- 1Department of Social Work, Education and Community Wellbeing, Northumbria University, Newcastle upon Tyne, United Kingdom
- 2Institute for Collective Place Leadership, Teesside University, Middlesbrough, United Kingdom
- 3Department of Mathematics Physics and Electrical Engineering, Northumbria University, Newcastle upon Tyne, United Kingdom
- 4School of Informatics, University of Edinburgh, Edinburgh, United Kingdom
- 5Newcastle University Translational & Clinical Research Institute, Newcastle upon Tyne, United Kingdom
- 6Department of Psychology, Northumbria University, Newcastle upon Tyne, United Kingdom
- 7Population Health Sciences Institute, Newcastle University, Newcastle upon Tyne, United Kingdom
- 8College of Medical, Veterinary & Life Sciences, University of Glasgow, Glasgow, United Kingdom
Objectives: To pilot and assess the feasibility of a fully remote effectiveness evaluation of a novel smartphone self-management app for people living with Sjögren disease (SjD), including evaluating trial procedures and app engagement.
Methods: We conducted a double-blind, randomised, fully-remote pilot feasibility of a self-management smartphone app (Sjogo) containing interactive components with an information-only control app. After completing onboarding procedures, participants were allocated to a trial arm following download from Apple App and Google Play stores. Participants completed symptoms and quality of life measures at baseline and (at two further timepoints (5–7 and 10–13 weeks) after download. Engagement with the app was measured with number and duration of logins.
Results: 996 participants downloaded Sjogo to their smartphone. 871 (87.45%) consented to take part in the study and 617 (61.95%) completed the onboarding procedures and baseline measures and were randomised to the full-version of the app (n = 318) or control-version (n = 299). In-app randomisation produced balanced groups. In week 1 engagement was higher in the intervention group m = 4.76 logins (S.D. 8.06) than the control group m = 3.47 (S.D. 2.75). At week 2 engagement dropped in both groups (intervention group m = 1.17, SD 4.56, control m = 0.40, SD 0.93). Outcome completion rates at subsequent timepoints were 36.63% (weeks 5–7) and 27.39% (weeks 10–13).
Conclusion: It is feasible to collect data fully remotely, automate trial procedures, and recruit participants to a randomised controlled trial of a self-management smartphone app for people with SjD through app stores. However, app engagement and outcome completion rates could be improved.
Introduction
Sjögren disease (SjD) is a common autoimmune disease with a prevalence rate of 65 per 100,000 and a female to male ratio incidence of 9–1 peaking around age 50 (1, 2). SjD is complex and while exocrinopathy causing ocular, oral and vaginal dryness is the main feature (3), SjD is also associated with fatigue, pain and sleep disturbances and impacts on daily activities and quality of life (4, 5). SjD has previously been defined as presenting independently [as primary Sjögren's Syndrome (SS)] or in association with another autoimmune disease, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE), and known as secondary SS (6).
Despite many SjD patients experiencing functional disability (7), current medical care mainly focusses on pharmacological interventions for classic symptoms which are only partially effective (8). Few non-pharmacological interventions exist to help people live well with their condition and improve quality of life (9).
Our previous work indicates that SjD patients need access to support outside of medical review appointments to empower them to self-manage symptoms of dryness, fatigue, pain and sleep disturbances (10, 11). Our qualitative work exploring potential evidence-based SjD interventions and their mode of delivery (11, 12) indicated that digital interventions, such as smartphone apps containing appropriate self-management support and behaviour change techniques (13), could be beneficial, especially for those lacking face-to-face symptom management support (12).
Self-management apps are a promising adjunct to clinical care for people with rheumatic diseases. They show high levels of usability and acceptability among young people with juvenile idiopathic arthritis (14) and favourable outcomes for rheumatoid arthritis patients (15). However, many apps designed, developed and evaluated by academics and practitioners are not made publicly available (16), and commercial apps may lack regulated, evidence-based content, such those targeting lower back pain (17). Consequently, people with rheumatic conditions, including SjD, often lack access to evidence-based apps for self-managing their symptoms outside the clinic.
The translation of rheumatology app intervention research for public benefit is further hindered by evaluation settings. Promising randomised controlled trials of self-management apps for rheumatic diseases (15) often lack external validity, not representing real-world populations and contexts. In-person trial procedures and participant payments and incentives may artificially boost engagement (18) leading to disappointing results in subsequent implementation trials. This is particularly likely for smartphone apps for which engagement is particularly low (19).
Pragmatic implementation-effectiveness trial designs can save time and improve efficiency by assessing real-world implementation alongside effectiveness (20). Early testing of adoption and engagement allows for further development and optimisation for the intended delivery setting. For self-management apps, this can mean through online marketplaces like the Apple App Store and Google Play, with increasing use by people with immune-mediated inflammatory diseases (21). These platforms have facilitated fully-remote pragmatic trials of public health apps (22, 23). To date, and to our knowledge, no self-management app for SjD has undergone feasibility testing in a pragmatic trial. A pilot feasibility design was chosen to test this complex intervention and the RCT protocol before potentially progressing to a fully powered trial (24).
In this study, we assessed the feasibility of a fully-remote and automated trial of a novel evidence-based self-management smartphone application for SjD (Sjogo). When developing the app, we followed the Medical Research Council guidance for complex intervention research (24, 25) and incorporated the European Alliance of Associations for Rheumatology recommendations on self-management in inflammatory arthritis and for developing self-management apps (26, 27). The app was based on self-determination theory (28) and informed by behaviour change techniques (29) and the previous British Society of Rheumatology iteration of the disease guideline (30). It was developed in collaboration with people with SjD who attended a series of eight design workshops (31), and informed by clinicians (rheumatologist, occupational therapist, rheumatology nurse specialist, health psychologist, sleep specialist and dentist). The app was developed with the aim of improving users' skills, knowledge and confidence in self-managing their disease (patient activation) (32) and their quality of life (QOL).
