- 1Research Department, Hippo Dx, Aarschot, Belgium
- 2Department of Otorhinolaryngology-Head and Neck Surgery, UZ Leuven, Leuven, Belgium
- 3Laboratory of Experimental Otorhinolaryngology, Department of Neurosciences, KU Leuven, Leuven, Belgium
- 4Allergy and Clinical Immunology Research Group, Department of Microbiology, Immunology & Transplantation, KU Leuven, Leuven, Belgium
- 5Department of General Internal Medicine, UZ Leuven, Leuven, Belgium
- 6Department of Otorhinolaryngology, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 7Department of Otorhinolaryngology, University of Ghent, Ghent, Belgium
Background: The skin prick test (SPT) is the gold standard for identifying allergic sensitization in individuals suspected of having an inhalant allergy. Recently, it was demonstrated that SPT using a novel skin prick automated test (SPAT) device showed increased reproducibility and tolerability compared to the conventional SPT, among other benefits.
Objective: This study aimed to evaluate prick location bias using the novel SPAT device.
Methods: A total of 118 volunteers were enrolled in this study and underwent SPATs with histamine (nine pricks) and glycerol control (one prick) solutions on the volar side of their forearms. Imaging of the skin reactions was performed using the SPAT device, and the physician determined the longest wheal diameter by visually inspecting the images using a web interface. Prick location bias was assessed along the medial vs. lateral and proximal vs. distal axes of the forearm.
Results: In total, 944 histamine pricks were analyzed. Four medial and four lateral histamine pricks were grouped, and wheal sizes were compared. The longest wheal diameters were not significantly different between the medial and lateral prick locations (p = 0.41). Furthermore, the pricks were grouped by two based on their position on the proximal–distal axis of the forearm. No significant difference was observed among the four groups of analyzed prick locations (p = 0.73).
Conclusion: The prick location on the volar side of the forearm did not influence wheal size in SPAT-pricked individuals.
Introduction
The skin prick test (SPT) and serum-specific IgE test are both commonly used to evaluate type I hypersensitivity in patients with suspected inhalant allergy (1). Previous studies indicated that SPT is a more sensitive diagnostic test than the serum-specific IgE test (2, 3). Recently, it has been shown that screening for sensitization to allergens using in vitro molecular tests exhibits lower sensitivity than the extract-based SPT (4).
Despite the clear clinical need for the SPT and the availability of guideline recommendations (1, 5, 6), a considerable variation in its application persists due to both operator- and device-dependent factors (7, 8). To address this challenge, a skin prick automated test (SPAT), which is a novel device to automate and standardize the SPT procedure, has been developed. The device performs the prick procedure and the imaging of the prick reaction. A previous study by Gorris et al. (9) showed that the SPAT is associated with reduced intrasubject variability and patient discomfort while maintaining high levels of sensitivity and specificity.
According to international guidelines, the SPT is performed on the volar side of the forearm or the back of the patient (1, 6). However, previous literature is inconclusive on whether prick location on different positions of the forearm impacts the outcome of the test result (10, 11). In addition, these studies were performed in rather small datasets. Therefore, we evaluated prick location bias using the SPAT device, eliminating other variables such as prick force, device, or operator.
Methods
Study design
The setup of the previous SPAT validation study (9), which involved nine pricks of histamine (10 mg/ml) as positive controls and one prick of glycerol-saline solution as a negative control, allowed us to address our research question. The study was approved by the institutional review board of UZ Leuven (S66403) and registered online at www.isrctn.com (ISRCTN14098475).
Recruitment
All study participants provided written informed consent before inclusion in the study. Healthy volunteers, irrespective of their atopic status, between 18 and 65 years old were eligible for the study.
The prick procedure was performed using the SPAT medical device (Hippo Dx, Aarschot, Belgium; Figure 1A). In brief, the study participants positioned their forearms against the foreseen location of the SPAT device after the operator started the testing procedure on the touch screen and the automated pricking procedure was started. A total of 10 pricks were performed simultaneously by the device on the volar side of the forearm.
