, this isolated compound was identified as formononetin (Figure 4C). In addition, we also isolated a second compound and identified as maackiain by 1H-NMR (Supplementary Figure S2). However, the isolation efficiency of maackiain is very low using our prep HPLC system. We, therefore focused on formononetin for further anti IgE effect and underlying mechanisms.
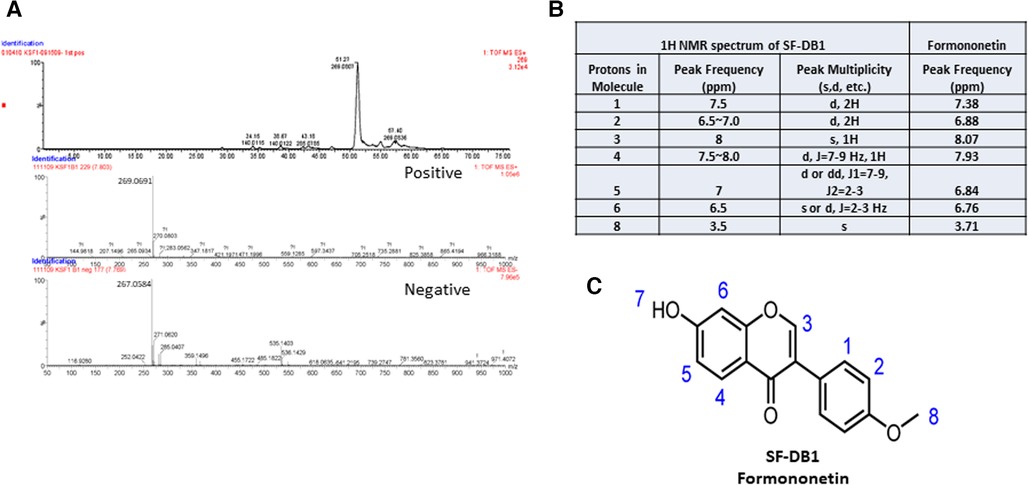
Figure 4. Characterization of isolated compound. (A) Mass spectra of SF-DB1 in both positive and negative mode. (B) 1H NMR spectra data of SF-DB1 (Formononetin). (C) Chemical structures of isolated compound, Formononetin.
Formononetin inhibits IgE production in U266 cells without affecting IgG in ARH-77 cells
To further examine the bioactivity of formononetin on IgE production, U266 cells were co-cultured with formononetin at concentrations (0.15, 0.31, 0.63, 1.25, 2.5, 5, 10, 20 µg/ml). The results showed that formononetin dose dependently inhibited the IgE production by U266 cells (Figure 5A, p < 0.05; p < 0.001). Formononetin significantly inhibited the IgE production in vitro at the concentration as low as 0.15 µg/ml. No significant toxicity was observed at concentrations lower than 1.25 µg/ml (Figure 5B). The IC50 value of formononetin was calculated as 0.16 µg/ml (Figure 5C). Having identified the compound formononetin, we obtained formononetin commercially from Sigma Aldrich, (St. Louis, MO), and tested at different concentrations (2.5, 5, 10, 20 µg/ml), using ELISA, we measured IgE, the results showed that formononetin dose dependently inhibited IgE production in U266 (Supplementary Figure S3A, p < 0.001). No significant cytotoxicity was observed as indicated by trypan blue measure (Supplementary Figure S3B) and cytotoxic measure using CCK-8 assay (Supplementary Figure S3C). To investigate if the inhibitory mechanism of formononetin is specific to IgE we cultured commercial formononetin with ARH-77 cells and measured the IgG production. Our result showed that formononetin did not significantly change the IgG production in the ARH-77 cells (Supplementary Figure S4).
