- 1NESMOS Department, Faculty of Medicine and Psychology, Pediatric Unit Sant’Andrea Hospital, “Sapienza” University, Rome, Italy
- 2Pediatric Allergology Unit, Sandro Pertini Hospital, Rome, Italy
- 3Allergology Service, Policlinico Casilino, Rome, Italy
- 4Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
- 5Institute for Biomedical Research and Innovation, Pediatric National Healthcare System, Turin, Italy
- 6TPS Production., Rome, Italy
- 7Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 8Department of Biology, Tor Vergata University, Rome, Italy
Background: Nasal provocation testing (NPT) is a reference methodology to identify the culprit allergen in patients with allergic rhinitis. Selecting the right allergen for NPT is particularly difficult in poly-sensitized patients with seasonal allergic rhinitis (SAR). Predictors of NPT outcomes may facilitate the proper use of this test or even substitute it.
Objective: To identify predictors of grass pollen NPT outcome from an array of clinical data, e-diary outcomes, and allergy test results in poly-sensitized pediatric patients with SAR.
Methods: Poly-sensitized, SAR patients with grass pollen allergy, participating in the @IT.2020 pilot project in Rome and Pordenone (Italy), participated in a baseline (T0) visit with questionnaires, skin prick testing (SPT), and blood sampling to measure total (ImmunoCAP, TFS, Sweden) and specific IgE antibodies to grass pollen extracts and their major allergenic molecules (ESEP, Euroimmun Labordiagnostika, Germany). During the pollen season, patients filled the AllergyMonitor® e-diary app measuring their symptoms, medication intake, and allergy-related well-being via the Visual Analogue Scale (VAS). After the pollen season (T1), patients answered clinical questionnaires and underwent a nasal provocation test (NPT) with grass pollen extract.
Results: We recruited 72 patients (age 14.3 ± 2.8 years, 46 males) sensitized to grass and/or other pollens, including olive (63; 87.5%) and pellitory (49; 68.1%). Patients positive to grass pollen NPT (61; 84.7%), compared to the negative ones, had worse VAS values in the e-diary, larger SPT wheal reactions, and higher IgE levels, as well as specific activity to timothy and Bermuda grass extracts, rPhl p 5 and nCyn d 1. A positive NPT to grass pollen was predicted by an index combining the specific activity of IgE towards Phl p 5 and Cyn d 1 (AUC: 0.82; p < 0.01; best cut-off ≥7.25%, sensitivity 70.5%, specificity: 90.9%). VAS results also predicted NPT positivity, although with less precision (AUC: 0.77, p < 0.01; best cut-off ≥7, sensitivity: 60.7%, specificity: 81.8%).
Conclusions: An index combining the specific activity of IgE to rPhl p 5 and nCyn d 1 predicted with moderate sensitivity and high specificity the outcome of a grass pollen NPT in complex, poly-sensitized pediatric patients with seasonal allergic rhinitis. Further studies are needed to improve the index sensitivity and to assess its usefulness for NPT allergen selection or as an alternative to this demanding test procedure.
Introduction
Allergic rhinitis (AR) is a widely prevalent condition that affects about 20% of the world population, particularly children and adolescents (1–3). Patients' discomfort is related to nasal and ocular symptoms (e.g., sneezing, rhinorrhea, nasal congestion, pruritus, tearing), systemic symptoms (e.g., fatigue, irritability, headache), and side effects of treatment (e.g., sedation by antihistamines). Severe AR symptoms lead to decreased productivity or absenteeism at school and have a negative impact on the quality of life in pediatric patients. Seasonal AR (SAR) does not only affect school performance but also participation in outdoor activities during the pollen season, and frequently coexists with asthma and other comorbidities (2, 3).
In a multicenter epidemiological study of 1,360 Italian children with seasonal allergic rhinitis, mono-sensitization was observed in only 6.2% of participants, while 84.9% were sensitized to ≥3 pollen, with timothy grass being the dominant allergenic pollen (89.6%) (4). Children with seasonal AR who cannot be sufficiently controlled with symptomatic drug treatment and preventive measures may benefit from allergen-specific immunotherapy (AIT), whose effectiveness entails the identification of the clinically relevant allergen (5). In accordance, molecular diagnostics (CRD) helps to identify, especially in poly-sensitized patients, genuine sensitization towards the major, specific molecules of pollen, thus making the AIT prescription more precise (6, 7). However, the sensitization profile expressed in the serum may diverge in some patients from the one provoking symptoms at a local level (3, 8). In these patients, testing the acute nasal response to the suspected allergen with an allergen-specific nasal provocation test (NPT) is required (3, 9). However, NPT is not easily applicable in daily clinical practice, as it is time consuming and requires preparatory actions (e.g., pausing treatments that may affect the results) and coordination (e.g., several appointments for poly-sensitized patients) (9, 10). Given these limitations, criteria have been developed to select the patients and optimal candidate allergens for NPT (9, 11). Nevertheless, diagnostic algorithms precisely predicting a positive or negative NPT outcome, aimed at reducing the need of this demanding and difficult test in daily practice, are essential. Extensive clinical research allowed predicting the outcome of oral provocation tests in food allergic patients (12–14), but similar studies, dedicated to NPT in seasonal AR, are still missing. An interesting research tool is that which assesses IgE-specific activity as the proportion of allergen-specific IgE to total IgE (15).
To fill this gap, we examined children with atopic sensitization to grass pollen, who participated in the pilot phase of the @IT.2020 study (16, 17). All children were thoroughly examined with a clinical questionnaire, pollen exposure and symptom monitoring during the pollen season, nasal cytology, skin prick test (SPT), serum-specific IgE to grass pollen extract and its molecules before undergoing an NPT to confirm or exclude a grass pollen allergy. We then extensively analyzed the data to find out which parameters or their algorithmic combination predicted a positive or negative outcome of the NPT.
Materials and methods
Study design
The @IT2020 pilot project is an observational clinical study on the impact of component-resolved diagnosis and digital symptom recording for the diagnosis of pollen allergy. In the context of this project, 101 participants, children aged 4–18 years suffering from seasonal allergic rhinitis, were recruited in the Sandro Pertini Hospital in Rome between November 2016 and February 2017. The detailed study protocol has been published previously (16, 17). Briefly, inclusion criteria were: (1) doctor’s diagnosis of seasonal allergic rhinitis/rhinoconjunctivitis; (2) residing within 30 km of the aerobiological station of the study center; (3) having no intention to change residence in the next 6 months; and (4) smartphone ownership (for children: parental smartphone). Exclusion criteria were: (1) previous allergen immunotherapy for any outdoor allergen; and (2) any other severe nonatopic chronic disease. Recruited patients underwent a first medical examination (T0), including skin prick testing (SPT), blood sampling, and clinical questionnaires. At the end of the visits, participants were instructed on the use of the AllergyMonitor® (AM) (TPS software production, Rome, Italy) mobile app to monitor their ocular, nasal, and lung symptoms, as well as medication intake and the impact of allergy symptoms on their well-being during an individual study period. After the monitoring period (i.e., pollination period of the suspected relevant allergen source), all participants underwent a second medical examination (T1), including a repetition of the initial clinical questionnaires focused on the past pollen season. Among the patients sensitized to grass pollen, the nasal provocation test (NPT) to grass allergens was proposed, and 82 agreed to participate. The local ethics committee approved the study design and procedures. All parents or guardians provided informed written consent to the clinical investigations.
