- 1Duke Institute for Health Innovation, Durham, NC, United States
- 2Mayo Clinic, Rochester, MN, United States
- 3OCHIN, Portland, OR, United States
- 4Division of Nephrology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States
- 5Academic Unit of Ophthalmology, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, United Kingdom
Editorial on the Research Topic
Surfacing best practices for AI software development and integration in healthcare
Introduction
The evidence supporting the mainstream use of artificial intelligence (AI) software in healthcare is rapidly mounting. Three systematic reviews of AI software randomized controlled trials (RCTs) were published in 2021 and 2022, including 95 studies across 29 countries (1–3). In the United States (US), the Centers for Medicare and Medicaid Services (CMS) is approving AI software systems for reimbursement through multiple payment mechanisms (4). In the United Kingdom (UK), the National Screening Committee is exploring the use of AI software in national cancer screening and has awarded £90 million to prospective multi-center trials (5, 6). Two large, multi-hospital studies showed the mortality benefit of early detection and treatment of inpatient deterioration and pneumonia (7, 8). However, despite advances in technology and policy, isolated success stories are not leading to efficient diffusion of validated AI software across settings.
A key barrier preventing efficient translation of AI software to new clinical settings is the lack of visibility into poorly characterized, yet critically important labor that Mary Gray and Siddharth Suri call “Ghost Work” (9). Ghost work is broadly described as the invisible labor that powers technology platforms. In healthcare, ghost work is carried out by front-line clinical and administrative staff working beyond the contours of technical AI systems to effectively integrate the technologies into local social environments. But while the brittleness of AI software systems over time and across sites is broadly recognized (10, 11), health systems develop strategies largely in silos. To fill this gap, we invited teams from health systems around the globe to contribute to the research topic “Surfacing Best Practices for AI Software Development and Integration in Healthcare (12).” The research topic was sponsored by Janssen Pharmaceuticals of Johnson & Johnson. In this editorial, we present a synthesis of the nine featured manuscripts and highlight strategies used across settings as well as future opportunities for development and partnership.
Methods
We conducted two primary analyses of the nine research topic manuscripts to identify key themes. We then complement the two primary analyses with details about the host institution, country, model use case, manuscript objectives, and key takeaways.
In the first primary analysis, we mapped the topics described in each manuscript to various stages of the AI software lifecycle. The four stages are defined as follows. First, problem definition and solution procurement describes the activities related to how organizations identify and prioritize problems and then allocate resources and personnel to pursue opportunities. Second, AI solution development and adaptation describes the activities related to how organizations either build technologies internally or adapt externally built tools. Third, technical and clinical integration describes the activities related to how organizations integrate AI solutions into legacy information technology systems and clinical workflows, roles, and responsibilities. Fourth, lifecycle management describes the activities related to maintenance, updating, and decommissioning of AI solutions used in clinical care. Each research topic manuscript could be mapped to multiple lifecycle stages.
In the second primary analysis, we reviewed biosketches, organization websites, and professional social media pages to map each research topic manuscript author to formal academic training across disciplines. Due to the large number of manuscript authors and broad range of formal training, we grouped disciplines into seven categories: engineering, computer science, and physics; statistics, biostatistics, and bioinformatics; business and management; public health and economics; biological or behavioral science; clinical doctorate; ethics or bioethics. Each author could be mapped to multiple academic disciplines.
