- 1Student Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 2School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 3Student Research Committee, International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 4Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
- 5Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 6Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Student Research Committee, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
- 8School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- 9School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Inflammatory bowel disease (IBD) is a term used for a variety of conditions involving persistent inflammation of the digestive system. Ulcerative colitis (UC) and Crohn’s disease (CD) are examples of IBD. There were some treatments like Amino salicylates, glucocorticoids, immunosuppressants, antibiotics, and surgery which have been used for treating IBD. However, the short and long-term disabling adverse effects, like nausea, pancreatitis, elevated liver enzymes, allergic reactions, and other life-threatening complications remain a significant clinical problem. On the other hand, herbal medicine, believed to be safer, cheaper, and easily available, has gained popularity for treating IBD. Nowadays, Ginger, the Rizhome of Z. officinale from the Zingiberaceae family, one of the most commonly used fresh spices and herbs, has been proposed as a potential option for IBD treatment. According to upper issues, IBD treatment has become one of the society’s concerns. So, this review aims to summarize the data on the yin and yang of ginger use in IBD treatment.
Introduction
CD and UC (examples of inflammatory bowel disorders) are relapsing, chronic, idiopathic, and remitting conditions of the gastrointestinal tract with an increasing incidence worldwide for which existing therapies are mostly limited by severe side effects (Chan and Giovannucci 2010; Terzić, Grivennikov et al., 2010; Sussman, Santaolalla et al., 2012). Typical clinical signs and symptoms of IBD, such as diarrhea, abdominal pain, hematochezia, as well as clinical signs of bleeding and intestinal obstruction, relying on the location of the disease, which may significantly reduce the patient’s quality of life. Despite the fact that the etiology of IBD is not well understood, both environmental factors and genetics are known as contributive risks (Fiocchi 1998). Cytokines, reactive oxygen metabolites, and eicosanoids are all inflammatory mediators with a role in the progression of the disease (Seo, Takata et al., 1995; Loguercio, D'Argenio et al., 1996; Carty, De Brabander et al., 2000). Amino salicylates, glucocorticoids, immunosuppressants, antibiotics, and surgery, in some cases, have been used for the treatment of IBD. However, the short and long-term disabling adverse effects like, nausea, pancreatitis, elevated liver enzymes, allergic reactions, and other life-threatening complications remain a significant clinical problem (Navaneethan and Lashner 2013). Furthermore, most of the medicines listed above need regular high-dose administration to achieve significant clinical effectiveness (Ensign et al., 2012; Moriasi et al., 2012; Wang and DuBois 2013; Dulai et al., 2014; Xiao et al., 2014; Zhang et al., 2015). In recent years, herbal therapies have shown beneficial effects in IBD patients (Ng et al., 2013). Nanoparticles have been used in treating IBD patients and in addition, it can be used to deliver low doses of medicines to special tissues and cell types while also reducing systemic adverse effects (Laroui et al., 2010; Laroui et al., 2011; Xiao et al., 2013; Morton et al., 2014; Xiao et al., 2014; Araújo et al., 2015; Han et al., 2015; Hansen et al., 2015; Ramishetti et al., 2015; Tang et al., 2015; Xiao et al., 2015; Xing et al., 2015).
Ginger, the Rizhome of Z. officinale from the Zingiberaceae family, is one of the most commonly used fresh spices and herbs, containing many active phenolic components such as Shogaol, Gingerol, and Zingerone (Algieri et al., 2015). These components have anti-inflammatory, antioxidative, and immunomodulatory properties (Mozaffari-Khosravi et al., 2016). Ginger has traditionally been used as a remedy for gingivitis, rheumatism, stroke, asthma, and diabetes (Afzal et al., 2001). Ginger has also been known as a “broad-spectrum anti-emetic” cause of its prevention of nausea resulting from postoperative courses, pregnancy, and motion sickness (Ali et al., 2008). Also, the efficacy of ginger as a remedy to improve IBD has been reported in many studies (Ensign et al., 2012; Ng et al., 2013; Xiao et al., 2014). Therefore, this review aims to summarize the results of studies on the potential efficacy or possible side effects of ginger in the IBD treatment.
Methods
We conducted a comprehensive search in online databases (PubMed, Google Scholar, Scopus, and Science Direct) published up to September 2021, to find studies investigating the effect of ginger in the treatment of IBD. No language limit was exerted. Our keywords included Ginger, Z. officinale, Gingerol, Zingerone, Inflammatory bowel diseases (IBDs), Ulcerative colitis, and Crohn’s disease.
Due to the importance of ginger and current investigations, we divided these studies into two parts: in vivo and in vitro studies.