The aim of the study was to pilot and assess the feasibility of a fully-remote automated effectiveness evaluation of the Sjogo self-management app for those living with SS, as it was conducted prior to the most recent guideline (4), which uses the term SjD. Specifically, we tested trial procedures: “in app” automatic randomisation at the point of download, recruitment rates and outcome completion (attrition rates), engagement in the app through recording the number of sessions each participant engaged in and the average length of each session.
Methods
Study design and setting
This intervention study was conducted during 2021. Ethical approvals were obtained from Northumbria University Ethics Committee in November 2020 (reference: 120.1849). The study protocol was prospectively registered at ClinicalTrials.gov (NCT04653935). The study was an automated, double-blind, two-arm, individually randomised pilot feasibility study of the Sjogo app, to test the feasibility of the trial procedures and participant engagement. The Sjogo app (Version 1.0) was released worldwide for 8 weeks on Android Play and Apple iOS app stores in January 2021. No incentives to participate in the study were provided to ensure it was representative of a real-world setting and that people would only download it and use it if they thought it may benefit them. Potential participants who downloaded the app were guided through in-app, fully automated study procedures (eligibility screening, informed consent, symptom and QOL measures). The overarching app contained 2 sub-apps. Participants who downloaded the app were automatically randomised (simple randomisation) after completing the onboarding procedures (including eligibility screening, consent and completion of baseline measures), to an information-version (control) or the full-version of the Sjogo app. They were asked to complete outcome measures at baseline (T1), 5 (T2) and 10 weeks (T3). Push notifications were triggered, and email reminders were sent at 5 weeks and 10 weeks after downloading the app. Participants had very little contact with the researchers but could contact them via email with any technical queries. The study design and reporting were in accordance with the Consolidated Standards of Reporting Trials (CONSORT) (33) the CONSORT EHealth Checklist (34) and the CONSORT extension for pilot and feasibility trials (35).
User involvement
People with SjD were involved at various stage of the trial, including the development of the smartphone app and its content at a series of user-centered workshops, and study design of the feasibility trial (31). We received input from people with SjD through a user-led organisation (North-East Sjögren's Syndrome Association) and a member of this organisation was a collaborator (MH).
Recruitment
Potential participants (adults over 18 with SjD) were alerted to the trial through social media (Twitter, now known as X) and through two UK-based patient support groups (via email newsletter and Facebook). The term SS was used during the study, as this term was commonly used by patient groups at the time of recruitment. From the advertisements, potential participants were guided to the Google Play or Apple iOS app stores where they were able to download the Sjogo app. The app store descriptor of Sjogo explained that the app was developed by researchers for people with primary or secondary SS as part of a study. The descriptor further stated that if participants were eligible, after consenting to take part, they would be randomised to receive either a control or a full version of the app. The recruitment process started on 30th December 2020 for Android users, when the Android version of the Sjogo app was published to Google Play Store. Recruitment started for iOS users when Sjogo was published to the Apple App Store on 5th January 2021.
Eligibility screening
Inclusion in the study was based on the following criteria: Being over 18, a diagnosis of SS by a doctor (with an option indicate either primary or secondary SS). Respondents who did not meet these criteria were thanked for their time but were unable to proceed any further within the app. Those who were under 18 and without a self-reported diagnosis of SS made by a clinician, were therefore excluded from the study.
Consent
Participants fulfilling the inclusion criteria were guided through the in-app participant information sheet, prior to reaching the area in the app where they could provide their consent to take part in the study. Potential participants were provided with contact information (email address) of the research team and were given the opportunity to ask any questions prior to consenting. Participants were free to withdraw from the study at any time without giving a reason.
Baseline data collection
As part of the onboarding procedures, the following baseline data was collected: sex, age, diagnosis of primary or secondary SS, years since diagnosis, device type (tablet or smartphone) and operating system (iOS or Android) and country from which the participant downloaded the app. Device identifiers were not collected.
Intervention
App description
Two versions of the app were developed: a full “active” version of the app and an “information only” control (See Supplementary Material S1). In brief, the active app contained multiple behaviour change techniques (theory driven strategies or methods used to modify behaviour) (29) within the following 5 components: About Sjögren's Syndrome (symptom and lifestyle information), Energy Management, Goal Setting, Managing Difficult Times and Assertiveness and Communication Skills. It included a retrospective activity diary for logging and appraising daily activities based on energy demand. This data was compiled into weekly energy charts for users to review and plan their activities. The active app also prompted users to set SMART goals based on their values and managed through an in-app prospective planning diary. The app also included guidance for managing flare-ups, relaxation and sleep techniques, and assertive and communication exercises.
The communication style within the app text was carefully considered with validating language, key points were framed as a dialogue to facilitate interactivity and reflection, and app components were accompanied by a treatment rationale to increase user buy-in (31).
The information-only control app was created solely from the “About Sjögren's Syndrome” section of the active app, where extensive information on the condition and symptoms was provided. It contained one behaviour change technique - Information about Antecedents (providing information about situations, events, emotions, cognitions which reliably predict performance of behaviour) (29).
Both active and control apps were stand-alone and apart from the in-app instructions on how to navigate the app, no additional training was given to users. The app was intended to be used ad libitum. No recommendations were provided regarding timings, frequency, or intensity of use.