AllergyMonitor® App
AllergyMonitor® (AM) (TPS software production, Rome, Italy) is a CE-certified and scientifically investigated smartphone application (app) (17–22) designed for the daily reporting of allergic symptoms of the eyes, nose, and lungs. Further, the users are asked to record the impact of allergic symptoms on their daily activities and sleep, as well as their daily medication intake. To facilitate the correct recording of medication intake, the individual treatment regimen was registered individually in the app by the study doctor via back-office technology (21). While the initial set-up for each user took approximately 5 min, daily recordings could be completed within 1–2 min. AllergyMonitor output records include the Rhinoconjunctivitis Total Symptoms Score (RTSS, 0–18) (23); the Combined Symptom and Medication Score (CSMS, 0–6) (24); and the Visual Analogue Scale (VAS, 0–10) (25). Daily records of RTSS and CSMS encompass nasal symptoms (sneezing, rhinorrhea, nasal pruritus, nasal congestion), ocular symptoms (itchy eyes, watery eyes), and medication intake (antihistaminic drugs, local steroids, systemic steroids). The VAS estimates the overall AR severity by asking “How do you feel in relation to your allergic symptoms today?”.
Grass pollen season
Season criteria of the European Academy of Allergy and Clinical Immunology (EAACI) (26) were adapted to the local setting, and for 2016 resulted in a whole grass pollen season from 13 April to 28 July, as well as a peak grass pollen season between 4 May and 28 June, as reported previously (22). Grass pollen concentrations ranged from 0 to 199 grains/m3 air. Based on aerobiological cut-off values for central Italy, daily pollen concentrations were also classified as low (<10/m3), medium (10–30/m3), and high (>30/m3) (27).
Skin prick tests
Skin prick tests were performed using a standard panel of commercial extracts (ALK-Abelló, Milan, Italy) of outdoor and indoor aeroallergens (Alternaria, Bermuda grass, birch, cat dander, cypress, dog dander, hazel, house dust mite, mugwort, olive tree, plane tree, ragweed, Russian thistle, timothy grass, and pellitory-of-the-wall). Histamine 0.1 mg/ml and glycerol solution were used as positive and negative controls, respectively. Morrow Brown needles were used to prick the skin, and the wheal reactions were read after 15 min. A wheal equal to or greater than 3 mm after subtraction of the negative control was regarded as positive.
IgE assays
During the T0 visit, patients underwent blood sampling. Serum total IgE was tested with ImmunoCAP-FEIA (Thermo Fisher Scientific, Phadia AB, Uppsala, Sweden), and the results were expressed in kU/L. Allergen-specific IgE antibodies were measured with a multiplex immunoblot assay (EUROLINE Southern European Profile Test EUROIMMUN Labordiagnostika AG, Lübeck, Germany) specifically designed for the in vitro identification of specific IgE to airborne allergen (pollen and Alternaria alternata) extracts in the Mediterranean area and their allergenic molecules (28). The ESEP included the following allergen extracts and native (n) and recombinant (r) molecules: birch (Betula verrucosa), rBet v 1, rBet v 2, rBet v 4, olive tree (Olea europaea), rOle e 1, cypressus (Cupressus arizonica), nCup a 1, Bermuda grass (Cynodon dactylon), rCyn d 1, timothy grass (Phleum pratense), rPhl p 1, rPhl p 5, rPhl p 4, rPhl p 7, rPhl p 12, mugwort (Artemisia vulgaris), nArt v 1, pellitory (Parietaria judaica), rPar j 2, Alternaria (Alternaria alternata), rAlt a 1 and a cross-reactive carbohydrate determinant (CCD) marker. The test is semi-quantitative, and results are expressed in kU/L. Values greater than 0.35 KU/L are considered positive. For simplicity, prefixes “n” and “r” for pollen molecules will be avoided in the text. The IgE-specific activity for grass-pollen extracts and molecules was calculated as their percentage fraction from total IgE, i.e., (specific IgE/total IgE) *100 (15). The combined IgE-specific activity was calculated as the sum of specific-IgE activities for extracts or molecules [e.g., (specific IgE to Phl p 5 + Cyn d 1/total IgE)*100].
Nasal provocation test
During the T1 visit, patients with positive SPT for grass pollen underwent an NPT according to international guidelines (9). Baseline Total Nasal Symptom Score (TNSS), Peak Nasal Inspiratory Flow (PNIF), and subjective evaluation of symptoms such as rhinorrhea, nasal obstruction, nasal itch, ocular itch, lacrimation, were used as parameters. The evaluation system used is that proposed by the otolaryngology section of the German Society of Allergology and Clinical Immunology (11). For a detailed description of the NPT protocol, please see the supplementary material.
Statistical analysis
Continuous variables were evaluated for normal distribution (K-S test) and reported as means ± SD or as medians and interquartile range (IQR). The Mann–Whitney U test was used to assess differences between two groups of continuous variables; contingency tables (χ2) with Fisher's correction were used to compare frequencies. Grass-allergen SPT variables were analyzed as the single mean-wheal size; specific IgE to extracts and molecular fractions were analyzed separately and as the sum of two or more fractions (kU/L), and as their IgE-specific activity (percentage fraction from total IgE). Individual AM daily records during the peak grass pollen season were assessed both as the maximum value and the coefficient of variation (CV = 100* SD/mean) for RTSS, CSMS, and VAS, during the days with high airborne grass-pollen concentrations (>30/m3). Receiver operating characteristic (ROC) curves were used to analyze the relationship between sensitivity and 1-specificity for predictors of a positive NPT; areas under curves (AUCs) were calculated. Logistic regression (enter method) was performed with the NPT outcome as a dependent variable against potentially influencing (independent) variables on the degree of allergen sensitization and symptom scores. Independent variables were selected based on significant differences between groups of NPT outcomes; the dependent variable had mutually exclusive and exhaustive categories (NPT; negative: 0, positive: 1). The Box-Tidwell test was used to estimate the linear relationship between continuous independent variables and the logit transformation of the dependent variable. Statistical software (SPSS version 27, Chicago, l) was used for calculations. Statistical significance was accepted for “P” values <.05.
