Abstract
Cross fostering piglets is a common management practise in the pig industry to manage large and heterogeneous litters, whereby piglets are moved from their biological litter to be reared by another sow. At present research has focused on immediate survival consequences and time of cross fostering, with little attention given to positive aspects of welfare such as social affiliations and the potential for positive interactions for these piglets such as play behaviour. The focus of our study was purely observational to record behaviour of piglets reared in either impoverished (farrowing crates) or enriched neonatal environments (PigSAFE pens) where fostering was practised as part of normal husbandry routines to promote piglet survival. We employed social network analysis to understand more about the behaviour of foster piglets in these environments and their acceptance into their new litter. In line with previous work, piglets exposed to enriched neonatal farrowing pens demonstrated more play behaviour than piglets reared in farrowing crates. We showed that pen piglets received and initiated more play invitations (higher degree centrality) than piglets in crates. We also found effects of cross fostering irrespective of neonatal environment. Non-foster piglets received and initiated more play behaviours (higher degree centrality) 2–3 weeks post-farrowing compared to piglets fostered into the litter and as such, fostered piglets remained isolated from play for the first few weeks of life. However, our data suggests this may be mitigated by neonatal environment; foster piglets reared in pens were better connected (betweenness centrality) within their foster litter than those fostered in crates. Our findings highlight the importance of the neonatal environment and its potential influence on the isolation of cross-fostered piglets and suggest that rearing in enriched neonatal environments may help mitigate against social isolation in early life of cross-fostered piglets, having obvious immediate, and long-term consequences for piglet welfare and behaviour. We also highlight the importance and application of methodologies such as social network analysis, for gaining more insight and understanding about the sociality of animal behaviour and its potential for measuring indicators of positive welfare, thus highlighting its application for veterinary science and animal behaviour and welfare research.
Introduction
A common management practise in the pig industry to help deal with large litter sizes and heterogeneous litters is to cross-foster, ideally shortly after birth. This involves moving neonatal piglets from their biological litter to be reared by another sow with teat space and with piglets of similar birth weight to those being fostered. If done correctly cross-fostering gives piglets enhanced survival prospects and offers the possibility to reduce further management interventions for piglets that are suffering from competition in large litters or those with low birth weights that are failing to compete for a productive teat with their larger littermates (Kirkden et al., 2013). However, the advent of super-prolific breeding programmes to achieve more than 35 pigs weaned per sow per year and improve production efficiency, means sows are increasingly giving birth to more piglets than they can rear, and such extreme breeding programmes have negative consequences for both sow and piglet welfare (Baxter et al., 2013; Rutherford et al., 2013). Surplus piglets require different management interventions to promote survival including; split suckling, cross-fostering, the use of nurse sows systems and early weaning and split weaning (Baxter et al., 2013). All of these methods including the additional handling they may involve, have documented short- and long-term negative effects (Baxter et al., 2013).
With regards to fostering, knowledge is centred around determining immediate survival prospects and the appropriate time to foster piglets based on colostrum availability, gut closure, and establishment of a stable teat order [for reviews see (Baxter et al., 2013; Alexopoulos et al., 2018)]. Behavioural observations of fostering practises have centred on the sow accepting the foster piglets (Weary et al., 1999) and conflict around suckling as teat order is re-established (Horrell, 1982). To our knowledge, there has been no attention given to social affiliations, and the potential for positive interactions for these piglets and as such the effects of fostering practises on indicators of positive welfare remain underexplored. One of the most positive and important social experiences for mammals, particularly young mammals, is play behaviour (Špinka et al., 2001; Lawrence et al., 2019). Play behaviour is multifunctional and social play is thought to establish bonds and help prepare young animals for social encounters in later life (Špinka et al., 2001). It is used as an animal-based indicator of welfare because it is purported to be associated with positive emotional experiences (Ahloy-Dallaire et al., 2018) and it often disappears when an animal's fitness is challenged (Held and Špinka, 2011). Fostered piglets face challenges, and one aim of our study was to focus on whether foster status impacts on sociality, particularly on play behaviour. In addition, we wanted to explore whether there are aspects of the neonatal environment that might mitigate against the potential negative impacts of fostering. It is known that more complex, enriched environments benefit pig welfare (van de Weerd and Day, 2009), including benefits for long-term health (Van Dixhoorn et al., 2016), growth (Brown et al., 2015), as well as on social and cognitive development (De Jonge et al., 1996; Martin et al., 2015). Therefore, the focus of our observational study was to record behaviour of piglets reared in either impoverished or enriched neonatal environments where fostering was practised as part of normal husbandry routines to promote piglet survival. To understand more about the behaviour of foster piglets in these environments and their acceptance into their new litter we employed social network analyses. More specifically we wanted to understand more about the sociality of piglet play and cohesion throughout the litter pre-weaning. Over recent years, the application of social network analyses has gained significant traction for animal behaviour and welfare science due to it providing a powerful tool for strengthening our understanding of the likely causes and consequences of animal sociality (Wey et al., 2008; Turner et al., 2020). It offers the ability to consider the connections beyond the dyadic level, extending to an animals' indirect social connections or “friends of friends” (Wey et al., 2008; Foister et al., 2018; Turner et al., 2020). This has had important welfare implications across a range of species including pigs, where, for example it has been used for predicting chronic aggression in commercial pig systems (Foister et al., 2018). However, the effects of cross-fostering on positive indicators such as the sociality of piglets within a litter remains to be elucidated and could have wide-reaching consequences for existing fostering practises, neonatal environments, piglet welfare, and behaviour later in life.
Methods and Materials
Ethical Approval
This project was reviewed and approved by SRUC's ethical review committee (ED AE 06/2009) and all routine animal management procedures were adhered to by trained staff.
