- 1Agriculture and Food, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Armidale, NSW, Australia
- 2School of Environmental and Rural Science, University of New England, Armidale, NSW, Australia
The weaning of beef calves in yards places multiple stressors on the animals, and environmental enrichment may help mitigate some of these stressors and improve animal welfare. This trial assessed the impacts of enrichment provision to beef calves during yard weaning using measures of biological functioning, behaviour, and affective state. Overall, calves utilised the brush more than the other provided enrichments, which were a hanging rope and a ball. Enrichment influenced the behaviours of calves during both an attention bias test, with enriched calves exhibiting behaviours associated with greater anxiety, and a novel object recognition test, with enriched calves spending less time interacting with objects. In their home pens, enriched calves performed more drinking and grooming behaviours. However, no significant differences were seen between treatments for body weight, faecal cortisol metabolites, and internal body temperature. Enrichment also did not influence any longer-term measurements of body weight, flight speed, or crush score. The study design was impacted by mud, requiring the regrouping of the animals. Thus, some results should be interpreted with caution. Overall, this study demonstrates that further work is required into the assessment of affective states for these animals as it could not be confirmed whether the results seen indicate that calf welfare was improved or impaired through enrichment provision.
1 Introduction
Environmental enrichment has been defined as a modification to a captive animal’s environment resulting in an improvement in its biological functioning. This can be indicated by improved physical health, which is a prerequisite for other measures of biological functioning such as increased lifetime reproductive success and inclusive fitness (Newberry, 1995). Effective enrichment also aims to increase the range of species-specific behavioural expression and the animal’s ability to cope with behavioural and physiological challenges (Shepherdson, 2003; Young, 2003; Riber et al., 2018). Across a production cycle for livestock animals, there may be periods of adversity where enrichment provision could be a management strategy to facilitate adaptability. In cattle, weaning from the mother is an example of a significant challenge experienced by young calves. In dairy cattle, separation from the mother typically occurs within hours of birth, with calves often housed individually before later being weaned from milk (Weary et al., 2008). In contrast, beef cattle are typically weaned at 5–8 months of age (Meat and Livestock Australia, 2020), both separation from the mother and weaning from milk occur simultaneously, and they are weaned as a group and housed together. Therefore, weaning is a multifactorial stressor on beef calves that includes social, nutritional, physical, and psychological stressors (Weary et al., 2008; Lynch et al., 2019). Enrichment provision during this period may help mitigate some of these stressors.
There have been multiple studies on the effects of environmental enrichments for dairy calves around weaning. However, as many dairy calves are individually housed, much of this research is focused on social enrichment. Calves are motivated for full physical contact with conspecifics (Ede et al., 2022). When housed in pairs or groups, they display fewer vocalisations during weaning, spend more time at a feeder, and display improved social skills and ability to cope with environmental stressors compared to single-housed calves, potentially due to greater cognitive development (reviewed in Mandel et al., 2016). When provided with physical enrichments such as brushes, chains, dry teats, and balls, dairy calves display reduced vocalisations following weaning (da Silva et al., 2022), less non-nutritive and cross-sucking (Zhang et al., 2021), reduced time being inactive (Velasquez-Munoz et al., 2019; Miranda et al., 2023), and more time playing (Pempek et al., 2017) compared to control calves. However, the impacts of these enrichments on other measures have been inconclusive, with reports of enrichment resulting in more time eating (Velasquez-Munoz et al., 2019; Miranda et al., 2023), reduced feed intake (da Silva et al., 2022), and no impact on starter feed intake (Pempek et al., 2017). The impacts of enrichment on biological functioning are also inconclusive for dairy calves, with studies showing both increased average daily gain (Pempek et al., 2017; Zhang et al., 2021) and no impact on weight gain (da Silva et al., 2022). However, enriched calves have shown higher plasma oxytocin levels than those unenriched (Miranda et al., 2023). Calves provided with physical enrichments also showed no differences compared to controls in the responses to novel environments and objects (Pempek et al., 2017; Zhang et al., 2021); however, enriched veal calves had a lower chance of avoiding an unfamiliar human, indicating reduced fear (Leruste et al., 2012). These inconsistent findings on the impacts of enrichment could be due to many factors. For example, mice have also shown contradictory outcomes of enrichment, which have been associated with strain, age, gender, and other housing attributes (reviewed in Bayne, 2018). Therefore, in cattle, the inconsistent findings could similarly be related to breed, age, gender, enrichment type (i.e., rotating brushes are preferred over stationary brushes: Strappini et al., 2021), and location (i.e., higher use if located close to feed: Foris et al., 2023).
To the best of the authors’ knowledge, while there have been studies looking at enrichment in beef cattle, no research has been specifically done during the weaning period. When presented with four different enrichments in a paddock environment, beef cattle performed either grooming behaviours or oral manipulations on each enrichment, indicating that these may be rewarding behaviours and can be stimulated through enrichment (Dickson et al., 2022). In addition, the loss of a grooming brush can impair the welfare of beef cattle (Dickson et al., 2024). Research to date has shown that enriched beef calves and steers display more playing and social behaviours (Bulens et al., 2014), reduced agonistic behaviour (Ninomiya and Sato, 2009; Park et al., 2020), stereotypic behaviour (Park et al., 2020), and time inactive, whilst also displaying increased affiliative behaviour (Ninomiya and Sato, 2009) and exploratory behaviours (Bruno et al., 2020) than control animals. Similar to studies on dairy calves, beef cattle have shown no impact on dry matter intake (Bruno et al., 2020), weight gain (Ninomiya and Sato, 2009; Ninomiya, 2019; Park et al., 2020), or hair cortisol concentrations (Park et al., 2020) when provided with enrichments. However, these studies were performed on weaned cattle in a pen or feedlot environment (Ninomiya, 2019; Bruno et al., 2020; Park et al., 2020) or on young calves, but not specifically during the weaning period (Ninomiya and Sato, 2009; Bulens et al., 2014). Overall, variable effects of enrichment have been observed in both dairy and beef, and further investigation is warranted regarding the benefits of enrichment for young beef calves weaned in groups.
The early-life environment influences the development of animals, which can have an impact on coping behaviours and adaptability, resulting in individuals that are functional within their environment. For example, individuals reared in an ‘unsafe’ environment are more likely to respond to unexpected or novel stimuli by freezing or finding cover, whilst individuals reared in a ‘safe’ environment may gain greater benefit from engaging in new situations and stimuli (Langenhof and Komdeur, 2018). The influence of early environmental enrichment on brain development has been extensively studied in rodents, with neuroanatomical changes observed in enriched animals, along with improvements in memory and learning and increased exploratory activity (reviewed in van Praag et al., 2000; Nithianantharajah and Hannan, 2006). In other animals, early-life enrichment has had conflicting results. For example, in pigs, early enrichment has impacted agonistic behaviours in later life by reducing (Munsterhjelm et al., 2009), increasing (Melotti et al., 2011), or having no effect (Statham et al., 2011) on their incidence and both reducing (sensitivity to reward loss: Luo et al., 2020) and not impacting (attention bias test: Luo et al., 2019) signs of anxiety. However, dairy calves enriched prior to weaning had improved recognition memory and were more likely to explore a novel object than a familiar object following weaning (Zhang et al., 2022). Compared to beef calves weaned in a paddock, calves weaned in yards show increased average daily weight gain and reduced morbidity when moved to a feedlot later in life (Fell et al., 1998). Despite this, it is unknown whether long-term impacts of enrichment will be seen at weaning for beef calves as weaning typically occurs later in life than in dairy calves and environmental enrichments may be less effective if implemented after specific preferences and behaviours have developed (Newberry, 1995).
The aim of the current study was to determine the impacts of environmental enrichment on the behaviour and welfare of beef calves during yard weaning, which involves weaning calves onto forage or other feed whilst housed in yards away from their mothers. It was predicted that the impacts on physiological functioning, namely, body temperature, weight, and faecal cortisol, would be small based on what previous studies on dairy calves and adult beef cattle have found. However, the provision of enrichments was expected to alter pen behaviours, specifically by decreasing agonistic and increasing affiliative interactions. If enrichment was successful at reducing stress, this would be reflected through improved temperament as measured by the crush score and flight speed, reduced anxiety in an attention bias test, along with reduced neophobia and improved cognition and memory, as assessed using a novel object recognition test.
2 Materials and methods
2.1 Ethical statement
The protocol and conduct of the study were approved by the CSIRO Agriculture Animal Ethics Committee (Armidale) under the New South Wales Animal Research Act 1985 (Animal Research Authority 22-07).
2.2 Animals and housing
2.2.1 Animals and treatments
A total of 48 mixed-sex Angus calves (approximately 7 months old) were used in the study. Calves were sorted into eight groups (six per group) balanced for sex, sire, weight, crush score, and flight speed (see Section 2.3.3 for a description of the crush score and flight speed assessments). Half of the groups were enriched and the other half non-enriched (control).
The enrichments chosen included a cattle brush (Redpath, Palmerston North, New Zealand) mounted with bottom bristles 1.1 m from the ground. A three-strand twisted sisal rope 10 mm thick and 5 m long was also used in enriched pens. The rope hung horizontally for approximately 2–2.5 m, adjacent to a 1-m length hung vertically with four knots down the length. Finally, a 25-in. ‘Jolly Mega Horse Ball’ (Horsemen’s Pride Inc., Streetsboro, OH, USA) with a blue and red soccer print cover was placed in each enriched pen. A photograph of each enrichment used is shown in Figure 1C. The brush was chosen as it is the preferred enrichment of beef steers (Dickson et al., 2022), the rope was chosen to potentially satisfy a suckling motivation of the young calves (De Passillé, 2001; Zobel et al., 2017), and the ball was chosen to encourage play behaviour (Bulens et al., 2014).