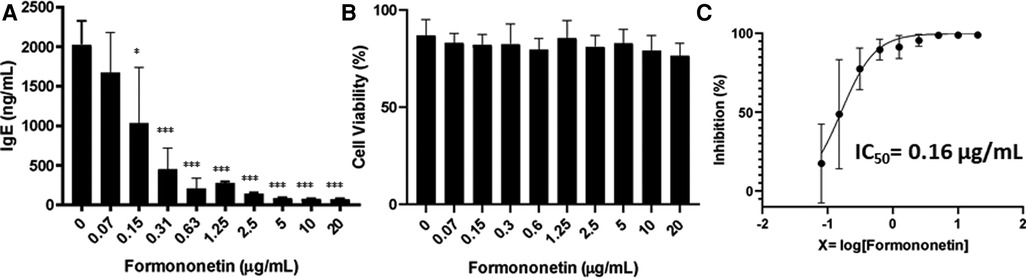
Figure 5. Formononetin inhibits IgE production in U266 cells in a dose dependent manner. (A) Inhibitory effect of formononetin on IgE production by U266 cells. (B) Cell viability of cultured U266 cells with or without formononetin. (C) Percentage of IgE inhibition vs. log [formononetin concentration] curve. *p < 0.05; **p < 0.01; ***p < 0.001. Data represents triplicate experiments.
Formononetin inhibits the XBP-1 and IgE heavy chain mRNA expression in U266 cells
IgE heavy chain transcription is a critical step for IgE isotype switching. To investigate the mechanism by which formononetin inhibits IgE production, mRNA expression of IgE heavy chain and XBP-1 was measured using real-time PCR. Human U266 cells were cultured with formononetin at 10 µg/ml, the total RNA in U266 cells was extracted and real time-PCR experiment was performed. Results showed that both the relative mRNA expression of IgE Heavy chain and XBP-1 were significantly downregulated by formononetin (Figure 6, p < 0.001) when compared to the untreated culture group.
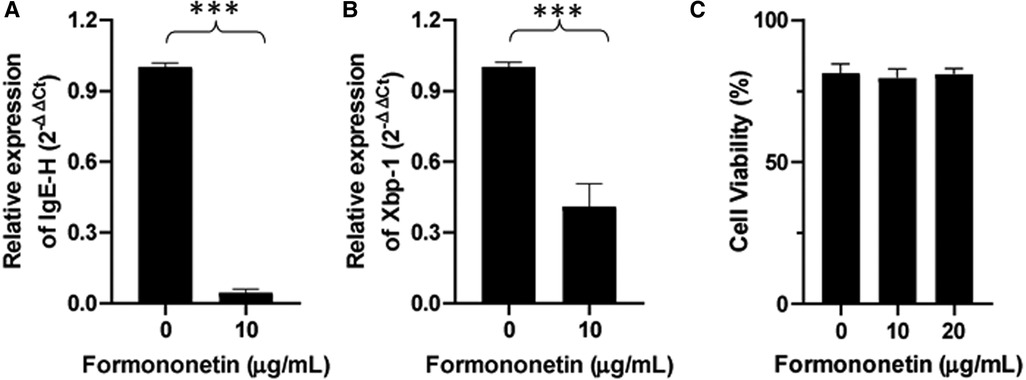
Figure 6. Formononetin inhibits the XBP-1 and IgE heavy chain mRNA expression in U266 cells. The relative expression level of IgE heavy chain (A) and XBP-1 (B) were determined by comparing with GAPDH mRNA expression. Fold change were calculated by the 2(−ΔΔCt) method. (C) Cell viability of formononetin on U266 cells at 10 and 20 µg/ml. Data are shown as the mean ± SEM. ***p < 0.001. Data represents triplicate experiments.
Formononetin inhibits the XBP-1 protein expression in U266 cells
The gene expression of XBP-1 was significantly decreased by formononetin, therefore we used western blotting to measure the effect of formononetin on the protein expression of XBP-1. Our results showed that formononetin significantly inhibited the protein expression of XBP-1 (Figure 7, p < 0.01).
