- 1Department of Allergy & Rhinology, Royal National ENT Hospital, London, United Kingdom
- 2Division of Immunity and Infection, University College, London, United Kingdom
- 3Paediatric Allergist, Red Cross Children's Hospital and University of Cape Town, Cape Town, South Africa
- 4Kidsallergy Centre, Cape Town, South Africa
- 5The European Forum for Research and Education in Allergy and Airway Diseases Scientific Expert Team Members, Brussels, Belgium
- 6Escuela de Doctorado UAM, Centro de Estudios de Posgrado, Universidad Autónoma de Madrid, Calle Francisco Tomás y Valiente, no 2, Ciudad Universitaria de Cantoblanco, Madrid, Spain
- 7The Allergy Clinic, Blairgowrie, Randburg, South Africa
- 8Department of Otorhinolaryngology, Head & Neck Surgery, and Audiology, Rigshospitalet, Copenhagen University, Copenhagen, Denmark
- 9Allergy, Royal Brompton Hospital, London, United Kingdom
- 10Otolaryngology-Department, Clinic Barcelona, Barcelona, Spain
- 11Otolaryngology-Department, University of Barcelona, Barcelona, Spain
- 12Otolaryngology Head and Neck Surgery, A. Gemelli University Hospital Foundation IRCCS, Rome, Italy
- 13Department of Respiratory Medicine & Allergology, Institute for Clinical Science, Skane University Hospital, Lund University, Lund, Sweden
- 14Department of Respiratory Medicine, First Faculty of Medicine, Charles University and Thomayer Hospital, Prague, Czech Republic
- 15Department Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
- 16Department of Microbiology Immunology & Transplantation, KU Leuven, Catholic University of Leuven, Leuven, Belgium
- 17Department of Rhinology and Skull Base Surgery, Guy’s and St Thomas’ Hospital NHS Foundation Trust, London, United Kingdom
- 18Department of Clinical Immunology and Allergology, University Teaching Hospital in Martin, Martin, Slovak Republic
- 19Department of Paediatrics, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, University Teaching Hospital in Martin, Martin, Slovak Republic
- 20Department of Pulmonology and Phthisiology, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, University Teaching Hospital in Martin, Martin, Slovak Republic
- 21Department of Dermatology, University of Zurich, Zurich, Switzerland
- 22Department of Dermatology, University Hospital of Zurich, Zurich, Switzerland
- 23Department of Pulmonology, STZ Centre of Excellence for Asthma, COPD and Respiratory Allergy, Franciscus Gasthuis & Vlietland, Rotterdam, Netherlands
- 24Rhinology Unit and Smell Clinic, ENT Department, Hospital Clínic, FRCB-IDIBAPS, Universitat de Barcelona, CIBERES, Barcelona, Spain
- 25Observational and Pragmatic Research Institute, Singapore, Singapore
- 26Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, United Kingdom
- 27Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain
- 28Department of Otorhinolarynogology and Head/Neck Surgery, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands
- 29Department of Otorhinolaryngology, Kuopio University Hospital and University of Eastern Finland, Kuopio, Finland
- 30Department of Allergy, Inflammation Center, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
- 31Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 32Department of Dermatology, Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark
- 33Department for Pediatric Pneumology and Immunology, Charite University Medicine, Berlin, Germany
- 34Department of Otorhinolaryngology-Head and Neck Surgery, University Hospitals, Leuven, Belgium
- 35Laboratory of Allergy and Clinical Immunology, University Hospitals Leuven, Leuven, Belgium
- 36Upper Airways Research Laboratory, Department of Head and Skin, Ghent University, Ghent, Belgium
The concept of pre-diabetes has led to provision of measures to reduce disease progression through identification of subjects at risk of diabetes. We previously considered the idea of pre-asthma in relation to allergic asthma and considered that, in addition to the need to improve population health via multiple measures, including reduction of exposure to allergens and pollutants and avoidance of obesity, there are several possible specific means to reduce asthma development in those most at risk (pre- asthma). The most obvious is allergen immunotherapy (AIT), which when given for allergic rhinitis (AR) has reasonable evidence to support asthma prevention in children (2) but also needs further study as primary prevention. In this second paper we explore the possibilities for similar actions in late onset eosinophilic asthma.
1 Introduction
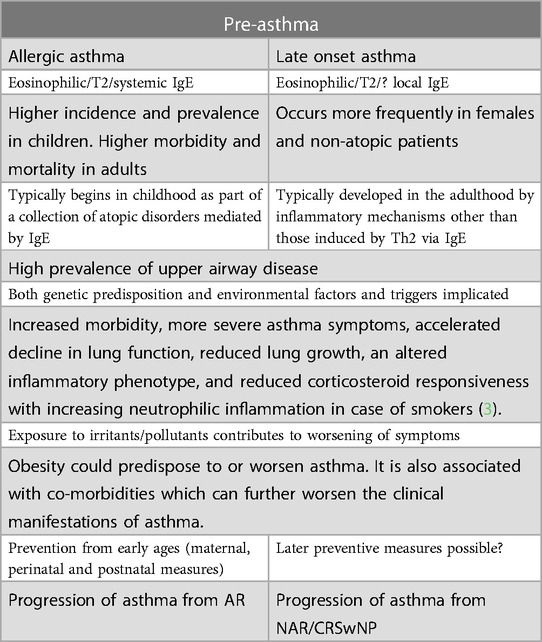
The concept of pre-diabetes has led to provision of measures to reduce disease progression through identification of subjects at risk of diabetes (1). We previously considered the idea of pre-asthma in relation to allergic asthma (2) (Table 1) and considered that, in addition to the need to improve population health via multiple measures, including reduction of exposure to allergens and pollutants and avoidance of obesity, there are several possible specific means to reduce asthma development in those most at risk (pre- asthma). The most obvious is allergen immunotherapy (AIT), which when given for allergic rhinitis (AR) has reasonable evidence to support asthma prevention in children (2) but also needs further study as primary prevention (4).

In this second paper we explore the possibilities for similar actions in late onset eosinophilic asthma.
2 Late onset eosinophilic type 2 asthma
2.1 Pathophysiology
Inflammatory mechanisms other than those induced by Th2 via IgE may be involved in the pathophysiology of late onset asthma which has multiple phenotypes (5) (Figure 1). Indeed, the proportion of such asthma attributable to atopy is usually less than 50% (7). Whilst non-eosinophilic asthma remains relatively under-explored (8), there is now more and better characterisation of eosinophilic asthma, which lacks systemic IgE, i.e., asthma with low levels of serum IgE and negative skin prick tests to common allergens. Asthma with a type 2 (T2) endotype is driven by alarmins, IL-25, IL-33, and thymic stromal lymphopoetin (TSLP) either through naïve T-cells or through ILC2 cells, and involves the cytokines IL-4, IL-5 and IL-13, as well as eosinophils and, sometimes, IgE (9).
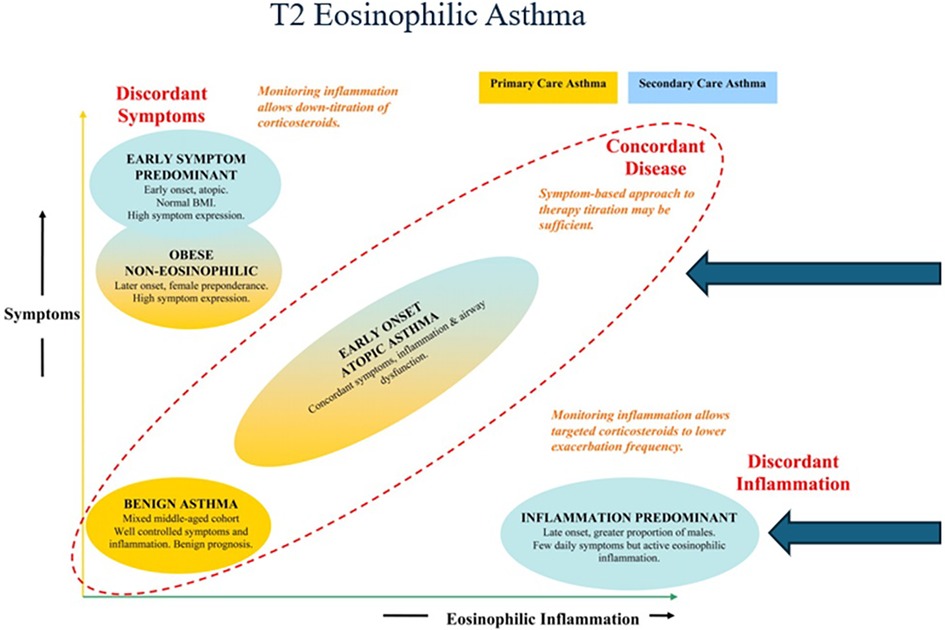
Figure 1. T2 eosinophilic asthma of late onset can be allergic or non—allergic as seen here in the asthma clusters described by Haldar et al. Adapted from Haldar et al. (6).
