- 1Department of Animal Science, Faculty of Natural and Agricultural Sciences, North-West University, Mafikeng, South Africa
- 2Food Security and Safety Focus Area, Faculty of Natural and Agricultural Sciences, North-West University, Mafikeng, South Africa
- 3School of Agricultural Sciences, Faculty of Agriculture and Natural Sciences, University of Mpumalanga, Nelspruit, South Africa
The utility of Moringa oleifera leaf meal (MOLM) as a source of biologically active substances and nutrients for Jumbo quail is limited by high concentrations of condensed tannins and fiber. Simultaneous application of polyethylene glycol (PEG), a tannin-binding compound, and exogenous fibrolytic multi-enzymes could ameliorate antinutritional effects of condensed tannins and fiber thus improving MOLM utilization in quail diets. This study investigated the effect of pre-treating dietary MOLM with PEG and fibrolytic enzymes on live performance, blood parameters, visceral organs, and carcass and meat quality characteristics in Jumbo quail. A total of 381, two-week-old quail chicks (57.5 ± 3.95 g live-weight) were randomly distributed to six dietary treatments replicated six times. The treatments were: T1 = a standard grower diet containing untreated MOLM (10%); T2 = a standard grower diet containing MOLM (10%) pre-treated with 5.4% PEG; and a standard grower diet containing MOLM (10%) pre-treated with 5.4% PEG and 1.25% (T3), 1.50% (T4), 1.75% (T5) and 2.0% (T6) fibrolytic multi-enzymes. Graded levels of enzymes did not induce linear or quadratic effects for overall feed intake, feed conversion efficiency, hematological, carcass, and meat quality parameters in response to increasing fibrolytic enzyme levels. However, weight gain in week 3 quadratically responded [R2 = 0.117, P = 0.043]. Three-week old birds reared on T3 had lower (p < 0.05) weight gains (40.9 g/bird) than those reared on the other treatment groups. Birds reared on T6 diet had longer caecum (14.1 cm) than those reared on T2, T3, T4 and T5 whose caeca lengths did not differ (P > 0.05). Birds reared on diet T5 had shorter small intestines (59.5 cm) than those reared on T1 and T2 diets. It can be concluded that simultaneous pre-treatment of dietary MOLM with PEG and fibrolytic enzymes did not improve live performance, blood parameters, and carcass and meat quality traits, but affected some visceral organ sizes in Jumbo quail.
Introduction
Sustainable Jumbo quail (Coturnix spp.) production has the potential to economically transform the poultry industry and contribute towards food and nutrition security for the rapidly growing human population. This is because the Jumbo quail has rapid growth rates, high meat-to-bone ratio, early sexual maturity (42 days of age), high reproductive rates, strong disease resistance, and low feed and space requirements (Mbhele et al., 2019; Mahlake et al., 2021). Thus, the use of phytogenic plant products in their diets would enhance feed utilization efficiency, thereby facilitating sustainable large-scale intensification for safe, healthy, and high-quality quail products (Mahlake et al., 2021). Phytogenic products such as Moringa oleifera leaf meal (MOLM) can be incorporated in Jumbo quail diets as a source of nutrients and functionally active substances (Ahmed and El-Rayes, 2019) such as protein, amino acids, omega−3 fatty acids, vitamins A and C, antioxidants, calcium, iron, potassium, and phosphorous (Onunkwo and George, 2015; Trigo et al., 2020). The antioxidant compounds (phenols, flavonoids, vitamin C, vitamin E, β-carotene, zinc, and selenium) in M. oleifera leaves are said to improve stability and the quality of meat products (Valeria and Williams, 2011). Moringa oleifera leaf meal has also been reported to reduce the activity of pathogenic bacteria and molds, thus has the potential to protect birds from subclinical infections and consequently boost their performance (Khan et al., 2022). Unfortunately, the high levels of condensed tannins (0.070 absorbance unit (AU)550 nm/200 mg) and fibre (157.5 g/kg DM) in MOLM reduces nutrient utilization, growth performance and meat quality (Mahfuz and Piao, 2019) especially when included beyond 25 g/kg in quail diets (Mulaudzi et al., 2019). Indeed, poultry birds reared on diets that have high concentrations of fiber and condensed tannins have been reported to suffer from metabolic disorders due to poor nutrient uptake (Khajali and Slominski, 2012). Undigested non-starch polysaccharides (NSP) constitute a large proportion of digesta in high fiber diets, which causes digestive upsets that result in sticky droppings and poor development in young growing birds (Desbruslais et al., 2021; Jha and Mishra, 2021). High levels of cellulose, pectin, and xylan in MOLM (David et al., 2012), causes the formation of high-molecular weight sticky clumps in the gastrointestinal tract of the birds, which delays digesta flow and ultimately affects growth performance (Wallace et al., 2010). High concentrations of condensed tannins have been linked to poor feed utilization efficiency, low growth rates, and reduced protein digestibility in poultry birds (Redondo et al., 2014; Choi and Kim, 2020). Thus, the combined use of a tannin-binding agent, polyethylene glycol (PEG), and fibrolytic enzymes could be an efficient strategy to ameliorate the antinutritional effects of condensed tannins and fiber for improved MOLM utilization, live performance, blood parameters, visceral organs, and carcass and meat quality traits in Jumbo quail. Pre-treatment of MOLM with exogenous fibrolytic enzymes could facilitate the breakdown of the cell wall matrix of moringa NSP that cannot be hydrolyzed by endogenous digestive enzymes, thus reducing their antinutritive effects (Mousa et al., 2022). On the other hand, the PEG could deactivate MOLM condensed tannins by forming PEG-tannin complexes, and as such increase nutrient bioavailability and digestibility (Hlatini et al., 2018). The hydroxyl groups in condensed tannins form hydrogen bonds with oxygen molecules from water-soluble PEG, which could explain the increase in nutrient digestibility of PEG-treated tannin-rich feeds (Abd El Tawab and Khattab, 2018). No studies have investigated the simultaneous application of PEG and exogenous fibrolytic multi-enzymes as a strategy to improve the utilization of MOLM in Jumbo quail diets. Furthermore, pre-treatment with both PEG and exogenous fibrolytic enzymes could facilitate the use of higher dietary inclusion levels of MOLM (e.g., 100 g/kg) allowing the birds to benefit from higher doses of the bioactive compounds in moringa. This study, therefore, evaluated the effect of pre-treating MOLM with PEG (5.4%) and different levels of fibrolytic enzymes on live performance, blood parameters, visceral organs, and carcass and meat quality traits in Jumbo quail. We hypothesized that pre-treating MOLM with PEG and fibrolytic enzymes would improve feed intake, physiological, and meat quality parameters of the birds.