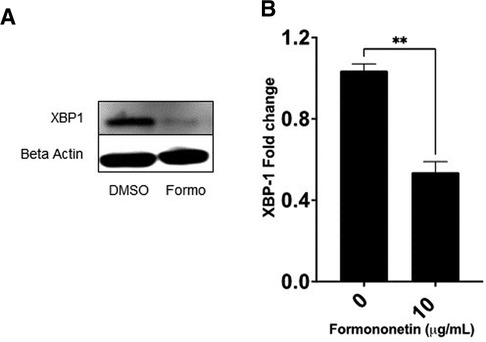
Figure 7. Formononetin inhibits protein XBP-1 protein expression. U266 cells were cultured with formononetin for 72 h and protein expression was determined using western blotting. (A) Western Bands (B) XBP-1 protein expression. Data represents triplicate experiments and expressed as mean ± SD. **p < 0.01 vs. control (0.1% DMSO).
Discussion
One of the herbal constituents of ASHMI, Sophora flavescens, was shown to be effective in reducing IgE. Sephora flavescens is an herb clinically used for the treatment of viral hepatitis, viral myocarditis, enteritis, gastrointestinal dysfunction, and skin diseases such as colpitis, psoriasis and eczema. SF was previously determined to have anti-inflammatory properties, interfering specifically with the TNF-α cytokine, which accounts for its traditional use in inflammatory-related disease (31). It was found that the main bioactive components of the herb are major alkaloids found to have antipyretic properties (32). However, alkaloids alone do not work to combat inflammatory response related to type I hypersensitivity and allergy related responses instigated through IgE. Therefore, it became important to investigate essential isoflavanoids in order to determine possible IgE inhibitory compounds. Further analysis using preparative HPLC showed that a subfraction of sephora flavescens was very effective in reducing IgE, and this fraction was identified using 1H-NMR analysis to be formononetin. Formononetin is a compound that belongs to a class of naturally occurring active compounds known as flavonoids. Flavonoids are an important class of natural products that have favorable biochemical and antioxidant, anti-inflammatory, anti-mutagenic and anti-carcinogenic effect associated with diseases such as cancer, alzheimer, atherosclerosis, and allergic diseases. Several studies have shown that flavonoids decrease IgE production. Therefore, it became important to investigate essential isoflavonoids in order to determine possible IgE inhibitory compounds. Formononetin isolated from Sephora flavescens one of the three main components of ASHMI is a compound that belongs to a class of naturally occurring flavonoid. Therefore, we sought to investigate the mechanism by which formononetin decreases IgE production using human B cell line U266.
Formononetin at a concentration of 20 µg/ml significantly decreased IgE production compared to zero treatment on U266 cells. This data is consistent with previous results obtained from different studies in which the whole herb extract was seen to decrease serum IgE level in mice. Our data suggested that formononetin is the main active compound responsible for IgE inhibition in SF. Cell viability results also showed that the formononetin has a high safety profile at the dose dependent concentration tested on U266 cells. Further study needs to be done to investigate the ability of formononetin to decrease IgE production in the PBMCs of patients with allergies.
IgE production requires class switch recombination between large and highly repetitive switch (S) regions. The human ɛ-germline gene promoter region consists of several promoters which includes STAT6 promoter region and NF-ĸB region. NF-ĸB binds to two sites in the ɛ-germline gene promoter and cooperates with STAT6 in synergistic action of transcription (39). The activity of STAT6 is highly influenced by NF-ĸB (33, 34). At the conclusion of Ig class switching, a B cell secretes one of the different classes of antibodies (35). Each of the three stages of class switching—germ line gene transcription, DNA recombination and B cell differentiation is very complex resulting in the activation of ɛ-germline gene transcription is essential (36).