As in nasal polyps, IgE may present locally, rather than systemically (10). Circumstantial evidence that both atopic and nonatopic asthma may be mediated by local IgE in human bronchial mucosa (11). Although late-onset eosinophilic asthma has traditionally been considered non-allergic in nature, it can be associated with allergic features and comorbidities (12). In some patients, there is evidence that inflammation is driven by toxins from Staphylococcus aureus (vide infra). The possibility that late onset asthma is partly an auto-immune disorder has also been suggested, with some patients having pathogenic sputum autoantibodies against autologous eosinophil proteins (e.g., eosinophil peroxidase) (13, 14).
2.2 Predisposing conditions
The T2 form of asthma occurs more frequently in females and non-atopic patients and, like allergic asthma, and it may also be preceded by nasal disease, e.g., predominantly non-allergic rhinitis and/ or chronic rhinosinusitis with nasal polyposis (CRSwNP) (15).
2.2.1 Non-allergic rhinitis (NAR)
NAR is defined as a chronic condition of the nasal mucosa that is not caused by allergy nor by an infectious agent, and subdivided into several groups, including gustatory, hormonal, drug-induced or idiopathic rhinitis. Typically, symptoms are triggered by irritants such as cigarette smoke, pollution, strong odors, physical exercise and/or changes in temperature and humidity. This induction of nasal symptoms by non-specific stimuli is called nasal hyperreactivity (NHR) and found in two thirds of patients with inflammatory conditions of the nasal and paranasal mucosa (16).
While allergic rhinitis is a well-established risk factor for asthma, the relationship between NAR and asthma is less clear. In the European Respiratory Health Survey NAR was associated with a nearly threefold risk of asthma development compared to subjects without rhinitis or atopy, only slightly lower than the risk for those young adults with allergic rhinitis (17). There is some evidence that individuals who have nasal symptoms of congestion and postnasal drip are most at risk (16). Another study found that NAR was associated with an increased risk of asthma exacerbations in individuals with comorbid asthma (18). Other studies have failed to confirm the link between NAR and comorbid asthma or have suggested that local allergic rhinitis has been misdiagnosed as non- allergic (19).
The relationship may be more complex and depend on factors such as the type and severity of NAR, which includes both inflammatory and neurogenic forms of rhinitis, as well as individual genetic and environmental factors. It is likely that inflammatory eosinophilic NAR, also called NARES, is the form of NAR, which is most associated with asthma development (20). Concomitant mast cells together with eosinophils in the nasal smears gave a particularly high risk for associated asthma (21).
2.2.2 Chronic rhinosinusitis
Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) is an inflammatory process affecting the lining of the nasal passages and sinuses. Individuals with CRSwNP are more likely to develop/have asthma than patients without polyposis (CRSsNP) (22) and are more likely to have severe disease (23). The prevalence of asthma in individuals with CRSwNP is estimated to be between 20% and 50% (24). The chronic inflammation associated with nasal polyps is variable, but those subjects with Type 2 eosinophilic polyps are highly likely to have concomitant asthma (25), particularly patients with allergic fungal rhinosinusitis (AFS) (26) or aspirin sensitivity (AERD). By extension, the proportion of comorbid asthma in patients with severe uncontrolled CRSwNP is very high and reaches up to ∼80% when biological therapy is indicated (27–29).
Indeed, approximately 20% of late onset asthmatics develop AERD or hypersensitivity to aspirin and other cyclooxygenase inhibitors (NSAID) (30). In a European study, most of the AERD patients developed upper airway symptoms prior to asthma (31). However, in an American study, with a majority of self-identifying, black study participants, the development of A-ERD was highly variable in onset and progression (32). Asthma occurred first in 50% of all participants, mainly in younger, female obese subjects. The “NSAID-sensitivity first” group was predominantly male odd's ratio (OR = 3.3), 95% confidence interval (CI) 1.5–7.4, p = 0.004) with exposure to pollutants, (OR = 4.4, CI 1.6–11.9, p = 0.003). A-ERD has evidence of additional eicosanoid dysregulation (33).
In a prospective study Sinus inflammation and chronic rhinosinusitis (CRS) [identified in two ways: validated text algorithm applied to sinus computerized tomography (CT) scan or two diagnoses] have been reported to be associated with a diagnosis of new-onset asthma in the following year (34).
3 Other risk factors for late onset T2 eosinophilic asthma
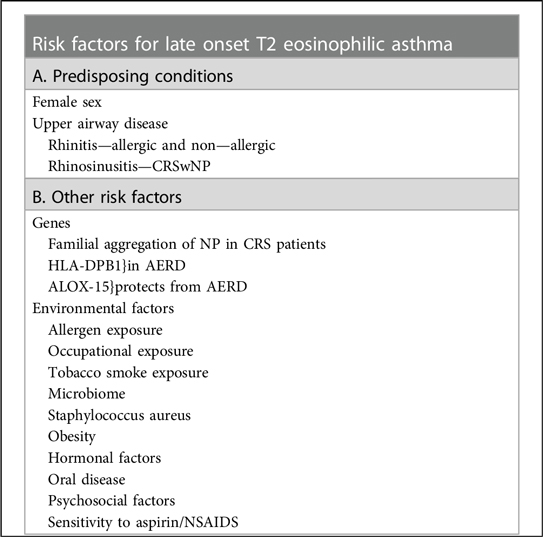
As in allergic asthma, there is evidence for both genetic and environmental influences (Box 1).

Abbreviations missing (CRSwNP, chronic rhinosinusitis with nasal polyps; NP, nasal polyposis; CRS, chronic rhinosinusitis; AERD, aspirin-exacerbated respiratory disease; NSAIDS, non-steroidal anti-inflammatory drugs).
3.1 Genes
3.1.1 Familial aggregation of NP in CRS patients
Familial aggregation of NP is demonstrated in CRS patients and correlates with disease severity (35, 36). A systematic review of all published data on genetic and epigenetic variations in CRSwNP since 2,000 identified over 150 genetic variants in 99 genes involved in pathogenesis. These were clustered into 8 main networks, linking genes involved in inflammation and immune response (e.g., MHC), cytokine genes (e.g., TNF), leukotriene metabolism, and the extracellular matrix. Eighty-nine miRNAs were also identified, associated mainly with the cell cycle, inflammation, and the immune response (37).
Genes related to Epithelial abnormalities, including filaggrin, were identified using whole exome sequencing in a small study (38).
3.1.2 HLA-DPB1
AERD has several genetic associations in an asthmatic population with HLA-DPB1 gene polymorphism the most susceptible factor for the risk of AERD (39). Multiple other genes identified as possibly relevant in AERD are noted in Dahlin et al. (40).
3.1.3 ALOX-15
Polyps from CRSwNP patients with AERD show elevated ALOX-15 expression, worse sinonasal disease and more operations (41) compared to patients without AERD.
The ALOX-15 mRNA expression level could distinguish between eosinophilic and non-eosinophilic CRSwNP, being significantly higher in eosinophilic ones (42).
Protection against AERD occurs with a loss of function mutation of ALOX-15 (43).
A promoter polymorphism enhances IgE responses to staphylococcal superantigens in adult asthmatics (44).
3.2 Environmental factors
3.2.1 Allergen exposure
Indoor mould exposure in the last year, especially involving Cladosporium species, was associated with asthma symptoms and bronchial responsiveness (OR range, 1.14–1.44) (45).
Sensitization to perennial aeroallergens was present in 68% of adults with severe asthma, many of whom were likely late onset asthmatics. The most prevalent sensitizations were to Dermatophagoides pteronyssinus, D. farinae, D. microceras, Aspergillus fumigatus, Staphylococcus aureus Toxic Shock Syndrome Toxin (TSST), and Candida albicans (46).
3.2.2 Occupational exposure
Exposure to certain substances in the workplace, such as irritants (47), chemicals, dust and fumes, can trigger asthma symptoms in adult life (48). The CONSTANCES cohort has shown that exposure to solvents and to irritants can trigger adult asthma (49, 50).
3.2.3 Exposure to environmental tobacco smoke
Smoking is a risk factor for eosinophilic asthma in adult life (51). The effects of smoking on the risks of atopic and non-atopic asthma differ and are modified by gender. In women, but no in men, the risk of atopic asthma was increased (adjusted OR 1.24, 95% CI 0.83–1.85) by smoking. Recent smoking cessation was related to increased risk of both atopic (aOR 4.91, CI2.26–10.65) and non-atopic (aOR 4.37, CI 1.87–10.21) asthma. Ceasing to smoke over 12 months ago was related to increased risk of non-atopic asthma (aOR 1.57, CI 1.08–2.28), mainly in men (aOR 2.03, CI 1.06–3.88) (52).