Materials and methods
Polyethylene glycol and enzyme treatment of moringa
The MOLM was supplied by Origin Organics Investments (PTY) LTD located in Centurion (Gauteng, South Africa), whereas the PEG (Mr 4000; H−(O−CH2−CH2)n−OH) was purchased from Agro-Enviro Solutions (Gauteng, South Africa). The fibrolytic enzyme mixture (Viscozyme® L), which is a multi-enzyme complex containing xylanase, cellulase, β-glucanase, arabanase, and hemicellulase, was bought from Sigma-Aldrich (Gauteng, South Africa). The multi-enzyme had an activity of 100 fungal β-glucanase per gram and a density of 1.2 g/ml. In our previous work, pre-treating dietary MOLM with 5.4% PEG maximized feed conversion efficiency in Jumbo quail (Mulaudzi et al., 2022a), however, pre-treatment with enzyme levels up to 1% did not maximize the performance of the birds (Mulaudzi et al., 2022b) suggesting higher enzyme levels may be effective. Thus, in this study, MOLM pre-treated with 5.4% PEG was further treated with 0, 1.25, 1.50, 1.75, or 2% fibrolytic enzymes before being incorporated (10% w/w) into a standard quail diet. A standard quail diet containing untreated MOLM (10% w/w) was also included as the sixth experimental diet. The PEG solution was made by dissolving 324 g of PEG in 6 L of distilled water. For the fibrolytic enzyme pre-treatment, 62.5, 75, 87.5 and 100 ml of Viscozyme® L were dissolved in the PEG solution described above and then sprayed on MOLM (6 kg per treatment). The untreated MOLM (6 kg) was sprayed with 6 L of distilled water only, and mixing was done by hand for all the samples. The untreated and treated MOLM samples were kept at an average room temperature of 25°C for 12 h to allow PEG and the enzymes time to react with MOLM tannins and fiber, respectively. Thereafter, the untreated and treated MOLM samples were air-dried to constant weight and then milled (Polymix PX-MFC 90 D, Kinematica AG, Switzerland) before diet formulation. The proximate composition (Table 1) of untreated and PEG and fibrolytic enzyme pre-treated MOLM was determined as described by Mulaudzi et al., (2022a) and Mulaudzi et al., (2022b).
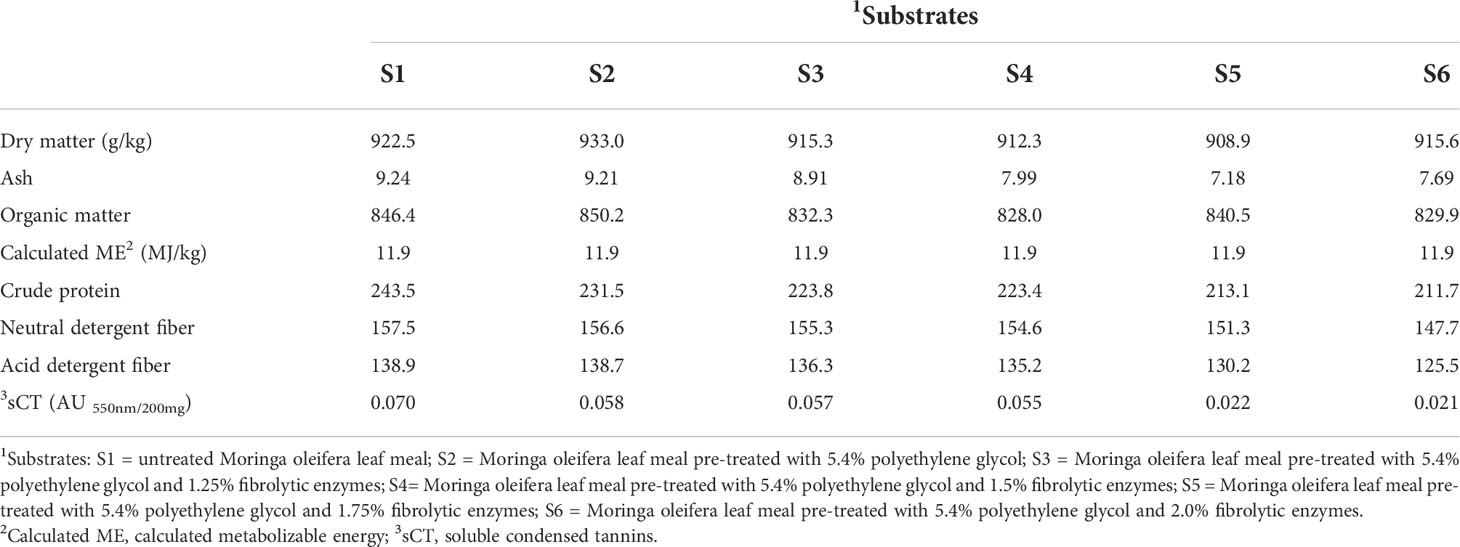
Table 1 Nutritional composition (g/kg DM, unless stated otherwise) of untreated and polyethylene glycol- and fibrolytic- enzyme pre-treated Moringa oleifera leaf meal.
Diet formulation
Six mash dietary treatments were formulated by hand to meet the nutritional requirements for grower quail as stated by the National Research Council (National Research Council, 2004) as follows: T1 = a standard grower diet containing untreated 10% MOLM; T2 = a standard grower diet containing 10% MOLM pre-treated with 5.4% PEG; and a standard grower diet containing 10% MOLM pre-treated with 5.4% PEG and 1.25% (T3), 1.50% (T4), 1.75% (T5) and 2% (T6) fibrolytic enzymes as shown in Table 2. The nutritional composition of the dietary treatments was determined as described in our previous work (Mulaudzi et al., 2022a; Mulaudzi et al., 2022b).
