- 1Department of Otolaryngology, National Taiwan University Hospital, Taipei, Taiwan
- 2College of Medicine, National Taiwan University, Taipei, Taiwan
Chronic rhinosinusitis (CRS) is one of the most common causes of inflammation of the olfactory system, warranting investigation of the link between chronic inflammation and the loss of olfactory function. Type 2 inflammation is closely related to the clinical features and disease mechanisms of olfactory dysfunction secondary to CRS. Patients with eosinophilic CRS, aspirin-exacerbated respiratory disease, and central compartment atopic disease report increased olfactory dysfunction. Increased levels of interleukin-(IL-)2, IL-5, IL-6, IL-10, and IL-13 in the mucus from the olfactory slit have been reported to be associated with reduced olfactory test scores. The influence of several cytokines and signaling transduction pathways, including tumor necrosis factor-α, nuclear factor-κB, and c-Jun N-terminal kinases, on olfactory signal processing and neurogenesis has been demonstrated. Corticosteroids are the mainstay treatment for olfactory dysfunction secondary to CRS. Successful olfaction recovery was recently demonstrated in clinical trials of biotherapeutics, including omalizumab and dupilumab, although the treatment effect may diminish gradually after stopping the use of the medications. Future studies are required to relate the complex mechanisms underlying chronic inflammation in CRS to dysfunction of the olfactory system.
Introduction
Olfactory dysfunction is one of the cardinal symptoms of chronic rhinosinusitis (CRS) (1–3). CRS has been reported to affect 13.4% of the American (4) and 10.9% of the European (5) general population, and up to 80% of CRS patients experience reduction or loss of smell (6), which significantly impedes quality of life (7–9). By phenotyping, CRS can be classified as chronic rhinosinusitis with nasal polyps (CRSwNP) and chronic rhinosinusitis without nasal polyps (CRSsNP). Olfactory dysfunction is observed more frequently in patients with CRSwNP (6, 10, 11), and olfaction may temporarily improve after surgery but deteriorate later (12). Olfactory dysfunction has been regarded as the consequence of obstructed air flow to the olfactory slit but increasing evidence has shown that inflammation in the olfactory neuroepithelium leads to dysfunction of the transduction of olfactory signals.
The olfactory neuroepithelium is located at the main air flow pathway in the nasal cavity and it continues with the respiratory epithelium. This spatial location makes the olfactory neuroepithelium easily susceptible to various inhaled substances, including viruses, molds, allergens, pollutants, and toxic materials. These epithelial stimulants may lead to recruitment of inflammatory cells, increased proinflammatory factors, and changes in ciliary function and secretion from goblet cells. Oral corticosteroids are the main treatment of olfactory dysfunction in patients with CRS. An initial rapid response may be followed by gradual diminishment of the treatment effect. Patients with olfactory dysfunction may eventually become corticosteroid-dependent, and long-term corticosteroid treatment may be accompanied by increasing side effects.
The critical issue is to understand the disease mechanism and find a suitable long-term treatment for olfactory dysfunction. In this review article, we present the clinical features related to olfactory dysfunction, investigate the influence of inflammation on neurogenesis and olfactory processing, and analyze the medical management of olfactory dysfunction secondary to chronic rhinosinusitis.
Olfactory Dysfunction and Chronic Rhinosinusitis Endotypes
The emerging view is that CRS is a heterogenous syndrome resulting from a dysfunctional interaction between various environmental factors and the host immune system (3). Extensive scientific evidence has been accumulated that justifies the differentiation of CRS by recognition of more detailed endotypes, i.e., definition by the presence of particular patterns of immune cells and/or biomarkers (3). The clinical dichotomization of CRSwNP vs. CRSsNP was initially indicated by a predominance of TH1 cells in CRSsNP patients and TH2 cells and eosinophils in CRSwNP patients (13, 14). However, this definition has proven difficult to apply in East Asia where a neutrophilic type of inflammation with involvement of other T-cell subsets, such as TH1 and TH17 cells, has been observed aside from eosinophil-dominant inflammation (15–18). The EPOS2020 categorizes primary CRS by endotype dominance into type 2 or non-type 2 (3). Type 2 inflammation is characterized by the presence of increased levels of cytokines interleukin-(IL-)4, IL-5, and IL-13, as well as activation and recruitment of eosinophils and mast cells.