Results
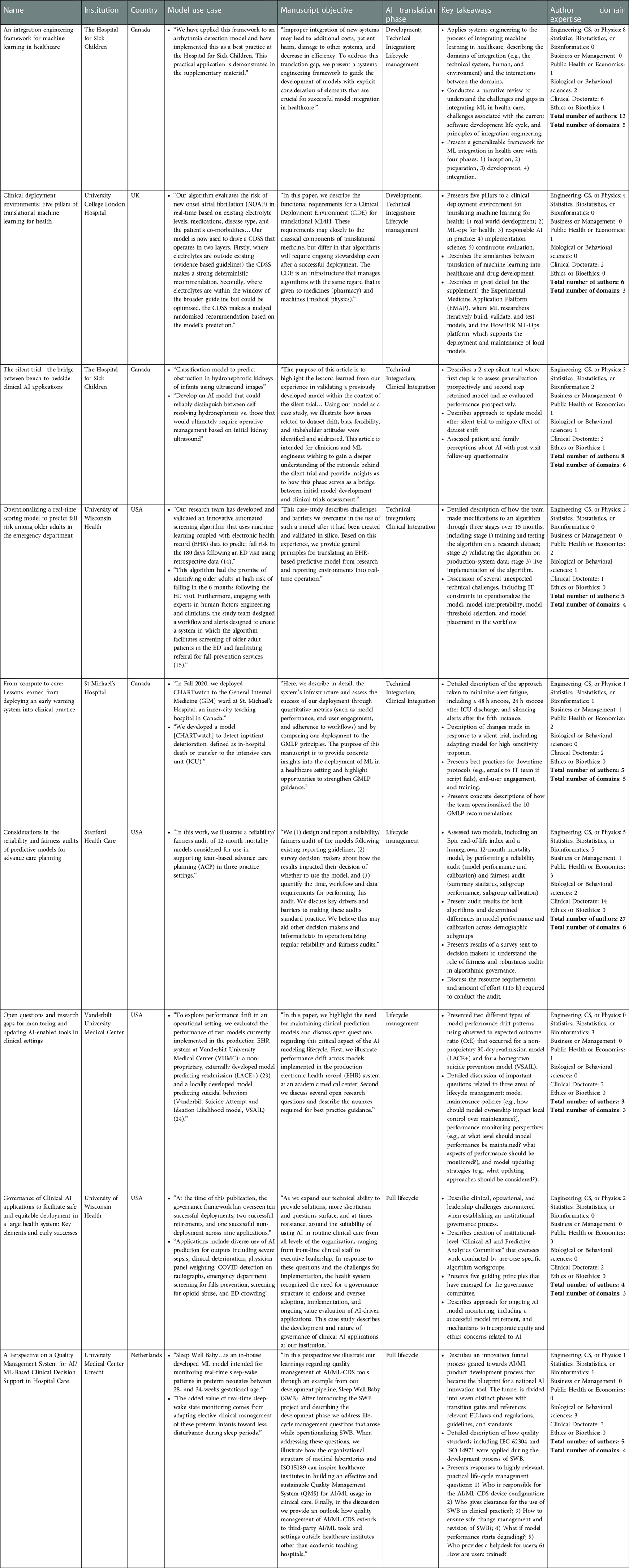
The research topic “Surfacing Best Practices for AI Software Development and Integration in Healthcare” features 9 manuscripts with 73 authors from 7 institutions across 4 countries. Two institutions published two manuscripts each, including The Hospital for Sick Children in Toronto, Canada and University of Wisconsin in Madison, Wisconsin, USA. The AI software use cases featured in the research topic include three pediatric applications (hydronephrosis due to obstruction, arrhythmia detection, and sleep-wake patterns in neonates), one mental health application (suicide prevention), three general adult applications (30-day readmission, inpatient deterioration, and new-onset atrial fibrillation), and two geriatrics applications (advance care planning, falls risk in the emergency department). One research topic manuscript describes an organizational governance framework that has overseen ten AI software integrations, two decommissions, and one decision to not integrate (Liao et al.). Additional information about the use cases and key takeaways are presented in Table 1.
AI software lifecycle stages
The research topic features manuscripts that contribute insights related to all four AI software lifecycle stages (problem definition and solution procurement, development and adaptation, technical and clinical integration, and lifecycle management). Two manuscripts describe programs that span all lifecycle stages, including the implementation of an AI quality management system at University Medical Center in Ultrecht, Netherlands and an AI organizational governance process at University of Wisconsin in Madison, USA. Two manuscripts present different frameworks for AI solution development, technical and clinical integration, and lifecycle management. A team from The Hospital for Sick Children in Toronto, Canada presents an approach that adopts language from systems engineering, while a team from University College London in the UK presents an approach that adopts language from therapeutics development (Assadi et al.). Two manuscripts present case studies focused on technical and clinical integration, including an adult deterioration model integrated at St. Michael's Hospital in Toronto, Canada, and a falls risk model integrated at University of Wisconsin in Madison, USA (Pou-Prom et al.). Lastly, three manuscripts present best practices related to specific lifecycle stages. A team from The Hospital for Sick Children in Toronto, Canada describes the use of AI software silent trials during technical integration (Kwong et al.), a team from Stanford Health Care in Palo Alto, USA describes reliability and fairness audits during lifecycle management (Lu et al.), and a team from Vanderbilt Health describes AI solution monitoring and updating during lifecycle management (Davis et al.).