Upon activation mature B cells undergo immunoglobulin class switch recombination and differentiate into antibody-secreting plasma cells (37). Antagonistic influences from two groups of transcription factors control the differentiation of B cells into plasma cells (38). The group of transcription factors that maintain B cell includes Pax5, Bach 2, and Bcl6 while the group of transcription factors that promote and facilitate plasma cell differentiation include Irf4, Blimp1 and XBP-1 (37). XBP-1 is a positively acting transcription factor in the CREB/ATF family that is expressed at a high level in plasma cells (37). XBP-1 acts downstream of Blimp-1 gene to regulate a broad complement of genes encoding ER-associated proteins, many of which are involved in protein secretion (38). XBP-1 induces physical expansion of the ER, in addition to increasing cell size, organelle biosynthesis, total protein synthesis. This shows that XBP-1 plays a critical role in the secretion of immunglobulins (38). XBP-1 is uniformly expressed in all multiple myeloma cell lines (38) (U266 cell is a multiple myeloma cell line). Because of the role of XBP-1 in secretion of immunoglobulin we decided to investigate if whether it is inhibited in human B cell line U266 cultured with formononetin. U266 cells were cultured with 20 µg/ml of formononetin, and the cells were harvested after 6 days for RNA isolation and RT-PCR was done to analyze gene expression levels. Our result showed that formononetin decreased the XBP-1 gene expression significantly when compared with untreated cell cultures. We believe that XBP-1 inhibition is another mechanism that formononetin uses in decreasing IgE secretion. This is the only study to show that formononetin inhibits XBP-1 in human B cell line U266 cells.
Activation of ɛ germline gene is an essential step towards IgE expression (35, 36), which leads to generation of ɛ mRNA transcripts after class switch recombination. The presence of IgEH transcripts in the B cell provides evidence that class switch recombination has occurred in the germline region. In our experiment, we used an IgE-producing myeloma cell line. This cell line already underwent class switch recombination to IgE. As such, the expression of IgE heavy chain in these cells is largely under control of the promoters located 5’ of the variable (V) gene and the enhancers located between the joining and constant genes in the IgH. Therefore, we used RT-PCR to compare the relative expression of IgE heavy chain transcripts in the U266 cultured with formononetin against untreated cultures. The result showed that there was a significant decrease in IgEH mRNA transcripts in the treated culture compared to untreated cultures. This finding indicates that formononetin may be a potential therapeutic in established IgE-mediated allergies such as food allergy. Studies are underway to investigate formononetin effect in peanut allergic murine model. Nevertheless, the limitation of this study include that we were unable to determine the effect of formononetin on IgE class switch recombination (CSR) using this cell line. Further studies are needed using a primary B cell line to investigate if indeed formononetin alters germline transcription and possibly prevents class switch recombination.
Conclusion
We, for the first time, demonstrated that Sophora flavescens, one of the three herbal constituents of ASHMI, is the major component which inhibited the IgE production. Formononetin, isolated from Sophora flavescens, significantly inhibited IgE production in vitro without altering cell viability. The IgE inhibitory mechanisms of formononetin might be through the regulation of XBP-1.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
X-ML designed the experiment. NY, IM, AM, KL, ZW, and SZ conducted the research work, wrote, and contributed to the writing of the manuscript. NY, IM, KL, ZW wrote up the data analysis. NY, IM and X-ML contributed to the discussion. IM, BL, JZ and X-ML revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institutes of Health grants # PO1 AT002647 and by Sean P Parker Foundation to X-ML.
Acknowledgments
We thank Ivan Lopez for his early work related to this manuscript. We also thank Henry Ehrlich for reading this manuscript.