Results
Study population
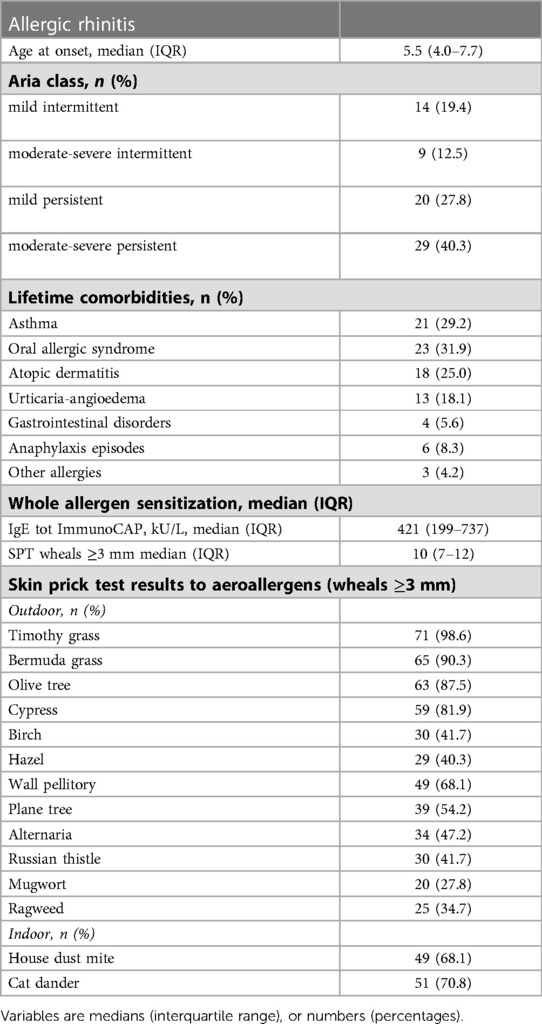
The present analysis includes 72 children and adolescents (14.3 ± 2.8 years, BMI 20.7 ± 3.8 kg/m2, BMI percentile 57.1 ± 30.3, M/F: 46/26) fulfilling the inclusion criteria for the @IT.2020 pilot study and with a complete dataset of assessments including the NPT. The male gender was slightly more frequent (63.4%), and the population was characterized by a predominantly persistent classification of AR symptoms as assessed by a retrospective questionnaire during T0 according to the Allergic Rhinitis and Its Impact on Asthma (ARIA) criteria; persistent symptoms were reported as moderate to severe in 40.3% (29/72) of patients in this session. The rate of patients with moderate-severe persistent symptoms increased to 75% (54/72, p < 0.01) at the final study visit (T1) when being asked the same questions concerning the past grass pollen season. Frequent allergic comorbidities were oral allergy syndrome, asthma, and atopic dermatitis. All participants were sensitized to grass pollen, with 98.6% having a positive SPT to timothy grass and 90.3% reacting to Bermuda grass; they also were frequently sensitized to other pollens, mostly olive, cypress, and wall pellitory (Table 1).
NPT outcomes
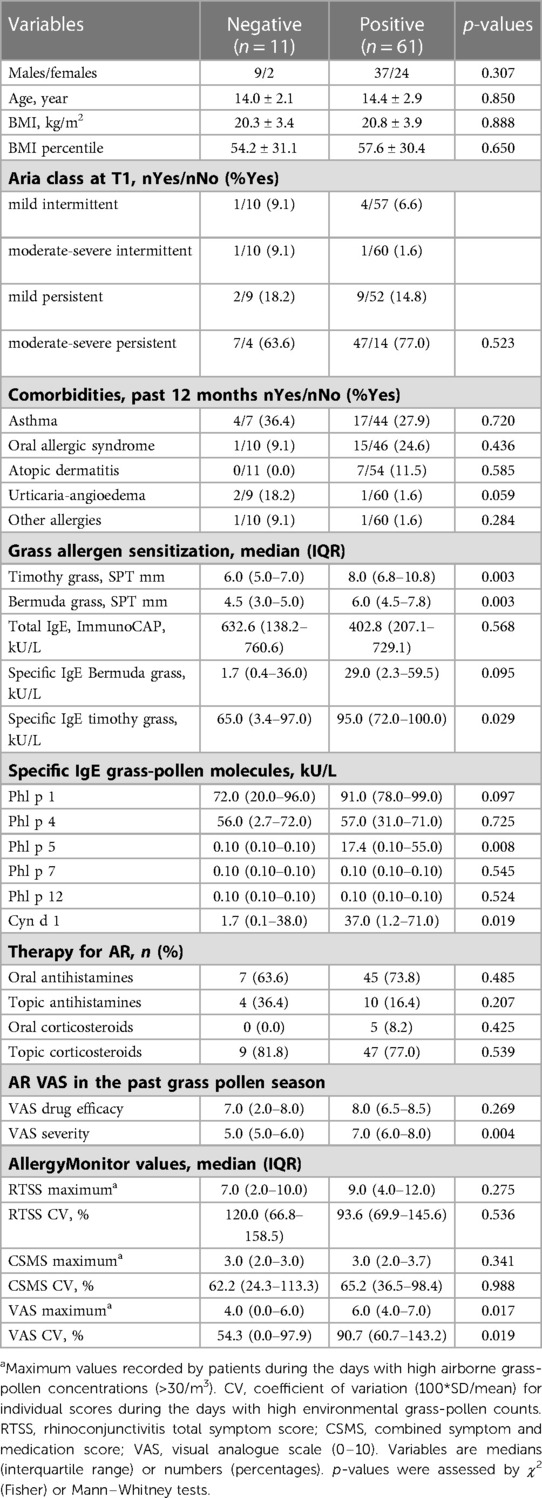
Of the 72 patients, 61 (84.7%) had a positive NPT to grass pollen extract. Patients' anthropometric data, comorbidities, and therapy for AR did not differ by NPT outcomes. All subjects were poly-sensitized to aeroallergens; the median (IQR) number of SPT wheal reactions ≥3 mm was similar in patients who had a positive or a negative NPT [10 (7–12) vs. 9 (8–10)], p = 0.937 by Mann–Whitney U test. Out of the 11 patients with a negative NPT, nine (81.8%) and three (27.3%) had no IgE to Phl p 5 and to Phl p 1, respectively. Four and two of these patients were highly sensitized, both in vivo (SPT) and in vitro (IgE), to pellitory and to olive, respectively. Only two of the 11 patients (18.2%) were highly sensitized to grass pollen, having IgE to Phl p 5, so that a negative NPT could not be easily explained.
IgE sensitization vs. NPT outcomes
Among the specific IgE to timothy grass molecules, only IgE to Phl p 5 predicted NPT outcomes. IgE levels to Phl p 5 > 0.35 KU/L were found in 39/61 (64.0%) of NPT-positive subjects vs. 2/11 (18.2%) of NPT-negative subjects (p = 0.007 by χ2 Fisher's corrected). IgE to Phl p 1 and IgE to Phl p 4 were observed in almost all the patients despite their NPT outcome; conversely, IgE to Phl p 7 and Phl p 12 were infrequently observed in both patient groups. Overall, patients with a positive NPT were more intensively sensitized to grass pollen than patients yielding negative NPT, as they had larger SPT wheal reactions to timothy grass and Bermuda grass, and higher specific IgE levels to timothy grass extract, to Phl p 5, and to Cyn d 1 (Table 2). This difference was even stronger when the specific activity of the above listed IgE antibodies was considered (Table 3).
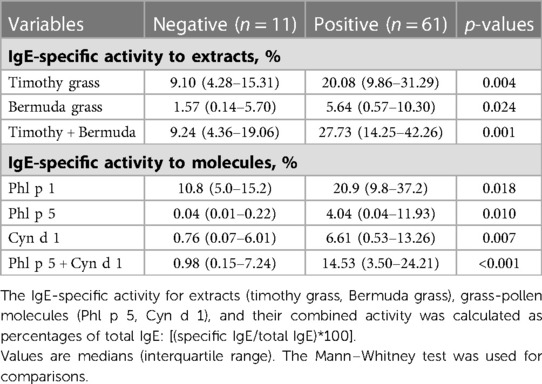
Table 3. Values of IgE-specific activity to grass-pollen extracts and molecules by outcomes of the nasal provocation test (NPT) with the undiluted grass pollen extract.