In vivo studies
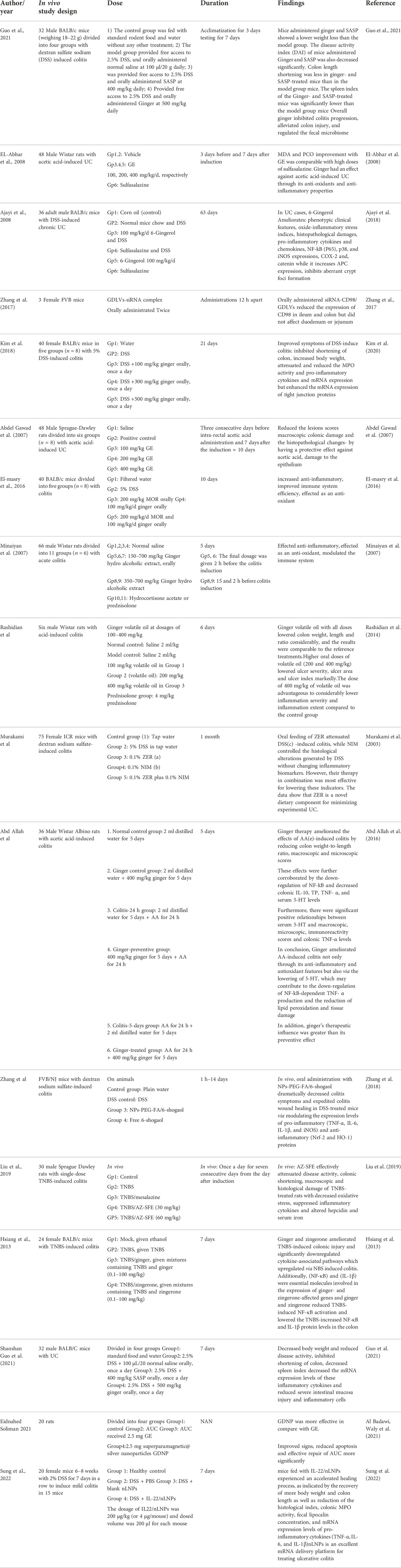
The efficacy of ginger has been shown in several in vivo and in vitro studies (Tables 1, 2). Studies demonstrated that T cells have a significant function in the immunological mechanisms of IBD. These data were concluded from genome-wide association studies, animal models of IBD, and some clinical trials. Inflammation is induced by Th1/Th17 cells that produce IFN-γ and IL-17; while TGF-β and IL-10 produced by Th2 have anti-inflammatory functions, for example, IL-10 inhibits pro-inflammatory cytokine expression by adaptive cells using STAT3-dependent signaling. Moreover, they have claimed that IL-17A enforces epithelial barrier function, so an inflammatory cytokine will be induced using an NF-κB dependent signaling (Gálvez 2014; Chen and Sundrud 2016).
In 2018, Chen et al. (2018) investigated the effect of the volatile of Amomum villosum (VOAV), a herbaceous plant in the ginger family, on the immunological function of T cells in IBD rats. They discovered that VOAV therapy reduced IL-17 and IFN- levels while increasing TGF- and IL-10 levels. Therefore, VOAV therapy reduced the level of pro-inflammatory cytokines and clearly repressed intestinal inflammation in IBD rats. These results were also supported in an in vivo part of a study by Zhang et al. In which they studied a particular population of nanoparticles that were derived from edible ginger (GDNPs 2) and analyzed their IBD targeting following oral administration. These particles contain high levels of bioactive elements of ginger like 6-gingerol and 6-shogaol. Using different mouse models, they claimed that oral use of GDNPs 2 reduced the pro-inflammatory cytokines like IL-10 and IL-22 (Zhang et al., 2016). Guo et al. (2021) evaluated the effect of ginger on improving symptoms of UC using a DSS-induced mice colitis model. Ginger inhibited colon shortening decreased INOs, IL-6, and the mRNA expression levels of inflammatory cytokines, Hematoxylin, and Eosin (H&E) staining of the colon tissues. Less severe intestinal mucosa injury and regulated the fecal microbiome compared to the control group. Reduced lesions and histopathological changes in the colon subsequent to the use of the ginger extract in rats with acetic acid-induced UC have also been reported (Abdel Gawad et al., 2007). Hsiang et al. (2013), for the first time, demonstrated that ginger and its constituent zingerone could improve TNBS-induced colitis in mice via modulation of IL-1β signaling pathway and NF-κB activity. Ginger plus zingerone therapy reduced the number of brown cells in the colon. They also substantially regulated numerous cytokine expressions, such as IL-6, IL-1β, interferon-c, IL-17, and tumor necrosis factor (TNF-α). Toll-like receptors (TLRs) were increased by TNBS, which resulted in the activation of inflammation-related pathways and the development of intestinal inflammation, while ginger and zingerone inhibited TLR signaling and alleviated TNBS-induced colitis in mice.
In 2014, Rashidian et al. (2014) realized that volatile ginger oil could significantly relieve colitis symptoms. The results showed that volatile ginger oil decreased the inflammation severity, inflammation extent, and crypt damage induced by acetic acid and involvement percentage in a dose-dependent manner. The reference drug used in their study was oral prednisolone, and it was used to portray the effectiveness of the test substance. They assumed that pretreatment for 5 days with this drug enhanced the absorption and systemic availability of the active drugs. The same results were found using a medium and high doses of volatile ginger oil. Their results indicate that ginger may be advantageous in the treatment of colon mucosal damage caused by IBD due to its anti-lipo per oxidative and anti-oxidant actions. These properties originate from its ability to scavenge free radicals and protect cell membranes from oxidants, and overall its anti-oxidant value Ajayi et al. (2018) showed that 6-Gingerol could Ameliorate phenotypic clinical features of UC, such as rectal bleeding and diarrhea, and substantially minimize the rate of reduction in body weight, colon length, and colon weight in mice, oxido-inflammatory stress indices level [such as MDA levels, MPO activity and NO concentration (Owumi et al., 2020), pro-inflammatory cytokines, and chemokines] which have an established role in the growth of UC (Landskron et al., 2014). Ginger could also decrease the expression of iNOS, P65, p38, and NF- kB, which catalyze the production of NO. Reduced histopathological damages were also reported to decrease colonic neutrophil infiltration and mucosal ulcerations (Morris et al., 2003).