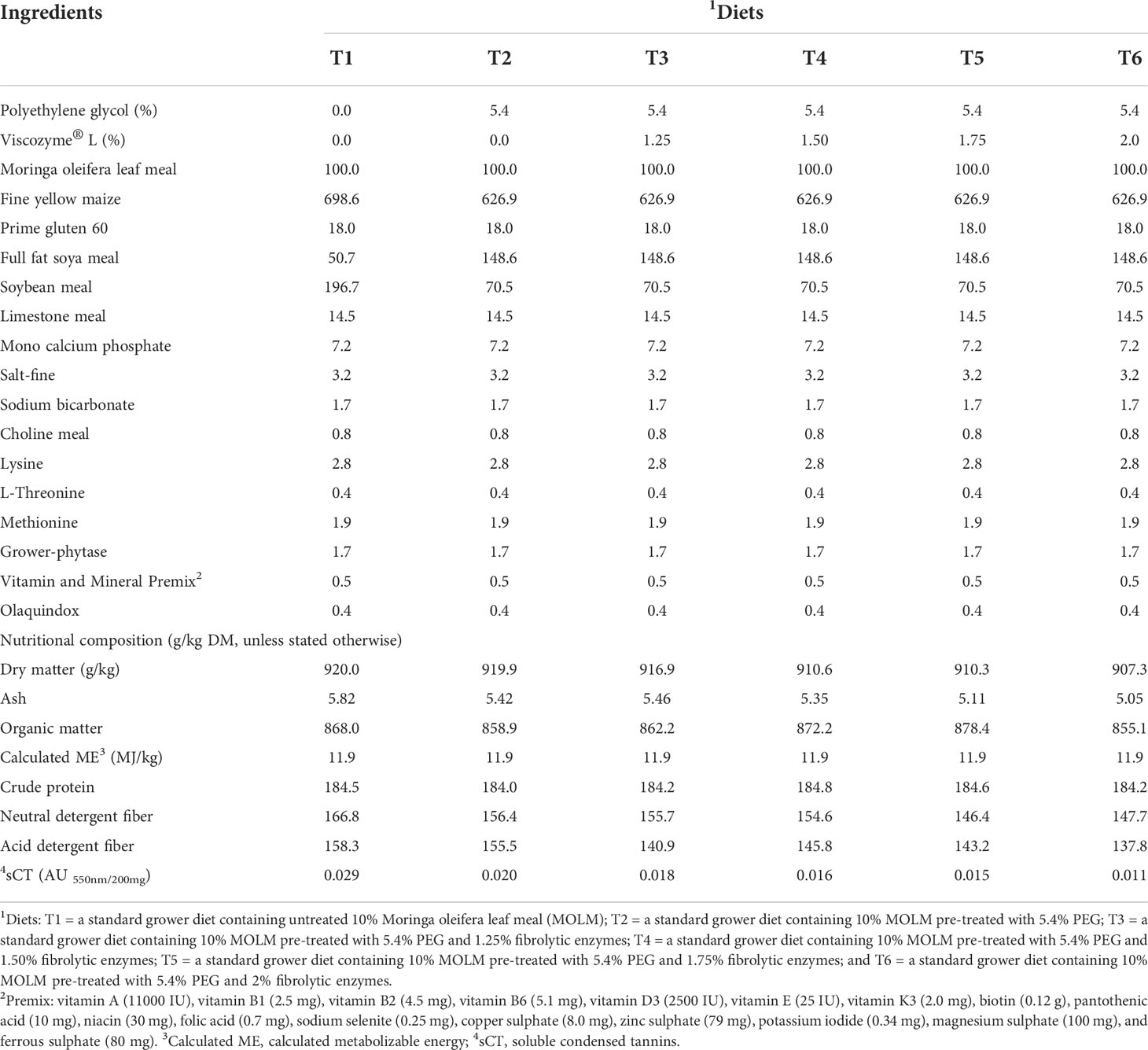
Table 2 Gross ingredient composition (g/kg as fed basis, unless stated otherwise) of the dietary treatments.
Feeding trial
The experiment was conducted in spring (September – October 2021) at North-West University Molelwane Farm (25°86′00” S; 25°64′32” E), South Africa. In a completely randomized design, 381 two-week old unsexed Jumbo quail chicks, purchased from Golden Quail Farm (Randfontein, South Africa), were evenly distributed to 36 replicate pens (experimental unit). The experimental diets were then randomly allocated to the experimental units such that each diet was replicated six times. The pens, each holding 10 or 11 chicks, were built using wire mesh (30 cm H × 60 cm W × 100 cm L) while polythene plastics were used as bedding. The birds were offered stress packs (consisting of soluble vitamins and electrolytes) for the first three days. The quail house had an average temperature of 30°C and humidity of 60%, which was monitored daily. The birds had unlimited access to the experimental diets, fresh and clean water for the entire feeding period, and natural lighting as a source of light (06h00 - 18h00). Initial live weights (57.5 ± 1.72 g) were recorded at two weeks of age and then measured weekly until six weeks of age by weighing all the birds in a pen to establish average weekly body weight gain (ABWG). From week 2 to week 6, the average weekly feed intake (AWFI) was calculated by subtracting the weight of feed refusals from the weight of feed supplied. Feed conversion efficiency (FCE) was calculated as a proportion of weight gain to feed intake. The growth performance data was adjusted using the six mortality values randomly observed from the treatment groups during the feeding trial.
Blood collection and analyses
At day 42 of age, all the birds in each experimental unit were weighed to determine final body weight (FBW). The birds per pen were then transferred to well-ventilated crates and then transported by road to a local poultry abattoir. Upon arrival, the birds were allowed to rest for a period of 1 – 2 hours with access to drinking water. Thereafter, the birds were stunned and slaughtered by cutting the jugular vein. While bleeding, 2 ml of fresh blood samples were randomly collected from two birds per experimental unit and immediately transferred into whole blood tubes (with EDTA) and serum tubes for analyses of hematological and serum biochemical parameters, respectively. The blood samples were handled, processed, and stored as described by Washington and van Hoosier (2012). Hematological parameters (erythrocytes, hematocrits, hemoglobin, mean corpuscular hemoglobin, mean corpuscular volume) were determined using the automated Hematology Analyzer (model no. 93-30001-01), whereas biochemical parameters (albumin, alkaline phosphatase (ALKP), alanine transaminase (ALT), amylase, blood glucose, calcium, creatinine, globulin, lipase, phosphorus and total protein) were analyzed using the automated Vet Test Chemistry Analyzer (model no. 89-92525-00) from IDEXX Laboratories (Pty) Ltd (Gauteng, South Africa), respectively.