The risk factors for olfactory dysfunction differed between CRS endotypes, and CRS patients with type 2 inflammation endotype reported loss or reduction of olfaction more frequently than those with non-type 2 CRS (19). Esoinophilic CRS is the most dominant type of type 2 CRS, and the association of esosinophilic CRS and olfactory dysfunction has been well-recognized (18, 20). The most apparent difference in computed tomography (CT) images of eosinophilic CRS compared to non-eosinophilic CRS images is an ethmoid sinus predominance pattern (21). CT images of the early stage of eosinophilic CRS show opacification of the posterior ethmoid sinus and the olfactory cleft (18). Mori et al. (19) identified olfactory cleft polyps, current smoking, serum IgE ≥400 IU/ml, ethmoid opacification, and asthma as independent risk factors for olfactory dysfunction in eosinophilic CRS. In non-eosinophilic CRS, only ethmoid opacification and olfactory cleft polyps were identified as independent risk factors for olfactory dysfunction. Aspirin-exacerbated respiratory disease (AERD) has also been identified as one of the independent factors of olfactory dysfunction in CRS patients (22, 23).
Central compartment atopic disease (CCAD) is another CRS subtypes of type 2 CRS. DelGaudio et al. (24) were the first to define CCAD as one of the subtypes of CRS in 2017 and associate CCAD with inhalant allergen sensitization. CCAD is characterized as an inflammation and edematous change of the central sinonasal compartment, including the middle turbinate, superior turbinate, and posteriosuperior nasal septum (24, 25). CCAD presents as a polypoid edema of the middle turbinate on endoscopic examination. A centrally limited sinus inflammation entity on the CT scan has been defined as having normal sinus mucosal or mucosal thickening involving only the floor or medial wall of the ethmoid sinuses (25, 26). We previously found that central-compartment-type CRS represented an eosinophilic/type 2 inflammation endotype, with elevated expression of IL-5 and IL-13 in the sinonasal tissues, and patients with this central-compartment subtype of CRS had more smell problems as major symptoms than patients with other CRS subtypes (27).
Mechanisms of Olfactory Dysfunction Secondary to CRS
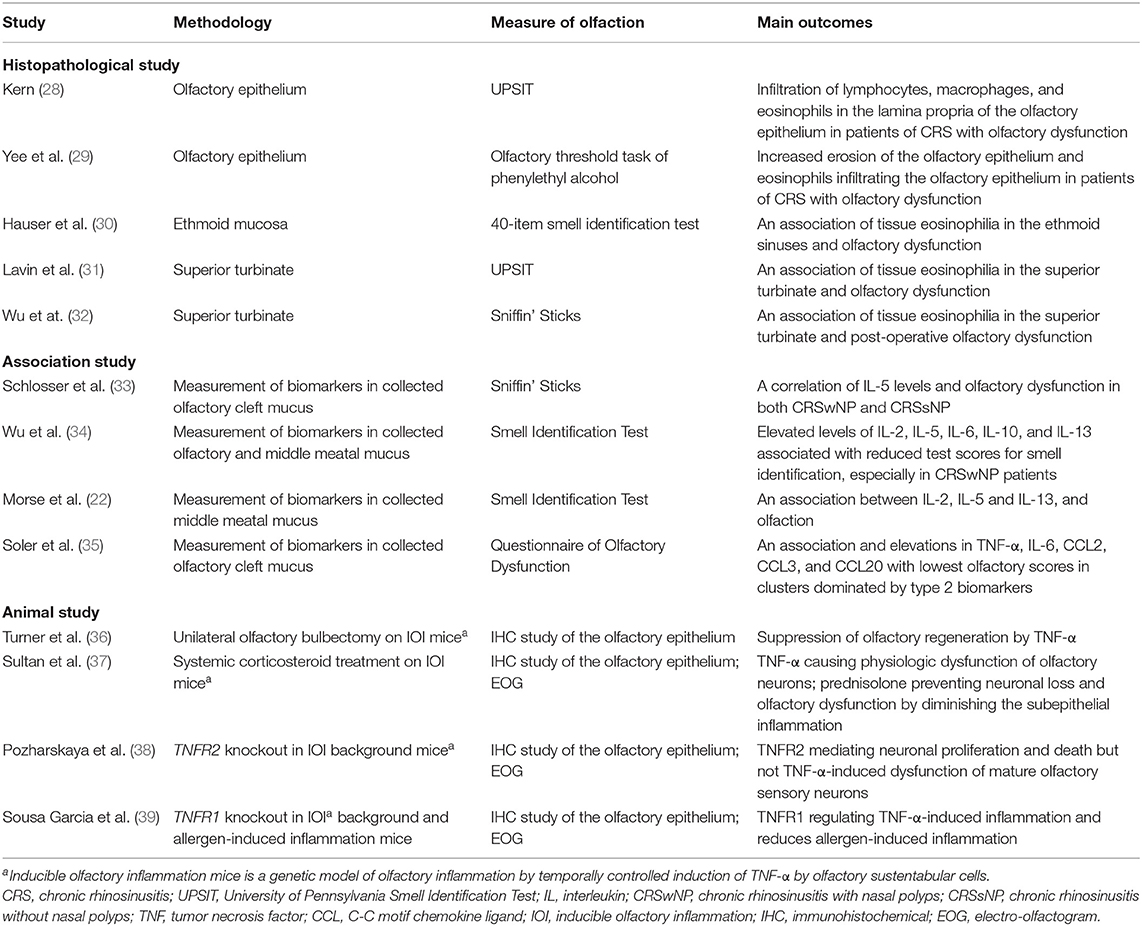
With advances in immunologic and histopathological studies, the rationale of olfactory dysfunction secondary to CRS is regarded not only as diminished access of odorant molecules to the neuroepithelium (conduction disorder) but also direct effects on the olfactory mucosa (sensory disorder) (10). Olfactory dysfunction due to chronic rhinosinusitis is relatively reversible compared to other causes. As short-term anti-inflammatory treatment can generally render rapid regain of olfactory function, the mechanism of olfactory dysfunction may be reversible after elimination of inflammation. The schematic diagram of the olfactory mucosa and the current evidence of the impact of inflammation on the peripheral olfactory system were illustrated in the Figure 1, and the possible mechanisms and molecules involved in the development of olfactory dysfunction were summarized in the Table 1.