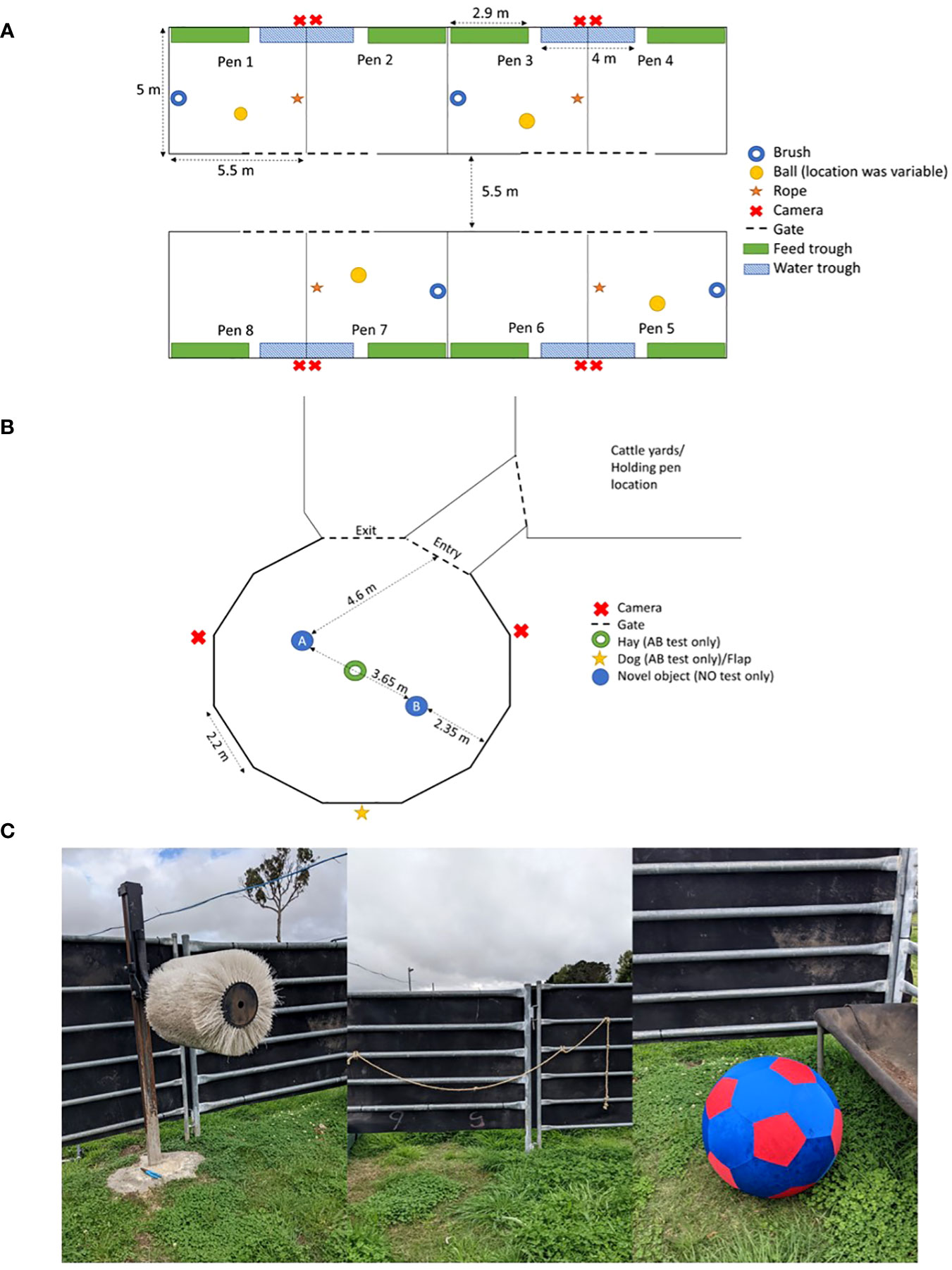
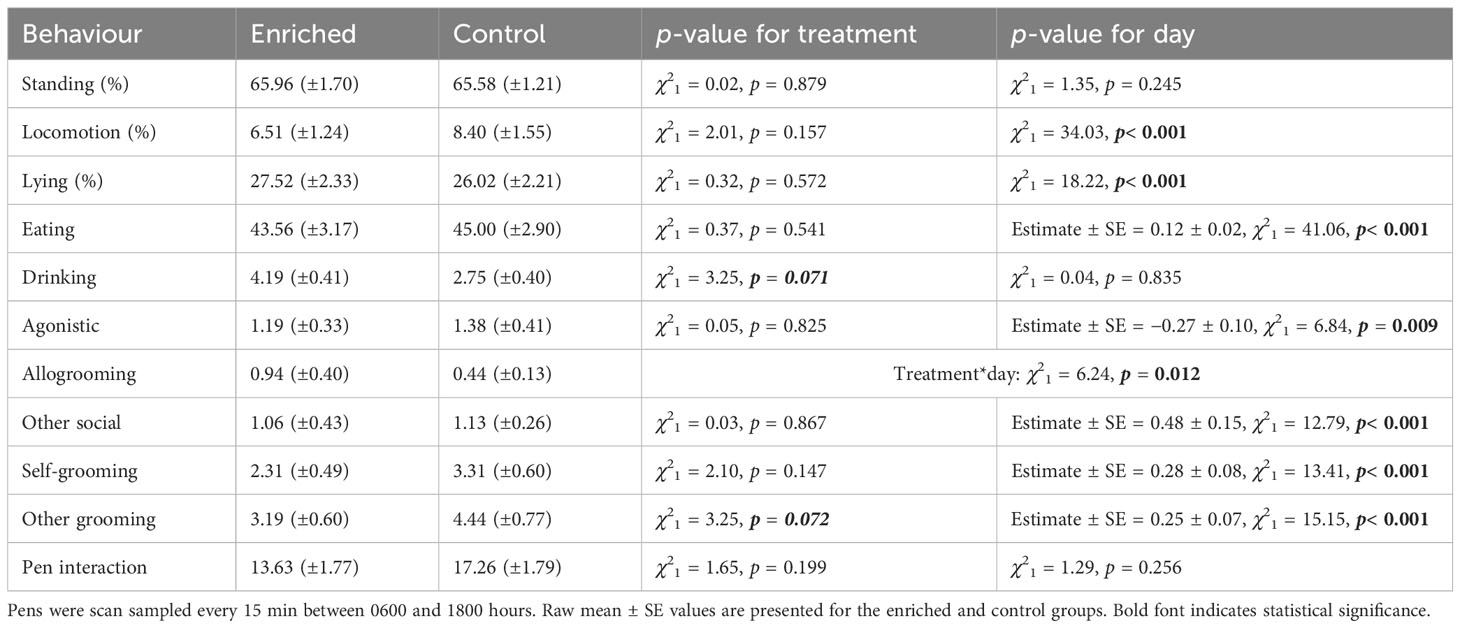
Figure 1 (A) Diagram of the home pens, including the locations of the water and feed troughs, cameras, and enrichments (i.e., brush, ball, and rope). (B) Diagram of the arena used for the attention bias (AB) and novel object recognition (NO) tests showing the entry and exit gates, the placement of the hay, and the flap where the dog was presented. (C) Photographs of the brush, rope, and ball used for the enriched groups. Diagrams are not to scale.
2.2.2 Facilities
The experiment was undertaken at CSIRO, FD McMaster Laboratory (Chiswick, Armidale, NSW, Australia) in April and May 2022, with follow-up testing done in September 2022.
The animal groups were housed in separate pens with all the behavioural testing facilities located adjacent to the home pens. Each pen was 27.5 m2 in size (5.5 × 5 m) (Figure 1A), which was sufficient as per the recommended guidelines of at least 4 m2 per animal (NSW Department of Primary Industries, n.d). Rubber matting (Andromeda Engineering, Moonbi, NSW, Australia) lined the panels between adjacent pens to prevent cattle physically interacting with other groups; however, calves could still see groups in pens across from them. All pens were constructed on pasture as, in Australia, weaning typically occurs outside with exposure to the elements.
The testing arena was accessed from the yard facilities and holding pens. This area was a dodecagon made using 2.2-m panels with rubber matting (Andromeda Engineering, Moonbi, NSW, Australia) surrounding the sides (Figure 1B).
Video cameras (Hikvision EXIR turret network camera DS-2CD2365G1-I) were installed above each home pen to record home pen behaviours (Figure 1A) and were connected to a network video recorder (Hikvision DS-7732NI-I4/16P NVR). Two GoPros (Hero5 Black and Hero7 Black) were installed on opposite sides above the testing arena (Figure 1B).
2.3 Experimental protocol
2.3.1 Regrouping
On day 10 of the experiment, all animals had to be removed from their original home pens due to unexpected excessive mud in these areas resulting from the wet weather. There was concern that the muddy pen substrate would prevent adequate lying. All control animals were grouped together, with all enriched animals also grouped together. The new pens were located next to the original home pens in nearby yards with gravel- and sand-based flooring, and enriched animals had access to the same enrichments as previously (Figure 2). However, all enrichments were supplied at a slightly reduced density (i.e., one enrichment/six cattle pre-mixing vs. one enrichment/eight cattle post-mixing) due to limited suitable locations to install the grooming brushes.
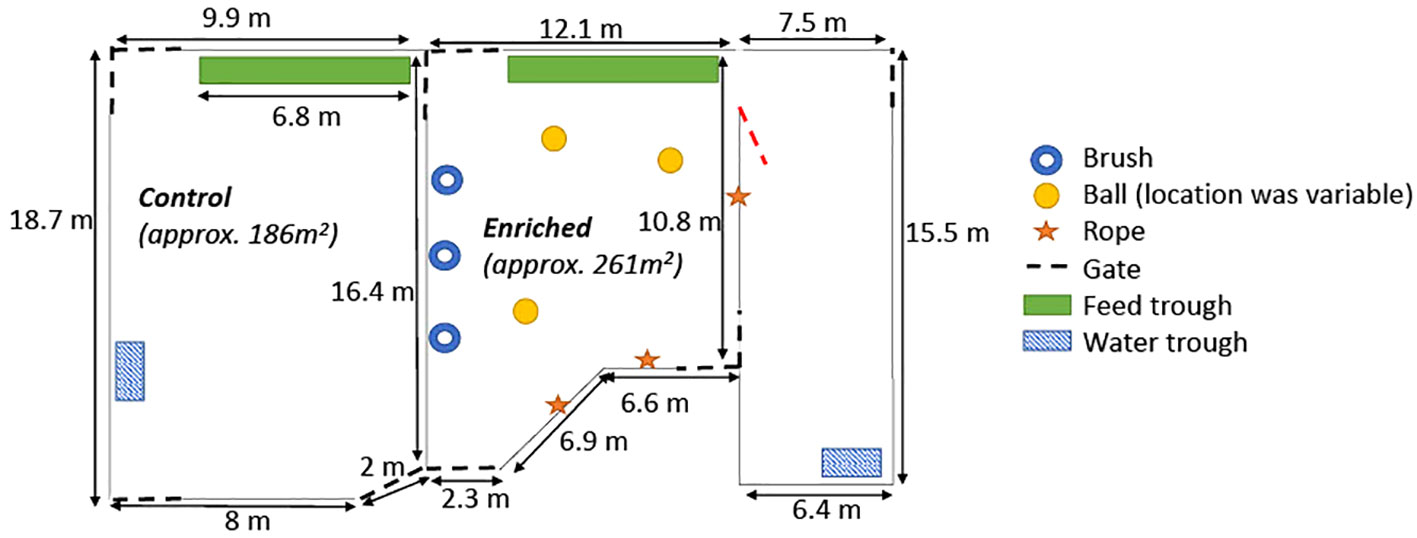
Figure 2 Diagram of the new home pens following regrouping as a result of unexpected mud, including placement of the food and water troughs and the enrichment items (i.e., brush, ball, and rope). Control calves were located in the larger pen on the left, whilst enriched calves had access to both smaller yards on the right. Note that the gate depicted in red remained open at all times. Diagram not to scale.
Due to this regrouping event, the experiment was divided into two stages: stage 1, which occurred before regrouping, and stage 2, which occurred following regrouping (Table 1).

Table 1 Daily activities and weather data recorded during weaning and during follow-up experimental days.
2.3.2 Order of events
On day 1 of the experiment, all calves were separated from their mothers and brought into the yards. They had previously been housed at pasture as grazing herds. Mothers were then housed in a separate paddock and were out of both visual and auditory range from the calves. The calves were blood sampled (for an alternate experiment), vaccinated with Ultravac® 7-in-1 (Zoetis Australia Pty Ltd, Rhodes, NSW, Australia) in the race, weighed using walk-over weigh scales, then held in a crush for the crush score and flight speed tests as they exited the crush (Ramage Engineering, Guyra, NSW, Australia) (further details on these measures are provided in Section 2.3.3). These measurements were used to sort calves into groups balanced approximately for sex, sire, weight, crush score, and flight speed. The calves were then brought into the crush again approximately 2 h later to be fitted with iButton temperature loggers (iButton DS1921H-F5; Embedded Data Systems, Lawrenceburg, KY, USA) in order to measure core body temperature. The iButtons were attached as described by Lea et al. (2008), in which the logger was attached using heat shrink plastic to a PVC rod (18 mm length × 8 mm diameter), which was fitted to a 120-mm length of a high-pressure rubber hose and attached to the tail with vet wrap. Temperature was recorded at 5-min intervals from 1400 hours on day 1 until 1635 hours on day 8 of the experiment. Individuals within each group were also marked on their side with the numbers 1–6 using livestock paint (Leader Products Pty Ltd, Craigieburn, VIC, Australia) before being drafted into their groups and moved to the experimental pens. Paint was reapplied as needed when animals were brought through the crush (days 3, 7, 8, 9, and 11). In stage 1, a single group was brought through the crush at a time in order of pen number (which alternated enriched and control calves). In stage 2, enriched calves were all brought through the crush and tested, followed by the control calves. A total of approximately 2–3 h was required for all events involving animals travelling through the race (i.e., faecal sampling, iButton fitting/removal, and weight, crush score, and flight speed measurements). Table 1 provides a description of the daily activities and the climate data during weaning and at the follow-up assessment approximately 5 months later.
All groups were fed the same amount twice daily, which consisted of hay and ‘starter’ pellets at ratios predetermined by farm staff, at approximately 0800–0900 and 1600–1700 hours. Water was provided ad libitum.
2.3.3 Crush score and flight speed
The crush score was determined by one trained researcher. The calf was held in the crush for approximately 10 s with the back ‘kick’ gate shut, but with the head free of the head bail, and scored on a 1–5 scale. The scoring system used was modified from Grandin (1993) and was as follows:
1: Very calm and still, may take one or two steps;
1.5: Slightly agitated (stepping, leaning back, or tail swishing), but settles;
2: Slightly agitated, but does not settle;
2.5: Agitated, crate may occasionally shake;
3: Agitated/restless, moving back and forth, shaking crate;
4: Continuous, very vigorous movement, chute shaking; and
5: Rearing, struggling violently, body twisting.
The flight speed was measured as the time taken to pass through two pairs of electric eyes (FarmTek Electronic Timers, Wylie, TX, USA) 1.8 m apart. The first pair of eyes was located approximately 0.9 m from the head bail.