In vitro studies
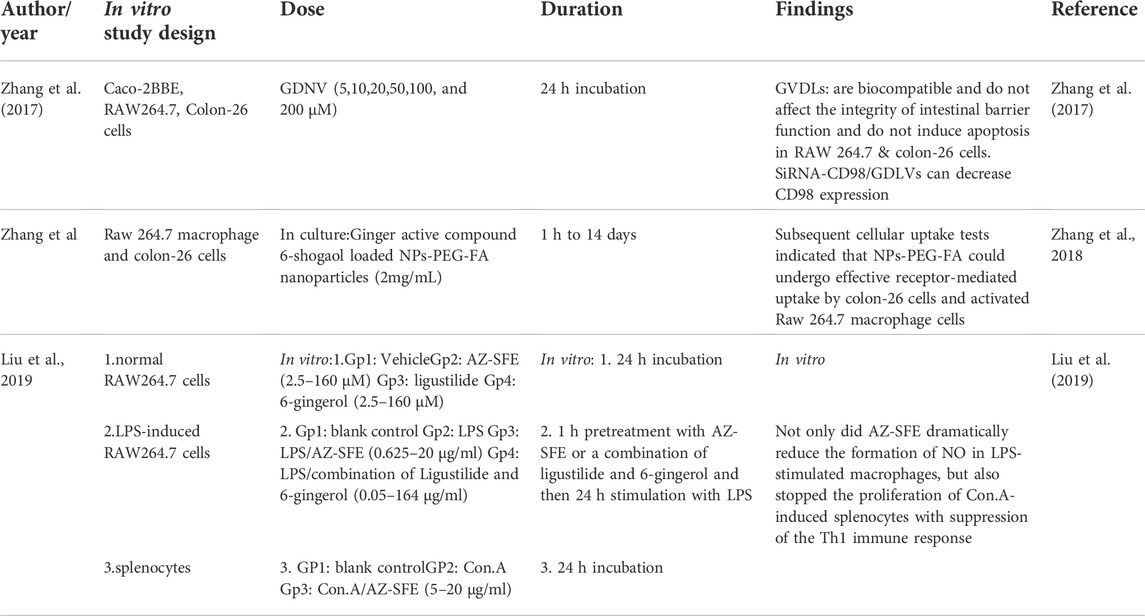
There are many in vitro studies that confirm the effect of ginger in the treatment of inflammatory bowel disease. In addition to the previous studies, in 2017, Zhang et al. (2017) used ginger-derived nano lipids loaded with siRNA as a new path for siRNA drug delivery that could have fewer side effects for UC treatment in both in vitro and in vivo studies. The in vitro part was done on Caco-2BBE, RAW264.7, and Colon-26 cells, which were incubated with Ginger-derived nano vectors (GDNV). The result showed that Ginger-derived lipid vehicles (GVDLs) are biocompatible and have fewer effects on the viabilities of cell lines compared with Lipid nanoparticles (currently the favored vehicle for therapeutic siRNA delivery that causes cell stress, inflammation, and apoptosis). In addition, siRNA-CD98/GDLVs can decrease CD98 expression. Also, their results indicated that GDLVs could be developed as a nontoxic siRNA-delivery vehicle. GDLVs/siRNA-FITC were also taken up by cells by high efficacy, which means it can be used as a siRNA-delivery vehicle without using toxic transfection reagents (like Lipofectamine). The results suggest that GDLVs/siRNA-FITC has the potential to shift the current paradigm of siRNA delivery away from artificially synthesized nanoparticles toward the use of nature-derived nano vehicles from edible plants.
Liu et al. (2019) did research about the anti-inflammatory characteristics of AZ-SFE in vitro by determining NO production on lipopolysaccharide (LPS)-induced RAW264.7 cells. LPS-induced RAW264.7 macrophages are typically used to assess the anti-inflammatory influence in vitro. The in vitro study augmented the investigation of the anti-colitis activity of AZ-SFE in the TNBS-induced rat model. They concluded that the following in vitro study showed the potential of AZ-SFE for mitigating colitis by cutting down the oxidative stress, inhibiting inflammatory mediators, impeding the Th1 immune response, and managing iron homeostasis. As a result, AZ-SFE derived from traditional Chinese herbs could be a promising supplement for recent IBD therapy, and the precise mechanism requires more investigation.
Oral drug delivery is the most appealing pathway for UC treatment, because it has many benefits. Adaptable Single-step surface-functionalizing method was used by Zhang et al. (2018) to prepare PLGA/PLA-PEG-FA nanoparticles loaded with the active ginger compound, 6-shogaol (NPs-PEG-FA/6-shogaol). They utilized both in vitro and in vivo models. The result indicated that NPs-PEG-FA showed great biocompatibility both in vitro and in vivo. Subsequent cellular uptake experiments exhibited that NPs-PEG-FA could undergo effective receptor-mediated uptake by colon-26 cells and activated Raw 264.7 macrophage cells. Finally, their study illustrated a convenient, orally administered 6-shogaol drug delivery system that effectively targets colitis tissue, reduces colitis symptoms, and expedites colitis wound repair.