AR symptoms vs. NPT outcomes
Patients with a positive NPT also perceived a higher impact of their AR symptoms on disease severity than their counterparts with a negative NPT. This difference was coherently observed not only retrospectively at T1, by the VAS for AR severity in the past grass pollen season, but also prospectively by the VAS measured during the high grass pollen season (Table 2). In contrast, RTSS and CSMS values did not differ among patients with different NPT outcomes.
Individual predictors of the NPT outcome
High diagnostic values on predicting a positive NPT were found for the combined IgE-specific activity to either timothy plus Bermuda grass extracts, or to Phl p 5 plus Cyn d 1 (Figure 1A). Less accurate predictors of a positive NPT were the SPT wheal size or the specific IgE level (or the IgE-specific activity), for a single grass-allergen extract or molecule (Table 4). ROC curves for the AR severity score (VAS) in the past grass pollen season fitted better than both the e-diary AR VAS records (CV and maximum VAS) during airborne grass-pollen concentrations >30/m3 (Figure 1B).
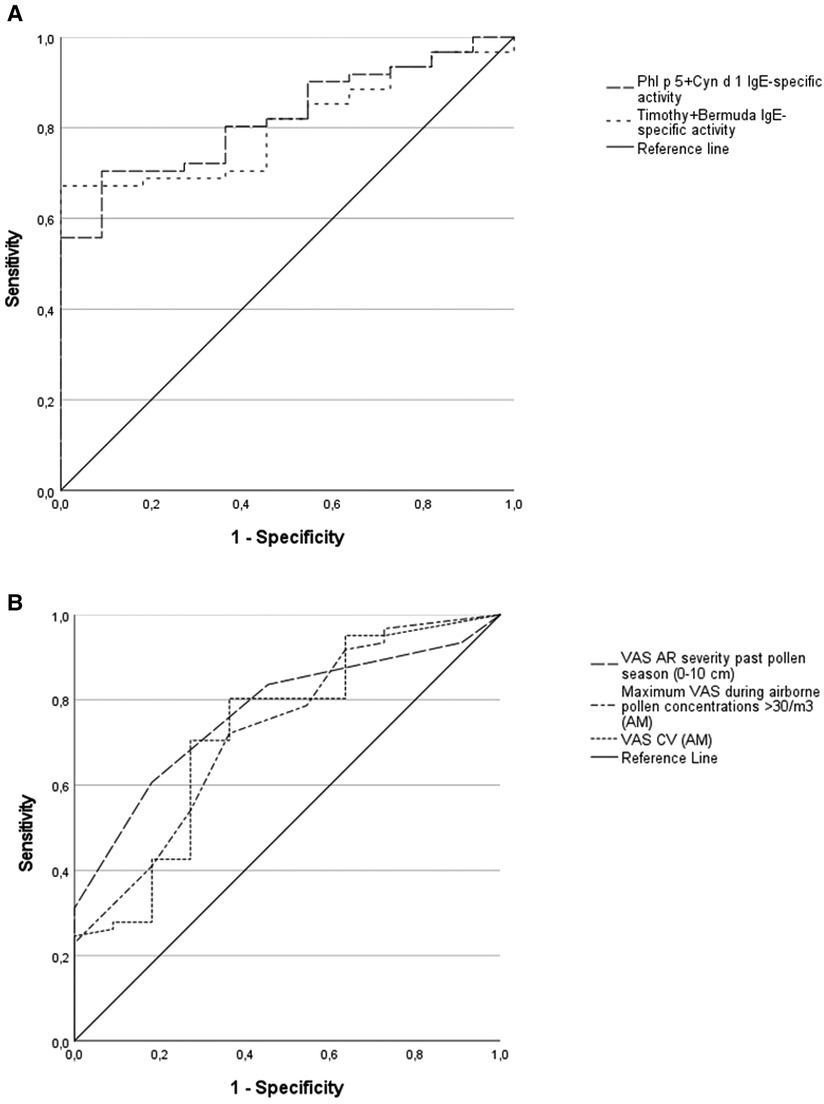
Figure 1. Receiver operating characteristic (ROC) curves for allergen sensitization and AR visual analogue scales (VAS) as predictors of the nasal provocation test (NPT) outcome. 1-A: combined IgE-specific activity to timothy plus Bermuda grass extracts, area under curve (AUC) = 0.81; combined IgE-specific activity to Phl p 5 plus Cyn d 1, AUC = 0.82; p < 0.01 for both. 1-B: VAS for AR severity in the past grass pollen season, AUC = 0.77, p < 0.01; maximum VAS recorded (AllergyMonitor, AM) during the days with high airborne grass-pollen counts (>30/m3), AUC = 0.73, p < 0.05; VAS coefficient of variance (VAS CV, AM) during the days with high airborne grass-pollen counts, AUC = 0.72, p < 0.05.
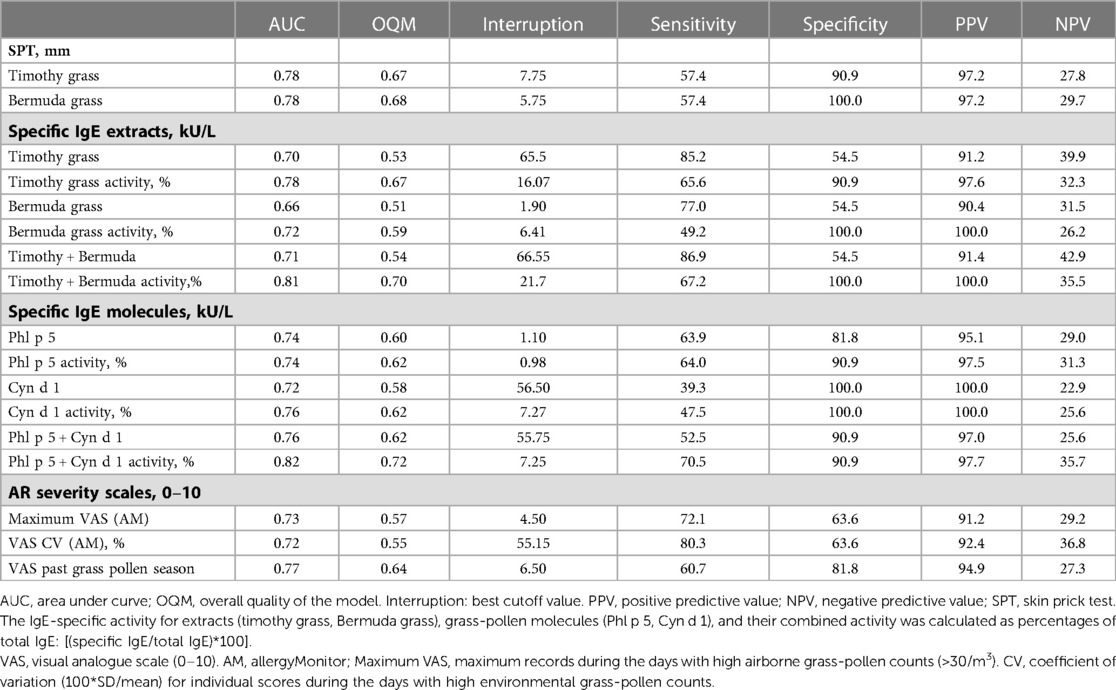
Table 4. ROC analysis for grass-pollen sensitization and symptom scores on AR severity by outcomes of the nasal provocation test (NPT).