Animals, Housing, and Husbandry
Data from a total of 142 piglets, bred from commercial cross-bred dams (Large White × Landrace) and sired by Pietrain boars were used. Animals were housed at the SRUC Pig Unit (Midlothian, Scotland) and were born and raised in two different neonatal environments. Out of 142 piglets, 66 were born in standard farrowing crates (Crates), and 76 piglets were born in PigSAFE pens, an enriched free farrowing system (Baxter et al., 2015) (Pen). Focal litters were produced from six sows in each neonatal environment (average parity was 3.6 in crates and 3.2 in pens) with mixed or single experience with neonatal environments, dependent on parity. Normal husbandry routines were performed with minimal experimental interference, as such at birth piglets were weighed as a litter and average birth litter weight recorded (Table 1) and sex of each piglet was recorded. Cross-fostering was performed in line with normal husbandry routines in order to improve piglet survival. It was not performed for the purposes of the experiment, therefore “natural variation” in numbers fostered in and out of litters occurred (Table 1) and within the Crate treatment four piglets were fostered into focal litters from two non-focal Crate litters (2 piglets per non-focal litter). Three litters did not experience any cross-fostering or removal of piglets and remained stable from birth. In total, two litters per neonatal environment had piglets cross-fostered, increasing their litter sizes. Where fostering was required, best practise was adopted: it was conducted within 24–48 h after birth within the neonatal environmental condition, but after the piglets had been with their biological mothers for at least 6 h to obtain maternal colostrum; chosen foster piglets were healthy and of good vigour and were of equivalent weight to that of piglets in the adoptive litter. In addition, although the risk of mixing aggression is low when cross-fostering piglets within 48 h after birth, it was decided not to foster single piglets into an established litter, meaning fostered piglets were fostered alongside at least one sibling and numbers fostered ranged from 2 to 6 in or out per litter. Given the requirement for all-in-all-out management, our two neonatal environment conditions could not be run simultaneously. Instead, the unit was managed across batches, whereby a batch of sows farrowed in farrowing crates and 3-weeks later a batch would farrow in PigSAFE. No mutilations were performed (i.e., all piglets kept intact tails and males were not castrated). All piglets received an intramuscular iron injection on day 3 post-farrowing (Gleptosil®, Alstoe Animal Health).
Table 1
| Neonatal Environment | Sow ID | Number of piglets born alive | Litter weight (kg) | Mean piglet birth weight (kg) | Number of piglets Fostered off (−) or on (+) | Total piglets at weaning | Weaned litter weight (kg) |
|---|---|---|---|---|---|---|---|
| Crate | BF67 | 14 | 18.5 | 1.32 | −2 | 10 | 92.0 |
| Crate | BF53 | 10 | 17.9 | 1.79 | 0 | 10 | 80.4 |
| Crate | BF37 | 3 | 6.7 | 2.23 | +4 | 7 | 70.0 |
| Crate | BF36 | 9 | 14.5 | 1.61 | +4 | 13 | 113.9 |
| Crate | BF24 | 13 | 18.1 | 1.29 | 0 | 11 | 92.7 |
| Crate | YF6311 | 17 | 22.0 | 1.22 | −2 | 11 | 92.3 |
| Pen | BF82 | 15 | 14.4 | 0.96 | 0 | 14 | 86.8 |
| Pen | BF46 | 10 | 19.8 | 1.98 | +3 | 11 | 116.3 |
| Pen | BF42 | 17 | 23.5 | 1.38 | −3 | 11 | 100.7 |
| Pen | BF23 | 16 | 24.4 | 1.53 | −3 | 12 | 106.4 |
| Pen | YF7072 | 4 | 8.4 | 2.10 | +6 | 10 | 99.1 |
| Pen | YF6368 | 14 | 20.5 | 1.37 | −3 | 11 | 99.4 |
Table outlining litter information, including neonatal farrowing environment and number of piglets fostered off (–) and on (+) to litters.
The Piglet and Sow Alternative Farrowing Environment, or PigSAFE (Pens), was developed based on the design criteria proposed by (Baxter et al., 2011) and described in detail in (Baxter et al., 2015). The pen measured 3.61m in length and 2.2 m in width (Figures 1A, 2A), with a basic nest area, with solid and insulated concrete flooring. The nest was equipped with sloping walls against which the sow can slide more slowly to ground level for suckling, which had a gap between the base and the floor to lower the risk of piglets being trapped and killed. The gap under the sloped wall was wide enough to allow piglets to move freely underneath. A floor heated, corner creep area (0.75 m2) with easy access from the nest was bedded with a thin layer of sawdust. A separate slatted dunging area was bounded by walls with barred panels to adjacent pens to discourage farrowing outside the nest and allow visual and oral-nasal contact between neighbouring sows and piglets later on during lactation. A feeding stall for the sow was included at one side of the pen, where the sow could be locked in to allow safe inspection or treatment of the piglets. However the feeding stall was only 0.50 m wide, bounded by solid sides, and therefore the sow could not be locked in for farrowing. The pen was designed to provide a more complex, less restrictive environment for both the sow and piglets, and when the piglets were 7 days old the wall separating the dunging passage from the nest area could be swung open facilitating greater hygiene and more contiguous space. Lighting was provided artificially between 0700 and 1600 daily, with night lights remaining on at a lower lux. For nesting, 2 kg of long-stemmed straw was maintained by daily replenishment (not cumulative) from 5 days prior to farrowing. This level was maintained until day +7 and then it was reduced to 1 kg of straw daily until weaning.
Figure 1

Schematic diagrams (not to scale) of (A) two side-by-side PigSAFE pens (Pens), and (B) a conventional farrowing crate (Crate) used to house farrowing and lactating sows in this study (adapted from: Baxter et al., 2015).
Figure 2
(A) PigSAFE Pen with sow and litter during lactation. (B) Farrowing Crate with sow and litter during lactation.