Randomisation
Consenting users that completed baseline measures were automatically randomised to either the active or control version of the app using simple randomisation within the app (1:1). Users were aware they were being randomised but were blinded to which version of the app they were allocated to. Participants were not stratified based on age or sex. After submitting their in-app baseline measures, the following screen participants saw depended on which version they had been assigned to: users arrived at either the main page of the active version (where they could access all 5 components), or the main page of the information-only control version. Investigators were also blinded to group allocation.
Measures
The outcomes collected were quality of life (ICECAP-A) (36), global symptom severity [EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) - including single 0–10 measurements of pain, dryness and general fatigue] (37), physical and mental fatigue (numeric visual analogue scale (VAS) based on the Profile of Fatigue and Dryness (PROFAD) (38), depression (numeric VAS), anxiety (numeric VAS), sleep (numeric VAS), impact of fatigue [Modified Fatigue Impact Scale-5-item version (MFIS-5)], Sleep Condition Indicator (SCI) (39) and patient activation (Patient Activation Measure 10 (PAM10) (32). Participants were asked to complete these measures at baseline (T1), 5-weeks (T2) and 10-weeks (T3) post app download. Further details of the selected measures are included in Supplementary Material S2. In addition, the number of participant logins to the app (sessions) and the length of each session were collated. Completion rates were calculated as the number of participants who completed all questions in all surveys at each time point.
At Weeks 5 and 10 the outcome measures became available to participants with a 2-week and 3-week data collection window at Timepoints 2 and 3 (T2 and T3) respectively to maximise engagement. The surveys were closed for analysis 13 weeks after the last user completed the onboarding and consent procedures. Completion rates were calculated as the number of participants who completed all questions in all surveys at each time point. Completion rates for T3 were calculated separately from T2 (independently not cumulatively).
Statistical analysis
Descriptive statistics were generated for demographic variables and engagement data. Continuous variables were described using means, standard deviation and interquartile range. Categorical variables were expressed as percentages. To examine if there are significant differences in users' demographic variables between the intervention and control versions, we used chi-square test for categorical variables and t-test for continuous ones, with critical level alpha = 5%. Statistical analyses were performed using Stata v17.
Results
Participants
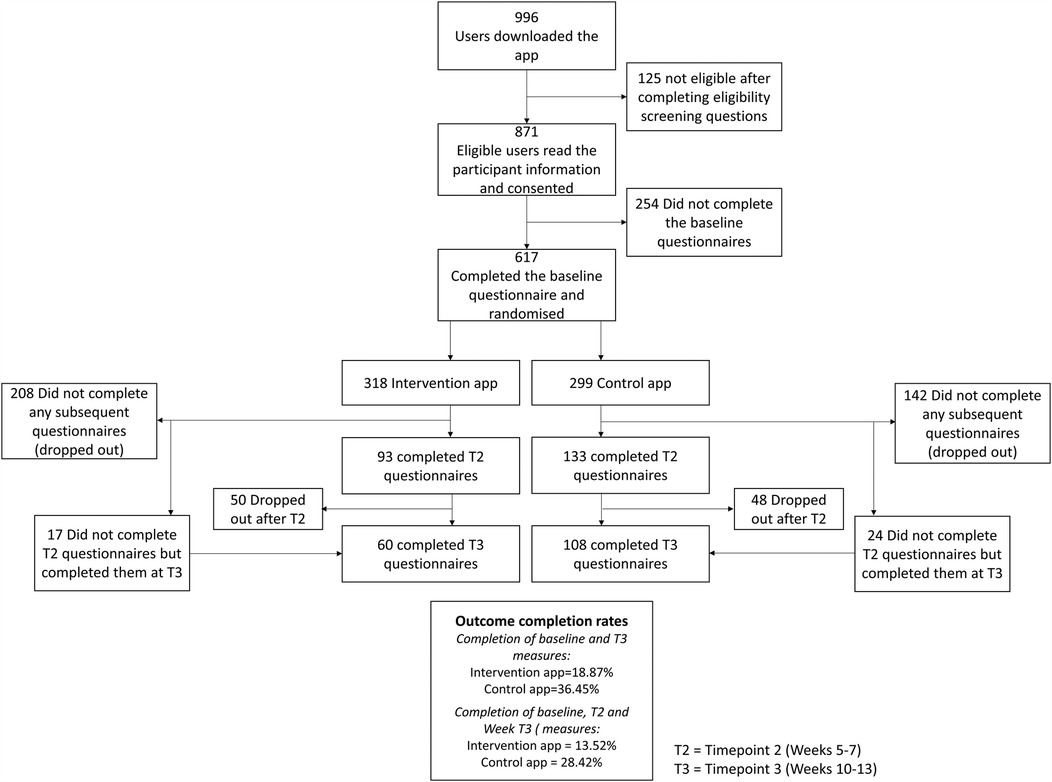
Over 8 weeks in January and February 2021, 996 participants from 33 countries downloaded Sjogo to their smartphone via the Google Play and iOS Apple Stores. Of these, 871 consented to take part in the study and 617 completed the onboarding procedures, completed the baseline measures, and were randomised to the full-version of the app (n = 318) or control-version (n = 299). The flowchart of participants is shown in Figure 1.
Participants were mostly iOS users (55.11%), female (95.62%), from the UK (54.62%) or USA (28.92%) with a mean age of 50.97 (SD = 13.75; Range = 18–84). A breakdown of demographic data for participants in each condition can be seen in Table 1.