2.3.4 Faecal samples
Faecal samples were collected opportunistically if an animal was noticed defecating whilst in the yards; otherwise, these were collected manually whilst the animal was in the race. The samples were placed into a mobile freezer upon collection (approximately −18°C) and stored until completely frozen. They were then dried in a drying oven at 60°C before being ground using a Retsch ZM 200 Ultra Centrifugal Mill with a 1-mm sieve. The samples were further processed for cortisol metabolites at the University of Western Australia using the method described by Campbell et al. (2019). A total of 2–3 h was required to collect all faecal samples each collection day, which amounted to approximately 15–20 min that each group was removed from their home pens.
2.3.5 Pen behaviours
Pen behaviours were analysed by a single observer using video recordings from the cameras depicted in Figure 1A. Days 2, 4, 5, and 9 were analysed between 0600 and 1800 hours using scan sampling every 15 min. Up to 10 s at each time period was observed to determine the predominant behaviour of each animal. Behaviours are presented in Table 2. Standing, lying, and locomotion were mutually exclusive events; however, a calf could also be engaged in another activity whilst performing one of these behaviours (e.g., lying and self-grooming).

Table 2 Ethogram of behaviours used during the attention bias test, novel object recognition test, and for pen behaviours, comparing beef calves in an enriched vs. a control environment.
2.3.6 Attention bias test
In animals, the housing environment and management practices influence the behaviours seen in an attention bias test (Luo et al., 2019; Verbeek et al., 2019). This is expected to be related to affective states such as anxiety (Lee et al., 2018; Monk et al., 2020), but could also be dependent on the personality of an individual (Luo et al., 2019). The attention bias protocol used in the current study was based on a pharmacologically validated test by Lee et al. (2018), who showed that cattle in an increased state of anxiety demonstrate increased attention towards a threat, in this case a dog. The dog was an unfamiliar Border Collie, and calves had no prior experience with dogs. Prior to the testing days, the grass of the arena was mown as short as possible, but grass was still present.
Animals were fed a half feed ration on the morning of testing and received the remainder of the feed once all the animals had been tested. Calves were fed 1 h prior to the start of testing, and testing lasted approximately 4 h. Groups of five to six animals were moved by familiar personnel into nearby holding yards, with the enriched and control animals kept separately. As the arena was located close to the holding yards, vocal but not visual contact was possible; however, no excessive noise was noted throughout the testing period, and all individuals were exposed to a similar level of noise. Enriched and control animals were tested in an alternating fashion, with the calves to be tested at specific time points randomly chosen.
Approximately 2 kg of hay was located in the centre of the arena (Figure 1B) and was refreshed as necessary between animals. The animals entered the arena individually, and once the entry gate was shut behind them, a flap in the rubber matting was opened to reveal the dog. A timer began and the test commenced once the calf made visual contact with the dog (i.e., the head was orientated directly towards the dog). From this point, the dog was visible for 10 s before the flap was lowered and the dog removed. The dog used for testing has been previously used in attention bias tests for livestock (both cattle and sheep) and was calm throughout testing. The dog barked on only one occasion and was otherwise silent. It was possible that the dog could be smelled by the calf. The test lasted 3 min, after which the calf was removed from the arena. All animals were held in nearby grassed areas to avoid mixing with the untested animals and requiring further handling. They were returned to their home pens after the completion of all testing, which totaled approximately 4 h.
Behaviours were later collated from video footage using The Observer XT (Noldus) by a single individual who was blind to the treatments. The behaviours recorded are described in Table 2.
2.3.7 Novel object recognition test
The novel object recognition test provides a measure of cognitive function, notably learning and memory, and compares the time spent exploring a novel versus a familiar object (Antunes and Biala, 2012). Animals tend to show a preference for novel objects, suggesting that the animal remembered the familiar object. The novel object recognition test requires both a familiarisation stage and a testing stage (Antunes and Biala, 2012).
For the familiarisation stage and the first 2 days of novel object recognition testing, the calves entered the holding pens and arena in the same fashion as for the attention bias test on each day. For the follow-up test, the testing order was random as the enriched and control animals were housed together following weaning. Each test lasted 7 min commencing from the time the entry gate was shut behind the animal. After the completion of the test, the calf was removed from the arena, housed in a nearby pastured area, and returned to their home pen after all the animals were tested. Each day required approximately 7–7.5 h to test all animals.
For the familiarisation stage, two orange traffic cones (75 cm tall) were used for all calves. For the testing stage, the procedures were the same as for the familiarisation stage, except two different objects were used. On testing day 1, one traffic cone (‘familiar’) and one white plastic chair (‘novel’) (California Comfort Resin Chair – White, Marquee, 555 × 840 × 580 mm) were used. The sides of the arena on which these objects were presented were switched every two animals. On testing day 2, the objects used were the cone (‘familiar’) and a black plastic planter box (‘novel’) (Charcoal Raised Garden Bed, Fountain Products, 700 × 375 × 655 mm). On this day, the cone was presented to individual calves on the opposite side to which it was shown the previous day.
For the follow-up test 5 months later (testing day 3), the cone was again used as the familiar object, and the novel object was a standing drink tray (Cooler Party Tub, Marquee, 400 × 770 × 400 mm). Steers and heifers from both the control and enriched groups were tested on separate days as, prior to this final test, all heifers were housed as a single group and all steers were also housed as a single group, both at pasture. The side on which the novel object was presented was randomly assigned for all animals.
Behaviours were later collated using The Observer XT (Noldus) and are described in Table 2. On all days, behaviours related to objects were measured (i.e., look or sniff object, and head or other interactions). On all testing days, each object was defined as either novel or familiar and either left or right based on their position when the calf first entered the arena. The familiarisation day also measured the time looking at and exploring the dog flap, exploring the arena, and eating/grazing and the time spent moving versus stationary. This was because this was the day following the attention bias test and the threat of the dog may have impacted vigilance and explorative behaviours. Each day was analysed by a single individual who was blind to the treatments.
2.4 Climate
Climate data (daily average temperature and rainfall) during weaning were recorded from a nearby onsite MEA weather station (Green Brain, Magill, SA, Australia) and are presented in Table 1. The average daily temperature during weaning was 11.2°C and daily rainfall was 0.9 mL. During the follow up testing, the average temperature was 9.6°C and rainfall was 0.1 mL.
2.5 Data and statistical analysis
All data analyses were performed in ‘R’ (R Core Team, 2022). Main effects were considered significant at p ≤ 0.05 and trends at an alpha level of 0.05< p< 0.10. Prior to model fitting, all interaction plots were visually examined; however, as none were significant, these were not included in later models. For all models, all logical two-way interactions were initially fit and tested, but only those that reached significance are presented.
2.5.1 Flight speed, crush score, and body weight
As calves were required to be regrouped on day 10, the flight speed, crush score, and body weight data were analysed in two stages: before and after regrouping. For both stages, the individual was the experimental unit. Linear mixed-effects models (LMMs) were fit for flight speed and body weight data using the ‘lme4’ package (Bates et al., 2015), whilst cumulative link mixed models (CLMMs) were fit for crush score using the ‘ordinal’ package (Christensen, 2022). Two flight speed scores on day 16 were removed from the analysis as the calves stopped before crossing the second pair of electric eyes and were thus outliers (both enriched). The weight of one animal also failed to be collected at follow-up testing (enriched).
Stage 1 fit data before regrouping and included the measurement on day 7 as the dependent variable and treatment (enriched vs. control), sex, and each individual’s original measure of crush score or flight speed on day 1 as the fixed effects, with random error of pen number (n = 8). Stage 2 fit data following regrouping, with the fixed effects as described for stage 1, in addition to day (day 16 vs. follow-up), and random effect of the individual. Model assumptions were checked using a visual assessment of Q–Q and residuals relative to the fitted values plots. Flight speed required log transformation to meet normality assumptions. Significance testing of the fixed effects for both stages was conducted using the ‘drop1’ function, with a likelihood ratio test.
2.5.2 Faecal cortisol metabolites
A total of seven faecal samples were not included in the analyses (day 8: one failed to be collected, one outlier; day 11: three failed to be collected, two outliers). Outliers were believed to be a result of processing error rather than natural individual variation. Faecal cortisol was also analysed in two stages due to regrouping, with individual as the experimental unit. Stage 1 was analysed using an LMM, with treatment, sex, and day (day 3 vs. day 8) as the fixed effects and individual nested within pen as the random effect. Model assumptions were checked as above (Section 2.5.1), as well as significance testing of the fixed effects. For stage 2 (day 11), the Mann–Whitney U test was utilised to compare treatment groups as the data were not normally distributed.
2.5.3 Body temperature
Data from four iButtons failed to import (two enriched, two control). In addition, on three occasions, an iButton became dislodged and therefore was excluded from the analysis during this time period. The average daily temperature was calculated for each individual and used as the response variable. An LMM was fit for temperature data, with fixed effects of treatment, sex, and day. The random effect was individual nested within pen, with individual as the experimental unit. The model diagnostics and testing of the fixed effects were performed as previously described in Section 2.5.1.
2.5.4 Pen behaviours
Data for standing, locomotion, and lying were expressed as a percentage of daily observations and analysed using an LMM, with pen as the experimental unit. The fixed effects were treatment and day number, with pen as the random effect. Locomotion required log transformation to meet normality assumptions. Other pen behaviours (see Table 2) were each analysed with separate Poisson generalised linear mixed models (GLMMs) using the same fixed and random effects as for locomotor activities. The use of enrichments was also analysed with a GLMM, with fixed effects of enrichment type (i.e., brush, rope, or ball) and day and random effect of pen. Only the enriched groups were included in this analysis. The model diagnostics and the testing of fixed effects were conducted as previously described in Section 2.5.1.
2.5.5 Attention bias test
Latency to become non-vigilant was analysed using a generalised linear model (GLM) with a log-transformed response variable as the residuals did not meet normality assumptions. The fixed effects included treatment, sex, and test order and were analysed as previously stated. Test order was included as a variable to account for responses that may be influenced by time, such as calf hunger and dog behaviour. As some cattle failed to move/step and eat hay, these latencies were analysed with Cox’s proportional hazards model through survival analysis using the ‘survival’ package (Therneau, 2022). Animals that failed to perform the behaviour within 180 s were treated as censored results, with fixed effects of treatment, sex, and test order.
The duration data (i.e., looking at threat, eating hay, vigilance, wall exploration, movement, ear position, and position in arena relative to flap) were analysed using linear models (LMs), with treatment, sex, and test order as fixed effects. Model diagnostics occurred as previously described in Section 2.5.1, whilst the testing of fixed effects was conducted using the ‘drop1’ function, with an F-test. Data for wall exploration, time at dog flap, and time on far side required square root transformation to meet normality assumptions.
A Fisher’s exact test was conducted for tail shaking data. However, only one instance of head shaking and vocalisations occurred, whilst there were no instances of escape attempts, urinations, or defecations; therefore, these behaviours were not included in statistical analyses.
2.5.6 Novel object recognition test
Data for the familiarisation day and testing phases (days 1 and 2 and follow-up) were analysed separately. A discrimination ratio (DR) was calculated for each animal on each day of the testing phase and reflects the duration of exploration of the novel compared to the familiar object, proportional to the time exploring both objects (Sivakumaran et al., 2018). The DR was calculated as:
Separate LMs were fitted for behaviours on the familiarisation day (i.e., exploring arena, exploring dog flap, looking at dog flap, eating/grazing, movement, looking at objects, and exploring objects). The fixed effects were treatment, sex, and test order, as well as object side for time spent looking and exploring objects. ‘Dog flap exploration’ required square root transformation to meet normality assumptions. Model diagnostics occurred as previously described (Section 2.5.1), whilst fixed effects were tested using the ‘drop1’ function, with an F-test.
For the testing phases, LMMs were fit for time spent looking at and exploring objects. For the purposes of analysis, the behaviours ‘sniff object’, ‘head interaction’, and ‘other interaction’ (Table 2) were combined into the behaviour ‘object exploration’. The fixed effects were treatment, day, object (familiar vs. novel), sex, test order, and object side, with the random effect of the individual. A square root transformation was required to meet normality assumptions for time spent looking at and exploring objects. DR was also fit with an LMM, with treatment, sex, day, and test order as fixed effects and individual as random effect. If an individual did not interact with either object, it was given a DR of “0” on that day (testing day 1: three enriched, one control; testing day 2: one enriched, two control). The model diagnostics and the testing of fixed effects were performed as previously described in Section 2.5.1. Calves were excluded from these analyses if they failed to interact with objects for a minimum of 4 s during the familiarisation phase. This was done to ensure that the individuals were familiar with this object. A similar criterion has also been used in novel object recognition tests for other species (e.g., Lueptow, 2017). A Fisher’s exact test was conducted to analyse the number of calves in each treatment that failed this criterion.
