- 1Department of Materials and Environmental Chemistry, Stockholm University, Stockholm, Sweden
- 2Advanced Multifunctional Materials Laboratory, Department of Chemistry, Faculty of Science, Assiut University, Assiut, Egypt
Cellulose-based materials have been advanced technologies that used in water remediation. They exhibit several advantages being the most abundant biopolymer in nature, high biocompatibility, and contain several functional groups. Cellulose can be prepared in several derivatives including nanomaterials such as cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), and TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical)-mediated oxidized cellulose nanofibrils (TOCNF). The presence of functional groups such as carboxylic and hydroxyls groups can be modified or grafted with organic moieties offering extra functional groups customizing for specific applications. These functional groups ensure the capability of cellulose biopolymers to be modified with nanoparticles such as metal-organic frameworks (MOFs), graphene oxide (GO), silver (Ag) nanoparticles, and zinc oxide (ZnO) nanoparticles. Thus, they can be applied for water remediation via removing water pollutants including heavy metal ions, organic dyes, drugs, and microbial species. Cellulose-based materials can be also used for removing microorganisms being active as membranes or antibacterial agents. They can proceed into various forms such as membranes, sheets, papers, foams, aerogels, and filters. This review summarized the applications of cellulose-based materials for water remediation via methods such as adsorption, catalysis, and antifouling. The high performance of cellulose-based materials as well as their simple processing methods ensure the high potential for water remediation.
Introduction
Water pollution represents a big challenge nowadays (WHO, 2019). Clean drinking water is highly required for safety and healthy life. WHO stated that half of the world’s population will face water-stressed demand by 2025 (WHO, 2019). It is estimated that 785 million people lack a basic drinking-water service (WHO, 2019). Clean drinking water decreases over time due to pollution. The air pollutions with toxic gases such as sulfur oxides and nitrogen oxide render the rainwater acidic. Sea and ocean water can be contaminated with several pollutants such as heavy metals, organic molecules (e.g., dyes, drugs, and antibiotics), and microplastics. WHO reported that 90% of the global population (6.8 billion people) require at least a basic service to obtain clean drinking water. The basic service to remove or mitigate water pollutants is highly required for clean drinking water.
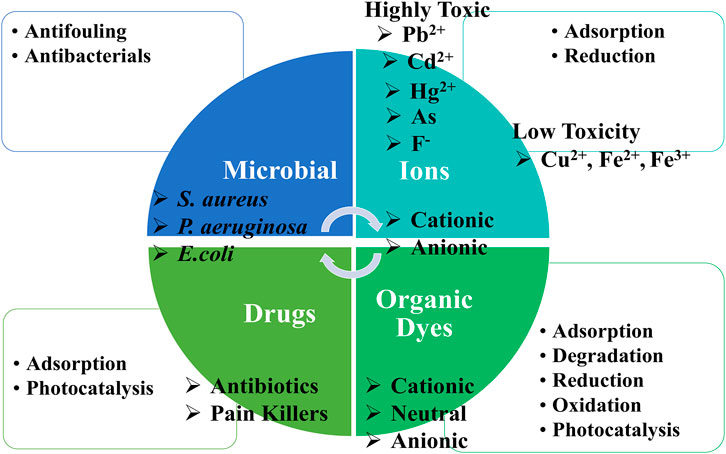
Water treatment aims to remove or mitigate the toxicity of pollutants that could be metal ions, organic dyes, drugs, or microorganism species (Figure 1). The pollutants can be inorganic (e.g., metal ions), organic (dyes, drugs, proteins, and antibiotics), or microorganisms (e.g., fungus, bacteria, and algae). They can be classified as degradable (e.g., dyes, proteins, and antibiotics), or non-degradable (e.g., metal ions). The toxicity of the water contaminants is varied and depends on several factors including the form of the pollutants, concentration, administration method, and exposure time. However, there are several highly toxic pollutants such as heavy metals of arsenic (As), lead (Pb), cadmium (Cd), and Mercury (Hg). The water pollutants were listed based on their toxicity by The Agency for Toxic Substances and Disease Registry and the United States Environmental Protection Agency (EPA), ‘2019 Priority List of Hazardous Substances’ in Reference (ATSDR, 2017). Drugs such as painkillers can be also toxic for humans (Khadir et al., 2020). It was reported that approximately 15–23% of ingested drugs such as Ibuprofen are excreted to water through urine (Tiwari et al., 2017).
Nanotechnology has been advanced in several applications including water treatment (Qu et al., 2013; Tan et al., 2015; Santhosh et al., 2016; Westerhoff et al., 2016). Nanomaterials can be applied for several methods such as chemical precipitation, adsorption, ion exchange, membrane, separation, filtration, coagulation/flocculation, flotation, catalysis, and electrochemical-based methods. Among the wide number of nanomaterials, cellulose-based materials are promising. Cellulose exhibits several advantages being an abundant and non-toxic chemical. The functional groups of cellulose ensure rational chemical modification via several methods and reagents (Wang, 2019). It can be fabricated with other biopolymers such as chitosan (Olivera et al., 2016; Thakur and Voicu, 2016), and inorganic nanoparticles (Qiu and Netravali, 2014; Oun et al., 2020; Yu et al., 2021) such as metal oxides (Oun et al., 2020). It enables the processing of the materials into commercial products such as membranes (Thakur and Voicu, 2016) and can be advanced engineering technologies (Wei et al., 2014).
This review summarizes the applications of cellulose-based nanomaterials and their hybrids for water treatments such as the removal of pollutants (e.g., heavy metals and organic dyes) and antifouling properties. The applications of cellulose-based materials for catalytic degrdaration, microbial removing via filtration and anti-microobial activity are also included. The key parameters affecting the performance of cellulose are covered.
Cellulose in Nanoscale
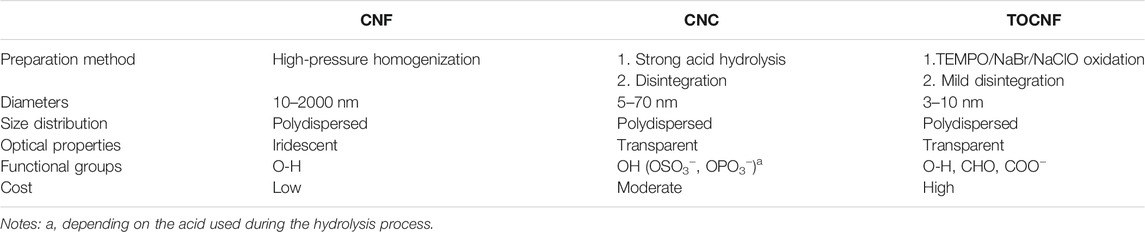
Cellulose, an abundant biopolymer with a production capacity of and approximately 700 billion tons annually (Pulidindi and Prakash, 2020) has been used since the time of ancient Egyptians, The global market indicates that the cellulose market’s size was estimated to be USD 211.68 billion in 2019 with an expected increase of compound annual growth rate (CAGR) by 2.9% from 2020 to 2026 (Bacakova et al., 2019). Cellulose is used for several important industries such as paper making, Cellophane, textiles (e.g., Viscose, and Rayon) as well as additives for food and pharmaceuticals. It can be used as raw material for the production of fuel sources such as cellulosic ethanol. Cellulose can be functionalized with carboxymethyl groups and form carboxymethyl cellulose (CMC) via replacing the primary hydroxyl groups, C6, using reagents such as chloroacetic acid via chemical reaction. The cellulose research is growing tremendously during since the 1990s for the advanced processing of cellulose-based materials in the nanoscale (Figure 2) (Carpenter et al., 2015; Rol et al., 2019). It can be prepared with different functional groups in different sizes and derivatives including cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), and TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical)-mediated oxidized cellulose nanofibrils (TOCNF). These nanoscaled entries can be prepared via several methods including mechanical, chemical, and biological processes (Xie et al., 2018).
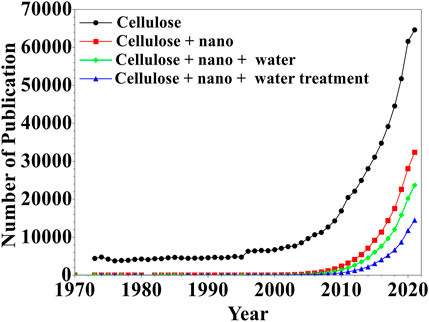
FIGURE 2. Evolution of cellulose and their applications for water treatment using searching words of “Cellulose”, “Cellulose + nano”, “Cellulose + nano + water”, “Cellulose + nano + water treatment”. Data extracted from SCOPUS, Nov. 2021.
The chemical treatments using acids create negatively charged groups on the surface of CNCs causing repulsion between CNCs and assist the mechanical disintegration using methods such as an ultrasonic homogenizer. TOCNF with a diameter of 3–4 nm and length of a few microns can be prepared via TEMPO-mediated oxidation of cellulose fibers in a mild aqueous environment (Saito et al., 2007; Isogai et al., 2011). The oxidation process produces fibrils with average carboxylate content of 1.0–1.8 mmol/g depending on the oxidation conditions such as TEMPO/NaBr/NaClO at pH 10 at room temperature (RT) or TEMPO/NaClO/NaClO2 at pH 6.8 at 60 °C. The oxidation process is selective at the position of C6-hydroxyl groups due to the steric hindrance of methyl groups of TEMPO molecules. TEMPO-mediated CNF was disintegrated via moderate mechanical treatment. TOCNF exhibits high crystallinity (65–95%), a high aspect ratio (>100), and high negative zeta potential in an aqueous solution (−75 mV, Table 1). It is worth mention that ecofriendly oxidation of microcrystalline celluloses (MCCs) using Fe2+/H2O2 oxidant offered CNCs with the highest carboxyl content of 2.2 mmol/g with a zeta potential of −41 mV (Fan et al., 2019). Different nanoscaled cellulose, have been employed for water purification motivated by the high abundance, high specific surface area, high surface-to-volume ratio, good mechanical properties in an aqueous medium, surface functional groups, and surface charge density of these nanomaterials (Table 1).
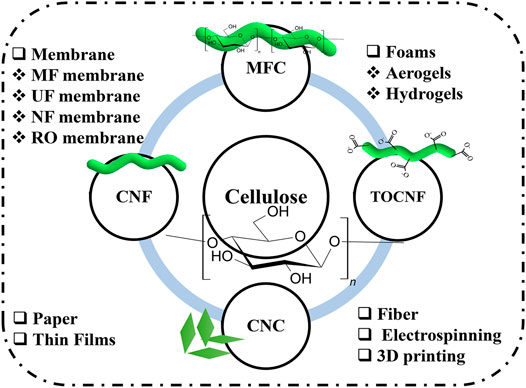
These cellulose nanomaterials can be used for the fabrication of thin films, papers and membranes (microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) (Klemm et al., 2011; Carpenter et al., 2015; Voisin et al., 2017; Nasrollahzadeh et al., 2021) (Figure 3) or as (fibers), and three-dimensional (3D) objects (foams, aerogels, beads, and 3D printing, Figure 3).
Cellulose are inherently hydrophilic and can be modified with hydrophobic or oleophilic moieties for the removal of organic pollutants such as oils and cyclohexenes (Zhang X. et al., 2014; Zhang Z. et al., 2014; Wang et al., 2014). It can be also modified with other biopolymers such as chitosan (Abdul Khalil et al., 2016; Olivera et al., 2016), polymers (Kumar et al., 2017; Patel et al., 2019; Aguilar-Sanchez et al., 2021), dendrimers (Zhao et al., 2015) and nanomaterials such as noble metallic nanoparticles (Johnson et al., 2011), carbon (Dong et al., 2021), carbon nanotubes (CNT) (Miyashiro et al., 2020), graphene oxide (GO) (Soliman et al., 2021), metal oxides (Foresti et al., 2017; Farooq et al., 2020; Oyewo et al., 2020), zeolites (Baghdad and Hasnaoui, 2020), and metal-organic frameworks (MOFs) (Abdelhamid and Mathew, 2021; Abdelhamid, 2021b; Nasser Abdelhamid and Mathew, 2021). The modifications via chemical and physical methods improve the mechanical properties of cellulose and enhance their performance towards water treatment.
Water Treatment Technologies Using Cellulose
The Agenda 2030 explicitly put forth clean water and sanitation as one of the Global Sustainable Development Goals (SDG #6), emphasizing that “more than 2 billion people are living with the risk of reduced access to freshwater resources and by 2050, at least one in four people is likely to live in a country affected by chronic or recurring shortages of fresh water.”
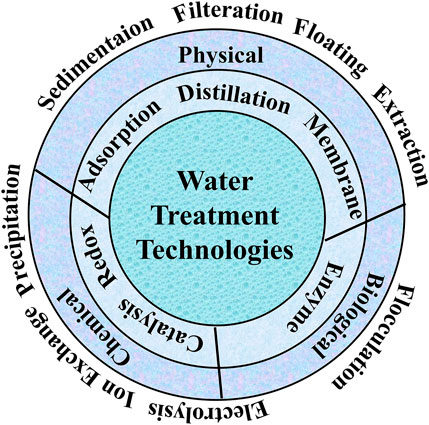
There are several methods to remove pollutants from water; classified as chemical, physical, and biological methods (Figure 4). Chemical methods can be such as precipitation (via chemical reaction, electrochemical), complexation, degradation (via chemical reaction (oxidation, reduction). The water treatment via physical methods includes adsorption, sedimentation, filtration, extraction, floating, and degradation via light (photocatalysis), or heat (pyrolysis). The biological methods including enzymatic degradation, and flocculation can be also used for the removal of pollutants such as organic contaminants. The judicious selection of the methods depends on several parameters including the type of the pollutants, concentration, the present form, interferences, and efficiencies. For instance, the water treatment methods that suit the removal of organic dyes could be unsuitable for the removal of heavy metals and vice versa. The selection of the removal method depends also on the concentration of the pollutants. As a rule of thumb, the high concentration of the pollutants such as heavy metals decreases the removal efficiency of the method such as adsorption. Thus, a simple method such as dilution improves the adsorption efficiency.
The section below shows the possibility for using cellulose based materials in the above mentioned water treatment technologies. Cellulose-based materials may circumvent some of the current challenges related to biological treatments and chemical treatments (e.g., multiple steps, addition of chemicals, secondary pollution, undesired side products or degradation products) and open new avenues for advanced technologies.
Chemical precipitation is an old method for the removal of metal ions and anionic species. This method involves the addition of chemical reagents that react with contaminating and form a solid that precipitates. Thus, the pollutants can be removed via decantation or filtration through filter paper or membrane. Several examples of precipitating agents including cellulose were reported for the co-precipitation of metal ions such as Cd2+ ions (Sharma et al., 2018), and Cu2+ ions (Zhu et al., 2017). The precipitates are usually filtrated through a commercial cellulosic filter paper such as Whatman® filter paper.
Coagulation or flocculation is an efficient method for water treatment. This method relies on the addition of a chemical coagulant that causes neutralization of the charged particles in water causing the aggregation and precipitation of the particles. It includes the addition of reagent such as cellulose, aluminum chloride, ferric chloride that causes coagulation for pollutants. This method can be used for the removal of metals, suspended solids, and total dissolved solids (TDS). However, it is limited to the formation of a large volume of alkaline sludge. The coagulant could be a source of secondary pollutants. Thus, the application of biopolymers such as cellulose as a coagulant is interesting (Liu H. et al., 2019; Ray and Iroegbu, 2021). Cellulose is eco-friendly biomaterials and requires a few steps (Nkalane et al., 2019). Sawdust-derived CNC was reported as a coagulant for Ni2+ and Cd2+ ions (Oyewo et al., 2019). It offered adsorption capacities of 956.6 mg/g and 2,207 mg/g for Ni2+ and Cd2+ ions, respectively (Oyewo et al., 2019). When modified with hexadecyltrimethylammonium bromide (HDTMA-Br) showing higher performance compared to a commercial coagulant such as R2T2 (Nkalane et al., 2019).
The adsorption process is based on the interaction between the pollutants (adsorbate) and the external surface of solid materials (adsorbent). This technique is the common method for the removal of pollutants such as metal ions and dyes. The interactions can take place via 1) physical forces: such as electrostatic, hydrogen bonds, van der Waals; and 2) chemical forces: such as ion exchange and complexation. The adsorption process offers several advantages such as high reversibility offering the reuse of spent adsorbent via desorption. Thus, the pollutants can be used and reconsidered as a source for chemicals. The adsorption process depends on the good matching between the adsorbent and adsorbate. Adsorbents with large surface area, porosity, and small particle size offer usually high adsorption efficiencies.