Figure 1. The illustration of the structure of the olfactory mucosa and the known inflammatory mechanisms leading to olfactory dysfunction. IL, interleukin; CCL, C-C motif chemokine ligand; TNF, tumor necrosis factor.
Change in Histopathologic Images of the Olfactory Epithelium in CRS
The respiratory mucosa is pseudostratified and ciliated with goblet cells, a highly vascular lamina propria and a thick basement membrane, whereas the olfactory mucosa is characterized by irregular cilia, a cellular lamina propria, Bowman's glands, and a thin basement membrane. The olfactory neuroepithelium contains three major cell types of the peripheral sensory system, including olfactory sensory neurons (OSNs), sustentacular cells, and basal cells (Figure 1). Sustentacular cells enwrap olfactory sensory neurons to maintain the integrity and function of OSNs. Basal cells are located along the basement membrane and capable of replenishing OSNs to maintain ongoing neurogenesis during adult life. OSNs and their progenitors are particularly susceptible to local immune mediators in the setting of rhinosinusitis (40, 41).
As we observed compromised integrity of the epithelium and infiltrating immune cells in the respiratory epithelium in cases of CRS, similar histopathology in the olfactory neuroepithelium may account for the dysfunction of the peripheral olfactory system (28). Yee et al. (29) studied the neuropathology of the olfactory mucosa in CRS and found a significant decrease in the percentage of normal olfactory neuroepithelium, a reduction of mature OSNs in CRS biopsy specimens, and a variety of epithelial changes, including intermixing of goblet cells, metaplasia to squamous-like cells, and erosion of the olfactory neuroepithelium. With continued inflammation in the olfactory mucosa, an abnormal epithelium and infiltration of lymphocytes, macrophages, and eosinophils in the lamina propria were identified (28). Eosinophilic infiltration may play a significant role in cases of olfactory disability, and CRS patients, especially those with nasal polyps, presenting with anosmia had the greatest amount of epithelial erosion and the highest density of eosinophil infiltrations (30). Moreover, increased eosinophils in the superior turbinate have been correlated with the degree of olfactory dysfunction and olfactory decline after sinus surgery (31, 32). Inflammation of the epithelium can affect olfactory neurogenesis, differentiation, and maturation of OSNs.
The Role of Cytokines and Immunologic Biomarkers
Researchers have worked on identifying types of cytokines or biomarkers in the olfactory neuroepithelium, and increased levels of several cytokines in the olfactory cleft have been correlated with olfactory dysfunction in CRS patients.