3.2.4 Microbiome
Microbial imbalance could be involved in the pathogenesis of upper and lower airway diseases, including asthma. The composition of the airway microbiome is susceptible to influences such as genetics, environmental exposures and medications. CRSwNP patient had reduced Corynebacterium and Dolosigranulum in their nasal samples compared to healthy controls. Bacterial genera such as Lactobacillus, Escherichia coli, Shigella, Turicibacter, Clostridium, Enterococcus, and Romboutsia were positively correlated with the severity of CRSwNP (53).
Viruses, rather than bacteria, constitute the largest proportion of the human microbiota. The lung virome has as yet been little studied. There is evidence of overabundance of cytomegalovirus (CMV) and Epstein Barr virus (EBV) in patients with asthma exacerbations, plus correlation with higher asthma severity, lower lung function and ACT scores. Conversely bacteriophage abundant in healthy controls was reduced in asthma, proportionally to severity (54).
3.2.4.1 Staphylococcus. aureus
S. aureus-Serum IgE specific to S. aureus enterotoxin (SA-IgE) has been linked to adult-onset asthma and to more severe asthma (55–57). Nasal S. aureus carriage was positively associated with asthma prevalence in meta-analysis of five cross-sectional studies (OR 1.19, 95% CI 1.06–1.34) in the general adult population. It was positively associated with asthma in another meta-analysis of 11 studies of CRS patients (OR 1.86, CI 1.18–2.95) (58).
Much stronger associations exist with asthma prevalence for S. aureus recovery from surgical tissue specimens from CRS patients (OR 40.4, CI 10.5–155) than for S. aureus recovery from swab samples (OR 1.21, CI 0.99–1.48) (57). CRSwNP–derived S. aureus biofilms showed thicker biomass, higher colony-forming units, and higher exoprotein production than those from controls (P < 0.05). CRS severity scores were positively correlated with S. aureus biofilm properties and numbers of inflammatory cells (59).
More recently correlation of CD3 + cell subsets in the sinonasal tissue of CRS and non-CRS control patients with CRS severity scores, in vitro-grown biofilm properties and virulence genes of the corresponding patient-derived S. aureus have been demonstrated. In tissue harbouring the Staphylococcal isolates carrying the lukFPV gene (Panton–Valentine Leukocidin, a leukotoxin which lyses cells of the leukocytic lineage and destroys neutrophils) CD4T cell counts were higher (60).
Staphylococcal superantigens, such as enterotoxins (SEA), are highly mitogenic and stimulate activity in many T lymphocytes, leading to substantial mediator and proinflammatory cytokine release (61, 62), intensifying the Th2 response in the tissue and diminishing the immunosuppressive activity of Tregs (63).
3.2.5 Obesity
A meta-analysis of several prospective studies involving more than 300,000 adults found a weight-response relationship between obesity and asthma. The risk of asthma in the overweight and in the obese groups compared with the lean group were OR 1.5 and OR 1.9, respectively (64, 65).
Arismendi and colleagues (65) note that obesity is a major modifiable risk factor for asthma, possibly acting via systemic inflammation, lung function alterations, metabolic dysregulation, microbiome changes, and epigenetic/genomic regulation. Adipose tissue is metabolically active, releasing pro-inflammatory cytokines, including leptin which induces the proliferation and survival of type 2 innate lymphoid cells (ILC2) and T helper 2 (Th2) cells, and also induces monocyte, CD4 + and CD8+ T cell activation.
Obese asthma patients are more likely to have a poor response to glucocorticoids. Vitamin D, which increases glucocorticoid effectiveness (66) is often low in obese subjects.
Several phenotypes of obesity-associated asthma exist. Patients with an earlier onset are likely to have T2 inflammation with more severe disease, while others may have a non-inflammatory form of asthma or neutrophilic disease (67–70).
3.2.6 Hormonal factors
Oral contraceptives may be associated with asthma development (71) whereas hormone replacement therapy was associated with a reduced risk of development of late onset asthma in menopausal women (72).
3.2.7 Oral disease
Adult asthmatics experience a higher risk for a major oral disease or oral-manifesting disease, but it is uncertain whether this is post or propter hoc (73).
3.2.8 Psychosocial factors
Stress, depression and traumatic events in childhood have been reported as risk factors for adult-onset asthma, but there may be reverse causation (74).
3.2.9 Poor quality sleep
The UK Biobank cohort involving 455,405 participants aged 38–73 years was employed in a large-scale prospective study looking at genes and sleep scores. Over the 10 years plus of follow-up 17.836 of these individuals were diagnosed with asthma. The hazard ratio (HR) for poor sleep compared to the low-risk group was 1.55 (95% CI: 1.45–1.65). Poor sleep was additive to high genetic susceptibility (HR (95% CI): 2.22 (1.97–2.49), p < 0.001) compared with the low-risk combination. Healthy sleep lowered the risk of asthma in all genetic susceptibility groups, HR being 0.63 (0.57–0.70) in the high-risk group. Risk analysis suggests that 19% of asthma cases could be prevented by improved sleep traits (75).
4 Possibilities for prevention
4.1 Allergen/occupational allergen exposure
Reduction of mould and house dust mites in homes by cleaning and proper ventilation should be encouraged. Wearing a face mask whilst cleaning might also be protective.
Proper precautions against exposure in relevant industries need to be enforced (76). Early identification of susceptible individuals (some of whom will initially develop rhinitis) by continued monitoring is advisable, with removal from subsequent exposure before irreversible asthma develops.
4.1.1 Possible role of allergen immunotherapy
Allergen immunotherapy in adults with allergic rhinitis may present a potential pathway to reduction to progression to asthma. Although the effect appears stronger in children (77), a retrospective cohort study following up 332 non-asthmatic adults with allergic rhinitis over 9 years, showed that allergen immunotherapy significantly reduced the development of new onset asthma [OR 0.53, (0.32–0.86)] (78).
4.2 Smoking
Continued discouragement of initiation of smoking of all kinds is necessary, together with improved education about the underlying reasons behind this (79).
4.3 Obesity
Weight loss and vitamin D improve hyporesponsiveness to corticosteroids in obese asthma (80). Bariatric surgery improved corticosteroid responses of peripheral monocytes in vitro in a small study in obese asthmatics and normalized their adiponectin/leptin ratio and vitamin D levels (80). Following the breakthrough with semaglutide (marketed as Wegovy or Ozempic), other similar glucagon-like peptide (GLP-1) weight loss drugs are entering the market. There is evidence that these drugs may reduce diabetes and cardiovascular complications of obesity. Studies are needed to investigate if late onset asthma is also decreased by the use of GLP-1 drugs, which can reduce inflammation in the liver, kidneys, heart and brain. As for yet, there is no evidence of any effect of GLP-1 drugs on asthma. However, since immune cells do not express a high frequency of GLP-1 receptors, the drugs may have limited or no effect on asthma (81). Studies are needed.
4.4 Progression of asthma from NAR
Several prior studies have shown an association between AR and development of asthma, but only one small study found that intranasal corticosteroids reduced the incidence of asthma in non-allergic rhinitis (82). Further research is needed to confirm these findings.
4.5 Progression of asthma from CRSwNP
There is currently limited scientific evidence for asthma prevention in CRSwNP patients. Reduction in the burden of upper airway inflammation might be preventative. In CRSwNP, burden can be reduced both medically (appropriate medical treatment and biologics, in severe uncontrolled patients) and surgically (endoscopic sinus surgery), but in most patients it recurs over time, being ameliorated in the long term by regular intranasal corticosteroids (83, 84). It is conceivable that those CRSwNP patients, who are excellent responders to anti-leukotrienes, might show reduced progression to asthma (85, 86). To date, this association has not been investigated.
There is some evidence that early surgery of CRSwNP is associated with less progression to asthma (87, 88).
Debulking surgery in CRSwNP, removing extensive polyp tissue reduces asthma symptoms and decreases the release of LTE4, the major leukotriene metabolite in AERD and useful in diagnosis of AERD (89, 90). Better CRSwNP control leads to better asthma control (91). However, long-term sizeable observational studies are needed to see if early polyp surgery is associated with reduced progression to asthma. Retrospective data may be available from existing records.
4.5.1 Possible role of aspirin desensitization
Patients with hypersensitivity to Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), including aspirin, show a significant increased risk of asthma [OR 5.5 (4.84–6.26)] (92) and an increased risk of uncontrolled asthma (93).
In those with AERD presenting with nasal polyposis and/or chronic rhinosinusitis, aspirin desensitization may present a possible opportunity for reduction of progression to asthma or reduction in asthma severity. A meta-analysis of aspirin desensitization in AERD showed a trend towards improved lung function and asthma symptom and medications scores, but additional RCTs are needed to fully assess this (94).
4.5.2 Effects of biologics
Monoclonal antibodies (mAbs) targeting IgE and cytokines implicated in the T2 inflammatory cascade of asthma have been developed (95–97).