The adsorption of heavy metal ions via ion-exchange materials such as cellulose and resins relies on the exchange between heavy metal ions and innocuous ions such as sodium (Na+) or potassium (K+). This method is strictly used for metal ions. Thus, it cannot remove other pollutants such as dyes or bacteria. Furthermore, the high concentration of metal ions such as Na+, and K+ in drinking water may affect the ecological system and causes a neurological disorder.
The separation of a mixture containing several liquids can be performed via methods such as funnel separation (simple separation of two immiscible liquids), distillation (depending on evaporation of liquids to gas at a different temperature depending on boiling points of each liquid), and pervaporation (is a processing method for the separation of mixtures of liquids via partial vaporization through a membrane). Zeolite 13 X/regenerated cellulosic membrane was reported for the separation of a mixture of water–glycerol via pervaporation (Dogan and Hilmioglu, 2010). The membrane displayed a flux and selectivity of 65 gm−2h−1 and 1,681, respectively for 90 wt% glycerol aqueous solution. A cellulose membrane with 20 wt% of zeolite 13X showed higher selectivity compared to cellophane or the pristine cellulosic membrane (Dogan and Hilmioglu, 2010).
Biological methods can be used for the removal of organic pollutants. These methods are widely used on an industrial scale. They depend on the use of a biological species such as bacteria, or other organisms that can break down organic compounds. They can be divided into aerobic (presence of oxygen) and anaerobic (absent of oxygen) processes. The process is effective for the removal of organic pollutants. However, they usually required a secondary treatment process such as dissolved air flotation (DAF) to remove material remaining after the primary treatment such as sedimentation. The process requires high energy. Thus, advanced technologies such as membrane aerated biofilm reactor (MABR) save 90% of the required energy for the aeration process offering a promising future.
Chemical reactions such as the advanced oxidation process (AOPs) become a well-known technique for water treatment. They are highly efficient for the removal of organic-based pollutants. They depend on the degradation of the organic-based contaminants into small fragments with less harmful effects. They can be applied without producing secondary waste. The degradation process can take place via a light source in the presence of a catalyst (i.e., photocatalysis reaction), heat (i.e., pyrolysis), ozone (O3, ozonation), or electron (ionization).
Water treatment requires several steps involving different methods. These stages may cause residual of the chemical added during the treatment. For example, the chemical disinfection step creates undesirable disinfection by-products (DBPs) such as chloroform (CHCl3) and high mutagenic and/or carcinogenic potential. Chemical reagents such as chloride or ozone may cause the formation of chlorinated organics, or oxo-anions species, respectively. Thus, the water quality may be decreased. Cellulose-based materials may circumvent some of the current challenges and open new avenues for advanced technologies.
The review is focusing is on three aspects of 1) Adsoprtion 2) Cataylsis and 3) Anti-microorganism including anti-fouling for water treatment using cellulose-based materials. Cellulose with and without nanomaterials are reviewed. The water treatment of pollutants such as metal ions, drug, dye, antibiotics, and microorganism are reported. The separation of oils using cellulose-based materials is also included.
Adsorption
Cellulose-Based Adsorbents for Metal Ions
Heavy metals are toxic and non-biodegradable pollutant prevalent in wastewater. Heavy metal ions can be removed from wastewater via several approaches such as adsorption, membrane filtration, precipitation, sedimentation, coagulation, flocculation, and electrochemical treatment (e.g., electro-precipitation). Among these methods, the adsorption process is promising due to low cost, high removal efficiency, and the adsorbed metals can be further used when it is needed.
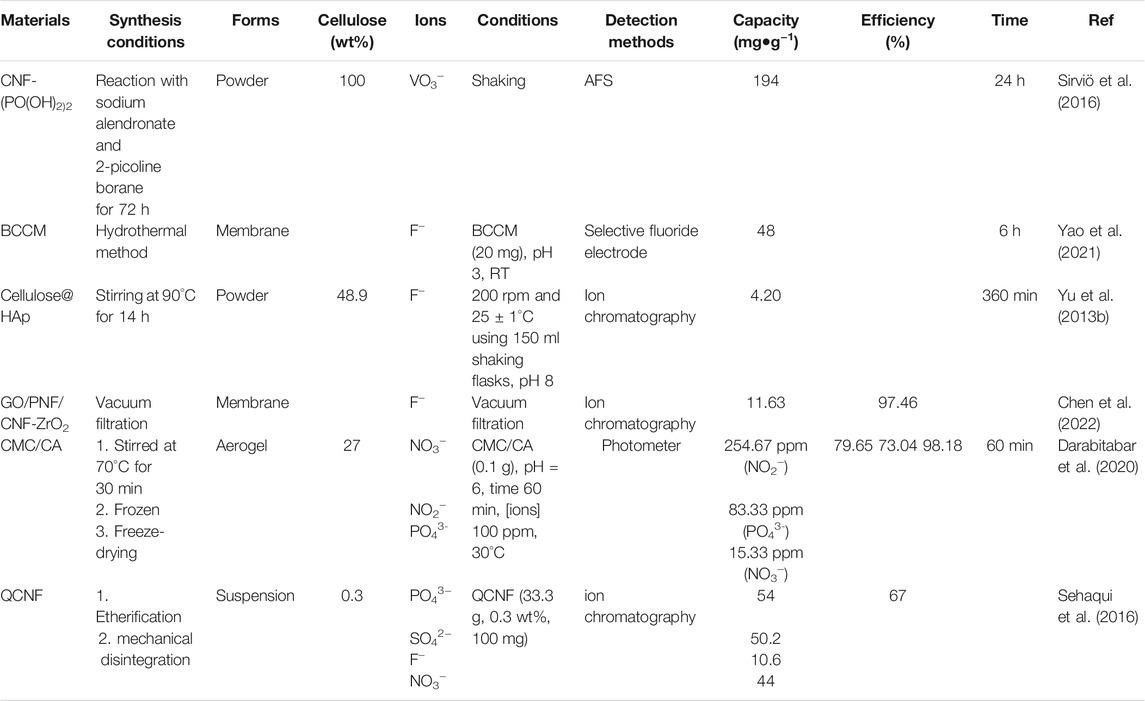
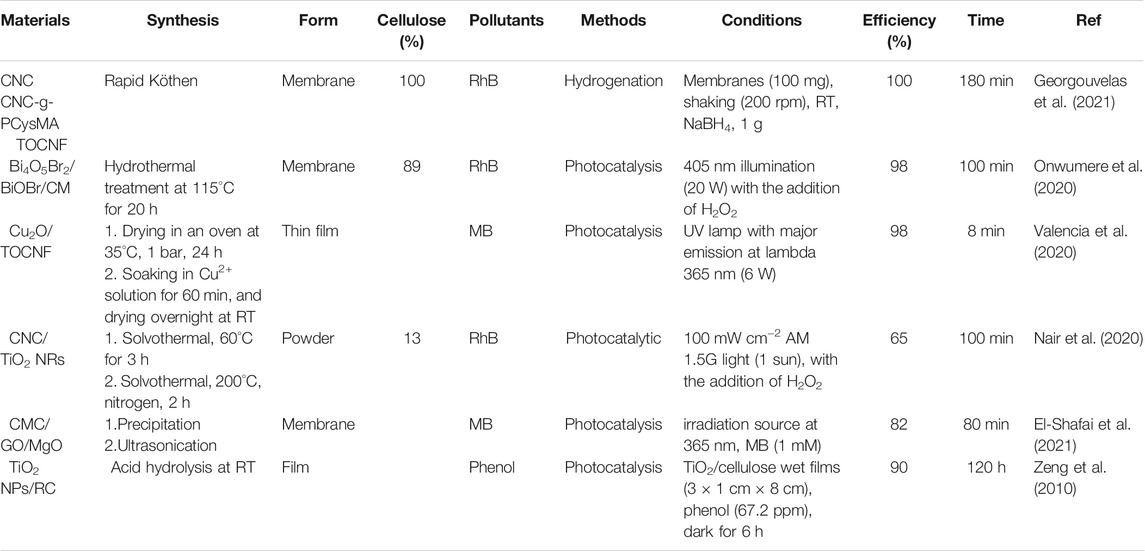
Cellulose-based materials were reported for the adsorption of metal ions such as UO22+ (Sharma et al., 2020a), As3+ (Chen et al., 2019; Sharma S. K. et al., 2020), Cu2+ (Qin et al., 2019), Cd2+ (Movaghgharnezhad et al., 2020), Cr6+ (Awang et al., 2019.; Reshmy et al., 2022), Pb2+ (Hernández-Francisco et al., 2020; Sharma P. R. et al., 2021), and Hg2+ (Chen et al., 2021). The functional groups of cellulose materials play an important role in the adsorption of metal ions. TOCNF showed higher adsorption toward Cu2+ ions and dye molecules compared to CNC (Zhu et al., 2020). The same observation was also reported for carboxylated CNCs that exhibited higher metal adsorption than non-modified CNFs (obtained through mechanical treatment) (Kardam et al., 2014). Cellulose nanofibrils (CNF) were modified with CMC (denoted as CNF-CMC) for the adsorption of Ag+, Cu2+, Pb2+, and Hg2+ ions due to the abundant negatively charged groups (e.g., hydroxyl and carboxyl groups) (Chen et al., 2016). Cellulose materials such as MFC can be grafted with methyl and phosphate functional for metal ions adsorption (Karim and Monti, 2021). CNC-containing O-SO3- functional group (modified during the hydrolysis using sulfuric acid) exhibited higher Ag+ adsorption capacity (34 mg/g) than CNF (obtained via mechanical grinding, 14 mg/g) (Liu et al., 2014). The hydroxyl group of CNC was modified with succinic anhydride (denoted as SCNCs) for high adsorption uptake of Pb2+ and Cd2+ ions (Yu et al., 2013a). SCNCs can be further modified with NaHCO3 to prepare sodium salt i.e. NaSCNCs. The sodium adsorbent i.e., NaSCNCs showed higher adsorption capacities compared to SCNCs (Table 2).
Cellulose can be functionalized by amination (Araki et al., 2001; Singh et al., 2014), xanthation (Pillai et al., 2013), sulfonation (Septevani et al., 2020), thiols (Choi et al., 2020), and phosphorylation (Sirviö et al., 2016) to ensure high adsorption capacity and strengthens the binding between the adsorbent and the metal ions species. Amino-functionalized bacterial cellulose using diethylenetriamine (EABC) was also reported (Shen et al., 2009). The presence of amino-functional groups enhanced the metal ion’s adsorption. The presence of functional groups such as carboxylic, amine, sulfonate, or phosphate ensures high adsorption toward cationic metal ions. The metal adsorption onto cellulose fibers is increased linearly with the increase of the content of these functional groups.
The nitro-oxidation method for CNF, denoted as NOCNF, using raw Australian spinifex grass via nitric acid and sodium nitrite was reported for metal adsorption (Sharma et al., 2018). The extracted NOCNF exhibited medium crystallinity with a crystallinity index (CI) of 53%. It showed surface charge, carboxylated content, and static contact angle of −68 mV, 0.86 mmol/g, and 38°, respectively. The material displayed exceptional high uptake of Cd2+ ions with a maximum adsorption capacity of 2,550 mg/g. The high adsorption capacity was explained due to the interactions between carboxylate groups of NOCNF and Cd2+ ions leading to cross-linking and precipitation as Cd(OH)2 nanocrystals (Sharma et al., 2018).
Cellulose adsorbents in the form of membranes, beads, foams etc are also reported. Cellulose-membranes were prepared using CNCs, TOCNF, or zwitterionic polymer grafted CNC (CNC-g-PCysMA) (Georgouvelas et al., 2021) and used for the adsorption of Au3+, Co2+, and Fe3+ ions (Table 2). The synthesis procedure is simple and offers the fabrication of a robust membrane with a diameter size of 200 mm using the sheet former method Rapid Köthen (Georgouvelas et al., 2021). The membrane exhibited high adsorption performance toward heavy metal ions with good adsorption capacities (Table 2).
Park et al. reported particles of poly (acryloyl hydrazide) (PAH)-grafted CNC (CNC-PAH) was synthesized via the atom transfer radical polymerization (ATRP) method for the adsorption of Cr6+ ions (Park et al., 2020). It showed a rapid adsorption rate with an adsorption capacity of 457.6 mg/g at room temperature (RT). The high adsorption capacity wsa attributed to the numerous amine groups in the grafted polymers (Park et al., 2020). CNC-PAH showed 20% higher adsorption capacity than that for poly (amidoamine)-grafted CNF (PAMAM-g-CNF) aerogel (377.4 mg/g) at similar conditions (Table 2) (Zhao et al., 2015).
TOCNF/calcium alginate beads and aerogels were reported for the adsorption of heavy metals such as Cu2+ ions (Fiol et al., 2019). TOCNF beads and aerogels exhibit removal efficiencies of 60%, and 94%, respectively. The adsorption of Cu2+ ions for the beads is mainly due to alginate. Thus, the adsorption capacity is decreased with the increase of TOCNF contents in the beads (Fiol et al., 2019). However, the highest adsorption Cu2+ capacity (374 mg/g) was reported using TOCNF-based membranes (Sludge–CNFSL/TEMPO–CNCBE) (Karim et al., 2017). The adsorption of Cu2+ ions can be improved via treatment with NaOH solutions, adjusting the pH of the solution, or adding polymers. TOCNF-polyethyleneimine (PEI) exhibited higher Cu2+ removal ability compared to the use of TOCNF alone (Zhang et al., 2016). It can be regenerated and reused after treatment with HCl for several cycles with an adsorption performance of 33 mg/g which represents 63% of the initial performance (Zhang et al., 2016).
Cellulose mixed with other biopolymers such as sodium alginate (SA) can be easily processed into beads. The biobased additive of SA play important role in the material’s processing as well as metal adsorption performance. Beads of carboxylated cellulose nanocrystal and sodium alginate was reported for the adsorption of toxic heavy metals such as Pb2+ ions (Hu et al., 2018). Alginate exhibits a significant contribution to the adsorption performance. The beads exhibit high adsorption efficiency with good recyclability (Hu et al., 2018). SA–CMC beads were also synthesized via blending and cross-linking in CaCl2 and FeCl3 solutions (Ren et al., 2016). The adsorption of Pb2+ takes place via physical, chemical, and electrostatic interactions (Ren et al., 2016).
The pH value of the solution plays important role in the adsorption process. The wastewater is usually acidic. Thus, cellulose can be used as an effective adsorbent compared to other adsorption that shows instability under acidic conditions. The metal adsorption onto cellulose nanofibers is usually performed in the pH values range between 3 and 7. The basic pH value (>7) is usually avoided to prevent the precipitation of the metal ions as hydroxide or oxides.
Nanocellulose Hybrids Adsorbent for Metal Ions
Nanohybrids material of nanocellulose with a seconr nanomaterial offer several advantages in water treatment (Wang, 2019).; for e.g., hybrid materials that show high mechanical properties and offer high adsorption capacities (Table 2). Nanocellulose-graphene oxide (GO) membranes were reported for the adsorption of metal ions (Cu2+ and Ag+) from aqueous solutions (Zhu et al., 2017, 2018; Valencia et al., 2019). The adsorption process was followed in real-time using in-situ small-angle X-ray scattering (SAXS) and reactive molecular dynamics (ReaxFF) computational simulations (Figure 5). The analysis of SAXS data suggested that the GO layers prevent the swelling and structural deformations of CNFs during the filtration of aqueous metal solutions. The adsorbed metal ions formed nanoparticles during the drying stage. The particle size of the formed nanoparticles is increased with the time of the drying process. The molecular dynamics simulations data confirm the motion of the metal (Cu2+ and Ag+) ions into the membrane for the formation of metal clusters during adsorption (Figure 5) (Zhu et al., 2018). The high adsorption performance of the prepared membrane is mainly due to the synergistic effect of GO and CNFs (Valencia et al., 2019). GO/carboxymethyl cellulose nanofibril (CMCNF)-Fe3+ exhibited high adsorption efficiency for Pb2+ with good adsorbent recovery (Yu et al., 2020). GO and cellulose can be self-assembled into continuous filament via interfacial nanoparticle complexation (Zhang et al., 2020).
