- Environmental Futures, School of Science, University of Wollongong, Wollongong, NSW, Australia
Application of hormone therapies to amphibians has increased substantially in recent years, though protocol development has predominantly focused on moderate- to large- bodied species, with fewer examples of application to small- or micro- bodied amphibians. The development of alternative methods of hormone administration that circumvent the need for animal injection stand to increase the diversity of species to benefit from hormone therapy, particularly species of smaller body size. The aim of the present study was to quantify the efficacy of different routes of hormone administration of gonadotropin releasing-hormone agonist (GnRHa) on spermiation in the common eastern froglet, Crinia signifera. Males were assigned to one of four experimental treatments; intranasal application, hormone injection, hormone bath or no hormone, and sperm-release was quantified (males spermiating, total sperm, sperm concentration and sperm viability) at 0, 2, 4, 6, 8, 10, and 12-hours. Sperm-release was highest in the hormone injection and hormone bath treatments, both resulting in 88% of males spermiating, and the highest mean total number and concentration of sperm. Intranasal application resulted in poor sperm-release, with only 25% of males spermiating, and very low total sperm and sperm concentration, statistically similar to the aspermic no-hormone treatment group. Sperm viability remained above 86% and did not differ significantly among treatments. Overall, we describe successful protocols for the hormonal induction of sperm-release in C. signifera. Our findings add to a growing body of evidence that topical hormone application offers a viable alternative to injection for amphibians, providing an effective pathway for the increased application of hormone therapies to small-bodied amphibian species.
1 Introduction
Unprecedented rates of biodiversity loss continue to impact life on earth and threaten ecosystem functioning across the globe. Amphibians are the most threatened vertebrate class, with over 40% of species threatened with extinction (IUCN, 2025). Conservation Breeding Programs (CBPs) have been established for a growing number of amphibian species globally, following recommendations outlined in the first Amphibian Conservation Action Plan in 2007 (Gascon et al., 2007), and two subsequent updated documents (Wren et al., 2015, 2024). The integration of reproductive technologies within CBPs has far-reaching benefits, including bolstering propagation, facilitating genetic management, and allowing for valuable genetic material to be stored and used well into the future. Reproductive technologies include, but are not limited to, hormone therapies, sperm cryopreservation, and assisted fertilization (AF). Fundamental to the application of reproductive technologies is the ability to collect viable gametes. Gamete collection affords conservation practitioners the ability to apply assisted fertilization protocols to breed specific male-female pairs, store genetic material in biobanks, and exchange genetic material to enhance genetic management (Silla and Byrne, 2019; Bolton et al., 2022). Application of hormone therapies to induce gamete release allows viable gametes to be collected from breeding stock, without compromising the future ability of reproductive adults to contribute to offspring recruitment in subsequent breeding events and seasons during their natural lifespan. As the application of reproductive technologies to assist amphibian conservation has expanded over the past three decades, the corresponding development of optimal hormone therapies has similarly increased, with hormone-induced gamete-release achieved in a growing number and diversity of species (Silla and Langhorne, 2022; Graham and Kouba, 2022; Clulow et al., 2022).
Exogenous reproductive hormones are typically administered to amphibians via injection, using fine-gauge single-use sterile syringes (Silla et al., 2021; Silla and Langhorne, 2022). Hormone injections are most commonly administered to anurans (frogs and toads) either intraperitoneally (IP injection; directed into the peritoneal cavity of the abdomen) or subcutaneously (SC injection; directed under the skin, typically into the dorsal lymph sac) (Calatayud et al., 2024; Silla and Langhorne, 2022). By contrast, hormone injections are normally administered to caudates (salamanders and newts) via intramuscular injection (IM; directed into the muscles of the hindlimb or between the shoulder blades) (Silla and Langhorne, 2022). Whilst intraperitoneal injection can be a safe and effective method of delivery, absorption occurs via mesenteric vessels, draining into the bloodstream and then passing through the liver before being circulated systemically (Turner et al., 2011). Therefore, hormone bioavailability may be limited when administered via this route, due to the first-pass effect of metabolism by the liver (Turner et al., 2011). Additionally, intraperitoneal injections are considered to be of moderate welfare risk to small-bodied amphibian species due to the chance of perforation of the gastrointestinal tract or bladder, even when performed by competently trained individuals (Lewis, 1966; Turner et al., 2011; Roth and Obringer, 2003). Subcutaneous injection is considered comparatively more suitable for administration of exogenous hormones to anurans of varying body size, including smaller bodied species, as there is less risk of inadvertent perforation of organs and this method is relatively easy to master with training (Turner et al., 2011; Silla et al., 2021). The speed and efficacy with which hormone absorption occurs following subcutaneous injection largely depends on the injection location. It is recommended that subcutaneous injection be administered into the posterior dorsal lymph sac to allow circulation via subcutis capillaries and the lymphatic system (Whitaker and Wright, 2001). Subcutaneous injections are not recommended for caudates due to the adherence of the skin to underlying muscle tissue; injection may, however, be accomplished via injection directly into the dorsal lymph sac (Whitaker and Wright, 2001). Dorsal lymph sac injections have been recommended for reproductive hormone administration (Silla and Langhorne, 2022), as well as by clinicians for the administration of medications, due to the rapid assimilation of drugs administered via this site (Whitaker and Wright, 2001). When considering intramuscular injection, this method is expected to result in uniform absorption due to the rich vascular supply to these regions, however, consideration needs to be given to the volume of hormone administered, as clinical guidelines recommend much smaller volumes compared to intraperitoneal or subcutaneous injections (Turner et al., 2011). In addition, intramuscular injections are only recommended for amphibians that are large enough to provide an adequate muscle mass (Whitaker and Wright, 2001).
Of the numerous studies to effectively apply hormone therapies to male amphibians over the past 50 years, most have employed species characterized by moderate- to large- body size, weighing from approximately eight to 210 grams body mass and measuring 40 to 150 mm snout-vent length (Supplementary Table S1). One notable outlier, the Chinese giant salamander, Andrias davidianus, has been administered reproductive hormones intramuscularly (Yongjie et al., 2017), with this species weighing as much as 50 kilograms and measuring over 400 mm in snout-vent length (Supplementary Table S1), making it the world’s largest amphibian species (Bolaños et al., 2024). By contrast, well over a thousand amphibian species are either micro- or small- bodied in size. Of the studies to administer hormones via injection to male amphibians, less than 20% have employed species weighing under three grams body mass (Supplementary Table S1). Notably, the smallest species to have been successfully injected with exogenous hormones is the Australian rattling frog, Crinia glauerti, weighing a mere 0.45 grams (Silla and Roberts, 2012). While injection methods have been used to safely and effectively induce spermiation in these species, extensive training in hormone injection is required to minimize any potential adverse impacts to animal welfare. Alternative hormone administration protocols that do not require injection may be of benefit to smaller amphibian species, particularly those in conservation breeding programs that do not have access to staff adequately trained in hormone injection.
Amphibians possess semi-permeable, highly vascularized skin surfaces, which allow for the uptake of chemicals percutaneously, making them ideal candidates for topical hormone application methods (Helmer and Whiteside, 2005; Llewelyn et al., 2016; Whitaker and Wright, 2001). The topical application of gonadotropin-releasing hormone agonist (GnRHa) to the ventral skin surface has been successfully employed to induce spermiation in American toads (Anaxyrus americanus) (Rowson et al., 2001; Obringer et al., 2000), Gulf coast toads (Incilius valliceps) (Rowson et al., 2001) and roseate frogs (Anstisia (formerly Geocrinia) rosea) (Silla et al., 2020), and to induce spawning in northern corroboree frogs (Pseudophryne pengilleyi) (Silla et al., 2018). In plethodontid salamanders, hormones may be applied topically to the nasolabial groove, a distinctive groove that extends from the lips to the nares which is highly vascularized and important for chemical communication (Dawley and Bass, 1989). The application of GnRHa to the nasolabial groove of Texas blind salamanders (Eurycea rathbuni) (Glass Campbell et al., 2021) and San Marcos salamanders (Eurycea nana) (Marcec-Greaves, unpublished data), species that are generally unsuited to injection due to their small body size and delicate anatomy, resulted in significantly improved spawning success. While the effective hormone dose required to elicit a spermiation response is generally higher compared to injection methods (Rowson et al., 2001; Silla et al., 2018, 2020), and is therefore a costly approach for hormone application to large amphibian species, topical application methods remain cost effective for small-bodied species, and offer a viable alternative approach for hormone administration.
