- 1Department of Biology, Institute of Pharmacy, Chemistry and Biology, Belgorod State National Research University, Belgorod, Russia
- 2Department of General Chemistry, Institute of Pharmacy, Chemistry and Biology, Belgorod State National Research University, Belgorod, Russia
In this mini-review, we describe the currently available literature concerning synthesis and applications of layered double hydroxides (LDHs) containing rare earth cations (RE-LDHs), focusing on the catalytic activity of those compounds. The lack of studies of some rare earth elements (REE) and the insufficient knowledge of their catalytic activity in the structure of LDHs indicate the need for further research.
Introduction
REE are a set of metals including lanthanides [lanthanum (La) by lutetium (Lu)]. Scandium (Sc) and yttrium (Y) are often included in the set.
REE have a well-known history of use as catalysts. Since the 1960s, REE-based catalytic materials found application in different fields from the petroleum chemistry industry to olefin polymerization (Zhan et al., 2014).
LDHs are a system of positively charged brucite-like octahedral layers alternating with an interlayer of anions and water molecules. Structural stability of LDHs is ensured by electrostatic interaction between hydroxide layers and interlayer anions (Cavani et al., 1991; Evans and Slade, 2006). The general formula of LDHs is [M(II)1−x M (III)x (HO−)2]x+ [An−x/n·yH2O]x− where M (II) and M (III) are cations of divalent and trivalent metals, respectively, and An− is an n-valent anion. LDHs thermal destruction forms another important compound—mixed metal oxides (MO). MO obtained as a result of LDHs calcination have a higher dispersion than mixtures obtained by the simple mechanical method and find wide application in catalysis (Xie et al., 2006; Mikulová et al., 2007).
Compositional flexibility is one of the most important properties of layered double hydroxides. The ability to include in their composition various cations both of divalent and trivalent metals, as well as anionic complexes in the interlayer space, makes it possible to create materials with unique characteristics. In recent years, interest has grown in the incorporation of REE into LDHs structure, which allows us to expect the appearance of new materials with promising properties on their basis, including new catalysts. Most often REE have a +3 charge and take place of tri-charged cations in the LDHs crystal lattice. It should be noted that samarium, europium, thulium, and ytterbium can be reduced to +2 at some conditions so one could expect them to play the role of double-charged cations too. Cerium, praseodymium, and terbium can be oxidized to +4 and that can lead to their anomalous behavior in various processes and the structure of LDHs as well.
The modification of LDHs with REE in the preparation step can lead to changes in various physicochemical properties. For instance, the basicity of the samples can increase in the presence of REE cations due to their low electronegativity (Zăvoianu et al., 2018). At the same time, the addition of REE ions not only reduces the crystallinity, but also expands the basal spacing and improves hydrophobicity and mechanical properties of the LDHs (Wang et al., 2012).
Although REE cations are included in the structure of LDHs mostly only partially and co-existed with another triply charged cation, several articles demonstrated the successful synthesis of binary RE-LDHs—Mg/Tb (Wang et al., 2017), Zn/Eu (Chen et al., 2018), Ca/Sc (Szabados et al., 2020), and Ni/La (Ensafi et al., 2016; Jiang et al., 2019). Our group is one of the first to synthesize Sc-containing LDHs (including the first binary Mg/Sc LDHs) (Vorontsova et al., 2007). Part of our studies is also dedicated to the synthesis and applications of Ce-containing LDHs (Golovin et al., 2020).
There is recently discovered and synthesized a new family of layered host compounds—layered rare earth hydroxides (LREHs) with typical structure [R4(OH)10(H2O)4]nAn (where R = RE ions, A = intercalated organic anions) (Gándara et al., 2006). LREHs were not included in our mini-review since this novel class of compounds requires separate consideration.
We carried out a thorough search and analysis of articles dedicated to RE-LDHs in the Scopus database preparing this mini-review. 216 articles were found in total, including 51 describing the application of LDHs and LDHs-derived MO in catalysis. We found that studies of RE-LDHs are unevenly distributed (Figure 1A)—most of the articles are devoted to Ce-LDHs, La-LDHs, and Eu-LDHs, while promethium (the only radioactive REE), holmium, and thulium are not represented at all, and lutetium is mentioned in one article where Hu et al. reported an unsuccessful attempt to incorporate it into Ni/Al LDHs (Hu et al., 2015).
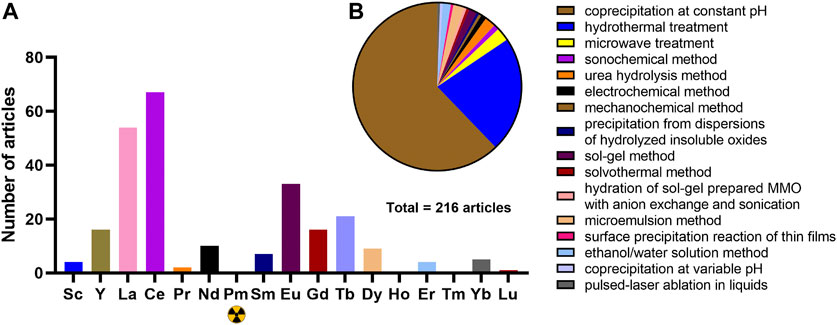
FIGURE 1. Distribution of the number of articles on RE-LDH in the Scopus database (A) and the distribution of methods for preparing RE-LDH in articles (B).