The conventional farrowing house consisted of rooms of six Crates, in rows of three back-to-back. Sows and litters were physically isolated from one another. Visual contact between neighbouring sows was possible when they were standing. Each crate had a solid-floored pen (2.47 m long × 1.50 m wide), with a centrally placed farrowing crate (1.82 × 0.50 m) and a front creep area (0.65 × 1.50 m) with underfloor heating for the piglets. The sow was restricted to the central area in the crate via parallel bars and a small, slatted area was at her rear for easy dung removal (Figures 1B, 2B). The piglets were able to move freely around. Apart from the creep area, which was permanently lit, artificial lighting was on between 07:00 and 16:00 h daily, and natural light was provided by windows in the farrowing house. In line with standard farm practise, upon entry into the farrowing house sows were given ~0.5 kg of long-stemmed straw for nest-building and this level was maintained post-farrowing until weaning, with straw being added daily after pens were mucked out.
When housed in Crates or Pens, sows were fed a standard pelleted lactation diet (17% CP, 13.75 MJ DE.kg−1) twice daily, at 0700 and 1530h. After farrowing, lactation diet (17% CP, 13.75 MJ DE.kg−1) was offered at a rate of three kg per day followed by 0.5 kg increments each day until seven kg and then followed by one kg increments each day up to a maximum of 12 kg until weaning. Throughout, all animals had ad libitum access to water. The overall farrowing room temperature in both farrowing houses was set at 20°C for the first week during and after farrowing, before being reduced to ~18°C for the remainder of lactation. Creep temperatures were also standardised across both systems using Dicam monitors (Farmex, Oxford, UK). Underfloor heating was set at 30°C, at 60% during and after farrowing and for the first seven days, then reduced to 50% after 7 days. Creeps in both environments were covered (i.e., roofed) and bedded. One week before weaning, piglets were introduced to solid feed (Compound pellet creep feed, Scotlean Pigs Ltd., primary diets—AB Abri Ltd., Yorks, UK) by scattering pellets within the creep area. Weaning occurred at 27 days old, during which piglets were removed from sows and underwent several management procedures [e.g., vaccination—(Ingelvac CircoFLEX®, Boehringer Ingelheim Vetmedica, Inc.) and ear tagging], were individually weighed (Table 1), then moved to weaner pens.
Experimental Data Acquisition
All focal litters (12 in total) from each neonatal environment were digitally video recorded (Low-lux B/W waterproof cameras: SK-2020XC/SO, RF Concepts Ltd, Belfast, Ireland and Geovision GV-DVR, ezCCTV Ltd, Herts, UK) continuously for 4 days post-farrowing. Piglet handling was minimal during this time in order not to disrupt managerial procedures (e.g., such as fostering) or maternal behaviour. Following the initial 4 days, between 08:05 and 08:55 h every day, piglets were picked up daily and individually labelled with a number on their backs in black permanent marker (Sharpie® Magnum chisel tip). The same markers were used across all piglets, litters and neonatal environments to ensure the smell of the marker did not have varying effects on behaviour. Focal sampling occurred hourly for every piglet for 3 mins from 0800 to 1600h on Mondays, Thursdays, and Sundays up until weaning, resulting in 288 mins per piglet. From focal sample periods, we recorded the number of play invitations and rejections between initiator and receiver piglets along with the corresponding individual piglet IDs to create an adjacency matrix for each litter. As defined in (Martin et al., 2015), a play invitation was defined as a locomotor or social play behaviour directed through face-to-face body orientation to another non-playing piglet, which are often repeated and are highly energetic, and repeated invitations were counted independently. A play invitation was only classed as a play invitation if it resulted in social play behaviour between the inviter and receiver and was therefore reciprocal in nature. A play rejection was defined as the piglet responding to a play invite from another piglet, by turning its head and body at least 90° away from the “inviting” piglet, without reciprocating play behaviour (Martin et al., 2015). Observers could not be blinded to neonatal environment but were blinded to individual piglets' foster status.
Social Network Analyses (SNA)
The number of play invitations between piglets in each litter were collated to create an adjacency matrix per litter, for each of the 4 weeks prior to weaning. From this, using R (v 4.0.3, R Core Team, 2020) and using the igraph package (Csardi and Nepusz, 2006), a directed (who to whom), weighted (frequency of play invitations) social network was built for each litter per week taking into account foster and non-foster piglets and network analysis attributes were computed. Network analysis attributes included betweenness centrality and degree centrality (in, out and freeman) and were obtained using the sna package (Butts, 2020). Outputs for each attribute (Table 2) were obtained for every piglet in each litter across the 4-week pre-weaning period and were classified according to foster-status and neonatal environment for statistical analyses.
Table 2
| Network attribute | Definition | Interpretation | References |
|---|---|---|---|
| Betweenness centrality | This measures the number of shortest paths that must go through a specific node. | How important a node (piglet) is in linking one part of the network to the other. i.e., who plays with groups of piglets who otherwise do not have a close connexion to each other. High betweenness centrality indicates that the piglet connected a number of otherwise unconnected piglets. | (Wey et al., 2008; Turner et al., 2020) |
| In degree centrality Out degree centrality Degree centrality (freeman) | A node's degree centrality is a count of how many edges (i.e., social interactions) it has. In a directed network these can be separated according to direction (in or out) | How many play invitations a single node (piglet) received. How many play invitations a single node (piglet) initiated. The total number of play invitations received and initiated for a given node (piglet). | (Wey et al., 2008; Canon Jones et al., 2010; Turner et al., 2020) |
Definitions of key network analyses attributes from social network theory and their interpretation in relation to our findings.