In a study by Henkin et al., (42) increased levels of IL-6 in nasal mucus, saliva, and plasma were reported in hyposmia patients due to various causes. IL-6 is a proinflammatory cytokine, and inflammation was proposed to play a role in the biochemical pathological process underlying hyposmia. Schlosser et al. (33) corrected the mucus defect in olfactory clefts by inserting a polyurethane sponge into each olfactory cleft under endoscopic guidance and correlated the threshold discrimination identification score of the Sniffin' Sticks test with the levels of secreted mediators. IL-5 levels were found to be inversely correlated with olfactory test scores in both CRSwNP and CRSsNP. In another study by Wu et al., mucus was collected from both the olfactory cleft and middle meatus of CRSwNP, CRSsNP, and control subjects to compare the expression of cytokines/chemokines and olfactory function. Elevated levels of IL-2, IL-5, IL-6, IL-10, and IL-13 were associated with reduced test scores for smell identification, especially in CRSwNP patients. A strength of the aforementioned study was that cytokine levels in the middle meatus were demonstrated to be compatible with those in the olfactory cleft, which has considerable clinical significance because secretion in the middle meatus is more applicable in clinical settings than secretion in the olfactory cleft (34). The same research group applied a hierarchal cluster analysis and machine learning algorithms to data from 110 patients to characterize inflammatory patterns and correlated these patterns with smell identification scores. Olfaction was found to be strongly correlated with levels of cytokines IL-5 and IL-13, whereas the mucus IL-12 levels, CT score, and AERD were independently associated with olfactory dysfunction in CRS patients (22). In another cluster analysis of olfactory cleft mucus biomarkers, including cytokines, chemokines, and growth factors, Soler et al. demonstrated that clusters dominated by IL-4, IL-5, IL-13, and IgE were associated with lowest olfactory scores and elevations in tumor necrosis factor-α (TNF-α), IL-6, and chemokines that promote monocyte/macrophage recruitment (C-C motif chemokine ligand [CCL]2, CCL3, CCL20) (35).
The Effect of Inflammation on Neurogenesis
Several cytokines have neurotoxic potential, and the effects of cytokines may mediate OSN function and regeneration. OSNs of abnormal morphologies and potential functional defects have been reported in CRS patients, along with increased numbers of immature neurons (43). However, the effect of cellular and molecular pathways and underlying mechanisms of CRS-associated inflammation on the function of the peripheral olfactory system remain incompletely understood. This gap in our knowledge may be due to the limited accessibility of human olfactory tissue and the difficulty of maintaining human olfactory neurons in standard cell cultures. Holbrook et al. (44) performed an autopsy study and used immunohistochemical analysis to compare the molecular phenotypes of olfactory epithelial cells between rodents and humans. The immunostaining patterns showed there were two distinct basal cell types, horizontal and globose, in both humans and rodents, and this similarity between experimental animals and humans could shed light on olfactory pathophysiology. The effect of inflammatory mediators on the apoptosis, differentiation, and proliferation of OSNs in animal models has been investigated.
TNF-α is a pleiotropic cytokine that has been universally associated with CRS, regardless of subtype or etiology (45). TNF-α plays a role in antigen-specific immunoglobulin E (IgE) production and Th2 cytokine production and modulates the migration of Th2 cells to inflammation sites (46–50). Lane et al. investigated a transgenic mouse model expressing TNF-α by sustentacular cells in the olfactory epithelium: TNF-α was found to directly affect olfactory neuron function and neuroepithelial regeneration, and the downstream mediators following infiltration of inflammatory cells contributed to histological damage to the olfactory neuroepithelium (36, 38, 40, 51). Sousa Garcia et al. demonstrated that genetic deletion of TNFR1 (tumor necrosis factor α receptor 1) in inducible olfactory and allergen-induced inflammation models prevented histological damage, reduced eosinophilic infiltration, and preserved the neuronal layer thickness. TNFR1 may be crucial in the development of inflammation-associated olfactory dysfunction (39). The acute inflammatory response may promote regeneration of olfactory neuroepithelium through the nuclear factor-κB (NF-κB) pathway (52), and c-Jun N-terminal kinases (JNK), the principal signaling molecules involved in the TNF-α apoptotic pathway, were found to be activated in neuroinflammation (53). The expression of TNF-α in the olfactory neuroepithelium is critical in the pathogenesis of olfactory dysfunction.
Rouyar et al. investigated the impact of type 2/Th2-driven inflammation on olfactory function in a mouse model of ECRS sensitized to house dust mites and Staphylococcus aureus enterotoxin B. The expression of IL-4, IL-5, IL-13 and total IgE in the olfactory epithelium was significantly increased in the group treated with house dust mites, and IL-13Rα1 and IL-4Rα mRNAs was detected in mature OSNs, globose basal cells, and horizontal basal cells, which are involved in OSN renewal. The transcriptomic and histology markers revealed a decrease in the number of immature OSNs that did not affect the sense of smell, as measured by electroolfactogram and animal behavioral food tests (54). The roles of the IL-4 and IL-13 pathways and their possible regulatory impacts on neurogenesis and homeostasis of olfactory neurons have not been determined. According to the olfactory vector hypothesis, some neurological disorders may be caused or accelerated by agents entering the central nervous system through the olfactory bulb via the olfactory mucosa (55). Mori et al. suggested that IL-4 and IL-13 cytokines may contribute to pathological mechanisms leading to the loss of dopaminergic neurons in Parkinson's disease (56). Further studies on neuroinflammation and damage in other brain regions could provide novel insights into the olfactory vector hypothesis and facilitate application of this hypothesis to the pathogenesis of possible central olfactory disorders (41).