Algorithm best predicting NPT outcome
We tested the hypothesis that the combination of multiple predictors in a diagnostic algorithm may improve our predictive capacity. When NPT outcomes were tested with two potential explanatory variables (degree of allergen sensitization and symptom scores), the best logistic model included the combined IgE-specific activity for Phl p 5 plus Cyn d 1 and the VAS for AR severity in the past grass pollen season (Table 5). The regression model was statistically significant (χ2 = 22.85, p = 0.000), explained 47% of the variance (Nagelkerke R square), and correctly classified 86.1% of cases. A raise in the combined IgE-specific activity for Phl p 5 plus Cyn d 1 and the VAS for AR severity in the past grass pollen season was associated with an increased likelihood of having a positive NPT outcome (Odds ratio 1.2 and 2.4, respectively) (Table 5). Based upon optimal cut-off values for ROC curves in Table 4, percentages of IgE-specific activity for Phl p 5 plus Cyn d 1 ≥7.25% or VAS scores for AR severity in the past pollen season ≥7, predicted the NPT outcome with a 93% sensitivity, 73% specificity, 95% PPV, and 67% NPV in our patients (Figure 2).
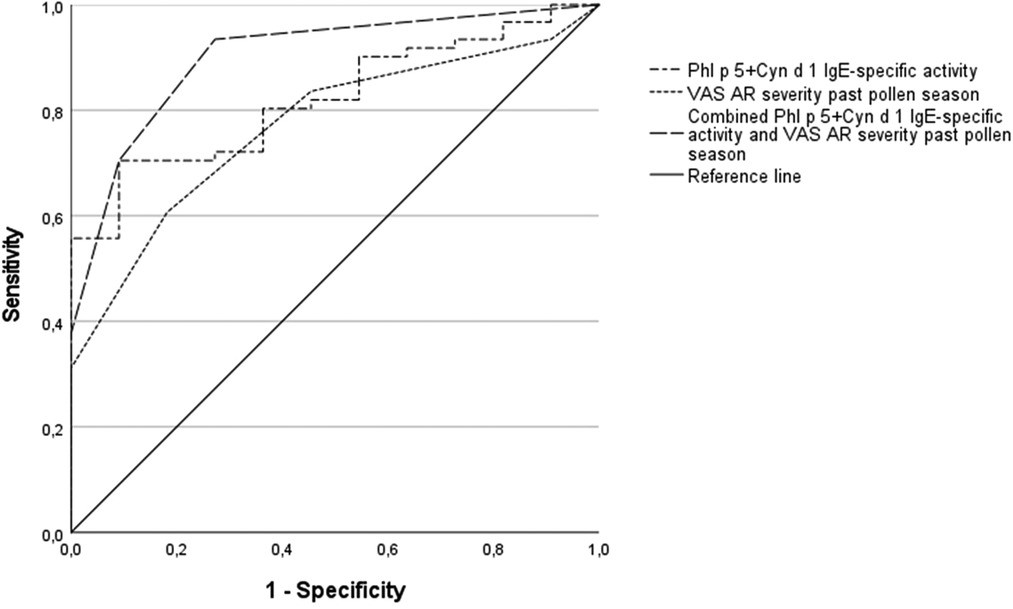
Figure 2. Receiver operating characteristic (ROC) curves for sensitization to combined grass molecules and AR visual analogue scales (VAS) as predictors of the nasal provocation test (NPT) outcome. Combined IgE-specific activity to Phl p 5 plus Cyn d 1, AUC = 0.82, p < 0.01; VAS for AR severity in the past grass pollen season, AUC = 0.77, p < 0.01; diagnostic algorithm using cut-off values for the combined IgE-specific activity (Phl p 5 plus Cyn d 1 ≥7.25%) or VAS scores for AR severity in the past pollen season ≥7, AUC = 0.90, p < 0.001.
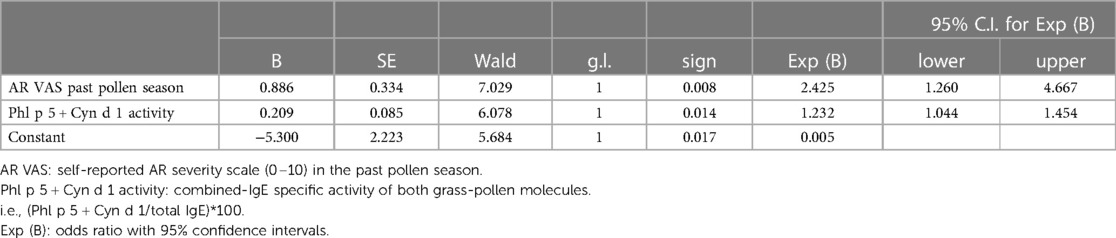
Table 5. Predictors of a positive nasal provocation test (NPT) with the undiluted grass pollen extract. Variables in the equation.
Discussion
In our study group of pediatric patients allergic to grasses, both the degree of specific allergen sensitization and self-reports of AR severity predicted the NPT outcome well; we also found that the combination of IgE-specific activity of Phl p 5 plus Cyn d 1 together with the VAS value of AR severity in the past grass pollen season predicted with a high sensitivity and moderate specificity the outcome of an NPT with grass pollen extract. In contrast, anthropometric characteristics, ARIA criteria, comorbidities, and medication use for AR did not predict the NPT outcome. To our knowledge, this is the first study investigating complex diagnostic algorithms, based on multiple variables, which may reduce the need to perform NPT in patients with seasonal allergic rhinitis due to grass pollen allergy.
Despite involving risk, the need for standardization, time consumption, and costs, NPT remains the reference test for assessing the clinical relevance of an allergen (9). A systematic review and meta-analysis including seven studies (430 patients) on several airborne allergens reported a pooled estimate for sensitivity and specificity for SPT results (“positive” or “negative”) of 85% and 77% respectively on predicting the NPT outcome (29); three of these studies testing timothy grass reported sensitivity between 68% and 97% and specificity between 70% and 86% (30–32). To note, different cut-off values for defining a “positive” SPT were used (29). The SPT-allergen wheal size and/or specific IgE levels have been found predictive of NPT results for several allergens such as Dermatophagoides pteronyssinus (D. pt.) (33, 34), cat (35, 36), and Salsola kali (37); in contrast, the SPT wheal size was found unrelated to timothy grass-titrated NPT outcomes in a study by Huss-Marp et al. (38). Notwithstanding the absent dose-response relationship, the authors could establish that specific IgE levels for timothy extract at the cut-off 0.35 kUA/L predicted dichotomized challenge outcomes (any reaction regardless of concentration vs. no reaction), with a sensitivity of 99% and specificity of 84% (38).
