- 1Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, United States
- 2Department of Biomedical Engineering, University of Alabama at Birmingham (UAB), Birmingham, AL, United States
Adeno-associated viruses (AAVs) have been well characterized and used to deliver therapeutic genes for diseases treatment in clinics and basic research. This study used the triple transient transfection of AAV-DJ/8 as a model expression system to develop and optimize the laboratory production of AAV for research and pre-clinical applications. Specifically, various production parameters, including host cell, transfection reagent, cell density, ratio of plasmid DNA and cells, gene size, and production mode, were tested to determine the optimal process. Our results showed that the adherent production using HEK 293AAV with calcium transfection generated the highest volumetric productivity of 7.86 × 109 gc/ml. The optimal suspensive production using HEK 293F had best AAV productivity of 5.78 × 109 gc/ml in serum-free medium under transfection conditions of transfection density of 0.4 × 106 cells/ml, plasmid DNA:cells ratio of 1.6 µg:106 cells and synthesized cationic liposomes as transfection reagent. The similar AAV productivity was confirmed at scales of 30–450 ml in shaker and/or spinner flasks. The in vitro transfection and in vivo infection efficiency of the harvested AAV-DJ/8 carrying luciferase reporter gene was confirmed using cell line and xenograft mouse model, respectively. The minimal or low purification recovery rate of AAV-DJ/8 in ion-exchange chromatography column and affinity column was observed in this study. In summary, we developed and optimized a scalable suspensive production of AAV to support the large-scale preclinical animal studies in research laboratories.
1 Introduction
Adeno-associated virus (AAV) is a small (20 nm), replication-defective, non-pathogenic parvovirus. The single-stranded DNA (ssDNA) genome with maximal size of 4.8 kb is encapsidated in non-enveloped capsid (Blessing et al., 2019). Hundreds of serotypes and variants have been isolated and characterized, which can infect both dividing and non-dividing cells of different tissues in human and other animals (Crosson et al., 2018). The chimeric AAV, such as AAV-DJ, has been constructed via capsid evolution of AAV2, 8 and 9 by Kay (Büning and Srivastava, 2019), Samulski (Li et al., 2008) and Schaffer (Koerber et al., 2008) Labs to deliver therapeutic gene to multiple cell types (El Andari and Grimm, 2021).
AAV has great potential as gene delivery vehicle due to the multiple advantages such as minimal immunogenicity, long-term transgene expression, no adverse events reported in clinics, high stability, and broad range of cell types to infect (Blessing et al., 2019; Hacker et al., 2020). European Medicines Agency (EMA) approved the first AAV-based gene therapy in 2012 to treat lipoprotein lipase deficiency (Ledley et al., 2014; van der Loo and Wright, 2016) and United States Food and Drug Administration (FDA) approved Luxturna in 2017 and Zolgensma in 2019 to treat rare inherited blindness and spinal muscular atrophy, respectively. There are more than 300 ongoing clinical studies to treat a wide variety of diseases or disorders, such as Alzheimer, Parkinson, cystic fibrosis, hemophilia B, HIV infection, Leber’s congenital amaurosis, arthritis, and cancers (Carter, 2005; Urabe et al., 2006; Chahal et al., 2014; Mietzsch et al., 2014). In addition to clinical applications, AAV is also an important research tool which can deliver reporter genes, CRISPR endonuclease or other genes for fundamental studies (Senís et al., 2014; Crosson et al., 2018).
Because of the broad applications of AAV in basic research and pre-clinical translational study, it is highly desired to develop a robust, scalable and Good Lab Practice (GLP) bioproduction process of AAV vector. Literature has reported multiple AAV production procedures using adherent and suspensive cultures. For example, Grieger et al. have developed a polyethylenimine (PEI)-facilitated three-plasmid transfection protocol by evaluating four ratios of helper, capsid and expression plasmids, two transfection densities and three host cell passage numbers, which generated volumetric productivity of 1 × 109–3.3 × 1010 vector genome (vg)/mL of AAV (serotypes 1–6, 8, and 9) (Grieger et al., 2016). Blessing et al. reported AAV2/9 productivity of 2 × 108 vg/ml in PEI-mediated three-plasmid transfection of suspensive HEK 293 culture (Blessing et al., 2019). Zhao et al. reported an AAV productivity of 3 × 1011 vg/ml of HEK 293T suspensive culture with PEI transfection (Zhao et al., 2020). In addition, calcium and lipofection have been used to deliver plasmids for adherent AAV production (Negrini et al., 2020). A developed dynamic mathematic model has indicated that the coordination between ssDNA replication and capsid synthesis is a key parameter to produce full capsid AAV (Nguyen et al., 2021). Despite these achievements, the low robustness of production process, high variation of titer and productivity and low biological activity of end product remain the major challenges in AAV production in research laboratory (Wright, 2008; Nguyen et al., 2021).
The objective of this study was to develop and optimize a scalable high-yield production process. Although both adherent and suspensive operation modes were evaluated and compared, we use suspensive production as model system to perform the process development. Various parameters such as host cell, transfection reagent, transfection cell density, plasmid DNA amount, insert gene size, and scalability were investigated to determine the optimal production process. The in vitro transfection and in vivo infection of produced AAV were confirmed using cancer cell line and xenograft mouse model. In addition to production, the purification methods were briefly evaluated but further development is needed. The results obtained in this study could benefit the in-house three-plasmid transient expression of AAV.
2 Materials and Methods
2.1 Plasmids for Adeno-Associated Viruses Vectors
The AAV-DJ/8 Helper Free Promoterless Expression System (Cell Biolabs, San Diego, CA, United States) was applied to construct AAV vector. The pAAV-cfos-NLuc-ACC plasmid (unpublished) was constructed by Professor Zhou Lab (UAB, United States). The blue luminescence-emitting Nanoluciferase (NLuc) reporter gene cloned from pNL-CMV-NLuc (Promega #N1091) and synthesized ACC genes (unpublished), total of ∼3.9 kb, were inserted into the pAAV-MCS vector (Cell Biolabs). The plasmid pAAV-CMV-eYFP was constructed by cloning CMV promoter cloned from pcDNA3.1-PsChR2-eYFP (Addgene #69057) (Govorunova et al., 2013; Ernst et al., 2019) and eYFP reporter gene from pcDNA3.1-PsChR2-eYFP, totoal of 1.1 kb, into the pAAV-MCS. The PCR primers are NLuc-forward: 5′- ACTCACTATAGGGAGACCCACACCATGGTCTTCACACTCG-3′, NLuc-reverse: 5′- TGCCTGATCCCGCCAGAATGCGTTCGCAC-3′, eYFP forward: 5′-GGTAGTAGCAATGCTAGTGAGCAAGGGC-3′, and eYFP reverse: 5′-ACCTTCGAACCGCGGGCCCTCTAGATTACTTGTACAGCTCGTCC-3′. The pHelper plasmid and pAAV-DJ/8 Rep-Cap plasmid (Cell Biolabs) were used to produce AAV-DJ8 serotype.