3 Results
3.1 Body weight
Treatment did not impact bodyweight gain (stage 1 model before regrouping: χ21 = 0.57, p = 0.450; stage 2 model after regrouping: χ21 = 1.21, p = 0.272) (Figure 3A). The body weight on day 1 of weaning was positively correlated with the individual’s weight on subsequent measurement days (stage 1 model: estimate ± SE = 0.67 ± 0.10, χ21 = 32.04, p< 0.001; stage 2 model: estimate ± SE = 0.75 ± 0.09, χ21 = 44.37, p< 0.001). Unsurprisingly, cattle were heavier during the follow-up testing than at the end of weaning on day 16 (stage 2 model: χ21 = 182.78, p< 0.001), whilst steers were heavier than heifers during the early stages of weaning (stage 1 model: χ21 = 5.69, p = 0.017; stage 2 model: χ21 = 3.57, p = 0.059) (Figure 3A).
Figure 3 (A–D) Raw data for body weight (A), flight speed (B), crush score (C), and faecal cortisol metabolites (D) of the enriched versus control beef calves during yard weaning and at follow-up testing 2 months later (A–C). A faster flight speed is associated with a smaller value. Calves were regrouped on day 10 due to unforeseen mud, with all the enriched calves grouped together and all the controls also grouped together. An asterisk indicates a significant difference between treatment groups.
3.2 Flight speed
Treatment only influenced flight speed on day 7, with enriched animals being faster than the controls (stage 1 model: χ21 = 4.44, p = 0.035; stage 2 model: χ21 = 0.02, p = 0.880) (Figure 3B). However, the flight speed on day 1 of weaning was positively correlated with the individual’s speed on subsequent days (stage 1 model: estimate ± SE = 0.59 ± 0.14, χ21 = 16.29, p< 0.001; stage 2 model: estimate ± SE = 0.43 ± 0.19, χ21 = 5.05, p = 0.025). Sex did not impact flight speed (stage 1 model: χ21 = 0.01, p = 0.938; stage 2 model: χ21 = 0.79, p = 0.374), and animals had a faster flight speed at the follow-up testing than at the end of weaning on day 16 (stage 2 model: χ21 = 9.22, p = 0.002) (Figure 3B).
3.3 Crush score
The crush score was not influenced by treatment (stage 1 model: χ21 = 3.08, p = 0.079; stage 2 model: χ21 = 1.05, p = 0.306), sex (stage 1 model: χ21 = 0.02, p = 0.886; stage 2 model: χ21 = 0.23, p = 0.633), or the individual’s original score on day 1 (stage 1 model: χ21 = 5.98, p = 0.050; stage 2 model: χ21 = 2.13, p = 0.344). However, calves had a lower crush score at the follow-up testing than at the end of weaning (stage 2 model: χ21 = 5.19, p = 0.023) (Figure 3C).
3.4 Faecal cortisol metabolites
Treatment had no impact on faecal cortisol metabolites (stage 1 model: χ21 = 1.12, p = 0.290; stage 2 model: Mann–Whitney U test = 200.5, p = 0.624). However, day 8 had lower cortisol metabolite concentrations than day 3 (stage 1 model: estimate ± SE = −46.05 ± 5.48, χ21 = 52.08, p< 0.001) regardless of sex (stage 1 model: χ21 = 0.45, p = 0.503) (Figure 3D).
3.5 Body temperature
Time decreased the internal body temperature (estimate ± SE = −0.09 ± 0.01, χ21 = 159.03, p< 0.001) regardless of treatment (χ21 = 0.14, p = 0.712) and sex (χ21 = 1.06, p = 0.304).
3.6 Pen behaviours
There was a significant interaction between treatment and day number, with enriched calves observed performing more allogrooming as time increased (p = 0.050) (Table 3; Figure 4A). Enrichment tended to increase drinking (p = 0.071) and ‘other’ grooming (e.g., grooming on pen fixtures) (p = 0.072), which also increased with time (p< 0.001) (Table 3; Figure 4B).
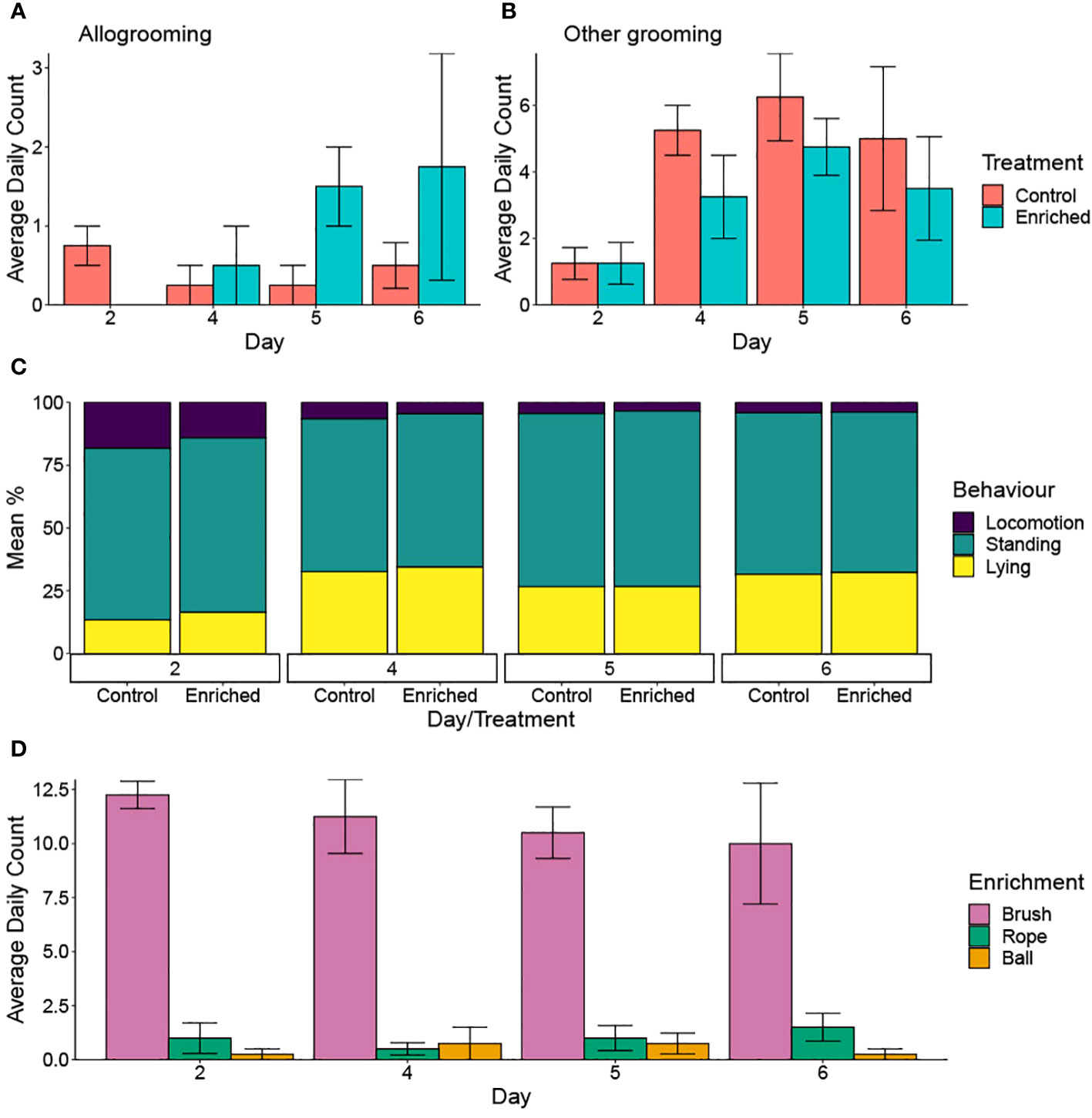
Figure 4 (A–D) Average frequency of the behaviours performed per pen of six calves. The pens were scan sampled every 15 min, between 0600 and 1800 hours. Mean ± SE values are presented for the enriched and control groups. Behaviours included allogrooming (A); other grooming (on pen fixtures excluding the brush) (B); locomotion, standing, and lying (C); and use of enrichments (enriched treatment only) (D). Note the different scales used for individual graphs.
Agonistic interactions decreased as the day number increased regardless of treatment group. Other social interactions, self-grooming, and eating also increased with time, but treatment was not significant. Calves displayed increased time spent lying and decreased locomotion following day 2 regardless of treatment group (Figure 4C; Table 3).
Interactions with pen fixtures (excluding enrichments) were not impacted by either treatment or day (both p > 0.05) (Table 3). The brush was the enrichment most commonly observed being used (χ22 = 262.12, p< 0.001) (Figure 4D), but day was not significant (χ21 = 0.51, p = 0.475).
3.7 Attention bias test
Treatment did not impact latency to become non-vigilant (χ21 = 0.09, p = 0.770); however, steers were slower than heifers (estimate ± SE = −0.56 ± 0.27, χ21 = 4.16, p = 0.041) (Figure 5A). The animals tested first had a longer latency to become non-vigilant than those tested last (estimate ± SE = −0.02 ± 0.01, χ21 = 4.01, p = 0.045).
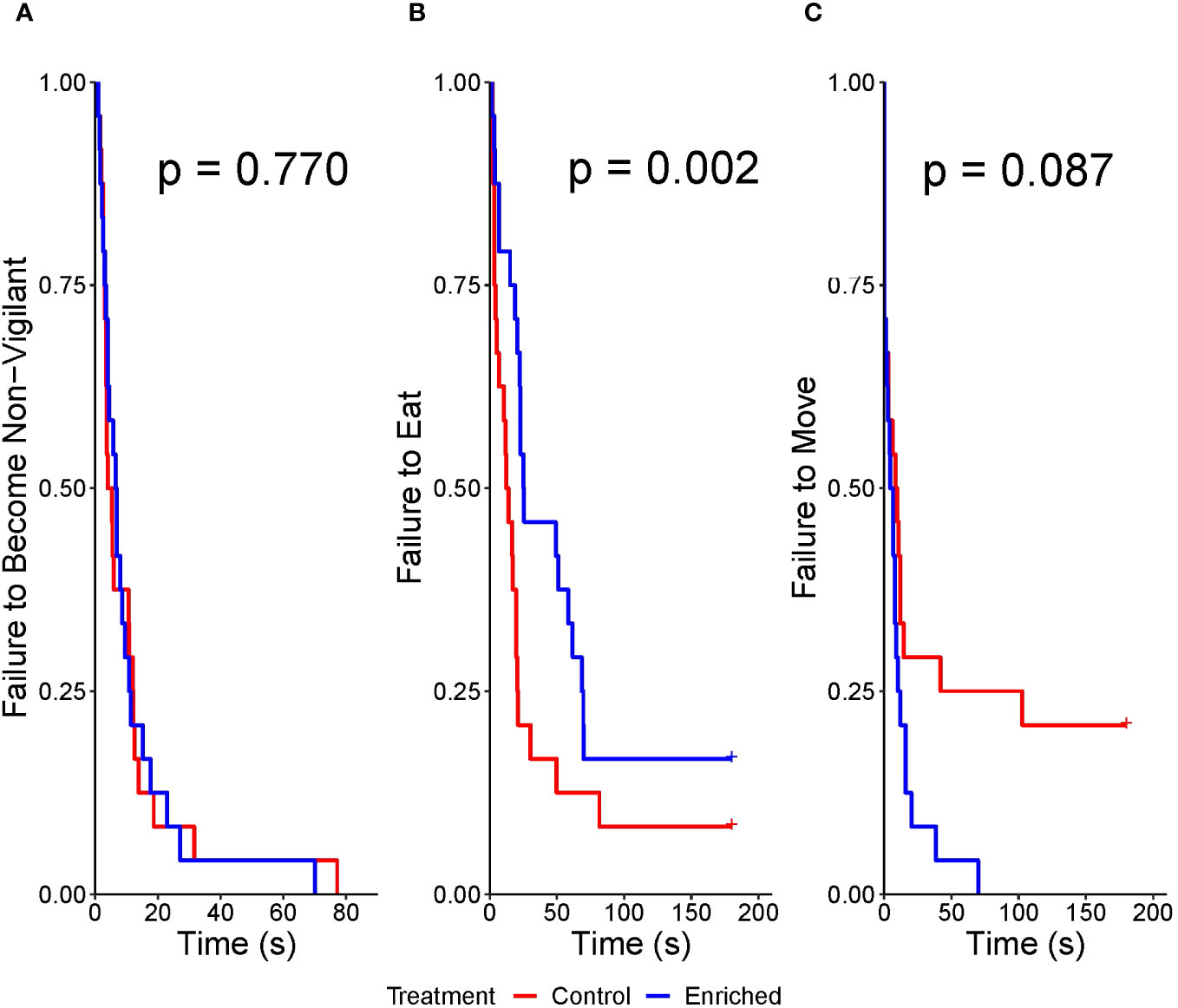
Figure 5 (A–C) Kaplan–Meier curves for the latency to first become non-vigilant (A), eat hay (B), and move (C) during the attention bias test by the enriched versus control beef calves. Note the different x-axis scales for latency to become non-vigilant.