Carcass, internal organs, and meat quality
All carcasses were weighed to determine hot carcass weight (HCW) and then re-weighed after being chilled in a cold room for 24 hours to determine cold carcass weight (CCW). The proportion of HCW on FBW was used to measure the carcass yield. All carcass parts (breast, wing, drumstick, and thigh) and internal organs (liver, gizzard, proventriculus, and small intestine) were weighed using a digital weighing scale (Explorer® EX224, OHAUS Corp, US) and expressed as a percentage of the HCW. Breast meat lightness (L*), redness (a*), and yellowness (b*) were taken 1 hour and 24 hours post-mortem using a color spectrophotometer (CM 2500c, Konika Minolta, Osaka, Japan) according to the Commission Internationale del’Eclairage (CIE, 1976). Hue angle and chroma values were calculated using a* and b* as described by Priolo et al. (2002). Meat pH was measured on the pectoralis major muscle 1 hour and 24 hours post-mortem using a pH-temperature meter (Corning Glass Works, Medfield, MA, USA), which was calibrated using standard pH solutions after each experimental unit. Breast meat samples were pre-weighed and then cooked to a core temperature of 75°C to determine cooking loss (Honikel, 1998). The filter-paper press method was used to assess the water holding capacity (WHC) of breast meat slices held under pressure of 60 kg in between two filter papers (Grau and Hamm, 1957). Drip loss was determined following the methods described by Honikel (1998) where breast meat slices are hanged at 1 – 4°C for 3 days.
Statistics
Regression analysis (Proc RSREG; SAS, 2010) was employed to evaluate the coefficients for linear and quadratic effects of all measured parameters in response to the increasing levels of fibrolytic enzymes. The T1 data were not used in the regression analysis. Weekly measured data (feed intake, weight gain, and FCE) were analyzed using repeated measures analysis option in the general linear model statement of SAS (2010) to determine interaction effects between diet and time (bird age in weeks). Data for overall feed intake, live performance, blood parameters, visceral organs, and carcass and meat quality traits were analyzed using one-way ANOVA (PROC GLM; SAS, 2010) with diet as the only factor. Significance was declared at P < 0.05 and treatment means were separated using the probability of difference option in SAS.
Results
Repeated measures analysis showed a significant week × diet interaction effect on ABWG (P = 0.001), but not on AWFI (P = 0.356) and FCE (P = 0.321). Table 3 shows that neither linear or quadratic effects (P > 0.05) were recorded for overall feed intake and FCE as enzyme levels increased. However, weight gain in three-week-old quail quadratically responded [y = 53.18 (± 4.63) – 0.198 ( ± 0.081) x + 0.000848 ( ± 0.000324) x2; R2 = 0.248, P = 0.016] to increasing enzyme pre-treatment levels. Similarly, dietary influences (P < 0.05) were only observed on weight gain in week 3. Three-week-old birds reared on diet T3 had lower (P < 0.05) weight gain compared to birds reared on the other treatment groups, whose weight gain did not differ (P > 0.05).

Table 3 Effect of pre-treating dietary Moringa oleifera leaf meal with polyethylene glycol and fibrolytic enzymes on growth performance in Jumbo quail.
Table 4 indicates that there were neither linear nor quadratic effects (P > 0.05) for all the blood parameters. Similarly, no significant dietary effects were observed on the blood parameters (P > 0.05) of Jumbo quail.
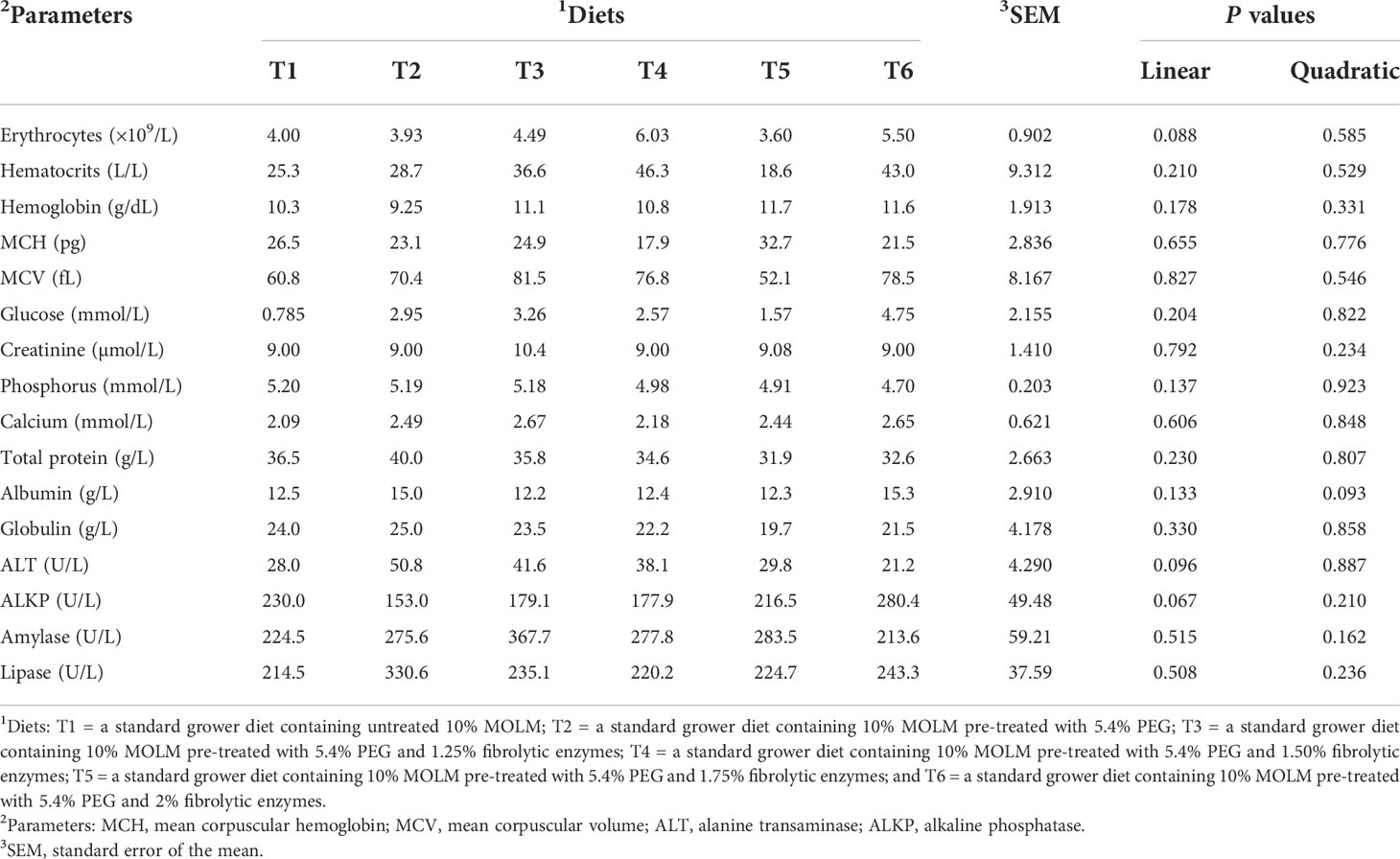
Table 4 Effect of pre-treating dietary Moringa oleifera leaf meal with polyethylene glycol and fibrolytic enzymes on blood parameters in Jumbo quail.