These biological drugs include omalizumab (anti-IgE), mepolizumab and reslizumab (anti-IL-5), benralizumab (antagonist of the α subunit of IL-5 receptor), and dupilumab (directed against the α subunit of IL-4 receptor which is a common receptor for IL-4 and IL-13). Tezepelumab is an anti-alarmin directed against thymic stromal lymphopoietin (TSLP).
Clinical trials and real-world studies have shown their efficacy in T2 asthma in reducing exacerbations, improving asthma control, pulmonary function, and withdrawing or at least reducing oral corticosteroid use (OCS) (98–100).
There is also evidence of effective action in CRSwNP and in subjects with both CRSwNP and asthma (101), with greater efficacy in those with severe asthma (102). However, more research is needed to determine whether any biologic, if given to CRS patients, can prevent asthma development (97).
Since IL-5 and eosinophils predominate in CRSwNP, one would expect anti-IL-5 to be an effective treatment. However, more effective in CRSwNP is treatment with the molecule directed against the IL4Rα (dupilumab) (103, 104). Dupilumab therapy can also reverse aspirin/NSAID sensitivity, protecting patients against unwonted drug-induced exacerbations (97, 105).
A registry of patients treated with biologics for CRSwNP needs to be established in order to investigate the effect of biologics on the progression of CRSwNP to asthma. EUFOREA is in the process of setting up such a data collection.
4.6 Microbiome
Chalermwatanachai and colleagues noted an abundance of Phylum Proteobacteria in patients with asthma—associated CRSwNP compared the those with CRSwNP alone in whom Staphylococci and Moraxella were less prevalent (53). Frequent use of antibiotics has led to increasing resistance rates, becoming now a major health concern. Novel therapeutic strategies, including anti-virulence treatments that directly or indirectly neutralize S. aureus toxins, are in development (106). In CRSwNP patients, S. aureus grows intramucosally and intracellularly in the polyp tissue (24). More than 600 proteins released by S. aureus, including virulence factors such as the enterotoxins, were identified in the upper airway mucosa of patients with CRSwNP by high-resolution mass spectrometry among these were (107).
Antibiotic therapy with macrolides over months improved CRSwNP and associated asthma symptoms in a small study (108). A larger macrolide study is ongoing (EudraCT Number: 2018-001100-11.) Doxycycline reduces polyp size in CRSwNP (109) and might also be preventative of asthma. Similarly Staphylococcal decolonization routines could be attempted in those with risk factors or asthma. Antibiotic use carries risks such as changes in nasal microbiome and antibiotic resistance (110).
A different approach, such as measures to disable toxin production (106), could prove preventative with fewer adverse effects, since Staphylococcal toxins appear relevant to disease causation. The use of highly bacteriophages to remove certain bacteria is under study (111).
4.7 Anti-oxidants
Oxidative stresses play a role in inflammatory airways diseases, and low antioxidant levels may be a risk factor for asthma inception (112). Diets deficient in anti-oxidant vitamins and minerals, including vitamins A, D, E, C as well as selenium and zinc, have been associated with increased asthma prevalence. Supplementation with such vitamins and minerals have shown promising results in some, but not all, studies and merit further study (113). Resveratrol, a plant derived bioactive polyphenol found naturally in red grapes, berries and certain nuts, and also commercially in supplements, has shown pleiotropic anti-inflammatory and anti-oxidative effects (114). Murine models have shown resveratrol to attenuate allergic asthma with potential therapeutic effects on respiratory system diseases (115). Studies supplementing resveratrol in patients at risk of asthma or with asthma are warranted.
4.8 Sleep medicine
Early detection and management of sleep disorders could be beneficial in reducing asthma incidence.
5 Discussion
Prevention of late onset eosinophilic asthma seems more challenging than prevention of allergic asthma, as many patients have no identifying atopic history, though some studies suggest the relevance of IgE, possibly on a local level (12, 116). General measures such as reduction of obesity plus careful checking for occupational or recreational exposures and sensitizations are relevant. As with allergic asthma, recent understanding of the role of highly processed foods in causing obesity would suggest that avoidance of such foods should be recommended. However, the issue of dietary healthy eating guidelines is sensible at a population level.
The existence of allergic fungal rhinosinusitis with small quantities of fungus in the sinuses causing extensive inflammation, high levels of IgE and associated asthma (allergic bronchopulmonary aspergillosis, ABPA) warrants a careful check for this in CRSwNP in relevant areas of the world, as surgical removal is a necessity. Mask-wearing in polluted areas plus saline nasal douching after exposure could conceivably reduce asthma incidence in those sensitive to moulds and fungi.
A family history of CRSwNP, particularly in the father, conveys a high risk for nasal polyp development (117). Similarly, a family history of asthma or aspirin/NSAID sensitivity increase the likelihood of AERD (38). In consequence, identification of pre-asthma, in which intermittent lower airways inflammation is occurring, would be possible using FeNO measurements in the offspring of such patients. Possible preventive measures could then include regular nasal douching to remove local contributory factors acting on the epithelium. This could be tried at the initial stages of non-allergic eosinophilic rhinitis, or at the first appearance of polyps. Supplemental treatment could be intranasal corticosteroids or a trial of inhibitors of staphylococcal toxin production, or their combination. Early polyp surgery improves the prognosis of CRSwNP (87) with fewer asthma subjects than in the late surgical group, so this should be encouraged. Subsequent regular therapy to reduce recurrence should again be intranasal corticosteroids and possibly a trial of inhibitors of staphylococcal toxin production. The use of monoclonal antibodies in CRSwNP should be recorded in databases so that these can be mined for useful data on asthma prevention and length of effectiveness.
There is a complex interplay between an individual's genetic predisposition and their exposure to various environmental triggers. This fact underscores the necessity of a holistic approach to prevention, considering both intrinsic and extrinsic factors. The possibilities for prevention extend beyond traditional interventions, suggesting innovative approaches such as the use of biologics targeting specific inflammatory pathways and the potential role of weight loss drugs in reducing the inflammatory burden associated with obesity, a known risk factor for asthma. The effect of healthy sleep, even in those of high genetic predisposition, is remarkable and deserves further prospective work.
The limitations and gaps in current knowledge, highlight the need for further research. This includes the need for large-scale observational studies to understand the long-term effectiveness of interventions like early surgery for CRSwNP and the use of monoclonal antibodies in preventing asthma development in patients with CRSwNP (Table 2). Upper airway disease, both AR and CRS, needs to be taken seriously. Since it is likely that effective AR and CRS treatment might prevent progression to asthma. Identification of some older subjects at risk of asthma followed by therapeutic intervention is now becoming a possibility.
In conclusion, this manuscript contributes significantly to the ongoing discourse on asthma prevention, offering valuable insights into the potential for reducing the incidence of late-onset eosinophilic asthma through a combination of early identification and management of precursor conditions, genetic and environmental risk factor modification, and innovative therapeutic interventions. The call for further research underscores the evolving nature of our understanding of asthma and the continuous quest for more effective preventive strategies.