Synthesis of Rare Earth Layered Double Hydroxides
Standard and simple coprecipitation at constant pH and hydrothermal synthesis remains the most common methods for the synthesis of RE-LDHs (Figure 1B). Adriana Urdă et al. synthesized Mg/AlLn LDH (Ln = Ce, Sm, Dy, and Yb) methane oxidation catalyst by coprecipitation from an appropriate nitrate solution (Urdă et al., 2013). Li et al. by hydrothermal synthesis obtained Ni/FeGd LDH for oxygen evolution (Li et al., 2021). This RE-LDH showed higher catalytic activity than Ni/Fe LDH and even commercial RuO2.
At the same time, methods for synthesizing RE-LDHs are not limited to the above-mentioned and include many variations that have advantages for certain purposes. In the work of Hunter et al. surfactant-free Ni/FeTiLa LDH was synthesized by pulsed-laser ablation in liquids, where nanoparticles are formed by very rapid cooling of a plasma comprised of elements from the solid ablation target and the surrounding liquid (Hunter et al., 2014). The addition of Ti4+ and La3+ ions enhanced the electrocatalytic water oxidation activity of these nanocatalysts. Jing et al. managed to obtain Ni/AlCe MO from LDHs precursors synthesized by the urea homogeneous precipitation method, which allows preparing material with a better crystallinity and control of the particle size (Jing et al., 2020). The resulting material showed high catalytic activity in steam reforming of glycerol for the production of hydrogen. Ensafi et al. synthesized Ni/La LDH with N-doped graphene using a sonochemical method during which the mixed solution of reagents was ultrasonicated for 2 h (Ensafi et al., 2016). The resulting catalyst was used in a hydrogen evolution reaction. Using an electrodeposition technique with a three-electrode system in potentiostatic mode Jadhav et al. synthesized Ni/FeCe LDH electrocatalyst, which outperformed bare Ni/Fe LDH in an overall water splitting (Jadhav et al., 2020). Mechanochemical synthesis route with direct milling certain amounts of chemicals in a mortar allowed Pavel et al. to modify Mg/Al LDH by La, which afforded higher cyclohexene conversions and the higher selectivity to epoxide than unmodified samples (Pavel et al., 2017). The addition of La preserves the epoxidation activity in the absence of the reconstruction effect and under the CO2 atmosphere.
Applications of Rare Earth Layered Double Hydroxides
There are frequent studies devoted to the luminescent properties of RE-LDHs. E.g., a single-phase Mg/AlTb LDH with various terbium contents was obtained by the hydrothermal method (Yanase et al., 2019). Green emission was observed in the samples with the correlation of the intensity increasing with the rise of terbium content. Then the nitrate ions were replaced by carbonates by ion exchange. It was found that the intensity of emission of the carbonate form increased with an increase in the concentration of CO32− in the solution. Thus, the resulting sample was able to capture carbonate ions dissolved in water and react to them. In another study, Mg/AlTb LDHs with different contents of the REE were obtained by the sol-gel method and then intercalated with terephthalate anions (Smalenskaite et al., 2018). The study of their luminescent properties showed that the inclusion of terephthalates increased the intensity of luminescence due to the sensitization effect. For Mg/AlTb LDHs, the change in their luminescent properties upon calcination was also studied (Chen et al., 2016). It was found that upon heating up to 600°C the luminescence intensity increased and then started to decrease.
The work describing the synthesis of Mg/Tb LDH with different cation ratios deserves special attention since studies on binary RE-LDHs are quite scarce (Wang et al., 2017). The synthesis was carried out by coprecipitation with aqueous ammonia followed by hydrothermal treatment. The authors managed to find out that obtained materials were capable of photoluminescence.
The luminescent properties of europium-containing LDHs are also being studied very actively. Mg/AlEu and Ca/AlEu LDHs were synthesized by coprecipitation and then calcined to obtain a mixture of oxides (including europium (II) doped) exhibiting red luminescence (Sonoyama et al., 2020). By calcining in a quartz tube in the presence of titanium powder it was possible to obtain Mg/AlEu and Ca/AlEu MO, which luminesced in the green and blue regions, respectively. Thus, stable phosphors were obtained for the three primary RGB colors.
RE-LDHs can be used to target drug delivery and magnetic resonance imaging (MRI) contrasting. Gadolinium-based contrast agents are among the most widely used materials for MRI, which is one of the world’s most recognized non-invasive methods used in clinical diagnostics (Usman et al., 2017). Usman et al. created a theranostic system for the delivery of both a therapeutic agent and a diagnostic agent for MRI based on Zn/AlGd LDHs (Usman et al., 2020). The drug was chlorogenic acid intercalated into LDHs and gadolinium ions added for contrast on MRI. Zn/AlDy LDH was also obtained and successfully intercalated with ibuprofen, folate, and gallate (Arratia-Quijada et al., 2016). The resulting LDH can also serve as a basis for the creation of theranostic systems. By simultaneously incorporating gadolinium and dysprosium cations in Zn/Al LDHs Andrade et al. obtained a compound with a better contrast effect for magnetic resonance imaging than commercial contrast agents (Nava Andrade et al., 2020).
There are works devoted to more specific properties of RE-LDHs. For instance, the friction properties of Mg/AlLa LDH, which was intercalated with dodecyl sulfate anion, were investigated (Li et al., 2015). According to the results of the experiments, the use of LDHs nanoparticles as an additive to lubricants made it possible to reduce friction and increase wear resistance in comparison with the base oil. The anticorrosive properties of cerium-containing LDHs were also studied (Zhang et al., 2017). The synthesis of Zn/Al and Zn/AlCe LDHs was carried out by the method of coprecipitation in a nitrogen atmosphere to prevent the ingress of carbonate ions into the sample. These anti-corrosion coatings showed decent results and can be used to protect metals and alloys from corrosion.