Enriched calves were less likely to eat than control calves [hazard ratio (HR) = 0.32, 95% CI = 0.16–0.65, z = −3.16, p = 0.002], steers had a shorter latency than heifers (HR = 2.81, 95% CI = 1.43–5.54, z = 2.99, p = 0.003), and animals tested later had a shorter latency to eat than those tested earlier (HR = 1.05, 95% CI = 1.02–1.07, z = 3.41, p< 0.001) (Figure 5B). Latency to move tended to be impacted by treatment (HR = 1.73, 95% CI = 0.92–3.24, z = 1.71, p = 0.087), but not sex (z = −1.05, p = 0.292) or test order (z = −1.04, p = 0.297) (Figure 5C).
Enriched and control calves did not differ in the time spent looking at the threat (i.e., dog and dog flap); ears backwards, asymmetrical, and axial; or the number of animals that tail swished (all p > 0.05) (Table 4). However, enriched animals spent longer being vigilant, having ears forward, exploring the arena, and walking and spent more time at the dog flap and on the far side of the arena. Conversely, control calves had longer durations of eating the hay, and this was also influenced by test order, with calves tested later spending longer time eating (estimate ± SE = 0.84 ± 0.39, p = 0.035). However, the interaction between treatment and test order was not significant (F1, 43 = 3.17, p = 0.082). Steers spent less time with ears asymmetrical (estimate ± SE = −4.60 ± 1.76, p = 0.009), but sex did not have an impact on any of the other measured behaviours (all p > 0.05) (Table 4).
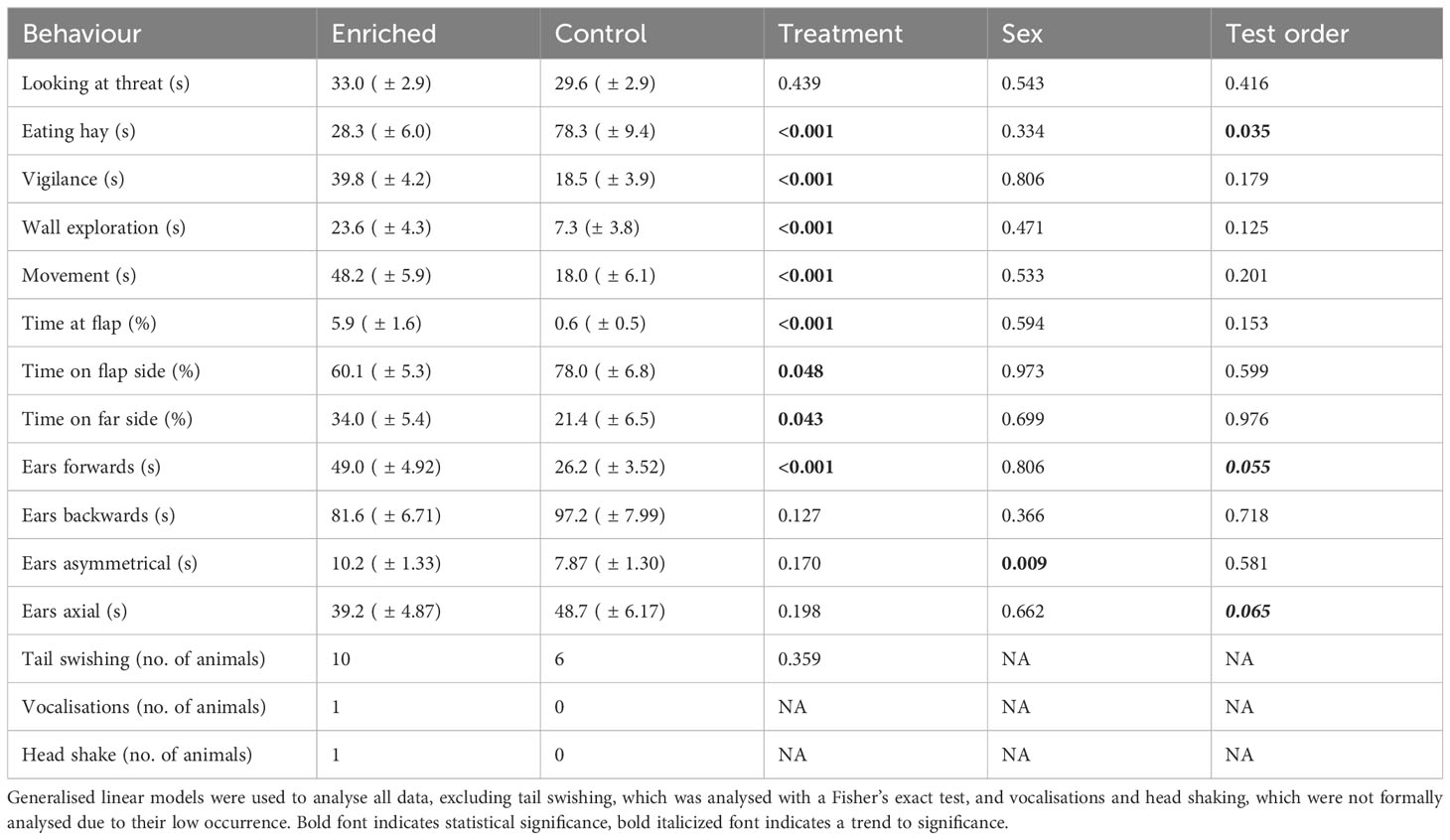
Table 4 Behaviours exhibited during the attention bias test comparing beef calves housed in either an enriched or a control environment during yard weaning.
3.8 Novel object recognition test
During the familiarisation phase, treatment influenced the amount of time spent exploring (sniffing + interacting) the objects, with enriched calves performing less exploring (β ± SE = −3.28 ± 1.59, F1, 44 = 4.26, p = 0.042) (Figure 6A), but did not influence the time spent looking at the objects (F1, 44 = 2.18, p = 0.144) (Figure 6B). The test order, side on which the objects were presented, and the sex of the calves did not influence either time spent looking at (F1, 44 = 0.02, p = 0.890; F1, 44 = 0.77, p = 0.381; and F1, 44 = 0.13, p = 0.719, respectively) or time exploring objects (F1, 44 = 0.53, p = 0.468, F1, 44 = 1.63, p = 0.205; and F1, 44 = 0.02, p = 0.890, respectively).
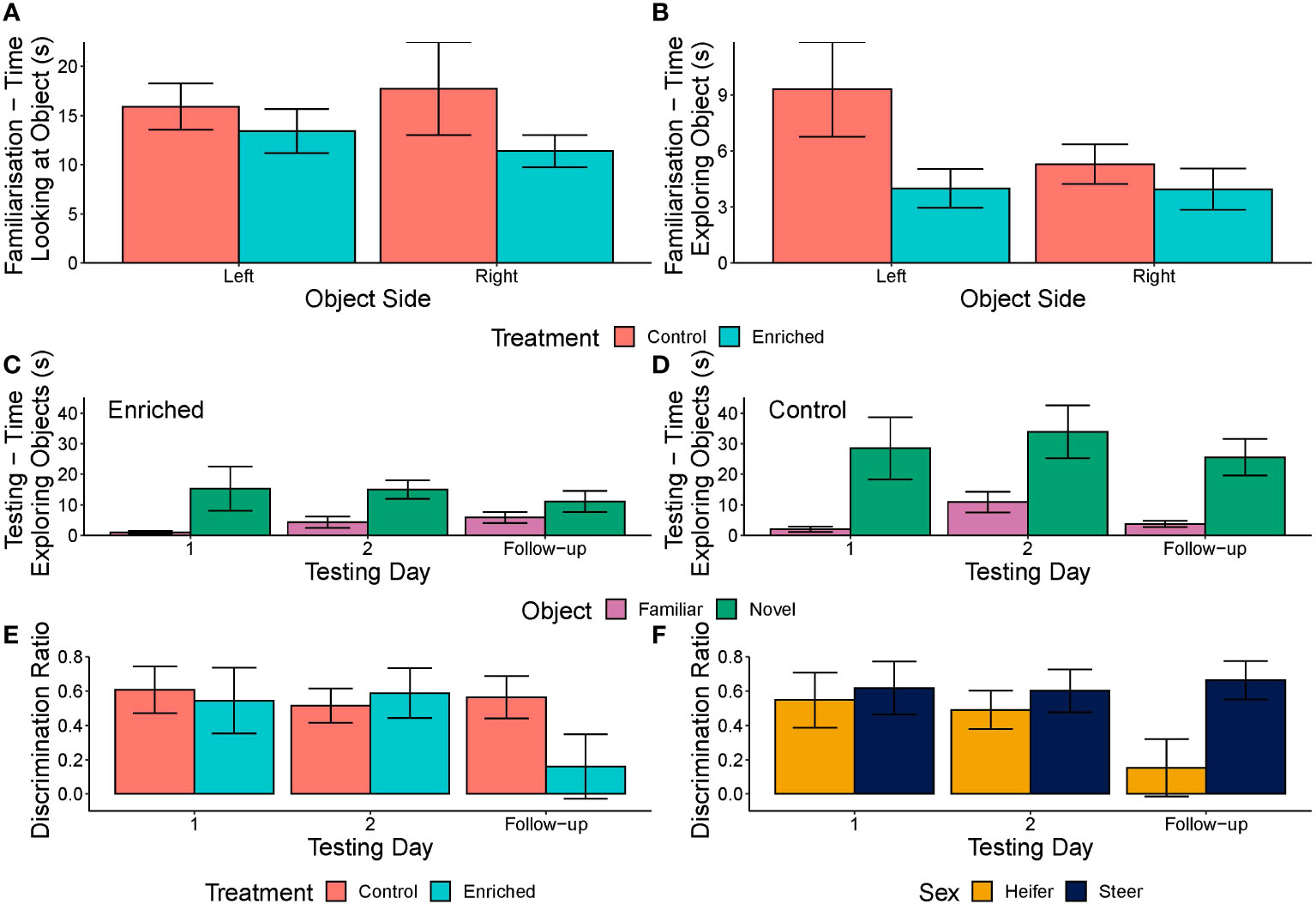
Figure 6 (A–F) Times spent looking at (A) or exploring (B) objects during the familiarisation stage of the novel object recognition test and times spent exploring novel and familiar objects during the testing stage of the novel object recognition test for both enriched (C) and control (D) beef calves during yard weaning. The discrimination ratio (mean ± SE) compared the enriched and control (E) and the steers and heifers (F) of beef calves that were yard weaned, calculated as the duration of exploration of the novel compared to the familiar object proportional to the time exploring both objects.
During the familiarisation stage, treatment did not significantly impact behaviours that were not directly related to the objects during the test (all p > 0.05) (Supplementary Table S1). Steers tended to graze (β ± SE = 50.42 ± 27.37, F1, 44 = 3.39, p = 0.072) and be stationary (β ± SE = −30.54 ± 15.41, F1, 44 = 3.86, p = 0.054) more than heifers during the first day of testing. Time spent exploring the arena also tended to increase with test order (β ± SE = 1.53 ± 0.77, F1, 44 = 3.95, p = 0.053).
Significantly more enriched than control calves were excluded from the analysis during the testing phase as they failed to meet the criterion of 4 s of interaction with objects during the familiarisation phase (nine enriched, two control; p = 0.036).