Conflict of interest
X-ML received research support to her institution from the National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM) # 1P01 AT002644725-01 “Center for Chinese Herbal Therapy (CHT) for Asthma”, and grant #1R01AT001495-01A1 and 2R01 AT001495-05A2, NIH/NIAID R43AI148039, Food Allergy Research and Education (FARE), Winston Wolkoff Integrative Medicine Fund for Allergies and Wellness, the Parker Foundation and Henan University of Chinese Medicine; received consultancy fees from FARE and Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Bayer Global Health LLC; received royalties from UpToDate; is an Honorary Professor of Chinese Medical University, Taichung, Taiwan; Henan University of Chinese Medicine Zhenzhou, China, and Professorial Lecture at Icahn School of Medicine at Mount Sinai, New York, NY, US; received travel expenses from the NCCAM and FARE; share US patent US7820175B2 (FAHF-2), US10500169B2 (XPP), US10406191B2 (S. Flavescens), US10028985B2 (WL); US11351157B2 (nanoBBR): take compensation from her practice at Center for Integrative Health and Acupuncture PC; US Times Technology Inc is managed by her related party; is a member of General Nutraceutical Technology LLC and Health Freedom LLC. NY received research support from the National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM), NIH/NIAID R43AI148039; shares US patent: US10500169B2 (XPP), US10406191B2 (S. Flavescens), US10028985B2 (WL); and is a member of General Nutraceutical Technology LLC and Health Freedom LLC; receives a salary from General Nutraceutical Technology LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2022.1056203/full#supplementary-material.
References
1. American College of Allergy Asthma & Immunology. Allergy facts. Available at: https://acaai.org/allergies/allergies-101/facts-stats/.
2. National Institute of Allergy and Infectious Diseases. Diseases & conditions. Available at: https://www.niaid.nih.gov/diseases-conditions?f%5B0%5D=disease%3A51.
3. Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. (2014) 14(4):247–59. doi: 10.1038/nri3632
4. Platts-Mills TA, Snajdr MJ, Ishizaka K, Frankland AW. Measurement of IgE antibody by an antigen-binding assay: correlation with PK activity and IgG and IgA antibodies to allergens. J Immunol. (1978) 120(4):1201–10. doi: 10.4049/jimmunol.120.4.1201
5. Janeway CA, Travers JP, Walport M, Shlomchik MJ. Immunobiology, 5th edition: The immune system in health and disease. New York: Garland Science (2001).
6. Hendeles L, Sorkness CA. Anti-immunoglobulin E therapy with omalizumab for asthma. Ann Pharmacother. (2007) 41(9):1397–410. doi: 10.1345/aph.1K005
7. Gauvreau GM, Arm JP, Boulet LP, Leigh R, Cockcroft DW, Davis BE, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol. (2016) 138(4):1051–9. doi: 10.1016/j.jaci.2016.02.027
8. Sheldon E, Schwickart M, Li J, Kim K, Crouch S, Parveen S, et al. Pharmacokinetics, pharmacodynamics, and safety of MEDI4212, an anti-IgE monoclonal antibody, in subjects with atopy: a phase I study. Adv Ther. (2016) 33(2):225–51. doi: 10.1007/s12325-016-0287-8
9. Harris JM, Maciuca R, Bradley MS, Cabanski CR, Scheerens H, Lim J, et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res. (2016) 17:29. doi: 10.1186/s12931-016-0347-2
10. Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, et al. Ige(+) memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. (2012) 13(4):396–404. doi: 10.1038/ni.2256