Pre-treatment of dietary MOLM with incremental levels of fibrolytic enzymes showed no significant linear or quadratic effects for carcass characteristics in Jumbo quail (Table 5). Similarly, no significant dietary effects were observed on all carcass traits. A linear decrease was observed for small intestine length [y = 64.75 (± 4.390) – 0.004 (± 0.077) x; R2 = 0.215, P = 0.028] and a quadratic effect was observed for caecum length [y = 15.37 (± 3.281) – 0.112 (± 0.058) x – 0.0005 (± 0.0002) x2; R2 = 0.182; P = 0.039] in response to incremental levels of fibrolytic enzymes. Birds reared on T6 diet had longer caeca (14.1 cm) than those reared on T2, T3, T4 and T5, whose caeca lengths did not differ (P > 0.05). The control diet T1 promoted similar (P > 0.05) caeca length as the T6 diet. Birds reared on diet T5 had shorter small intestines (59.5 cm) than those reared on T1 and T2 diets. The T2 diet promoted similar (P > 0.05) small intestine lengths as the T1, T3, T4 and T6 diets.

Table 5 Effect of pre-treating dietary Moringa oleifera leaf meal with polyethylene glycol and fibrolytic enzymes on carcass characteristics and size of internal organs (%HCW, unless stated otherwise) in Jumbo quail.
Table 6 indicates that there were neither linear nor quadratic effects (P > 0.05) for all the meat quality parameters. Similarly, no significant dietary effects were observed on meat quality parameters (P > 0.05).
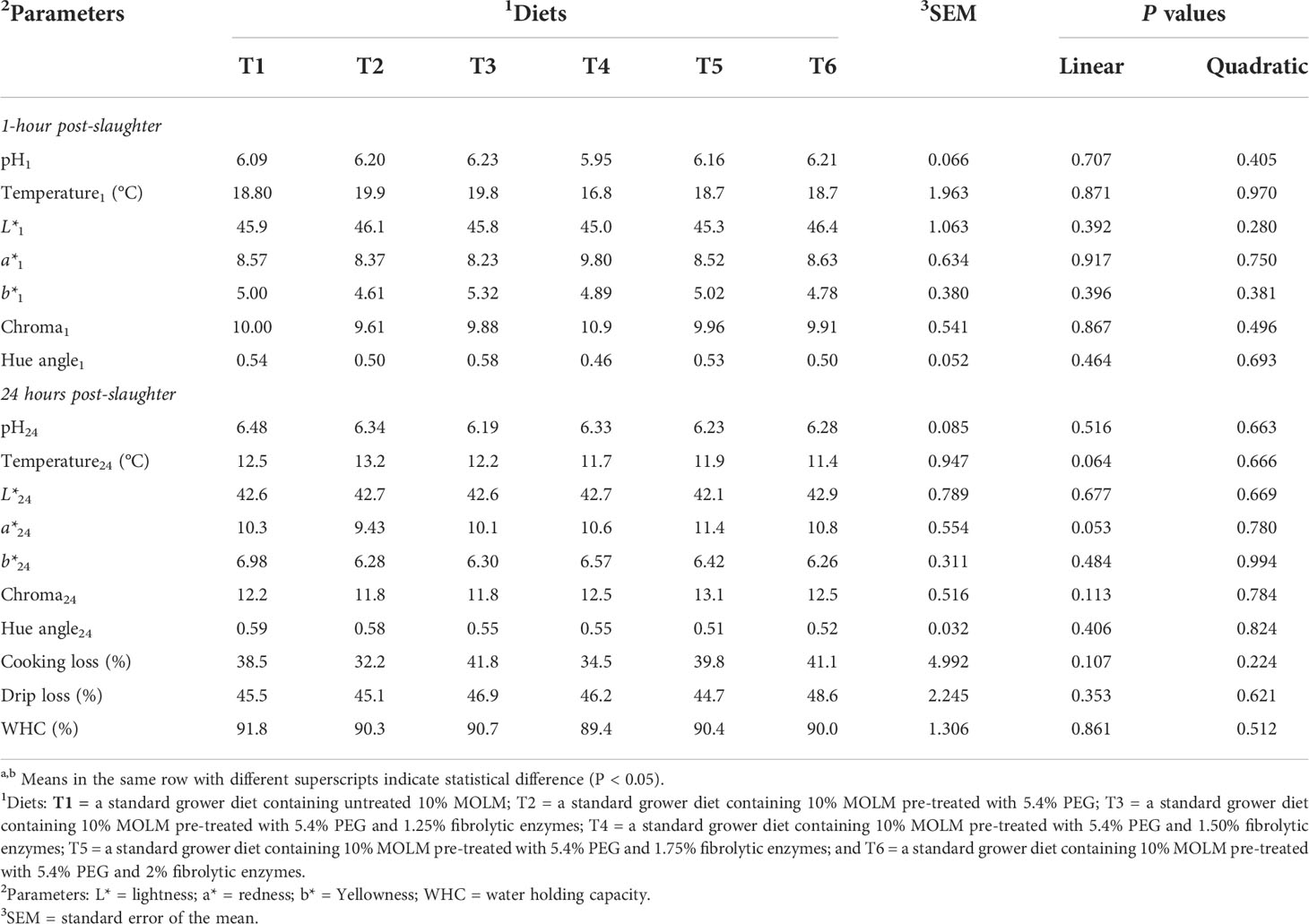
Table 6 Effect of pre-treating dietary Moringa oleifera leaf meal with polyethylene glycol and fibrolytic enzymes on breast meat quality parameters in Jumbo quail.