During the testing phase, enriched calves interacted with objects less than the controls (β ± SE = −0.75 ± 0.37, χ21 = 4.41, p = 0.036) (Figures 6C, D), but treatment did not influence the time looking at objects (χ21 = 0.78, p = 0.378). Novel objects were looked at and interacted with more than the familiar objects regardless of treatment (β ± SE = 1.00 ± 0.19, χ21 = 25.86, p< 0.001; β ± SE = 2.38 ± 0.29, χ21 = 59.24, p< 0.001, respectively). Calves interacted with objects on day 2 and follow-up testing more than on day 1 (χ22 = 9.76, p = 0.008) (Figures 6C, D), and the day number similarly influenced the time looking at objects (χ22 = 6.55, p = 0.038). Steers also looked at and interacted with objects more than heifers (β ± SE = 0.55 ± 0.21, χ21 = 6.57, p = 0.010; β ± SE = 0.81 ± 0.36, χ21 = 5.11, p = 0.024, respectively), and animals increased object exploration (β ± SE = 0.03 ± 0.01, χ21 = 6.48, p = 0.011), but not looking at objects (χ21 = 0.10, p = 0.757), with ascending test order, but the side on which the object was presented was not significant for either looking at or exploring objects (χ21 = 0.08, p = 0.772; χ21 = 1.71, p = 0.190, respectively).
There was no significant effect of treatment (χ21 = 0.73, p = 0.394), test day (χ22 = 0.97, p = 0.616), or test order (χ21 = 0.43, p = 0.512) on the DR (Figure 6E). However, sex was significant, with steers having a higher DR than heifers (β ± SE = 0.23 ± 0.12, χ21 = 3.85, p = 0.050), particularly at follow-up testing (Figure 6F).
4 Discussion
This study aimed to determine the impacts of environmental enrichment on the behaviour and welfare of beef calves during yard weaning. No significant impacts of enrichment on body temperature, weight, faecal cortisol metabolites, or crush score were observed. However, the unavoidable limitation of regrouping partway through weaning potentially impacted the results. In addition, it is possible that the relatively short weaning period was not long enough to have a greater impact on the chosen measures. However, control calves tended to groom more on pen fixtures, whilst enriched calves performed more allogrooming with time. Differences in behaviour were observed in both the attention bias and novel object recognition tests.
4.1 Crush score and flight speed
The crush score and flight speed were examined in the current study as it was hypothesised that appropriate environmental enrichment could reduce fearfulness, as has been observed in other species such as chickens (Campbell et al., 2021; Dumontier et al., 2022). Cattle with high crush scores and flight speed are associated with higher physiological measures of stress (Curley et al., 2006; Lees et al., 2020) and are thought to be more fearful (Grandin, 2019). On day 7, enriched animals had a faster flight speed than the controls. This is contrary to predictions of enrichments reducing fearfulness and also contrasts with previous work revealing that veal calves housed in an enriched environment show reduced fear of humans (Leruste et al., 2012). In support of this finding, enriched goats exhibit increased fearfulness by maintaining a further distance from handlers than the controls and emitting more vocalisations during handling compared to the controls (Miranda-de la Lama et al., 2013). Enriched calves also showed more variation in flight speed at the end of weaning on day 16 compared to control calves (Figure 3B). This might be due to differing impacts of the regrouping and enrichment based on an individual’s personality type. This has been seen in chickens, in which enriched hens show fewer correlations in personality testing over time, suggesting a more adaptable personality type (Campbell et al., 2021). However, no lasting significant impact of treatment was seen for either crush score or flight speed. This may be because all calves were handled regularly throughout the weaning period, and repeated handling can improve the temperament and handling ability of cattle (Ceballos et al., 2016; Parham et al., 2019).
4.2 Biological measures (cortisol, body weight, and body temperature)
Although it was hypothesised that the impact of enrichment on the measures of biological functioning would be small, no significant effect of treatment on faecal cortisol metabolites, body weight, and internal body temperature was found. This is in line with previous studies showing no impact of grooming brush access on the cortisol concentrations (Ishiwata et al., 2006; Matković et al., 2020; Park et al., 2020) and weight gain (Ishiwata et al., 2006; Ninomiya, 2019; Park et al., 2020) of cattle. It is possible that the stress induced by weaning masked any potential impacts of enrichment on these biological measures as weaning causes physiological responses such as increased cortisol, protein, and urea concentrations and neutrophil/lymphocyte ratio in calves, which are reduced with time (Kim et al., 2011; Lynch et al., 2011). This is reflected in the current study, with both body temperature and faecal cortisol metabolites reducing with time regardless of treatment. In addition to the stress of weaning, calves were also exposed to regrouping and increased handling for sampling purposes. As these are known to be stressful to cattle (regrouping: Bouissou et al., 2001; von Keyserlingk et al., 2008; handling: Grandin, 1997), they could have acted as additional stressors and might have masked any benefits of enrichments on these biological measures.
4.3 Pen behaviours
The brush was used five to six times more than either the rope or ball, and the ball appeared to be the least favoured enrichment. Previous studies reported conflicting preferences between a brush and a hanging rope, with beef steers at pasture utilising a brush more (Dickson et al., 2022), weaned group-housed dairy calves preferring a rope (Strappini et al., 2021), and pair-housed dairy calves using both enrichments for similar total durations (Zobel et al., 2017). However, these differences might be due to the age of the calves as younger calves may have a stronger motivation to suckle and therefore utilise a rope allowing for oral manipulation.
Control calves groomed on pen structures more than enriched calves, but only by a small amount (3.19 vs. 4.44 daily average observations), potentially because the brush provided an additional opportunity for enriched calves to groom. Allogrooming was also impacted by the provision of enrichment, with enriched calves displaying more allogrooming with increasing day number in the first week of weaning. Previous studies reported conflicting results, with a brush or other grooming objects increasing social behaviours (Bulens et al., 2014), decreasing (Park et al., 2020; Meneses et al., 2021), or not impacting (Kohari et al., 2007; Horvath and Miller-Cushon, 2019) allogrooming behaviours. However, the use of brushes is thought to trigger allogrooming in horses (Lansade et al., 2022). This link between brush use and allogrooming is worth exploring as allogrooming is thought to be a beneficial behaviour that establishes and maintains social relationships, supports herd stability (Sato et al., 1993; Šárová et al., 2016), and is associated with a positive affective state (Laister et al., 2011).
Unexpectedly, enriched calves were observed drinking water more than the control calves. However, the volume of water consumed per pen was not recorded, and thus it cannot be confirmed whether this difference is biologically relevant. This same result was not seen for eating behaviours. However, feed was delivered twice daily rather than supplied ad libitum and therefore was relatively quickly consumed. It is possible that the enrichments provided in the current study encouraged consummatory behaviours, manifested as increased drinking, but the underlying mechanisms are unknown as the study was not designed to specifically address this.
A major limitation of the analysis of pen behaviours is that the experimental unit was the pen, in order to avoid pseudoreplication; therefore, increasing the number of replicates might show greater behavioural differences between the enriched and control calves. A more complete analysis of pen behaviours was also limited by the number of days of observations as calves were frequently handled and were away from their home pens, which might have disrupted diurnal behavioural patterns when returned. Furthermore, cameras were not installed in the pens the calves were moved to on day 10, so it is unknown whether the impacts of enrichment continued past this point.
4.4 Attention bias test
Contrary to the hypothesis that enrichment would reduce anxiety as measured using an attention bias test, the results from the current study indicate that enriched calves displayed more anxiety during testing. This was evidenced by the longer latency to eat, as well as less time spent eating, more time being vigilant, and more time moving. Other studies on beef cattle (Lee et al., 2018; Somarriba et al., 2019), dairy heifers (Kremer et al., 2021), and sheep (Monk et al., 2018, 2020) support this interpretation of less eating, greater vigilance, and a higher amount of locomotion representing more anxious animals. This could be due to the enrichments causing increased competition within the home pens as a valued resource. Alternatively, it is possible that the enriched calves experienced a negative contrast by transitioning from their enriched environment to the relatively bare testing arena, whilst control calves may have encountered greater novelty. This theory is supported by studies in a range of species in which control animals showed a more positive affective state during an attention bias test (pigs: Luo et al., 2019) or operant conditioning tasks (mice: Mitchell et al., 2012) than the enriched animals. Similarly, control mink paid greater attention towards a range of stimuli than the enriched mink, suggesting that the non-enriched animals were bored (Meagher et al., 2017). Overall, these results indicate that enriched calves were experiencing greater anxiety during the attention bias test. However, it is unknown whether this is due to control calves experiencing a more positive state during testing due to the novelty involved (i.e., they were in a more negative state in their home pens) or due to the enrichments having a negative impact on the affective states of calves that persisted during testing (e.g., increased competition in the home pens).
Enriched calves spent longer exploring the walls of the arena during the attention bias test, which could reflect increased curiosity. Alternatively, it could be due to increased motivation to reunite with herd mates as ‘social’ dairy heifers had contact with the arena walls during an attention bias test more than ‘non-social’ calves (Kremer et al., 2021). The same study also looked at positive and negative housing conditions, but treatment did not significantly impact wall contacts (Kremer et al., 2021). As treatment was significant in the current study, it is possible that enrichment improved social bonds. This is supported by the increased allogrooming in the pen environment for enriched calves only, as previously discussed. Enriched calves were also located within one body length of the dog flap for an average of five times longer than the control calves. Along with increased curiosity, as previously discussed, this could also signify reduced levels of fear.
Enriched calves spent longer with ears in the forward position than the controls, but no difference was seen between treatments for the backwards, axial, or asymmetrical ear postures. This conflicts with a previous study, in which cattle treated with the anxiogenic drug m-chlorophenylpiperazine (m-CPP), and therefore presumed to be anxious, spent more time with their ears in a backwards posture (Lee et al., 2018). Alternatively, backwards ear postures are also thought to be indicative of a positive affective state in dairy cattle, although the treatment in this study was gentle stroking (Proctor and Carder, 2014), and this ear posture did not differ between treatment groups in the current study. It is possible that enriched calves displayed more forward ear postures simply due to increased vigilance. However, in sheep, a forward ear posture has been associated with negative affect resulting from social separation (Reefmann et al., 2009). This supports the previous statement of increased wall explorations indicating a stronger motivation to reunite with herd mates.
Overall, there have been inconsistencies in the findings of previous attention bias tests, which limits the interpretation of the current study. A review on the effectiveness of the attention bias test to assess affective states in sheep suggests that this could be due to not only differences in the test methodology but also the interactions between emotions (short-term states), moods (longer term), and personality (Monk et al., 2023). The current attention bias testing methodology did discriminate between enriched and control calves, albeit contrary to the initial hypothesis, supporting its potential for future use in assessing affective states following refinement.
4.5 Novel object recognition test
Although relatively understudied in cattle, the novel object recognition test shows promise in identifying differences in the cognitive abilities of beef calves. As hypothesised, novel objects were interacted with by the calves significantly more than familiar objects during the testing stages, indicating familiar object recognition. Enrichment reduced the amount of time calves spent interacting with objects throughout the novel object recognition test. This conflicts with previous reports of no differences in the responses to novel objects and environments seen between enriched and control calves (Pempek et al., 2017; Zhang et al., 2021), as well as the duration exploring novel objects between enriched and control pigs (Krugmann et al., 2019). Moreover, significantly more enriched than control calves were excluded from the analysis during the testing stages of the novel object recognition test as they failed to meet the requirements of interacting with the objects for a minimum of 4 s during the familiarisation stage. Although this might limit the interpretation of the data from the testing days, previous literature has shown that enriched parrots explored novel objects presented in their home pens less than the control parrots (Meehan and Mench, 2002), and enriched mink similarly explored a range of stimuli less than the control mink (Meagher et al., 2017). This suggests that control animals may be bored, which aligns with the theory of control calves experiencing boredom in their home pens.
4.6 General discussion
It is possible that the enrichments used in the current study were not appropriate. Both the brush and rope have been used in previous studies (e.g., Zobel et al., 2017; Strappini et al., 2021) and were used by calves during the first 9 days of weaning. However, the ball was observed as being used very infrequently (a total of eight instances over the observation days) and anecdotally appeared to be bumped into more than directly played with. This might be due to the ball being not as biologically relevant to the calves as the other enrichments. A brush allows for grooming, which may allow the calves to stay clean by removing dirt and ectoparasites, and has also been suggested as a method of de-arousal (Spruijt et al., 1992). As calves are weaned, they may experience a strong suckling motivation (De Passillé, 2001), which may be satisfied through the provision of a hanging rope. In contrast, the ball was chosen to encourage play behaviour (e.g., hanging balls used by Bulens et al., 2014), as has been anecdotally observed. Play behaviours have been shown to reduce during weaning for beef calves (Enríquez et al., 2010) and other stressful time periods for a range of animals (e.g., castration in lambs: Thornton and Waterman-Pearson, 2002; cold weather in pigs: Newberry et al., 1988; insufficient food in deer fawns: Muller-Schwarze et al., 1982). However, as the balls used in the current study were brightly coloured and were not fixed in position and could be moved by wind or other calves, it is possible that these balls were perceived by calves as an additional stressor due to sudden movements. In addition, the calves were initially housed under relatively high stocking densities (approximately 4.5 m2 per calf), which could have decreased play behaviours (Jensen and Kyhn, 2000) and the ability to interact with the ball. Therefore, it might be beneficial to study the impacts of providing single forms of enrichment to calves at weaning under different stocking densities to determine which enrichments are most beneficial and whether this changes with space allowance.