2.2 Cell Lines, Media, and Culture
The embryonic human kidney (HEK) 293AAV cell line (Cell Biolabs, #AAV-100), which was cloned and selected from parental 293 cell line (transformed with human adenovirus type 5 DNA), was used to produce AAV in adherent culture. The HEK 293F cell line (Gibco, Buffalo, NY, United States) that was adapted to serum-free medium was used to produce AAV in suspensive culture. The human triple-negative breast cancer (TNBC) cell line MDA-MB-468 (GenTarget, San Diego, CA, United States) was used to test the in vitro transfection capability of AAV and was also used to establish the tumor xenograft animal model to validate the in vivo infection.
All basal media, nutrient supplements and other reagents used in this study were purchased from Thermo Fisher Scientific (Waltham, MA, United States) or Gibco (Grand Island, NY, United States) unless otherwise specified. The HEK 293AAV cells were cultivated in DMEM (high glucose) supplemented with 10% fetal bovine serum (FBS, v/v), 0.1 mM MEM Non-Essential Amino Acids (NEAA), 2 mM L-glutamine, and 100 IU penicillin and 100 μg/ml streptomycin (i.e., 1% Pen-Strep) in T75 flasks. The HEK 293F cells were maintained in chemically defined (CD) FreeStyle 293 Expression Medium (Gibco) supplemented with 4 mM GlutaMAX in 125 or 250-ml shaker flasks on an orbital shaker at 135 rpm. The TNBC MDA-MB-468 cells were maintained in DMEM/F12 medium supplemented with 4 g/L glucose, 4 mM L-glutamine, 10% FBS (v/v) and 1% P/S in T-flasks. All the cell cultures were incubated in a humidified incubator (Caron, Marietta, OH) with set points of 37°C and 5% CO2.
2.3 Adeno-Associated Viruses Production Using Adherent Culture
The adherent AAV production was performed in 150-mm petri dish. Specifically, the 30 ml of complete DMEM medium (same formulation as seed culture) was seeded with HEK 293AAV at viable cell density (VCD) of 0.1 × 106 cells/ml. When the confluence reached 95%, spent medium was removed and replaced with fresh complete medium. The cells were transfected with pAAV-cfos-NLuc-ACC, pHelper, and pAAV-DJ/8 Rep-Cap plasmids with transfection reagent of 25 mM of Ca2+, lipofectamine 3,000 (Gibco), or liposomes synthesized in house. 1) Calcium transfection: The 0.33 ml of 2.5 M CaCl2, 30 µg of each plasmid (total of 120 µg), and 0.1 µM of TE buffer were mixed to reach final volume of 1.5 ml. The Ca2+/DNA mixture was added to 1.5 ml of 2X HBS buffer slowly and dropwise to make 3 ml of Ca2+/DNA/HBS mixture, which was added to the 30-ml HEK 293AAV culture. The transfected cells were incubated for 4–6 h, followed with medium exchange and incubation for 48–60 h, and harvested for AAV lysis. 2) Lipofectamine transfection: 10 µg of each plasmid (total of 30 µg) and 60 µL of P3000 reagent were added into 300 µL of Opti-MEM medium to prepare the DNA-P3000 mixture. The 45 µL of Lipofectamine 3,000 was added into 300 µL of Opti-MEM medium to prepare Lipofectamine 3,000 mixture. The 600-µL complete DNA-Lipofectamine 3,000 mixture was incubated for 15 min at room temperature and used to transfect the HEK 293AAV cells. Medium exchange was performed at 6 h post transfection. The transfected HKE 293AAV cells were harvested and centrifuged at 48–60 h for further AAV clarification. 3) Cationic liposomes transfection: We synthesized cationic liposomes following similar synthetic procedure of neutral liposomes (Si et al., 2021) with modifications: mixing 7 µmole 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 7 µmole 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and 10 ml of chloroform and evaporating at 60°C and 50 rpm for 1 h. The generated liposomes were analyzed using NanoSight, which showed concentration of 3.2 × 1011 particles/ml and mean size of 92.6 ± 0.4 nm. Total of 30 µg three plasmids (10 µg each plasmid) were mixed with 900 µL of cationic liposomes, incubated on ice for 30 min, and mixed with Opti-MEM medium with volume ratio of 1:1. The HEK 293AAV cells in petri-dish were transfected with the liposomes-DNA complex and incubated for 48–60 h before AAV harvest.
2.4 Adeno-Associated Viruses Production Using Suspension Culture
The suspensive AAV production was performed in shaker flasks or spinner flasks. The Freestyle 293 medium was inoculated with HEK 293F cells at seeding VCD of 0.1–0.3 × 106 cells/ml and incubated for 20–24 h before transfection. Different plasmid:cell ratios of 1.6, 3.3, and 6.6, and three transfection reagents of Polyethylenimine HCI (PEI) MAX, TurboFect and our synthesized liposomes were evaluated and compared in suspensive AAV production. The following formulation was designed for 30-ml HEK 293F culture. 1) PEI transfection: Total of 30 µg plasmids (10 µg each plasmid) were mixed with 120 µL of 1.6 mg/ml PEI MAX and incubated at 37°C for 10 min, which was used to transfect HEK 293F cells. 2) TurboFect transfection: The same formulation of TurboFect-DNA transfection complex as PEI was prepared for suspension transfection. 3) Cationic liposomes transfection: The same transfection reagent formulation as in adherent AAV production was used to transfect HEK 293F cells.
2.5 Adeno-Associated Viruses Harvest and Clarification
In the end of AAV production, cell pellets were collected with centrifugation of the culture broth at 1,000 g and 4°C for 10 min. The cell pellets were re-suspended using lysis buffer (50 mM TrisCl, 150 mM NaCl, 2 mM MgCl2, pH 8.0) with volume of 1/30 of production culture volume. Then cell lysis was processed through three cycles of freezing/thawing (30-min freezing in ethanol/dry ice and 15-min in 37°C water bath thawing each cycle). The 50 U/ml benzonase and 5 μg/ml RNase A were added into AAV lysate and incubated in shaker at 37°C and 150 rpm for 60 min. Then 0.5% sodium deoxycholate was added to further treat the lysis solution for 30 min. The AAV supernatant was collected after centrifugation at 3,000×g and 4°C for 20 min, and filtered using 0.45 and 0.22 μm PES membrane to remove cell debris.