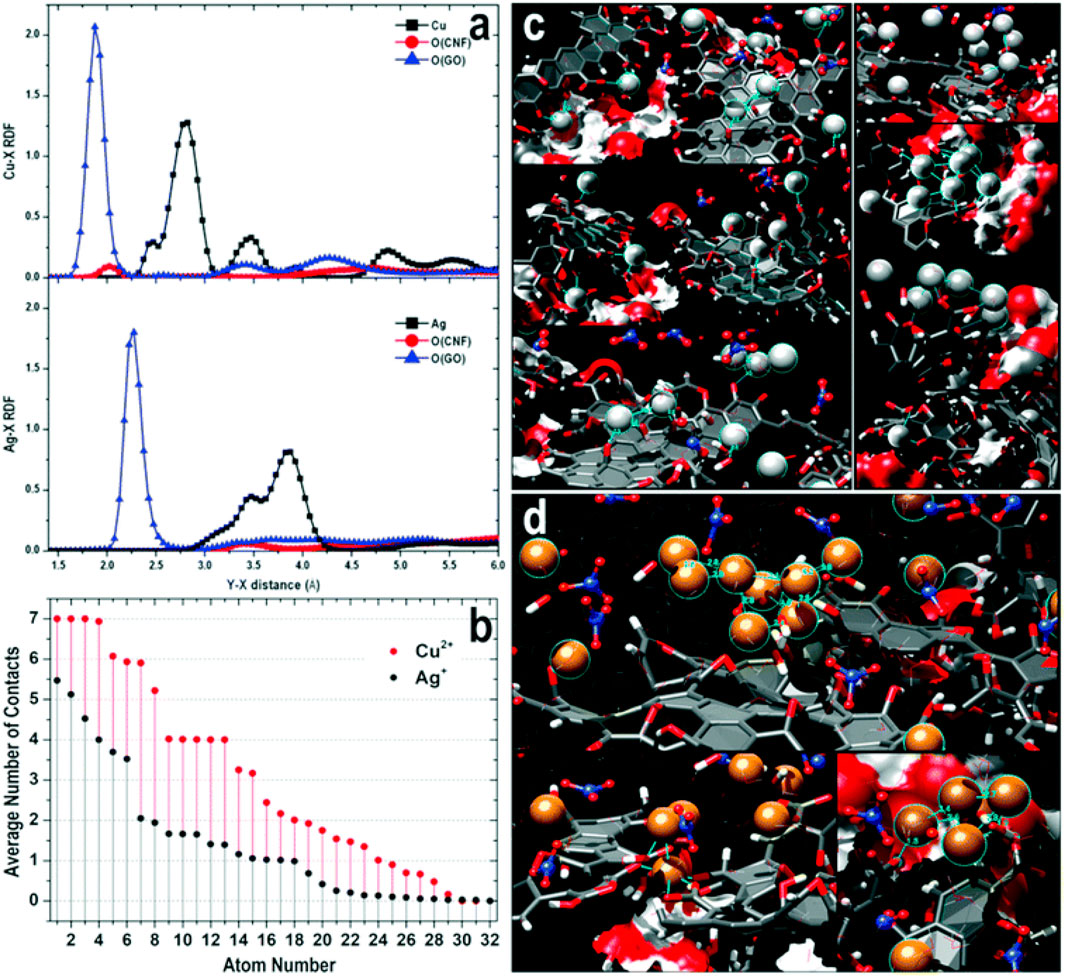
FIGURE 5. (A) Normalized radial distribution functions (RDFs) of the adsorption of Cu2+ and Ag + on CNF–GO, (B) the Average number of metal ions found around each metal ion (within 7 Å) during the simulation; The total number of metal ions is 32 in each case, (C) Ag + ions are represented with light grey spheres and NO3− ions are rendered with ball and stick models (blue nitrogen atoms), (D) Adsorption of Cu2+ ions on CNF–GO (layer model). Cu2+ ions are represented with orange spheres and NO3− ions are rendered with ball and stick models (blue nitrogen atoms). CNFs are rendered through red (oxygen) and white (hydrogen) surface patches (carbon—light grey), and GO is displayed through dark grey stick models with filled aromatic rings. Figure reprinted from Ref. (Valencia et al., 2019). This Open Access Article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
Cellulose-metal oxide nanocomposites have been reported for the adsorption of metal ions. A hybrid membrane consisting of cellulose membrane (CM) and Bi4O5Br2/BiOBr nanosheets were reported for metal ions adsorption of Ni2+ and Co2+ ions (Onwumere et al., 2020). The synthesized membrane contains mixed materials of BiOBr (JCPDS-01-085-0862) and Bi4O5Br2 (JCPDS-37–0699) (Onwumere et al., 2020). The adsorption capacities of Co2+ and Ni2+ were 28.7 mg/g and 29.7 mg/g, respectively using CM; 37.3 mg/g and 30.2 mg/g, respectively for CM/Bi4O5Br2/BiOBr (Onwumere et al., 2020). Cellulose/ZrO2 composite showed selective adsorption toward Ni2+ (Khan et al., 2013).
Rice husk and sugarcane bagasse derived nanocellulose iron oxide nanocomposites (NIONs) showed adsorption of As3+ ions (Baruah et al., 2020). Fe2O3/CNs were synthesized via the solvothermal method for the adsorption maximal amount of As3+ and As6+ ions (Dong et al., 2020). It showed adsorption capacities of 13.866 and 15.712 mg/g at pH of 7 and 3, respectively (Dong et al., 2020). Magnetic attapulgite (ATP)/chitosan/BC nanofibrils (BCNs) were reported for the adsorption of Pb2+, Cu2+, and Cr6+ (Chen et al., 2020). The material can be separated using an external magnetic field. It can be recycled several times with only less than an 8% decrease after five cycles of the adsorption-desorption process (Chen et al., 2020). The use of nanomaterials such as magnetic nanoparticles offered several advantages such as simple separation via a simple external magnetic field (Haniffa et al., 2021). The hybrid materials exhibit the advantages of both entities. They showed also good mechanical properties and high adsorption performance (Table 2).
Cellulose-MOFs (denoted as CelloMOF) composites are widely reported for metal ions adsorption (Xiao et al., 2021). A foam consisting of MIL-100(Fe) and BC nanofibers was reported for As3+ ions adsorption with a maximum adsorption capacity of 4.81 mg/g (Ashour et al., 2020). The presence of MOF materials such as zeolitic imidazolate frameworks (ZIF-8) enhanced the adsorption efficiency for ZnO nanorods loaded a cotton fabric (Schelling et al., 2020). ZIF-8@ZnO@cotton and ZnO@cotton showed an uptake efficiency of 70 and 38%, respectively (Schelling et al., 2020). The high adsorption efficiency is mainly due to the large surface area of MOF materials. Magnetic cellulose nanocrystals (MCNC) and a Zn-based MOF were used for the adsorption of Pb2+ ions (Wang et al., 2017). MCNC@Zn-MOF and MCNC showed adsorption capacities of 558.66 mg/g and 92.24 mg/g, respectively (Wang et al., 2017). Cellulose improves also the adsorption performance of MOF materials. A composite of ZIF-8 and BC offered 1.2 times higher adsorption capacity toward Pb2+ ions compared to pure ZIF-8 (Ma X. et al., 2019). Cellulose offers also a MOF-based material with a lightweight and porous structure (Bo et al., 2018; Ma S. et al., 2019; Bahmani et al., 2020). Cellulose-MOFs materials can be prepared as aerogels (Zhu et al., 2016; Lei et al., 2019; Li J. et al., 2020), filter paper (Hashem et al., 2019), substrate (Gu et al., 2020), membrane (Yang et al., 2018), and foams (Liu et al., 2021). Further information for CelloMOF for water treatment can be found in the Review (Abdelhamid and Mathew, 2022).
Removal of Anionic Pollutants
Water contamination with anionic species, such as fluoride (F−), sulfate (SO42-), nitrate (NO3−), nitrite (NO2−), and phosphate (PO43-), affect human’s health. Anionic species such as F− are highly risky due to the penetration of human skin and dissolving the bone and teeth (convert hydroxyapatite (Ca10(PO4)6(OH)2) to form fluorapatite (Ca5(PO4)3F)). WHO guidelines stated that the daily drinking water containing F− ions should be not more than 1.5 ppm. Drinking of water-containing ions such as sulfate (>600 ppm) results in laxative effects. Anionic pollutants such as sulfate, nitrate, nitrite, and phosphate ion are very water-soluble species render their removal a tedious task.
Anionic pollutants can be removed from water via several methods including precipitation and adsorption. The use of anionic adsorbents such as cellulose renders the adsorption of anionic species inefficient (Table 3). Thus, cationic cellulose or cellulose-based nanocomposites are widely used for the adsorption of anionic pollutants (Table 3). Cationic cellulose nanofibers can be an effective adsorbent for anions e.g., nitrate and fluoride. (Table 3). A study reported that the adsorption capacity of anionic dyes via quaternized cellulose nanofibrils using trimethylammonium chloride (TMAC) was increased with the increasing of TMAC’s content on cellulose nanofibrils (Pei et al., 2013). Cationic cellulose nanofibers containing positively charged quaternary ammonium groups (QCNF) were synthesized from waste pulp residues (Sehaqui et al., 2016). The synthesis procedure involved the etherification of the pulp using glycidyl trimethylammonium chloride followed by mechanical disintegration. The produced material has a cationic charge content of 1.2 mmol/g. QCNF showed selective adsorption for multivalent ions e.g., PO43− and SO42− compared to monovalent ions e.g., F− and NO3− (Sehaqui et al., 2016).
Cerium oxide (CeO2) nanoparticles were in-situ grown into biomass recyclable cellulose membrane (BCCM) for the adsorption of fluoride from industrial wastewater (Table 3) (Yao et al., 2021). BCCM showed maximum adsorption of 48.0 mg/g for F− ions (Yao et al., 2021). The membrane exhibited high adsorption capacity compared to cellulose/hydroxyapatite (HAp, Table 3) (Yu et al., 2013b). GO/CNFs/ZrO2 self-assembled peptide nanofibers (PNFs), denoted as GO/PNF/CNF-ZrO2, were synthesized via a biomineralization process for the adsorption of F− ions (Chen et al., 2022). The membrane offered selectivity of 95.39% with maximum adsorption of 11.63 mg/g. The adsorption capacity increases with the increase of ZrO2 content in the biohybrid membrane (Chen et al., 2022). Interestedly, the material exhibits high sustainability according to the Overall Sustainability Footprint (OSF, considering the performance, cost, and environmental effects) method with an OSF value of 83% compared to other materials (Chen et al., 2022).
CMC/citric acid aerogel was reported for removal of anionic pollutants of nitrate, nitrite, phosphate (Darabitabar et al., 2020). The aerogels exhibited a high specific surface area with high porosity (low density). They showed high adsorption capacity for nitrite (NO2−), nitrate (NO3−), and phosphate (PO43−) with a capacity of 79.65, 73.04, and 98.18 ppm, respectively (Darabitabar et al., 2020).
Dye Removal Using Nanocellulose Materials
Organic dyes are widely used for several industries including paper, textile, and dying processes. The world production of dyes exceeds hundreds of tonnes (ca. 7 × 105 metric tons/year) annually (Carmen and Daniel, 2012). The water-soluble dyes are usually leaked to water sources including groundwater. Thus, it can be considered a critical pollutant for drinking water. For instance, there is more than 20% of the used dyes released into the water during the application process such as dyeing. The organic dyes are toxic for humans, marine, and microorganisms. The toxicity depends on the charge of the dye (cationic dyes are higher toxicity than anionic dyes), functional groups present in the dye’s molecules, concentration, and the uptake procedure. Organic dyes are typically removed from water via several processes including adsorption, filtration, encapsulation, and degradation (Figure 6).
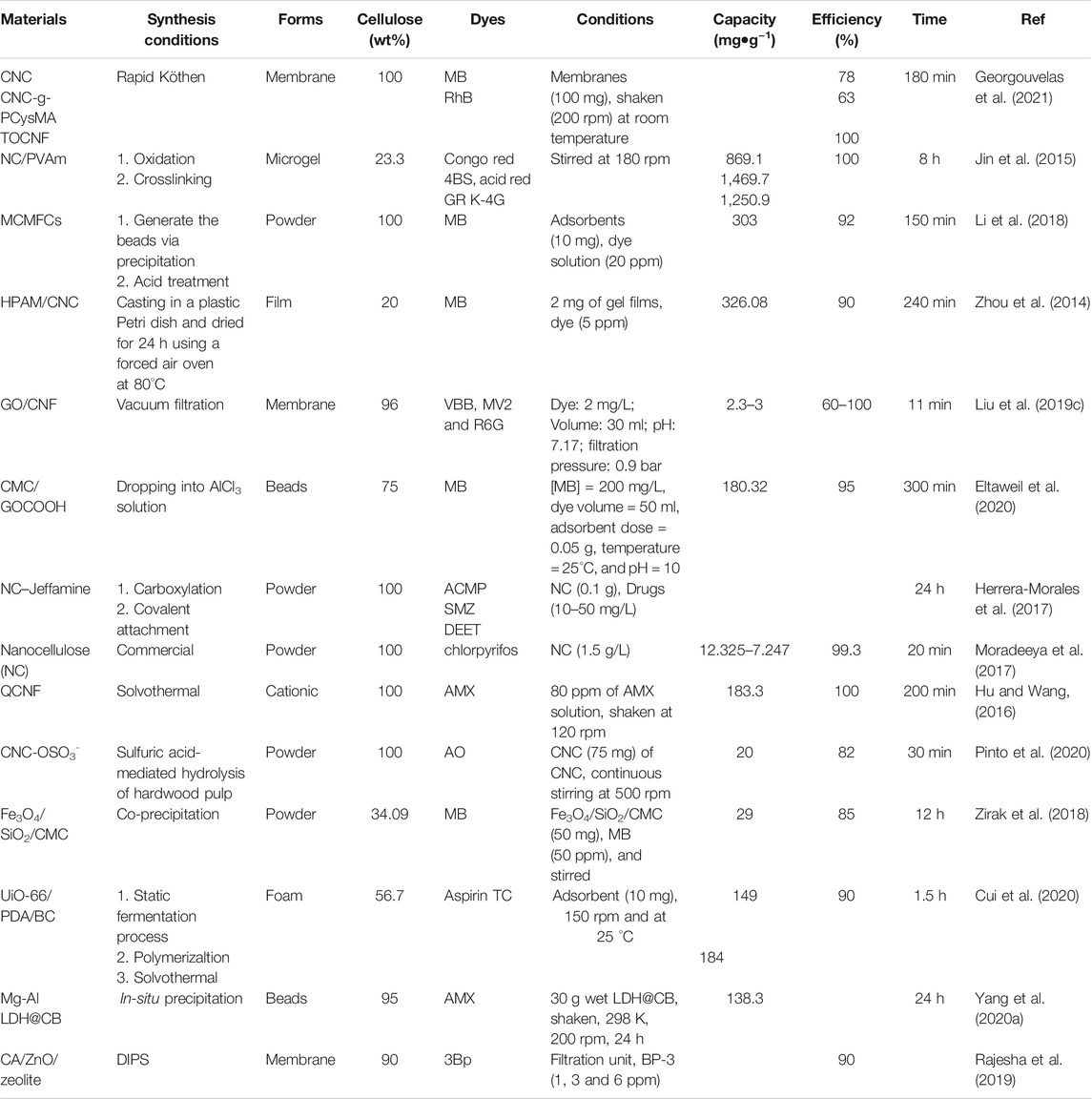
Cellulose enables high adsorption efficiencies for organic dyes (Table 4). Anionic forms of CNCs (sulfated and carboxylated) were investigated for the adsorption of cationic dye called Auramine O (AO) (Pinto et al., 2020). Thermodynamic values indicated that the process of the adsorption is spontaneous due to the electrostatic interactions. Amino-functional cellulose enables the adsorption of anionic dyes under acidic conditions. Nanocellulose modified polyvinylamine (PVAm) under acidic conditions showed high adsorption of anionic dyes; congo red 4BS, acid red GR, and Reactive light yellow K-4G with maximum adsorption capacities of 869.1, 1,469.7, and 1,250.9 mg/g, respectively (Jin et al., 2015). The high adsorption capacity could be due to the chemisorption of these species (Jin et al., 2015). Cationic cellulose exhibits high adsorption performance for anionic organic dyes such as dyes, and antibiotics (Table 4). Cellulose from flax noil (CFN) was functionalized with triethylamine to obtain quaternary ammonium salt CNF (QCFN) for the adsorption of amoxicillin (AMX) (Hu and Wang, 2016). QCNF showed an adsorption capacity of 183.3 mg/g of AMX (Hu and Wang, 2016).
Electrospun fibers consisting of cellulose acetate (CA) and CNC with different wt% ratio of CA:CNC (Goetz et al., 2018) exhibited rejection efficiency of 20–56% for particles of 0.5–2.0 μm. They showed a high rejection of 80–99% for dyes (Goetz et al., 2018). All-cellulose layered membranes containing CNC, TOCNF, or CNC-g-PCysMA were reported for the adsorption of organic dyes such as rhodamine B (RhB) and methylene blue (MB) (Georgouvelas et al., 2021). The membranes were fabricated via a sheet former Rapid-Köthen (Figure 7). They exhibited high flux ranging from 3,417 to 11,742 L h−1•m−2•bar−1. They can remove MB via adsorption with adsorption efficiencies of 26%, 21%, and 35% for CNC, CNC-g-PCysMA, and TOCNF, respectively (Figure 7). They can be also used as a catalyst for dye decolorization via hydrogenation using sodium borohydride (NaBH4). The membrane shows a decolorization efficiency of 100% toward RhB (Georgouvelas et al., 2021). However, this process is reversible due to the incomplete degradation of the dye (Figure 7).