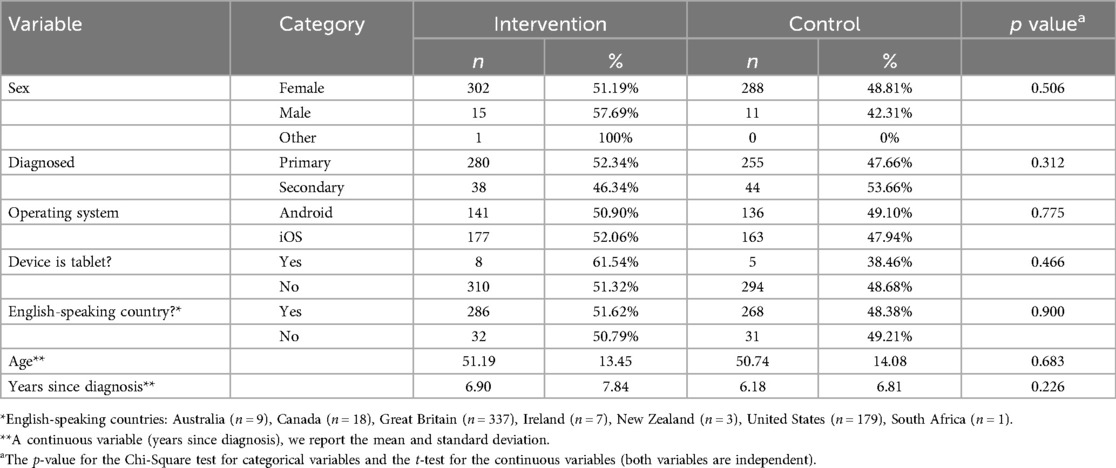
Table 1. In-app automatic randomisation of participants based on participants’ characteristics at baseline.
Feasibility of in-app automatic randomisation
The automatic randomisation of participants can be seen in Table 1. Participants in each condition did not significantly differ on age, sex, diagnosis type (primary SS or secondary SS) or years since diagnosis (all p > 0.20). Additionally, participants in each condition did not significantly differ on operating system or device type used to access the app (p > 0.20).
Outcome completion rates
Overall outcome completion rates were 36.63% at T2 and 27.39% at T3. For the full version, completion rates were 29.24% at T2 and 13.52% at T3, while the control version had rates of 44.48% at T2 and 28.42% at T3. Some participants ignored T2 prompts but went on to complete outcomes when prompted again at T3. Eighty-three participants (23.71%) were considered dropouts, having not completed any outcomes after baseline. Dropout rates were higher in the intervention group (n = 208) than the control group (n = 142). Participants who dropped out were significantly younger (M = 49.33, SD = 13.79) than those who remained (M = 53.12, SD = 13.43, p = 0.0003). There were no sex differences (p = 0.68) or differences in PAM scores (p = 0.46) between dropouts and those who remained. Those with higher ESSPRI scores were more likely to drop out (M = 6.48, SD = 1.73) compared to those who stayed (M = 6.10, SD = 1.73, p = 0.0067). No differences in individual symptom scores were observed between those who dropped out and those who remained.
Changes in outcomes from baseline
Mean scores for each outcome measure at each time point (baseline, T2 and T3) are reported in Table 2.
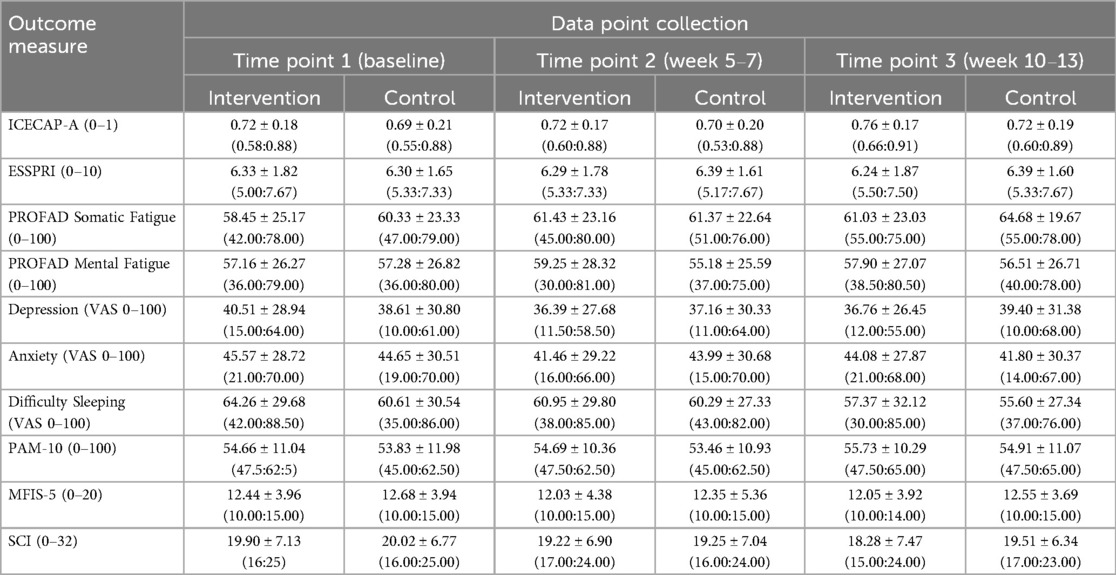
Table 2. Mean scores for each outcome measure across three data point collections (time points 1, 2 and 3). The numbers presented are the mean ± standard deviation, and the inter-quartile range (p25 and p75 in the parenthesis).
Engagement with the Sjogo app
Participants in the intervention group logged into the Sjogo app more frequently (M = 11.78, SD = 37.60) than controls (M = 6.97, SD = 7.11, p = 0.03). They also used the app for a longer total duration (M = 57.56 min, SD = 160.5) compared to the control group (M = 33.35 min, SD = 32.20, p = 0.01).