11. Chu SY, Yeter K, Kotha R, Pong E, Miranda Y, Phung S, et al. Suppression of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody that coengages B cell antigen receptor complex and fcγ receptor IIb inhibitory receptor. Arthritis Rheumatol. (2014) 66(5):1153–64. doi: 10.1002/art.38334
12. Yanagihara Y, Kiniwa M, Ikizawa K, Shida T, Matsuura N, Koda A. Suppression of IgE production by IPD-1151 T (suplatast tosilate), a new dimethylsulfonium agent: (2). regulation of human IgE response. Jpn J Pharmacol. (1993) 61(1):31–9. doi: 10.1254/jjp.61.31
13. Yoshida M, Aizawa H, Inoue H, Matsumoto K, Koto H, Komori M, et al. Effect of suplatast tosilate on airway hyperresponsiveness and inflammation in asthma patients. J Asthma. (2002) 39(6):545–52. doi: 10.1081/jas-120004925
14. Sano Y, Suzuki N, Yamada H, To Y, Ogawa C, Ohta K, et al. Effects of suplatast tosilate on allergic eosinophilic airway inflammation in patients with mild asthma. J Allergy Clin Immunol. (2003) 111(5):958–66. doi: 10.1067/mai.2003.1415
15. Tamaoki J, Kondo M, Sakai N, Aoshiba K, Tagaya E, Nakata J, et al. Effect of suplatast tosilate, a Th2 cytokine inhibitor, on steroid-dependent asthma: a double-blind randomised study. Tokyo joshi-idai asthma research group. Lancet. (2000) 356(9226):273–8. doi: 10.1016/s0140-6736(00)02501-0
16. Shioya T, Satake M, Sano M, Kagaya M, Watanabe A, Sato K, et al. Effect of suplatast tosilate, a Th2 cytokine inhibitor, on cough variant asthma. Eur J Clin Pharmacol. (2002) 58(3):171–6. doi: 10.1007/s00228-002-0468-z
17. Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. (2005) 116(3):517–24. doi: 10.1016/j.jaci.2005.05.029
18. Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Li XM, et al. Safety and tolerability of an antiasthma herbal formula (ASHMI) in adult subjects with asthma: a randomized, double-blinded, placebo-controlled, dose-escalation phase I study. J Altern Complement Med. (2009) 15(7):735–43. doi: 10.1089/acm.2008.0543
19. Srivastava K, Teper AA, Zhang TF, Li S, Walsh MJ, Huang CK, et al. Immunomodulatory effect of the antiasthma Chinese herbal formula MSSM-002 on TH2 cells. J Allergy Clin Immunol. (2004) 113(2):268–76. doi: 10.1016/j.jaci.2003.10.062
20. Srivastava K, Zhang T, Yang N, Sampson H, Li XM. Anti-Asthma simplified herbal medicine intervention-induced long-lasting tolerance to allergen exposure in an asthma model is interferon-gamma, but not transforming growth factor-beta dependent. Clin Exp Allergy. (2010) 40(11):1678–88. doi: 10.1111/j.1365-2222.2010.03545.x
21. Chen ML, Lin BF. Effects of triterpenoid-rich extracts of ganoderma tsugae on airway hyperreactivity and Th2 responses in vivo. Int Arch Allergy Immunol. (2007) 143(1):21–30. doi: 10.1159/000098222
22. Wen MC, Huang CK, Srivastava KD, Zhang TF, Schofield B, Sampson HA, et al. Ku-Shen(Sophora Flavescens ait), a single Chinese herb, abrogates airway hyperreactivity in a murine model of asthma. J Allergy Clin Immunol. (2004) 113(2):218. doi: 10.1016/j.jaci.2004.01.237
23. Yang N, Patil S, Zhuge J, Wen MC, Bolleddula J, Doddaga S, et al. Glycyrrhiza uralensis flavonoids present in anti-asthma formula, ASHMI™, inhibit memory Th2 responses in vitro and in vivo. Phytother Res. (2013) 27(9):1381–91. doi: 10.1002/ptr.4862
24. China TSPCoTPsRo. Pharmacopoeia of the people's republic of China. Vol 1. Beijing: People's Medical Publishing House (2005).