It is possible to obtain multifunctional LDHs. For example, the preparation of Mg/AlEu LDH with glycine and Fe3O4 nanoparticles in the interlayer space was described (Wang et al., 2010). Magnetic measurements showed that the obtained sample had paramagnetic properties at room temperature, and the excitation and emission spectra exhibited the presence of fluorescence.
Catalytic Activity
The possibility of using RE-LDHs and the related MO as catalysts is being studied by numerous authors.
One of the frequent subjects is the application of RE-LDHs as photocatalysts. Single-phase Zn/AlCe LDHs samples with different cerium contents were obtained by coprecipitation from nitrates of the corresponding metals (Suárez-Quezada et al., 2016). The authors confirm the co-existence of Ce3+ and Ce4+ species and report that the inclusion of cerium leads to an improvement in the photocatalytic properties of hydrotalcite-like materials. Presumably, this LDH promotes the separation of the photogenerated electron-hole pairs where Ce4+ acts as electron scavenger, facilitating the electron transfer toward adsorbed O2 and an accumulation of holes, increasing the generation of radicals OH•. Comparing the results of the phenol photodegradation using Zn/Al and Zn/AlCe LDHs, they concluded that the sample with 5% cerium content showed the best result. Sarkarat et al. synthesized Zn/NiTiLa LDHs by hydrothermal method and evaluated their MO for the photodegradation of NOx (Sarkarat et al., 2013). They found that doping of lanthanum in LDH structures led to poor crystallinity, prevented the formation of pure zinc titanate phase, and increased specific surface areas. However, Zn/NiTiLa LDH calcined at 400°C showed the best photocatalytic activity for the decomposition of NOx among the prepared samples. Khodam et al. synthesized Co/AlNd LDH by coprecipitation, though the resulting material contained extraneous phases (Khodam et al., 2018). The MO were obtained by calcination. The study of the photocatalytic properties was carried out in the reaction with the dye AR 14. It was found that the incorporation of Nd into the crystal lattice of LDH and its annealing leads to an increase in the absorption of light and a decrease in the band gap. Moreover, doping and annealing reduce the photoinduced recombination of charge carriers and contribute to the efficiency of their separation due to the trapping of photoexcited electrons in the conduction band. This catalyst can be used in several cycles.
It should be noted that MO do not always exhibit higher catalytic activity as compared to their LDH precursors. Andrade et al. showed that Zn/AlDy LDH demonstrated better catalytic activity in photodegradation of sulfamethoxazole than derived MO and even commercial ZnO and P-25 TiO2 photocatalysts (Andrade et al., 2020).
Another field of interest is using RE-LDHs and their MO in esterification and transesterification reactions. Liao et al. synthesized Ca/AlRE (where REE were La, Ce, and Y) LDHs via coprecipitation and used their MO as solid basic catalysts for dimethyl carbonate synthesis by transesterification of methanol with propylene carbonate (Liao et al., 2017). However, it was found that CaMgAl MO showed higher catalytic activity in this process than MO derived from RE-LDHs. In the article of Bálsamo et al. La and Ce were incorporated into Mg/Al LDHs by coprecipitation and wet impregnation methods and corresponding MO were used as catalysts to produce high-valued derivatives of biodiesel by-product (Bálsamo et al., 2020). The sample with Ce incorporated by the impregnation method exhibited the best selective monoglycerides yield of 77% attributed to the higher density of medium basic sites. Binary Ca/Sc LDH was successfully obtained by Szabadoc et al. and tested as a catalyst in the transesterification reactions of dimethyl carbonate with glycerol (Szabados et al., 2020). The stability of this sample as well as Ca/In LDHs were the highest within the investigated compounds. In the oleic acid esterification with methanol under soft reaction conditions, ZnAlLa MO from respective LDH reached conversions of 75% to the ester after 15 min and higher than 88% after 1 h of reaction (Tzompantzi et al., 2013).
Several articles on RE-LDHs are devoted to the dry reforming of methane. Cao et al. investigated the promotional effects of REE (Sc, Y, Ce, and Pr) on NiMgAl MO derived from LDHs (Cao et al., 2016). Compared with unmodified catalysts, the RE promoted catalysts, especially with the Ce or Pr, showed improved catalytic performance in terms of both catalytic stability and coke resistance. Authors supposed that either the addition of Ce or Pr could increase the amount of strong basic sites and the coexistence of redox pairs (Ce3+/Ce4+, Pr3+/Pr4+) could contribute to the enhancement of redox properties and formation of oxygen vacancies. Taherian et al. studied the impact of the Sm incorporation on the Ni/MgAl LDHs catalytic activity in both dry and steam reforming of methane at 700°C (Taherian et al., 2021). The obtained catalyst showed the highest conversion of methane (72%) and stability without any carbon formation due to the strong metal-support interaction which inhibited the sintering and the scaffold structure. As a result, the mass transportation of feedstock and products was increased.
The catalytic activity of RE-LDHs and MO is also investigated in hydrogenation and dehydrogenation reactions. Han et al. tested Cu/MgFe LDH derived MO for higher alcohol synthesis via carbon monoxide hydrogenation (Han et al., 2015). The results showed that Ce promotion mainly contributed to the formation of tetrahedrally coordinated copper species, which favored the enhancement of the total alcohol selectivity. Mitran et al. prepared LnMgAl mixed oxide catalysts (Ln = Ce, Sm, Dy, Yb) from LDH precursors and tested them in the oxidative dehydrogenation of propane (Mitran et al., 2009). The best yields of propene were obtained with Dy and Sm promoted catalysts. A linear correlation between the catalyst basicity and the propene selectivity was observed. No correlation between the reducibility of the RE cation and the catalytic performance was observed.