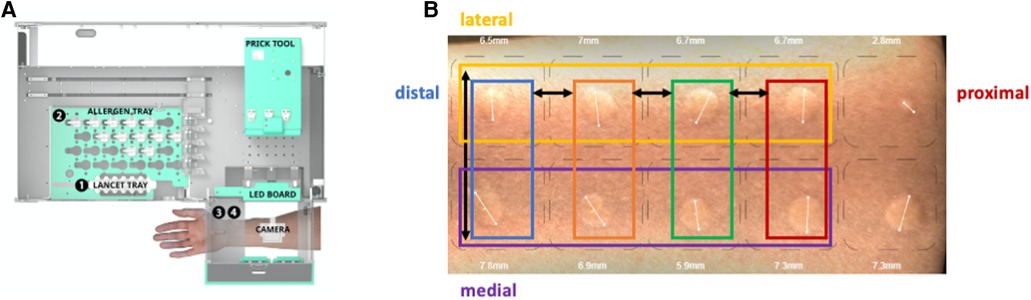
Figure 1. Study setup. (A) Inside view of the SPAT medical device with the prick tool moving first to position 1, the lancet tray, to collect the lancets, then moving to position 2, the allergen tray, to collect the allergens from the vials, and finally moving to position 3, where the arm is positioned for the prick procedure. After 15 min, the arm is positioned at position 4, where the camera is also located, taking 35 images of the volar side of the arm. (B) Representative image of the skin reaction 15 min after a skin prick automated test (SPAT). The prick locations on the volar side of the arm are indicated.
After 15 min, the SPAT device was utilized to conduct imaging of the skin reaction, and the physician analyzed the images of the skin reactions in a web interface to determine the longest wheal diameter.
Statistics
The Shapiro–Wilk test was used to evaluate normality. The Mann–Whitney or Kruskal–Wallis test was employed to conduct between-group comparisons of nonparametric data. A p-value of <0.05 was considered statistically significant.
Results
Data were collected from 118 volunteers, with each individual undergoing eight pricks (four rows on the proximal–distal axis and two rows on the medial–lateral axis) (Figure 1B). Prick location bias was assessed along the medial vs. lateral axis and proximal vs. distal axis on the volar side of the forearm. The histamine pricks were categorized into groups of two or four depending on their position along the proximal–distal or medial–lateral axis of the forearm, respectively (Figure 1A).
In total, 944 histamine pricks were analyzed. The longest wheal diameters were not significantly different between medial [median with interquartile range: 7.5 mm (6.5–8.3)] and lateral [7.5 mm (6.7–8.3)] prick locations (p = 0.41, Figure 2A). Furthermore, considering the proximal–distal axis, no significant difference was observed among the four groups [7.4 mm (6.7–8.3), 7.5 mm (6.7–8.4), 7.5 mm (6.7–8.4), 7.5 mm (6.5–8.2)] of prick locations analyzed (p = 0.73, Figure 2B).
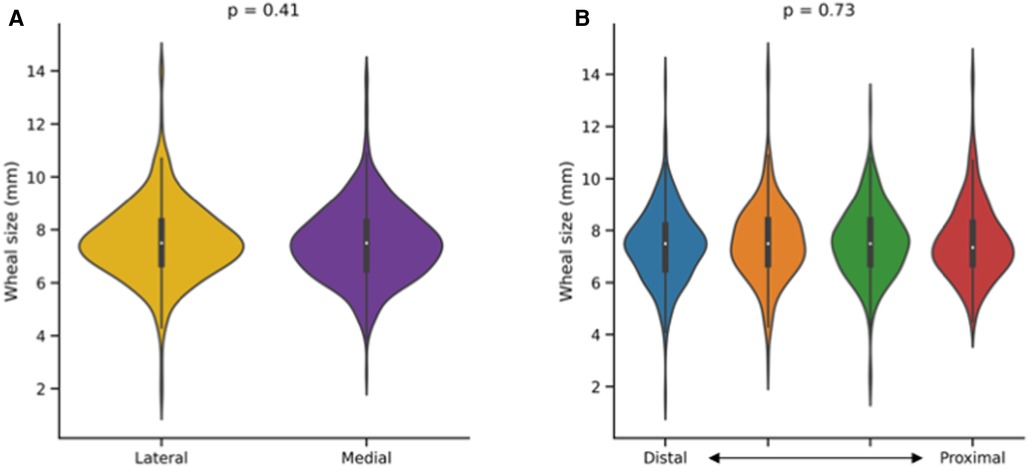
Figure 2. Impact of prick location on the outcome of a skin prick automated test (SPAT). Wheal sizes (longest wheal diameter) were compared along the lateral–medial (A) and proximal–distal (B) axes of the forearm. Mann–Whitney and Kruskal–Wallis tests were used for between-group comparisons.