Discussion
Moringa oleifera leaf meal (MOLM) can supplement Jumbo quail diets with bioactive substances and nutrients required for optimum production. However, the utility of the meal is limited by high levels of fiber and condensed tannins. In our previous study, the inclusion of dietary MOLM beyond 25 g/kg in quail diets had adverse effects on feed utilization efficiency and growth performance traits (Mulaudzi et al., 2019). Higher dietary MOLM inclusion levels in Jumbo quail diets would promote sustainable intensification of the birds but require pre-treatment to ameliorate the antinutritional activities of fiber and condensed tannins. In the current study, repeated measures analysis revealed a significant diet x week interaction effect on weight gain only, indicating that as the birds grew older the ranking of dietary treatments in terms of weight gain changed. It is not clear why the birds fed with diet T3 had lower weight. Indeed, no dietary differences were observed in weeks 4, 5, and 6. Thus, the reasons for the reduced weight gain in the T3 group are unknown. In addition, pre-treatment of MOLM with PEG and fibrolytic enzymes had no effect on overall feed intake and feed conversion efficiency, signifying that both additives did not improve the feed value of MOLM. This further suggests that there is no benefit in pre-treating high dietary MOLM (100 g/kg) levels with PEG and enzymes as it does not enhance growth performance traits in Jumbo quail. Similar findings were reported by Kumanda et al. (2019), who reported a lack of improvement on growth performance of broiler chickens upon pre-treatment of red grape pomace waste with PEG and fibrolytic enzymes. Similarly, Lee et al. (2014) and Abolade (2016) did not observe any significant improvement on growth performance parameters of broiler chickens reared on enzyme-supplemented diets. Although these feed additives have been proven to individually improve nutrient utilization and growth performance (Kaczmarek et al., 2015; Aziz ur Rahman et al., 2021), it should be noted that different methods of application could account for variation in their modes of action and ameliorative activities. This could be the reason why there are inconsistent results reported in literature regarding their effectiveness in improving poultry performance.
The evaluation of blood parameters is critical in determining the impact of untreated and PEG- and fibrolytic enzyme-pretreated MOLM on the pathophysiological and health status of the birds (Saki et al., 2017). Because dietary MOLM contains antinutritional components such as condensed tannins and insoluble fibers, it was expected that their inclusion at a rate of 100 g/kg would have a negative impact on hematological and biochemical parameters. However, the selected blood indices measured in this study revealed that untreated and PEG- and enzyme-treated MOLM had no effect on the birds’ pathophysiology and health status, with all the blood values falling within the values reported for healthy quail (Mbhele et al., 2019; Mahlake et al., 2021). The lack of negative effects suggests that higher dietary inclusion levels of MOLM do not compromise the well-being of Jumbo quail. Indeed, Cañedo-Castro et al. (2019) reported that serum biochemical indices provide useful information on the health and nutritional status of animals fed non-conventional feed ingredients. This further indicates that the Jumbo quail did not suffer from nutritional deficiencies. Moreover, liver enzymes such as ALKP and ALT were unaffected signifying that the birds did not suffer from diet-induced toxicosis (Bona et al., 2018).
Changes in gastrointestinal morphology associated with variation in the concentration of dietary fiber have been reported (Jha et al., 2019). Supplementation with fibrolytic multi-enzyme tended to reduce the fiber content of the diets, which could explain the linear decrease in the lengths of small intestines. The negative quadratic response observed for caecum length could also point to the ineffectiveness of the enzyme to improve fiber utilization by the birds. No dietary influences were observed on the sizes of liver, gizzard, and proventriculus, which was also surprising given that the diet containing untreated MOLM tended to contain high levels of fiber and condensed tannins. The profitability of poultry production has been strongly related to the improvements in carcass yield and composition. Thus, understanding factors that affect carcass characteristics is important for the success of any poultry enterprise. Unfortunately, knowledge of such factors in quail birds is limited (Dyubele et al., 2010). The supplementation with PEG and enzymes had no effect on carcass parameters, which is consistent with the findings of Alefzadeh et al. (2016) and Taheri and Shirzadegan (2017), who reported no significant differences in carcass traits of broilers reared on diets supplemented with exogenous enzymes.
The appearance, texture, and sensory characteristics of poultry and other meat products all contribute to their overall consumer acceptability (Muchenje et al., 2009; Yu et al., 2017). The color of the meat is the most important indicator when consumers buy meat products and is the major factor that affects consumer acceptance of the meat (Muchenje et al., 2009). Consumers tend to reject the products based on color variations from the expected norm. However, no color variations were observed in all the treatment groups, suggesting that PEG and fibrolytic enzymes do not affect the appearance of the meat. Meat pH is primarily related to the biochemical state of the muscle at the time of slaughter, resulting in the onset of rigor mortis (Ding et al., 2022). Chan et al. (2011) discovered a correlation between the pH of the meat and its color, with lighter meat indicating low pH, while dark meat indicating a high pH value. Kralik et al. (2018) reported that low pH in broiler meat stimulates the oxidation of myoglobin (pink color) and oxyhemoglobin (red color) to metamyoglobin (brown meat color). However, the dietary treatments in this study had no effect on meat pH, which further confirm that the addition of PEG and enzymes does not alter post-mortem pH. Water holding capacity refers to the ability of the meat to retain water when external forces such as cutting, grinding, and pressing are applied (Bowker and Zhuang, 2015). The degree of water lost or retained depends mainly on the pH of the tissue for example a higher pH will result in less water loss, while a lower pH value would determine the amount of water retained by the meat (Warner, 2017). When the pH is low in chicken meat it will lead to protein denaturation, which will further reduce the WHC of the meat (Singh and Deshpande, 2018). Thus, the lack of dietary differences on meat pH could explain why the meat had similar water-binding potential. Indeed, no differences were observed on cooking loss and drip loss, which further indicate that PEG and enzyme supplementation do not compromise the organoleptic (juiciness, tenderness, texture, smell and color) and sensorial qualities (Maison et al., 2016; Choi et al., 2019) of quail meat.
Conclusion
Pre-treatment of dietary M. oleifera leaf meal with 5.4% PEG and up to 2% fibrolytic enzymes do not improve live performance, blood, carcass and meat quality parameters, but affects some visceral organ sizes in Jumbo quail. In addition, an optimum could not be determined using the growth performance data, indicating a need to investigate levels beyond 2.0% of the fibrolytic enzyme to generate non-linear responses. Information on M. oleifera leaf meal treated with polyethylene glycol and fibrolytic enzymes is limited thus further studies are required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The feeding trial, handling and slaughtering of the birds were approved (NWU-01884-19-S5) by the Animal Production Research Ethics Committee of the North-West University.