Human studies
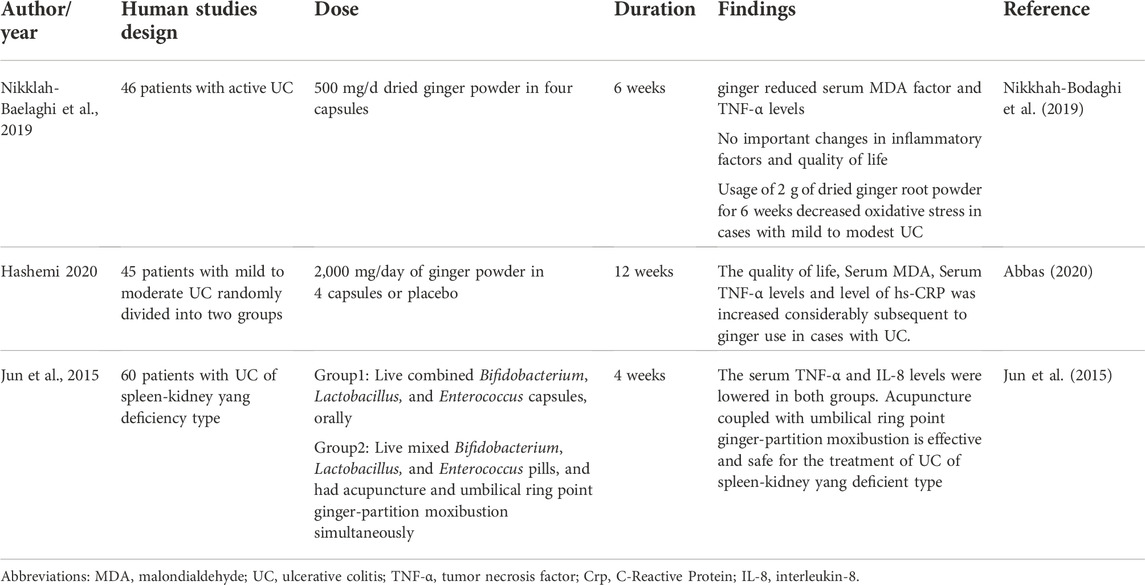
Chronic inflammation and Oxidative stress play a key role in ulcerative colitis (UC) onset and severity. A randomized clinical trial in Iran by Bodaghi et al. (2019) examined the short-term effects of ginger on UC and patient’s quality of life.
Forty-six patients with active mild to modest UC randomly consumed 500 mg/day of dried ginger powder in four capsules or identical placebo capsules for 6 weeks. They examined the quality of life, and serum levels of Total anti-oxidant capacity (TAC), High-sensitivity C-reactive protein (hs-CRP), TNF-α, Malondialdehyde (MDA), and nuclear factor kappa B (NF-κB) and the results showed ginger decreased TNF-α and serum MDA factor levels. There were no important changes in inflammatory factors or quality of life. It was concluded that consumption of 2 g of dried ginger root powder for 6 weeks reduces oxidative stress in patients with mild to moderate UC. A 2021 meta-analysis of ginger supplementation on biomarkers of oxidative stress such as glutathione peroxidase (GPx), malondialdehyde (MDA), and total antioxidant capacity (TAC) (Sheikhhossein et al., 2021) showed that ginger supplementation reduced MDA and increased GPx but the outcomes demonstrated that no significant changes in TAC activities. Also, according to the study by Atashak et al. (2011a), Atashak et al. (2011b) ginger un take compared to placebo did not result in a significant change in serum glucose, lipid, MDA and TAC levels.
Another double-blind and randomized clinical trial by Hashemi et al. (Abbas 2020) observed the relationship between ginger and UC. Forty-five patients with mild to modest ulcerative colitis who were separated into two groups received 2,000 mg/day of ginger powder in four capsules or a similar placebo for 12 weeks, and oxidative stress and inflammatory indices were evaluated. The result indicated that the Serum TNF-α levels, Serum MDA, level of hs-CRP, and quality of life were increased considerably after ginger use in cases with UC. This randomized placebo-controlled trial indicated that ginger supplementation of 2000 mg daily could enhance the inflammatory signs of the disease.
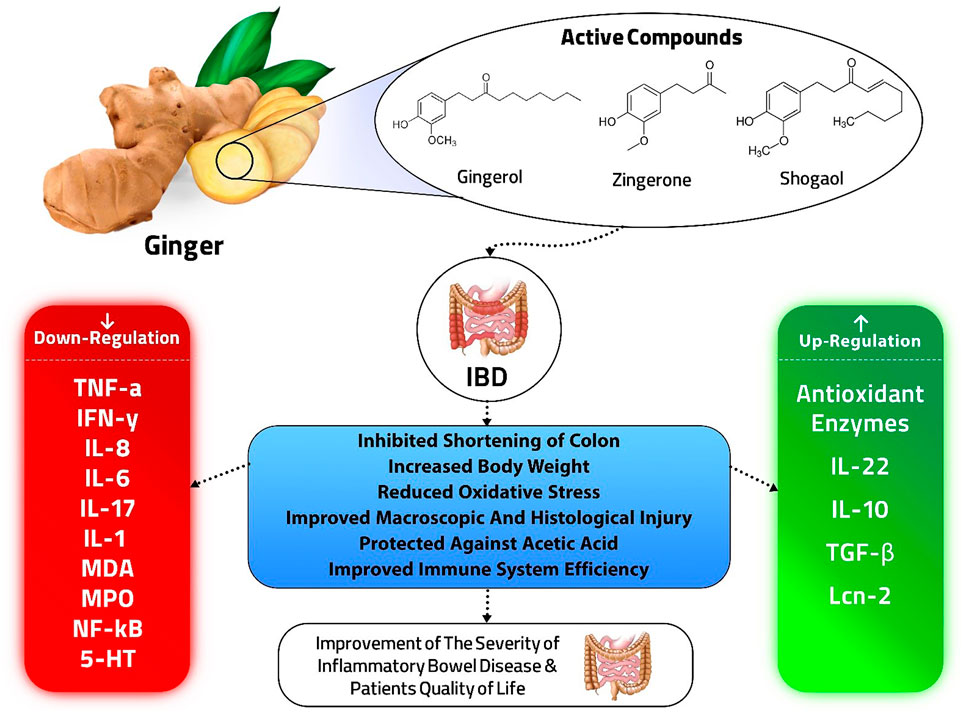
Jun et al. (2015) investigated the therapeutic effect of acupuncture combined with umbilical ring point ginger-partition moxibustion for ulcerative colitis of the spleen-kidney yang deficiency type and its impact on related inflammatory factors in ulcerative colitis of the spleen-kidney yang deficiency type. After therapy, serum TNF- and IL-8 levels were shown to be lower in both the control groups and the treatment group, and the reduction in the treatment group surpassed the control group. Acupuncture coupled with ginger-partition moxibustion at the umbilical ring point was shown to be efficacious and safe in the treatment of UC (Table 3), but the number, quality and the overall sample size is too small for definitive conclusions; on the other hand, there is evidence that moxibustion is an inconclusive treatment for UC (Lee et al., 2010). The benefits of ginger in IBD are summarized in Figure 1.