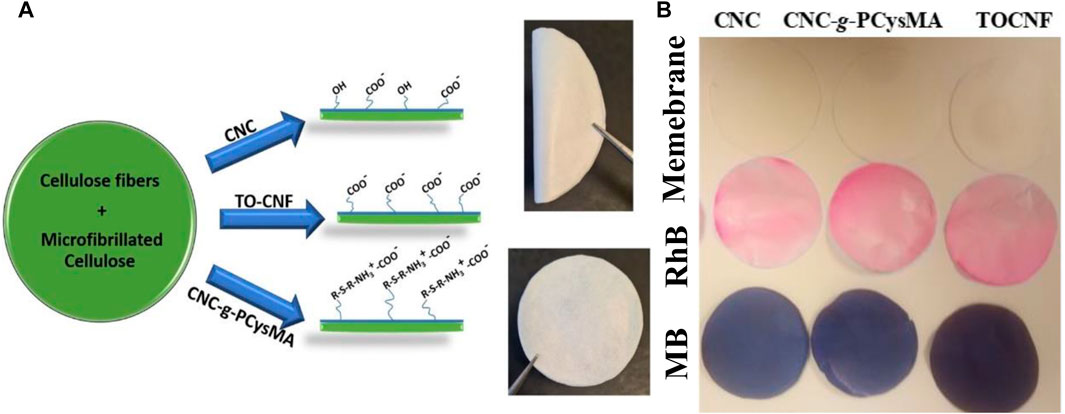
FIGURE 7. (A) Schematic representation for the synthesis of all-cellulose membranes, and photographs of CNC membranes (51 mm diameter) that can be flipped and (B) the membrane before and after adsorption of organic dyes, RhB and MB. Figure reprinted from Ref (Georgouvelas et al., 2021). This Open Access Article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
Cellulose was modified with several polymers such as polypiperazine (Yu et al., 2010), and hydrolyzed polyacrylamide (HPAM) and was used successfully to remove MB. (Zhou et al., 2014). Pore-forming agents or porogen as CaCO3 nanoparticles can be also added during the formation of cellulose/MFC composites (denoted as MCMFCs, Figure 8) (Li et al., 2018) and removed in acidic conditions after the formation of MCMFCs composite (Li et al., 2018) to tune the porosity.

FIGURE 8. Schematic representation of MCMFCs. Figure reprinted with permission from Ref. (Li et al., 2018). Copyright belongs to the American Chemical Society (ACS, 2018).
Nanocellulose-based membrane with GO was synthesized via vacuum filtration of CNF suspension and GO solution, successively (Figure 9) (Liu et al., 2019c). The synthesized membranes exhibited ultrahigh flux of 18,123 L m−2•h−1•bar−1 with excellent mechanical properties in both dry and wet forms. They showed an efficient rejection rate of positively and negatively charges organic dyes (92–99%) due to electrostatic repulsion, hydrophobic nature, and size exclusion properties (Liu et al., 2019c). The membrane showed removal efficiency of 98.8, 97.6, and 92.3% for Victoria blue B (VBB), Methyl Violet 2 B (MV2), and Rhodamine 6 G (Rh6 G), respectively (Liu et al., 2019c). TOCNF/GO was also used for the coating of a polyvinylidene difluoride (PVDF) substrate in using the blade coating technique (Liu et al., 2019b). The pristine free-standing TOCNF film exhibits poor permeability. On the other side, hybrid coating with a TOCNF:GO ratio of 100:1 offered water permeability of 816 ± 3.4 L m−2 •h−1 •bar−1. The membranes showed higher permeability compared to most of the common polymer-based membranes. They offered high retention efficiency of 82–99% for dyes (VBB, Rhodamine B (RhB), Methyl Orange (MO), Rh6G, Methyl Blue (MeB), and Methylene Blue (MB)) (Liu et al., 2019b).
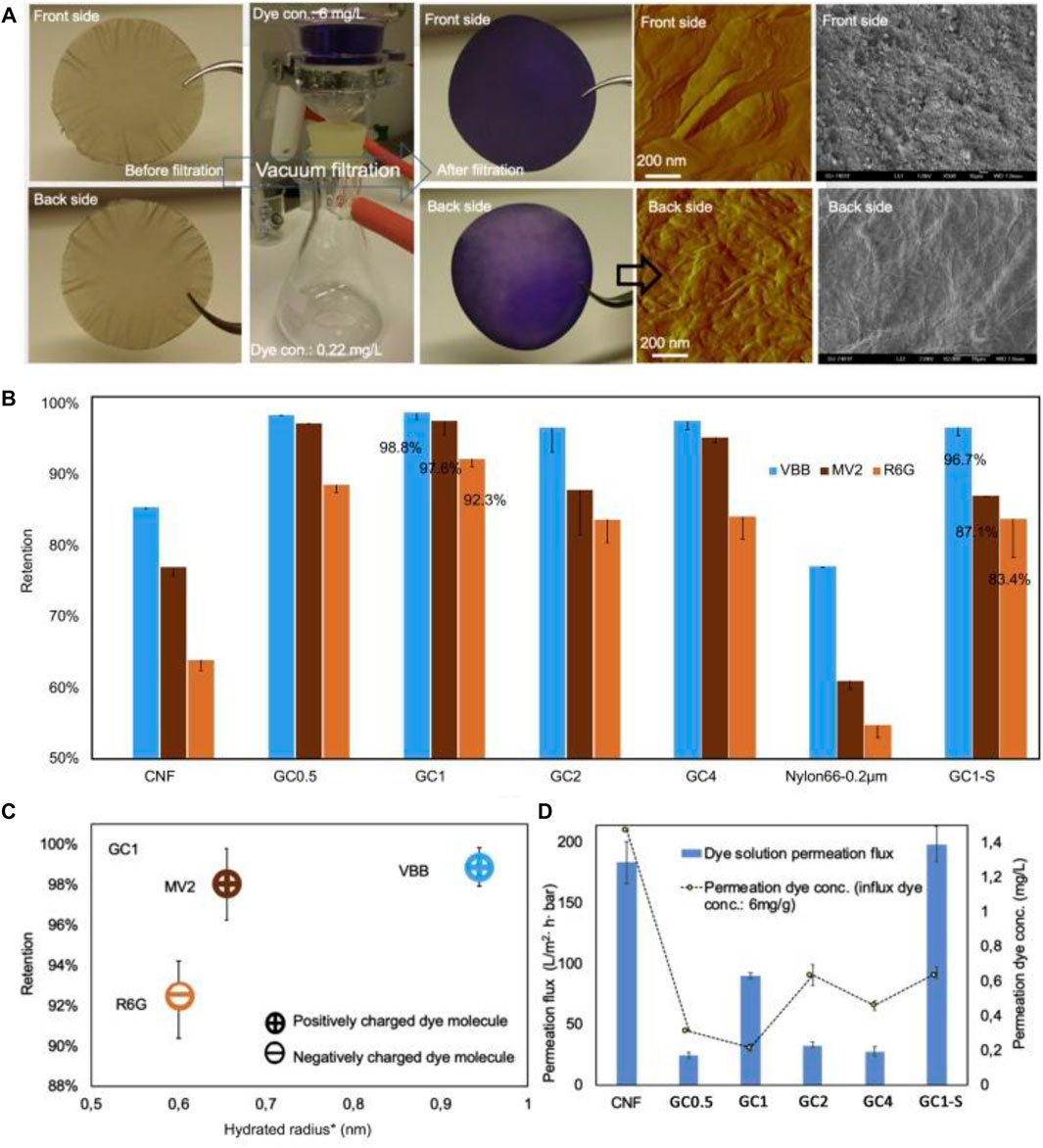
FIGURE 9. Dye filtration performance, (A) Photograph and micrograph of GO-CNF1 membrane after dye filtration. (B) Retention of dye molecules by the membranes. (C) Retention performance of GO-CNF1 membrane, as a function of hydrated radius for the probe dye molecules with different charges; (D) permeation of dye solution through the membranes. Figure reprinted with permission from Ref. (Liu et al., 2019c). Copyrights belong to Elsevier (2019).
Microbeads consisting of CMC/carboxylated graphene oxide (GOCOOH) were fabricated via dropping at room temperature into AlCl3 solution (3%) for the adsorptive removal of a cationic dye such as MB (Eltaweil et al., 2020). The adsorption process of MB dye onto CMC/GOCOOH microbeads is an exothermic process indicating the high affinity of the synthesized beads toward MB molecules. The process exhibited maximum capacity at pH 10. The cellulose beads can be recycled for nine repetitive cycles (Eltaweil et al., 2020). Zwitterionic cellulose-based aerobeads containing anionic (HKUST-1) and cationic (ZIF-67) MOFs (denoted as HKUST-1&ZIF-67@CNF) was reported for the adsorption of diclofenac (DIC) and methyl orange (MO) (KarzarJeddi et al., 2020). The aerobeads exhibited adsorption capacities of 121.20 mg/g and 49.21 mg/g toward DIC and MO, respectively (KarzarJeddi et al., 2020).
Removal of Other Organic Pollutants
Water contamination with organic pollutants is widely spread nowadays. The organic pollutants can be small molecules such as oils, organic dyes, pharmaceuticals, antibiotics, nitro-compounds, cosmetic products, and drugs. They can be classified as miscible/soluble and immiscible water organic pollutants. The immiscible organic pollutants such as oils can be easily removed via the separation of organic layers from aqueous layers. In contrast, removing water-miscible/soluble organic pollutants is a tedious task. The water-soluble organic pollutants can be removed via adsorption, or degradation to small fragments via advanced approaches such as photolysis via UV-Visible photocatalysis, ozonation, and oxidation/reduction.
Nanocellulose-based materials were reported for the adsorption of other pollutants such as pharmaceuticals, drugs, and antibiotics (Table 4). Nanocellulose (NC) was grafted with a block copolymer Jeffamine ED 600, denoted as NC–Jeffamine for the adsorption of paracetamol (acetaminophen, ACMP), sulfamethoxazole (SMZ), and N, N-diethyl-meta-toluamide (DEET) (Herrera-Morales et al., 2017). The adsorption takes place via electrostatic interactions. NC was also reported for the adsorption of an insecticide called chlorpyrifos (Moradeeya et al., 2017). The experimental data, as well as computational results, indicated that NC exhibited effective removal of chlorpyrifos via adsorption from aqueous solution (Table 4) (Moradeeya et al., 2017).
Cellulose-inorganic nanoparticles hybrid materials were reported for the adsorption of dye, drug, and cosmetic products (Table 4). A core-shell of CMC coated Fe3O4@SiO2 (Fe3O4/SiO2/CMC) was synthesized via chemical co-precipitation for the removal of MB (Zirak et al., 2018). A soft foam consisting of MOF (UiO-66), polydopamine (PDA), and bacterial cellulose (BC), UiO-66/PDA/BC, was reported for the adsorption active pharmaceuticals compounds such as aspirin (AS) and tetracycline hydrochloride (TC) (Cui et al., 2020). A cellulose bead (CB) with Mg-Al layered double hydroxide (Mg-Al LDH) was synthesized via the in-situ co-precipitation method for the adsorption of Amoxicillin (AMX) (Yang C. et al., 2020). The material showed an adsorption capacity of 138.3 mg/g (Yang C. et al., 2020). Cellulose acetate (CA)/zinc oxide (ZnO)-Zeolite membrane was synthesized using diffusion induced phase separation (DIPS) method for the removal of benzophenone-3 (Bp-3), a sunscreen agent, from water (Rajesha et al., 2019). The membrane offered 98% rejection of Bp-3 in neutral pH.
Oil-Water Separation
Ships transport several billion gallons of petroleum oil every year. In 2020 only, OPEC announced the refinery capacity of 12.2 million barrels per calendar day. Several million gallons of petroleum oils are spilled in the sea and ocean during transportation. The contamination of water with oils harms the aquatic life of animals and other species. Oils are usually immiscible with water and float on the top layer of water preventing the penetration of sunlight into the water and decreasing the underwater photosynthesis process. Thus, several materials including lignocellulose (Fürtauer et al., 2021), polypropylene (Teas et al., 2001), silica, zeolites, and natural sorbents (Adebajo et al., 2003) were reported for cleaning up the oil spill. Cellulose-based materials were reported as adsorbents, flocculants, and separation membranes for oil/water (Peng et al., 2020).
Cellulose is a hydrophilic material. However, it can be modified to be super-hydrophobic. It was modified with hexadecyltrimethoxysilane (HTMS) via chemical vapor deposition (CVD) for the separation of oils (Shang et al., 2021). The materials were fabricated into aerogel with a water contact angle of 151°. It exhibited high adsorption performance for various oil with a capacity of 77–226 g/g with excellent recyclability over 30 cycles. It showed an adsorption capacity of 226 g/g for chloroform with high selectivity over other tested oils (such as olive oil, gasoline, and silicone oil), and solvents (such as n-hexane, cyclohexane, toluene, 1,2-dichloromethane, chloroform (CHCl3), dimethyl sulfoxide (DMSO), and DMF (dimethylformamide)) (Shang et al., 2021).
Degradation of Organic Pollutants Using Nanocellulose-Based Catalysis
Dye molecules can be degraded into small molecules via several reactions including chemical reactions (reduction, and oxidation), and photocatalysis (Table 5). The degradation process can be achieved via chemical reactions such as redox reaction i.e. reduction, and oxidation process. The chemical process requires reducing or oxidizing agents. The cellulose-based materials are mainly working as catalysts to enhance the redox reaction. They can be also conjugated with photocatalysts such as TiO2, CuO, ZnO.etc for degradation under UV and visible light i.e., photocatalysis (Table 5).
All cellulose-based membranes were reported for the adsorption and decolorization of organic dyes such as MB (Figure 7). They showed adsorption efficiencies of 26, 21, and 35% for CNC, CNC-g-PCysMA, and TOCNF, respectively (Georgouvelas et al., 2021). However, they can effectively decolorize the dyes with an efficiency of 100% for dye such as RhB using NaBH4 as a reducing agent. The high decolorization efficiency in the presence of cellulose membranes was due to the improvement in the self-hydrolysis of NaBH4 (Abdelhamid, 2021a). Cellulose-based membranes showed 1.5-3 fold higher hydrogenation efficiency compared to self-hydrolysis of NaBH4 (Georgouvelas et al., 2021).
Cellulose-MOFs (denoted as CelloMOF) were reported as an effective catalyst for dye degradation via the chemical reduction using NaBH4 as reducing agents (Nasser Abdelhamid and Mathew, 2021). CelloMOF materials showed high selectivity toward anionic dye adsorption such as MeB with a removal efficiency of 100% (Figure 10). They can be also used as a catalyst for other dyes via hydrogenation. They exhibited complete removal of dye via degradation that was confirmed using analytical techniques such as mass spectrometry. The materials can be implemented into a cellulosic filter paper (Figure 10). They can selectively remove the dye from a mixture with good recyclability and excellent efficiency (Figure 10). They are promising for further exploration.

FIGURE 10. a) Dye solution of (A) RhB, MeB, and the mixture (RhB and MeB) before and after separation via filtration using (B) conventional filter paper (FP) and CelloZIF-8 loaded FP, (C) filter paper after separation and drying, and (D) recyclability. Figure reprinted from Ref. (Nasser Abdelhamid and Mathew, 2021). This Open Access Article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
Dye degradation via photocatalysis using cellulose-based materials was also reported (Table 5). Metal oxide nanoparticles can be crystallized spontaneously into carboxylated cellulose nanofibrilbased films such as TOCNF during the adsorption of Cu2+ metal ions from water (Valencia et al., 2020). The adsorption process leads to the growth process of Cu2O without the need for any further chemicals or experimental conditions such as temperature. The formation of nanoparticles on the TOCNF films is simple and easy (Valencia et al., 2020). The formed Cu2O/TOCNF thin film was further used as a photocatalyst for the removal of organic dyes such as MB via synergistic adsorption and photocatalytic degradation (Table 5) (Valencia et al., 2020). The photocatalysis showed effective removal of almost all organic dyes within a short time (Table 5) (Valencia et al., 2020). Photocatalytic degradation of a dye such as RhB was reported using Bi4O5Br2/BiOBr/CM as a photocatalyst (Onwumere et al., 2020). The photocatalyst showed almost complete removal of the dye after 100 min under 405 nm illumination (20 W) with the addition of an oxidant such as hydrogen peroxide (H2O2, Table 5) (Onwumere et al., 2020). In-situ growth of TiO2 nanorods (NRs) and Au nanocrystals (NCs) was reported using CNCs (Figure 11) (Nair et al., 2020). CNC/TiO2 NRs/Au NCs were investigated as photocatalysts for the degradation of RhB dye in the presence of H2O2 (Figure 11). The presence of plasmonic nanoparticles such as Au improved the performance of the photocatalyst leading to high dye degradation (Figure 11) (Nair et al., 2020).