Statistical Analyses
All statistical analyses were conducted in R (v 4.0.3, R Core Team, 2020) through R Studio (Version 1.3.1093, RStudio, PBC, 2009–2020). Generalised linear mixed models (GLMMs) via the glmmTMB package (Brooks et al., 2017) and general linear mixed models (GLMs) via the lme4 package (Bates et al., 2014) were used to identify factors which may affect network analysis attributes. Model fit was determined by examination of residuals via the DHARMa package (Hartig, 2021) and appropriate error distributions set for GLMMs. Fixed factors included neonatal environment (2 levels: Crate or Pen), foster status (2 levels: foster or non-foster) and week post-farrowing (4 levels: week 1–4) and all models included litter size as an offset to account for differences in litter size and the potential limitations this may have on network analysis attributes. We assessed significance of explanatory variables with the ANOVA function in the car package (Fox and Weisberg, 2018), with statistical significance based on p < 0.05 threshold. Following maximal model approaches (Zuur et al., 2009), all possible interactions between primary fixed effects were included in models and according to step-wise regression reduction, insignificant non-primary research fixed factor interactions were removed. Pairwise comparisons were reported using estimated marginal means via the emmeans package, with P values adjusted for multiple comparisons used the Tukey method (Lenth, 2021). All models included the unique piglet ID nested within litter as a random effect to account for repeated piglet measures and non-independence of piglets from the same litter. Litter refers to the litter in which animals are fostered into rather than the litter they were born into.
Results
Effects of Fostering
In our directed social play network, we found clear effects of fostering on all three measures of degree centrality (in = receiving play invitations, out = giving play invitations and freeman = total number of play invitations). Non-foster piglets both received [In: 8.8, p = 0.003] and initiated [Out: 31.6, p < 0.0001] more play invitations [Freeman (total): 27.1, p < 0.0001] than foster piglets overall. The effects of fostering were influenced by week post farrowing [Freeman: 30.0, p < 0.0001; In: 20.4, p < 0.0001; Out: 21.0, p < 0.0001] where foster piglets had lower degree centrality than non-foster piglets at weeks 2 and 3 irrespective of neonatal environment (Figures 3A–C).
Figure 3
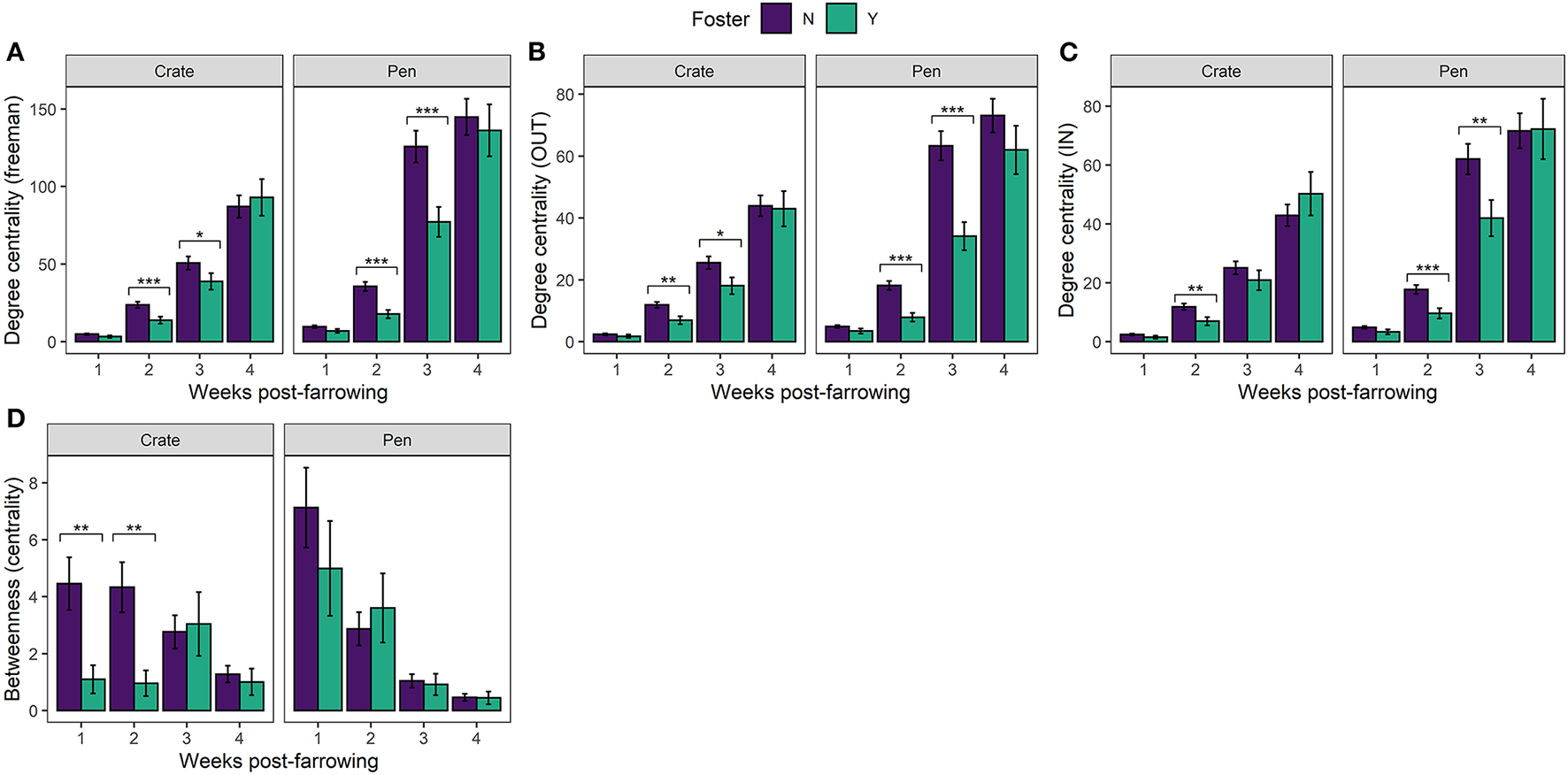
Mean (±SEM) (A) Degree centrality (freeman) (B) Degree centrality (out) (C) Degree centrality (in) (D) Betweenness centrality (Farrowing Crate or PigSAFE Pen) and foster status (foster or non-foster). Statistical significance denoted *p < 0.05, **p < 0.01, ***p < 0.001.