Team composition
In some ways, the research topic authorship teams are similar. All manuscripts feature interdisciplinary teams at academic health centers and graduate students and clinical trainees made significant contributions as co-authors. All manuscripts include clinical and technical expert co-authors. And lastly, all manuscripts build on prior work from authorship teams who have previously published AI solution validation studies.
In other ways, the research topic authorship teams are heterogeneous. The smallest teams were a group of three clinicians and informaticians at Vanderbilt Health who describe AI software monitoring and updating challenges and a group of four engineers, public health experts, and clinicians who describe the AI software organizational governance model at University of Wisconsin. The largest team was a group of twenty-seven engineers, bioinformaticians, managers, public health experts, biological science experts, and clinicians who conducted fairness and robustness audits of multiple models at Stanford Health Care. All teams included experts with formal training in at least three of the disciplines listed in Table 1 and two teams included experts with formal training in six disciplines. Among the 73 authors who contributed to the research topic, two perspectives were unique. There was a single AI ethics expert from The Hospital for Sick Children in Toronto, Canada and there was a senior data scientist at University Medical Center in Utrecht, Netherlands who is also a clinical microbiologist who has implemented and audited laboratory quality management systems.
Discussion
The research topic “Surfacing Best Practices for AI Software Development and Integration in Healthcare” features a remarkably diverse set of insights and learnings from teams around the globe integrating and using AI software into practice (12). Throughout the research topic, teams consistently describe responses to unexpected challenges encountered in the transition from conducting AI software research to translating a technology into practice. The success of AI in healthcare hinges on the ability to adapt and transition from research into routine clinical practice. Sharing challenges, failures and describing promising approaches that were implemented in real-world settings can inform teams around the globe looking to advance the use of AI software in healthcare.
Across the research topic, consensus emerged around three important AI software integration practices. First, many teams highlighted the importance of simulating AI software performance in local, operational settings prior to initial use in clinical care. One method discussed in multiple articles involved the operationalization of a “silent trial,” during which bedside clinicians are initially blinded to the AI software as it is prospectively applied on operational data. While not novel, consensus is emerging around the importance of this activity (13–15). Silent trials can alert AI software developers to potential patient safety risks, bias, or integration concerns prior to clinical testing in a manner that minimizes risk to patients. Another article described the creation of a synthetic clinical deployment environment that anticipates real-world clinical decision making (Harris et al.).
Second, many teams highlighted the importance of AI software governance and management. Articles highlighted the importance of transdisciplinary teams and the need to assign responsibility and accountability to oversee AI software performance and appropriate use. One team used international standards to create a quality management system for AI software lifecycle management (Bartels et al.). Manuscripts in the research topic build upon existing frameworks and broaden the focus from AI software manufacturers to humans within health systems who oversee AI software used in clinical settings. The frameworks complement national efforts to equip the healthcare workforce to effectively adopt AI (16).
Lastly, many teams highlighted the importance of ongoing AI software monitoring and auditing. Some articles used existing standards for evaluating AI, including Health Canada/FDA/MHRA Joint Statement on 10 guiding principles for Good Machine Learning Practices (GMLP), however real-world experience led to additional recommendations, such as emphasizing user engagement, utilizing a silent trial, and creating downtime protocols. Another team described periodic reliability and fairness audits that went beyond quantitative comparison of AI software performance across demographic subgroups to also include stakeholder interviews to better understand the impact of the AI software.
While consensus emerged on the themes described above, the research topic did surface divergent perspectives on the importance of interpretability and explainability of AI software. For example, the teams at University of Wisconsin and University College London explicitly promote the use of explainable models. One team explained that “a desire to ensure we had an interpretable model further influenced our choice to pursue regression rather than tree-based models (Engstrom et al.).” The other team explained that “most AI models that operate as “black-box models” are unsuitable for mission-critical domains, such as healthcare, because they pose risk scenarios where problems that occur can remain masked and therefore undetectable and unfixable” (Harris et al.). This perspective offers a contrasting view from prior work examining the use of “black-box models” in clinical care (17), the limitations of current explainability methods (18), and the approach of regulators at the U.S. Food and Drug Administration (19). The research topic exposes the urgent need for research and policies that help organizations understand whether or not to prioritize AI software interpretability and explainability.