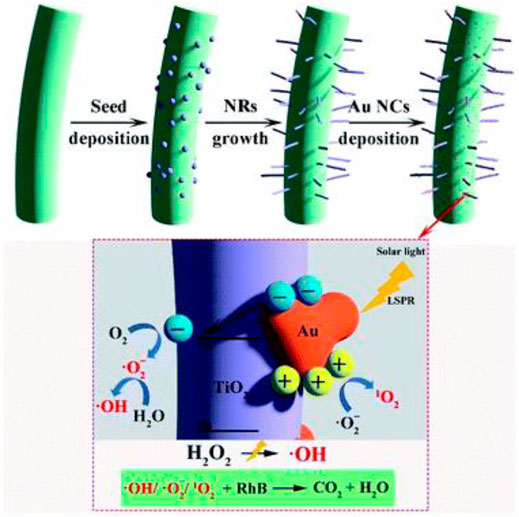
FIGURE 11. Schematic representation of seed-mediated TiO2 NR synthesis, Au NCs deposition, and suggested photocatalytic process for dye degradation (Nair et al., 2020). This Open Access Article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
A membrane of CMC/GO/magnesium oxide (MgO) nanoparticle (CMC/GO/MgO) was prepared for photocatalytic antifouling (El-Shafai et al., 2021). It can be used as an antifouling membrane due to the generation of electrons, and reactive oxygen species (ROS). Thus, it can be used for the oxidation of organic pollutants (El-Shafai et al., 2021). Photodegradation of phenol was reported using TiO2 NPs/regenerated cellulose (RC) (Table 5) (Zeng et al., 2010). TiO2 NPs/RC exhibited higher efficiency compared to RC and TiO2 NPs (Zeng et al., 2010). MOF@viscose exhibited self-cleaning properties for organic dyes (Emam et al., 2018).
Water Decontamination From Microbial Species: Anti-Fouling and Anti-Bcterial Properties of Cellulose-Based Materials
Microbial species such as bacteria, fungus, and algae cause a high risk to human health, especially if the contaminants are pathogen species. Pathogens microorganisms are usually removed via the addition of an oxidant such as ferric chloride, chlorine throughout the water treatment stages. The role of the oxidants, such as chlorine (Cl2), chlorine dioxide (ClO2), and ozone (O3), is to inactivate the biological activity of the pathogens preventing their replication.
Different grades of nanocellulose have shown favorable performance with respect to antifouling and antibacterial properties. Nanocellulose with residual lignin (Valencia et al., 2019) as well as TOCNF (Aguillar-Sanchez et al., 2021) exhibit inherently antibacterial, antioxidant, and antifouling properties.
TOCNF and poly (vinyl alcohol) (PVA) were used for the coating of polyethersulfone (PES) substrate (Aguilar-Sanchez et al., 2021). The membrane was used for antifouling application. TOCNF/PVA@PES membrane showed high resistance to bacterial Escherichia coli (E. coli) colonization. It prevents biofilm formation for 18 h offering high antifouling performance (Aguilar-Sanchez et al., 2021). Micro/nanocellulose membrane grafted with zwitterionic poly (cysteine methacrylate) (PCysMA) acts as an antibacterial agent (Valencia et al., 2018). The synthesized membranes exhibited excellent antifouling properties (Valencia et al., 2018). They showed a reduction efficiency of 85% in the biofilm formation of Staphylococcus aureus (S. aureus) (Valencia et al., 2018). The electrospun nanofibrous scaffolds functionalized using nanocellulose can remove waterborne bacteria at 2–3 times higher flux compared to that of commercial microfiltration) -based membranes (e.g., Millipore GS9035) (Ma et al., 2011).
Cellulose-inorganic hybrids exhibited high antibacterial performance. Nanofiltration (NF) membrane consisting of CNC/silver (CNC/Ag) into a polyamide layer was fabricated using the interfacial polymerization (IP) method (Xu et al., 2020). The membrane with 0.01 wt% CNC/Ag showed a high pure water permeability of 25.4 L m-2 h−1 bar−. It offered high antifouling with flux recovery of 92.6% using humic acid and antibacterial activity of 99.4% against E. coli viability (Xu et al., 2020). A composite nanofiber of cellulose acetate (CA) and polysulfone (PSf) with 0.1 wt% ZnO exhibited high antibacterial activity against E. coli (Mousa et al., 2020).
Cellulose-MOFs materials are promising for water decontamination from microbial species including bacteria, fungus, and algae. Superhydrophobic polydimethylsiloxane (PDMS)/ZIF-8@cotton showed excellent antibacterial activity against bacteria (Yang Y. et al., 2020). Different metal-based MOFs e.g., ZIF(Ni), ZIF-8(Zn), and ZIF-67(Co) were used for the modification of cotton (Emam et al., 2020). The materials were investigated as antibacterial fabric against S. aureus, Bacillus Cereus (B.cerus), E. Coli, and Candida albicans (C. Albicans) (Emam et al., 2020). Almost all reported MOFs exhibited antibacterial activity indicating their potential for water decontamination. Cellulose paper (CP)/cellulose nanofibrils (CNF)/ZIF-67 offered high antibacterial against E. coli (Qian et al., 2018). The antibacterial activities against E. coli were increased with the increase of the loading of ZIF-67 MOF (Qian et al., 2018). Copper-based MOFs such as HKUST-1 loaded TEMPO-oxidized corncobs (OCBs) exhibited higher antibacterial activity compared to Zn-based MOF such as ZIF-8/OCBs (Duan et al., 2019). HKUST-1/OCBs and ZIF-8/OCBs showed antibacterial efficiencies of 90.2%, and 44.8% against E. Coli, respectively (Duan et al., 2019).
Cationic cellulose materials can be used as flocculants agents to collect and harvest microalgae. Cationic CNC (QCNC) was synthesized via modification with cationic groups such as pyridinium (PYR) or methylimidazolium (MIM) (Blockx et al., 2019). The degree of substitution (DS) values were 0.08–0.34 and 0.10–0.29 for PYR and MIM, respectively. The materials were used as flocculants for harvesting the freshwater microalgae Chlorella Vulgaris with an efficiency of higher than 95% (Blockx et al., 2019). They exhibited several advantages such as low-cost and low energy requirements (Blockx et al., 2019). QCNC was also reported as flocculant for Nannochloropsis oculata (Verfaillie et al., 2020). It required a low dose (11 mg/L) compared to chitosan (35 mg/L). However, the maximum flocculation efficiency for QCNC (90%) is lower than chitosan (95%) (Verfaillie et al., 2020). The interactions are mainly due to the interaction between the negative charge membrane of bacterial and positive charge on QCNC and chitosan (Abdelhamid and Wu, 2013a, 2013b; Gopal et al., 2013; Abdelhamid et al., 2019).
Conclusion and Perspectives
Cellulose in nanoscale, without and with chemical modifications or in combination with other nanomaterials as graphene oxide, metal-organic frameworks (MOFs), TiO2 nanowires have demonstrated high efficiency for water treatment. They can be used as adsorbents, size exclusion membranes, and catalysts. Nanocellulose can be fabricated into different forms such as foams, paper, sheets, and filters to satisfy the customer’s requirements. They can be used for removing water pollutants such as metal ions, dyes, drugs, and microbial species.
The advantages and disadvantages of cellulose based water treatment are listed below:
- Cellulose has the advantage of being available bioresources source which can be abundant and cheap (Bhattacharya et al., 2008; Chen et al., 2011). It can be reproduced from waste via recycling (Mohamed et al., 2015). H0wever the current methods for the production of nanocellulose are still in the laboratory or pilot-scale production. Thus, further investigations for industrial production scale for cellulose in nanoscale and cationic cellulose are highly required for industrial-scale water treatment.
- Cellulose based materials exhibits high adsorption efficiency especially for the low concentration level of heavy metals (Zhou et al., 2020). Key parameters affecting adsorption using cellulose-based materials include pollutants properties such as charge, ionic radii, and compositions as well as presence of interfering species, functional groups of cellulose, adsorption conditions as well as adsorbent forms and properties. The adsorption efficiency usually decreases with concentration. Thus, extra steps such as dilution are required to achieve high adsorption performance.
- The direct interaction between cellulose and metal ions leads to the precipitation of metal ions into oxides. Several studies reported the precipitation of the metal ions after incubation with cellulose. For example, Cd2+ ions (Sharma et al., 2018), and Cu2+ ions (Valencia et al., 2019) were precipitated as Cd(OH)2 nanocrystals onto NOCNF and Cu2O onto TOCNF, respectively. The mechanism of the precipitation is still unclear and requires further investigation. The functional groups of cellulose such as hydroxyl groups enable direct synthesis of nanoparticles without the need for external agents. Thus, they can be used for the synthesis of nanoparticles such as Pt NPs (Johnson et al., 2011; Lin et al., 2011), ZnO NPs (Chen et al., 2013), Ag NPs (Wu et al., 2008; Ifuku et al., 2009; Elayaraja et al., 2017), Pd and Au NPs (Zhang et al., 2018). The synthesis requires the addition of the heavy metal ions to the cellulose suspension without any additional reducing agents. The reduction ability of cellulose is mainly attributed to the electron-rich hydroxyl groups. The adsorption of heavy metal ions into cellulose can be further converted to advanced materials such as hybrid nanocomposite of cellulose-nanoparticles.
- The integration of inorganic nanoparticles with cellulose brings several advantages such as preventing agglomeration and aggregation ensuring high colloidal stability (Shang et al., 2021). The use of inorganic nanoparticles such as magnetic nanoparticles offers simple separation of the materials after water purification via an external magnet. Inorganic nanoparticles enable the applications of cellulose for methods such as catalysis (Kaushik and Moores, 2016), and photocatalysis (Mohamed et al., 2017). However, the risk for nanoparticles on the human and ecological environment is questionable. Several reports prove cytotoxicity and ecotoxicity for common inorganic nanomaterials. For instance, Cu NPs (Manna et al., 2012), TiO2 NPs (Trouiller et al., 2009), and ZnO NPs (Ma et al., 2013) can induce hepatic dysfunction; DNA damage and inflammation in mice; generation of reactive oxygen species, respectively. The intrinsic toxicity of nanoparticles can be mitigated via the formation of strong interactions in the nanocomposite or via further modification that prevents the dissolution of nanoparticles into ions.
- Cellulose can be performed into several forms such as filters (Li C. et al., 2020), membranes (Sharma et al., 2020b) and is facilitated by their capability to perform reactions such as cross-linking (Liang et al., 2020). The processing technologies of cellulose-based materials into commercial products become san essential step for commercial use of this technology. The current methods are still in the infancy and require further exploration.
- Applications of cellulose for other water treatment methods including desalination (conversion of seawater into drinking water) should be further investigated. The electrochemical methods used to a very limited extent because of the low conductivity of cellulose. However, the creation of new functional groups inside cellulose may enhance the electric properties of cellulose enable high electrochemical performance for water remediation.
- Cellulose-based materials offer recycling possibility. For eg., adsorbed metal ions can be easily removed after adsorption by washing under acidic conditions (Sehaqui et al., 2014) which opens up possibilities for recovery of valuable metals and reuse of membranes. However, the regeneration of the materials required in some cases harsh conditions such as the use of acid, and environmentally unfriendly reagents. The regeneration step is essential for saving resources of cellulose-based materials. Further investigations for the regeneration procedure with high efficiencies are highly required. Celluose based water treatment products offer possibilities for biodegradation (Barbosa et al., 2020), however the biodegradation route depends on the nature and toxicity of the pollutant.
Author Contributions
The manuscript is prepared with contributions form both authors. HA was responsible for the writing and AM contributed with conceptualisation and editing.
Funding
This project is funded by The Swedish Foundation for Strategic Environmental Research (Mistra) under MISTRA TerraClean (project no. 2015/31). Open access publication is funded by Stockholm University Library.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhamid, H. N. (2021a). A Review on Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energ. 46, 726–765. doi:10.1016/j.ijhydene.2020.09.186
Abdelhamid, H. N., El-Bery, H. M., Metwally, A. A., Elshazly, M., and Hathout, R. M. (2019). Synthesis of CdS-Modified Chitosan Quantum Dots for the Drug Delivery of Sesamol. Carbohydr. Polym. 214, 90–99. doi:10.1016/j.carbpol.2019.03.024
Abdelhamid, H. N., and Mathew, A. P. (2022). Cellulose-Metal Organic Frameworks (CelloMOFs) Hybrid Materials and Their Multifaceted Applications: A Review. Coord. Chem. Rev. 451, 214263. doi:10.1016/j.ccr.2021.214263
Abdelhamid, H. N., and Mathew, A. P. (2021). In-situ Growth of Zeolitic Imidazolate Frameworks into a Cellulosic Filter Paper for the Reduction of 4-nitrophenol. Carbohydr. Polym. 274, 118657. doi:10.1016/j.carbpol.2021.118657
Abdelhamid, H. N., and Wu, H.-F. (2013a). Multifunctional Graphene Magnetic Nanosheet Decorated with Chitosan for Highly Sensitive Detection of Pathogenic Bacteria. J. Mater. Chem. B 1, 3950–3961. doi:10.1039/c3tb20413h
Abdelhamid, H. N., and Wu, H.-F. (2013b). Probing the Interactions of Chitosan Capped CdS Quantum Dots with Pathogenic Bacteria and Their Biosensing Application. J. Mater. Chem. B 1, 6094–6106. doi:10.1039/c3tb21020k
Abdelhamid, H. N. (2021b). Zeolitic Imidazolate Frameworks (ZIF-8) for Biomedical Applications: A Review. Cmc 28, 7023–7075. doi:10.2174/0929867328666210608143703
Abdul Khalil, H. P. S., Saurabh, C. K., Adnan, A. S., Nurul Fazita, M. R., Syakir, M. I., Davoudpour, Y., et al. (2016). A Review on Chitosan-Cellulose Blends and Nanocellulose Reinforced Chitosan Biocomposites: Properties and Their Applications. Carbohydr. Polym. 150, 216–226. doi:10.1016/j.carbpol.2016.05.028
Adebajo, M. O., Frost, R. L., Kloprogge, J. T., Carmody, O., and Kokot, S. (2003). Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 10, 159–170. doi:10.1023/A:1027484117065
Aguilar-Sanchez, A., Jalvo, B., Mautner, A., Rissanen, V., Kontturi, K. S., Abdelhamid, H. N., et al. (2021). Charged Ultrafiltration Membranes Based on TEMPO-Oxidized Cellulose Nanofibrils/poly(vinyl Alcohol) Antifouling Coating. RSC Adv. 11, 6859–6868. doi:10.1039/D0RA10220B
Araki, J., Wada, M., and Kuga, S. (2001). Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(ethylene Glycol) Grafting. Langmuir 17, 21–27. doi:10.1021/la001070m
Ashour, R. M., Abdel-Magied, A. F., Wu, Q., Olsson, R. T., and Forsberg, K. (2020). Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers 12, 1104. doi:10.3390/polym12051104
ATSDR (2017). Substance Priority List | ATSDR. Agency Toxic Subst. Dis. Regist., 1–11. Available at: https://www.atsdr.cdc.gov/spl/index.html#2019spl (Accessed September 1, 2021).