Investigations of RE-LDHs and their calcined products catalytic activity are not limited to the aforementioned topics but some directions are presented by a single article. It indicates the underdevelopment of such areas of research and requires further development. E.g., Ni et al. reported the successful effect of REE (Y, La, and Ce) on the performance of Mg/Al (REE) LDH derived catalysts for ammonia synthesis (Ni et al., 2018). The activity of ammonia synthesis was remarkably improved for the catalyst doped with Y. Ce-containing MgAl LDHs with graphene oxide was proposed by Stamate et al. as a multifunctional catalyst in two different types of organic transformations: Knoevenagel condensation (cinnamic acid synthesis) and one-pot cascade oxidation-Knoevenagel condensation (2-benzoyl-3-phenylacrylonitrile synthesis) (Stamate et al., 2021). Cota et al. investigate the catalytic activity of Mg/AlLa LDH derived MO for isomerization of 2,3-dimethyl-1-butene to 2,3-dimethyl-2-butene (Cota et al., 2016). The results of their study indicated that not only the basicity but also accessibility to the active sites controlled the catalytic activity.
The overall list of studies on the catalytic activity of RE-LDHs can be found in Table 1.
Perspective
Several RE cations (promethium, holmium, thulium, and lutetium) mentioned above are not incorporated in the LDH structure yet. The radioactivity and extreme rarity of promethium make such work exceedingly difficult but it seems possible with the other three REE. Another novel research area is the synthesis and study of binary RE-LDHs, including consideration of the possibility to use samarium, europium, thulium, and ytterbium as double-charged cations. Also, there are no studies devoted to Eu- and Er-LDHs catalytic activity, even though Eu-LDHs are one of the most discussed in the articles.
Obviously, there are still many applications for the RE-LDHs to test in the vast area of catalysis and this topic deserves the attention of scientists in the coming years.
Author Contributions
ES prepared the table and figure, drafted the manuscript with OL, and collaborated with SG in data search.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrade, K. N., Arízaga, G. G. C., Bautista, E., and Rodríguez-González, V. (2020). Dysprosium Doped Double Layered Hydroxide as an Efficient Catalyst for Photooxidation of Pharmaceutical Pollutants. J. Taiwan Inst. Chem. Eng. 113, 293–301. doi:10.1016/j.jtice.2020.08.030
Arratia-Quijada, J., Sánchez Jiménez, C., Gurinov, A., Pérez Centeno, A., Ceja Andrade, I., and Carbajal Arízaga, G. G. (2016). Dysprosium-containing Layered Double Hydroxides Nanoparticles Intercalated with Biologically Active Species as an Approach for Theranostic Systems. Mater. Sci. Eng. B 203, 7–12. doi:10.1016/j.mseb.2015.10.004
Bálsamo, N., Mendieta, S., Heredia, A., and Crivello, M. (2020). Nanoclays as Dispersing Precursors of La and Ce Oxide Catalysts to Produce High-Valued Derivatives of Biodiesel By-Product. Mol. Catal. 481, 110290. doi:10.1016/j.mcat.2019.01.010
Cao, Y., Li, H., Zhang, J., Shi, L., and Zhang, D. (2016). Promotional Effects of Rare Earth Elements (Sc, Y, Ce, and Pr) on NiMgAl Catalysts for Dry Reforming of Methane. RSC Adv. 6, 112215–112225. doi:10.1039/C6RA19139H
Cavani, F., Trifirò, F., and Vaccari, A. (1991). Hydrotalcite-type Anionic Clays: Preparation, Properties and Applications. Catal. Today 11, 173–301. doi:10.1016/0920-5861(91)80068-K
Chen, Y., Bao, Y., Yu, Z., Yang, G., and Wang, X. (2016). Photoluminescence of Tb-Doped MgAl-LDHs Depending on Phase Transition Caused by Annealing. J. Rare Earths 34, 36–44. doi:10.1016/S1002-0721(14)60575-5
Chen, Y., Zhang, K., Wang, X., and Zheng, F. (2018). Study on a Novel Binary Zn N Eu Layered Double Hydroxide with Excellent Fluorescence. J. Fluoresc. 28, 259–268. doi:10.1007/s10895-017-2188-x
Cota, I., Ramírez, E., Medina, F., Layrac, G., Tichit, D., and Gérardin, C. (2016). Influence of the Preparation Route on the Basicity of La-Containing Mixed Oxides Obtained from LDH Precursors. J. Mol. Catal. A: Chem. 412, 101–106. doi:10.1016/j.molcata.2015.11.022
Dib, H., El Khawaja, R., Rochard, G., Poupin, C., Siffert, S., and Cousin, R. (2020). CuAlCe Oxides Issued from Layered Double Hydroxide Precursors for Ethanol and Toluene Total Oxidation. Catalysts 10, 870. doi:10.3390/catal10080870