Engagement with both apps was highest in the first week (Figure 2) and declined thereafter, with the largest drop between weeks 1 and 2. In the intervention group, 318 participants accessed Sjogo an average of 4.76 times (SD = 8.06) in Week 1, including an outlier who accessed it 101 times. By Week 2, the average number of accesses fell to 1.17 times (SD = 4.56). In the control group, 299 participants accessed the information-only app an average of 3.47 times (SD = 2.75) in Week 1, dropping to 0.40 times (SD = 0.93) in Week 2.
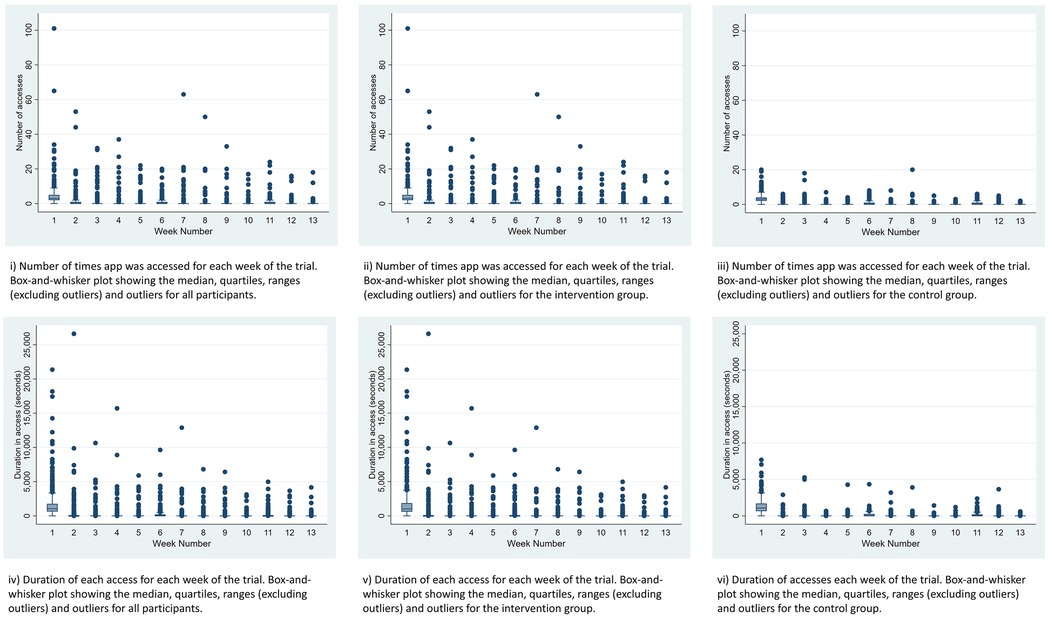
Figure 2. Box plots showing the number of accesses and duration of access for both apps, the intervention app and the control app for the duration of the study.
No adverse events were reported by any participants during the study.
Discussion
This study is the first to assess the feasibility of a fully remote effectiveness evaluation of a smartphone self-management app (Sjogo) for those living with SS.
We have demonstrated the feasibility of recruiting many participants with SS to a self-management app study via the Google Play and Apple App Stores. With minimal advertising, we achieved nearly 1,000 downloads, with over 600 users consenting and providing baseline measures. This efficient methodology demonstrates significant demand for a “direct-to-consumer” SS self-management intervention from app stores. The low entry criteria—offering a free app with information and self-management features and no in-person visits—potentially also contributed to the high recruitment (40).
The efficient recruitment suggests a future effectiveness trial could be well-powered; however, our outcome completion rates seemed low in comparison to similar studies (41). The study took place during the COVID-19 pandemic and it possible that participants were distracted with associated events which may in part have affected engagement with the study. Interestingly, completion rates at T2 and T3 were higher in the control group than in the intervention group, contrary to other self-management app studies (42). The control app contained no interactivity beyond information; however, a touchable prompt requesting participants to complete the measures, provided some interactivity (43) and may explain the greater response rates in the control group. Alternatively, simple information might have been sufficient for some participants, and it is possible they stopped engaging with the app after they had accessed it. In the intervention group, prompts may have increased user burden or led to notification fatigue (44) potentially causing participants to ignore or disable notifications.
Our fully-automated randomisation produced two well-balanced groups, similar in sex and average age of onset to the wider SS population (2). Younger participants were more likely to drop out of both study arms, possibly because the app was predominantly developed with older SS patients (31). Participants with greater ESSPRI scores were more likely to drop out, but the difference was not clinically meaningful (45), and there were no differences in individual symptom scores. Interestingly, there was no difference in PAM scores between those who dropped out and those who did not, indicating similar motivation to self-manage their condition (46), making it an unlikely reason for drop out.
Engagement was greater with the intervention app compared with the control, possibly as we co-developed it with people with SS (31) and it contained richer content, and interactive features. However, engagement sharply dropped in both groups at week two and continued to decline, similar to other studies (19). This drop could in part be due to both versions achieving desired outcomes, reducing the need for continued use (40). Alternatively, they may not have been engaging enough to overcome the typically low engagement rates for smartphone app interventions (19). Features that promote sustained engagement include gamification (47) in-app social support with peers/coaches (40) and data sharing with health professionals (48). These features were either not desired by participants during Sjogo app development (e.g., gamification) (31) or were outside the project's scope (e.g., social/health professional support). While a full trial is feasible with the current app version, incorporating social support and/or additional access to self-management coaching may potentially reduce attrition rates in a future full-scale effectiveness study. A qualitative process evaluation with a sample of participants may provide more insights which could be addressed in a future iteration of the app to try and improve engagement.