Awang, N. A., Wan Salleh, W. N., Ismail, A. F., Yusof, N., Aziz, F., and Jaafar, J. (2019). Adsorption Behavior of Chromium(VI) onto Regenerated Cellulose Membrane. Ind. Eng. Chem. Res. 58, 720–728. doi:10.1021/acs.iecr.8b02366
Bacakova, L., Pajorova, J., Bacakova, M., Skogberg, A., Kallio, P., Kolarova, K., et al. (2019). Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 9, 164. doi:10.3390/nano9020164
Baghdad, K., and Hasnaoui, A. M. (2020). Zeolite-cellulose Composite Membranes: Synthesis and Applications in Metals and Bacteria Removal. J. Environ. Chem. Eng. 8, 104047. doi:10.1016/j.jece.2020.104047
Bahmani, E., Seyyed Zonouzi, H., Koushkbaghi, S., Keyvani Hafshejani, F., Fassadi Chimeh, A., and Irani, M. (2020). Electrospun Polyacrylonitrile/cellulose acetate/MIL-125/TiO2 Composite Nanofibers as an Efficient Photocatalyst and Anticancer Drug Delivery System. Cellulose 27, 10029–10045. doi:10.1007/s10570-020-03459-1
Barbosa, R. F. S., Souza, A. G., Maltez, H. F., and Rosa, D. S. (2020). Chromium Removal from Contaminated Wastewaters Using Biodegradable Membranes Containing Cellulose Nanostructures. Chem. Eng. J. 395, 125055. doi:10.1016/j.cej.2020.125055
Baruah, J., Chaliha, C., Kalita, E., Nath, B. K., Field, R. A., and Deb, P. (2020). Modelling and Optimization of Factors Influencing Adsorptive Performance of Agrowaste-Derived Nanocellulose Iron Oxide Nanobiocomposites during Remediation of Arsenic Contaminated Groundwater. Int. J. Biol. Macromolecules 164, 53–65. doi:10.1016/j.ijbiomac.2020.07.113
Bhattacharya, D., Germinario, L. T., and Winter, W. T. (2008). Isolation, Preparation and Characterization of Cellulose Microfibers Obtained from Bagasse. Carbohydr. Polym. 73, 371–377. doi:10.1016/j.carbpol.2007.12.005
Blockx, J., Verfaillie, A., Eyley, S., Deschaume, O., Bartic, C., Muylaert, K., et al. (2019). Cationic Cellulose Nanocrystals for Flocculation of Microalgae: Effect of Degree of Substitution and Crystallinity. ACS Appl. Nano Mater. 2, 3394–3403. doi:10.1021/acsanm.9b00315
Bo, S., Ren, W., Lei, C., Xie, Y., Cai, Y., Wang, S., et al. (2018). Flexible and Porous Cellulose Aerogels/zeolitic Imidazolate Framework (ZIF-8) Hybrids for Adsorption Removal of Cr(IV) from Water. J. Solid State. Chem. 262, 135–141. doi:10.1016/j.jssc.2018.02.022
Cárdenas Bates, I. I., Loranger, É., Mathew, A. P., and Chabot, B. (2021). Cellulose Reinforced Electrospun Chitosan Nanofibers Bio-Based Composite Sorbent for Water Treatment Applications. Cellulose 28, 4865–4885. doi:10.1007/s10570-021-03828-10.1007/s10570-021-03828-4
Carmen, Z., and Daniel, S. (2012). “Textile Organic Dyes - Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents - A Critical Overview,” in Organic Pollutants Ten Years after the Stockholm Convention - Environmental and Analytical Update. Editor T. Puzyn (London, UK: InTech). doi:10.5772/32373
Carpenter, A. W., de Lannoy, C.-F., and Wiesner, M. R. (2015). Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 49, 5277–5287. doi:10.1021/es506351r
Chen, B., Zheng, Q., Zhu, J., Li, J., Cai, Z., Chen, L., et al. (2016). Mechanically strong Fully Biobased Anisotropic Cellulose Aerogels. RSC Adv. 6, 96518–96526. doi:10.1039/C6RA19280G
Chen, H., Sharma, S. K., Sharma, P. R., Chi, K., Fung, E., Aubrecht, K., et al. (2021). Nitro-oxidized Carboxycellulose Nanofibers from Moringa Plant: Effective Bioadsorbent for Mercury Removal. Cellulose 28, 8611–8628. doi:10.1007/s10570-021-04057-5
Chen, H., Sharma, S. K., Sharma, P. R., Yeh, H., Johnson, K., and Hsiao, B. S. (2019). Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose-Cysteine Microscale and Nanoscale Fibers. ACS Omega 4, 22008–22020. doi:10.1021/acsomega.9b03078
Chen, S., Zhou, B., Hu, W., Zhang, W., Yin, N., and Wang, H. (2013). Polyol Mediated Synthesis of ZnO Nanoparticles Templated by Bacterial Cellulose. Carbohydr. Polym. 92, 1953–1959. doi:10.1016/j.carbpol.2012.11.059
Chen, X., Cui, J., Xu, X., Sun, B., Zhang, L., Dong, W., et al. (2020). Bacterial Cellulose/attapulgite Magnetic Composites as an Efficient Adsorbent for Heavy Metal Ions and Dye Treatment. Carbohydr. Polym. 229, 115512. doi:10.1016/j.carbpol.2019.115512
Chen, X., Yu, J., Zhang, Z., and Lu, C. (2011). Study on Structure and thermal Stability Properties of Cellulose Fibers from rice Straw. Carbohydr. Polym. 85, 245–250. doi:10.1016/j.carbpol.2011.02.022
Chen, Y., Yang, G., Liu, B., Kong, H., Xiong, Z., Guo, L., et al. (2022). Biomineralization of ZrO2 Nanoparticles on Graphene Oxide-Supported Peptide/cellulose Binary Nanofibrous Membranes for High-Performance Removal of Fluoride Ions. Chem. Eng. J. 430, 132721. doi:10.1016/j.cej.2021.132721
Choi, H. Y., Bae, J. H., Hasegawa, Y., An, S., Kim, I. S., Lee, H., et al. (2020). Thiol-functionalized Cellulose Nanofiber Membranes for the Effective Adsorption of Heavy Metal Ions in Water. Carbohydr. Polym. 234, 115881. doi:10.1016/j.carbpol.2020.115881
Cui, J., Xu, X., Yang, L., Chen, C., Qian, J., Chen, X., et al. (2020). Soft Foam-like UiO-66/Polydopamine/Bacterial Cellulose Composite for the Removal of Aspirin and Tetracycline Hydrochloride. Chem. Eng. J. 395, 125174. doi:10.1016/j.cej.2020.125174
Darabitabar, F., Yavari, V., Hedayati, A., Zakeri, M., and Yousefi, H. (2020). Novel Cellulose Nanofiber Aerogel for Aquaculture Wastewater Treatment. Environ. Techn. Innovation 18, 100786. doi:10.1016/j.eti.2020.100786
Dogan, H., and Hilmioglu, N. D. (2010). Zeolite-filled Regenerated Cellulose Membranes for Pervaporative Dehydration of Glycerol. Vacuum 84, 1123–1132. doi:10.1016/j.vacuum.2010.01.043
Dong, F., Xu, X., Shaghaleh, H., Guo, J., Guo, L., Qian, Y., et al. (2020). Factors Influencing the Morphology and Adsorption Performance of Cellulose Nanocrystal/iron Oxide Nanorod Composites for the Removal of Arsenic during Water Treatment. Int. J. Biol. Macromolecules 156, 1418–1424. doi:10.1016/j.ijbiomac.2019.11.182
Dong, Y.-D., Zhang, H., Zhong, G.-J., Yao, G., and Lai, B. (2021). Cellulose/carbon Composites and Their Applications in Water Treatment - a Review. Chem. Eng. J. 405, 126980. doi:10.1016/j.cej.2020.126980
Duan, C., Meng, X., Liu, C., Lu, W., Liu, J., Dai, L., et al. (2019). Carbohydrates-rich Corncobs Supported Metal-Organic Frameworks as Versatile Biosorbents for Dye Removal and Microbial Inactivation. Carbohydr. Polym. 222, 115042. doi:10.1016/j.carbpol.2019.115042
El-Shafai, N. M., Beltagi, A. M., Ibrahim, M. M., Ramadan, M. S., and El-Mehasseb, I. (2021). Enhancement of the Photocurrent and Electrochemical Properties of the Modified Nanohybrid Composite Membrane of Cellulose/graphene Oxide with Magnesium Oxide Nanoparticle (GO@CMC.MgO) for Photocatalytic Antifouling and Supercapacitors Applications. Electrochimica Acta 392, 138989. doi:10.1016/j.electacta.2021.138989
Elayaraja, S., Zagorsek, K., Li, F., and Xiang, J. (2017). In Situ synthesis of Silver Nanoparticles into TEMPO-Mediated Oxidized Bacterial Cellulose and Their Antivibriocidal Activity against Shrimp Pathogens. Carbohydr. Polym. 166, 329–337. doi:10.1016/j.carbpol.2017.02.093
Eltaweil, A. S., Elgarhy, G. S., El-Subruiti, G. M., and Omer, A. M. (2020). Carboxymethyl Cellulose/carboxylated Graphene Oxide Composite Microbeads for Efficient Adsorption of Cationic Methylene Blue Dye. Int. J. Biol. Macromolecules 154, 307–318. doi:10.1016/j.ijbiomac.2020.03.122
Emam, H. E., Abdelhamid, H. N., and Abdelhameed, R. M. (2018). Self-cleaned Photoluminescent Viscose Fabric Incorporated Lanthanide-Organic Framework (Ln-MOF). Dyes Pigm. 159, 491–498. doi:10.1016/j.dyepig.2018.07.026
Emam, H. E., Darwesh, O. M., and Abdelhameed, R. M. (2020). Protective Cotton Textiles via Amalgamation of Cross-Linked Zeolitic Imidazole Frameworks. Ind. Eng. Chem. Res. 59, 10931–10944. doi:10.1021/acs.iecr.0c01384
Fan, X.-M., Yu, H.-Y., Wang, D.-C., Mao, Z.-H., Yao, J., and Tam, K. C. (2019). Facile and Green Synthesis of Carboxylated Cellulose Nanocrystals as Efficient Adsorbents in Wastewater Treatments. ACS Sustain. Chem. Eng. 7, 18067–18075. doi:10.1021/acssuschemeng.9b05081
Farooq, A., Patoary, M. K., Zhang, M., Mussana, H., Li, M., Naeem, M. A., et al. (2020). Cellulose from Sources to Nanocellulose and an Overview of Synthesis and Properties of Nanocellulose/zinc Oxide Nanocomposite Materials. Int. J. Biol. Macromolecules 154, 1050–1073. doi:10.1016/j.ijbiomac.2020.03.163
Fiol, N., Vásquez, M. G., Pereira, M., Tarrés, Q., Mutjé, P., and Delgado-Aguilar, M. (2019). TEMPO-oxidized Cellulose Nanofibers as Potential Cu(II) Adsorbent for Wastewater Treatment. Cellulose 26, 903–916. doi:10.1007/s10570-018-2106-7
Foresti, M. L., Vázquez, A., and Boury, B. (2017). Applications of Bacterial Cellulose as Precursor of Carbon and Composites with Metal Oxide, Metal Sulfide and Metal Nanoparticles: A Review of Recent Advances. Carbohydr. Polym. 157, 447–467. doi:10.1016/j.carbpol.2016.09.008
Fürtauer, S., Hassan, M., Elsherbiny, A., Gabal, S. A., Mehanny, S., and Abushammala, H. (2021). Current Status of Cellulosic and Nanocellulosic Materials for Oil Spill Cleanup. Polymers 13, 2739. doi:10.3390/polym13162739
Georgouvelas, D., Abdelhamid, H. N., Li, J., Edlund, U., and Mathew, A. P. (2021). All-cellulose Functional Membranes for Water Treatment: Adsorption of Metal Ions and Catalytic Decolorization of Dyes. Carbohydr. Polym. 264, 118044. doi:10.1016/j.carbpol.2021.118044
Goetz, L. A., Naseri, N., Nair, S. S., Karim, Z., and Mathew, A. P. (2018). All Cellulose Electrospun Water Purification Membranes Nanotextured Using Cellulose Nanocrystals. Cellulose 25, 3011–3023. doi:10.1007/s10570-018-1751-1
Gopal, J., Abdelhamid, H. N., Hua, P.-Y., and Wu, H.-F. (2013). Chitosan Nanomagnets for Effective Extraction and Sensitive Mass Spectrometric Detection of Pathogenic Bacterial Endotoxin from Human Urine. J. Mater. Chem. B 1, 2463. doi:10.1039/c3tb20079e
Gu, Y., Wang, Y., Li, H., Qin, W., Zhang, H., Wang, G., et al. (2020). Fabrication of Hierarchically Porous NH2-MIL-53/wood-carbon Hybrid Membrane for Highly Effective and Selective Sequestration of Pb2+. Chem. Eng. J. 387, 124141. doi:10.1016/j.cej.2020.124141
Haniffa, M. A. C. M., Munawar, K., Chee, C. Y., Pramanik, S., Halilu, A., Illias, H. A., et al. (2021). Cellulose Supported Magnetic Nanohybrids: Synthesis, Physicomagnetic Properties and Biomedical Applications-A Review. Carbohydr. Polym. 267, 118136. doi:10.1016/j.carbpol.2021.118136
Hashem, T., Ibrahim, A. H., Wöll, C., and Alkordi, M. H. (2019). Grafting Zirconium-Based Metal-Organic Framework UiO-66-NH2 Nanoparticles on Cellulose Fibers for the Removal of Cr(VI) Ions and Methyl Orange from Water. ACS Appl. Nano Mater. 2, 5804–5808. doi:10.1021/acsanm.9b01263
Hernández-Francisco, E., Bonilla-Cruz, J., Márquez-Lamas, U., Suárez-Jacobo, Á., Longoria-Rodríguez, F., Rivera-Haro, J., et al. (2020). Entangled Cellulose Nanofibrils/nanosheets Derived from Native Mexican Agave for Lead(II) Ion Removal. Cellulose 27, 8785–8798. doi:10.1007/s10570-020-03373-6
Herrera-Morales, J., Morales, K., Ramos, D., Ortiz-Quiles, E. O., López-Encarnación, J. M., and Nicolau, E. (2017). Examining the Use of Nanocellulose Composites for the Sorption of Contaminants of Emerging Concern: An Experimental and Computational Study. ACS Omega 2, 7714–7722. doi:10.1021/acsomega.7b01053
Hu, D., and Wang, L. (2016). Adsorption of Amoxicillin onto Quaternized Cellulose from Flax Noil: Kinetic, Equilibrium and Thermodynamic Study. J. Taiwan Inst. Chem. Eng. 64, 227–234. doi:10.1016/j.jtice.2016.04.028
Hu, Z.-H., Omer, A. M., Ouyang, X. k., and Yu, D. (2018). Fabrication of Carboxylated Cellulose Nanocrystal/sodium Alginate Hydrogel Beads for Adsorption of Pb(II) from Aqueous Solution. Int. J. Biol. Macromolecules 108, 149–157. doi:10.1016/j.ijbiomac.2017.11.171