Dinari, M., Momeni, M. M., and Ghayeb, Y. (2016). Photodegradation of Organic Dye by ZnCrLa-Layered Double Hydroxide as Visible-Light Photocatalysts. J. Mater. Sci. Mater. Electron. 27, 9861–9869. doi:10.1007/s10854-016-5054-8
Djebarri, B., Gonzalez-Delacruz, V. M., Halliche, D., Bachari, K., Saadi, A., Caballero, A., et al. (2014). Promoting Effect of Ce and Mg Cations in Ni/Al Catalysts Prepared from Hydrotalcites for the Dry Reforming of Methane. Reac Kinet Mech. Cat 111, 259–275. doi:10.1007/s11144-013-0646-2
do Nascimento, L. A., Barroso-Martín, L., Peçanha, S. R. S., Arias, S., Santos, B. S., Pacheco, J. G. A., et al. (2021). NiAlCe Mixed Oxides Obtained from Layered Double Hydroxides Applied to Anisole Hydrodeoxygenation. Catal. Today. doi:10.1016/j.cattod.2021.08.026
Dou, L., Fu, M., Gao, Y., Wang, L., Yan, C., Ma, T., et al. (2021). Efficient Sulfur Resistance of Fe, La and Ce Doped Hierarchically Structured Catalysts for Low-Temperature Methanation Integrated with Electric Internal Heating. Fuel 283, 118984. doi:10.1016/j.fuel.2020.118984
Ensafi, A. A., Nabiyan, A., Jafari-Asl, M., Dinari, M., Farrokhpour, H., and Rezaei, B. (2016). Galvanic Exchange at Layered Doubled hydroxide/N-Doped Graphene as an In-Situ Method to Fabricate Powerful Electrocatalysts for Hydrogen Evolution Reaction. Energy 116, 1087–1096. doi:10.1016/j.energy.2016.10.048
Evans, D. G., and Slade, R. C. T. (2006). “Structural Aspects of Layered Double Hydroxides” in Layered Double Hydroxides. (Berlin/Heidelberg: Springer-Verlag), 1–87. doi:10.1007/430_005
Fu, Y., Ning, F., Xu, S., An, H., Shao, M., and Wei, M. (2016). Terbium Doped ZnCr-Layered Double Hydroxides with Largely Enhanced Visible Light Photocatalytic Performance. J. Mater. Chem. A. 4, 3907–3913. doi:10.1039/C5TA10093C
Gándara, F., Perles, J., Snejko, N., Iglesias, M., Gómez-Lor, B., Gutiérrez-Puebla, E., et al. (2006). Layered Rare-Earth Hydroxides: A Class of Pillared Crystalline Compounds for Intercalation Chemistry. Angew. Chem. Int. Ed. 45, 7998–8001. doi:10.1002/anie.200602502
Gao, L.-G., Gao, Y.-Y., Song, X.-L., and Ma, X.-r. (2018). A Novel La3+-Zn2+-Al3+-MoO42− Layered Double Hydroxides Photocatalyst for the Decomposition of Dibenzothiophene in Diesel Oil. Pet. Sci. Techn. 36, 850–855. doi:10.1080/10916466.2018.1447957
Gao, Z., Xie, R., Fan, G., Yang, L., and Li, F. (2017). Highly Efficient and Stable Bimetallic AuPd over La-Doped Ca-Mg-Al Layered Double Hydroxide for Base-free Aerobic Oxidation of 5-Hydroxymethylfurfural in Water. ACS Sustain. Chem. Eng. 5, 5852–5861. doi:10.1021/acssuschemeng.7b00573
Golovin, S. N., Yapryntsev, M. N., Ryltsova, I. G., Veligzhanin, A. A., and Lebedeva, O. E. (2020). Novel Cerium-Containing Layered Double Hydroxide. Chem. Pap. 74, 367–370. doi:10.1007/s11696-019-00877-9
Han, X., Fang, K., and Sun, Y. (2015). Effects of Metal Promotion on CuMgFe Catalysts Derived from Layered Double Hydroxides for Higher Alcohol Synthesis via Syngas. RSC Adv. 5, 51868–51874. doi:10.1039/C5RA05846E
Hu, M., Zuo, S., Yang, R., Zhang, H., Yan, Y., and Lei, L. (2015). Modification of Lutetium Hydroxide for the Structural and Electrochemical Stability of Ni-Al Layered Double Hydroxide. J. Solid State. Electrochem. 19, 671–683. doi:10.1007/s10008-014-2651-4
Hunter, B. M., Blakemore, J. D., Deimund, M., Gray, H. B., Winkler, J. R., and Müller, A. M. (2014). Highly Active Mixed-Metal Nanosheet Water Oxidation Catalysts Made by Pulsed-Laser Ablation in Liquids. J. Am. Chem. Soc. 136, 13118–13121. doi:10.1021/ja506087h
Iqbal, K., Iqbal, A., Kirillov, A. M., Wang, B., Liu, W., and Tang, Y. (2017). A New Ce-Doped MgAl-LDH@Au Nanocatalyst for Highly Efficient Reductive Degradation of Organic Contaminants MgAl-LDH@Au Nanocatalyst for Highly Efficient Reductive Degradation of Organic Contaminants. J. Mater. Chem. A. 5, 6716–6724. doi:10.1039/C6TA10880F
Jadhav, H. S., Roy, A., Desalegan, B. Z., and Seo, J. G. (2020). An Advanced and Highly Efficient Ce Assisted NiFe-LDH Electrocatalyst for Overall Water Splitting. Sustain. Energ. Fuels 4, 312–323. doi:10.1039/C9SE00700H
Jiang, S., Liu, Y., Xie, W., and Shao, M. (2019). Electrosynthesis of Hierarchical NiLa-Layered Double Hydroxide Electrode for Efficient Oxygen Evolution Reaction. J. Energ. Chem. 33, 125–129. doi:10.1016/j.jechem.2018.08.010