Author contributions
AM, CM, and VM designed the study. AM conducted the experiment and analyzed the data. CM and VM supervised the project. AM, CM, and VM were equally involved in the write-up of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the South African National Research Foundation (NRF (ZA), grant number: 121399). The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El Tawab A. M., Khattab M. S. A. (2018). Utilization of polyethylene glycol and tannase enzyme to reduce the negative effect of tannins on digestibility, milk production and animal performance. Asian. J. Anim. Vet. Adv. 13, 201–209. doi: 10.3923/ajava.2018.201.209
Abolade O. J. (2016). Effect of protease supplementation on the performance of laying chickens fed marginally deficient protein diet. J. Manage. Stud. 2 (11), 20–29. doi: 10.4025/actascianimsci.v37i1.22830
Ahmed W. F., El-Rayes T. K. (2019). Effect of using Moringa oleifera leaves on productive performance and some physiological parameters of Japanese quail. Egypt. Poult. Sci. J. 39 (1), 193–205. doi: 10.21608/epsj.2019.29811
Alefzadeh T., Bouyeh M., Van den Hoven R., Seidavi A., Laudadio V., Tufarelli V. (2016). Effect of dietary dried orange (Citrus sinensis) peel powder and exogenous multienzymes on growth and carcass traits and ileal microflora of broiler chickens. Pak. J. Zool. 48 (6), 1891–1897. doi: 10.7482/0003-9438-56-002
Aziz ur Rahman M., Jamal U., Anwar U., Bilal M. Q., Riaz M., Hussain M., et al. (2021). Effects of potato peels inclusion with exogenous enzymes in broiler diet on growth performance, nutrients digestibility and carcass characteristics. Sci. Prog. 104 (4), 1–14. 00368504211061972. doi: 10.1177/00368504211061972
Bona L., Van Staaveren N., Pokharel B. B., Van Krimpen M., Harlander-Matauschek A. (2018). The effect of low protein energy-rich diets on plasma hepatic markers, hepatic damage, and discrimination reversal learning in young female chicks. Front. Vet. Sci. 5. doi: 10.3389/fvets.2018.00107
Bowker B., Zhuang H. (2015). Relationship between water-holding capacity and protein denaturation in broiler breast meat. J. Poult. Sci. 94 (7), 1657–1664. doi: 10.3382/ps/pev120
Cañedo-Castro B., Piñón-Gimate A., Carrillo S., Ramos D., Casas-Valdez M. (2019). Prebiotic effect of ulva rigida meal on the intestinal integrity and serum cholesterol and triglyceride content in broilers. J. Appl. Phycol. 31, 3265–3273. doi: 10.1007/s10811-019-01785-x
Chan J. T., Omana D. A., Betti M. (2011). Effect of ultimate pH and freezing on the biochemical properties of proteins in turkey breast meat. Food Chem. 127 (1), 109–117. doi: 10.1016/j.foodchem.2010.12.095
Choi Y. M., Garcia L. G., Lee K. (2019). Correlations of sensory quality characteristics with intramuscular fat content and bundle characteristics in bovine longissimus thoracis muscle. Food Sci. Anim. Resour. 39 (2), 197–208. doi: 10.5851/kosfa.2019.e15
Choi J., Kim W. K. (2020). Dietary application of tannins as a potential mitigation strategy for current challenges in poultry production: A review. Animals 10 (12), 2389. doi: 10.3390/ani10122389
CIE (1976). Recommendations on uniform color spaces, color-difference equations, psychometric color terms; supplement no. 2 to CIE publication no. 15 (E-1.3.1.) 1978, 1971/(TC-1-3) (Paris, France: Commission Internationale del’Eclairage).
David L. S., Vidanarachchi J. K., Samarasinghe K., Cyril H. W., Dematawewa C. M. B. (2012). Effects of moringa based feed additives on the growth performance and carcass quality of broiler chicken. Trop. Agric. Res. 24 (1), 12–20. doi: 10.4038/tar.v24i1.7985
Desbruslais A., Wealleans A., Gonzalez-Sanchez D., Di Benedetto M. (2021). Dietary fibre in laying hens: a review of effects on performance, gut health and feather pecking. World Poult. Sci. J. 77 (4), 797–823. doi: 10.1080/00439339.2021.1960236
Ding Z., Wei Q., Liu C., Zhang H., Huang F. (2022). The quality changes and proteomic analysis of cattle muscle postmortem during rigor mortis. Foods 11 (2), 217. doi: 10.3390/foods11020217
Dyubele N. L., Muchenje V., Nkukwana T. T., Chimonyo M. (2010). Consumer sensory characteristics of broiler and indigenous chicken meat: A south African example. Food Qual. Prefer. 21 (7), 815–819. doi: 10.1016/j.foodqual.2010.04.005
Grau R., Hamm R. (1957). About the water-binding capacity of the mammalian muscle. II. communication. Z Lebensm Unters Briskly. 105, 446. doi: 10.1007/BF01126901
Hlatini V. A., Ncobela C. N., Zindove T. J., Chimonyo M. (2018). Use of polyethylene glycol to improve the utilisation of leguminous leaf meals in pigs: a review. S. Afr. J. Anim. Sci. 48 (4), 609–620. doi: 10.4314/sajas.v48i4.2
Honikel K. O. (1998). Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49, 447–570. doi: 10.1016/s0309-1740(98)00034-5
Jha R., Fouhse J. M., Tiwari U. P., Li L., Willing B. P. (2019). Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 6. doi: 10.3389/fvets.2019.00048
Jha R., Mishra P. (2021). Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechnol. 12, 51. doi: 10.1186/s40104-021-00576-0
Kaczmarek S. A., Bochenek M., Samuelsson A. C., Rutkowski A. (2015). Effects of glyceryl polyethylene glycol ricinoleate on nutrient utilisation and performance of broiler chickens. Arch. Anim. Nutr. 69 (4), 285–296. doi: 10.1080/1745039x.2015.1061722
Khajali F., Slominski B. A. (2012). Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 91 (10), 2564–2575. doi: 10.3382/ps.2012-02332
Khan R. U., Naz S., Raziq F., Qudratullah Q., Khan N. A., Laudadio V., et al. (2022). Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet. Environ. Sci. pollut. Res. 29, 32594–32604. doi: 10.1007/s11356-022-19241-8
Kralik G., Kralik Z., Grčević M., Hanžek D. (2018). Quality of chicken meat. Anim. Husb. Nutr. 63. doi: 10.5772/intechopen.72865
Kumanda C., Mlambo V., Mnisi C. M. (2019). Valorization of red grape pomace waste using polyethylene glycol and fibrolytic enzymes: Physiological and meat quality responses in broilers. Animals 9 (10), 779. doi: 10.3390/ani9100779