Conclusion and future perspective
In inflammatory conditions of IBD, the migration of inflammatory cells into the colon, such as neutrophils, results in the production of oxidative stress, ROS, and lipid peroxidation. These events affect cell macromolecules and create imbalances in membrane integrity, which can resulting in chronic inflammation, and ulcers (Ajayi et al., 2015). Due to its spasmolytic properties, which are mediated by calcium channel blockade, ginger also has protective effects against UC (Ghayur and Gilani 2005). By inhibiting the peroxidation of lipids, p38 expression, and ginger active compounds also reduce MDA, hydrogen peroxide, and protein carbonyl in a dose-dependent manner (Zhang et al., 2016; Ajayi et al., 2018). A ginger supplement greatly decreased MDA but did not affect TAC. As a result, it may drastically lower the combined SCCAIQ and IBDQ scores (Nikkhah-Bodaghi et al., 2019).
Human studies have some experimental restrictions, such as the high expense of colonoscopies and tissue biopsies. Patients also reported a few minor side effects from taking ginger supplements, such as heartburn and a strong odor. It appears that the supplementation’s dosage and duration were too low to result in a noticeable improvement in UC patients, but it improved some aspects of oxidative stress and disease activity. Altogether, more clinical trials using various ginger supplementation doses and durations are required (Nikkhah-Bodaghi et al., 2019).
In summary, ginger and its components efficiently treated IBD by targeting the inflamed intestinal mucosa, blocking damaging factors such as IFN-γ, IL-17, and TNFα despite promoting healing factors like IL-22, IL-10, and TGF-β. Also, ginger had no known side effects and no known herb or drug interactions. These natural products can easily be developed for comprehensive production and may act as an effective therapeutic strategy for preventing and treating IBD. However, further experiments are required to evaluate the safety and efficacy of ginger in IBD treatment, to evaluate the effect of different dosages and durations, to check its regulatory effects on the gut microbiota and, to realize if genetic diversity and environmental agents can influence these activities. Most importantly, RCTs must be designed to study the effect of ginger on Crohn’s disease. The result of these studies may open up a novel path in gastrointestinal disorders treatment.
Author contributions
Study concept and design: NiD. Acquisition of data: TM, SO-T, SN, NiD, ZT, and MP. Drafting of the Manuscript: FS, FM, AO, NoD, MV, FN, and SD. Critical revision of the manuscript for important intellectual content: SN. Study supervision: NiD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
APC, adenomatous polyposis coli; AUC, acute ulcerative colitis; AZ-SFE, supercritical fluid extract of Angelica sinensis and Z. officinale roscoe; CD, Crohn’s disease; Con.A, concanavalin A; COX-2, cyclooxygenase-2; DSS, dextran sulfate sodium; GDNP, ginger loaded nanoparticles; GDNPs 1, ginger derived nanoparticles (8/30%); GDNPs 2, ginger derived nanoparticles (30/45%); GDNV, ginger-derived nano vectors; GE, ginger extract; GVDLs, ginger-derived lipid vehicles; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; Lcn-2, lipocalin-2; LPS, lipopolysaccharide; MAPK, mitogen activated protein kinase; MDA, malondialdehyde; MOR, Moringa oleifera; MPO, myeloperoxidase; MTT, [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide]; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NIM, nimesulide: a selective COX-2 inhibitor; NO, nitrogen monoxide; PCO, protein carbonyl; TNBS, trinitrobenzene sulfonic acid; TNF-α, tumor necrosis factor; UC, ulcerative colitis; ZER, zerumbone: a sesquiterpenoid with very large amounts detected in rhizomes.