Discussion
In the present study, we showed that the prick location on the volar side of the forearm did not influence the wheal size in SPAT-pricked individuals. Previous studies predominantly consist of older studies that have shown inconsistent results. In their study, Swain and Becker (10) conducted intradermal testing utilizing similar doses of histamine and observed a significant increase in wheal sizes in proximity to the elbow compared with the wrist. Several other studies have demonstrated differences in skin sensitivity to allergens on the arm, exhibiting a similar pattern with the cubital fossa being more reactive than the sites near the wrist (12–15).
These reports differ from the findings of Clarke et al. (11), who investigated skin reactions to common aeroallergens in 35 individuals with asthma using conventional skin prick testing and did not observe any significant influence of the prick location on the patient's forearm. Furthermore, the study conducted by Demoly et al. (16) could not detect a significant difference in skin reactivity to histamine when comparing the medial and lateral sides of the forearm in a sample of eight healthy volunteers. Since these studies were performed in small datasets or several decades ago, we argue that it is not possible to draw any firm conclusions.
In addition, it should be noted that exogenous histamine induces wheals directly by binding to histamine 1 and 4 receptors, whereas allergens first require binding to allergen-specific IgE and subsequent mast cell degranulation to release endogenous histamine (17). Although the expected skin reactions may therefore be different, the use of exogenous histamine allows easy evaluation of intrasubject variations of skin reactions.
Our current study is more robust compared to the previous reports in several aspects. Most importantly, performing a skin test using the SPAT device excludes the influence of operator-dependent factors such as prick force and human errors, often leading to false-positive or false-negative results (8). Second, the number of tested individuals and thus the number of pricks being analyzed are approximately 3–4 times higher. Third, our study was performed with a concentration of 10 mg/ml of histamine, which is currently widely used as a positive control in skin tests.
In conclusion, we are convinced that the use of an automated SPT for skin testing, as demonstrated in this study with the SPAT device, will play a significant role in establishing a standardized approach to allergy testing in future clinical practice.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset is not published online. Requests to access these datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethische Commissie Onderzoek UZ/KU Leuven. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: conceptualization, writing – original draft, writing – review and editing. SG: conceptualization, funding acquisition, writing – review and editing. SU: formal analysis, writing – review and editing. WB: formal analysis, writing – review and editing. MJ: supervision, writing – review and editing. RS: conceptualization, writing – review and editing. RD: formal analysis, writing – review and editing. DL: conceptualization, funding acquisition, writing – review and editing. LV: conceptualization, supervision, writing – review and editing. PH: conceptualization, supervision, writing – review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by a VLAIO grant (HBC.2021.1170).