25. Yang N, Liang B, Srivastava K, Zeng J, Zhan J, Brown L, et al. The Sophora flavescens flavonoid compound trifolirhizin inhibits acetylcholine induced airway smooth muscle contraction. Phytochemistry. (2013) 95:259–67. doi: 10.1016/j.phytochem.2013.07.023
26. Yang N, Wang J, Liu C, Song Y, Zhang S, Zi J, et al. Berberine and limonin suppress IgE production by human B cells and peripheral blood mononuclear cells from food-allergic patients. Ann Allergy Asthma Immunol. (2014) 113(5):556–564.e4. doi: 10.1016/j.anai.2014.07.021
27. Drewinko B, Mars W, Stragand JJ, Henderson SD, Latreille J, Barlogie B, et al. ARH-77, an established human IgG-producing myeloma cell line. II. Growth kinetics, clonogenic capacity, chalone production, xenogeneic transplantations, and response to melphalan. Cancer. (1984) 54(9):1893–903. doi: 10.1002/1097-0142(19841101)54:9%3C1893::aid-cncr2820540920%3E3.0.co;2-e
28. Kagawa K, Nakano A, Miki H, Oda A, Amou H, Takeuchi K, et al. Inhibition of TACE activity enhances the susceptibility of myeloma cells to TRAIL. PLoS One. (2012) 7(2):e31594.22389670
29. Wang ZZ, Li K, Maskey AR, Huang W, Toutov AA, Yang N, et al. A small molecule compound berberine as an orally active therapeutic candidate against COVID-19 and SARS: A computational and mechanistic study. Faseb j. (2021) 35(4):e21360.33749932
30. Zheng L, Wang M, Ibarra-Estrada E, et al. Investigation of chemomarkers of astragali radix of different ages and geographical origin by NMR profiling. Molecules. (2015) 20(2):3389–405. doi: 10.3390/molecules20023389
31. Zhou H, Lutterodt H, Cheng Z, Yu LL. Anti-Inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J Agric Food Chem. (10 2009) 57(11):4580–5. doi: 10.1021/jf900340b
32. The State Pharmacopoeia Commission of The People's Republic of China. Pharmacopoeia of the people's republic of China. Vol 1. Beijing: People's Medical Publishing House (2005).
33. Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. (2011) 50(1):87–96. doi: 10.1007/s12026-011-8205-2
34. Tinnell SB, Jacobs-Helber SM, Sterneck E, Sawyer ST, Conrad DH. STAT6, NF-kappaB and C/EBP in CD23 expression and IgE production. Int Immunol. (1998) 10(10):1529–38. doi: 10.1093/intimm/10.10.1529
35. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. (2003) 3(9):721–32. doi: 10.1038/nri1181
36. Oettgen HC. Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of epsilon germline transcripts. Curr Opin Immunol. (2000) 12(6):618–23. doi: 10.1016/s0952-7915(00)00153-9
37. Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. (2008) 26:261–92. doi: 10.1146/annurev.immunol.26.021607.090248
Keywords: asthma, antiasthma simplified herbal medicine intervention (ASHMI), formononetin, IgE inhibition, IgE heavy chain, XBP-1
Citation: Yang N, Musa I, Maskey AR, Li K, Wang Z, Liang B, Zhang S, Zhan J and Li X (2023) Formononetin isolated from Sophorae flavescentis inhibits B cell-IgE production by regulating ER-stress transcription factor XBP-1. Front. Allergy 3:1056203. doi: 10.3389/falgy.2022.1056203
Received: 28 September 2022; Accepted: 12 December 2022;
Published: 1 February 2023.
Edited by:
Claudio Rhyner, Head of Vaccine Development SIAF, SwitzerlandReviewed by:
Willem Van De Veen, University of Zurich, SwitzerlandMar Martin-Fontecha, Complutense University of Madrid, Spain
© 2023 Yang, Musa, Maskey, Li, Wang, Liang, Zhang, Zhan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu-Min Li WGl1TWluX0xpQG55bWMuZWR1
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Therapies and Therapeutic Targets, a section of the journal Frontiers in Allergy
 Nan Yang1,2,†
Nan Yang1,2,† Ibrahim Musa
Ibrahim Musa Anish R. Maskey
Anish R. Maskey Zhenzhen Wang
Zhenzhen Wang Banghao Liang
Banghao Liang Jixun Zhan
Jixun Zhan Xiu-Min Li
Xiu-Min Li