Lee K. W., Choi E. J., Moon S. T., Lee H., Kang C. W., An B. K. (2014). Evaluation of dietary multiple enzyme preparation (Natuzyme) in laying hens. Asian-australas. J. Anim. Sci. 27 (2), 1749–1754. doi: 10.5713/ajas.2014.14294
Mahfuz S., Piao X. S. (2019). Application of moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 9 (7), 431. doi: 10.3390/ani9070431
Mahlake S. K., Mnisi C. M., Lebopa C., Kumanda C. (2021). The effect of green tea (Camellia sinensis) leaf powder on growth performance, selected hematological indices, carcass characteristics and meat quality parameters of jumbo quail. Sustainability 13 (13), 7080. doi: 10.3390/su13137080
Maison A., Abdullah B., Muradov M., Korostynska O., Al-Shamma A., Bjarnadottir S. T., et al. (2016). Theoretical basis and application for measuring pork loin drip loss using microwave spectroscopy. Sensors 16, 1–13. doi: 10.3390/s16020182
Mbhele F. G. T., Mnisi C. M., Mlambo V. (2019). A nutritional evaluation of insect meal as a sustainable protein source for jumbo quails: Physiological and meat quality responses. Sustainability 11 (23), 6592. doi: 10.3390/su11236592
Mousa G. A., Allak M. A., Shehata M. G., Hashem N. M., Hassan O. G. (2022). Dietary supplementation with a combination of fibrolytic enzymes and probiotics improves digestibility, growth performance, blood metabolites, and economics of fattening lambs. Animals 12 (4), 476. doi: 10.3390/ani12040476
Muchenje V., Dzama K., Chimonyo M., Strydom P. E., Hugo A., Raats J. G. (2009). Some biochemical aspects pertaining to beef eating quality and consumer health: A review. Food Chem. 112, 279–289. doi: 10.1016/j.foodchem.2008.05.103
Mulaudzi A., Mnisi C. M., Mlambo V. (2019). Dietary Moringa oleifera leaf meal improves growth performance but not haemo-biochemical and meat quality parameters in female Japanese quails. Pak. J. Nutr. 18, 953–960. doi: 10.3923/pjn.2019.953.960
Mulaudzi A., Mnisi C. M., Mlambo V. (2022a). Effect of pre-treating dietary Moringa oleifera leaf powder with fibrolytic enzymes on physiological and meat quality parameters in jumbo quail. Poultry 1 (2), 54–65. doi: 10.3390/poultry1020006
Mulaudzi A., Mnisi C. M., Mlambo V. (2022b). Enhancing the utility of dietary Moringa oleifera leaf meal for sustainable jumbo quail (Coturnix sp.) production. Sustainability 14 (9), 5067. doi: 10.3390/su14095067
National Research Council (2004). Nutrient requirements of poultry. 9th ed (Washington, DC, USA: NRC; National Academy Press).
Onunkwo D. N., George O. S. (2015). Effects of leaf meal on the growth performance and carcass characteristics of broiler birds. J. Agric. Vet. Sci. 8, 63–66. doi: 10.9790/2380-08326366
Priolo A., Micol D., Agabriel J., Prache S., Dransfield E. (2002). Effect of grass or concentrate feeding systems on lamb carcass and meat quality. Meat Sci. 62, 179–185. doi: 10.1016/s0309-1740(01)00244-3
Redondo L. M., Chacana P. A., Dominguez J. E., Fernandez Miyakawa M. E. D. (2014). Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 5. doi: 10.3389/fmicb.2014.00118
Saki A. A., Goudarzi S. M., Ranjbaran M., Ahmadi A., Khoramabadi V. (2017). Evaluation of biochemical parameters and productive performance of Japanese quail in response to the replacement of soybean meal with canola meal. Acta Sci. Anim. Sci. 39, 51–56. doi: 10.4025/actascianimsci.v39i1.31487
Singh R. K., Deshpande D. (2018). Thermally induced changes in quality of chicken breast meat protein fractions. J. Nutr. Food Sci. 8, 709. doi: 10.4172/2155-9600.1000709
Taheri H. R., Shirzadegan K. (2017). Multiple-enzyme supplementation on digestive traits, carcass characteristics, blood lipid parameters and growth performance of broilers fed a wheat-based diet. Asian-Australas. J. Anim. Sci. 30 (9), 1285–1291. doi: 10.5713/ajas.16.0415
Trigo C., Castello M. L., Ortola M. D., Garcia-Mares F. J., Desamparados Soriano M. (2020). Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 10 (1), 31. doi: 10.3390/foods10010031
Valeria V., Williams P. (2011). Improving meat quality through natural antioxidants. Chil. J. Agric. Res. 71, 2. doi: 10.4067/S0718-58392011000200017
Wallace R. J., Oleszek W., Franz C., Hahn I., Baser K. H. C., Mathe A., et al. (2010). Dietary plant bioactives for poultry health and productivity. Br. Poult. Sci. 51, 461–487. doi: 10.1080/00071668.2010.506908
Warner R. D. (2017). “The eating quality of meat-IV water-holding capacity and juiciness,” in Lawrie’s meat science (Sawston, United Kingdom: Woodhead Publishing), 419–459. doi: 10.1016/B978-0-08-100694-8.00014-5
Washington I. M., van Hoosier G. (2012). Clinical biochemistry and haematology (Seattle, WA, USA: University of Washington), 59–91. doi: 10.1016/B978-0-12-380920-9.00003-1
Yu Q. P., Feng D. Y., Xiao J., Wu F., He X. J., Xia M. H., et al. (2017). Studies on meat color, myoglobin content, enzyme activities, and genes associated with oxidative potential of pigs slaughtered at different growth stages. Asian-Australas. J. Anim. Sci. 30 (12), 1739–1750. doi: 10.5713/ajas.17.0005
Keywords: blood parameters, fiber, fibrolytic enzymes, growth, meat quality, polyethylene glycol, quail, tannins
Citation: Mulaudzi A, Mnisi CM and Mlambo V (2022) Simultaneous pre-treatment of dietary Moringa oleifera leaf meal with polyethylene glycol and fibrolytic enzymes: Effect on growth performance, physiological indices, and meat quality parameters in jumbo quail. Front. Anim. Sci. 3:960233. doi: 10.3389/fanim.2022.960233
Received: 02 June 2022; Accepted: 15 August 2022;
Published: 02 September 2022.
Edited by:
Aristide Maggiolino, University of Bari Aldo Moro, ItalyReviewed by:
Felicitas Mukumbo, Rwanda Institute for Conservation Agriculture (RICA), RwandaIfeanyi Ogbuewu, Federal University of Technology Owerri, Nigeria
Copyright © 2022 Mulaudzi, Mnisi and Mlambo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caven Mguvane Mnisi, MjMyNTc1MzlAbnd1LmFjLnph
 Anzai Mulaudzi
Anzai Mulaudzi Caven Mguvane Mnisi
Caven Mguvane Mnisi Victor Mlambo
Victor Mlambo