Future directions
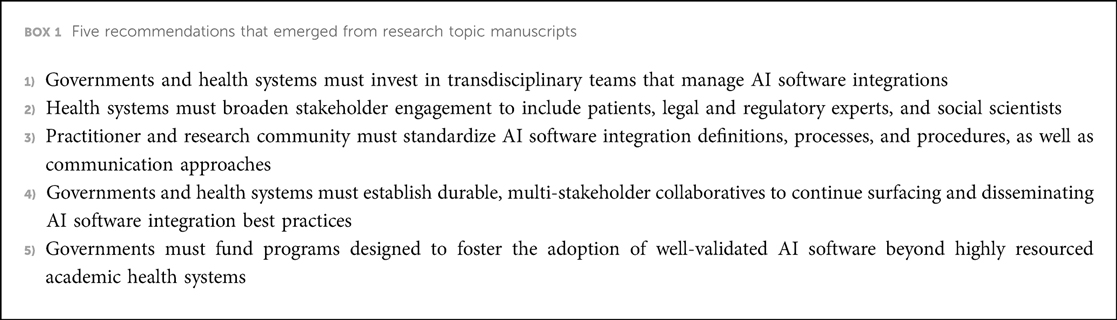
The research topic reveals five important opportunities to advance AI software integration in health care, summarized in Box 1. First, governments and health systems must invest in building and sustaining transdisciplinary teams that manage AI software integrations. Best practices did not emerge from the heroic acts of individual scientists, but rather from transdisciplinary teams of experts working with health systems. These types of roles are often funded through health system operations and require significant investment.
Second, health systems must broaden stakeholder engagement throughout the AI software lifecycle. Unfortunately, only a single instance of direct patient engagement was described in the research topic, occurring at The Hospital for Sick Children in Toronto, Canada. Otherwise, there was limited patient and community engagement. And while the research topic authors were diverse, there was minimal representation of legal and regulatory experts and social scientists. These perspectives are crucial to ensure that AI software integration aligns with rapidly evolving regulations, and unintended consequences of AI software integration and use are anticipated, identified, and mitigated.
Third, there is an urgent need to develop and formalize standard AI software integration definitions, processes, and procedures as well as communication approaches (20). The research topic features teams that used language from different disciplines to describe AI software integration, including drug discovery, systems engineering, and international quality management standards. While it's important to build upon existing work across disciplines, the multiplicity of terms creates unnecessary ambiguity and confusion. Precisely defined steps and procedures need to be specified for rapid diffusion of more mature best practices, such as the “silent trial”.
Fourth, durable, multi-stakeholder collaboratives are needed to continue surfacing and disseminating AI software integration best practices. Efforts that we are directly involved in to achieve this aim are the Health AI Partnership (21) to disseminate best practices across health systems and the development of AI software reporting standards, including DECIDE-AI (22), CONSORT-AI (23), STARD-AI (24), and SPIRIT-AI (25).
Fifth, the research topic highlights the importance of fostering the adoption of well-validated AI software beyond highly resourced academic health systems. Persistence of the status quo, where AI software is best integrated within settings with the most expertise, will undermine the potential benefit of AI software. Business models and public sector programs must be designed to enable academic health systems to support smaller under-resourced settings that do not have the internal capabilities to utilize AI software most effectively. One research topic manuscript described a promising approach: “For smaller entities, such as a single general practitioner, this effort [to establish an AI software quality management system] seems unfeasible. In this situation, complete dependence on the manufacturer is imaginable, making it difficult to establish truly safe performance. Again, inspiration can be found in the regional services of medical laboratories that very often provide access to competences and resources for safe application of diagnostics. Regional AI labs could provide services for the development, acquisition, and quality control of AI/ML for smaller healthcare institutes including general practitioners (Bartels et al.).” Programs that test different approaches of regional, multi-institutional support are urgently needed to ensure equitable diffusion of AI software.