It is also possible that, as the yard environment itself was novel, the addition of novel enrichments created an environment that was too stimulating. This means that the enrichments may have added an extra stressor instead of reducing stressors. It is possible that this novelty could be reduced by presenting the enrichments to calves in their paddocks, either from birth or in the weeks leading up to weaning. It is unknown whether this would increase the utilisation of the enrichments and enhance any potential benefits, which poses an interesting avenue for exploration. An additional source of stress could be the competition over the enrichment resources, which may have been made worse following the regrouping event as the calves were required to establish a new social hierarchy. However, competition over enrichments such as brushes is relatively low, with a displacement occurring less than once per hour for dairy calves (Reyes et al., 2022), although a higher ratio of bushes to calves was provided by Reyes et al. (2022) than in the current study.
5 Conclusion
Overall, the provision of environmental enrichments during yard weaning altered the behaviours of beef calves during both a novel object recognition test, with enriched animals exploring the objects less than the controls, and an attention bias test, with enriched animals eating less, but spending more time vigilant, exploring the arena, moving, and with ears forwards. Enrichment also increased the drinking and allogrooming behaviours in home pens in comparison to control calves. Although there were no identified differences in biological functioning between groups, the results of the current study highlight the difficulties in the assessment of affective states in animals as multiple interpretations could be reached with regard to the behavioural test results. More investigation into which enrichments were responsible for these changes would be valuable and could confirm whether the overall welfare of the calves was improved or impaired through enrichment provision. Finally, it is possible that the stress experienced by the calves during both weaning and regrouping may have overruled any positive enrichment effects.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the CSIRO Agriculture Animal Ethics Committee (Armidale). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ED: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JM: Conceptualization, Methodology, Supervision, Writing – review & editing. CL: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. DC: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ED was supported by an Australian Government Research Training Program (RTP) Scholarship through the University of New England, Armidale, NSW, Australia.
Acknowledgments
Thank you to Troy Kalinowski, Jim Lea, Sue Belson, and Tim Dyall for their assistance with data collection.
Conflict of interest
The authors declare that this study received funding from Meat and Livestock Australia Ltd., project number P.PSH.0807. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2024.1364259/full#supplementary-material
References
Antunes M., Biala G. (2012). The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 110. doi: 10.1007/S10339-011-0430-Z
Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Bayne K. (2018). Environmental enrichment and mouse models: Current perspectives. Anim. Models Exp. Med. 1, 82–90. doi: 10.1002/ame2.12015
Bouissou M. F., Boissy A., Neindre P., Veissier I. (2001). The social behaviour of cattle. In Keeling L. J, Gonyou H. W. (Eds), Social Behaviour in Farm Animals (CABI: International, Wallingford, UK), 113–145. doi: 10.1079/9780851993973.0113
Bruno K., Desocio E., White J., Wilson B. K. (2020). Effect of environmental enrichment devices on behavior of individually housed beef heifers. Trans. Anim. Sci. 4, 1–10. doi: 10.1093/TAS/TXAA220
Bulens A., Beirendonck S. V., Thielen J. V., Driessen B. (2014). The effect of environmental enrichment on the behaviour of beef calves. In Mouniet L., Veissier I. (Eds) Proceedings of the 6th International Conference on the Assessment of Animal Welfare at Farm and Group Level, Clermont-Ferrand, France. Available at: https://www.researchgate.net/publication/271012698_The_effect_of_environmental_enrichment_on_the_behaviour_of_beef_calves.
Campbell D. L. M., Lea J. M., Keshavarzi H., Lee C. (2019). Virtual fencing is comparable to electric tape fencing for cattle behavior and welfare. Front. Veterinary Sci. 6. doi: 10.3389/FVETS.2019.00445
Campbell D. L. M., Whitten J. M., Slater E., Lee C. (2021). Rearing enrichments differentially modified hen personality traits and reduced prediction of range use. Anim. Behav. 179, 97–109. doi: 10.1016/j.anbehav.2021.06.024
Ceballos M. C., Góis K. C. R., Sant’Anna A. C., Paranhos Da Costa M. J. R. (2016). Frequent handling of grazing beef cattle maintained under the rotational stocking method improves temperament over time. Anim. Production Sci. 58, 307–313. doi: 10.1071/AN16025
Christensen R. H. B. (2022). Ordinal - Regression Models for Ordinal Data. Available at: https://cran.r-project.org/package=ordinal.
Curley K. O., Paschal J. C., Welsh T. H., Randel R. D. (2006). Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 84, 3100–3103. doi: 10.2527/JAS.2006-055
da Silva M. D., da Silva A. P., Coelho M. G., Poczynek M., de Toledo A. F., Virgínio Junior G. F., et al. (2022). Evaluation of different liquid diets associated with environmental enrichment in the performance and behaviour of dairy calves. Trop. Anim. Health Production 54, 1–9. doi: 10.1007/s11250-022-03331-3
De Passillé A. M. (2001). Sucking motivation and related problems in calves. Appl. Anim. Behav. Sci. 72, 175–187. doi: 10.1016/S0168-1591(01)00108-3
Dickson E. J., Campbell D. L. M., Lee C., Lea J. M., McDonald P. G., Monk J. E. (2022). Beef cattle preference and usage of environmental enrichments provided simultaneously in a pasture-based environment. Animals 12, 3544. doi: 10.3390/ANI12243544
Dickson E., Monk J. E., Lee C., McDonald P. G., Narayan E., Campbell D. L. M. (2024). Loss of a grooming enrichment impacts physical, behavioural, and physiological measures of welfare in grazing beef cattle. Animal 18, 101091. doi: 10.1016/j.animal.2024.101091
Dumontier L., Janczak A. M., Smulders T. V., Moe R. O., Vas J., Nordgreen J. (2022). Early life environment and adult enrichment: Effects on fearfulness in laying hens. Appl. Anim. Behav. Sci. 256, 105750. doi: 10.1016/j.applanim.2022.105750
Ede T., Weary D. M., von Keyserlingk M. A. G. (2022). Calves are socially motivated. JDS Commun. 3, 44–48. doi: 10.3168/JDSC.2021-0132
Enríquez D. H., Ungerfeld R., Quintans G., Guidoni A. L., Hötzel M. J. (2010). The effects of alternative weaning methods on behaviour in beef calves. Livestock Sci. 128, 20–27. doi: 10.1016/j.livsci.2009.10.007
Fell L. R., Walker K. H., Reddacliff L. A., Davies L., Vallance H. J., House J. R., et al. (1998). Effects of yard weaning and pre-feedlot vaccination on feedlot performance of Bos Taurus steers. Anim. Production Aust. 22, 173–176.
Foris B., Sadrzadeh N., Krahn J., Weary D. M., von Keyserlingk M. A. G. (2023). The effect of placement and group size on the use of an automated brush by groups of lactating dairy cattle. Animals 13, 760. doi: 10.3390/ani13040760
Grandin T. (1993). Behavioral agitation during handling of cattle is persistent over time. Appl. Anim. Behav. Sci. 36, 1–9. doi: 10.1016/0168-1591(93)90094-6
Grandin T. (1997). Assessment of stress during handling and transport. J. Anim. Sci. 75, 249–257. doi: 10.2527/1997.751249x
Grandin T. (2019). “The effects of both genetics and previous experience on livestock behaviour, handling and temperament,” in Livestock Handling and Transport. Ed. Grandin T. (Wallingford, UK: CAB International), 58–79. doi: 10.1079/9781786399151.0058
Horvath K. C., Miller-Cushon E. K. (2019). Characterizing grooming behavior patterns and the influence of brush access on the behavior of group-housed dairy calves. J. Dairy Sci. 102, 3421–3430. doi: 10.3168/jds.2018-15460
Ishiwata T., Uetake K., Abe N., Eguchi Y., Tanaka T. (2006). Effects of an environmental enrichment using a drum can on behavioral, physiological and productive characteristics in fattening beef cattle. Anim. Sci. J. 77, 352–362. doi: 10.1111/j.1740-0929.2006.00359.x
Jensen M. B., Kyhn R. (2000). Play behaviour in group-housed dairy calves, the effect of space allowance. Appl. Anim. Behav. Sci. 67, 35–46. doi: 10.1016/S0168-1591(99)00113-6
Kim M.-H., Yang J.-Y., Upadhaya S. D., Lee H.-J., Yun C.-H., Ha J. K. (2011). The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J. Veterinary Sci. 12, 151–157. doi: 10.4142/jvs.2011.12.2.151
Kohari D., Kosako T., Fukasawa M., Tsukada H. (2007). Effect of environmental enrichment by providing trees as rubbing objects in grassland: Grazing cattle need tree-grooming. Anim. Sci. J. 78, 413–416. doi: 10.1111/j.1740-0929.2007.00455.x
Kremer L., Bus J. D., Webb L. E., Bokkers E. A. M., Engel B., van der Werf J. T. N., et al. (2021). Housing and personality effects on judgement and attention biases in dairy cows. Sci. Rep. 11, 22984. doi: 10.1038/s41598-021-01843-w
Krugmann K., Warnken F., Krieter J., Czycholl I. (2019). Are behavioral tests capable of measuring positive affective states in growing pigs? Animals 9, 274. doi: 10.3390/ANI9050274
Laister S., Stockinger B., Regner A. M., Zenger K., Knierim U., Winckler C. (2011). Social licking in dairy cattle-Effects on heart rate in performers and receivers. Appl. Anim. Behav. Sci. 130, 81–90. doi: 10.1016/j.applanim.2010.12.003
Langenhof M. R., Komdeur J. (2018). Why and how the early-life environment affects development of coping behaviours. Behav. Ecol. Sociobiology 72, 1–32. doi: 10.1007/s00265-018-2452-3
Lansade L., Lemarchand J., Reigner F., Arnould C., Bertin A. (2022). Automatic brushes induce positive emotions and foster positive social interactions in group-housed horses. Appl. Anim. Behav. Sci. 246, 105538. doi: 10.1016/J.APPLANIM.2021.105538
Lea J. M., Niemeyer D. D. O., Reed M. T., Fisher A. D., Ferguson D. M. (2008). Development and validation of a simple technique for logging body temperature in free-ranging cattle. Aust. J. Exp. Agric. 48, 741–745. doi: 10.1071/EA07422
Lee C., Cafe L. M., Robinson S. L., Doyle R. E., Lea J. M., Small A. H., et al. (2018). Anxiety influences attention bias but not flight speed and crush score in beef cattle. Appl. Anim. Behav. Sci. 205, 210–215. doi: 10.1016/j.applanim.2017.11.003
Lees A. M., Salvin H. E., Colditz I. G., Lee C. (2020). The influence of temperament on body temperature response to handling in angus cattle. Animals 10, 172. doi: 10.3390/ani10010172
Leruste H., Bokkers E. A. M., Heutinck L. F. M., Wolthuis-Fillerup M., van der Werf J. T. N., Brscic M., et al. (2012). Evaluation of on-farm veal calves’ responses to unfamiliar humans and potential influencing factors. Animal 6, 2003–2010. doi: 10.1017/S1751731112001346
Lueptow L. M. (2017). Novel object recognition test for the investigation of learning and memory in mice. J. Visualized Experiments 126, 55718. doi: 10.3791/55718
Luo L., Reimert I., de Haas E. N., Kemp B., Bolhuis J. E. (2019). Effects of early and later life environmental enrichment and personality on attention bias in pigs (Sus scrofa domesticus). Anim. Cogn. 22, 959–972. doi: 10.1007/S10071-019-01287-w
Luo L., Reimert I., Graat E. A. M., Smeets S., Kemp B., Bolhuis J. E. (2020). Effects of early life and current housing on sensitivity to reward loss in a successive negative contrast test in pigs. Anim. Cogn. 23, 121–130. doi: 10.1007/s10071-019-01322-w
Lynch E. M., McGee M., Doyle S., Earley B. (2011). Effect of post-weaning management practices on physiological and immunological responses of weaned beef calves. Ir. J. Agric. Food Res. 50, 161–174. doi: 10.2307/41549249
Lynch E., Mcgee M., Earley B. (2019). Weaning management of beef calves with implications for animal health and welfare. J. Appl. Anim. Res. 47, 167–175. doi: 10.1080/09712119.2019.1594825
Mandel R., Whay H. R., Klement E., Nicol C. J. (2016). Invited review: Environmental enrichment of dairy cows and calves in indoor housing. J. Dairy Sci. 99, 1695–1715. doi: 10.3168/jds.2015-9875
Matković K., Šimić R., Lolić M., Ostović M. (2020). The effects of environmental enrichment on some welfare indicators in fattening cattle, housed at different stocking densities. Veterinarski Arhiv 90, 575–582. doi: 10.24099/vet.arhiv.1170
Meagher R. K., Campbell D. L. M., Mason G. J. (2017). Boredom-like states in mink and their behavioural correlates: A replicate study. Appl. Anim. Behav. Sci. 197, 112–119. doi: 10.1016/J.APPLANIM.2017.08.001
Meat and Livestock Australia. (2020). Weaner Management in Northern Beef Herds. Available at: https://publications.mla.com.au/login/eaccess?elink=LWSKULS3cmsjsP6ZfaS9.