Conflict of interest
SS, SG, RD, DL, and LV hold shares of Hippocreates. PH has a first-line relative who holds shares of Hippocreates. SS, SG, RD, and DL are employees of Hippocreates.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test—European standards. Clin Transl Allergy. (2013) 3:3. doi: 10.1186/2045-7022-3-3
2. van der Zee JS, de Groot H, van Swieten P, Jansen HM, Aalberse RC. Discrepancies between the skin test and IgE antibody assays: study of histamine release, complement activation in vitro, and occurrence of allergen-specific IgG. J Allergy Clin Immunol. (1988) 82(2):270–81. doi: 10.1016/0091-6749(88)91011-1
3. Tschopp JM, Sistek D, Schindler C, Leuenberger P, Perruchoud AP, Wüthrich B, et al. Current allergic asthma and rhinitis: diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and phadiatop). results from 8329 randomized adults from the SAPALDIA study. Swiss study on air pollution and lung diseases in adults. Allergy. (1998) 53(6):608–13. doi: 10.1111/j.1398-9995.1998.tb03937.x
4. Gureczny T, Heindl B, Klug L, Wantke F, Hemmer W, Wöhrl S. Allergy screening with extract-based skin prick tests demonstrates higher sensitivity over in vitro molecular allergy testing. Clin Transl Allergy. (2023) 13(2):e12220. doi: 10.1002/clt2.12220
5. Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. (2012) 67(1):18–24. doi: 10.1111/j.1398-9995.2011.02728.x
6. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. (2008) 100(3 Suppl 3):S1–148. doi: 10.1016/s1081-1206(10)60305-5
7. Werther RL, Choo S, Lee KJ, Poole D, Allen KJ, Tang MLK. Variability in skin prick test results performed by multiple operators depends on the device used. World Allergy Organ J. (2012) 5(12):200–4. doi: 10.1097/WOX.0b013e31827e6513
8. Topal SG, Karaman BF, Aksungur VL. Variables affecting interpretation of skin prick test results. Indian J Dermatol Venereol Leprol. (2017) 83(2):200–4. doi: 10.4103/0378-6323.192956
9. Gorris S, Uyttebroek S, Backaert W, Jorissen M, Schrijvers R, Thompson MJ, et al. Reduced intra-subject variability of an automated skin prick test device compared to a manual test. Allergy. (2022) 78(5):1366–8. doi: 10.1111/all.15619
10. Swain HH, Becker EL. Quantitative studies in skin testing: V. The whealing reactions of histamine and ragweed pollen extract. J Allergy (Cairo). (1952) 23(5):441–51. doi: 10.1016/0021-8707(52)90008-7
11. Clarke CW, Mitchell J, Nunn AJ, Pepys J. Reproducibility of prick skin tests to five common allergens. Clin Experiment Allergy. (1982) 12(1):1–8. doi: 10.1111/j.1365-2222.1982.tb03120.x
12. Schloss O. Allergy in infants and children. Am J Dis Child. (1920) 19(6):433–54. doi: 10.1001/archpedi.1920.01910240021003
13. Alexander H, Harter J, McConnel F. Observations on the formation of wheals. II. Comparison of wheals induced by allergens and by histamine. Proc Soc Exper Biol Med. (1930) 27(6):484–86. doi: 10.1381/00379727-27-4815
14. Bowman K. Pertinent factors influencing comparative skin tests on the arm. J Allergy. (1935) 7(1):39–53. doi: 10.1016/S0021-8707(35)90034-X
15. Becker E, Rappaport B. Quantitative studies in skin testing. II. The form of the dose-response curve utilizing a quantitative response. J Allergy Clin Immunol. (1948) 19(5):317–28. doi: 10.1016/0021-8707(48)90112-9
16. Demoly P, Bousquet J, Manderscheid JC, Dreborg S, Dhivert H, Michel FB. Precision of skin prick and puncture tests with nine methods. J Allergy Clin Immunol. (1991) 88(5):758–62. doi: 10.1016/0091-6749(91)90183-O
Keywords: skin prick test, allergy diagnosis, standardization, histamine, wheal
Citation: Seys SF, Gorris S, Uyttebroek S, Backaert W, Jorissen M, Schrijvers R, Daems R, Loeckx D, Van Gerven L and Hellings PW (2023) Evaluation of skin prick location on the forearm using a novel skin prick automated test device. Front. Allergy 4:1289031. doi: 10.3389/falgy.2023.1289031
Received: 5 September 2023; Accepted: 13 October 2023;
Published: 1 November 2023.
Edited by:
Julie Weidner, AstraZeneca, SwedenReviewed by:
Roxana Mincheva, University of Gothenburg, SwedenTatsuro Nakamura, Rakuno Gakuen University, Japan
© 2023 Seys, Gorris, Uyttebroek, Backaert, Jorissen, Schrijvers, Daems, Loeckx, Van Gerven and Hellings. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sven F. Seys c3Zlbi5zZXlzQGhpcHBvLWR4LmNvbQ==
 Sven F. Seys
Sven F. Seys Senne Gorris1
Senne Gorris1 Mark Jorissen
Mark Jorissen Rik Schrijvers
Rik Schrijvers Laura Van Gerven
Laura Van Gerven