References
Abbas, H. S. (2020). The effects of ginger on quality of life, disease activity index in people with ulcerative colitis. Mazandaran university of medical sciences. Mazandaran, Iran,
Abd Allah, E. S., Makboul, R., and Mohamed, A. O. (2016). Role of serotonin and nuclear factor-kappa B in the ameliorative effect of ginger on acetic acid-induced colitis. Pathophysiology 23 (1), 35–42. doi:10.1016/j.pathophys.2015.12.001
Abdel Gawad, H., Hammad, L., and El-Abhar, H. (2007). Amelioration of acetic acid-induced colitis in rats by oral administration of ginger extract. Bull. Egypt. Soc. Physiological Sci. 27 (1), 221–240. doi:10.21608/besps.2007.37138
Abdel Gawad, H., Hammad, L., and El-Abhar, H. (2007). "Amelioration of acetic acid-induced colitis in rats by oral administration of ginger extract." 27(1): 221–240. doi:10.21608/besps.2007.37138
Afzal, M., Al-HaDiDi, D., MenonM., , Pesek, J., and Dhami, M. S. (2001). Ginger: An ethnomedical, chemical and pharmacological review. Drug Metabol. Drug Interact. 18 (3-4), 159–190. doi:10.1515/dmdi.2001.18.3-4.159
Ajayi, B., Adedara, I. A., and Farombi, E. O. (2018). Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum. Exp. Toxicol. 37 (10), 1054–1068. doi:10.1177/0960327117751235
Ajayi, B. O., Adedara, I. A., and Farombi, E. O. (2015). Pharmacological activity of 6-gingerol in dextran sulphate sodium-induced ulcerative colitis in BALB/c mice. Phytother. Res. 29 (4), 566–572. doi:10.1002/ptr.5286
Al Badawi, M. H., Waly, N., Eid, M., and Soliman, N. (2021). Histopathological impact of Ginger loaded nanoparticle versus ginger extract as a novel therapy of experimentally induced acute ulcerative colitis. Egypt. J. Histology 0, 0. doi:10.21608/ejh.2021.68124.1448
Algieri, F., Rodriguez-Nogales, A., Rodriguez-Cabezas, M. E., Risco, S., Ocete, M. A., and Galvez, J. (2015). Botanical drugs as an emerging strategy in inflammatory bowel disease: A review. Mediat. Inflamm. 2015, 179616. doi:10.1155/2015/179616
Ali, B. H., Blunden, G., Tanira, M. O., and Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (zingiber officinale roscoe): A review of recent research. Food Chem. Toxicol. 46 (2), 409–420. doi:10.1016/j.fct.2007.09.085
Araújo, F., Shrestha, N., Shahbazi, M. A., Liu, D., Herranz-Blanco, B., Makila, E. M., et al. (2015). Microfluidic assembly of a multifunctional tailorable composite system designed for site specific combined oral delivery of peptide drugs. ACS Nano 9 (8), 8291–8302. doi:10.1021/acsnano.5b02762
Atashak, S., (2011b). Effects of ginger supplementation and resistance training on lipid profiles and body composition in obese men. J. Med. Plants Res. 5. doi:10.5897/JMPR.9000524
Atashak, S., Peeri, M., Jafari, A., and Azarbayjani, M. A. (2011a). Obesity-related cardiovascular risk factors after long- term resistance training and ginger supplementation. J. Sports Sci. Med. 10, 685–691.
Bodaghi, M., Maleki, I., Agah, S., and Hekmatdoost, A., (2019). Short term effects of ginger on quality of life, disease activity index, inflammatory and oxidative stress factors in ulcerative colitis. Tehran Univ. Med. J. 76, 748–756.
Carty, E., De BrabanderM., , Feakins, R. M., and Rampton, D. S. (2000). Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut 46 (4), 487–492. doi:10.1136/gut.46.4.487
Chan, A. T., and Giovannucci, E. L. (2010). Primary prevention of colorectal cancer. Gastroenterology 138 (6), 20292029–20292043. doi:10.1053/j.gastro.2010.01.057
Chen, M. L., and Sundrud, M. S. (2016). Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm. Bowel Dis. 22 (5), 1157–1167. doi:10.1097/MIB.0000000000000714
Chen, Z., Ni, W., Yang, C., Zhang, T., Lu, S., Zhao, R., et al. (2018). Therapeutic effect of Amomum villosum on inflammatory bowel disease in rats. Front. Pharmacol. 9, 639. doi:10.3389/fphar.2018.00639
Dulai, P. S., Siegel, C. A., Colombel, J. F., Sandborn, W. J., and Peyrin-Biroulet, L. (2014). Systematic review: Monotherapy with antitumour necrosis factor α agents versus combination therapy with an immunosuppressive for IBD. Gut 63 (12), 1843–1853. doi:10.1136/gutjnl-2014-307126
El-Abhar, H. S., Hammad, L. N. A., and Gawad, H. S. A. (2008). Modulating effect of ginger extract on rats with ulcerative colitis. J. Ethnopharmacol. 118 (3), 367–372. doi:10.1016/j.jep.2008.04.026
El-masry, A., Daba, M-.H., and El-Karef, A., (2016). Possible effects of moringa oleifera versus ginger (Zingiber officinalis) on experimental colitis in mice, British Journal of Medicine and Medical Research, 16, 1–19. doi:10.9734/BJMMR/2016/26312
Ensign, L. M., Cone, R., and Hanes, J. (2012). Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 64 (6), 557–570. doi:10.1016/j.addr.2011.12.009
Fiocchi, C. (1998). Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 115 (1), 182–205. doi:10.1016/s0016-5085(98)70381-6
Gálvez, J. (2014). Role of Th17 cells in the pathogenesis of human IBD, ISRN Inflamm, 2014, 928461.doi:10.1155/2014/928461
Ghayur, M. N., and Gilani, A. H. (2005). Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig. Dis. Sci. 50 (10), 1889–1897. doi:10.1007/s10620-005-2957-2
Guo, S., Geng, W., Chen, S., Wang, L., Rong, X., Wang, S., et al. (2021). Ginger alleviates DSS-induced ulcerative colitis severity by improving the diversity and function of gut microbiota. Front. Pharmacol. 12, 632569. doi:10.3389/fphar.2021.632569
Guo, S., Geng, W., Chen, S., Wang, L., Rong, X., Wang, S., et al. (2021). Ginger alleviates DSS-induced ulcerative colitis severity by improving the diversity and function of gut microbiota. Front. Pharmacol. 12, 632569. doi:10.3389/fphar.2021.632569
Han, S., Cheng, Q., Wu, Y., Zhou, J., Long, X., Wei, T., et al. (2015). Effects of hydrophobic core components in amphiphilic PDMAEMA nanoparticles on siRNA delivery. Biomaterials 48, 45–55. doi:10.1016/j.biomaterials.2015.01.026
Hansen, A. E., Petersen, A. L., Henriksen, J. R., Boerresen, B., Rasmussen, P., Elema, D. R., et al. (2015). Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 9 (7), 6985–6995. doi:10.1021/acsnano.5b01324
Hsiang, C.-Y., Lo, H. Y., Huang, H. C., Wu, S. L., and Ho, T. Y. (2013). Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-κB activity and interleukin-1β signalling pathway. Food Chem. 136 (1), 170–177. doi:10.1016/j.foodchem.2012.07.124
Jun, H., Muxi, L., and Zhenzhen, M., (2015). Therapeutic effect of acupuncture combined with umbilical ring point ginger-partition moxibustion for ulcerative colitis of spleen-kidney yang deficiency type and its influence on related inflammatory factors. J. Guangzhou Univ. Traditional Chin. Med. (4), 687–689.