Treatment of Olfactory Dysfunction in Chronic Rhinosinusitis
Despite limited effective treatment choices for olfactory dysfunction, olfactory dysfunction related to CRS is regarded as a treatable trait. In contrast to other non-CRS-related olfactory dysfunctions, corticosteroids are an effective mainstay treatment for the management of smell problems secondary to CRS (57). Endoscopic sinus surgery can aid the recovery of olfactory function (58). Sinus surgery can remove the diseased tissues and improve the nasal ventilation, and it has been reported that current septoplasty can increase the likelihood of achieving normal olfaction (59). A meta-analysis reported by Zhao et al. (60) demonstrated that endoscopic sinus surgery may be beneficial olfactory dysfunction The olfactory function, assessed by Sniffin' Sticks total score, discrimination score and identification score, University of Pennsylvania Smell Identification Test, and Visual Analougue Scale, improved in the patients of CRSwNP. However, the results of olfaction function after surgery were reported to be inconsistent in the CRSsNP and non-classified CRS patients. The inflammatory natures of CRS are decisive in the olfactory outcomes after the surgery. Comparing to the relatively well-sustained olfaction in non-eosinophilic CRS patients, the improvement of olfaction deteriorated with time among eosinophilic CRS patients (61). Administration of adjuvant medical therapy post-operatively may aid the continued recovery of olfactory function.
Biotherapeutic agents targeting type 2 inflammation were recently introduced into the treatment of CRS (62). The biologics dupilumab (63–65), omalizumab (66, 67), and mepolizumab (68) have been shown to improve olfaction based on symptom scores and olfaction tests. Dupilumab, blocking the shared receptor component of IL-4 and IL-13, can even improve olfaction function in patients who previously underwent more than 3 surgeries (69, 70). Successful treatment with biologics may imply that type 2 inflammation plays a role in the disease mechanism of olfactory dysfunction in CRS. A transcriptomic study of respiratory epithelial, immune, and stromal cell types and subsets in the ethmoid sinus of patients with CRS showed that epithelial stem cells may contribute to the persistence of inflammatory disease by serving as repositories for allergic memories, and a shift from interferon-α (IFN-α)/IFN-γ-induced genes to IL-4/IL-13-induced genes was demonstrated to be correlated with disease severity. A comparison of epithelial cells scraped before and 6 weeks after dupilumab treatment showed that this IL-4 receptor α-subunit blocker can modify basal and secretory cell states in vivo (71). The role of type 2 inflammation-mediated barrier dysfunction in the olfactory neuroepithelium remains to be determined.
Conclusion
Evidence from clinical features, experimental investigations, and treatment responses shows that type 2 inflammation may play an important role in olfactory dysfunction secondary to CRS. In-depth research on the link between the olfactory transduction pathway and inflammation is warranted. The olfactory system and frontline of the respiratory immune system share the same airway passage in the nose, and understanding the impact of epithelial barrier dysfunction, localized inflammation, and aggregation of immunocytes on the olfactory neuroepithelium may shed light on the management of olfactory dysfunction. An effective and sustained treatment for patients with olfactory dysfunction secondary to CRS can significantly improve patients' quality of life.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by grants #110-S5159 and #111-X0039 from National Taiwan University Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, et al. Smell and taste disorders, a study of 750 patients from the university of pennsylvania smell and taste center. Arch Otolaryngol Head Neck Surg. (1991) 117:519–28. doi: 10.1001/archotol.1991.01870170065015
2. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. (2011) 128:693–707; quiz 708–699. doi: 10.1016/j.jaci.2011.08.004
3. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. (2020) 58(Suppl. S29):1–464. doi: 10.4193/Rhin20.600
4. Pleis JR, Barnes PM. A comparison of respiratory conditions between multiple race adults and their single race counterparts: an analysis based on American Indian/Alaska Native and white adults. Ethn Health. (2008) 13:399–415. doi: 10.1080/13557850801994839
5. Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GALEN study. Allergy. (2011) 66:1216–23. doi: 10.1111/j.1398-9995.2011.02646.x
6. Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler M, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. (2017) 127:309–20. doi: 10.1002/lary.26316
7. Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. (2012) 20:29–32. doi: 10.1097/MOO.0b013e32834dfb3d
8. Soler ZM, Smith TL, Alt JA, Ramakrishnan VR, Mace JC, Schlosser J, et al. Olfactory-specific quality of life outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol. (2016) 6:407–13. doi: 10.1002/alr.21679
9. Philpott CM, Boak D. The impact of olfactory disorders in the United kingdom. Chem Senses. (2014) 39:711–8. doi: 10.1093/chemse/bju043
10. Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinology. (2016) 56:1–30. doi: 10.4193/Rhin16.248
11. Rombaux P, Huart C, Levie P, Cingi C, Hummel T. Olfaction in chronic rhinosinusitis. Curr Allergy Asthma Rep. (2016) 16:41. doi: 10.1007/s11882-016-0617-6
12. Sakuma Y, Ishitoya J, Komatsu M, Shiono O, Hirama M, Yamashita Y, et al. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. (2011) 38:583–8. doi: 10.1016/j.anl.2011.01.007
13. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. (2006) 61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x
14. Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. (2008) 121:1435–41, 1441.e1–3. doi: 10.1016/j.jaci.2008.02.018
15. Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee S, et al. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. (2007) 137:925–30. doi: 10.1016/j.otohns.2007.07.036
16. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. (2008) 122:961–8. doi: 10.1016/j.jaci.2008.07.008
17. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. (2009) 124:478–84, 484.e1–2. doi: 10.1016/j.jaci.2009.05.017
18. Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. (2010) 59:239–45. doi: 10.2332/allergolint.10-RAI-0231
19. Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. (2019) 7:2812–20.e3. doi: 10.1016/j.jaip.2019.05.009
20. Mori E, Matsuwaki Y, Mitsuyama C, Okushi T, Nakajima T, Moriyama, et al. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx. (2013) 40:465–9. doi: 10.1016/j.anl.2012.12.005
21. Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC study. Allergy. (2015) 70:995–1003. doi: 10.1111/all.12644
22. Morse JC, Shilts MH, Ely KA, Li P, Sheng Q, Huang LC, et al. Patterns of olfactory dysfunction in chronic rhinosinusitis identified by hierarchical cluster analysis and machine learning algorithms. Int Forum Allergy Rhinol. (2019) 9:255–64. doi: 10.1002/alr.22249
23. Gudziol V, Michel M, Sonnefeld C, Koschel D, Hummel T. Olfaction and sinonasal symptoms in patients with CRSwNP and AERD and without AERD: a cross-sectional and longitudinal study. Eur Arch Otorhinolaryngol. (2017) 274:1487–93. doi: 10.1007/s00405-016-4366-x
24. DelGaudio JM, Loftus PA, Hamizan AW, Harvey RJ, Wise SK. Central compartment atopic disease. Am J Rhinol Allergy. (2017) 31:228–34. doi: 10.2500/ajra.2017.31.4443
25. Hamizan AW, Loftus PA, Alvarado R, Ho J, Kalish L, Sacks R, et al. Allergic phenotype of chronic rhinosinusitis based on radiologic pattern of disease. Laryngoscope. (2018) 128:2015–21. doi: 10.1002/lary.27180
26. Siroux V, Ballardini N, Soler M, Lupinek C, Boudier A, Pin I, et al. The asthma-rhinitis multimorbidity is associated with IgE polysensitization in adolescents and adults. Allergy. (2018) 73:1447–58. doi: 10.1111/all.13410
27. Lin YT, Lin CF, Liao CK, Chiang BL, Yeh TH. Clinical characteristics and cytokine profiles of central-compartment-type chronic rhinosinusitis. Int Forum Allergy Rhinol. (2021) 11:1064–73. doi: 10.1002/alr.22759
28. Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. (2000) 110:1071–7. doi: 10.1097/00005537-200007000-00001