Effects of Neonatal Environment
In support of previous findings (Martin et al., 2015) we found overarching effects of neonatal environment, whereby compared to Crates, piglets reared in Pens showed higher degree centrality, thus played more overall [Freeman: 32.2, p < 0.0001; In: 32.8, p < 0.0001; Out: 40.4, p < 0.0001]. We also found clear effects of time (weeks post-farrowing), whereby play behaviour increased [Freeman: 4468.4, p < 0.0001; In: 2600.9., p < 0.0001; Out: 2998.4, p < 0.0001] and piglets became more connected [Betweenness centrality: 233.3, p < 0.0001] across weeks irrespective of neonatal environment and foster status.
Interaction Between Fostering and Environment Effects
We found a significant effect of fostering status between our two neonatal environments [Betweenness centrality: 4.18, p = 0.04], which differed across time [Betweenness centrality significant three-way interaction: 8.9, p = 0.03131]. In Crates, non-foster piglets were better connectors within the network compared to foster piglets at weeks 1 and 2 post-farrowing, but not at later timepoints (Figure 3D). Interestingly, the same effects of fostering on betweenness centrality were not observed with Pen reared piglets. Instead, we found no effect of foster status, across any time point post-farrowing between foster and non-foster piglets (Figure 3D). This combined with results examining degree centrality, suggests that although foster piglets have lower degree centrality than non-foster piglets, they remain equally good connectors within the network. This finding suggests that all foster piglets are interacting with the majority of non-foster piglets in the Pen neonatal environment, rather than the suggestion of segregation between foster and non-foster piglets which appears to be the case in Crates at weeks 1 and 2. This finding is further supported by the visualisation of individual social networks between our two neonatal environments across time (Figure 4).
Figure 4
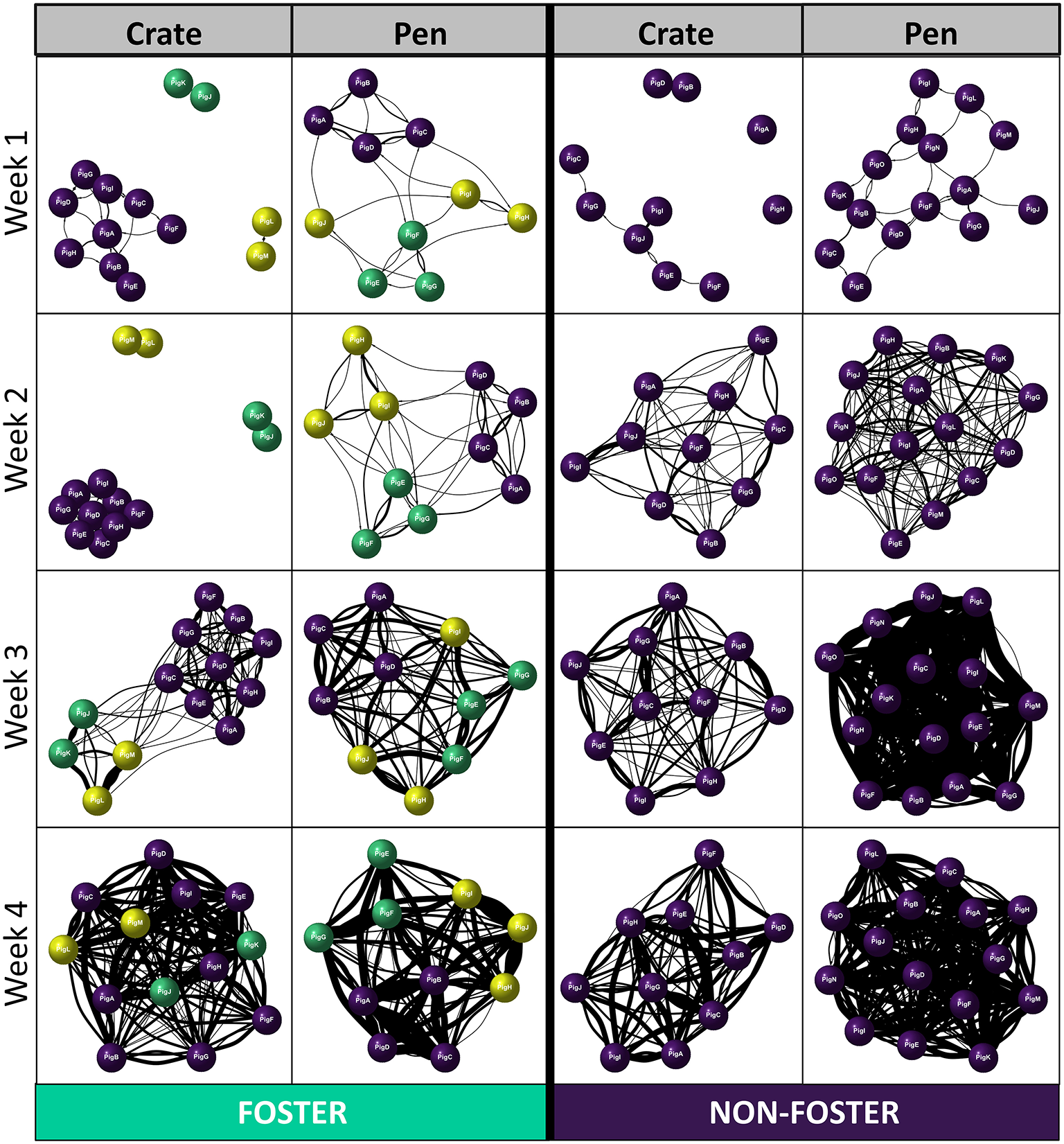
Examples of directed Social Network Analyses (SNA) across the two neonatal environments (Crate and Pen), separated according to week post-farrowing and foster status (Foster or Non-foster). Purple nodes represent non-foster piglets, green and yellow represent foster piglets; same colour denotes siblings from the same original litter. Thickness of the edges (lines) represents a greater number of play invitations between individuals.