In addition to the topical application of hormones, intranasal application offers another minimally invasive alternative to injection, though this method has only been trialed in one amphibian species to date, the Fowler’s toad (Anaxyrus fowleri) (Julien et al., 2019). Hormones applied intranasally may be able to reach the pituitary, where key GnRH receptors are located, through passive diffusion across the olfactory mucosa into the subarachnoid space or cerebrospinal fluid where it can then be circulated (Lochhead and Thorne, 2012). However, connectivity between the nares and mouth means that hormones applied intranasally may also be swallowed, and subsequently enter the bloodstream through digestive processes (Julien et al., 2019), which may decrease hormone efficiency (Turner et al., 2011). Intranasal application of GnRHa at the optimal dose, administered by pipetting directly into the nares, resulted in a high (>90%) spermiation response in Fowler’s toads (Julien et al., 2019). This result highlights the potential for intranasal administration to induce gamete-release in amphibians; however, it is important to note that the size and structure of the nasal cavity may influence the efficacy of this approach among species, justifying further investigation.
The continued development and application of hormone therapies to amphibians is imperative to safeguard amphibian biodiversity, with 2,873 species currently threatened with extinction (IUCN, 2025), and less than 100 species with established hormone therapies to induce gamete release. Access to large numbers of threatened species for research is often limited. Where this is the case, employing closely related model species can be valuable for initial protocol development, bridging the gap between protocol development and application, and expediting the application of these technologies to related threatened species. For example, sperm collection and cryopreservation protocols developed in Fowler’s toads were successfully applied to threatened anuran species: Chiricahua leopard frogs (Lithobates chiricahuensis), Puerto Rican crested toads (Peltophryne lemur), and Houston toads (Anaxyrus houstonensis) (Burger et al., 2023). Additionally, an ovulation protocol initially developed in Günther’s toadlets (Pseudophryne guentheri) was successfully applied to three related threatened species: Bibron’s toadlets (Pseudophryne bibronii), northern corroboree frogs and southern corroboree frogs (Pseudophryne corroboree) (Silla and Byrne, 2021). Utilizing closely related, non-threatened model species provides a means of rigorously testing effective protocols with larger sample sizes, with the aim of applying and refining these in threatened species (Bolton et al., 2022). The genus Crinia comprises a group of micro-bodied frog species, with 94% of species in the genus less than 1 gram in body mass and less than 30 mm in snout-vent length. Of these species, approximately 30% are threatened or in decline, including two endangered species: the streambank froglet, C. riparia and Sloane’s froglet C. sloanei. Within this genus, the common eastern froglet, C. signifera, is a common species found throughout southeastern Australia, and is of a similar size to related threatened species, making this species an ideal model for developing hormone therapies. The aim of this study was to empirically test protocols for the hormonal induction of sperm release in the common eastern froglet, C. signifera. Specifically, this study aimed to investigate the effect of the route of administration (intranasal application, hormone injection or hormone bath) on the spermiation response of males over a 12-hour sampling period.
2 Methods
2.1 Ethical note
All procedures were performed following approval by the University of Wollongong’s Animal Ethics Committee (approval number: AEPR22/12) in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes 2013 (updated in 2021). Animal collection and scientific research was authorized by the New South Wales National Parks and Wildlife Service (approval number: SL102672).
2.2 Study species and population
The common eastern froglet, C. signifera is a small (14 to 29 mm snout-vent-length) frog species of the family Myobatrachidae (Figure 1A). The species has a wide distribution throughout south-eastern mainland Australia and Tasmania. Males aggregate in shallow ephemeral or permanent water bodies and typically call to attract females from sunset to sunrise (though daylight calling activity is also observed during cooler months) (Lemckert, 2001). Amplexus is inguinal, and egg deposition aquatic. Male calling and breeding behaviors are known to occur throughout the year, with peak activity occurring between July to November (Austral winter to spring) (Lemckert, 2001).
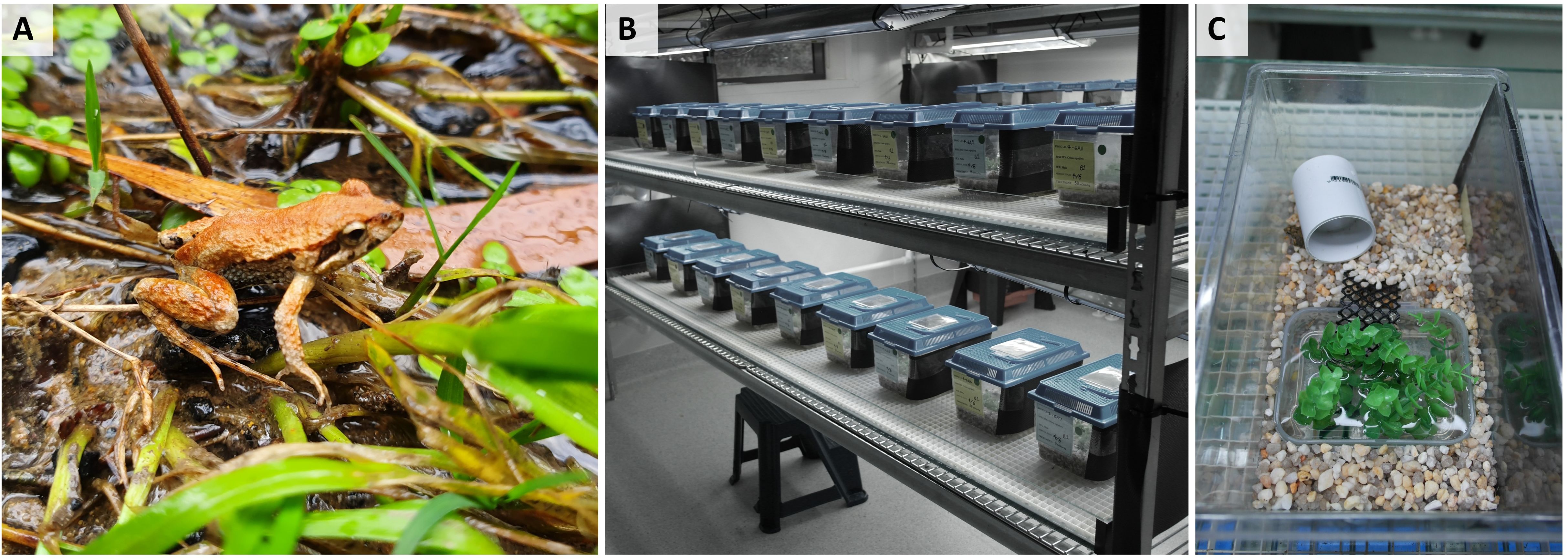
Figure 1. Study species and captive environment used to house study animals. Shown is (A) an adult male common Eastern froglet, Crinia signifera in the field, (B) set-up of captive housing within the Ecological Research Facility (ERF) at the University of Wollongong, and (C) individual housing containers used to house study animals. Photographs A and B courtesy of A. Silla and C courtesy of Z. Anastas.
Male study animals were collected from a single, large population, located adjacent to the Ecological Research Facility (ERF) at the University of Wollongong (UOW), Australia (34.4048°S, 150.8717°E). This location is a known breeding site for C. signifera, characterized by a large open grassland containing a natural, ephemeral drainage line and swampland. All animals were released to their site of capture within the breeding population located at UOW following experimental procedures.