Data availability statement
All relevant data is contained within the article: The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
SG: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing, Validation. GC: Conceptualization, Investigation, Resources, Supervision, Writing – review & editing. CD: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – review & editing. MM: Conceptualization, Investigation, Supervision, Writing – review & editing. BV: Conceptualization, Investigation, Supervision, Writing – review & editing. SG: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. B-SM: Conceptualization, Investigation, Supervision, Writing – review & editing. DE: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. DZ: Conceptualization, Data curation, Supervision, Writing – review & editing. HC: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing. JM: Writing – review & editing, Investigation, Supervision. JP: Investigation, Methodology, Supervision, Writing – review & editing. KJ: Investigation, Supervision, Validation, Writing – review & editing. MJ: Investigation, Resources, Supervision, Writing – review & editing. PD: Methodology, Resources, Supervision, Writing – review & editing. QS: Investigation, Methodology, Resources, Supervision, Writing – review & editing. RS: Investigation, Methodology, Validation, Writing – review & editing. T-SS: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. SB: Formal Analysis, Investigation, Methodology, Writing – review & editing. TJ: Investigation, Methodology, Supervision, Writing – review & editing. WU: Investigation, Methodology, Supervision, Writing – review & editing. HP: Investigation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
DC: Serves as academic manager at the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA) and as review editor at Frontiers in Allergy, rhinology section. MM: Honoraria for speaker and advisory boards: Sanofi, Glenmark, P&G, Immunotek, Cipla, Thermo-Scientific, Lancet laboratories. EUFOREA expert panel member. VB: Is recipient of consultancy/lecture fees or unrestricted research grants from Sanofi/Regeneron, Novartis, GSK, AZ, ALK Abello and BORK-npc. GS: has received speakers fees from ALK-Abello, Meda and Glenpharm, and received honoraria for participation in an advisory group from ALK-Abello. MB-S: Speaker honorarium from GSK Spain, Viatris Spain, Sanofi Spain, WIDEX Spain, Bionorica, Germany. ED: Received fee for consultation, speaker activity, advisory board by Sanofi, Regeneron, GSK, Novartis and AstraZeneca. ZD: In the past 3 years, received speaker or consultant honoraria and/or served on advisory boards at: Antabio, Arcede, Biosion, Foresee Pharmaceuticals, Galenushealth, GlaxoSmithKline, Hippo-Dx, QPS-Netherlands, Sanofi-Genzyme-Regeneron, and Springer Media all outside the submitted work. From 2012 to 2020 she acted as Director Respiratory & Allergy Research at QPS-Netherlands; in 2019-ongoing, QPS-Netherlands received a European grant from ERA4TB and funding Foresee Pharmaceuticals for early respiratory studies. CH: Ad boards—GSK, Sanofi Regeneron, Lilly. MJ: Has received consulting fees (ALK-Abello, Stallergenes-Greer, Takeda, Zentiva); honoraria for lectures, presentations (ALK-Abello, Stallergenes-Greer, Takeda, Zentiva, Mundipharma, AstraZeneca, SOBI, Chiesi, CSL Behring, Novartis, Benela, Pfizer, Viatris); support for attending meetings and/or travel (ALK-Abello, Stallergenes-Greer, Takeda, Novartis, Sanofi Pasteur) and honoraria for participation on Advisory Boards (ALK-Abello, Stallergenes-Greer, Chiesi, Novartis, SOBI, Pfizer, Sanofi Genzyme/Pasteur). JK: Jasper Kappen reports grants and/or personal fees from ALK, Chiesi, GSK, Novartis, AstraZeneca, Sanofi, Boerhinger, Teva, Viatris, Stallergen, Abbot, all outside the submitted work. JM: Joaquim Mullol is or has been member of national and international scientific advisory boards, consulting, received fees for lectures, and grants for research projects or clinical trials from AstraZeneca, Genentech-Roche, Glenmark, GSK, LETI, Lilly, Menarini, MSD, Mitsubishi-Tanabe, NOUCOR/Uriach Group, Novartis, OPTINOSE, Proctor & Gamble, Regeneron Pharmaceuticals Inc., Sanofi-Genzyme, UCB Pharma, and Viatris/MEDA Pharma. DP: David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Teva Pharmaceuticals; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. SQ: Speaking, lecture and consulting fees from Allergy Therapeutics, ALK, AstraZeneca, Chiesi, GSK, Mundipharma, Novartis, Sanofi-Genzyme, and Teva. SR: Has acted as a consultant and/or advisory board member for Sanofi, GSK, and Novartis. The department of Otorhinolaryngology and Head/Neck Surgery of the Amsterdam UMC has received research funding from Sanofi, GSK, and Novartis. ST-S: Reports consultancies for ALK-Abelló, AstraZeneca, ERT, GSK, Novartis, Sanofi, and Roche Products outside the submitted work, as well as grant of GSK outside the submitted work. BS: Lyra Therapeutics: consultant, Stryker: Consultant, Neurent: consultant, MCSP: consultant and American Rhinologic Society: VP of Development and Strategy. JT: Jacob Thyssen previously as an advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co, Pfizer, Regeneron, and Sanofi-Genzyme; a speaker for AbbVie, Almirall, Eli Lilly & Co, Pfizer, Regeneron, and Sanofi Genzyme; and received research grants from Pfizer, Regeneron, and Sanofi Genzyme. He currently holds a shared position between the university and LEO Pharma where he receives a salary and holds stock options. UW: Consulting for VIATRIS, lecture fees from ALK Germany. PH: Is recipient of consultancy/lecture fees or unrestricted research grants from Sanofi/Regeneron, Novartis, GSK, Medtronic and Viatris.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABPA, allergic bronchopulmonary aspergillosis; AERD, aspirin-exacerbated respiratory disease; AFS, allergic fungal rhinosinusitis; CI, confidence interval; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyposis; CRSwNP, chronic rhinosinusitis with nasal polyposis; NAR, non-allergic rhinitis; NARES, non-allergic rhinitis with eosinophilic syndrome; OR, odd's ratio; T2, type 2; TSLP, thymic stromal lymphoprotein; TSST, toxic shock syndrome toxin.
References
1. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes prevention program research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346(6):393–403. doi: 10.1056/NEJMoa012512
2. Scadding GK, McDonald M, Backer V, Scadding G, Bernal-Sprekelsen M, Conti DM, et al. Pre-asthma: a useful concept for prevention and disease-modification? A EUFOREA paper. Part 1—allergic asthma. Front. Allergy. (2024) 4:1291185. doi: 10.3389/falgy.2023.1291185
3. Turan N, van der Veen TA, Draijer C, Fattahi F, Ten Hacken NH, Timens W, et al. Neutrophilic asthma is associated with smoking, high numbers of IRF5+, and low numbers of IL10+ macrophages. Front Allergy. (2021) 2:676930. doi: 10.3389/falgy.2021.676930
4. Alviani C, Roberts G, Mitchell F, Martin J, Zolkipli Z, Michaelis LJ, et al. Primary prevention of asthma in high-risk children using HDM SLIT: assessment at age 6 years. J Allergy Clin Immunol. (2020) 145(6):1711–3. doi: 10.1016/j.jaci.2020.01.048
5. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. (2014) 133(6):1557–63.e5. doi: 10.1016/j.jaci.2013.10.011
6. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. (2008) 178(3):218–24. doi: 10.1164/rccm.200711-1754OC
7. Pearce N, Douwes J, Beasley R. Is allergen exposure the major primary cause of asthma? Thorax. (2000) 55(5):424–31. doi: 10.1136/thorax.55.5.424
8. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. (2002) 57(7):643–8. doi: 10.1136/thorax.57.7.643
9. Bachert C, Luong AU, Gevaert P, Mullol J, Smith SG, Silver J, et al. The unified airway hypothesis: evidence from specific intervention with anti-IL-5 biologic therapy. J Allergy Clin Immunol Pract. (2023) 11(9):2630–41. doi: 10.1016/j.jaip.2023.05.011