Jing, F., Liu, S., Wang, R., Li, X., Yan, Z., Luo, S., et al. (2020). Hydrogen Production through Glycerol Steam Reforming over the NiCexAl Catalysts. Renew. Energ. 158, 192–201. doi:10.1016/j.renene.2020.05.044
Jirátová, K., Mikulová, J., Klempa, J., Grygar, T., Bastl, Z., and Kovanda, F. (2009). Modification of Co-mn-al Mixed Oxide with Potassium and its Effect on Deep Oxidation of VOC. Appl. Catal. A: Gen. 361, 106–116. doi:10.1016/j.apcata.2009.04.004
Khan, A. A., Tahir, M., and Mohamed, A. R. (2022). Constructing S-Scheme Heterojunction of Carbon Nitride Nanorods (G-CNR) Assisted Trimetallic CoAlLa LDH Nanosheets with Electron and Holes Moderation for Boosting Photocatalytic CO2 Reduction under Solar Energy. Chem. Eng. J. 433, 133693. doi:10.1016/j.cej.2021.133693
Khodam, F., Amani-Ghadim, H. R., Aber, S., Amani-Ghadim, A. R., and Ahadzadeh, I. (2018). Neodymium Doped Mixed Metal Oxide Derived from CoAl-Layered Double Hydroxide: Considerable Enhancement in Visible Light Photocatalytic Activity. J. Ind. Eng. Chem. 68, 311–324. doi:10.1016/j.jiec.2018.08.002
Li, E., Xu, Z. P., and Rudolph, V. (2009). MgCoAl-LDH Derived Heterogeneous Catalysts for the Ethanol Transesterification of Canola Oil to Biodiesel. Appl. Catal. B: Environ. 88, 42–49. doi:10.1016/j.apcatb.2008.09.022
Li, J., and Yang, Y. J. (2018). New Type Ternary NiAlCe Layered Double Hydroxide Photocatalyst for Efficient Visible-Light Photoreduction of CO2 into CH4New Type Ternary NiAlCe Layered Double Hydroxide Photocatalyst for Efficient Visible-Light Photoreduction of CO 2 into CH 4. Mater. Res. Express 5, 026204. doi:10.1088/2053-1591/aaaf0d
Li, M., Li, H., Jiang, X., Jiang, M., Zhan, X., Fu, G., et al. (2021). Gd-induced Electronic Structure Engineering of a NiFe-Layered Double Hydroxide for Efficient Oxygen Evolution. J. Mater. Chem. A. 9, 2999–3006. doi:10.1039/D0TA10740A
Li, S., Qin, H., Zuo, R., and Bai, Z. (2015). Friction Properties of La-Doped Mg/Al Layered Double Hydroxide and Intercalated Product as Lubricant Additives. Tribology Int. 91, 60–66. doi:10.1016/j.triboint.2015.06.012
Liao, Y., Li, F., Dai, X., Zhao, N., and Xiao, F. (2017). Solid Base Catalysts Derived from Ca-M-Al (M = Mg, La, Ce, Y) Layered Double Hydroxides for Dimethyl Carbonate Synthesis by Transesterification of Methanol with Propylene Carbonate. Chin. J. Catal. 38, 1860–1869. doi:10.1016/S1872-2067(17)62898-5
Liu, G., Yang, J., and Xu, X. (2020). Synthesis of Biodiesel from Waste Cooking Oil Catalyzed by β-cyclodextrin Modified Mg-Al-La Composite Oxide. RSC Adv. 10, 26358–26363. doi:10.1039/D0RA05307D
Mikulová, Z., Čuba, P., Balabánová, J., Rojka, T., Kovanda, F., and Jirátová, K. (2007). Calcined Ni-Al Layered Double Hydroxide as a Catalyst for Total Oxidation of Volatile Organic Compounds: Effect of Precursor Crystallinity. Chem. Pap. 61, 103–109. doi:10.2478/s11696-007-0006-7
Mitran, G., Urda, A., Tanchoux, N., Fajula, F., and Marcu, I.-C. (2009). Propane Oxidative Dehydrogenation over Ln-Mg-Al-O Catalysts (Ln = Ce, Sm, Dy, Yb). Catal. Lett. 131, 250–257. doi:10.1007/s10562-009-0057-1
Mo, S., Li, S., Li, J., Peng, S., Chen, J., and Chen, Y. (2016). Promotional Effects of Ce on the Activity of Mn Al Oxide Catalysts Derived from Hydrotalcites for Low Temperature Benzene Oxidation. Catal. Commun. 87, 102–105. doi:10.1016/j.catcom.2016.09.017
Nava Andrade, K., Carbajal Arízaga, G. G., and Rivera Mayorga, J. A. (2020). Effect of Gd and Dy Concentrations in Layered Double Hydroxides on Contrast in Magnetic Resonance Imaging. Processes 8, 462. doi:10.3390/pr8040462
Ni, J., Jing, B., Lin, J., Lin, B., Zhao, Z., and Jiang, L. (2018). Effect of Rare Earth on the Performance of Ru/MgAl-LDO Catalysts for Ammonia Synthesis. J. Rare Earths 36, 135–141. doi:10.1016/j.jre.2017.07.011
Nivangune, N. T., Ranade, V. V., and Kelkar, A. A. (2017). MgFeCe Ternary Layered Double Hydroxide as Highly Efficient and Recyclable Heterogeneous Base Catalyst for Synthesis of Dimethyl Carbonate by Transesterification. Catal. Lett. 147, 2558–2569. doi:10.1007/s10562-017-2146-x
Pavel, O. D., Zăvoianu, R., Bîrjega, R., Angelescu, E., and Pârvulescu, V. I. (2017). Mechanochemical versus Co-precipitated Synthesized Lanthanum-Doped Layered Materials for Olefin Oxidation. Appl. Catal. A: Gen. 542, 10–20. doi:10.1016/j.apcata.2017.05.012