29. Yee KK, Pribitkin EA, Cowart BJ, Vainius AA, Klock CT, Rosen D, et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. Am J Rhinol Allergy. (2010) 24:110–20. doi: 10.2500/ajra.2010.24.3435
30. Hauser LJ, Chandra RK, Li P, Turner JH. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. Int Forum Allergy Rhinol. (2017) 7:957–62. doi: 10.1002/alr.21994
31. Lavin J, Min JY, Lidder AK, Huang JH, Kato A, Lam K, et al. Superior turbinate eosinophilia correlates with olfactory deficit in chronic rhinosinusitis patients. Laryngoscope. (2017) 127:2210–8. doi: 10.1002/lary.26555
32. Wu D, Li Y, Bleier BS, Wei Y. Superior turbinate eosinophilia predicts olfactory decline in patients with chronic rhinosinusitis. Ann Allergy Asthma Immunol. (2020) 125:304–10.e1. doi: 10.1016/j.anai.2020.04.027
33. Schlosser RJ, Mulligan JK, Hyer JM, Karnezis TT, Gudis DA, Soler M, et al. Mucous cytokine levels in chronic rhinosinusitis-associated olfactory loss. JAMA Otolaryngol Head Neck Surg. (2016) 142:731–7. doi: 10.1001/jamaoto.2016.0927
34. Wu J, Chandra RK, Li P, Hull BP, Turner JH. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. Laryngoscope. (2018) 128:E304–10. doi: 10.1002/lary.27112
35. Soler ZM, Schlosser RJ, Bodner TE, Alt JA, Ramakrishnan VR, Mattos JL, et al. Endotyping chronic rhinosinusitis based on olfactory cleft mucus biomarkers. J Allergy Clin Immunol. (2021) 147:1732–41.e1. doi: 10.1016/j.jaci.2021.01.021
36. Turner JH, Liang KL, May L, Lane AP. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am J Rhinol Allergy. (2010) 24:336–40. doi: 10.2500/ajra.2010.24.3498
37. Sultan B, May LA, Lane AP. The role of TNF-α in inflammatory olfactory loss. Laryngoscope. (2011) 121:2481–6. doi: 10.1002/lary.22190
38. Pozharskaya T, Liang J, Lane AP. Regulation of inflammation-associated olfactory neuronal death and regeneration by the type II tumor necrosis factor receptor. Int Forum Allergy Rhinol. (2013) 3:740–7. doi: 10.1002/alr.21187
39. Sousa Garcia D, Chen M, Smith AK, Lazarini PR, Lane AP. Role of the type I tumor necrosis factor receptor in inflammation-associated olfactory dysfunction. Int Forum Allergy Rhinol. (2017) 7:160–8. doi: 10.1002/alr.21855
40. Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. (2010) 30:2324–9. doi: 10.1523/JNEUROSCI.4507-09.2010
41. Imamura F, Hasegawa-Ishii S. Environmental toxicants-induced immune responses in the olfactory mucosa. Front Immunol. (2016) 7:475. doi: 10.3389/fimmu.2016.00475
42. Henkin RI, Schmidt L, Velicu I. Interleukin 6 in hyposmia. JAMA Otolaryngol Head Neck Surg. (2013) 139:728–34. doi: 10.1001/jamaoto.2013.3392
43. Yee KK, Pribitkin EA, Cowart BJ, Rosen D, Feng P, Rawson E, et al. Analysis of the olfactory mucosa in chronic rhinosinusitis. Ann N Y Acad Sci. (2009) 1170:590–5. doi: 10.1111/j.1749-6632.2009.04364.x
44. Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. (2011) 121:1687–701. doi: 10.1002/lary.21856
45. Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. (2000) 14:367–73. doi: 10.2500/105065800779954329
46. Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. (1997) 186:1737–47. doi: 10.1084/jem.186.10.1737
47. Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grencis K, et al. Tumor necrosis factor alpha is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J Exp Med. (1999) 190:953–62. doi: 10.1084/jem.190.7.953
48. Iwasaki M, Saito K, Takemura M, Sekikawa K, Fujii H, Yamada Y, et al. TNF-alpha contributes to the development of allergic rhinitis in mice. J Allergy Clin Immunol. (2003) 112:134–40. doi: 10.1067/mai.2003.1554
49. Bradding P, Mediwake R, Feather IH, Madden J, Church MK, Holgate ST, et al. TNF alpha is localized to nasal mucosal mast cells and is released in acute allergic rhinitis. Clin Exp Allergy. (1995) 25:406–15. doi: 10.1111/j.1365-2222.1995.tb01071.x
50. Bachert C, Wagenmann M, Hauser U. Proinflammatory cytokines: measurement in nasal secretion and induction of adhesion receptor expression. Int Arch Allergy Immunol. (1995) 107:106–8. doi: 10.1159/000236945
51. Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. (2010) 24:192–6. doi: 10.2500/ajra.2010.24.3460