2.3 Study animals
A total of 32 wild-caught male frogs were collected on October 19, 2022, during Austral spring following a period of light rainfall when calling activity increased. Collections were performed at night, between 17:00 and 21:00 hours. Frogs were caught by hand after tracking their vocalizations, and each individual was then confirmed to be male by checking for the presence of a darkened vocal sac. Immediately after capture, frogs were placed in an individual sealed plastic zip-lock bag containing three Kimwipes wetted with water from the sight of capture. At the end of the collection period, male frogs were immediately transported on foot to an isolated constant-temperature room within the ERF (total distance ≈ 130 m). Adult males employed for this study ranged in mass from 0.45 to 0.93 grams (mean mass ± SEM = 0.71 ± 0.019 g, n=32).
2.4 Animal husbandry
Males were housed individually in ventilated plastic terraria (27 x 17 x 16.5 cm, Lx W x H; Mayvic, Lansvale, NSW, Australia) (Figure 1B). Enclosures contained a layer of aquarium gravel (2 cups), a 300 mL rectangular plastic water dish (10 x 6 x 5 cm, L x W x H), and a section of PVC piping (3 x 5 cm, D x L) placed on top of the gravel (Figure 1C). Each terrarium also contained equal-sized lengths of plastic aquarium plant (6 x 6 x 7 cm, L x W x H) and a section of plastic lattice (9 x 4 cm, L x W) placed in the water dish, to provide additional refuge and facilitate movement of the frogs out of the water (Figure 1C). For drainage, each terrarium had 8 holes (~0.5cm D) in the base. The right side of each terrarium was covered in black dampcourse (16 × 9 cm, L x H) to prevent visual contact between adjacent males.
Frogs were held in an artificially illuminated constant temperature room maintained at 22°C with a 13:11 hour light: dark cycle. These conditions were chosen to reflect natural field conditions during Austral spring. Artificial broad spectrum UV lighting was provided using Reptisun 34” high output fluorescent strip bulbs (10.0 UVB output; Pet Pacific Pty Ltd, Sydney) suspended approximately 20 cm above the terraria. Frogs were monitored daily while in captivity and fed once per week on a diet of live hatchling (first instar) crickets (Acheta domesticus) that were dusted immediately prior to feeding with calcium powder plus D3 (Repti calcium; Zoo Med, USA). Food was withheld for three days prior to experimental procedures to reduce fecal contamination of spermic urine samples. Terraria were thoroughly flushed with reverse osmosis (RO) water (Sartorius Stedim biotech, Australia) (~1000 mL/terrarium) once a week. Additionally, water was changed in all water dishes in each terrarium twice weekly, and water dishes were topped up with RO water on days that water was not changed. Frogs remained in captivity for a total duration of two weeks before being returned to the field to their site of collection. Experiments commenced four days following field collection, with a brief acclimation period imposed in order to minimize the potential effects of collection stress on the efficacy of hormone treatment. Experiments were conducted over four days on October 23, 24, 28 and 29, 2022. Eight frogs were sampled on each experimental day, with even representation of each experimental treatment on each experimental day (n=2/treatment/day).
2.5 Hormonal induction of spermiation
To determine the effect of the route of hormone administration (GnRHa; leuprolide acetate, Sigma-Aldrich, L0399) on spermiation outcomes, 32 frogs were assigned to one of four experimental treatments (n = 8 per treatment); T1 no-hormone, T2 intranasal application, T3 hormone injection, or T4 hormone bath (Figure 2). Note that frogs were exposed to one experimental treatment only and were not reused during the course of the present study, nor were they used for any experiments prior to the experiments presented herein. Frogs were randomly assigned to experimental treatments, and no significant difference (ANOVA: F3,28 = 0.7188, p = 0.5491) in the weight of animals was detected between treatment groups (mean mass ± SEM = 0.71 ± 0.019 g). The no-hormone group (T1) was handled in the same way as individuals in treatment 4, but were administered no hormone; T2 were administered 2 μg GnRHa in 100μl of simplified amphibian ringer (SAR) via adjustable micropipettor with single-use tip directly into the opening of the nares; T3 were administered 2 μg GnRHa in 100 μL of SAR via subcutaneous injection into the dorsal lymph sac using an ultra-fine 30-gauge single-use syringe; and T4 received 2 μg GnRHa/100 μL distilled water in a 1 mL hormone bath for topical absorption (total hormone in solution = 20 μg GnRHa). Hormones administered via hormone bath were diluted to the correct concentration using distilled water used as an alternative to SAR in order to promote hormone uptake across the epidermis. Following hormone administration, frogs were placed in individual holding containers (4.5 x 6.0 cm D x H) for the duration of spermic urine collection. Holding containers contained 1 mL of distilled water for all treatments with the exception of T4 which contained 1 mL of hormone solution. Two hours post administration (PA), two Kimwipe tissues and 9 mL of distilled water were added to all containers to ensure adequate hydration to allow urine collection at every sampling time PA.
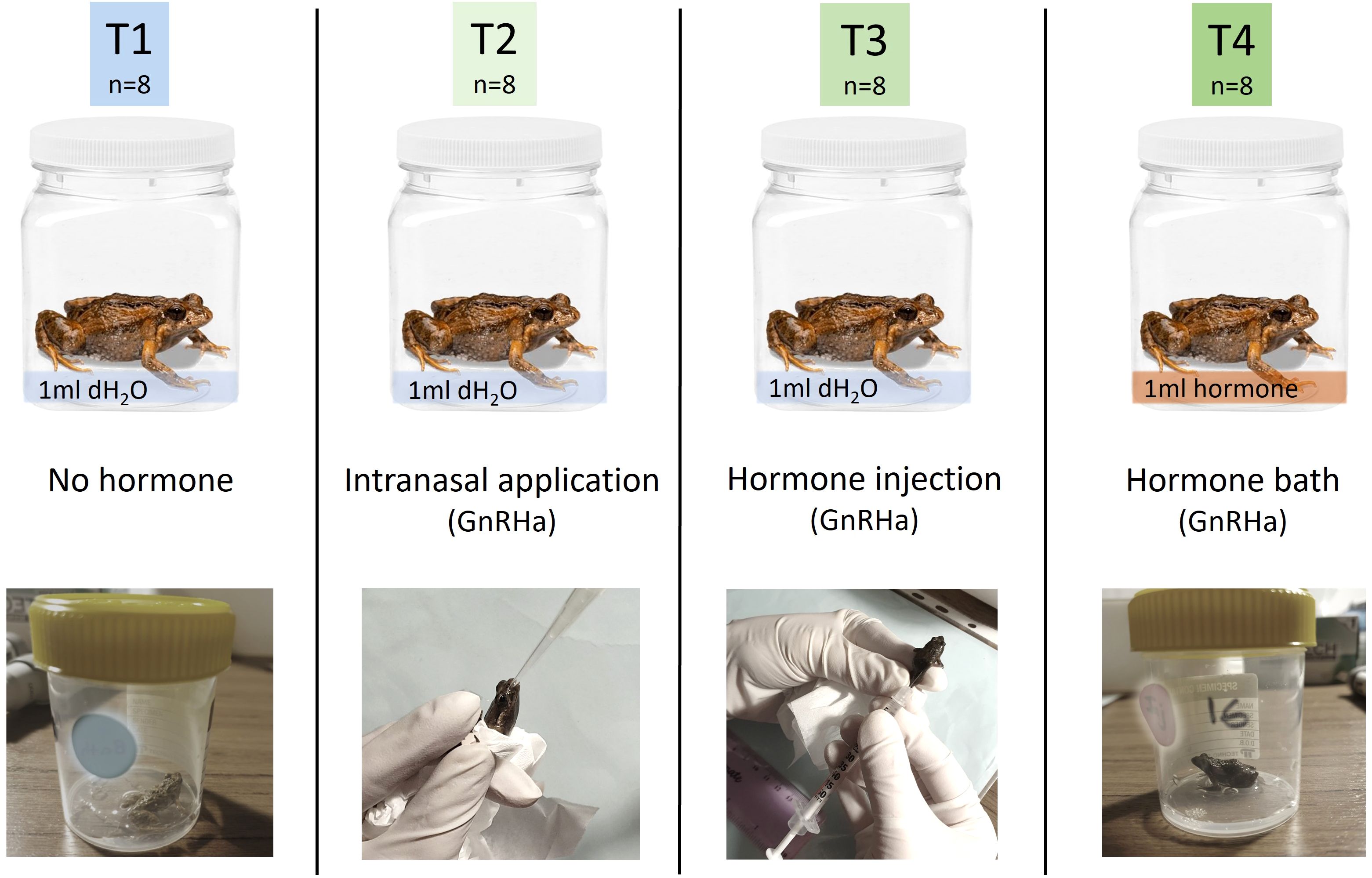
Figure 2. Experimental treatments used to test the effect of the route of hormone administration on spermiation outcomes in the common eastern froglet. Individual males (n=32) were randomly assigned to one of four treatments (n=8/treatment): T1 no-hormone, T2 intranasal application (2 μg GnRHa in 100 μL), T3 subcutaneous hormone injection (2 μg GnRHa in 100 μL), or T4 hormone bath (2 μg GnRHa in 100 μL x 1mL; total hormone in bath= 20 μg GnRHa). dH2O, distilled water; GnRHa, gonadotropin releasing-hormone agonist.