2.6 Adeno-Associated Viruses Titration and Storage
To extract ssDNA from AAV, 1 µL of 2000 U/ml DNase I (BioLabs), 2 µL of 10X DNase I reaction buffer, 12 µL of nuclease free water, and 5 µL of AAV sample were added into a 0.2-ml PCR tube. The mixture was incubated at 37°C for 30 min and followed with DNase I inactivation at 70°C for 10 min. The sample was further digested with 1 µL of 2 mg/ml Proteinase K by incubation at 50°C for 1 h and deactivation at 95°C for 20 min. Finally, the extracted ssDNA was diluted by 10 folds with nuclease free water and titrated using RT-PCR with primers of NLuc forward: 5′-ATTGTCCTGAGCGGTGAAA-3′, NLuc reverse: 5′-CACAGGGTACACCACCTTAAA-3′, eYFP forward: 5′-GCACAAGCTGGAGTACAACTA-3′, and eYFP reverse: 5′-TGTTGTGGCGGATCTTGAA-3′. The raw AAV or purified AAV was buffer exchanged in 1x PBS, 5% Sorbitol, and 350 mmol/L NaCl, and stored at −80°C for long term.
2.7 In Vitro Transfection and In Vivo Infection Analysis
The animal study conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals published (Publication No. 85‐23), with the approved animal protocol of IACUC-21949 by the Institutional Biosafety Committee at University of Alabama at Birmingham.
In in vitro evaluation, the MDA-MB-468 cancer cells were seeded in 2 ml of complete DMEM/F12 medium in 6-well plate with seeding VCD of 0.05 × 106 cells/ml. After 24-h incubation, the cells were infected with the raw AAV carrying NLuc at multiplicity of infection (MOI) of 10,000 in triplication with mock infection with PBS as control. Two days after infection, the fresh growth medium containing 30 µM of substate ViviRen was used to replace spent medium. The In Vivo Imaging System (IVIS) Lumina Series III (PerkinElmer, Waltham, MA, United Staes) was applied to take the bioluminescence images of infected cells at emission wavelength of 460 nm.
To evaluate the in vivo infection capability of produced AAV, 5 × 106 human TNBC MDA-MB-468 cells were subcutaneously (s.c.) injected into the 6-week-old NSG (NOD scid gamma) female mice (Jackson Laboratory, Bar Harbor, ME) to establish xenograft model. Total of 1 × 1010 gc AAV was intravenously (i.v.) injected and mice were maintained for 7 days to allow NLuc expression, followed by i.p. injection of 37 µg ViviRen substrate. The live-animal image was captured using IVIS system at excitation/emission wavelength of 660/710 nm. The bioluminescence intensity was recorded with exposure time of 5 s to monitor peak excitation.
2.8 Statistical Analysis
The replication numbers (n) used in this study was >4 and all experimental data were presented as mean ± standard error of the mean (SEM). Two-tailed Student’s t tests were used to determine the probability of significance between groups and comparison was performed using a one-way ANOVA followed by post-hoc (Dunnett’s) analysis. The statistical significance of **p value <0.05 was considered for all tests.
3 Results and Discussion
3.1 Adeno-Associated Viruses Biomanufacturing
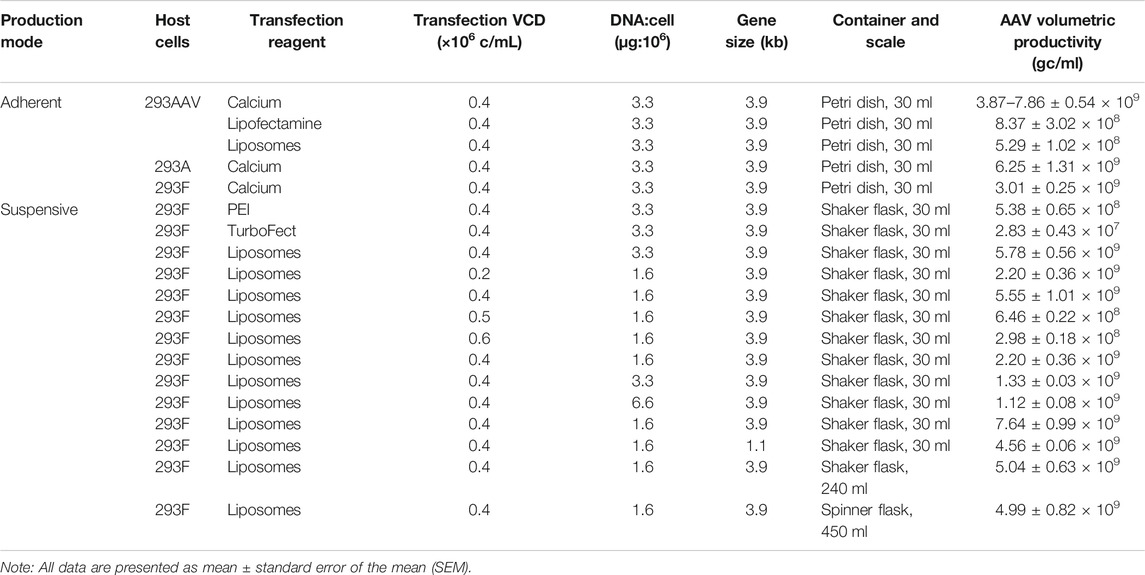
The overview of the developed AAV biomanufacturing process is detailed in Figure 1, including both adherent production, suspensive production, purification, and storage. In adherent operation mode, the HEK 293AAV cells were used to seed multiple petri-dishes for AAV production. The calcium solution that showed the highest transfection efficiency and AAV productivity was applied to deliver the three helper-free expression system plasmids, including the pAAV plasmid expressing NLuc reporter gene (Figure 2A) or eYFP reporter gene (Figure 2B), to HEK 293AAV cells. The key parameters that we identified in calcium transfection were the pH of transfection complex, medium exchange before transfection, and medium exchange post transfection. In suspensive operation mode, the HEK 293F cells were seeded in CD medium in shaker flasks or spinner flasks for AAV production. The synthesized cationic liposomes identified as the best transfection reagent was used to deliver plasmids to 293F cells. The key parameters that we identified in suspensive production were the transfection cell density and ratio among liposomes, plasmid and cell number.