Sarkarat, M., Komarneni, S., Rezvani, Z., Wu, X., Yin, S., Sato, T., et al. (2013). Multi-cationic Layered Double Hydroxides: Calcined Products as Photocatalysts for Decomposition of NOx. Appl. Clay Sci. 80-81, 390–397. doi:10.1016/j.clay.2013.07.002
Smalenskaite, A., Salak, A. N., Ferreira, M. G. S., Skaudzius, R., and Kareiva, A. (2018). Sol-gel Synthesis and Characterization of Hybrid Inorganic-Organic Tb(III)-terephthalate Containing Layered Double Hydroxides. Opt. Mater. 80, 186–196. doi:10.1016/j.optmat.2018.04.048
Sonoyama, N., Takagi, K., Yoshida, S., Ota, T., Kimilita, P. D., and Ogasawara, Y. (2020). Optical Properties of the Europium (II) and (III) Ions Doped Metal Oxides Obtained from Sintering Layered Double Hydroxides, and Their fine Structures. Appl. Clay Sci. 186, 105440. doi:10.1016/j.clay.2020.105440
Stamate, A.-E., Pavel, O. D., Zăvoianu, R., Brezeştean, I., Ciorȋță, A., Bȋrjega, R., et al. (2021). Ce-Containing MgAl-Layered Double Hydroxide-Graphene Oxide Hybrid Materials as Multifunctional Catalysts for Organic Transformations. Materials 14, 7457. doi:10.3390/ma14237457
Suárez-Quezada, M., Romero-Ortiz, G., Suárez, V., Morales-Mendoza, G., Lartundo-Rojas, L., Navarro-Cerón, E., et al. (2016). Photodegradation of Phenol Using Reconstructed Ce Doped Zn/Al Layered Double Hydroxides as Photocatalysts. Catal. Today 271, 213–219. doi:10.1016/j.cattod.2016.01.009
Sun, C., Świrk, K., Wierzbicki, D., Motak, M., Grzybek, T., and Da Costa, P. (2021). On the Effect of Yttrium Promotion on Ni-Layered Double Hydroxides-Derived Catalysts for Hydrogenation of CO2 to Methane. Int. J. Hydrogen Energ. 46, 12169–12179. doi:10.1016/j.ijhydene.2020.03.202
Świrk, K., Gálvez, M. E., Motak, M., Grzybek, T., Rønning, M., and Da Costa, P. (2018). Dry Reforming of Methane over Zr- and Y-Modified Ni/Mg/Al Double-Layered Hydroxides. Catal. Commun. 117, 26–32. doi:10.1016/j.catcom.2018.08.024
Szabados, M., Adél Ádám, A., Traj, P., Muráth, S., Baán, K., Bélteky, P., et al. (2020). Mechanochemical and Wet Chemical Syntheses of CaIn-Layered Double Hydroxide and its Performance in a Transesterification Reaction Compared to Those of Other Ca2M(III) Hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based Hydrotalcites. J. Catal. 391, 282–297. doi:10.1016/j.jcat.2020.07.038
Taherian, Z., Shahed Gharahshiran, V., Khataee, A., and Orooji, Y. (2021). Anti-coking Freeze-Dried NiMgAl Catalysts for Dry and Steam Reforming of Methane. J. Ind. Eng. Chem. 103, 187–194. doi:10.1016/j.jiec.2021.07.032
Taherian, Z., Shahed Gharahshiran, V., Khataee, A., and Orooji, Y. (2022). Synergistic Effect of Freeze-Drying and Promoters on the Catalytic Performance of Ni/MgAl Layered Double Hydroxide. Fuel 311, 122620. doi:10.1016/j.fuel.2021.122620
Tzompantzi, F. J., Carrera, Y., Morales-Mendoza, G., Valverde-Aguilar, G., and Mantilla, A. (2013). ZnO-Al2O3-La2O3 Layered Double Hydroxides as Catalysts Precursors for the Esterification of Oleic Acid Fatty Grass at Low Temperature. Catal. Today 212, 164–168. doi:10.1016/j.cattod.2012.12.017
Tzompantzi, F., Mendoza-Damián, G., Rico, J. L., and Mantilla, A. (2014). Enhanced Photoactivity for the Phenol Mineralization on ZnAlLa Mixed Oxides Prepared from Calcined LDHs. Catal. Today 220-222, 56–60. doi:10.1016/j.cattod.2013.07.014
Urdă, A., Popescu, I., Cacciaguerra, T., Tanchoux, N., Tichit, D., and Marcu, I.-C. (2013). Total Oxidation of Methane over Rare Earth Cation-Containing Mixed Oxides Derived from LDH Precursors. Appl. Catal. A: Gen. 464-465, 20–27. doi:10.1016/j.apcata.2013.05.012
Usman, M. S., Hussein, M. Z., Fakurazi, S., and Ahmad Saad, F. F. (2017). Gadolinium-based Layered Double Hydroxide and Graphene Oxide Nano-Carriers for Magnetic Resonance Imaging and Drug Delivery. Chem. Cent. J. 11, 47. doi:10.1186/s13065-017-0275-3
Usman, M. S., Hussein, M. Z., Kura, A. U., Fakurazi, S., Masarudin, M. J., and Ahmad Saad, F. F. (2020). Chlorogenic Acid Intercalated Gadolinium-Zinc/Aluminium Layered Double Hydroxide and Gold Nanohybrid for MR Imaging and Drug Delivery. Mater. Chem. Phys. 240, 122232. doi:10.1016/j.matchemphys.2019.122232
Velu, S., and Suzuki, K. (2003). Selective Production of Hydrogen for Fuel Cells via Oxidative Steam Reforming of Methanol over CuZnAl Oxide Catalysts: Effect of Substitution of Zirconium and Cerium on the Catalytic Performance. Top. Catal. 22, 235–244. doi:10.1023/A:1023576020120