2.6 Collection and assessment of spermic urine
A urine sample was collected from each male immediately prior to the administration of hormones (0 hours) and in all cases was aspermic. Following hormone administration, samples were collected at 2, 4, 6, 8, 10, and 12 hours PA. Samples were collected by gently inserting the tip of a 50-μL glass microcapillary tube (fire polished and cooled) into the opening of the cloaca until urination was achieved. For each sample, spermic urine volume was measured, and the sample immediately prepared for the assessment of sperm concentration and sperm viability. Note that sperm motility was not formally quantified, though observed across all treatments and sampling times, as many samples fell below the minimum volume required for computer assisted sperm analysis (CASA). Sperm concentration was quantified using an Improved Neubauer Hemocytometer chamber (Bright Line, Optik Labor). The number of spermatozoa present in 25 quadrats was recorded (where sperm concentration was low, <15 spermatozoa in 25 quadrats, the number of sperm in the entire sample was determined to improve accuracy) and sperm numbers were used to calculate total sperm concentration. Sperm viability was quantified by adding 4-μL of a 1:50 dilution of SYBR-14, followed by 1-μL of propidium iodide (Invitrogen, L-7011, Thermo Fisher Scientific, Melbourne, Australia) to the spermic urine sample. The sample was incubated in the dark for 5 min following the addition of each solution. Wet mounts were prepared, and the proportion of viable sperm was evaluated under a fluorescent microscope to determine proportion live/dead.
2.7 Statistical analyses
The number of males spermiating was compared between the four treatments using a Fisher's exact test, and the number of males spermiating was then compared for each pairwise combination of treatments using two-tailed Fisher’s exact tests. Any males releasing less than, or equal to 4 sperm cells in total across all sampling times were categorized as ‘non-spermiating’ when comparing the number of males spermiating between treatment groups. For all variables, assumptions of normality and homogeneity of variances were first tested prior to all other analyses, using Shapiro-Wilk goodness of fit tests and Brown-Forsythe tests, respectively. Regression analyses were conducted to determine if the response variables (total number of sperm released, sperm concentration, or sperm viability) could be predicted by male body mass. To assess the effect of treatment on response variables, non-parametric Kruskal-Wallis tests were conducted. Post-hoc treatment comparisons were made using Wilcoxon matched-pair tests. To assess the effect of treatment and sampling time on response variables, a linear mixed effects (LME) model fitted with restricted maximum likelihood (REML) was performed, where sampling time (2, 4, 6, 8, 10 or 12 h), treatment, and the interaction between sampling time and treatment were fixed categorical effects, male ID was a random effect, and the response variable was either total number of sperm released, sperm concentration, or sperm viability. Male body mass was not included in any of the LME models presented as male body mass did not have a significant effect on any of the response variables when included in the model as a co-variate. Statistical analyses were performed using JMP Pro 17.0 software package (SAS Institute Inc. Cary, NC, USA). For all tests in this study, statistical significance was accepted at p < 0.05.
3 Results
Urine samples were collected from each male prior to (0h), and at 2, 4, 6, 8, 10 and 12 hours post hormone administration (PA). Mean urine volume was 9.53 ± 0.58 µL (mean ± SEM) at each sampling time, and total urine volume across all sampling times PA was 64.41 ± 6.93 µL (mean ± SEM).
The number of males spermiating differed significantly among treatment groups (Fisher’s exact test, p < 0.05; Table 1), with the number of males spermiating significantly lower in both the no hormone treatment (T1) and the intranasal application treatment (T2) compared to the hormone injection and hormone bath treatments (T3 and T4; two-tailed Fisher’s exact tests, p < 0.05; Table 1). Overall, the total number of sperm released differed significantly among treatment groups (Kruskal-Wallis test, p < 0.05; Table 1), with mean total sperm released significantly higher in both the hormone injection and hormone bath treatments compared to the control and intranasal application treatments (Wilcoxon matched-pair tests, p < 0.05; Table 1). Similarly, sperm concentration differed significantly among treatments groups (Kruskal-Wallis test, p < 0.05; Table 1), with mean sperm concentration significantly higher in both the hormone injection and hormone bath treatments compared to the no hormone and intranasal application treatments (Wilcoxon matched-pair tests, p < 0.05; Table 1). Sperm viability did not differ significantly among treatment groups (Kruskal-Wallis test, p > 0.05; Table 1).
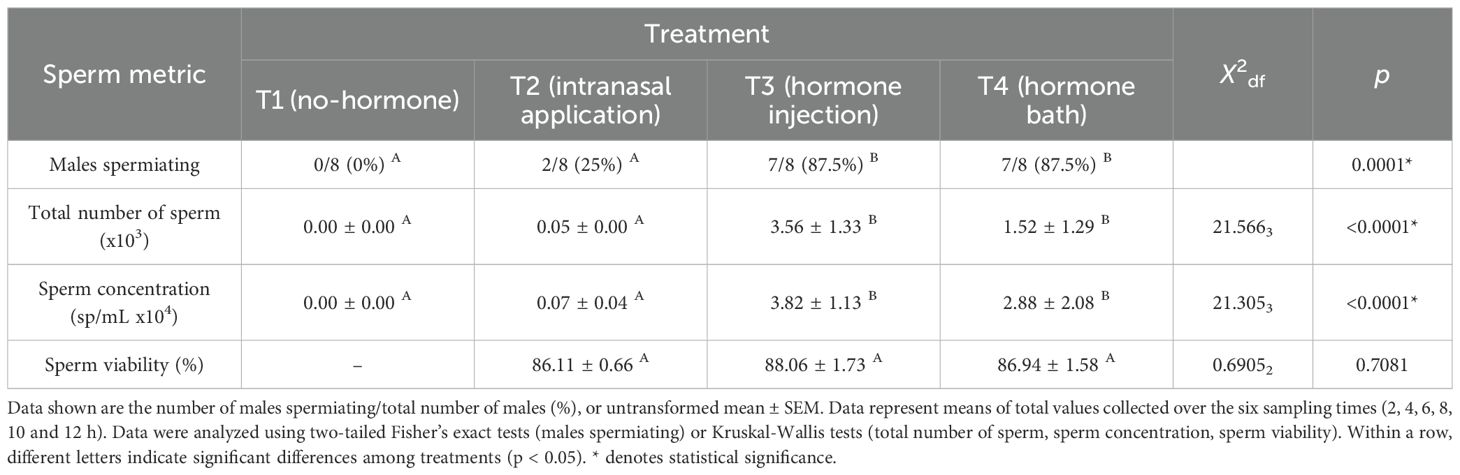
Table 1. Spermiation response of males administered GnRHa via four different routes of administration (T1, no hormone; T2, intranasal application; T3, hormone injection; T4, hormone bath) (n = 8 per treatment).