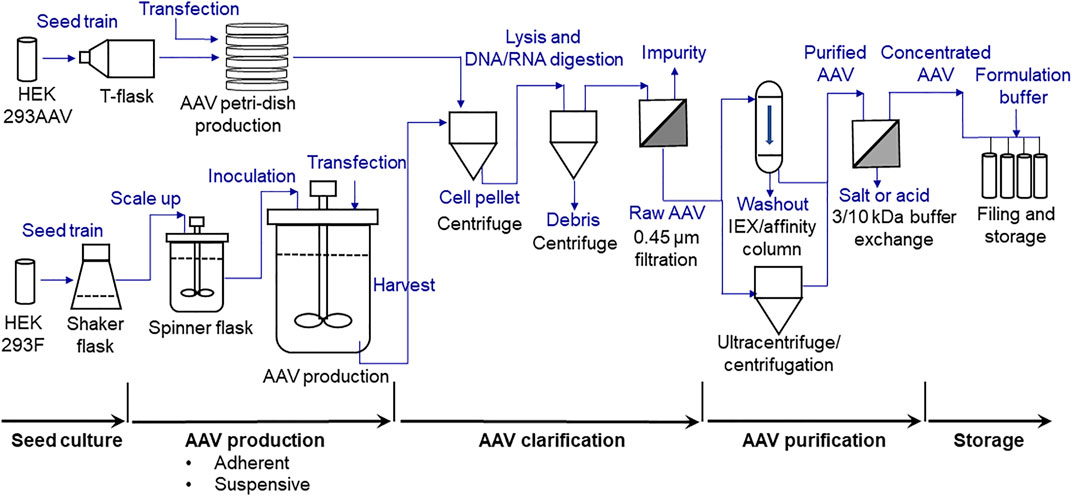
FIGURE 1. Process flow diagram (PFD) of scalable high-yield production of AAV, including seed culture preparation, adherent and suspensive productions, clarification, purification (ultracentrifugation used in this study) and storage.
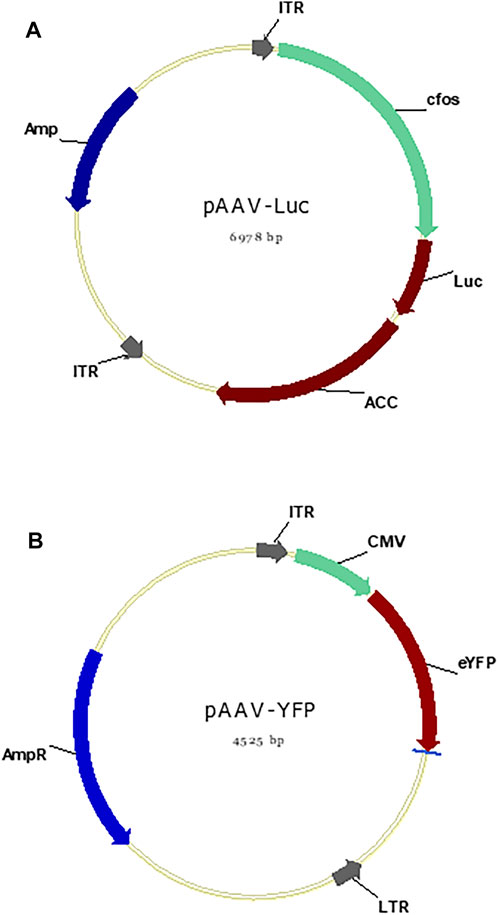
FIGURE 2. pAAV plasmid maps. (A) The plasmid map of pAAV-cfos-NLuc-ACC containing cfos promoter and NLuc reporter gene and fused gene, total of 6.978 kb. (B) The plasmid map of pAAV-cMV-eYFP containing CMV promoter and YFP reporter gene, total of 4.525 kb.
The cell pellets harvested in the end of AAV production were processed to release AAV through freezing/thawing in lysis buffer. The supplement of sodium deoxycholate, benzonase, and RNase A could further treat AAV lysis and remove DNA/RNA impurities. The raw AAV supernatant post centrifugation was filtered using 0.45 and 0.22 μm PES filters to remove cell debris. The well-developed iodixanol gradient ultracentrifugation could be used to purify AAV product, which could achieve high purity and recovery rate despite the low purification capacity and long processing procedure (Zolotukhin et al., 1999; Strobel et al., 2015). The ion-exchange chromatography column (Brument et al., 2002; Lock et al., 2012; Potter et al., 2014; Qu et al., 2015) or affinity chromatography column (Wu and Ataai, 2000; Grieger et al., 2006; Arakawa et al., 2007; Potter et al., 2014) can be used to purify some serotypes of AAVs. The buffer exchange and AAV concentration was performed using 3 or 10 kDa MWCO concentrator. The purified AAV was stored at −80°C freezer for long-term storage.
In this study, we focused on the process development and optimization of AAV-DJ/8 production and validation of the infection bioactivity. The purification and storage were also briefly tested and discussed.
3.2 Effect of Production Mode, Transfection Reagent and Host Cell
During the AAV bioprocessing optimization, we first evaluated the effects of production mode (i.e., adherent and suspensive), transfection reagents and host cells on AAV packing and production. As described in Figure 3A, the calcium-facilitated transfection of pAAV-cfos-NLuc-ACC, pHelper, and pAAV-DJ/8 Rep-Cap plasmids generated the highest volumetric productivity of 3.87 × 109 gc/ml using HEK 293AAV cells in petri dishes. The transfections with lipofectamine 3,000 and in-house synthesized cationic liposomes resulted in AAV productivity of 8.37 × 108 gc/ml and 5.29 × 108 gc/ml, respectively. The titer of raw AAV lysis were 5.45 × 1010 gc/ml, 1.98 × 1010 gc/ml, and 1.09 × 1010 gc/ml for these three transfection reagents. The comparison among three host cells showed that the HEK 293AAV cells had higher AAV productivity of 7.86 × 109 gc/ml and titer of 4.89 × 1011 gc/ml than HEK 293A with productivity of 6.25 × 109 gc/ml and titer of 1.67 × 1011 gc/ml and HEK 293F cells with productivity of 3.01 × 109 gc/ml and titer of 1.34 × 1011 gc/ml using calcium transfection in petri dish culture (Figure 3B). Three transfection reagents of PEI MAX, TurboFect and liposomes were investigated in suspensive AAV production. As shown in Figure 3C, the liposomes generated the highest AAV productivity of 5.78 × 109 gc/ml and titer of 9.84 × 1010 gc/ml by HEK 293F in shaker flasks. The PEI produced AAV with productivity of 5.38 × 108 gc/ml and titer of 5.38 × 109 gc/ml, and TurboFect generated productivity of 1.70 × 108 gc/ml and titer of 2.83 × 107 gc/ml.