10. Ying S, Humbert M, Meng Q, Pfister R, Menz G, Gould HJ, et al. Local expression of epsilon germline gene transcripts and RNA for the epsilon heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. (2001) 107(4):686–92. doi: 10.1067/mai.2001.114339
11. Wilson DR, Merrett TG, Varga EM, Smurthwaite L, Gould HJ, Kemp M, et al. Increases in allergen-specific IgE in BAL after segmental allergen challenge in atopic asthmatics. Am J Respir Crit Care Med. (2002) 165(1):22–6. doi: 10.1164/ajrccm.165.1.2010112
12. Quirce S, Heffler E, Nenasheva N, Demoly P, Menzies-Gow A, Moreira-Jorge A, et al. Revisiting late-onset asthma: clinical characteristics and association with allergy. J Asthma Allergy. (2020) 13:743–52. doi: 10.2147/JAA.S282205
13. Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. (2018) 10(5):428–47. doi: 10.4168/aair.2018.10.5.428
14. Gevaert P, Han JK, Smith SG, Sousa AR, Howarth PH, Yancey SW, et al. The roles of eosinophils and interleukin-5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. (2022) 12(11):1413–23. doi: 10.1002/alr.22994
15. Castillo JA, Plaza V, Rodrigo G, Juliá B, Picado C, Fernández C, et al. Chronic rhinosinusitis with nasal polyps and allergic rhinitis as different multimorbid treatable traits in asthma. J Allergy Clin Immunol Glob. (2023) 2(4):100134. doi: 10.1016/j.jacig.2023.100134
16. Hellings PW, Klimek L, Cingi C, Agache I, Akdis C, Bachert C, et al. Non-allergic rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy. (2017) 72(11):1657–65. doi: 10.1111/all.13200
17. Shaaban R, Zureik M, Soussan D, Neukirch C, Heinrich J, Sunyer J, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. (2008) 372(9643):1049–57. doi: 10.1016/S0140-6736(08)61446-4
18. Shin YS, Tse K, Lee YJ. The association between non-allergic rhinitis and asthma exacerbations: a systematic review and meta-analysis. J Asthma. (2018) 55(12):1292–302. doi: 10.1080/02770903.2017.1421197
19. Rondón C, Bogas G, Barrionuevo E, Blanca M, Torres MJ, Campo P. Nonallergic rhinitis and lower airway disease. Allergy. (2017) 72(1):24–34. doi: 10.1111/all.12988
20. De Corso E, Seccia V, Ottaviano G, Cantone E, Lucidi D, Settimi S, et al. Clinical evidence of type 2 inflammation in non-allergic rhinitis with eosinophilia syndrome: a systematic review. Curr Allergy Asthma Rep. (2022) 22(4):29–42. doi: 10.1007/s11882-022-01027-0
21. Gelardi M, Maselli del Giudice A, Fiorella ML, Fiorella R, Russo C, Soleti P, et al. Non-allergic rhinitis with eosinophils and mast cells constitutes a new severe nasal disorder. Int J Immunopathol Pharmacol. (2008) 21(2):325–31. doi: 10.1177/039463200802100209
22. Khan A, Vandeplas G, Huynh TMT, Joish VN, Mannent L, Tomassen P, et al. The global allergy and asthma European network (GALEN rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. (2019) 57(1):32–42. doi: 10.4193/Rhin17.255
23. ten Brinke A, Grootendorst DC, Schmidt JT, de Bruïne FT, van Buchem MA, Sterk PJ, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. (2002) 109(4):621–6. ISSN 0091-6749. doi: 10.1067/mai.2002.122458
24. Khalid AN, Hunt JG, Hamilos DL. Inflammatory endotypes in chronic rhinosinusitis with nasal polyps and asthma. Ann Am Thorac Soc. (2011) 8(4):347–55. doi: 10.1513/AnnalsATS.201011-173OC
25. Wang X, Sima Y, Zhao Y, Zhang N, Zheng M, Du K, et al. Endotypes of chronic rhinosinusitis based on inflammatory and remodeling factors. J Allergy Clin Immunol. (2023) 151(2):458–68. doi: 10.1016/j.jaci.2022.10.010
26. Philpott CM, Erskine S, Hopkins C, Kumar N, Anari S, Kara N, et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK national chronic rhinosinusitis epidemiology study. Respir Res. (2018) 19(1):129. doi: 10.1186/s12931-018-0823-y
27. De Corso E, Pasquini E, Trimarchi M, La Mantia I, Pagella F, Ottaviano G, et al. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): a multicentric observational phase IV real-life study (DUPIREAL). Allergy. (2023) 78(10):2669–83. doi: 10.1111/all.15772
28. Chuang CC, Guillemin I, Bachert C, Lee SE, Hellings PW, Fokkens WJ, et al. Two-year results of tapered dupilumab for CRSwNP demonstrates enduring efficacy established in the first 6 months. Allergy. (2023) 78(10):2684–97. doi: 10.1111/all.15796
29. Haxel BR, Hummel T, Fruth K, Lorenz K, Gunder N, Nahrath P, et al. Real-world-effectiveness of biological treatment for severe chronic rhinosinusitis with nasal polyps. Rhinology. (2022) 60(6):435–43. doi: 10.4193/Rhin22.129
30. Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax. (2000) 55:S42–4. doi: 10.1136/thorax.55.suppl_2.S42
31. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE investigators. European network on aspirin-induced asthma. Eur Respir J. (2000) 16:432–6. doi: 10.1034/j.1399-3003.2000.016003432.x
32. Dages KN, Sofola-James O, Sehanobish E, Regula P, Chen C-C, Chiarella SE, et al. Sex, ethnicity, BMI, and environmental exposures associated with NSAID-exacerbated respiratory disease symptom sequence. J Allergy Clin Immunol Pract. (2023). doi: 10.1016/j.jaip.2023.07.035
33. Mullol J, Boyce J, Dahlén SE, Dahlén B, Picado C, Bobolea I. Eicosanoid dysregulation and type 2 inflammation in AERD. J Allergy Clin Immunol. (2021) 148(5):1157–60. doi: 10.1016/j.jaci.2021.08.015
34. Schwartz BS, Pollak JS, Bandeen-Roche K, Hirsch AG, Lehmann AE, Kern RC, et al. Sinus inflammation and chronic rhinosinusitis are associated with a diagnosis of new onset asthma in the following year. Allergy. (2023). doi: 10.1111/all.15771
35. Cohen NA, Widelitz JS, Chiu AG, Palmer JN, Kennedy DW. Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol Head Neck Surg. (2006) 134(4):601–4. doi: 10.1016/j.otohns.2005.11.042
36. Greisner WA 3rd, Settipane GA. Hereditary factor for nasal polyps. Allergy Asthma Proc. (1996) 17(5):283–6. doi: 10.2500/108854196778662192
37. Martin MJ, Garcia-Sanchez A, Estravis M, Gil-Melcón M, Isidoro-Garcia M, Sanz C, et al. Genetics and epigenetics of nasal polyposis: a systematic review. J Investig Allergol Clin Immunol. (2021) 31(3):196–211. doi: 10.18176/jiaci.0673
38. Jerschow E, Dubin R, Chen CC, iAkushev A, Sehanobish E, Asad M, et al. Aspirin-exacerbated respiratory disease is associated with variants in filaggrin, epithelial integrity, and cellular interactions. J Allergy Clin Immunol Glob. (2024) 3(2):100205. doi: 10.1016/j.jacig.2024.100205
39. Park BL, Kim TH, Kim JH, Bae JS, Pasaje CF, Cheong HS, et al. Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. Hum Genet. (2013) 132:313–21. doi: 10.1007/s00439-012-1247-2
40. Dahlin A, Weiss ST. Genetic and epigenetic components of aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. (2016) 36(4):765–89. doi: 10.1016/j.iac.2016.06.010
41. Stevens WW, Staudacher AG, Hulse KE, Carter RG, Winter DR, Abdala-Valencia H, et al. Activation of the 15-lipoxygenase pathway in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. (2021) 147:600–12. doi: 10.1016/j.jaci.2020.04.031
42. Imoto Y, Takabayashi T, Sakashita M, Kato Y, Yoshida K, Kidoguchi M, et al. Enhanced 15-lipoxygenase 1 production is related to periostin expression and eosinophil recruitment in eosinophilic chronic rhinosinusitis. Biomolecules. (2020) 10:1–13. doi: 10.3390/biom10111568
43. Kristjansson RP, Benonisdottir S, Davidsson OB, Oddsson A, Tragante V, Sigurdsson JK, et al. A loss-of-function variant in ALOX15 protects against nasal polyps and chronic rhinosinusitis. Nat Genet. (2019) 51:267–76. doi: 10.1038/s41588-018-0314-6
44. Muluk NB, Altın F, Cingi C. Role of superantigens in allergic inflammation: their relationship to allergic rhinitis, chronic rhinosinusitis, asthma, and atopic dermatitis. Am J Rhinol Allergy. (2018) 32(6):502–17. doi: 10.1177/1945892418801083