Ifuku, S., Tsuji, M., Morimoto, M., Saimoto, H., and Yano, H. (2009). Synthesis of Silver Nanoparticles Templated by TEMPO-Mediated Oxidized Bacterial Cellulose Nanofibers. Biomacromolecules 10, 2714–2717. doi:10.1021/bm9006979
Isogai, A., Saito, T., and Fukuzumi, H. (2011). TEMPO-oxidized Cellulose Nanofibers. Nanoscale 3, 71–85. doi:10.1039/c0nr00583e
Jin, L., Sun, Q., Xu, Q., and Xu, Y. (2015). Adsorptive Removal of Anionic Dyes from Aqueous Solutions Using Microgel Based on Nanocellulose and Polyvinylamine. Bioresour. Techn. 197, 348–355. doi:10.1016/j.biortech.2015.08.093
Johnson, L., Thielemans, W., and Walsh, D. A. (2011). Synthesis of Carbon-Supported Pt Nanoparticle Electrocatalysts Using Nanocrystalline Cellulose as Reducing Agent. Green. Chem. 13, 1686. doi:10.1039/c0gc00881h
Kardam, A., Raj, K. R., Srivastava, S., and Srivastava, M. M. (2014). Nanocellulose Fibers for Biosorption of Cadmium, Nickel, and lead Ions from Aqueous Solution. Clean. Techn Environ. Pol. 16, 385–393. doi:10.1007/s10098-013-0634-2
Karim, Z., Hakalahti, M., Tammelin, T., and Mathew, A. P. (2017). In Situ TEMPO Surface Functionalization of Nanocellulose Membranes for Enhanced Adsorption of Metal Ions from Aqueous Medium. RSC Adv. 7, 5232–5241. doi:10.1039/c6ra25707k
Karim, Z., and Monti, S. (2021). Microscopic Hybrid Membranes Made of Cellulose-Based Materials Tuned for Removing Metal Ions from Industrial Effluents. ACS Appl. Polym. Mater. 3, 3733–3746. doi:10.1021/acsapm.1c00105
KarzarJeddi, M., Laitinen, O., Mahkam, M., and Liimatainen, H. (2020). Zwitterionic Hybrid Aerobeads of Binary Metal Organic Frameworks and Cellulose Nanofibers for Removal Anionic Pollutants. Mater. Des. 196, 109106. doi:10.1016/j.matdes.2020.109106
Kaushik, M., and Moores, A. (2016). Review: Nanocelluloses as Versatile Supports for Metal Nanoparticles and Their Applications in Catalysis. Green. Chem. 18, 622–637. doi:10.1039/C5GC02500A
Khadir, A., Motamedi, M., Negarestani, M., Sillanpää, M., and Sasani, M. (2020). Preparation of a Nano Bio-Composite Based on Cellulosic Biomass and Conducting Polymeric Nanoparticles for Ibuprofen Removal: Kinetics, Isotherms, and Energy Site Distribution. Int. J. Biol. Macromolecules 162, 663–677. doi:10.1016/j.ijbiomac.2020.06.095
Khan, S. B., Alamry, K. A., Marwani, H. M., Asiri, A. M., and Rahman, M. M. (2013). Synthesis and Environmental Applications of cellulose/ZrO2 Nanohybrid as a Selective Adsorbent for Nickel Ion. Composites B: Eng. 50, 253–258. doi:10.1016/j.compositesb.2013.02.009
Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., et al. (2011). Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 50, 5438–5466. doi:10.1002/anie.201001273
Kumar, R., Sharma, R. K., and Singh, A. P. (2017). Cellulose Based Grafted Biosorbents - Journey from Lignocellulose Biomass to Toxic Metal Ions Sorption Applications - A Review. J. Mol. Liquids 232, 62–93. doi:10.1016/j.molliq.2017.02.050
Lei, C., Gao, J., Ren, W., Xie, Y., Abdalkarim, S. Y. H., Wang, S., et al. (2019). Fabrication of Metal-Organic Frameworks@cellulose Aerogels Composite Materials for Removal of Heavy Metal Ions in Water. Carbohydr. Polym. 205, 35–41. doi:10.1016/j.carbpol.2018.10.029
Li, C., Ma, H., Venkateswaran, S., and Hsiao, B. S. (2020a). Highly Efficient and Sustainable Carboxylated Cellulose Filters for Removal of Cationic Dyes/heavy Metals Ions. Chem. Eng. J. 389, 123458. doi:10.1016/j.cej.2019.123458
Li, J., Tan, S., and Xu, Z. (2020b). Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal. Nanomaterials 10, 1114. doi:10.3390/nano10061114
Li, Y., Xiao, H., Pan, Y., and Wang, L. (2018). Novel Composite Adsorbent Consisting of Dissolved Cellulose Fiber/Microfibrillated Cellulose for Dye Removal from Aqueous Solution. ACS Sustain. Chem. Eng. 6, 6994–7002. doi:10.1021/acssuschemeng.8b00829
Liang, L., Bhagia, S., Li, M., Huang, C., and Ragauskas, A. J. (2020). Cross‐Linked Nanocellulosic Materials and Their Applications. ChemSusChem 13, 78–87. doi:10.1002/cssc.201901676
Lin, X., Wu, M., Wu, D., Kuga, S., Endo, T., and Huang, Y. (2011). Platinum Nanoparticles Using wood Nanomaterials: Eco-Friendly Synthesis, Shape Control and Catalytic Activity for P-Nitrophenol Reduction. Green. Chem. 13, 283–287. doi:10.1039/C0GC00513D
Liu, H., Liu, Z., Hui, L., Liu, H., Liu, P., Zhang, F., et al. (2019a). Cationic Cellulose Nanofibers as Sustainable Flocculant and Retention Aid for Reconstituted Tobacco Sheet with High Performance. Carbohydr. Polym. 210, 372–378. doi:10.1016/j.carbpol.2019.01.065
Liu, J., Hao, D., Sun, H., Li, Y., Han, J., Fu, B., et al. (2021). Integration of MIL-101-NH2 into Cellulosic Foams for Efficient Cr(VI) Reduction under Visible Light. Ind. Eng. Chem. Res. 60, 12220–12227. doi:10.1021/acs.iecr.1c01777
Liu, P., Milletto, C., Monti, S., Zhu, C., and Mathew, A. P. (2019b). Design of Ultrathin Hybrid Membranes with Improved Retention Efficiency of Molecular Dyes. RSC Adv. 9, 28657–28669. doi:10.1039/C9RA04435C
Liu, P., Sehaqui, H., Tingaut, P., Wichser, A., Oksman, K., and Mathew, A. P. (2014). Cellulose and Chitin Nanomaterials for Capturing Silver Ions (Ag+) from Water via Surface Adsorption. Cellulose 21, 449–461. doi:10.1007/s10570-013-0139-5
Liu, P., Zhu, C., and Mathew, A. P. (2019c). Mechanically Robust High Flux Graphene Oxide - Nanocellulose Membranes for Dye Removal from Water. J. Hazard. Mater. 371, 484–493. doi:10.1016/j.jhazmat.2019.03.009
Ma, H., Burger, C., Hsiao, B. S., and Chu, B. (2011). Ultra-fine Cellulose Nanofibers: New Nano-Scale Materials for Water Purification. J. Mater. Chem. 21, 7507. doi:10.1039/c0jm04308g
Ma, H., Williams, P. L., and Diamond, S. A. (2013). Ecotoxicity of Manufactured ZnO Nanoparticles - A Review. Environ. Pollut. 172, 76–85. doi:10.1016/j.envpol.2012.08.011
Ma, S., Zhang, M., Nie, J., Tan, J., Song, S., and Luo, Y. (2019a). Lightweight and Porous Cellulose-Based Foams with High Loadings of Zeolitic Imidazolate Frameworks-8 for Adsorption Applications. Carbohydr. Polym. 208, 328–335. doi:10.1016/j.carbpol.2018.12.081
Ma, X., Lou, Y., Chen, X.-B., Shi, Z., and Xu, Y. (2019b). Multifunctional Flexible Composite Aerogels Constructed through In-Situ Growth of Metal-Organic Framework Nanoparticles on Bacterial Cellulose. Chem. Eng. J. 356, 227–235. doi:10.1016/j.cej.2018.09.034
Manna, P., Ghosh, M., Ghosh, J., Das, J., and Sil, P. C. (2012). Contribution of Nano-Copper Particles Toin Vivoliver Dysfunction and Cellular Damage: Role of IκBα/NF-Κb, MAPKs and Mitochondrial Signal. Nanotoxicology 6, 1–21. doi:10.3109/17435390.2011.552124
Miyashiro, D., Hamano, R., and Umemura, K. (2020). A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 10, 186. doi:10.3390/nano10020186
Mohamed, M. A., Abd Mutalib, M., Mohd Hir, Z. A., Zain, M. F., Jeffery Minggu, L., Awang, N. A., et al. (2017). An Overview on Cellulose-Based Material in Tailoring Bio-Hybrid Nanostructured Photocatalysts for Water Treatment and Renewable Energy Applications. Int. J. Biol. Macromolecules 103, 1232–1256. doi:10.1016/j.ijbiomac.2017.05.181
Mohamed, M. A., Salleh, W. N. W., Jaafar, J., Ismail, A. F., Abd. Mutalib, M., and Jamil, S. M. (2015). Feasibility of Recycled Newspaper as Cellulose Source for Regenerated Cellulose Membrane Fabrication. J. Appl. Polym. Sci. 132, n/a. doi:10.1002/app.42684
Moradeeya, P. G., Kumar, M. A., Thorat, R. B., Rathod, M., Khambhaty, Y., and Basha, S. (2017). Nanocellulose for Biosorption of Chlorpyrifos from Water: Chemometric Optimization, Kinetics and Equilibrium. Cellulose 24, 1319–1332. doi:10.1007/s10570-017-1197-x
Mousa, H. M., Alfadhel, H., and Abouel Nasr, E. (2020). Engineering and Characterization of Antibacterial Coaxial Nanofiber Membranes for Oil/Water Separation. Polymers 12, 2597. doi:10.3390/polym12112597
Movaghgharnezhad, S., Mirabi, A., Toosi, M. R., and Rad, A. S. (2020). Synthesis of Cellulose Nanofibers Functionalized by Dithiooxamide for Preconcentration and Determination of Trace Amounts of Cd(II) Ions in Water Samples. Cellulose 27, 8885–8898. doi:10.1007/s10570-020-03388-z
Nair, S. S., Chen, J., Slabon, A., and Mathew, A. P. (2020). Converting Cellulose Nanocrystals into Photocatalysts by Functionalisation with Titanium Dioxide Nanorods and Gold Nanocrystals. RSC Adv. 10, 37374–37381. doi:10.1039/D0RA05961G
Nasrollahzadeh, M., Sajjadi, M., Iravani, S., and Varma, R. S. (2021). Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 251, 116986. doi:10.1016/j.carbpol.2020.116986
Nasser Abdelhamid, H., and Mathew, A. P. (2021). Cellulose-zeolitic Imidazolate Frameworks (CelloZIFs) for Multifunctional Environmental Remediation: Adsorption and Catalytic Degradation. Chem. Eng. J. 426, 131733. doi:10.1016/j.cej.2021.131733
Nkalane, A., Oyewo, O. A., Leswifi, T., and Onyango, M. S. (2019). Application of Coagulant Obtained through Charge Reversal of Sawdust-Derived Cellulose Nanocrystals in the Enhancement of Water Turbidity Removal. Mater. Res. Express 6, 105060. doi:10.1088/2053-1591/ab3b49
Olivera, S., Muralidhara, H. B., Venkatesh, K., Guna, V. K., Gopalakrishna, K., and Kumar K., Y. (2016). Potential Applications of Cellulose and Chitosan Nanoparticles/composites in Wastewater Treatment: A Review. Carbohydr. Polym. 153, 600–618. doi:10.1016/j.carbpol.2016.08.017
Onwumere, J., Pia̧tek, J., Budnyak, T., Chen, J., Budnyk, S., Karim, Z., et al. (2020). CelluPhot: Hybrid Cellulose−Bismuth Oxybromide Membrane for Pollutant Removal. ACS Appl. Mater. Inter. 12, 42891–42901. doi:10.1021/acsami.0c12739
Oun, A. A., Shankar, S., and Rhim, J.-W. (2020). Multifunctional Nanocellulose/metal and Metal Oxide Nanoparticle Hybrid Nanomaterials. Crit. Rev. Food Sci. Nutr. 60, 435–460. doi:10.1080/10408398.2018.1536966
Oyewo, O. A., Elemike, E. E., Onwudiwe, D. C., and Onyango, M. S. (2020). Metal Oxide-Cellulose Nanocomposites for the Removal of Toxic Metals and Dyes from Wastewater. Int. J. Biol. Macromolecules 164, 2477–2496. doi:10.1016/j.ijbiomac.2020.08.074
Oyewo, O. A., Mutesse, B., Leswifi, T. Y., and Onyango, M. S. (2019). Highly Efficient Removal of Nickel and Cadmium from Water Using Sawdust-Derived Cellulose Nanocrystals. J. Environ. Chem. Eng. 7, 103251. doi:10.1016/j.jece.2019.103251
Park, S.-H., Shin, S. S., Park, C. H., Jeon, S., Gwon, J., Lee, S.-Y., et al. (2020). Poly(acryloyl Hydrazide)-Grafted Cellulose Nanocrystal Adsorbents with an Excellent Cr(VI) Adsorption Capacity. J. Hazard. Mater. 394, 122512. doi:10.1016/j.jhazmat.2020.122512
Patel, D. K., Dutta, S. D., and Lim, K.-T. (2019). Nanocellulose-based Polymer Hybrids and Their Emerging Applications in Biomedical Engineering and Water Purification. RSC Adv. 9, 19143–19162. doi:10.1039/C9RA03261D
Pei, A., Butchosa, N., Berglund, L. A., and Zhou, Q. (2013). Surface Quaternized Cellulose Nanofibrils with High Water Absorbency and Adsorption Capacity for Anionic Dyes. Soft Matter 9, 2047. doi:10.1039/c2sm27344f
Peng, B., Yao, Z., Wang, X., Crombeen, M., Sweeney, D. G., and Tam, K. C. (2020). Cellulose-based Materials in Wastewater Treatment of Petroleum Industry. Green. Energ. Environ. 5, 37–49. doi:10.1016/j.gee.2019.09.003
Pillai, S. S., Deepa, B., Abraham, E., Girija, N., Geetha, P., Jacob, L., et al. (2013). Biosorption of Cd(II) from Aqueous Solution Using Xanthated Nano Banana Cellulose: Equilibrium and Kinetic Studies. Ecotoxicology Environ. Saf. 98, 352–360. doi:10.1016/j.ecoenv.2013.09.003
Pinto, A. H., Taylor, J. K., Chandradat, R., Lam, E., Liu, Y., Leung, A. C. W., et al. (2020). Wood-based Cellulose Nanocrystals as Adsorbent of Cationic Toxic Dye, Auramine O, for Water Treatment. J. Environ. Chem. Eng. 8, 104187. doi:10.1016/j.jece.2020.104187
Pulidindi, K., and Prakash, A. (2020). Cellulose Market Size. GMI4854, 375. https://www.gminsights.com/industry-analysis/cellulose-market.