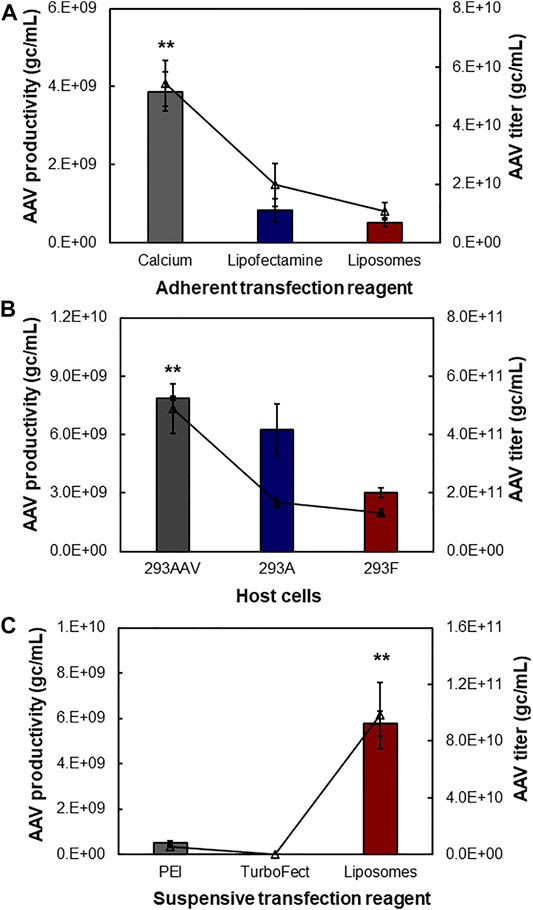
FIGURE 3. Effect of transfection reagent, operation mode and host cell. The three-plasmid (pAAV, pHelper, and pAAV-DJ/8 Rep-Cap) transfection was used to produce AAV. (A) Adherent AAV production mediated with three transfection reagents including calcium, lipofectamine and liposomes. The HEK 293AAV cells were cultivated in 30-ml high-glucose DMEM medium supplemented with 10% FBS, 0.1 mM MEM NEAA, 2 mM L-glutamine and 1% Pen-Strep in petri dish (data represent mean ± SEM, n = 4). (B) Effect of host cells on AAV production in adherent cultures (n = 2). Three host cells, i.e. HEK 293AAV, 293A, and 293F, were tested with calcium transfection. (C) Suspensive AAV production mediated with three transfection reagents including PEI, TurboFect and liposomes. The HEK 293F cells were cultivated in 30-ml chemical defined FreeStyle medium supplemented with 4 mM GlutaMAX at agitation speed of 135 rpm (n = 4). **p ≤ 0.05.
This study showed that calcium had higher transfection efficiency than other reagents in adherent AAV production, but it’s hard to use calcium to perform suspension transfection and medium exchange pre-and post-transfection. The cationic liposomes demonstrated high transfection efficiency in suspensive AAV production and the low cost of liposomes could significantly reduce the AAV production cost as compared to PEI and TurboFect. Moreover, the AAV productivity using HEK 293F and liposomes was only ∼25% lower than that using HEK 293AAV and calcium. Therefore, the suspensive AAV production with HEK 293F and cationic liposomes was performed to optimize AAV production.
3.3 Transfection Optimization: Viable Cell Density, Ratio of DNA:Cell and Gene Size
To further optimize the suspensive AAV production, we tested the effects of transfection cell density, ratio between plasmid DNA and transfected cells, and size of insert gene in the AAV-DJ/8 Helper Free Promoterless Expression System. In this study, the suspensive HEK 293F was transfected with synthesized liposomes. First, the transfection VCD of 0.4 × 106 cells/ml of HEK 293F with pAAV carrying 3.9-kb insert gene produced the highest productivity of 5.55 × 109 gc/ml and titer of 3.33 × 1010 gc/ml, which was significantly higher than that of VCD of 0.2, 0.5, and 0.6 × 106 cells/ml with productivity of 0.29–2.20 × 109 gc/ml and titer of 0.18–1.32 × 1010 gc/ml (Figure 4A). Second, different ratios of plasmid DNA and transfected cells were examined, including 1.6, 3.3, and 6.6 µg:106 cells. The AAV production showed that ratio of 1.6 generated higher productivity (2.20 × 109 gc/ml) and titer (1.32 × 1010 gc/ml) (Figure 4B). Third, the AAV production using pAAV carrying 3.9 kb of insert gene was higher than that using pAAV carrying 1.1 kb of insert, i.e., productivity of 7.64 vs. 4.56 × 109 gc/ml and titer of 9.84 vs. 1.37 × 1010 gc/ml (Figure 4C). The maximum insert of AAV-DJ/8 expression system is 3.9 kb, including promoter and gene of interest, so we tested a large insert cfos-NLuc-ACC with size of 3.9 kb and small insert CMV-eYFP with size of 1.1 kb in this study.
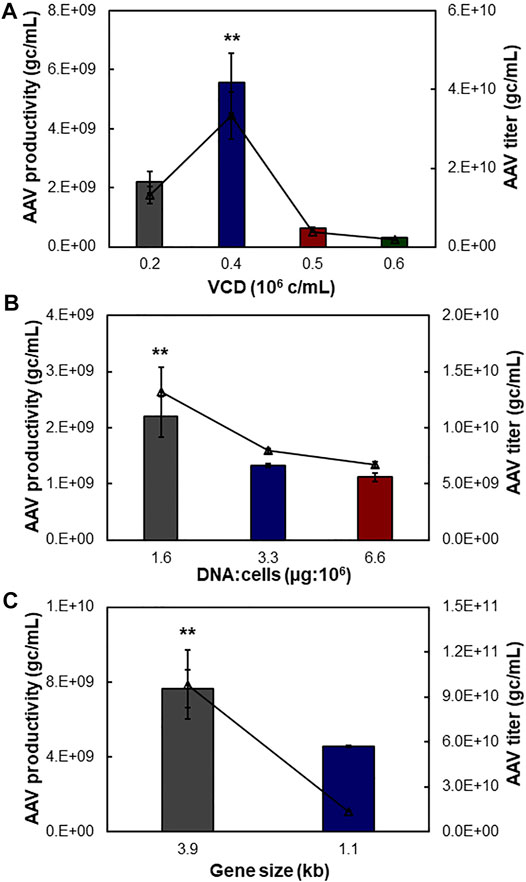
FIGURE 4. Process optimization of suspensive AAV production. The pAAV plasmid carrying 3.9 kb of NLuc fused genes was used to transfect HEK 293F cells with duplication. (A) Effect of transfection viable cell density (VCD) on AAV production. (B) Effect of ratio of plasmid DNA (µg) and host cell number (106 cells). (C) Effect of gene size cloned in pAAV expression plasmid. Both 3.9 and 1.1 kb of insert genes were tested. **p ≤ 0.05.