45. Zock JP, Jarvis D, Luczynska C, Sunyer J, Burney P, European Community Respiratory Health Survey (ECRHS). Housing characteristics, reported mold exposure, and asthma in the European community respiratory health survey. J Asthma. (2007) 44(4):257–64. doi: 10.1080/02770900701203427
46. Schleich F, Maury E, Bachert C, Hanon S, Michel O, Jansen M, et al. Epidemiology of sensitization to perennial aeroallergens in adults with severe asthma in Belgium. The BEIgE study. Allergy. (2023) 78(10):2774–7. doi: 10.1111/all.15785
47. Kogevinas M, Zock JP, Jarvis D, Kromhout H, Lillienberg L, Plana E, et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II). Lancet. (2007) 370(9584):336–41. doi: 10.1016/S0140-6736(07)61164-7
48. Bakke PS, Baste V, Hanoa R, Gulsvik A. Prevalence of obstructive lung disease in a general population: relation to occupational title and exposure to some airborne agents. Thorax. (1991) 46(12):863–70. doi: 10.1136/thx.46.12.863
49. Sit G, Letellier N, Iwatsubo Y, Goldberg M, Leynaert B, Nadif R, et al. Occupational exposures to organic solvents and asthma symptoms in the CONSTANCES cohort. Int J Environ Res Public Health. (2021) 18(17):9258. doi: 10.3390/ijerph18179258
50. Sit G, Orsi L, Iwatsubo Y, Brigitte Dananché, Florence Orsi, Marcel Goldberg, et al. Chronic occupational exposures to irritants and asthma in the CONSTANCES cohort. Occup Environ Med. (2024). doi: 10.1136/oemed-2023-109100
51. Klein DK, Silberbrandt A, Frøssing L, Hvidtfeldt M, von Bülow A, Nair P, et al. Impact of former smoking exposure on airway eosinophilic activation and autoimmunity in patients with severe asthma. Eur Respir J. (2022) 60(4):2102446. doi: 10.1183/13993003.02446-2021
52. Lajunen TK, Jaakkola JJK, Jaakkola MS. Different effects of smoking on atopic and non-atopic adult-onset asthma. Clin Transl Allergy. (2021) 11(8):e12072. doi: 10.1002/clt2.12072
53. Chalermwatanachai T, Vilchez-Vargas R, Holtappels G, Lacoere T, Jáuregui R, Kerckhof FM, et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci Rep. (2018) 8(1):7926. doi: 10.1038/s41598-018-26327-2
54. Choi S, Sohn KH, Jung JW, Kang MG, Yang MS, Kim S, et al. Lung virome: new potential biomarkers for asthma severity and exacerbation. J Allergy Clin Immunol. (2021) 148(4):1007–1015.e9. doi: 10.1016/j.jaci.2021.03.017
55. Bachert C, van Steen K, Zhang N, Holtappels G, Cattaert T, Maus B, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. (2012) 130(2):376–81.e8. doi: 10.1016/j.jaci.2012.05.012
56. Pastacaldi C, Lewis P, Howarth P. Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy. (2011) 66(4):549–55. doi: 10.1111/j.1398-9995.2010.02502.x
57. Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. (2014) 44(4):553–62. doi: 10.1111/cea.12239
58. Kim YC, Won HK, Lee JW, Sohn KH, Kim MH, Kim TB, et al. Staphylococcus aureus nasal colonization and asthma in adults: systematic review and meta-analysis. J Allergy Clin Immunol Pract. (2019) 7(2):606–615.e9. doi: 10.1016/j.jaip.2018.08.020
59. Shaghayegh G, Cooksley C, Bouras GS, Panchatcharam BS, Idrizi R, Jana M, et al. Chronic rhinosinusitis patients display an aberrant immune cell localization with enhanced S aureus biofilm metabolic activity and biomass. J Allergy Clin Immunol. (2023) 151(3):723–736.e16. ISSN 0091-6749. doi: 10.1016/j.jaci.2022.08.031
60. Shaghayegh G, Cooksley C, Bouras G, Nepal R, Houtak G, Panchatcharam BS, et al. Staphylococcus aureus biofilm properties and chronic rhinosinusitis severity scores correlate positively with total CD4+ T-cell frequencies and inversely with its Th1, Th17 and regulatory cell frequencies. Immunology. (2023) 170(1):120–33. doi: 10.1111/imm.13655
61. Bachert C, van Zele T, Gevaert P, De Schrijver L, Van Cauwenberge P. Superantigens and nasal polyps. Curr Allergy Asthma Rep. (2003) 3(6):523–31. doi: 10.1007/s11882-003-0065-y
62. Heaton T, Mallon D, Venaille T, Holt P. Staphylococcal enterotoxin induced IL-5 stimulation as a cofactor in the pathogenesis of atopic disease: the hygiene hypothesis in reverse? Allergy. (2003) 58(3):252–6. doi: 10.1034/j.1398-9995.2003.00088.x
63. Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. (2004) 113(4):756–63. doi: 10.1016/j.jaci.2004.01.772
64. Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. (2007) 175(7):661–6. doi: 10.1164/rccm.200611-1717OC
65. Arismendi E, Bantulà M, Picado C. Obese asthma syndrome: much work to do. Arch Bronconeumol. (2023) 59(8):473–5. (English, Spanish). doi: 10.1016/j.arbres.2023.02.012
66. Bantulà M, Arismendi E, Tubita V, Roca-Ferrer J, Mullol J, de Hollanda A, et al. Effect of obesity on the expression of genes associated with severe asthma-a pilot study. J Clin Med. (2023) 12(13):4398. doi: 10.3390/jcm12134398
67. Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. (2011) 127(6):1486–93.e2. doi: 10.1016/j.jaci.2011.03.036
68. Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. (2011) 128(3):508–15.e1–2. doi: 10.1016/j.jaci.2011.06.009
69. Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, et al. Cluster analysis of obesity and asthma phenotypes. PLoS One. (2012) 7(5):e36631. doi: 10.1371/journal.pone.0036631
70. Carr TF, Kraft M. Use of biomarkers to identify phenotypes and endotypes of severeasthma. Ann Allergy Asthma Immunol. (2018) 121(4):414–20. doi: 10.1016/j.anai.2018.07.029
71. Nwaru BI, Pillinger R, Tibble H, Shah SA, Ryan D, Critchley H, et al. Hormonal contraceptives and onset of asthma in reproductive-age women: population-based cohort study. J Allergy Clin Immunol. (2020) 146(2):438–46. doi: 10.1016/j.jaci.2020.02.027
72. Shah SA, Tibble H, Pillinger R, McLean S, Ryan D, Critchley H, et al. Hormone replacement therapy and asthma onset in menopausal women: national cohort study. J Allergy Clin Immunol. (2021) 147(5):1662–70. doi: 10.1016/j.jaci.2020.11.024
73. Lemmetyinen R, Karjalainen J, But A, Renkonen R, Pekkanen J, Haukka J, et al. Diseases with oral manifestations among adult asthmatics in Finland: a population-based matched cohort study. BMJ Open. (2021) 11(12):e053133. doi: 10.1136/bmjopen-2021-053133
74. Ilmarinen P, Tuomisto LE, Phenotypes KH, Factors R. And mechanisms of adult-onset asthma. Mediators Inflamm. (2015) 2015:514868. doi: 10.1155/2015/514868
75. Xiang B, Hu M, Yu H. Highlighting the importance of healthy sleep patterns in the risk of adult asthma under the combined effects of genetic susceptibility: a large-scale prospective cohort study of 455 405 participants. BMJ Open Respir Res. (2023) 10:e001535. doi: 10.1136/bmjresp-2022-001535
76. Cullinan P, Muñoz X, Suojalehto H, Agius R, Jindal S, Sigsgaard T, et al. Occupational lung diseases: from old and novel exposures to effective preventive strategies. Lancet Respir Med. (2017) 5(5):445–55. doi: 10.1016/S2213-2600(16)30424-6
77. Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, Shamji M, Agache I, et al. Allergen immunotherapy for asthma prevention: a systematic review and meta-analysis of randomized and non-randomized controlled studies. Allergy. (2022) 77(6):1719–35. doi: 10.1111/all.15295
78. Polosa R, Al-Delaimy WK, Russo C, Piccillo G, Sarvà M. Greater risk of incident asthma cases in adults with allergic rhinitis and effect of allergen immunotherapy: a retrospective cohort study. Respir Res. (2005) 6(1):153. doi: 10.1186/1465-9921-6-153
79. Jayes L, Haslam PL, Gratziou CG, Powell P, Britton J, Vardavas C, et al. Smokehaz: systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest. (2016) 150(1):164–79. doi: 10.1016/j.chest.2016.03.060
80. Bantulà M, Tubita V, Roca-Ferrer J, Mullol J, Valero A, Bobolea I, et al. Weight loss and vitamin D improve hyporesponsiveness to corticosteroids in obese asthma. J Investig Allergol Clin Immunol. (2023) 33(6):464–73. doi: 10.18176/jiaci.0861
81. Lenharo M. Obesity drugs have another superpower: taming inflammation. Nature. Available online at: https://www.nature.com/articles/d41586-024-00118-4 (Accessed January 26, 2024).