The total number of sperm released differed significantly both between hormone administration treatments (LME; F28 = 3.250, p = 0.037) and over time (LME; F140 = 2.315, p = 0.047), but the hormone administration treatment-by-time interaction was not significant (LME; F140 = 1.261, p = 0.235). The total number of sperm was consistently highest in the hormone injection treatment (T3), followed by the hormone bath treatment (T4), with peak sperm release occurring at 10-hours PA and 12-hours PA, respectively (Figure 3A). Similarly, sperm concentration differed significantly, both between hormone administration treatments (LME; F28 = 3.533, p = 0.027) and over time (LME; F140 = 2.354, p = 0.044), but the hormone administration treatment-by-time interaction was not significant (LME; F140 = 1.341, p = 0.186). Sperm concentration was consistently highest in the hormone injection treatment (T3), followed by the hormone bath (T4), except at 12-hours PA, where it was highest in the hormone bath treatment (T4) (Figure 3B). Sperm concentration peaked at 8-hours PA for the hormone injection treatment (T3) and at 12-hours PA for the hormone bath treatment (T4) (Figure 3B). Sperm viability did not differ significantly between hormone administration treatments (LME; F2 = 0.008, p = 0.992) nor over time (LME; F5 = 0.580, p = 0.715).
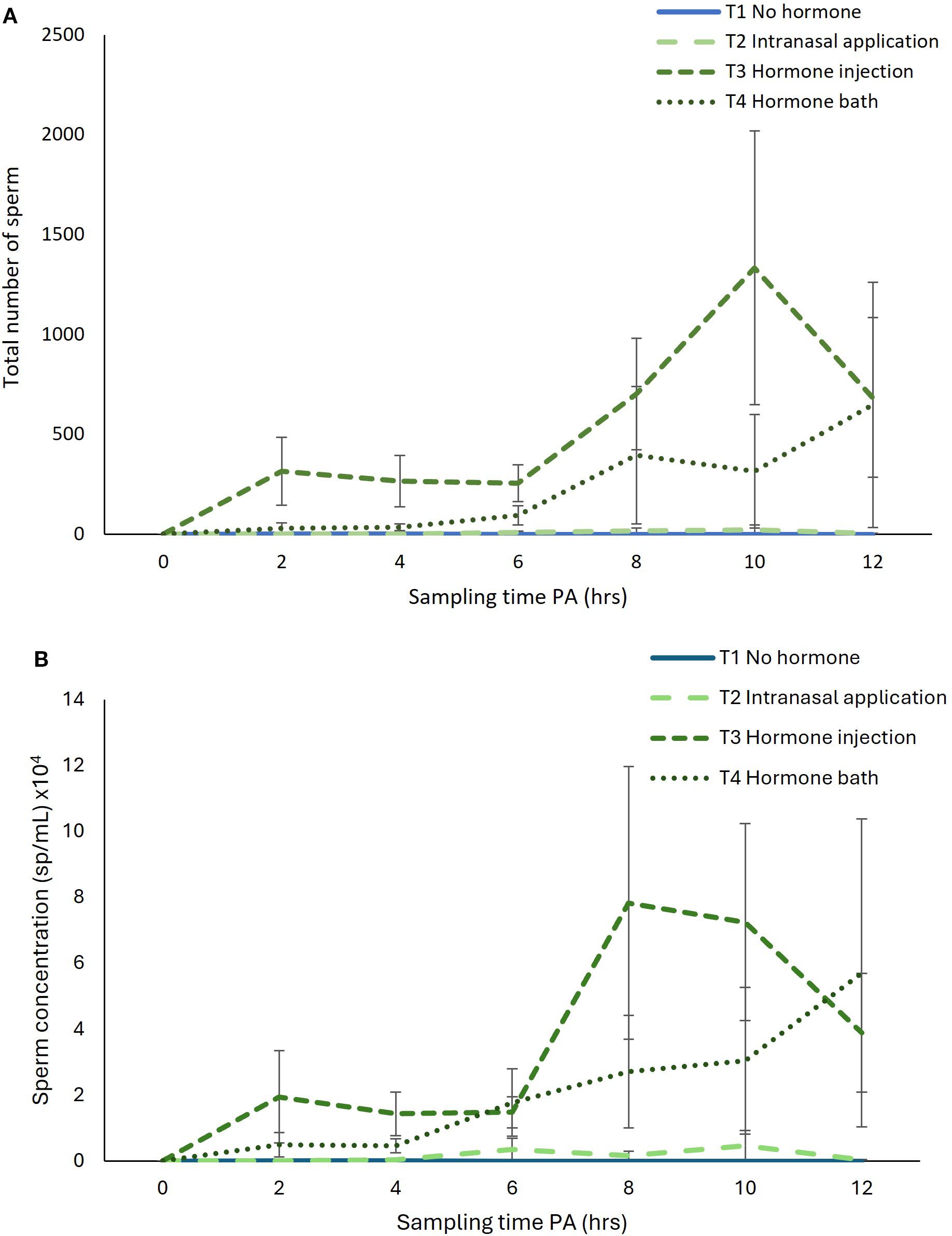
Figure 3. Effect of sampling time post-administration of hormone (PA) on (A) total number of sperm released and (B) sperm concentration over a 12-hour period post-administration of GnRHa, via four experimental treatments: T1 no-hormone, T2 intranasal application, T3 hormone injection, and T4 hormone bath. Data shown are untransformed mean ± SEM (n = 8 frogs per treatment).
4 Discussion
Reproductive technologies are increasingly being incorporated into conservation breeding programs to facilitate amphibian conservation. The investigation of alternative routes of hormone administration to induce spermiation requires further research. This study quantified the effect of various routes of GnRHa administration (intranasal, injection, hormone bath, and no-hormone) on the number of males spermiating, sperm concentration, and sperm viability of male common eastern froglets.
The route of hormone administration significantly affected the spermiation response of common eastern froglets. While sperm viability didn’t differ among treatments, both the number of males spermiating and sperm concentrations were significantly higher in the hormone injection and hormone bath treatment groups, compared to the intranasal and no-hormone treatment groups. Subcutaneous injection into the area of the posterior dorsal lymph sac allows circulation via subcutis capillaries and the lymphatic system (Whitaker and Wright, 2001), which has the benefit of bypassing metabolism in the liver, thus maximizing efficacy (Turner et al., 2011). Subcutaneous injection of reproductive hormones has been shown to be a safe and reliable method for inducing spermiation in a diversity of amphibian species (Silla and Langhorne, 2022; Silla et al., 2021), including a number of micro anuran species weighing as little as 0.45 g (rattling frog, Crinia glauerti; Silla and Roberts, 2012; Supplementary Table S1). However, safely administering injections to such small amphibians requires adequate training given the increased risk to animal well-being. Amphibians are a diverse taxon, with large variation in body size and well over a thousand species measuring below 30 mm in length or weighing less than three grams in body mass. The smallest known vertebrate species is an amphibian, the Brazilian flea toad (Brachycephalus pulex), with males measuring just over 7 mm in length (Bolaños et al., 2024), followed closely behind by another amphibian, the New Guinea Amau frog (Paedophryne amauensis) (measuring 7.70 mm SVL) (Rittmeyer et al., 2012). In Australia alone, over 100 frog species (≈43% of described species) have males that measure less than or equal to 30 mm, including 16 Crinia species, 14 Cophixalus species (Anstis, 2017) and five newly described Assa species of pouched frogs (Hoser, 2020; Mahony et al., 2021). Australia’s smallest anuran species is the rattling nursery frog (Cophixalus hosmeri) measuring only 14 mm in length and 0.27 g in weight (Anstis, 2017). While Australia has no native caudates, globally, a number of very small caudate species, such as plethodontids, may similarly be unsuitable for standard injection methods, because they can weigh less than 1 g and are particularly sensitive to handling due to their delicate structure and reliance on their skin for respiration (Glass Campbell et al., 2021). Given the extent of the amphibian biodiversity crisis, development of hormone therapies and collection and biobanking of amphibian sperm is urgently needed to preserve global amphibian biodiversity. The development of alternative methods of hormone application, such as topical application, stand to be of immense value to increase the number of small to micro species where sperm samples are effectively collected and preserved.