HEK 293F cells have been widely used to transiently produce antibody or other recombinant protein. Literature has reported that the plasmid transfection and protein expression level are affected by the transfection VCD and ratio of plasmid and cells (Grieger et al., 2016). This study showed that the transfection cell density and plasmid amount can be optimized to improve the suspensive AAV production. Interestingly, we found that the size of insert gene had no obvious correlation to AAV production in suspensive culture. The identified optimal transfection conditions, i.e. transfection VCD of 0.4 × 106 cells/ml, plasmid and HEK 293F cell ratio of 1.6, and synthesized cationic liposomes, were used in suspensive AAV production process.
3.4 Process Scalability
The scalability of our developed suspensive AAV production process was evaluated in both shaker flask and spinner flask, which showed consistent AAV productivity. Specifically, the identified process parameters in 30-ml shaker flask suspension production (Table 1), i.e. transfection VCD of 0.4 × 106 cells/ml of HEK 293F cells, liposomes as transfection reagent of 6.9-kb pAAV-Luc, and DNA:cells ratio of 1.6 µg:106 cells, were applied in the scalability evaluation in 240-ml of production culture in shaker flask at 37°C and agitation 100 rpm and 450-ml production culture in spinner flask at 37°C and agitation 70 rpm. As presented in Figure 5, the 240-ml production culture in 1-L shaker flask and 450-ml culture in 1-L spinner flask produced similar level of AAV with volumetric productivity of 5.04 × 109 gc/ml and 4.99 × 109 gc/ml and titer of 3.02 × 1010 gc/ml and 2.84 × 1010 gc/ml, respectively. During the liposomes-mediated plasmid transfection in shaker and spinner flasks, the cultures were kept in static status for half hour after adding transfection complex. Medium exchange is not necessary pre- and post-transfections. The successful scale-up of AAV production in spinner flask indicated the possibility to produce large-scale AAV in stirred-tank bioreactor.
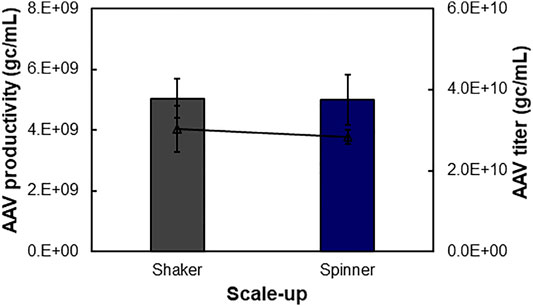
FIGURE 5. Scalability of suspensive AAV production process. AAV production can be scaled up from 30 to 450 ml in shaker flasks at 37°C and agitation 100 rpm and spinner flasks at 37°C and agitation 70 rpm.
3.5 Formulation Buffer and Storage Condition
In this study, the raw or purified AAV was concentrated and exchanged to formulation buffer (1x PBS, 5% sorbitol, 350 mmol/L NaCl). It is found that AAV is stable with this storage condition and the functional titer change is <5% between pre-freezing and post-thawing.
3.6 Infection Bioactivity
In addition to genome copy-based titration, it’s very important to evaluate the bilogical activity of AAV carrying the NLuc reporter gene. In this study, we transfected the TNBC MDA-MB-468 cells using AAV in 6-well plate. As shown in Figure 6A, the high-level expression of NLuc gene was detected in the cancer cells with IVIS imaging, but no bioluminecent signal in the cells with mock infection (i.e., no AAV). This result confirmed the in vitro transfection and bioactivity of the produced AAV. The kinetic profile of in vivo NLuc bioluminecence, which was excited at wavelength of 470 nm, demosntrated the peak signal at ∼15 min post substrate induction followed with signal reduction by 67% wuthin 60 min. We further tested the in vivo infection efficiency by administrating AAV into the TNBC MDA-MB-468 xengraft models via tail vein. As described in Figure 6B, we detected the functional expression of NLuc gene in TNBC xenograft, which demonstrated the effective in vivo infection and gene expression that was delivered by AAV. Moreover, the application of cancer-specific promoter cfos resulted in gene expression in tumor xenograft. To overcome the challenge of possible low resolution of live-animal IVIS imaging, we will further validate the AAV biodistribution through RT-PCR analysis of the important organs to detect the mRNA level of delivered gene in future.
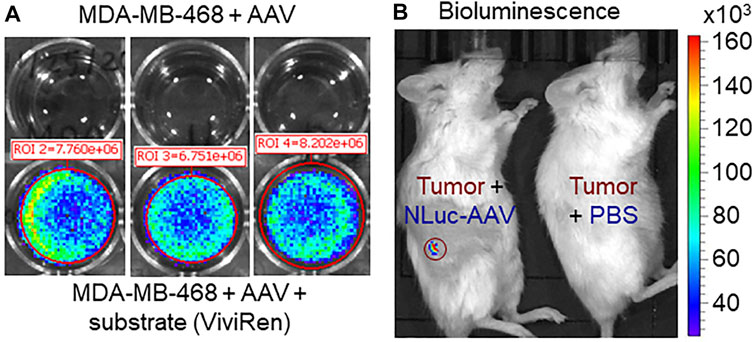
FIGURE 6. Evaluation of AAV transfection/infection capability. (A) In vitro transfection and gene expression of AAV in TNBC MDA-MB-468 cells. Multiplicity of infection (MOI): 10,000. Live-cell In Vivo Imaging System (IVIS) image was taken 3 days after transfection. (B) In vivo infection and gene expression of AAV in TNBC xenograft model. Live-animal IVIS image was taken 7 days post AAV injection.
3.7 Challenges in Adeno-Associated Viruses Biomanufacturing and Future Work
This study compared and optimized both adherent production and suspensive production of AAV as summarized in Table 1. The results showed that these two operation modes could generate AAV with similar productivity and titer, which is doable for small-scale production. However, the large-amount AAV needs scalable suspensive production in stirred-tank container or bioreactor with optimal transfection conditions. For example, the agitation speed could affect the transfection efficiency significantly and might need optimization after adding transfection complex. The in-house synthesized cationic liposomes have size distribution of 92.6 ± 0.4 nm and titer of 3.2 × 1011 particles/ml. The particle size of the cationic liposomes could be further optimized in order to improve the suspensive transfection efficiency. Although the AAV-DJ/8 with an engineered capsid to achieve high in vivo infection efficiency was used as model system, the developed bioprocess could be used to generate different serotypes.