Vorontsova, O. A., Saenko, R. N., and Lebedeva, O. E. (2007). Scandium-containing Layered Hydroxides. Russ. J. Inorg. Chem. 52, 1662–1665. doi:10.1134/S0036023607110046
Wang, J., Zhou, J., Li, Z., Liu, Q., Yang, P., Jing, X., et al. (2010). Design of Magnetic and Fluorescent Mg-Al Layered Double Hydroxides by Introducing Fe3O4 Nanoparticles and Eu3+ Ions for Intercalation of glycine. Mater. Res. Bull. 45, 640–645. doi:10.1016/j.materresbull.2010.01.006
Wang, L., Li, B., Zhao, X., Chen, C., and Cao, J. (2012). Effect of Rare Earth Ions on the Properties of Composites Composed of Ethylene Vinyl Acetate Copolymer and Layered Double Hydroxides. PLoS One 7, e37781. doi:10.1371/journal.pone.0037781
Wang, W., Miao, L., Wu, K., Chen, G., Huang, Y., and Yang, Y. (2019). Hydrogen Evolution in the Dehydrogenation of Methylcyclohexane over Pt/Ce Mg Al O Catalysts Derived from Their Layered Double Hydroxides. Int. J. Hydrogen Energ. 44, 2918–2925. doi:10.1016/j.ijhydene.2018.12.072
Wang, X., Chen, Y., Zhou, H., and Zhang, K. (2017). Structure and Photoluminescence of a New Binary Mg/Tb Layered Double Hydroxide. Appl. Clay Sci. 150, 184–191. doi:10.1016/j.clay.2017.09.025
Wani, A. A., Khan, A. M., Manea, Y. K., and Salem, M. A. S. (2021). Enhanced Photocatalytic Degradation of Organic Dyes from Aqueous Environment Using Neodymium-Doped Mesoporous Layered Double Hydroxide. J. Rare Earths. doi:10.1016/j.jre.2021.09.007
Xie, F., Ma, L., Gan, M., He, H., Hu, L., Jiang, M., et al. (2019). One-pot Construction of the Carbon Spheres Embellished by Layered Double Hydroxide with Abundant Hydroxyl Groups for Pt-Based Catalyst Support in Methanol Electrooxidation. J. Power Sourc. 420, 73–81. doi:10.1016/j.jpowsour.2019.02.088
Xie, W., Peng, H., and Chen, L. (2006). Calcined Mg-Al Hydrotalcites as Solid Base Catalysts for Methanolysis of Soybean Oil. J. Mol. Catal. A: Chem. 246, 24–32. doi:10.1016/j.molcata.2005.10.008
Xie, X., An, X., Wang, X., and Wang, Z. (2003). Preparation, Characterization and Application of ZnAlLa-hydrotalcite-like Compounds. J. Nat. Gas Chem. 12 (4), 259–263. doi:10.1016/S1003-9953-2003-12-4-259-263
Yanase, I., Horiuchi, Y., and Kobayashi, H. (2019). Photoluminescence Changes of Tb-Substituted Layered Double Hydroxides Caused by Capturing Carbonate Ions in Water. Mater. Res. Bull. 110, 207–213. doi:10.1016/j.materresbull.2018.10.021
Zăvoianu, R., Bîrjega, R., Angelescu, E., and Pavel, O. D. (2018). Effect of Hydration Temperature on the Structure Reconstruction of Mg Al Y Layered Materials. Comptes Rendus Chim. 21, 318–326. doi:10.1016/j.crci.2017.07.002
Zhan, W., Guo, Y., Gong, X., Guo, Y., Wang, Y., and Lu, G. (2014). Current Status and Perspectives of Rare Earth Catalytic Materials and Catalysis. Chin. J. Catal. 35, 1238–1250. doi:10.1016/S1872-2067(14)60189-3
Zhang, F., Zhang, X., Hao, Z., Jiang, G., Yang, H., and Qu, S. (2018). Insight into the H2S Selective Catalytic Oxidation Performance on Well-Mixed Ce-Containing Rare Earth Catalysts Derived from MgAlCe Layered Double Hydroxides. J. Hazard. Mater. 342, 749–757. doi:10.1016/j.jhazmat.2017.09.014
Zhang, M., Zhao, Y., Liu, Q., Yang, L., Fan, G., and Li, F. (2016). A La-Doped Mg-Al Mixed Metal Oxide Supported Copper Catalyst with Enhanced Catalytic Performance in Transfer Dehydrogenation of 1-decanol. Dalton Trans. 45, 1093–1102. doi:10.1039/C5DT03217B
Keywords: rare earth elements, layered double hydroxides, mixed metal oxides, metal cation doping, heterogeneous catalysis
Citation: Seliverstov ES, Golovin SN and Lebedeva OE (2022) Layered Double Hydroxides Containing Rare Earth Cations: Synthesis and Applications. Front. Chem. Eng. 4:867615. doi: 10.3389/fceng.2022.867615
Received: 01 February 2022; Accepted: 16 February 2022;
Published: 09 March 2022.
Edited by:
Octavian-Dumitru Pavel, University of Bucharest, RomaniaReviewed by:
Xingyun Li, Qingdao University, ChinaCopyright © 2022 Seliverstov, Golovin and Lebedeva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga E. Lebedeva, b2xlYmVkZXZhQGJzdS5lZHUucnU=
†These authors have contributed equally to this work and share first authorship
 Evgeniy S. Seliverstov
Evgeniy S. Seliverstov Sergei N. Golovin
Sergei N. Golovin Olga E. Lebedeva
Olga E. Lebedeva