82. Menezes-Martins LF, Vianna P, Viegas-Júnior OA, et al. Intranasal corticosteroids and the prevention of asthma onset in non-allergic rhinitis patients: a pilot randomized controlled trial. J Asthma. (2019) 56(9):954–62. doi: 10.1080/02770903.2018.1506672
83. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. (2020) 58(2):82–111. doi: 10.4193/Rhin20.601
84. Rimmer J, Fokkens W, Chong LY, Hopkins C. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev. (2014) 12:CD006991. doi: 10.1002/14651858.CD006991.pub2
85. Ragab S, Parikh A, Darby YC, Scadding GK. An open audit of montelukast, a leukotriene receptor antagonist, in nasal polyposis associated with asthma. Clin Exp Allergy. (2001) 31(9):1385–91. doi: 10.1046/j.1365-2222.2001.01160.x
86. De Corso E, Anzivino R, Galli J, Baroni S, Di Nardo W, De Vita C, et al. Antileukotrienes improve naso-ocular symptoms and biomarkers in patients with NARES and asthma. Laryngoscope. (2019) 129(3):551–7. doi: 10.1002/lary.27576
87. Hopkins C, Rimmer J, Lund VJ. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Prospective findings from the national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Rhinology. (2015) 53(1):10–7. doi: 10.4193/Rhino13.217
88. Plaza V, del Cuvillo A, Soto-Retes L. Functional endoscopic Sinus surgery for nasal polyposis in asthma patients: impact on bronchial inflammation. Arch Bronconeumol:56(6):403–5. doi: 10.1016/j.arbres.2019.12.029
89. Jerschow E, Edin ML, Chi Y, Hurst B, Abuzeid WM, Akbar NA, et al. Sinus surgery is associated with a decrease in aspirin-induced reaction severity in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract. (2019) 7(5):1580–8. doi: 10.1016/j.jaip.2018.12.014
90. Divekar R, Hagan J, Rank M, Park M, Volcheck G, O'Brien E, et al. Diagnostic utility of urinary LTE4 in asthma, allergic rhinitis, chronic rhinosinusitis, nasal polyps, and aspirin sensitivity. J Allergy Clin Immunol Pract. (2016) 4(4):665–70. doi: 10.1016/j.jaip.2016.03.004
91. Huang CC, Wang CH, Fu CH, Huang CC, Chang PH, Chen IW, et al. The link between chronic rhinosinusitis and asthma: a questionnaire-based study. Medicine (Baltimore). (2016) 95(31):e4294. doi: 10.1097/MD.0000000000004294
92. Makowska JS, Burney P, Jarvis D, Keil T, Tomassen P, Bislimovska J, et al. Respiratory hypersensitivity reactions to NSAIDs in Europe: the global allergy and asthma network (GA2 LEN) survey. Allergy. (2016) 71(11):1603–11. doi: 10.1111/all.12941
93. Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. (2014) 133(6):1549–56. doi: 10.1016/j.jaci.2013.10.006
94. Eraso I, Sangiovanni S, Morales EI, Fernández-Trujillo L. Aspirin desensitization in NSAID-exacerbated respiratory disease and its outcomes in the clinical course of asthma: a systematic review of the literature and meta-analysis. PLoS One. (2021) 16(3):e0247871. doi: 10.1371/journal.pone.0247871
95. Atanasio A, Orengo JM, Sleeman MA, Stahl N. Biologics as novel therapeutics for the treatment of allergy: challenges and opportunities. Front Allergy. (2022) 3:1019255. doi: 10.3389/falgy.2022.1019255
96. Striz I, Golebski K, Strizova Z, Loukides S, Bakakos P, Hanania NA, et al. New insights into the pathophysiology and therapeutic targets of asthma and comorbid chronic rhinosinusitis with or without nasal polyposis. Clin Sci (Lond). (2023) 137(9):727–53. doi: 10.1042/CS20190281
97. Kolkhir P, Akdis CA, Akdis M, Bachert C, Bieber T, Canonica GW, et al. Type 2 chronic inflammatory diseases: targets, therapies and unmet needs. Nat Rev Drug Discov. (2023) 22(9):743–67. doi: 10.1038/s41573-023-00750-1
98. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. (2014) 371(13):1189–97. doi: 10.1056/NEJMoa1403291
99. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. (2018) 378(26):2475–85. doi: 10.1056/NEJMoa1804093
100. Canonica GW, Rottoli P, Bucca C, Zappa MC, Michetti G, Macciocchi B, et al. Improvement of patient-reported outcomes in severe allergic asthma by omalizumab treatment: the real life observational PROXIMA study. World Allergy Organ J. (2018) 11(1):33. doi: 10.1186/s40413-018-0214-3
101. Barroso B, Valverde-Monge M, Alobid I, Olaguibel JM, Rial MJ, Quirce S, et al. Improvement in olfaction in patients with CRSwNP and severe asthma taking anti-IgE and anti-IL-5 biologics: a real-life study. J Investig Allergol Clin Immunol. (2023) 33(1):37–44. doi: 10.18176/jiaci.0812
102. Wechsler ME, Scelo G, Larenas-Linnemann DES, Torres-Duque CA, Maspero J, Tran TN, et al. Association between T2-related comorbidities and effectiveness of biologics in severe asthma. Am J Respir Crit Care Med. (2024) 209(3):262–72. doi: 10.1164/rccm.202305-0808OC
103. Oykhman P, Paramo FA, Bousquet J, Kennedy DW, Brignardello-Petersen R, Chu DK. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol. (2022) 149(4):1286–95. doi: 10.1016/j.jaci.2021.09.009
104. Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. (2019) 394(10209):1638–50. doi: 10.1016/S0140-6736(19)31881-1
105. Schneider S, Poglitsch K, Morgenstern C, Quint T, Gangl K, Sinz C, et al. Dupilumab increases aspirin tolerance in NSAID-exacerbated respiratory disease. Eur Respir J. (2023) 61(3):2201335. doi: 10.1183/13993003.01335-2022
106. Ahmad-Mansour N, Loubet P, Pouget C, Dunyach-Remy C, Sotto A, Lavigne JP, et al. Staphylococcus aureus toxins: an update on their pathogenic properties and potential treatments. Toxins (Basel). (2021) 13(10):677. doi: 10.3390/toxins13100677
107. Schmidt F, Meyer T, Sundaramoorthy N, Michalik S, Surmann K, Depke M, et al. Characterization of human and Staphylococcus aureus proteins in respiratory mucosa by in vivo- and immunoproteomics. J Proteomics. (2017) 155:31–9. doi: 10.1016/j.jprot.2017.01.008
108. Ragab S, Scadding GK, Lund VJ, Saleh H. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J. (2006) 28(1):68–74. doi: 10.1183/09031936.06.00043305
109. Van Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. (2010) 125(5):1069–1076.e4. ISSN 1874-3919. doi: 10.1016/j.jaci.2010.02.020
110. Davis MF, McCormack MC, Matsui EC. Growing concerns with Staphylococcus aureus and asthma: new territory for an old foe? J Allergy Clin Immunol Pract. (2019) 7(2):616–7. doi: 10.1016/j.jaip.2018.10.003
111. Tzani-Tzanopoulou P, Skliros D, Megremis S, Xepapadaki P, Andreakos E, Chanishvili N, et al. Interactions of bacteriophages and bacteria at the airway mucosa: new insights into the pathophysiology of asthma. Front Allergy. (2021) 1:617240. doi: 10.3389/falgy.2020.617240
112. Hartert TV, Deng X, Hartman TJ, Wen W, Yang G, Gao YT, et al. The Shanghai women’s asthma and allergy study: objectives, design, and recruitment results. Am J Epidemiol. (2008) 167(11):1387–96. doi: 10.1093/aje/kwn057
113. Zhu LY, Ni ZH, Luo XM, Wang XB. Advance of antioxidants in asthma treatment. World J Respirol. (2017) 7(1):17–28. doi: 10.5320/wjr.v7.i1.17
114. Zhu XD, Lei XP, Dong WB. Resveratrol as a potential therapeutic drug for respiratory system diseases. Drug Des Devel Ther. (2017) 11:3591–8. doi: 10.2147/DDDT.S148868
115. Alharris E, Alghetaa H, Seth R, Chatterjee S, Singh NP, Nagarkatti M, et al. Resveratrol attenuates allergic asthma and associated inflammation in the lungs through regulation of miRNA-34a that targets FoxP3 in mice. Front Immunol. (2018) 9:2992. doi: 10.3389/fimmu.2018.02992
116. Ediger D, Günaydın FE, Erbay M, Pekbak G. Can omalizumab be an alternative treatment for non-atopic severe asthma? A real-life experience with omalizumab. Tuberk Toraks. (2023) 71(1):24–33. (English). doi: 10.5578/tt.20239904
Keywords: late onset asthma, non-allergic rhinitis, chronic rhinosinusitis with nasal polyps, eosinophils, mast cells, virulence genes, S. aureus biofilm
Citation: Scadding G. K., Gray C., Conti D. M., McDonald M., Backer V., Scadding G., Bernal-Sprekelsen M., De Corso E., Diamant Z., Hopkins C., Jesenak M., Johansen P., Kappen J., Mullol J., Price D., Quirce S., Reitsma S., Toppila-Salmi S., Senior B., Thyssen J. P., Wahn U. and Hellings P. W. (2024) Pre-asthma: a useful concept? A EUFOREA paper. Part 2—late onset eosinophilic asthma. Front. Allergy 5:1404735. doi: 10.3389/falgy.2024.1404735
Received: 21 March 2024; Accepted: 23 April 2024;
Published: 15 May 2024.
Edited by:
Maciej Kupczyk, Medical University of Lodz, PolandReviewed by:
Arzu Yorgancıoğlu, Manisa Celal Bayar University, TürkiyeAnna Lewandowska-Polak, Medical University of Lodz, Poland
© 2024 Scadding, Gray, Conti, McDonald, Backer, Scadding, Bernal-Sprekelsen, De Corso, Diamant, Hopkins, Jesenak, Johansen, Kappen, Mullol, Price, Quirce, Reitsma, Toppila-Salmi, Senior, Thyssen, Wahn and Hellings. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. M. Conti, Y29udGkuZGllZ29tYXJjZWxvQGdtYWlsLmNvbQ==
 G. K. Scadding
G. K. Scadding C. Gray
C. Gray D. M. Conti
D. M. Conti M. McDonald
M. McDonald V. Backer
V. Backer G. Scadding9
G. Scadding9 Z. Diamant
Z. Diamant M. Jesenak
M. Jesenak P. Johansen
P. Johansen J. Kappen
J. Kappen J. Mullol
J. Mullol D. Price
D. Price S. Quirce
S. Quirce S. Reitsma
S. Reitsma S. Toppila-Salmi
S. Toppila-Salmi P. W. Hellings
P. W. Hellings