Sensitization to multiple molecular allergen components has been found predictive of the acute response to the specific mucosal challenge. Darsow et al., in 101 adult patients with timothy grass allergy, analyzed IgE against eight molecules (Phl ps: 1, 2, 4, 5b, 6, 7, 11, and 12). Increased numbers of sensitizations to these molecules (cut-off 0.35 kUA/L) predicted NPT and conjunctival provocation test (CPT) outcomes (39). We found that only IgE to Phl p 5 was significantly more frequent (64.0%) in NPT-positive subjects than in NPT-negative subjects (18.2%); other serological parameters, such as IgE to Phl p 1 and Phl p 4 (both very frequent) or IgE to Phl p 7 and Phl p 12 (both relatively infrequent) did not add any further power to our prediction capacity of NPT outcomes. Phl p 5, a prototypic member for the group 5 allergen molecules of grass pollen, is an important molecule, whose potent allergenicity is probably due to its multiple, independent IgE epitopes (40). Grass pollen allergic patients with IgE to Phl p 5 were shown to have a higher risk of developing asthma, with an increasing prevalence of sensitization to this molecule towards adulthood (41, 42). Consistent with these previous observations, our study adds a new, hitherto unrecognized property of sensitization to Phl p 5, which confirms the diagnostic relevance of in vitro molecular testing for a complete phenotype analysis of the patients with grass pollen allergy (43), as well as standardization of grass pollen AIT preparation guided by their content of Phl p 5 (44).
Estimates of the IgE-specific activity as the proportion of allergen-specific IgE to total IgE have been suggested as more appropriate than allergen-specific IgE levels for calculating the degree of allergen sensitization and, ultimately, its impact on clinical symptoms (28, 45). We calculated the IgE-specific activity for main grass-pollen extracts but also for grass-pollen molecules, an approach that (to our knowledge) has not been used before to assess NPT predictors in poly-sensitized patients. Our results confirm the relevance of IgE-specific activity as a novel parameter that may be introduced in routine clinical practice (12, 28, 46, 47). However, it would be important to test whether such a valuable performance can be replicated not only in other populations of grass pollen allergic patients, but also among patients mainly sensitized to other pollen (e.g., birch, pellitory, olive).
Certainly, the severity of symptoms upon allergen exposure does not depend solely on IgE-specific activity, but also on other immunological parameters, host factors, and environmental factors, particularly regarding exposure to other, co-seasonal allergenic pollen (45). Hence, we temporally restricted our analysis on the season segment characterized by high airborne grass-pollen concentrations (peak pollen season) to minimize the confounding effect of overlapping airborne allergens (e.g., olive, pellitory), to which many of our patients were also co-sensitized. The relevance of peak pollen seasons and so-called “high days” has been already highlighted by previous studies (48, 49) and included in consensus statements of the EAACI (25). Our study offers an additional reason to keep in consideration those guidelines and focus on high pollination periods, especially when examining patients with poly-sensitization to co-seasonal pollen.
Contrasting results have been reported on the relationship between clinical history (self-reported AR symptoms) and NPT outcomes. AR severity, as assessed by the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) score, increased with the dose of titrated Dpt-NPT (50), but another index of AR severity, the Total Nasal Symptom Score (TNSS) did not relate with the allergen concentration to elicit a positive D. pt.-NPT (34). Similarly, the VAS referring to the most recent pollen season was found unrelated to results from titrated-NPT for grasses (51). In contrast, we found that both retrospective self-reported nasal symptoms/VAS and prospectively daily reported VAS values predicted NPT outcomes. Not surprisingly, we also found in this population sample a close relationship between retrospective and prospective assessments for symptom severity of seasonal AR (52). The assessment of exposure-related symptoms may also serve as a prognostic marker for NPT-outcomes in patients suffering from local allergic rhinitis. However, future studies will be needed to carefully evaluate this potential.
A relevant advancement in our study, compared to previous ones, is that the combination of biological (atopic sensitization to grass pollen) and clinical (retrospective and prospective disease severity scores) methods is an essential strategy to win a better prediction power. This approach replicates what has been already repeatedly demonstrated in patients with food allergies, where diagnostic algorithms predicting the outcome of oral provocation tests with the culprit food are more efficient if they combine both biological and clinical information (53, 54, 14).
We must acknowledge some limitations of our study protocol. First, we performed the NPT with undiluted allergen extract rather than allergen titration; hence we could not establish a sensitivity threshold to allergens and quantitative response through a dose-finding process (9, 55). However, our procedure is in line with the recommendation for qualitative outcomes (positive vs. negative) expressed in recently published international guidelines (8). Second, we examined only 11 NPT-negative patients. However, we selected patients for a “real-life” clinical study in pediatric patients with seasonal AR symptoms and allergen poly-sensitization including grass pollen, whose symptoms justified the NPT procedure. Moreover, the observed associations and predictions were all examined for their statistical significance, so that this limitation may apply, if at all, only to the negative outcomes, not to the positive ones. The small sample size of NPT-negative patients may have affected the statistical power and generalizability of the findings as observed for the low negative predictive value and specificity of our predictive algorithm.
In conclusion, the combined IgE-specific activity for main allergen molecules of timothy and Bermuda grass together with the retrospective and prospective VAS score for AR severity helps to predict the outcome of allergen-specific NPT for grass pollen extracts in pediatric patients of a Mediterranean country sensitized to multiple pollen with overlapping seasonality. Measuring these parameters can help appropriate NPT prescription and even reduce the need for time-consuming NPT in such patients.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato Etico “Lazio 2” of Ospedale Sandro Pertini, Rome. Comitato Etico Regionale Unico, Pordenone. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
ST, SA, SP, VV, AT, MAB, PMM and SD: were actively involved in the implementation of the @IT.2020 pilot study and data collection. MB, ST and PMM: established the concept and analysis plan. MB and MM: analyzed the data and prepared the first manuscript draft, all co-authors were actively involved in the discussion and several critical review rounds of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
SA was supported by the EAACI Fellowship Award of the European Academy of Allergy and Clinical Immunology. The study was supported by an unrestricted grant from EUROIMMUN (grant no 118583). The Informatics Platform AllergyCARD and the app AllergyMonitor® were kindly provided by TPS Production, Rome, Italy.
Acknowledgments
We thank the entire team and all participants of the @IT.2020 pilot study.
Conflict of interest
PMM reports grants and personal fees from EUROIMMUN AG, during the conduct of the study; and grants and personal fees from Thermo Fisher Scientific, and personal fees from Hycor Biomedical Inc, outside the submitted work. ST is cofounder of TPS Production. SP reports personal fees from TPS Production. SD reports personal fees from OMRON Healthcare, dbv Technologies, Allergopharma and Bencard Allergie outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2023.1186353/full#supplementary-material.