The present study contributes to a small but growing body of literature demonstrating the effectiveness of topical hormone application methods, with the spermiation response of males administered GnRHa via a hormone bath equal to the response of males administered the hormone via subcutaneous injection. Frogs have highly permeable, vascularized skin surfaces (particularly the ventral abdominal surface), allowing for the topical absorption of a range of chemicals, which are then rapidly circulated (Llewelyn et al., 2016). This high permeability allows relatively low molecular weight chemicals including GnRHa (~ 1.2 kDa) to be effectively absorbed (Llewelyn et al., 2016). Of note, the reproductive hormone hCG, another hormone used to induce gamete-release in various amphibian species, has a much higher molecular weight (~ 36 kDa), and previous research has shown a lack of response from males following the topical application of this hormone (Silla et al., 2020). Consistent with the results of the present study, previous research has reported high success in response to the topical application of GnRHa. Topical application of 100 µg GnRHa to the ventral surface of American toads and Gulf Coast toads resulted in 75% of males spermiating (Rowson et al., 2001), with mean sperm concentration for American toads in response to topical administration comparable to previous reports following intraperitoneal injection of GnRHa (Obringer et al., 2000). Similarly, 100 µg GnRHa applied to the ventral surface of roseate frogs resulted in 100% of males spermiating, and sperm concentrations were higher than those observed for males administered GnRHa via subcutaneous injection (Silla et al., 2020). In the northern corroboree frog, 25 µg/g bodyweight GnRHa applied to the ventral surface of both males and females resulted in successful spawning in 77% of pairs (Silla et al., 2018). In addition to the successful topical application of GnRHa to the ventral abdominal surface of anurans, the topical application of hormones to the nasolabial groove area of caudates has been successful. In a critically endangered plethodontid, the Texas blind salamander, administration of 25 µg/g bodyweight GnRHa to the nasolabial groove area on the head resulted in 92% spawning success (Glass Campbell et al., 2021), with similar success also observed in the endangered San Marcos salamander (Marcec-Greaves, pers. comm.). Overall, these studies demonstrate the value of topical application of GnRHa to induce gamete-release or spawning in amphibians. It is important to note that skin permeability and absorption kinetics may differ both across regions and among species. In anurans, the skin of the ventral surface is generally more permeable, however regional skin thickness, vascularization, and epidermal sculpturing varies across species (Llewelyn et al., 2019b, 2018, 2019; Toledo and Jared, 1993). Future studies would benefit from elucidating permeability across skin regions in a greater diversity of species, as well as assessing absorption kinetics of GnRHa and its bioactivity following topical application. Of note, while the topical application of GnRHa often requires a greater concentration or volume of hormone to be administered per individual compared to injection methods, these protocols remain a cost-effective approach for small species.
The present study also quantified the effectiveness of another alternative GnRHa administration method to injection: intranasal application. Herein, we demonstrate that the intranasal application of GnRHa resulted in a poor spermiation response (25% of males spermiating), which, statistically, was not significantly different from the no-hormone treatment group (0% of males spermiating). Intranasal application of reproductive hormones has only been trialed in one other anuran species to date, the Fowler’s toad, with the study reporting 93% of males spermiating in response to the optimal dose of GnRHa (Julien et al., 2019). The speed and efficacy of hormone absorption via the nasal cavity of anurans may be influenced by the surface area of the nasal cavity as well as interspecific differences in the structure and function of the olfactory system and capacity for chemical detection and uptake (Jungblut et al., 2021). Additionally, amphibian brain structures associated with olfaction have been found to differ morphologically across species (Manzano et al., 2017), highlighting the diversity of olfactory systems and differences in the relative importance of chemical communication. Therefore, some amphibian species may possess specialized nasal anatomy and high vascularization that facilitate chemotaxis, making them more amenable to intranasal hormone application. Additionally, little is known about the absorption pathways or the pharmacokinetics of GnRHa when administered intranasally. Further research is required to better understand how anatomical structures can support hormone absorption, and to assess hormone bioavailability once absorbed. In addition, future research would benefit from quantifying the response of anurans to intranasal hormone application across a diversity of species in concert with characterization of the species’ nasal anatomy and olfactory system. Such research would allow for enhanced capacity to predict which anuran groups are likely to respond favorably to intranasal hormone application.
In addition to determining the overall total quantity and quality of sperm released in response to different routes of hormone administration, it is imperative to quantify peak periods of sperm release in order to target collection of samples of the highest quality and concentration. Identifying peak collection periods will enhance the success of subsequent reproductive technologies, including assisted fertilizations, where success has been correlated with sperm concentration (Silla, 2013; Edwards et al., 2004) and total percentage sperm motility (Burger et al., 2022; Dziminski et al., 2009). The collection of optimal quality and concentration of sperm is particularly important where samples are to be cryopreserved, as the freeze-thaw process results in a reduction of viability and fertilization capacity (Hobbs et al., 2023; Howard et al., 2025; Lampert et al., 2023; Burger et al., 2022). In the present study, sperm viability did not differ among treatment groups, but total sperm and concentration was significantly higher in the hormone injection and hormone bath treatments. Additionally, the sperm-release response of males over time following hormone administration differed according to the route of hormone administration. Of the two treatments successfully inducing the release of high numbers of sperm, males in the hormone injection treatment group released the highest total number of sperm 10-hrs post hormone administration, with both total sperm released and sperm concentration peaking around 8-10-hours PA before declining at 12-hours PA. In contrast, males in the hormone bath treatment exhibited a more gradual increase in total sperm and sperm concentration over time, with sperm-release continuing to increase at 12-hours PA. Future studies administering GnRHa topically in our study species would benefit from extending the sampling period beyond 12-hours post hormone administration to specifically determine when sperm-release peaks and declines.
Overall, the results from this study shed light on the efficacy of alternative hormone administration routes to induce sperm-release in a small-bodied anuran, the common eastern froglet. The present study is an important starting point towards developing gamete-release protocols for additional small-bodied amphibian species, which have been underrepresented in the development of amphibian hormone therapies to date. The study adds to a growing body of research highlighting the potential for topical hormone administration to successfully induce gamete-release in amphibians. It is important to note that a limitation of the study was that only a single hormone dose per treatment was administered. Further research is required to ascertain the optimal hormone dose to apply via each administration route by establishing dose-relationship curves (see Silla and Langhorne, 2022). Moving forward, research would also benefit from quantifying additional sperm fertility metrics including motility (percent motility and velocity), acrosome integrity, and fertilization capacity (through assisted fertilizations). Additional sperm quality metrics were not possible in the present study due to small spermic urine volumes released from our study species, which only allowed us to quantify sperm viability. Additional metrics could be quantified from study species with higher sample volumes. Finally, the field would also benefit from advanced molecular studies quantifying absorption, metabolism, and the half-life of GnRHa in vivo following administration via each of the alternative hormone administration routes, to better understand pharmacokinetics and pharmacodynamics of GnRHa in amphibians.