52. Chen M, Reed RR, Lane AP. Acute inflammation regulates neuroregeneration through the NF-kappaB pathway in olfactory epithelium. Proc Natl Acad Sci U S A. (2017) 114:8089–94. doi: 10.1073/pnas.1620664114
53. Victores AJ, Chen M, Smith A, Lane AP. Olfactory loss in chronic rhinosinusitis is associated with neuronal activation of c-Jun N-terminal kinase. Int Forum Allergy Rhinol. (2018) 8:415–20. doi: 10.1002/alr.22053
54. Rouyar A, Classe M, Gorski R, Bock MD, Le-Guern J, Roche S, et al. Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy. (2019) 74:549–59. doi: 10.1111/all.13559
55. Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. (2008) 63:7–15. doi: 10.1002/ana.21327
56. Mori S, Maher P, Conti B. Neuroimmunology of the Interleukins 13 and 4. Brain Sci. (2016) 6:18. doi: 10.3390/brainsci6020018
57. Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinol Suppl. (2017) 54:1–30. doi: 10.4193/Rhino16.248
58. Federspil PA, Wilhelm-Schwenk R, Constantinidis J. Kinetics of olfactory function following endonasal sinus surgery for nasal polyposis. Rhinology. (2008) 46:184–7. Available online at: https://www.rhinologyjournal.com/Abstract.php?id=704
59. Mattos JL, Soler ZM, Schlosser RJ, Mace JC, Alt JA, Ramakrishnan VR, et al. Olfactory function after surgical treatment of CRS: a comparison of CRS patients to healthy controls. Am J Rhinol Allergy. (2021) 35:391–8. doi: 10.1177/1945892420960671
60. Zhao R, Chen K, Tang Y. Olfactory changes after endoscopic sinus surgery for chronic rhinosinusitis: A meta-analysis. Clin Otolaryngol. (2021) 46:41–51. doi: 10.1111/coa.13639
61. Oka H, Tsuzuki K, Takebayashi H, Kojima Y, Daimon T, Sakagami, et al. Olfactory changes after endoscopic sinus surgery in patients with chronic rhinosinusitis. Auris Nasus Larynx. (2013) 40:452–7. doi: 10.1016/j.anl.2012.12.001
62. Hellings PW, Verhoeven E, Fokkens WJ. State-of-the-art overview on biological treatment for CRSwNP. Rhinology. (2021) 59:151–63. doi: 10.4193/Rhin20.570
63. Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. (2016) 315:469–79. doi: 10.1001/jama.2015.19330
64. Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. (2019) 394:1638–50. doi: 10.1016/S0140-6736(19)31881-1
65. Fujieda S, Matsune S, Takeno S, Asako M, Takeuchi M, Fujita H, et al. The effect of dupilumab on intractable chronic rhinosinusitis with nasal polyps in Japan. Laryngoscope. (2021) 131:E1770–7. doi: 10.1002/lary.29230
66. Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. (2020) 146:595–605. doi: 10.1016/j.jaci.2020.05.032
67. Gevaert P, Saenz R, Corren J, Han JK, Mullol J, Lee SE, et al. Long-term efficacy and safety of omalizumab for nasal polyposis in an open-label extension study. J Allergy Clin Immunol. (2021). doi: 10.1016/j.jaci.2021.07.045. [Epub ahead of print].
68. Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. (2017) 140:1024–31.e14. doi: 10.1016/j.jaci.2017.05.044
69. Mullol J, Bachert C, Amin N, Desrosiers M, Hellings PW, Han JK, et al. Olfactory outcomes with dupilumab in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. (2021). doi: 10.1016/j.jaip.2021.09.037. [Epub ahead of print].
70. Hopkins C, Wagenmann M, Bachert C, Desrosiers M, Han JK, Hellings PW, et al. Efficacy of dupilumab in patients with a history of prior sinus surgery for chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. (2021) 11:1087–101. doi: 10.1002/alr.22780
Keywords: olfactory dysfunction, smell, chronic rhinosinusitis, inflammation, cytokines
Citation: Lin Y-T and Yeh T-H (2022) Studies on Clinical Features, Mechanisms, and Management of Olfactory Dysfunction Secondary to Chronic Rhinosinusitis. Front. Allergy 3:835151. doi: 10.3389/falgy.2022.835151
Received: 14 December 2021; Accepted: 01 February 2022;
Published: 04 March 2022.
Edited by:
Peter Kenneth Smith, Griffith University, AustraliaReviewed by:
Cristobal Langdon Montero, Hospital Clínic de Barcelona, SpainCopyright © 2022 Lin and Yeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Te-Huei Yeh, dGVodWVpeWVoQG50dS5lZHUudHc=
 Yi-Tsen Lin
Yi-Tsen Lin Te-Huei Yeh
Te-Huei Yeh