3.8 Purification Challenges and Future Work
This study aimed to optimize the AAV production bioprocessing, so we did not focus on purification strategies. However, we performed a quick test and evaluation of two chromatography purification methods following published protocols to purify AAV-DJ/8. The ion-exchange purification using liquid chromatography system (Bio-Rad, Hercules, CA, United States) equipped with Cytiva HiTrap™ Q Sepharose XL IEX column (Cytiva, Marlborough, MA, United States) (Brument et al., 2002; Lock et al., 2012; Potter et al., 2014; Qu et al., 2015) was performed, but the recovery rate was lower than 10% and the purity was low. The affinity purification with Cytiva HiTrap™ AVB Sepharose column (Wu and Ataai, 2000; Grieger et al., 2006; Arakawa et al., 2007; Potter et al., 2014) was also tested, and the recovery rate was less than 1% or close to 0%. The failure to use AVB column to isolate AAV-DJ/8 was also reported by Andari and Grimm (El Andari and Grimm, 2021) and Nass et al. (Nass et al., 2018) due to the serotypes evolution. The traditional iodixanol gradient ultracentrifugation with Beckman Coulter L-100K ultracentrifuge (Beckman Coulter, Brea, CA) (Zolotukhin et al., 1999; Strobel et al., 2015) achieved the highest recovery rate and purity although the operation took a long time and was hard to scale up. We will further develop and optimize the chromatography-based AAV purification protocol in future study.
4 Conclusion
In this study, we developed a scalable robust suspensive AAV production process. The host cells, transfection reagents, transfection conditions (viable cell density, plasmid DNA and gene size), and culture containers were evaluated to determine the optimal process parameters. Furthermore, the scalability and robustness of the developed biomanufacturing was confirmed in both shaker flask and spinner flask. The produced AAV showed high transfection/infection capability in cancer cell lines and tumor xenograft models. Despite the promising results, we need to further optimize the liposomes transfection of suspensive cells and also develop an efficient AAV purification process for large-scale suspensive AAV production.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the animal study conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals published (Publication No. 85-23), with the approved animal protocol of IACUC-21949 by the Institutional Biosafety Committee at University of Alabama at Birmingham.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by DoD BCRP W81XWH2110066 (XL) and W81XWH2110067 (LZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Small Animal Imaging Facility at University of Alabama at Birmingham (UAB) for the IVIS imaging.
References
Arakawa, T., Ejima, D., Tsumoto, K., Ishibashi, M., and Tokunaga, M. (2007). Improved Performance of Column Chromatography by Arginine: Dye-Affinity Chromatography. Protein Expr. Purif. 52, 410–414. doi:10.1016/j.pep.2006.10.005
Blessing, D., Vachey, G., Pythoud, C., Rey, M., Padrun, V., Wurm, F. M., et al. (2019). Scalable Production of AAV Vectors in Orbitally Shaken HEK293 Cells. Mol. Ther. - Methods Clin. Development 13, 14–26. doi:10.1016/j.omtm.2018.11.004
Brument, N., Morenweiser, R., Blouin, V., Toublanc, E., Raimbaud, I., Chérel, Y., et al. (2002). A Versatile and Scalable Two-step Ion-Exchange Chromatography Process for the Purification of Recombinant Adeno-Associated Virus Serotypes-2 and -5. Mol. Ther. 6, 678–686. doi:10.1006/mthe.2002.0719
Büning, H., and Srivastava, A. (2019). Capsid Modifications for Targeting and Improving the Efficacy of AAV Vectors. Mol. Ther. - Methods Clin. Development 12, 248–265. doi:10.1016/j.omtm.2019.01.008
Carter, B. J. (2005). Adeno-associated Virus Vectors in Clinical Trials. Hum. Gene Ther. 16, 541–550. doi:10.1089/hum.2005.16.541
Chahal, P. S., Schulze, E., Tran, R., Montes, J., and Kamen, A. A. (2014). Production of Adeno-Associated Virus (AAV) Serotypes by Transient Transfection of HEK293 Cell Suspension Cultures for Gene Delivery. J. Virol. Methods 196, 163–173. doi:10.1016/j.jviromet.2013.10.038
Crosson, S. M., Dib, P., Smith, J. K., and Zolotukhin, S. (2018). Helper-free Production of Laboratory Grade AAV and Purification by Iodixanol Density Gradient Centrifugation. Mol. Ther. - Methods Clin. Development 10, 1–7. doi:10.1016/j.omtm.2018.05.001
El Andari, J., and Grimm, D. (2021). Production, Processing, and Characterization of Synthetic AAV Gene Therapy Vectors. Biotechnol. J. 16, e2000025. doi:10.1002/biot.202000025
Ernst, P., Xu, N., Qu, J., Chen, H., Goldberg, M. S., Darley-Usmar, V., et al. (2019). Precisely Control Mitochondria with Light to Manipulate Cell Fate Decision. Biophysical J. 117, 631–645. doi:10.1016/j.bpj.2019.06.038
Govorunova, E. G., Sineshchekov, O. A., Li, H., Janz, R., and Spudich, J. L. (2013). Characterization of a Highly Efficient Blue-Shifted Channelrhodopsin from the marine Alga Platymonas Subcordiformis. J. Biol. Chem. 288, 29911–29922. doi:10.1074/jbc.m113.505495
Grieger, J. C., Choi, V. W., and Samulski, R. J. (2006). Production and Characterization of Adeno-Associated Viral Vectors. Nat. Protoc. 1, 1412–1428. doi:10.1038/nprot.2006.207
Grieger, J. C., Soltys, S. M., and Samulski, R. J. (2016). Production of Recombinant Adeno-Associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector from the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol. Ther. 24, 287–297. doi:10.1038/mt.2015.187