Conclusion
The research topic “Surfacing Best Practices for AI Software Development and Integration in Healthcare” successfully surfaced best practices from 7 organizations across 4 countries. All teams were based at academic health systems and had previously published AI software validation studies. The research topic features insights across the AI software integration lifecycle and contributing authors represent diverse domains of expertise. There was consensus around the importance of local evaluations of AI software in a “silent trial”, establishing organizational governance structures for AI software, and monitoring of technologies post-integration. However, the research topic also exposed limitations of current work and we present five recommendations to further advance AI software integration across settings. We hope our work informs AI software developers and policy makers and contributes to future efforts to broadly engage stakeholders in multi-institutional learning collaboratives.
Author contributions
MPS and DV wrote the first draft. All authors contributed to both the subsequent drafting and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
MPS and SB are co-inventors of intellectual property licensed by Duke University to Clinetic, Inc. and Cohere-Med, Inc. MPS and SB hold equity in Clinetic, Inc. MPS and SB receive funding from the Gordon and Betty Moore Foundation, Patrick J McGovern Foundation, and NIH. KS's institution receives grant funding from Teva Pharmaceuticals and Blue Cross Blue Shield of Michigan for unrelated work, and KS serves on an advisory board for Flatiron Health. XL receives funding from the Wellcome Trust, the National Institute of Health Research/ NHSX/Health Foundation, the Alan Turing Institute, the MHRA, and NICE.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou Q, Chen ZH, Cao YH, Peng S. Clinical impact and quality of randomized controlled trials involving interventions evaluating artificial intelligence prediction tools: a systematic review. Npj Digit Med. (2021) 4(1):154. doi: 10.1038/s41746-021-00524-2
2. Plana D, Shung DL, Grimshaw AA, Saraf A, Sung JJY, Kann BH. Randomized clinical trials of machine learning interventions in health care. JAMA Netw Open. (2022) 5(9):e2233946. doi: 10.1001/jamanetworkopen.2022.33946
3. Lam TYT, Cheung MFK, Munro YL, Lim KM, Shung D, Sung JJY. Randomized controlled trials of artificial intelligence in clinical practice: systematic review. J Med Internet Res. (2022) 24(8):e37188. doi: 10.2196/37188
4. Parikh RB, Helmchen LA. Paying for artificial intelligence in medicine. Npj Digit Med. (2022) 5(1):63. doi: 10.1038/s41746-022-00609-6
5. Taylor-Phillips S, Seedat F, Kijauskaite G, Marshall J, Halligan S, Hyde C, et al. UK National screening committee’s approach to reviewing evidence on artificial intelligence in breast cancer screening. Lancet Digital Heal. (2022) 4(7):e558–65. doi: 10.1016/S2589-7500(22)00088-7
6. The Artificial Intelligence in Health and Care Award. Available at: https://transform.england.nhs.uk/ai-lab/ai-lab-programmes/ai-health-and-care-award/ (Accessed January 21, 2023).
7. Dean NC, Vines CG, Carr JR, Rubin JG, Webb BJ, Jacobs JR, et al. A pragmatic stepped-wedge, cluster-controlled trial of real-time pneumonia clinical decision support. Am J Resp Crit Care. (2022) 205(11):1330–6. doi: 10.1164/rccm.202109-2092OC
8. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. (2020 Nov 12) 383(20):1951–60. doi: 10.1056/NEJMsa2001090
10. Finlayson SG, Subbaswamy A, Singh K, Bowers J, Kupke A, Zittrain J, et al. The clinician and dataset shift in artificial intelligence. New Engl J Med. (2021) 385(3):283–6. doi: 10.1056/NEJMc2104626
11. Wong A, Otles E, Donnelly JP, Krumm A, McCullough J, DeTroyer-Cooley O, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. (2021) 181(8):1065–70 . doi: 10.1001/jamainternmed.2021.2626
12. Surfacing Best Practices for AI Software Development and Integration in Healthcare. Available at: https://www.frontiersin.org/research-topics/28021/surfacing-best-practices-for-ai-software-development-and-integration-in-healthcare (Accessed January 21, 2023).