5 Conclusions
Reproductive technologies can assist conservation breeding programs to reach propagation targets and genetic management goals. Fundamental to the application of assisted fertilization, cryopreservation, and the biobanking of genetic resources, is the efficient collection of high-quality sperm at sufficiently high numbers. Overall, the present study reports on the successful collection of common eastern froglet sperm following administration of GnRHa either via subcutaneous injection or hormone bath, and that intranasal hormone application was ineffective. Results reported herein have important implications for the conservation of amphibians because they expand the development of assisted reproductive technologies. Specifically, the development of alternative hormone administration protocols that do not require injection stand to increase the diversity of amphibian species to benefit from hormone therapies by providing a safe and effective method for hormone administration to small amphibian species. Knowledge gained from our study may assist conservation efforts for two closely related small-bodied endangered species, Sloane’s frog, C. sloanei, and the streambank frog, C. riparia, and will further inform the conservation breeding programs of other threatened amphibian species globally.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by University of Wollongong’s Animal Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZA: Writing – original draft, Writing – review & editing, Formal analysis. PB: Project administration, Formal analysis, Supervision, Writing – review & editing. AS: Formal analysis, Data curation, Methodology, Project administration, Investigation, Writing – review & editing, Supervision, Funding acquisition, Writing – original draft, Conceptualization.
Author’s note
Additional procedural contributions: AS was responsible for conceptualization of the study and acquisition of funding. AS acquired animal ethics approvals and scientific licenses. AS and PB were responsible for project supervision and administration. AS collected the study animals. ZA was responsible for animal monitoring and husbandry prior to experimental procedures, and AS and ZA were responsible for animal monitoring during experimental procedures. AS prepared and administered all hormone solutions, collected the spermic urine samples, and obtained all data. ZA, AS and PB were responsible for data analysis and interpretation. ZA and AS wrote the manuscript with contributions from PB. Each author contributed to the review and editing of the manuscript and approved the submitted version.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Research was funded by the Australian Research Council, Discovery Early Career Researcher Award (DE210100812) awarded to AS. Publication of this article was financially supported by the Holsworth Wildlife Research Endowment—Equity Trustees Charitable Foundation & the Ecological Society of Australia awarded to ZA. Preparation of the manuscript was conducted while ZA was in receipt of an Australian Government Research Training Program (AGRTP) Scholarship.
Acknowledgments
The authors would like to acknowledge the Traditional Custodians and knowledge holders of Dharawal Country, the lands on which this research was conducted. We acknowledge their connection to Country and are united in our dedication to the preservation of species on these lands.
Conflict of interest
The authors declare that the research was conducted in the absence of any relationships that could be construed as a potential conflict of interest.
The author AS declared that they were an editorial board member for Frontiers, at the timeof submission. This had no impact on the peer review process andthe final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/famrs.2025.1615858/full#supplementary-material
References
Bolaños W. H., Dias I. R., and Solé M. (2024). Zooming in on amphibians: Which is the smallest vertebrate in the world? Zool. Scr. 53, 414–418. doi: 10.1111/zsc.12654
Bolton A. L., Mooney A., Pettit M. T., Bolton A. E., Morgan L., Drake G. J., et al. (2022). Resurrecting biodiversity: advanced assisted reproductive technologies and biobanking. Reprod. Fertil. 3, R121–R146. doi: 10.1530/RAF-22-0005
Burger I. J., Chen L.-D., Lampert S. S., Kouba C. K., Barber D., Smith D., et al. (2023). Applying sperm collection and cryopreservation protocols developed in a model amphibian to three threatened anuran species targeted for biobanking management. Biol. Conserv. 277, 109850. doi: 10.1016/j.biocon.2022.109850
Burger I. J., Lampert S. S., Kouba C. K., Morin D. J., and Kouba A. J. (2022). Development of an amphibian sperm biobanking protocol for genetic management and population sustainability. Conserv. Physiol. 10, coac032–coac032. doi: 10.1093/conphys/coac032
Calatayud N., Howell L., Upton R., Tapley B., Johnson K., Browne R., et al. (2024). “Amphibian assisted reproductive technologies and biobanking,” in Amphibian Conservation Action Plan: A status review and roadmap for global amphibian conservation. Eds. Wren S., Borzée A., Marcec-Greaves R., and Angulo A. (IUCN/SSC Amphibian Specialist Group, Gland, Switzerland).
Clulow S., Clulow J., Marcec-Greaves R., Della Togna G., and Calatayud N. E. (2022). Common goals, different stages: the state of the ARTs for reptile and amphibian conservation. Reprod. Fert. Dev. 34, i–ix. doi: 10.1071/RDv34n5_FO
Dawley E. M. and Bass A. H. (1989). Chemical access to the vomeronasal organs of a plethodontid salamander. J. Morphol. 200, 163–174. doi: 10.1002/jmor.1052000206
Dziminski M. A., Roberts J. D., Beveridge M., and Simmons L. W. (2009). Sperm competitiveness in frogs: slow and steady wins the race. Proc. R. Soc B: Biol. Sci. 276, 3955–3961. doi: 10.1098/rspb.2009.1334
Edwards D. L., Mahony M. J., and Clulow J. (2004). Effect of sperm concentration, medium osmolality and oocyte storage on artificial fertilization success in a myobatrachid frog (Limnodynastes tasmaniensis). Reprod. Fert. Dev. 16, 347–354. doi: 10.1071/RD02079
Gascon C., Collins J. P., Moore R. D., Church D. R., Mckkay J. E., and Mendelson Iii J. R. (2007). Amphibian Conservation Action Plan (Gland, Switzerland: IUCN/SSC Amphibian Specialist Group).
Glass Campbell L., Anderson K., and Marcec-Greaves R. (2021). Topical application of hormone gonadotropin-releasing hormone (GnRH-A) stimulates reproduction in the endangered Texas blind salamander (Eurycea rathbuni). Conserv. Sci. Pract. 4 (3). doi: 10.1111/csp2.609
Graham K. M. and Kouba C. K. (2022). “Ultrasonography to assess female reproductive status and inform hormonally induced ovulation,” in Reproductive Technologies and Biobanking for the Conservation of Amphibians. Eds. : Silla A. J., Kouba A. J., and Heatwole H. (CSIRO Publishing, Melbourne, Australia).
Helmer P. and Whiteside D. (2005). “Amphibian anatomy and physiology,” in Clinical Anatomy and Physiology of Exotic Species. Ed. O'malley B. (Elsevier Saunders, Edinburgh, UK).
Hobbs R., Upton R., Calatayud N., Silla A., Daly J., Mcfadden M., et al. (2023). Cryopreservation cooling rate impacts post-thaw sperm motility and survival in Litoria booroolongensis. Animals 13, 3014. doi: 10.3390/ani13193014
Hoser R. T. (2020). Four new species of frog in the genus Assa from eastern Australia. Aust. J. Herpetol. 47, 57–63.
Howard M. S., Byrne P. G., O’Brien J. K., Hobbs R. J., and Silla A. J. (2025). Antioxidant supplementation of cryopreservation extenders improves post-thaw sperm viability in the red-crowned toadlet, Pseudophryne australis. Front. Amphib. Reptile Sci. 3. doi: 10.3389/famrs.2025.1525965
IUCN (2025). IUCN Red List of Threatened Species. Available online at: https://www.iucnredlist.org/ (Accessed April 4, 2025).