Hacker, U. T., Bentler, M., Kaniowska, D., Morgan, M., and Büning, H. (2020). Towards Clinical Implementation of Adeno-Associated Virus (AAV) Vectors for Cancer Gene Therapy: Current Status and Future Perspectives. Cancers (Basel) 12, 1889–1918. doi:10.3390/cancers12071889
Koerber, J. T., Jang, J.-H., and Schaffer, D. V. (2008). DNA Shuffling of Adeno-Associated Virus Yields Functionally Diverse Viral Progeny. Mol. Ther. 16, 1703–1709. doi:10.1038/mt.2008.167
Ledley, F. D., McNamee, L. M., Uzdil, V., and Morgan, I. W. (2014). Why Commercialization of Gene Therapy Stalled; Examining the Life Cycles of Gene Therapy Technologies. Gene Ther. 21, 188–194. doi:10.1038/gt.2013.72
Li, W., Asokan, A., Wu, Z., Van Dyke, T., DiPrimio, N., Johnson, J. S., et al. (2008). Engineering and Selection of Shuffled AAV Genomes: A New Strategy for Producing Targeted Biological Nanoparticles. Mol. Ther. 16, 1252–1260. doi:10.1038/mt.2008.100
Lock, M., Alvira, M. R., and Wilson, J. M. (2012). Analysis of Particle Content of Recombinant Adeno-Associated Virus Serotype 8 Vectors by Ion-Exchange Chromatography. Hum. Gene Ther. Methods 23, 56–64. doi:10.1089/hgtb.2011.217
Mietzsch, M., Grasse, S., Zurawski, C., Weger, S., Bennett, A., Agbandje-McKenna, M., et al. (2014). OneBac: Platform for Scalable and High-Titer Production of Adeno-Associated Virus Serotype 1-12 Vectors for Gene Therapy. Hum. Gene Ther. 25, 212–222. doi:10.1089/hum.2013.184
Nass, S. A., Mattingly, M. A., Woodcock, D. A., Burnham, B. L., Ardinger, J. A., Osmond, S. E., et al. (2018). Universal Method for the Purification of Recombinant AAV Vectors of Differing Serotypes. Mol. Ther. - Methods Clin. Development 9, 33–46. doi:10.1016/j.omtm.2017.12.004
Negrini, M., Wang, G., Heuer, A., Björklund, T., and Davidsson, M. (2020). AAV Production Everywhere: A Simple, Fast, and Reliable Protocol for In-House AAV Vector Production Based on Chloroform Extraction. Curr. Protoc. Neurosci. 93, e103. doi:10.1002/cpns.103
Nguyen, T. N. T., Sha, S., Hong, M. S., Maloney, A. J., Barone, P. W., Neufeld, C., et al. (2021). Mechanistic Model for Production of Recombinant Adeno-Associated Virus via Triple Transfection of HEK293 Cells. Mol. Ther. - Methods Clin. Development 21, 642–655. doi:10.1016/j.omtm.2021.04.006
Potter, M., Lins, B., Mietzsch, M., Heilbronn, R., Van Vliet, K., Chipman, P., et al. (2014). A Simplified Purification Protocol for Recombinant Adeno-Associated Virus Vectors. Mol. Ther. - Methods Clin. Development 1, 14034. doi:10.1038/mtm.2014.34
Qu, W., Wang, M., Wu, Y., and Xu, R. (2015). Scalable Downstream Strategies for Purification of Recombinant Adeno- Associated Virus Vectors in Light of the Properties. Cpb 16, 684–695. doi:10.2174/1389201016666150505122228
Senís, E., Fatouros, C., Grosse, S., Wiedtke, E., Niopek, D., Mueller, A.-K., et al. (2014). CRISPR/Cas9-mediated Genome Engineering: an Adeno-Associated Viral (AAV) Vector Toolbox. Biotechnol. J. 9, 1402–1412. doi:10.1002/biot.201400046
Si, Y., Zhang, Y., Ngo, H. G., Guan, J. S., Chen, K., Wang, Q., et al. (2021). Targeted Liposomal Chemotherapies to Treat Triple-Negative Breast Cancer. Cancers (Basel) 13. doi:10.3390/cancers13153749
Strobel, B., Miller, F. D., Rist, W., and Lamla, T. (2015). Comparative Analysis of Cesium Chloride- and Iodixanol-Based Purification of Recombinant Adeno-Associated Viral Vectors for Preclinical Applications. Hum. Gene Ther. Methods 26, 147–157. doi:10.1089/hgtb.2015.051
Urabe, M., Nakakura, T., Xin, K.-Q., Obara, Y., Mizukami, H., Kume, A., et al. (2006). Scalable Generation of High-Titer Recombinant Adeno-Associated Virus Type 5 in Insect Cells. J. Virol. 80, 1874–1885. doi:10.1128/jvi.80.4.1874-1885.2006
van der Loo, J. C. M., and Wright, J. F. (2016). Progress and Challenges in Viral Vector Manufacturing. Hum. Mol. Genet. 25, R42–R52. doi:10.1093/hmg/ddv451
Wright, J. F. (2008). Manufacturing and Characterizing AAV-Based Vectors for Use in Clinical Studies. Gene Ther. 15, 840–848. doi:10.1038/gt.2008.65
Wu, N., and Ataai, M. M. (2000). Production of Viral Vectors for Gene Therapy Applications. Curr. Opin. Biotechnol. 11, 205–208. doi:10.1016/s0958-1669(00)00080-x
Zhao, H., Lee, K.-J., Daris, M., Lin, Y., Wolfe, T., Sheng, J., et al. (2020). Creation of a High-Yield AAV Vector Production Platform in Suspension Cells Using a Design-Of-Experiment Approach. Mol. Ther. - Methods Clin. Development 18, 312–320. doi:10.1016/j.omtm.2020.06.004
Keywords: AAV, scalable bioproduction, adherent, suspensive, validation
Citation: Guan J-S, Chen K, Si Y, Kim T, Zhou Z, Kim S, Zhou L and Liu X (2022) Process Improvement of Adeno-Associated Virus Production. Front. Chem. Eng. 4:830421. doi: 10.3389/fceng.2022.830421
Received: 07 December 2021; Accepted: 10 January 2022;
Published: 28 January 2022.
Edited by:
Shang-Tian Yang, The Ohio State University, United StatesReviewed by:
Luis H. Reyes, University of Los Andes, ColombiaXiaoping Bao, Purdue University, United States
Copyright © 2022 Guan, Chen, Si, Kim, Zhou, Kim, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoguang Liu, bWxpdUB1YWIuZWR1
†These authors have contributed equally to this work
 Jia-Shiung Guan
Jia-Shiung Guan Kai Chen
Kai Chen Yingnan Si2
Yingnan Si2 Taehyun Kim
Taehyun Kim Seulhee Kim
Seulhee Kim Lufang Zhou
Lufang Zhou Xiaoguang Liu
Xiaoguang Liu