13. McCradden MD, Anderson JA, Stephenson EA, Drysdale E, Erdman L, Goldenberg A, et al. A research ethics framework for the clinical translation of healthcare machine learning. Am J Bioeth. (2022) 22(5):8–22. doi: 10.1080/15265161.2021.2013977
14. Wiens J, Saria S, Sendak MP, Ghassemi M, Liu VX, Doshi-Velez F, et al. Do no harm: a roadmap for responsible machine learning for health care. Nat Med. (2019) 25:1337–40. doi: 10.1038/s41591-019-0548-6
15. Sendak MP, Ratliff W, Sarro D, Alderton E, Futoma J, Gao M, et al. Real-World integration of a sepsis deep learning technology into routine clinical care: implementation study. JMIR Med Inform. (2020) 8(7):e15182. doi: 10.2196/15182
16. Horizon Scanning. Available at: https://digital-transformation.hee.nhs.uk/building-a-digital-workforce/dart-ed/horizon-scanning (Accessed January 21, 2023).
17. Sendak MP, Elish MC, Gao M, Futoma J, Ratliff W, Nichols M, et al. The human body is a black box”: supporting clinical decision-making with deep learning. FAT* ‘20: conference on fairness, accountability, and transparency; vol. 44 (2020). p. 99–109. Available from: https://dl.acm.org/doi/pdf/10.1145/3351095.3372827?download=true
18. Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit Health. (2021) 3(11):e745–50. doi: 10.1016/S2589-7500(21)00208-9
19. Ross C. A “disaster”, or a “clear path” forward?: New FDA guidance on AI in medicine sparks strong reactions. STAT News. (2022). Available from: https://www.statnews.com/2022/09/28/fda-artificial-intelligence-tools-regulation-oversight/ (Accessed January 21, 2023).
20. Sendak MP, Gao M, Brajer N, Balu S. Presenting machine learning model information to clinical end users with model facts labels. npj Digit Med. (2020) 3(41):1–4. doi: 10.1038/s41746-020-0253-3.32219182
21. Duke Institute for Health Innovation. Health AI Partnership: an innovation and learning network for health AI software. (2021). Available from: https://dihi.org/health-ai-partnership-an-innovation-and-learning-network-to-facilitate-the-safe-effective-and-responsible-diffusion-of-health-ai-software-applied-to-health-care-delivery-settings/ (Accessed January 21, 2023).
22. Vasey B, Clifton DA, Collins GS, Denniston AK, Faes L, Geerts BF, et al. DECIDE-AI: new reporting guidelines to bridge the development-to-implementation gap in clinical artificial intelligence. Nat Med. (2021) 27(2):186–7. doi: 10.1038/s41591-021-01229-5
23. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK, Keane P. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. (2020) 26:1364–74. doi: 10.1038/s41591-020-1034-x
24. Sounderajah V, Ashrafian H, Aggarwal R, Fauw JD, Denniston AK, Greaves F, et al. Developing specific reporting guidelines for diagnostic accuracy studies assessing AI interventions: the STARD-AI steering group. Nat Med. (2020) 26(6):807–8. doi: 10.1038/s41591-020-0941-1
Keywords: health innovation, health policy, technology regulation, technology diffusion, machine learning, artificial intelligence
Citation: Sendak M, Vidal D, Trujillo S, Singh K, Liu X and Balu S (2023) Editorial: Surfacing best practices for AI software development and integration in healthcare. Front. Digit. Health 5:1150875. doi: 10.3389/fdgth.2023.1150875
Received: 25 January 2023; Accepted: 6 February 2023;
Published: 21 February 2023.
Edited and Reviewed by:
Daniel B. Hier, Missouri University of Science and Technology, United States© 2023 Sendak, Vidal, Trujillo, Singh, Liu and Balu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Sendak bWFyay5zZW5kYWtAZHVrZS5lZHU=
Specialty Section: This article was submitted to Health Informatics, a section of the journal Frontiers in Digital Health
 Mark Sendak
Mark Sendak David Vidal
David Vidal Sylvia Trujillo
Sylvia Trujillo Karandeep Singh
Karandeep Singh Xiaoxuan Liu
Xiaoxuan Liu Suresh Balu
Suresh Balu