Qian, L., Lei, D., Duan, X., Zhang, S., Song, W., Hou, C., et al. (2018). Design and Preparation of Metal-Organic Framework Papers with Enhanced Mechanical Properties and Good Antibacterial Capacity. Carbohydr. Polym. 192, 44–51. doi:10.1016/j.carbpol.2018.03.049
Qin, F., Fang, Z., Zhou, J., Sun, C., Chen, K., Ding, Z., et al. (2019). Efficient Removal of Cu2+ in Water by Carboxymethylated Cellulose Nanofibrils: Performance and Mechanism. Biomacromolecules 20, 4466–4475. doi:10.1021/acs.biomac.9b01198
Qiu, K., and Netravali, A. N. (2014). A Review of Fabrication and Applications of Bacterial Cellulose Based Nanocomposites. Polym. Rev. 54, 598–626. doi:10.1080/15583724.2014.896018
Qu, X., Alvarez, P. J. J., and Li, Q. (2013). Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 47, 3931–3946. doi:10.1016/j.watres.2012.09.058
Rajesha, B. J., Vishaka, V. H., Balakrishna, G. R., Padaki, M., and Nazri, N. A. M. (2019). Effective Composite Membranes of Cellulose Acetate for Removal of Benzophenone-3. J. Water Process Eng. 30, 100419. doi:10.1016/j.jwpe.2017.06.003
Ray, S. S., and Iroegbu, A. O. C. (2021). Nanocellulosics: Benign, Sustainable, and Ubiquitous Biomaterials for Water Remediation. ACS Omega 6, 4511–4526. doi:10.1021/acsomega.0c06070
Ren, H., Gao, Z., Wu, D., Jiang, J., Sun, Y., and Luo, C. (2016). Efficient Pb(II) Removal Using Sodium Alginate-Carboxymethyl Cellulose Gel Beads: Preparation, Characterization, and Adsorption Mechanism. Carbohydr. Polym. 137, 402–409. doi:10.1016/j.carbpol.2015.11.002
Reshmy, R., Philip, E., Madhavan, A., Pugazhendhi, A., Sindhu, R., Sirohi, R., et al. (2022). Nanocellulose as green Material for Remediation of Hazardous Heavy Metal Contaminants. J. Hazard. Mater. 424, 127516. doi:10.1016/j.jhazmat.2021.127516
Rol, F., Belgacem, M. N., Gandini, A., and Bras, J. (2019). Recent Advances in Surface-Modified Cellulose Nanofibrils. Prog. Polym. Sci. 88, 241–264. doi:10.1016/j.progpolymsci.2018.09.002
Saito, T., Kimura, S., Nishiyama, Y., and Isogai, A. (2007). Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 8, 2485–2491. doi:10.1021/bm0703970
Santhosh, C., Velmurugan, V., Jacob, G., Jeong, S. K., Grace, A. N., and Bhatnagar, A. (2016). Role of Nanomaterials in Water Treatment Applications: A Review. Chem. Eng. J. 306, 1116–1137. doi:10.1016/j.cej.2016.08.053
Schelling, M., Kim, M., Otal, E., Aguirre, M., and Hinestroza, J. P. (2020). Synthesis of a Zinc-Imidazole Metal-Organic Framework (ZIF-8) Using ZnO Rods Grown on Cotton Fabrics as Precursors: Arsenate Absorption Studies. Cellulose 27, 6399–6410. doi:10.1007/s10570-020-03216-4
Sehaqui, H., de Larraya, U. P., Liu, P., Pfenninger, N., Mathew, A. P., Zimmermann, T., et al. (2014). Enhancing Adsorption of Heavy Metal Ions onto Biobased Nanofibers from Waste Pulp Residues for Application in Wastewater Treatment. Cellulose 21, 2831–2844. doi:10.1007/s10570-014-0310-7
Sehaqui, H., Mautner, A., Perez de Larraya, U., Pfenninger, N., Tingaut, P., and Zimmermann, T. (2016). Cationic Cellulose Nanofibers from Waste Pulp Residues and Their Nitrate, Fluoride, Sulphate and Phosphate Adsorption Properties. Carbohydr. Polym. 135, 334–340. doi:10.1016/j.carbpol.2015.08.091
Septevani, A. A., Rifathin, A., Sari, A. A., Sampora, Y., Ariani, G. N., Sudiyarmanto, , et al. (2020). Oil palm Empty Fruit bunch-based Nanocellulose as a Super-adsorbent for Water Remediation. Carbohydr. Polym. 229, 115433. doi:10.1016/j.carbpol.2019.115433
Shang, Q., Chen, J., Hu, Y., Yang, X., Hu, L., Liu, C., et al. (2021). Facile Fabrication of Superhydrophobic Cross-Linked Nanocellulose Aerogels for Oil-Water Separation. Polymers 13, 625. doi:10.3390/polym13040625
Sharma, P. R., Chattopadhyay, A., Sharma, S. K., Geng, L., Amiralian, N., Martin, D., et al. (2018). Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 6, 3279–3290. doi:10.1021/acssuschemeng.7b03473
Sharma, P. R., Sharma, S. K., Borges, W., Chen, H., and Hsiao, B. S. (2020a). Remediation of UO22+ from Water by Nitro-Oxidized Carboxycellulose Nanofibers: Performance and Mechanism Washington: American Chemical Society, 269–283. doi:10.1021/bk-2020-1352.ch013
Sharma, P. R., Sharma, S. K., Lindström, T., and Hsiao, B. S. (2020b). Nanocellulose‐Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 4, 1900114. doi:10.1002/adsu.201900114
Sharma, P. R., Sharma, S. K., Nolan, M., Li, W., Kundal, L., and Hsiao, B. S. (2021b). Sequential Oxidation on Wood and its Application in Pb2+ Removal from Contaminated Water. Polysaccharides 2, 245–256. doi:10.3390/polysaccharides2020017
Sharma, S. K., Sharma, P. R., Chen, H., Johnson, K., Zhan, C., Wang, R., et al. (2020c). Cellulose-Supported Nanosized Zinc Oxide: Highly Efficient Bionanomaterial for Removal of Arsenic from Water, 253–267. doi:10.1021/bk-2020-1352.ch012
Shen, W., Chen, S., Shi, S., Li, X., Zhang, X., Hu, W., et al. (2009). Adsorption of Cu(II) and Pb(II) onto Diethylenetriamine-Bacterial Cellulose. Carbohydr. Polym. 75, 110–114. doi:10.1016/j.carbpol.2008.07.006
Singh, K., Arora, J. K., Sinha, T. J. M., and Srivastava, S. (2014). Functionalization of Nanocrystalline Cellulose for Decontamination of Cr(III) and Cr(VI) from Aqueous System: Computational Modeling Approach. Clean. Techn Environ. Pol. 16, 1179–1191. doi:10.1007/s10098-014-0717-8
Sirviö, J. A., Hasa, T., Leiviskä, T., Liimatainen, H., and Hormi, O. (2016). Bisphosphonate Nanocellulose in the Removal of Vanadium(V) from Water. Cellulose 23, 689–697. doi:10.1007/s10570-015-0819-4
Soliman, M., Sadek, A. A., Abdelhamid, H. N., and Hussein, K. (2021). Graphene Oxide-Cellulose Nanocomposite Accelerates Skin Wound Healing. Res. Vet. Sci. 137, 262–273. doi:10.1016/j.rvsc.2021.05.013
Tan, K. B., Vakili, M., Horri, B. A., Poh, P. E., Abdullah, A. Z., and Salamatinia, B. (2015). Adsorption of Dyes by Nanomaterials: Recent Developments and Adsorption Mechanisms. Separat. Purif. Techn. 150, 229–242. doi:10.1016/j.seppur.2015.07.009
Teas, C., Kalligeros, S., Zanikos, F., Stournas, S., Lois, E., and Anastopoulos, G. (2001). Investigation of the Effectiveness of Absorbent Materials in Oil Spills Clean up. Desalination 140, 259–264. doi:10.1016/S0011-9164(01)00375-7
Thakur, V. K., and Voicu, S. I. (2016). Recent Advances in Cellulose and Chitosan Based Membranes for Water Purification: A Concise Review. Carbohydr. Polym. 146, 148–165. doi:10.1016/j.carbpol.2016.03.030
Tiwari, B., Sellamuthu, B., Ouarda, Y., Drogui, P., Tyagi, R. D., and Buelna, G. (2017). Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater Using Biological Approach. Bioresour. Techn. 224, 1–12. doi:10.1016/j.biortech.2016.11.042
Trouiller, B., Reliene, R., Westbrook, A., Solaimani, P., and Schiestl, R. H. (2009). Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability In Vivo in Mice. Cancer Res. 69, 8784–8789. doi:10.1158/0008-5472.CAN-09-2496
Valencia, L., Kumar, S., Jalvo, B., Mautner, A., Salazar-Alvarez, G., and Mathew, A. P. (2018). Fully Bio-Based Zwitterionic Membranes with superior Antifouling and Antibacterial Properties Prepared via Surface-Initiated Free-Radical Polymerization of Poly(cysteine Methacrylate). J. Mater. Chem. A. 6, 16361–16370. doi:10.1039/C8TA06095A
Valencia, L., Kumar, S., Nomena, E. M., Salazar-Alvarez, G., and Mathew, A. P. (2020). In-Situ Growth of Metal Oxide Nanoparticles on Cellulose Nanofibrils for Dye Removal and Antimicrobial Applications. ACS Appl. Nano Mater. 3, 7172–7181. doi:10.1021/acsanm.0c01511
Valencia, L., Monti, S., Kumar, S., Zhu, C., Liu, P., Yu, S., et al. (2019). Nanocellulose/graphene Oxide Layered Membranes: Elucidating Their Behaviour during Filtration of Water and Metal Ions in Real Time. Nanoscale 11, 22413–22422. doi:10.1039/C9NR07116D
Verfaillie, A., Blockx, J., Praveenkumar, R., Thielemans, W., and Muylaert, K. (2020). Harvesting of marine Microalgae Using Cationic Cellulose Nanocrystals. Carbohydr. Polym. 240, 116165. doi:10.1016/j.carbpol.2020.116165
Voisin, H., Bergström, L., Liu, P., and Mathew, A. (2017). Nanocellulose-Based Materials for Water Purification. Nanomaterials 7, 57. doi:10.3390/nano7030057
Wang, D. (2019). A Critical Review of Cellulose-Based Nanomaterials for Water Purification in Industrial Processes. Cellulose 26, 687–701. doi:10.1007/s10570-018-2143-2
Wang, N., Ouyang, X.-K., Yang, L.-Y., and Omer, A. M. (2017). Fabrication of a Magnetic Cellulose Nanocrystal/Metal-Organic Framework Composite for Removal of Pb(II) from Water. ACS Sustain. Chem. Eng. 5, 10447–10458. doi:10.1021/acssuschemeng.7b02472
Wang, X., Yeh, T.-M., Wang, Z., Yang, R., Wang, R., Ma, H., et al. (2014). Nanofiltration Membranes Prepared by Interfacial Polymerization on Thin-Film Nanofibrous Composite Scaffold. Polymer 55, 1358–1366. doi:10.1016/j.polymer.2013.12.007
Wei, H., Rodriguez, K., Renneckar, S., and Vikesland, P. J. (2014). Environmental Science and Engineering Applications of Nanocellulose-Based Nanocomposites. Environ. Sci. Nano 1, 302–316. doi:10.1039/C4EN00059E
Westerhoff, P., Alvarez, P., Li, Q., Gardea-Torresdey, J., and Zimmerman, J. (2016). Overcoming Implementation Barriers for Nanotechnology in Drinking Water Treatment. Environ. Sci. Nano 3, 1241–1253. doi:10.1039/C6EN00183A
WHO (2019). Drinking-water. Available at: https://www.who.int/en/news-room/fact-sheets/detail/drinking-water (Accessed January 16, 2021).
Wu, M., Kuga, S., and Huang, Y. (2008). Quasi-One-Dimensional Arrangement of Silver Nanoparticles Templated by Cellulose Microfibrils. Langmuir 24, 10494–10497. doi:10.1021/la801602k
Xiao, Z., Zhou, J., Fan, L., Li, Y., He, Y., Wang, Y., et al. (2021). Controllable Preparation of Cu-MOF-Coated Carboxyl Filter Paper for Simultaneous Removal of Organic Dye and Metal Ions. Ind. Eng. Chem. Res. 60, 7311–7319. doi:10.1021/acs.iecr.1c00140
Xie, H., Du, H., Yang, X., and Si, C. (2018). Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 1–25. doi:10.1155/2018/7923068
Xu, C., Chen, W., Gao, H., Xie, X., and Chen, Y. (2020). Cellulose Nanocrystal/silver (CNC/Ag) Thin-Film Nanocomposite Nanofiltration Membranes with Multifunctional Properties. Environ. Sci. Nano 7, 803–816. doi:10.1039/C9EN01367A
Yang, C., Wang, L., Yu, Y., Wu, P., Wang, F., Liu, S., et al. (2020a). Highly Efficient Removal of Amoxicillin from Water by Mg-Al Layered Double Hydroxide/cellulose Nanocomposite Beads Synthesized through In-Situ Coprecipitation Method. Int. J. Biol. Macromolecules 149, 93–100. doi:10.1016/j.ijbiomac.2020.01.096
Yang, W., Wang, J., Yang, Q., Pei, H., Hu, N., Suo, Y., et al. (2018). Facile Fabrication of Robust MOF Membranes on Cloth via a CMC Macromolecule Bridge for Highly Efficient Pb(II) Removal. Chem. Eng. J. 339, 230–239. doi:10.1016/j.cej.2018.01.126
Yang, Y., Guo, Z., Huang, W., Zhang, S., Huang, J., Yang, H., et al. (2020b). Fabrication of Multifunctional Textiles with Durable Antibacterial Property and Efficient Oil-Water Separation via In Situ Growth of Zeolitic Imidazolate Framework-8 (ZIF-8) on Cotton Fabric. Appl. Surf. Sci. 503, 144079. doi:10.1016/j.apsusc.2019.144079
Yao, G., Zhu, X., Wang, M., Qiu, Z., Zhang, T., and Qiu, F. (2021). Controlled Fabrication of the Biomass Cellulose–CeO2 Nanocomposite Membrane as Efficient and Recyclable Adsorbents for Fluoride Removal. Ind. Eng. Chem. Res. 60, 5914–5923. doi:10.1021/acs.iecr.1c00012
Yu, H., Hong, H.-J., Kim, S. M., Ko, H. C., and Jeong, H. S. (2020). Mechanically Enhanced Graphene Oxide/carboxymethyl Cellulose Nanofibril Composite Fiber as a Scalable Adsorbent for Heavy Metal Removal. Carbohydr. Polym. 240, 116348. doi:10.1016/j.carbpol.2020.116348
Yu, J., Wang, A. C., Zhang, M., and Lin, Z. (2021). Water Treatment via Non-membrane Inorganic Nanoparticles/cellulose Composites. Mater. Today. doi:10.1016/j.mattod.2021.03.024
Yu, S., Liu, M., Ma, M., Qi, M., Lü, Z., and Gao, C. (2010). Impacts of Membrane Properties on Reactive Dye Removal from Dye/salt Mixtures by Asymmetric Cellulose Acetate and Composite Polyamide Nanofiltration Membranes. J. Memb. Sci. 350, 83–91. doi:10.1016/j.memsci.2009.12.014
Yu, X., Tong, S., Ge, M., Wu, L., Zuo, J., Cao, C., et al. (2013a). Adsorption of Heavy Metal Ions from Aqueous Solution by Carboxylated Cellulose Nanocrystals. J. Environ. Sci. 25, 933–943. doi:10.1016/S1001-0742(12)60145-4
Yu, X., Tong, S., Ge, M., and Zuo, J. (2013b). Removal of Fluoride from Drinking Water by Cellulose@hydroxyapatite Nanocomposites. Carbohydr. Polym. 92, 269–275. doi:10.1016/j.carbpol.2012.09.045
Zeng, J., Liu, S., Cai, J., and Zhang, L. (2010). TiO2 Immobilized in Cellulose Matrix for Photocatalytic Degradation of Phenol under Weak UV Light Irradiation. J. Phys. Chem. C 114, 7806–7811. doi:10.1021/jp1005617
Zhang, K., Ketterle, L., Järvinen, T., Lorite, G. S., Hong, S., and Liimatainen, H. (2020). Self-assembly of Graphene Oxide and Cellulose Nanocrystals into Continuous Filament via Interfacial Nanoparticle Complexation. Mater. Des. 193, 108791. doi:10.1016/j.matdes.2020.108791
Zhang, K., Shen, M., Liu, H., Shang, S., Wang, D., and Liimatainen, H. (2018). Facile Synthesis of Palladium and Gold Nanoparticles by Using Dialdehyde Nanocellulose as Template and Reducing Agent. Carbohydr. Polym. 186, 132–139. doi:10.1016/j.carbpol.2018.01.048
Zhang, N., Zang, G.-L., Shi, C., Yu, H.-Q., and Sheng, G.-P. (2016). A Novel Adsorbent TEMPO-Mediated Oxidized Cellulose Nanofibrils Modified with PEI: Preparation, Characterization, and Application for Cu(II) Removal. J. Hazard. Mater. 316, 11–18. doi:10.1016/j.jhazmat.2016.05.018
Zhang, X., Zhao, J., Cheng, L., Lu, C., Wang, Y., He, X., et al. (2014a). Acrylic Acid Grafted and Acrylic Acid/sodium Humate Grafted Bamboo Cellulose Nanofibers for Cu 2+ Adsorption. RSC Adv. 4, 55195–55201. doi:10.1039/C4RA08307E
Zhang, Z., Sèbe, G., Rentsch, D., Zimmermann, T., and Tingaut, P. (2014b). Ultralightweight and Flexible Silylated Nanocellulose Sponges for the Selective Removal of Oil from Water. Chem. Mater. 26, 2659–2668. doi:10.1021/cm5004164
Zhao, J., Zhang, X., He, X., Xiao, M., Zhang, W., and Lu, C. (2015). A Super Biosorbent from Dendrimer Poly(amidoamine)-Grafted Cellulose Nanofibril Aerogels for Effective Removal of Cr( <scp>vi</scp> ). J. Mater. Chem. A. 3, 14703–14711. doi:10.1039/C5TA03089G
Zhou, C., Wu, Q., Lei, T., and Negulescu, I. I. (2014). Adsorption Kinetic and Equilibrium Studies for Methylene Blue Dye by Partially Hydrolyzed Polyacrylamide/cellulose Nanocrystal Nanocomposite Hydrogels. Chem. Eng. J. 251, 17–24. doi:10.1016/j.cej.2014.04.034
Zhou, H., Zhu, H., Xue, F., He, H., and Wang, S. (2020). Cellulose-based Amphoteric Adsorbent for the Complete Removal of Low-Level Heavy Metal Ions via a Specialization and Cooperation Mechanism. Chem. Eng. J. 385, 123879. doi:10.1016/j.cej.2019.123879
Zhu, C., Liu, P., and Mathew, A. P. (2017). Self-Assembled TEMPO Cellulose Nanofibers: Graphene Oxide-Based Biohybrids for Water Purification. ACS Appl. Mater. Inter. 9, 21048–21058. doi:10.1021/acsami.7b06358
Zhu, C., Monti, S., and Mathew, A. P. (2018). Cellulose Nanofiber–Graphene Oxide Biohybrids: Disclosing the Self-Assembly and Copper-Ion Adsorption Using Advanced Microscopy and ReaxFF Simulations. ACS Nano 12, 7028–7038. doi:10.1021/acsnano.8b02734
Zhu, C., Monti, S., and Mathew, A. P. (2020). Evaluation of Nanocellulose Interaction with Water Pollutants Using Nanocellulose Colloidal Probes and Molecular Dynamic Simulations. Carbohydr. Polym. 229, 115510. doi:10.1016/j.carbpol.2019.115510
Zhu, H., Yang, X., Cranston, E. D., and Zhu, S. (2016). Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal-Organic-Framework Particles for Separations Applications. Adv. Mater. 28, 7652–7657. doi:10.1002/adma.201601351
Keywords: water treatment, adsorption, catalysis, (Nano)cellulose, cellulose hybrid
Citation: Abdelhamid HN and Mathew AP (2021) Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 3:790314. doi: 10.3389/fceng.2021.790314
Received: 06 October 2021; Accepted: 17 November 2021;
Published: 02 December 2021.
Edited by:
Benjamin Hsiao, Stony Brook University, United StatesReviewed by:
Vaishakh Nair, National Institute of Technology, Karnataka, IndiaZeinab Abbas Jawad, Qatar University, Qatar
Copyright © 2021 Abdelhamid and Mathew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hani Nasser Abdelhamid, aGFueS5hYmRlbGhhbWlkQGF1bi5lZHUuZWc=; Aji P. Mathew, YWppLm1hdGhld0BtbWsuc3Uuc2U=
 Hani Nasser Abdelhamid
Hani Nasser Abdelhamid Aji P. Mathew
Aji P. Mathew