References
1. Passali D, Cingi C, Staffa P, Passali F, Muluk NB, Bellussi ML. The international study of the allergic rhinitis survey: outcomes from 4 geographical regions. Asia Pac Allergy. (2018) 8:e7. Published 2018 Jan 25. doi: 10.5415/apallergy.2018.8.e7
2. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. (2011) 378:2112–22. doi: 10.1016/S0140-6736(11)60130-X
3. Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. (2018) 8:108–352. doi: 10.1002/alr.22073
4. Dondi A, Tripodi S, Panetta V, Asero R, Businco AD, Bianchi A, et al. Italian pediatric allergy network (I-PAN). pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. (2013) 24:742–51. doi: 10.1111/pai.12136
5. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. (2018) 73:765–98. doi: 10.1111/all.13317
6. Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. (2011) 66:775–83. doi: 10.1111/j.1398-9995.2011.02565.x
7. Rodríguez-Domínguez A, Berings M, Rohrbach A, Huang HJ, Curin M, Gevaert P, et al. Molecular profiling of allergen-specific antibody responses may enhance success of specific immunotherapy. J Allergy Clin Immunol. (2020) 146:1097–108. doi: 10.1016/j.jaci.2020.03.029
8. Fokkens W, Desrosiers M, Harvey R, Hopkins C, Mullol J, Philpott C, et al. EPOS2020: development strategy and goals for the latest European position paper on rhinosinusitis. Rhinology. (2019) 57:162–8. doi: 10.4193/Rhin19.080
9. Augé J, Vent J, Agache I, Airaksinen L, Campo Mozo P, Chaker A, et al. EAACI position paper on the standardization of nasal allergen challenges. Allergy. (2018) 73:1597–608. doi: 10.1111/all.13416
10. Jang TY, Kim YH. Nasal provocation test is useful for discriminating allergic, nonallergic, and local allergic rhinitis. Am J Rhinol Allergy. (2015) 29:e100–4. doi: 10.2500/ajra.2015.29.4214
11. Riechelmann H, Bachert C, Goldschmidt O, Hauswald B, Klimek L, Schlenter WW, et al. German society for allergology and clinical immunology (ENT section); working team for clinical immunology. Application of the nasal provocation test on diseases of the upper airways. Position paper of the German society for allergology and clinical immunology (ENT section) in cooperation with the working team for clinical immunology. Laryngorhinootologie. (2003) 82(3):183–8. doi: 10.1055/s-2003-38411
12. Gupta RS, Lau CH, Hamilton RG, Donnell A, Newhall KK. Predicting outcomes of oral food challenges by using the allergen-specific IgE-total IgE ratio. J Allergy Clin Immunol Pract. (2014) 2:300–5. doi: 10.1016/j.jaip.2013.12.006
13. Calvani M, Anania C, Caffarelli C, Martelli A, Del Giudice MM, Cravidi C, et al. Food allergy: an updated review on pathogenesis, diagnosis, prevention and management. Acta Biomed. (2020) 91(11-S):e2020012. doi: 10.23750/abm.v91i11-S.10316
14. Foong RX, Santos AF. Biomarkers of diagnosis and resolution of food allergy. Pediatr Allergy Immunol. (2021) 32:223–33. doi: 10.1111/pai.13389
15. Hamilton RG, MacGlashan DW, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res. (2010) 47:273–84. doi: 10.1007/s12026-009-8160-3
16. Arasi S, Castelli S, Di Fraia M, Villalta D, Tripodi S, Perna S, et al. @IT2020: an innovative algorithm for allergen immunotherapy prescription in seasonal allergic rhinitis. Clin Exp Allergy. (2021) 51:821–8. doi: 10.1111/cea.13867
17. Di Fraia M, Tripodi S, Arasi S, Dramburg S, Castelli S, Villalta D, et al. Adherence to prescribed E-diary recording by patients with seasonal allergic rhinitis: observational study. J Med Internet Res. (2020) 22(3):e16642. doi: 10.2196/16642
18. Costa C, Menesatti P, Brighetti MA, Travaglini A, Rimatori V, Di Rienzo Businco A, et al. Pilot study on the short-term prediction of symptoms in children with hay fever monitored with e-health technology. Eur Ann Allergy Clin Immunol. (2014) 46:216–25. PMID: 25398165.25398165
19. Bianchi A, Tsilochristou O, Gabrielli F, Tripodi S, Matricardi PM. The smartphone: a novel diagnostic tool in pollen allergy? J Investig Allergol Clin Immunol. (2016) 26(3):204–7. doi: 10.18176/jiaci.0060
20. Florack J, Brighetti MA, Perna S, Pizzulli A, Pizzulli A, Tripodi S, et al. Comparison of six disease severity scores for allergic rhinitis against pollen counts a prospective analysis at population and individual level. Pediatr Allergy Immunol. (2016) 27(4):382–90. doi: 10.1111/pai.12562
21. Tripodi S, Giannone A, Sfika I, Pelosi S, Dramburg S, Bianchi A, et al. Digital technologies for an improved management of respiratory allergic diseases: 10 years of clinical studies using an online platform for patients and physicians. Ital J Pediatr. (2020) 46:105. doi: 10.1186/s13052-020-00870-z
22. Dramburg S, Perna S, Di Fraia M, Tripodi S, Arasi S, Castelli S, et al. Heterogeneous validity of daily data on symptoms of seasonal allergic rhinitis recorded by patients using the e-diary AllergyMonitor®. Clin Transl Allergy. (2021) 11(10):e12084. doi: 10.1002/clt2.12084
23. Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a world allergy organization (WAO) taskforce. Allergy. (2007) 62(3):317–24. doi: 10.1111/j.1398-9995.2006.01312.x
24. Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. European academy of allergy and clinical immunology. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy. (2014) 69(7):854–67. doi: 10.1111/all.12383
25. Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Méchin H, Daures JP, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. (2007) 62(4):367–72. doi: 10.1111/j.1398-9995.2006.01276.x
26. Pfaar O, Bastl K, Berger U, Buters J, Calderon MA, Clot B, et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen-induced rhinoconjunctivitis - an EAACI position paper. Allergy. (2017) 72(5):713–22. doi: 10.1111/all.13092
27. ISPRA. POLLnet-Linee guida per il monitoraggio aerobiologico, Manuali e Linee Guida 151/2017, ISBN 978-88-448-0820-4. Available at: POLLnet - Linee guida per il monitoraggio aerobiologico - Certifico Srl.