Meehan C. L., Mench J. A. (2002). Environmental enrichment affects the fear and exploratory responses to novelty of young Amazon parrots. Appl. Anim. Behav. Sci. 79, 75–88. doi: 10.1016/S0168-1591(02)00118-1
Melotti L., Oostindjer M., Bolhuis J. E., Held S., Mendl M. (2011). Coping personality type and environmental enrichment affect aggression at weaning in pigs. Appl. Anim. Behav. Sci. 133, 144–153. doi: 10.1016/j.applanim.2011.05.018
Meneses X. C. A., Park R. M., Ridge E. E., Daigle C. L. (2021). Hourly activity patterns and behaviour-based management of feedlot steers with and without a cattle brush. Appl. Anim. Behav. Sci. 236, 105241. doi: 10.1016/j.applanim.2021.105241
Miranda C. O., Lima M. L. P., Filho A. E. V., Salles M. S. V., Simili F. F., Negrão J. A., et al. (2023). Benefits of tactile stimulation and environmental enrichment for the welfare of crossbred dairy calves. J. Appl. Anim. Res. 51, 130–136. doi: 10.1080/09712119.2022.2162531
Miranda-de la Lama G. C., Pinal R., Fuchs K., Montaldo H. H., Ducoing A., Galindo F. (2013). Environmental enrichment and social rank affects the fear and stress response to regular handling of dairy goats. J. Veterinary Behav. 8, 342–348. doi: 10.1016/J.JVEB.2013.03.001
Mitchell E. N., Marston H. M., Nutt D. J., Robinson E. S. J. (2012). Evaluation of an operant successive negative contrast task as a method to study affective state in rodents. Behav. Brain Res. 234, 155–160. doi: 10.1016/J.BBR.2012.06.016
Monk J. E., Belson S., Colditz I. G., Lee C. (2018). Attention bias test differentiates anxiety and depression in sheep. Front. Behav. Neurosci. 12. doi: 10.3389/FNBEH.2018.00246
Monk J. E., Campbell D. L. M., Lee C. (2023). Future application of an attention bias test to assess affective states in sheep. Anim. Production Sci. 63, 523–534. doi: 10.1071/AN22260
Monk J. E., Lee C., Dickson E., Campbell D. L. M. (2020). Attention bias test measures negative but not positive affect in sheep: A replication study. Animals 10, 1314. doi: 10.3390/ANI10081314
Muller-Schwarze D., Stagge B., Muller-Schwarze C. (1982). Play behavior: persistence, decrease, and energetic compensation during food shortage in deer fawns. Science 215, 85–87. doi: 10.1126/SCIENCE.215.4528.85
Munsterhjelm C., Peltoniemi O. A. T., Heinonen M., Hälli O., Karhapää M., Valros A. (2009). Experience of moderate bedding affects behaviour of growing pigs. Appl. Anim. Behav. Sci. 118, 42–53. doi: 10.1016/j.applanim.2009.01.007
Newberry R. C. (1995). Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 44, 229–243. doi: 10.1016/0168-1591(95)00616-Z
Newberry R. C., Wood-Gush D. G. M., Hall J. W. (1988). Playful behaviour of piglets. Behav. Processes 17, 205–216. doi: 10.1016/0376-6357(88)90004-6
Ninomiya S. (2019). Grooming device effects on behaviour and welfare of Japanese Black fattening cattle. Animals 9, 186. doi: 10.3390/ani9040186
Ninomiya S., Sato S. (2009). Effects of “Five freedoms” environmental enrichment on the welfare of calves reared indoors. Anim. Sci. J. 80, 347–351. doi: 10.1111/j.1740-0929.2009.00627.x
Nithianantharajah J., Hannan A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709. doi: 10.1038/nrn1970
NSW Department of Primary Industries Weaning Beef Calves. (n.d.) Available at: https://www.dpi.nsw.gov.au/animals-and-livestock/beef-cattle/husbandry/general-management/weaning-beef-calves.
Parham J. T., Tanner A. E., Barkley K., Pullen L., Wahlberg M. L., Swecker W. S., et al. (2019). Temperamental cattle acclimate more substantially to repeated handling. Appl. Anim. Behav. Sci. 212, 36–43. doi: 10.1016/J.APPLANIM.2019.01.001
Park R. M., Schubach K. M., Cooke R. F., Herring A. D., Jennings J. S., Daigle C. L. (2020). Impact of a cattle brush on feedlot steer behavior, productivity and stress physiology. Appl. Anim. Behav. Sci. 228, 104995. doi: 10.1016/j.applanim.2020.104995
Pempek J. A., Eastridge M. L., Proudfoot K. L. (2017). The effect of a furnished individual hutch pre-weaning on calf behavior, response to novelty, and growth. J. Dairy Sci. 100, 4807–4817. doi: 10.3168/jds.2016-12180
Proctor H. S., Carder G. (2014). Can ear postures reliably measure the positive emotional state of cows? Appl. Anim. Behav. Sci. 161, 20–27. doi: 10.1016/j.applanim.2014.09.015
R Core Team. (2022). R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing). Available at: https://www.r-project.org/.
Reefmann N., Bütikofer Kaszàs F., Wechsler B., Gygax L. (2009). Ear and tail postures as indicators of emotional valence in sheep. Appl. Anim. Behav. Sci. 118, 199–207. doi: 10.1016/J.APPLANIM.2009.02.013
Reyes F. S., Gimenez A. R., Anderson K. M., Miller-Cushon E. K., Dorea J. R., Van Os J. M. C. (2022). Impact of stationary brush quantity on brush use in group-housed dairy heifers. Animals 12, 972. doi: 10.3390/ani12080972
Riber A. B., Van De Weerd H. A., De Jong I. C., Steenfeldt S. (2018). Review of environmental enrichment for broiler chickens. Poultry Sci. 97, 378–396. doi: 10.3382/ps/pex344
Šárová R., Gutmann A. K., Špinka M., Stěhulová I., Winckler C. (2016). Important role of dominance in allogrooming behaviour in beef cattle. Appl. Anim. Behav. Sci. 181, 41–48. doi: 10.1016/j.applanim.2016.05.017
Sato S., Tarumizu K., Hatae K. (1993). The influence of social factors on allogrooming in cows. Appl. Anim. Behav. Sci. 38, 235–244. doi: 10.1016/0168-1591(93)90022-H
Shepherdson D. J. (2003). Environmental enrichment: Past, present and future. Int. Zoo Yearbook 38, 118–124. doi: 10.1111/j.1748-1090.2003.tb02071.x
Sivakumaran M. H., Mackenzie A. K., Callan I. R., Ainge J. A., O’Connor A. R. (2018). The discrimination ratio derived from novel object recognition tasks as a measure of recognition memory sensitivity, not bias. Sci. Rep. 8, 11579. doi: 10.1038/S41598-018-30030-7
Somarriba M., Lonis W., Roehe R., Macrae A., Dewhurst R. J., Duthie C.-A., et al. (2019). “The effects of a composite chronic stress treatment on fear responses and attention bias in beef cattle,” in Proceedings of the 53rd Congress of the ISAE. Eds. Newberry R. C., Braastad B. O. (Wageningen, Netherlands: Wageningen Academic Publishers), 142. doi: 10.3920/978-90-8686-889-6
Spruijt B. M., Van Hooff J. A. R. A. M., Gispen W. H. (1992). Ethology and neurobiology of grooming behavior. Physiol. Rev. 72, 825–852. doi: 10.1152/physrev.1992.72.3.825
Statham P., Green L., Mendl M. (2011). A longitudinal study of the effects of providing straw at different stages of life on tail-biting and other behaviour in commercially housed pigs. Appl. Anim. Behav. Sci. 134, 100–108. doi: 10.1016/j.applanim.2011.08.009
Strappini A. C., Monti G., Sepúlveda-Varas P., de Freslon I., Peralta J. M. (2021). Measuring calves’ usage of multiple environmental enrichment objects provided simultaneously. Front. Veterinary Sci. 0. doi: 10.3389/FVETS.2021.698681
Therneau T. (2022). A Package for Survival Analysis in R (R package version 3.3-1). Available at: https://cran.r-project.org/package=survival%3E.
Thornton P. D., Waterman-Pearson A. E. (2002). Behavioural responses to castration in lambs. Anim. Welfare 11, 203–212. doi: 10.1017/S0962728600028153
van Praag H., Kempermann G., Gage F. H. (2000). Neural consequences of enviromental enrichment. Nat. Rev. Neurosci. 1, 191–198. doi: 10.1038/35044558
Velasquez-Munoz A., Manriquez D., Paudyal S., Solano G., Han H., Callan R., et al. (2019). Effect of a mechanical grooming brush on the behavior and health of recently weaned heifer calves. BMC Veterinary Res. 15, 284. doi: 10.1186/s12917-019-2033-3
Verbeek E., Colditz I., Blache D., Lee C. (2019). Chronic stress influences attentional and judgement bias and the activity of the HPA axis in sheep. PloS One 14, e0211363. doi: 10.1371/journal.pone.0211363
von Keyserlingk M. A. G., Olenick D., Weary D. M. (2008). Acute behavioral effects of regrouping dairy cows. J. Dairy Sci. 91, 1011–1016. doi: 10.3168/jds.2007-0532
Weary D. M., Jasper J., Hötzel M. J. (2008). Understanding weaning distress. Appl. Anim. Behav. Sci. 110, 24–41. doi: 10.1016/j.applanim.2007.03.025
Young R. J. (2003). Environmental Enrichment for Captive Animals (Oxford, UK: Blackwell Science Ltd). doi: 10.1002/9780470751046
Zhang C., Juniper D. T., McDonald R., Parsons S., Meagher R. K. (2022). Holstein calves’ preference for potential physical enrichment items on different presentation schedules. J. Dairy Sci. 105, 8316–8327. doi: 10.3168/JDS.2021-21715
Zhang C., Juniper D. T., Meagher R. K. (2021). Effects of physical enrichment items and social housing on calves’ growth, behaviour and response to novelty. Appl. Anim. Behav. Sci. 237, 105295. doi: 10.1016/j.applanim.2021.105295
Keywords: beef calves, environmental enrichment, weaning, welfare, affective state
Citation: Dickson EJ, Monk JE, Lee C and Campbell DLM (2024) Environmental enrichment during yard weaning alters the performance of calves in an attention bias and a novel object recognition test. Front. Anim. Sci. 5:1364259. doi: 10.3389/fanim.2024.1364259
Received: 02 January 2024; Accepted: 05 March 2024;
Published: 27 March 2024.
Edited by:
E. Tobias Krause, Friedrich-Loeffler-Institute, GermanyReviewed by:
Margret Vonholdt-Wenker, Friedrich Loeffler Institute, GermanyTemple Grandin, Colorado State University, United States
Copyright © 2024 Dickson, Monk, Lee and Campbell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily J. Dickson, ZW1pbHkuZGlja3NvbkBjc2lyby5hdQ==
 Emily J. Dickson
Emily J. Dickson Jessica E. Monk
Jessica E. Monk Caroline Lee
Caroline Lee Dana L. M. Campbell
Dana L. M. Campbell