Limitations and directions for future research
A fully-automated trial of different versions of an app provided an efficient way to implement double-blind testing. To support blinding, we took care not to reveal app features associated with only the intervention version when advertising. However, future studies could measure all users' expectations of improvement to understand the effectiveness of blinding procedures (49).
As with many pragmatic trials aiming for high ecological and external validity, there are trade-offs around control and internal validity. For example, we relied on self-report of a SS diagnosis to take part in the study, which was not verified by the researchers. Furthermore, the achieved sample was dominated by middle-aged women from English-speaking countries (mainly the UK and USA). While this generally reflects age and sex demographics of the SjD population (2), any future trial should seek to reach and recruit the broadest range of SjD participants to ensure generalisability.
In line with the CONSORT guidance for feasibility and pilot trials (35), we did not assess the app's effectiveness, as this pilot feasibility study was not powered for this. Effectiveness, in terms of impact on symptom management, in both the short and longer term, needs to be examined in further studies. Our control condition was a different version of the app that we developed, not an existing intervention like a paper booklet. The appropriate control condition for digital therapeutic apps is an ongoing debate (50). Comparing a static low-maintenance version of the app, with a complex, feature-rich version could reveal whether the latter's added effort is justified.
High loss to follow-up was observed, particularly in the intervention group, indicating possible differential attrition bias. This may have been partly due to the greater interactivity with the intervention app in comparison to the text-based control app, which may have caused an element of “notification fatigue” (51) in the intervention group. Another possible factor may have been the number of outcome measures participants were asked to complete. We have conducted qualitative interviews with participants from both groups, and the analysis of these process evaluation data will give more insights into streamlining the app and the trial procedures. However, prioritising key quality of life measures in future trials may help reduce respondent burden.
Whilst our app was developed for an English-speaking audience and mainly involved participants from high-income countries (UK and USA), future intervention development work would be required to adapt this app for diverse groups before any future context-specific feasibility study or evaluation of effectiveness.
Conclusions
It is feasible to recruit participants to a fully remote RCT of a self-management smartphone app for SjD. While trial procedures were successful, outcome completion needs improvement. Researchers should account for high early attrition rates. Enhancing app features desired by people with SjD may boost both app engagement and outcome completion.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Northumbria University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because informed consent was provided digitally within the app during the onboarding process.
Author contributions
KH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Conceptualization, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. EP: Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. JV: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing. DL: Formal analysis, Writing – review & editing. JM: Writing – original draft, Writing – review & editing. TR: Conceptualization, Writing – review & editing. JE: Conceptualization, Writing – review & editing. VD: Conceptualization, Writing – review & editing. EM: Conceptualization, Methodology, Writing – review & editing. CM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by a grant from Versus Arthritis (ref: 22026).
Acknowledgments
We would like to thank the following clinicians who reviewed the app content: Dr Elizabeth Price, Dr Helen Cartner and Julie Norris. In addition, we are grateful for the input and support from the Northeast Sjögren's Syndrome Association, particularly Michelle Harrison, and the British Sjögren's Syndrome Association. We would like to thank Parvin Asadzadeh Birjandi for her work on developing and maintaining the app during the trial period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1549093/full#supplementary-material
References
1. Holdgate N, St Clair EW. Recent advances in primary Sjogren’s syndrome. F1000Res. (2016) 5:1–10. doi: 10.12688/f1000research.8352.1
2. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjogren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74(11):1983–9. doi: 10.1136/annrheumdis-2014-205375
3. Mariette X, Criswell LA. Primary Sjogren’s syndrome. N Engl J Med. (2018) 379(1):97. doi: 10.1056/NEJMcp1702514
4. Price EJ, Benjamin S, Bombardieri M, Bowman S, Carty S, Ciurtin C, et al. British Society for rheumatology guideline on management of adult and juvenile onset Sjögren disease. Rheumatology. (2025) 64(2):409–39. doi: 10.1093/rheumatology/keae152
5. Mardale DA, Opriș-Belinski D, Bojincă V, Bojincă M, Mazilu D, Păsăran E, et al. The physical and psychosocial impact of fatigue among patients with Sjogren’s syndrome: a systematic review. J Clin Med. (2024) 13(6):1537. doi: 10.3390/jcm13061537
6. Felten R, Meyer A, Gottenberg J-E. Non-primary Sjogren’s syndrome: secondary or associated? Joint Bone Spine. (2023) 90(2):105502. doi: 10.1016/j.jbspin.2022.105502
7. Schoon H, Slack E, Pearce M, Ng WF, Hackett KL. Activity interference in patients with Sjögren’s syndrome: a cross-sectional study of 149 patients in the UK. Rheumatology. (2022) 61(10):4065–75. doi: 10.1093/rheumatology/keac053
8. Fox RI, Fox CM, Gottenberg JE, Dörner T. Treatment of Sjögren’s syndrome: current therapy and future directions. Rheumatology (Oxford). (2021) 60(5):2066–74. doi: 10.1093/rheumatology/kez142
9. Hackett KL, Deane KH, Strassheim V, Deary V, Rapley T, Newton JL, et al. A systematic review of non-pharmacological interventions for primary Sjogren’s syndrome. Rheumatology (Oxford). (2015) 54(11):2025–32. doi: 10.1093/rheumatology/kev227
10. Hackett KL, Deane KHO, Newton JL, Deary V, Bowman SJ, Rapley T, et al. Mixed-methods study identifying key intervention targets to improve participation in daily living activities in primary Sjogren’s syndrome patients. Arthritis Care Res (Hoboken). (2018) 70(7):1064–73. doi: 10.1002/acr.23536
11. Hackett KL, Deary V, Deane KH, Newton JL, Ng WF, Rapley T. Experience of sleep disruption in primary Sjogren’s syndrome: a focus group study. Br J Occup Ther. (2018) 81(4):218–26. doi: 10.1177/0308022617745006
12. Hackett KL. Developing a non-pharmacological intervention model to improve function and participation in people with primary Sjögren’s syndrome. Institute of Cellular Medicine, Newcastle University: Unpublished (2017).