Discussion
In the present study we observed the effects of cross-fostering on the sociality of piglet play and the influence of neonatal environment. We found evidence supporting previous findings (De Jonge et al., 1996; Brown et al., 2015; Martin et al., 2015; Weller et al., 2019) that piglets exposed to enriched neonatal environments, such as PigSAFE Pens, demonstrate more active play behaviour, through more directed play invitations, than piglets born into standard farrowing crates with limited space and enrichment provision. Piglets kept under enriched conditions (pens), both received and initiated more play invitations (higher degree centrality values) than piglets kept under relatively barren conditions (Crates), and therefore despite piglets having significantly more space and opportunity to avoid engaging in social play in Pens, we found that piglets actively chose to play more. We also found effects of cross-fostering whereby fostered piglets received and initiated fewer play invitations (lower degree centrality) 2–3 weeks post-farrowing compared to native non-foster piglets, irrespective of neonatal environment. As such, fostered piglets remained isolated from play for the first few weeks of life, however our data suggests this may be mitigated by neonatal environment. We found that foster piglets exposed to Pens were better connected (betweenness centrality) within the litter than those fostered in Crates.
Early-life environment has been shown to influence post-weaning aggression, with piglets reared under enriched conditions able to form dominance relationships more easily than those reared under barren conditions (De Jonge et al., 1996; Olsson et al., 1999). Establishing dominance is an important “peace-keeping” strategy in pigs, and despite initial aggressive encounters it can lead to stability in a group (Meese and Ewbank, 1973). It is possible that early play behaviour is influential in the prevalence of aggressive behaviour post-weaning and for speed of establishing dominance and stability (D'Eath, 2005). Martin et al. (2015) found that less play pre-weaning is associated with higher chronic aggression and greater injurious lesions and more play is associated with enriched neonatal environments. Therefore, the finding that foster piglets remain socially isolated from play could result in poorer outcomes post-weaning and into adulthood, through greater aggression and instability of the social hierarchy which may also have detrimental effects on pig health (Kanaan et al., 2008; Van Dixhoorn et al., 2016).
Our work suggests that the provision of an enriched neonatal environment could mitigate against the social isolation of foster piglets in play. Despite foster piglets receiving and initiating less play invitations than non-foster piglets overall, we found that fostered piglets reared in enriched Pens were equally as connected (betweenness centrality) as those who were non-fostered, whereas piglets fostered in farrowing Crates were not. When combined with the results assessing the number of play invitations (degree centrality), our findings suggest that foster piglets remain equally well connected within the network and are interacting with the majority of non-foster piglets when kept in enriched free-farrowing conditions such as Pens. In contrast fostered piglets reared in farrowing crates were less connected within the network for the first two weeks of life and were segregated from non-foster piglet play. Taken together, our findings suggest that piglets reared in enriched pens partake in more play behaviour irrespective of foster status, which as discussed may lead to enhanced social stability and lower aggression in later life than those reared in standard farrowing crates. However, given that this was an observational study, whereby fostering was conducted as part of routine husbandry, rather than a full systematically controlled study, several limitations, and caveats must be considered. First, we cannot fully rule out the potential confound of time (batch) and other potentially unmeasured factors associated with batch on piglet play, although numerous previous studies suggest batch is not a significant factor (Bolhuis et al., 2005; Chaloupkov et al., 2007; Martin et al., 2015; Yang et al., 2018). Second, given that the selection of foster piglets was not random (i.e., piglet vigour and weight were considered), we should be wary of extrapolating our findings. Finally, we have relatively low power as a consequence of the study being purely observational where foster piglets were only fostered as part of routine husbandry and not driven by experimental reasons resulting in relatively low numbers of foster piglets overall, as well as litters containing fosters. Therefore, taken together, although it is highly feasible that neonatal environment and foster status are causing these differences in play behaviour, our findings must be considered with these limitations in mind.
Our work is timely given the increased interest in prohibiting the use of farrowing crates (cf. European Citizen Initiative (ECI) by the “End the Cage Age” campaign and subsequent EU hearing; UK Pig Husbandry (Farrowing) Bill (2021); announcements by NZ and Germany to phase out crate use by 2027 and 2036 respectively) in favour of higher welfare alternatives that satisfy the biological needs of both the sows and piglets. Changing to higher welfare systems involves significant costs (Guy et al., 2012), however welfare enhancements can have short- and long-term benefits impacting on the performance and health of offspring (Brown et al., 2015; Van Dixhoorn et al., 2016) that could offset such investments.
Our work suggests that the provision of enriched neonatal environments and early life socialisation through piglet play may help compensate for early-life challenges, such as fostering, which involves social disruption (separation from the mother and biological siblings) during a sensitive developmental period. Fostering of piglets has become increasingly common on farms as a result of increased litter size and necessary early interventions by staff to save supernumerary piglets (Baxter et al., 2013). Who and when to foster primarily involves decisions about teat availability and piglet size but rarely considers kinship and the potential for social detriments to fosters. In this study we did consider the welfare of foster piglets prior to fostering and did not foster single piglets into a new litter, it is possible solo fostering would have resulted in more pronounced social exclusion. Given the caveats of our observational study, future research should focus on a controlled experiment systematically controlling cross-fostering techniques and neonatal environment to fully determine effective integration of foster piglets and environmental attributes contributing to increased play. Such a study would help inform possible refinement for routine practises for the cross-fostering of piglets to make evidence-based improvements to piglet welfare.