28. Di Fraia M, Arasi S, Castelli S, Dramburg S, Potapova E, Villalta D, et al. A new molecular multiplex IgE assay for the diagnosis of pollen allergy in mediterranean countries: a validation study. Clin Exp Allergy. (2019) 49:341–9. doi: 10.1111/cea.13264
29. Nevis IF, Binkley K, Kabali C. Diagnostic accuracy of skin-prick testing for allergic rhinitis: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. (2016) 12:20. doi: 10.1186/s13223-016-0126-0
30. Krouse JH, Sadrazodi K, Kerswill K. Sensitivity and specificity of prick and intradermal testing in predicting response to nasal provocation with timothy grass antigen. Otolaryngol Head Neck Surg. (2004) 131(3):215–9. doi: 10.1016/j.otohns.2004.03.024
31. Pepys J, Roth A, Carroll KB. RAST, skin and nasal tests and the history in grass pollen allergy. Clin Allergy. (1975) 5(4):431–42. doi: 10.1111/j.1365-2222.1975.tb01882.x
32. Petersson G, Dreborg S, Ingestad R. Clinical history, skin prick test and RAST in the diagnosis of birch and timothy pollinosis. Allergy. (1986) 41(6):398–407. doi: 10.1111/j.1398-9995.1986.tb00319.x
33. Haxel BR, Huppertz T, Boessert P, Bast F, Fruth K. Correlation of skin test results and specific immunoglobulin E blood levels with nasal provocation testing for house-dust mite allergies. Am J Rhinol Allergy. (2016) 30(1):60–4. doi: 10.2500/ajra.2016.30.4262
34. Xiao H, Jia Q, Zhang H, Zhang L, Liu G, Meng J. The importance of nasal provocation testing in the diagnosis of dermatophagoides pteronyssinus-induced allergic rhinitis. Am J Rhinol Allergy. (2022) 36(2):191–7. doi: 10.1177/19458924211037913
35. Zarei M, Remer CF, Kaplan MS, Staveren AM, Lin CK, Razo E, et al. Optimal skin prick wheal size for diagnosis of cat allergy. Ann Allergy Asthma Immunol. (2004) 92(6):604–10. doi: 10.1016/S1081-1206(10)61425-1
36. Al-Ahmad M, Jusufovic E, Arifhodzic N, Nurkic J, Hanoun AL. Sensitization to cat: when is nasal challenge needed? Int Arch Allergy Immunol. (2019) 179(2):108–13. doi: 10.1159/000496835
37. Al-Ahmad M, Jusufovic E, Arifhodzic N. Which skin prick test wheal size detects true allergy to Salsola kali? Eur Ann Allergy Clin Immunol. (2021) 53(5):228–33. doi: 10.23822/EurAnnACI.1764-1489.161
38. Huss-Marp J, Darsow U, Brockow K, Pfab F, Weichenmeier I, Schober W, et al. Can immunoglobulin E-measurement replace challenge tests in allergic rhinoconjunctivits to grass pollen? Clin Exp Allergy. (2011) 41(8):1116–24. doi: 10.1111/j.1365-2222.2011.03745.x
39. Darsow U, Brockow K, Pfab F, Jakob T, Petersson CJ, Borres MP, et al. Allergens. Heterogeneity of molecular sensitization profiles in grass pollen allergy–implications for immunotherapy? Clin Exp Allergy. (2014) 44(5):778–86. doi: 10.1111/cea.12303
40. Levin M, Rotthus S, Wendel S, Najafi N, Källström E, Focke-Tejkl M, et al. Multiple independent IgE epitopes on the highly allergenic grass pollen allergen Phl p 5. Clin Exp Allergy. (2014) 44(11):1409–19. doi: 10.1111/cea.12423
41. Savi E, Peveri S, Incorvaia C, Dell'Albani I, Marcucci F, Di Cara G, et al. Association between a low IgE response to Phl p 5 and absence of asthma in patients with grass pollen allergy. Clin Mol Allergy. (2013) 11(1):3. doi: 10.1186/1476-7961-11-3
42. Sekerkova A, Polackova M, Striz I. Detection of Phl p 1, Phl p 5, Phl p 7 and Phl p 12 specific IgE antibodies in the sera of children and adult patients allergic to Phleum pollen. Allergol Int. (2012) 61(2):339–46. doi: 10.2332/allergolint.11-OA-0372
43. Testera-Montes A, Jurado R, Salas M, Eguiluz-Gracia I, Mayorga C. Diagnostic tools in allergic rhinitis. Front Allergy. (2021) 2:721851. doi: 10.3389/falgy.2021.721851
44. Tripodi S, Frediani T, Lucarelli S, Macrì F, Pingitore G, Di Rienzo Businco A, et al. Molecular profiles of IgE to phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. (2012) 129(3):834–9.e8. doi: 10.1016/j.jaci.2011.10.045
45. Hamilton RG. Provocation tests with objective measures remain more diagnostic than surrogate immunoglobulin E antibody measures of sensitization. Clin Exp Allergy. (2011) 41(8):1048–9. doi: 10.1111/j.1365-2222.2011.03752.x
46. Hemmings O, Niazi U, Kwok M, James LK, Lack G, Santos AF. Peanut diversity and specific activity are the dominant IgE characteristics for effector cell activation in children. J Allergy Clin Immunol. (2021) 148(2):495–505.e14. doi: 10.1016/j.jaci.2021.02.029
47. Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. (2018) 29(8):841–9. doi: 10.1111/pai.12969
48. Bastl K, Kmenta M, Berger UE. Defining pollen seasons: background and recommendations. Curr Allergy Asthma Rep. (2018) 18(12):73. doi: 10.1007/s11882-018-0829-z
49. Hoffmann TM, Acar Şahin A, Aggelidis X, Arasi S, Barbalace A, Bourgoin A, et al. “Whole” vs. “fragmented” approach to EAACI pollen season definitions: a multicenter study in six southern European cities. Allergy. (2020) 75(7):1659–71. doi: 10.1111/all.14153
50. Wanjun W, Qiurong H, Yanqing X, Mo X, Nili W, Jing L. Responsiveness of nasal provocation testing-but not skin test and specific immunoglobulin E blood level-correlates with severity of allergic rhinitis in dermatophagoides species-sensitized patients. Am J Rhinol Allergy. (2018) 32(4):236–43. doi: 10.1177/1945892418779435
51. Senti G, Vavricka BM, Graf N, Johansen P, Wüthrich B, Kündig TM. Evaluation of visual analog scales for the assessment of symptom severity in allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol. (2007) 98(2):134–8. doi: 10.1016/S1081-1206(10)60685-0
52. Dramburg S, Perna S, Di Fraia M, Tripodi S, Arasi S, Castelli S, et al. Prospective (e-diary) vs retrospective (ARIA) measures of severity in allergic rhinoconjunctivitis: an observational compatibility study. Allergy. (2022). doi: 10.1111/all.15499
53. DunnGalvin A, Daly D, Cullinane C, Stenke E, Keeton D, Erlewyn-Lajeunesse M, et al. Highly accurate prediction of food challenge outcome using routinely available clinical data. J Allergy Clin Immunol. (2011) 127(3):633–9.e1-3. doi: 10.1016/j.jaci.2010.12.004
54. DunnGalvin A, Segal LM, Clarke A, Alizadehfar R, Hourihane JO. Validation of the cork-southampton food challenge outcome calculator in a Canadian sample. J Allergy Clin Immunol. (2013) 131(1):230–2. doi: 10.1016/j.jaci.2012.08.051
Keywords: seasonal allergic rhinitis, nasal provocation test (NPT), pollen allergy, component-resolved diagnostics, precision medicine, e-Diary
Citation: Barreto M, Tripodi S, Arasi S, Landi M, Montesano M, Pelosi S, Potapova E, Sfika I, Villella V, Travaglini A, Brighetti MA, Matricardi PM and Dramburg S (2023) Factors predicting the outcome of allergen-specific nasal provocation test in children with grass pollen allergic rhinitis. Front. Allergy 4:1186353. doi: 10.3389/falgy.2023.1186353
Received: 14 March 2023; Accepted: 8 May 2023;
Published: 26 May 2023.
Edited by:
Hasan Bayram, Koç University, TürkiyeReviewed by:
Darío Antolín-Amérigo, Ramón y Cajal University Hospital, SpainTugce Yakut, Koç University Hospital, Türkiye
Leyla Pur Ozyigit, University Hospitals of Leicester NHS Trust, United Kingdom
© 2023 Barreto, Tripodi, Arasi, Landi, Montesano, Pelosi, Potapova, Sfika, Villella, Travaglini, Brighetti, Matricardi and Dramburg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Dramburg c3RlcGhhbmllLmRyYW1idXJnQGNoYXJpdGUuZGU=
 M. Barreto
M. Barreto S. Tripodi
S. Tripodi S. Arasi
S. Arasi M. Landi
M. Landi M. Montesano
M. Montesano S. Pelosi
S. Pelosi E. Potapova
E. Potapova I. Sfika
I. Sfika V. Villella2
V. Villella2 P. M. Matricardi
P. M. Matricardi S. Dramburg
S. Dramburg