Kim, U., Koroukian, S., Statler, A., and Rose, J. (2020). The effect of Medicaid expansion among adults from low-income communities on stage at diagnosis in those with screening-amenable cancers. Cancer 126 (18), 4209–4219. doi:10.1002/cncr.32895
Landskron, G., De la Fuente, M., Thuwajit, P., Thuwajit, C., and Hermoso, M. A. (2014). Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 149185. doi:10.1155/2014/149185
Laroui, H., Dalmasso, G., Nguyen, H. T. T., Yan, Y., Sitaraman, S. V., and Merlin, D. (2010). Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 138 (3), 843–842.
Laroui, H., Theiss, A. L., Yan, Y., Dalmasso, G., Nguyen, H. T. T., Sitaraman, S. V., et al. (2011). Functional TNFα gene silencing mediated by polyethyleneimine/TNFα siRNA nanocomplexes in inflamed colon. Biomaterials 32 (4), 1218–1228. doi:10.1016/j.biomaterials.2010.09.062
Lee, D. H., Kim, J. I., Lee, M. S., Choi, T. Y., Choi, S. M., and Ernst, E. (2010). Moxibustion for ulcerative colitis: A systematic review and meta-analysis. BMC Gastroenterol. 10, 36. doi:10.1186/1471-230X-10-36
Liu, J., Yu, L., Lan, H., Zhang, Y., Liu, X., et al. (2019a). Supercritical fluid extract of Angelica sinensis and zingiber officinale roscoe ameliorates TNBS-induced colitis in rats. Int. J. Mol. Sci. 20 (15), 3816. doi:10.3390/ijms20153816
Liu, J., Yu, L., Mo, N., Lan, H., Zhang, Y., Liu, X., et al. (2019b). Supercritical fluid extract of Angelica sinensis and Zingiber officinale roscoe ameliorates TNBS-induced colitis in rats. Int. J. Mol. Sci. 20 (15), 3816. doi:10.3390/ijms20153816
Loguercio, C., D'ArGenio, G., Delle CaveM., , Cosenza, V., Della ValleN., , Mazzacca, G., et al. (1996). Direct evidence of oxidative damage in acute and chronic phases of experimental colitis in rats. Dig. Dis. Sci. 41 (6), 1204–1211. doi:10.1007/BF02088238
Minaiyan, M., Ghannadi, A., Mahzouni, P., and Nabi-Meibodi, M. (2007). Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) hydroalcoholic extract on acetic acid-induced acute colitis in rats. Res. Pharm. Sci. 3.
Moriasi, C., Subramaniam, D., Awasthi, S., Ramalingam, S., and Anant, S. (2012). Prevention of colitis-associated cancer: Natural compounds that target the IL-6 soluble receptor. Anticancer. Agents Med. Chem. 12 (10), 1221–1238. doi:10.2174/187152012803833080
Morris, K. R., Lutz, R. D., Choi, H. S., Kamitani, T., Chmura, K., and Chan, E. D. (2003). Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect. Immun. 71 (3), 1442–1452. doi:10.1128/iai.71.3.1442-1452.2003
Morton, S. W., Lee, M. J., Deng, Z. J., Dreaden, E. C., Siouve, E., Shopsowitz, K. E., et al. (2014). A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci. Signal. 7 (325), 44. doi:10.1126/scisignal.2005261
Mozaffari-Khosravi, H., Naderi, Z., Dehghan, A., Nadjarzadeh, A., and Fallah Huseini, H. (2016). Effect of ginger supplementation on proinflammatory cytokines in older patients with osteoarthritis: Outcomes of a randomized controlled clinical trial. J. Nutr. Gerontol. Geriatr. 35 (3), 209–218. doi:10.1080/21551197.2016.1206762
Murakami, A., Hayashi, R., Tanaka, T., Kwon, K. H., Ohigashi, H., Safitri, R., et al. (2003). Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: Separately and in combination. Biochem. Pharmacol. 66 (7), 1253–1261. doi:10.1016/s0006-2952(03)00446-5
Navaneethan, U., and Lashner, B. A. (2013). Effects of immunosuppression and liver transplantation on inflammatory bowel disease in patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 11 (5), 524–525. doi:10.1016/j.cgh.2013.01.020
Ng, S. C., Lam, Y. T., Tsoi, K. K. F., Chan, F. K. L., Sung, J. J. Y., and Wu, J. C. Y. (2013). Systematic review: The efficacy of herbal therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 38 (8), 854–863. doi:10.1111/apt.12464
Nikkhah-Bodaghi, M., Maleki, I., Agah, S., and Hekmatdoost, A. (2019). Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 43, 1–6. doi:10.1016/j.ctim.2018.12.021
Owumi, S. E., Nwozo, S. O., Effiong, M. E., and Najophe, E. S. (2020). Gallic acid and omega-3 fatty acids decrease inflammatory and oxidative stress in manganese-treated rats. Exp. Biol. Med. 245 (9), 835–844. doi:10.1177/1535370220917643
Ramishetti, S., Kedmi, R., Goldsmith, M., Leonard, F., Sprague, A. G., Godin, B., et al. (2015). Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano 9 (7), 6706–6716. doi:10.1021/acsnano.5b02796
Rashidian, A., Mehrzadi, S., Ghannadi, A. R., Mahzooni, P., Sadr, S., and Minaiyan, M. (2014). Protective effect of ginger volatile oil against acetic acid-induced colitis in rats: A light microscopic evaluation. J. Integr. Med. 12 (2), 115–120. doi:10.1016/S2095-4964(14)60011-X