13. Whitehead L, Seaton P. The effectiveness of self-management Mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res. (2016) 18(5):e97. doi: 10.2196/jmir.4883
14. Cai RA, Beste D, Chaplin H, Varakliotis S, Suffield L, Josephs F, et al. Developing and Evaluating JIApp: Acceptability and Usability of a Smartphone App System to Improve Self-Management in Young People With Juvenile Idiopathic Arthritis (2017).
15. Rodríguez Sánchez-Laulhé P, Luque-Romero LG, Barrero-García FJ, Biscarri-Carbonero Á, Blanquero J, Suero-Pineda A, et al. An exercise and educational and self-management program delivered with a smartphone app (CareHand) in adults with rheumatoid arthritis of the hands: randomized controlled trial. JMIR Mhealth Uhealth. (2022) 10(4):e35462. doi: 10.2196/35462
16. Najm A, Gossec L, Weill C, Benoist D, Berenbaum F, Nikiphorou E. Mobile health apps for self-management of rheumatic and musculoskeletal diseases: systematic literature review. JMIR Mhealth Uhealth. (2019) 7(11):e14730. doi: 10.2196/14730
17. Didyk C, Lewis LK, Lange B. Effectiveness of smartphone apps for the self-management of low back pain in adults: a systematic review. Disabil Rehabil. (2021) 44(25):7781–90. doi: 10.1080/09638288.2021.2005161
18. Pratap A, Neto EC, Snyder P, Stepnowsky C, Elhadad N, Grant D, et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit Med. (2020) 3(1):21. doi: 10.1038/s41746-020-0224-8
19. Baumel A, Muench F, Edan S, Kane JM. Objective user engagement with mental health apps: systematic search and panel-based usage analysis. J Med Internet Res. (2019) 21(9):e14567. doi: 10.2196/14567
20. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. (2012) 50(3):217–26. doi: 10.1097/MLR.0b013e3182408812
21. Romero-Jimenez R, Escudero-Vilaplana V, Chamorro-De-Vega E, Ais-Larisgoitia A, Lobato Matilla ME, Herranz-Alonso A, et al. The characteristics and functionalities of Mobile apps aimed at patients diagnosed with immune-mediated inflammatory diseases: systematic app search. J Med Internet Res. (2022) 24(3):e31016. doi: 10.2196/31016
22. Volkova E, Li N, Dunford E, Eyles H, Crino M, Michie J, et al. “Smart” RCTs: development of a smartphone app for fully automated nutrition-labeling intervention trials. JMIR Mhealth Uhealth. (2016) 4(1):e23. doi: 10.2196/mhealth.5219
23. BinDhim NF, McGeechan K, Trevena L. Smartphone smoking cessation application (SSC app) trial: a multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid ‘app’. BMJ Open. (2018) 8(1):e017105. doi: 10.1136/bmjopen-2017-017105
24. Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. Br Med J. (2021) 374:n2061. doi: 10.1136/bmj.n2061
25. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: New guidance (2006) (09/11/2016). Available at: https://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ (Accessed December 12, 2024).
26. Nikiphorou E, Santos EJF, Marques A, Böhm P, Bijlsma JW, Daien CI, et al. 2021 EULAR recommendations for the implementation of self-management strategies in patients with inflammatory arthritis. Ann Rheum Dis. (2021) 80(10):1278. doi: 10.1136/annrheumdis-2021-220249
27. Najm A, Nikiphorou E, Kostine M, Richez C, Pauling JD, Finckh A, et al. EULAR points to consider for the development, evaluation and implementation of mobile health applications aiding self-management in people living with rheumatic and musculoskeletal diseases. RMD Open. (2019) 5(2):e001014. doi: 10.1136/rmdopen-2019-001014
28. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. (2000) 55(1):68–78. doi: 10.1037/0003-066X.55.1.68
29. Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. (2013) 46(1):81–95. doi: 10.1007/s12160-013-9486-6
30. Price EJ, Rauz S, Tappuni AR, Sutcliffe N, Hackett KL, Barone F, et al. The British Society for Rheumatology guideline for the management of adults with primary Sjögren’s syndrome. Rheumatology (Oxford). (2017) 56(10):1643–7. doi: 10.1093/rheumatology/kex375
31. McCallum C, Campbell M, Vines J, Rapley T, Ellis J, Deary V, et al. A smartphone app to support self-management for people living with Sjögren’s syndrome: qualitative co-design workshops. JMIR Human Factors. (2024) 11:e54172. doi: 10.2196/54172
32. Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. (2005) 40(6 Pt 1):1918–30. doi: 10.1111/j.1475-6773.2005.00438.x
33. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Br Med J. (2010) 340:c332. doi: 10.1136/bmj.c332
34. Eysenbach G. CONSORT-EHEALTH: implementation of a checklist for authors and editors to improve reporting of web-based and mobile randomized controlled trials. Stud Health Technol Inform. (2013) 192:657–61. doi: 10.3233/978-1-61499-289-9-657
35. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Br Med J. (2016) 355:i5239. doi: 10.1136/bmj.i5239
36. Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res. (2012) 21(1):167–76. doi: 10.1007/s11136-011-9927-2