Julien A. R., Kouba A. J., Kabelik D., Feugang J. M., Willard S. T., and Kouba C. K. (2019). Nasal administration of gonadotropin releasing hormone (GnRH) elicits sperm production in Fowler’s toads (Anaxyrus fowleri). BMC Zool. 4, 1–10. doi: 10.1186/s40850-019-0040-2
Jungblut L. D., Reiss J. O., and Pozzi A. G. (2021). Olfactory subsystems in the peripheral olfactory organ of anuran amphibians. Cell Tissue Res. 383, 289–299. doi: 10.1007/s00441-020-03330-6
Lampert S. S., Burger I. J., Julien A. R., Gillis A. B., Kouba A. J., Barber D., et al. (2023). Sperm cryopreservation as a tool for amphibian conservation: Production of F2 generation offspring from cryo-produced F1 progeny. Animals 13, 53. doi: 10.3390/ani13010053
Lemckert F. (2001). The influence of micrometeorological factors on the calling activity of the frog Crinia signifera (Anura: Myobatrachidae). Aust. Zool. 31, 625–631. doi: 10.7882/AZ.2001.009
Llewelyn V. K., Berger L., and Glass B. D. (2016). Percutaneous absorption of chemicals: developing an understanding for the treatment of disease in frogs. J. Vet. Pharmacol. Ther. 39, 109–121. doi: 10.1111/jvp.2016.39.issue-2
Llewelyn V. K., Berger L., and Glass B. D. (2018). Regional variation in percutaneous absorption in the tree frog Litoria caerulea. Environ. Toxicol. Pharmacol. 60, 5–11. doi: 10.1016/j.etap.2018.03.019
Llewelyn V. K., Berger L., and Glass B. D. (2019a). Effects of skin region and relative lipophilicity on percutaneous absorption in the toad Rhinella marina. Environ. Toxicol. Chem. 38, 361–367. doi: 10.1002/etc.4302
Llewelyn V. K., Berger L., and Glass B. D. (2019b). Percutaneous absorption between frog species: Variability in skin may influence delivery of therapeutics. J. Vet. Pharmacol. Ther. 43, 91–95. doi: 10.1111/jvp.12824
Lochhead J. J. and Thorne R. G. (2012). Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 64, 614–628. doi: 10.1016/j.addr.2011.11.002
Mahony M. J., Hines H. B., Mahony S., Moses B., Catalano S. R., Myers S., et al. (2021). A new hip-pocket frog from mid-eastern Australia (Anura: Myobatrachidae: Assa). Zootaxa 5057, 451–486. doi: 10.11646/zootaxa.5057.4.1
Manzano A. S., Herrel A., Fabre A. C., and Abdala V. (2017). Variation in brain anatomy in frogs and its possible bearing on their locomotor ecology. J. Anat. 231, 38–58. doi: 10.1111/joa.2017.231.issue-1
Obringer A. R., O'Brien J. K., Saunders R. L., Yamamoto K., and Kikuyama S. (2000). Characterization of the spermiation response, luteinizing hormone release and sperm quality in the American toad (Bufo americanus) and the endangered Wyoming toad (Bufo baxteri). Reprod. Fert. Dev. 12, 51–58. doi: 10.1071/RD00056
Rittmeyer E. N., Allison A., Gründler M. C., Thompson D. K., and Austin C. C. (2012). Ecological guild evolution and the discovery of the world's smallest vertebrate. PloS One 7, e29797. doi: 10.1371/journal.pone.0029797
Roth T. L. and Obringer A. R. (2003). “Reproductive research and the worldwide amphibian extinction crisis,” in Reproductive Science and Integrated Conservation. Eds. Holt W. V., Pickard A. R., Rodger J. C., and Wildt D. E. (Cambridge University Press, Cambridge, UK).
Rowson A. D., Obringer A. R., and Roth T. L. (2001). Non-invasive treatments of luteinizing hormone-releasing hormone for inducing spermiation in American (Bufo americanus) and Gulf Coast (Bufo valliceps) toads. Zoo Biol. 20, 63–74. doi: 10.1002/zoo.v20:2
Silla A. J. (2013). Artificial fertilization in a terrestrial toadlet (Pseudophryne guentheri): effect of medium osmolality, sperm concentration and gamete storage. Reprod. Fertil. Dev. 25, 1134–1141. doi: 10.1071/RD12223
Silla A. J. and Byrne P. G. (2019). The role of reproductive technologies in amphibian conservation breeding programs. Annu. Rev. Anim. Biosci. 7, 499–519. doi: 10.1146/annurev-animal-020518-115056
Silla A. J. and Byrne P. G. (2021). Hormone-induced ovulation and artificial fertilization in four terrestrial-breeding anurans. Reprod. Fert. Dev. 33, 615. doi: 10.1071/RD20243
Silla A. J., Calatayud N. E., and Trudeau V. L. (2021). Amphibian reproductive technologies: approaches and welfare considerations. Conserv. Physiol. 9, coab011–coab011. doi: 10.1093/conphys/coab011
Silla A. J. and Langhorne C. J. (2022). “Protocols for hormonally induced spermiation, and the cold storage, activation, and assessment of amphibian sperm,” in Reproductive Technologies and Biobanking for the Conservation of Amphibians. Eds. Silla A. J., Kouba A. J., and Heatwole H. (CSIRO Publishing, Melbourne, Australia).
Silla A. J., Mcfadden M., and Byrne P. G. (2018). Hormone-induced spawning of the critically endangered northern corroboree frog Pseudophryne pengilleyi. Reprod. Fertil. Dev. 30, 1352–1358. doi: 10.1071/RD18011
Silla A. J. and Roberts J. D. (2012). Investigating patterns in the spermiation response of eight Australian frogs administered human chorionic gonadotropin (hCG) and luteinizing hormone-releasing hormone (LHRHa). Gen. Comp. Endocrinol. 179, 128–136. doi: 10.1016/j.ygcen.2012.08.009
Silla A. J., Roberts J. D., and Byrne P. G. (2020). The effect of injection and topical application of hCG and GnRH agonist to induce sperm-release in the roseate frog, Geocrinia rosea. Conserv. Physiol. 8, 1–coaa104. doi: 10.1093/conphys/coaa104
Toledo R. C. and Jared C. (1993). Cutaneous adaptations to water balance in amphibians. Comp. Biochem. Physiol. – Part A: Physiol. 105, 593–608. doi: 10.1016/0300-9629(93)90259-7
Turner P. V., Brabb T., Pekow C., and Vasbinder M. A. (2011). Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 50, 600–613.
Whitaker B. R. and Wright K. M. (2001). “Clinical Techniques,” in Amphibian medicine and captive husbandry. Eds. Wright K. M. and Whitaker B. R. (Krieger Pub. Co, Malabar, FL, USA).
Wren S., Angulo A., Meredith H., Kielgast J., Dos Santos L., and Bishop P. (2015). Amphibian conservation action plan (Gland, Switzerland: IUCN/SSC Amphibian Specialist Group).
Wren S., Borzée A., Marcec-Greaves R., and Angulo A. (2024). Amphibian conservation action plan: A status review and roadmap for global amphibian conservation (Gland, Switzerland: IUCN/SSC Amphibian Specialist Group).
Keywords: amphibian, sperm release, sperm, hormone therapy (HT), spermiation induction, topical, reproductive technologies, anuran
Citation: Anastas ZM, Byrne PG and Silla AJ (2025) Hormonal induction of spermiation in the common eastern froglet: testing alternative routes of hormone administration. Front. Amphib. Reptile Sci. 3:1615858. doi: 10.3389/famrs.2025.1615858
Received: 22 April 2025; Accepted: 11 September 2025;
Published: 16 October 2025.
Edited by:
José Luis Ros-Santaella, Czech University of Life Sciences Prague, CzechiaReviewed by:
Samadhan Phuge, Savitribai Phule Pune University, IndiaIsabella Burger, University of North Carolina at Chapel Hill, United States
Maura Lane, Yale University, United States
Copyright © 2025 Anastas, Byrne and Silla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aimee J. Silla, YXNpbGxhQHVvdy5lZHUuYXU=
†These authors have contributed equally to this work
 Zara M. Anastas
Zara M. Anastas Phillip G. Byrne
Phillip G. Byrne Aimee J. Silla
Aimee J. Silla