Conclusions
Our findings highlight the importance of the neonatal environment and its influence on the isolation of cross-fostered piglets. We provide more evidence for enhanced welfare of piglets reared using a free farrowing and lactation pen compared to standard commercial farrowing crates. It is possible that providing enriched neonatal environments may help mitigate against social isolation in early life of cross-fostered piglets. Furthermore, we highlight the benefit of adopting methodologies and techniques from other scientific disciplines and further advocate the use of social network theory and its application to existing animal welfare and husbandry practises. This approach helped quantify the complex relationship occurring between neonatal environment with existing cross-fostering practises, helping us understand more about their impact on positive welfare indicators such as piglet play and their subsequent influence for animal welfare.
Funding
The Roslin Institute is funded by a BBSRC Institute Strategic Program Grant BB/P013759/1. SRUC receives funding from the Scottish Government.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This project was reviewed and approved by SRUC's ethical review committee (ED AE 06/2009) and all routine animal management procedures were adhered to by trained staff.
Author contributions
JM and EB contributed to the conception and experimental design of the study. JM conducted the data collection and practical work. JC and JM conducted all data processing and analysis. JC wrote the first draught of the manuscript, with JM and EB making substantial edits to the manuscript, both in substance and style. All authors contributed to the manuscript revision and final editing.
Acknowledgments
The authors would like to thank technical and farm staff for assistance with the project, especially Sarah Ison. The work was conducted as part of a student dissertation associated with the MSc. in Applied Animal Behaviour and Animal Welfare at the University of Edinburgh. We would also like to thank the Department for Environment, Food, and Rural Affairs (Defra) of the UK for funding the PigSAFE project (AW0143) which developed the neonatal environment (PigSAFE pens).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Ahloy-DallaireJ.EspinosaJ.MasonG. (2018). Play and optimal welfare: does play indicate the presence of positive affective states?Behav. Processes156, 3–15. 10.1016/j.beproc.2017.11.011
2
AlexopoulosJ. G.LinesD. S.HallettS.PlushK. J. (2018). A review of success factors for piglet fostering in lactation. Animals8:38. 10.3390/ani8030038
3
BatesD.MächlerM.BolkerB.WalkerS. (2014). Fitting linear mixed-effects models using lme4:i01. 10.18637/jss.v067.i01
4
BaxterE. M.AdeleyeO. O.JackM. C.FarishM.IsonS. H.EdwardsS. A. (2015). Achieving optimum performance in a loose-housed farrowing system for sows: the effects of space and temperature. Appl. Anim. Behav. Sci.143, 135–149. 10.1016/j.applanim.2015.05.004
5
BaxterE. M.JarvisS.SherwoodL.FarishM.RoeheR.LawrenceA. B.et al. (2011). Genetic and environmental effects on piglet survival and maternal behaviour of the farrowing sow. Appl. Anim. Behav. Sci.130, 28–41. 10.1016/j.applanim.2010.11.020
6
BaxterE. M.RutherfordK. M. D.D'EathR. B.ArnottG.TurnerS. P.SandøeP.et al. (2013). The welfare implications of large litter size in the domestic pig II: management factors. Anim. Welf.22, 219–238. 10.7120/09627286.22.2.219
7
BolhuisJ. E.SchoutenW. G. P.SchramaJ. W.WiegantV. M. (2005). Behavioural development of pigs with different coping characteristics in barren and substrate-enriched housing conditions. Appl. Anim. Behav. Sci.93, 213–228. 10.1016/j.applanim.2005.01.006
8
BrooksM. E.KristensenK.van BenthemK. J.MagnussonA.BergC. W.NielsenA.et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J.9, 378–400. 10.32614/RJ-2017-066
9
BrownS. M.KlaffenböckM.NevisonI. M.LawrenceA. B. (2015). Evidence for litter differences in play behaviour in pre-weaned pigs. Appl. Anim. Behav. Sci.172, 17–25. 10.1016/j.applanim.2015.09.007
10
ButtsC. T. (2020). Package ‘sna’: tools for social network analysis. 35, 1–243.
11
Canon JonesH. A.HansenL. A.NobleC.DamsgardB.BroomD. M.PearceG. P. (2010). Social network analysis of behavioural interactions influencing fin damage development in Atlantic salmon (salmo salar) during feed-restriction. Appl. Anim. Behav. Sci.127, 139–151. 10.1016/j.applanim.2010.09.004
12
Chaloupkov,áH.IllmannG.Barto,šL.ŠpinkaM. (2007). The effect of pre-weaning housing on the play and agonistic behaviour of domestic pigs. Appl. Anim. Behav. Sci.103, 25–34. 10.1016/j.applanim.2006.04.020
13
CsardiG.NepuszT. (2006). The igraph software package for complex network research. Int. J. Complex Syst.1695, 1–9.
14
De JongeF. H.BokkersE. A. M.SchoutenW. G. P.HelmondF. A. (1996). Rearing piglets in a poor environment: developmental aspects of social stress in pigs. Physiol. Behav.60, 389–396. 10.1016/S0031-9384(96)80009-6
15
D'EathR. B. (2005). Socialising piglets before weaning improves social hierarchy formation when pigs are mixed post-weaning. Appl. Anim. Behav. Sci.93, 199–211. 10.1016/j.applanim.2004.11.019
16
FoisterS.Doeschl-WilsonA.RoeheR.ArnottG.BoyleL.TurnerS. (2018). Social network properties predict chronic aggression in commercial pig systems. PLoS ONE13:122. 10.1371/journal.pone.0205122
17
FoxJ.WeisbergS. (2018). An R companion to applied regression. 3rd ed. New York, NY: SAGE Publications Inc.