37. Seror R, Ravaud P, Mariette X, Bootsma H, Theander E, Hansen A, et al. EULAR Sjögren’s syndrome patient reported Index (ESSPRI): development of a consensus patient index for primary sjögren’s syndrome. Ann Rheum Dis. (2011) 70(6):968. doi: 10.1136/ard.2010.143743
38. Bowman SJ, Hamburger J, Richards A, Barry RJ, Rauz S. Patient-reported outcomes in primary Sjögren’s syndrome: comparison of the long and short versions of the profile of fatigue and discomfort—sicca symptoms inventory. Rheumatology. (2008) 48(2):140–3. doi: 10.1093/rheumatology/ken426
39. Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The sleep condition indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open. (2014) 4(3):e004183. doi: 10.1136/bmjopen-2013-004183
40. Amagai S, Pila S, Kaat AJ, Nowinski CJ, Gershon RC. Challenges in participant engagement and retention using Mobile health apps: literature review. J Med Internet Res. (2022) 24(4):e35120. doi: 10.2196/35120
41. Meyerowitz-Katz G, Ravi S, Arnolda L, Feng X, Maberly G, Astell-Burt T. Rates of attrition and dropout in app-based interventions for chronic disease: systematic review and meta-analysis. J Med Internet Res. (2020) 22(9):e20283. doi: 10.2196/20283
42. Torous J, Lipschitz J, Ng M, Firth J. Dropout rates in clinical trials of smartphone apps for depressive symptoms: a systematic review and meta-analysis. J Affect Disord. (2020) 263:413–9. doi: 10.1016/j.jad.2019.11.167
43. Shi SW, Kalyanam K. Touchable apps: exploring the usage of touch features and their impact on engagement. J Interact Mark. (2018) 44(1):43–59. doi: 10.1016/j.intmar.2018.06.001
44. Kushlev K, Proulx J, Dunn EW. “Silence your phones” smartphone notifications increase inattention and hyperactivity symptoms. Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems (2016).
45. Cooper C, Wratten S, Williams-Hall R, Bookman AAM, Ndife B, Hueber W, et al. Qualitative research with patients and physicians to assess content validity and meaningful change on ESSDAI and ESSPRI in Sjögren’s. Rheumatol Ther. (2022) 9(6):1499–515. doi: 10.1007/s40744-022-00487-0
46. Hibbard J, Gilburt H. Supporting People to Manage Their Health: An introduction to Patient Activation. London: The King’s Fund (2014).
47. Fernandez MP, Bron GM, Kache PA, Larson SR, Maus A, Gustafson D Jr, et al. Usability and feasibility of a smartphone app to assess human behavioral factors associated with tick exposure (the tick app): quantitative and qualitative study. JMIR Mhealth Uhealth. (2019) 7(10):e14769. doi: 10.2196/14769
48. Mendiola MF, Kalnicki M, Lindenauer S. Valuable features in Mobile health apps for patients and consumers: content analysis of apps and user ratings. JMIR mHealth UHealth. (2015) 3(2):e40. doi: 10.2196/mhealth.4283
49. Boot WR, Simons DJ, Stothart C, Stutts C. The pervasive problem with placebos in psychology: why active control groups are not sufficient to rule out placebo effects. Perspect Psychol Sci. (2013) 8(4):445–54. doi: 10.1177/1745691613491271
50. Lutz J, Offidani E, Taraboanta L, Lakhan SE, Campellone TR. Appropriate controls for digital therapeutic clinical trials: a narrative review of control conditions in clinical trials of digital therapeutics (DTx) deploying psychosocial, cognitive, or behavioral content. Front Digit Health. (2022) 4:823977. doi: 10.3389/fdgth.2022.823977
Keywords: Sjögren's syndrome, Sjögren disease, smartphone app, feasibility, selfmanagement, tools
Citation: Hackett KL, Campbell M, Pakpahan E, Vines J, Lendrem D, McCready J, Rapley T, Ellis J, Deary V, McColl E and McCallum C (2025) A pragmatic double blind remote pilot feasibility randomised controlled trial of a self-management app for people with Sjögren disease. Front. Digit. Health 7:1549093. doi: 10.3389/fdgth.2025.1549093
Received: 20 December 2024; Accepted: 19 May 2025;
Published: 3 June 2025.
Edited by:
Yang Gong, University of Texas Health Science Center at Houston, United StatesReviewed by:
Kausik Basak, JIS Institute of Advanced Studies and Research, IndiaRegina Reyes, National Institute of Dental and Craniofacial Research (NIH), United States
Copyright: © 2025 Hackett, Campbell, Pakpahan, Vines, Lendrem, McCready, Rapley, Ellis, Deary, McColl and McCallum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katie L. Hackett, a2F0ZS5oYWNrZXR0QG5vcnRodW1icmlhLmFjLnVr
†ORCID:
Katie L. Hackett
orcid.org/0000-0003-0249-9434
Miglena Campbell
orcid.org/0000-0003-0999-944X
Eduwin Pakpahan
orcid.org/0000-0002-0058-1808
John Vines
orcid.org/0000-0003-4051-3356
Dennis Lendrem
orcid.org/0000-0001-6268-5509
Jemma McCready
orcid.org/0000-0002-9997-6956
Tim Rapley
orcid.org/0000-0003-4836-4279
Jason Ellis
orcid.org/0000-0002-8496-520X
Vincent Deary
orcid.org/0000-0001-6115-9259
Elaine McColl
orcid.org/0000-0001-8300-3204
Claire McCallum
orcid.org/0000-0001-7801-1330
 Katie L. Hackett
Katie L. Hackett Miglena Campbell2,†
Miglena Campbell2,† Eduwin Pakpahan
Eduwin Pakpahan Dennis Lendrem
Dennis Lendrem Tim Rapley
Tim Rapley Jason Ellis
Jason Ellis Claire McCallum
Claire McCallum