Seo, H. G., Takata, I., NakaMuraM., , Tatsumi, H., SuzuKi, K., FuJii, J., et al. (1995). Induction of nitric oxide synthase and concomitant suppression of superoxide dismutases in experimental colitis in rats. Arch. Biochem. Biophys. 324 (1), 41–47. doi:10.1006/abbi.1995.9932
Sheikhhossein, F., Borazjani, M., Jafari, A., Askari, M., Vataniyan, E., Gholami, F., et al. (2021). Effects of ginger supplementation on biomarkers of oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 45, 111–119. doi:10.1016/j.clnesp.2021.07.010
Sussman, D. A., Santaolalla, R., Strobel, S., Dheer, R., and Abreu, M. T. (2012). Cancer in inflammatory bowel disease: Lessons from animal models. Curr. Opin. Gastroenterol. 28 (4), 327–333. doi:10.1097/MOG.0b013e328354cc36
Tang, L., Tong, R., Coyle, V. J., Yin, Q., Pondenis, H., Borst, L. B., et al. (2015). Targeting tumor vasculature with aptamer-functionalized doxorubicin-polylactide nanoconjugates for enhanced cancer therapy. ACS Nano 9 (5), 5072–5081. doi:10.1021/acsnano.5b00166
Terzić, J., Grivennikov, S., Karin, E., and Karin, M. (2010). Inflammation and colon cancer. Gastroenterology 138 (6), 21012101–21012114. doi:10.1053/j.gastro.2010.01.058
Wang, D., and DuBois, R. N. (2013). The role of anti-inflammatory drugs in colorectal cancer. Annu. Rev. Med. 64, 131–144. doi:10.1146/annurev-med-112211-154330
Xiao, B., Laroui, H., Ayyadurai, S., Viennois, E., Charania, M. A., Zhang, Y., et al. (2013). Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials 34 (30), 7471–7482. doi:10.1016/j.biomaterials.2013.06.008
Xiao, B., Laroui, H., Viennois, E., Ayyadurai, S., Charania, M. A., Zhang, Y., et al. (2014). Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 146 (5), 1289–1219.
Xiao, B., Si, X., Zhang, M., and Merlin, D. (2015). Oral administration of pH-sensitive curcumin-loaded microparticles for ulcerative colitis therapy. Colloids Surf. B Biointerfaces 135, 379–385. doi:10.1016/j.colsurfb.2015.07.081
Xiao, B., Yang, Y., Viennois, E., Zhang, Y., Ayyadurai, S., Baker, M., et al. (2014). Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle. J. Mat. Chem. B 2 (11), 1499–1508. doi:10.1039/C3TB21564D
Xing, X., Zhang, B., Wang, X., Liu, F., Shi, D., and Cheng, Y. (2015). An "imaging-biopsy" strategy for colorectal tumor reconfirmation by multipurpose paramagnetic quantum dots. Biomaterials 48, 16–25. doi:10.1016/j.biomaterials.2015.01.011
Zhang, M., Viennois, E., Prasad, M., Zhang, Y., Wang, L., Zhang, Z., et al. (2016). Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101, 321–340. doi:10.1016/j.biomaterials.2016.06.018
Zhang, M., Wang, X., Han, M. K., Collins, J. F., and Merlin, D. (2017). Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine (Lond) 12 (16), 1927–1943. doi:10.2217/nnm-2017-0196
Zhang, M., Xu, C., Liu, D., Han, M. K., Wang, L., and Merlin, D. (2018). Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohns Colitis 12 (2), 217–229. doi:10.1093/ecco-jcc/jjx115
Keywords: Z. officinale, ginger, ulcerative colitis, inflammatory bowel disease, natural compound
Citation: Sadeghi Poor Ranjbar F, Mohammadyari F, Omidvar A, Nikzad F, Doozandeh Nargesi N, Varmazyar M, Dehghankar S, Vosoughian F, Olangian-Tehrani S, Nanbakhsh S, Mansourian T, Deravi N, Tutunchian Z, Salahi M, Poudineh M and Ghayyem H (2022) Zingiber officinale (Ginger) as a treatment for inflammatory bowel disease: A review of current literature. Front. Drug. Discov. 2:1043617. doi: 10.3389/fddsv.2022.1043617
Received: 13 September 2022; Accepted: 21 November 2022;
Published: 08 December 2022.
Edited by:
Rajeev K. Tyagi, Institute of Microbial Technology (CSIR), IndiaReviewed by:
Chengxin Sun, Zunyi Medical University, ChinaJoshua R. Sacher, Photys Therapeutics, United States
Copyright © 2022 Sadeghi Poor Ranjbar, Mohammadyari, Omidvar, Nikzad, Doozandeh Nargesi, Varmazyar, Dehghankar, Vosoughian, Olangian-Tehrani, Nanbakhsh, Mansourian, Deravi, Tutunchian, Salahi, Poudineh and Ghayyem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niloofar Deravi, bmlsb29mYXJkZXJhdmlAc2JtdS5hYy5pcg==
†These authors have contributed equally to this work
 Fatemeh Sadeghi Poor Ranjbar1†
Fatemeh Sadeghi Poor Ranjbar1† Fatemeh Mohammadyari
Fatemeh Mohammadyari Farhad Nikzad
Farhad Nikzad Nooria Doozandeh Nargesi
Nooria Doozandeh Nargesi Tina Mansourian
Tina Mansourian Niloofar Deravi
Niloofar Deravi Mohadeseh Poudineh
Mohadeseh Poudineh