18
GuyJ. H. J.CainP. J. P.SeddonY. M. Y.BaxterE. M.EdwardsS. A. (2012). Economic evaluation of high welfare indoor farrowing systems for pigs. Anim. Welf.21, 19–24. 10.7120/096272812X13345905673520
19
HartigF. (2021). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.1. 0.
20
HeldS. D. E.ŠpinkaM. (2011). Animal play and animal welfare. Anim. Behav.81, 891–899. 10.1016/j.anbehav.2011.01.007
21
HorrellR. I. (1982). Immediate behavioural consequences of fostering 1-week-old piglets. J. Agric. Sci.99, 329–336. 10.1017/S0021859600030100
22
KanaanV. T.PajorE. A.LayD. C.RichertB. T.GarnerJ. P. (2008). A note on the effects of co-mingling piglet litters on pre-weaning growth, injuries and responses to behavioural tests. Appl. Anim. Behav. Sci.110, 386–391. 10.1016/j.applanim.2007.05.002
23
KirkdenR. D.BroomD. M.AndersenI. L. (2013). Invited review: Piglet mortality: management solutions. J. Anim. Sci.91, 3361–3389. 10.2527/jas.2012-5637
24
LawrenceA. B.VigorsB.SandøeP. (2019). What is so positive about positive animal welfare?—a critical review of the literature. Animals9:783. 10.3390/ani9100783
25
LenthR. V. (2021). emmeans: estimated marginal means, aka least-squares means. Am. Statistic.34, 216-221. 10.1080/00031305.1980.10483031
26
MartinJ. E.IsonS. H.BaxterE. M. (2015). The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Appl. Anim. Behav. Sci.163:22. 10.1016/j.applanim.2014.11.022
27
MeeseG. B.EwbankR. (1973). The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav.21, 326–334. 10.1016/S0003-3472(73)80074-0
28
OlssonI. A. S.De JongeF. H.SchuurmanT.HelmondF. A. (1999). Poor rearing conditions and social stress in pigs: repeated social challenge and the effect on behavioural and physiological responses to stressors. Behav. Processes46, 201–215. 10.1016/S.0376-6357(99)00036-4
29
R Core Team (2020). R: A language and environment for statistical computing.
30
RutherfordK. M. D.BaxterE. M.D'EathR. B.TurnerS. P.ArnottG.RoeheR.et al. (2013). The welfare implications of large litter size in the domestic pig I: Biologica factors. Anim. Welf.22, 199–218. 10.7120/09627286.22.2.199
31
ŠpinkaM.NewberryR. C.BekoffM. (2001). Mammalian play: training for the unexpected. Q. Rev. Biol.76, 141–168. 10.1086/393866
32
TurnerS. P.WellerJ. E.CamerlinkI.ArnottG.ChoiT.Doeschl-WilsonA.et al. (2020). Play fighting social networks do not predict injuries from later aggression. Sci. Rep.10, 1–16. 10.1038/s41598-020-72477-7
33
van de WeerdH. A.DayJ. E. L. (2009). A review of environmental enrichment for pigs housed in intensive housing systems. Appl. Anim. Behav. Sci.116, 1–20. 10.1016/j.applanim.2008.08.001
34
Van DixhoornI. D. E.ReimertI.MiddelkoopJ.BolhuisJ. E.WisselinkH. J.KoerkampP. W. G. G.et al. (2016). Enriched housing reduces disease susceptibility to co-infection with porcine reproductive and respiratory virus (PRRSV) and actinobacillus pleuropneumoniae (A. Pleuropneumoniae) in young pigs. PLoS One11:e0161832. 10.1371/journal.pone.0161832
35
WearyD. M.ApplebyM. C.FraserD. (1999). Responses of piglets to early separation from the sow. Appl. Anim. Behav. Sci.63, 289–300. 10.1016/S0168-1591(99)00021-0
36
WellerJ. E.CamerlinkI.TurnerS. P.FarishM.ArnottG. (2019). Socialisation and its effect on play behaviour and aggression in the domestic pig (Sus scrofa). Sci. Rep.9, 1–11. 10.1038/s41598-019-40980-1
37
WeyT.BlumsteinD. T.ShenW.JordánF. (2008). Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav.75, 333–344. 10.1016/j.anbehav.2007.06.020
38
YangC. H.KoH. L.SalazarL. C.LlonchL.MantecaX.CamerlinkI.et al. (2018). Pre-weaning environmental enrichment increases piglets' object play behaviour on a large scale commercial pig farm. Appl. Anim. Behav. Sci.202, 7–12. 10.1016/j.applanim.2018.02.004
39
ZuurA. F.IenoE. N.WalkerN.SavelievA. A.SmithG. M. (2009). Mixed effects models and extensions in ecology with R.6, 399–422. 10.1007/978-0-387-87458-6
Summary
Keywords
free farrowing, pigs, social play behavior, animal welfare, social network analysis, cross fostering
Citation
Clarkson JM, Baxter EM and Martin JE (2021) Who Plays With Whom: Farrowing Environment Influences Isolation of Foster Piglets in Play. Front. Anim. Sci. 2:724080. doi: 10.3389/fanim.2021.724080
Received
11 June 2021
Accepted
27 August 2021
Published
22 September 2021
Volume
2 - 2021
Edited by
Ruth Catriona Newberry, Norwegian University of Life Sciences, Norway
Reviewed by
Jamie Ahloy-Dallaire, Laval University, Canada; Marek Spinka, Czech University of Life Sciences Prague, Czechia
Updates
Copyright
© 2021 Clarkson, Baxter and Martin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica E. Martin jessica.martin@ed.ac.uk
This article was submitted to Animal Welfare and Policy, a section of the journal Frontiers in Animal Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.